Abstract
Decades of research have highlighted the importance of optimal stimulation of cortical dopaminergic receptors, particularly the D1R receptor (D1R), for prefrontal-mediated cognition. This mechanism is particularly relevant to the cognitive deficits in schizophrenia, given the abnormalities in cortical dopamine (DA) neurotransmission and in the expression of D1R. Despite the critical need for D1R-based therapeutics, many factors have complicated their development and prevented this important therapeutic target from being adequately interrogated. Challenges include determination of the optimal level of D1R stimulation needed to improve cognitive performance, especially when D1R expression levels, affinity states, DA levels, and the resulting D1R occupancy by DA, are not clearly known in schizophrenia, and may display great interindividual and intraindividual variability related to cognitive states and other physiological variables. These directly affect the selection of the level of stimulation necessary to correct the underlying neurobiology. The optimal mechanism for stimulation is also unknown and could include partial or full agonism, biased agonism, or positive allosteric modulation. Furthermore, the development of D1R targeting drugs has been complicated by complexities in extrapolating from in vitro affinity determinations to in vivo use. Prior D1R-targeted drugs have been unsuccessful due to poor bioavailability, pharmacokinetics, and insufficient target engagement at tolerable doses. Newer drugs have recently become available, and these must be tested in the context of carefully designed paradigms that address methodological challenges. In this paper, we discuss how a better understanding of these challenges has shaped our proposed experimental design for testing a new D1R/D5R partial agonist, PF-06412562, renamed CVL-562.
Keywords: D1 receptor, cognition, schizophrenia, D1 partial agonism
Dopamine-Dependent Prefrontal Cortical Cognition in Schizophrenia
Cognitive deficits are major contributors to the loss of function in patients with schizophrenia (SCZ). While antipsychotic drugs have been largely successful at controlling the positive symptoms of the disorder, we still lack any therapies for the cognitive deficits. Among these, prefrontal cortex (PFC)-mediated cognitive deficits, including poor attention, working memory (WM) and executive function represent major challenges, as they predict poor functioning1–3 (and see Van Snellenberg et al, this volume). Over the years, hypotheses regarding the pathophysiological basis of these symptoms emphasized the role of the dorsolateral prefrontal cortex (DLPFC) and related circuitry4,5 and the role of dopamine (DA). These studies showed that the high activity-associated polymorphism (Val allele) of the DA metabolism enzyme catechol-O-methyltransferase (COMT) gene6 predicts WM deficits, that patients with drug-induced or idiopathic Parkinson’s disease (PD) present deficits on prefrontal cortical tasks7,8 and that monkeys with selective DA lesions in the DLPFC exhibit prefrontal cognitive dysfunction (for reviews, see9,10 and Cho et al, this volume).
In SCZ, postmortem and in vivo functional studies suggest alterations in the cytoarchitecture and function of the PFC (for review, see11). Decreased DA neuron terminals,12 and decreased cerebrospinal fluid homovanillic acid (HVA), a marker for cortical presynaptic DA activity13 have been described. In vivo imaging studies using an amphetamine challenge to examine displacement of a cortically detectable D2 receptor (D2R) radiotracer showed a deficit in cortical and extrastriatal DA release.14 Furthermore, improving the DA deficit with amphetamine or apomorphine administration is associated with improved performance on frontal tasks.15,16 This dopaminergic deficit may exacerbate the PFC inhibitory tuning deficits described in SCZ.17 The main mediators of DA function in the PFC are D1 and D5 receptors (D1Rs/D5Rs). D1Rs are present on spines of distal dendrites of glutamatergic cells, whereas D5Rs are located on the proximal dendrites. Furthermore, D1R and D5R modulate distinct populations of GABAergic interneurons18 and play a role in fine-tuning the inhibition of glutamatergic cells and the overall excitability of PFC. A deficiency in DA could result in suboptimal D1R stimulation of GABAergic interneurons and further deficits in inhibitory tuning. Here we describe the rationale for using a newly developed compound, the PF-06412562, a D1R/D5R partial agonist, to address the cortical DA deficit and suboptimal D1R stimulation as well as the associated deficits in inhibitory tuning of PFC connectivity in early phase patients with SCZ.
The Role of Optimal D1R Stimulation in Mediating Dopamine-Dependent Prefrontal Cortical Cognition
Preclinical studies in rodents and nonhuman primates (NHPs) have highlighted the role of the D1R in cognition by showing an inverted U-shaped curve relating spatial WM performance to D1R stimulation.19–22 Iontophoretic application of D1R antagonists in the DLPFC impairs WM performance in monkeys.19 In aged monkeys and in catecholamine-depleted monkeys, infusion of the full D1R agonists A77636 and SKF81297 partially reverses deficits in spatial WM.20,23 Amy Arnsten’s work24,25 clarified the molecular and circuitry basis of this inverted U by showing that varying degrees of D1R stimulation have differential effects on the firing of prefrontal cortical “delay” cells in layer III of the DLPFC. The persistent firing of these cells during the delay component of a spatial WM task maintains the representation of the object of interest. In the absence of D1R stimulation, delay cells exhibit little firing. Low levels of D1R stimulation can be excitatory, resulting in phosphorylation of NMDA (N-methyl-d-aspartate) receptors and their trafficking into the synapse, producing noisy firing. With more optimal stimulation, hyperpolarization-activated cyclic nucleotide–gated (HCN) potassium channels located near D1R on dendritic spines of pyramidal cells open, to suppress irrelevant inputs, while NMDA receptors in the synaptic membrane maintain and strengthen relevant connections. These combined effects gate out “noise,” sharpening the signal. At higher DA concentrations, as during stress, excess D1R stimulation causes excessive HCN channel opening, nonspecific suppression of delay cell firing, and impairment of WM. In addition, D1Rs modulate inhibitory interneurons, which can further sharpen the signal-to-noise ratio in prefrontal cortical function. Specific models have been proposed to explain how activation of these receptors might play a role in flexibility and persistent activity needed for adequate cognitive performance26,27 by regulating the balance between excitation and inhibition in the PFC. Furthermore, these effects are likely to take place during development and may especially become prominent during the transition to puberty, by enhancing the activity of GABAergic interneurons, in addition to stimulating pyramidal cells, a process that is deficient in certain animal models of SCZ.28,29 Another effect of D1R stimulation is to reinforce signaling in the D1R expressing medium spiny neurons or Go pathway across the cortico-basal ganglia thalamocortical loops. This facilitation may affect processes across different functional domains, but the exact effect on symptoms remains speculative at this point.
The Challenges of Targeting Optimal D1R Stimulation in Schizophrenia
Despite the clinical and preclinical lines of evidence supporting a role for optimal stimulation of D1R by DA for prefrontal cortical function in SCZ, developing D1R-based therapeutics has been challenging for many reasons related to the target as well as the therapeutic intervention. We will discuss these here, starting with the fundamental challenge of interindividual variation in the level of D1R stimulation. This depends on multiple variables, including baseline prefrontal DA availability, determined by levels of storage, release, and clearance, baseline levels of D1R, their affinity for DA, and resulting occupancy by DA, their potential interactions with other partners in the membrane such as NMDA receptors, and other intracellular factors that may affect their trafficking and signaling, as well as broader indirect effects on the underlying microcircuitry that determine the downstream result of their stimulation, including availability of D2R and their contribution to the balance of prefrontal cortical firing levels. Another challenge is the inherent complexity of the dynamics of DA release during cognitive tasks. Reproducing the dynamic range of stimulation needed for cognitive performance with a pharmacological intervention is a challenge. Furthermore, developing safe and brain penetrant D1R drugs that provide the proper amount of target engagement, if this level can be estimated, without causing peripheral side effects, has not been possible until recently.
Challenge 1: Prefrontal Cortical D1R Is Not a Static Target
The PFC receives dopaminergic innervation from nuclei within the ventral tegmental area (VTA) and parts of the dorsal substantia nigra (SN) pars compacta (SNc).30 “Dorsal tier” DA neurons, a band along the SNc and contiguous regions of the VTA and retrorubral field (RRF), project to cerebral cortex, as well as ventromedial striatum, pallidum, amygdala, extended amygdala, and thalamus. The ventral tier DA cells, including the densocellular region of the SNc and DA cell columns within the pars reticulata (SNr), project to the dorsal striatum.31–33 These DA neurons have different intrinsic properties and afferents regulating spike activity, synthesis, release or reuptake of DA, and postsynaptic effects.31–34 DA neurons that project to the PFC receive direct excitatory inputs from the PFC neurons they selectively synapse onto, suggesting that cortical pathology could directly affect the function of the DA mesocortical pathway.35
In light of the postmortem report of decreased prefrontal DA innervation, and abnormal HVA levels, as well as the importance of DA tone to WM performance, we used in vivo imaging with positron emission tomography (PET) to examine the levels of prefrontal DA storage and release, levels of D1R, and other DAergic parameters. For prefrontal DA studies, we used [11C]FLB457, a high-affinity radiotracer for the D2R, combined with amphetamine challenge, to measure in vivo DA release in the cortex and other extrastriatal regions in patients with SCZ compared to healthy controls (HC). These measures are made by comparing the level of binding of the radiotracer to D2R before and after a pharmacological challenge that results in increased perisynaptic levels of DA. The difference in radiotracer binding relates to the magnitude of amphetamine-induced DA release.36 We showed significant blunting of DA release throughout the cortex in SCZ. DA release in the DLPFC was significantly positively associated with WM-related BOLD activation, suggesting a relationship between blunted release and deficits of frontal cortical function.14 Another report using the same tracer combined with a stress test to elicit DA release also showed blunted release in cortex in patients compared to controls.37 [18F]DOPA was also used to assess synthesis and storage capacity in extrastriatal regions38–41 but these reports of [18F]DOPA in the cortex are not interpretable due to low signal.42
D2R availability in SCZ was shown to be normal in prefrontal,14,43–45 occipital,14,44 parietal,14,44 entorhinal,46 anterior cingulate14,43,47 (except for44), and insular14,46 cortices. A meta-analysis (excluding14) found no differences in the temporal cortex.48 One study reported lower binding in uncus47 while another did not.14
Studies of prefrontal cortical D1R availability in SCZ yielded inconsistent results of increases,49–51 decreases,52 or no change,53 as summarized previously.48 Using [11C]NNC112, a tracer with higher cortical signal-to-noise ratio, we reported an increase in DLPFC D1R associated with severity of WM impairment.49 We postulated that the increase is related to compensatory upregulation in response to chronic deficits in DA tone as observed in DA-depleted rats54 and across COMT genotypes in healthy humans.55 In a second SCZ cohort, we observed higher D1R levels only in antipsychotic naïve patients, but not in antipsychotic free previously medicated patients.50 Furthermore, the duration of antipsychotic free interval positively correlated with higher binding in previously treated patients. Studies in NHP indicated a clear downregulation of D1R in the cortex after a few months of exposure to D2R antagonists.56 In order to reconcile the discrepancies across radiotracers, we studied a small set of subjects with both [11C]NNC112 and [11C]SCH2339057,58 and showed that the direction of difference between patients and controls was independent of the tracer used, suggesting that the discrepancies in the literature could be related to cohort effects. While both tracers bind to cortical 5HT2A receptors in vivo,59,60 this lack of selectivity for D1R did not explain the discrepancies in results in SCZ across tracers. Taken together these findings suggest that D1R levels are upregulated in SCZ, and that the upregulation is related to the illness itself and may be normalized by chronic antipsychotic administration. Thus, antipsychotic exposure and chronicity could explain the discrepancies across studies.
In summary, extensive imaging data suggest that D1Rs in the cortex in SCZ are understimulated due to a deficit in DA storage capacity and release, and that they may upregulate in expression in response to lack of stimulation, and can be normalized, at least in expression levels, by antipsychotic exposure. These PET findings have implications for the design of therapeutic interventions targeting the D1R receptor in SCZ, as they suggest changes in expression during the course of treatment. Thus, D1R interventions may need to involve different strategies in first-episode patients compared to chronic patients, and adopting the same approach in both sets of patients in initial proof of concept studies may be counterproductive. The data suggest that in early phase of the illness D1R may be more sensitive to stimulation due to higher expression. This effect may decrease with treatment over time. In addition, D1R expression decreases with age.61 For these reasons, targeting the early phase of the disease may provide a more favorable therapeutic window for cognitive-enhancing effects than later stages of the disease.
Challenge 2: Partial Agonism or Allosteric Modulation?
Of the 5 DA receptor subtypes, D1R and D5R are both positively coupled to cAMP and have high homology, whereas D2R, D3R, and D4R decrease intracellular cAMP and differ structurally from D1R and D5R. Relative to D1R, D5R is expressed at much lower levels in the human and rodent brains.62 To date, no ligands with significant selectivity for D1R vs D5R have emerged, and thus imaging studies and pharmacological studies measure contributions of both receptors. The distinct biological roles of D5R continue to be difficult to study in vivo, and insights are either at the expression level or are derived from studying the phenotype of knockout or knockdown animals.63,64 The results of these genetic modifications suggest potential differential roles,65,66 but owing to the well-established limitations of such studies, including developmental compensations, their relevance for guiding therapeutics remains an open question.
Beyond the classical concepts of receptor agonism and antagonism, there is now appreciation for more nuanced forms of G protein-coupled receptor (GPCR) activation. A D1R partial agonist elicits less than complete activation of D1R receptor signaling cascades, even at full occupancy.67,68 Endogenous tone of DA is estimated to result in modest to low tonic activation of D1R signaling in healthy individuals.69,70 This is particularly true in the cortex where high receptor reserve and low D1R occupancy are expected, consistent with the low doses of D1R agonists that have pro-cognitive effects. Given the specifics of D1R microcircuitry and the desire to avoid over-activation and the descending arm of the Yerkes-Dodson inverted U (see Cho et al, this volume), a partial agonist approach may be particularly appropriate. An additional nuance relates to biased agonism or functional selectivity, through which agonists acting at the same receptor can differentially activate downstream effectors, resulting in divergent functional effects.67,68,71 While functional selectivity can lead to differential activation of G protein isoforms, it can also manifest as differential G protein activation relative to arrestin recruitment and arrestin-dependent signaling.72,73 Certain consequences of D1R activation such as rapid D1R tolerance74–76 have been linked to specific signaling cascades; therefore, it is reasonable to consider that functionally selective D1R ligands could represent a strategy to increase the therapeutic margin between desired effects on cognitive or motor function and problems such as tolerance or other adverse effects. Further progress in this area will, however, require a better understanding of the signaling pathways linked to therapeutic actions as opposed to side effects.
An alternative approach is modulation of D1R signaling via D1R-selective positive allosteric modulators (PAMs). Augmented signaling via a PAM is dependent on the release of endogenous DA. While a theoretical advantage of this approach is being able to enhance the effect of endogenous DA released in a normal temporal pattern associated with behavior rather than tonic D1R activation, one potential disadvantage is lack of effect due to deficits in endogenous DA release in extrastriatal regions. D1R PAM’s from Eli Lilly (LY3154207) and Astellas (ASP4345) have both entered the clinical study.77,78 Both compounds demonstrate pharmacodynamic activity on laboratory endpoints in phase 1 studies79 and are now the focus of phase 2 studies examining impact on cognition in Parkinson’s dementia and SCZ.
Historically, one view, now discredited, that emerged from early preclinical work postulated that D1R antagonism might be useful in SCZ.80,81 The D1R antagonist ecopipam was tested in SCZ,63 obesity,82 and drug abuse.83–85 Adverse or undesired effects including cognitive deficits and amotivational states were prevalent in these studies. These results spurred even greater interest in potentiating D1R signaling as a therapeutic strategy.
Challenge 3: Pharmacodynamic Profile of First-Generation D1R Agonists
Dihydrexidine was one of the first compounds to display good D1R agonist potency and efficacy, as well as some selectivity over D2R, marking an important and early discovery, along with a related variant discovered by chemists at SK&F86–88). Dihydrexidine and other selective D1R agonist compounds from this era were active in preclinical disease models. By the late 1980s, 4 selective D1R agonists were advanced into small phase 1 clinical studies,89–93 yielding a mix of tantalizing clinical observations and frustrating technical limitations. DA is known for poor oral bioavailability and rapid metabolism in the blood, and each of these compounds was derived from DA and retained its common catecholamine structural motif. Despite structural variations across the compounds and even efforts to develop prodrugs, nasal formulations, and chiral variants, they all ultimately suffered from clinical limitations rooted in their common DA core.94,95 To bypass low oral bioavailability, studies employed i.v. or subcutaneous formulations, but their rapid clearance greatly limited the time window for therapeutically effective exposure. Full agonist activation of D1Rs in the renal vasculature drives vasodilatory action, leading to hypotension.96,97 Thus, achieving sufficient activation of D1R in the brain without causing large peripherally mediated changes in blood pressure presented immense technical challenges.
Nevertheless, dihydrexidine (DAR-0100) or its active enantiomer (DAR-0100A) were used in several studies in SCZ spectrum disorders. George et al gave a single subcutaneous dose of DAR-0100 to 20 individuals with stable SCZ, immediately measured BOLD fMRI during WM performance, and observed significant enhancement to prefrontal (and non-prefrontal) perfusion compared to placebo.98 Administration of DAR-0100A subcutaneously to 16 unmedicated individuals with diagnosed schizotypal personality disorder resulted in fairly large and statistically significant improvement on the Paced Auditory Serial Addition Test (PASAT) task and also on the N-back, though some caveats in the small dataset were noted for the latter task.99,100 A trial of DAR-0100A in patients with SCZ101 used doses of 0.5 mg, 15 mg, or placebo in antipsychotic-treated SCZ over 5 consecutive days followed by 9 days without treatment, and an additional 5 days of treatment. Study drug was administered as a 30 min. i.v. infusion, due to its poor oral bioavailability and rapid peripheral clearance. Subjects were tested for cognitive effects 5, 15, and 90 days from the start of treatment. Assessments included WM tasks performed during fMRI scanning, the NIMH MATRICS102 and the Cogstate Schizophrenia Battery.103 The fMRI results and many of the cognitive measures failed to show an effect of treatment, although there were modest improvements in attention and some aspects of WM on the MATRICS and Cogstate. A primary limitation of generalizing these findings was the restriction to doses attaining very low receptor occupancy due to the pharmacokinetic properties and side effect profile of DAR-0100A.
New Generation D1R Partial Agonists
Recently, important breakthroughs yielded new direct D1R agonists and D1R PAMs, offering new opportunities for D1R agonist therapeutic research. A targeted discovery program at Pfizer identified a novel chemical scaffold with functional and selective D1R agonist pharmacology without catecholamine or ergot structural motifs.104 Initial screening led to high-quality compounds designed to have favorable pharmacokinetics and avoid significant blood pressure effects. The novel compounds were optimized to be partial agonists of D1R-mediated G protein activation of the cAMP signaling pathway but to produce less recruitment of β-arrestin and receptor desensitization pathways. In a comprehensive series of studies, the Pfizer team confirmed that PF-6142, a prototypical non-catechol D1R agonist, has a similar acute behavioral efficacy profile to prior D1R agonists, including a pro-cognitive profile in rodents through reversal of the disruptive effects of the NMDA receptor antagonist MK-801 on paired-pulse facilitation.105–107 Consistent with the lack of psychosis-related side effects in studies of D1R agonists to date, PF-6142 had no impact on the efficacy of risperidone in the mouse paired-pulse facilitation or rat conditioned avoidance response models. Like A77636 and other prior D1R full agonists, PF-6142 reduced hallucinatory behaviors and reversed the ketamine disruption of the spatial delayed response (SDR) task performance at extremely low doses.106 In line with numerous preclinical and prior clinical studies demonstrating D1R agonist-driven reduction in PD motor symptoms, a compound from this series demonstrated extremely robust efficacy in a MPTP model of PD.107 Efficacy was maintained for many hours after dosing and over 3 days of consecutive dosing. This is in contrast to short duration of effect and tachyphylaxis observed with catechol-based D1R agonists76 and suggests that the combination of improved pharmacokinetics, reduced receptor desensitization, and partial agonism effectively overcomes a potential loss of efficacy with extended dosing.
Wang et al showed that iontophoretic application of the moderately potent non-catechol D1R agonist, PF-3628, produced an inverted U dose-response curve on the firing of DLPFC delay cells in aged monkeys, increasing persistent firing at low to moderate doses, with reduced efficacy at higher doses. The excitatory effects of PF-3628 were reversed by the D1R antagonist, SCH23390, consistent with drug actions at the D1R family of receptors (D1R/D5R).108
Overall, the relevant preclinical profile of these new selective D1R ligands is fully consistent with that of prior compounds that have established the therapeutic potential of this target for treating cognitive deficits in SCZ.
Clinical Studies With the Novel D1R Partial Agonists
In 2018, Papapetropoulos et al reported results from a clinical study with the D1R selective partial agonist PF-0641256 in individuals with PD showing a good pharmacokinetic profile and statistically significant improvement on motor scores.109 Following this study, 1 of 2 dose levels of PF-06412562 or placebo was given for 5–7 days to 77 healthy individuals identified based on low performance on a WM task. During the dosing period, the participants completed a range of cognitive assessments with and without fMRI imaging. The drug was safe and well-tolerated, but no drug-related improvements in task performance were identified. Overall, results from the cognitive and motivation-related endpoints were variable.110 Arce et al reported a conceptually related study conducted in 95 individuals with SCZ who were on stable antipsychotic therapy. In this study, 1 of 3 doses of PF-06412562 or placebo was given orally over 15 days and participants completed assessments of cognition and motivation as well as functional imaging. The drug was safe and well-tolerated but did not show any benefits over placebo on any assessment, nor any statistically significant changes on the prespecified fMRI analysis.111 The authors noted a number of caveats with the experimental methodology used in the study, as well as potential post-study impact of treatment on the MATRICS that warrants follow-up. Another study in healthy volunteers with low capacity for WM showed minimal improvements in WM across all groups including placebo.110
In order to study the role of varying levels of D1R activation on goal- and risk-based decision making, Soutschek et al used several doses of PF-06412562 and placebo in a double-blind study of 120 healthy young volunteers.112,113 The data suggest that D1R activation increased the willingness to exert physical effort for reward and a reduced preference for risky outcomes. Importantly, this study also identified baseline-dependent impact of D1R activation on Pavlovian-to-instrumental transfer and on reversal learning. Specifically, higher doses of PF-06412562 improved reversal learning only in individuals with low baseline WM functioning. See table 1 for a summary of D1R agonist trials in SCZ spectrum disorders.
Table 1.
D1R Agonist Trials in Schizophrenia Spectrum Disorders
| Publication | Year | D1R Agonist | Design | Sample Size | Primary Outcome Measure | Results |
|---|---|---|---|---|---|---|
| Arce et al111 | 2019 | PF-06412562 | Placebo, 3 mg, 9 mg, and 45 mg twice daily, 15 days add on | N = 95, SCZ | MATRICS | Improvement in all groups including placebo |
| Girgis et al101 | 2016 | DAR-0100A | Placebo, 0.5 mg, 15 mg subacute | N = 49, SCZ | MATRICS, N-back | No group differences |
| Rosell et al99 | 2015 | DAR-0100A | Placebo, 15 mg, 3 days | N = 16, SPD | Working memory tasks | Improvement |
| George et al98 | 2007 | DAR-0100 | Placebo, 20 mg single dose | N = 20 SCZ | Prefrontal perfusion | Increased perfusion |
| Davidson et al114 | 1990 | SKF 38393 | Placebo, 250 mg twice a day 1 month, add on to haldol | N = 10 SCZ | BPRS, WCST, AIMS | Mixed results |
Note: AIMS, Autonomic Involuntary Movement Scale; BPRS, Brief Psychiatric Rating Scale; SCZ, schizophrenia; SPD, schizotypal personality disorder, MATRICS cognitive battery; WCST, Wisconsin Card Sort Task.
Taken together, the data showing low- and post-dose effects and the existence of inverted U phenomenon in vivo clearly indicate the importance of the underlying dopaminergic state for determining dose-response, and suggest that maximizing pro-cognitive or pro-motivational effects of D1R stimulation requires individual- or disease-state-specific dosing. We are currently testing 4 doses of this drug, now known as CVL-562, and a placebo, in an acute challenge paradigm, to examine the effects on the DLPFC and its computationally modeled microcircuitry during performance of a spatial WM task, in patients with SCZ who are in the first 5 years of the illness. Patients can be either drug-free or on stable doses of antipsychotics without substantial D1R affinity during the study.
Measuring In Vivo Occupancy of a D1R Agonist Drug
For successful development and testing of novel drugs, PET imaging is necessary to probe receptor occupancy to demonstrate target engagement and, ideally, a dose-occupancy relationship, in vivo. However, in vivo imaging of D1R occupancy by an agonist presents many challenges. The PET radiotracers that have been used to date to measure D1R in vivo, [11C]SCH23390 and [11C]NNC112, are antagonists at D1R. An agonist tracer, [18F]MNI-968 has recently been developed,115 but has not been widely disseminated or broadly characterized. While it is relatively straightforward to measure competition between unlabeled antagonists and antagonist radiotracers,116 measurement of receptor occupancy by unlabeled agonists with antagonist tracers presents several challenges. Theoretical considerations and experimental evidence suggest that receptors will be configured in high- and low-affinity states for agonists, according to whether they are coupled to G proteins or not,117 whereas the antagonist tracer will be unaffected by the agonist affinity state and have similar affinity for all receptors. The result will be that the agonist will only compete effectively at a subset of the receptors to which the tracer binds, leading to apparent occupancy that is lower than would be seen if the tracer and competitor were competing for the same pool of receptors, ie, if the tracer were an agonist.118 While there is some controversy as to whether the multiple affinity states observed in ligand binding experiments to guanosine-5’-triphosphate (GTP)-depleted brain membranes would translate to detectable effects in the in vivo setting with endogenous GTP,119,120 it has been observed, eg, that endogenous DA, released via pharmacological stimulation, causes more displacement of D2R/D3R agonist radiotracers than of antagonist tracers.121,122 An additional concern is that agonists may induce receptor trafficking, and radiotracer affinity for internalized receptors may be different than for surface-bound receptors,123 although this will be tracer dependent. Finally, even at low concentrations, agonists may produce undesirable pharmacological effects, limiting the dose range.124
We performed PET imaging in anesthetized NHP to test D1R occupancy by DAR-0100A,97,125 which has functional efficacy comparable to that of DA,126 using the radiotracer [11C]NNC112, to inform testing of DAR-0100A in humans. Quantification of the tracer in the cortex is confounded by binding to 5-HT2A receptors,59,60 but the signal in the striatum, where occupancy was assessed, is exclusively due to D1R binding, as striatal 5-HT2AR levels are very low. DAR-0100A was administered as an i.v. infusion, in doses ranging from 1.5 mg/kg to 9 mg/kg. The maximum dose was limited, as systemically administered DAR-0100A lowered blood pressure; reductions by as much as 40% were observed at the higher doses. Measured D1R receptor occupancy was 35% at the highest doses. This dose-limiting side effect, also observed in preliminary human data, constrained the maximal dose in the human trial to 15 mg.101 Extrapolation of the concentration-occupancy curve from the NHP study to humans suggested that this dose would lead to D1R occupancy <1%, thus severely limiting the range of receptor occupancy over which the cognitive effects of DAR-0100A could be tested. This illustrates the importance of linking dose testing to D1R occupancy studies in order to interpret behavioral effects and design future studies.
Dosing Strategies for D1R Agonist Trials
The optimal administration paradigm for a D1R agonist remains to be determined. Various administration protocols have been tried across the trials described earlier. Chronic intermittent administration of the D1R agonist ABT-431a at very low doses in a NHP model of cognitive deficits induced by chronic administration of haloperidol has been tested.127 In this study, Castner et al demonstrated that long-term reduction of the deficits could be achieved by sensitizing D1R with very low and repeated doses. In the clinical trial of PF-06412562, administered daily for 15 days to patients with SCZ, one intriguing observation was the improvement over placebo noted for the highest dose 10 days after withdrawal of the drug. This improvement was not present earlier after acute dosing of the drug.111 Another shorter regimen of repeated administration of DAR-0100A, described earlier, was not successful in humans,101 Because these chronic administration regimens involved different drugs and different duration and repetition, it is difficult to determine the best paradigm at this point. For these reasons we opted in our current trial with PF-06412562 for acute dosing, to be followed up with chronic administration in a future trial if our primary outcome measure of DLPFC and its computationally modeled microcircuitry during spatial WM and fMRI shows a dose effect.
Conclusions
We have reprised the rationale and summarized the historical challenges in developing D1R targeted therapeutics and applying them to SCZ. These relate to the dynamic complexity of the target itself, the difficulty in knowing baseline DA function in patients, the difficulty in developing appropriate pharmaceuticals, and the complexity in selecting a target outcome measure to probe the results. This latter is described in more detail by Van Snellenberg et al (this issue). However, with the benefit of a clinically viable D1R selective compound, we have now devised an approach that we believe will allow us an informative testing of this mechanism. Our study with the D1R partial agonist, CVL-562, testing 4 doses and a placebo, in an acute challenge paradigm, in patients with SCZ who are in the first 5 years of the illness will provide a dose-response relationship at low to moderate receptor occupancy that can be used as proof of concept for further development and testing in subacute or chronic administration paradigms (figure 1).
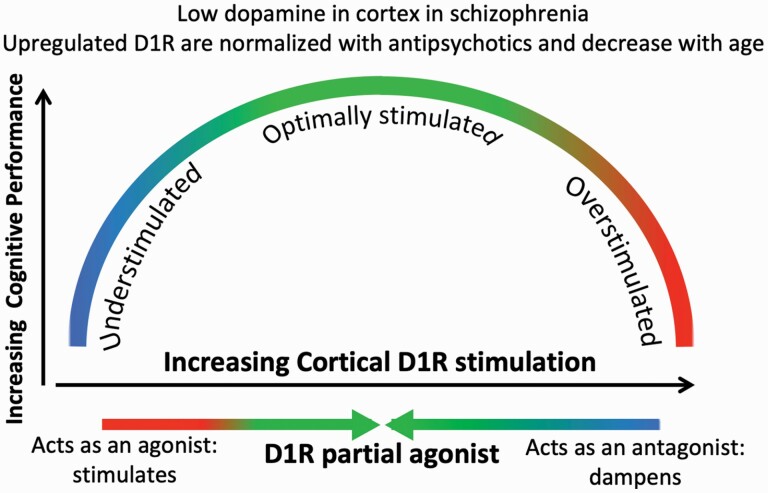
Fig. 1.
Conceptual representation of the role of cortical D1R stimulation on cognitive performance and the hypothesized effect of a D1R partial agonist in SCZ. The partial agonist will increase cortical D1R stimulation in DA-deficient states (left arm of inverted U) but decrease excess stimulation (right arm of inverted U) by occupying D1R and reducing access by endogenous DA. Note: DA, dopamine; SCZ, schizophrenia.
Currently, in addition to our test of the Pfizer/Cerevel D1R/D5R partial agonist in acute phase SCZ, Eli Lilly and Astellas are testing D1R PAMs in SCZ. It remains unclear which D1R augmentation approach might be more favorable for cognitive enhancement in SCZ: the typically more potent circuitry activation and independence from endogenous tone requirement of a direct agonist, or the more dynamic state-dependent enhancement of endogenous DA activation by a PAM. Nevertheless, these novel therapeutic developments offer hope for new therapeutics in SCZ, to address the long-standing cognitive challenges that prevent patients from resuming normal lives. In particular, our design, presented over the next few papers, builds on a vast knowledge of the complex biological and circuitry effects of the target, the underlying biology and circuitry in the disease, and a sophisticated use of cognitive testing and neuroinformatics, to optimize the detection of a signal from a wide range of D1R occupancy and stimulation.
TRANSCENDS Group Members
Yale University School of Medicine: Deepak D’Souza, Vinod Srihari, Ralitza Gueorguieva, Prashant Patel, Kimberlee Forselius-Bielen, Jing Lu, Audrey Butler, Geena Fram, Yvette Afriyie-Agyemang, Alexandria Selloni, Laura Cadavid, Sandra Gomez-Luna, Aarti Gupta, Rajiv Radhakrishnan, Ali Rashid, Ryan Aker, Philisha Abrahim, Anahita Bassir Nia, Toral Surti. Columbia University, College of Physicians and Surgeons/New York State Psychiatric Institute: Lawrence S. Kegeles, Marlene Carlson, Terry Goldberg, James Gangwisch, Erinne Benedict, Preetika Govil, Stephanie Brazis, Megan Mayer, Nathalie de la Garrigue. Stony Brook University Renaissance School of Medicine: Natalka Fallon, Topaz Baumvoll, Sameera Abeykoon, Greg Perlman, Kelly Bobchin. University of Pennsylvania, Perelman School of Medicine: Mark Elliott, Lyndsay Schmidt, Sage Rush, Allison Port, Zac Heffernan, Nina Laney, Jenna Kantor, Thomas Hohing.
Acknowledgments
Anissa Abi-Dargham: Consultant to Sunovion, Otsuka, Merck, Neurocrine. Stock options in Systems 1 Bio and in Terran Biosciences. Mark Slifstein: Consultant to Neurocrine Bioscience, Inc. Consultant to Curasen Therapeutics, Inc. Alan Anticevic: Technology Advisory Board Member for RBNC Therapeutics; Co-founder Manifest Sciences. Clara Fontaineau: Consult for RBNC Therapeutics. Ragy Girgis: Within the last 3 years, consulting work for IMS Expert Services, Noble Insights, and Fowler White Burnett, as well as royalties from Wipf and Stock Book, and Routledge/Taylor. Joshua Kantrowitz: Received consulting payments within the last 24 months from AlphaSights, Charles River Associates, Medscape, Putnam, techspert.io, Third Bridge, MEDACorp, Parexel, GroupH, Simon Kucher, ECRI Institute, ExpertConnect, Parexel, Schlesinger Group, CelloHealth, Acsel Health, Strafluence, Guidepoint, L.E.K. and System Analytic. He serves on the MedinCell Psychiatry and Karuna Mechanism of Action (MOA) Advisory Boards. He has conducted clinical research supported by the NIMH, Sunovion, Roche, Alkermes, Cerevance, Corcept, Takeda, Taisho, Lundbeck, Boehringer Ingelheim, NeuroRX, and Teva within the last 24 months. Dr. Kantrowitz was a co-investigator on a study that receives lumateperone and reimbursement for safety testing for an investigator-initiated research from Intra-Cellular Therapies Inc. He owns a small number of shares of common stock from GSK. John Krystal: Has received consulting payments from AstraZeneca Pharmaceuticals, Biogen, Biomedisyn Corporation, Bionomics, Boehringer Ingelheim International, COMPASS Pathways, Concert Pharmaceuticals, Epiodyne, EpiVario, Heptares Therapeutics, Janssen Research & Development, Otsuka America Pharmaceutical, Perception Neuroscience Holdings, Spring Care, Sunovion Pharmaceuticals, Takeda Industries, and Taisho Pharmaceutical. He has served on advisory boards for Bioasis Technologies, Biohaven Pharmaceuticals, BioXcel Therapeutics, BlackThorn Therapeutics, Cadent Therapeutics, Cerevel Therapeutics, EpiVario, Eisai, Lohocla Research Corporation, Novartis Pharmaceuticals Corporation, and PsychoGenics. He is a co-sponsor of a patent for the intranasal administration of ketamine for the treatment of depression and for the treatment of suicide risk that was licensed by Janssen Pharmaceuticals; has a patent related to the use of riluzole to treat anxiety disorders that was licensed by Biohaven Pharmaceuticals; has stock or stock options in Biohaven Pharmaceuticals, Blackthorn Therapeutics, Luc Therapeutics, Cadent Pharmaceuticals, Terran Biosciences, Spring Healthcare, and Sage Pharmaceuticals. He serves on the Board of Directors of Inheris Pharmaceuticals. He receives compensation for serving as editor of the journal Biological Psychiatry. John Murray: Technology Advisory Board Member for RBNC Therapeutics; Co-founder of Manifest Sciences. Zailyn Tamayo: Consult for RBNC Therapeutics. David Gray: Employee and shareholder of Cerevel Therapeutics (which owns rights to CVL-562 and has other D1 agonists in development). Jeffrey Lieberman: Dr. Lieberman neither accepts nor receives any personal financial remuneration for consulting, speaking, or research activities from any pharmaceutical, biotechnology, or medical device companies. He receives support administered through Columbia University and the Research Foundation for Mental Hygiene in the form of funding and medication supplies for investigator-initiated research from Denovo, Taisho, Sunovion, and Genentech, and for company-sponsored phase II, III, and IV studies from Alkermes, Allergan and Boehringer Ingelheim. However, none of this research support contributes to his institutional compensation. He is a consultant to or member of the advisory board of Intracellular Therapies, Pear Therapeutics, and Gilgamesh Therapeutics for which he receives no remuneration. He is a paid consultant for Signant, a clinical research services organization, and holds a patent from Repligen that neither has nor currently yields any royalties. The other authors declare no conflict of interest.
Contributor Information
TRANSCENDS Group:
Deepak D’Souza, Vinod Srihari, Ralitza Gueorguieva, Prashant Patel, Kimberlee Forselius-Bielen, Jing Lu, Audrey Butler, Geena Fram, Yvette Afriyie-Agyemang, Alexandria Selloni, Laura Cadavid, Sandra Gomez-Luna, Aarti Gupta, Rajiv Radhakrishnan, Ali Rashid, Ryan Aker, Philisha Abrahim, Anahita Bassir Nia, Toral Surti, Lawrence S Kegeles, Marlene Carlson, Terry Goldberg, James Gangwisch, Erinne Benedict, Preetika Govil, Stephanie Brazis, Megan Mayer, Nathalie de la Garrigue, Natalka Fallon, Topaz Baumvoll, Sameera Abeykoon, Greg Perlman, Kelly Bobchin, Mark Elliott, Lyndsay Schmidt, Sage Rush, Allison Port, Zac Heffernan, Nina Laney, Jenna Kantor, and Thomas Hohing
Funding
National Institute of Mental Health [1U01MH121766-01 to J.K. and A.A (MPI)], 09/01/19 – 08/31/22.
References
- 1. Saykin AJ, Shtasel DL, Gur RE, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51(2):124–131. [DOI] [PubMed] [Google Scholar]
- 2. Elvevåg B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14(1):1–21. [PubMed] [Google Scholar]
- 3. Riley EM, McGovern D, Mockler D, et al. Neuropsychological functioning in first-episode psychosis – evidence of specific deficits. Schizophr Res. 2000;43(1):47–55. [DOI] [PubMed] [Google Scholar]
- 4. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. [DOI] [PubMed] [Google Scholar]
- 5. Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43(2):114–124. [DOI] [PubMed] [Google Scholar]
- 6. Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowen F, Kamienny R, Burn M, Yahr N. Parkinsonism: effect of levodopa treatment on concept formation. Neurology 1975;25:701–704. [DOI] [PubMed] [Google Scholar]
- 8. Stern Y, Langston JW. Intellectual changes in patients with MPTP-induced parkinsonism. Neurology. 1985;35(10):1506–1509. [DOI] [PubMed] [Google Scholar]
- 9. Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull. 1997;23(3):437–458. [DOI] [PubMed] [Google Scholar]
- 10. Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205(4409):929–932. [DOI] [PubMed] [Google Scholar]
- 11. Weinberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1495–1503. [DOI] [PubMed] [Google Scholar]
- 12. Akil M, Pierri JN, Whitehead RE, et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156(10):1580–1589. [DOI] [PubMed] [Google Scholar]
- 13. Weinberger DR, Berman KF, Illowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. III. A new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry. 1988;45(7):609–615. [DOI] [PubMed] [Google Scholar]
- 14. Slifstein M, van de Giessen E, Van Snellenberg J, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72(4):316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daniel DG, Weinberger DR, Jones DW, et al. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci. 1991;11(7):1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RS, Grasby PM. Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature. 1995;378(6553):180–182. [DOI] [PubMed] [Google Scholar]
- 17. Krystal JH, Anticevic A, Yang GJ, et al. Impaired tuning of neural ensembles and the pathophysiology of schizophrenia: a translational and computational neuroscience perspective. Biol Psychiatry. 2017;81(10):874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glausier JR, Khan ZU, Muly EC. Dopamine D1 and D5 receptors are localized to discrete populations of interneurons in primate prefrontal cortex. Cereb Cortex. 2009;19(8):1820–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71(2):515–528. [DOI] [PubMed] [Google Scholar]
- 20. Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl). 1994;116(2):143–151. [DOI] [PubMed] [Google Scholar]
- 21. Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376(6541):572–575. [DOI] [PubMed] [Google Scholar]
- 22. Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17(21):8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnt J, Hyttel J, Sánchez C. Partial and full dopamine D1 receptor agonists in mice and rats: relation between behavioural effects and stimulation of adenylate cyclase activity in vitro. Eur J Pharmacol. 1992;213(2):259–267. [DOI] [PubMed] [Google Scholar]
- 24. Arnsten AF, Jin LE. Molecular influences on working memory circuits in dorsolateral prefrontal cortex. Prog Mol Biol Transl Sci. 2014;122:211–231. [DOI] [PubMed] [Google Scholar]
- 25. Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76(1):223–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21(10):3628–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74(1):1–58. [DOI] [PubMed] [Google Scholar]
- 28. O’Donnell P. Cortical disinhibition in the neonatal ventral hippocampal lesion model of schizophrenia: new vistas on possible therapeutic approaches. Pharmacol Ther. 2012;133(1):19–25. [DOI] [PubMed] [Google Scholar]
- 29. O’Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37(3):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8(4):321–345. [DOI] [PubMed] [Google Scholar]
- 31. Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96(3):451–474. [DOI] [PubMed] [Google Scholar]
- 33. Bentivoglio M, Morelli M. The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. In: Dunnett SB, Bentivoglio M, Björklund A, Hökfelt T, eds. Handbook of Chemical Neuroanatomy. Vol. 21. Amsterdam: Elsevier. 2005:1–107. [Google Scholar]
- 34. Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav. 2002;77(4–5):513–517. [DOI] [PubMed] [Google Scholar]
- 36. Narendran R, Jedema HP, Lopresti BJ, et al. Imaging dopamine transmission in the frontal cortex: a simultaneous microdialysis and [11C]FLB 457 PET study. Mol Psychiatry. 2014;19(3):302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schifani C, Tseng HH, Kenk M, et al. Cortical stress regulation is disrupted in schizophrenia but not in clinical high risk for psychosis. Brain. 2018;141(7):2213–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elkashef AM, Doudet D, Bryant T, Cohen RM, Li SH, Wyatt RJ. 6-(18)F-DOPA PET study in patients with schizophrenia. Positron emission tomography. Psychiatry Res. 2000;100(1):1–11. [DOI] [PubMed] [Google Scholar]
- 39. Kumakura Y, Cumming P, Vernaleken I, et al. Elevated [18F]fluorodopamine turnover in brain of patients with schizophrenia: an [18F]fluorodopa/positron emission tomography study. J Neurosci. 2007;27(30):8080–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lindström LH, Gefvert O, Hagberg G, et al. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(β-11C) DOPA and PET. Biol Psychiatry. 1999;46(5):681–688. [DOI] [PubMed] [Google Scholar]
- 41. Nozaki S, Kato M, Takano H, et al. Regional dopamine synthesis in patients with schizophrenia using L-[β-11C]DOPA PET. Schizophr Res. 2009;108(1–3):78–84. [DOI] [PubMed] [Google Scholar]
- 42. Cropley VL, Fujita M, Bara-Jimenez W, et al. Pre- and post-synaptic dopamine imaging and its relation with frontostriatal cognitive function in Parkinson disease: PET studies with [11C]NNC 112 and [18F]FDOPA. Psychiatry Res. 2008;163(2):171–182. [DOI] [PubMed] [Google Scholar]
- 43. Talvik M, Nordstrom AL, Olsson H, Halldin C, Farde L. Decreased thalamic D2/D3 receptor binding in drug-naive patients with schizophrenia: a PET study with [11C]FLB 457. Int J Neuropsychopharmacol. 2003;6(4):361–370. [DOI] [PubMed] [Google Scholar]
- 44. Suhara T, Okubo Y, Yasuno F, et al. Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Arch Gen Psychiatry. 2002;59(1):25–30. [DOI] [PubMed] [Google Scholar]
- 45. Glenthoj BY, Mackeprang T, Svarer C, et al. Frontal dopamine D2/3 receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biol Psychiatry. 2006;60(6):621–629. [DOI] [PubMed] [Google Scholar]
- 46. Kegeles LS, Slifstein M, Xu X, et al. Striatal and extrastriatal dopamine D2/D3 receptors in schizophrenia evaluated with [18F]fallypride positron emission tomography. Biol Psychiatry. 2010;68(7):634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kessler RM, Woodward ND, Riccardi P, et al. Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol Psychiatry. 2009;65(12):1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kambeitz J, Abi-Dargham A, Kapur S, Howes OD. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. Br J Psychiatry. 2014;204(6):420–429. [DOI] [PubMed] [Google Scholar]
- 49. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abi-Dargham A, Xu X, Thompson JL, et al. Increased prefrontal cortical D1 receptors in drug naive patients with schizophrenia: a PET study with [11C]NNC112. J Psychopharmacol. 2012;26(6):794–805. [DOI] [PubMed] [Google Scholar]
- 51. Hirvonen J, van Erp TG, Huttunen J, et al. Brain dopamine D1 receptors in twins discordant for schizophrenia. Am J Psychiatry. 2006;163(10):1747–1753. [DOI] [PubMed] [Google Scholar]
- 52. Okubo Y, Suhara T, Suzuki K, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385(6617):634–636. [DOI] [PubMed] [Google Scholar]
- 53. Karlsson P, Farde L, Halldin C, Sedvall G. PET study of D1 dopamine receptor binding in neuroleptic-naive patients with schizophrenia. Am J Psychiatry. 2002;159(5):761–767. [DOI] [PubMed] [Google Scholar]
- 54. Guo N, Hwang DR, Lo ES, Huang YY, Laruelle M, Abi-Dargham A. Dopamine depletion and in vivo binding of PET D1 receptor radioligands: implications for imaging studies in schizophrenia. Neuropsychopharmacology. 2003;28(9):1703–1711. [DOI] [PubMed] [Google Scholar]
- 55. Slifstein M, Kolachana B, Simpson EH, et al. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Mol Psychiatry. 2008;13:821–827. [DOI] [PubMed] [Google Scholar]
- 56. Lidow MS, Elsworth JD, Goldman-Rakic PS. Down-regulation of the D1 and D5 dopamine receptors in the primate prefrontal cortex by chronic treatment with antipsychotic drugs. J Pharmacol Exp Ther. 1997;281(1):597–603. [PubMed] [Google Scholar]
- 57. Kosaka J, Takahashi H, Ito H, et al. Decreased binding of [11C]NNC112 and [11C]SCH23390 in patients with chronic schizophrenia. Life Sci. 2010;86(21–22):814–818. [DOI] [PubMed] [Google Scholar]
- 58. Poels EM, Girgis RR, Thompson JL, Slifstein M, Abi-Dargham A. In vivo binding of the dopamine-1 receptor PET tracers [11C]NNC112 and [11C]SCH23390: a comparison study in individuals with schizophrenia. Psychopharmacology (Berl). 2013;228(1):167–174. [DOI] [PubMed] [Google Scholar]
- 59. Slifstein M, Kegeles LS, Gonzales R, et al. [11C]NNC 112 selectivity for dopamine D1 and serotonin 5-HT2A receptors: a PET study in healthy human subjects. J Cereb Blood Flow Metab. 2007;27(10):1733–1741. [DOI] [PubMed] [Google Scholar]
- 60. Ekelund J, Slifstein M, Narendran R, et al. In vivo DA D1 receptor selectivity of NNC 112 and SCH 23390. Mol Imaging Biol. 2007;9(3):117–125. [DOI] [PubMed] [Google Scholar]
- 61. Abi-Dargham A, Xu X, Thompson JL, et al. Increased prefrontal cortical D1 receptors in drug naive patients with schizophrenia: a PET study with [11C]NNC112. J Psychopharmacol. 2011;6:794–805. [DOI] [PubMed] [Google Scholar]
- 62. Sunahara RK, Guan HC, O’Dowd BF, et al. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350(6319):614–619. [DOI] [PubMed] [Google Scholar]
- 63. Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl). 2008;200(1):117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ciliax BJ, Nash N, Heilman C, et al. Dopamine D5 receptor immunolocalization in rat and monkey brain. Synapse. 2000;37(2):125–145. [DOI] [PubMed] [Google Scholar]
- 65. Carr GV, Maltese F, Sibley DR, Weinberger DR, Papaleo F. The dopamine D5 receptor is involved in working memory. Front Pharmacol. 2017;8:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Castello J, Cortés M, Malave L, et al. The Dopamine D5 receptor contributes to activation of cholinergic interneurons during L-DOPA induced dyskinesia. Sci Rep. 2020;10(1):2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Urban JD, Clarke WP, von Zastrow M, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. [DOI] [PubMed] [Google Scholar]
- 68. Yang Y, Lee S-M, Imamura F, Gowda K, Amin S, Mailman RB. D1 dopamine receptors intrinsic activity and functional selectivity affect working memory in prefrontal cortex. Molecular Psychiatry. 2021;26(2):645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Christian BT, Lehrer DS, Shi B, et al. Measuring dopamine neuromodulation in the thalamus: using [F-18]fallypride PET to study dopamine release during a spatial attention task. Neuroimage. 2006;31(1):139–152. [DOI] [PubMed] [Google Scholar]
- 70. Volkow ND, Fowler JS, Gatley SJ, et al. PET evaluation of the dopamine system of the human brain. J Nucl Med. 1996;37(7):1242–1256. [PubMed] [Google Scholar]
- 71. Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9(5):373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Correll CC, McKittrick BA. Biased ligand modulation of seven transmembrane receptors (7TMRs): functional implications for drug discovery. J Med Chem. 2014;57(16):6887–6896. [DOI] [PubMed] [Google Scholar]
- 73. Donthamsetti P, Gallo EF, Buck DC, et al. Arrestin recruitment to dopamine D2 receptor mediates locomotion but not incentive motivation. Mol Psychiatry. 2020;25(9):2086–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Goulet M, Grondin R, Blanchet PJ, Bédard PJ, Di Paolo T. Dyskinesias and tolerance induced by chronic treatment with a D1 agonist administered in pulsatile or continuous mode do not correlate with changes of putaminal D1 receptors in drug-naive MPTP monkeys. Brain Res. 1996;719(1–2):129–137. [DOI] [PubMed] [Google Scholar]
- 75. Ryman-Rasmussen JP, Griffith A, Oloff S, et al. Functional selectivity of dopamine D1 receptor agonists in regulating the fate of internalized receptors. Neuropharmacology. 2007;52(2):562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lin CW, Bianchi BR, Miller TR, et al. Persistent activation of the dopamine D1 receptor contributes to prolonged receptor desensitization: studies with A-77636. J Pharmacol Exp Ther. 1996;276(3):1022–1029. [PubMed] [Google Scholar]
- 77. Desai A, Benner L, Wu R, et al. Phase 1 randomized study on the safety, tolerability, and pharmacodynamic cognitive and electrophysiological effects of a dopamine D1 receptor positive allosteric modulator in patients with schizophrenia. Neuropsychopharmacology. 2020;46:1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wilbraham D, Biglan KM, Svensson KA, Tsai M, Kielbasa W. Safety, tolerability, and pharmacokinetics of mevidalen (LY3154207), a centrally acting dopamine D1 receptor-positive allosteric modulator (D1PAM), in healthy subjects. Clin Pharmacol Drug Dev. 2020;4:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Biglan K, Ardayfio P, Kielbasa W, Svensson K. A D1 receptor positive allosteric modulator (LY3154207) enhances wakefulness in sleep deprived healthy volunteers (P3.6-041). Neurology. 2019;92(15 Supplement):P3.6-041. [Google Scholar]
- 80. Bourne JA. SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS Drug Rev. 2001;7(4):399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Den Boer JA, van Megen HJ, Fleischhacker WW, et al. Differential effects of the D1-DA receptor antagonist SCH39166 on positive and negative symptoms of schizophrenia. Psychopharmacology (Berl). 1995;121(3):317–322. [DOI] [PubMed] [Google Scholar]
- 82. Astrup A, Greenway FL, Ling W, et al. ; Ecopipam Obesity Study Group . Randomized controlled trials of the D1/D5 antagonist ecopipam for weight loss in obese subjects. Obesity (Silver Spring). 2007;15(7):1717–1731. [DOI] [PubMed] [Google Scholar]
- 83. Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl). 2001;155(4):330–337. [DOI] [PubMed] [Google Scholar]
- 84. Nann-Vernotica E, Donny EC, Bigelow GE, Walsh SL. Repeated administration of the D1/5 antagonist ecopipam fails to attenuate the subjective effects of cocaine. Psychopharmacology (Berl). 2001;155(4):338–347. [DOI] [PubMed] [Google Scholar]
- 85. Chausmer AL, Smith BJ, Kelly RY, Griffiths RR. Cocaine-like subjective effects of nicotine are not blocked by the D1 selective antagonist ecopipam (SCH 39166). Behav Pharmacol. 2003;14(2):111–120. [DOI] [PubMed] [Google Scholar]
- 86. Kaiser C, Jain T. Dopamine receptors: functions, subtypes and emerging concepts. Med Res Rev. 1985;5(2):145–229. [DOI] [PubMed] [Google Scholar]
- 87. Lovenberg TW, Brewster WK, Mottola DM, et al. Dihydrexidine, a novel selective high potency full dopamine D-1 receptor agonist. Eur J Pharmacol. 1989;166(1):111–113. [DOI] [PubMed] [Google Scholar]
- 88. Pendleton RG, Samler L, Kaiser C, Ridley PT. Studies on renal dopamine receptors with a new agonist. Eur J Pharmacol. 1978;51(1):19–28. [DOI] [PubMed] [Google Scholar]
- 89. Blanchet PJ, Fang J, Gillespie M, et al. Effects of the full dopamine D1 receptor agonist dihydrexidine in Parkinson’s disease. Clin Neuropharmacol. 1998;21(6):339–343. [PubMed] [Google Scholar]
- 90. Braun A, Fabbrini G, Mouradian MM, Serrati C, Barone P, Chase TN. Selective D-1 dopamine receptor agonist treatment of Parkinson’s disease. J Neural Transm. 1987;68(1–2):41–50. [DOI] [PubMed] [Google Scholar]
- 91. Michaelides MR, Hong Y, DiDomenico S Jr, et al. (5aR,11bS)-4,5,5a,6,7,11b-hexahydro-2-propyl-3-thia-5-azacyclopent-1-ena[c]-phenanthrene-9,10-diol (A-86929): a potent and selective dopamine D1 agonist that maintains behavioral efficacy following repeated administration and characterization of its diacetyl prodrug (ABT-431). J Med Chem. 1995;38(18):3445–3447. [DOI] [PubMed] [Google Scholar]
- 92. Tsui JK, Wolters EC, Peppard RF, Calne DB. A double-blind, placebo-controlled, dose-ranging study to investigate the safety and efficacy of CY 208-243 in patients with Parkinson’s disease. Neurology. 1989;39(6):856–858. [DOI] [PubMed] [Google Scholar]
- 93. Giardina WJ, Williams M. Adrogolide HCl (ABT-431; DAS-431), a prodrug of the dopamine D1 receptor agonist, A-86929: preclinical pharmacology and clinical data. CNS Drug Rev. 2001;7(3):305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Buchanan RW, Freedman R, Javitt DC, Abi-Dargham A, Lieberman JA. Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophr Bull. 2007;33(5):1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mailman R, Huang X, Nichols DE. Parkinson’s disease and D1 dopamine receptors. Curr Opin Investig Drugs. 2001;2(11):1582–1591. [PubMed] [Google Scholar]
- 96. Granda ML, Schroeder FA, Borra RH, et al. First D1-like receptor PET imaging of the rat and primate kidney: implications for human disease monitoring. Am J Physiol Renal Physiol. 2014;307(1):F116–F121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Slifstein M, Suckow RF, Javitch JA, Cooper T, Lieberman J, Abi-Dargham A. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab. 2011;31(1):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. George MS, Molnar CE, Grenesko EL, et al. A single 20 mg dose of dihydrexidine (DAR-0100), a full dopamine D1 agonist, is safe and tolerated in patients with schizophrenia. Schizophr Res. 2007;93(1-3):42–50. [DOI] [PubMed] [Google Scholar]
- 99. Rosell DR, Zaluda LC, McClure MM, et al. Effects of the D1 dopamine receptor agonist dihydrexidine (DAR-0100A) on working memory in schizotypal personality disorder. Neuropsychopharmacology. 2015;40(2):446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Thompson JL, Rosell DR, Slifstein M, et al. Prefrontal dopamine D1 receptors and working memory in schizotypal personality disorder: a PET study with [11C]NNC112. Psychopharmacology (Berl). 2014;231(21):4231–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Girgis RR, Van Snellenberg JX, Glass A, et al. A proof-of-concept, randomized controlled trial of DAR-0100A, a dopamine-1 receptor agonist, for cognitive enhancement in schizophrenia. J Psychopharmacol. 2016;30(5):428–435. [DOI] [PubMed] [Google Scholar]
- 102. Marder SR, Fenton W. Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72(1):5–9. [DOI] [PubMed] [Google Scholar]
- 103. Pietrzak RH, Olver J, Norman T, Piskulic D, Maruff P, Snyder PJ. A comparison of the CogState Schizophrenia Battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Battery in assessing cognitive impairment in chronic schizophrenia. J Clin Exp Neuropsychol. 2009;31(7):848–859. [DOI] [PubMed] [Google Scholar]
- 104. Gray DL, Allen JA, Mente S, et al. Impaired β-arrestin recruitment and reduced desensitization by non-catechol agonists of the D1 dopamine receptor. Nat Commun. 2018;9(1):674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Davoren JE, Nason D, Coe J, et al. Discovery and lead optimization of atropisomer D1 agonists with reduced desensitization. J Med Chem. 2018;61(24):11384–11397. [DOI] [PubMed] [Google Scholar]
- 106. Kozak R, Kiss T, Dlugolenski K, et al. Characterization of PF-6142, a novel, non-catecholamine dopamine receptor D1 agonist, in murine and nonhuman primate models of dopaminergic activation. Front Pharmacol. 2020;11(1005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Young D, Popiolek M, Trapa P, et al. D1 agonist improved movement of Parkinsonian nonhuman primates with limited dyskinesia side effects. ACS Chem Neurosci. 2020;11(4):560–566. [DOI] [PubMed] [Google Scholar]
- 108. Wang M, Datta D, Enwright J, et al. A novel dopamine D1 receptor agonist excites delay-dependent working memory-related neuronal firing in primate dorsolateral prefrontal cortex. Neuropharmacology. 2019;150:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Papapetropoulos S, Liu W, Duvvuri S, Thayer K, Gray DL. Evaluation of D1/D5 partial agonist PF-06412562 in Parkinson’s disease following oral administration. Neurodegener Dis. 2018;18(5–6):262–269. [DOI] [PubMed] [Google Scholar]
- 110. Balice-Gordon R, Honey GD, Chatham C, et al. A neurofunctional domains approach to evaluate D1/D5 dopamine receptor partial agonism on cognition and motivation in healthy volunteers with low working memory capacity. Int J Neuropsychopharmacol. 2020;23(5):287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Arce E, Balice-Gordon R, Duvvuri S, et al. A novel approach to evaluate the pharmacodynamics of a selective dopamine D1/D5 receptor partial agonist (PF-06412562) in patients with stable schizophrenia. J Psychopharmacol. 2019;33(10):1237–1247. [DOI] [PubMed] [Google Scholar]
- 112. Soutschek A, Gvozdanovic G, Kozak R, et al. Dopaminergic D1 receptor stimulation affects effort and risk preferences. Biol Psychiatry. 2020;87(7):678–685. [DOI] [PubMed] [Google Scholar]
- 113. Soutschek A, Kozak R, de Martinis N, et al. Activation of D1 receptors affects human reactivity and flexibility to valued cues. Neuropsychopharmacology. 2020;45(5):780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Davidson M, Harvey PD, Bergman RL, et al. Effects of the D-1 agonist SKF-38393 combined with haloperidol in schizophrenic patients. Arch Gen Psychiatry. 1990;47(2):190–191. [DOI] [PubMed] [Google Scholar]
- 115. Tamagnan G, Barret O, Algille D, et al. In vivo characterization of the first agonist dopamine D1 receptors PET imaging tracer [18F] MNI-968 in humans. Schizophr Bull. 2018;44:S176. [Google Scholar]
- 116. Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. [DOI] [PubMed] [Google Scholar]
- 117. Sibley DR, De Lean A, Creese I. Anterior pituitary receptors: demonstration of interconvertible high and low affinity states of the D2 dopamine receptor. J Biol Chem. 1982;257:6351–6361. [PubMed] [Google Scholar]
- 118. Slifstein M, Abi-Dargham A. Is it pre- or postsynaptic? Imaging striatal dopamine excess in schizophrenia. Biol Psychiatry. 2018;83(8):635–637. [DOI] [PubMed] [Google Scholar]
- 119. Sibley DR, Mahan LC, Creese I. Dopamine receptor binding on intact cells. Absence of a high-affinity agonist-receptor binding state. Mol Pharmacol. 1983;23(2):295–302. [PubMed] [Google Scholar]
- 120. Skinbjerg M, Sibley DR, Javitch JA, Abi-Dargham A. Imaging the high-affinity state of the dopamine D2 receptor in vivo: fact or fiction? Biochem Pharmacol. 2012;83(2):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Shotbolt P, Tziortzi AC, Searle GE, et al. Within-subject comparison of [11C]-(+)-PHNO and [11C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab. 2012;32(1):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Narendran R, Mason NS, Laymon CM, et al. A comparative evaluation of the dopamine D2/3 agonist radiotracer [11C](−)-N-propyl-norapomorphine and antagonist [11C]raclopride to measure amphetamine-induced dopamine release in the human striatum. J Pharmacol Exp Ther. 2010;333(2):533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Guo N, Guo W, Jiang M, et al. Impact of D2 receptor internalization on binding affinity of positron emission tomography radiotracers. Neuropsychopharmacology. 2010;35(3):806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mizrahi R, Houle S, Vitcu I, Ng A, Wilson AA. Side effects profile in humans of (11)C-(+)-PHNO, a dopamine D2/3 agonist ligand for PET. J Nucl Med. 2010;51(3):496–497. [DOI] [PubMed] [Google Scholar]
- 125. Knoerzer TA, Nichols DE, Brewster WK, Watts VJ, Mottola D, Mailman RB. Dopaminergic benzo[a]phenanthridines – resolution and pharmacological evaluation of the enantiomers of dihydrexidine, the full efficacy D1 dopamine-receptor agonist. J Med Chem. 1994;37(15):2453–2460. [DOI] [PubMed] [Google Scholar]
- 126. Mottola DM, Brewster WK, Cook LL, Nichols DE, Mailman RB. Dihydrexidine, a novel full efficacy d1-dopamine receptor agonist. J Pharmacol Exp Ther. 1992;262(1):383–393. [PubMed] [Google Scholar]
- 127. Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020–2022. [DOI] [PubMed] [Google Scholar]