Abstract
Schizophrenia (SZ) and bipolar disorder (BD) share some similarities in terms of genetic-risk genes and abnormalities of gray-matter structure in the brain, but white matter (WM) abnormalities have not been studied in depth. We undertook a comparative multimodal meta-analysis to identify common and disorder-specific abnormalities in WM structure between SZ and BD. Anisotropic effect size-signed differential mapping software was used to conduct a comparative meta-analysis of 68 diffusion tensor imaging (DTI) and 34 voxel-based morphometry (VBM) studies comparing fractional anisotropy (FA) and white matter volume (WMV), respectively, between patients with SZ (DTI: N = 1543; VBM: N = 1068) and BD (DTI: N = 983; VBM: N = 518) and healthy controls (HCs). The bilateral corpus callosum (extending to the anterior and superior corona radiata) showed shared decreased WMV and FA in SZ and BD. Compared with BD patients, SZ patients showed remarkable disorder-specific WM abnormalities: decreased FA and increased WMV in the left cingulum, and increased FA plus decreased WMV in the right anterior limb of the internal capsule. SZ patients showed more extensive alterations in WM than BD cases, which may be the pathophysiological basis for the clinical continuity of both disorders. The disorder-specific regions in the left cingulum and right anterior limb of the internal capsule provided novel insights into both disorders. Our study adds value to further understanding of the pathophysiology, classification, and differential diagnosis of SZ and BD.
Keywords: bipolar disorder, diffusion tensor imaging, schizophrenia, voxel-based morphometry, white matter microstructure, fiber crossing
Introduction
Schizophrenia (SZ) and bipolar disorder (BD) are leading causes of disability worldwide.1 They are classified as two distinct disorders under the current diagnosis framework.2 However, the associations between SZ and BD have garnered increasing interest. Studies in families3 and twins4 have shown that both disorders aggregate in families, and there is additional evidence showing semblable changes in gene expression in both conditions.5 In addition, psychotic symptoms, such as hallucinations or delusions, are not only the typical symptoms of SZ but also occur in extreme manic episodes of bipolar-I disorder.6,7Diagnostic and Statistical Manual of Mental Disorders (DSM-5) has addressed the differential diagnosis by adding a dimensional assessment of transverse symptom severity.2 Nevertheless, the symptomatic overlaps between SZ and BD might indicate that both disorders could share a common pathological mechanism and that the distinct clinical phenotypes of the two diseases should have different pathological bases. Thus, further studies comparing the similarities and differences in neural mechanisms between these two disorders would be valuable for understanding the underlying pathophysiological basis of the clinical spectrum of psychosis. They would also contribute to a clearer diagnosis and classification, thereby enabling guidance of “precision medicine” to improve the prognosis.
An accumulating amount of evidence on impaired white matter (WM) tracts and abnormal structural and functional connectivity8,9 across brain regions have indicated the dysconnectivity between brain regions in SZ and BD.10,11 WM alterations have provided remarkable insights into the possible pathophysiology or causes of these disorders. WM abnormalities, in general, include white matter volume (WMV) deficits or disruption of the microstructure of WM pathways.12 Voxel-based morphometry (VBM) is a whole-brain, automatic method employed to characterize WMV in separate regions quantitatively.13,14 Moreover, diffusion tensor imaging (DTI) is used to assess WM microstructure.15 Importantly, fractional anisotropy (FA) derived from DTI can reflect fiber density, axonal diameter, and myelination in WM, which is thought to be a general measure to observe the microstructure of WM fiber tracts in the brain.16
The coupling of WMV and FA has been suggested to enhance the understanding of fiber morphometry.17,18 Specifically, WMV and FA are positively associated in areas where fiber tracts are organized in parallel. However, in the case of fiber crossing, the association between WMV and FA would become negative. In the two tracts crossing the midline of WM areas, one is thicker (i.e., dominant) and the other(s) thinner (i.e., non-dominant) in healthy controls (HCs). The predominance of the dominant tract forces water to flow mainly in only one direction (i.e., that of the dominant tract), which translates to relatively high FA. Following this hypothesis, Radua et al.17 demonstrated that an increase in the thickness of a non-dominant tract (e.g., increase of fiber crossing) could lead to increased WMV and decreased FA in patients with obsessive-compulsive disorder. Thus, coordinates-based imaging meta-analysis with novel multimodal methods for a combination of different imaging modalities in the same meta-analysis could offer insights that are not apparent from any given imaging modality alone.19
Studies have explored the shared and distinct WM abnormalities in BD and SZ.20,21 On the one hand, DTI and VBM have shown that both disorders share WM abnormalities in the internal capsule, uncinate fasciculus, and anterior thalamic radiation regions, and there were no differences in WM abnormalities between these two disorders.22,23 Conversely, studies have suggested that SZ showed significantly lower FA in the left external capsule, right thalamo-occipital, thalamo-parietal tracts,11 fronto-occipital tracts,24 temporal and occipital WM25 in comparison with BD and HC groups. A VBM study reported that SZ displayed more extensive structural alterations in WMV relative to that in BD.26 Such inconsistent findings have been driven by small sample size, the methods used, sample characteristics, and imaging modalities.
A meta-analysis is a useful tool to integrate the results from existing studies in an unbiased way.27 Several meta-analyses have been conducted to explore the WM abnormalities in BD and SZ, respectively, which limits understanding of the common pathophysiological basis of the clinical continuum of psychosis.28,29 A timely meta-analysis covering studies on the structure of the whole brain using magnetic resonance imaging (MRI) showing WM abnormalities in SZ and BD could reliably identify shared or disorder-specific WM abnormalities between these two illnesses. Such data could provide a reference for future differential diagnoses and treatment development.
In this comparative multimodal meta-analysis of abnormalities of WM structure, we aimed to identify the shared and distinct WM abnormalities in BD and SZ. We hypothesized that both disorders would show significantly decreased WMV and FA in fibers associated with psychotic symptoms, cognition, and emotion relative to that seen in HCs. Moreover, across the spectrum of psychiatric disorders, SZ is regarded as a more serious mental disorder than BD, and the pathological process common to both disorders might be expressed more strongly in SZ.20,30 Therefore, we hypothesized that the regions of decreased WMV and FA would be more extensive in SZ compared with that of BD. In addition to common WM abnormalities, we expected a disorder-specific WM abnormality in SZ and BD to be found using multimodal analyses.
Materials and Methods
Literature Searching and Selection of Studies
Our meta-analysis was conducted following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).31 A comprehensive literature search was conducted using Pubmed and Web of Science databases from inception to February 2020 to identify WM studies of VBM and DTI in SZ and BD. The keywords used for the search are detailed in Supplementary Material.
Articles were considered to be eligible if: (i) studies were published in English; (ii) studies were original work (not reviews or meta-analyses); (iii) the overall number of samples in the study was >10; (iv) studies reported the result of voxel-based analysis (VBA) or tract-based spatial statistics (TBSS) or VBM; (v) studies compared patients with SZ or BD and HCs, and reported the alteration of FA or WMV (because the head motion has less of an effect on FA compared with other diffusion metrics, such as mean diffusivity)32; (vi) studies reported the group difference across the whole brain. Studies were excluded if peak coordinates could not be retrieved after contacting the authors.
Meta-analyses Using Anisotropic Effect Size-Signed Differential Mapping (AES-SDM)
Differences in regional WMV or FA were conducted using AES-SDM software (www.sdmproject.com/), which has specific templates for WMV and FA.33,34 Voxel-based meta-analytic methods have been described.35,36 Briefly, ES-SDM uses effect sizes to combine reported peak coordinates that are extracted from databases with statistical parametric maps. This is done to recreate effect-size maps and an effect-size variance map of the differences in WMV or FA between patients and controls. AES-SDM uses an anisotropic non-normalized Gaussian kernel to optimize the recreation of an effect-size map. Meta-analytic WM maps are based on a WM template. Moreover, meta-analytical calculations are based on random-effects models, and account for sample size, inter-study variability, and between-study heterogeneity.36
First, separate analyses within each group (SZ or BD) were conducted to investigate the alteration of WMV and FA with their respective HCs. We adopted the DTI-fractional anisotropy template (non-TBSS) in ES-SDM to combine VBA and TBSS studies. Next, a voxel-wise quantitative comparison was undertaken to assess abnormalities of WMV and FA (relative to HCs) between SZ and BD. Default SDM thresholds were used (voxel P < .005, peak height z > 1, cluster extent = 10 voxels). Then, to detect regions showing shared abnormalities in both disorders/modalities, following the approach described previously,37,38 we used the multimodal analysis section of AES-SDM to conduct conjunction/multimodal analysis.
Meta-regression was conducted within the patient group to examine the effect of potential confounding variables on abnormalities in WMV and FA when the available study data were >10, and only regions found in the main between-group analysis were included.35,36 Furthermore, the main analyses were complemented with additional analyses: (a) analyses of DTI subgroups in the group using identical motion-correction software; (b) analyses of SZ subgroups in the group which excluded first-episode samples; (c) analyses of age- and sex-matched subgroups; (d) “jackknife” sensitivity analyses to test the replicability of results by repeating analyses iteratively; (e) funnel plots and Egger’s test to assess the publication bias.
Results
Included Studies
We included: 48 DTI experiments comparing SZ patients (1543) with HCs (1612); 32 DTI experiments comparing BD cases (983) with HCs (1163); 24 VBM experiments comparing SZ patients (1068) with HCs (1073); 14 VBM experiments comparing BD patients (518) with HCs (651) (Supplementary tables S1–S4). Group differences in demographics are presented in Supplementary materials. To ensure that the group differences of the meta-analysis were not due to differences in sex or age, analyses of age- and sex-matched subgroups were done (table 1).
Table 1.
Demographic Information of Meta-analysis samples
| SZ | BD | |||
|---|---|---|---|---|
| Healthy controls | Patients | Healthy controls | Patients | |
| Total study sample | ||||
| DTI | ||||
| N | 1612 | 1543 | 1163 | 983 |
| Female | 673 | 580 | 576 | 542 |
| Mean age | 30.69 | 30.36 | 34.78 | 36.99 |
| VBM | ||||
| N | 1073 | 1068 | 651 | 518 |
| Female | 416 | 413 | 374 | 277 |
| Mean age | 29.50 | 30.25 | 35.17 | 35.99 |
| Age and sex-matched subsample | ||||
| DTI | ||||
| N | 873 | 763 | 615 | 541 |
| Female | 369 | 291 | 307 | 311 |
| Mean age | 31.87 | 31.46 | 32.6 | 34.73 |
| VBM | ||||
| N | 683 | 688 | 446 | 402 |
| Female | 272 | 275 | 258 | 217 |
| Mean age | 30.58 | 31.25 | 34.96 | 36.18 |
Regional Abnormalities in FA
DTI in SZ Patients
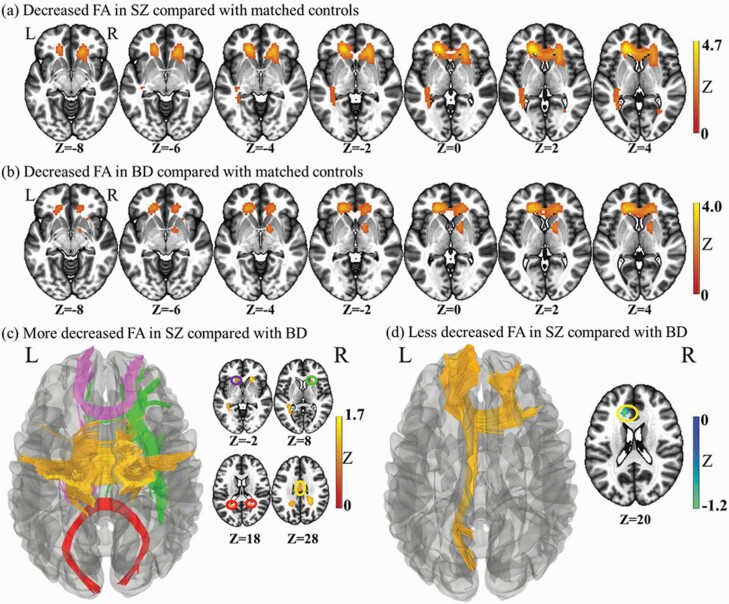
SZ patients, relative to HCs, showed decreased FA in the corpus callosum (extending to the left anterior corona radiata) and right posterior thalamic radiation (Supplementary table S5, figure 1a). No regions with increased FA were observed in SZ cases compared with those in HCs.
Fig. 1.
Whole brain meta-analysis of alterations in schizophrenia and bipolar disorder white matter fractional anisotropy. (a) Two-dimensional (2-D) representation of the significant clusters in the schizophrenia analysis. (b) Two-dimensional (2D) representation of the significant clusters in the bipolar disorder analysis. (c) Three-dimensional (3D) illustration of the more deceased FA in schizophrenia compared with bipolar disorder. The fibers of genu of corpus callosum are in purple, body in orange, and splenium in red. The green parts are the right Anterior limb of internal capsule. (d) Three-dimensional (3D) representation of the less deceased FA in schizophrenia compared with bipolar disorder. The yellow is the left anterior and superior corona radiata. The blue is the left external capsule extending to the posterior limb and Retrolenticular part of internal capsule and cerebral peduncle.
DTI in BD Cases
Compared with HCs, BD patients showed reduced FA in four clusters: the corpus callosum (extending to the anterior corona radiata), right anterior limb of the internal capsule, left posterior thalamic radiation (extending to posterior corona radiata), and right external capsule (Supplementary table S5, figure 1b). No regions with increased FA were found in BD compared with those in HCs.
Comparison of FA Differences Between SZ and BD Patients
Compared with BD patients, SZ patients (relative to respective control groups) revealed greater FA in the genu of the corpus callosum extending to the left anterior corona radiata, and decreased FA in five clusters: (i) left posterior thalamic radiation extending to the left posterior corona radiata and splenium of the corpus callosum and left retrolenticular part of the internal capsule; (ii) the splenium of the corpus callosum extending to the right posterior corona radiata; (iii) the right anterior corona radiata extending to the right anterior limb of the internal capsule and right external capsule; (iv) the body of the corpus callosum: (v) the genu of the corpus callosum (Supplementary table S5, figures 1c and 1d). Decreased FA in the splenium of the corpus callosum extending to the right posterior corona radiata was found upon meta-analyses of age- and sex-matched subgroups.
Conjunction Analyses
Conjunction analyses revealed the shared impairments of FA in patient groups relative to HCs to be the corpus callosum extending to the left and right anterior corona radiata, and the left posterior thalamic radiation extending to the left posterior corona radiata (Supplementary table S5). These impairments were retained in meta-analysis of age- and sex-matched subgroups.
Regional Differences in WMV
VBM in SZ Patients
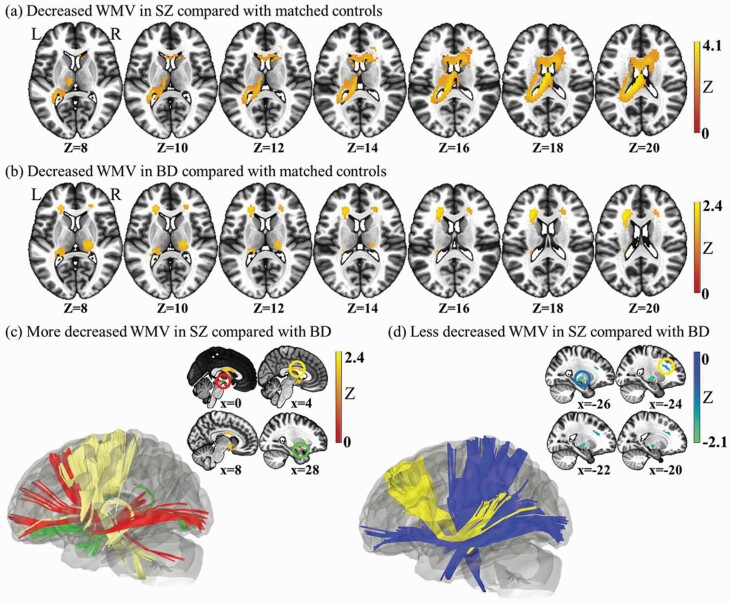
SZ patients, relative to HCs, showed reduced WMV in three clusters: (i) the body of the corpus callosum extending to the left posterior corona radiata: (ii) the left external capsule extending to the left uncinate fasciculus; (iii) the right external capsule extending to the right uncinate fasciculus (Supplementary table S6, figure 2a). No regions with increased WMV were found. Global WM was decreased significantly compared with that in HCs.
Fig. 2.
Whole brain meta-analysis of alterations in schizophrenia and bipolar disorder white matter volume. (a) Two-dimensional (2D) representation of the significant clusters volume reduction in the schizophrenia analysis. (b) Two-dimensional (2D) representation of the significant clusters volume reduction in the bipolar disorder analysis. (c) Three-dimensional (3D) illustration of the more deceased WMV in schizophrenia compared with bipolar disorder. Red indicates right anterior limb of internal capsule. Yellow is the body of corpus callosum. Green parts are right uncinate fasciculus. (d) Three-dimensional (3D) representation of the less deceased WMV in SZ compared with BD. The yellow is the fibers of corpus callosum. The blue is the left pons tracts.
VBM in BD Patients
BD patients, relative to HCs, showed reduced WMV in five clusters; (i) the left anterior and superior corona radiata; (ii) the left external capsule extending to the left retrolenticular part of the internal capsule; (iii) the right posterior limb and retrolenticular part of the internal capsule; (iv) the right anterior corona radiata; (ii) the left cingulum (cingulate gyrus) (Supplementary table S6, Figure 2b). No regions with increased WMV were found in the BD group compared with that in HCs. Global WMV was not significantly different between BD cases and HCs.
Comparison of WMV Differences Between SZ and BD Cases
Compared with BD patients, SZ patients (relative to respective control groups) revealed greater WMV in the left external capsule extending to the posterior limb and retrolenticular part of the internal capsule and cerebral peduncle, the left anterior and superior corona radiate (Supplementary figure 2c), and less WMV in the right anterior limb of the internal capsule, the right uncinate fasciculus extending to the external capsule, and the body of the corpus callosum (Supplementary table S6, figure 2d). Greater WMV in the left anterior and superior corona radiata and decreased WMV in the right uncinate fasciculus extending to the external capsule were found in meta-analyses of age- and sex-matched subgroups.
Conjunction Analyses
Conjunction analyses showed that the corpus callosum extending to the corona radiata, the right posterior limb and retrolenticular part of the internal capsule, and the left external capsule extending to the uncinate fasciculus, were the regions where both patient groups showed significantly decreased WMV (Supplementary table S6). Besides the left external capsule extending to the uncinate fasciculus, all other regions survived under meta-analysis of age- and sex-matched subgroups.
Multimodal Analyses
Multimodal Analyses in SZ
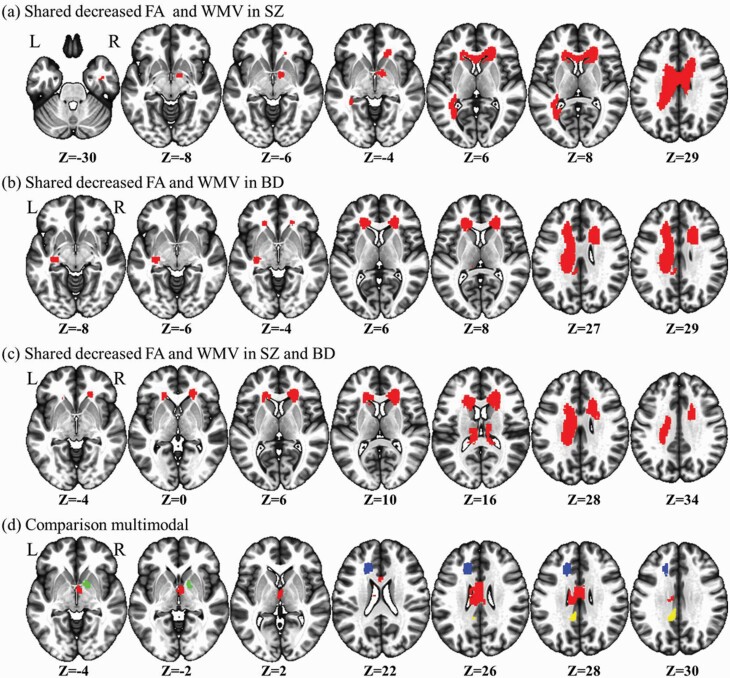
Relative to HCs, multimodal analyses revealed that SZ patients showed decreases in FA and WMV in the body and genu of the corpus callosum extending to the right anterior corona radiata, and the right anterior limb of the internal capsule (Supplementary table S7, figure 3a). The right anterior limb of the internal capsule did not survive meta-analysis of age- and sex-matched subgroups.
Fig. 3.
Multimodal meta-analysis of alterations in schizophrenia and bipolar disorder white matter. (a) The results for shared FA and WMV reduction in the schizophrenia analysis. (b) The results for shared FA and WMV reduction in the bipolar disorder analysis. (c) The common significant clusters between schizophrenia and bipolar disorder. (d) The comparison multimodal results for disorder-specific white matter alterations. Relative to bipolar disorder, blue indicates less reduction in FA and WMV in schizophrenia, green indicates less FA reduction but more WMV reduction, and yellow indicates more FA reduction but less reduction in WMV, and red indicates more reduction in FA and WMV.
Multimodal Analyses in BD
Multimodal analyses in BD patients, relative to that in HCs, revealed shared reductions in FA and WMV in three clusters: (i) the body of the corpus callosum extending to the left corona radiata; (ii) the body, and genu of the corpus callosum extending to the right anterior and superior corona radiata; (iii) the left retrolenticular part of the internal capsule extending to the left sagittal stratum and external capsule (Supplementary table S7, figure 3b). All of these features survived meta-analysis of age- and sex-matched subgroups.
Comparison of Multimodal Analyses Between SZ and BD Cases
Multimodal analyses between BD cases and SZ patients revealed shared reductions in FA and WMV of the WM bundle, including: (i) the left corpus callosum extending to the left anterior and superior corona radiata; (ii) the body and genu of the corpus callosum extending to the right anterior and superior corona radiata; (iii) the splenium of the corpus callosum (Supplementary table S7, figure 3c). The splenium of the corpus callosum was not present in the meta-analysis of age- and sex-matched subgroups.
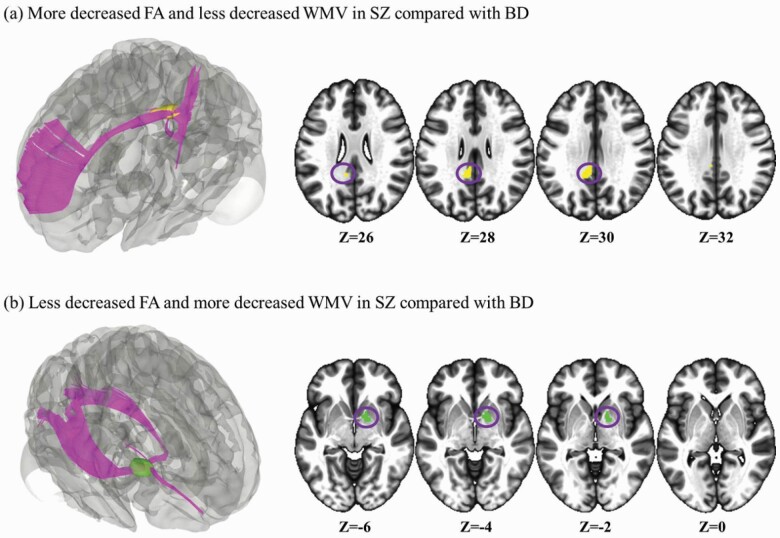
Multimodal comparison between BD cases and SZ patients (relative to HCs) showed that increased FA and WMV in the genu of the corpus callosum extending to left anterior corona radiata was disorder-specific in SZ patients compared with that in BD patients. Disorder-specific increased FA (but decreased WMV) was seen in the right anterior limb of the internal capsule in SZ cases relative to that in BD patients. Decreased FA (but increased WMV) was disorder-specific in the left cingulum in SZ patients relative to that in BD cases. Compared with BD cases, SZ patients showed greater reductions in FA and WMV in the body of the corpus callosum (figures 3d and 4). The genu of the corpus callosum extending to left anterior corona radiata and the body of the corpus callosum did not survive in the comparison of age- and sex-matched subgroups.
Fig 4.
Three-dimensional (3D) illustration of comparison multimodal analysis for disorder-specific white matter abnormalities. (a) Clusters showing significant effects are in yellow. (b) Clusters showing significant effects are in green.
Assessment of A Publication Bias and Heterogeneity
The Egger’s test was non-significant (P > .05) but only one that the left external capsule extending to the retrolenticular part of the internal capsule (peak coordinate: −32, −14, −6; Z = −2.34; P < .001) in the analysis of BD-VBM was significant (Egger’s test: bias = 2.26; t = 2.23; P = .046). Funnel plots revealed that no result was driven by only one study (Supplementary figures S6 and S7).
Complementary Analyses
The results of DTI subgroup analyses in the group of using FSL software motion correction approaches and SZ subgroup analyses in the group of excluding of first episode samples were quite consistent with the findings of the main analyses (Supplementary tables S19 and S20). Jackknife sensitivity analyses showed that our findings were robust and reliable (Supplementary tables S12–15).
Discussion
This is the first comparative multimodal meta-analysis of abnormalities in WM structure in SZ and BD. We integrated VBM studies and DTI studies and compared the shared or disorder-specific alteration of WM microstructure between SZ and BD. Given group differences in age distribution and sex distribution in the included studies, only findings that were retained in meta-analyses of age- and sex-matched subgroups are discussed primarily. The meta-analysis showed that BD and SZ shared reduced WMV and FA in the corpus callosum extending to the left anterior and superior corona radiata, and the body and genu of the corpus callosum extending to the right anterior and superior corona radiata. Moreover, compared with BD patients, SZ cases showed remarkable disorder-specific WM abnormalities: decreased FA (but increased WMV) in the left cingulum and increased FA (but decreased WMV) in the right anterior limb of the internal capsule.
Multimodal analyses showed that SZ cases and BD patients shared reductions in WMV and FA in the corpus callosum extending to the anterior and superior corona radiata. Specifically, studies have demonstrated that abnormalities in the corpus callosum are associated with voice hallucinations in SZ patients.39,40 Dong et al.41 showed that the genu of the corpus callosum connects the bilateral frontal cortices and shared reduced FA between BD cases and SZ cases relative to that in HCs. The bilateral frontal cortices were identified as having a common deficit area in SZ cases and BD patients suffering from delusion.42 Moreover, the WM fibers that pass through the splenium of the corpus callosum are diverse. They project bilaterally to three distinct brain regions (occipital, parietal, and temporal lobes43–45), a defect of which could contribute to abnormalities in posterior interhemispheric connectivity in patients. Damage to the temporal, occipital, and parietal lobes has been demonstrated to be related to psychotic symptoms (e.g., hallucinations, delusions).42,46 One possibility is that this deficit could reflect the psychotic functioning common in SZ and BD.30 However, in our comparative meta-analysis, relative to BD cases, SZ patients showed more severe WM alterations in the splenium of the corpus callosum. The degree of change may have been due to the inclusion of more patients with non-psychotic BD.
In addition, the anterior callosal fibers connect the bilateral frontal cortices, including the cortices associated with several cognitive domains (e.g., working memory, attention, and inhibitory control47,48). Importantly, cognitions such as working memory, attention, and inhibitory control are deficits in SZ and BD.47,48 An analysis of callosal thickness showed a positive association in the splenium of the corpus callosum for intellectual scores.49 Thus, shared alterations in the corpus callosum might also account for the cognition deficits in SZ and BD. Furthermore, the comparative meta-analysis showed that SZ was associated with more extensive deficits compared with that in BD (figures 1 and 2). SZ patients showed more severe alterations in WMV in the right uncinate fasciculus extending to the external capsule. The uncinate fasciculus is the largest of the three fiber bundles connecting the frontal and temporal lobes,50 dysfunctions of which may underlie impairments in memory, language, and social-emotional processing in SZ and BD.51 A meta-analysis of four psychiatric disorders indicated that a reduction in FA of the uncinate fasciculus was specific to SZ.52 However, our results showed that the WMV reduction of SZ was more severe than BD in the uncinate fasciculus. The reduction of FA in the corpus callosum (which is closely related to cognition) showed greater effects in SZ in a large-scale prospective meta-analysis.53 Studies have also indicated that SZ shows more severe cognitive impairment30,54 compared with that in BD. Therefore, more extensive and severe degeneration of WM may indicate more severe cognitive deficits.
In addition to the corpus callosum, we identified the shared WM abnormalities in the anterior and superior corona radiata, and the WMV reduction of the left anterior corona radiata was more serious in BD than that in SZ. The corona radiata includes descending and ascending fibers with the thalamus and cerebral cortex. Morphological and functional studies have indicated that the corona radiata is closely related to the medial prefrontal cortex and anterior cingulate, which are involved in the top–down regulation systems that organize emotion processing.55,56 The corona radiata is also the pathway interconnecting the anterior insula,57 which has consistent gray-matter loss across mental illnesses, and which is related to emotional and executive dysfunction in disorders.55,58 Impairment of emotional processing and executive function has also been observed in BD and SZ.59,60 Damage to the corona radiata may indicate impairment of emotional processing and executive function. In particular, BD showed a more severe and stable reduction in WMV in the left anterior corona radiata, relative to that in SZ. The anterior corona radiata contains reciprocal connections between the anterior thalamus and prefrontal cortex, and plays a part in the neural circuitry of emotion regulation.55 Thus, more severe damage to the anterior corona radiata may indicate more serious emotional impairment, which is consistent with BD-prominent emotional symptoms.
Disorder-specific increased FA but decreased WMV was seen in the right anterior limb of the internal capsule in SZ cases compared with that in BD patients. The alteration of the anterior limb of the internal capsule in BD has been demonstrated.61 The anterior limb of the internal capsule contains the anterior thalamic peduncle, which connects the medial and anterior thalamic nuclei with the prefrontal cortex and the cingulate gyrus.62 Dysconnectivity in the thalamo-cortical loop (the intercept point of which is represented by the internal capsule) might be involved specifically in the pathophysiological development of cognitive dysfunctions observed in SZ.63
Decreased FA (but increased WMV) was found in the left cingulum (including the WM of the cingulate gyrus) in SZ cases relative to BD patients. The left cingulum is part of the limbic system. The latter is a group of interconnected cortical and subcortical structures linking visceral states and emotion to cognition and behavior.64 WM dysconnectivity within the limbic system may lead to a range of cognitive and emotional problems that are found frequently in SZ and BD.54,65 The current study extended a previous large-scale DTI meta-analysis that indicated SZ and BD feature comparable changes in the limbic system, such as the fornix and cingulum.52 Furthermore, the disorder-specific left cingulum, decreased FA but increased WMV in the SZ cases relative to BD patients, might indicate the SZ showed more fiber crossings than BD in the left cingulum, according to the fiber crossing hypothesis.17 This disorder-specific WM alteration may also be due to changes in membrane permeability or the presence of non-axonal components (e.g., cells, vessels, interstitial fluid). Importantly, FA can reflect myelination in WM, and lithium and antipsychotic agents influence the structure of cell membranes and myelin sheaths,66,67 which may also affect increases and decreases in fiber crossing. Furthermore, meta-regression analysis showed that the reduction in FA was associated with the frequency of medication use, which may affect WM microstructure. The impact of medication on WM remains an area of research for the future. In summary, disorder-specific regions provided novel insights into distinguishing BD from SZ.
Our study had five main limitations. First, our study was based primarily on peak coordinates rather than “raw” statistical brain maps, which may have caused a bias in the results.17 Second, the results of meta-regression analyses should be viewed cautiously because they were driven by a small number of studies or did not overlap with the between-group differences found in the main analyses.17 Third, although motion correction or minimizing of head-motion approaches were reported in all DTI studies but one15 (Supplementary tables S1 and S2), different motion-correction software (e.g., FSL, DTIPrep, SPM) can affect neuroimaging findings substantially.68 We encourage researchers in this field to make great efforts to minimize head motion (e.g., by using foam pads, dental rests, or bite bars)69–71 and use standardized motion-correction software. Four, the influence of medication was complicated: we employed the frequency of medication use for meta-regression and not the type of drug. Five, our meta-analysis focused on WM alterations. To minimize the impact of head motion, we used only FA as the DTI metric.32 Non-use of other DTI indices (e.g., mean, radial, or axial diffusivity) or advanced diffusion-MRI measures (e.g., from diffusion kurtosis imaging, free-water imaging, neurite orientation dispersion, and density imaging) was a limitation of our meta-analysis. It would be valuable to include more indices and focus on gray matter in future investigations.
Conclusions
This meta-analysis showed that BD and SZ share widespread abnormalities in WM. There may be a pathophysiological basis for these seemingly similar behavioral phenotypes and the clinical continuity of both disorders. SZ and BD differ with regard to the left cingulum and right anterior limb of the internal capsule. The current study would be beneficial for understanding the pathophysiology, classification, and differential diagnoses of SZ and BD.
Funding
This study was supported by the National Natural Science Foundation of China (ref: 31900806). The funding organizations played no further role in study design, data collection, analysis and interpretation, and paper writing.
Conflict of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Supplementary Material
References
- 1. Spencer L James, Degu Abate, Kalkidan Hassen Abate, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) Nov 10 2018; 392(10159):1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 3. Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P. A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry. 2002;159(4):539–545. [DOI] [PubMed] [Google Scholar]
- 5. Shao L, Vawter MP. Shared gene expression alterations in schizophrenia and bipolar disorder. Biol Psychiatry. 2008;64(2):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kerner B. Toward a Deeper Understanding of the Genetics of Bipolar Disorder. Front Psychiatry. 2015;6:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McIntyre RS, Berk M, Brietzke E, et al. Bipolar disorders. Lancet (London, England) Dec 5 2020;396(10265):1841–1856. [DOI] [PubMed] [Google Scholar]
- 8. Karcher NR, Rogers BP, Woodward ND. Functional connectivity of the striatum in schizophrenia and psychotic bipolar disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(11):956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jimenez AM, Riedel P, Lee J, Reavis EA, Green MF. Linking resting-state networks and social cognition in schizophrenia and bipolar disorder. Hum Brain Mapp. 2019;40(16):4703–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cetin-Karayumak S, Di Biase MA, Chunga N, et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol Psychiatry. 2020;25(12):3208–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joo SW, Kim H, Jo YT, Yoon W, Kim Y, Lee J. Shared and distinct white matter abnormalities in schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2021;108:110175. [DOI] [PubMed] [Google Scholar]
- 12. Duval T, Stikov N, Cohen-Adad J. Modeling white matter microstructure. Funct Neurol. 2016;31(4):217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 14. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–821. [DOI] [PubMed] [Google Scholar]
- 15. Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. [DOI] [PubMed] [Google Scholar]
- 16. Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. Journal of molecular neuroscience: MN 2008;34(1):51–61. [DOI] [PubMed] [Google Scholar]
- 17. Radua J, Grau M, van den Heuvel OA, et al. Multimodal voxel-based meta-analysis of white matter abnormalities in obsessive-compulsive disorder. Neuropsychopharmacology. 2014;39(7):1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yin RH, Tan L, Liu Y, et al. Multimodal voxel-based meta-analysis of white matter abnormalities in Alzheimer’s disease. J Alzheimers Dis. 2015;47(2):495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Radua J, Romeo M, Mataix-Cols D, Fusar-Poli P. A general approach for combining voxel-based meta-analyses conducted in different neuroimaging modalities. Curr Med Chem. 2013;20(3):462–466. [PubMed] [Google Scholar]
- 20. Cui Y, Dong J, Yang Y, et al. White matter microstructural differences across major depressive disorder, bipolar disorder and schizophrenia: a tract-based spatial statistics study. J Affect Disord. 2020;260:281–286. [DOI] [PubMed] [Google Scholar]
- 21. Sutcubasi B, Metin SZ, Erguzel TT, Metin B, Tas C, Arikan MK, Tarhan N. Anatomical connectivity changes in bipolar disorder and schizophrenia investigated using whole-brain tract-based spatial statistics and machine learning approaches. Neur Comput Appl. 2019;31(9SI):4983–4992. [Google Scholar]
- 22. Kumar J, Iwabuchi S, Oowise S, Balain V, Palaniyappan L, Liddle PF. Shared white-matter dysconnectivity in schizophrenia and bipolar disorder with psychosis. Psychol Med. 2015;45(4):759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sussmann J, Lymer K, McKirdy J, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Schizophr Res. 2008;11(1):11–18. [DOI] [PubMed] [Google Scholar]
- 24. Sui J, Pearlson G, Caprihan A, et al. Discriminating schizophrenia and bipolar disorder by fusing fMRI and DTI in a multimodal CCA+ joint ICA model. Neuroimage. 2011;57(3):839–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson D, Ardekani BA, Burdick KE, et al. Overlapping and distinct gray and white matter abnormalities in schizophrenia and bipolar I disorder. Bipolar Disord. 2013;15(6):680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watson DR, Anderson JM, Bai F, et al. A voxel based morphometry study investigating brain structural changes in first episode psychosis. Behav Brain Res. 2012;227(1):91–99. [DOI] [PubMed] [Google Scholar]
- 27. Müller VI, Cieslik EC, Laird AR, et al. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. 2018;84:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Favre P, Pauling M, Stout J, et al. ; ENIGMA Bipolar Disorder Working Group . Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega- and meta-analyses across 3033 individuals. Neuropsychopharmacology. 2019;44(13):2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang C, Li L, Hu X, et al. Psychoradiologic abnormalities of white matter in patients with bipolar disorder: diffusion tensor imaging studies using tract-based spatial statistics. J Psychiatry Neurosci. 2019;44(1):32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sorella S, Lapomarda G, Messina I, Frederickson JJ, Siugzdaite R, Job R, Grecucci A. Testing the expanded continuum hypothesis of schizophrenia and bipolar disorder. Neural and psychological evidence for shared and distinct mechanisms. NeuroImage Clinical 2019;23:101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kong XZ. Association between in-scanner head motion with cerebral white matter microstructure: a multiband diffusion-weighted MRI study. PeerJ. 2014;2:e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Radua J, Via E, Catani M, Mataix-Cols D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 2011;41(7):1539–1550. [DOI] [PubMed] [Google Scholar]
- 34. Peters BD, Szeszko PR, Radua J, et al. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38(6):1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195(5):393–402. [DOI] [PubMed] [Google Scholar]
- 36. Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27(8):605–611. [DOI] [PubMed] [Google Scholar]
- 37. Radua J, Romeo M, Mataix-Cols D, Fusar-Poli P. A general approach for combining voxel-based meta-analyses conducted in different neuroimaging modalities. Curr Med Chem. 2013;20(3):462–466. [PubMed] [Google Scholar]
- 38. Carlisi CO, Norman LJ, Lukito SS, Radua J, Mataix-Cols D, Rubia K. Comparative multimodal meta-analysis of structural and functional brain abnormalities in autism spectrum disorder and obsessive-compulsive disorder. Biol Psychiatry. 2017;82(2):83–102. [DOI] [PubMed] [Google Scholar]
- 39. Hubl D, Koenig T, Strik W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61(7):658–668. [DOI] [PubMed] [Google Scholar]
- 40. Dias AM. The integration of the glutamatergic and the white matter hypotheses of schizophrenia’s etiology. Curr Neuropharmacol. 2012;10(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dong D, Wang Y, Chang X, et al. Shared abnormality of white matter integrity in schizophrenia and bipolar disorder: a comparative voxel-based meta-analysis. Schizophr Res. 2017;185:41–50. [DOI] [PubMed] [Google Scholar]
- 42. Song J, Han DH, Kim SM, et al. Differences in gray matter volume corresponding to delusion and hallucination in patients with schizophrenia compared with patients who have bipolar disorder. Neuropsychiatr Dis Treat. 2015;11:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knyazeva MG. Splenium of corpus callosum: patterns of interhemispheric interaction in children and adults. Neural Plast. 2013;2013:639430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598(1-2):143–153. [DOI] [PubMed] [Google Scholar]
- 45. Delvenne JF, Scally B, Bunce D, Burke MR. Splenium tracts of the corpus callosum degrade in old age. Neurosci Lett. 2021;742:135549. [DOI] [PubMed] [Google Scholar]
- 46. Qiu L, Yan H, Zhu R, et al. Correlations between exploratory eye movement, hallucination, and cortical gray matter volume in people with schizophrenia. BMC Psychiatry. 2018;18(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–483. [DOI] [PubMed] [Google Scholar]
- 48. Perlini C, Marini A, Garzitto M, et al. Linguistic production and syntactic comprehension in schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2012;126(5):363–376. [DOI] [PubMed] [Google Scholar]
- 49. Westerhausen R, Friesen CM, Rohani DA, et al. The corpus callosum as anatomical marker of intelligence? A critical examination in a large-scale developmental study. Brain Struct Funct. 2018;223(1):285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex Jan 2012;48(1):82–96. [DOI] [PubMed] [Google Scholar]
- 51. Ho NF, Li Hui Chong P, Lee DR, Chew QH, Chen G, Sim K. The amygdala in schizophrenia and bipolar disorder: a synthesis of structural MRI, diffusion tensor imaging, and resting-state functional connectivity findings. Harv Rev Psychiatry. 2019;27(3):150–164. [DOI] [PubMed] [Google Scholar]
- 52. Koshiyama D, Fukunaga M, Okada N, et al. ; COCORO . White matter microstructural alterations across four major psychiatric disorders: mega-analysis study in 2937 individuals. Mol Psychiatry. 2020;25(4):883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kelly S, Jahanshad N, Zalesky A, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23(5):1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am J Psychiatry. 2013;170(3):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wei Y, Chang M, Womer FY, et al. Local functional connectivity alterations in schizophrenia, bipolar disorder, and major depressive disorder. J Affect Disord. 2018;236:266–273. [DOI] [PubMed] [Google Scholar]
- 57. Nomi JS, Schettini E, Broce I, Dick AS, Uddin LQ. Structural connections of functionally defined human insular subdivisions. Cereb Cortex. 2018;28(10):3445–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lisa M. McTeague, Ph.D, Benjamin M.Rosenberg, M.A.James W.Lopez, B.S.et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiatry 2020;177(5):411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aparicio A, Santos JL, Jiménez-López E, Bagney A, Rodríguez-Jiménez R, Sánchez-Morla EM. Emotion processing and psychosocial functioning in euthymic bipolar disorder. Acta Psychiatr Scand. 2017;135(4):339–350. [DOI] [PubMed] [Google Scholar]
- 60. Yang C, Zhang T, Li Z, et al. The relationship between facial emotion recognition and executive functions in first-episode patients with schizophrenia and their siblings. BMC Psychiatry. 2015;15:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ren S, Chang M, Yin Z, et al. Age-related alterations of white matter integrity in adolescents and young adults with bipolar disorder. Front Psychiatry. 2019;10:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhou SY, Suzuki M, Hagino H, et al. Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biol Psychiatry. 2003;54(4):427–436. [DOI] [PubMed] [Google Scholar]
- 63. Rosenberger G, Nestor PG, Oh JS, et al. Anterior limb of the internal capsule in schizophrenia: a diffusion tensor tractography study. Brain Imaging Behav. 2012;6(3):417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Catani M, Dell’acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37(8):1724–1737. [DOI] [PubMed] [Google Scholar]
- 65. Acuff HE, Versace A, Bertocci MA, et al. ; LAMS Consortium . White matter—emotion processing activity relationships in youth offspring of bipolar parents. J Affect Disord. 2019;243:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1–7. [DOI] [PubMed] [Google Scholar]
- 67. Steiner J, Martins-de-Souza D, Schiltz K, et al. Clozapine promotes glycolysis and myelin lipid synthesis in cultured oligodendrocytes. Front Cell Neurosci. 2014;8:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kreilkamp BA, Zacà D, Papinutto N, Jovicich J. Retrospective head motion correction approaches for diffusion tensor imaging: effects of preprocessing choices on biases and reproducibility of scalar diffusion metrics. J Magn Reson Imaging. 2016;43(1):99–106. [DOI] [PubMed] [Google Scholar]
- 69. Aoki Y, Cortese S, Castellanos FX. Research review: diffusion tensor imaging studies of attention-deficit/hyperactivity disorder: meta-analyses and reflections on head motion. J Child Psychol Psychiatry. 2018;59(3):193–202. [DOI] [PubMed] [Google Scholar]
- 70. Maclaren J, Herbst M, Speck O, Zaitsev M. Prospective motion correction in brain imaging: a review. Magn Reson Med. 2013;69(3):621–636. [DOI] [PubMed] [Google Scholar]
- 71. Zaitsev M, Maclaren J, Herbst M. Motion artifacts in MRI: a complex problem with many partial solutions. J Magn Reson Imaging. 2015;42(4):887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.