Abstract
The biological mechanisms underlying emotional distress in HIV infection are likely to be complex but remain understudied. We investigated whether dysbiotic signatures in the gut microbiome of persons living with HIV (PLWH) are associated with their emotional status. We retrospectively examined the gut microbiome and clinical evaluation of 129 adults (94 PLWH and 35 HIV−) enrolled at UC San Diego’s HIV Neurobehavioral Research Program. A subset of participants (32 PLWH vs. 13 HIV−) underwent an emotional assessment using the NIH Toolbox Emotion Battery summarized by three composite scores (negative affect, social satisfaction, and psychological well-being). We then sequenced the 16S rDNA V3-V4 regions from stool and performed taxonomic assignment using CLC Microbial Genomics Module. The gut microbiota profiles were evaluated in relation to participants’ emotional assessment. All analyses were done in R statistical software. We found that the relative abundance of aerotolerant bacteria was significantly higher in PLWH (p < 0.01) and was associated with a lifetime major depression diagnosis independently of HIV status (p = 0.05). Moreover, PLWH experienced significantly worse psychological well-being (p = 0.02), less social satisfaction (p = 0.03), and more negative affect (p = 0.02). Higher levels of aerotolerant bacteria were associated with worse psychological well-being (rho = −0.35, p = 0.02), less social satisfaction (r = − 0.42, p < 0.01), and more negative affect (rho = 0.46, p < 0.01). The association of aerotolerant bacteria with social satisfaction and negative affect was independent of HIV status (p < 0.05, for both). The over-representation of aerotolerant bacteria in the gut may reflect worse oxidative stress and barrier defects and may contribute to emotional distress during HIV infection.
Keywords: Viral infection, Microbiome, Emotions, Aerotolerant bacteria, Emotional distress
Introduction
It is estimated that more than 36 million people globally are living with HIV infection. The development of effective combination antiretroviral therapy (cART) has extended the lifespan of those infected with HIV and effectively shifted HIV infection from an acute to a chronic disease. People living with HIV (PLWH) are now reaching advanced age, which is associated with an increased incidence of comorbidities including cardiovascular disease, metabolic syndrome, liver and kidney disease, “accelerated” aging, neurocognitive impairment (Guihot et al. 2011; Kelley et al. 2009; Marin et al. 2009; Sauce et al. 2011). While cART effectively suppresses plasma viral load and promotes restoration of circulating CD4+ cell counts, the gut immune compartment remains deficient in several regards. CD4+ T cells in the gut are predominantly of a memory phenotype and more prone to activation by cytokines and gut microbial pro-inflammatory antigens such as lipopolysaccharide (LPS) (Catalfamo et al. 2012; Ciccone et al. 2010). Th17 cells play an important role in maintaining immune homeostasis and gut barrier integrity, limiting exposure to microbially encoded antigens that contribute to the observed chronic systemic inflammation (Bixler and Mattapallil 2013). HIV-infected individuals display disturbance of homeostasis in the gut as the result of depletion of gut Th17 cells and CD4+ Treg in circulation, and there is an increase in FOXP3+ Treg in the gut (Kanwar et al. 2010; Nilsson et al. 2006; Shaw et al. 2011). This imbalance has been attributed to altered tryptophan catabolism by indoleamine 2,3-dioxygenase produced by plasmacytoid dendritic cells (DCs) (Favre et al. 2010; Miller and Bhardwaj 2013).
Defects in the gut immune compartment directly impact gut barrier function, a hallmark of HIV infection (Hunt et al. 2014; Jiang et al. 2009). While it remains controversial what specifically causes reduced barrier integrity, the increased levels of circulating LPS represent a significant consequence of its occurrence (Marchetti et al. 2013). LPS derived from γ-Proteobacteria are highly antigenic and pro-inflammatory. Furthermore, LPS has direct negative effects on barrier integrity, thereby reinforcing chronic systemic inflammation. Translocation of LPS is strongly correlated with disease progression, morbidity, and mortality (Kuller et al. 2008; Rodger et al. 2009; Sandler et al. 2011).
The observed chronic immune activation and gut barrier defects in HIV-infected individuals have prompted several studies that evaluated potential dysbiosis of gut microbiota. Virtually, all studies have identified bacterial taxa that are over- and under-represented in cohorts of PLWH; however, these results are often contradictory with respect to the key taxa identified and the direction of change observed. These differences may be explained by small sample sizes, variations in geography and ancestry of the cohorts, and difficulties in controlling for confounding variables such as diet and lifestyle. A key limitation associated with most published studies of gut microbiota in PLWH is the lack of comprehensive clinical data that would allow sub-categorization of PLWH based on age, anti-retroviral treatment, duration of infection, prior severe immunosuppression, and others. Despite these limitations, certain microbiota signatures have been reported by multiple studies, including a reduction in bacterial diversity, Bacteroides spp. and an increased relative abundance of Prevotella spp. (Dillon et al. 2014; Lozupone et al. 2013; Mutlu et al. 2014; Vujkovic-Cvijin et al. 2013; Wommack and Ravel 2013). The increased proportion of Prevotella spp. has been associated with increased activation of CD40 expressing CD1c+ mDC (Dillon et al. 2016). The prevalence of Bacteroides spp. has been associated with the abundance of GALT iNKT cell populations and IL-4 production (Paquin-Proulx et al. 2017).
With the rise in use of cART, the increase in lifespan in PLWH has shed light on potential comorbidities including general and social anxiety and depression (Gaynes et al. 2015; Pence et al. 2006; Tesfaw et al. 2016). HIV-infected homosexual and bisexual men, for example, experienced hospitalization for anxiety and mood disorders 10% more than uninfected homosexual and bisexual men (Moore et al. 2016). They also show a 4% increase in readmission when compared with the average adult in the USA. Neuropsychiatric illnesses can contribute to and/or be worsened by a generally negative quality of life. Feelings of shame have been linked to loneliness, which itself correlates with health-related social aspects, in infected men and women (Vincent et al. 2017). Depression, in particular, has been shown to account for associations between HIV-related shame and emotional well-being. HIV-related stigma is not only related with anxiety, but also increases in anxious arousal (Brandt et al. 2017; Kamen et al. 2015). PLWH also report increased levels of early life stress when compared with HIV-negative adults (Pereira and Canavarro 2012). Such early life stress has been linked to lower amygdala response which in turn has been correlated to increases in neuropsychiatric symptoms such as anxiety, alexithymia, and depression.
An increasing number of studies have linked the gut microbiota with emotion and mood disorders (Jenkins et al. 2016; Petra et al. 2015; Sylvia and Demas 2018). However, there are no studies to date that focus on this association considering HIV status. We therefore examined whether dysbiotic signatures associated with HIV+ infection was correlated with different emotional states in a well-characterized cohort.
Methods
Study participants and sample collection
We retrospectively examined the gut microbiome of 129 adults (94 PLWH and 35 HIV−) with prospectively collected clinical samples and evaluations in the HIV Neurobehavioral Research Program cohorts at the University of California, San Diego (UCSD). Stool, sociodemographic, neuromedical, and clinical variables were collected for each participant. Lifetime major depression disorder (LT MDD) and history of substance use were assessed using structured interviews (First et al. 2002; Hasin et al. 1996; Ustun et al. 1997) that follows the Diagnostic and Statistical Manual-IV edition criteria (American Psychiatry Association, 1994). Substance use disorder was defined as meeting the criteria for either abuse or dependence for any of the following substances: alcohol, cannabis, opioids, methamphetamine, cocaine, sedatives, and hallucinogens. In addition, a subset of participants (32 PLWH and 13 HIV−) underwent emotional assessment summarized in three composite scores assessed by the NIH Toolbox Emotion Battery (NIHTB-EB) (Babakhanyan et al. 2018). The study was approved by the UCSD Institutional Review Board and all participants provided informed consent. A summary of the study design is shown in Fig. 1.
Fig. 1.
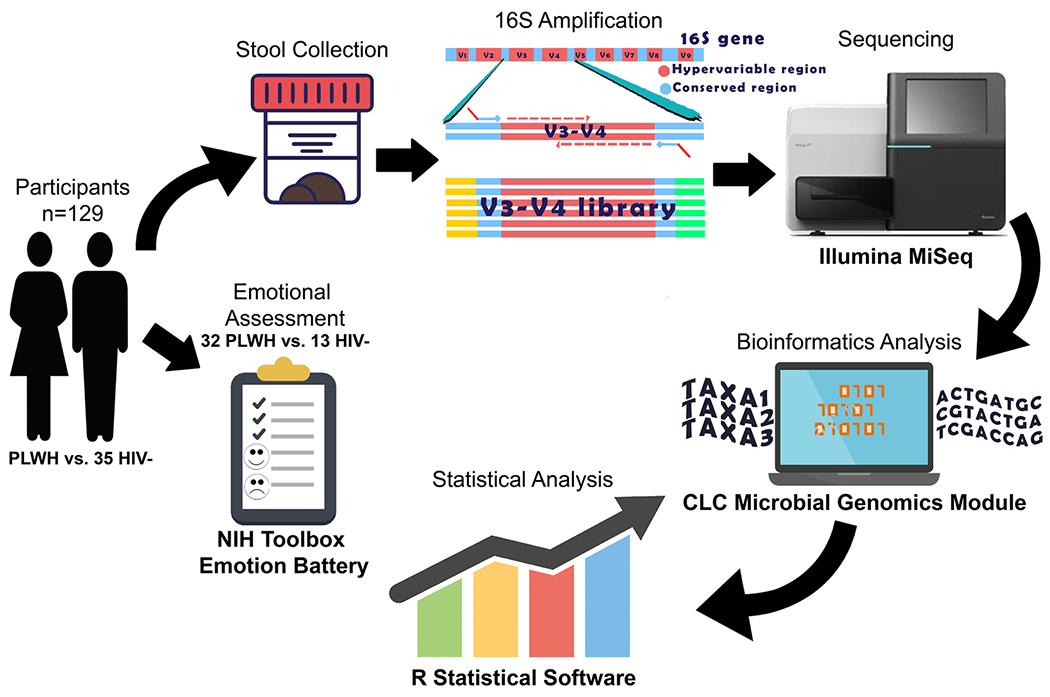
Summary of study methods. Stool and clinical evaluation were collected from 129 adults (94 PLWH and 35 HIV−). A subset of participants (32 PLWH vs. 13 HIV−) underwent emotional assessment using the NIH toolbox emotion battery. The hypervariable region V3-V4 was amplified from stool DNA and sequenced using the Illumina MiSeq. Bioinformatics and statistical analyses were performed using the CLC Microbial Genomics Module and R statistical Software, respectively
Microbial DNA isolation
Genomic DNA was isolated from 200 mg of stool using the QIAamp DNA stool kit (Qiagen) per manufacturer protocol with the inclusion of an additional step of bead beating for 5 min using the Thermo FastPrep instrument (MP Bio) to ensure uniform lysis of bacterial cells.
16S rDNA sequence analysis.
Multiplexed 16S rDNA libraries were prepared using standard 16S metagenomic sequencing library protocols from Illumina, using V3-V4 region of 16S rDNA for target amplification. We performed paired end reads (250 bp each end) sequencing using the Illumina MiSeq platform. Subsequent analysis was done in CLC Microbial Genomics Module 2.5 (Qiagen) and R. Paired end reads were merged (mismatch cost: 2, minimum score: 8, gap cost: 3, maximum unaligned end mismatches: 0) and trimmed to the same length. Additional quality filter steps were applied to exclude short reads, sequences with poor quality scores, and chimeras. We enumerated taxa using each unique 16S rDNA sequence (differing by 1 or more base pair) by BLAST using the NCBI 16S rRNA database (Bacteria and Archaea) to identify best matches to taxa at the genus and species levels based on percent identity. Alpha and beta diversity measures were calculated using Qiime 2(Caporaso et al. 2010) and used to perform principal coordinates analysis (PCoA) on unweighted and weighted UniFrac distances (Lozupone and Knight 2005) and for the pairwise PERMANOVA tests.
Emotion battery.
The NIH Toolbox Emotion Battery (NIHTB-EB) was used to measure emotional states. Its 17 individual scales yield three composites: (1) negative affect, (2) social satisfaction, and (3) psychological well-being (Babakhanyan et al. 2018). The negative affect composites include aspects of anger, fear/anxiety, sadness/depression, and perceived stress. Social satisfaction includes emotional and instrumental support from others, quality of friendships, loneliness, perceived hostility, and perceived rejection. Psychological well-being encompasses life satisfaction, meaning and purpose in life, and positive affect. Raw scores for each composite and individual scales were converted to census-weighted standard scores (T-scores) based on published data (Babakhanyan et al. 2018). The T-scores represent a participant’s emotional health compared with the average English-speaking adult in the USA. The three composites and individual scales were treated as separate continuous outcome variables for the purposes of analyses.
Statistical analyses.
All statistical analyses were performed using R statistical software. Association of categorical variables was assessed using a chi-squared test. A Mann-Whitney or t test was used to assess statistical difference in emotional states or taxonomic relative abundance between study groups (PLWH vs. HIV−) according to normality of variables evaluated by a Shapiro test with a significance of p < 0.05. Differences in beta-diversity were assessed with a PERMANOVA test. We performed univariate fixed effects linear models to determine the relationship between each taxon with emotional and clinical variables. Multivariate fixed effect linear or logistic regression models using HIV status as a covariate were used to determine independent associations between emotional states and the relative abundance of bacterial taxa while adjusting for HIV status and other covariates.
Results
Participant’s characteristics
A summary of the socio-demographical and clinical variables is provided (Table 1). Study participants were 129 adults, 52% of whom were white, 83% male, and the median age and education were 52 and 14 years, respectively. HIV+ subjects (n = 94) had a median estimated duration of infection (EDI) of 20.2 years, with 95% of the participants receiving antiretroviral therapy (ART) and 90% with undetectable levels of HIV RNA in plasma. PLWH were more likely to be men than HIV− participants (p < 0.01), while there were no differences with respect to ethnicity/race, age, and level of education between PLWH and HIV− subjects. Fifty-two percent of all participants reported lifetime major depression disorder, and LT MDD was associated with HIV infection (p < 0.01).
Table 1.
Participants’ socio-demographical and clinical characteristics
| Variables | All participants (n = 129) | HIV+ (n = 94) | HIV− (n = 35) | p valuea |
|---|---|---|---|---|
| Gender (M/F) | 107:22 | 84:10 | 23:12 | < 0.01 |
| Race (W/NW) | 65:64 | 48:46 | 17:18 | 0.06 |
| Age | 54 (48–62) | 53.5 (49, 61) | 55 (46.5, 67.5) | 0.45 |
| Education | 14 (12–16) | 14 (12, 16) | 14 (12, 16) | 0.90 |
| LT MDD (Y/N)b | 66:61 | 56:36 | 10:25 | < 0.01 |
| LT SUD (Y/N)b | 89:38 | 73:19 | 16:19 | < 0.01 |
| EDI (years) | - | 20.2 (12.7, 26.4) | - | - |
| Nadir CD4 (cells/μL) | - | 123 (18.8, 324.3) | - | - |
| CD4 absolute (cells/μL) | - | 612 (430, 792) | - | - |
| CD4 percent (%) | - | 25.8 (32.1, 40.3) | - | - |
| CD8 absolute (cells/μL) | - | 797 (545.5, 1102) | - | - |
| CD8 percent (%) | - | 41.7 (34.5, 53.4) | - | - |
| CD4 CD8 ratio | - | 0.76 (0.49, 1.18) | - | - |
| % on ART | - | 95% | - | - |
| % RNA levels undetectable | - | 90% | - | - |
Characteristics of all of our participants
M male, F female, W White, NW not White, LT MDD lifetime major depression disorder diagnosis, LT SUD lifetime substance use disorder diagnosis, EDI estimated duration of infection
The p value of a double tailed chi-squared or Mann-Whitney test. There was a significant association between biological sex, LT MDD, LT SUD, and HIV status in relation to HIV serostatus
Missing data of 2 participants in the LT MDD and LT SUD variables that did not underwent structured interviews to asses LT MDD and LT SUD
Microbiome signatures by HIV status
The profiling of fecal microbiota by 16S rDNA sequencing of 94 PLWH and 35 HIV− subjects allowed the identification of multiple dysbiotic signatures distinguishing HIV+ microbiota. PLWH had significantly increased beta diversity in the fecal microbiota measured by weighted and unweighted UniFrac (p = 0.02 and p < 0.01, respectively) than HIV− microbiota. The patterns that emerged defined statistically significant differences in functionally coherent and taxonomically related species. We discerned four significant alterations in HIV+ microbiota. First, we identified a number of aerotolerant taxa, many but not all of which are normal residents of oral microbiota but not typically observed in “healthy” gut communities (Supplementary Table 1). The relative abundance of these taxa individually and collectively is over-represented in HIV+ microbiota (p < 0.01, Fig. 2a). Additionally, several Bifidobacterium spp. were under-represented, as was the aggregate relative abundance of all members of this genus in HIV+ subjects (p = 0.03, Supplementary Fig. 1a). More striking was the under-representation of all Bacteroides spp. profiled (p < 0.01, Supplementary Fig. 1b). The median relative abundance of Bacteroides was 28% in HIV− and 5% in HIV+ microbiota. Finally, another striking signature was evident in all Prevotella spp. profiled. These taxa are uniformly increased in relative abundance in HIV+ microbiota. Prevotella median abundance was near detection limits in HIV− microbiota but is increased to over 20% in HIV+ microbiota (p = 0.015, Supplementary Fig. 1c). A dominant driver of this difference was the differential abundance of P. copri, a gut microbiota resident that was over-represented in HIV+ subjects.
Fig. 2.
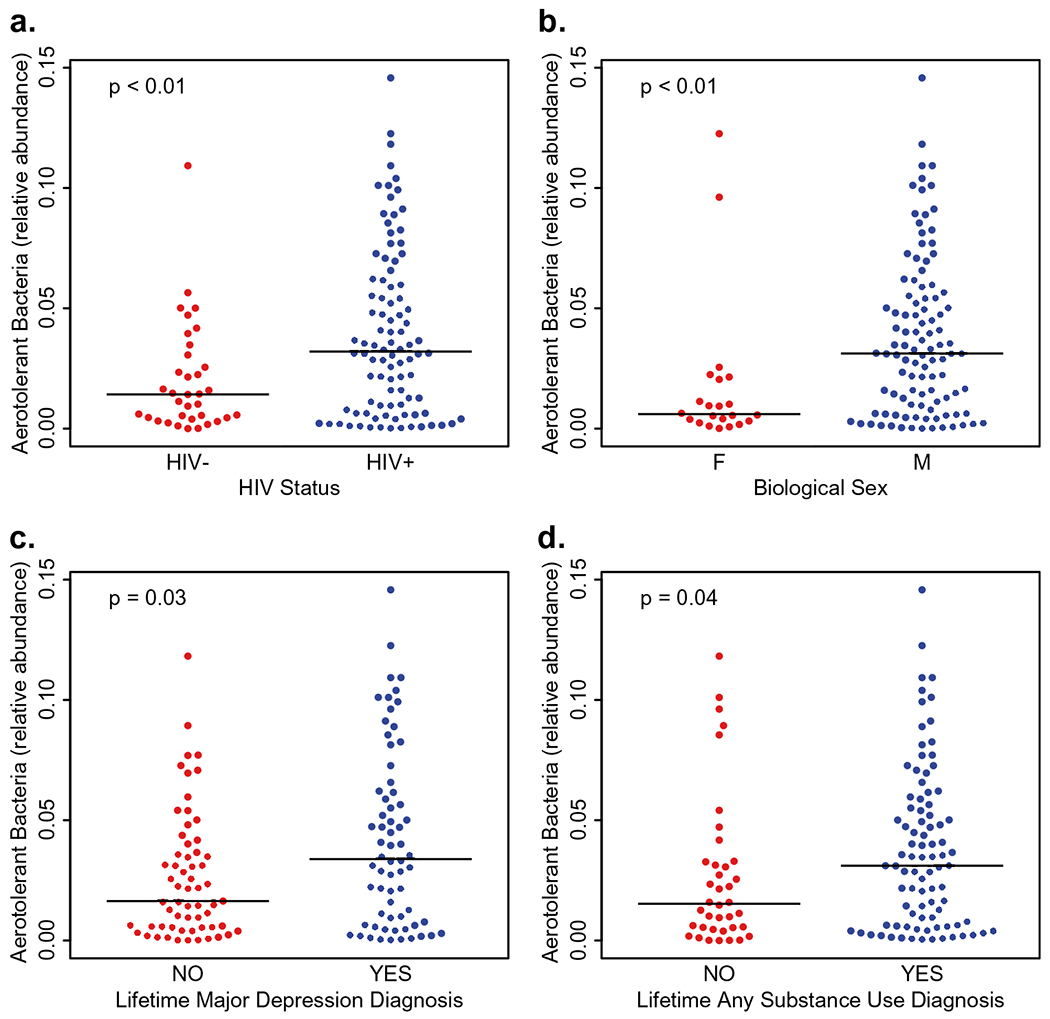
Levels of aerotolerant bacteria in the gut were significantly higher in a PLWH, b males, c subjects with a lifetime diagnosis of major depressive disorder [LT-MDD], and d subjects with a lifetime diagnosis substance use disorder [LT-SUD]
We further explored these signatures and noted that among all participants, males displayed a significantly higher relative abundance of aerotolerant bacteria and Prevotella when compared with females (p < 0.01 for both, Fig. 2b and Supplementary Fig. 2a). Additionally, males had significantly lower levels of the Bacteroides than females (p < 0.01) while there was no statistical difference in Bifidobacterium based on biological sex (Supplementary Fig. 2b, c). These associations remained significant after adjusting for HIV status with the exception of the genus Bifidobacterium, for which HIV status was the main effect of the association, and the genus Prevotella, for which biological sex was the driver of the association (Supplementary Table 2). (See Table 3)
Table 3.
Multivariate association of emotional states with levels of oral bacteria adjusting for HIV status
| Term | Negative affect |
Psychological well-being |
Social satisfaction |
|||
|---|---|---|---|---|---|---|
| Coefficient estimate (Std. error) | p value | Coefficient estimate (Std. error) | p value | Coefficient estimate (Std. error) | p value | |
| Aerotolerant Bacteria | 117.8 (± 47.2) | 0.02 | − 83.1 (± 49.8) | 0.10 | − 111.6 (± 51.2) | 0.03 |
| HIV Status | 6.4 (± 3.3) | 0.06 | 6.7 (± 3.5) | 0.06 | − 4.6 (± 3.6) | 0.21 |
Multivariate association of emotional states with levels of oral bacteria adjusting for HIV status. Negative affect and social satisfaction remained significant after adjusting for HIV status
Microbiome signatures and lifetime major depression disorder
We also evaluated the levels of these bacterial signatures among participants with and without a diagnosis of LT MDD in the whole study cohort. The levels of aerotolerant bacteria were significantly higher in subjects with LT MDD (p = 0.03, Fig. 2c) and there was a trend for lower levels of Bacteroides and Bifidobacterium (p = 0.06 for both, Supplementary Fig. 3a, b). While we also observed an increase in Prevotella in subjects with lifetime MDD, it did not reach significant levels (Supplementary Fig. 3c). A logistic regression model examining the association of these bacterial signatures on LT MDD adjusting for HIV status showed a borderline significant association between aerotolerant bacterial and LT MDD (p = 0.05, Supplementary Table 3).
Microbiome signatures and lifetime substance use disorder
Since we observed there was an association between HIV status and LT MDD, we further evaluated the levels of bacterial signatures associated with HIV status in participants with and without a LT SUD diagnosis in all subjects. The relative abundance of aerotolerant bacteria in participants with a LT SUD diagnosis was significantly higher (p = 0.04, Fig. 2d) than those without LT SUD. Additionally, the Prevotella and Bacteroides genera was significantly higher in participants with a LT SUD diagnosis (p < 0.01 for both, Supplementary Fig. 4a, b) while there was no statistical difference in the levels of Bifidobacterium genus (Supplementary Fig. 4c). In a multivariate logistic regression model investigating the association of LT SUD and the levels of the bacterial signatures while adjusting for HIV status, the Prevotella and Bacteroides genera remained associated with LT SUD (p = 0.02 and p < 0.01, respectively), while aerotolerant bacteria did not (Supplementary Table 3).
NIH toolbox emotion battery
A subset of the study participants composed of 32 PLWH and 13 HIV− adults underwent emotional assessment using the NIHTB-EB. All clinical and socio-demographical variables of this subset of participants are summarized in Table 2. PLWH had an average EDI of 20.7 years, 87% were on ART with 85% of these having undetectable levels of HIV RNA in plasma. PLWH reported significantly more negative affect (p = 0.02), less social satisfaction (p = 0.03), and less psychological well-being (p = 0.02) when compared with HIV− subjects (Fig. 3a–c). When investigating the differences on the individual emotion domains comprising these composites by HIV status, PLWH reported increased loneliness (p = 0.01) and less friendship (p < 0.01) within the domain of social satisfaction; increased sadness (p = 0.02), perceived stress (p = 0.03), and fear affect (p = 0.06) within the domain of negative affect; and reduced general life satisfaction (p = 0.01) and meaning and purpose (p = 0.02) within the domain of psychological well-being (Supplementary Table 4).
Table 2.
Clinical and socio-demographical characteristics in subset of participants with emotional assessment
| Variables | HIV+ (n = 32) | HIV− (n = 13) | p value* |
|---|---|---|---|
| Gender (M/F) | 28:4 | 8:5 | 0.05 |
| Race (W/NW) | 16:16 | 7:6 | 1 |
| Age | 51 (47.5, 56.5) | 54 (46, 58) | 0.76 |
| Education | 13 (12, 16) | 14 (13, 16) | 0.17 |
| EDI (years) | 20.7 (11.8, 26.1) | - | - |
| Nadir CD4 (cells/μL) | 93.5 (20.3, 240) | - | - |
| CD4 absolute (cells/μL) | 586.5 (416.8, 798) | - | - |
| CD4 percent (%) | 31.5 (25.9, 43.2) | - | - |
| CD8 absolute (cells/μL) | 786 (586.5, 1039.3) | - | - |
| CD8 percent (%) | 41.6 (38.7, 56.3) | - | - |
| CD4 CD8 ratio | 0.7 (0.5, 1.2) | - | - |
| % on ART | 87% | - | - |
| % RNA levels undetectable | 85% | - | - |
Clinical and socio-demographical characteristics in subset of participants with emotional assessment
M male, F female, W White, NW not White, EDI estimated duration of infection
The p value of a double tailed chi-squared or Mann-Whitney test
Fig. 3.
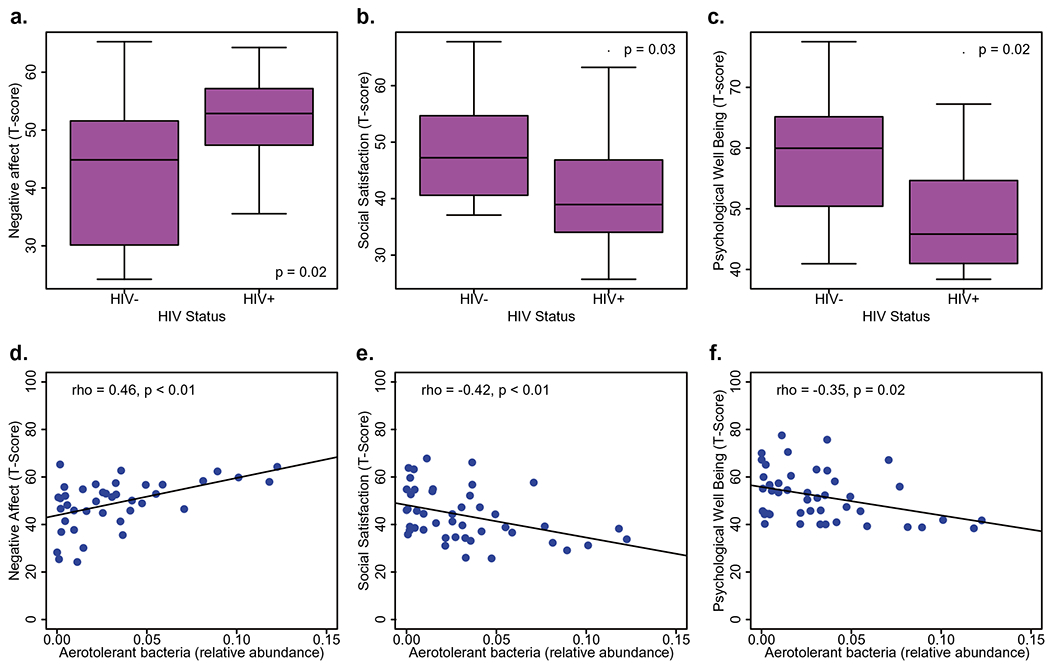
PLWH reported significantly more negative affect a, less social satisfaction b, and less psychological well-being c when compared with HIV− subjects. Higher levels of aerotolerant bacteria were associated with more negative affect d, less social satisfaction e, and less psychological well-being f
Microbiome associated with emotional disturbance
The overall coherence of the HIV dysbiotic signatures suggests the possibility of relating the functional potential of each bacterial group to clinical parameters of interest. We hypothesized that the dysbiotic signatures observed in HIV+ microbiota will be linked to variables pertaining to worse emotional status. To test this hypothesis, we analyzed relationships between the relative abundance of each signature and variables of emotion battery testing. Higher levels of aerotolerant bacteria were correlated with worse psychological well-being (rho = − 0.35, p = 0.02). Less abundant Bacteroides was associated with worse negative affect (rho = − 0.36, p = 0.03). By contrast, higher levels of aerotolerant bacteria and Prevotella were associated with increased negative affect (rho = 0.46, p < 0.01; rho = 0.35, p = 0.03, respectively). The relative abundance of Bifidobacterium trended to positive correlation with social satisfaction (rho = 0.28; p = 0.06), whereas both aerotolerant species and Prevotella were negatively correlated (rho = − 0.42; p < 0.01; rho = − 0.34; p = 0.02, respectively). These results are shown in Fig. 3d–f and Supplementary Table 5.
We examined individual domains within the Emotion Toolbox that reinforced the relevance of the dysbiotic signatures observed by HIV status. We noted higher levels of aerotolerant bacteria (rho = 0.31, p = 0.04) and lower levels of Bacteroides were associated with more fear affect (rho = − 0.30, p < 0.05) More abundant Bacteroides was positively correlated with friendship (rho = 0.37, p = 0.01), whereas higher levels of Lactobacillus, Prevotella, and aerotolerant species were negatively correlated (rho = − 0.34, p = 0.02; rho = − 0.47, p < 0.01; rho = − 0.38, p = 0.01, respectively). Higher levels of Prevotella were significantly correlated with more perceived hostility (rho = 0.30, p = 0.05). Less abundant Bacteroides and Bifidobacterium were correlated with more feelings of loneliness (rho = − 0.34, p = 0.02 for both), whereas more abundant Lactobacillus, Proteobacteria, aerotolerant bacteria, and Prevotella were correlated with more loneliness (rho = 0.31, p = 0.04; rho = 0.33, p = 0.03; rho = 0.50, p < 0.01; rho = 0.43, p < 0.01, respectively). Higher levels of Bacteroides were associated with more meaning and purpose (rho = 0.37, p = 0.01), whereas lower levels Prevotella, Lactobacillus, and aerotolerant species were also associated with more meaning and purpose (rho = − 0.37, p = 0.01; rho = − 0.30, p = 0.04; rho = − 0.30, p = 0.04). Similarly, Bacteroides were negatively correlated with sadness, i.e., depressed mood (rho = − 0.34, p = 0.02), whereas Prevotella and aerotolerant bacteria were positively correlated (rho = 0.39, p < 0.01; rho = 0.35, p = 0.02, respectively). The relative abundance of aerotolerant bacteria was negatively correlated with positive affect (rho = − 0.31, p = 0.04). Finally, aerotolerant bacteria were associated with perceived stress (rho = 0.42, p < 0.01). All results are summarized in Fig. 4 and Supplementary Table 5.
Fig. 4.
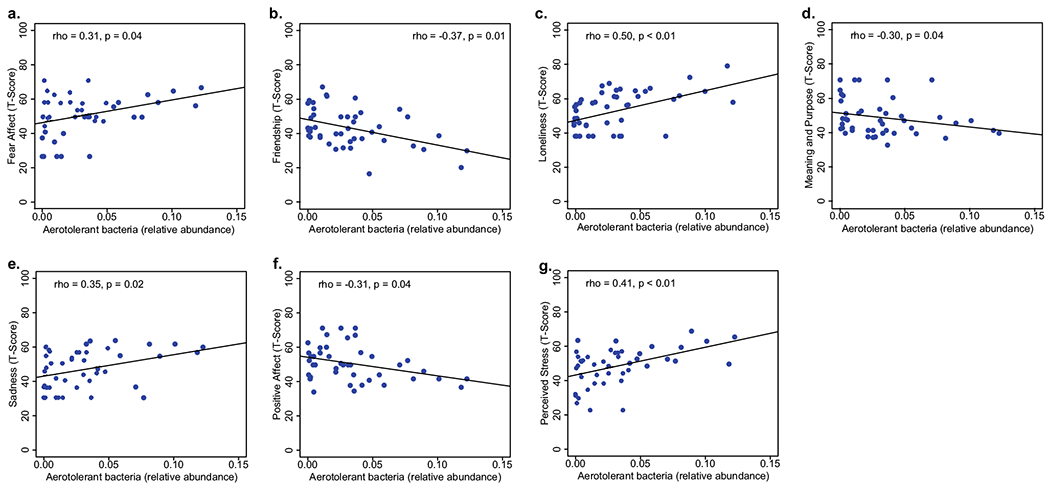
a Higher levels of aerotolerant bacteria were associated with more fear affect, b less friendship, c more loneliness, d less meaning and purpose, e more sadness, f less positive affect, and g more perceived stress
Since both HIV status and microbiome signatures showed associations with emotional states, we constructed a multivariate fixed-effect regression model to determine the independent effects of HIV and the microbiome, and any interaction effect associated with emotional states. The aerotolerant bacteria remained significantly associated with negative affect (p = 0.02) and social satisfaction (p = 0.03) after adjusting for HIV status (p = 0.06, p = 0.21, respectively, Table 3), while only HIV status trended for an association with psychological well-being. None of the emotional states showed any significant interaction between HIV status and the microbiome.
Discussion
Interest in the gut microbiota as a factor in PLWH disease progression and co-morbidities has increased in recent years. A number of studies have reported that HIV+ microbiota is dysbiotic in multiple taxa. Our results agree with several previous reports but also highlight novel signatures of HIV microbiota. As detailed in other studies (Dillon et al. 2016; Kaur et al. 2018), we noted an increased relative abundance of Prevotella spp., driven primarily by increased P. copri, the most dominant natural resident of the gut microbiota. One report to date has noted the increased relative abundance of aerotolerant microbes in PLWH (Dubourg et al. 2016; Gootenberg et al. 2017), in agreement with our analyses. We were unable to identify statistically significant differences in Faecalibacterium prausnitzii or other butyrate producers in this cohort. In summary, our efforts highlight a coherent set of HIV+ microbiota signatures. The over-representation of aerotolerant microbiota in PLWH is likely to reflect persistent oxidative stress in the gut environment of some HIV-infected individuals. Increased oxidative stress is expected to increase local concentrations of oxygen in the gut, providing a positive selective force for aerotolerant species. Our finding that the over-representation of aerotolerant species is potentially gender specific suggests that additional or alternative factors may contribute to the increased relative abundance of these taxa in HIV+ fecal microbiota. However, a key limitation while performing sex-based analysis and comparisons is the small sample size. Results from the NIH Toolbox Emotion Battery where not examined on the basis of sex in view of the fact that the male subset cohort is significantly bigger than the female subset size and this may preclude any conclusion from the NIH Toolbox Emotion Battery regarding sex.
Findings from this study support the conclusion that dysbiotic signatures in HIV+ microbiota are not neutral to host CNS function. The results repeatedly identify the increased relative abundance of Prevotella and aerotolerant species in association with variables of emotional and social experiences. Conversely, the reduced relative abundance of Bacteroides spp., in HIV+ microbiota, was associated with worse emotions. While not definitive evidence of causality, the coherent relationships of these taxa on host phenotype suggest that their functional impact may be significant and worthy of follow-up study to verify. It was somewhat surprising that the relative abundance of Bifidobacterium and γ-Proteobacteria were not as uniformly associated with the variables assessed here, given the positive relationships in the literature concerning Bifidobacterium and the pro-inflammatory potential of LPS derived from Proteobacteria that is well recognized source of neuroinflammation (Qin et al. 2007; Zhao et al. 2019).
A number of studies have evaluated the relationship between gut microbiota composition and various psychiatric disorders, including depression (Maes et al. 2008, 2012, 2013). Indeed, transplantation of gut microbiota from patients with MDD confers depression-like behaviors to recipient mice (Kelly et al. 2016; Zheng et al. 2016). In a meta-analysis of studies investigating the relationship between gut microbiota and MDD, the relative abundance of Bifidobacterium was reduced compared with control subjects, consistent with our findings in PLWH, whereas the relative abundance of Bacteroides and Prevotella were mixed across studies (Cheung et al. 2019). This variability may relate to differences in inclusion/exclusion criteria employed or methods used for microbiota analysis.
We sought to understand whether the increased prevalence of aerotolerant microbes is a passive reflection of host gut dysfunction or may negatively impact host physiology and CNS function. Our results suggest that the increased prevalence of aerotolerant microbes, including most Prevotella spp. observed (except P. copri, a normal gut resident), is associated with negative consequences on emotional and social function as assessed by the NIH Toolbox Emotion Battery. Aerotolerant microbes have been previously documented in a number of disease contexts including necrotizing enterocolitis (Mai et al. 2011), irritable bowel syndrome (Carroll et al. 2012), inflammatory bowel disease (Frank et al. 2007), and HIV (Dubourg et al. 2016). Given that increased oxidative stress contributes to impaired CNS function on neurodegenerative diseases (Dumitrescu et al. 2018), it remains unclear to what extent the presence of oral and/or aerotolerant microbiota in the gut of PLWH is limited to a consequence of oxidative stress or a contributing factor to HIV-associated emotional deficits.
Limitations of this study include a relatively small sample size and the cross-sectional design. In addition, there is observed variability (outliers) in taxa in both PLWH and uninfected controls, possibly reflecting other factors (lifestyle, diet, genetics, and environmental factors) that could be influencing the abundance of these taxa. Since the focus of this study was restricted to the differences in the median values between our groups, we used non-parametric comparative statistical tests to avoid any bias in our analysis. It is noteworthy that while some participants had tendencies of sharing higher or lower values in different emotional variables, the extreme case values were not always the same participants. Future research should evaluate the effects of interventions such as probiotics on gut microbial flora and mood. Metabolomics may help clarify the molecular mechanisms by which microbial abundance and diversity influence emotional state.
Supplementary Material
Acknowledgments
Special thanks to Carlos Solá-Morlá, Jamie L. Ortíz-Santiago, Jeannette L. Salgado, and André Beauchamp-Saalman.
Funding
The project described was supported by the NIH, National Institute of Mental Health R25 MH108389; K23 MH105297; R01 MH107345; U01 MH83506; P30 MH62512; K24 MH097673, and National Institute of Drug Abuse P50 DA26306. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This publication was also supported by the Comprehensive Cancer Center of the UPR (a public corporation of the Government of Puerto Rico created in virtue of Law 230 of August 26, 2004 as amended). The content is entirely the responsibility of the authors and does not necessarily represent the official views of the Comprehensive Cancer Center UPR.
Footnotes
Supplementary information The online version contains supplementary material available at https://doi.org/10.1007/s13365-020-00933-1.
References
- American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC [Google Scholar]
- Babakhanyan I, McKenna BS, Casaletto KB, Nowinski CJ, Heaton RK (2018) National Institutes of Health Toolbox Emotion Battery for English- and Spanish-speaking adults: normative data and factor-based summary scores. Patient Relat Outcome Meas 9:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixler SL, Mattapallil JJ (2013) Loss and dysregulation of Th17 cells during HIV infection. Clin Dev Immunol 2013:852418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt CP, Paulus DJ, Jardin C, Heggeness L, Lemaire C, Zvolensky MJ (2017) Examining anxiety sensitivity as an explanatory construct underlying HIV-related stigma: relations to anxious arousal, social anxiety, and HIV symptoms among persons living with HIV. J Anxiety Disord 48:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y (2012) Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 24(6):521–530, e248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalfamo M, Le Saout C, Lane HC (2012) The role of cytokines in the pathogenesis and treatment of HIV infection. Cytokine Growth Factor Rev 23(4–5):207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, Sublette ME (2019) Systematic review of gut microbiota and major depression. Front Psychiatry 10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone EJ, Read SW, Mannon PJ, Yao MD, Hodge JN, Dewar R, Chairez CL, Proschan MA, Kovacs JA, Sereti I (2010) Cycling of gut mucosal CD4+ T cells decreases after prolonged antiretroviral therapy and is associated with plasma LPS levels. Mucosal Immunol 3(2):172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, Landay AL, Robertson CE, Frank DN, Wilson CC (2014) An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol 7(4):983–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SM, Lee EJ, Kotter CV, Austin GL, Gianella S, Siewe B, Smith DM, Landay AL, McManus MC, Robertson CE, Frank DN, McCarter MD, Wilson CC (2016) Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. Mucosal Immunol 9(1):24–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourg G, Lagier JC, Hue S, Surenaud M, Bachar D, Robert C, Michelle C, Ravaux I, Mokhtari S, Million M, Stein A, Brouqui P, Levy Y, Raoult D (2016) Gut microbiota associated with HIV infection is significantly enriched in bacteria tolerant to oxygen. BMJ Open Gastroenterol 3(1):e000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu L, Popescu-Olaru I, Cozma L, Tulba D, Hinescu ME, Ceafalan LC, Gherghiceanu M, Popescu BO (2018) Oxidative stress and the microbiota-gut-brain axis. Oxid Med Cell Longev 2018:2406594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, Huang Y, Douek DC, Brenchley JM, Martin JN, Hecht FM, Deeks SG, McCune JM (2010) Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med 2(32):32ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, BW WJ (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. (SCID-I/P). New York State Psychiatric; New York, NY USA [Google Scholar]
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 104(34):13780–13785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, O’Donnell J, Nelson E, Heine A, Zinski A, Edwards M, McGuinness T, Riddhi MA, Montgomery C, Pence BW (2015) Psychiatric comorbidity in depressed HIV-infected individuals: common and clinically consequential. Gen Hosp Psychiatry 37(4):277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootenberg DB, Paer JM, Luevano JM, Kwon DS (2017) HIV-associated changes in the enteric microbial community: potential role in loss of homeostasis and development of systemic inflammation. Curr Opin Infect Dis 30(1):31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guihot A, Bourgarit A, Carcelain G, Autran B (2011) Immune reconstitution after a decade of combined antiretroviral therapies for human immunodeficiency virus. Trends Immunol 32(3):131–137 [DOI] [PubMed] [Google Scholar]
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J (1996) Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry 153(9):1195–1201 [DOI] [PubMed] [Google Scholar]
- Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, Deeks SG, Meinert CL, Van Natta ML, Jabs DA, Lederman MM (2014) Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 210(8):1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP (2016) Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 8 (1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI, Deeks SG, Douek DC, Brenchley JM (2009) Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 199(8):1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen C, Arganbright J, Kienitz E, Weller M, Khaylis A, Shenkman T, Smith S, Koopman C, Gore-Felton C (2015) HIV-related stigma: implications for symptoms of anxiety and depression among Malawian women. Afr J AIDS Res 14(1):67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar B, Favre D, McCune JM (2010) Th17 and regulatory T cells: implications for AIDS pathogenesis. Curr Opin HIV AIDS 5(2):151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur US, Shet A, Rajnala N, Gopalan BP, Moar PDH, Singh BP, Chaturvedi R, Tandon R (2018) High Abundance of genus Prevotella in the gut of perinatally HIV-infected children is associated with IP-10 levels despite therapy. Sci Rep 8(1):17679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, Crane HM, Willig J, Mugavero M, Saag M, Martin JN, Deeks SG (2009) Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 48(6):787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, C OB, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG (2016) Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82:109–118 [DOI] [PubMed] [Google Scholar]
- Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD, Group ISS (2008) Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71(12):8228–8235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE (2013) Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe 14(3):329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC (2008) The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 29(1):117–124 [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, Berk M (2012) Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord 141(1):55–62 [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, Berk M, Geffard M, Bosmans E (2013) In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr Scand 127(5):344–354 [DOI] [PubMed] [Google Scholar]
- Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J (2011) Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE 6(6):e20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti G, Tincati C, Silvestri G (2013) Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev 26(1):2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin B, Thiebaut R, Bucher HC, Rondeau V, Costagliola D, Dorrucci M, Hamouda O, Prins M, Walker S, Porter K, Sabin C, Chene G (2009) Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS 23(13):1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Bhardwaj N (2013) Dendritic cell dysregulation during HIV-1 infection. Immunol Rev 254(1):170–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CL, Grulich AE, Prestage G, Gidding HF, Jin F, Petoumenos K, Zablotska IB, Poynten IM, Mao L, Law MG, Amin J (2016) Hospitalization for anxiety and mood disorders in HIV-infected and -uninfected gay and bisexual men. J Acquir Immune Defic Syndr 73(5):589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, French A, Demarais P, Sun Y, Koenig L, Cox S, Engen P, Chakradeo P, Abbasi R, Gorenz A, Burns C, Landay A (2014) A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog 10(2):e1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, Shearer GM, Andersson J, Chougnet C (2006) HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 108(12):3808–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin-Proulx D, Ching C, Vujkovic-Cvijin I, Fadrosh D, Loh L, Huang Y, Somsouk M, Lynch SV, Hunt PW, Nixon DF, SenGupta D (2017) Bacteroides are associated with GALT iNKT cell function and reduction of microbial translocation in HIV-1 infection. Mucosal Immunol 10(1):69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Miller WC, Whetten K, Eron JJ, Gaynes BN (2006) Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the Southeastern United States. J Acquir Immune Defic Syndr 42(3):298–306 [DOI] [PubMed] [Google Scholar]
- Pereira M, Canavarro MC (2012) Quality of life and emotional distress among HIV-positive women during transition to motherhood. Span J Psychol 15(3):1303–1314 [DOI] [PubMed] [Google Scholar]
- Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC (2015) Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther 37(5):984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55(5):453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D, Skoutelis A, Goetz MB, Phillips AN, Group, I.S.f.M.o.A.T.S (2009) Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis 200(6):973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC, Group I.S.S (2011) Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 203(6):780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauce D, Larsen M, Fastenackels S, Pauchard M, Ait-Mohand H, Schneider L, Guihot A, Boufassa F, Zaunders J, Iguertsira M, Bailey M, Gorochov G, Duvivier C, Carcelain G, Kelleher AD, Simon A, Meyer L, Costagliola D, Deeks SG, Lambotte O, Autran B, Hunt PW, Katlama C, Appay V (2011) HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood 117(19):5142–5151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JM, Hunt PW, Critchfield JW, McConnell DH, Garcia JC, Pollard RB, Somsouk M, Deeks SG, Shacklett BL (2011) Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers. J Virol 85(21):11422–11434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia KE, Demas GE (2018) A gut feeling: microbiome-brain-immune interactions modulate social and affective behaviors. Horm Behav 99:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaw G, Ayano G, Awoke T, Assefa D, Birhanu Z, Miheretie G, Abebe G (2016) Prevalence and correlates of depression and anxiety among patients with HIV on-follow up at Alert Hospital, Addis Ababa. Ethiopia BMC Psychiatry 16(1):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustun B, Compton W, Mager D, Babor T, Baiyewu O, Chatterji S, Cottler L, Gogus A, Mavreas V, Peters L, Pull C, Saunders J, Smeets R, Stipec MR, Vrasti R, Hasin D, Room R, Van den Brink W, Regier D, Blaine J, Grant BF, Sartorius N (1997) WHO Study on the reliability and validity of the alcohol and drug use disorder instruments: overview of methods and results. Drug Alcohol Depend 47(3):161–169 [DOI] [PubMed] [Google Scholar]
- Vincent W, Fang X, Calabrese SK, Heckman TG, Sikkema KJ, Hansen NB (2017) HIV-related shame and health-related quality of life among older. HIV-positive adults J Behav Med 40(3):434–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, Hernandez RD, Lederman MM, Huang Y, Somsouk M, Deeks SG, Hunt PW, Lynch SV, McCune JM (2013) Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 5(193):193ra191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wommack KE, Ravel J (2013) Microbiome, demystifying the role of microbial communities in the biosphere. Microbiome 1(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, Lu D, Wei W, Wang Y, Li H, Fu Y, Zhu L (2019) Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep 9(1):5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P (2016) Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21(6):786–796 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


