Abstract
Cortical thickness reductions are evident in schizophrenia (SZ). Associations between antipsychotic medications (APMs) and cortical morphometry have been explored in SZ patients. This raises the question of whether the reconfiguration of morphological architecture by APM plays potential compensatory roles for abnormalities in the cerebral cortex. Structural magnetic resonance imaging was obtained from 127 medication-naive first-episode SZ patients and 133 matched healthy controls. Patients received 12 weeks of APM and were categorized as responders (n = 75) or nonresponders (NRs, n = 52) at follow-up. Using surface-based morphometry and structural covariance (SC) analysis, this study investigated the short-term effects of antipsychotics on cortical thickness and cortico-cortical covariance. Global efficiency was computed to characterize network integration of the large-scale structural connectome. The relationship between covariance and cortical thinning was examined by SC analysis among the top-n regions with thickness reduction. Widespread cortical thickness reductions were observed in pre-APM patients. Post-APM patients showed more reductions in cortical thickness, even in the frontotemporal regions without baseline reductions. Covariance analysis revealed strong cortico-cortical covariance and higher network integration in responders than in NRs. For the NRs, some of the prefrontal and temporal nodes were not covariant between the top-n regions with cortical thickness reduction. Antipsychotic effects are not restricted to a single brain region but rather exhibit a network-level covariance pattern. Neuroimaging connectomics highlights the positive effects of antipsychotics on the reconfiguration of brain architecture, suggesting that abnormalities in regional morphology may be compensated by increasing interregional covariance when symptoms are controlled by antipsychotics.
Keywords: antipsychotics, first-episode schizophrenia, structural covariance network, brain connectivity, cortical thickness, magnetic resonance imaging, treatment response
Introduction
Schizophrenia (SZ) has been hypothesized to be a neurodevelopmental disorder, ie, typically characterized by onset in childhood.1 Structural magnetic resonance imaging (MRI) studies suggest widespread brain abnormalities even in the earliest stages of SZ.2 Studies have revealed progressive reductions in gray matter volume with longer illness duration, beginning in the thalamus and progressing to the frontal regions and then to the temporal and occipital lobes.3 Additionally, coordinated gray matter loss exhibits a pattern of irregular topographic distribution across cerebral cortices in SZ.4 The pattern of disturbance in morphology may suggest a coordinated pattern of structural reorganization in SZ.5
Structural covariance network (SCN) analysis is frequently employed to assess the network-level disturbances in structural connections between brain regions in SZ.6 Cortico-cortical covariance, established by structural covariance (SC), reflects group-level covarying relationships in brain structure between cortical regions. SC has been associated with some types of brain connections, such as the anatomical connectivity of white matter fiber tractography or the functional connectivity of synchronous neuronal activation.6,7 In SZ, reduced SCs were identified across cortices in frontal, temporal, and parietal regions.4 Frontal-temporal areas also exhibit cortical thinning in SZ, possibly due to overpruning of synapses during adolescence.8 Increased frontal-temporal covariance has been associated with greater auditory hallucination severity,9 which is one of the most common symptoms in SZ. Graph theoretical studies reported reduced integrity of the SCN in SZ,10 suggesting a low network efficiency of brain topological organization. A plausible mechanism to interpret SC is that synapses between distant neurons can have a mutually trophic or protective effect on neuronal development, leading to strong covariance in their morphology (“common fate”), such as similar gray matter volume changes during development, degeneration, or other plastic changes.6 Thus, we can expect that when antipsychotics induce cortical reorganization, regional changes are likely to exhibit a pattern of organized distribution instead of a random pattern.5
The association of antipsychotic medications (APMs) with cortical morphometry has been explored in SZ studies. A meta-analysis of longitudinal studies involving 1155 SZ patients and 911 healthy subjects showed higher loss of cortical total volume, which was related to cumulative antipsychotic intake.11 In addition to the reduction in cortical total volume, findings associated with APM effects focused on frontal, temporal, and parietal lobes.12 Reduced volumes in subcortical regions were associated with symptomatic improvement following APM treatment.13 Although these studies have identified APM effects on certain brain regions, they ignored the existence of coordinated relationships between brain regions. The SC may reflect developmental coordination or synchronized maturation between brain regions.5,6 Differences in interregional covariance in SZ compared to healthy controls (HCs) may reflect developmentally mediated dysconnectivity and play a critical role in the pathophysiologic trajectory of brain abnormalities in SZ.5,6 Recent work has used SC analysis to examine brain topological reorganization in medicated patients with SZ.14 They found that treatment nonresponders (NRs) exhibited worse network integration (measured by global efficiency) than responders.14 This raises the question of whether the reconfiguration of the structural architecture by APM-induced morphological plasticity plays potential compensatory functions for abnormalities in the cerebral cortex, which is primed for relief of clinical symptoms in SZ. This hypothesis should be investigated in a large cohort of individuals with medication-naive first-episode SZ (FES) who are less confounded by illness duration and previous treatments. In addition, the short-term effects of APM on brain morphometry, structural connectivity, and networks are evaluated by a longitudinal design.
To address this issue, we estimated the brain morphometry (region-wise cortical thickness) and large-scale SCN in 127 medication-naive FES patients and 133 healthy subjects. Patients were grouped according to their psychiatric symptom relief at follow-up after 12 weeks of APM. The aim of this study was to explore (1) whether the APM effects were not constrained within a single brain region but exhibited a topographical distribution across brain regions and (2) whether cortico-cortical covariance inferred from the SCN could explain the extent and location of cortical thickness reductions following APM. We hypothesized that APM responders would show enhanced integration in the SCN and stronger cortico-cortical covariance among regions with cortical thickness reductions compared with NRs.
Methods
Participants
This study recruited 127 patients with medication-naive FES (SZ group) who were diagnosed using the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) from Shanghai Mental Health Center and 133 HCs (HC group) matched for age, gender, education, and handedness. MRI was scanned at baseline for all subjects and at follow-up after 12 weeks of APM for only patients. Following the baseline scanning, patients received second-generation antipsychotics. At follow-up, patients were grouped as responders (SR group, n = 75) and NRs (NR group, n = 52) by the criterion of less than 50% Positive and Negative Syndrome Scale (PANSS) reduction for nonresponse.15 More details are described in table 1 and Supplementary Materials. The Institutional Review Board of Shanghai Mental Health Center approved the study. All subjects/patients signed written informed consent forms.
Table 1.
Demographic and Clinical Data of Participants
| (A) | Schizophrenia | Healthy Controls | P Value |
|---|---|---|---|
| Subject number | 127 | 133 | |
| Age (years) | 24.6 (7.0) | 23.7 (5.9) | .254a |
| Gender (male/female) | 63/64 | 66/67 | .998b |
| Education (years) | 12.9 (2.9) | 13.5 (2.8) | .110a |
| Handness (right) | 127 | 133 | |
| TIV (cm3) | 1480.5 (148.8) | 1476.9 (133.0) | .838a |
| Cortical thickness (mm) | 2.71 (0.11) | 2.74 (0.09) | .045a |
| Illness duration (months) | 13.4 (16.0) | — | — |
| CPZ (mg/d) | 402.8 (188.9) | — | — |
| Baseline PANSS | |||
| Positive score | 24.0 (5.1) | — | — |
| Negative score | 19.1 (6.7) | — | — |
| General score | 42.4 (6.9) | — | — |
| Total score | 85.8 (12.9) | — | — |
| 12-Week PANSS | |||
| Positive score | 12.6 (4.1) | — | — |
| Negative score | 14.4 (4.8) | — | — |
| General score | 28.8 (5.7) | — | — |
| Total score | 55.8 (12.5) | — | — |
| PANSS reduction (%) | 53.0 (21.1) | — | — |
| (B) | Responders | Nonresponders | P Value |
| Subject number | 75 | 52 | |
| Age (years) | 25.3 (6.6) | 23.6 (7.4) | .176a |
| Gender (male/female) | 35/40 | 28/24 | .426b |
| Education (years) | 13.4 (2.9) | 12.3 (2.7) | .033a |
| Handness (right) | 75 | 52 | |
| TIV (cm3) | 1478.1 (147.6) | 1483.9 (152.0) | .832a |
| Cortical thickness (mm) | 2.72 (0.11) | 2.71 (0.11) | .710a |
| Illness duration (months) | 13.2 (15.0) | 13.7 (17.4) | .871a |
| CPZ (mg/d) | 406.2 (197.9) | 397.9 (176.9) | .810a |
| Baseline PANSS | |||
| Positive score | 24.6 (5.0) | 23.2 (5.3) | .125a |
| Negative score | 18.4 (6.6) | 20.2 (6.7) | .146a |
| General score | 43.6 (7.2) | 40.8 (6.0) | .020a |
| Total score | 86.9 (14.1) | 84.1 (10.9) | .221a |
| 12-Week PANSS | |||
| Positive score | 11.0 (3.0) | 14.9 (4.3) | <.001a |
| Negative score | 12.0 (3.6) | 17.8 (4.3) | <.001a |
| General score | 26.0 (4.6) | 32.8 (4.9) | <.001a |
| Total score | 49.0 (9.2) | 65.7 (9.6) | <.001a |
| PANSS reduction (%) | 66.7 (12.8) | 33.3 (13.7) | <.001a |
Note: CPZ, chlopromazine equivalents; PANSS, Positive and Negative Syndrome Scale; TIV, total intracranial volume.
a P values were obtained by using 2-sample t tests.
b P values were obtained by using the chi-square test.
Image Acquisition, Processing, and Cortical Thickness Estimation
T1-weighted images were collected by a 3-Tesla MRI (Siemens MR B17). Using the Computational Anatomy Toolbox (http://www.neuro.uni-jena.de/cat/), cortical thickness was estimated in 360 regions of interest (ROIs) based on HCP Multi-Modal Parcellation16 (Supplementary table S1). Details about image acquisition and processing are described in Supplementary Materials.
Statistical Analysis
Demographic Differences
Demographic and clinical information differences were tested using t tests for continuous variables between the SZ and HC groups and between the 2 patient groups. The chi-square test was used for the categorical variables.
Cortical Thickness Differences
At baseline, we compared the cortical thickness between all SZ patients and HCs using a 2-sample t test. ANOVA and post hoc t tests were performed to compare the differences among the SR, NR, and HC groups. To examine cortical thickness changes after APM, 2-way repeated-measures ANOVA and post hoc paired t tests were conducted in the SR and NR groups. The univariate statistical test was implemented independently for each of 360 cortical regions while controlling for age, square of age, gender, education years, and total intracranial volume (TIV).4 To correct for multiple comparisons and to minimize the bias of data distribution, a permutation-based procedure was considered for controlling the familywise error (FWE) rate17 (Supplementary Materials).
Associations With Treatment Outcomes
To examine the relationships between cortical thickness changes and treatment outcomes, the differences between follow-up and baseline were extracted from each region that exhibited significant cortical thickness changes following APM. A linear stepwise regression analysis was performed to assess the association between these regional changes and PANSS total score reductions in each patient group.
Structural Covariance Network
SC analysis has been widely used to characterize interregional structural connectivity between brain regions6 based on the assumption that intersubject differences in cortical morphology covary across individuals between regions that may be anatomically connected.18 To investigate the regional covariance in cortical thickness change, the SCN was constructed using the cortical thickness change between baseline and follow-up. Cortical thickness change was corrected by regressing out the effects of gender, education, age, the square of age, and TIV by a regression model.6 Pearson’s correlation coefficients were calculated across the subjects in each group between pairwise ROIs and were further transformed into z values. To reduce the correlations of false positives, false discovery rate correction was performed (P < .000001).
To investigate the integration of SCN, global efficiency was used for measuring integration. The global efficiency of a network is denoted by the averaged inverse shortest path length and has been regarded as a superior measure of integration.19 The formula of global efficiency is described as , where dij is the shortest path length between nodes i and j. The null hypothesis of equality in the global efficiency of the SCN between the SR and NR groups was tested using a permutation test (Supplementary Materials).
SC in Top-n Regions With Cortical Thickness Reduction
To investigate the impact of varying severity of cortical thickness change at follow-up, we analyzed the SC in top-n regions with cortical thickness change. The methodology has been reported in a prior study.4 First, regions were ranked from highest to lowest according to the severity of cortical thickness changes following APM, which was assessed by Cohen’s d effect size. Subsequently, the averaged SC among the top-n regions with the highest cortical thickness changes (ie, SCtop-n) was calculated using the thickness change values. The SCtop-n value reflects the SC of the subnetwork composed of the first n regions. The mean SC was also computed in a set of randomly chosen n regions (ie, SCrand-n). Randomly chosen sets were repeated at 5000, thus generating a distribution of SCs of n random regions. According to the location of the actual SCtop-n within the distribution of SCrand-n values, a P value was obtained for the null hypothesis of equality in mean SC among top-n regions and random regions. This analysis was repeated independently for n = 2 to the maximum threshold (ie, 360) to explore the impact of varying severity of cortical thickness changes (ie, a larger n included regions with smaller cortical thickness reductions). As n increased, continuous values of mean SC among the top-n regions were computed and could be plotted as a function of n. The area under the curve (AUC) of this plot was used to calculate a global P value for all n values. To determine which subnetwork composed of the first n top regions exhibited the strongest SC compared to the randomly chosen n regions, the SCtop-n was transformed into a z score (zSCtop-n) by subtracting the average of SCrand-n values and then dividing it by the standard deviation of SCrand-n values.
Reproducibility and Ancillary Analysis
Reproducibility and ancillary analysis are provided in Supplementary Materials.
Results
Cortical Thickness Baseline Comparisons
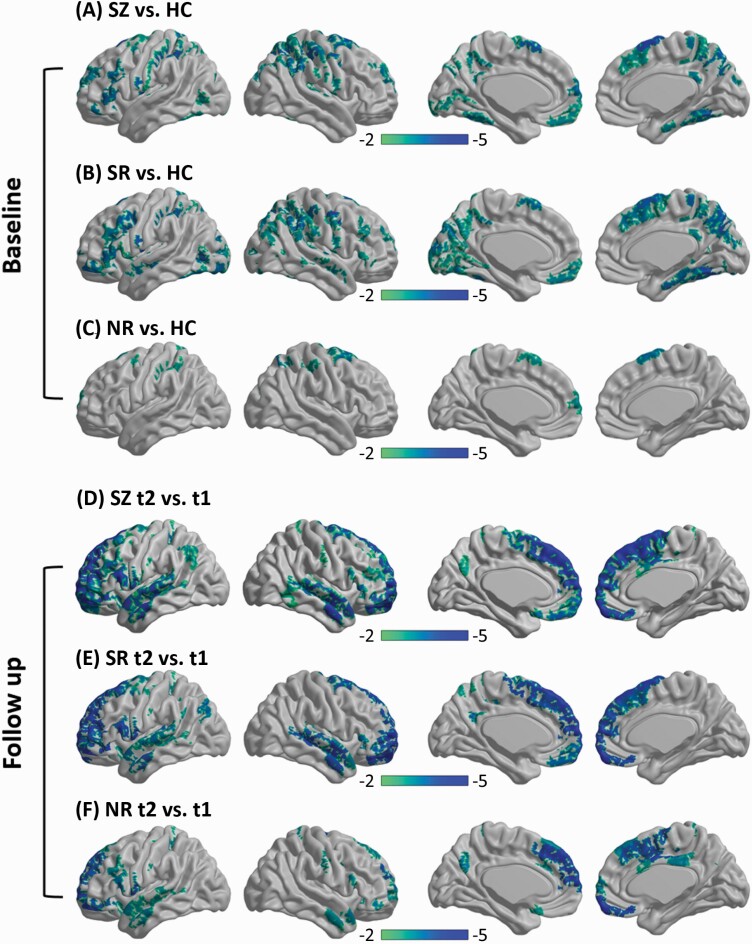
Compared with the HCs, a total of 73, 90, and 12 regions were observed to present significantly thinner cortical thickness in the baseline SZ (combined SRt1 and NRt1), SR, and NR groups, respectively (FWE-corrected P < .05). Increased cortical thickness was not found in the 3 patient groups. In the SR group, thinner cortical thickness illustrated a distributed pattern involving frontal, parietal, temporal, and occipital lobes as well as posterior cingulate cortex (figure 1). In the NR group, thinner cortical thickness was observed in the frontal and parietal cortex (figure 1). Details of regions with significantly thinner cortical thickness are described in Supplementary tables S2–S6.
Fig. 1.
Cortical thickness comparisons. At baseline, thinner cortical thickness was shown in the 3 patient groups (A, all SZ patients; B, SR patients; C, NR patients) than in the HC group. At the follow-up (t2), cortical thickness reductions were observed in the patient groups (D, all SZ patents; E, SR patients; F, NR patients) compared with the baseline (t1). Regions for which the null hypothesis was rejected after controlling the FWE rate at 5% are shown in color maps. Color bar represents the t values. Note: FWE, familywise error; HC, healthy control; NR, nonresponder; SR, responder; SZ, schizophrenia.
Cortical Thickness Changes
Compared with the baseline, the SR group at follow-up exhibited more extensive cortical thickness changes (only reductions) in the medial frontal cortex and right temporal regions, although cortical thickness changes were found in extensive regions (all SZ patient groups, n = 85; SR group, n = 73; NR group, n = 43) for both patient groups (FWE-corrected P < .05) (figure 1). Regions with significant cortical thickness changes at follow-up and the effect sizes are listed in Supplementary tables S7–S9. The interaction effect between group and time was not evident after correction for multiple comparisons.
Associations Between Cortical Thickness Changes and Treatment Outcomes
Regression analysis found a significant relationship between the cortical thickness change in the left area-45 of the inferior frontal cortex and PANSS total reduction (T = 2.37, P < .05), which was only observed in the SR group (Supplementary figure S1). In addition, a supplementary analysis (Supplementary Materials) indicated that the relationship was not affected by gender, age, education, TIV, chlopromazine, illness duration, or baseline PANSS total scores.
Structural Covariance Network
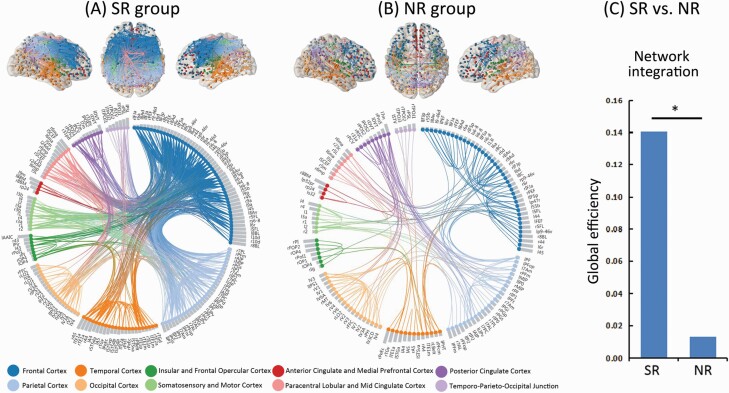
Figures 2A and B show surviving SC connections after correction for multiple comparisons (false discovery rate-corrected P < .000001) for the SR and NR groups, respectively. A larger number of surviving connections were observed in the SR group than in the NR group by the permutation test (P = .020). In the network of the SR group, the majority of these connections (63.04%) were associated with frontal and parietal cortices, as shown in Supplementary figure S2. The null hypothesis of equality in network integration between the SR and NR groups was rejected, indicating superior global efficiency in the SR group (P = .029) (figure 2C).
Fig. 2.
Cortico-cortical covariance based on the amount of cortical thickness thinning over the follow-up period is shown by brain connectivity maps and a circular connectogramb for the SR group (panel A) and NR group (panel B) after multiple comparison correction with the false discovery rate corrected, P < .000001. (C) The null hypothesis of equality in network integration between the SR and NR groups was rejected by the permutation test (*P < .05), indicating superior global efficiency in the SR group compared with the NR group. Note: NR, nonresponder; SR, responder. Brain regions are coloured on the brain connectivity maps according to cortex classifications (described below the circular connectogram). Brain regions are grouped on the circular connectogram according to cortex classifications (described below the circular connectogram). Within each subgroup, regions are ranked according to the severity of cortical thickness reductions at the follow-up, assessed by Cohen’s d effect size (ie, gray column adjacent to nodes). Brain connectivity maps and circular connectograms were produced by using NeuroMArVL (http://immersive.erc.monash.edu.au/neuromarvl).
SC in Top-n Regions With Cortical Thickness Reduction
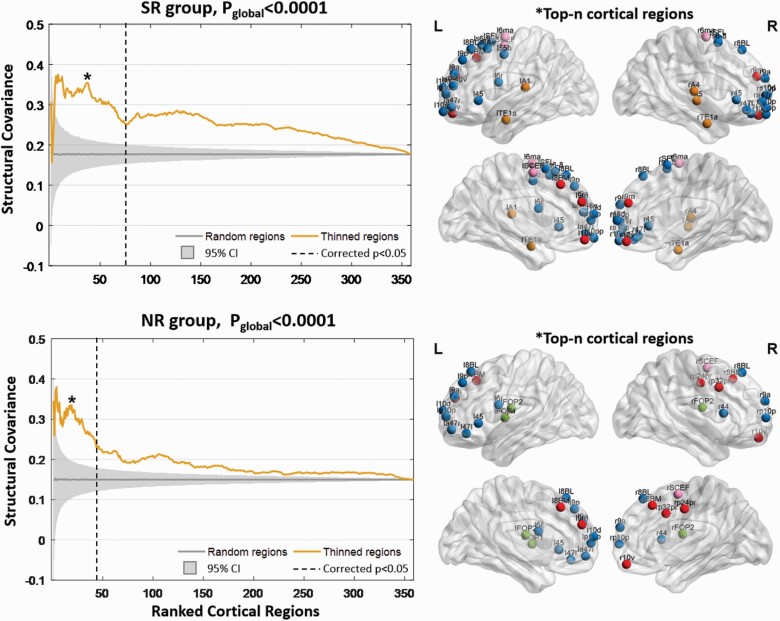
Figure 3 illustrates the SC among the top-n regions with the greatest extent of cortical thickness reduction. In figure 3, the majority of SCtop-n values (the yellow line) exceed the shaded area (ie, 95% confidence intervals of the average SCrand-n values) (global P < .0001), indicating that the SC between pairs of top-n regions was stronger than randomly chosen regions for both patient groups. For both groups, the SC among the top-n regions was found to be increased with the increased severity of cortical thickness reductions induced by antipsychotics.
Fig. 3.
Structural covariance among the ranked regions with cortical thickness reductions in the SR and NR groups. The horizontal axis shows the cortical regions that ranked from high to low according to the severity of cortical thickness reductions using Cohen’s d effect size. The dashed vertical line denotes the boundary between regions with and without significantly reduced cortical thickness after the antipsychotic medications (FWE-corrected P < .05). The vertical axis measures the structural covariance, which is computed as the Pearson’s correlation coefficient between 2 paired regions across the population of each group. The yellow line represents the mean structural covariance among the top-n ranked regions, ie, SCtop-n. The gray line indicates the mean structural covariance among the 5000 randomly chosen n regions (ie, SCrand-n). The shaded areas show the 95% confidence intervals across the permutated data, ie, the 5000 SCrand-n values. The null hypothesis of equality in the area under the curve (AUC) between SCtop-n and SCrand-n is rejected by the permutation test (Pglobal < .0001). Values of n marked with a star represent the strongest structural covariance (SCtop-n) compared to SCrand-n of the randomly chosen regions, which is determined by the SCtop-nz score (ie, subtracting the average of SCrand-n values and then dividing it by the standard deviation of SCrand-n values). The brain node maps on the right side indicate the spatial distributions of the top-n regions at the maximum SCtop-nz score. Nodes are coloured according to cortex classifications (described in figure 2). Note: FWE, familywise error; NR, nonresponder.
In the NR group, the first 43 ranked regions showed decreased cortical thickness after treatment; when n = 23, the zSCtop-n was highest, indicating that the subnetwork composed of the first 23 top regions showed the strongest SC. The AUC at n = 23 was 69.36. The top 23 regions were located within the frontal and anterior cingulate cortex (figure 3). In the SR group, the first 73 ranked regions exhibited reduced cortical thickness at the follow-up. When n = 39, zSCtop-n was highest, showing that the subnetwork composed of the top 39 regions exhibited the strongest SC; the AUC at n = 39 was 91.67. The 39 top regions were located within the frontal and temporal regions and anterior cingulate cortex, especially for the right prefrontal and temporal regions, which were only observed in the SR group (figure 3).
Finally, SC analysis in the top-n regions was also performed in the HC group based on their raw cortical thickness scores (Supplementary figure S3), which indicated that these regions with thickness changes following APM were strongly connected in the HC group.
Reproducibility and Ancillary Analysis
The ancillary analysis indicated that the SC results were repeatable (Supplementary figure S4). The group differences in baseline symptoms did not influence the SCN (Supplementary figure S5). The global efficiency difference between the SR and NR was not driven by the correlation strength (Supplementary figure S6). The between-group differences in the top-n region SC did not result from potential geometric effects or hemispheric disproportion (Supplementary figures S7 and S8). Details are provided in Supplementary Materials.
Discussion
This study comprehensively investigated the short-term effects of atypical antipsychotics on brain morphometry changes in FES. First, thinner cortical thickness was observed in the frontal, parietal, temporal, occipital lobes, and the posterior cingulate cortex in FES patients. Second, the effects of atypical antipsychotics were not constrained within a single brain region but exhibited a topographic distribution (ie, network level) across specific regions with cortical thickness reductions. The network-level assumption was demonstrated by the subsequent SCN analysis, indicating stronger cortico-cortical covariance and higher network integration in APM responders. Third, SC analysis among the top-n regions suggested an association between stronger covariance and thinner cortical thickness. Collectively, these findings support a potential network-level regulatory mechanism of antipsychotics on brain structure, suggesting that the reconfiguration of morphological architecture induced by APM plays potential compensatory functions for cortical abnormalities.
Thinner Cortical Thicknesses Are Found Even in the Early Stage of Medication-Naive Patients With FES
Although thinner cortical thickness is a well-established feature in SZ, the degree of thinning has been demonstrated to vary across different illness stages.4 For example, FES patients show subtle cortical thinning mainly in the frontal and temporal cortices.20 In chronic SZ, pronounced reductions spread to parietal and occipital lobes.21 Individuals with treatment-resistant SZ exhibit more widespread cortical thickness thinning.22 The inconsistency may have a variety of possible reasons, such as illness duration, medication history, psychotic symptoms, and so on. In this study, thinner cortical thickness was observed in the frontal, parietal, temporal, and occipital lobes as well as the posterior cingulate cortex in medication-naive FES patients. Interestingly, individuals who respond to treatment showed more widespread cortical thickness reductions at baseline than NRs, suggesting the potential association between pathological neuroanatomical changes and response to antipsychotic treatment.23 Supporting this result, relationships were found between volumes in frontal and medial temporal structures and treatment outcome.24 These findings support the existence of observable changes in morphometry of the cerebral cortex even in the SZ early stage.
Cortical Thickness Reduction and Improved Structural Network Integrity Are the Short-Term Effects of Atypical Antipsychotics
Accumulating evidence has demonstrated that APM can modulate brain morphology. More loss in gray matter volume has been reported in APM patients regardless of use of different analytic methods.11 Similarly, animal studies provide evidence of gray matter volume loss in macaque monkeys25 and rodent exposure to antipsychotics.26 The loss in volume can be explained by fewer glial cells and higher densities of neurons or increased myelination of fibers near the gray-white matter boundary27 and has been linked with neuroinflammatory models.28,29 Conversely, some studies reported that APM increased gray matter volume in the prefrontal and occipital cortex in SZ.30 The inconsistency may be related to the illness stage, symptom severity, antipsychotic type, term of treatment, definition of response, and analytic methods. Consistent with previous studies,31 this study observed widespread cortical thickness reductions regardless of treatment response. It is understandable when we assume that progressive neuroanatomical alterations are partly associated with the disease itself.32 Nonetheless, cortical thickness reductions were found following APM only in the response group, suggesting that morphological changes in specific areas may be due to antipsychotics; however, the effect of concomitant illness progression during APM could not be completely ruled out. A previous study found cortical thinning over time in relation to altered SC and unremitted psychotic symptoms in FES,33 suggesting that it is unclear whether APMs or persistent psychotic symptoms are predominantly driving these changes in the current study.
In addition, the effects of antipsychotics on brain structure have been further demonstrated to occur not independently within constrained cortical locations but rather exhibit a topographically coherent pattern. This topographical pattern has been frequently linked with structural changes across development from childhood to early adulthood and continues across the lifespan.34 Abnormalities in structural covary patterns have been indicated to be involved in SZ pathophysiological processes and are associated with cortical gene expression in therapeutic targets and early brain development.35 Using a graph theoretical approach on the SCN, Palaniyappan et al measured the structurally coherent pattern and found reduced global efficiency in SZ, indicating weaker structural network integrity.5 Hence, improved structural network integrity and, more strongly, cortico-cortical covariance in responders highlight the potentially positive effects of antipsychotics on brain morphometry by means of connectomics rather than traditionally interpreted as a detrimental effect if cortical thickness reduction was explored in isolation.
Evidence Supports the Link Between Stronger Covariance and Thinner Cortical Thickness
This study observed increased cortico-cortical covariance between regions with thinner cortical thickness in FES patients, suggesting a link between cortical covariance and morphological alteration. Supporting this finding, recent studies reported that cortical covariance could shape and constrain the spatial distribution of cortical pathology in SZ.4,36 A possible explanation is that local pathological processes, including abnormal neuronal signaling and neurotransmitter release, may propagate to distant regions by cortico-cortical covariance.4 In FES patients, abnormalities in morphology are related to disruptions in normal maturation and plasticity of higher-order regions, such as the prefrontal, parietal, and temporal cortices,37 which have also presented pathological decreases in these regions in pre-APM patients. Notably, stronger SC between thinned regions was found in the responders after taking APM. In addition, more connections among the right prefrontal and temporal regions were found in the covariance network of responders compared with that of NRs, indicating better reconfiguration of morphological architecture among the key nodes in APM responders. These findings suggest that structural abnormalities of the cerebral cortex in FES are balanced by the reconfiguration of morphological architecture and increasing interregional covariance, possibly reflecting a compensation process5 when symptoms are controlled by antipsychotics.
Limitations
First, although the 12-week follow-up investigated the short-term effect of APM on brain structure, the long-term persistence of antipsychotics is still unknown. Due to the lack of follow-up in HCs, we cannot estimate cortical thickness changes in controls and SCN differences between patients and controls; thus, normal neurodevelopment or neurodegeneration progression cannot be neglected. Second, the definition of treatment response is based on a cutoff of 50% PANSS reduction. Other cutoff points (ie, <30%, 30%–50%, and 50%–100%) are also used. Third, the choice of APMs is clinically led. The current sample size is not enough to compare the effects of different antipsychotic types. In addition, the adipogenic effect of antipsychotics is a potential bias, as weight gain is evident in FES patients after medication treatment. It is also difficult to rule out the possible effects of variations in brain microstructure (ie, myelination, iron, and water content) on cortical thickness reductions.38 Due to the lack of cognitive assessments, the relationships between the structural network and cognitive deficits in SZ were not examined.
Conclusions
This study demonstrated that antipsychotic-induced changes in brain structures occurred not independently within constrained cortical locations but rather exhibited a network-level pattern. The relationship between increased covariance and cortical thinning suggests that abnormalities in cortical morphology may be compensated by increasing interregional covariance when symptoms are controlled by antipsychotics. Patients who responded to medications showed stronger cortico-cortical covariance and higher network integration after APM, highlighting the potentially positive effects of antipsychotics on the reconfiguration of brain morphological architecture by means of connectomics.
Supplementary Material
Acknowledgments
We are grateful to all the participants in this study. Our thanks also go to the American Journal Experts Editing (https://www.aje.com/) for language assistance of the manuscript. All authors report no biomedical financial interests or potential conflicts of interest.
Funding
This work was partly supported by Ministry of Science and Technology of China, National Key R&D Program of China grant (2016YFC1306800), grants from the National Natural Science Foundation of China (61933003, U2033217, 81771822, 81861128001, and 81771925), the CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-5-039), and the Project funded by China Postdoctoral Science Foundation (BX2021078).
References
- 1. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17(12):1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wheeler AL, Wessa M, Szeszko PR, et al. Further neuroimaging evidence for the deficit subtype of schizophrenia: a cortical connectomics analysis. JAMA Psychiatry. 2015;72(5):446–455. [DOI] [PubMed] [Google Scholar]
- 3. Jiang Y, Luo C, Li X, et al. Progressive reduction in gray matter in patients with schizophrenia assessed with MR imaging by using causal network analysis. Radiology. 2018;287(2):633–642. [DOI] [PubMed] [Google Scholar]
- 4. Wannan CMJ, Cropley VL, Chakravarty MM, et al. Evidence for network-based cortical thickness reductions in schizophrenia. Am J Psychiatry. 2019;176(7):552–563. [DOI] [PubMed] [Google Scholar]
- 5. Palaniyappan L, Hodgson O, Balain V, Iwabuchi S, Gowland P, Liddle P. Structural covariance and cortical reorganisation in schizophrenia: a MRI-based morphometric study. Psychol Med. 2019;49(3):412–420. [DOI] [PubMed] [Google Scholar]
- 6. Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14(5):322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gong G, He Y, Chen ZJ, Evans AC. Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. Neuroimage. 2012;59(2):1239–1248. [DOI] [PubMed] [Google Scholar]
- 8. van Erp TGM, Walton E, Hibar DP, et al. ; Karolinska Schizophrenia Project . Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry. 2018;84(9):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Modinos G, Vercammen A, Mechelli A, Knegtering H, McGuire PK, Aleman A. Structural covariance in the hallucinating brain: a voxel-based morphometry study. J Psychiatry Neurosci. 2009;34(6):465–469. [PMC free article] [PubMed] [Google Scholar]
- 10. Spreng RN, DuPre E, Ji JL, et al. Structural covariance reveals alterations in control and salience network integrity in chronic schizophrenia. Cereb Cortex. 2019;29(12):5269–5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vita A, De Peri L, Deste G, Barlati S, Sacchetti E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry. 2015;78(6):403–412. [DOI] [PubMed] [Google Scholar]
- 12. Tarcijonas G, Sarpal DK. Neuroimaging markers of antipsychotic treatment response in schizophrenia: an overview of magnetic resonance imaging studies. Neurobiol Dis. 2019;131:104209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tronchin G, Akudjedu TN, Ahmed M, et al. Progressive subcortical volume loss in treatment-resistant schizophrenia patients after commencing clozapine treatment. Neuropsychopharmacology. 2020;45(8):1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palaniyappan L, Marques TR, Taylor H, et al. Globally efficient brain organization and treatment response in psychosis: a connectomic study of gyrification. Schizophr Bull. 2016;42(6):1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boter H, Peuskens J, Libiger J, et al. ; EUFEST study group . Effectiveness of antipsychotics in first-episode schizophrenia and schizophreniform disorder on response and remission: an open randomized clinical trial (EUFEST). Schizophr Res. 2009;115(2–3):97–103. [DOI] [PubMed] [Google Scholar]
- 16. Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Groppe DM, Urbach TP, Kutas M. Mass univariate analysis of event-related brain potentials/fields I: a critical tutorial review. Psychophysiology. 2011;48(12):1711–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zalesky A, Pantelis C, Cropley V, et al. Delayed development of brain connectivity in adolescents with schizophrenia and their unaffected siblings. JAMA Psychiatry. 2015;72(9):900–908. [DOI] [PubMed] [Google Scholar]
- 19. Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3(2):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schultz CC, Koch K, Wagner G, et al. Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 2010;116(2–3):204–209. [DOI] [PubMed] [Google Scholar]
- 21. Goldman AL, Pezawas L, Mattay VS, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66(5):467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zugman A, Gadelha A, Assunção I, et al. Reduced dorso-lateral prefrontal cortex in treatment resistant schizophrenia. Schizophr Res. 2013;148(1–3):81–86. [DOI] [PubMed] [Google Scholar]
- 23. Palaniyappan L, Marques TR, Taylor H, et al. Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry. 2013;70(10):1031–1040. [DOI] [PubMed] [Google Scholar]
- 24. Bodnar M, Malla AK, Joober R, et al. Neural markers of early remission in first-episode schizophrenia: a volumetric neuroimaging study of the parahippocampus. Psychiatry Res. 2012;201(1):40–47. [DOI] [PubMed] [Google Scholar]
- 25. Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649–1661. [DOI] [PubMed] [Google Scholar]
- 26. Vernon AC, Natesan S, Crum WR, et al. Contrasting effects of haloperidol and lithium on rodent brain structure: a magnetic resonance imaging study with postmortem confirmation. Biol Psychiatry. 2012;71(10):855–863. [DOI] [PubMed] [Google Scholar]
- 27. Bartzokis G, Lu PH, Raven EP, et al. Impact on intracortical myelination trajectory of long acting injection versus oral risperidone in first-episode schizophrenia. Schizophr Res. 2012;140(1–3):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carter CS, Bullmore ET, Harrison P. Is there a flame in the brain in psychosis? Biol Psychiatry. 2014;75(4):258–259. [DOI] [PubMed] [Google Scholar]
- 29. Konopaske GT, Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology. 2007;32(6):1216–1223. [DOI] [PubMed] [Google Scholar]
- 30. Goghari VM, Smith GN, Honer WG, et al. Effects of eight weeks of atypical antipsychotic treatment on middle frontal thickness in drug-naïve first-episode psychosis patients. Schizophr Res. 2013;149(1–3):149–155. [DOI] [PubMed] [Google Scholar]
- 31. Ahmed M, Cannon DM, Scanlon C, et al. Progressive brain atrophy and cortical thinning in schizophrenia after commencing clozapine treatment. Neuropsychopharmacology. 2015;40(10):2409–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37(8):1680–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lepage M, Makowski C, Bodnar M, Chakravarty MM, Joober R, Malla AK. Do unremitted psychotic symptoms have an effect on the brain? A 2-year follow-up imaging study in first-episode psychosis. Schizophr Bull Open. 2020;1(1):sgaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khundrakpam BS, Reid A, Brauer J, et al. ; Brain Development Cooperative Group . Developmental changes in organization of structural brain networks. Cereb Cortex. 2013;23(9):2072–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu F, Tian H, Li J, Li S, Zhuo C. Altered voxel-wise gray matter structural brain networks in schizophrenia: association with brain genetic expression pattern. Brain Imaging Behav. 2019;13(2):493–502. [DOI] [PubMed] [Google Scholar]
- 36. Shafiei G, Markello RD, Makowski C, et al. Spatial patterning of tissue volume loss in schizophrenia reflects brain network architecture. Biol Psychiatry. 2020;87(8):727–735. [DOI] [PubMed] [Google Scholar]
- 37. Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull. 2011;37(3):504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lorio S, Kherif F, Ruef A, et al. Neurobiological origin of spurious brain morphological changes: a quantitative MRI study. Hum Brain Mapp. 2016;37(5):1801–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.