Abstract
The wide application of additive manufacturing in dentistry implies the further investigation into oral micro-organism adhesion and biofilm formation on vat-photopolymerization (VP) dental resins. The surface characteristics and microbiological analysis of a VP dental resin, printed at resolutions of 50 μm (EG-50) and 100 μm (EG-100), were evaluated against an auto-polymerizing acrylic resin (CG). Samples were evaluated using a scanning electron microscope, a scanning white-light interferometer, and analyzed for Candida albicans (CA) and Streptococcus mutans (SM) biofilm, as well as antifungal and antimicrobial activity. EG-50 and EG-100 exhibited more irregular surfaces and statistically higher mean (Ra) and root-mean-square (rms) roughness (EG-50-Ra: 2.96 ± 0.32 µm; rms: 4.05 ± 0.43 µm/EG-100-Ra: 3.76 ± 0.58 µm; rms: 4.79 ± 0.74 µm) compared to the CG (Ra: 0.52 ± 0.36 µm; rms: 0.84 ± 0.54 µm) (p < 0.05). The biomass and extracellular matrix production by CA and SM and the metabolic activity of SM were significantly decreased in EG-50 and EG-100 compared to CG (p < 0.05). CA and SM growth was inhibited by the pure unpolymerized VP resin (48 h). EG-50 and EG-100 recorded a greater irregularity, higher surface roughness, and decreased CA and SM biofilm formation over the CG.
Keywords: digital technologies, biomaterials, poly methyl methacrylate resin, photo-polymerizable resin, surface properties, antifungal activity, antibacterial activity
1. Introduction
The oral cavity is constituted of a complex and diverse microbiota that influences oral and systemic health [1], such as Streptococcus mutans, a major cariogenic bacterial species [2], and Candida albicans, a fungal species able to act as an opportunistic pathogen leading to mucosal and disseminated infections [3] under conditions that trigger the imbalance of the oral and immune system homeostasis [4,5,6]. Thus, the introduction of restorative or prosthetic dental materials into the oral environment might provide such an imbalance, thereby maximizing biofilm formation [7]. Recently, products manufactured with three-dimensional (3D) printing have become popular in dentistry, being increasingly used due to the ease of acquisition, processing, and manipulation of the images obtained through intraoral scanning [8,9].
The additive-manufacturing technologies: vat-photopolymerization, power bed fusion, binder jetting, material extrusion, direct energy deposition, material jetting and sheet lamination enable the application to substrates such as polymers, metals and ceramics, yielding different results regarding accuracy, precision and trueness, thus providing diverse and wide applications [10,11,12]. Digital-light-processing (DLP) technology is one of the first 3D-printing processes, which is categorized according to ISO/ASTM 52900/15(E) as a vat-photopolymerization (VP) process, wherein the photopolymerizable liquid is selectively cured by light projection of the object to be printed through a small projector or mirrors that display a single image at one time [13]. Each layer is photoactivated so that the resolution of the item to be printed is directly related to the number of projectors and mirrors used in the technique. That way, at each activation, solid layers of small cubic blocks, referred to as voxels, originate from the interaction of the light with the resin of origin, until the object is completely formed [10,14,15,16,17].
Dental crowns and prostheses, surgical guides and components, orthodontic aligners and appliances, as well as courseware for research and teaching, represent a reality arising from digital dentistry [10,18,19]. However, certain parameters need to be observed in order to obtain the best results from printing techniques applied in dentistry, such as the thickness of each photoactivated layer, base design, post-processing, and storage media, which are all factors that are known to influence the accuracy of 3D dental models [20]. As for printing resolutions, Shim et al. [21] and Unkovskiy et al. [22] demonstrated, through in vitro studies, that greater accuracy is obtained when objects are printed in a 90° orientation in both stereolithography (SLA) and DLP techniques. Alternatively, Hada in 2020 [23] reported that the impression in a 45° orientation presented superior accuracy in relation to 0° and 90° orientations. Shim et al. [21] also demonstrated, recently, that the printing orientation also influences the microbiological colonization capacity, as significantly lower levels of C. albicans were detected on objects printed in a 90° orientation rather than 45° and 0° orientations.
With respect to biocompatibility and activity against potential pathogens, photopolymers have been evaluated [24,25,26] regarding the antibacterial capacity of natural polymers [27] and the addition of antimicrobial agents or drugs in the synthetic polymer matrix [28,29,30]. Bloukh et al. [31] emphasized that the microbial-resistance phenomenon is a serious matter that threatens humanity’s existence, mainly due to the indiscriminate use of antibiotic drugs. In dentistry, the development of alternative antimicrobial properties of surgical sutures [32], dental implants [33,34], restorative and denture materials [35,36] are relevant contributors to the mitigation or cessation of the use of antibiotics [37] in clinical practice.
Although there is an exponential growth in the application of additive manufacturing in dentistry, the knowledge regarding the comparison of VP dental resins to auto-polymerizing acrylic-resin systems, which are standardly applied to the manufacture of dental devices, is limited. Therefore, the present research focused on the investigation of a dental VP resin regarding surface characteristics, microbiological colonization, and antifungal/antimicrobial activities against an auto-polymerizing acrylic-resin system as the control. The null hypothesis of our study comprised: (i) the surface characteristics and biofilm formation by C. albicans and S. mutans of a VP dental resin for additive manufacturing will be similar to an auto-polymerizing acrylic resin, (ii) the 3D-printing resolution will not affect the surface characteristics and microbiological colonization of VP dental resins, and (iii) C. albicans and S. mutans growth will not be inhibited by the unpolymerized VP dental resin.
2. Materials and Methods
2.1. Materials
The sample of this study consisted of 300 specimens (4 mm in diameter × 1 mm in height) (Figure 1), divided into three groups (n = 100): control group (CG), constituted of auto-polymerizing acrylic resin, and experimental groups containing VP additive-manufacturing dental resins printed at resolutions of 50 μm (EG-50) and 100 μm (EG-100) (Table 1).
Figure 1.
Specimen characteristics. (A): CG, auto-polymerizing acrylic resin; (B): EG-50, VP additive-manufacturing dental resin printed at 50 μm resolution; (C): EG-100, VP additive-manufacturing dental resin printed at 100 μm resolution.
Table 1.
Auto-polymerizing acrylic and VP additive-manufacturing resins used in the study.
| Product | Component | Manufacturer |
|---|---|---|
| Auto-polymerizing acrylic-resin system Ortho Class |
Methyl Methacrylate Monomer, DMT, Crosslink, Methyl Ethyl Methacrylate Copolymer | Clássico São Paulo, SP, Brazil |
| VP additive-manufacturing dental resin COSMOS Splint |
Oligomers, Monomers, Photoinitiators, Stabilizer, Pigment | Yller Pelotas, RS, Brazil |
The CG was manufactured with an auto-polymerizing acrylic-resin system (OrtoClass, São Paulo-SP, Brazil) by the same operator, with the aid of prefabricated molds of addition silicone (3 M®, São Paulo, SP, Brazil) according to the manufacturer’s recommendations. To reduce the chances of air-bubble occurrence, the specimens were fabricated by an incremental-build-up technique and immersed in a pan with negative pressure (Protécni, Araraquara, SP, Brazil) during the polymerization phase. Then, the finishing of the specimens was performed sequentially using 400, 600, and 1200 g sandpapers in a Politriz metallographic machine (APL4, Arotec, Cotia, SP, Brazil) at a speed of 300 rpm/60 s for each sandpaper per specimen [38].
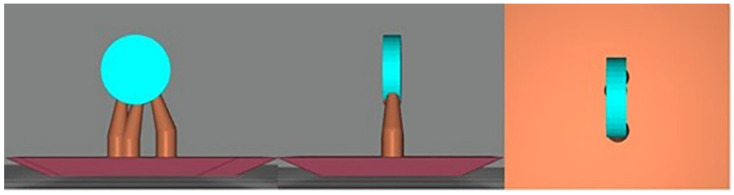
In the experimental groups, EG-50 and EG-100, the specimens were designed using open-source CAD software (Meshmixer v. 3.5, Autodesk, Inc., San Rafael, CA, USA) (Figure 2) with a 90° print orientation. 3D specimens were manufactured in a dental resin for additive manufacturing (Cosmos Splint, Yller, Pelotas, RS, Brazil) in a 3D printer (PHOTON S, Anycubic 3D Printing, Shenzhen, Guangdong) using digital-light-processing technology (DLP/LCD) with printing resolutions of 50 and 100 μm. The residual surface monomers on the 3D-printed specimens were cleaned using a wipe and isopropyl alcohol. Postpolymerization was performed in accordance with the manufacturer’s instructions using a UV-light polymerization unit (CureDen; DENTIS). The support structures were removed using low-speed rotary instruments (Beltec, Araraquara, SP, Brazil).
Figure 2.
CAD design of the VP additive-manufacturing dental resin sample with 90° printing orientation (Meshmixer v. 3.5, Autodesk, Inc., CA, USA).
2.2. Analysis of Surface Characteristics
For the purpose of surface characterization, samples from each group were coated with gold particles and analyzed using a scanning electron microscope (Quanta FEG 250, FEI, Eindhoven, The Netherlands) at ×250, ×500 and ×1000 magnifications.
Morphology and surface roughness were evaluated in three samples per group, in duplicate, using a non-contact, three-dimensional, scanning white-light interferometer Zygo NewView 7100 (Zygo, Middlefield, CA, USA) using ×20 magnification lenses. The mean roughness (Ra), root mean square (rms), and mean roughness of the 3rd peak and valley (R3z) were used, with an area of 40 μm × 40 μm. The mean roughness (Ra) was quantified from the arithmetic mean of the absolute values and the ordinate distance of the points of the roughness profile in relation to the mean line within the measurement area. The root-mean-square (rms) deviation was defined as the square root of the mean of the ordinates’ squares of the effective profile in relation to the mean line within the measurement path. Finally, the mean roughness of the 3rd peak and valley (R3z) was obtained by the arithmetic mean of the partial roughness values corresponding to each of the five modules.
2.3. Microbiological Analysis
Prior to the beginning of microbiological analysis, the specimens were sterilized by ultraviolet (UV) light for 30 min [39] and then allocated to cell-culture microplates with 96 wells each (Kasvi®, São José dos Pinhais, Paraná, Brazil) wherein 1 × 106 Candida albicans yeasts and 1 × 106 colony-forming units (CFUs) of Streptococcus mutans were added. The systems were kept in an oven (Quimis®, Diadema, Brazil) at a controlled temperature of 37 °C ± 1 °C for 24, 48, and 72 h, in brain heart infusion (BHI). After each of the different timepoints, the resins were re-allocated into new 96-well plates, and the biomass, extracellular matrix, and metabolic activity were quantified in triplicate.
The biomass formed by different micro-organisms was evaluated according to the method described by Peeters (2008) [40]. The culture media were discarded and the biomass was fixed with 100% methanol (200 µL) for 15 min. The systems were dried at room temperature for 5 min and then a solution containing 0.4% crystal violet (200 µL) was added, and the plates were incubated for 20 min. To remove excess dye, the systems were washed again with PBS. Subsequently, the biomass was decolorized with 30% acetic acid (200 µL) for 5 min. The bleach solution (100 µL) was transferred to another 96-well plate, and the absorbance was measured at 590 nm using a SpectraMax M3 microplate reader (Molecular Devices, San Jose, CA, USA).
The extracellular matrix was quantified according to the method described by Choi in 2015 [41]. The culture medium was discarded and a solution containing 0.1% safranin (200 µL) at room temperature was added to the wells for 5 min. To remove excess dye, the system was washed with PBS. Subsequently, the extracellular matrix was decolorized with 30% acetic acid (200 µL) for 5 min. The bleach solution (100 µL) was transferred to another 96-well plate, and the absorbance was measured at 530 nm using a SpectraMax M3 microplate reader.
The metabolic activity of the microbial biomass formed after each incubation time was evaluated by reducing XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT; Sigma-Aldrich, Saint Louis, MO, USA). A solution of XTT at a final concentration of 200 µg/mL and 0.04 mM of menadione was added to the wells for 4 h in the absence of light. After this time, the color change was measured using a SpectraMax M3 microplate reader at a wavelength of 492 nm [36].
2.4. Antifungal and Antimicrobial Activity Testing
The antifungal and antimicrobial activities of unpolymerized VP resin were evaluated at concentrations of 100%, 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, 10%, and 0%, diluted in BHI medium according to modified versions of the CLSI M27-A3 and CLSI M07-A9 (2012) for yeasts and bacteria, respectively. To each of the wells (96-well plate), 1 × 103 yeasts of C. albicans and 1 × 104 CFU of S. mutans were added. The following controls were performed: (i) pure BHI medium, (ii) pure unpolymerized VP resin, and (iii) BHI medium with micro-organisms. The plates were visually read after incubation at 37 °C for 48 h [42,43].
2.5. Statistical Analysis
The statistical analysis was performed using the Statistical Package for Social Science IBM SPSS software for Windows (version 20.0, SPSS Inc., Chicago, IL, USA). The adherence to the normal curve was assessed with the Shapiro–Wilk test. After verifying the normal distribution, the control- and experimental-group comparisons of surface roughness and microbiological data were performed using the one-way analysis of variance (ANOVA) followed by Tukey’s and Dunnett’s multiple-comparisons tests, respectively. In all of the analyses, the significance level was set at 5%.
3. Results
3.1. Surface Characteristics
3.1.1. Scanning Electron Microscope (SEM)
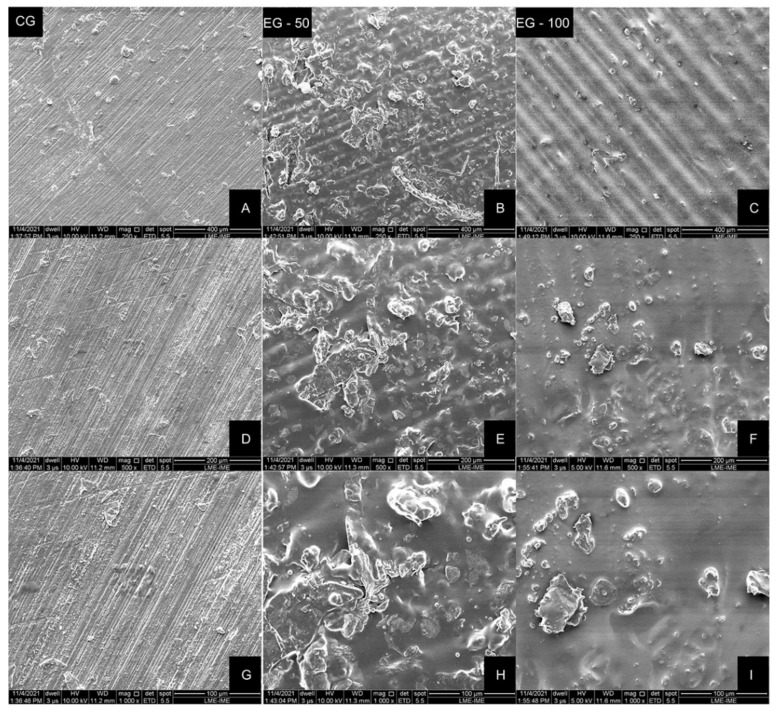
SEM images of the auto-polymerizing acrylic and VP resin samples are illustrated in Figure 3. According to the analysis of the images, a flat but grooved surface was observed in the CG, with clear notches resulting from the polishing sandpapers. A distinguishable, corrugated and more irregular surface was noticed in the experimental groups (EG-50 and EG-100), which might be attributed to the photopolymers’ layer-deposition process (Figure 3).
Figure 3.
Representative SEM images of specimens from each group. (A, D and G), CG—auto-polymerizing acrylic resin. (B, E and H)—EG-50, VP dental resin printed with 50 μm resolution. (C, F and I)—EG-100, VP dental resin printed with 100 μm resolution. Magnifications: (A–C), ×250; (D–F), ×500; (G–I), ×1000.
3.1.2. Surface Roughness
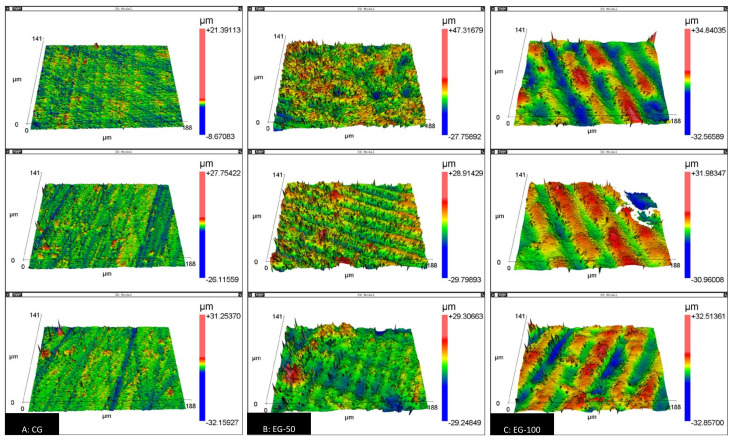
The surface-roughness data are presented in Table 2. Intergroup comparisons indicated a statistically significant difference in all of the roughness parameters evaluated (p < 0.05). The experimental groups, EG-50 (Ra: 2.96 ± 0.32 µm; rms: 4.05 ± 0.43 µm) and EG-100 (Ra: 3.76 ± 0.58 µm; rms: 4.79 ± 0.74 µm), recorded the highest mean roughness and root mean square, compared to the control group (Ra: 0.52 ± 0.36 µm; rms: 0.84 ± 0.54 µm). As for R3z, a statistically significant difference was observed only in EG-100 compared to CG (EG-100: 58,324.17 ± 4936.48 nm; CG: 31,022.08 ± 16,470.44 nm) (Table 2). Images of the three-dimensional morphology of the samples from each group are shown in Figure 4.
Table 2.
Descriptive statistics (mean and standard deviation) of the surface roughness analysis.
| Groups | Ra (µm) | p-Value | rms (µm) | p-Value | R3z (nm) | p-Value |
|---|---|---|---|---|---|---|
| CG | 0.52 ± 0.36 a | <0.001 | 0.84 ± 0.54 a | <0.001 | 31,022.08 ± 16,470.44 a | 0.042 |
| EG-50 | 2.96 ± 0.32 b | 4.05 ± 0.43 b | 53,966.22 ± 6866.32 ab | |||
| EG-100 | 3.76 ± 0.58 b | 4.79 ± 0.74 b | 58,324.17 ± 4936.48 b |
Ra, mean roughness; rms, root mean square; R3z, mean roughness of the 3rd peak and valley. CG, acrylic-resin group; EG-50, VP resin printed with 50 μm resolution; EG-100, VP resin printed with 100 μm resolution. Distinct letters indicate statistically significant difference with ANOVA/Tukey test (α = 0.05).
Figure 4.
Three-dimensional surface-morphology images obtained with a scanning white-light interferometer (scan area: 40 μm2) (A): CG, auto-polymerizing acrylic resin; (B): EG-50, VP resin printed with 50 μm resolution; (C): EG-100, VP resin printed with 100 μm resolution.
3.2. Quantification and Kinetics of Biofilm Formation
The biomass, metabolic-activity, and extracellular-matrix data of the micro-organisms C. albicans and S. mutans that were evaluated in the different study groups are presented in Figure 5, Figure 6 and Figure 7, respectively.
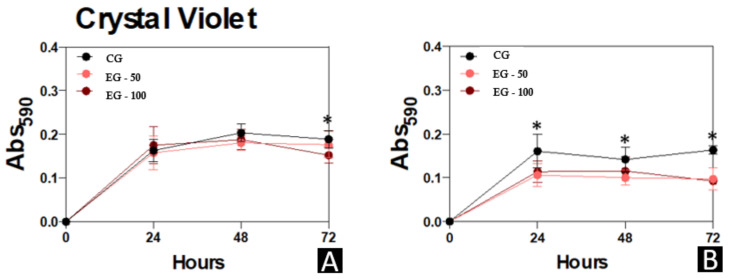
Figure 5.
Biofilm formation by C. albicans and S. mutans for 24, 48, and 72 h at 37 °C. (A), C. albicans. (B), S. mutans. At each time point evaluated, the systems were processed to detect biomass by incorporating a crystal-violet solution at 590 nm. Results are expressed as the mean ± standard deviation of three independent replicates. * p < 0.05.
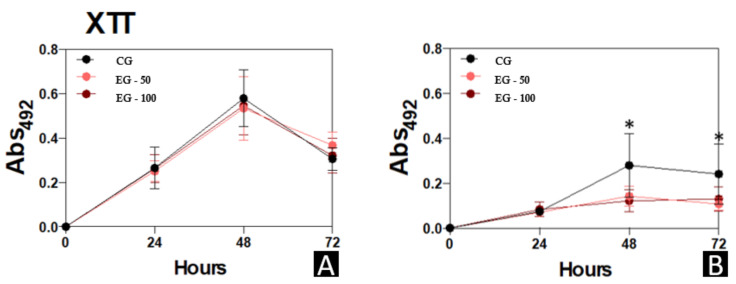
Figure 6.
Biofilm formation by C. albicans and S. mutans for 24, 48, and 72 h at 37 °C. (A), C. albicans. (B), S. mutans. At each time point evaluated, the systems were processed to detect metabolic activity by reducing XTT in menadione by viable cells at 492 nm. Results are expressed as the mean ± standard deviation of three independent replicates. * p < 0.05.
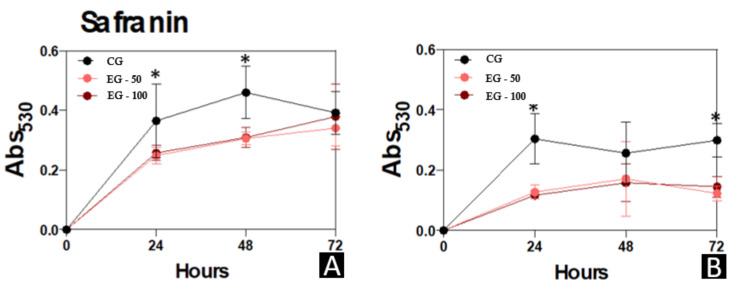
Figure 7.
Extracellular matrix in biofilm formed by C. albicans and S. mutans for 24, 48, and 72 h at 37 °C. (A), C. albicans. (B), S. mutans. mutans. At each time point evaluated, the systems were processed to detect extracellular matrix by staining with safranin at 530 nm. Results are expressed as the mean ± standard deviation of three independent replicates. * p < 0.05.
A significantly higher quantification of the biomass of C. albicans was observed in CG in relation to EG-100 at 72 h (p < 0.05). The CG samples also presented a statistically greater quantification of the biomass of S. mutans compared to the experimental groups (EG-50 and EG-100) at 24 h and 72 h (p < 0.05), and to EG-50 at 48 h (p < 0.05) (Figure 5).
No significant differences were observed in the metabolic activity of C. albicans among the study groups. However, a statistically higher metabolic activity of S. mutans was observed in CG compared to EG-50 and EG-100 at 48 h (p < 0.05), and in CG compared to EG-50 at 72 h (p < 0.05) (Figure 6).
CG presented a higher extracellular matrix production of biofilm formation by C. albicans in relation to EG-50 and EG-100 at 24 h (p < 0.05) and 48 h (p < 0.01). Also, the biofilm formed by S. mutans recorded increased records of extracellular matrix production in CG compared to EG-50 and EG-100 at 24 h (p < 0.001) and 72 h (p ≤ 0.001) (Figure 7).
3.3. Antifungal and Antimicrobial Activity Testing
Antifungal and antimicrobial activity testing indicated that, with the exception of pure unpolymerized VP resin (100%), all of the VP-resin dilutions and control systems of BHI medium with micro-organisms presented C. albicans and S. mutans growth after a 48 h incubation period at 37 °C.
4. Discussion
The wide application of additive-manufacturing resources in dentistry implies the further investigation into the capacity of oral micro-organisms to adhere and form biofilm on vat-photopolymerization (VP)-resin substrates. This in vitro study analyzed the surface characteristics of a VP dental resin for additive manufacturing, printed at resolutions of 50 and 100 μm, in order to determine whether there would be a difference in the surface characteristics and microbiological activity compared to an auto-polymerizing acrylic resin. This investigation directly applies to the manufacture of intraoral devices that usually remain in the oral cavity for a long period of time [4,5].
The analysis of the images obtained with SEM (Figure 3) and with the scanning white-light interferometer (Figure 4) reveals the surface grooves caused by the sequential finishing with the sandpaper strips in the CG, and the printing traces of each resin layer deposition in the EG-50 and EG-100.
When investigating the surface quality of a material-jetting additive-manufacturing process, Udroiu et al. [44] observed that the platen orientation and finishing type were influential factors of the surface roughness, of which their best interaction produced roughness values (Ra) of up to 0.5 μm. On the other hand, previous studies reported Ra values ranging from 0.87 to 5.77 µm [45,46,47,48], which were attributed to the methodological difference of the experiments, such as diverse materials, printers, additive-manufacturing technologies, printing parameters, build orientations, and measurement techniques. In the present study, the VP resin samples recorded Ra values that were approximately six to eight times higher (EG-50: 2.9 μm and EG-100: 3.7 μm) compared to the auto-polymerizing acrylic-resin samples (CG: 0.5 μm). Despite the fact that the surface roughness of 3D printed objects has been previously related to the thickness of each layer deposition [49,50], the Ra parameter in the present study was not significantly influenced by the layer thicknesses of 50 and 100 μm.
The literature shows that an increased surface roughness above the Ra threshold of 0.2 μm [51] facilitates a greater microbiological colonization [52,53] owing to features such as the greater area available for micro-organisms adhesion, the protection from shear forces and the chemical changes that favor physicochemical interactions [54]. Conversely, despite the higher average Ra values, EG-50 and EG-100 recorded a decreased C. albicans and S. mutans biofilm formation in comparison to CG. These findings may be attributed to the influence of the surface energy and the charge of the photopolymers for additive manufacturing on micro-organism colonization and growth [7,55]. Cell-surface charge is essential for modulating microbial adhesion and aggregation [56], and since Steiner et al. [57] presented their discoveries of peptides’ antibacterial activity against both bacteria and eukaryotic cells, these proteins have been employed in polymeric structures. A common feature is the combination of amino acids with polar and non-polar side chains, leading to amphiphilic structures in these peptides [25].
The negative surface charge of S. mutans and C. albicans, which is attributed to the phospholipids and teichoic acids of gram-positive bacteria and the sialic acid of the Candida cell wall [58], is attracted by the positive surface charge of the auto-polymerizing acrylic resin and photopolymers. However, positively, neutral and negatively charged functional groups can vary depending on the polymer’s composition [25,59,60,61].
There is also a strong correlation between attractive hydrophobic and repulsive electrostatic forces with C. albicans’ ability to adhere to polymeric surfaces, which are important in the initial adherence to the dental resin, providing means for further attachment and colonization [62,63]. The poly methyl methacrylate has monomer units exposed on its surface that interact with the hydrophobic domains, thus favoring the immediate adhesion of the fungi [63,64]. Secondarily to hydrophobic forces, there are electrostatic interactions that favor the adhesion process of micro-organisms [65].
Another factor that may influence microbial colonization is the orientation of the object at the time of photopolymer printing. In 2020, Shim et al. [21] demonstrated that objects printed with a 90° orientation, as well as those printed in the present study, had significantly lower levels of colonization by C. albicans than those printed at 0° and 45°. In addition, the 90° printing orientation was chosen as it allowed for less contact with the supporting pillar of the specimen during the printing process, thereby reducing the interference on the sample’s surface.
The potential of microbiological growth inhibition by the photopolymers should also be highlighted. Many authors have previously discussed the benefits of introducing components with antibacterial potential to additive manufacturing, especially against micro-organisms that have, to some degree, bacterial resistance [25,66,67,68]. The use of phytochemical agents has also become a prominent alternative strategy against bacteria and fungi. Moussaoui et al. [69] showed promising results of the antibacterial, antifungal and antioxidant power of Lepidium sativum seed oil. Meanwhile, the selection of antimicrobial components needs to be done carefully, as there is a great similarity between human cells and fungi (both eukaryotic organisms), which hampers the use of a substance that only has selective toxicity for pathogens [70].
The antifungal and antimicrobial potential of photopolymers would be crucial in dentistry with regards to the manufacturing of several intraoral devices such as prostheses and orthodontic retainers. The photopolymer evaluated in the present study inhibited the growth of S. mutans and C. albicans in its pure unpolymerized form for a period of 48 h. However, once diluted, even at the lowest concentration of BHI (90% photopolymer and 10% BHI), there was a positive microbiological growth. Therefore, further investigation is necessary to evaluate whether the inhibition of microbiological growth in the pure unpolymerized photopolymer is due to the lack of BHI medium nutrients or to a soluble component with antifungal and antimicrobial properties that loses its potential of action when in contact with the BHI medium.
5. Conclusions
Within the limitation of this in vitro study, the following conclusions can be drawn:
VP dental-resin samples recorded a greater irregularity, higher surface roughness, and decreased C. albicans and S. mutans biofilm formation over the auto-polymerizing acrylic resin.
Surface characteristics and microbiological colonization of VP resins were not significantly affected by the 3D-printing resolutions of 50 and 100 μm.
VP dental resin inhibited C. albicans and S. mutans growth only in its pure, unpolymerized form for an incubation period of 48 h.
Acknowledgments
The authors would like to acknowledge the contribution of Mariana Braz Herzog in this study.
Abbreviations
| Abs | Absorbance |
| BHI | Brain Heart Infusion |
| CA | Candida albicans |
| CG | Control group |
| CFU | Colony forming units |
| DLP | Digital light processing technology |
| EG-50 | 3D resin experimental group with 50 μm resolution |
| EG-100 | 3D resin experimental group with 100 μm resolution |
| µL | Microliter |
| μ m | Micrometer |
| mm | Millimeter |
| nm | Nanometer |
| n | Sample size |
| rpm | Rotations per minute |
| Ra | Surface roughness |
| rms | Root mean square |
| R3z | Roughness of the 3 rd peak and valley |
| SEM | Scanning electron microscope |
| SM | Streptococcus mutans |
| 3D | Three-dimensional |
| UV | Ultraviolet |
| VP | Vat photopolymerization |
| XTT | 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide |
Author Contributions
Conceptualization, P.L.E.O. and A.C.R.d.C.; methodology, E.O.S., T.P.d.M., C.N.E. and A.C.R.d.C.; formal analysis, T.P.d.M. and A.C.R.d.C.; investigation, E.O.S., T.P.d.M. and A.C.R.d.C.; resources, P.L.E.O. and A.C.R.d.C.; data curation, T.P.d.M. and A.C.R.d.C.; writing—original draft preparation, E.O.S. and A.C.R.d.C.; writing—review and editing, P.L.E.O., T.P.d.M., A.L.S.d.S., C.N.E., S.-H.C. and A.C.R.d.C.; visualization, E.O.S. and A.C.R.d.C.; supervision, A.C.R.d.C.; project administration, S.-H.C. and A.C.R.d.C.; funding acquisition, S.-H.C. and A.C.R.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001 and was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health &Welfare, the Ministry of Food and Drug Safety) (Project Number: KMDF_PR_20200901_0067-01) and was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI20 C0611).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Y., Wang X., Li H., Ni C., Du Z., Yan F. Human oral microbiota and its modulation for oral health. Biomed. Pharm. 2018;99:883–893. doi: 10.1016/j.biopha.2018.01.146. [DOI] [PubMed] [Google Scholar]
- 2.Lemos J.A., Quivey R.G., Koo H., Abranches J. Streptococcus mutans: A new Gram-positive paradigm? Microbiology. 2013;159:436–445. doi: 10.1099/mic.0.066134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masuoka J. Surface glycans of Candida albicans and other pathogenic fungi: Physiological roles, clinical uses, and experimental challenges. Clin. Microbiol. Rev. 2004;17:281–310. doi: 10.1128/CMR.17.2.281-310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zijnge V., van Leeuwen M.B., Degener J.E., Abbas F., Thurnheer T., Gmur R., Harmsen H.J. Oral biofilm architecture on natural teeth. PLoS ONE. 2010;5:e9321. doi: 10.1371/journal.pone.0009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabin N., Zheng Y., Opoku-Temeng C., Du Y., Bonsu E., Sintim H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015;7:493–512. doi: 10.4155/fmc.15.6. [DOI] [PubMed] [Google Scholar]
- 6.Marcotte H., Lavoie M.C. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol. Mol. Biol. Rev. 1998;62:71–109. doi: 10.1128/MMBR.62.1.71-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao Y., Huang X., Zhou X., Li M., Ren B., Peng X., Cheng L. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int. J. Mol. Sci. 2018;19:3157. doi: 10.3390/ijms19103157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S.Y., Shin Y.S., Jung H.D., Hwang C.J., Baik H.S., Cha J.Y. Precision and trueness of dental models manufactured with different 3-dimensional printing techniques. Am. J. Orthod. Dentofac. Orthop. 2018;153:144–153. doi: 10.1016/j.ajodo.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Camargo I.F., Manetti L.P., Zeczkowski M., Sundfeld Neto D., Pini N.I.P., Mori A.A., Ferrairo B.M., Lima F.F. Sistemas cad/cam e suas aplicações na odontologia: Revisão da literatura. REVISTA UNINGÁ. 2018;55:221–228. [Google Scholar]
- 10.Revilla-León M., Özcan M. Additive Manufacturing Technologies Used for Processing Polymers: Current Status and Potential Application in Prosthetic Dentistry. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2019;28:146–158. doi: 10.1111/jopr.12801. [DOI] [PubMed] [Google Scholar]
- 11.Nath S.D., Nilufar S. An Overview of Additive Manufacturing of Polymers and Associated Composites. Polymers. 2020;12:2719. doi: 10.3390/polym12112719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagac M., Hajnys J., Ma Q.-P., Jancar L., Jansa J., Stefek P., Mesicek J. A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3D Printing. Polymers. 2021;13:598. doi: 10.3390/polym13040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ISO/ASTM 52900:2015(E) Standard Terminology for Additive Manufacturing—General Principles—Terminology. ASTM Internacional; West Conshohocken, PA, USA: 2015. [DOI] [Google Scholar]
- 14.Voet V.S.D., Strating T., Schnelting G.H.M., Dijkstra P., Tietema M., Xu J., Woortman A.J.J., Loos K., Jager J., Folkersma R. Biobased Acrylate Photocurable Resin Formulation for Stereolithography 3D Printing. ACS Omega. 2018;3:1403–1408. doi: 10.1021/acsomega.7b01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawood A., Marti Marti B., Sauret-Jackson V., Darwood A. 3D printing in dentistry. Br. Dent J. 2015;219:521–529. doi: 10.1038/sj.bdj.2015.914. [DOI] [PubMed] [Google Scholar]
- 16.Salmoria G.V., Ahrens C.H., Beal V.E., Pires A.T.N., Soldi V. Evaluation of post-curing and laser manufacturing parameters on the properties of SOMOS 7110 photosensitive resin used in stereolithography. Mater. Des. 2009;30:758–763. doi: 10.1016/j.matdes.2008.05.016. [DOI] [Google Scholar]
- 17.Borrello J., Nasser P., Iatridis J., Costa K.D. 3D Printing a Mechanically-Tunable Acrylate Resin on a Commercial DLP-SLA Printer. Addit. Manuf. 2018;23:374–380. doi: 10.1016/j.addma.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhargav A., Sanjairaj V., Rosa V., Feng L.W., Fuh Yh J. Applications of additive manufacturing in dentistry: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018;106:2058–2064. doi: 10.1002/jbm.b.33961. [DOI] [PubMed] [Google Scholar]
- 19.Javaid M., Haleem A. Current status and applications of additive manufacturing in dentistry: A literature-based review. J. Oral Biol. Craniofacial Res. 2019;9:179–185. doi: 10.1016/j.jobcr.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etemad-Shahidi Y., Qallandar O.B., Evenden J., Alifui-Segbaya F., Ahmed K.E. Accuracy of 3-Dimensionally Printed Full-Arch Dental Models: A Systematic Review. J. Clin. Med. 2020;9:3357. doi: 10.3390/jcm9103357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shim J.S., Kim J.E., Jeong S.H., Choi Y.J., Ryu J.J. Printing accuracy, mechanical properties, surface characteristics, and microbial adhesion of 3D-printed resins with various printing orientations. J. Prosthet. Dent. 2020;124:468–475. doi: 10.1016/j.prosdent.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 22.Unkovskiy A., Schmidt F., Beuer F., Li P., Spintzyk S., Kraemer Fernandez P. Stereolithography vs. Direct Light Processing for Rapid Manufacturing of Complete Denture Bases: An In Vitro Accuracy Analysis. J. Clin. Med. 2021;10:1070. doi: 10.3390/jcm10051070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hada T., Kanazawa M., Iwaki M., Arakida T., Soeda Y., Katheng A., Otake R., Minakuchi S. Effect of Printing Direction on the Accuracy of 3D-Printed Dentures Using Stereolithography Technology. Materials. 2020;13:3405. doi: 10.3390/ma13153405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palza H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015;16:2099–2116. doi: 10.3390/ijms16012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-Henríquez C.M., Sarabia-Vallejos M.A., Rodríguez Hernandez J. Antimicrobial Polymers for Additive Manufacturing. Int. J. Mol. Sci. 2019;20:1210. doi: 10.3390/ijms20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin C.H., Lin Y.M., Lai Y.L., Lee S.Y. Mechanical properties, accuracy, and cytotoxicity of UV-polymerized 3D printing resins composed of Bis-EMA, UDMA, and TEGDMA. J. Prosthet. Dent. 2020;123:349–354. doi: 10.1016/j.prosdent.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Sultan S., Siqueira G., Zimmermann T., Mathew A.P. 3D printing of nano-cellulosic biomaterials for medical applications. Curr. Opin. Biomed. Eng. 2017;2:29–34. doi: 10.1016/j.cobme.2017.06.002. [DOI] [Google Scholar]
- 28.Van Hengel I.A.J., Tierolf M., Valerio V.P.M., Minneboo M., Fluit A.C., Fratila-Apachitei L.E., Apachitei I., Zadpoor A.A. Self-defending additively manufactured bone implants bearing silver and copper nanoparticles. J. Mater. Chem. B. 2020;8:1589–1602. doi: 10.1039/C9TB02434D. [DOI] [PubMed] [Google Scholar]
- 29.Yue J., Zhao P., Gerasimov J.Y., van de Lagemaat M., Grotenhuis A., Rustema-Abbing M., van der Mei H.C., Busscher H.J., Herrmann A., Ren Y. 3D-Printable Antimicrobial Composite Resins. Adv. Funct. Mater. 2015;25:6756–6767. doi: 10.1002/adfm.201502384. [DOI] [Google Scholar]
- 30.Garcia C., Gallardo A., López D., Elvira C., Azzahti A., Lopez-Martinez E., Cortajarena A.L., González-Henríquez C.M., Sarabia-Vallejos M.A., Rodríguez-Hernández J. Smart pH-Responsive Antimicrobial Hydrogel Scaffolds Prepared by Additive Manufacturing. ACS Appl. Bio Mater. 2018;1:1337–1347. doi: 10.1021/acsabm.8b00297. [DOI] [PubMed] [Google Scholar]
- 31.Haj Bloukh S., Edis Z., Abu Sara H., Alhamaidah M.A. Antimicrobial Properties of Lepidium sativum L. Facilitated Silver Nanoparticles. Pharmaceutics. 2021;13:1352. doi: 10.3390/pharmaceutics13091352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syukri D.M., Nwabor O.F., Singh S., Ontong J.C., Wunnoo S., Paosen S., Munah S., Voravuthikunchai S.P. Antibacterial-coated silk surgical sutures by ex situ deposition of silver nanoparticles synthesized with Eucalyptus camaldulensis eradicates infections. J. Microbiol. Methods. 2020;174:105955. doi: 10.1016/j.mimet.2020.105955. [DOI] [PubMed] [Google Scholar]
- 33.Choi S.H., Jang Y.S., Jang J.H., Bae T.S., Lee S.J., Lee M.H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J. Appl. Biomater. Funct. Mater. 2019;17:2280800019847067. doi: 10.1177/2280800019847067. [DOI] [PubMed] [Google Scholar]
- 34.Trindade L.A., de Araújo Oliveira J., de Castro R.D., de Oliveira Lima E. Inhibition of adherence of C. albicans to dental implants and cover screws by Cymbopogon nardus essential oil and citronellal. Clin. Oral Investig. 2015;19:2223–2231. doi: 10.1007/s00784-015-1450-3. [DOI] [PubMed] [Google Scholar]
- 35.Ferrando-Magraner E., Bellot-Arcís C., Paredes-Gallardo V., Almerich-Silla J.M., García-Sanz V., Fernández-Alonso M., Montiel-Company J.M. Antibacterial Properties of Nanoparticles in Dental Restorative Materials. A Systematic Review and Meta-Analysis. Medicine. 2020;56:55. doi: 10.3390/medicina56020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AlMojel N., AbdulAzees P.A., Lamb E.M., Amaechi B.T. Determining growth inhibition of Candida albicans biofilm on denture materials after application of an organoselenium-containing dental sealant. J. Prosthet. Dent. 2021. in press. [DOI] [PubMed]
- 37.Edis Z., Haj Bloukh S., Ibrahim M.R., Abu Sara H. “Smart” Antimicrobial Nanocomplexes with Potential to Decrease Surgical Site Infections (SSI) Pharmaceutics. 2020;12:361. doi: 10.3390/pharmaceutics12040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos D.M., Nagay B.E., da Silva E.V., Bonatto Lda R., Sonego M.V., Moreno A., Rangel E.C., da Cruz N.C., Goiato M.C. In vitro analysis of different properties of acrylic resins for ocular prosthesis submitted to accelerated aging with or without photopolymerized glaze. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;69:995–1003. doi: 10.1016/j.msec.2016.07.081. [DOI] [PubMed] [Google Scholar]
- 39.Yagi N., Mori M., Hamamoto A., Nakano M., Akutagawa M., Tachibana S., Takahashi A., Ikehara T., Kinouchi Y. Sterilization using 365 nm UV-LED; Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Lyon, France. 22–26 August 2007; pp. 5842–5845. [DOI] [PubMed] [Google Scholar]
- 40.Peeters E., Nelis H.J., Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods. 2008;72:157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Choi N.Y., Kang S.Y., Kim K.J. Artemisia princeps Inhibits Biofilm Formation and Virulence-Factor Expression of Antibiotic-Resistant Bacteria. BioMed Res. Int. 2015;2015:239519. doi: 10.1155/2015/239519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.M27-43 . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts M27-A3. 3rd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2008. [Google Scholar]
- 43.M 07-A9 . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically:M07-A9. 9th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2012. [Google Scholar]
- 44.Udroiu R., Braga I.C., Nedelcu A. Evaluating the Quality Surface Performance of Additive Manufacturing Systems: Methodology and a Material Jetting Case Study. Materials. 2019;12:995. doi: 10.3390/ma12060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnold C., Monsees D., Hey J., Schweyen R. Surface Quality of 3D-Printed Models as a Function of Various Printing Parameters. Materials. 2019;12:1970. doi: 10.3390/ma12121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Revilla-León M., Jordan D., Methani M.M., Piedra-Cascón W., Özcan M., Zandinejad A. Influence of printing angulation on the surface roughness of additive manufactured clear silicone indices: An in vitro study. J. Prosthet. Dent. 2021;125:462–468. doi: 10.1016/j.prosdent.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Revilla-León M., Al-Haj Husain N., Methani M.M., Özcan M. Chemical composition, surface roughness, and ceramic bond strength of additively manufactured cobalt-chromium dental alloys. J. Prosthet. Dent. 2021;125:825–831. doi: 10.1016/j.prosdent.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Aravind Shanmugasundaram S., Razmi J., Mian M.J., Ladani L. Mechanical Anisotropy and Surface Roughness in Additively Manufactured Parts Fabricated by Stereolithography (SLA) Using Statistical Analysis. Materials. 2020;13:2496. doi: 10.3390/ma13112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Qahtani A.S., Tulbah H.I., Binhasan M., Abbasi M.S., Ahmed N., Shabib S., Farooq I., Aldahian N., Nisar S.S., Tanveer S.A., et al. Surface Properties of Polymer Resins Fabricated with Subtractive and Additive Manufacturing Techniques. Polymers. 2021;13:77. doi: 10.3390/polym13234077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez M., Medina-Sánchez G., García-Collado A., Gupta M., Carou D. Surface Quality Enhancement of Fused Deposition Modeling (FDM) Printed Samples Based on the Selection of Critical Printing Parameters. Materials. 2018;11:1382. doi: 10.3390/ma11081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bollen C.M., Lambrechts P., Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1997;13:258–269. doi: 10.1016/s0109-5641(97)80038-3. [DOI] [PubMed] [Google Scholar]
- 52.Teughels W., Van Assche N., Sliepen I., Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006;17:68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 53.Anselme K., Davidson P., Popa A.M., Giazzon M., Liley M., Ploux L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010;6:3824–3846. doi: 10.1016/j.actbio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Scheuerman T.R., Camper A.K., Hamilton M.A. Effects of Substratum Topography on Bacterial Adhesion. J. Colloid Interface Sci. 1998;208:23–33. doi: 10.1006/jcis.1998.5717. [DOI] [PubMed] [Google Scholar]
- 55.Engel A.S., Kranz H.T., Schneider M., Tietze J.P., Piwowarcyk A., Kuzius T., Arnold W., Naumova E.A. Biofilm formation on different dental restorative materials in the oral cavity. BMC Oral Health. 2020;20:162. doi: 10.1186/s12903-020-01147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahimou F., Denis F.A., Touhami A., Dufrêne Y.F.J.L. Probing microbial cell surface charges by atomic force microscopy. Langmuir. 2002;18:9937–9941. doi: 10.1021/la026273k. [DOI] [Google Scholar]
- 57.Steiner H., Hultmark D., Engström A., Bennich H., Boman H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292:246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 58.Kozmos M., Virant P., Rojko F., Abram A., Rudolf R., Raspor P., Zore A., Bohinc K. Bacterial Adhesion of Streptococcus mutans to Dental Material Surfaces. Molecues. 2021;26:1152. doi: 10.3390/molecules26041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du Y., Xu J., Sakizadeh J.D., Weiblen D.G., McCormick A.V., Francis L.F. Modulus- and Surface-Energy-Tunable Thiol-ene for UV Micromolding of Coatings. ACS Appl. Mater. Interfaces. 2017;9:24976–24986. doi: 10.1021/acsami.7b06339. [DOI] [PubMed] [Google Scholar]
- 60.Park S.E., Periathamby A.R., Loza J.C. Effect of surface-charged poly(methyl methacrylate) on the adhesion of Candida albicans. J. Prosthodont Off. J. Am. Coll. Prosthodont. 2003;12:249–254. doi: 10.1016/s1059-941x(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 61.Yang N., Zhang D.D., Li X.D., Lu Y.Y., Qiu X.H., Zhang J.S., Kong J. Topography, Wettability, and Electrostatic Charge Consist Major Surface Properties of Intraocular Lenses. Curr. Eye Res. 2017;42:201–210. doi: 10.3109/02713683.2016.1164187. [DOI] [PubMed] [Google Scholar]
- 62.Minagi S., Miyake Y., Inagaki K., Tsuru H., Suginaka H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infect. Immun. 1985;47:11–14. doi: 10.1128/iai.47.1.11-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klotz S.A., Drutz D.J., Zajic J.E. Factors governing adherence of Candida species to plastic surfaces. Infect. Immun. 1985;50:97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collier T.O., Jenney C.R., DeFife K.M., Anderson J.M. Protein adsorption on chemically modified surfaces. Biomed. Sci. Instrum. 1997;33:178–183. [PubMed] [Google Scholar]
- 65.Klotz S.A. The contribution of electrostatic forces to the process of adherence of Candida albicans yeast cells to substrates. FEMS Microbiol. Lett. 1994;120:257–262. doi: 10.1111/j.1574-6968.1994.tb07042.x. [DOI] [PubMed] [Google Scholar]
- 66.Turner R.D., Wingham J.R., Paterson T.E., Shepherd J., Majewski C. Use of silver-based additives for the development of antibacterial functionality in Laser Sintered polyamide 12 parts. Sci. Rep. 2020;10:892. doi: 10.1038/s41598-020-57686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Hengel I.A.J., Putra N.E., Tierolf M., Minneboo M., Fluit A.C., Fratila-Apachitei L.E., Apachitei I., Zadpoor A.A. Biofunctionalization of selective laser melted porous titanium using silver and zinc nanoparticles to prevent infections by antibiotic-resistant bacteria. Acta Biomater. 2020;107:325–337. doi: 10.1016/j.actbio.2020.02.044. [DOI] [PubMed] [Google Scholar]
- 68.Van Hengel I.A.J., Riool M., Fratila-Apachitei L.E., Witte-Bouma J., Farrell E., Zadpoor A.A., Zaat S.A.J., Apachitei I. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials. 2017;140:1–15. doi: 10.1016/j.biomaterials.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 69.El Moussaoui A., Jawhari F.Z., Almehdi A.M., Elmsellem H., Fikri Benbrahim K., Bousta D., Bari A. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens.L. Bioorganic Chem. 2019;93:103337. doi: 10.1016/j.bioorg.2019.103337. [DOI] [PubMed] [Google Scholar]
- 70.Dantas K.N.M., Andrade L.R., Lisboa E., Santana V.L., Santos A.L.S., Mello T.P., Sangenito L.S., Lima Á.S., Fricks A.T., Begnami A.F., et al. Antimycotic nail polish based on humic acid-coated silver nanoparticles for onychomycosis. J. Chem. Technol. Biotechnol. 2021;96:2208–2218. doi: 10.1002/jctb.6676. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.