Abstract
Background:
Considering the effect of beclomethasone on allergic rhinitis or nasal polyps, it has been attempted to find the best method of using this drug to have the maximum effect and increase the patients' satisfaction. Therefore, the aim of this study was to compare the efficacy of beclomethasone aerosol and aqueous nasal sprays in the patients with nasal polyps.
Materials and Methods:
This double-blind randomized clinical trial was conducted on 60 patients with nasal polyps. The patients were divided into two groups. The first group (beclomethasone dipropionate aqueous [BD-AQ] group) was treated with daily two puffs of beclomethasone aqueous nasal spray 50 μg in each nostril, and the second group (beclomethasone dipropionate aerosol [BD-A] group) was treated with two puffs of aerosol beclomethasone 50 μg in each nostril daily for 6 months. At the beginning of the study, the sino-nasal outcome test-22 (SNOT-22) and Lund-Mackay scores were recorded after the evaluation of disease status and the severity of symptoms.
Results:
The results of this study demonstrated that the mean changes in Lund-Mackay and SNOT-22 scores (83 ± 6.30 and 4.25 ± 31.60, respectively) in the BD-A group were significantly higher than the BD-AQ group (2.01 ± 3.87and 9.83 ± 24.13, respectively) (P < 0.05), but there was no significant difference in the patients' satisfaction between the two groups (P > 0.05).
Conclusion:
According to the results of this study, patients with nasal polyps showed a significant improvement following both the interventions, but the disease severity in the BD-A group was significantly higher than the BD-AQ group based on the mean values of Lund-Mackay score.
Keywords: Aerosols, Blood-Aqueous Barrier, beclomethasone, nasal polyps
Introduction
Nasal polyps are benign masses caused by various diseases in the nasal cavity and originated from the nose and the sinus mucous membranes and the outer wall of the nose. The pathogenesis of polyps is often unknown.[l, 2]
Various diseases, such as malignancies and cystic fibrosis, can cause polyps, but the most common cause is a chronic inflammation of the nose and sinuses. Nearly 25%–30% of all patients with chronic rhinitis also have nasal polyps.[3] Chronic inflammation results in excessive growth of sinuses and nasal tissue, with an overall prevalence of 1%–4% in the US population.[4]
The nasal polyp is usually bilateral, starting from the ethmoid sinus and projecting to the nasal space near the middle turbinate.[5] The clinical manifestations of this disease are anterior or posterior rhinorrhea, nasal congestion, hyposmia, feeling of pressure, and pain (if it lasts more than 12 weeks), but none of the above symptoms have a high sensitivity and specificity for diagnosis of nasal polyps.[1] The gold standard for diagnosis of inflammatory nasal polyp is endoscopy and computed tomography (CT) scans.[6]
On the other hand, treatments available for patients with chronic nasal and sinusitis inflammation and polyps are limited. Topical corticosteroids and topical saline are recommended as primary therapies for these patients.[7] In addition, intranasal corticosteroids (INSs) reduce the size and number of polyps and symptoms.[8] Previous systematic review studies have demonstrated the efficacy of intranasal steroids.[9,10,11]
To this end, beclomethasone dipropionate is a synthetic glucocorticoid that has a potent, topical, and anti-inflammatory function in the respiratory tract. As an inhaled preventer medication, it is effective in the management of asthma and rhinitis and is one of the important drugs used in the treatment of these diseases.
It has been used for several years for the treatment of rhinitis in oral and nasal spray forms, and aerosol and aqueous forms are the most commonly used forms. Aerosols are not really sprays. The aerosol form consists of two components: the drug and the propellant. The propellant's role is to provide sufficient force to pull the drug out of the container. As the percentage of propellant increases, the extracted drug splits into smaller particles and decreasing percentage of propellant increases the wetness.[12] However, in the form of nasal sprays (aqueous, hydroalcoholic, suspension, or emulsions), unlike the aerosol, there is no propellant and the force required to exit the drug is provided by charging or manually. Noteworthy in these types of sprays is the small size of the sprayed particles, which can lead to drug accumulation in the lungs and rapid clearance of the drug from the environment.[12]
Now, with regard to the way in which the drug is administered to cover the nasal mucosa and their various effects and complications, recently, many researchers have focused on evaluating and choosing the best method for administration of these effective drugs to reduce or prevent nasal symptoms of allergic rhinitis, and they have yielded contradictory results. Some previous studies have reported administration of beclomethasone dipropionate (aqueous) (BD-AQ) and others reported administration of beclomethasone dipropionate (aerosol) (BD-A) in the treatment of chronic rhinosinusitis and allergic rhinitis.[13,14,15] However, some patients may not be able to use pressurized aerosol because of the unpleasant feeling, although it seems that this form of medication may be more effective. The aim of this study was to compare aqueous and aerosol spray forms of beclomethasone in patients with nasal polyps.
Materials and Methods
This study is a double-blind randomized clinical trial. The study sample consisted of 70 patients with nasal polyps diagnosed by physical examinations, description, and endoscopic diagnosis of nasal polyposis by the otorhinolaryngologist.
These patients were selected using census method from the patients with nasal polyps referred to Alzahra and Kashani Hospitals in Isfahan during 2018–2019.
Inclusion criteria included being 18–55 years old, developing inflammatory nasal polyps, and satisfied to participate in the study. In case of previous allergy to beclomethasone, pregnancy or breastfeeding, smoking, severe renal, heart, liver, or neurologic diseases, vascular collagen, malignancy, untreated bacterial or fungal infection, history of trauma and facial and nasal fracture, use of systemic or topical steroids during 30 days before the study, use of nasal decongestant, antihistamine, cromolyn sodium, or anticholinergics during 7 days before the study, use of cardiovascular drugs, neuroleptics, hormones, or any drug that causes allergic rhinitis, the participants did not enter the study.
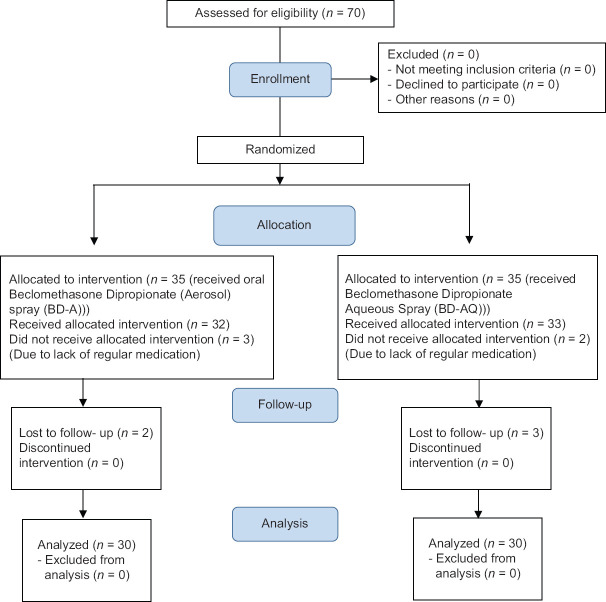
In addition, if they did not want to participate in this study and no irregular use of drug and lack of follow-up to assess the patient's condition, they were excluded from the study. In this study, 5 cases from both the first and second groups were excluded due to the lack of regular use of drug and no referral for further follow-up, and the study was performed on 60 patients [Figure 1].
Figure 1.
Consort patient flow diagram
After Isfahan University of Medical Sciences Ethics Committee approval (Code: IR.MUI.MED.REC.1398.503) and obtaining clinical trial code (IRCT20171030037093N32; https://fa.irct.ir/trial/45230) and written consent from patients, they were divided into two groups using random allocation software.
The age and sex of patients were initially recorded, and the disease status and severity of symptoms were assessed by sino-nasal outcome test-22 (SNOT-22) and Lund-Mackay scoring system questionnaires.
The SNOT-22 is a 22-item questionnaire of 0 (asymptomatic) to 5 (most severe symptom) ranging.[16] In this questionnaire, patients described their health status in the last 2 weeks by showing the severity of symptoms and their quality of life by indicating the importance of various domains (including physical problems, functional limitations, and emotional consequences of rhinosinusitis). This questionnaire has also been validated and approved in Iran.[17]
The Lund-Mackay scoring system is also completed using sinus CT scan. In this questionnaire, anterior ethmoidal, posterior ethmoidal, maxillary, frontal, and sphenoid sinuses and eustachian tubes are evaluated in terms of involvement. Scores on the sinus items are 0 (no abnormality), 1 (partial opacification), and 2 (complete opacification), and the eustachian tubes scoring items are 0 (no obstruction) and 2 (occlusion). The score of this scale indicates the severity of symptoms.[18]
Patients in the first group were treated with BD-AQ spray 50 μg/with the dose of two puffs per day in each nostril for 2 months, and the second group received oral BD-A spray 50 μg/with the dose of two puffs per day in each nostril for 2 months. Patients were advised not to take any drug other than this medicine. It should be noted that for the use of beclomethasone oral spray, nasal spray interface was installed on oral spray.
To apply the terms of a double-blind study, therapeutic sprays were placed in two groups in closed packs with codes A and B. The first group was assigned packs containing code A, and the second group was given packs with code B. In addition, the conditions for prescribing the drug in both the groups at an equal dose (50 μg) of two puffs per day for 2 months were determined.
Immediately after completion of treatment, patients' satisfaction (use of medicine and recovery rate) was assessed based on 0 (no satisfaction) to 10 (completely satisfied) score. The scores for SNOT-22 and Lund-Mackay were again evaluated and recorded.
Finally, the collected data were analyzed by SPSS software, version 22 [SPSS - Chicago, IL, United States]. Mean, standard deviation, frequency, and percentage of frequency were used to indicate the variables. In addition, according to the results of Kolmogorov–Smirnov test for abnormal distribution of data, Mann–Whitney test was used for comparing the mean of quantitative variables between the two groups and Wilcoxon test for comparing the mean of quantitative variables before and after the intervention in each group. Chi-square test was used to compare the sex distribution between the two groups, and independent t-test was used to compare the mean age of the patients between the two groups. Significance level was considered <0.05 in all analyzes.
Results
In the present study, 16 (53.3%) males and 14 (46.7%) females with a mean age of 46.40 ± 13.15 years participated in the BD-AQ group and 16 (53.3%) males and 14 (46.7%) females were in the BD-AQ group with a mean age of 46.30 ± 14.28 years (P > 0.05) [Table 1].
Table 1.
Age and sex of the patients
| Characteristics | BD-AQ group (n=30) | BD-A group (n=30) | P |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 16 (53.3) | 16 (53.3) | 1.00* |
| Female | 14 (46.7) | 14 (46.7) | |
| Age (years) | 46.40±13.15 | 46.30±14.28 | 0.978** |
*Used of Chi-square test, **Used of independent sample t-test. BD-AQ: Beclomethasone dipropionate aqueous, BD-A: Beclomethasone dipropionate aerosol
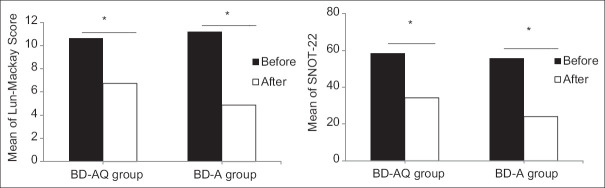
Evaluation of disease status with SNOT-22 questionnaire and results of sinus CT scan based on Lund-Mackey scoring system showed that Lund-Mackey and SNOT-22 scores were not significantly different before treatment (P > 0.05). However, after treatment, Lund-Mackey score was significantly lower in the BD-A group with a mean of 4.53 ± 2.89 than the BD-AQ group with a mean of 6.73 ± 4.10 (P = 0.041). In addition, the changes of the Lund-Mackey and SNOT-22 scores in the BD-A group with a mean of 6.30 ± 2.83 and 31.60 ± 4.25 were higher than the BD-AQ group with a mean of 3.87 ± 2.01 and 24.13 ± 9.83 (P < 0.05). In addition, patients' satisfaction in the BD-AQ group with a mean of 8.20 ± 1.27 compared to the BD-A group with a mean of 7.73 ± 1.44 was not significantly different (P = 0.197) [Table 2 and Figure 2].
Table 2.
Determination and comparison of the mean scores of sino-nasal outcome test-22 and Lund-Mackay and patient satisfaction in the two groups
| Variables | BD-AQ group (n=30) | BD-A group (n=30) | P* |
|---|---|---|---|
| Lund-Mackay score | |||
| Before | 10.60±3.65 | 11.17±4.47 | 0.688 |
| After | 6.73±4.10 | 4.53±2.89 | 0.041 |
| Changes | 3.87±2.01 | 6.30±2.83 | 0.001 |
| SNOT-22 | |||
| Before | 58.33±13.15 | 55.60±10.19 | 0.534 |
| After | 34.20±21.48 | 24.00±13.60 | 0.104 |
| Changes | 24.13±9.83 | 31.60±4.25 | 0.002 |
| Satisfaction | 8.20±1.27 | 7.73±1.44 | 0.197 |
*Used of Mann-Whitney test. BD-AQ: Beclomethasone dipropionate aqueous, BD-A: Beclomethasone dipropionate aerosol, SNOT: Sino-nasal outcome test
Figure 2.
Mean of sino-nasal outcome test-22 and Lund-Mackay score before and after intervention in both the groups. *A significance level of <0.001 from Wilcoxon test indicates a significant decrease in symptoms and severity of disease after intervention in both the groups
It should be noted that in this study, only two patients in the BD-A group developed mild (no bleeding) nose stuffiness and no complication was observed in the BD-AQ group.
Discussion
Our results indicated that both interventions were significantly affected the improvement of the disease status and reducing the severity of disease in the patients with nasal polyps based on the mean scores of SNOT-22 and Lund-Mackay, but the mean Lund-Mackay score in the BD-A group was significantly lower than the BD-AQ group. In addition, the mean scores of SNOT-22 and Lund-Mackay were significantly decreased in the BD-A group as compared with the BD-AQ group. In this study, both methods of administration had a significant effect in the patients with nasal polyps, but the effect of beclomethasone aerosol nasal spray was significantly higher than the beclomethasone aqueous nasal spray.
The results of the present study are in line with the study of Moller et al., who compared the efficacy of drug delivery to the sinuses and lungs following administration of the aqueous and aerosol nasal sprays using technetium scan, which revealed that the aerosol nasal spray slightly delivered the drugs to the lungs, and it was more effectively transferred the drugs to the sinuses than the aqueous spray.[13]
Furthermore, the results obtained from a study conducted by Ly et al. indicated that in the aerosols with the pressurized chamber and the aqueous models using the pump, the drugs were delivered outside the pump. On the other hand, aqueous nasal spray may release different doses of medication after each use due to the intensity and speed of the pump charged. In addition, the intensity of drug delivery to nasal aerosols is lower than the aqueous nasal spray, and the dose of the drug released in the aqueous nasal spray also varies due to charging the spray pump.[15]
Another study investigated the effect of beclomethasone aerosol nasal spray on the patients with allergic rhinitis as compared with mometasone furoate and propionate aqueous nasal sprays, and the results showed that the stability of the drug in the nasal cavity and drug delivery to the nasal mucosa in the nasal aerosol were higher than those in the aqueous nasal spray, leading to an increased risk of its side effects.[14]
In fact, due to the higher pressure and the higher dispersion rates of the drug in the nasal aerosols, the drug particles have a longer contact time and longer residence time in the nasal mucosa, which will, therefore, have a greater effect.
In addition, Luskin et al. investigated the efficacy of both aqueous and aerosol nasal sprays in the treatment of allergic rhinitis.[19] In another study, a comparison was made between the effects of triamcinolone acetonide aerosol spray and fluticasone propionate aqueous on patients with allergic rhinitis, and the results suggested that no statistically significant difference was observed between the two groups with respect to reduced symptoms of allergic rhinitis.[20]
However, two points are worth noting in this regard. First, previous studies have compared the efficacy of beclomethasone aerosol and aqueous nasal sprays, and the results obtained from these studies showed that the beclomethasone aerosol nasal spray had greater efficacy compared to beclomethasone aqueous nasal spray, which were consistent with those of the present study. In addition, the mean Lund-Mackay score significantly improved in patients after the administration of beclomethasone aerosol nasal spray compared to beclomethasone aqueous nasal spray, but no significant difference in reducing the mean SNOT-22 scores was observed between the two groups. Second, previous studies have applied different methods such as symptom observation, endoscopy, CT scan, and technetium scan to determine the improvement rate of a patient's symptoms. In the present study, the most accurate method of calculating the improvement rate using endoscopy and CT scan was evaluated after abstaining Lund-Mackay scores. In addition, the severity of the disease symptoms was assessed by the SNOT-22, and few studies have paid attention to it, which can be considered as one of the strengths of the current study.
Our results showed that there was no significant difference between the two groups in terms of the patients' satisfaction. In other words, beclomethasone aerosol nasal spray may cause unpleasant sensations in some patients, but the two groups exhibited similar profiles of overall satisfaction.
According to the previous studies, the efficacy of the drug has been a major factor contributing to the choice of drug for patients with allergic rhinitis[21,22,23] as 99% of patients reported the drug's efficacy as a key or positive factor for the prioritization of selecting an INS.[21] In fact, the negative factors were the major cause of patients' complaints, but the lack of effectiveness remained the most common primary reason for patients requesting physicians to change their medication. However, some patients are unable or reluctant to use a pressurized aerosol. The INS spray is formulated based on an aqueous mixture (the beclomethasone aqueous nasal spray) which may be considered as an alternative method of the administration in these patients.[24]
It is, therefore, assumed that the patients by considering their preferences tend to change how to use INS spray based on sensory perceptions and efficacy. However, in the present study, given that there were no statistically significant differences in the patients' satisfaction with the drug use and its efficacy, it is possible to conclude that both methods have been accepted by the patients, and thus, the researchers using further research can determine the most effective drug and recommend the best method of administering the drug to a patient.
One of the limitations of the present study included the short follow-up period, but previous studies have evaluated the therapy protocols for both allergic rhinitis and nasal polyps between 2 weeks and 6 months.
Conclusion
According to our results, patients with nasal polyps exhibited a significant improvement after using the beclomethasone aerosol and aqueous nasal sprays, and there was no significant difference in health status between the treatment groups based on the mean SNOT-22 score, but based on the mean Lund-Mackay scores, the severity of symptoms was significantly decreased in the group treating with beclomethasone aerosol nasal spray as compared with the group treating with beclomethasone aqueous nasal spray.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Small P, Kim H. Allergic rhinitis. Allergy Asthma Clin Immunol. 2011;7(Suppl 1):S3. doi: 10.1186/1710-1492-7-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103–15. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- 3.Stevens WW, Schleimer RP, Kern RC. Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2016;4:565–72. doi: 10.1016/j.jaip.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–2. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 5.Steinke JW, Payne SC, Chen PG, Negri J, Stelow EB, Borish L. Etiology of nasal polyps in cystic fibrosis: Not a unimodal disease. Ann Otol Rhinol Laryngol. 2012;121:579–86. doi: 10.1177/000348941212100904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batra PS, Tong L, Citardi MJ. Analysis of comorbidities and objective parameters in refractory chronic rhinosinusitis. Laryngoscope. 2013;123(Suppl 7):S1–11. doi: 10.1002/lary.24418. [DOI] [PubMed] [Google Scholar]
- 7.Peters AT, Spector S, Hsu J, Hamilos DL, Baroody FM, Chandra RK, et al. Diagnosis and management of rhinosinusitis: A practice parameter update. Ann Allergy Asthma Immunol. 2014;113:347–85. doi: 10.1016/j.anai.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Fokkens W, Reitsma S. New delivery forms of nasal corticosteroids. J Allergy Clin Immunol. 2019;143:87–8. doi: 10.1016/j.jaci.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Chong LY, Head K, Hopkins C, Philpott C, Schilder AG, Burton MJ. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011996. doi: 10.1002/14651858.CD011996.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalish L, Snidvongs K, Sivasubramaniam R, Cope D, Harvey RJ. Topical steroids for nasal polyps. Cochrane Database Syst Rev. 2012;12:CD006549. doi: 10.1002/14651858.CD006549.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Snidvongs K, Thanaviratananich S. Update on Intranasal Medications in Rhinosinusitis. Curr Allergy Asthma Rep. 2017;17:47. doi: 10.1007/s11882-017-0720-3. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni VS, Shaw C. Essential Chemistry for Formulators of Semisolid and Liquid Dosages. eBook, Academic Press; 2015. [Google Scholar]
- 13.Moller W, Saba GK, Haussinger K, Becker S, Keller M, Schuschnig U. Nasally inhaled pulsating aerosols: Lung, sinus and nose deposition. Rhinology. 2011;49:286–91. doi: 10.4193/Rhino10.268. [DOI] [PubMed] [Google Scholar]
- 14.Leach CL, Kuehl PJ, Chand R, McDonald JD. Nasal deposition of HFA-beclomethasone, aqueous fluticasone propionate and aqueous mometasone furoate in allergic rhinitis patients. J Aerosol Med Pulm Drug Deliv. 2015;28:334–40. doi: 10.1089/jamp.2014.1180. [DOI] [PubMed] [Google Scholar]
- 15.Ly J, Morales A, Salas K, Cabrera J, Dalvi M, Zeng ZM. Qnasl® non-aqueous nasal aerosol delivers softer sprays than aqueous Flonase®, Nasacort AQ®, and Nasonex®. J Allergy Clin Immunol. 2013;131:AB39. [Google Scholar]
- 16.Feng AL, Wesely NC, Hoehle LP, Phillips KM, Yamasaki A, Campbell AP, et al. A validated model for the 22-item Sino-Nasal Outcome Test subdomain structure in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7:1140–8. doi: 10.1002/alr.22025. [DOI] [PubMed] [Google Scholar]
- 17.Naghdi S, Anjeie F, Nakhostin Ansari N, Fathali MJ. Development, cultural adaptation, reliability and validity of Persian version of Sino-Nasal outcome test in chronic rhinosinusitis: A brief report. Tehran Univ Med J. 2013;70(11):735–740. [Google Scholar]
- 18.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117:S35–40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 19.Luskin AT, Blaiss MS, Farrar JR, Settipane R, Hayden ML, Stoloff S, et al. Is there a role for aerosol nasal sprays in the treatment of allergic rhinitis: A white paper. Allergy Asthma Proc. 2011;32:168–77. doi: 10.2500/aap.2011.32.3438. [DOI] [PubMed] [Google Scholar]
- 20.Small P, Houle PA, Day JH, Briscoe M, Gold M, Brodarec I, et al. A comparison of triamcinolone acetonide nasal aerosol spray and fluticasone propionate aqueous solution spray in the treatment of spring allergic rhinitis. J Allergy Clin Immunol. 1997;100:592–5. doi: 10.1016/s0091-6749(97)70160-x. [DOI] [PubMed] [Google Scholar]
- 21.Kaliner MA. Patient preferences and satisfaction with prescribed nasal steroids for allergic rhinitis. Allergy Asthma Proc. 2001;22:S11–5. [PubMed] [Google Scholar]
- 22.Storms WW. Introduction: Patient preference of inhaled nasal corticosteroids. Allergy Asthma Proc. 2001;22:S1–3. [PubMed] [Google Scholar]
- 23.Neffen H, Mello JF, Jr, Sole D, Naspitz CK, Dodero AE, Garza HL, et al. Nasal allergies in the Latin American population: Results from the Allergies in Latin America survey. Allergy Asthma Proc. 2010;31(Suppl 1):S9–27. doi: 10.2500/aap.2010.31.3347. [DOI] [PubMed] [Google Scholar]
- 24.Dunn AM, Wilson RS, Baggott PJ. A comparison of beclomethasone dipropionate aqueous nasal spray and beclomethasone dipropionate pressurized nasal spray in the management of seasonal rhinitis. Postgrad Med J. 1984;60:404–6. doi: 10.1136/pgmj.60.704.404. [DOI] [PMC free article] [PubMed] [Google Scholar]