Abstract
Objective
This study aimed to provide insight into the efficacy of cognitive-behavioral therapy for psychosis (CBTp) in patients with “clinical high risk of psychosis (CHR-P)”.
Methods
Major scientific databases were searched up to April 17, 2020. Randomized controlled trials in CHR-P individuals, comparing CBTp with needs-based interventions (NBI, including treatment as usual or nonspecific control treatment) were included, following PRISMA guidelines. The primary outcome (efficacy) was transition to psychosis by 6 months, 12 months, 24 months, and over 24 months. Secondary outcomes were change in attenuated psychotic symptoms, depression, distress, improvements in functioning, and quality of life.
Results
Ten randomized controlled studies met inclusion criteria. The comparisons included 1128 participants. CBTp was significantly more efficacious in reducing rate of transition to psychosis by 6 months (after post-hoc sensitivity analysis) (relative risk [RR] = 0.44, 95% confidence interval [CI]: 0.26, 0.73), 12 months (RR = 0.44, 95% CI: 0.30, 0.64), 12 months (RR = 0.46, 95%CI: 0.30, 0.69), and over 24 months (RR = 0.58, 95% CI: 0.35, 0.95) after treatment, compared with those receiving NBI. CBTp was also associated with more reduced attenuated psychotic symptoms by 12 months (SMD = −0.17, 95% CI: −0.33, −0.02) and by 24 months (SMD = −0.24, 95% CI: −0.43, −0.06). No beneficial effects on functioning, depression, quality of life, or distress were observed favoring CBTp.
Conclusions
CBTp is effective in reducing both psychosis transition rates and attenuated psychotic symptoms for the prodromal stage of psychosis. It is a promising intervention at the preventative stage.
Keywords: psychosis, CBTp, cognitive-behavioral therapy, meta-analysis, systematic review, clinical high risk, distress, quality of life, functioning
Introduction
Psychosis is a serious mental health condition with a high global disease burden.1 The unsatisfying prognosis for psychosis has led to the development of early detection and intervention services. In early intervention for psychosis, patients with “clinical high risk of psychosis (CHR-P)” are identified and treated to postpone and prevent the transition to a first psychotic episode.2 Among CHR-P, about 20% are at risk of transition (developing psychosis) within 2 years.3
Cognitive-behavioral therapy for psychosis (CBTp) is a highly recommended first-line treatment for CHR-P individuals in current international guidelines (eg. the National Institute for Health and Care Excellence, NICE, and the European Psychiatric Association, EPA).4,5 The effectiveness of CBTp has been tested in high-income, western cultures such as North America, Europe and Australia. These trials showed evidence that the clinical outcomes, such as transition6 and attenuated psychotic symptoms7,8 of CHR-P population could be improved by CBTp. Some previous meta-analyses have supported the results demonstrated in these trials.6,9,10 However, some other meta-analyses and reviews have questioned the effectiveness of CBTp, reporting negative results when comparing the efficacy of different interventions in preventing transition to psychosis,11 alleviating the severity of positive symptoms12,13 and negative symptoms,14 improving social functioning15 quality of life,10 and acceptability of treatments.11
The latest pairwise meta-analysis published by the Cochrane group concluded that “there is no convincing, unbiased, high-quality evidence to suggest that any type of intervention is of value” for CHR-P people,16 whereas CBTp is one intervention with evidence to supporting its efficacy. According to some of the latest meta-analyses, CBTp has positive impact on some clinical outcomes for CHR-P individuals, even if the differences were not significant. For example, Devoe et al. claimed that CBTp demonstrated a slight trend at reducing attenuated positive psychotic symptoms at long-term follow-up compared to controls.13 On the other hand, the results of this meta-analyses might be influenced by the comparably small number of trials in this field of study and the low-quality of evidence under evaluation, which was also reported in the latest Cochrane systematic review by Bosnjak et al.16 It is necessary to conduct an updated meta-analysis reviewing the comprehensive effectiveness of CBTp for delaying transition and reducing symptoms in subjects with CHR-P, as there are now recently published relevant studies that should be included. In fact, several randomized controlled trials (RCTs) conducted in China, on the effectiveness of CBTp among CHR-P patients, have shown a positive effect.17–19 These articles were not included in the recent meta-analysis of RCTs of CBT in CHR-P, because they were published in the Chinese language and are not well-known to Western researchers. Moreover, these trials were published after the latest Cochrane review, which was updated on August 2017. These trials are useful to extend the current knowledge of the efficacy of CBTp in CHR-P individuals.
We therefore aimed to conduct a systematic review and meta-analysis of RCTs of CBTp in CHR-P, including RCTs in the Chinese language, to determine whether evidence shows that CBTp improves the clinical outcomes of young people at risk of developing psychosis, by comparing the short- and long-term efficacy of this intervention with usual or nonspecific control treatment. The study focuses on 2 main aspects: first, whether CBTp is associated with a significantly reduced rate of transition to psychosis; and secondly, whether CBTp is associated with improved overall symptoms, functioning, and quality of life. We also examined acceptability, as indicated by dropout rate.
Methods
The review protocol was registered in advance with the International Prospective Register of Systematic Reviews (PROSPERO), Protocol No: CRD42020175513.20
Search Strategy and Selection Criteria
A systematic literature search conducted in February 2020 identified 510 articles for potential inclusion. The following databases were included in the search: MEDLINE via Pubmed, EMBASE and PsycINFO via OVID, The Cochrane Library, Chinese Biomedical Literature Database (CBM), China Knowledge Resource Integrated Database (CNKI), VIP Database and Wanfang Database.
Searches were unrestricted regarding language and whether material was published or unpublished. The OpenGrey database (http://www.opengrey.eu) was used to identify unpublished material from the gray literature. Reference lists of published meta-analyses were also examined.
Multiple searches were conducted using the following terms and combinations of terms:
(Prodromal Symptoms OR prodrom* OR ultra-high risk OR clinical high risk OR at risk mental state OR genetic high risk) OR risk of progression OR progression to first-episode OR basic symptoms AND (psychosis) AND (Cognitive Behavio* or “Cognitive Behavioral Therapy) OR CBT) AND (Randomized Controlled Trial OR random* OR RCT)
According to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement,21 the inclusion criteria were constructed using the PICOS acronym: Participants (P): patients with CHR-P according to validated assessments, ie, Comprehensive Assessment of At-Risk Mental States (CAARMS),22 Structured Interview for Psychosis-risk Syndromes (SIPS),23,24 Positive and Negative Syndrome Scale (PANSS),25 or Brief Psychiatric Rating Scale (BPRS).26 Intervention (I): individualized CBTp. (C): Need based interventions (NBI), including treatment as usual (TAU) or a nonspecific control treatment (ie, supportive therapy, monitoring, case management). Outcomes (O): primary outcome: rate of transition to psychosis; secondary outcome: change in attenuated psychotic symptoms, functioning, depression, distress and quality of life. Study design (S): RCT.
We excluded studies (a) focusing on a specific subgroup of patients such as those with a comorbid substance disorder; (b) lacking sufficient data to perform the essential meta-analytical computations; (c) applying inappropriate randomization methods (eg, allocation by alternation or by availability of the intervention). (d) presenting duplicated data (ie, data for the same outcome at the same time point)—in this case, we extracted the data from the study with the largest sample size.
CBTp was defined according to the criteria of the National Institute of Health and Clinical Excellence5: (a) links are established between patients thoughts, feelings or actions and their current or past symptoms and functioning, (b) patient perceptions, beliefs or reasoning are reevaluated in relation to target symptoms.
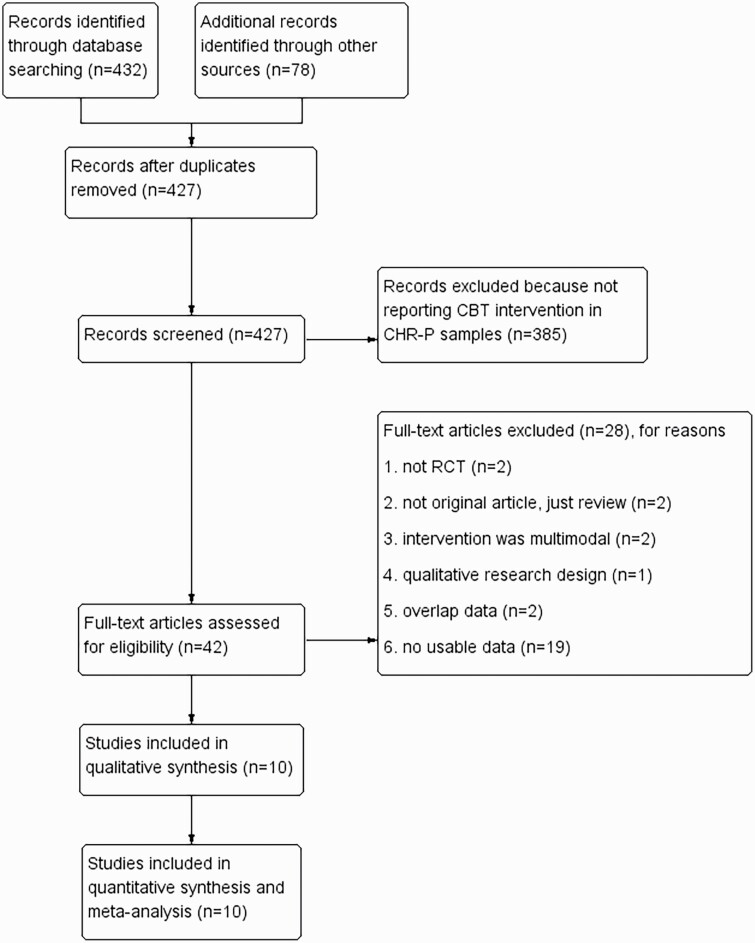
In line with the PRISMA guidance,27 2 researchers (Y.C.Z. and T.T.X.) independently conducted the screening of the search results. Figure 1 demonstrates the flow of study selection. After removing duplicate articles, 2 researchers reviewed the remaining articles independently according to the selection and exclusion criteria, deciding whether the full-text article should be reviewed. The eligible articles which have been screened with full-text reports and met the selection standard were included in our review. Disagreements were discussed with a third researcher (Y.K.Z.) and resolved by consensus.
Fig. 1.
Description of the selection process of studies to be included in the review.
Outcome Measures and Data Extraction
Due to the variable effect of time on clinical outcomes in some studies,17,28 analyses for time-dependent outcomes were conducted. The primary outcome was transition to psychosis. Secondary outcomes were attenuated psychotic symptoms, depression, distress, and improvements in functioning and quality of life. Results with similar follow-up time point (6 months, 12 months, with one 14-month data included, 24 months and more than 24 months after the treatment initiated) were grouped up for analysis.
The psychotic symptoms measures used in studies included: CAARMS, PANSS, BPRS, SOPS. The depression measures used were the Beck Depression Inventory (BDI),29 Brief Symptom Inventory (BSI30) depression, Hamilton Depression Rating Scale (HDRS31), and Calgary Depression Scale (CDSS32). The distress measure used was the CAARMS distress subscale.
For functioning, studies used a variety of clinician-assessed questionnaires, including the Global Assessment of Functioning scale (GAF33); the Social and Occupational Functioning Assessment Scale (SOFAS34). GAF scores were preferred when more than 1 questionnaire was used to measure functioning on a study.
The quality of life measures used in studies included: the Quality of Life Scale (QLS35); the Manchester Short Assessment of Quality of Life (MANSA36).
All-cause discontinuation and Intention-to-Treat (ITT) datasets regarding clinical outcomes were extracted independently by 2 authors (Y.C.Z. and T.T.X.) and checked for consistency mutually. Attempts were made to contact the authors of the study in cases of missing or unusable data.
Assessment of Bias
The Cochrane Risk of Bias tool was applied to assess the risk of bias in each study.37 Using the standardized criteria,37 each study was rated as to whether it was at high, low or unclear risk of bias across 6 specific domains, including random sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessments, incomplete outcome data, and selective outcome reporting. An overall risk of bias classification of high, low or unclear was produced after these domains were assessed. The overall rating of low risk was assigned when none of the 6 domains was found to be at high risk and if 3 or less domains were found to be at unclear risk. The overall rating of moderate risk was assigned when one domain was found to be at high risk; or no domains were found to be at high risk but 4 or more were found to be at unclear risk. In all other cases, the studies were rated as having an overall high risk of bias.38
Meta-analysis
In this meta-analysis, we defined the primary outcome (transition to psychosis) measure as relative risk (RR) and corresponding 95% confidence intervals (CIs), using the Mantel-Haenszel test. RR with 95%CI would be calculated through observed event data according to the method provided by Tierney et al.39
The absolute risk difference (RD) and the numbers needed to treat (NNT) were calculated only if the RR was significant. The NNT was calculated as the inverse of the product of the relative risk reduction (RRR).40
For continuous data, standardized mean difference (SMD) were used when the outcome were measured using different instruments.41 All outcomes are reported with 95% CIs.
A random effects model with a restricted-information maximum likelihood estimate was performed when I2 > 30%, or else a fixed-effects model was used.42 Cohen’s guideline for magnitude of effect: −0.2 to be small, −0.5 to be medium, −0.8 to be large.
Study heterogeneity was measured by visual inspection of forest plot, by using Q test and I2 statistic. An I2 value of 0–40% suggests that heterogeneity may not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% represents considerable heterogeneity.40 Sensitivity analyses were only performed when the heterogeneity was above moderate (I2 ≥ 30%) and at least 4 studies were available for that comparison.43 Potential factors which generated heterogeneity (clinic, methodology, and statistics) would be identified and discussed. Funnel plots were used to assess publication bias in the analysis. At least 10 trials are thought to be sufficient to ensure adequate power for funnel-plot tests.44 All analyses were 2 tailed and the significance level was set at 0.05.
All the above statistical analyses were performed with the help of The Review Manager, Version 5.3 (http://www.cochrane.org).
Results
Study Inclusion and Basic Characteristics of Study
The process of selecting studies is demonstrated in figure 1 (PRISMA diagram). Our database search yielded 510 resulting publications. After removing duplicates, 427 peer-reviewed studies were screened, of which 385 were excluded after abstract and title review. The full-text publications or reports for each of these were traced. A further 29 were then excluded as they did not meet our eligibility criteria. All duplicated data in the cited papers were excluded. Ten RCTs reported in 14 published papers met all inclusion criteria.
Study Characteristics and Treatment
The characteristics and baseline demographics for all studies are given in table 1. Table 1 presents details on studies, including sample sizes, country, number of sessions, treatment duration, measurement time points, dropout-rates, and measurement for primary and key secondary outcomes.
Table 1.
Characteristics of Included RCT Study
| No. | Study | Study arms | Manual | Cohort, country | Total N | Male (%) | Mean age (y) | Study design | Treatment duration (mo) | Number of sessions | Follow-up time points (months) | Entry criteria | Transition criterion | Measurement for primary and key secondary outcomes | Dropout rate (%)a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Addington et al7 | CBT NBI |
CBT-F | ADAPT-Canada | 51 | 71 | 20.9 | SB-RCT | 6 | 20 | 6/12/18 | SIPS | POPS | SOPS positive, GAF, CDSS | 31/39/45 |
| 2 | Bechdolf et al52 | CBT NBI |
IPI | EIPS Germany | 128 | 63 | 26.0 | SB-RCT | 12 | 25 | 12/24 | IRAOS, ERIraosb | PANSS | PANSS, MADRS, GAF | 16/37 |
| 3 | Han et al17 | CBT NBI |
CBT-H | China | 60 | 57 | 21.2 | RCT | 9 | 14 | 3/6 | SIPS | PANSS | PANSS total | NA |
| 4 | McGorry et al45 Yung et al46 |
CBT+RIS CBT+NBI NBI | CBT-M | PACE clinic, Austrialia | 115 | 39 | 18.1 | SB-RCT | 12 | 15 | 6/12 | CAARMS | CAARMS | BPRS total, GAF, HDRS, QLS total | 19/35 |
| 5 | Morrison et al,49,50 | CBT NBI |
CBT-F | EDIE-UK, 5 sites | 60 | 67 | 22 | SB-RCT | 6 | 26 | 12/36 | PANSS | PANSS | NA | 27/55 |
| 6 | Morrison et al8 | CBT NBI |
CBT-F | EDIE-2-UK, 5 sites | 288 | 63 | 20.7 | SB-RCT | 6 | 26 | 6/12/24 | CAARMS | CAARMS | CAARMS severity, GAF, BDI, MANSA | 32/35/43 |
| 7 | Pozza et al29 | CBT NBI |
CBT-V | Italy, 6 sites | 58 | 67 | 25.7 | SB-RCT | 7 | 30 | 14/28 | CAARMS | SCID-I, PANSS | NA | 22/22 |
| 8 | Stain et al51 | CBT NBI |
CBT-F | Australia | 57 | 40 | 16.5 | SB-RCT | 6 | 26 | 6/12 | CAARMS | CAARMS | CAARMS intensity, GAF, BSI depression, QLS interpersonal | 40/53 |
| 9 | Sun et al18,19 | CBT NBI |
CBT-S | China | 110 | 59 | 28.8 | RCT | 6 | 12 | 3/6/12/18 | SIPS | SOPS | PANSS | 10 |
| 10 | van der Gaag et al47 Ising et al48 |
CBT+NBI NBI | CBT-V | EDIE-NL, Netherland | 201 | 49 | 22.7 | SB-RCT | 6 | 26 | 6/12/18/48 | CAARMS | CAARMS, SCAN | CAARMS intensity, SOFAS, BDI, MANSA | 15/25/30/41 |
Note: Abbreviation: RIS: risperidone; NBI: needs-based therapy; RCT: randomized controlled trial; SB-RCT: single-blind randomized controlled trial; Questionnaires: BDI: Beck Depression Inventory; BPRS: Brief Psychiatry Rating Scale; BSI: Brief Symptom Inventory; CAARMS: Comprehensive Assessment of the At Risk Mental State; CDSS: Calgary Depression Scale; COPS: Criteria of Prodromal States; ERIraos: Early Recognition Inventory; GAF: Global Assessment of Functioning; HDRS: Hamilton Depression Rating Scale; IRAOS: the Interview for the Retrospective Assessment of the Onset of Schizophrenia; MANSA: Manchester Short Assessment of Quality of Life; MADRS: Montgomery Asberg Depression Rating Scale; PANSS: the Positive and Negative Syndrome Scale; POPS: The Presence of Psychotic Symptoms; QLS: Quality of Life Scale; SCAN: Schedules for Clinical Assessment in Neuropsychiatry; SCID-I: Structured Clinical Interview for DSM-IV; SIPS: the Structured Interview for Prodromal Symptoms; SOFAS: Social and Occupational Functioning Assessment Scale; SOPS: scale of prodromal symptoms.
aThe dropout rates at each follow-up time points were recorded in the table.
bHaFner H, Maurer K, Ruhrmann S, et al. Early detection and secondary prevention of psychosis: facts and visions*[J]. Eur Arch Psychiatry Clin Neurosci, 2004, 254(2):117–128.
The included trials enrolled 1128 participants with a median sample size of 112 (range 51–288), and with a mean age of 22.33 years, and of whom 57.5% were male (table 1). The countries of origin of the publications were Great Britain (2 studies), Canada (1 study), Australia (2 studies), Italy (1 study), Germany (1 study), Netherlands (1 study), and China (2 studies).
In order to define the entry criteria of prodromal stage of psychosis, 5 studies utilized the comprehensive assessment of at-risk mental states (CAARMS)22 as the screening instrument; 3 studies has applied the Structured Interview for Prodromal Symptoms (SIPS); 1 study made this assessment based on the Positive and Negative Syndrome Scale (PANSS) cutoff scores.
Among the 10 included studies, 2 of which made comparisons between the effectiveness of CBTp together with NBI and NBI alone,45–48 while the others made comparisons between the effectiveness of CBTp and NBI only.7,8,17–19,28,49–52 NBI includes monitoring,8,18,19,49,50 supportive therapy,7,17,28,45,46,52 treatment as usual47,48 and non-directive reflective listening.51
For intervention, all studies incorporated elements of CBT, including normalization, cognitive restructuring, and behavioral experiments. 4 studies8,48–51 applied the CBT protocol developed by Morrison and French et al.53 (CBT-F). 2 studies,28,47,48 one of them from Early Detection, Intervention and Evaluation (EDIE) trial, have utilized the CBT model developed by Van der Gaag et al. (CBT-V), which was enriched from CBT-F by adding psychoeducation on how dopamine supersensitivity may affect perception and thinking. One study of the Personal Assessment and Crisis Evaluation (PACE) Clinic applied the manual developed by McGorry et al. (CBT-M), with 4 modules including stress management, positive symptoms, negative symptoms/depression and other comorbidity.54 One study has delivered the Integrated psychological intervention (IPI), with individual CBT as its core module.52 The Chinese studies have applied CBT manuals developed by their research groups (CBT-H, CBT-S).17–19
Transition to psychosis was defined with several instruments, including Scale of Prodromal symptom (SOPS),19,55 The Presence of Psychotic Symptoms (POPS),7,56 diagnosis of psychotic disorders/bipolar disorders using the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV)28,57; PANSS25 symptom severity17,49,50,52 and CAARMS22 symptom severity.8,45–48,51 One study assessed the transition with the Schedules for Clinical Assessment in Neuropsychiatry (SCAN).47,48,58
The treatment duration of CBTp ranged from 6 to 12 (mean = 7.29, median = 6) months. The number of CBTp sessions ranged from 12 to 30 sessions (mean = 21.47, median = 26).
Compliance with the intervention was reported in 9 studies, with dropout rates ranging from 10 to 53%. The mean follow-up period across studies was 20 months (SD = 11.56), ranging from 6 months to 48 months.
Risk of Bias
The method of randomization, particularly the random sequence, was unclear in one of the 10 studies. Five studies provided information on the blinding of assessors. Three studies have complete outcome data. Only 2 studies had an unclear overall risk, and the remaining 8 studies had overall high risk. The full risk of bias assessment is presented in supplementary figure 7.
Result of Meta-analysis
Primary Outcome: Effect of CBTp on Transition Rates
Transition by 6 Months
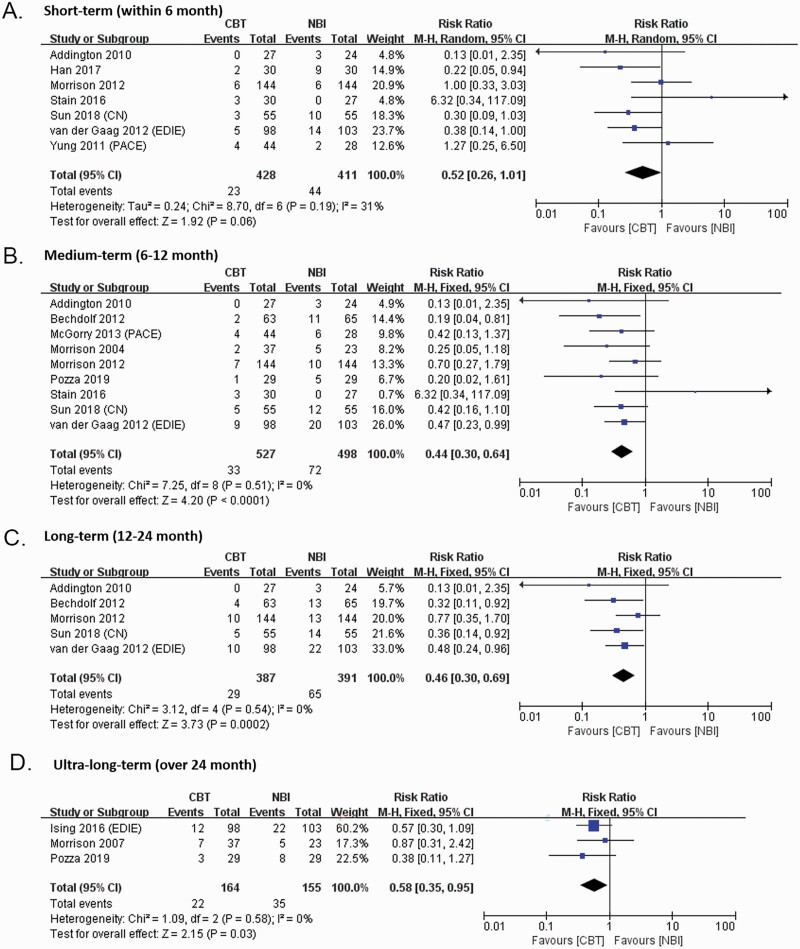
Seven trials contributed to this outcome, providing a total of 839 participants (428 received CBTp and 411 were in the control condition).
Of the 7 trials, 5 compared CBTp to NBI, while the remaining compared CBTp plus NBI to NBI. No difference was observed (RR = 0.52, 95% CI: 0.26, 1.01, P = 0.06 just failed to meet the criterion for statistical significance) in the fixed-effects analysis by 6 months. The studies were moderately heterogeneous (Q = 8.70, df = 6, P = .19) with an I2 value of 31 (figure 2).
Fig. 2.
Forest plot for post-intervention rate of transition to psychosis.
The result of leave-one-out sensitivity analyses showed that the statistical significance was dependent on the presence of the study by Stain et al.51 The magnitude and precision of effect favoring CBTp by 6 months was enhanced if we excluded this study, according to fixed-effects analyses (6 studies, RR = 0.44, 95% CI: 0.26, 0.73, P = .002) (supplementary figure 1). The NNT for one person to avoid transition to psychosis (by 6 months) was 19 (95% CI: 11, 60; based on an RD of –0.053).
Transition by 12 Months
Nine trials provided usable data from 1025 participants (527 received CBTp and 498 were in the control condition). Of the 9 trials, 7 compared CBTp to NBI, while the remaining 2 compared CBTp plus NBI to NBI. The pooled effect size for transition rates at 6–12 months across 1025 samples was 0.44 (95%CI: 0.30, 0.64, P < .0001, positive sign indicates CBTp better than control). The studies were low heterogeneous (Q = 7.25, df = 8, P = .51) with an I2 value of 0 (figure 2). The NNT was 12 (95% CI: 8, 22; based on an RD of –0.0820).
Transition by 24 Months
Five trials provided usable data from 778 participants (387 received CBTp and 391 were in the control condition). Of the 5 trials, 4 compared CBTp to NBI, while in the other one compared CBTp plus NBI with NBI. The effect size was significant at 0.46 (95% CI: 0.30, 0.69, P = .0002); and showed low heterogeneity (Q = 3.12, df = 4, P = 0.54; I2 = 0). The NNT was 11 (95% CI: 7, 22; based on an RD of –0.046).
Transition Over 24 Months
Three trials provided usable data from 319 participants (164 received CBTp and 155 were in the control condition). Of the 3 trials, 2 compared CBTp to NBI, while in the other one compared CBTp plus NBI with NBI. The effect size was significant at 0.58 (95%CI: 0.35, 0.95, p = 0.03); and showed low heterogeneity (Q = 1.09, df = 2, P = 0.58; I2 = 0). The NNT was 11 (95% CI: 6, 130; based on an RD of –0.0917).
Effects on Secondary Outcomes
We also explored the effects of CBTp on attenuated psychotic symptoms (by 6 months: 7 studies; 12 months: 7 studies; 24 months: 4 studies), functioning (by 6 months: 5 studies; 12 months: 6 studies; 24 months: 3 studies), depression (by 6 months: 4 studies; 12 months: 5 studies; 24 months: 3 studies), distress (by 6 months: 3 studies; 12 months: 3 studies; 24 months: 2 studies), and quality of life (by 6 months: 4 studies; 12 months: 4 studies; 24 months: 2 studies).
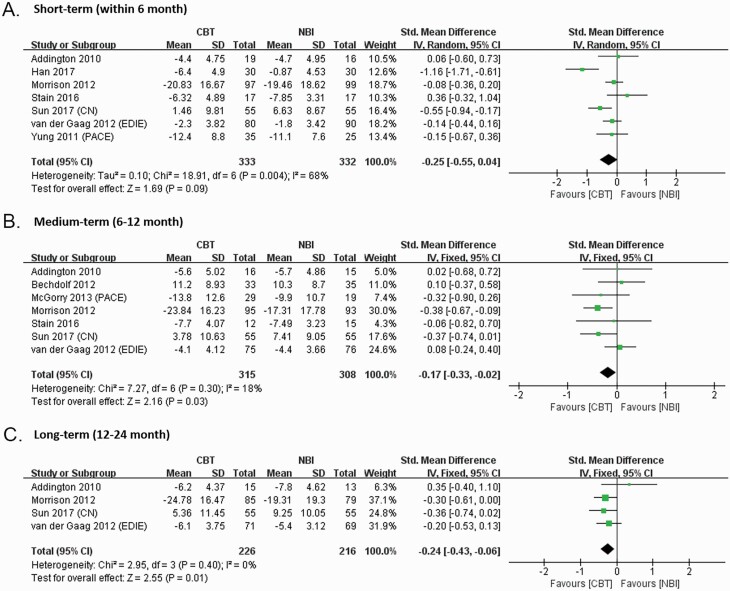
Post-intervention between-group effects on all secondary outcomes were in the small/medium to large range but none were statistically significant by 6 months (attenuated psychotic symptoms: SMD = −0.25, 95% CI: −0.55, 0.04; 7 studies, N = 665; functioning: SMD = −0.05, 95% CI: −0.23, 0.12; 5 studies, N = 492; depression: SMD = 0.18, 95% CI: −0.39, 0.75; 4 studies, N = 458; distress: SMD = −0.11, 95% CI: −0.46, 0.23; 3 studies, N = 399; quality of life: SMD = 0.01, 95% CI: −0.24, 0.26; 4 studies, N = 455) (figure 3, supplementary figure 3–6). Leave-one-out sensitivity analyses showed that the effect on attenuated psychotic symptoms was influenced by one study; after removing the study of Han et al., the magnitude and precision of the effect was reduced, although still without statistical significance (SMD = −0.15, 95% CI: −0.31, 0.01; 6 studies, N = 605) (supplementary figure 2).
Fig. 3.
Forest plot for post-intervention between-group effect sizes on attenuated psychotic symptoms (SOPS positive score, BPRS total score, PANSS total score, CAARMS severity score), the difference scores of post-treatment minus pretreatment (higher score = worse).
However, at follow-up (by 12 months and 24 months), effects on attenuated psychotic symptoms were significant (by 12 months: SMD = −0.17, 95% CI: −0.33, −0.02; 7 studies, N = 623; by 24 months: SMD = −0.24, 95% CI: −0.43, −0.06; 4 studies, N = 442) (figure 3). The effects of the other outcomes remained non-significant (by 12 months: functioning: SMD = 0.31, 95% CI: −0.06, 0.68; 6 studies, N = 549; depression: SMD = −0.01, 95% CI: −0.32, 0.29; 5 studies, N = 482; quality of life: SMD = 0.05, 95% CI: −0.15, 0.24; 4 studies, N = 414; by 24 months: functioning: SMD = 0.15, 95% CI: −0.06, 0.37; 3 studies, N = 332; depression: SMD = −0.00, 95% CI: −0.63, 0.62; 2 studies, N = 332; distress: SMD = −5.16, 95% CI: −11.06, 0.73; 2 studies, N = 304; quality of life: SMD = 4.38, 95% CI: − 1.51, 10.27; 2 studies, N = 304) (supplementary figures 3–6), although the effect on distress outcomes by 12 months only just failed to meet the criteria for statistical significance (SMD = −0.20, 95% CI: −0.41, 0.01; 3 studies, N = 366) (supplementary figure 5). There were too few studies with measures of secondary outcomes to warrant moderation analysis.
Discussion
Previous meta-analyses have not achieved robust agreement on whether CBTp reduces the transition rate of CHR (vide supra). Six meta-analyses have examined the impact of CBTp on transition,6,9–11,16,59 and one meta-analysis has reviewed the effects of CBTp on attenuated psychosis symptoms,13 while ours is the first to examine the impact of CBTp across a broader range of clinical outcomes (including non-symptomatic outcomes) with the latest data including non-westerner samples. Our work has also analyzed the pooled effect sizes of the secondary outcomes in individual studies, which is not included in the latest Cochrane reports.16
Interpretation of the Results
The results of the primary outcome in this meta-analysis are consistent with 4 previous meta-analyses,6,9,10,59 suggesting that CBTp could prevent or delay of the onset of psychosis by 12 months and 24 months. CBTp is associated with a significantly reduced rate of transition to first-episode psychosis by 6 months (after post-hoc sensitivity analysis), 12 months, 24 months, and over 24 months compared with those receiving NBI. The number of participants needed to treat to avoid transition by 6 months, 12 months, 24 months, and over 24 months are respectively 19, 12, 11, 11, which indicates that the effect of the therapy does not decline across the time.
CBTp also showed a small but robust superiority in reducing attenuated positive symptoms 12 months (−0.17) and 24 months (−0.24) after treatment. The effect by 6 months was not robust even after post-hoc sensitivity analysis.
Furthermore, the results revealed that CBTp did not significantly improve functioning, depression, or quality of life. These findings accord with earlier smaller analyses of functioning and quality of life, by Hutton et al10 and the Cochrane Collaboration,16 both of which found no evidence of CBTp showing significant superiority for these outcomes.
Implication From This Research
The current number of trials remains quite small (10 studies), and fewer trials have provided data about secondary outcomes such as distress, functioning and quality of life. There were even fewer studies that provided efficacy results at long-term follow-up. Future studies with longer follow-up periods are in need, in order to provide more useful data and adequate power, especially in terms of secondary outcomes. Possible adverse effects of CBTp were not directly examined in this study, but according to the results of secondary outcomes, CBTp is unlikely to aggravate clinical symptoms (eg, depression), functioning or quality of life. It is suggested that self-harm, suicidal ideation, and behaviors should be included in the assessment of side effects in future studies, considering the evidence that self-harm and suicidality are highly prevalent in the CHR population.60
The study of Stain et al. was found causing the heterogeneity of transition by 6 months. The results of this study showed that the control condition, NDRL, resulted in a lower transition rate and a significantly higher reduction in distress associated with psychotic symptoms when compared with CBTp.51 However, this trial was underpowered because of the small sample size (N = 57) and high dropout rate (6 months: 40%; 12 months: 53%). The lack of a treatment effect for CBTp might also be due to the difficulty younger adolescents have in fully engaging in the intervention, considering that the average age of participants in this trial (16 years) was lower than the mean age of the sample included in the other trials (23.03 years). Other possibilities (such as the potential ceiling effect cause by the better overall functioning and milder psychotic symptoms of the sample, when compared with other trials) were also discussed in the study.51 Future research could compare the clinical outcomes among different subgroups of CHR after treatment with CBTp. Strict inclusion criteria are needed, or the required sample size should be larger.
Compared with the latest study of the Cochrane Collaboration,16 this meta-analysis has included 3 new trials (4 published papers), 1 from Italy28 and 2 from China.17–19 According to the results of quality assessment, the study of Pozza et al. has unclear risk of bias, while the 2 trials from China have high risk. The problem of low-quality could be avoided with adequate study design and a larger number of participants. The latest Cochrane study suggests the sample size should be 300, in order to detect a difference in groups of 15%.16 Considering this standard, there is just one trial,8 which is also the most rigorously conducted, that has met the demand of sample size.
The Chinese studies have utilized CBTp with a smaller number of sessions (mean = 13), when compared with other studies (mean = 25.1). NICE has suggested that CBTp should be delivered with a minimum of 16 planned sessions,5 however, evidence is lacking about the effectiveness of low-intensity CBTp (defined as CBTp interventions with less than 16 sessions of face-to-face contact time) among CHR population. Although studies have been conducted in patients with psychotic disorders, the results are inconsistent.61,62 The minimal dose of CBTp for CHR is still open.
Moreover, the manuals of the Chinese studies were developed by the local specialist. Although the description of the models used met the inclusion criteria of this study, the specific contents of these manuals were not clear. The study of Hans et al. was found causing the heterogeneity of the change in attenuated psychotic symptoms by 6 months, which might be related with the factors discussed above. In order to ensure that a practical and evidence-based treatment could be rolled out in China, with its specific culture and a lack of training for mental health professionals,63 a carefully designed manual is required, including skilled training and supervision.
On the basis of this review, further research, especially high-quality research in non-Western countries, is clearly warranted to determine the benefits of CBTp amongst CHR population. Valuable information, such as which subgroups of CHRs may benefit from CBTp, the predictive indicators of the effectiveness of the intervention, the minimal dose of CBTp for CHR, the results of secondary outcomes (such as side effect) and the therapeutic factors of the treatment is also needed.
Limitations of This Study
The results of this meta-analysis should be interpreted with caution due to several limitations. The transition criteria varied among the studies, which might influence the result of transition rates. Although the random effects model and standard mean differences were used, significant heterogeneity exists in some secondary outcomes, which might be related with the heterogeneity of samples selected by different entry criteria. The heterogeneity of results might also be influenced by therapists who offered the treatments - however, few studies provided descriptions. Most of the RCTs included were at high/unclear risk of bias, which would influence the overall quality of our meta-analysis. This problem has been partially controlled through assessment of biases and sensitivity analyses. Adverse effects and cost-effectiveness were not analyzed in this study because these were infrequently reported and could not be combined for meta-analysis. Furthermore, the limited number of trials with usable outcome data precludes most of the tests for publication bias (eg,funnel-plot tests44).
Conclusion
Our meta-analysis presents updated data assessing the impact of CBTp on the transition rate, functioning, quality of life and distress reduction in CHR-P individuals. While the case for beneficial effects on functioning, depression, quality of life and distress appears, from studies to date, to be weak, current evidence does support the hypothesis that CBTp could significantly reduce transition rates by 12 months, 24 months, and over 24 months. Benefits could also be observed in reducing attenuated psychotic symptoms by 12 months and 24 months, with less robust evidence.
Supplementary Material
Acknowledgments
We thank all the participants for their volunteering to our study. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
We have been supported by the following grants: Ministry of Science and Technology of China, National Key R&D Program of China (2016YFC1306800), National Natural Science Foundation of China (81671329, 81671332, 81971251), Science and Technology Commission of Shanghai Municipality (19441907800, 19ZR1445200, 17411953100, 16JC1420200, No.2018SHZDZX01, 19410710800, 19411969100), The Clinical Research Center at Shanghai Mental Health Center (CRC2018ZD01, CRC2018ZD04, CRC2018YB01, CRC2019ZD02), Shanghai Clinical Research Center for Mental Health (19MC1911100), Funds for talents by Shanghai Mental Health Center (2019-QH-04), and Shanghai Mental Health Center Foundations (2019-YJ-07).
References
- 1. Charlson FJ, Ferrari AJ, Santomauro DF, et al. Global epidemiology and burden of schizophrenia: findings from the Global Burden of Disease Study 2016. Schizophr Bull. 2018;44(6):1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marshall M, Rathbone J. Early intervention for psychosis. The Cochrane database of systematic reviews. 2011;6:Cd004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fusar-Poli P, Cappucciati M, Borgwardt S, et al. Heterogeneity of psychosis risk within individuals at clinical high risk: a meta-analytical stratification. JAMA Psychiatry. 2016;73(2):113–120. [DOI] [PubMed] [Google Scholar]
- 4. EPGW G. Australian Clinical Guidelines for Early Psychosis. 2nd ed. Melbourne, Australia: Orygen: The National Centre of Excellence in Youth Mental Health; 2010. [Google Scholar]
- 5.National Collaborating Centre for Mental Health (UK). Psychosis and Schizophrenia in Adults: Treatment and Management: Updated Edition 2014. London, UK: National Institute for Health and Care Excellence; 2014. [PubMed] [Google Scholar]
- 6. Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ. 2013;346:f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophr Res. 2011;125(1):54–61. [DOI] [PubMed] [Google Scholar]
- 8. Morrison AP, French P, Stewart SL, et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ. 2012;344:e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okuzawa N, Kline E, Fuertes J, et al. Psychotherapy for adolescents and young adults at high risk for psychosis: a systematic review. Early Interv Psychiatry. 2014;8(4):307–322. [DOI] [PubMed] [Google Scholar]
- 10. Hutton P, Taylor PJ. Cognitive behavioural therapy for psychosis prevention: a systematic review and meta-analysis. Psychol Med. 2014;44(3):449–468. [DOI] [PubMed] [Google Scholar]
- 11. Davies C, Cipriani A, Ioannidis JPA, et al. Lack of evidence to favor specific preventive interventions in psychosis: a network meta-analysis. World Psychiatry. 2018;17(2):196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies C, Radua J, Cipriani A, et al. Efficacy and acceptability of interventions for attenuated positive psychotic symptoms in individuals at clinical high risk of psychosis: a network meta-analysis. Front Psychiatry. 2018;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devoe DJ, Farris MS, Townes P, Addington J. Attenuated psychotic symptom interventions in youth at risk of psychosis: a systematic review and meta-analysis. Early Interv Psychiatry. 2019;13(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Devoe DJ, Peterson A, Addington J. Negative symptom interventions in youth at risk of psychosis: a systematic review and network meta-analysis. Schizophr Bull. 2018;44(4):807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Devoe DJ, Farris MS, Townes P, Addington J. Interventions and social functioning in youth at risk of psychosis: a systematic review and meta-analysis. Early Interv Psychiatry. 2019;13(2):169–180. [DOI] [PubMed] [Google Scholar]
- 16. Bosnjak Kuharic D, Kekin I, Hew J, Rojnic Kuzman M, Puljak L. Interventions for prodromal stage of psychosis. Cochrane Database of Systematic Reviews. 2019;2019(11):CD012236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han Haibin WT, Liu Xiaocui, Liu Jie, Cheng Yaling, Tian Bo. A comparative study of cognitive behavioral therapy for social function in patients with schizophrenia at high risk. Journal of Qiqihar University of Medicine. 2017;38(19):2262–2264. [Google Scholar]
- 18. Sun Xirong SD, Zhang Jie, Tong Jie, et al. The effect of cognitive behavioral therapy on psychiatric symptoms of the”Ultra-high-risk”Psychosis population. Chin J Health Psychol. 2017;25(9):1281–1285. [Google Scholar]
- 19. Sun Xirong SD, Zhang Jie, Tong Jie, et al. Effect of cognitive behavior therapy on the symptoms and conversion of ultra-high-risk of schizophrenia. Chinese Journal of Behavioral Medicine and Brain Science. 2018;27(2):121–126. [Google Scholar]
- 20. Zheng Y. Cognitive behavioural therapy for prodromal stage of psychosis - outcomes for transition, functioning, distress and quality of life: a meta-analysis. PROSPERO. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 2005;39(11-12):964–971. [DOI] [PubMed] [Google Scholar]
- 23. McGlashan TH, Walsh BC, Woods SW.. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
- 24. Fusar-Poli P, Cappucciati M, Rutigliano G, et al. Towards a standard psychometric diagnostic interview for subjects at ultra high risk of psychosis: CAARMS versus SIPS. Psychiatry J. 2016;2016:7146341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 26. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. 1988;24:97–99. [Google Scholar]
- 27. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. [DOI] [PubMed] [Google Scholar]
- 28. Pozza A, Dèttore D. Modular cognitive-behavioral therapy for affective symptoms in young individuals at ultra-high risk of first episode of psychosis: Randomized controlled trial. J Clin Psychol. 2020;76(3):392–405. [DOI] [PubMed] [Google Scholar]
- 29. Rosner RI. Beck Depression Inventory (BDI). New Jersey: John Wiley & Sons, Inc.; 2015. [Google Scholar]
- 30. Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 31. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary depression scale. The British journal of psychiatry Supplement. 1993;22:39–44. [PubMed] [Google Scholar]
- 33. Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF). Br J Psychiatry. 1995;166(5):654–659. [DOI] [PubMed] [Google Scholar]
- 34. Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149(9):1148–1156. [DOI] [PubMed] [Google Scholar]
- 35. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388–398. [DOI] [PubMed] [Google Scholar]
- 36. Priebe S, Huxley P, Knight S, Evans S. Application and results of the Manchester Short Assessment of Quality of Life (MANSA). Int J Soc Psychiatry. 1999;45(1):7–12. [DOI] [PubMed] [Google Scholar]
- 37. Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Furukawa TA, Salanti G, Atkinson LZ, et al. Comparative efficacy and acceptability of first-generation and second-generation antidepressants in the acute treatment of major depression: protocol for a network meta-analysis. BMJ Open. 2016;6(7):e010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 41. Cooper H, Hedges LV. The handbook of research synthesis. BMJ. 1994;309(3):458. [Google Scholar]
- 42. Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. 2005;30(3):261–293. [Google Scholar]
- 43. Turner DT, van der Gaag M, Karyotaki E, Cuijpers P. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171(5):523–538. [DOI] [PubMed] [Google Scholar]
- 44. Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176(8):1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McGorry PD, Nelson B, Phillips LJ, et al. Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: twelve-month outcome. J Clin Psychiatry. 2013;74(4):349–356. [DOI] [PubMed] [Google Scholar]
- 46. Yung AR, Phillips LJ, Nelson B, et al. Randomized controlled trial of interventions for young people at ultra high risk for psychosis: 6-month analysis. J Clin Psychiatry. 2011;72(4):430–440. [DOI] [PubMed] [Google Scholar]
- 47. van der Gaag M, Nieman DH, Rietdijk J, et al. Cognitive behavioral therapy for subjects at ultrahigh risk for developing psychosis: a randomized controlled clinical trial. Schizophr Bull. 2012;38(6):1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ising HK, Kraan TC, Rietdijk J, et al. Four-Year follow-up of cognitive behavioral therapy in persons at ultra-high risk for developing psychosis: The Dutch Early Detection Intervention Evaluation (EDIE-NL) Trial. Schizophr Bull. 2016;42(5):1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morrison AP, French P, Parker S, et al. Three-year follow-up of a randomized controlled trial of cognitive therapy for the prevention of psychosis in people at ultrahigh risk. Schizophr Bull. 2007;33(3):682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry. 2004;185:291–297. [DOI] [PubMed] [Google Scholar]
- 51. Stain HJ, Bucci S, Baker AL, et al. A randomised controlled trial of cognitive behaviour therapy versus non-directive reflective listening for young people at ultra high risk of developing psychosis: The detection and evaluation of psychological therapy (DEPTh) trial. Schizophr Res. 2016;176(2-3):212–219. [DOI] [PubMed] [Google Scholar]
- 52. Bechdolf A, Wagner M, Ruhrmann S, et al. Preventing progression to first-episode psychosis in early initial prodromal states. Br J Psychiatry. 2012;200(1):22–29. [DOI] [PubMed] [Google Scholar]
- 53. French P, Morrison AP.. Early Detection and Cognitive Therapy for People at High Risk of Developing Psychosis: A Treatment Approach. New Jersey: John Wiley & Sons; 2004. [Google Scholar]
- 54. Phillips LJFS. Changing PACE: psychological interventions in the presychotic phase. In: Gleeson J, McGorry PD, eds. Psychological Interventions in Early Psychosis A treatment Handbook. Chichester, UK: John Wiley & Sons; 2004:23–40. [Google Scholar]
- 55. Miller TJ, Zipursky RB, Perkins D, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. II. Baseline characteristics of the “prodromal” sample. Schizophr Res. 2003;61(1):19–30. [DOI] [PubMed] [Google Scholar]
- 56. McGlashan TH, Zipursky RB, Perkins D, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. I. Study rationale and design. Schizophr Res. 2003;61(1):7–18. [DOI] [PubMed] [Google Scholar]
- 57. Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 58. Petrangeli L, Nicolaou S. [A training course of SCAN use (Schedules for Clinical Assessment in neuropsychiatry)]. Epidemiol Psichiatr Soc. 1997;6(3):215–217. [DOI] [PubMed] [Google Scholar]
- 59. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1-3):56–62. [DOI] [PubMed] [Google Scholar]
- 60. Taylor PJ, Hutton P, Wood L. Are people at risk of psychosis also at risk of suicide and self-harm? A systematic review and meta-analysis. Psychol Med. 2015;45(5):911–926. [DOI] [PubMed] [Google Scholar]
- 61. Hazell CM, Hayward M, Cavanagh K, Strauss C. A systematic review and meta-analysis of low intensity CBT for psychosis. Clin Psychol Rev. 2016;45:183–192. [DOI] [PubMed] [Google Scholar]
- 62. Lincoln TM, Jung E, Wiesjahn M, Schlier B. What is the minimal dose of cognitive behavior therapy for psychosis? An approximation using repeated assessments over 45 sessions. Eur Psychiatry. 2016;38:31–39. [DOI] [PubMed] [Google Scholar]
- 63. Li W, Zhang L, Luo X, et al. A qualitative study to explore views of patients’, carers’ and mental health professionals’ to inform cultural adaptation of CBT for psychosis (CBTp) in China. BMC Psychiatry. 2017;17(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.