Abstract
Jatropha integerrima Jacq., family: Euphorbiaceae, is used in India and subtropical Africa to treat different skin conditions. In this study we evaluated the anti-inflammatory activity of J. integerrima leaves extract (JILE) using rat paw edema model. The extract was administered orally (200 and 400 mg/kg) or applied topically as creams at 2.5, 5, and 10% strength. Four hours post-treatment, maximum reduction of edema volume by 63.09% was observed after oral administration of JILE (400 mg/kg) as compared to indomethacin with 60.43%. The extract anti-inflammatory effect was accompanied by a decrease in NO, prostaglandin PGE2, TNF-α and PKC levels by 19, 29.35, 16.9, and 47.83%, respectively. Additionally, topical applications of JILE showed dose dependent reduction in paw edema and resulted in normalized levels of PGE2, TNF-α, and PKC when used as 10% cream. Signs of inflammations were reduced or absent from paw tissue of animals receiving JILE either orally or topically. Finally, liquid chromatography/mass spectrometry analysis of JILE resulted in the annotation of 133 metabolites including 24 diterpenoids, 19 flavonoids, 10 phenolic acid conjugates, 8 cyclic peptides, 6 phytosterols, 4 sesquiterpenes, and 4 coumarins. Several of the annotated metabolites have known anti-inflammatory activity including vitexin, isovitexin, fraxitin, scopeltin, stigmasterol, and many diterpenoidal derivatives.
Keywords: anti-inflammatory, diterpenoids, cyclic peptide, Euphorbiaceae, hydroxyl fatty acids Jatropha integerrima, vitexin, UPLC/MS-MS
1. Introduction
The genus Jatropha (family Euphorbiaceae) has a wide distribution in tropical and subtropical regions especially South America, West Africa, India, and Southeast Asia [1,2]. Many members of the genus were used medicinally in their indigenous countries such as J. gossypifolia, J. curcas, J. chevalieri, and J. multifida [3]. Leaves and latex from Jatropha plants are especially useful in treating skin conditions such as ulcers, blisters, eczema and also to accelerate wound healing [4,5,6]. The genus is rich in bioactive secondary metabolites especially diterpenoids of tigliane, lathyrane, and jatrophane skeletons which exist mainly as esters [7,8]. However, among more than 175 Jatropha species, only few species were chemically investigated. As a result of these investigations, many structurally unique and bioactive phytochemicals were identified including flavonoids, cyclic peptides, lignans, and diterpenes [1,3].
Jatropha integerrima Jacq (syn. Jatropha pandurifolia And.), also known as spicy jatropha, is cultivated around the world as an ornamental shrub due to its showy bright red flowers. Its leaves are used as poultice in India and Bangladesh to treat different conditions including eczema, pruritus and skin warts [9,10]. Phytochemical investigation of J. integerrima identified coumarins [11], cyclic peptides [12], neolignans [13], and several novel diterpenes with different biological activities [14,15]. More than 16 new compounds were isolated from J. integerrima in the past decade alone [13,14,15], indicating a rich metabolome that is yet to be fully explored. Recent pharmacological investigation of the plant showed promising antimicrobial activity of extracts and essential oils obtained from seeds and leaves [16,17] and strong antioxidant activity of the flowers extract [18].
Based on the accumulated literature and the known folk use of J. integerrima, this study was performed to investigate the anti-inflammatory effect of J. integerrima leaves extract (JILE) when administered orally or applied topically. Anti-inflammatory activity was assessed in rat paw edema model through measuring edema volumes and levels of inflammatory mediators nitric oxide (NO), tumor necrosis factor-α (TNF-α), prostaglandin E2 (PGE2), and protein kinase C (PKC) in addition to examining histopathological features of paw tissue. Furthermore, ultra-performance liquid chromatography coupled to high resolution mass spectrometry (UPLC-MS) was used to identify secondary metabolites in JILE through tandem mass fragmentation to assist with identifying metabolites that can contribute to the anti-inflammatory activity of the plant.
2. Results
2.1. Acute Toxicity Study
Rats treated with J. integerrima leaves extract (JILE) at a high dose of 5 g/kg did not show any skin abnormalities or changes in respiratory, circulatory, and somatomotor activities as well as behavior pattern.
2.2. Evaluation of Oral Anti-Inflammatory Effect of Jatropha integerrima Leaves Extract
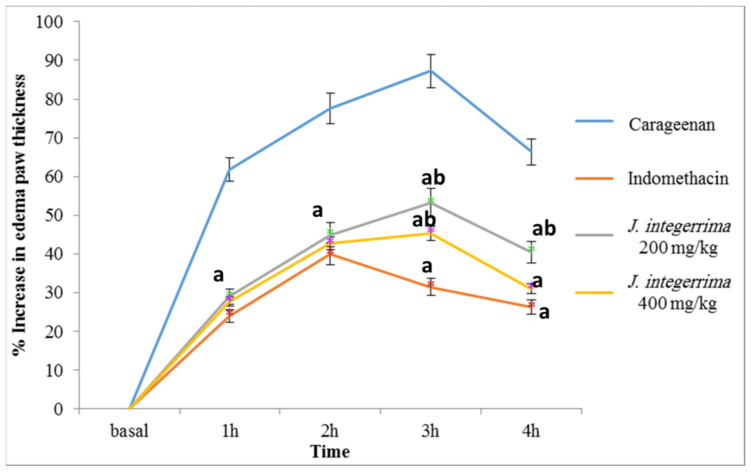
The subplanter injection of 100 μL of 1% sterile carrageenan into the rat hind paw elicited an inflammation manifested by swelling, erythema and a time-dependent increase in paw edema by 61.82, 77.58, 87.24%, and 66.37% after 1, 2, 3, and 4 h after injection, as compared with precarrageenan control values, Figure 1. This observed response to carrageenan injection follows the same pattern of maximum inflammation after 3 h as previously reported [19].
Figure 1.
Effect of oral administration of ethanol extract of Jatropha integerrima leaves on edema volume. One way ANOVA and Fishers LSD comparison test were used, p < 0.05; a: significantly different from carrageenan control group at respective time point; b: significantly different from indomethacin group at respective time point.
Pretreatment with JILE (200 mg/kg and 400 mg/kg, orally) showed a significant inhibition of edema formation by 53.09% and 55.17% after 1 h, 42.15% and 44.87%, after 2 h, 39.04% and 48.03% after 3 h, and 39.01% and 63.90% after 4 h, respectively, as compared with carrageenan control group at the same time. In the same assay, indomethacin treatment (25 mg/kg) resulted in a 61.17%, 48.56%, 63.9%, and 60.43% reduction of edema volume, as compared with that of carrageenan group after 1, 2, 3, and 4 h postcarrageenan injection, respectively, Figure 1.
By the end of the experiment, serum NO level increased by 44% in animals that received only carrageenan as compared with normal control group. Animals that were pre-treated with indomethacin and J. integerrima at 200 mg/kg and 400 mg/kg had significantly decreased serum NO levels by 17%, 13%, and 19%, respectively, as compared with that of animals that received carrageenan only (p < 0.05), Table 1.
Table 1.
Effect of oral administration of Jatropha integerrima leaves extract (JILE) on different inflammation biomarkers.
| Control | Carrageenan | Indomethacin 25 mg/kg |
JILE 200 mg/kg |
JILE 400 mg/kg |
|
|---|---|---|---|---|---|
| NO (µmol/L) |
16.00 ± 1.29 | 23.00 ± 0.676 a | 19.03 ± 0.3 ab | 19.9 ± 0.08 ab | 18.55 ± 0.39 b |
| PGE2 (pg/mL) | 251.28 ± 4.16 | 498.8 ± 37.04 a | 354.88 ± 5.36ab | 514.88 ± 5.36 a | 352.4 ± 23.52 ab |
| TNF-α (pg/mL) | 1581.92 ± 10.88 | 2194.56 ± 99.52 a | 1804.8 ± 7.84 ab | 2104.96 ± 4.16 a | 1823.68 ±0.16 ab |
| PKC (pg/mL) | 870.4 ± 99.705 | 1505.35 ± 5.78 a | 842.35 ± 153.85 b | 805.8 ± 62.985 b | 785.4 ± 23.29 b |
Data were expressed as mean ± SD. Statistical analysis was carried out by one-way ANOVA, followed by Tukey’s HSD test for multiple comparisons. a: significantly different from normal control (Saline), b: significantly different from carrageenan control at p < 0.05.
Similarly, after 4 h following carrageenan injection, serum levels of inflammation mediators such as PGE2, TNF-α, and PKC were elevated by 98.5%, 38.75%, and 72.94%, respectively, as compared with that of normal control group. Animals pretreated with indomethacin had their levels of PGE2 decreased by 28.85%, TNF-α by 17.76%, and PKC by 44%. Meanwhile, pretreatment with JILE at 200 mg/kg caused insignificant changes in serum levels of TNF-α and PGE2 (p > 0.05) but reduced PKC by 46.47%, Table 1. Meanwhile, pretreatment with JILE at a dose of 400 mg/kg decreased PGE2 by 29.35%, TNF-α by 16.90% and PKC by 47.83%, as compared with carrageenan control group resulting in restoring serum levels of NO and PKC to their basal levels and were not significantly different from the effect observed with indomethacin treatment (p > 0.05).
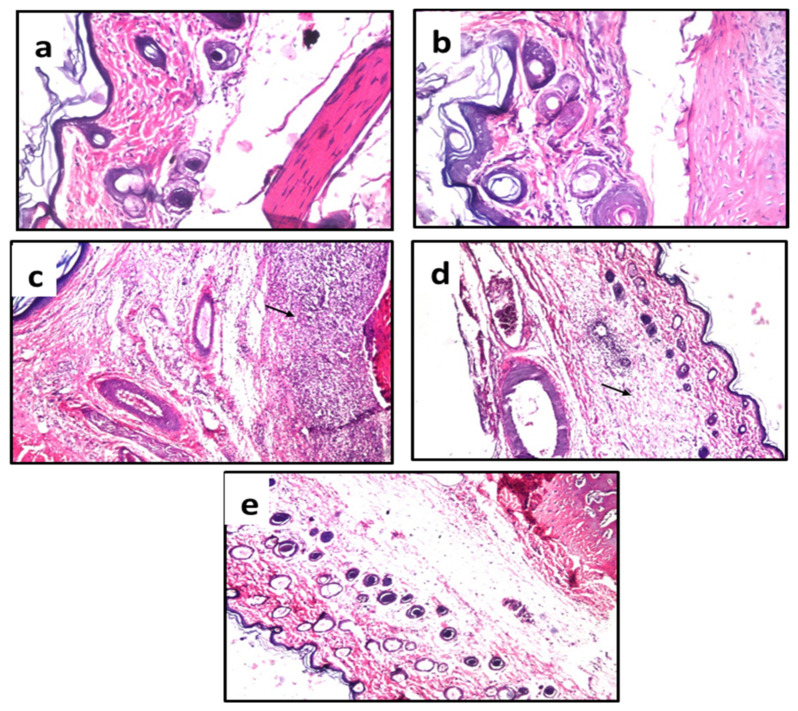
The paw tissue in the healthy group and the group treated with carrageenan and indomethacin displayed normal histological features (Figure 2a,b), while the carrageenan treated group showed a few inflammatory cells infiltration in the dermal tissue and massive inflammatory cell infiltration in the subcutaneous tissue (Figure 2c). Mild focal inflammatory aggregation in subcutaneous tissue was noticed in the group treated with JILE (200 mg/kg) with normal dermal tissue (Figure 2d) while the dermal and subcutaneous tissues appeared intact in the group treated with JILE at 400 mg/kg, (Figure 2e).
Figure 2.
Photomicrographs of subcutaneous and dermal tissues of treated rats. (a) control group; (b) indomethacin group; (c) carrageenan group; (d) group receiving oral 200 mg/kg of J. integerrima leaves extract; and (e) group receiving oral 400 mg/kg of J. integerrima leaves extract. Arrows refer to inflammatory cell infiltration.
2.3. Evaluation of Topical Anti-Inflammatory Activity of Jatropha integerrima Leaves Extract
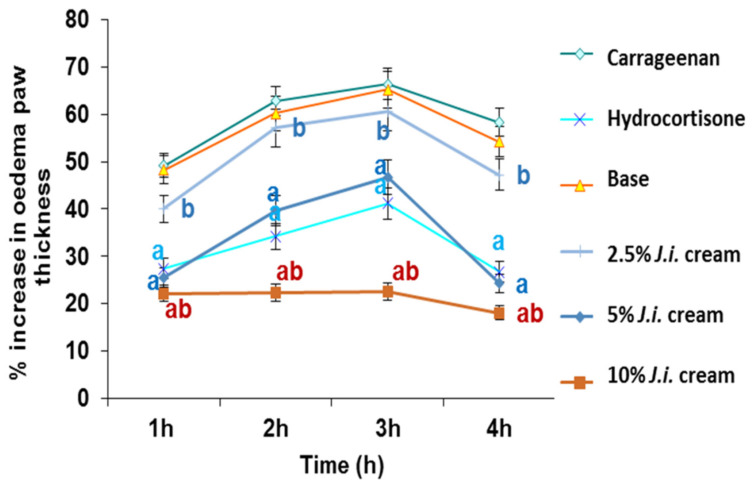
Rats given J. integerrima cream in the skin irritation test showed no sensitivity or irritation. The subplantar injection of carrageenan into the rat hind paw elicited an inflammation as previously mentioned in Section 2.2. Topical application of base non-medicated cream and 2.5% strength JILE cream before carrageenan injection showed no significant inhibition of edema formation at all-time points as compared with that of carrageenan group, Figure 3. Meanwhile topical application of higher strength JILE creams (5% and 10%) showed a significant inhibition of edema formation at all-time points especially after 4 h by 58.27% and 68.96%, respectively, as compared with that of untreated animals. In addition, the effect produced upon application of 10% JILE cream was superior to standard hydrocortisone cream (p < 0.05), Figure 3.
Figure 3.
Effect of topical administration of Jatropha integerrima cream on edema volume in rat hind paw model. One way ANOVA and Fishers LSD comparison test* were used in data analysis, p < 0.05; a significantly different from carrageenan control value at respective time point. b significantly different from hydrocortisone group value at respective time point.
Four hours postcarrageenan injection, inflammatory mediators were measured. An increased serum NO levels by 39.66% and 35.81% were observed in carrageenan treated animals and in animals treated with base cream, respectively, as compared with that of healthy control group. Animals that were treated with hydrocortisone cream and JILE cream at 2.5%, 5% and 10% showed significantly decreased serum NO levels by 14.84%, 10.92%, 17.04%, and 18.04%, respectively, as compared with that of untreated animals, Table 2.
Table 2.
Effect of topical administration of ethanol extract of Jatropha integerrima cream on different inflammation biomarkers.
| Control | Carrageenan | Hydrocortisone 1% Cream |
Base | JILE 2.5% Cream |
JILE 5% Cream |
JILE 10% Cream |
|
|---|---|---|---|---|---|---|---|
| NO (µmol/L) | 17.9 ± 0.7 | 25 ± 0.7 a | 21.29 ± 0.6 ab | 24.31 ± 0.1 a | 22.27 ± 0.59 ab | 20.74 ± 0.58 ab | 20.49 ± 0.08 ab |
| PGE2 (pg/mL) | 215.4 ± 1.5 | 286.2 ± 0.4 a | 223.4 ± 0.2 b | 284.4 ± 1.8 a | 256.4 ± 1.4 ab | 243.4 ± 1.1 ab | 216.4 ± 1.1 b |
| TNF-α (pg/mL) | 1561.9 ± 13.6 | 1903.9 ± 62.9 a | 1615.9 ± 15.68 b | 1857.9 ± 78.1 a | 1804.5 ± 23.7 a | 1779.9 ± 4.4 ab | 1609.9 ± 22.3 b |
| PKC (pg/mL) | 854.9 ± 22.8 | 1259.9 ± 4.4 a | 854.98 ± 15.1 b | 1251.9 ± 26.4 a | 965.98 ± 9.8 ab | 924.9 ± 11.1 ab | 839.9 ± 2.2 b |
Data were expressed as mean ± SD. Statistical analysis was carried out by one-way ANOVA followed by Tukey’s HSD test for multiple comparisons. a: Significantly different from normal control (Saline) at p < 0.05. b: Significantly different from carrageenan control at p < 0.05.
As expected for animals receiving carrageenan only or treated with base cream, levels of inflammatory mediators were all elevated. An increase of serum levels by 32.86% and 32.03% in case of PGE2, 21.89% and 18.95% for TNF-α, and 47.37% and 46.43% in case of PKC were observed in animals that received no treatment or base cream only, respectively. Treatment with hydrocortisone resulted in a decrease in serum levels of PGE2 by 21.94%, TNF-α by 15.12%, and PKC by 32.13% when compared with that of untreated group. Meanwhile, pretreatment with cream containing 2.5% of JILE decreased PGE2 and PKC levels by 10.41% and 23.32%, respectively, while animals treated with cream containing 5% JILE decreased PGE2, TNF-α and PKC by 14.95%, 6.51%, and 26.58%, respectively, as compared with that of carrageenan group. Meanwhile, animals that received cream containing 10% of extract showed normalized serum levels of TNF-α, PKC, and PGE2, as compared with that of control group, as did treatment with hydrocortisone cream, Table 2.
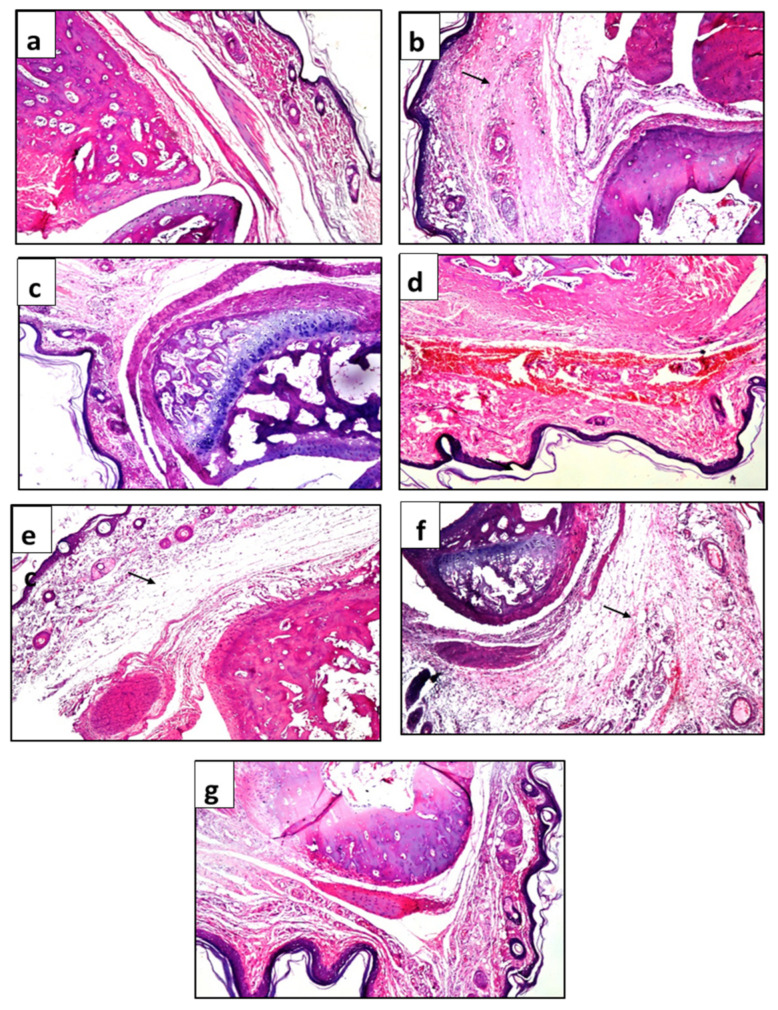
The paw tissue in the healthy group and group treated with carrageenan and hydrocortisone displayed normal histological features (Figure 4a,c), except for few inflammatory cells in the hydrocortisone group. Animals that were injected with carrageenan but received no further treatment showed massive inflammatory cell infiltration in the subcutaneous tissue (Figure 4b). Hemorrhages, inflammatory cells, and hyalinization were noticed in animals treated with base cream only (Figure 4d). Edema with inflammatory cells infiltration was detected in animals treated with creams containing 2.5% and 5% of JILE (Figure 4e,f), while animals treated with 10% JILE cream showed few inflammatory cells in subcutaneous tissue, and the skin layer remained intact, Figure 4g.
Figure 4.
Photomicrographs of subcutaneous and dermal tissues of treated rats. (a) healthy animals; (b) animals treated with carrageenan only; (c) 1% hydrocortisone cream; (d) base cream; (e) 2.5% JILE cream; (f) 5% JILE cream; (g) 10% JILE cream. Arrows refer to inflammatory cell infiltration.
2.4. UPLC-MS Analysis of Jatropha integerrima Extract
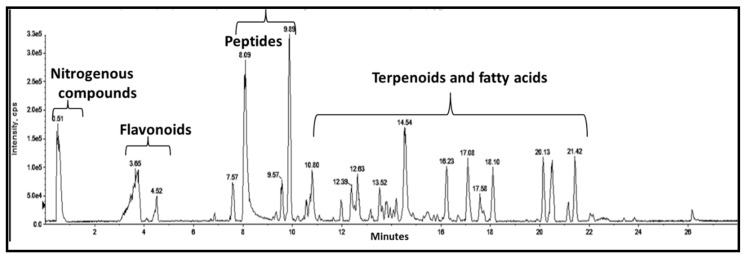
A total of 133 compounds were annotated in the extract representing different primary and secondary metabolites. Flavonoids and amino acids were eluted between 0–6 min, cyclic peptides were eluted from 9–11 min while diterpenoids, sterols, fatty acids, and fatty acid glycerides were eluted at the late part of the chromatographic run, Figure 5.
Figure 5.
Base peak chromatogram of the UPLC/MS analysis of J. integerrima leaves extract.
2.4.1. Phenolic Compounds
Analysis of the UPLC/MS chromatogram resulted in the identification of 19 flavonoids, 10 phenolic acid conjugates, 4 coumarins, and 1 lignan. Flavonoids occurred mainly as C-linked hexoses (11 flavonoids) which were readily identified by the neutral loss of 120 Da, Figure S1, as observed in peaks 10, 11, 13, 14, 19, 22, 24, 29, 30, 32 and 35 (Table 3, Figure 6). Most of the C-glycosides were derivatives of apigenin, including isoorientin (peak 11), vitexin (peak 13), and isovitexin (peak 14), which are reported here for the first time from J. integerrima. Vitexin (C-8 glycoside) was differentiated from its structural isomer isovitexin (C-6) by the intensity of the fragment ions at m/z 313 and 283, produced due to the cleavage of the C-attached sugar, Figure S2.
Table 3.
Annotated metabolites in leaves extract of Jatropha integerrima using UPLC/MS-Q-TOF analysis.
| Peak No. | tR (min) | Experimental m/z [M + H]+ | Molecular Formula | Error (ppm) | Fragments (m/z) | Tentative Identification |
|---|---|---|---|---|---|---|
| Flavonoids | ||||||
| 10 | 0.981 | 595.1656 | C27H30O15 | 3.4 | 577, 433, 379, 367, 313, 283 | Isovitexin-O-hexoside |
| 11 | 2.68 | 449.1076 | C21H20O11 | 2.3 | 431,383, 353, 339, 329, 299 | Isoorientin |
| 13 | 2.915 | 433.1116 | C21H20O10 | 3.2 | 415, 379, 367, 337, 313, 283 | Vitexin |
| 14 | 3.13 | 433.1116 | C21H20O10 | 3.2 | 415, 379, 337, 323, 313, 283 | Isovitexin |
| 16 | 3.4 | 449.1076 | C21H20O11 | 2.9 | 431, 395, 287 | Kaempferol O hexoside |
| 19 | 3.67 | 560.1788 | C27H29NO12 | 4.5 | 445, 427,409, 325, 295 | Methylisovitexin proline |
| 20 | 3.912 | 595.1672 | C27H30O15 | 2.2 | 287, 271 | Kaempferol O-rutinoside |
| 22 | 4.05 | 771.2151 | C37H38O18 | 2.6 | 675, 651,433,415, 313, 283, 177 | Vitexin ferulate-O-hexoside |
| 24 | 4.123 | 771.2111 | C37H38O18 | −2.6 | 675, 651,433,415, 313, 283, 177 | Isovitexin ferulate hexoside |
| 25 | 4.253 | 579.1708 | C27H30O14 | −0.4 | 433,417,399,351,321 297, 271 | Apigenin O-rhamnoside-O-hexoside |
| 26 | 4.396 | 433.1155 | C21H20O11 | 4.6 | 271 | Apigenin-O-hexoside |
| 27 | 4.44 | 579.1717 | C27H30O14 | −1.5 | 271.0597 | Apigenin-O-rutinoside |
| 29 | 5.213 | 553.1329 | C28H24O12 | −1 | 433, 415,397,337,313, 295, 283, | Vitexin p hydroxy benzoate |
| 30 | 5.253 | 877.2175 | C30H40O20 | 1.2 | 859, 757, 739, 455, 379, 325 | Jatrophenol I/II/III |
| 32 | 5.942 | 877.219 | C30H40O20 | 0.5 | 859, 445, 427, 409,379,349,325 | Jatrophenol I/II/III |
| 33 | 6.06 | 639.1772 | C32H30O14 | 2.1 | 415, 271,207 | Apigenin sinapoyl hexose |
| 34 | 6.13 | 609.159576 | C31H28O13 | 2.4 | 489, 433, 397, 313, 283, 177 | Apigenin ferulate-C-hexoside |
| 35 | 6.215 | 736.2208 | C33H36NO15 | 3.8 | 621,585,427,391,325, 295,177 | Methylisovitexin proline ferulate ester |
| 58 | 11.1 | 225.091 | C15H12O2 | 0.0 | 197, 165, 105 | Flavanone |
| Phenolic Acid Conjugates | ||||||
| 21 | 3.98 | 417.1347 | C18H24O11 | −4.4 | 237,109 | Cinnamic glycerol hexoside |
| 23 | 4.06 | 503.1766 | C22H30O13 | 1.4 | 485, 467, 383 | Methyl-O-feruloylquinate diacetate |
| 52 | 9.9 | 261.1852 | C17H24O2 | 1.1 | 243, 201, 147, 119 | Octyl cinnamic acid |
| 56 | 10.57 | 221.1537 | C14H20O2 | 5.0 | 203, 161, 151, 133, 123, 105 | 4-Heptylbenzoic acid |
| 74 | 13 | 291.1958 | C18H26O3 | 1.1 | 193, 123 | Octyl-4-methoxycinnamate |
| 76 | 13.1 | 235.17 | C15H22O2 | 3.2 | 179,123,57 | Octyl benzoate |
| 106 | 19.5 | 401.3422 | C27H44O2 | 2.0 | 191, 177, 137 | Eicosenoyl benzoate |
| 118 | 21.4 | 391.2837 | C24H38O4 | 1.2 | 167,149 | Tetradecyl ferulate |
| 128 | 22.17 | 419.3153 | C26H42O4 | 0.7 | 275, 293, 275,177, 127 | Hexadecyl ferulate |
| 129 | 23.4 | 463.3793 | C29H50O4 | 2.4 | 445, 417, 177,139 | Nonadecyl ferulate |
| Coumarins | ||||||
| 12 | 2.9 | 193.0492 | C10H18O4 | 137, 77,53 | Scopoletin | |
| 15 | 3.31 | 223.0595 | C11H10O5 | 2.7 | 149, 207, 121 | Fraxidin |
| 28 | 5.081 | 501.159 | C22H28O13 | 2.5 | 339, 321, 177, 209 | Methylhydroxycoumarin dihexoside |
| 42 | 9.2 | 223.0738 | C15H10O2 | 5.7 | 177, 149, 121 | 4-Phenyl coumarin |
| Nitrogenous Compounds | ||||||
| 1 | 0.465 | 266.1604 | C11H23NO6 | 2.2 | 248, 230, 116,104, 87 | Choline hexoside |
| 4 | 0.515 | 104.106585 | C5H13NO | 0.9 | 56.04, 58.06, 59.07, 60.07, 71 | Choline |
| 5 | 0.515 | 116.070497 | C5H9NO2 | 0.9 | 70 | L-Proline |
| 6 | 0.541 | 144.100864 | C7H13NO2 | 0 | 58, 84 | Stachydrine |
| 7 | 0.6 | 138.0549 | C7H7NO2 | 0.4 | 136, 94,92,79,66 | Amino benzoic acid |
| 8 | 0.65 | 213.1241 | C10H16N2O3 | 2.3 | 195, 177,135,121 | Prolylproline |
| 9 | 0.975 | 146.060114 | C9H7NO | 1.0 | 51, 65, 77, 91, 117, 118, 128 | Indole carboxyaldehyde |
| 73 | 12.96 | 270.3153 | C18H39N | 0.8 | 158 | Octadecylamine |
| Cyclic Peptides | ||||||
| 43 | 9.51 | 722.4826 | C36H63N7O8 | 1.3 | 623, 510, 379, 280, 183 | Cyclo (Val-leu-Leu-Val-Ser-Leu-Pro) |
| 45 | 9.59 | 809.5482 | C40H72N8O9 | −1.8 | 722, 623, 605, 510, 280, 211 | Cyclo (ABA-Val-leu-Leu-Val-Ser-Leu-Val) |
| 46 | 9.6 | 767.5383 | C38H70N8O8 | −1.5 | 722, 704, 623, 510, 379 | Cyclo (MeLys-leu-Leu-Val-Ser-Leu-Val) |
| 47 | 9.7 | 652.4030 | C31H53N7O8 | 0.24 | 634,521, 381, 268, 181 | Integerrimacyclopeptide B |
| 49 | 9.84 | 782.4596 | C39H59N9O8 | 4.8 | 669, 651, 355, 284 | Cyclogossine A |
| 53 | 10.05 | 781.4595 | C40H60N8O8 | 1.5 | 763, 668, 650, 555, 468 | Integrimide A |
| 54 | 10.53 | 767.5031 | C37H66N8O9 | 0.7 | 749, 654, 636, 523, 394 | Integerrimacyclopeptide A |
| 55 | 10.55 | 668.430 | C32H57N7O8 | −3.2 | 650, 555, 537, 424,367,284,171 | Cyclo (Leu-Leu-Gly-Thr-Leu-Ala-Val) |
| Diterpenes | ||||||
| 31 | 5.814 | 317.2112 | C20H28O3 | 0.6 | 299, 271, 231, 175, 173 | Cleistanthol/ Jatrointelone C |
| 37 | 6.88 | 291.1951 | C18H26O3 | 1.3 | 255, 167 | Triihydroxy-13- methylpodocarpane-triene |
| 41 | 9.11 | 317.2110 | C20H28O3 | −0.06 | 299, 271,231,175 | Jatrointelone C/ Cleistanthol |
| 51 | 9.9 | 275.2008 | C18H26O2 | 0.88 | 257,239, 173,159,119 | Dihydroxy-13- methylpodocarpane-triene |
| 59 | 11.36 | 301.21539 | C20H28O2 | 2.7 | 283 239 227 218 185 | Spruceanol |
| 61 | 11.95 | 275.2016 | C18H26O2 | 3.8 | 257, 173, 131, 91 | Dihydroxy methylpodocarpane-8,10,13-triene isomer |
| 63 | 11.98 | 315.1936 | C20H26O3 | 5.9 | 231, 199, 133,123,81 | Multifidone |
| 67 | 12.4 | 411.2355 | C22H34O7 | 5.4 | 351, 333, 369 | Excolabdone C/ Isoforskolin |
| 68 | 12.6 | 289.179 | C18H24O3 | −2.8 | 221, 205 | Methyl podocarpate |
| 70 | 12.7 | 671.3041 | C36H46O12 | −3.1 | 621, 593, 331 | Premyrsinol propanoate-benzoate-triacetate |
| 90 | 15.8 | 321.2428 | C20H32O3 | 1.2 | 275, 257 | Jatrodagricaine A |
| 94 | 17.57 | 609.2703 | C34H40O10 | 0.5 | 591, 531, 515, 273, 123 | Diterpene benzoate triacetate |
| 96 | 18 | 415.2391 | C25H34O5 | 1.8 | 369, 313, 295 | Deoxyingenol angelate |
| 101 | 18.18 | 651.2815 | C36H42O11 | 1.5 | 633, 601, 573, 483, 283 | Diterpene benzoate tetracetate |
| 103 | 18.65 | 593.2741 | C34H40O9 | −0.7 | 547, 533, 461, 447 | Diterpene benzoate triacetate |
| 104 | 18.85 | 575.3942 | C34H54O7 | 0.1 | 309, 177 | Phorbol-12-Myristate |
| 110 | 20.15 | 695.307 | C38H46O12 | 1.1 | 649, 563, 517 | Diterpene benzoate pentacetate |
| 112 | 20.37 | 653.2981 | C36H44O11 | 2.4 | 609, 575,565, 549,503, 521 | Euphorbiaproliferin F or isomer |
| 113 | 20.4 | 637.3002 | C36H44O10 | −1.6 | 619, 587, 559 | Peditithin H or isomer |
| 114 | 20.52 | 637.3018 | C36H44O10 | 0.8 | 619, 587,559, 473 | Peditithin H or isomer |
| 117 | 21.1 | 621.3045 | C36H44O9 | −1.8 | 561, 533,461,433, 193 | Diterpene benzoate triacetate |
| 119 | 21.46 | 431.2432 | C25H34O6 | 0.9 | 231,165 | Ingenol mebutate |
| 123 | 21.9 | 665.3349 | C38H48O10 | 4.3 | 619, 587, 559 | Diterpene dibenzoate diacetate |
| 126 | 22.03 | 681.3265 | C38H48O11 | −0.6 | 635, 593, 549, 503 | Diterpene dibenzoate diacetate |
| Sesquiterpenoids | ||||||
| 57 | 10.9 | 219.1751 | C15H22O | 3.0 | 203 | Unidentified sesquiterpenoid |
| 60 | 11.6 | 253.179 | C15H24O3 | 3.2 | 197, 179, 141, 151 | Ilicic Acid |
| 75 | 13.06 | 235.1700 | C15H22O2 | 3.2 | 179, 123,57 | 4-Patchoulen-15-oic acid |
| 122 | 21.65 | 235.1697 | C15H22O2 | 1.9 | 179, 81 | Oxo-hydroxyguai-diene |
| Triterpenoids | ||||||
| 107 | 20.03 | 441.3736 | C30H48O2 | 2 | 423 287 235 189 149 | Oxoamyrin |
| 116 | 20.9 | 439.3573 | C30H46O2 | 0.6 | 301, 233, 173, 149, 121 | Dioxo-olean-12-ene |
| Fatty Acids and Their Conjugates | ||||||
| 38 | 7.46 | 293.2125 | C18H28O3 | 4.7 | 275, 257, 213, 195, 155 | Oxo-phytodienoic acid |
| 44 | 9.53 | 767.5324 | C43H74O11 | 2.6 | 623, 511 | GlcADG (34:3) |
| 62 | 11.95 | 293.2113 | C18H28O3 | 0.6 | 275, 257, 147, 133 | Oxo-phytodienoic acid |
| 64 | 12.26 | 319.2256 | C20H30O3 | −5.3 | 301 | Oxo-eicosatetraenoic acid |
| 65 | 12.4 | 313.2362 | C18H32O4 | 3.6 | 295,277,151,95,81 | Hydroperoxy-octadecadienoic acid |
| 66 | 12.4 | 295.2278 | C18H30O3 | 3.5 | 277,179,149,151, 137, 119 | Oxo-octadecadienoic acid |
| 69 | 12.63 | 277.2166 | C18H28O2 | 1.4 | 259, 135,149, 121, 107 | Octadecatetraenoic acid |
| 71 | 12.85 | 295.2276 | C18H30O3 | 2.8 | 277, 259, 231, 165 | Hydroxy-Octadecatrienoic |
| 72 | 12.86 | 351.2539 | C21H34O4 | 2.9 | 277, 259, 179, 149,133 | MG 18:4 |
| 77 | 13.48 | 520.3395 | C26H50NO7P | −0.9 | 502,184,104 | LPC 18:2 |
| 78 | 13.5 | 295.2278 | C18H30O3 | 6.2 | 277,179,149,151, 137,119 | Hydroxy linoleinc acid |
| 79 | 13.53 | 279.232 | C18H30O2 | 0.5 | 149, 95, 81 | Linolenic acid |
| 80 | 13.7 | 465.2623 | C22H41O8P | 1.22 | 447,311,237, 155 | PA: (10:1/ 9:0) |
| 81 | 13.76 | 313.2734 | C19H36O3 | 1.0 | 295, 277, 165, 123, 95 | Oxononadecanoic acid |
| 82 | 13.8 | 331.2899 | C19H38O4 | 1.9 | 313, 257, 239, 123 | 2-Monopalmitin MG 16:0 |
| 83 | 13.94 | 295.2278 | C18H30O3 | 3.5 | 277,179,149,151, 137, 119 | Hydroxy-octadecatrienoic acid |
| 84 | 14.08 | 467.2784 | C22H44O8 P | 2.17 | 393,313,239,155 | PA (10:0/ 9:0) |
| 85 | 14.1 | 295.2265 | C18H30O3 | 0.9 | 277, 179, 151 | Hydroxylinolenic acid |
| 86 | 14.18 | 496.3403 | C24H50NO7P | −0.34 | 478,184,125, 104, 86 | LPC 16:0 |
| 87 | 14.3 | 522.3580 | C26H52NO7P | 3.89 | 504,184,150, 104 | LPC 18:1 |
| 88 | 15.3 | 291.1945 | C18H26O3 | 3.3 | 273 249 203 147 | Oxo-octadecatetraenoic acid |
| 89 | 15.37 | 723.5084 | C41H70O10 | 5.2 | 177, 133,89 | MGDG 16:2, 16:2 |
| 91 | 16.095 | 524.3709 | C26H54NO7P | −1.36 | 506,184,104 | LPC 18:0 |
| 92 | 16.19 | 305.2483 | C20H32O2 | 2.6 | 259, 149, 135 | Arachidonic acid |
| 93 | 17.1 | 307.2631 | C20H34O2 | 0.4 | 329, 307 | Eicosatrienoic acid |
| 95 | 17.64 | 323.2590 | C20H34O3 | 2.0 | 305, 277, 179,151 | Hydroxylinoleinic acic ethyl ester |
| 97 | 18.06 | 331.2846 | C19H38O4 | 0.9 | 313, 257, 239 | 1-Monopalmitin MG 16:0 |
| 98 | 18.07 | 699.5008 | C39H70O10 | 4.6 | 625, 607, 429 | MGDG 16:2, 14:0 |
| 99 | 18.07 | 353.2665 | C21H36O4 | 4.0 | 2-Monolinolenin MG 18:3 | |
| 100 | 18.08 | 745.483 | C43H68O10 | −5.5 | 699, 625, 415, 295 | MGDG 16:3, 18:4 |
| 102 | 18.24 | 782.5670 | C44H80NO8P | −3.8 | 765,307 | Phosphatidylcholine (18:2/18:2) |
| 108 | 20.1 | 359.3145 | C21H42O4 | 3.0 | 341, 285, 267, 123 | Monosteirin |
| 109 | 20.13 | 755.5713 | C43H78O10 | 3.9 | 681,663,443,323 | MGDG 18:2/16:0 |
| 111 | 20.15 | 381.2996 | C23H40O4 | 0.9 | none | MG 20:3 |
| 115 | 20.89 | 549.4527 | C34H60O5 | 2.5 | 531, 513, 287, 189, 121 | DG 14:1/17:2 |
| 121 | 21.6 | 313.2723 | C19H36O3 | 4.5 | 295, 277, 149, 133 | Ricinoleate methyl esters |
| 125 | 22 | 613.4828 | C39H64O5 | 0.2 | 595, 335, 261 | Dilinolenin DG 18:3/18:3 |
| 132 | 25.1 | 591.4920 | C37H66O5 | −6.3 | 573, 335,313,261 | DG 18:3/16:0 |
| Phytosterols | ||||||
| 105 | 19.36 | 445.3630 | C29H48O3 | −4.6 | 427, 341, 185 | Oxo-hydroxy sitosterol |
| 120 | 21.55 | 413.3792 | C29H48O | 2.07 | 395, 159 | Stigmasterol |
| 124 | 21.9 | 429.3723 | C29H48O2 | 0.9 | 411 | Stigmast-4-en-6beta-ol-3-one |
| 127 | 22.15 | 461.3616 | C29H48O4 | 2.0 | 443, 401, 383, 187 | Trihydroxystigmastan-6-one ene |
| 130 | 23.5 | 415.3964.8 | C29H50O | 4.8 | 397, 341 | Sitosterol |
| 131 | 23.7 | 429.3729 | C29H48O2 | 0.2 | 219, 205, 165 | Stigmastane 3,6 dione |
| Jasmonates | ||||||
| 36 | 6.263 | 265.1433 | C15H20O4 | 0.5 | 247, 219,207,205,167,99 191 | Abscisic acid |
| 39 | 7.55 | 181.122163 | C11H16O2 | 9.4 | 163,135, 121, 107, 99, 93 | Jasmorolone |
| 40 | 8.6 | 255.1498 | C13H20O3 | 5.7 | 195, 179, 143,137 | Methyljasmonate |
| Miscellaneous Compounds | ||||||
| 2 | 0.47 | 365.1069 | C14H20O11 | 2.6 | 203, 185 | Ethyl aconitate hexoside |
| 3 | 0.48 | 527.1584 | C20H30O16 | 4.8 | 365, 347, 203, 185 | Ethyl aconitate dihexoside |
| 17 | 3.56 | 197.1168 | C11H16O3 | −2.1 | 179,161,135, 107 | Loliolide/Epiloliolide |
| 18 | 3.63 | 197.1170 | C11H16O3 | −1.2 | 179, 161, 133, 105, 91 | Loliolide/Epiloliolide |
| 48 | 9.8 | 409.166 | C24H24O6 | 3.5 | 289, 121, 119 | Benzyl shikonin |
| 50 | 9.88 | 387.1793 | C22H26O6 | −2.3 | 267, 147, 121, 105 | Eudesmin/epieudesmin |
| 133 | 25.45 | 417.3652 | C28H48O2 | 0.3 | 191, 151 | Tocopherol |
DG: diglyceride; LPC: lysophosphatidylcholine; MG: monoglyceride; MGDG: monoglyceridedi galactoside; PA: phosphatidic acid.
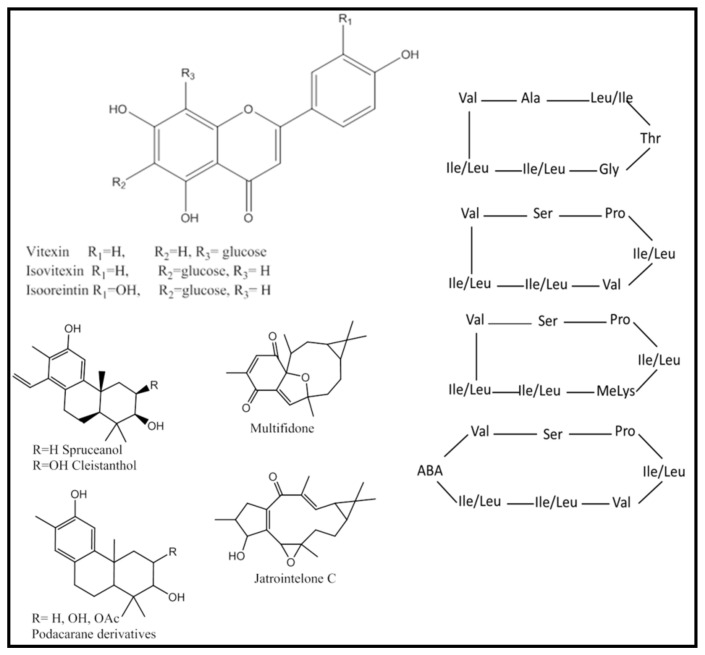
Figure 6.
Structures of selected compounds annotated in J. integerrima extract including flavonoids, peptides, and diterpenoids.
Two C-glycosides dimers were observed at 5.25 and 5.94 min (peaks of 30 and 32, respectively) with [M + H]+ at m/z 877.218 for C30H41O20 and were assigned to the isomeric jatrophenols I/II/III previously isolated from J. multifida [20]; they were also detected in two other Jatropha species [21]. These are dimers of isovitexin connected by a methylene bridge [22], Figure S3. Fragmentation of these dimers resulted in loss of isovitexin monomer (−432 Da) and appearance of methylisovitexin fragment ion at m/z = 445, Table 3, Figure S3. Two other peaks (peaks 19 and 35) produced the same fragment ion (m/z = 445) with [M+H]+ observed at m/z 560.1788 (for C27H30NO12) and 736.2208 (for C33H37NO15), respectively. Both peaks showed loss of proline amino acid as indicated by the neutral loss of 115 Da for C5H9NO2, Figure S4 and S5 and produced methylisovitexin fragment ions at m/z = 445 and 427. Therefore, peak 19 and 35 were annotated as methylisovitexin proline and methylisovitexin proline ferulate. To the best of our knowledge, this is the first report of isovitexin proline derivatives.
Meanwhile, flavonoids O-glycosides were characterized by the neutral loss of 162 Da for hexoses (peaks 10, 16, 22, 26), Figure S6, and 146 for rhamnosyl residue (peak 25) [23], Figure S7, Table 3.
Phenolic acids in J. integerrima extract were detected only as conjugated molecules substituted with medium or long aliphatic chains, as seen in peaks 52, 56, 74, 76,106, 118, 128, and 129. Among the annotated phenolic acid esters, only tetradecyl ferulate (peak 118) was previously reported in the genus and was isolated from both J. curcas [24] and J. multifida [25].
2.4.2. Nitrogenous Compounds
Eight simple nitrogenous compounds were annotated in J. integerrima extract, including choline and its glycoside (peaks 4 and 1, respectively). Other simple nitrogenous compounds included proline (peak 5) and its betaine derivative stachydrine (peak 6) in addition to proylproline (peak 8).
Apart from the simple nitrogenous compounds, 8 cyclic peptides (hepta-and octa-peptides) were observed between retention time of 9.5–10.5 min. Five cyclic peptides were previously isolated from the latex of J. integerrima [12,26,27] including integerrimide A (peak 53, [M + H]+ at m/z 781.4595 calculated for C40H61N8O8) [12], integrimmacyclopeptide A (peak 54, [M + H]+ at m/z 767.5031 calculated for C37H67N8O9), and integrrimacyclopeptide B (peak 47, [M + H]+ at m/z 652.4030 calculated for C31H54N7O8). Additionally, four cyclic peptides, which were not previously reported, were annotated in peaks 43, 45, 46, and 55, Figure 6. The amino acid sequence of these peptides was tentatively determined based on the high-resolution mass of their fragment ions Table S1, Figures S8–S11. These new cyclic peptides consisted mainly of hydrophobic residues including leucine or isoleucine (neutral loss of 113 Da, corresponding to C6H11NO) and valine (neutral loss of −99 Da corresponding to C5H9NO), Table S1, Figure 6.
2.4.3. Terpenoids
The most abundant group of secondary metabolites detected in J. integerrima leaves was that of terpenoids including sesquiterpenes (4 metabolites), diterpenes (24 metabolites) and two triterpenoids. To date, only free diterpenoids or their monoacetyl derivatives were reported in J. integerrima [14,15,28]. In this study, free diterpenoids were eluted first at retention time 5–11 min and were of cleistanthane, lathyrane, and podocarpane scaffold containing two or three oxygen atoms (peaks 31, 37, 41, 51, 59, 61, 63, and 68, Table 3, Figure 6, Figures S12–S14).
Meanwhile, diterpenoids esters were eluted at retention time 12–22 min and were mostly esters of jatrophane, phorbol, and ingenol. Acetate esters were predominant and were readily identified by the neutral loss of acetic acid (−60 Da) as seen in peaks 67, 70, 94, 112, 113, 114, and 117, while the presence of angelate ester (peak 96) was inferred from the neutral loss of 102 Da. Additionally esterification with benzoic acid was observed in late eluting peaks as indicated by the increase in double bond equivalent (14–16). These were observed in peaks 103 (C34H40O9), 110 (C38H46O12), 113,114 (C36H44O10), 117 (C36H44O9), 123 (C38H48O10), and 126 (C38H48O11) which showed multiple loss of acetate (−60 Da) and fragment ion at m/z 123 for benzoic acid, Figures S15 and S16. This substitution pattern of acetate and benzoate esters of a highly oxygenated skeleton has never reported before in J. integerrima. However, among Euphorbiaceae plants, molecules with highly oxygenated jatrophane, esterified with benzoic and acetic acid, were isolated from Pedilanthus tithymaloides and are designated as peditithin [29], and from Euphobia sanctae-catharinae are designated as euphosantiananes, [30,31]. Due to lack of extensive fragmentation of these high molecular weight esters, the exact carbon skeleton of these diterpenes could not be identified.
Among sesquiterpenes annotated in this study, an oxo-hydroxyguai-diene derivative was annotated (C15H22O2, peak 122), which was previously isolated from J. integerrima [11]. Two triterpenoids were annotated, namely, oxoamyrin and dioxo-olean-12-ene.
2.4.4. Fatty Acids and Their Conjugates
Fatty acids were detected as free fatty acid, fatty acid glycerides, phospholipids, and glycolipids, which together accounted for 38 metabolites. Unsaturated fatty acids C-18 were most abundant including oxo-phytodienoic (C18H28O3), linolenic (C18H30O2), hydroxylinoleinic (C18H30O3), oxo-octadecadienoic (C18H30O3) and ricinoleic acids. Several fatty acids derivatives were annotated in the extract including monoglyceride (MG), diglycerides (DG), monogalactosyl diglycerides (MGDG), phosphatidic acid esters (PA), and lysophasphatidyl choline (LPC).
Monoglycerides showed two consecutive loss of water (−18 Da), in addition to loss of −92 Da corresponding to loss of glycerol moiety (peaks 82, 108, and 111, Figure S17). Meanwhile, phosphatidic acid esters displayed a characteristic loss of 154 Da and appearance of a fragment ion at 155 amu corresponding to phosphopropionic acid (C3H7O5P) as seen in peaks 80 and 84, Table 3, Figure S18. Annotated phosphatidic acid esters were diglyceride with medium chain length (C-9 and C-10) and were observed in peaks 80 and 84. Fatty acid esters containing choline showed characteristic choline fragment at m/z 104.106 as in peaks 86, 87, and 91, Figure S19. Additionally, monogalactosyl diglycerides MGDG were identified in peaks 89, 98, 100, and 109, mainly as monoglycerides of C-16 or C-18 fatty acids.
2.4.5. Phytosterols and Other Compounds
Six phytosterols were annotated in the UPLC/MS chromatogram of J. integerrima including β-sitosterol, stigmasterol, stigmastane 3,6 dione and stigmast-4-en-6beta-ol-3-one, which all were previously reported in the genus Jatropha and the latter was isolated from J. integerrima. [11]. Three jasmonates (peaks 36, 39, and 40), two terpene lactones (peaks 17, 18), and tocopherol (peak 133) were also among annotated compounds.
2.5. Quantitative Determination of Secondary Metabolites
To gain possible insights into the relative abundance of secondary metabolites in J. integerrima extract, spectrophotometric methods were used to estimate the abundance of certain classes of secondary metabolites. Leaves’ extract of J. integerrima showed low abundance of phenolic compounds and flavonoids at 70.4 ± 0.4 mg GAE and 10.7 ± 0.1 mg QE per gram of extract, respectively. Meanwhile, results from vanillin/sulfuric assay indicated a high terpenoidal content of 149.7 mg UAE per gram of the extract.
3. Discussion
Leaves of Jatropha integerrima were used in South-East Asia (particularly in India and Bangladesh) for treatment of some inflammatory conditions such as arthritis and eczema. Other Jatropha species have well documented anti-inflammatory activity as proven by folk use, in vitro and in vivo assays [4,32,33]. Our investigation showed that Jatropha integerrima leaves extract (JILE) possess an anti-inflammatory effect when used in rat paw edema model. Both oral administration and topical application of the extract were able to reduce signs of inflammation (edema volume) and levels of inflammatory mediators (NO, TNF-α, PKC, and PGE2). Inflammation induced by carrageenan increased paw edema thickness and was associated with elevated levels of NO, TNF-α, PKC, and PGE2 after 4 h, as previously shown in other studies [34]. The involvement of NO and PGs in the modulation of inflammation is well established [35]. Lipid peroxidation initiated by the product of the reaction of NO with superoxide (peroxynitrite) liberates arachidonic acid from the cell membrane activating PGE2, which is one of the strongest inflammatory mediators [36]. Moreover, TNF-α induces NO synthesis by activating inducible nitric oxide synthase iNOS and augments the responses of neutrophils to inflammatory stimuli [37,38]. Activation of PKC mediates the activation of NF-κB, and secretion of TNF-α, IL-6, and IL-10 through TLR2/1 [39]; this may explain the elevation of PKC that provoked the increase in TNF-α serum level in our work.
Treatment with JILE decreased levels of these inflammatory mediators in a dose dependent manner and restored their basal levels at higher treatment dose of 400 mg/kg (per oral route) and using 10% strength cream (after topical application). Additionally, normal tissue structure was observed in animals receiving JILE treatment especially with topical application of 5% or 10% cream or using an oral dose of 400 mg/kg confirming the ability of JILE extract to inhibit inflammatory response.
The metabolomics profile of J. integerrima identified many compounds with known anti-inflammatory activity. For example, 19 flavonoidal compounds were detected in the extract which were mainly apigenin derivatives. Apigenin and its C-glycosides (vitexin and isovitexin) are known anti-inflammatory compounds that protect cells against oxidative stress and inflammation [40,41,42]. The major flavonodial compound in the extract (vitexin) was shown to reduce the levels of inflammatory cytokines such as IL4, IL5, and IL13 by 64%, 95%, and 65% in an induced asthma model at concentration of 1 mg/kg [43].
In addition to flavonoids, coumarins such as scopoletin and fraxidin have anti-inflammatory action mediated by their antioxidant potential [44,45,46]. Scopoletin can reduce inflammation and level of PGE2 in LPS stimulated cell lines [47].
Finally, nonpolar compounds such as triterpenes, sterols, and diterpenes are usually main contributors to anti-inflammatory activity of plant extracts. As reported previously, the nonpolar n-hexane fraction of Jatropha curcas extract had a better anti-inflammatory activity when tested in vitro compared to the polar fraction [33]. Additionally, J.integerrima extract was enriched with oxo and hydroxyl fatty acids (13 compounds, Table 3), which received attention recently due to their potential as anti-inflammatory agents and their indigenous role in regulating body inflammatory response [48].
4. Conclusions
These results suggest that the ethanol extract of the leaves of Jatropha integerrima possess a significant anti-inflammatory effect, both through oral administration and topical application. This effect can be attributed to its high content of flavonoids, terpenoids, and oxygenated fatty acids, which are represented in the metabolomics profile of the extract by 65 compounds. Our findings encourage further investigation of the rich metabolome of J. integerrima to identify novel anti-inflammatory compounds.
5. Material and Methods
5.1. Plant Material
Fresh leaves of Jatropha integerrima Jacq. were collected from plants cultivated at the Medicinal, Aromatic and Poisonous Plants Experimental Station of the Faculty of Pharmacy, Cairo University (Giza, Egypt). Plant identity was confirmed by Therese Labib, consultant of plant taxonomy at the Ministry of Agriculture and Orman Botanical Garden (Giza, Egypt). Vouchered herbarium specimen (code numbers 582018III) was deposited at the Pharmacognosy Department, Faculty of Pharmacy, Cairo University.
5.2. Preparation of Plant Extract
Fresh leaves were air dried in shade and reduced to powder. The powdered leaves (1 kg) were extracted with absolute ethanol by cold maceration till exhaustion (4 × 5 L). The solvent was then removed by vacuum distillation at a temperature not exceeding 40 °C, and the dried ethanol extract was kept in an air-tight container at 4 °C till use.
5.3. Determination of Total Phenolic Content
Total phenolic content of J. integerrima leaves extract was carried out using the Folin–Ciocalteu method following the optimized assay procedures described by Blainski, Lopes, and de Mello [49]. The total phenolic content was expressed as mg gallic acid equivalent (GAE)/g of extract using a standard calibration curve of gallic acid (20–200 μg/mL).
5.4. Determination of Flavonoid Content
Total flavonoid content was determined by measuring the yellow color developed upon reacting flavonoids with AlCl3 reagent according to optimized assay conditions described by Silva et al. [50]. The total flavonoid content was expressed in mg quercetin equivalent (QE)/g of extract based on pre-established calibration curve of quercetin (0.1–0.7 mg/mL).
5.5. Determination of Steroidal/Terpenoidal Content
Spectrophotometric estimation of total steroidal and/or terpenoid content was carried out based on the chromogenic reaction produced upon treatment of the extract with vanillin/ sulfuric acid reagent according to the protocol developed by V. Le et al. [51]. The color developed was measured at λ = 544 nm, and total steroid and/or terpenoid content was expressed as ursolic acid equivalent (UAE mg/g of extract) as deduced from a pre-established calibration curve using standard ursolic acid (20–160 μg/mL).
5.6. Ultra-Performance Liquid Chromatography Analysis
Liquid chromatography instrument (Sciex, TripleTOF 5600+, Framingham, MA, USA) was used for chromatographic separation. A reversed phase C18 analytical column (Waters, Xbridge C18, 2.1 × 50 mm, 3.5 µm particle size) was used at 40 °C. Leaves extract (50 mg) was dissolved in 1 mL of water: methanol: acetonitrile (50:25:25) and centrifuged for 5 min at 10,000 rpm followed by filtration through a membrane disc filter. Twenty microliters were diluted to 1 mL and 10 µL of this solution was injected into the system. Gradient elution was carried out at a flow rate of 0.3 mL/min using eluent A (0.1% formic acid in deionized water) and eluent B (100% acetonitrile). Elution was performed according to the following gradient: 10% B, 0–1 min; 10%–90% B, 1–25 min; 90%–10% B, 25–28 min.
5.7. High Resolution Quadrpole-Time of Flight Mass Analysis
UPLC system was coupled to electrospray ion source with quaderpole-time of flight mass analyzer (ESI-QTOF, Framingham, MA, USA). MS analysis was performed in positive ion mode; cone voltage, 30 eV; capillary voltage, 3 kV; desolvation temperature, 450 °C; cone gas flow, 50 L/h and desolvation gas flow, 900 L/h. Time of flight mass scan (TOF-MS, Framingham, MA, USA) was controlled using Analyst TF 1.7.1 and was recorded over m/z range 50–1000. TOF MS/MS scan was done in information dependent acquisition over the same mass range. UPLC-MS/MS data were processed with PeakView 2.2 (Framingham, MA, USA) for data extraction.
5.8. Anti-Inflammatory Assay
5.8.1. Animals
Adult male Wister albino rats (7–8 weeks old, weighing 130–180 g) were housed in standard cages (10 rats/cage) under pathogen-free conditions and maintained at controlled room temperature (21–24 °C) with a 40–60% relative humidity and under normal dark-light cycles. All animals had free access to rat chew diet and water ad libitum. All procedures were approved by the Animal Care Committee of the National Research Centre.
5.8.2. Acute Toxicity Study
Rats were divided into two groups of 12 rats each (6 males and 6 females). The extract of J. integerrima was suspended in distilled water and given orally to rats of the first group in a single dose of 5 g/kg. The control group received the same volumes of distilled water. The percentage mortality for the extract was recorded for 24 h. Animals were observed for 14 days, for any changes in skin, fur, respiratory, circulatory, central nervous system, somatomotor activity, and behavior pattern. Particular observation for tremors, convulsions, salivation, diarrhea, lethargy, sleep, and coma were also recorded.
5.8.3. Carrageenan Induced Paw Edema
Anti-inflammatory activity was evaluated using carrageenan induced paw edema. Paw swelling was elicited by subplantar injection of 100 μL of 1% sterile lambda carrageenan suspension in saline into the right hind paw [35]. Contralateral paw received an equal volume of saline solution. The edema component of inflammation was quantified by measuring hind footpad immediately before carrageenan injection and 1–4 h after carrageenan injection with a micrometer caliber [52]. Edema was expressed as a percentage of change from control (predrug) values. Rats, to be injected with carrageenan, were divided into nine groups each of 10 animals. Group 1 (control group): where rats received oral saline (0.2 mL/rat); group 2: rats were given indomethacin (25 mg/kg); groups 3 and 4: rats received the extract of J. integerrima (200 mg/kg and 400 mg/kg), respectively. Indomethacin and the extracts were given orally 60 min before the injection of the carrageenan suspension.
5.8.4. Skin Irritation Test
Twelve rats were divided into two groups (six rats each), namely, control and test groups. Hairs of rat hind paw were shaved, and rats of different groups were kept in separate cages for seven days. An amount (0.3 g) of 10% cream was placed on the shaved skin (4 cm2) for rats of the test group while the other group received only base cream. The area was covered with a cotton bandage and any sensitivities were assessed and graded.
5.8.5. Topical Anti-Inflammatory Activity
The base cream was prepared according to recipe by Franyoto et al.( 2018) [53] containing stearic acid (142 g), glycerine (100 g), sodium tetraborate (2.5 g), triethanolamine (10 g), methyl paraben (0.1 g) and 750 mL of distilled water. The base cream was then mixed with the ethanol extract of the leaves of Jatropha integerrima at three different concentrations (2.5%, 5%, and 10%) [53].
Before edema induction as described in Section 5.8.3, rats were divided into six groups each of 10 rats. Group 1 was left untreated, while animals in group 2 were given a single topical dose (0.3 g) of market product of hydrocortisone (1%). Group 3 rats received a single topical dose (0.3 g) of base cream, while animals in groups 4, 5, and 6 received 0.3 g of the cream extract of J. integerrima at concentrations of 2.5%, 5%, and 10%, respectively. An amount of 0.3 g of the tested cream was used 30 min before injection of the carrageenan suspension, and it was gently rubbed 50 times with the index finger. A separate group of healthy animals were kept as a control group. Edema was expressed as a percentage change from control (predrug) values [54].
5.8.6. Blood Samples and Biochemical Analysis
Four hours after carrageenan injection and immediately after measuring edema volume, animals were anesthetized with urethane (1.5 g/kg; i.p.) and blood samples were taken from the abdominal aorta and used for determination of PGE2 using Abnova ELISA Kit, TNF-α using Cusabio ELISA Kit, and PKC by enzyme linked immunoassay (ELISA) technique using standard kits (Glory Science Co., Ltd, Louisiana, LA, USA).
5.8.7. Histological Examination
Paws were fixed in 10% formalin solution and dehydrated in ascending grades of alcohol and embedded in paraffin. Sections at four-micron thickness were taken and stained with hematoxylin and eosin (H&E).
5.9. Statistical Analysis
All the values are presented as mean ± standard error of the means (SE). Comparisons between different groups were carried out using one-way analysis of variance (ANOVA), followed by Tukey’s HSD test for multiple comparisons. Graph pad Prism software, version 5 (GraphPad Software Inc., San Diego, CA, USA), was used to carry out statistical tests. The difference was considered significant when p < 0.05.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants11020218/s1, Figure S1: MS/MS spectrum of peak 29: Vitexin-p-hydroxybenzoate; Figure S2: Fragmentation pattern highlighting different fragmentation of the two structural isomer vitexin and isovitaxin based on the intensity of 313 and 283 fragment ions; Figure S3: MS/MS spectrum of peaks 30 and 32: Jatrophenol I/II/III; Figure S4: MS/MS spectrum of peak 35: Proline methylisovitexin; Figure S5: MS/MS spectrum of peak 35: Methylisovitexin proline ferulate; Figure S6: MS/MS spectrum of peak 26: Apigenin-O-hexoside; Figure S7: MS/MS spectrum of peak 25: Apigenin-O-hexoside-O-rhamnoside; Figure S8: MS/MS spectrum of peak 43: A cycloheptapeptide; Figure S9: MS/MS spectrum of peak 45: A cyclooctapeptide; Figure S10: MS/MS spectrum of peak 46: A cycloheptapeptide; Figure S11: MS/MS spectrum of peak 46: A cycloheptapeptide; Figure S12: MS/MS spectrum of peak 59: Spruceanol; Figure S13: MS/MS spectrum of peak 61; Figure S14: MS/MS for peak 67: Isoforskolin; Figure S15: MS/MS for peak 114: Premyrsinol/peditithin derivatives; Figure S16: MS/MS of peak 125: Premyrsinol/peditithin derivative; Figure S17: MS/MS of peak 82: Monopalmitin; Figure S18: MS/MS of peak 84: Phosphatidic acid (10:0/9:0); Figure S19: MS/MS of peak 86: Lysophosphatidylcholine (16:0/0:0); Table S1: Analysis of high resolution MS/MS Q-TOF fragments for newly identified cyclic peptides.
Author Contributions
Conceptualization: S.M.E.-Z. and A.M.S.; methodology, A.H.E.; software, E.A.M.; validation, A.A.A.S. and A.H.E.; formal analysis, A.H.E. and E.A.M.; investigation, A.H.E., E.A.M. and A.A.A.S.; resources, S.M.E.-Z. and A.M.S.; data curation, E.A.M.; writing—original draft preparation, E.A.M. and A.A.A.S.; writing—review & editing, A.H.E. and S.M.E.-Z.; supervision, A.M.S. and S.M.E.-Z. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data related to this article are presented in this manuscript or available as supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cavalcante N.B., Diego da Conceição Santos A., Guedes da Silva Almeida J.R. The genus Jatropha (Euphorbiaceae): A review on secondary chemical metabolites and biological aspects. Chem. Biol. Interact. 2020;318:108976. doi: 10.1016/j.cbi.2020.108976. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan N., Palanisamy K., Mulpuri S. Jatropha: Phytochemistry, Pharmacology, and Toxicology. In: Mulpuri S., Carels N., Bahadur B., editors. Jatropha, Challenges for a New Energy Crop. Springer; Singapore: 2019. pp. 415–435. [Google Scholar]
- 3.Sabandar C.W., Ahmat N., Jaafar F.M., Sahidin I. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): A review. Phytochemistry. 2013;85:7–29. doi: 10.1016/j.phytochem.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Salim M.N., Masyitha D., Harris A., Balqis U., Iskandar C.D., Hambal M. Darmawi Anti-inflammatory activity of Jatropha curcas Linn. latex in cream formulation on CD68 expression in mice skin wound. Vet. World. 2018;11:99–103. doi: 10.14202/vetworld.2018.99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdulkarim B., Al-Maqtari T., Al-Doaiss A.A., Dhabali A.A.H. Antioxidant, antimicrobial and wound healing potential of Jatropha variegata -An interesting plant endemic to Yemen. Pakistan J. Biol. Sci. 2020;23:1581–1590. doi: 10.3923/pjbs.2020.1581.1590. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Hernandez A.B., Alarcon-Aguilar F.J., Garcia-Lorenzana M., Rodriguez-Monroy M.A., Canales-Martinez M.M. Jatropha neopauciflora Pax latex exhibits wound-healing effect in normal and diabetic mice. J. Evid.-Based Integr. Med. 2021;26:2515690X2098676. doi: 10.1177/2515690X20986762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verardo G., Baldini M., Ferfuia C., Gorassini A. Rapid and selective screening for toxic phorbol esters in Jatropha curcas seed oil using high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Chromatogr. A. 2019;1597:63–75. doi: 10.1016/j.chroma.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Roach J.S., Devappa R.K., Makkar H.P.S., Becker K. Isolation, stability and bioactivity of Jatropha curcas phorbol esters. Fitoterapia. 2012;83:586–592. doi: 10.1016/j.fitote.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Akhter A., Rahman M.S., Ahsan M. Preliminary antimicrobial and cytotoxic activities of n-hexane extract of Jatropha pandurifolia. Lat. Am. J. Pharm. 2008;27:918–921. [Google Scholar]
- 10.Wagh V.V., Jain A.K. Ethnopharmacological survey of plants used by the Bhil and Bhilala ethnic community in dermatological disorders in Western Madhya Pradesh, India. J. Herb. Med. 2020;19:100234. doi: 10.1016/j.hermed.2018.09.005. [DOI] [Google Scholar]
- 11.Sutthivaiyakit S., Mongkolvisut W., Prabpai S., Kongsaeree P. Diterpenes, sesquiterpenes, and a sesquiterpene−coumarin conjugate from Jatropha integerrima. J. Nat. Prod. 2009;72:2024–2027. doi: 10.1021/np900342b. [DOI] [PubMed] [Google Scholar]
- 12.Mongkolvisut W., Sutthivaiyakit S., Leutbecher H., Mika S., Klaiber I., Möller W., Rösner H., Beifuss U., Conrad J. Integerrimides A and B, cyclic heptapeptides from the latex of Jatropha integerrima. J. Nat. Prod. 2006;69:1435–1441. doi: 10.1021/np0602012. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J.-Y., Cheng B., Zheng Y.-J., Dong Z., Lin S.-L., Tang G.-H., Gu Q., Yin S. Enantiomeric neolignans and sesquineolignans from Jatropha integerrima and their absolute configurations. RSC Adv. 2015;5:12202–12208. doi: 10.1039/C4RA15966G. [DOI] [Google Scholar]
- 14.Zhang D.-B., Wang Z., Liang Y.-N., Yu J.-G., Zhang Z., Liu S.-J., Zhang Z., Song Z.-X., Tang Z.-S., Duan D.-Z. Jatrophainolides A–C, new cembrane-type diterpenoids with PTP1B inhibitory activity from the root bark of Jatropha integerrima. Phytochem. Lett. 2020;36:166–170. doi: 10.1016/j.phytol.2020.02.007. [DOI] [Google Scholar]
- 15.Zhu J.-Y., Lou L.-L., Guo Y.-Q., Li W., Guo Y.-H., Bao J.-M., Tang G.-H., Bu X.-Z., Yin S. Natural thioredoxin reductase inhibitors from Jatropha integerrima. RSC Adv. 2015;5:47235–47243. doi: 10.1039/C5RA07274C. [DOI] [Google Scholar]
- 16.Mohammed A.E., Al-Keridis L.A., Rahman I., Alotaibi M.O., Suliman R.S., Alrajhi A.M., Elobeid M.M., Alothman M.R., Alhomaidi E.A., Korany S.M. Silver Nanoparticles Formation by Jatropha integerrima and LC/MS-QTOF-Based Metabolite Profiling. Nanomaterials. 2021;11:2400. doi: 10.3390/nano11092400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eshilokun A.O., Kasali A.A., Ogunwande I.A., Walker T.M., Setzer W.N. Chemical composition and antimicrobial studies of the essential oils of Jatropha integerrima Jacq (leaf and seeds) Nat. Prod. Commun. 2007;2:853–855. doi: 10.1177/1934578X0700200813. [DOI] [Google Scholar]
- 18.Xu D.-P., Zhou Y., Zheng J., Li S., Li A.-N., Li H.-B. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of Jatropha integerrima by response surface methodology. Molecules. 2016;21:18. doi: 10.3390/molecules21010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cong H.H., Khaziakhmetova V.N., Zigashina L.E. Rat paw oedema modeling and NSAIDs: Timing of effects. Int. J. Risk Saf. Med. 2015;27:S76–S77. doi: 10.3233/JRS-150697. [DOI] [PubMed] [Google Scholar]
- 20.Marzouk M.S., Moharram F.A., Haggag E.G., El-Batran S.M., Mahmoud I.I., Ibrahim R.R. Novel biflavone diglycosides and biological activity of Jatropha multifida leaves. Chem. Nat. Compd. 2012;48:765–770. doi: 10.1007/s10600-012-0377-z. [DOI] [Google Scholar]
- 21.Zengin G., Mahomoodally M.F., Sinan K.I., Ak G., Etienne O.K., Sharmeen J.B., Brunetti L., Leone S., Di Simone S.C., Recinella L., et al. Chemical composition and biological properties of two Jatropha species: Different parts and different extraction methods. Antioxidants. 2021;10:792. doi: 10.3390/antiox10050792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moharram F., Marzouk M., Haggag E., El-Batran S., Ibrahim R. Biological examination and novel biflavone di-C-glycosides from Jatropha multifida L. leaves. Planta Med. 2007;73:P048. doi: 10.1055/s-2007-986830. [DOI] [Google Scholar]
- 23.Mekky R.H., Thabet M.M., Rodríguez-Pérez C., Elnaggar D.M.Y., Mahrous E.A., Segura-Carretero A., Abdel-Sattar E. Comparative metabolite profiling and antioxidant potentials of seeds and sprouts of three Egyptian cultivars of Vicia faba L. Food Res. Int. 2020;136:109537. doi: 10.1016/j.foodres.2020.109537. [DOI] [PubMed] [Google Scholar]
- 24.Ravindranath N., Ravinder Reddy M., Ramesh C., Ramu R., Prabhakar A., Jagadeesh B., Das B. New lathyrane and podocarpane diterpenoids from Jatropha curcas. Chem. Pharm. Bull. 2004;52:608–611. doi: 10.1248/cpb.52.608. [DOI] [PubMed] [Google Scholar]
- 25.Das B., Ravikanth B., Laxminarayana K., Ramarao B., Raju T.V. New Macrocyclic diterpenoids from Jatropha multifida. Chem. Pharm. Bull. 2009;57:318–320. doi: 10.1248/cpb.57.318. [DOI] [PubMed] [Google Scholar]
- 26.Welé A., Baraguèye C., Ndiaye W., Fall D., Ndoye I., Diop Y., Dubosq L., Bodo B. [Cytotoxic activity of two cyclic peptides from the latex of Jatropha integerrima Euphorbiaceae] Dakar Med. 2007;52:209–215. [PubMed] [Google Scholar]
- 27.Idrissa N., Adama D., Mamadou B., Rokhaya S., Yoro T., Alassane W., Djibril F. Novel cytotoxic cycloheptapeptide from the Latex of Jatropha integerrima. J. Chem. Pharm. Res. 2016;8:135–139. [Google Scholar]
- 28.Sutthivaiyakit S., Mongkolvisut W., Ponsitipiboon P., Prabpai S., Kongsaeree P., Ruchirawat S., Mahidol C. A novel 8,9-seco-rhamnofolane and a new rhamnofolane endoperoxide from Jatropha integerrima roots. Tetrahedron Lett. 2003;44:3637–3640. doi: 10.1016/S0040-4039(03)00704-4. [DOI] [Google Scholar]
- 29.Zhu J., Wang R., Lou L., Li W., Tang G., Bu X., Yin S. Jatrophane diterpenoids as modulators of P-glycoprotein-dependent multidrug resistance (MDR): Advances of structure-activity relationships and discovery of promising MDR reversal agents. J. Med. Chem. 2016;59:6353–6369. doi: 10.1021/acs.jmedchem.6b00605. [DOI] [PubMed] [Google Scholar]
- 30.Hegazy M.-E., Hamed A., Ibrahim M., Talat Z., Reda E., Abdel-Azim N., Hammouda F., Nakamura S., Matsuda H., Haggag E., et al. Euphosantianane A–D: Antiproliferative premyrsinane diterpenoids from the endemic Egyptian plant Euphorbia sanctae-catharinae. Molecules. 2018;23:2221. doi: 10.3390/molecules23092221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elshamy A.I., Mohamed T.A., Al-Rowaily S.L., Abd-ElGawad A.M., Dar B.A., Shahat A.A., Hegazy M.-E.F. Euphosantianane E–G: Three new premyrsinane type diterpenoids from Euphorbia sanctae-catharinae with Contribution to chemotaxonomy. Molecules. 2019;24:2412. doi: 10.3390/molecules24132412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mujumdar A., Misar A. Anti-inflammatory activity of Jatropha curcas roots in mice and rats. J. Ethnopharmacol. 2004;90:11–15. doi: 10.1016/j.jep.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Othman A.R., Abdullah N., Ahmad S., Ismail I.S., Zakaria M.P. Elucidation of in-vitro anti-inflammatory bioactive compounds isolated from Jatropha curcas L. plant root. BMC Complement. Altern. Med. 2015;15:11. doi: 10.1186/s12906-015-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abd El-Alim S.H., Kassem A.A., Basha M., Salama A. Comparative study of liposomes, ethosomes and transfersomes as carriers for enhancing the transdermal delivery of diflunisal: In vitro and in vivo evaluation. Int. J. Pharm. 2019;563:293–303. doi: 10.1016/j.ijpharm.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Salvemini D., Wang Z.-Q., Bourdon D.M., Stern M.K., Currie M.G., Manning P.T. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur. J. Pharmacol. 1996;303:217–220. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- 36.Mariotto S., Suzuki Y., Persichini T., Colasanti M., Suzuki H., Cantoni O. Cross-talk between NO and arachidonic acid in inflammation. Curr. Med. Chem. 2007;14:1940–1944. doi: 10.2174/092986707781368531. [DOI] [PubMed] [Google Scholar]
- 37.Salim T., Sershen C.L., May E.E. Investigating the role of TNF-α and IFN-γ activation on the dynamics of iNOS gene expression in LPS stimulated macrophages. PLoS ONE. 2016;11:e0153289. doi: 10.1371/journal.pone.0153289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saini R., Singh S. Inducible nitric oxide synthase: An asset to neutrophils. J. Leukoc. Biol. 2019;105:49–61. doi: 10.1002/JLB.4RU0418-161R. [DOI] [PubMed] [Google Scholar]
- 39.Loegering D.J., Lennartz M.R. Protein kinase C and toll-like receptor signaling. Enzyme Res. 2011;2011:1–7. doi: 10.4061/2011/537821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He M., Min J.-W., Kong W.-L., He X.-H., Li J.-X., Peng B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia. 2016;115:74–85. doi: 10.1016/j.fitote.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Peng Y., Gan R., Li H., Yang M., McClements D.J., Gao R., Sun Q. Absorption, metabolism, and bioactivity of vitexin: Recent advances in understanding the efficacy of an important nutraceutical. Crit. Rev. Food Sci. Nutr. 2021;61:1049–1064. doi: 10.1080/10408398.2020.1753165. [DOI] [PubMed] [Google Scholar]
- 42.Ginwala R., Bhavsar R., Chigbu D.I., Jain P., Khan Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants. 2019;8:35. doi: 10.3390/antiox8020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venturini C.L., Macho A., Arunachalam K., de Almeida D.A.T., Rosa S.I.G., Pavan E., Balogun S.O., Damazo A.S., de Oliviera Martins D.T. Vitexin inhibits inflammation in murine ovalbumin-induced allergic asthma. Biomed. Pharmacother. 2018;97:143–151. doi: 10.1016/j.biopha.2017.10.073. [DOI] [PubMed] [Google Scholar]
- 44.Whang W.K., Park H.S., Ham I., Oh M., Namkoong H., Kim H.K., Hwang D.W., Hur S.Y., Kim T.E., Park Y.G., et al. Natural compounds, fraxin and chemicals structurally related to fraxin protect cells from oxidative stress. Exp. Mol. Med. 2005;37:436–446. doi: 10.1038/emm.2005.54. [DOI] [PubMed] [Google Scholar]
- 45.Jamuna S., Karthika K., Paulsamy S., Thenmozhi K., Kathiravan S., Venkatesh R. Confertin and scopoletin from leaf and root extracts of Hypochaeris radicata have anti-inflammatory and antioxidant activities. Ind. Crops Prod. 2015;70:221–230. doi: 10.1016/j.indcrop.2015.03.039. [DOI] [Google Scholar]
- 46.Ding Z., Dai Y., Hao H., Pan R., Yao X., Wang Z. Anti-Inflammatory effects of scopoletin and underlying mechanisms. Pharm. Biol. 2008;46:854–860. doi: 10.1080/13880200802367155. [DOI] [Google Scholar]
- 47.Kim H.-J., Jang S.I., Kim Y.-J., Chung H.-T., Yun Y.-G., Kang T.-H., Jeong O.-S., Kim Y.-C. Scopoletin suppresses pro-inflammatory cytokines and PGE2 from LPS-stimulated cell line, RAW 264.7 cells. Fitoterapia. 2004;75:261–266. doi: 10.1016/j.fitote.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 48.Kolar M.J., Konduri S., Chang T., Wang H., McNerlin C., Ohlsson L., Härröd M., Siegel D., Saghatelian A. Linoleic acid esters of hydroxy linoleic acids are anti-inflammatory lipids found in plants and mammals. J. Biol. Chem. 2019;294:10698–10707. doi: 10.1074/jbc.RA118.006956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blainski A., Lopes G., de Mello J. Application and analysis of the Folin Ciocalteu Method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013;18:6852–6865. doi: 10.3390/molecules18066852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva Mathias M., Rodrigues de Oliveira R. Differentiation of the phenolic chemical profiles of Cecropia pachystachya and Cecropia hololeuca. Phytochem. Anal. 2019;30:73–82. doi: 10.1002/pca.2791. [DOI] [PubMed] [Google Scholar]
- 51.Le A.V., Parks E.S., Nguyen M.H., Roach P.D. Improving the vanillin-sulphuric acid method for quantifying total saponins. Technologies. 2018;6:84. doi: 10.3390/technologies6030084. [DOI] [Google Scholar]
- 52.Salama A., Kotb R., Shaker R. Effect of treatment durability and coloration of coated cotton fabrics on antibacterial, UV-blocking, healing and anti-inflammatory properties. J. Chem. Pharm. Res. 2015;7:181–193. [Google Scholar]
- 53.Franyoto Y.D., Kusmita L., Mutmainah, Angrena R.D. Total flavonoid content and formulation antioxidant cream stem of Jatropha multifida L. J. Phys. Conf. Ser. 2018;1025:012130. doi: 10.1088/1742-6596/1025/1/012130. [DOI] [Google Scholar]
- 54.Pashmforosh M., Rajabi Vardanjani H., Rajabi Vardanjani H., Pashmforosh M., Khodayar M.J. Topical anti-inflammatory and analgesic activities of Citrullus colocynthis extract cream in rats. Medicina (B. Aires) 2018;54:51. doi: 10.3390/medicina54040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data related to this article are presented in this manuscript or available as supplementary material.