Abstract
Purpose: Neural mobilization (NM) is often used to treat nerve-related conditions, and its use is reasonable with nerve-related neck and arm pain (NNAP). The aims of this study were to establish the effect of NM on the pain, function, and quality of life (QOL) of patients with NNAP and to establish whether high catastrophizing and neuropathic pain influence treatment outcomes. Method: A randomized controlled trial compared a usual-care (UC; n = 26) group, who received cervical and thoracic mobilization, exercises, and advice, with an intervention (UCNM; n = 60) group, who received the same treatment but with the addition of NM. Soft tissue mobilization along the tract of the nerve was used as the NM technique. The primary outcomes were pain intensity (rated on the Numerical Pain Rating Scale), function (Patient-Specific Functional Scale), and QOL (EuroQol-5D) at 3 weeks, 6 weeks, 6 months, and 12 months. The secondary outcomes were the presence of neuropathic pain (using the Neuropathic Diagnostic Questionnaire) and catastrophizing (Pain Catastrophising Scale). Results: Both groups improved in terms of pain, function, and QOL over the 12-month period (p < 0.05). No between-groups differences were found at 12 months, but the UCNM group had significantly less pain at 6 months (p = 0.03). Patients who still presented with neuropathic pain (p < 0.001) and high pain catastrophizing (p = 0.02) at 6- and 12-mo follow-ups had more pain. Conclusions: Both groups had similar improvements in function and QOL at 12-month follow-up. The UCNM group had significantly less pain at 6-month follow-up and a lower mean pain rating at 12-month follow-up, although the difference between groups was not significant. Neuropathic pain is common among this population and, where it persisted, patients had more pain and functional limitations at 12-mo follow-up.
Key Words: neck pain, physiotherapy modalities, neuralgia
Mots-clés : : douleur cervicale, modalités de physiothérapie, névralgie
Abstract
Objectif : la mobilisation neurale (MN) est souvent utilisée pour traiter des douleurs d’origine nerveuse, et il est raisonnable d’y recourir pour le traitement des douleurs cervico-brachiales d’origine nerveuse (DCBN). La présente étude visait à établir l’effet de la MN sur la douleur, la fonction et la qualité de vie (QdV) des patients présentant des DCBN et à déterminer si la catastrophisation élevée et la douleur neuropathique influent sur les résultats des traitements. Méthodologie : un essai aléatoire et contrôlé comparant un groupe recevant des soins habituels (SH; n = 26; mobilisation cervicale et thoracique, exercices et conseils) à un groupe d’intervention (SHMN; n = 60; même traitement, mais avec l’ajout d’une MN). Les chercheurs ont utilisé la mobilisation des tissus mous le long de la voie nerveuse comme technique MN. Les résultats primaires étaient l’intensité de la douleur (classée d’après l’échelle d’évaluation numérique de la douleur), la fonction (échelle fonctionnelle propre au patient) et la QdV (EuroQol-5D) au bout de trois et de six semaines, de six et de 12 mois. Les résultats secondaires étaient la présence de douleur neuropathique (selon le questionnaire de diagnostic neuropathique) et la catastrophisation (échelle de catastrophisation de la douleur). Résultats : l’état des deux groupes s’est amélioré sur le plan de la douleur, de la fonction et de la QdV au cours de la période de 12 mois (p < 0,05). Les deux groupes ne présentaient aucune différence au bout de 12 mois, mais le groupe SHMN ressentait nettement moins de douleurs au bout de six mois (p = 0,03). Les patients qui présentaient encore des douleurs neuropathiques (p < 0,001) et une catastrophisation élevée de la douleur (p = 0,02) au moment du suivi (à six et 12 mois) ressentaient davantage de douleur. Conclusions : les deux groupes présentaient une amélioration semblable de la fonction et de la QdV au suivi de 12 mois. Le groupe SHMN présentait considérablement moins de douleur au suivi de six mois et une classification moyenne de la douleur plus basse au suivi de 12 mois, même si la différence entre les groupes n’était pas significative. La douleur neuropathique est courante au sein de cette population, et lorsqu’elle persistait, les patients présentaient plus de douleur et de limitations fonctionnelles au suivi de 12 mois.
Introduction
Neck pain is a common and often debilitating musculoskeletal complaint.1 Different terms are used to describe neck and arm pain, such as cervico-brachial pain, nerve-related neck and arm pain (NNAP), and cervical radiculopathy,2–4 and these terms are often used interchangeably.4 Neck and arm pain is associated with shoulder pain or pain radiating down the arm, and it can include headache.4 Patients with NNAP are more disabled than patients with only neck pain.5
Neural mobilization
Neural mobilization (NM) is used to assess an increased mechano-sensitivity of the nervous system and to restore altered homeostasis in and around the nervous system using movement or palpation.6 In NNAP, neural tissue sensitivity to mechanical stimulus plays a role.7 One case report of a patient with cervico-brachial pain identified the use of NM along the tract of the nerve, which resulted in an improvement in pain and disability.8 There is evidence that targeting the neural structures of patients with NNAP can improve pain and that it could therefore be considered to help in managing NNAP.9
Neuropathic pain and pain catastrophizing
Neuropathic pain is often present among this population.10 It is consistently linked to high levels of pain, disability, poor quality of life (QOL), and poor response to treatment.11,12 Because neuropathic pain can affect treatment outcomes, it is important to establish its presence among these patients. Psychosocial factors such as pain catastrophizing and fear avoidance beliefs also play an important role in treatment outcomes;13,14 they are risk factors for developing first-onset neck pain and, if severe, have been shown to be important determinants of poor recovery.15
Aims of This Study
The aims of this study were to establish the effect of NM on pain, function, and QOL of patients with acute or subacute NNAP, as well as the influence of neuropathic pain and high pain catastrophizing on pain, function, and QOL.
Methods
We undertook a randomized controlled trial comparing an intervention group (usual care [UC] plus NM [UCNM]) with a UC group in four private practices in Pretoria, South Africa (trial registration no. PACTR201303000500157). Ethical approval for the study was obtained from the Human Research Ethics Committee of the University of the Witwatersrand. All study participants gave written, informed consent before participating in the study.
Therapists
Four physiotherapists in four separate private physiotherapy practices treated the patients. All had a postgraduate qualification in orthopaedic manual therapy with knowledge of NM and had on average 7 years of experience. We screened patients for eligibility and undertook a full clinical evaluation, including neural conduction testing – for example, muscle power, sensation, and reflex testing – and the physical examination part of the Neuropathic Diagnostic Questionnaire (DN4) and the Upper Limb Neurodynamic Test (ULNT1). We also administered the baseline set of questionnaires.
Participants
Participants were aged older than 18 years and had NNAP as evidenced by a physical examination and patient history of a local cause of nerve-related pain.7 The examination included active movement dysfunction, passive movement dysfunction, adverse responses to neurodynamic tests, mechanical allodynia on palpation of nerve trunks, and evidence from the physical examination of a local cause of the nerve-related pain.7 Moreover, participants needed to have pain of recent onset (£12 wk), recurrent or first incident, and a positive ULNT1. The ULNT1 was considered positive if a patient’s pain was reproduced or partially reproduced by the test and changed by structural differentiation.16
Participants were excluded if they had surgery or recent fractures of the cervical spine, serious neurological signs, rheumatoid arthritis, neurological disease, stroke, cerebral palsy, carcinoma, or any other red flags.
Treatment
The treatment of the UC group consisted of posterior–anterior and unilateral posterior–anterior mobilization of the cervical and thoracic spine, exercises, and advice to stay active. Exercises were as described by Gross and colleagues.17 The UCNM group received UC plus NM. The NM used was as described by Butler:18 a gentle soft tissue mobilization of the neural container or interface “along the tract”18 (p. 380) of the nerve – directly, where the nerve is palpable, and indirectly, where it lies deeper. The treatment concentrated on areas where the nerve was mechano-sensitive to palpation; it was done from the hand or elbow (depending on the patient’s area of pain) and followed up along the arm, first rib, and scalene and into the neck. Mobilization was first done in a position where the nerve was in a non-tensioned position, not provoking any of the patient’s symptoms. The principles of NM were used to progress treatment – that is, to commence treatment in the acute phase with the nerve in a neutral position and to progress into a more tensioned position as pain and irritability improved. The number of treatments for both groups was determined by the treating physiotherapist and was recorded. More information on the study design has been published in Basson and colleagues.19
Outcome measures
We measured pain with the Numeric Pain Rating Scale (NPRS). Its reliability has been established as good (rs = 0.72–0.78),20 and it is as sensitive to change as the visual analogue scale, which is considered the gold standard.21 The NPRS consists of an 11-point scale on which patients are asked to rate their pain from 0 (no pain) to 10 (worst pain possible).
We then measured function using the Patient-Specific Functional Scale (PSFS). It has excellent reliability (r = 0.92) and validity (rs = 0.73–0.83) compared with the Neck Disability Index.22 The PSFS is a valid, reliable, and responsive measure of function for patients with upper extremity problems.23 Patients are asked to nominate three to five activities that are difficult to perform and rate them on an 11-point scale ranging from 0 (unable to perform activity) to 10 (able to perform activity as before). The total of the three ratings of a participant’s nominated activities was used for analysis.
We measured QOL using the EuroQol-5D (EQ-5D) instrument. It has two sections: the first part consists of five sections (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). Each section is rated by three descriptions ranging from I have no problem to I am unable. The second section of the questionnaire has a 20-centimetre visual analogue scale with best imaginable health state at one end and worst imaginable health state at the other. Its intra-class correlation coefficient reliability is 0.82, which is excellent.24 Its test–retest reliability ranges between 69.8% and 99.7% for the EQ-5D dimensions, and the k coefficient is 0.67.25
Neuropathic pain was measured with the DN4. The DN4 consists of two sections, an interview and an examination. The interview is composed of two questions, the first regarding the characteristics of the pain (e.g., burning) and the second regarding associated symptoms (such as pins and needles) to which the respondent answers either yes or no. The examination tests for hypoesthesia to touch and prick in the painful area as well as whether brushing aggravates the pain. Each positive response is scored a point, with a total possible score of 10. A patient with a score of 4 out of 10 or higher on the DN4 can be diagnosed with neuropathic pain.26 The sensitivity of the test at a cutoff of 4 in population with neck and arm pain is 88% (range 71.0%–96.5%), but the specificity is low at 41% (range 31.5%–51.4%).27 Interrater reliability has ks between 0.70 and 0.96.26
The Pain Catastrophising Scale (PCS) identifies patients with high pain catastrophizing. A score higher than 24 of 52 on the PCS signifies a patient with high pain catastrophizing.28 The internal consistency of the PCS’s three subscales, Rumination, Magnification, and Helplessness is Cronbach’s αs = 0.85, 0.75, and 0.85, respectively.29 The criterion of reliability correctly identified 77.1% of the cases.29
Participants completed a demographic questionnaire, which documented their age, gender, duration of symptoms, previous neck pain, injury or insidious onset, education, occupation, exercise, presence of headache or dizziness, and an indication of the area of pain on a body chart.
Randomization
Blocked randomization with a 2:1 ratio in blocks of six was done using a computer random number generator. A research assistant (qualified physiotherapist) was blinded to group allocation and conducted all follow-up measurements. A second research assistant was an administrative person and was responsible for informing the physiotherapists of a patient’s number and treatment allocation by telephone. Treatment (group) allocation was given to participating physiotherapists after all baseline measurements had been taken, thus ensuring concealed allocation. The physiotherapists involved in the study then received an envelope with the consent form, patient information leaflet, treatment recording sheet, and all outcome measures.
Sample size
We determined that a sample size of 68 UCNM participants and 34 UC participants – for a total sample size of 102 patients – had 90% power to detect a clinically relevant difference of 2 in NPRS score between the UC and UCNM groups at 6 weeks. The data points that were analyzed were raw scores. A SD of 2.05 was assumed, as derived from the effect size and as published by Bolton and Wilkinson.30 It was inflated by √2 because the difference between the two groups was of interest. We made our calculations using nQuery Advisor, Version 7 (Statistical Solutions Ltd., Boston). A dropout rate of 15%–20% was assumed.
Participants were recruited from February 2012 to October 2014, and data collection was completed in November 2015. Although the a priori sample size had been determined to be 102, we stopped recruiting patients at 86 because recruitment was so difficult, which reduced the power of the sample size from 90% to 85%.
Statistical analysis
The primary goal of this study was to assess whether, 6 weeks after treatment began, the UCNM group’s NPRS score was reduced compared with that of the UC group. The mean, SD, and 95% CI were reported for each group for the continuous variables NPRS, PSFS, and EQ-5D. Catastrophizing (yes and no) and neuropathic pain (yes and no) categorical data were summarized using frequencies and percentages. The NPRS, PSFS, and EQ-5D scores of the two treatment groups were compared for between-groups differences at 6 weeks using an analysis of covariance with baseline scores, catastrophizing (yes and no), and neuropathic pain (yes and no) as covariates. The same covariates were used in the linear mixed-model analysis. For the total assessment period of 12 months, we assessed the interaction between measurements and timeframes with respect to NPRS, PSFS, and EQ-5D scores using a linear mixed-model analysis; for these calculations, we used Stata, Version 15 (StataCorp LLP, College Station, TX).
The linear mixed model included main effects and interaction. When we found a significant interaction, we interpreted the main effects accordingly: by group (UC and UCNM) and time (baseline and 3 weeks, baseline and 6 weeks, baseline and 6 months, baseline and 12 months). Effect sizes were expressed as Cohen’s d for the NPRS. The pattern and manner with which the study data became missing is detailed in Figure 1. The missing data were not random but could be fully accounted for by variables for which there was complete information.31 An intention-to-treat analysis was performed; to match with this pattern of missing data, multiple imputation procedures were used.31 One-sided testing was done at the 0.05 level of significance.
Figure 1 .
Flow diagram of participant recruitment and follow-up.
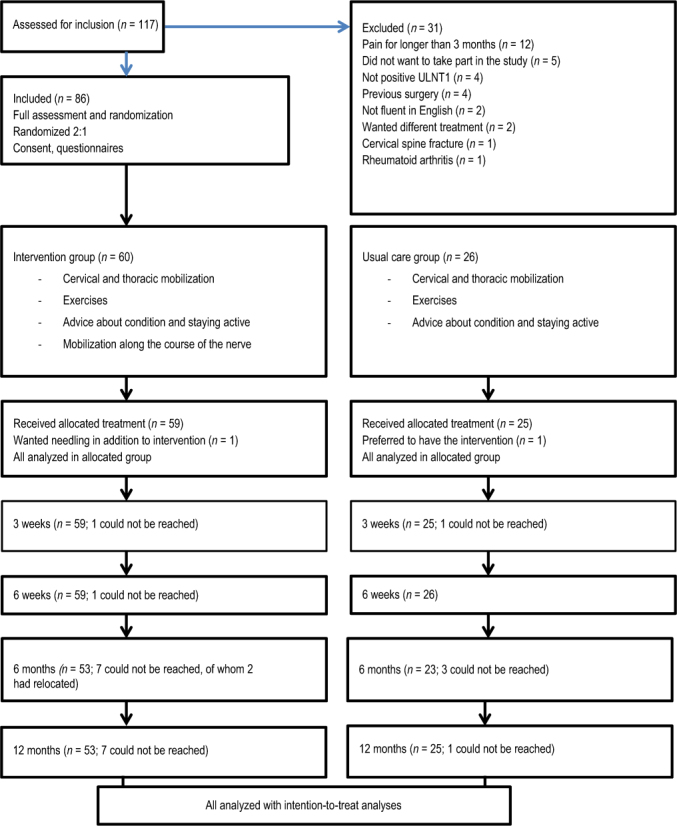
ULNT1 = Upper Limb Neurodynamic Test.
n = numbers in parentheses.
Results
Baseline
Figure 1illustrates the recruitment of patients and their follow-up. Table 1 provides the participants’ demographic information. Groups were comparable at baseline, with more women (65; 76%) than men (21; 24%). The measurement time points are shown in Table 2.
Table 1 .
Patient Characteristics
| Characteristic | No. of patients (%)* |
|
|---|---|---|
| Intervention group (n = 60) | Usual-care group (n = 26) | |
| Age, y, mean (SD) | 46.47 (14.09) | 48.61 (13.64) |
| Duration of pain, d, mean (SD) | 30.19 (27.39) | 23.46 (22.90) |
| Hours sitting/d, mean (SD) | 6.72 (2.96) | 7.23 (2.97) |
| Previous pain | 49 (81.67) | 21 (80.77) |
| Pain due to injury or accident | 20 (33.33) | 8 (30.77) |
| Regular exercise | 39 (65.00) | 13 (50.00) |
| Headache | 35 (58.53) | 15 (57.69) |
| Dizziness | 17 (28.33) | 4 (15.38) |
| Paraesthesia | 32 (53.33) | 14 (53.85) |
| Education | ||
| < 12 y | 0 | 0 |
| 12 y | 12 (20.00) | 6 (23.08) |
| College or university | 48 (80.00) | 20 (76.92) |
Unless otherwise indicated.
Table 2 .
Outcome Measures and Measurement Time Points
| Measure | Baseline | 3 wk | 6 wk | 6 mo | 12 mo |
|---|---|---|---|---|---|
| NPRS | ✔ | ✔ | ✔ | ✔ | ✔ |
| PSFS | ✔ | ✔ | ✔ | ✔ | ✔ |
| EQ-5D | ✔ | ✔ | ✔ | ✔ | ✔ |
| DN4 | ✔ | – | – | ✔ | ✔ |
| PCS | ✔ | – | – | ✔ | ✔ |
| Demographics | ✔ | – | – | – | – |
| Neural conduction | ✔ | – | – | – | – |
Note: Dash indicates not measured at this time point.
NPRS = Numerical Pain Rating Scale; PSFS = Patient-Specific Functional Scale; EQ-5D = EuroQual-5D; DN4 = Diagnostic Neuropathic Questionnaire; PCS = Pain Catastrophising Scale.
Treatment
The mean number of treatments for both groups was four. We found no significant differences between the groups with regard to the number of treatments they received: UCNM group, 3.92 (SD 1.78); UC group, 4.69 (SD 2.34); p = 0.10.
Pain
At 6 weeks, there was no statistically significant difference between the two groups (p = 0.34; see Figure 2 and Table 3). The entire sample had a statistically significant improvement on the NPRS from baseline to 12 months, with a large effect size (d = 6.89; 95% CI: 5.71, 8.07; p < 0.001). However, the UCNM group had significantly less pain at 6 months than the UC group (p = 0.033). At all the other time points, there was no statistically significant difference between the groups (p ≥ 0.05). At 12 months, 50% (30) of participants in the UCNM group were pain free, whereas only 38.4% (10) of the UC participants were pain free (p = 0.32). Figure 2 shows the changes in the NPRS scores over the study period, and Table 4 illustrates the effect sizes. The mixed effects model for the NPRS is shown in the Appendix.
Figure 2 .
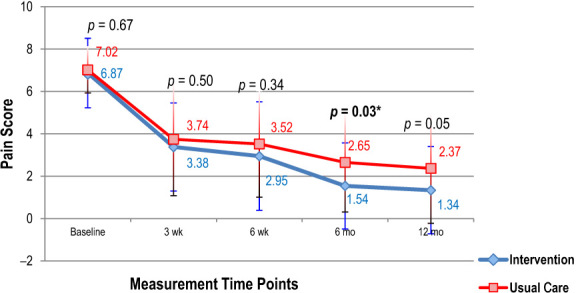
Difference in measurements on the Numerical Pain Rating Scale between the two groups from baseline to 12-month follow-up.
Table 5 .
Pain, Function, and QOL for Neuropathic Pain, Non-Neuropathic Pain, High Pain Catastrophizing, and Low Pain Catastrophizing across the Entire Sample (N = 86)
| Time point | Neuropathic pain (DN4 score ≥ 4) |
Non-neuropathic pain (DN4 score < 4) |
p-value | 95% CI | ||
|---|---|---|---|---|---|---|
| No. (%) of patients | Mean (SD) score | No. (%) of patients | Mean (SD) score | |||
| NPRS | ||||||
| Baseline | 45 (52) | 14.33 (6.34) | 41 (48) | 13.20 (6.53) | 0.41 | −1.0, −0.3 |
| 6 mo | 13 (15) | 3.71 (1.87) | 65 (76) | 1.38 (2.14) | < 0.001* | −3.5, −1.0 |
| 12 mo | 11 (13) | 3.23 (2.21) | 67 (78) | 1.38 (2.14) | 0.01* | −3.2, −0.4 |
| PSFS | ||||||
| Baseline | 45 (52) | 23.85 (4.16) | 41 (48) | 13.20 (6.53) | 0.41 | −3.9, −1.6 |
| 6 mo | 13 (15) | 23.91 (4.39) | 73 (85) | 26.21 (5.36) | 0.14 | −0.8, −5.5 |
| 12 mo | 11 (13) | 23.91 (4.39) | 77 (89) | 27.15 (4.85) | 0.04* | 0.1, −6.4 |
| EQ-5D | ||||||
| Baseline | 45 (52) | 70.40 (19.12) | 41 (48) | 74.63 (17.78) | 0.29 | −3.7, −12.2 |
| 6 mo | 13 (15) | 82.00 (14.05) | 73 (85) | 85.61 (10.46) | 0.29 | −3.2, −10.4 |
| 12 mo | 11 (13) | 79.82 (12.05) | 77 (89) | 86.11 (11.11) | 0.09 | −1.0, −13.6 |
|
| ||||||
| High pain catastrophizing (PCS > 24) |
Low pain catastrophizing (PCS ≤ 24) |
|||||
| No. (%) of patients | Mean (SD) score | No. (%) of patients | Mean (SD) score | |||
|
| ||||||
| NPRS | ||||||
| Baseline | 23 (27) | 7.30 (1.00) | 63 (73) | 6.80 (1.60) | 0.13 | −1.3, −0.2 |
| 6 mo | 4 (5) | 4.25 (3.90) | 82 (95) | 1.70 (2.00) | 0.02* | −4.7, −0.4 |
| 12 mo | 7 (8) | 3.60 (2.70) | 79 (92) | 1.47 (2.10) | 0.02* | −3.8, −0.4 |
| PSFS | ||||||
| Baseline | 23 (27) | 12.35 (6.40) | 63 (73) | 14.3 (6.4) | 0.21 | −1.1, −5.1 |
| 6 mo | 4 (5) | 24.25 (4.50) | 82 (95) | 25.8 (5.3) | 0.56 | −3.8, −7.0 |
| 12 mo | 7 (8) | 25.14 (5.50) | 79 (92) | 26.8 (4.9) | 0.40 | −2.2, −5.5 |
| EQ-5D | ||||||
| Baseline | 23 (27) | 61.96 (22.90) | 63 (73) | 75.8 (15.1) | 0.002* | 5.3, −22.4 |
| 6 mo | 4 (5) | 87.00 (8.70) | 82 (95) | 85.0 (11.3) | 0.73 | −13.5, −9.5 |
| 12 mo | 7 (8) | 84.86 (8.70) | 79 (92) | 85.1 (11.7) | 0.96 | −8.8, −9.3 |
QOL = quality of life; DN4 = Diagnostic Neuropathic Questionnaire; NPRS = Numerical Pain Rating Scale; PSFS = Patient-Specific Functional Scale; EQ-5D = EuroQoL-5D instrument; PCS = Pain Catastrophising Scale.
p < 0.05.
Table 3 .
Differences in Pain, Function, and Quality of Life at Measurement Time Points
| Outcome and time point | Mean (SD) |
p-value | 95% CI | |
|---|---|---|---|---|
| UCNM | UC | |||
| NPRS | ||||
| Baseline | 6.9 (1.6) | 7.0 (1.1) | 0.67 | −0.5, 0.8 |
| 3 wk | 3.4 (2.1) | 3.7 (2.6) | 0.50 | −0.6, 1.5 |
| 6 wk | 2.9 (2.6) | 3.5 (2.5) | 0.34 | −0.6, 1.8 |
| 6 mo | 1.5 (1.9) | 2.6 (2.5) | 0.033 | 0.1, 2.2 |
| 12 mo | 1.3 (1.6) | 2.4 (3.0) | 0.05 | −0.1, 2.0 |
| PSFS | ||||
| Baseline | 13.6 (6.5) | 14.3 (6.3) | 0.65 | −2.3, 3.7 |
| 3 wk | 20.9 (6.8) | 20.7 (7.5) | 0.86 | −3.7, 3.1 |
| 6 wk | 22.7 (7.5) | 22.3 (7.2) | 0.81 | −3.9, 3.1 |
| 6 mo | 25.3 (5.4) | 25.4 (4.9) | 0.82 | −2.7, 2.5 |
| 12 mo | 26.4 (5.8) | 26.7 (4.1) | 0.99 | −2.7, 2.5 |
| EQ-5D | ||||
| Baseline | 72.4 (19.6) | 72.4 (15.8) | 0.99 | −8.7, 8.6 |
| 3 wk | 80.7 (11.0) | 79.1 (15.2) | 0.45 | −8.1, 3.6 |
| 6 wk | 84.1 (11.1) | 83.0 (11.8) | 0.70 | −6.3, 4.3 |
| 6 mo | 85.3 (11.1) | 83.7 (11.3) | 0.37 | −8.1, 3.0 |
| 12 mo | 86.1 (10.0) | 83.2 (13.5) | 0.20 | −9.0, 1.9 |
UCNM = usual care + neural mobilization; UC = usual care; NPRS = Numerical Pain Rating Scale; PSFS = Patient-Specific Functional Scale; EQ-5D = EuroQol 5D instrument.
Function
No significant differences were found between the groups’ PSFS scores at the 6-week follow-up or at any other time point (p > 0.05; see Table 3). The average PSFS score was 14.3 at baseline for the UC group; the interaction was not significant, and only the main effects of time were significant, increasing over time from baseline. Both groups improved significantly over time, with a large effect size (d = 2.87; 95% CI: 2.22, 3.52; p < 0.001). At the 12-month follow-up, 46.67% (28) of the UCNM group and 34.61% (9) of the UC group had no functional limitations as measured by the PSFS using initial nominated activities.
Quality of life
No significant differences were found in terms of the state of health between the groups at 6 weeks (p = 0.70). The average EQ-5D score was 72.4 at baseline for the UC group; the interaction was not significant, and only the main effects of time were significant, increasing over time from baseline. Both groups improved significantly in health-related QOL (p < 0.001) over the 12-month period, with a large effect size (d = 1.38; 95% CI: 0.87, 1.91; see Table 3). All domains, with the exception of mobility, improved from baseline to 12 months with no statistically significant differences between the groups (p > 0.05).
Neuropathic pain
The presence of neuropathic pain was assessed across the entire population rather than between groups. A total of 45 (52.32%) patients (UCNM, 32; UC, 13) were identified as having neuropathic pain at baseline. At 12 months, 11 (12.79%) patients (UCNM, 7; UC, 4) were classified as having neuropathic pain.
We found no statistically significant difference between those with and those without neuropathic pain at baseline (p = 0.95). At 6 months and 12 months, patients who still presented with neuropathic pain (11) had significantly more pain than those who did not (p < 0.05).
At baseline and 6 months, patients with and without neuropathic pain were the same in terms of function (p > 0.05). At 12 months, patients who still presented with neuropathic pain were more limited in function (p = 0.04). There were no significant differences in state of health, as measured by the EQ-5D, between the patients with neuropathic pain and those without at any time point (p > 0.05). Table 5 illustrates these findings.
Table 4 .
Effect Sizes for Pain over Measurement Time Points
| Time point | UCNM |
UC |
||||
|---|---|---|---|---|---|---|
| Cohen d | ES | 95% CI | Cohen d | ES | 95% CI | |
| Baseline–3 wk | 1.8‡ | 0.7 | −2.5, −1.2 | 1.6‡ | 0.6 | −2.5, −0.7 |
| 3–6 wk | 0.2 | 0.1 | −0.6, −0.5 | 0.1 | 0.1 | −0.9, −0.6 |
| 6 wk–6 mo | 0.6† | 0.3 | −1.1, −0.1 | 0.4* | 0.1 | −1.1, −0.0 |
| 6–12 mo | 0.1 | 0.1 | −0.6, 0.5 | 0.1 | 0.1 | −0.9, −0.6 |
| Baseline–6 mo | 3.0‡ | 0.8 | −3.7, −2.3 | 2.4‡ | 0.8 | −3.3, −1.3 |
| Baseline–12 mo | 3.0‡ | 0.8 | −3.8, −2.3 | 2.4‡ | 0.8 | −3.4, −1.4 |
Small (d = 0.2).
Medium (d = 0.5).
Large (d = 0.8).
UCNM = usual care + neural mobilization; UC = usual care; ES = effect size.
Pain catastrophizing
The presence of high pain catastrophizing was assessed across the entire sample rather than between groups. A total of 23 (26.74%) of all participants (UCNM, 18; UC, 5) were identified as patients with high pain catastrophizing. At 12 months, only 8.14% (7) of the participants (UCNM, 5; UC, 2) could still be classified as patients with high pain catastrophizing. At 6 and 12 months, those patients who could still be classified with high pain catastrophizing reported significantly more pain than those who could not (p < 0.05). There were no significant differences at any time point between those with high pain catastrophizing and those with low pain catastrophizing in terms of function (p > 0.05).
At baseline, those with high pain catastrophizing had a significantly lower state of health than those without catastrophizing (p = 0.002). At 6 months and 12 months, there were no significant differences between the two groups (p > 0.05). Table 5 illustrates these findings.
Discussion
Both the UC and the UCNM groups improved significantly at the primary outcome point (6 weeks) in terms of pain, function, and QOL, but there were no between-groups differences. However, the UCNM group had significantly less pain at 6 months than the UC group. Patients with persistent neuropathic pain reported more pain and lower function than patients without neuropathic pain. Patients with high pain catastrophizing reported worse QOL at baseline, and those who persisted in high pain catastrophizing had significantly more pain at follow-up than those who did not.
The pain in both groups improved significantly from baseline to 3-week follow-up, and this improvement can be expected considering natural recovery.32 However, at 6 months there was a significant difference between the groups, favouring the UCNM group, and this can only be ascribed to the addition of NM to the treatment. Although the between-groups difference was no longer statistically significant at 12-month follow-up, it still favoured the UCNM. It is possible that the sample size did not have sufficient power to adequately illustrate the difference as a result of variability in the data because the mean difference remained essentially the same.
Improvement is expected within the first month, after which levels of pain and disability remain the same up to 12 months.32 In contrast to the expected natural course of neck pain at the 6-month follow-up, the UCNM group kept on improving from 6 weeks to 6 months at a moderate level. The effect size for improvement in pain for the UCNM group was moderate (0.6) and small (0.4) for the UC group. The effect size difference in pain for both groups was large (>2) over the 12-month period, which is different than the expected natural course of neck pain.
The primary outcome point was 6 weeks; at that point we expected to see a between-groups difference; therefore, the significant difference between the groups at 6 months was unexpected. It is possible that some of the effects seen here can be ascribed to positive neurophysiological changes because the only difference in treatment was the addition of NM. NM has been associated with positive neurophysiological effects such as decreased intraneural oedema and nociceptive flexion reflex threshold, regardless of the techniques used. In further support of NM’s neurophysiological effects, NM has been shown to modulate the expression of endogenous opioids in rats and improved fluid dispersion in the nerves of unembalmed cadavers.33–36
Recently, a systematic review described moderate improvement with joint mobilization in people with acute neck pain at long-term follow-up.37 Similarly, exercise improves pain and function at short- and long-term follow-up.38 The fact that both groups received the same exercises and joint mobilization could partly explain why there was no longer a significant difference between the groups at 12-month follow-up.
Function normally improves over the first 6 weeks after the onset of neck pain with no further improvement up to 12 months.39 Contrary to this, both groups improved up to 12 months. The combination of mobilization and exercise, which both groups received, seems to have had a positive effect on function. This supports guideline recommendations.40,41 The addition of NM did not seem to add to treatment outcome for function. Because functional limitations play an important role in this population, it is important that clinicians measure function when assessing patients with NNAP pain.
An improvement in pain and function can lead to an improvement in health-related QOL.42 Therefore, the significant improvement in pain and function in both groups could partly explain the similar improvement in QOL in both groups. People with musculoskeletal diseases have significantly lower QOL than the general population.43 Measuring the QOL of patients with neck pain is important, considering the high burden of neck pain.1
The incidence of neuropathic pain in musculoskeletal conditions is under-recognised,10 and the high percentage of patients in our study with a predominantly neuropathic pain component supports this finding. The presence of referred pain of neural origin10 and the high incidence of negative neurological changes could partly account for this because neuropathic pain is present when there is a lesion or disease of the nervous system.44 Neuropathic pain is associated with higher pain, disability, and poor QOL.11,12
The presence of persistent neuropathic pain among the patients in this study had negative implications for pain and function; this finding is similar to those for patients with chronic pain conditions with a predominantly neuropathic pain component.45,46 However, the number of patients who were classified with neuropathic pain at 12-month follow-up was significantly lower than at baseline. It seems that a multimodal intervention was effective in reducing the number of patients with neuropathic pain. Because neuropathic pain is common with acute or subacute NNAP and is a risk factor for the development of chronic pain,47 early and effective treatment is very important. It is also important to assess for the presence of neuropathic pain and to address this in the acute phase because our study shows that treatment can decrease the presence of neuropathic pain in the acute or subacute phase. Surprisingly, we found no significant difference in health-related QOL between patients with neuropathic pain and those without. This contrasts with the findings of other studies, which have shown neuropathic pain to have a negative impact on QOL.11,46 However, they were conducted in a chronic population and not in an acute or subacute group.
Pain catastrophizing is linked to high pain reports, disability, poor QOL, and poor treatment outcomes.14,48 At 12-month follow-up, only a few patients could still be classified with high pain catastrophizing; however, only one of them was pain-free. The presence of persistent catastrophizing had a negative influence on pain. Reassessment at 1-month follow-up has been suggested because improvements in psychosocial parameters are expected in the first month.49 If pain catastrophizing is still present once pain has decreased, other treatment options, such as multidisciplinary therapy, should be considered.50
We found no significant difference in function and QOL between those with high pain catastrophizing and those without. Other studies have found that catastrophizing has a negative impact on function and QOL.51 The low number of patients identified as catastrophizers limits the confidence in our results; it could account for the difference in findings,52 and so they should be interpreted with caution.
This study had a number of limitations. The fact that the a priori sample size could not be reached could have resulted in a lack of statistical power, and this may, in part, explain the lack of significant findings at 12-month follow-up. The fact that we included patients up to 12 weeks after the onset of pain makes it possible that we included those who had already transitioned to chronic pain, and this could partly explain the poor outcome in pain and function at the 12-month follow-up. The small number of patients with neuropathic pain and high pain catastrophizing at 12 months makes it difficult to draw any firm conclusions about these populations. The presence of neuropathic pain and pain catastrophizing was measured only at baseline, 6 months, and 12 months; measuring at 3 weeks and 6 weeks may have added valuable information.
Conclusion
Both groups had similar improvements at 12-month follow-up. Adding NM was effective because pain was reduced sooner in the UCNM group. Neuropathic pain is common in this population, and those with persistent neuropathic pain had more pain and functional limitations at follow-up.
Key Messages
What is already known on this topic
Nerve-related neck and arm pain (NNAP) is common, and optimal management strategies are still not clear.
What this study adds
Neural mobilization improved pain significantly at 6 months in the usual-care plus neural mobilization group compared with the usual-care group and could be considered in the management of patients with NNAP. Neuropathic pain is common in the acute or subacute population, and neuropathic pain that persisted at follow-up resulted in more pain and poor function.
Appendix: Mixed Model Effects for the NPRS
Model with main effects and interactions
nprs | Coef. Std. Err. z P>|z| [95% Conf. Interval]
+
grp |
Protocol | .1492308 .5046627 0.30 0.767 -.83989 1.138352
|
time |
1 | -3.473351 .325351 -10.68 0.000 -4.111027 -2.835675
2 | -3.930978 .325351 -12.08 0.000 -4.568654 -3.293302
3 | -5.369511 .336087 -15.98 0.000 -6.02823 -4.710793
4 | -5.515637 .336087 -16.41 0.000 -6.174356 -4.856919
|
grp#time |
Protocol#1 | .3049474 .5941792 0.51 0.608 -.8596224 1.469517
Protocol#2 | .434824 .5894295 0.74 0.461 -.7204367 1.590085
Protocol#3 | .9756845 .6107481 1.60 0.110 -.2213598 2.172729
Protocol#4 | .8081861 .6002527 1.35 0.178 -.3682877 1.98466
|
_cons | 6.87 .2774845 24.76 0.000 6.32614 7.41386
Random-effects Parameters | Estimate Std. Err. [95% Conf. Interval]
+
ptnumber: Identity |
var(_cons) | 1.479397 .336609 .9471306 2.310786
+
var(Residual) | 3.140461 .2502112 2.686429 3.671229
LR test vs. linear model: chibar2(01) = 55.73 Prob >= chibar2 = 0.0000
Model with interactions only
nprs | Coef. Std. Err. z P>|z| [95% Conf. Interval]
+
grp#time |
Intervention#1 | -3.473351 .325351 -10.68 0.000 -4.111027 -2.835675
Intervention#2 | -3.930978 .325351 -12.08 0.000 -4.568654 -3.293302
Intervention#3 | -5.369511 .336087 -15.98 0.000 -6.02823 -4.710793
Intervention#4 | -5.515637 .336087 -16.41 0.000 -6.174356 -4.856919
Protocol#0 | .1492308 .5046627 0.30 0.767 -.83989 1.138352
Protocol#1 | -3.019173 .5102021 -5.92 0.000 -4.01915 -2.019195
Protocol#2 | -3.346923 .5046627 -6.63 0.000 -4.336044 -2.357802
Protocol#3 | -4.244596 .5226561 -8.12 0.000 -5.268983 -3.220209
Protocol#4 | -4.55822 .5103523 -8.93 0.000 -5.558492 -3.557948
|
_cons | 6.87 .2774845 24.76 0.000 6.32614 7.41386
Random-effects Parameters | Estimate Std. Err. [95% Conf. Interval]
+
ptnumber: Identity |
var(_cons) | 1.479397 .336609 .9471306 2.310786
+
var(Residual) | 3.140461 .2502112 2.686429 3.671229
LR test vs. linear model: chibar2(01) = 55.73 Prob >= chibar2 = 0.0000
References
- 1. Hoy D, March L, Woolf A, et al. The global burden of neck pain: estimates from the Global Burden of Disease 2010 Study. Ann Rheum Dis. 2014;73(7):1309–15. 10.1136/annrheumdis-2013-204431. Medline:24482302 [DOI] [PubMed] [Google Scholar]
- 2. Allison GT, Nagy BM, Hall T. A randomized clinical trial of manual therapy for cervico-brachial pain syndrome – a pilot study. Man Ther. 2002;7(2):95–102. 10.1054/math.2002.0453. Medline:12151246 [DOI] [PubMed] [Google Scholar]
- 3. Nee RJ, Vincenzino B, Jull GA, et al. Neural tissue management provides immediate clinically relevant benefits without harmful effects for patients with nerve related neck and arm pain: a randomised trial. J Physiother. 2012;58(1):23–31. 10.1016/s1836-9553(12)70069-3. Medline:22341379 [DOI] [PubMed] [Google Scholar]
- 4. Salt E, Wright C, Kelly S, et al. A systematic literature review on the effectiveness of non-invasive therapy for cervicobrachial pain. Man Ther. 2011;16(1):53–65. 10.1016/j.math.2010.09.005. Medline:21075037 [DOI] [PubMed] [Google Scholar]
- 5. Sarquis LMM, Coggon D, Ntani G, et al. Classification of neck/shoulder pain in epidemiological research: a comparison of personal and occupational characteristics, disability, and prognosis among 12,195 workers from 18 countries. Pain. 2016;157(5):1028–36. 10.1097/j.pain.0000000000000477. Medline:26761390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coppieters MW, Nee R. Neurodynamic management of the peripheral nervous system. In: Jull G, Moore AP, Falla D, Lewis D, McCarthy C, Sterling M, eds. Grieveʼs modern musculoskeletal physiotherapy. 4th ed. Edinburgh: Elsevier; 2015. p. 287–97 [Google Scholar]
- 7. Hall TM, Elvey RL. Nerve trunk pain: physical diagnosis and treatment. Man Ther. 1999;4(2):63–73. 10.1054/math.1999.0172. Medline:10509060 [DOI] [PubMed] [Google Scholar]
- 8. Costello M. Treatment of a patient with cervical radiculopathy using thoracic spine thrust manipulation, soft tissue mobilization and exercise. J Man Manip Ther. 2009;16(3):129–35. 10.1179/jmt.2008.16.3.129. Medline:19119401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Basson A, Olivier B, Ellis RF, et al. The effectiveness of neural mobilization for neuromusculoskeletal conditions: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2017;47(9):593–615. 10.2519/jospt.2017.7117. Medline:28704626 [DOI] [PubMed] [Google Scholar]
- 10. Tampin B, Briffa NK, Goucke R, et al. Identification of neuropathic pain in patients with neck/upper limb pain: application of a grading system and screening tools. Pain. 2013;154(12):2813–22. 10.1016/j.pain.2013.08.018. Medline:23973362 [DOI] [PubMed] [Google Scholar]
- 11. Smith BH, Torrance N, Bennett MI, et al. Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clin J Pain. 2007;23(2):143–9. 10.1097/01.ajp.0000210956.31997.89. Medline:17237663 [DOI] [PubMed] [Google Scholar]
- 12. Sterling M, Pedler A. A neuropathic pain component is common in acute whiplash and associated with a more complex clinical presentation. Man Ther. 2009;14(2):173–9. 10.1016/j.math.2008.01.009. Medline:18358761 [DOI] [PubMed] [Google Scholar]
- 13. Pool JJM, Ostelo RWJG, Knol D, et al. Are psychological factors prognostic indicators of outcome in patients with sub-acute neck pain? Man Ther. 2010;15(1): 111–16. 10.1016/j.math.2009.08.001. Medline:19717327 [DOI] [PubMed] [Google Scholar]
- 14. Verhagen AP, Karels CH, Schellingerhout JM, et al. Pain severity and catastrophising modify treatment success in neck pain patients in primary care. Man Ther. 2010;15(3):267–72. 10.1016/j.math.2010.01.005. Medline:20138562 [DOI] [PubMed] [Google Scholar]
- 15. Kim R, Wiest C, Clark K, et al. Identifying risk factors for first-episode neck pain: a systematic review. Musculoskelet Sci Pract. 2018;33:77–83. 10.1016/j.msksp.2017.11.007. Medline:29197234 [DOI] [PubMed] [Google Scholar]
- 16. Nee RJ, Vicenzino B, Jull GA, et al. A novel protocol to develop a prediction model that identifies patients with nerve-related neck and arm pain who benefit from the early introduction of neural tissue management. Contemp Clin Trials. 2011;32(5):760–70. 10.1016/j.cct.2011.05.018. Medline:21718803 [DOI] [PubMed] [Google Scholar]
- 17. Gross AR, Haines T, Goldsmith CH, et al. Knowledge to action: a challenge for neck pain treatment. J Orthop Sports Phys Ther. 2009;39(5):351–63. 10.2519/jospt.2009.2831. Medline:19521013 [DOI] [PubMed] [Google Scholar]
- 18. Butler DS. The sensitive nervous system. 1st ed. Adelaide, SA: Noigroup Publications; 2000. [Google Scholar]
- 19. Basson C, Stewart A, Mudzi W. The effect of neural mobilisation on cervico-brachial pain: design of a randomised controlled trial. BMC Musculoskelet Disord. 2014;15(1):419. 10.1186/1471-2474-15-419. Medline:25492697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Good M, Stiller C, Zauszniewski JA, et al. Sensation and distress of pain scales: reliability, validity, and sensitivity. J Nurs Meas. 2001;9(3):219–38. 10.1891/1061-3749.9.3.219. Medline:11881266 [DOI] [PubMed] [Google Scholar]
- 21. Holdgate A, Asha S, Craig J, et al. Comparison of a verbal numeric rating scale with the visual analogue scale for the measurement of acute pain. Emerg Med (Fremantle). 2003;15(5–6):441–6. 10.1046/j.1442-2026.2003.00499.x. Medline:14992058 [DOI] [PubMed] [Google Scholar]
- 22. Westaway MD, Stratford PW, Binkley M. The Patient-Specific Functional Scale: validation of its use in persons with neck dysfunction. J Orthop Sports Phys Ther. 1998;27(5):331–8. 10.2519/jospt.1998.27.5.331. Medline:9580892 [DOI] [PubMed] [Google Scholar]
- 23. Cleland JA, Fritz JM, Whitman JM. The reliability and construct validity of the Neck Disability Index and Patient Specific Functional Scale in patients with cervical radiculopathy. Spine. 2006;31(5):598–602. 10.1097/01.brs.0000201241.90914.22. Medline:16508559 [DOI] [PubMed] [Google Scholar]
- 24. Coons SJ, Rao S, Keininger DL, et al. A comparative review of generic quality-of-life instruments. Pharmacoeconomics. 2000;17(1):13–35. 10.2165/00019053-200017010-00002. Medline:10747763 [DOI] [PubMed] [Google Scholar]
- 25. Ravens-Sieberer U, Wille N, Badia X, et al. Feasibility, reliability, and validity of the EQ-5D-Y: results from a multinational study. Qual Life Res. 2010;19(6):887–97. 10.1007/s11136-010-9649-x. Medline:20401552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1):29–36. 10.1016/j.pain.2004.12.010. Medline:15733628 [DOI] [PubMed] [Google Scholar]
- 27. Epping R, Verhagen AP, Hoebink EA, et al. The diagnostic accuracy and test-retest reliability of the Dutch PainDETECT and the DN4 screening tools for neuropathic pain in patients with suspected cervical or lumbar radiculopathy. Musculoskelet Sci Pract. 2017;30:72–9. 10.1016/j.msksp.2017.05.010. Medline:28600958 [DOI] [PubMed] [Google Scholar]
- 28. Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524–32. 10.1037//1040-3590.7.4.524 [DOI] [Google Scholar]
- 29. Osman A, Barrio FX, Gutierrez PM, et al. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23(4):351–65. 10.1023/a:1005548801037. Medline:10984864 [DOI] [PubMed] [Google Scholar]
- 30. Bolton JE, Wilkinson RC. Responsiveness of pain scales: a comparison of three pain intensity measures in chiropractic patients. J Manipulative Physiol Ther. 1998;21(1):1–7. Medline:9467094. [PubMed] [Google Scholar]
- 31. Pedersen AB, Mikkelsen EM, Cronin-Fenton D, et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. 2017;9:157–66. 10.2147/clep.s129785. Medline:28352203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vasseljen O, Woodhouse A, Bjørngaard JH, et al. Natural course of acute neck and low back pain in the general population: the HUNT study. Pain. 2013;154(8):1237–44. 10.1016/j.pain.2013.03.032. Medline:23664654 [DOI] [PubMed] [Google Scholar]
- 33. Schmid AB, Elliott JM, Strudwick MW, et al. Effect of splinting and exercise on intraneural edema of the median nerve in carpal tunnel syndrome – an MRI study to reveal therapeutic mechanisms. J Orthop Res. 2012;30(8):1343–50. 10.1002/jor.22064. Medline:22231571 [DOI] [PubMed] [Google Scholar]
- 34. Sterling M, Pedler A, Chan C, et al. Cervical lateral glide increases nociceptive flexion reflex threshold but not pressure or thermal pain thresholds in chronic whiplash associated disorders: a pilot randomised controlled trial. Man Ther. 2010;15(2):149–53. 10.1016/j.math.2009.09.004. Medline:19884037 [DOI] [PubMed] [Google Scholar]
- 35. Santos FM, Grecco LH, Pereira MG, et al. The neural mobilization technique modulates the expression of endogenous opioids in the periaqueductal gray and improves muscle strength and mobility in rats with neuropathic pain. Behav Brain Funct. 2014;10:19. 10.1186/1744-9081-10-19. Medline:24884961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gilbert KK, Smith MP, Sobczak S, et al. Effects of lower limb neurodynamic mobilization on intraneural fluid dispersion of the fourth lumbar nerve root: an unembalmed cadaveric investigation. J Man Manip Ther. 2015;23(5):239–45. 10.1179/2042618615y.0000000009. Medline:26955255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gross A, Langevin P, Burnie SJ, et al. Manipulation and mobilisation for neck pain contrasted against an inactive control or another active treatment. Cochrane Database Syst Rev. 2015;9:CD004249. 10.1002/14651858.cd004249.pub4. Medline:26397370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gross AR, Paquin JP, Dupont G, et al. Exercises for mechanical neck disorders: a Cochrane Review update. Man Ther. 2016;24:25–45. 10.1016/j.math.2016.04.005. Medline:27317503 [DOI] [PubMed] [Google Scholar]
- 39. Hush JM, Lin CC, Michaleff ZA, et al. Prognosis of acute idiopathic neck pain is poor: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2011;92(5):824–9. 10.1016/j.apmr.2010.12.025. Medline:21458776 [DOI] [PubMed] [Google Scholar]
- 40. Childs JD, Cleland JA, Elliott JM, et al. Neck pain: clinical practice guidelines linked to the International Classification of Functioning, Disability, and Health from the Orthopedic Section of the American Physical Therapy Association. J Orthop Sports Phys Ther. 2008;38(9):A1–A34. 10.2519/jospt.2008.0303. Medline:18758050 [DOI] [PubMed] [Google Scholar]
- 41. Côté P, Wong JJ, Sutton D, et al. Management of neck pain and associated disorders: a clinical practice guideline from the Ontario Protocol for Traffic Injury Management (OPTIMa) collaboration. Eur Spine J. 2016;25(7):2000–22. 10.1007/s00586-016-4467-7. Medline:26984876 [DOI] [PubMed] [Google Scholar]
- 42. Hoffman DL, Sadosky A, Dukes EM, et al. How do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy? Pain. 2010;149(2):194–201. 10.1016/j.pain.2009.09.017. Medline:20303665 [DOI] [PubMed] [Google Scholar]
- 43. Picavet HSJ, Hoeymans N. Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann Rheum Dis. 2004;63(6):723–9. 10.1136/ard.2003.010769. Medline:15140781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Finnerup N, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606. 10.1097/j.pain.0000000000000492. Medline:27115670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berger A, Dukes EM, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J Pain. 2004;5(4):143–9. 10.1016/j.jpain.2003.12.004. Medline:15106126 [DOI] [PubMed] [Google Scholar]
- 46. Smart KM, Blake C, Staines A, et al. Self-reported pain severity, quality of life, disability, anxiety and depression in patients classified with “nociceptive,” “peripheral neuropathic” and “central sensitisation” pain: the discriminant validity of mechanisms-based classifications of low back (±leg) pain. Man Ther. 2012;17(2):119–25. 10.1016/j.math.2011.10.002. Medline:22074733 [DOI] [PubMed] [Google Scholar]
- 47. Doth AH, Hansson PT, Jensen MP, et al. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain. 2010;149(2):338–44. 10.1016/j.pain.2010.02.034. Medline:20227832 [DOI] [PubMed] [Google Scholar]
- 48. Geelen CC, Kindermans HP, van den Bergh JP, et al. Perceived physical activity decline as a mediator in the relationship between pain catastrophizing, disability, and quality of life in patients with painful diabetic neuropathy. Pain Pract. 2017;17(3):320–8. 10.1111/papr.12449. Medline:27006136 [DOI] [PubMed] [Google Scholar]
- 49. Wirth B, Humphreys BK, Peterson C. Importance of psychological factors for the recovery from a first episode of acute non-specific neck pain – a longitudinal observational study. Chiropr Man Therap. 2016;24(1):1–10. 10.1186/s12998-016-0090-2. Medline:26985362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Volker G, van Vree F, Wolterbeek R, et al. Long-term outcomes of multidisciplinary rehabilitation for chronic musculoskeletal pain. Musculoskeletal Care. 2017;15(1):59–68. 10.1002/msc.1141. Medline:27098842 [DOI] [PubMed] [Google Scholar]
- 51. Arnow BA, Blasey CM, Constantino MJ, et al. Catastrophizing, depression and pain-related disability. Gen Hosp Psychiatry. 2011;33(2):150–6. 10.1016/j.genhosppsych.2010.12.008. Medline:21596208 [DOI] [PubMed] [Google Scholar]
- 52. Hackshaw A. Small studies: strengths and limitations. Eur Respir J. 2008;32(5):1141. 10.1183/09031936.00136408. Medline:18978131 [DOI] [PubMed] [Google Scholar]


