Abstract
Objective
Cognitive impairment in schizophrenia predicts functional outcomes and is largely unresponsive to pharmacology or psychotherapy; it is thus a critical unmet treatment need. This article presents the impact of remotely completed, intensive, targeted auditory training (AT) vs control condition computer games (CG) in a double-blind randomized trial in young adults with recent-onset schizophrenia.
Method
Participants (N = 147) were assessed for cognition, symptoms, and functioning at baseline, post-intervention, and at 6-month follow-up. All participants were provided with laptop computers and were instructed to complete 40 hours remotely of training or computer games. An intent-to-treat analysis (N = 145) was performed using linear mixed models with time modeled as a continuous variable. Planned contrasts tested the change from baseline to post-training, baseline to 6-month follow-up, and post-training to 6-month follow-up.
Results
Global Cognition, which had improved in the AT group relative to the CG group at post-training, showed durable gains at 6-month follow-up in an omnibus group-by-time interaction test (F(1,179) = 4.80, P = .030), as did Problem-Solving (F(1,179) = 5.13, P = .025), and Speed of Processing improved at trend level significance (F(1,170) = 3.80, P = .053). Furthermore, the AT group showed significantly greater improvement than the CG group in positive symptoms (F(1,179) = 4.06, P = .045).
Conclusions
These results provide the first evidence of durable cognitive gains and symptom improvement at follow-up of cognitive training (CT) in early schizophrenia completed independently and remotely. While functioning did not show significant improvement, these findings suggest that intensive targeted CT of auditory processing is a promising component of early intervention to promote recovery from psychosis.
Keywords: recent-onset psychosis, first-episode psychosis, cognitive training, cognitive remediation
Introduction
Cognitive deficits associated with schizophrenia are prominent features of the disorder and are critical treatment targets.1 While positive symptoms often respond to antipsychotic treatment, cognitive deficits do not and are more closely associated with real-world outcomes.2,3 Cognitive impairment is present by the first psychotic episode and effective treatments for cognition are necessary to promote full recovery from psychosis.4,5
Cognitive training (CT) and remediation strategies emerged as a promising treatment for schizophrenia, demonstrating small to moderate effect sizes across cognitive domains in trials with participants with persistent schizophrenia.6 However, only a small number of CT studies have been conducted in the early phase of schizophrenia, despite recognition that early intervention is now the evidence-based treatment of choice.7 Further, only 7 randomized controlled trials of CT in first-episode and early-onset psychosis included a follow-up assessment.8–14 In each of these studies, CT interventions included one-to-one delivery by a therapist, trainer, or teacher, and/or group-based interventions. Five out of the 7 studies showed durable cognitive gains at follow-up; 10–14 one study did not find durable gains,8 and one did not assess cognition at follow-up.9 The results on symptoms and functioning at follow-up were mixed. Some studies showed improvement in symptoms11 or functioning,9 other studies found improvement in both symptoms and functioning in both the active and control groups,10,13 and 3 studies showed no improvement.8,12,14
In our previous research on targeted CT of auditory processing, individuals with persistent schizophrenia demonstrated gains in global cognition, verbal working memory, verbal learning, and verbal memory. Cognitive gains were associated with neural activity improvements and improved functioning 6 months later.15,16 This training targets early auditory perceptual processes and more complex auditory/verbal working memory operations to improve the temporally detailed resolution of auditory cortical representations and downstream verbal learning and memory processes that are known to be impaired in schizophrenia.17 In our previous report of our first 86 participants with recent-onset schizophrenia, we found that 40 hours of remote auditory training induced significant gains in global cognition, verbal memory, and problem-solving relative to a computer games control condition.18
It is axiomatic that ultimately—in order to maximize recovery for young individuals—CT must be delivered as one component of a comprehensive treatment program. However, to make meaningful scientific progress, we must establish the longer-term efficacy of specific forms of training on specific cognitive domains, independent of the effects of adjunctive psychosocial treatments. Furthermore, with the current health crisis, testing the effects of technologies that allow for remote delivery is more critical now than ever.
In this study, we posed the following questions: Can a precisely defined course of targeted training of auditory processing and auditory/verbal working memory generate enduring cognitive improvement when delivered remotely? What is the pattern of improvement? Are symptoms and functioning improved at 6-month follow-up?
We previously reported the results immediately post-training of our double-blind randomized controlled trial (RCT) of targeted auditory training (AT) vs control computer games (CG), completed by 86 individuals with recent-onset schizophrenia remotely on laptop computers.18Herein, we report the results in our final sample (N = 147), and the effects 6 months after the intervention. Our primary hypothesis was that individuals would demonstrate greater durable improvement in cognition in the AT vs CG group from baseline to the 6-month follow-up. Secondary hypotheses tested whether symptoms and functioning showed significant group-by-time interactions from baseline to the 6-month follow-up.
Methods
Participants
Participants (n = 147) were randomized to the study protocol in our university-based early psychosis research programs at University of California, San Francisco, and University of California, Davis (ClinicalTrials.gov NCT00694889). Of these, 104 completed 8-week post-training assessments, and 77 completed a 6-month follow-up assessment. Participants were recruited via presentations/flyers and clinician referrals, and met the following inclusion/exclusion criteria: (1) Diagnosis of schizophrenia, schizophreniform, or schizoaffective disorder; (2) Onset of the first psychotic episode within past 5 years; (3) Good general physical health (eg, not acutely ill or experiencing a severe/chronic illness that would impede the ability to complete study activities); (4) Age 12–35 years; (5) Fluent in English; (6) IQ > 70; (7) No neurological disorder; and (8) No substance dependence in the past year. No participant had inpatient treatment for at least 3 months and no medication changes for at least 1 month prior to participation (17 participants did not take psychiatric medications).
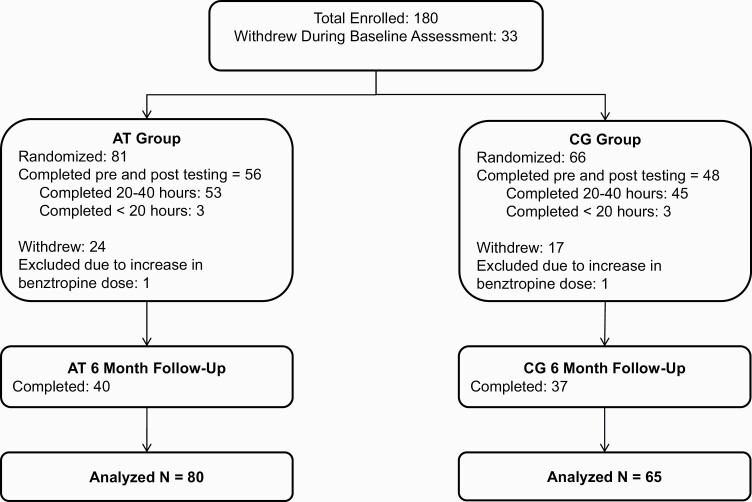
Participants aged 18 and older gave written informed consent; those younger than age 18 provided assent, with written parental/legal guardian consent. Baseline assessments were conducted prior to randomization. Participants were stratified by IQ, gender, and symptom severity, and randomly assigned to AT or CG conditions (CONSORT diagram in figure 1). Participants were loaned laptop computers that tracked training to complete the intervention remotely, except for one participant who preferred to train in the laboratory. Participants were asked to complete 40 hours of training (1 hour/day, 5 days/week, for 8 weeks), followed by post-training and 6-month follow-up assessments (ie, approximately 8 months total from baseline).
Fig. 1.
CONSORT diagram of participants with recent-onset schizophrenia who received computerized auditory training (AT) and participants who played computer games (CG).
Participants were contacted 1–2 times per week by phone to discuss progress. Coaching was provided if a participant indicated difficulty completing the recommended number of hours/week (eg, goal-setting, discussion of scheduling, setting an alarm, and using reminders). At a “check-in” appointment, after every 10 sessions completed, the same coaching was provided and participants were paid $5 for each completed hour, $20 for every 10 sessions, and $30 after 40 hours, as well as $20 per assessment appointment. Mean training hours for participants who completed post-training assessments was 35.50 hours (SD = 9.17) across both groups (table 1). During the training period, if a participant dropped out, attempts to reengage him/her were made through calls and a letter. During the trial, participants received unrestricted treatment by outside providers or clinic personnel not involved in the study (eg, case management, psychotherapy, and adjustments in medications as clinically indicated). See table 1 for demographic characteristics and table 2 for medications.
Table 1.
Baseline Characteristics of Subjects With Recent-Onset Schizophrenia Randomized to Computerized Auditory Training or Computer Games
| Auditory Training (N = 80) | Computer Games (N = 65) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t or χ 2 | dc | P | |
| Male/femalea | 61/19 | 47/18 | 0.29 | 1 | .59 | ||
| Age (range 16-35) | 21.69 | 4.00 | 20.51 | 3.48 | −1.87 | 143 | .06 |
| Education | 12.74 | 1.78 | 12.73 | 2.13 | −0.010 | 142 | .99 |
| WASI IQ | 103.21 | 12.20 | 102.34 | 14.87 | −0.39 | 143 | .70 |
| PANSS totalb | 58.28 | 12.15 | 61.29 | 15.44 | 1.32 | 143 | .19 |
| Strauss carpenter | 7.96 | 2.35 | 8.09 | 2.64 | 0.31 | 143 | .76 |
| Global functioning role | 4.74 | 2.17 | 4.92 | 2.11 | 0.52 | 143 | .61 |
| Global functioning social | 5.69 | 1.25 | 5.68 | 1.42 | −0.048 | 143 | .96 |
| Hours of training | 27.20 | 14.88 | 29.94 | 14.75 | 1.11 | 143 | .27 |
| Training intensity (hours per week) | 3.43 | 1.75 | 3.57 | 1.49 | 0.42 | 143 | .68 |
| Months of psychosis (range 1-60)d | 21.54 | 17.02 | 23.14 | 17.86 | 0.55 | 140 | .59 |
Note: WASI, Wechsler Abbreviated Scale of Intelligence. Bold indicates P value is at trend-level statistical significance.
aPearson’s Chi-Square test.
bPositive and Negative Syndrome Scale.
cMissing data: one participant missing education and 3 missing months of psychosis.
dFrom first psychotic episode to study entry.
Table 2.
Medication Regimens of Study Participants
| AT (N = 79)a | CG (N = 62)a | Test Statistic | P | |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Antipsychotic medicationb | ||||
| First generation only (N) | 3 (4%) | 1 (2%) | X 2(1) = 0.54 | .46 |
| Second generation only (N) | 58 (73%) | 49 (79%) | X 2(1) = 2.79 | .095 |
| First and second generations (N) | 2 (3%) | 5 (8%) | X 2(1) = 2.25 | .13 |
| No antipsychotic (N) | 16 (20%) | 7 (11%) | X 2(1) = 2.04 | 0.15 |
| Other psychiatric medication | ||||
| Antidepressants or mood stabilizers (N) | 27 (34%) | 23 (37%) | X 2(1) = 0.13 | .72 |
| Benzodiazepines (N) | 12 (15%) | 10 (16%) | X 2(1) = 0.023 | .88 |
| Other medication measures | ||||
| Chlorpromazine equivalentsc | 295.25 (290.82) | 369.94 (382.13) | t(114) = 1.194 | .24 |
| Changes in medication while in studyd | ||||
| Baseline—Post (N = 103) | 28 (52%) | 31 (63%) | X 2(1) = 1.37 | .24 |
| Post—Follow-up (N = 68) | 23 (66%) | 21 (64%) | X 2(1) = 0.032 | .86 |
Note: AT, auditory training group; CG, computer games control group.
aFour subjects missing medication data.
bFirst-generation antipsychotic medication = haloperidol, loxapine, perphenazine, thiothixene, and trifluoperazine. Second-generation antipsychotic medication = asenapine, aripiprazole, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone.
cMean and SD of Chlorpromazine Equivalents.19
dChange in medication type, class, or dose.
CT Program and Computer Games Control Condition
The CT program was provided by Posit Science, Inc and was described previously.20 It consists of computerized exercises designed to improve speed and accuracy of auditory information processing while engaging auditory and verbal working memory (eg, distinguishing between frequency modulation “sweeps” of auditory stimuli, identifying increasingly long arrays of open and closed syllables in spatial and sequential contexts). The exercises contain stimulus sets spanning the acoustic organization of speech. This training approach is based on evidence that schizophrenia is characterized by widespread disturbances in frontotemporal neural systems subserving auditory processing and verbal memory (eg, 21). Exercises adaptively adjust difficulty level to maintain an 80%–85% correct performance rate to engage users in a frequent reward schedule and drive successful learning. Correct trials are rewarded with points and animations. In each session, participants work with 4 of the 6 exercises for 15 minutes per exercise. Adherence is monitored by data upload.
The CG condition allows for maintenance of a double-blind trial design and controls for effects of computer exposure, contact with research personnel, payments, and nonspecific engagement of attention, executive functions, and motivation. Control participants rotated through a series of 16 commercially available games (supplemental table 1) for the same number of hours as AT participants, playing 4–5 games per session.
Randomization and Blinding
Assessment staff were blind to group assignment. Although participants were initially consented to be randomized to either AT or CG, they were not informed of their assignment or that one condition was a control condition. Investigators treated all participants as if both conditions were interventional. Randomization was 2:1 AT:CG at the study start to ensure sufficient experience with the AT intervention and was then reversed to result in roughly equivalent groups by study end (1.2:1).
Assessment Procedures
Cognitive assessors were trained and monitored across sites on manualized assessment procedures by the same senior researcher (M.F.) to ensure cross-site consistency (the measurement and treatment research to improve cognition in schizophrenia [MATRICS] battery showed an intra-class correlation of 0.88 in a multi-site RCT).22 Clinical assessors were trained and observed by expert supervisors at each site (R.L., J.D.R., and T.A.N.). Participant eligibility was determined in regular reliability rounds. Inter-rater reliability was calculated from staff ratings of training tapes, with an average intra-class correlation across sites of 0.83 for symptom ratings and an average kappa value of 0.95 for diagnostic agreement.
Eligibility diagnoses were determined using the Structured Clinical Interview for DSM-IV (SCID-IV). Symptoms and functioning were assessed with the Positive and Negative Syndrome Scale (PANSS),23 Strauss Carpenter Outcome Scale,24 and Global Functioning: Role and Social Scales.25 An abbreviated battery of MATRICS-recommended measures was administered (table 3).26The Tower Test from the Delis-Kaplan Executive Function System (D-KEFS) was used in place of neuropsychological assessment battery (NAB) Mazes.27In addition to the immediate recall trials of the Hopkins Verbal Learning Test-Revised (HVLT-R) and the Brief Visuospatial Memory Test-Revised (BVMT-R), the delayed recall trials were also administered, which are not part of the MATRICS battery. Raw scores were converted to z-scores using age-appropriate normative data provided in testing manuals, and age-appropriate, published normative data for Trails A,28 Category Fluency,29 and the BVMT-R.30 All primary outcome measures were distinct and independent from tasks practiced during training. Alternate forms of the HVLT-R and BVMT-R were administered and counterbalanced at each time point.
Table 3.
Cognitive, Symptom, and Functioning Scores by Condition, Before, After, and 6 months Post-Intervention
| Outcome Measuresa | Auditory Training (AT; N = 80) | Computer Games (CG; N = 65) | Fb | P | Baseline to Follow-Up Contrast | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size | |||||||||||
| Baseline | Post | Follow-up | Baseline | Post | Follow-up | 95% CI | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | dd | Lower | Upper | |||
| Global Cognitionc | −0.99 (0.81) | −0.70 (0.89) | −0.58 (0.93) | −0.90 (0.84) | −0.92 (0.98) | −0.81 (1.05) | 4.80 | .030* | 0.38 | −0.03 | 0.77 |
| Problem-Solvingc | −0.47 (0.77) | 0.19 (0.87) | 0.46 (1.00) | −0.36 (0.76) | −0.16 (0.96) | −0.09 (0.94) | 5.13 | .025* | 0.62 | 0.12 | 1.16 |
| Speed of Processingc | −0.77 (0.92) | −0.50 (1.01) | −0.34 (1.14) | −0.65 (0.82) | −0.62 (1.19) | −0.70 (1.22) | 3.80 | .053 | 0.55 | −0.08 | 1.22 |
| Working Memory | −0.41 (0.83) | −0.19 (1.00) | −0.30 (1.16) | −0.47 (1.06) | −0.46 (1.05) | −0.60 (0.89) | 0.00 | .978 | 0.25 | −0.23 | 0.76 |
| Verbal Learning | −1.72 (1.53) | −1.59 (1.42) | −1.09 (1.20) | −1.57 (1.33) | −1.94 (1.81) | −1.52 (1.66) | 2.49 | .116 | 0.40 | −0.01 | 0.82 |
| Verbal Memory | −1.79 (1.74) | −1.50 (1.54) | −1.51 (1.55) | −1.41 (1.47) | −1.78 (1.76) | −1.44 (1.83) | 2.40 | .123 | 0.19 | −0.22 | 0.65 |
| Visual Learning | −1.29 (1.56) | −1.01 (1.68) | −0.94 (1.58) | −1.16 (1.50) | −1.09 (1.66) | −0.91 (1.93) | 0.70 | .403 | 0.07 | −0.40 | 0.53 |
| Visual Memory | −1.23 (1.74) | −0.97 (1.78) | −0.86 (1.60) | −1.35 (1.79) | −1.14 (1.85) | −0.93 (1.24) | 0.00 | .989 | −0.04 | −0.50 | 0.35 |
| PANSS Totale | 58.23 (12.15) | 54.45 (11.80) | 52.38 (11.55) | 61.29 (15.44) | 56.60 (14.77) | 59.89 (13.63) | 1.65 | .201 | −0.40 | −0.81 | 0.00 |
| Positive Symptomsc,e | 12.79 (4.34) | 10.71 (3.32) | 10.35 (3.09) | 13.45 (5.09) | 11.67 (4.32) | 12.65 (4.20) | 4.06 | .045* | −0.41 | −0.80 | −0.02 |
| Negative Symptoms | 16.11 (6.24) | 16.77 (5.53) | 15.53 (4.91) | 17.00 (6.49) | 16.98 (6.15) | 17.54 (5.92) | 0.04 | .844 | −0.18 | −0.63 | 0.20 |
| Symptoms of General Psychopathology | 29.40 (6.75) | 26.96 (6.67) | 26.50 (6.80) | 30.85 (8.17) | 27.96 (7.41) | 29.70 (8.24) | 0.51 | .477 | −0.23 | −0.64 | 0.18 |
| Global Functioning Role | 4.74 (2.17) | 4.75 (2.39) | 5.13 (2.33) | 4.92 (2.11) | 4.60 (2.46) | 4.46 (2.62) | 1.71 | .192 | 0.40 | 0.00 | 0.82 |
| Global Functioning Social | 5.69 (1.25) | 5.80 (1.21) | 5.85 (1.47) | 5.68 (1.42) | 5.82 (1.62) | 5.86 (1.53) | 0.56 | .454 | −0.02 | −0.45 | 0.41 |
| Strauss Carpenter | 7.96 (2.35) | 8.16 (2.33) | 8.25 (2.72) | 8.09 (2.64) | 8.42 (2.76) | 8.35 (2.54) | 0.28 | .599 | 0.01 | −0.36 | 0.43 |
Note: PANSS, Positive and Negative Syndrome Scale; CI, confidence interval; NS, not significant. Bold values indicate statistical significance or at trend-level significance.
aGlobal Cognition (average z-score across all measures); Speed of Processing (Trail Making Test Part A; Category Fluency Animal Naming); Working Memory (Letter-Number Span; WMS-III Spatial Span); Verbal Learning and Verbal Memory (HVLT-R Immediate and Delayed Recall); Visual Learning and Visual Memory (BVMT-R Immediate and Delayed Recall); and Problem-Solving (D-KEFS Tower Test).
bMixed model with repeated measures, condition-by-time interaction.
cChange from baseline to post-training group contrasts: Global Cognition (P = .0014), Problem-Solving (P < .001), Speed of Processing (P = .09), Working Memory (P = .006), and Positive symptoms (P = .51). Change from baseline to follow-up group contrasts: Global Cognition (P < .001), Problem-Solving (P < .001), Speed of Processing (P = .02), and Positive symptoms (P = .0017). Change from post-training to follow-up group contrasts: NS.
dCohen’s d effect sizes = mean change scores of AT and CG groups (follow-up minus baseline)/pooled baseline standard deviations. AT N = 40 and CG N = 37.
eUntransformed means and standard deviations are shown. F, p, and d values were computed according to the transformed data. *P < .05.
Statistical Analyses
Based on previous findings that medication-induced anticholinergic burden adversely affected training response,31 we excluded 2 participants (one per condition) who were prescribed an increased dose of benztropine mesylate (Cogentin) during the study. Statistical analysis of primary and secondary outcomes was conducted using linear mixed models on all remaining randomized participants (N = 145) (see figure 1). This modeling approach enables estimation of change in repeated measures over time in the presence of missing data, without excluding participants with missing data.32 We modeled the effect of treatment condition (AT or CG) on individual outcomes as a linear function of time, treatment condition, and treatment by time interaction as fixed effects, with participant as a random effect. Prior studies and preliminary visual inspection of our own data suggest that a higher magnitude of change in the outcome is achieved in the early phases of training and the rate of change reduces over time. Therefore, we modeled time as a continuous variable and transformed to the log scale (natural log of time in days since baseline +1). For random effects, we included both random intercept and random slope terms. As an alternative modeling strategy, we also built linear mixed models with time treated as a categorical variable (baseline, post-training, and 6-month follow-up), where time, treatment condition, and treatment by time interaction are fixed effects, and participant is a random effect (results presented in supplementary material). Both sets of linear mixed models were built with and without age as an independent variable, since age showed a trend toward a statistically significant difference between groups at baseline. The loglikelihood ratio test was conducted to test if inclusion of age improved model fit. Finally, planned contrasts compared between-group change from baseline to post-training, baseline to 6-month follow-up, and post-training to 6-month follow-up (time treated as categorical). Given that these analyses were planned, we did not correct for Type I error.
All variables were screened, outlying values ± 2.5 SD from the mean were Winsorized (<1% of the data), and PANSS symptom scores were transformed with either a square root or log base 10. Pearson’s chi-Square was used to test for group differences in attrition rate. Effect sizes were computed as the difference between the change (follow-up vs baseline) in the CG group vs the AT group divided by the pooled standard deviation at baseline, as recommended in the study of Morris.33 Measures are listed in table 3. Linear mixed models were conducted using R 3.5.0 and fitted with the nlme (Linear and Nonlinear Mixed Effects Models) package. All other analyses were performed in SPSS 23.
Results
Adherence to CT via Laptop
Twenty-four out of the 80 (30%) AT participants withdrew from the study during training compared with 17 of the 65 (26.2%) CG participants, a nonsignificant difference, X2 (1, N = 145) = 0.26, P = .61. Another 16 (29%) AT participants failed to return for follow-up assessments, compared with 11 (23%) of CG participants, also a nonsignificant difference X2 (1, N = 104) = 0.43, P = .51.
We compared participants across conditions who completed the intervention to those who withdrew and those who completed 6-month follow-up assessments to those who did not (supplemental table 2). All baseline differences were nonsignificant with the exception of lower PANSS Positive Symptom ratings in intervention completers (M = 12.33, SD = 4.30) relative to participants who withdrew during the intervention (M = 15.00, SD = 5.11) (t (143) = 3.19, P = .002), and lower working memory in 6-month completers (M = −0.65, SD = 1.0) compared with those who withdrew before final follow-up (M = −0.18, SD = 0.79) (t (143) = 4.7, P = .002). There were no differences between treatment groups in baseline demographics, cognition, functioning, or medication regimens (tables 1 and 2).
Primary Cognitive Outcome Measures
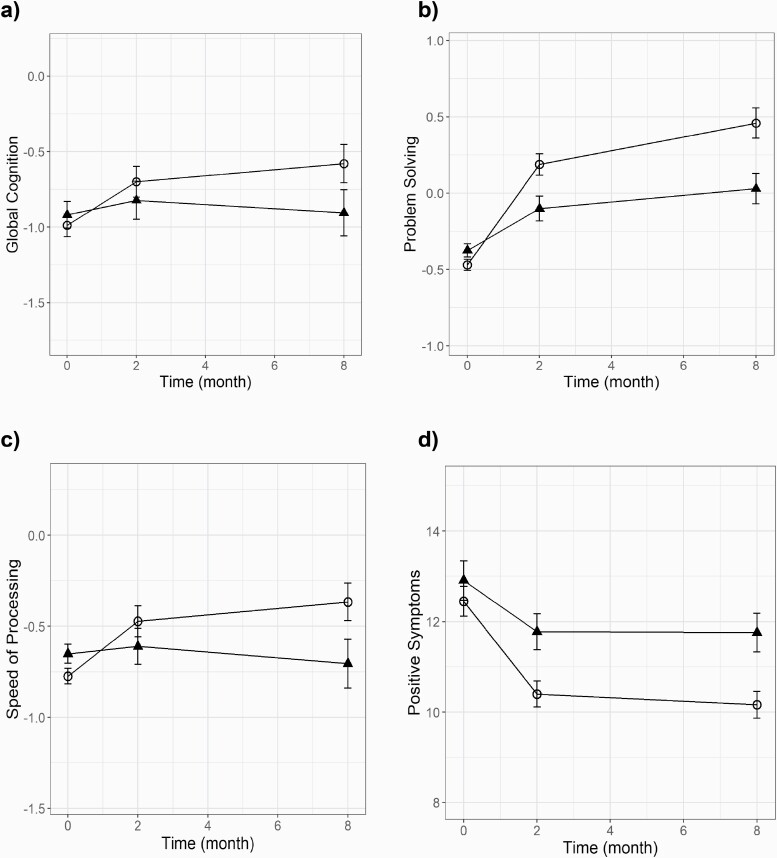
Linear mixed model results showed significant condition-by-time interactions with greater improvement in the AT group relative to the CG group in Global Cognition (F(1,179) = 4.80, P = .03), Problem-Solving (F(1,179) = 5.13, P = .025), and Speed of Processing at trend level significance (F(1,170) = 3.80, P = .053) (table 3, figure 2, and supplemental figure 1). Age was excluded from the final model since the loglikelihood ratio test indicated age did not improve model fit. With time treated as a categorical variable, results were the same with AT showing significant gains relative to CG in Global Cognition and Problem-Solving (see supplemental Results).
Fig. 2.
Model predicted baseline, post-training, and follow-up scoresa in (a) Global Cognition, (b) Problem-Solving, (c) Speed of Processing, and (d) PANSS Positive Symptom Ratings. Circle represents the AT group and triangle represents the CG group. Linear mixed model results of significant condition-by-time interactions. aModel estimated means and standard errors. Note: AT, auditory training group; CG, computer games control group; PANSS, Positive and Negative Syndrome Scale.
Planned group contrasts of the change from baseline to post-training showed significantly greater improvement in the AT group relative to the CG group in Global Cognition (P = .0014), Problem-Solving (P < .001), Working Memory (P = .006), and Speed of Processing at trend level significance (P = .09) The contrast from baseline to 6-month follow-up revealed significantly more improvement in the AT group than the CG group on measures of Global Cognition (P < .001), Problem-Solving (P < .001), and Speed of Processing (P = .02). Planned group contrasts of the change from post-training to 6-month follow-up were not significant.
Secondary Hypotheses
Linear mixed model results showed a significant condition-by-time interaction in Positive Symptoms (F(1,179) = 4.06, P = .045), with the AT group showing a greater decrease relative to the CG group. With time treated as a categorical variable, results were the same with AT showing significant improvement in Positive Symptoms relative to CG (see supplemental Results). We also explored the individual items of the PANSS Positive Symptom subscale (see supplemental Results).
The Positive Symptoms group contrast from baseline to post-training was not significant (P = 0.51), whereas the contrast from baseline to follow-up was significant (P = .0017). Within-group contrasts showed a significant decrease in positive symptoms in the AT group (baseline M = 12.79, SD = 4.34; follow-up M = 10.35, SD = 3.09) relative to the CG group (baseline M = 13.45, SD = 5.09; follow-up M = 12.65, SD = 4.20) (table 3). Planned group contrasts of change from post-training to 6-month follow-up were not significant. No other cognitive domains, symptoms, or functional outcomes showed statistically significant condition-by-time interactions at follow-up.
Effect Sizes of the Intervention
Effect sizes based on data from baseline to the 6-month follow-up were in the small range for Global Cognition (d = 0.38) and in the medium range for Problem-Solving (d = 0.62), and Speed of Processing (d = 0.55). The effect size on positive symptoms was in the small range (d = −0.41) with AT showing a greater decrease relative to the CG group.
Discussion
Intensive Targeted CT Generates Enduring Gains 6 Months Later
This is the first double-blind RCT of CT completed independently—without one-to-one coaching or group sessions—to demonstrate durable cognitive gains and improvement in symptoms after a significant follow-up period in the recent-onset schizophrenia patients. We previously demonstrated gains in global cognition, verbal memory, and problem-solving immediately after intervention in the first 86 individuals with recent-onset schizophrenia who completed 20–40 hours of targeted CT of auditory processing compared with individuals who completed 20–40 hours of computer games.18 Herein, in our final sample of 147 individuals, we demonstrate that gains in global cognition and problem-solving are maintained in the AT group compared with controls, with a trend toward statistically significant gains in speed of processing (P = .053).
In planned contrasts from baseline to follow-up, these findings were also significant. Longer-term impacts of training on more executive/global functions in this population (as compared to verbal functions) may be due to reduced statistical power from attrition. It may also indicate that training generalizes more easily to higher-order prefrontally mediated operations earlier in the course of schizophrenia (possible neural mechanisms are described by Dale et al.).34
Our results of durable cognitive gains are consistent with 5 of the 7 randomized controlled trials of first-episode and the early-onset psychosis that included a follow-up assessment.10–14 In each of these studies, CT interventions included one-to-one delivery by a therapist, trainer, or teacher, and/or group-based interventions. To the best of our knowledge, this is the first study to demonstrate durable cognitive gains in the recent-onset psychosis from a computerized CT intervention completed independently and remotely from home.
The nonsignificant verbal memory decline observed in the CG group returned close to baseline performance at the 6-month follow-up. This was also seen in our prior studies using nearly identical visually intensive CG.20,35 As suggested in those reports, individuals with psychosis may experience competition for neural resources in a “limited capacity” verbal memory system when exposed to many hours of CG which place intensive demands on visual processing.
Symptom Improvement
Positive symptoms also improved by 6-month follow-up in the AT group relative to the CG group. This improvement may represent direct neural system benefits of training, as the disrupted neural functioning that characterizes schizophrenia is restored to more adaptive patterns.15,17 We did not find significant symptom improvement immediately after the training. It was only at the 6-month follow-up that decreases in positive symptoms were statistically significant in the AT group relative to the CG group.
These results are consistent with Ostergaard who found improvement in positive symptoms in first-episode psychosis patients at 8-month follow-up in the CT group relative to a control group, which was not evident at post-training.11 Effects on symptoms at follow-up in other studies of first-episode and early-onset psychosis are mixed, with some studies showing no improvement,8,12,14 and others showing improvement in both active and control groups.10,13
Sleeper Effect
Our results suggest a similar “sleeper effect” in that the group-by-time interactions in our final sample for Speed of Processing and Positive Symptoms were not statistically significant from baseline to post-training, but are significant from baseline to the follow-up. This “sleeper effect” is also consistent with Best et al36 and Bowie et al37 who found continued improvements after treatment in 70 individuals with a schizophrenia spectrum disorder. For example, Best et al36 compared 6 weeks of perceptual training vs executive training, using a combination of exercises from Happy Neuron and Posit Science BrainHQ (some of which were used in the present study). Immediately after training, the perceptual training group showed improved EEG mismatch negativity and improved neurocognition; however, these improvements did not persist at follow-up. In contrast, the effects of executive training did not emerge until 12 weeks after the end of treatment, when improvements in neurophysiology, neurocognition, and functioning were observed. Further, both groups showed significant improvement in positive and negative symptoms from baseline to the follow-up.
“Sleeper effects” are defined as a delayed response to an intervention and have been observed in a number of prior cognitive remediation studies where participants have been followed up 3–12 months after treatment completion (eg, 11,16,36–38). Most often, the delayed improvements in symptoms, cognition, or functioning are associated with cognitive and/or neural system changes observed immediately after the intervention, suggesting that those individuals who benefit from initial cognitive improvement are in some way able to harness those neurocognitive improvements to more fully engage with their environments in the subsequent months.15,16,39
Although the exact mechanisms of action for these sleeper effects have not yet been studied, this process likely potentiates the effects of other psychosocial interventions due to an individual’s improved cognitive abilities.37 It may also facilitate an individual’s ability to engage with more cognitively and functionally stimulating activities in their outside lives,36 and it may help them to be able to better focus on and remember their medications.11 The specific 6-month improvement we observed in positive symptoms may be due to this latter effect on medication adherence behavior. It may also be due to salutary changes in thalamic volume, cortical thinning, corollary discharge function, and/or auditory processing efficiency we have observed after training.40–43 None of these potential mechanisms are independent of one another and all are likely to be mutually reinforcing. Clinically, it suggests that CT, like physical training, can reinforce other health-promoting behaviors in subsequent months—and clinical programs could make explicit use of this principle. Whether additional booster training sessions would be needed after the 6-12 month period is presently unknown.
Our results of improvements in cognition and positive symptoms 6 months after treatment are consistent with previous findings and significant given that this is the first follow-up study of the effects of a cognitive intervention completed independently and remotely by participants with recent-onset schizophrenia. While functioning showed no improvement, our findings indicate that CT provided without the aid of a therapist or group sessions can improve cognition and positive symptoms, and may provide a synergistic effect when added to other psychosocial and vocational interventions.37
Limitations
A limitation of the present study is a high attrition rate and lack of follow-up data on all participants. Dropout was similar across both interventions, suggesting that software content was roughly acceptable to both groups. Although we treated both groups as active interventions, participants may have been able to guess whether they were in the AT or CG conditions as we did not formally check the blind. Attrition rates were greater than what was reported in persistent schizophrenia,20 but similar to studies with clinical-high-risk individuals from our own group,35 and others44—suggesting that younger individuals are harder to engage in intensive training. Certainly, to translate these promising findings into real-world interventions, our field must develop appropriate supports to help young individuals meet the highly effortful and time-intensive demands of targeted CT, similar to approaches designed to promote adherence to other effortful health-enhancing behaviors such as regular physical exercise.45 While payment was a moderate motivator for some, it was insufficient as the sole motivator for most.
Finally, while the results in our final sample are broadly consistent with our interim report, there are some differences in the results in 2 of the 15 outcomes: (1) In the interim analysis of baseline to post-training, Verbal Memory was statistically significant, and in the final analysis this contrast was not significant; (2) Working Memory, which was not significant in the interim analysis, is statistically significant in our final sample. These differences are likely due to differences in the sample sizes and analyses used.
Conclusions and Future Directions
The results of our current study demonstrate, for the first time, that CT completed independently and remotely can induce cognitive gains that endure beyond the training period in recent-onset schizophrenia, and that training improves later positive symptoms. Future directions should address (1) far transfer and generalization—how do we enhance impact on real-world functioning? (2) dosage—how much training is needed by first-episode individuals? (3) durability— how long do changes last? and would booster sessions increase effects and durability? (4) mechanisms—what neural changes are associated with behavioral change? (5) personalization—which training exercises work best for whom? and (6) what supports are required for real-world implementation and how does that impact motivation? Finally, the addition of social cognition, vocational training, or other psychosocial interventions may also be required to more directly affect social and role functioning and enhance the overall effect sizes.46 Given the importance of cognition to later functioning, our results suggest that CT should be included in first-episode coordinated specialty care to promote full recovery from psychotic disorders.
Supplementary Material
Acknowledgments
We would like to acknowledge Kevin Delucchi, PhD, for consultation on statistical analyses. The cognitive training software used in this study was supplied to the first and last authors free of charge by Posit Science Inc, a company with a commercial interest in the cognitive training software used in this study. None of the authors have any financial interest in Posit Science Inc. The Authors have declared that there are no conflicts of interest in relation to the subject of this study. Portions of this article were presented at the International Congress on Schizophrenia Research in Colorado Springs, CO, March 28–April 1, 2015, and the International Early Psychosis Association meeting in Milan, Italy, October 19–22, 2016.
Funding
This work was supported by the Stanley Medical Research Institute (06TAF-972), the Laszlo N. Family Tauber Foundation, the National Institute of Mental Health (5R01MH081051), the NIH National Center for Advancing Translational Sciences (UL1TR002494), and the San Francisco DVA Medical Center.
References
- 1. Insel TR. Rethinking schizophrenia. Nature. 2010;468(7321):187–193. [DOI] [PubMed] [Google Scholar]
- 2. Nuechterlein KH, Subotnik KL, Green MF, et al. . Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr Bull. 2011;37 (suppl 2):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sakurai H, Bies RR, Stroup ST, et al. . Dopamine D2 receptor occupancy and cognition in schizophrenia: analysis of the CATIE data. Schizophr Bull. 2013;39(3):564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGorry P. Transition to adulthood: the critical period for pre-emptive, disease-modifying care for schizophrenia and related disorders. Schizophr Bull. 2011;37(3):524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495–506. [DOI] [PubMed] [Google Scholar]
- 6. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. [DOI] [PubMed] [Google Scholar]
- 7. Kane JM, Robinson DG, Schooler NR, et al. . Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drake RJ, Day CJ, Picucci R, et al. . A naturalistic, randomized, controlled trial combining cognitive remediation with cognitive-behavioural therapy after first-episode non-affective psychosis. Psychol Med. 2014;44(9):1889–1899. [DOI] [PubMed] [Google Scholar]
- 9. Eack SM, Greenwald DP, Hogarty SS, Keshavan MS. One-year durability of the effects of cognitive enhancement therapy on functional outcome in early schizophrenia. Schizophr Res. 2010;120(1–3):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kidd SA, Herman Y, Virdee G, et al. . A comparison of compensatory and restorative cognitive interventions in early psychosis. Schizophr Res Cogn. 2020;19:100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Østergaard Christensen T, Vesterager L, Krarup G, et al. . Cognitive remediation combined with an early intervention service in first episode psychosis. Acta Psychiatr Scand. 2014;130(4):300–310. [DOI] [PubMed] [Google Scholar]
- 12. Puig O, Penadés R, Baeza I, et al. . Cognitive remediation therapy in adolescents with early-onset schizophrenia: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2014;53(8):859–868. [DOI] [PubMed] [Google Scholar]
- 13. Ueland T, Rund BR. Cognitive remediation for adolescents with early onset psychosis: a 1-year follow-up study. Acta Psychiatr Scand. 2005;111(3):193–201. [DOI] [PubMed] [Google Scholar]
- 14. Wykes T, Newton E, Landau S, Rice C, Thompson N, Frangou S. Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophr Res. 2007;94(1–3):221–230. [DOI] [PubMed] [Google Scholar]
- 15. Subramaniam K, Luks TL, Garrett C, et al. . Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. Neuroimage. 2014;99:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophr Bull. 2010;36(4):869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. 2015;16(9):535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fisher M, Loewy R, Carter C, et al. . Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr Bull. 2015;41(1):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ragland JD, Gur RC, Raz J, et al. . Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. Am J Psychiatry. 2001;158(7):1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keefe RS, Fox KH, Harvey PD, Cucchiaro J, Siu C, Loebel A. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr Res. 2011;125(2–3):161–168. [DOI] [PubMed] [Google Scholar]
- 23. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 24. Strauss JS, Carpenter WT Jr. The prediction of outcome in schizophrenia. II. Relationships between predictor and outcome variables: a report from the WHO international pilot study of schizophrenia. Arch Gen Psychiatry. 1974;31(1):37–42. [DOI] [PubMed] [Google Scholar]
- 25. Cornblatt BA, Auther AM, Niendam T, et al. . Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33(3):688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nuechterlein KH, Green MF, Kern RS, et al. . The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. [DOI] [PubMed] [Google Scholar]
- 27. Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004;10(2):301–303. [DOI] [PubMed] [Google Scholar]
- 28. Strauss E, Sherman EMS, Spreen O.. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York: Oxford University Press; 2006. [Google Scholar]
- 29. Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14(2):167–177. [PubMed] [Google Scholar]
- 30. Smerbeck AM, Parrish J, Yeh EA, et al. . Regression-based pediatric norms for the brief visuospatial memory test: revised and the symbol digit modalities test. Clin Neuropsychol. 2011;25(3):402–412. [DOI] [PubMed] [Google Scholar]
- 31. Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9):1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gałecki A, Burzykowski T. Linear mixed-effects models using R. In: Casella G, Fienberg SE, Olkin I, eds. Springer Texts in Statistics. New York: Springer; 2013. [Google Scholar]
- 33. Morris SB. Estimating effect sizes from Pretest-Posttest-Control Group designs. Organ Res Methods. 2008;11(2):364–386. [Google Scholar]
- 34. Dale CL, Brown EG, Fisher M, et al. . Auditory cortical plasticity drives training-induced cognitive changes in schizophrenia. Schizophr Bull. 2016;42(1):220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loewy R, Fisher M, Schlosser DA, et al. . Intensive auditory cognitive training improves verbal memory in adolescents and young adults at clinical high risk for psychosis. Schizophr Bull. 2016;42 (suppl 1):S118–S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Best MW, Milanovic M, Iftene F, Bowie CR. A randomized controlled trial of executive functioning training compared with perceptual training for schizophrenia spectrum disorders: effects on neurophysiology, neurocognition, and functioning. Am J Psychiatry. 2019;176(4):297–306. [DOI] [PubMed] [Google Scholar]
- 37. Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for schizophrenia: effects on cognition, functional competence, and real-world behavior. Am J Psychiatry. 2012;169(7):710–718. [DOI] [PubMed] [Google Scholar]
- 38. Miley K, Fisher M, Nahum M, et al. . Six month durability of targeted cognitive training supplemented with social cognition exercises in schizophrenia. Schizophr Res Cogn. 2020;20:100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73(4):842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramsay IS, Fryer S, Boos A, et al. . Response to targeted cognitive training correlates with change in thalamic volume in a randomized trial for early schizophrenia. Neuropsychopharmacology. 2018;43(3):590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramsay IS, Fryer S, Roach BJ, et al. . Response to targeted cognitive training may be neuroprotective in patients with early schizophrenia. Psychiatry Res Neuroimaging. 2021;312:111285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roach BJ, Ford JM, Biagianti B, et al. . Efference copy/corollary discharge function and targeted cognitive training in patients with schizophrenia. Int J Psychophysiol. 2019;145:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Biagianti B, Fisher M, Loewy R, et al. . Specificity and durability of changes in auditory processing efficiency after targeted cognitive training in individuals with recent-onset psychosis. Front Psychiatry. 2020;11:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piskulic D, Barbato M, Liu L, Addington J. Pilot study of cognitive remediation therapy on cognition in young people at clinical high risk of psychosis. Psychiatry Res. 2015;225(1–2):93–98. [DOI] [PubMed] [Google Scholar]
- 45. Miller KL. Patient centered care: a path to better health outcomes through engagement and activation. NeuroRehabilitation. 2016;39(4):465–470. [DOI] [PubMed] [Google Scholar]
- 46. Nahum M, Fisher M, Loewy R, et al. . A novel, online social cognitive training program for young adults with schizophrenia: a pilot study. Schizophr Res Cogn. 2014;1(1):e11–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.