Abstract
Early successful conception of postpartum dairy cows is crucial in determining the optimum reproductive efficiency and profitability in modern dairy farming. Due to the inherent high production potential of modern dairy cows, the extra stress burden of peri-parturient events, and associated endocrine and metabolic changes causes negative energy balance (NEBAL) in postpartum cows. The occurrence of NEBAL is associated with excessive fat mobilization in the form of non-esterified fatty acids (NEFAs). The phenomenon of NEFA mobilization furthers with occurrence of ketosis and fatty liver in postpartum dairy cows. High NEFAs and ketones are negatively associated with health and reproductive processes. An additional burden of hypocalcemia, ruminal acidosis, and high protein metabolism in postpartum cows presents further consequences for health and reproductive performance of postpartum dairy cows. This review intends to comprehend these major nutritional metabolic alterations, their mechanisms of influence on the reproduction process, and relevant mitigation strategies.
Keywords: dairy cow, parturition, fertility, metabolic disorders, reproductive performance, ketosis, milk fever, fatty liver
1. Introduction
Modern large-scale dairy farming has emerged during recent decades. There is a gross improvement in milk production, mainly attributed to intensive selection and improved nutrition. However, a constant downward trend is observed for these high-yielding cows’ reproductive performance (RP) [1,2,3]. This decline in RP may be due to increased time to the first insemination, poor exhibition of estrus behavior, increased days open, decreased success rate of artificial inseminations (AIs), and high culling rates due to poor RP [4,5].
There are several factors contributing to the decline in RP, including genetic factors, heat stress, and disease-related causes, to name a few [6,7,8,9]. There exists a negative correlation between milk production and reproduction, as high milk production is maintained at the expense of reproductive health [10]. Enormous nutritional consumption by the mammary system causes an alteration in the physiology of reproduction. A lactating high-yielding cow consumes a great deal of glucose and suffers from a state of negative energy balance (NEBAL) during the early postpartum period [11,12]. With the selection for high milk yield in sires used for breeding, we see a narrowing of the genetic base of major breeds throughout the world and resultant inbreeding and, together with the intensification of dairy farming, difficulties of postpartum care increased. During the post-parturient period, severe NEBAL is manifested by clinical or subclinical metabolic diseases [13]. Hence, the disease factor is the single most common cause of RP decline in dairy cows. Besides a number of infectious diseases [14], milk fever, ketosis, and other nutritional alterations contribute to RP decline [15]. Nutritional metabolic alterations during the post-parturient period affect the development and dominance of follicles on the ovaries and subsequent ovulation, while reproductive tract diseases can directly affect fertilization, embryo/fetal development, implantation, and placental development [9,16].
Thus, maintaining reproductive efficiency is an obvious challenge presented through a longer post-parturient recovery period, silent estrus, lower conception rates, and early pregnancy (within 60 d) loss [2,4,5]. These conditions and alterations mechanistically affect the body’s three major regulatory systems: the nervous system, endocrine system, and circulatory and immune system. About two thirds of reproductive disorders are encountered in the first month postpartum. Collectively, these factors directly or indirectly affect the development of follicles, the embryo/fetus, and placenta, which in turn affect cows’ RP [17,18,19]. This review aims to comprehend all these events, their interrelationships, and consequences towards fertility decline and poor reproductive performance. The main focus will be the major nutritional metabolic disorders and their complex relationship with the fertility outcomes in dairy cows. Additionally, it will elaborate on various dietary mitigation strategies helpful to improve the postpartum welfare and RP of cows.
2. Nutritional Characteristics, Metabolic Diseases, and Reproduction
Ruminants such as cattle ferment feed to fulfill their energy requirement and gluconeogenesis in the liver is central to lactose synthesis in the mammary gland. During the peri-parturient period, there are fluctuations in the dry matter intake (DMI). A pronounced decline in DMI is observed in the last 10 days of parturition, followed by a marked increase afterwards, but this is presumably not sufficient to fulfill the increased nutrient and energetic demand of postpartum dairy cows [20,21,22]. The post-parturient lactation peak is generally observed after 4 to 6 weeks, while the DMI peak arrives at 8 to 10 weeks. Hence, cows are in NEBAL at least 50 d postpartum. A recent study has related low prepartum DMI and energy balance with postpartum indigestion and metabolic disorders [23]. The same group of researchers associated low prepartum DMI with postpartum reproductive disorders, while postpartum reproductive problems were associated with low postpartum DMI [24]. Therefore, the phenomenon of adequate feed intake and body energy balance during the perinatal period is of immense importance for health and reproductive processes.
In addition to DMI, the body condition score (BCS) is widely used to assess the energy balance, health, and reproductive outcomes in postpartum dairy cows [25]. High prepartum BCS is associated with increased risk of postpartum metabolic problems [23], while other studies show that low prepartum BCS is responsible for prepartum metabolic and reproductive disorders [26]. The reason for this conflict in studies is essentially due to the postpartum energetic metabolism changes related to fat mobilization [27], where higher prepartum BCS (obesity) is associated with postpartum metabolic disorders and low reproductive efficiency [28,29]. Additionally, a prepartum BCS loss of 0.5 points or more could negatively affect perinatal blood Ca levels and predispose cows to the risk of ketosis and delayed conception [30]. Furthermore, high prepartum BCS was associated with lower calf body weights [31]. It is concluded that prepartum low BCS and over-conditioning (higher BCS) are both unfavorable for postpartum reproductive efficiency. The over-conditioned cows had lower mRNA levels of TNFα and higher mRNA levels of peroxisome proliferator-activated receptor gamma (PPARγ) in adipose tissues during postpartum, while the phosphorylated protein kinase B (AKT) pathway related to extensive metabolic shifts through downstream signaling of insulin in adipose tissue was also upregulated [27]. The phenomenon of high inflammatory cytokine signaling and AKT signaling pathway upregulation synergistically enhance the energetic metabolism [32,33,34]. It can be proposed that energy balance monitoring through serum metabolites can help to predict the postpartum nutritional physiology and its ultimate repercussions on reproductive performance [30,35].
Postpartum NEBAL is characterized by low blood glucose and insulin and high ketone bodies and non-esterified fatty acid (NEFA) concentrations [36,37,38,39]. However, NEBAL combined with nutritional metabolic diseases such as ketosis will delay the recovery of the uterus and normal reproductive process, leading to prolonged time to first service and increased calving intervals. In the presence of NEBAL, there is an increased incidence of nutritional metabolic disorders [40]. The literature confirms that postpartum nutritional metabolic diseases are the major causes of post-parturient reproduction disorders in dairy cows [41]. Nutritional metabolic diseases are complex “production diseases”, with a high incidence rate in modern dairy cows. In addition to the direct economic losses caused by reduced milk production, they can also have a long-term negative impact on the physiology of cows, especially their reproductive efficiency [42,43,44]. A retrospective study of 7500 perinatal Holstein cows showed that within 21 d after parturition, about 1/3 of the cows presented with at least one subclinical or clinical metabolic disease. Furthermore, the 45 d fertilization rate decreased by 7%, milk yield decreased by 14%, and the culling rate increased from 22.6% (no clinical disease) to 35.7% (one clinical incidence) and 53.8% (more than three clinical incidences) for the cows suffering with metabolic diseases [45]. Another study indicated a 26% incidence rate of ketosis during the observation period of 60 days postpartum [46]. A study covering 12 countries from four continents found that the average subclinical ketosis prevalence in the first 21 days postpartum was 24.1%, ranging from 8.3% to 40.1% [47]. Another study based on commercial dairy farm data reported a higher incidence of 56% for clinical or subclinical metabolic and reproductive diseases (ketosis, hypocalcemia, metritis, and mastitis) in the first 3 weeks postpartum [48].
Initial post-parturient lactation is maintained at the expense of a decline in reproductive performance [10]. The mechanism involving underlying NEBAL can be explained by low insulin levels causing an abundance of growth hormones (GHs), which in turn mobilize NEFAs. However, at the same time, there is a decrease in hepatic GH receptor abundance, preventing negative feedback through IGF-1 [38,49], while the presence of NEFAs is consistently behind the low insulin via catecholamines [50]. As lipolysis helps the lactation demand, this is also the major cause of low BCS in postpartum cows. Thus, postpartum BCS is an indirect measurement of fat metabolism and correlates with the energy balance of cows [51]. These aforementioned metabolic alterations mediated by complex endocrine changes have further consequences for ovulation, oocytes, and the corpus luteum [38,49,52,53,54]. Further connections of these changes with reproductive performance include low concentrations of insulin and insulin growth factor 1 (IGF-1) causing follicular biochemical changes in ovaries and thus influencing luteinizing hormone (LH) and estradiol (E2) secretion [52,53,55]. This decrease in LH and E2 secretions in turn ultimately leads to delayed resumption of the estrus cycle [56]. On the other hand, NEBAL is also shown to be associated with low progesterone concentrations, which negatively influence the early pregnancy outcome [57,58]. Carrying the discussion further, a high concentration of blood NEFAs is shown to be negatively associated with the developmental competence of fertilized oocytes [59,60]. Generally, an increase above 0.55 mmol/L of plasma NEFA levels is regarded as a manifestation of serious postpartum NEBAL [61,62]. Postpartum NEBAL and high NEFA concentrations have shown evidence for higher levels of inflammation characterized by cytokines and Toll-like receptor (TLR) expression leading to alterations in uterine functions [63,64,65]. Furthermore, NEFA exerts cytotoxic effects at cellular levels and is attributed to immunosuppression [66,67]. As NEBAL in itself possesses implications for early reproductive recovery, it is also obvious that postpartum NEBAL can be regarded as a root cause of various postpartum production diseases. Based on the discussions in this review, Figure 1 summarizes various metabolic and endocrine mechanisms contributing to the decline in post-parturient reproductive efficiency of dairy cows. A score of mitigation strategies have been advised to minimize extreme postpartum NEBAL incidence. Dry cow management should be oriented at the feeding of energy-rich diets over the duration of 3–4 weeks prepartum, in order to support fetal growth, the decline in DMI, and peri-parturient events [68]. This phenomenon of increasing the energy content of the diet can also be supported by the facts of decreased DMI intake and rumination time during the last 3 weeks of gestation [21,24,69]. However, over-conditioning of prepartum cows is not desirable and leads to postpartum metabolic disorders [41,70]. A prepartum controlled energy diet near the calculated requirements essentially averted the risk of postpartum NEBAL [70,71]. An increased energy prepartum diet is shown to enhance fat accumulation, decrease DMI, and increase the risk of health problems in postpartum dairy cows [68,70,72]. Similarly, others have shown that an increased energy diet prepartum can lead to increased NEFA mobilization and triglyceride (TG) accumulation in the liver of postpartum cows [73], while a controlled energy diet prepartum is shown to improve the immune function of postpartum dairy cows [74]. Therefore, careful feeding management through monitoring of feed energy content and BCS assessment of prepartum cows constitute an essential key to perinatal transition success.
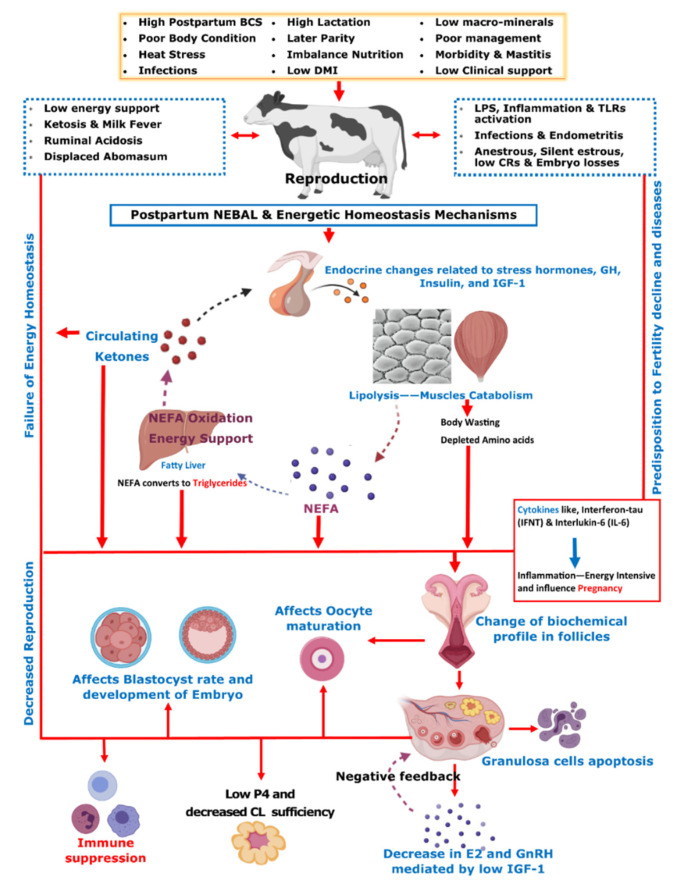
Figure 1.
On the basis of contents discussed in the manuscript, this summary chart illustrates post-parturient metabolic alterations resulting in the decline in reproductive performance in dairy cows. The postpartum dairy cow is over-stressed due to parturition labor, lactation demands, possible exposure to heat stress, reduced dry matter intake (DMI), uterine involution, and initiation of the reproductive cycle. Due to these problems, post-parturient dairy cows usually suffer from a negative energy balance (NEBAL). NEBAL leads to endocrine and metabolic alterations initiated by low insulin, high glucose consumption, decreased insulin growth factor (IGF-1), and high growth hormone (GH) activity, leading to high non-esterified fatty acid (NEFA) response. NEFA is oxidized in the liver for energy support, leading to ketosis, and ultimately results in the development of fatty liver due to the accumulation of triglycerides (TGs). A higher prepartum body condition score (BCS) is determined as a predisposing factor for extensive mobilization of body fat reserves in the form of NEFA. Fat mobilization and protein metabolism lead to the depletion of the most required fatty acids and amino acids for reproduction and body well-being. These changes are the primary cause of secondary metabolic diseases such as hypocalcemia, ruminal acidosis, and displaced abomasum. Furthermore, they ultimately cause changes in the biochemical profile of the ovarian follicles, contained oocyte, developing embryo, corpus luteum (CL), and uterus, which ultimately result in low conception rates (CRs), whereas they also trigger endocrine changes at the pituitary–hypothalamus-ovary axis, including changes in estrogen (E2), gonadotrophins (GnRH), luteinizing hormone (LH), and progesterone (P4). Additionally, these aforementioned changes also influence the immune system of postpartum dairy cows through activation of LPS, cytokines, and Toll-like receptors (TLRs). These phenomena predispose the cows to infections and inflammatory conditions and thus contribute to the decline in CRs.
3. Fatty Liver
Fatty liver (liver lipidosis) is a secondary peri-parturient metabolic disorder with high subclinical prevalence characterized by high TG accumulation in the liver [75,76]. Behind the backdrop of a variety of peri-parturient events and NEBAL, excessive NEFA mobilization is the major cause of fatty liver, mainly due to obesity and decreased DMI of parturition dairy cows [76,77]. The liver serves as the destination for NEFAs derived from fat mobilization, where they are oxidized into acetyl-CoA, which then condenses with oxaloacetate in the TCA cycle. During states of high NEFA delivery, much acetyl-CoA diverts from the TCA cycle to ketogenesis, producing β-hydroxy-butyric acid (BHBA), acetyl-acetic acid, and acetone. Fatty liver develops when the liver uptake of NEFA exceeds the oxidation and secretion capacity of the liver, where excess NEFAs are re-esterified to form TGs in the liver and are associated with decreased metabolic functions of the liver [75,78]. High TGs are shown to alter glucose metabolism in early pregnancy and can cause pre-eclampsia [79,80]. A postpartum fatty liver incidence rate of 56% has been reported in previous studies [79,80], while the probability of pregnancy is shown to be 30% lower for cows with higher contents of liver TGs [80]. The presence of inevitable NEBAL-associated metabolic changes and inflammatory condition-associated increases in cytokine levels are the major culprits behind the development of fatty liver [27,81,82]. As a direct outcome of NEBAL, fatty liver causes inhibition of gluconeogenesis, promotes ketosis, and decreases immune functions [77,83]. As fatty liver compromises liver function, it directly influences the reproductive system through the decline in NEFA oxidation as an energetic source, and indirectly influences the RP through the disposition of postpartum cows to ketosis and allied complications.
Regarding the occurrence of NEBAL and mobilization of NEFA, a manifestation of the fatty liver leading to ketosis, it is important to discuss ameliorating approaches. A variety of fatty acid supplementations have been used to try to modulate high NEFA responses during postpartum NEBAL [84]. Dietary supplementation of 3% to 4% vegetable oil has been associated with the improvement of postpartum reproductive performance [85,86]. A study shows that feeding saturated fatty acids increased the risk of fatty liver, while feeding unsaturated fatty acids sources such as flaxseed (3.3% and 11.0% of the dry matter in prepartum and postpartum diets, respectively) during the transition period could be a useful strategy to increase liver concentrations of glycogen and decrease liver TGs after calving [87]. Prepartum feeding of canola and sunflower (8% of DMI) is shown to have positive effects on energetic metabolism in postpartum cows, however, the transition from prepartum to lactation appeared to be the main driver of changes in energetic metabolism [88]. An interesting study shows that postpartum hydrogenated TG supplementation exerted more positive effects on hepatic lipid and glycogen metabolism than palm oil supplementation [89]. Contrary to high prepartum fat supplementation, postpartum high fat feeding is shown to increase TGs and decrease cholesterol levels, while switching from high prepartum fat to low postpartum fat supplementation increased cholesterol levels, feed intake, and milk production [90]. Feeding of unsaturated fatty acids (UFAs) is shown to improve the ovarian follicle biochemical profile, improve progesterone (P4) during the luteal cycle, and modulate prostaglandins during early pregnancy [5,91,92]. Polyunsaturated fatty acids (PUFAs), such as fish oil-based rumen protected supplementation, are the best in the class of dietary fats, which are shown to improve ovarian follicle growth, increase insemination success, and are helpful in the prevention of pregnancy losses [93,94]. Peroxisome proliferator activated receptors (PPARs) have an integral role in embryonic development and early pregnancy through lipid metabolism support. Due to their reported activation through NEFA and other fatty acids of dietary origin and subsequent coordination of lipid metabolism, studies have tried to enhance their expression and found that dietary fatty acids are helpful in this regard [33,95,96]. In conclusion, several nutritional supplementation-based mitigation strategies have been devised to avert the risk of fatty liver. However, a clear direction of the type of fat supplementation, its timing, and the duration of supplementations may constitute a future avenue of studies in complex in vivo trials.
4. The Impact of Ketosis on Reproductive Efficiency of Dairy Cattle
Ketosis refers to a nutritional metabolic disease of the blood circulation in which ketone bodies exceed normal physiological levels, and the excessive accumulation of fatty acids in the liver is the main cause of ketosis. Ketosis is a common metabolic disease around the post-parturient period [97], and later parity cows are more susceptible to the incidence of ketosis [98]. Predisposing factors of ketosis are similar to the ones causing postpartum NEBAL, NEFA mobilization, and hepatic lipidosis, discussed in the earlier sections. The liver oxidizes this NEFA and produces ketone bodies, and circulating NEFA and ketone bodies assist in overcoming the dairy cows’ energy requirements when suffering from NEBAL [39,53,99,100]. The more severe the NEBAL in the post-perinatal period of cows, the more NEFAs are transferred to the liver, leading to rapid increases in concentrations of ketone bodies such as BHBA, and eventually leading to different degrees of ketosis in cows. Since BHBA concentrations in the serum can directly reflect the extent of ketosis, they are often used as an important indicator for the diagnosis of ketosis.
Studies have found that the incidence of ketosis in the first 2 postnatal months is 2% to 15%. However, the incidence of subclinical ketosis during the same period could be as high as 40% to 60%. In addition, studies show that high-yielding cattle may have higher incidences of ketosis, due to more severe NEBAL in the post-parturient period [13,101,102]. Ketosis causes a decrease in the quantity and quality of milk, besides a decline in fertility in dairy cows. In one study, the economic losses of each cow with subclinical ketosis were averaged to be about USD 51, and the economic losses of clinical ketosis were about USD 232 per cow, while the long-term economic losses of subclinical ketosis in later parity cows can even reach USD 213 per head [103,104]. It affects the ovarian activity, the uterus and fallopian tubes, fertilization, and early and late embryo development [105,106,107,108,109]. A study considering the prenatal NEFA concentration and its relationship with metabolic hormones showed that cows in the prenatal high NEFA group were less likely to resume the estrus cycle than cows in the low NEFA group, suggesting that high concentrations of NEFA inhibited the recovery of postpartum ovulation [15].
4.1. Ketosis and Ovarian Dynamics
Postpartum ovarian follicle recovery or the initiation of development is the basis of the cow’s normal reproduction resumption. With the development of follicles, the amount of E2 secretion is increased, causing signs of estrus and triggering the LH surge for ovulation. Therefore, it directly determines the first postpartum service and the fertilization rate. Studies show that cows with subclinical ketosis delayed the exhibition of estrus and the duration was relatively shorter when compared to healthy cows. This suggests that ketosis affects the development of follicles and the normal synthesis and secretion of E2 in cows after calving [110]. NEFA accumulates in the follicle fluid, changing the composition of its metabolite profile, directly affecting the energy metabolism level of granulosa cells and causing apoptosis [111]. Studies show that, when compared with healthy cows, there is a decrease in time to first ovulation and 60 d postpartum conception rates in the cows with postnatal high blood NEFA and postpartum subclinical ketosis, respectively [105,112]. The high concentration of NEFA and BHBA in the blood circulation of diseased cows decreases and degrades the insulin-like growth factor (IGF-1) [113]. IGF-1 has a direct impact on the GnRH activity and LH activity, causing reduced E2 secretion. This phenomenon leads to failure of the follicular dominance and subsequent ovulation, delaying the time to first ovulation. In contrast, studies show that if follicles ovulate, the embryo quality remains poor. A decrease in IGF-1 concentration also leads to a decrease in progesterone secretion, and in that case, if initial pregnancy is confirmed, there is a greater chance of early embryonic death. Therefore, ketosis has long-lasting effects on follicles, ova, and embryonic development [59,112]. In conclusion, ketosis has a longer cycle of effects on cow reproduction and may involve follicle development to embryonic implantation.
4.2. Ketosis Association with Oocyte Maturation and Implantation
Metabolic disorders are manifested through changes in metabolites in blood circulation; therefore, the composition of follicle fluid is altered. Studies have confirmed a significant increase in BHBA and NEFA concentrations in ketosis cow follicle fluid [111]. A comprehensive study found high NEFA and BHBA, but lower glutathione, in the ovarian follicle fluid of cows suffering from liver diseases. Moreover, BHBA supplementation affected the concentration-dependent decrease in oocyte maturation, while the effect on blastocyst rate was significant [114]. In the presence of high NEFA, similar results are shown in cattle and human studies, where the follicular dominance and time to ovulation and blastocyst rate were reduced, respectively [115,116].
Early embryo development and implantation are the most complex events in developmental biology. Fertilized oocytes journey through the development and blastocyst stage towards the preimplantation stage. The development of a preimplantation embryo is of paramount importance, as it is a prerequisite for maternal identification, uterus implantation, and gestation. Studies have shown that the lipids nourish the embryo, which is regulated by PPARγ. In this context, PPARγ activity is essential for the extension and survival of embryos, and changing the concentration or composition of fatty acids in tissue fluids can potentially alter the development of fertilized oocytes [117]. An increase in the concentration of NEFA in blood, follicles, and in the uterus during ketosis may affect the elongation and survival of preimplanted embryos by affecting the metabolism of fatty acids [44,118]. A correct balance between inflammatory and anti-inflammatory reactions in the uterus is required for early embryo attachment and implantation, and an active immune system regulates this delicate balance [119,120]. Immune suppression in cows suffering from ketosis is also an important factor affecting embryo implantation. It can increase postpartum cows’ susceptibility to bacterial pathogens due to low resistance and thus increase the risk of uterine inflammation and endometritis [121,122]. Therefore, ketosis may affect the implantation of early embryos through alteration of the endometrial fatty acid metabolism and immune suppression, resulting in decreased fertility in cows. Given the importance of ketosis, feeding high-quality concentrates accompanied with prevention of over-feeding in later gestational stages can avert the occurrence of NEBAL and associated metabolic diseases [68,123]. Feeding of a glucogenic diet comprising starch in early lactation stages and lipogenic diets in later stages of breeding can improve the reproductive process [124].
5. The Impact of Hypocalcemia (Milk Fever) on Reproductive Efficiency of Dairy Cattle
Postpartum cows face the challenge of increasing demand for minerals, especially calcium, to support early lactation [125,126]. Increased prolactin, parathyroid hormone (PTH), and calcitriol levels are mainly involved in calcium homeostasis during the perinatal period [127,128,129]. Studies have shown that serum calcium drops by 9 h before and returns to the normal range by about 72 h after birth [130]. Lactation is the main cause of low blood calcium in cows after parturition, as the demand increases rapidly and calcium consumption is higher than the absorption, so hypocalcemia occurs. The incidence of hypocalcemia is high in smaller breeds with high milk production such as Jerseys [127]. BCS ≥ 3.00 and summer calving have been associated with higher risk of subclinical hypocalcemia on day 1 of parturition, where day 1 incidence cows took 32 days longer to get pregnant than normo-calcemic cows [131]. Most high-producing cows develop some degree of hypocalcemia (SCH) on the first day after calving, but only if the blood calcium concentration drops to a certain level, which can disrupt neuromuscular function and is detrimental. Hypocalcemia is divided into subclinical (total calcium concentration of 1.4 to 2.0 mmol/L) or clinical (total calcium concentration < 1.4 mmol/L), accompanied by mental restlessness, anorexia, mild paralysis, and even death. Compared to primiparous cows, later parity cows are relatively more susceptible to developing clinical symptoms of milk fever [132,133]. Hypocalcemia is a risk factor for causing ketosis, displaced abomasum, mastitis, retained placenta, and uterine prolapse [134] and thereby presents a greater chance of culling. The variable average incidence of milk fever is reported by different studies, ranging from 7.2% [135] to 21% [136].
Studies show that hypocalcemia negatively affected the recovery of ovarian function during the voluntary waiting period, reduced the rate of pregnancy after the voluntary waiting period, and reduced the pregnancy rate after first service [137]. Cows with chronic subclinical hypocalcemia are shown to have even more pronounced impaired reproductive function [137]. Subclinical hypocalcemia diagnosed on postpartum day 1 is shown to be responsible for low fertility rates, while diagnosis at both day 1 and day 7 was related to health issues in dairy cows [131]. Retained fetal membranes and uterine inflammation are typically associated with subclinical hypocalcemia [138,139]. Similarly, another study found that hypocalcemia had a negative effect on reproductive performance through a significant increase in the time to the first conception after birth and a higher risk of culling. However, they found that hypocalcemia had no effect on first postpartum service; this could be attributed to the husbandry practices [135]. Contrary to this, higher postpartum serum calcium concentrations are associated with higher serum total cholesterol, albumin, and glucose concentrations, a lower rate of placental retention, and clinical endometritis [140]. Abnormality in calcium homeostasis is also determined as a contributing factor towards arrested follicular development and acyclic ovaries [141]. About 50% of freshly calved multiparous cows are believed to suffer from hypocalcemia [142,143]. Together with blood fatty acid profile, serum calcium levels are useful to predict the incidence of displaced abomasum [144]. Given its association with the incidence of other reproductive diseases, the post-parturient blood calcium level is of paramount importance. Several studies show that blood calcium levels after 24 h of calving are positively associated with metritis [145].
Clinical milk fever cases should be treated with an intravenous infusion of calcium gluconate (23%, 500 mL = 10.8 g of calcium) [146]. Prepartum feeding of a diet with a negative dietary cation–anion difference (DCAD) produces a mild metabolic acidosis in prepartum cows which is demonstrated to be helpful to avert the risk of developing milk fever [134]. From −21 d prepartum, reducing DCAD (starting from −7.4 mmol/100 g to −16 mmol/100 g of dry matter) is shown to avert the risk of hypocalcemia and associated reproductive problems [147,148,149,150]. An alternative to negative DCAD is the feeding of low-calcium diets (<20 g per day) prepartum, which can improve calcium homeostasis [127,151]. Low circulating magnesium concentrations are associated with low blood calcium levels in dairy cows [152,153]. Phosphorous status also influence a cow’s ability to regulate calcium concentrations in the blood around parturition, where reduced prepartum dietary phosphorus intake can increase perinatal circulating calcium concentrations [154]. High-calcium forage such as alfalfa should be exchanged for low-calcium forages such as corn silage to improve calcium levels postpartum [155]. Dietary zeolite (sodium aluminum silicate) during the last 2 weeks prepartum improved the circulatory calcium levels [134,156]. Serotonin (5-hydroxytryptamine, 5-HT) has been shown to improve calcium homeostasis in postpartum dairy cows [157]. The combined use of negative DCAD (−55 v. +14 mmol/kg DM) with the supplementation of 1 mg/kg body weight of 5-hydroxy-l-tryptophan (5-HTP) prior to parturition resulted in additional increases in calcium concentrations compared to negative DCAD or 5-HTP alone [158]. These major mitigation approaches discussed here can be effectively employed to decrease the risk of hypocalcemia in dairy cows.
6. Ruminal Acidosis and Reproductive Efficiency of Dairy Cattle
The rumen contains a dense and diverse microbiota involved in digestion, and dietary organic compounds are hydrolyzed and fermented into volatile fatty acids (VFAs) and gases. These VFAs are absorbed into circulation and support around 70% of the energy supply. The concentrate ratio of the feed is increased to overcome postpartum lactation demands and NEBAL; the irrational increase in this concentrated feed can easily lead to rumen acidosis in postpartum cows. Ruminal acidosis is a nutritional metabolic disease in the high-yielding dairy cow that affects normal fermentation due to the decline in rumen pH, caused by feeding energy-rich diets. The post-calving period is high metabolic activity time, and cows’ ability to adapt is over-stressed [39]. In general, ruminal acidosis can be divided into subacute acidosis (pH is 5.2 to 5.6) and acute acidosis (pH below 5). Subacute acidosis is characterized by repeated bouts of pH decline, while acute ruminal acidosis is mainly characterized by lactic acid accumulation with a persistent pH drop and clinical manifestations [159,160]. The incidence rate of subacute acidosis ranges from 11% to 26% [161], and it can severely impact feeding behavior and DMI, milk quality and yield and cause indigestion and reproductive incapacity due to nutritional deficiency [162,163,164]. Ruminal acidosis affects the ruminal microbiome and causes endotoxemia, having negative consequences for the whole body system. Circulating lipopolysaccharides (LPSs) can reach the ovarian follicular fluid and can affect the ovaries’ neuroendocrine axis, both of which have negative consequences for the RP of dairy cows [11,165]. Circulatory LPSs are shown to suppress the GnRH and LH activity, and can also reduce the synthesis of PGF2 alpha [166,167,168]. Fluctuations in feeding behavior characterized by low feeding time and high intake per feeding are the contributing factors in the occurrence of ruminal acidosis [169]. Since maintaining proper nutritional support and gut health is very important in the speedy recovery of postpartum reproductive health and ovarian cyclicity, ruminal acidosis should be a greater concern for the postpartum cow. Earlier we discussed that feeding UFAs is helpful for postpartum energy balance and reproduction, and their feeding can have negative impact on rumen microbiota [170]. This negative impact can be minimized in diets with the inclusion of high forage content, which maximizes ruminal biohydrogenation [171]. On the other hand, essential consumption of a concentrate-rich diet acts conversely by decreasing biohydrogenation [171,172]. Therefore, prepartum feeding adjustments to incorporate all the ingredients contained in the postpartum feed is advised to avoid ruminal acidosis and successful energy-rich feed adaptation of postpartum cows [173]. Probiotics of yeast origin (Saccharomyces cerevisiae and Aspergillus orizae) are shown to modulate rumen function, avert risk of acidosis, and improve fertility in postpartum cows [173,174]. In conclusion, a careful increase in concentrates with due vigilance for the risk of acidosis and feeding dietary bicarbonates seem to be important in overcoming the problem of ruminal acidosis [175,176].
7. Effect of High-Protein Diet on Reproductive Performance
Modern dairy cows produce high milk quantities due to continuous genetic improvement. In order to fulfill the high energy and protein demand of lactation, the protein levels in the cow diet are also increasing. A high proportion of dietary protein, though helpful for lactation yield [177,178], is negatively associated with RP [179]. The proteins in the diet are divided into rumen degradable and non-degradable proteins. Higher rumen degradable protein feeding can disturb the nitrogen cycle, leading to high ammonia and subsequent increases in the blood urea content. Indeed, a high level of rumen non-degradable protein cannot be completely digested in the jejunum and thus should be degraded by microbial flora in the large intestine and transformed into ammonia. The phenomenon could be further promoted by a low level of non-structural carbohydrates in the diet [180]. A meta-analysis found 43% lower odds of reproduction success in cows where plasma urea nitrogen was 19.3 mg/dL or where urea was ≥420 mg/L in the milk compared with lower urea values, where high concentrations of urea nitrogen were negatively associated with reproductive capacity and affect the pituitary and ovarian function of cows and uterine physiology [181]. Studies report a negative correlation of milk urea content of postpartum cows with first postpartum service and conception rates. Another study showed that an increase in milk urea content from 12.5 mg/dL to 13.5 mg/dL caused a 5% decrease in the fertility rate of cows [182]. A high-protein diet and its metabolism generate oxidative stress in the body, affecting reproductive performance [183,184]. Oxidative stress, immune response, heat stress, and changes in gluconeogenesis deplete amino acids, decreasing the availability of essential amino acids [185,186,187]. Extensive protein metabolism may possess negative implications for reproductive performance and lead to depletion of amino acids for oxidative purposes but certain amino acids are essential for reproductive health and pregnancy [188,189]. Therefore, a balanced protein diet and supplementation of certain amino acids such as lysine, arginine, phenylalanine, and tyrosine may be useful for enhancing metabolic status and fertility outcomes of postpartum cows [185,190,191]. Hence, in this context, feeding controlled crude protein and supplementing cotton seed in feed can have better impacts on fertility [192]. Controlled rumen degradable and non-degradable proteins help to provide essential amino acid support and prevent their catabolism [193].
Before concluding this review, a summary chart comprising the nutritional mitigation and postpartum reproductive performance enhancement approaches is presented in Figure 2. This chart is based upon the recommendations discussed in this study and our previous studies [9,187]. Prepartum feeding of a controlled energy diet can help to avoid postpartum NEBAL and improve fertility [68,70], where the inclusion of short-chopped wheat straw or low-quality grass hays can be helpful in this regard [71,194]. The supplementation of the most limited methionine and lysine through rumen-protected means is shown to improve postpartum protein metabolism, improve ovarian follicular biochemical profile [195], conception rate [196,197], and embryonic development [198]. Dietary fat and trace mineral supplementations are also helpful in this regard [199,200]. Monensin supplementation significantly reduces the incidence of subclinical ketosis [201]. Injections of growth hormones such as recombinant bovine somatotropins (rbSTs) are also helpful in improving the metabolic profile and immunity of postpartum dairy cows [202,203]. Besides these nutritional support strategies, the practice of timed AI protocols is demonstrated to be helpful in improving the reproductive outcome of postpartum cows [204,205]. Adoption of synchronized AI protocols effectively makes up for the endocrine alterations and improves follicular ovarian dynamics and thus is effective for breeding success. Employment of embryo transfer technology can effectively bypass the initial reproductive processes and is helpful for enhanced reproductive outcomes [206]. Furthermore, injecting post-AI GnRH and progesterone is also helpful to support the corpus lutea and increase conception rates [207,208].
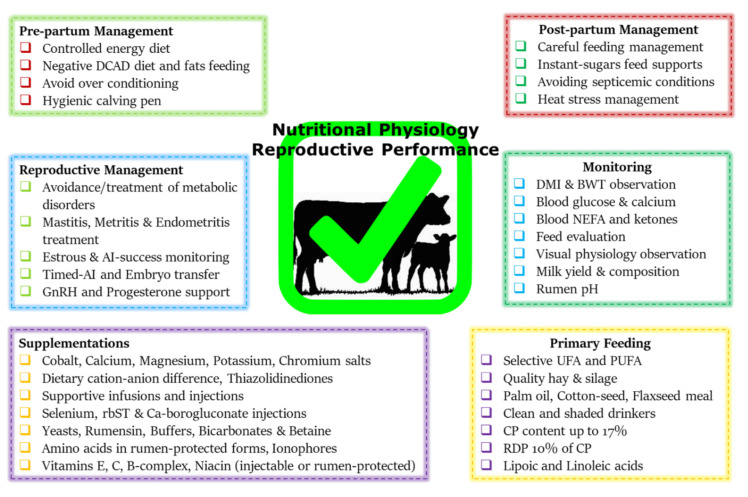
Figure 2.
This summary chart encompasses nutritional mitigation and postpartum reproductive management support strategies. Postpartum management should be based upon the advice of dairy extension workers and/or veterinarians, with special care for appropriate feeding practices. (Abbreviations: DCAD, dietary cation–anion difference; DMI, dry matter intake; BWT, body weight; UFA, unsaturated fatty acid; PUFA, poly-unsaturated fatty acid; CP, crude protein; RDP, rumen-degradable protein; inj., injection; rbST, generic somatotropin; AI, artificial insemination; GnRH, gonadotrophin.) This figure is based upon the mitigation charts of our previous studies [9,187] and the recommendations given in this study.
8. Conclusions
Postpartum metabolic disorders debilitate dairy cows and predispose them to a decline in postpartum reproductive efficiency. Perinatal NEBAL appears to be the major culprit behind the occurrence of post-parturient metabolic diseases and related conditions. Prepartum BCS and postpartum lipid mobilization are the two factors closely associated with NEBAL. A controlled energy diet starting from −21 d is helpful in successful perinatal transition of dairy cows. While doing so, attention must be paid to avoid over-conditioning during the prepartum period. There is enough literature about the management of perinatal cows to avoid the occurrence of NEBAL and excessive NEFA mobilization. However, controversy still exists about the supplementations of fats, their type, and quantity fed. Therefore, further research involving complex farm trials about fat supplementation would help move in the right direction. NEFA and ketone interactions with the ovarian–hypothalamus–pituitary axis, oocytes, and developing embryos at the system biology level can bring up exciting knowledge and insights. A score of nutritional mitigation strategies are available and future discoveries will help to maximize the welfare and reproductive efficiency of postpartum dairy cows.
Acknowledgments
We highly appreciate Geoffrey Kirton, advisor to “Cowinfo” forum at China Agricultural University, for his technical help and suggestions during the write-up of this review paper in addition to his help in English language proofreading.
Author Contributions
A.S., M.Z.K., Z.A., L.H., Q.U. and Y.W. (Yajing Wang) contributed significantly to this manuscript through literature collection, writing, revision. Y.W. (Yachun Wang) and H.Z. carried out final edits and validation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This effort was possible due to the authors’ financial assistance from the following projects: China Agriculture Research System of MOF and MARA and the Program for Changjiang Scholars and Innovation Research Teams in Universities (IRT–15R62).
Conflicts of Interest
All the authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dobson H., Smith R., Royal M., Knight C., Sheldon I. The high-producing dairy cow and its reproductive performance. Reprod. Domest. Anim. 2007;42((Suppl. S2)):17–23. doi: 10.1111/j.1439-0531.2007.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucy M.C. Reproductive Loss in High-Producing Dairy Cattle: Where Will It End? J. Dairy Sci. 2001;84:1277–1293. doi: 10.3168/jds.S0022-0302(01)70158-0. [DOI] [PubMed] [Google Scholar]
- 3.Pryce J.E., Royal M.D., Garnsworthy P.C., Mao I.L. Fertility in the high-producing dairy cow. Livest. Prod. Sci. 2004;86:125–135. doi: 10.1016/S0301-6226(03)00145-3. [DOI] [Google Scholar]
- 4.Lucy M.C. Symposium review: Selection for fertility in the modern dairy cow—Current status and future direction for genetic selection. Dairy Sci. 2019;102:3706–3721. doi: 10.3168/jds.2018-15544. [DOI] [PubMed] [Google Scholar]
- 5.Thatcher W.W., Bilby T.R., Bartolome J.A., Silvestre F., Staples C.R., Santos J.E.P. Strategies for improving fertility in the modern dairy cow. Theriogenology. 2006;65:30–44. doi: 10.1016/j.theriogenology.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson J.S., Call E.P. Reproductive Disorders in the Periparturient Dairy Cow. J. Dairy Sci. 1988;71:2572–2583. doi: 10.3168/jds.S0022-0302(88)79846-X. [DOI] [PubMed] [Google Scholar]
- 7.Fourichon C., Seegers H., Malher X. Effect of disease on reproduction in the dairy cow: A meta-analysis. Theriogenology. 2000;53:1729–1759. doi: 10.1016/S0093-691X(00)00311-3. [DOI] [PubMed] [Google Scholar]
- 8.Mohtashamipour F., Dirandeh E., Ansari-pirsaraei Z., Colazo M.G. Postpartum health disorders in lactating dairy cows and its associations with reproductive responses and pregnancy status after first timed-AI. Theriogenology. 2020;141:98–104. doi: 10.1016/j.theriogenology.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Sammad A., Umer S., Shi R., Zhu H., Zhao X., Wang Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. 2020;104:978–986. doi: 10.1111/jpn.13257. [DOI] [PubMed] [Google Scholar]
- 10.Häggman J., Christensen J.M., Mäntysaari E.A., Juga J. Genetic parameters for endocrine and traditional fertility traits, hyperketonemia and milk yield in dairy cattle. Animal. 2019;13:248–255. doi: 10.1017/S1751731118001386. [DOI] [PubMed] [Google Scholar]
- 11.Toledo-Alvarado H., Cecchinato A., Bittante G. Fertility traits of Holstein, Brown Swiss, Simmental, and Alpine Grey cows are differently affected by herd productivity and milk yield of individual cows. J. Dairy Sci. 2017;100:8220–8231. doi: 10.3168/jds.2016-12442. [DOI] [PubMed] [Google Scholar]
- 12.Song Y., Wang Z., Zhao C., Bai Y., Xia C., Xu C. Effect of negative energy balance on plasma metabolites, minerals, hormones, cytokines and ovarian follicular growth rate in Holstein dairy cows. J. Vet. Res. 2021;65:361–368. doi: 10.2478/jvetres-2021-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McArt J.A.A., Nydam D.V., Oetzel G.R. Epidemiology of subclinical ketosis in early lactation dairy cattle. J. Dairy Sci. 2012;95:5056–5066. doi: 10.3168/jds.2012-5443. [DOI] [PubMed] [Google Scholar]
- 14.Yoo H.S. Infectious causes of reproductive disorders in cattle. J. Reprod. Dev. 2010;56:S53–S60. doi: 10.1262/jrd.1056S53. [DOI] [PubMed] [Google Scholar]
- 15.Miqueo E., Chiarle A., Giuliodori M.J., Relling A.E. Association between prepartum metabolic status and resumption of postpartum ovulation in dairy cows. Domest. Anim. Endocrinol. 2019;69:62–67. doi: 10.1016/j.domaniend.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Roth Z., Meiden R., Braw-Tal R., Wolfenson D. Immediate and delayed effects of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows. J. Reprod. Fertil. 2000;120:83–90. doi: 10.1530/jrf.0.1200083. [DOI] [PubMed] [Google Scholar]
- 17.LeBlanc S.J., Lissemore K.D., Kelton D.F., Duffield T.F., Leslie K.E. Major advances in disease prevention in dairy cattle. J. Dairy Sci. 2006;89:1267–1279. doi: 10.3168/jds.S0022-0302(06)72195-6. [DOI] [PubMed] [Google Scholar]
- 18.LeBlanc S.J., Duffield T.F., Leslie K.E., Bateman K.G., Keefe G.P., Walton J.S., Johnson W.H. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. 2002;85:2223–2236. doi: 10.3168/jds.S0022-0302(02)74302-6. [DOI] [PubMed] [Google Scholar]
- 19.Vergara C.F., Döpfer D., Cook N.B., Nordlund K.V., McArt J.A.A., Nydam D.V., Oetzel G.R. Risk factors for postpartum problems in dairy cows: Explanatory and predictive modeling. J. Dairy Sci. 2014;97:4127–4140. doi: 10.3168/jds.2012-6440. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Báez J., Risco C.A., Chebel R.C., Gomes G.C., Greco L.F., Tao S., Thompson I.M., do Amaral B.C., Zenobi M.G., Martinez N., et al. Association of dry matter intake and energy balance prepartum and postpartum with health disorders postpartum: Part II. Ketosis and clinical mastitis. J. Dairy Sci. 2019;102:9151–9164. doi: 10.3168/jds.2018-15879. [DOI] [PubMed] [Google Scholar]
- 21.Hayirli A., Grummer R.R., Nordheim E.V., Crump P.M. Animal and dietary factors affecting feed intake during the prefresh transition period in Holsteins. J. Dairy Sci. 2002;85:3430–3443. doi: 10.3168/jds.S0022-0302(02)74431-7. [DOI] [PubMed] [Google Scholar]
- 22.Grummer R.R., Mashek D.G., Hayirli A. Dry matter intake and energy balance in the transition period. Vet. Clin. N. Am.-Food Anim. Pract. 2004;20:447–470. doi: 10.1016/j.cvfa.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Báez J., Risco C.A., Chebel R.C., Gomes G.C., Greco L.F., Tao S., Toledo I.M., do Amaral B.C., Zenobi M.G., Martinez N., et al. Investigating the Use of Dry Matter Intake and Energy Balance Prepartum as Predictors of Digestive Disorders Postpartum. Front. Vet. Sci. 2021;8:1016. doi: 10.3389/fvets.2021.645252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Báez J., Risco C.A., Chebel R.C., Gomes G.C., Greco L.F., Tao S., Thompson I.M., do Amaral B.C., Zenobi M.G., Martinez N., et al. Association of dry matter intake and energy balance prepartum and postpartum with health disorders postpartum: Part I. Calving disorders and metritis. J. Dairy Sci. 2019;102:9138–9150. doi: 10.3168/jds.2018-15878. [DOI] [PubMed] [Google Scholar]
- 25.Hoedemaker M., Prange D., Gundelach Y. Body condition change ante- and postpartum, health and reproductive performance in German Holstein Cows. Reprod. Domest. Anim. 2009;44:167–173. doi: 10.1111/j.1439-0531.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 26.Chebel R.C., Mendonça L.G.D., Baruselli P.S. Association between body condition score change during the dry period and postpartum health and performance. J. Dairy Sci. 2018;101:4595–4614. doi: 10.3168/jds.2017-13732. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F., Li D., Wu Q., Sun J., Guan W., Hou Y., Zhu Y., Wang J. Prepartum body conditions affect insulin signaling pathways in postpartum adipose tissues in transition dairy cows. J. Anim. Sci. Biotechnol. 2019;10:38. doi: 10.1186/s40104-019-0347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roche J.R., Meier S., Heiser A., Mitchell M.D., Walker C.G., Crookenden M.A., Riboni M.V., Loor J.J., Kay J.K. Effects of precalving body condition score and prepartum feeding level on production, reproduction, and health parameters in pasture-based transition dairy cows. J. Dairy Sci. 2015;98:7164–7182. doi: 10.3168/jds.2014-9269. [DOI] [PubMed] [Google Scholar]
- 29.Pires J.A.A., Delavaud C., Faulconnier Y., Pomiès D., Chilliard Y. Effects of body condition score at calving on indicators of fat and protein mobilization of periparturient Holstein-Friesian cows. J. Dairy Sci. 2013;96:6423–6439. doi: 10.3168/jds.2013-6801. [DOI] [PubMed] [Google Scholar]
- 30.Çolakoğlu H.E., Yazlık M.O., Pekcan M., Kaya U., Kaçar C., Vural M.R., Kurt S., Yildirim M.M., Bas A., Küplülü Ş. Impact of prepartum body condition score loss on metabolic status during the transition period and subsequent fertility in Brown Swiss dairy cows. J. Vet. Res. 2019;63:375. doi: 10.2478/jvetres-2019-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alharthi A.S., Coleman D.N., Alhidary I.A., Abdelrahman M.M., Trevisi E., Loor J.J. Maternal body condition during late-pregnancy is associated with in utero development and neonatal growth of Holstein calves. J. Anim. Sci. Biotechnol. 2021;12:44. doi: 10.1186/s40104-021-00566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy N.M., Potteti H.R., Vegiraju S., Chen H.J., Tamatam C.M., Reddy S.P. PI3K-AKT Signaling via Nrf2 Protects against Hyperoxia-Induced Acute Lung Injury, but Promotes Inflammation Post-Injury Independent of Nrf2 in Mice. PLoS ONE. 2015;10:e0129676. doi: 10.1371/journal.pone.0129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bionaz M., Chen S., Khan M.J., Loor J.J. Functional role of PPARs in ruminants: Potential targets for fine-tuning metabolism during growth and lactation. PPAR Res. 2013;2013:684159. doi: 10.1155/2013/684159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinsyu A., Bamba S., Kurihara M., Matsumoto H., Sonoda A., Inatomi O., Andoh A., Takebayashi K., Kojima M., Iida H., et al. Inflammatory cytokines, appetite-regulating hormones, and energy metabolism in patients with gastrointestinal cancer. Oncol. Lett. 2020;20:1469–1479. doi: 10.3892/ol.2020.11662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celeska I., Janevski A., Dzadzovski I., Ulchar I., Kirovski D. The dynamics of biochemical parameters in blood of clinically healthy Holstein cows from day 5 before to day 60 after calving. Maced. Vet. Rev. 2015;38:189–193. doi: 10.14432/j.macvetrev.2015.07.049. [DOI] [Google Scholar]
- 36.Gross J.J., Bruckmaier R.M. Review: Metabolic challenges in lactating dairy cows and their assessment via established and novel indicators in milk. Animal. 2019;13:s75–s81. doi: 10.1017/S175173111800349X. [DOI] [PubMed] [Google Scholar]
- 37.Bach À. Effects of nutrition and genetics on fertility in dairy cows. Reprod. Fertil. Dev. 2019;31:40–54. doi: 10.1071/RD18364. [DOI] [PubMed] [Google Scholar]
- 38.Bauman D.E., Bruce Currie W. Partitioning of Nutrients During Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J. Dairy Sci. 1980;63:1514–1529. doi: 10.3168/jds.S0022-0302(80)83111-0. [DOI] [PubMed] [Google Scholar]
- 39.Sundrum A. Metabolic Disorders in the Transition Period Indicate that the Dairy Cows’ Ability to Adapt is Overstressed. Animals. 2015;5:978–1020. doi: 10.3390/ani5040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdelli A., Raboisson D., Kaidi R., Ibrahim B., Kalem A., Iguer-Ouada M. Elevated non-esterified fatty acid and β-hydroxybutyrate in transition dairy cows and their association with reproductive performance and disorders: A meta-analysis. Theriogenology. 2017;93:99–104. doi: 10.1016/j.theriogenology.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Cardoso F.C., Kalscheur K.F., Drackley J.K. Symposium review: Nutrition strategies for improved health, production, and fertility during the transition period. J. Dairy Sci. 2020;103:5684–5693. doi: 10.3168/jds.2019-17271. [DOI] [PubMed] [Google Scholar]
- 42.Galligan D. Economic assessment of animal health performance. Vet. Clin. N. Am.-Food Anim. Pract. 2006;22:207–227. doi: 10.1016/j.cvfa.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Ceciliani F., Lecchi C., Urh C., Sauerwein H. Proteomics and metabolomics characterizing the pathophysiology of adaptive reactions to the metabolic challenges during the transition from late pregnancy to early lactation in dairy cows. J. Proteomics. 2018;178:92–106. doi: 10.1016/j.jprot.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Ribeiro E.S., Gomes G., Greco L.F., Cerri R.L.A., Vieira-Neto A., Monteiro P.L.J., Lima F.S., Bisinotto R.S., Thatcher W.W., Santos J.E.P. Carryover effect of postpartum inflammatory diseases on developmental biology and fertility in lactating dairy cows. J. Dairy Sci. 2016;99:2201–2220. doi: 10.3168/jds.2015-10337. [DOI] [PubMed] [Google Scholar]
- 45.Carvalho M.R., Peñagaricano F., Santos J.E.P., DeVries T.J., McBride B.W., Ribeiro E.S. Long-term effects of postpartum clinical disease on milk production, reproduction, and culling of dairy cows. J. Dairy Sci. 2019;102:11701–11717. doi: 10.3168/jds.2019-17025. [DOI] [PubMed] [Google Scholar]
- 46.Garzón-Audor A., Oliver-Espinosa O. Incidence and risk factors for ketosis in grazing dairy cattle in the Cundi-Boyacencian Andean plateau, Colombia. Trop. Anim. Health Prod. 2019;51:1481–1487. doi: 10.1007/s11250-019-01835-z. [DOI] [PubMed] [Google Scholar]
- 47.Brunner N., Groeger S., Canelas Raposo J., Bruckmaier R.M., Gross J.J. Prevalence of subclinical ketosis and production diseases in dairy cows in Central and South America, Africa, Asia, Australia, New Zealand, and Eastern Europe. Transl. Anim. Sci. 2019;3:84–92. doi: 10.1093/tas/txy102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sepúlveda-Varas P., Weary D.M., Noro M., Von Keyserlingk M.A.G. Transition Diseases in Grazing Dairy Cows Are Related to Serum Cholesterol and Other Analytes. PLoS ONE. 2015;10:e0122317. doi: 10.1371/journal.pone.0122317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucy M.C. Regulation of Ovarian Follicular Growth by Somatotropin and Insulin-Like Growth Factors in Cattle. J. Dairy Sci. 2000;83:1635–1647. doi: 10.3168/jds.S0022-0302(00)75032-6. [DOI] [PubMed] [Google Scholar]
- 50.Bauman D.E., Vernon R.G. Effects of Exogenous Bovine Somatotropin on Lactation. Annu. Rev. Nutr. 1993;13:437–461. doi: 10.1146/annurev.nu.13.070193.002253. [DOI] [PubMed] [Google Scholar]
- 51.Ghanem M.E., Tezuka E., Sasaki K., Takahashi M., Yamagishi N., Izaike Y., Osawa T. Correlation of blood metabolite concentrations and body condition scores with persistent postpartum uterine bacterial infection in dairy cows. J. Reprod. Dev. 2016;62:457–463. doi: 10.1262/jrd.2015-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beam S.W., Butler W.R. Effects of energy balance on follicular development and first ovulation in postpartum dairy cows. J. Reprod. Fertil. Suppl. 1999;54:411–424. doi: 10.1530/biosciprocs.4.032. [DOI] [PubMed] [Google Scholar]
- 53.Rhoads R.P., Kim J.W., Leury B.J., Baumgard L.H., Segoale N., Frank S.J., Bauman D.E., Boisclair Y.R. Insulin Increases the Abundance of the Growth Hormone Receptor in Liver and Adipose Tissue of Periparturient Dairy Cows. J. Nutr. 2004;134:1020–1027. doi: 10.1093/jn/134.5.1020. [DOI] [PubMed] [Google Scholar]
- 54.Jorritsma R., Wensing T., Kruip T.A.M., Vos P.L.A.M., Noordhuizen J.P.T.M. Metabolic changes in early lactation and impaired reproductive performance in dairy cows. Vet. Res. 2003;34:11–26. doi: 10.1051/vetres:2002054. [DOI] [PubMed] [Google Scholar]
- 55.Butler W.R. Nutritional effects on resumption of ovarian cyclicity and conception rate in postpartum dairy cows. BSAP Occas. Publ. 2001;26:133–145. doi: 10.1017/S0263967X00033644. [DOI] [Google Scholar]
- 56.Castro N., Kawashima C., van Dorland H.A., Morel I., Miyamoto A., Bruckmaier R.M. Metabolic and energy status during the dry period is crucial for the resumption of ovarian activity postpartum in dairy cows. J. Dairy Sci. 2012;95:5804–5812. doi: 10.3168/jds.2012-5666. [DOI] [PubMed] [Google Scholar]
- 57.Villa-Godoy A., Hughes T.L., Emery R.S., Chapin L.T., Fogwell R.L. Association Between Energy Balance and Luteal Function in Lactating Dairy Cows. J. Dairy Sci. 1988;71:1063–1072. doi: 10.3168/jds.S0022-0302(88)79653-8. [DOI] [PubMed] [Google Scholar]
- 58.Spicer L.J., Tucker W.B., Adams G.D. Insulin-Like Growth Factor-I in Dairy Cows: Relationships Among Energy Balance, Body Condition, Ovarian Activity, and Estrous Behavior. J. Dairy Sci. 1990;73:929–937. doi: 10.3168/jds.S0022-0302(90)78749-8. [DOI] [PubMed] [Google Scholar]
- 59.Leroy J.L.M.R., Vanholder T., Mateusen B., Christophe A., Opsomer G., de Kruif A., Genicot G., Van Soom A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction. 2005;130:485–495. doi: 10.1530/rep.1.00735. [DOI] [PubMed] [Google Scholar]
- 60.Leroy J.L.M.R., Vanholder T., Van Knegsel A.T.M., Garcia-Ispierto I., Bols P.E.J. Nutrient Prioritization in Dairy Cows Early Postpartum: Mismatch Between Metabolism and Fertility? Reprod. Domest. Anim. 2008;43:96–103. doi: 10.1111/j.1439-0531.2008.01148.x. [DOI] [PubMed] [Google Scholar]
- 61.Seifi H.A., Gorji-Dooz M., Mohri M., Dalir-Naghadeh B., Farzaneh N. Variations of energy-related biochemical metabolites during transition period in dairy cows. Comp. Clin. Path. 2007:253–258. doi: 10.1007/s00580-007-0682-2. [DOI] [Google Scholar]
- 62.Ospina P.A., Nydam D.V., Stokol T., Overton T.R. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010;93:1596–1603. doi: 10.3168/jds.2009-2852. [DOI] [PubMed] [Google Scholar]
- 63.Wathes D.C., Fenwick M., Cheng Z., Bourne N., Llewellyn S., Morris D.G., Kenny D., Murphy J., Fitzpatrick R. Influence of negative energy balance on cyclicity and fertility in the high producing dairy cow. Theriogenology. 2007;68:S232–S241. doi: 10.1016/j.theriogenology.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Velázquez M.M.L., Peralta M.B., Angeli E., Stassi A.F., Gareis N.C., Durante L., Cainelli S., Salvetti N.R., Rey F., Ortega H.H. Immune status during postpartum, peri-implantation and early pregnancy in cattle: An updated view. Anim. Reprod. Sci. 2019;206:1–10. doi: 10.1016/j.anireprosci.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y., Li X., Zhang H., Zhao Z., Peng Z., Wang Z., Liu G., Li X. Non-Esterified Fatty Acids Over-Activate the TLR2/4-NF-Κb Signaling Pathway to Increase Inflammatory Cytokine Synthesis in Neutrophils from Ketotic Cows. Cell. Physiol. Biochem. 2018;48:827–837. doi: 10.1159/000491913. [DOI] [PubMed] [Google Scholar]
- 66.Bradford B.J., Yuan K., Farney J.K., Mamedova L.K., Carpenter A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015;98:6631–6650. doi: 10.3168/jds.2015-9683. [DOI] [PubMed] [Google Scholar]
- 67.Wankhade P.R., Manimaran A., Kumaresan A., Jeyakumar S., Ramesha K.P., Sejian V., Rajendran D., Varghese M.R. Metabolic and immunological changes in transition dairy cows: A review. Vet. World. 2017;10:1367. doi: 10.14202/vetworld.2017.1367-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Douglas G.N., Overton T.R., Bateman H.G., Dann H.M., Drackley J.K. Prepartal Plane of Nutrition, Regardless of Dietary Energy Source, Affects Periparturient Metabolism and Dry Matter Intake in Holstein Cows. J. Dairy Sci. 2006;89:2141–2157. doi: 10.3168/jds.S0022-0302(06)72285-8. [DOI] [PubMed] [Google Scholar]
- 69.Sammad A., Luo H., Qiu W., Galindez J.M., Wang Y., Guo G., Huang X., Wang Y. Automated monitoring of seasonal and diurnal variation of rumination behaviour: Insights into thermotolerance management of Holstein cows. Biosyst. Eng. 2021 doi: 10.1016/j.biosystemseng.2021.12.002. [DOI] [Google Scholar]
- 70.Cardoso F.C., LeBlanc S.J., Murphy M.R., Drackley J.K. Prepartum nutritional strategy affects reproductive performance in dairy cows. J. Dairy Sci. 2013;96:5859–5871. doi: 10.3168/jds.2013-6759. [DOI] [PubMed] [Google Scholar]
- 71.Janovick N.A., Drackley J.K. Prepartum dietary management of energy intake affects postpartum intake and lactation performance by primiparous and multiparous Holstein cows1. J. Dairy Sci. 2010;93:3086–3102. doi: 10.3168/jds.2009-2656. [DOI] [PubMed] [Google Scholar]
- 72.Drackley J.K., Cicela T.M., LaCount D.W. Responses of primiparous and multiparous holstein cows to additional energy from fat or concentrate during summer. J. Dairy Sci. 2003;86:1306–1314. doi: 10.3168/jds.S0022-0302(03)73714-X. [DOI] [PubMed] [Google Scholar]
- 73.Ji P., Osorio J.S., Drackley J.K., Loor J.J. Overfeeding a moderate energy diet prepartum does not impair bovine subcutaneous adipose tissue insulin signal transduction and induces marked changes in peripartal gene network expression. J. Dairy Sci. 2012;95:4333–4351. doi: 10.3168/jds.2011-5079. [DOI] [PubMed] [Google Scholar]
- 74.Graugnard D.E., Bionaz M., Trevisi E., Moyes K.M., Salak-Johnson J.L., Wallace R.L., Drackley J.K., Bertoni G., Loor J.J. Blood immunometabolic indices and polymorphonuclear neutrophil function in peripartum dairy cows are altered by level of dietary energy prepartum. J. Dairy Sci. 2012;95:1749–1758. doi: 10.3168/jds.2011-4579. [DOI] [PubMed] [Google Scholar]
- 75.Grummer R.R. Etiology of Lipid-Related Metabolic Disorders in Periparturient Dairy Cows. J. Dairy Sci. 1993;76:3882–3896. doi: 10.3168/jds.S0022-0302(93)77729-2. [DOI] [PubMed] [Google Scholar]
- 76.Ingvartsen K.L. Feeding- and management-related diseases in the transition cow: Physiological adaptations around calving and strategies to reduce feeding-related diseases. Anim. Feed Sci. Technol. 2006;126:175–213. doi: 10.1016/j.anifeedsci.2005.08.003. [DOI] [Google Scholar]
- 77.Bobe G., Young J.W., Beitz D.C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004;87:3105–3124. doi: 10.3168/jds.S0022-0302(04)73446-3. [DOI] [PubMed] [Google Scholar]
- 78.Drackley J.K. ADSA foundation scholar award: Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999;82:2259–2273. doi: 10.3168/jds.S0022-0302(99)75474-3. [DOI] [PubMed] [Google Scholar]
- 79.Shen Y., Chen L., Yang W., Wang Z. Exploration of serum sensitive biomarkers of fatty liver in dairy cows. Sci. Rep. 2018;8:13574. doi: 10.1038/s41598-018-31845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jorritsma R., Jorritsma H., Schukken Y.H., Wentink G.H. Relationships between fatty liver and fertility and some periparturient diseases in commercial Dutch dairy herds. Theriogenology. 2000;54:1065–1074. doi: 10.1016/S0093-691X(00)00415-5. [DOI] [PubMed] [Google Scholar]
- 81.Trevisi E., Amadori M., Bakudila A.M., Bertoni G. Metabolic changes in dairy cows induced by oral, low-dose interferon-alpha treatment. J. Anim. Sci. 2009;87:3020–3029. doi: 10.2527/jas.2008-1178. [DOI] [PubMed] [Google Scholar]
- 82.Loor J.J., Everts R.E., Bionaz M., Dann H.M., Morin D.E., Oliveira R., Rodriguez-Zas S.L., Drackley J.K., Lewin H.A. Nutrition-induced ketosis alters metabolic and signaling gene networks in liver of periparturient dairy cows. Physiol. Genom. 2007;32:105–116. doi: 10.1152/physiolgenomics.00188.2007. [DOI] [PubMed] [Google Scholar]
- 83.Overton T.R., Waldron M.R. Nutritional management of transition dairy cows: Strategies to optimize metabolic health. J. Dairy Sci. 2004;87:E105–E119. doi: 10.3168/jds.S0022-0302(04)70066-1. [DOI] [Google Scholar]
- 84.Bionaz M., Vargas-Bello-Pérez E., Busato S. Advances in fatty acids nutrition in dairy cows: From gut to cells and effects on performance. J. Anim. Sci. Biotechnol. 2020;111:110. doi: 10.1186/s40104-020-00512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomas M.G., Bao B., Williams G.L. Dietary Fats Varying in Their Fatty Acid Composition Differentially Influence Follicular Growth in Cows Fed Isoenergetic Diets. J. Anim. Sci. 1997;75:2512–2519. doi: 10.2527/1997.7592512x. [DOI] [PubMed] [Google Scholar]
- 86.Staples C.R., Burke J.M., Thatcher W.W. Influence of Supplemental Fats on Reproductive Tissues and Performance of Lactating Cows. J. Dairy Sci. 1998;81:856–871. doi: 10.3168/jds.S0022-0302(98)75644-9. [DOI] [PubMed] [Google Scholar]
- 87.Petit H.V., Palin M.F., Doepel L. Hepatic Lipid Metabolism in Transition Dairy Cows Fed Flaxseed. J. Dairy Sci. 2007;90:4780–4792. doi: 10.3168/jds.2007-0066. [DOI] [PubMed] [Google Scholar]
- 88.Zapata R.C., Salehi R., Ambrose D.J., Chelikani P.K. Effects of prepartum fat supplementation on plasma concentrations of glucagon-like peptide-1, peptide YY, adropin, insulin, and leptin in periparturient dairy cows. J. Dairy Sci. 2015;98:6876–6885. doi: 10.3168/jds.2014-9283. [DOI] [PubMed] [Google Scholar]
- 89.Karcagi R.G., Gaál T., Wágner L., Husvéth F. Effect of various dietary fat supplementations on liver lipid and glycogen of high-yielding dairy cows in the peripartal period. Acta Vet. Hung. 2008;56:57–70. doi: 10.1556/avet.56.2008.1.6. [DOI] [PubMed] [Google Scholar]
- 90.Karimian M., Khorvash M., Forouzmand M.A., Alikhani M., Rahmani H.R., Ghaffari M.H., Petit H.V. Effect of prepartal and postpartal dietary fat level on performance and plasma concentration of metabolites in transition dairy cows. J. Dairy Sci. 2015;98:330–337. doi: 10.3168/jds.2013-7577. [DOI] [PubMed] [Google Scholar]
- 91.Santos J.E.P., Bilby T.R., Thatcher W.W., Staples C.R., Silvestre F.T. Long Chain Fatty Acids of Diet as Factors Influencing Reproduction in Cattle. Reprod. Domest. Anim. 2008;43:23–30. doi: 10.1111/j.1439-0531.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 92.Milojevic V., Sinz S., Kreuzer M., Chiumia D., Marquardt S., Giller K. Partitioning of fatty acids into tissues and fluids from reproductive organs of ewes as affected by dietary phenolic extracts. Theriogenology. 2020;144:174–184. doi: 10.1016/j.theriogenology.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 93.Silvestre F.T., Carvalho T.S.M., Francisco N., Santos J.E.P., Staples C.R., Jenkins T.C., Thatcher W. Effects of differential supplementation of fatty acids during the peripartum and breeding periods of Holstein cows: I. Uterine and metabolic responses, reproduction, and lactation. J. Dairy Sci. 2011;94:189–204. doi: 10.3168/jds.2010-3370. [DOI] [PubMed] [Google Scholar]
- 94.Zachut M., Arieli A., Moallem U. Incorporation of dietary n-3 fatty acids into ovarian compartments in dairy cows and the effects on hormonal and behavioral patterns around estrus. Reproduction. 2011;141:833–840. doi: 10.1530/REP-10-0518. [DOI] [PubMed] [Google Scholar]
- 95.Ribeiro E.S. Symposium review: Lipids as regulators of conceptus development: Implications for metabolic regulation of reproduction in dairy cattle1. J. Dairy Sci. 2018;101:3630–3641. doi: 10.3168/jds.2017-13469. [DOI] [PubMed] [Google Scholar]
- 96.Busato S., Bionaz M. The interplay between non-esterified fatty acids and bovine peroxisome proliferator-activated receptors: Results of an in vitro hybrid approach. J. Anim. Sci. Biotechnol. 2020;11:91. doi: 10.1186/s40104-020-00481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Esposito G., Irons P.C., Webb E.C., Chapwanya A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014;144:60–71. doi: 10.1016/j.anireprosci.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 98.Nazeer M., Kumar S., Jaiswal M., Mishra A., Upmanyu G., Kumar P., Kumar S.A. Prevalence and Clinical Manifestations of Ketosis in Cows in and Around Bikaner. Int. J. Curr. Microbiol. Appl. Sci. 2019;8:1554–1560. doi: 10.20546/ijcmas.2019.803.179. [DOI] [Google Scholar]
- 99.Galster A.D., Clutter W.E., Cryer P.E., Collins J.A., Bier D.M. Epinephrine plasma thresholds for lipolytic effects in man: Measurements of fatty acid transport with [l-13C]palmitic acid. J. Clin. Investig. 1981;67:1729–1738. doi: 10.1172/JCI110211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Melendez P., Marin M.P., Robles J., Rios C., Duchens M., Archbald L. Relationship between serum nonesterified fatty acids at calving and the incidence of periparturient diseases in Holstein dairy cows. Theriogenology. 2009;72:826–833. doi: 10.1016/j.theriogenology.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Duffield T. Subclinical ketosis in lactating dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 2000;16:231–253. doi: 10.1016/S0749-0720(15)30103-1. [DOI] [PubMed] [Google Scholar]
- 102.Oetzel G.R. Monitoring and testing dairy herds for metabolic disease. Vet. Clin. N. Am.-Food Anim. Pract. 2004;20:651–674. doi: 10.1016/j.cvfa.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 103.Staufenbiel R., Arndt G., Schröder U., Gelfert C.C. Body condition and metabolic stability as the basis for high milk yield and undisturbed fertility in dairy cows—A contribution for deduction of reference values. Dtsch. Tierarztl. Wochenschr. 2004;111:214–220. [PubMed] [Google Scholar]
- 104.Mostert P.F., Bokkers E.A.M., Van Middelaar C.E., Hogeveen H., De Boer I.J.M. Estimating the economic impact of subclinical ketosis in dairy cattle using a dynamic stochastic simulation model. Animal. 2018;12:145–154. doi: 10.1017/S1751731117001306. [DOI] [PubMed] [Google Scholar]
- 105.Diskin M.G., Mackey D.R., Roche J.F., Sreenan J.M. Effects of nutrition and metabolic status on circulating hormones and ovarian follicle development in cattle. Anim. Reprod. Sci. 2003;78:345–370. doi: 10.1016/S0378-4320(03)00099-X. [DOI] [PubMed] [Google Scholar]
- 106.Pushpakumara P.G.A., Gardner N.H., Reynolds C.K., Beever D.E., Wathes D.C. Relationships between transition period diet, metabolic parameters and fertility in lactating dairy cows. Theriogenology. 2003;60:1165–1185. doi: 10.1016/S0093-691X(03)00119-5. [DOI] [PubMed] [Google Scholar]
- 107.Chapinal N., LeBlanc S.J., Carson M.E., Leslie K.E., Godden S., Capel M., Santos J.E.P., Overton M.W., Duffield T.F. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J. Dairy Sci. 2012;95:5676–5682. doi: 10.3168/jds.2011-5132. [DOI] [PubMed] [Google Scholar]
- 108.Shin E.K., Jeong J.K., Choi I.S., Kang H.G., Hur T.Y., Jung Y.H., Kim I.H. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows. Theriogenology. 2015;84:252–260. doi: 10.1016/j.theriogenology.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 109.Lüttgenau J., Purschke S., Tsousis G., Bruckmaier R.M., Bollwein H. Body condition loss and increased serum levels of nonesterified fatty acids enhance progesterone levels at estrus and reduce estrous activity and insemination rates in postpartum dairy cows. Theriogenology. 2016;85:656–663. doi: 10.1016/j.theriogenology.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 110.Rutherford A.J., Oikonomou G., Smith R.F. The effect of subclinical ketosis on activity at estrus and reproductive performance in dairy cattle. J. Dairy Sci. 2016;99:4808–4815. doi: 10.3168/jds.2015-10154. [DOI] [PubMed] [Google Scholar]
- 111.Aardema H., van Tol H.T.A., Vos P.L.A.M. An overview on how cumulus cells interact with the oocyte in a condition with elevated NEFA levels in dairy cows. Anim. Reprod. Sci. 2019;207:131–137. doi: 10.1016/j.anireprosci.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 112.Walsh S.W., Williams E.J., Evans A.C.O. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011;123:127–138. doi: 10.1016/j.anireprosci.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Piechotta M., Mysegades W., Ligges U., Lilienthal J., Hoeflich A., Miyamoto A., Bollwein H. Antepartal insulin-like growth factor 1 and insulin-like growth factor binding protein 2 concentrations are indicative of ketosis in dairy cows. J. Dairy Sci. 2015;98:3100–3109. doi: 10.3168/jds.2014-8885. [DOI] [PubMed] [Google Scholar]
- 114.Sarentonglaga B., Ogata K., Taguchi Y., Kato Y., Nagao Y. The developmental potential of oocytes is impaired in cattle with liver abnormalities. J. Reprod. Dev. 2013;59:168–173. doi: 10.1262/jrd.2012-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Valckx S.D.M., Arias-Alvarez M., De Pauw I., Fievez V., Vlaeminck B., Fransen E., Bols P.E.J., Leroy J.L.M.R. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: A descriptive cross-sectional study. Reprod. Biol. Endocrinol. 2014;12:13. doi: 10.1186/1477-7827-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aardema H., Gadella B.M., van de Lest C.H.A., Brouwers J.F.H.M., Stout T.A.E., Roelen B.A.J., Vos P.L.A.M. Free fatty acid levels in fluid of dominant follicles at the preferred insemination time in dairy cows are not affected by early postpartum fatty acid stress. J. Dairy Sci. 2015;98:2322–2336. doi: 10.3168/jds.2014-7970. [DOI] [PubMed] [Google Scholar]
- 117.Cheng Y., Liu S., Lin R., Wang J., Peng T., Zhang Q., Cheng H. Plasma and amniotic fluid PPARγ is involved in the lipid metabolism of maternal–fetal interface cells. J. Matern. Neonatal Med. 2018;31:2656–2664. doi: 10.1080/14767058.2017.1350641. [DOI] [PubMed] [Google Scholar]
- 118.Furukawa E., Chen Z., Ueshiba H., Wu Y., Chiba H., Yanagawa Y., Katagiri S., Nagano M., Hui S.P. Postpartum cows showed high oocyte triacylglycerols concurrently with high plasma free fatty acids. Theriogenology. 2021;176:174–182. doi: 10.1016/j.theriogenology.2021.09.034. [DOI] [PubMed] [Google Scholar]
- 119.Campanile G., Baruselli P.S., Limone A., D’Occhio M.J. Local action of cytokines and immune cells in communication between the conceptus and uterus during the critical period of early embryo development, attachment and implantation – Implications for embryo survival in cattle: A review. Theriogenology. 2021;167:1–12. doi: 10.1016/j.theriogenology.2021.02.020. [DOI] [PubMed] [Google Scholar]
- 120.van Mourik M.S.M., Macklon N.S., Heijnen C.J. Embryonic implantation: Cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J. Leukoc. Biol. 2009;85:4–19. doi: 10.1189/jlb.0708395. [DOI] [PubMed] [Google Scholar]
- 121.Lacasse P., Vanacker N., Ollier S., Ster C. Innovative dairy cow management to improve resistance to metabolic and infectious diseases during the transition period. Res. Vet. Sci. 2018;116:40–46. doi: 10.1016/j.rvsc.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 122.Aleri J.W., Hine B.C., Pyman M.F., Mansell P.D., Wales W.J., Mallard B., Fisher A.D. Periparturient immunosuppression and strategies to improve dairy cow health during the periparturient period. Res. Vet. Sci. 2016;108:8–17. doi: 10.1016/j.rvsc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 123.Drackley J.K., Cardoso F.C. Prepartum and postpartum nutritional management to optimize fertility in high-yielding dairy cows in confined TMR systems. Animal. 2014;8:5–14. doi: 10.1017/S1751731114000731. [DOI] [PubMed] [Google Scholar]
- 124.Garnsworthy P.C., Sinclair K.D., Webb R. Integration of physiological mechanisms that influence fertility in dairy cows. Animal. 2008;2:1144–1152. doi: 10.1017/S1751731108002358. [DOI] [PubMed] [Google Scholar]
- 125.Goff J.P. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 2008;176:50–57. doi: 10.1016/j.tvjl.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 126.Zhang F., Nan X., Wang H., Guo Y., Xiong B. Research on the applications of calcium propionate in dairy cows: A review. Animals. 2020;10:1336. doi: 10.3390/ani10081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hernández-Castellano L.E., Hernandez L.L., Bruckmaier R.M. Review: Endocrine pathways to regulate calcium homeostasis around parturition and the prevention of hypocalcemia in periparturient dairy cows. Animal. 2020;14:330–338. doi: 10.1017/S1751731119001605. [DOI] [PubMed] [Google Scholar]
- 128.Özçelik R., Bruckmaier R.M., Hernández-Castellano L.E. Prepartum daylight exposure increases serum calcium concentrations in dairy cows at the onset of lactation. J. Anim. Sci. 2017;95:4440–4447. doi: 10.2527/jas2017.1834. [DOI] [PubMed] [Google Scholar]
- 129.Wysolmerski J.J. Osteocytic osteolysis: Time for a second look? Bonekey Rep. 2012;1:229. doi: 10.1038/bonekey.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Megahed A.A., Hiew M.W.H., El Badawy S.A., Constable P.D. Plasma calcium concentrations are decreased at least 9 hours before parturition in multiparous Holstein-Friesian cattle in a herd fed an acidogenic diet during late gestation. J. Dairy Sci. 2018;101:1365–1378. doi: 10.3168/jds.2017-13376. [DOI] [PubMed] [Google Scholar]
- 131.Umaña Sedó S., Rosa D., Mattioli G., Luzbel de la Sota R., Giuliodori M.J. Associations of subclinical hypocalcemia with fertility in a herd of grazing dairy cows. J. Dairy Sci. 2018;101:10469–10477. doi: 10.3168/jds.2017-14242. [DOI] [PubMed] [Google Scholar]
- 132.Saborío-Montero A., Vargas-Leitón B., Romero-Zúñiga J.J., Sánchez J.M. Risk factors associated with milk fever occurrence in grazing dairy cattle. J. Dairy Sci. 2017;100:9715–9722. doi: 10.3168/jds.2017-13065. [DOI] [PubMed] [Google Scholar]
- 133.Chiwome B., Kandiwa E., Mushonga B., Sajeni S., Habarugira G. A study of the incidence of milk fever in Jersey and Holstein cows at a dairy farm in Beatrice, Zimbabwe. J. S. Afr. Vet. Assoc. 2017;88:6. doi: 10.4102/jsava.v88i0.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.DeGaris P.J., Lean I.J. Milk fever in dairy cows: A review of pathophysiology and control principles. Vet. J. 2008;176:58–69. doi: 10.1016/j.tvjl.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 135.Venjakob P.L., Borchardt S., Heuwieser W. Hypocalcemia—Cow-level prevalence and preventive strategies in German dairy herds. J. Dairy Sci. 2017;100:9258–9266. doi: 10.3168/jds.2016-12494. [DOI] [PubMed] [Google Scholar]
- 136.Lean I.J., DeGaris P.J., McNeil D.M., Block E. Hypocalcemia in dairy cows: Meta-analysis and dietary cation anion difference theory revisited. J. Dairy Sci. 2006;89:669–684. doi: 10.3168/jds.S0022-0302(06)72130-0. [DOI] [PubMed] [Google Scholar]
- 137.Caixeta L.S., Ospina P.A., Capel M.B., Nydam D.V. Association between subclinical hypocalcemia in the first 3 days of lactation and reproductive performance of dairy cows. Theriogenology. 2017;94:1–7. doi: 10.1016/j.theriogenology.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 138.Correa M.T., Erb H., Scarlett J. Path Analysis for Seven Postpartum Disorders of Holstein Cows. J. Dairy Sci. 1993;76:1305–1312. doi: 10.3168/jds.S0022-0302(93)77461-5. [DOI] [PubMed] [Google Scholar]
- 139.Markusfeld O. Periparturient Traits in Seven High Dairy Herds. Incidence Rates, Association with Parity, and Interrelationships Among Traits. J. Dairy Sci. 1987;70:158–166. doi: 10.3168/jds.S0022-0302(87)79990-1. [DOI] [PubMed] [Google Scholar]
- 140.Jeong J.K., Kang H.G., Kim I.H. Associations between serum calcium concentration and postpartum health and reproductive performance in dairy cows. Anim. Reprod. Sci. 2018;196:184–192. doi: 10.1016/j.anireprosci.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 141.Thys-Jacobs S., Donovan D., Papadopoulos A., Sarrel P., Bilezikian J.P. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids. 1999;64:430–435. doi: 10.1016/S0039-128X(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 142.Reinhardt T.A., Lippolis J.D., McCluskey B.J., Goff J.P., Horst R.L. Prevalence of subclinical hypocalcemia in dairy herds. Vet. J. 2011;188:122–124. doi: 10.1016/j.tvjl.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 143.Neves R.C., Leno B.M., Stokol T., Overton T.R., McArt J.A.A. Risk factors associated with postpartum subclinical hypocalcemia in dairy cows. J. Dairy Sci. 2017;100:3796–3804. doi: 10.3168/jds.2016-11970. [DOI] [PubMed] [Google Scholar]
- 144.Chapinal N., Carson M., Duffield T.F., Capel M., Godden S., Overton M., Santos J.E.P., LeBlanc S.J. The association of serum metabolites with clinical disease during the transition period. J. Dairy Sci. 2011;94:4897–4903. doi: 10.3168/jds.2010-4075. [DOI] [PubMed] [Google Scholar]
- 145.Rodríguez E.M., Arís A., Bach A. Associations between subclinical hypocalcemia and postparturient diseases in dairy cows. J. Dairy Sci. 2017;100:7427–7434. doi: 10.3168/jds.2016-12210. [DOI] [PubMed] [Google Scholar]
- 146.Oetzel G.R. Parturient paresis and hypocalcemia in ruminant livestock. Vet. Clin. N. Am. Food Anim. Pract. 1988;4:351–364. doi: 10.1016/S0749-0720(15)31053-7. [DOI] [PubMed] [Google Scholar]
- 147.Leno B.M., Ryan C.M., Stokol T., Kirk D., Zanzalari K.P., Chapman J.D., Overton T.R. Effects of prepartum dietary cation-anion difference on aspects of peripartum mineral and energy metabolism and performance of multiparous Holstein cows. J. Dairy Sci. 2017;100:4604–4622. doi: 10.3168/jds.2016-12221. [DOI] [PubMed] [Google Scholar]
- 148.Weich W., Block E., Litherland N.B. Extended negative dietary cation-anion difference feeding does not negatively affect postpartum performance of multiparous dairy cows. J. Dairy Sci. 2013;96:5780–5792. doi: 10.3168/jds.2012-6479. [DOI] [PubMed] [Google Scholar]
- 149.Lopera C., Zimpel R., Vieira-Neto A., Lopes F.R., Ortiz W., Poindexter M., Faria B.N., Gambarini M.L., Block E., Nelson C.D., et al. Effects of level of dietary cation-anion difference and duration of prepartum feeding on performance and metabolism of dairy cows. J. Dairy Sci. 2018;101:7907–7929. doi: 10.3168/jds.2018-14580. [DOI] [PubMed] [Google Scholar]
- 150.Ryan K.T., Guadagnin A.R., Glosson K.M., Bascom S.S., Rowson A.D., Steelman A.J., Cardoso F.C. Increased dietary calcium inclusion in fully acidified prepartum diets improved postpartum uterine health and fertility when fed to Holstein cows. Theriogenology. 2020;142:338–347. doi: 10.1016/j.theriogenology.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 151.Thilsing-Hansen T., Jørgensen R.J., Enemark J.M.D., Larsen T. The effect of zeolite a supplementation in the dry period on periparturient calcium, phosphorus, and magnesium homeostasis. J. Dairy Sci. 2002;85:1855–1862. doi: 10.3168/jds.S0022-0302(02)74259-8. [DOI] [PubMed] [Google Scholar]
- 152.Kronqvist C., Emanuelson U., Spörndly R., Holtenius K. Effects of prepartum dietary calcium level on calcium and magnesium metabolism in periparturient dairy cows. J. Dairy Sci. 2011;94:1365–1373. doi: 10.3168/jds.2009-3025. [DOI] [PubMed] [Google Scholar]
- 153.Kronqvist C., Emanuelson U., Tråvén M., Spãrndly R., Holtenius K. Relationship between incidence of milk fever and feeding of minerals during the last 3 weeks of gestation. Animal. 2012;6:1316–1321. doi: 10.1017/S175173111200033X. [DOI] [PubMed] [Google Scholar]
- 154.Wächter S., Cohrs I., Golbeck L., Wilkens M.R., Grünberg W. Effects of restricted dietary phosphorus supply to dry cows on periparturient calcium status. J. Dairy Sci. 2022;105:748–760. doi: 10.3168/jds.2021-20726. [DOI] [PubMed] [Google Scholar]
- 155.Martín-Tereso J., Martens H., Deiner C., Van Laar H., Den Hartog L.A., Verstegen M.W.A. Pre-calving feeding of rumen-protected rice bran to multiparous dairy cows improves recovery of calcaemia after calving. J. Dairy Res. 2016;83:281–288. doi: 10.1017/S002202991600039X. [DOI] [PubMed] [Google Scholar]
- 156.Khachlouf K., Hamed H., Gdoura R., Gargouri A. Effects of dietary Zeolite supplementation on milk yield and composition and blood minerals status in lactating dairy cows. J. Appl. Anim. Res. 2019;47:54–62. doi: 10.1080/09712119.2018.1563548. [DOI] [Google Scholar]
- 157.Laporta J., Moore S.A.E., Peters M.W., Peters T.L., Hernandez L.L. Short communication: Circulating serotonin (5-HT) concentrations on day 1 of lactation as a potential predictor of transition-related disorders. J. Dairy Sci. 2013;96:281–288. doi: 10.3168/jds.2013-6718. [DOI] [PubMed] [Google Scholar]
- 158.Slater C.J., Endres E.L., Weaver S.R., Cheng A.A., Lauber M.R., Endres S.F., Olstad E., DeBruin A., Crump P.M., Block E., et al. Interaction of 5-hydroxy-L-tryptophan and negative dietary cation-anion difference on calcium homeostasis in multiparous peripartum dairy cows. J. Dairy Sci. 2018;101:5486–5501. doi: 10.3168/jds.2017-13938. [DOI] [PubMed] [Google Scholar]
- 159.Sato S. Pathophysiological evaluation of subacute ruminal acidosis (SARA) by continuous ruminal pH monitoring. Anim. Sci. J. 2016;87:168–177. doi: 10.1111/asj.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Steele M.A., AlZahal O., Hook S.E., Croom J., McBride B.W. Ruminal acidosis and the rapid onset of ruminal parakeratosis in a mature dairy cow: A case report. Acta Vet. Scand. 2009;51:39. doi: 10.1186/1751-0147-51-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kleen J.L., Upgang L., Rehage J. Prevalence and consequences of subacute ruminal acidosis in German dairy herds. Acta Vet. Scand. 2013;55:48. doi: 10.1186/1751-0147-55-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McCann J.C., Luan S., Cardoso F.C., Derakhshani H., Khafipour E., Loor J.J. Induction of subacute ruminal acidosis affects the ruminal microbiome and epithelium. Front. Microbiol. 2016;7:701. doi: 10.3389/fmicb.2016.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zebeli Q., Ghareeb K., Humer E., Metzler-Zebeli B.U., Besenfelder U. Nutrition, rumen health and inflammation in the transition period and their role on overall health and fertility in dairy cows. Res. Vet. Sci. 2015;103:126–136. doi: 10.1016/j.rvsc.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 164.Plaizier J.C., Krause D.O., Gozho G.N., McBride B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008;176:21–31. doi: 10.1016/j.tvjl.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 165.Heidari M., Kafi M., Mirzaei A., Asaadi A., Mokhtari A. Effects of follicular fluid of preovulatory follicles of repeat breeder dairy cows with subclinical endometritis on oocyte developmental competence. Anim. Reprod. Sci. 2019;205:62–69. doi: 10.1016/j.anireprosci.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 166.Lüttgenau J., Herzog K., Strüve K., Latter S., Boos A., Bruckmaier R.M., Bollwein H., Kowalewski M.P. LPS-mediated effects and spatio-temporal expression of TLR2 and TLR4 in the bovine corpus luteum. Reproduction. 2016;151:391–399. doi: 10.1530/REP-15-0520. [DOI] [PubMed] [Google Scholar]
- 167.Haziak K., Herman A.P., Tomaszewska-Zaremba D. Effects of central injection of anti-LPS antibody and blockade of TLR4 on GnRH/LH secretion during immunological stress in anestrous ewes. Mediators Inflamm. 2014;2014:867170. doi: 10.1155/2014/867170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Haziak K., Herman A.P., Wojtulewicz K., Pawlina B., Paczesna K., Bochenek J., Tomaszewska-Zaremba D. Effect of CD14/TLR4 antagonist on GnRH/LH secretion in ewe during central inflammation induced by intracerebroventricular administration of LPS. J. Anim. Sci. Biotechnol. 2018;9:52. doi: 10.1186/s40104-018-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kadzere C., Murphy M., Silanikove N., Maltz E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002;77:59–91. doi: 10.1016/S0301-6226(01)00330-X. [DOI] [Google Scholar]
- 170.Jenkins T.C. Lipid Metabolism in the Rumen. J. Dairy Sci. 1993;76:3851–3863. doi: 10.3168/jds.S0022-0302(93)77727-9. [DOI] [PubMed] [Google Scholar]
- 171.Doreau M., Ferlay A. Digestion and utilisation of fatty acids by ruminants. Anim. Feed Sci. Technol. 1994;45:379–396. doi: 10.1016/0377-8401(94)90039-6. [DOI] [Google Scholar]
- 172.Bauman D.E., Griinari J.M. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutr. 2003;23:203–227. doi: 10.1146/annurev.nutr.23.011702.073408. [DOI] [PubMed] [Google Scholar]
- 173.Jouany J.P. Optimizing rumen functions in the close-up transition period and early lactation to drive dry matter intake and energy balance in cows. Anim. Reprod. Sci. 2006;96:250–264. doi: 10.1016/j.anireprosci.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 174.Kamada H. Effects of selenium-rich yeast supplementation on the plasma progesterone levels of postpartum dairy cows. Asian-Australas. J. Anim. Sci. 2017;30:347–354. doi: 10.5713/ajas.16.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wildman C.D., West J.W., Bernard J.K. Effect of dietary cation-anion difference and dietary crude protein on performance of lactating dairy cows during hot weather. J. Dairy Sci. 2007;90:1842–1850. doi: 10.3168/jds.2006-546. [DOI] [PubMed] [Google Scholar]
- 176.Erdman R.A. Dietary Buffering Requirements of the Lactating Dairy Cow: A Review. J. Dairy Sci. 1988;71:3246–3266. doi: 10.3168/jds.S0022-0302(88)79930-0. [DOI] [Google Scholar]
- 177.Wang F., Shi H., Wang S., Wang Y., Cao Z., Li S. Amino Acid Metabolism in Dairy Cows and their Regulation in Milk Synthesis. Curr. Drug Metab. 2019;20:36–45. doi: 10.2174/1389200219666180611084014. [DOI] [PubMed] [Google Scholar]
- 178.Butler W.R. Nutritional interactions with reproductive performance in dairy cattle. Anim. Reprod. Sci. 2000;60–61:449–457. doi: 10.1016/S0378-4320(00)00076-2. [DOI] [PubMed] [Google Scholar]
- 179.Tamminga S. The effect of the supply of rumen degradable protein and metabolisable protein on negative energy balance and fertility in dairy cows. Anim. Reprod. Sci. 2006;96:227–239. doi: 10.1016/j.anireprosci.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 180.Campanile G., Di Palo R., Infascelli F., Gasparrini B., Neglia G., Zicarelli F., D’Occhio M.J. Influence of rumen protein degradability on productive and reproductive performance in buffalo cows. Reprod. Nutr. Dev. 2003;43:557–566. doi: 10.1051/rnd:2004008. [DOI] [PubMed] [Google Scholar]
- 181.Raboisson D., Albaaj A., Nonne G., Foucras G. High urea and pregnancy or conception in dairy cows: A meta-analysis to define the appropriate urea threshold. J. Dairy Sci. 2017;100:7581–7587. doi: 10.3168/jds.2016-12009. [DOI] [PubMed] [Google Scholar]
- 182.Kananub S., Pechkerd P., VanLeeuwen J., Stryhn H., Arunvipas P. Evaluation of influence of milk urea nitrogen on reproductive performance in smallholder dairy farms. Aust. Vet. J. 2020;98:375–379. doi: 10.1111/avj.12946. [DOI] [PubMed] [Google Scholar]
- 183.Mohanty P., Ghanim H., Hamouda W., Aljada A., Garg R., Dandona P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am. J. Clin. Nutr. 2002;75:767–772. doi: 10.1093/ajcn/75.4.767. [DOI] [PubMed] [Google Scholar]
- 184.Agarwal A., Aponte-Mellado A., Premkumar B.J., Shaman A., Gupta S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Guo J., Gao S., Quan S., Zhang Y., Bu D., Wang J. Blood amino acids profile responding to heat stress in dairy cows. Asian-Australas. J. Anim. Sci. 2018;31:47–53. doi: 10.5713/ajas.16.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Baumgard L.H., Rhoads R.P. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013;1:311–337. doi: 10.1146/annurev-animal-031412-103644. [DOI] [PubMed] [Google Scholar]
- 187.Sammad A., Wang Y.J., Umer S., Lirong H., Khan I., Khan A., Ahmad B., Wang Y. Nutritional physiology and biochemistry of dairy cattle under the influence of heat stress: Consequences and opportunities. Animals. 2020;10:793. doi: 10.3390/ani10050793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gao H. Amino acids in reproductive nutrition and health. Adv. Exp. Med. Biol. 2020;1265:111–131. doi: 10.1007/978-3-030-45328-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Elango R., Ball R.O. Protein and Amino Acid Requirements during Pregnancy. Adv. Nutr. 2016;7:839S–844S. doi: 10.3945/an.115.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ennis M., Lim K., Ball R., Pencharz P., Courtney-Martin G., Elango R. Dietary Phenylalanine and Tyrosine Requirements in Healthy Human Pregnancy. Curr. Dev. Nutr. 2020;4 doi: 10.1093/cdn/nzaa054_050. [DOI] [Google Scholar]
- 191.Di Costanzo A., Spain J.N., Spiers D.E. Supplementation of Nicotinic Acid for Lactating Holstein Cows Under Heat Stress Conditions. J. Dairy Sci. 1997;80:1200–1206. doi: 10.3168/jds.S0022-0302(97)76048-X. [DOI] [PubMed] [Google Scholar]
- 192.Hansen P.J. Hidden factors affecting fertility. Adv. Dairy Technol. Proc. West. Can. Dairy Semin. 2007;19:339–349. [Google Scholar]
- 193.Kaufman J.D., Pohler K.G., Mulliniks J.T., Ríus A.G. Lowering rumen-degradable and rumen-undegradable protein improved amino acid metabolism and energy utilization in lactating dairy cows exposed to heat stress. J. Dairy Sci. 2018;101:386–395. doi: 10.3168/jds.2017-13341. [DOI] [PubMed] [Google Scholar]
- 194.Havekes C.D., Duffield T.F., Carpenter A.J., DeVries T.J. Effects of wheat straw chop length in high-straw dry cow diets on intake, health, and performance of dairy cows across the transition period. J. Dairy Sci. 2020;103:254–271. doi: 10.3168/jds.2019-17033. [DOI] [PubMed] [Google Scholar]
- 195.Acosta D.A.V., Rivelli M.I., Skenandore C., Zhou Z., Keisler D.H., Luchini D., Corrêa M.N., Cardoso F.C. Effects of rumen-protected methionine and choline supplementation on steroidogenic potential of the first postpartum dominant follicle and expression of immune mediators in Holstein cows. Theriogenology. 2017;96:1–9. doi: 10.1016/j.theriogenology.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 196.Skenandore C.S., Velasco Acosta D.A., Zhou Z., Rivelli M.I., Corrêa M.N., Luchini D.N., Cardoso F.C. Effects of rumen-protected methionine and choline supplementation on vaginal discharge and uterine cytology of Holstein cows. Int. J. Vet. Sci. Med. 2017;5:1–7. doi: 10.1016/j.ijvsm.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stella S.L., Velasco-Acosta D.A., Skenandore C., Zhou Z., Steelman A., Luchini D., Cardoso F.C. Improved uterine immune mediators in Holstein cows supplemented with rumen-protected methionine and discovery of neutrophil extracellular traps (NET) Theriogenology. 2018;114:116–125. doi: 10.1016/j.theriogenology.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 198.Acosta D.A.V., Denicol A.C., Tribulo P., Rivelli M.I., Skenandore C., Zhou Z., Luchini D., Corrêa M.N., Hansen P.J., Cardoso F.C. Effects of rumen-protected methionine and choline supplementation on the preimplantation embryo in Holstein cows. Theriogenology. 2016;85:1669–1679. doi: 10.1016/j.theriogenology.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 199.Palmquist D.L., Jenkins T.C. Fat in Lactation Rations: Review. J. Dairy Sci. 1980;63:1–14. doi: 10.3168/jds.S0022-0302(80)82881-5. [DOI] [PubMed] [Google Scholar]
- 200.Bicalho M.L.S., Lima F.S., Ganda E.K., Foditsch C., Meira E.B.S., Machado V.S., Teixeira A.G.V., Oikonomou G., Gilbert R.O., Bicalho R.C. Effect of trace mineral supplementation on selected minerals, energy metabolites, oxidative stress, and immune parameters and its association with uterine diseases in dairy cattle. J. Dairy Sci. 2014;97:4281–4295. doi: 10.3168/jds.2013-7832. [DOI] [PubMed] [Google Scholar]
- 201.Mammi L.M.E., Guadagnini M., Mechor G., Cainzos J.M., Fusaro I., Palmonari A., Formigoni A. The Use of Monensin for Ketosis Prevention in Dairy Cows during the Transition Period: A Systematic Review. Animals. 2021;11:1988. doi: 10.3390/ani11071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Silva P.R.B., Machado K.S., Da Silva D.N.L., Moraes J.G.N., Keisler D.H., Chebel R.C. Effects of recombinant bovine somatotropin during the periparturient period on innate and adaptive immune responses, systemic inflammation, and metabolism of dairy cows. J. Dairy Sci. 2015;98:4449–4464. doi: 10.3168/jds.2014-8959. [DOI] [PubMed] [Google Scholar]
- 203.Wheelock J.B., Rhoads R.P., VanBaale M.J., Sanders S.R., Baumgard L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010;93:644–655. doi: 10.3168/jds.2009-2295. [DOI] [PubMed] [Google Scholar]
- 204.Hansen P.J., Aréchiga C.F. Strategies for managing reproduction in the heat-stressed dairy cow. J. Anim. Sci. 1999;77((Suppl. 2)):36–50. doi: 10.2527/1997.77suppl_236x. [DOI] [PubMed] [Google Scholar]
- 205.Cartmill J.A., El-Zarkouny S.Z., Hensley B.A., Rozell T.G., Smith J.F., Stevenson J.S. An Alternative AI Breeding Protocol for Dairy Cows Exposed to Elevated Ambient Temperatures before or after Calving or Both. J. Dairy Sci. 2001;84:799–806. doi: 10.3168/jds.S0022-0302(01)74536-5. [DOI] [PubMed] [Google Scholar]
- 206.Stewart B.M., Block J., Morelli P., Navarette A.E., Amstalden M., Bonilla L., Hansen P.J., Bilby T.R. Efficacy of embryo transfer in lactating dairy cows during summer using fresh or vitrified embryos produced in vitro with sex-sorted semen. J. Dairy Sci. 2011;94:3437–3445. doi: 10.3168/jds.2010-4008. [DOI] [PubMed] [Google Scholar]
- 207.López-Gatius F., Santolaria P., Martino A., Delétang F., De Rensis F. The effects of GnRH treatment at the time of AI and 12 days later on reproductive performance of high producing dairy cows during the warm season in northeastern Spain. Theriogenology. 2006;65:820–830. doi: 10.1016/j.theriogenology.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 208.Friedman E., Voet H., Reznikov D., Wolfenson D., Roth Z. Hormonal treatment before and after artificial insemination differentially improves fertility in subpopulations of dairy cows during the summer and autumn. J. Dairy Sci. 2014;97:7465–7475. doi: 10.3168/jds.2014-7900. [DOI] [PubMed] [Google Scholar]