Abstract
Background: Acute respiratory failure is the most important organ dysfunction of COVID-19 patients. While non-invasive ventilation (NIV) and high-flow nasal cannula (HFNC) oxygen are frequently used, efficacy and safety remain uncertain. Benefits and harms of awake prone positioning (APP) in COVID-19 patients are unknown. Methods: We searched for randomized controlled trials (RCTs) comparing HFNC vs. NIV and APP vs. standard care. We meta-analyzed data for mortality, intubation rate, and safety. Results: Five RCTs (2182 patients) were identified. While it remains uncertain whether HFNC compared to NIV alters mortality (RR: 0.92, 95% CI 0.65–1.33), HFNC may increase rate of intubation or death (composite endpoint; RR 1.22, 1.03–1.45). We do not know if HFNC alters risk for harm. APP compared to standard care probably decreases intubation rate (RR 0.83, 0.71–0.96) but may have little or no effect on mortality (RR: 1.08, 0.51–2.31). Conclusions: Certainty of evidence is moderate to very low. There is no compelling evidence for either HFNC or NIV, but both carry substantial risk for harm. The use of APP probably has benefits although mortality appears unaffected.
Keywords: respiratory failure, non-invasive ventilation, high-flow nasal cannula, awake prone positioning, COVID-19, systematic review
1. Introduction
As of November 2021, there have been more than 250 million confirmed SARS-CoV-2 cases with more than five million deaths globally [1]. Although many infections present with no or mild symptoms, more severe cases can be characterized by hypoxemia, which then often leads to hospitalization. Reports on large registry datasets found hospitalization rates of 14.0% and the need for ICU admission in 2.3–5% of all confirmed cases [2,3]. Among the most dangerous manifestations of the SARS-CoV-2 infection is the progression of respiratory insufficiency to acute respiratory distress syndrome (ARDS). Out of all hospitalized patients, 17% were found to require invasive mechanical ventilation in an analysis of health insurance data [4].
To alleviate the burden of disease for both patients and healthcare systems during this pandemic, researchers have been eager to find the best strategies for the therapeutic management of COVID-19-associated respiratory failure. Early on, COVID-ARDS was suspected to be pathophysiologically and morphologically different from known (infectious or non-infectious) ARDS [5,6]. However, later and larger studies did not confirm these early theories [7,8,9]. As a result, the principles of respiratory therapy for COVID-19 associated respiratory failure were similar to those of “conventional” ARDS [10].
After the initial trend towards early intubation to reduce aerosols, in the context of an ongoing global health crisis and in an alleged attempt to spare resources by both questioning work-intensive procedures and preventing disease progression as early as possible, research also started focusing on non-invasive ventilation (NIV) and high-flow nasal cannula oxygen administration. NIV can be applied by a face mask or helmet, both of which have to be fixed tightly to prevent pressure loss. A continuous positive airway pressure is applied and shall prevent collapse of the alveoli and atelectasis. HFNC promises to increase patient comfort, require less attention by healthcare professionals than conventional non-invasive ventilation, and may help avoid invasive ventilation, too [11,12]. Non-invasive ventilation strategies have been found to reduce mortality and lower the risk of intubation [13]. So far, no evidence-based recommendations favor either HFNC or conventional non-invasive ventilation (NIV) [14]. Awake prone positioning (APP) was hypothesized to lead to similar physiological improvements (e.g., reduction of ventilation-perfusion mismatches) as prone positioning in sedated, intubated patients [15,16], but perhaps do so in an earlier stage of the disease, thus slowing down or avoiding progression to more severe conditions. Case series and non-randomized controlled trials (non-RCTs) suggested feasibility of APP and a potential clinical benefit for patients with hypoxemic respiratory failure [17,18,19]. For both research questions (i.e., the value of HFNC or NIV and APP in COVID-19-associated respiratory failure), we prospectively registered protocols for systematic reviews on the matter. Here, we report and analyze the available evidence on HFNC vs. NIV and APP vs. standard of care (SoC) on COVID-19-associated respiratory failure. We provide meta-analyses for pre-defined, patient-centered core outcomes and assess certainty of evidence and possible resulting recommendations using systematic, validated approaches.
2. Methods
To assess the efficacy of awake prone positioning (APP) and the use of high-flow nasal oxygen or non-invasive ventilation (NIV), respectively, on COVID-19-induced respiratory failure, we included randomized controlled trials. Non-RCT designs were not eligible in case that RCT(s) on the matter were available. Thus, we excluded controlled, non-randomized studies and observational studies. We included full-text journal publications, preprint articles, abstracts publications and results published in trial registries if they provided information in sufficient detail. The methodology was predefined and registered in two separate PROSPERO protocols with any deviations pointed out below (APP/SoC: CRD42021261862 [20]; HFNC/NIV: CRD42021230825 [21])
We conducted two separate systematic searches for each topic in the following sources from initiation of those databases to the date of search without restrictions on the language of publication:
Cochrane COVID-19 Study Register (COVID-19.cochrane.org), comprising Cochrane Central Register of Controlled Trials, MEDLINE (PubMed), Embase, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, and medRxiv (last searched on 26 October 2021 for both topics);
WHO COVID-19 Global literature on coronavirus disease (https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov) (last searched on 26 October 2021 for both topics);
Web of Science (Science Citation Index Expanded and Emerging Sources Citation Index) (last searched on 1 March 2021 for NIV and 7 June 2021 for APP);
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (last searched on 1 March 2021 for NIV and 7 June 2021 for APP).
For detailed search strategies, see Appendix A. We identified other potentially eligible studies or ancillary publications by searching the reference lists of included studies, relevant systematic reviews and meta-analyses.
We included studies investigating adult patients with severe respiratory failure due to COVID-19 infection according to the WHO clinical progression scale [22]. This includes patients with the need for high-flow nasal oxygen, non-invasive ventilation, or invasive ventilation. COVID-19 infection had to be confirmed by reverse-transcription polymerase chain reaction (RT-PCR) or highly suspected by clinical presentation of the patient.
With regard to awake proning, full prone or 135° prone positioning were acceptable interventions. We did not define any restrictions on the duration of proning per day. Standard of care as the comparator was defined as 90° or supine positioning and otherwise all diagnostic and therapeutic interventions considered necessary at the treating physician’s discretion.
With regard to non-invasive ventilation, we deviated from the initially registered protocol: We focused on the highly relevant question from daily clinical practice about the efficacy and safety of HFNC (intervention) compared to NIV (standard care) as a non-invasive strategy of respiratory support. We did not include trials comparing different ventilator settings within HFNC or NIV, nor comparing simple oxygen insufflation or invasive ventilation to either HFNC or NIV. Additionally, we excluded comparisons of NIV vs. awake proning because there is no rationale not to combine both. The outcome set and the analysis specifications were adapted to the latest version of the APP protocol [20].
We evaluated core outcomes in accordance with the Core Outcome Measures in Effectiveness Trials (COMET) initiative for COVID-19 patients [22]. These outcomes were in part modified to better represent the nature of the non-pharmacological interventions investigated in this meta-analysis. Additional outcomes specific to our research question were also added. The following outcome set served as the basis for our analyses:
Main outcomes:
-
-
All-cause mortality at day 28, day 60, and time-to-event, and at hospital discharge
-
-Clinical status at day 28, day 60, and up to the longest follow-up, including:
-
-worsening of clinical status: patients with clinical deterioration or death
-
-improvement of clinical status: patients discharged alive without clinical deterioration or death
-
-quality of life, including fatigue and neurological status, assessed with standardized scales at up to 7 days, up to 28 days and longest follow-up available
-
-
-
-
Serious adverse events during the study period, defined as number of patients with any event
-
-
Adverse events (any grade) during the study period, defined as the number of patients with any event
Additional outcomes:
-
-Clinical status at day 28, day 60 and up to longest follow up, including:
-
-worsening of clinical status: new need for invasive mechanical ventilation
-
-improvement of clinical status:
-
-weaning or liberation from invasive mechanical ventilation
-
-liberation from supplemental oxygen in surviving patients
-
-ventilator-free days
-
-duration to liberation from invasive mechanical ventilation
-
-duration to liberation from supplemental oxygen
-
-
-
-
-
-
Admission to intensive care unit at day 28
-
-
Duration of hospitalization
-
-
Skin lesions from proning measures
Two review authors independently identified studies possibly eligible for this review. If encountering disagreement, we consulted a third review author and resolved all discrepancies by discussion. Further, two review authors independently extracted data using a standardized data extraction form.
Two review authors independently assessed the risk of bias for RCTs using the Cochrane “Risk of Bias” tool 2 (RoB2) [23]. The effect of interest is the effect of assignment at baseline, regardless of whether the interventions were received as intended (intention-to-treat). Risk of bias was assessed for all results which were identified as one of the predefined outcomes of this review. The overall risk of bias for each outcome was summarized according to RoB2 guidance.
Random effects meta-analyses were performed with RevMan 5, as far as the patient characteristics (disease severity) and interventions (standard care and treatment settings) were comparable across studies. Dichotomous outcomes were recorded as number of events and total patient number in all study groups. Pooled risk ratios (RR) and 95% confidence intervals (95% CI) were calculated. Continuous outcomes were recorded as mean, standard deviation and total number of patients in each group. Comparisons of continuous outcomes were reported as the difference of means (MD) and the corresponding 95% CI. Statistical heterogeneity was defined as p < 0.1 for the Chi2 test of heterogeneity or I2 ≥ 50%. We had planned to explore heterogeneity by subgroup analysis to calculate RR or MD in conjunction with the corresponding CI for each subgroup if sufficient studies had been available (at least 10 studies per outcome). For the current review, there were not enough studies available.
Certainty of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. Summary of findings tables were created using the MAGICapp software (Norwegian MAGIC Evidence Ecosystem Foundation powered by UserVoice Inc.; MAGICapp. Brønnøysund, Norway) for the proning part and GRADEpro APP (GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2021. Available from gradepro.org (accessed on 6 January 2022)) for the HFNC/NIV part. According to the five domains used in the GRADE approach, we downgraded the certainty of evidence of each individual outcome as follows:
-
-
serious (−1) or very serious (−2) risk of bias
-
-
serious (−1) or very serious (−2) inconsistency
-
-
serious (−1) or very serious (−2) uncertainty about directness
-
-
serious (−1) or very serious (−2) imprecision of the data
-
-
serious (−1) or very serious (−2) probability of reporting bias
The grade of evidence is then assigned one of the labels “high”, “moderate”, “low”, or “very low”.
3. Results
3.1. Study Selection
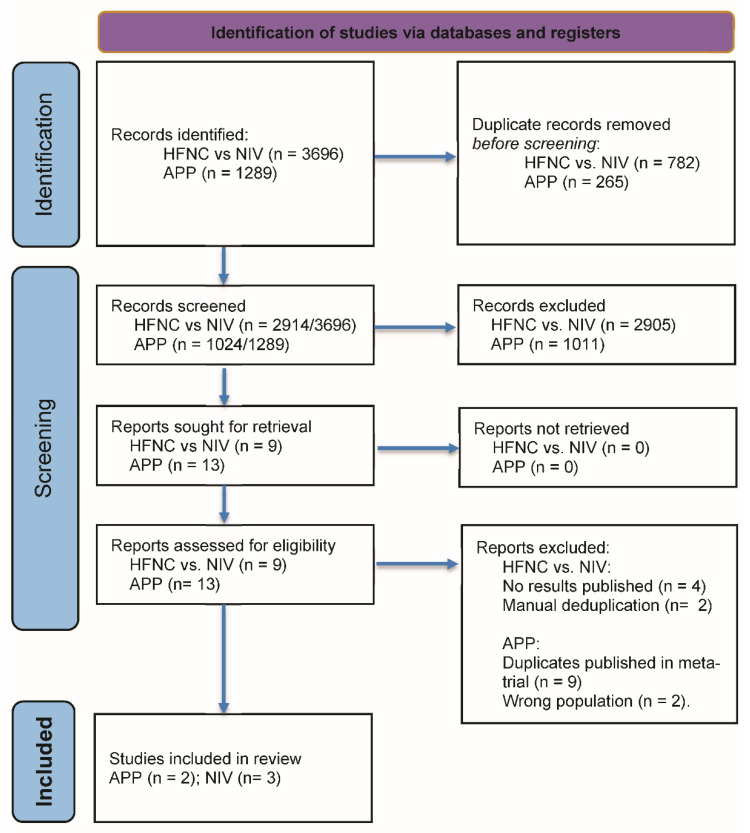
The literature search produced 4985 (HFNC vs. NIV: 3696/APP: 1289) records and after deduplication resulted in 3938 (HFNC vs. NIV: 2914/APP: 1024) records. After title and abstract screening, 22 records (APP: 13/HFNC vs. NIV: 9) were identified as potential RCTs. We retrieved 22 full-text articles including registry entries (HFNC vs. NIV: 9/APP: 13). Of those, 17 records were excluded (HFNC vs. NIV: 4, no results published; 2 duplicates/APP: 2, wrong population; 9, meta-trial), resulting in 5 records (APP: 2/HFNC vs. NIV: 3) for data extraction. The detailed search process is outlined in Figure 1.
Figure 1.
PRISMA flow diagram. HFNC: high-flow nasal cannula, NIV non-invasive ventilation, APP: awake prone-positioning; Adapted from: [24].
3.2. Study Characteristics
3.2.1. HFNC vs. NIV
We included three unblinded RCTs for the direct comparison of HFNC vs. NIV. Data from the largest study by Perkins et al., a pragmatic multicenter multinational adaptive three-armed RCT with 797 patients allocated to either HFNC or NIV, is currently available from a preprint version only [25]. We omitted patients from the “O2-insufflation only” arm of this preprint. Apart from that, there were two smaller conventional two-armed RCTs: the Italian multicenter study by Grieco et al. [26] and the Indian single-center study by Nair et al. [27], both allocating 109 patients and available as peer-reviewed publications.
All three trials enrolled hospitalized patients with similar disease severity at the verge of WHO stages 5 and 6, i.e., with severe respiratory failure (means of PaO2/FiO2 ratio ranging from 102 to 139 and tachypnea present) but invasive ventilation not yet imminent. All included RCTs conducted standard care according to their local clinical practice: dexamethasone was given (Grieco, Perkins), as well as remdesivir (Grieco) and since January 2021 Tocilizumab (Perkins). Every study allowed or encouraged awake prone positioning for both treatment groups, although Perkins (HFNC group: 58.3% vs. NIV group: 54.5%), Grieco (HFNC group: 60% vs. NIV group: 0%), and Nair (no exact data on APP rate; HFNC group: “almost all[...]” vs. NIV group: “[...]noncompliant[...]”) reported substantial differences of APP rate between HFNC and NIV groups, which could have introduced bias. Overall, there were no relevant differences between the treatment groups in terms of adherence to the intervention protocol (relative number of patients that received the intended intervention: Perkins: 92.1% HFNC vs. 91.6% NIV; Grieco: 87% HFNC vs. 91% NIV; Nair: not reported).
The recruitment periods overlapped well, patient characteristics including disease severity with PaO2/FiO2 ratios were similar across studies (indicating moderate ARDS) and standard care and treatment settings were sufficiently homogenous.
Of our predefined main outcomes, all three studies reported in-hospital mortality. Other outcomes were presented on different scales or for different time points. Table 1 summarizes the characteristics and intervention details of the included studies along with the reported outcomes.
Table 1.
Study characteristics. AE: adverse event, APP: awake prone-positioning, CPAP: continuous positive airway pressure, FiO2: inspiratory fraction of oxygen, HFNC: high-flow nasal cannula, ICU: intensive care unit, IQR: inter-quartile range, NIV: non-invasive ventilation, PaCO2: carbon dioxide partial pressure, PaO2: oxygen partial pressure, PEEP: positive end-exspiratory pressure, RR: relative risk, SD: standard deviation, WHO: world health organization.
| Authors | Population | Intervention | Comparator | Outcomes | Results per Endpoint |
|---|---|---|---|---|---|
| High-flow nasal cannula vs. non-invasive ventilation | |||||
| Perkins et al. [25] Standard care in both arms: Dexamethasone and Tocilizumab added as standard care in June 2020 and January 2021, respectively Recruitment: from April 2020 to May 2021 with ca. 90% of patients recruited between September 2020 and March 2021 |
n = 797 (allocated) age (years): mean 57.6 (SD 13.0) in HFNC, 56.7 (SD 12.5) in CPAP sex: 65.2% male vs. 68.4% male comorbidities: none in 34% vs. 39%, hypertension, morbid obesity, type 2 diabetes requiring medication, chronic lung disease clinical status at enrolment: inpatients not intubated with hypoxemic respiratory failure PaO2/FiO2 ratio 139 vs. 132, resp. rate 25 vs. 26 |
n = 417 HFNC Local policies, and clinical discretion informed decisions regarding choice of device, set-up, titration, and discontinuation of treatment, mean flow 51 L/min adherence to intended treatment protocol: 92.1% |
n = 380 NIV Local policies, and clinical discretion informed decisions regarding choice of device, set-up, titration, and discontinuation of treatment, mean PEEP 9.5 (SD 8.4) adherence to intended treatment protocol: 91.6% |
Endotracheal intubation or death within 30 days In-hospital mortality 30-day mortality Endotracheal intubation within 30 days Mean length of stay in hospital (SD) Serious adverse event (as no. of patients with at least one) Adverse events (as no. of patients with at least one) Adverse events (as total no. of events per patients) |
HFNC 184/414 vs. NIV 137/377: RR 1.22 (1.03 to 1.45) 88/404 vs. 72/364: RR 1.10 (0.83 to 1.45) 78/415 vs. 63/378: RR 1.13 (0.83 to 1.52) 170/414 vs. 126/377: RR 1.23 (1.02 to 1.48) 18.3 (20.0) vs. 16.4 (17.5) 0/417 vs. 7/380: RR 0.06 (0.00 to 1.06) 86/417 vs. 130/380: RR 0.60 (0.48 to 0.76) 157/417 vs. 200/380 |
| Grieco et al. [26] Standard care in both arms: Dexamethasone 100%, Remdesivir 81%. Sedation allowed but discouraged (continuous infusion 18%HFNC group vs. 37% in the helmet group). Awake proning allowed, 60% in HFNC vs. 0% in NIV underwent proning. Other aspects according to the clinical practice in each institution. Recruitment: from October 2020 to December 2020 |
n = 109 (allocated) age (years): 63 (IQR 55–69) (intervention) 66 (IQR 57–72) (control) sex: male 84% vs. 77% comorbidities: hypertension, diabetes mell., smoking, immunocompromised state, history of cancer clinical status at enrolment: inpatients not intubated with hypoxemic respiratory failure with PaO2/FiO2 ratio equal or below 200: PaO2/FiO2 ratio 102 vs. 105, PaCO2 34 vs. 34 mmHg, resp. rate 28 vs. 28 |
n = 55 HFNC for at least 48 h with 60 L/min and FiO2 titrated to SpO2 goal 92%. Stepwise weaning allowed when FiO2 was equal to or lower than 40% and respiratory rate lower than 25/min adherence to intended treatment protocol: received by 87% for 48 h or until intubation, one participant crossed over, other participants were weaned early according to the trial protocol |
n = 55 Helmet NIV for at least 48 h with initial PS 10–12 mbar and PEEP of 10–12 mbar and FiO2 titrated to SpO2 goal 92%. Stepwise weaning allowed when FiO2 was equal to or lower than 40% and respiratory rate lower than 25/min adherence to intended treatment protocol: received by 91% for 48 h or until intubation, 4% received it at least 16 h per day, 4% interrupted treatment, 2% did not receive the allocated treatment |
Days free of respiratory support within 28 days Endotracheal intubation within 28 days 28-day mortality 60-day mortality In-hospital mortality Adverse events (predefined selection of AEs relevant in respiratory and intensive care medicine, as total no. of events per patients) |
Mean (SD) in HFNC vs. NIV: 15 (11) vs. 13 (11) with MD 2 days (95% CI, −2 to 6) 28/55 vs. 16/54: RR 1.72 (1.06 to 2.79) 10/55 vs. 8/54: RR 1.23 (0.52 to 2.87) 12/55 vs. 13/54: RR 0.91 (0.46 to 1.80) 14/55 vs. 13/54: RR 1.06 (0.55 to 2.04) 70/55 vs. 37/54 |
| Nair et al. [27] Standard care in both arms: clinical management and drug therapy as per institutional protocols without further specification except: awake proning was encouraged, but only in almost all HFNC participants regularly performed. Recruitment: from August 2020 to December 2020 |
n = 109 (allocated) age (years): HFNC 57 (IQR 48–65) vs. NIV 57.5 (IQR 47–64) sex: male 80% vs. 64.8% comorbidities: Diabetes mell., hypertension, coronary heart disease, chronic kidney disease clinical status at enrolment: hospitalised, not intubated with hypoxemic respiratory failure despite O2 insufflation: PaO2/FiO2 ratio 105 vs. 111, PaCO2 34 vs. 32 mmHg, resp. rate 30 vs. 31 |
n = 55 HFNC through large-bore binasal prongs with a high-flow heated humidifier device, initial gas flow 50 L/min and FIO2 of 1.0, flow and FIO2 were subsequently adjusted between 30–60 L/min and 0.5–1.0, to maintain SpO2 of 94% or more. adherence to intended treatment protocol: Not quantified, but “Increased subject compliance and ease with which awake proning could be facilitated in HFNC group could have influenced the outcome in favor of the latter.” |
n = 54 NIV with either mask/helmet device connected to an ICU ventilator, PS of 10–20 cm H2O (aim of obtaining an expired tidal volume of 7–10 mL per kg of predicted body weight), PEEP 5–10 cm H2O, FIO2 0.5–1.0 titrated to target SpO2 > 94%. adherence to intended treatment protocol: Not quantified, but “Although NIV was initiated with PS 10–20 cm H2O, most of the subjects required PS) 5 cm H2O, [...]. Subjects on NIV usually felt claustrophobic and frequently complained of dry mouth, leading to repeated detachments of the oxygenation interface. Moreover, most of the subjects on NIV were not compliant.” The incompliance with NIV could have been even higher than expected due to aggressive initial ventilator settings. |
Intubation or death Intubation within 7 days In-hospital mortality Ventilator-free days at 28 d |
HR 0.51 (95% CI 0.28–0.94, p = 0.03) 15/55 vs. 25/54: RR 0.59 (0.35 to 0.99) 16/55 vs. 25/54: RR 0.63 (0.38 to 1.04); HR 0.54 (0.29 to 1.01) median 28.0 (IQR 27–28) vs. 27.5 (27–28) These data are implausible to us, given that 27% vs. 46% of patients were intubated within 7 days and the respective Kaplan-Meier estimator in the publication. This might be due to an unreported particular definition of this complex endpoint prone to misinterpretation. |
| awake prone positioning | |||||
| Ehrmann et al. [28] Recruitment: 4 April 2020–26 January 2021 |
n = 1121 | n = 564 | n = 557 | intubation or death | RR 0.86 (95% CI 0.75 to 0.98) |
| age (years) 61.5 ± 13.3 (intervention) 60.7 ± 13.3 (control) |
(awake) prone positioning for as long as possible | unrestricted (self) positioning except prone | 28-day mortality | RR 0.87 (0.71 to 1.07) | |
| comorbidities diabetes mellitus, chronic heart disease, obesity |
intubation within 28 days | RR 0.83 (0.71 to 0.96) | |||
| clinical status inpatient treatment, need for HFNC or NIV, no mechanical ventilation (i.e., WHO clinical progression scale 6) |
hospitalization: length of hospital stay (censored at 28 days) | difference of means −0.2 days (−1.35 to 0.96) | |||
| skin lesions | RR 0.5 (0.16 to 1.56) | ||||
| weaning from HFNC (time to event in days) | difference of means -0.9 days (0.35 to 1.45) | ||||
| Rosén et al. [29] Recruitment: 7 October 2020–7 February 2021 |
n = 75 | n = 36 | n = 39 | intubation within 30 days | RR 1.00 (0.53 to 1.90) |
| age (years) 65 (IQR 53–74; intervention) 65 (IQR 55–70; control) |
(awake) prone positioning ≥16 h/day | unrestricted (self) positioning except prone | 30-day mortality | RR 2.17 (0.58 to 8.03) | |
| comorbidities diabetes mellitus, arterial hypertension, obesity |
hospitalization: length of hospital stay (censored after 30 days) | difference of means −2.0 days (−7.16 to 3.16) | |||
| clinical status inpatient treatment, need for HFNC or NIV, no mechanical ventilation (i.e., WHO clinical progression scale 6) |
skin lesions | RR 0.24 (0.06 to 1.04) | |||
| days free of HFNC | difference of means 2.0 days (0.13 to 3.87) | ||||
3.2.2. APP
We included two RCTs on APP in this systematic review. The study by Ehrmann et al. [28] was designed and conducted as a prospective meta-analysis of six individual multicenter RCTs. As its results were reported and published in a combined publication, we did not assess the individual national RCTs in this systematic review. The second included multicenter RCT by Rosén et al. [29] contributed 75 of the overall 1196 patients included in this review. In both trials, participants were treated in an in-hospital setting. The intervention of APP intended to last for as long as possible in the trial by Ehrmann, whereas Rosén defined a proning duration of at least 16 h per day. This goal was not met (median prone time 9.0 h, IQR 4.4–10.6). Moreover, participants in the control group were also exposed to prone positioning for a median of 3.4 h per day (IQR 1.8–8.4). In the trial by Ehrmann, durations of proning varied widely between the participating countries. Mean proning duration in Spain was 1.7 h/day (SD 1.2), whereas in the Mexican sub-trial patients spent 9.0 (3.2) hours in a prone position. Proning in the control groups occurred only to a very small extent.
Of our predefined main outcomes, both studies reported mortality at day 28 as well as need for intubation until day 28. Table 1 summarizes the characteristics of the included studies and the reported outcomes.
3.3. Risk of Bias
3.3.1. HFNC vs. NIV
We assessed risk of bias and methodological quality for both RCTs using the RoB2 tool. In the overall bias assessment, we rated no outcome at low risk, three outcomes with some concerns and 14 with high risk of bias. This was mostly due to the unblinded study design affecting deviations from intended intervention (Perkins, Nair, one outcome Grieco), but also problems in the randomization process (Nair), selection of the reported result (Grieco). Detailed assessment of risk of bias for each outcome is summarized in Table 2.
Table 2.
Risk of bias assessment of the included studies per outcome. D1: randomization process, D2: deviations from the intended interventions, D3: missing outcome data, D4: measurement of the outcome, D5: selection of the reported results, HFNC high-flow nasal cannula, NIV: non-invasive ventilation.
| Study | Outcome | D1 | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|---|
| APP | |||||||
| Ehrmann et al. [28] | Intubation or death at 28 days |

|

|

|

|

|

|
| Ehrmann et al. | Mortality rate at 28 days |

|

|

|

|

|

|
| Ehrmann et al. | Need for intubation within 28 days |

|

|

|

|

|

|
| Ehrmann et al. | Hospital length of stay (censored at 28 days) |

|

|

|

|

|

|
| Ehrmann et al. | Occurrence of skin lesions |

|

|

|

|

|

|
| Ehrmann et al. | Weaning of HFNC (time to event) |

|

|

|

|

|

|
| Rosén et al. [29] | Mortality rate at day 30 |

|

|

|

|

|

|
| Rosén et al. | Need for intubation within 30 days |

|

|

|

|

|

|
| Rosén et al. | Occurrence of skin lesions |

|

|

|

|

|

|
| Rosén et al. | Hospital length of stay (censored at 30 days) |

|

|

|

|

|

|
| Rosén et al. | Days free of HFNC |

|

|

|

|

|

|
| HFNC vs. NIV | |||||||
| Grieco et al. [26] | Mortality rate at 28 days/60 days |

|

|

|

|

|

|
| Grieco et al. | In-hospital mortality |

|

|

|

|

|

|
| Grieco et al. | Intubation up to day 28 |

|

|

|

|

|

|
| Grieco et al. | Respirator-free days at 30 days |

|

|

|

|

|

|
| Grieco et al. | Hospital length of stay |

|

|

|

|

|

|
| Grieco et al. | Adverse events |

|

|

|

|

|

|
| Perkins et al. [25] | Intubation or death at 30 days |

|

|

|

|

|

|
| Perkins et al. | In-hospital mortality |

|

|

|

|

|

|
| Perkins et al. | Mortality rate at 30 days |

|

|

|

|

|

|
| Perkins et al. | Hospital length of stay |

|

|

|

|

|

|
| Perkins et al. | Intubation up to day 30 |

|

|

|

|

|

|
| Perkins et al. | Serious adverse events |

|

|

|

|

|

|
| Perkins et al. | Adverse events |

|

|

|

|

|

|
| Nair et al. [27] | In-hospital mortality |

|

|

|

|

|

|
| Nair et al. | Intubation rate at 7 days |

|

|

|

|

|

|
| Nair et al. | Intubation or death |

|

|

|

|

|

|
| Nair et al. | Hospital length of stay |

|

|

|

|

|

|
 low risk,
low risk,  some concerns,
some concerns,  high risk.
high risk.
3.3.2. APP
In total, the studies contributed 11 relevant outcomes. Due to the nature of the interventions, neither patients nor healthcare providers were able to be blinded in the included trials, which led at least to some concerns in every reported outcome. In the overall bias assessment, we rated one outcome at low risk, five outcomes with some concerns and five with high risk of bias. The outcomes we rated to be at high risk of bias were all from Rosén et al. due to the high amount of awake prone positioning in the control group. Detailed assessment of risk of bias for each outcome is summarized in Table 2.
3.4. Effects of the Intervention
3.4.1. HFNC vs. NIV in COVID-19 Acute Respiratory Failure
The main outcome of in-hospital mortality (to longest follow-up) was reported by the three included RCTs (RR 0.92; 95% CI 0.65–1.33, 986 patients from 3 studies, very low certainty), while two of the studies further reported mortality up to day 30 (RR 1.14; 95% CI 0.86–1.51, 902 patients from 2 studies, very low certainty), hence we do not know whether HFNC compared to NIV increases or decreases the risk of death. We downgraded certainty of evidence for both mortality outcomes due to serious risk of bias, serious imprecision, and indirectness.
Perkins provided data for another main outcome, intubation or death at day 28. HFNC compared to NIV may increase intubation or death (RR 1.22, 95% CI 1.03–1.45, 791 patients from 1 study). Certainty was low due to serious risk of bias and serious indirectness. Perkins and Grieco reported intubation within 28 and 30 days. We do not know if HFNC compared to NIV increases the risk of intubation (RR 1.34, 95% CI 1.00–1.80, 900 participants from 2 studies, very low certainty). Downgrading to very low certainty of evidence was due to serious risk of bias, imprecision, and indirectness. Adverse events were reported by Perkins and Grieco, although a predefined selection of events relevant in respiratory and intensive care medicine was made by the latter. As we deemed this selection still useful, we extracted AEs from both trials in the only way the reporting allowed to facilitate comparison: total events per patient in each arm. We do not know if HFNC vs. NIV increases or decreases AEs (Perkins: NIV 200/380 vs. HFNC 157/417; Grieco: 37/54 vs. 70/55, very low certainty). The downgrading to very low certainty is based on serious risk of bias, inconsistency, and indirectness. Length of Hospital stay with a follow-up of 30 days was reported by Perkins and may be longer in the HFNC group (MD 1.9, 95% CI 0.75 less to 4.55 more; baseline 16.4 in NIV group, low certainty). We downgraded due to serious risk of bias, imprecision, and indirectness. Respirator-free days defined as free from invasive ventilation, HFNC, and NIV in one study may have been decreased by HFNC (MD 2.0 days, 95% CI 6.16 less to 2.13 more, with baseline 15 days in the NIV group, low certainty). We downgraded here for very serious imprecision.
3.4.2. APP in COVID-19 Acute Respiratory Failure
The main outcome of mortality at 28 days was reported by both studies (Rosén reported mortality at 30 days). Prone positioning may have little to no effect on mortality at 28 days (RR 1.08, 95% CI 0.51–2.31, 1196 participants from two studies). The certainty of evidence is low for this outcome due to serious risk of bias and serious imprecision.
Ehrmann provided data for another main outcome, the combined risk of intubation or death until day 28. APP probably decreases the risk for this outcome (RR 0.86, 95% CI 0.75–0.98, 1121 participants from 1 study). Certainty of evidence was moderate due to serious risk of bias.
With moderate certainty of evidence due to serious risk of bias, prone position compared to standard of care probably decreases the rate of intubation (RR 0.83, 95% CI 0.71–0.96, 1196 participants from 2 studies) and probably increases the time of weaning of HFNC slightly (MD 0.9 days, 95% CI 0.35–1.45, 1121 participants from 1 study), but probably has little or no effect on length of hospital stay (difference of means −0.2 days, 95% CI −1.35–0.96, 1196 participants from 2 studies). With low certainty of evidence due to serious risk of bias and imprecision, APP may decrease the days free of HFNC slightly (difference of means 2 days, 95% CI 0.13–3.87, 50 participants from 1 study). With regard to the occurrence of skin lesions due to the intervention, we are uncertain whether APP increases or decreases the risk for this outcome (RR 0.5, 95% CI 0.16–1.56, 1196 participants from 2 studies).
Effects of the interventions are detailed in the summary of findings tables, Table 3 for HNFC vs. NIV and Table 4 for APP.
Table 3.
Effects of HFNC vs. NIV on predefined outcomes. RR: relative risk; CI: confidence interval; HFNC: high-flow nasal cannula; NIV: non-invasive ventilation.
| Outcome | Results | Absolute Effect Estimates | Certainty of Evidence | |
|---|---|---|---|---|
| NIV | HFNC | |||
| Mortality: in-hospital (up to longest follow-up) | RR: 0.92 (95% CI 0.65–1.33) 986 patients from 3 studies |
233 per 1000 |
214 per 1000 |
very low due to serious risk of bias, serious imprecision and indirectness |
| difference: 19 less per 1000 (95% CI 82 less to 77 more) | ||||
| Mortality: up to day 30 (follow-up 28 to 30 days) | RR: 1.14 (95% CI 0.86–1.51) 902 patients from 2 studies |
164 per 1000 |
187 per 1000 |
very low due to serious risk of bias, serious imprecision and indirectness |
| difference: 23 more per 1000 (95% CI 23 less to 84 more) | ||||
| Intubation or death (follow-up 30 days) | RR 1.22 (95% CI 1.03–1.45) 791 patients from 1 study |
363 per 1000 |
443 per 1000 |
low due to serious risk of bias and indirectness |
| difference: 80 more per 1000 (95% CI 11 more to 164 more) | ||||
| Intubation (follow-up 28 to 30 days) | RR 1.34 (95% CI 1.00–1.80) 900 patients from 2 studies |
329 per 1000 |
441 per 1000 |
very low due to serious risk of bias, serious imprecision and indirectness |
| difference: 112 more per 1000 (95% CI 0 more to 263 more) | ||||
| Serious adverse events (follow-up 30 days) |
RR 0.06 (95% CI 0.00–1.06) 797 patients from 1 study |
18 per 1000 |
1 per 1000 |
very low due to serious imprecision, serious risk of bias and indirectness |
| difference 17 less per 1000 (95% CI 18 less to 2 more) | ||||
| Adverse events (follow-up 30 days) | 906 patients from 2 studies | Perkins: NIV 200/380 vs. HFNC 157/417 (as no. events in total) Grieco: 37/54 vs. 70/55 |
very low due to serious risk of bias, inconsistency and indirectness |
|
| Length of hospital stay (censored at 30 days) |
difference of means +1.90 days 768 patients from 1 study |
16.4 days | 18.3 days | low due to serious risk of bias, serious imprecision and indirectness |
| difference: 1.90 days more (95% CI 0.75 less to 4.55 more) | ||||
| Respiratory-support-free days: no invasive ventilation, HFNC, or NIV (follow-up 28 days) | difference of means 2 days 109 patients from 1 study |
15 (11) | 13 (11) | low due very serious imprecision |
| difference: 2.0 days more (95% CI 6.16 less to 2.13 more) | ||||
Table 4.
Summary of findings—effects of awake prone positioning on predefined outcomes. RR: relative risk; CI: confidence interval; SoC: standard of care; APP: awake prone positioning; HFNC: high-flow nasal cannula.
| Outcome | Results | Absolute Effect Estimates | Certainty of Evidence | |
|---|---|---|---|---|
| SoC | APP | |||
| All-cause mortality (28 days) | RR: 1.08 (95% CI 0.51–2.31) 1196 patients from 2 studies |
227 per 1000 |
245 per 1000 |
low due to serious risk of bias and serious imprecision |
| difference: 18 more per 1000 (95% CI 111 less to 297 more) | ||||
| Intubation or death at 28 days | RR 0.86 (95% CI 0.75–0.98) 1121 patients from 1 study |
461 per 1000 |
396 per 1000 |
moderate due to serious risk of bias |
| difference: 65 less per 1000 (95% CI 115 less to 9 less) | ||||
| Intubation within 28 days | RR 0.83 (95% CI 0.71–0.96) 1196 patients from 2 studies |
396 per 1000 |
329 per 1000 |
moderate due to serious risk of bias |
| difference: 67 less per 1000 (95% CI 115 less to 16 less) | ||||
| Hospital length of stay (censored at 28 days) |
difference of means −0.2 days 1196 patients from 2 studies |
16.6 days | 16.4 days | moderate due to serious risk of bias |
| difference: 0.2 days less (95% CI 1.35 less to 0.96 more) | ||||
| Days free of HFNC within 30 days | difference of means 2 days 50 patients from 1 study |
24 days | 26 days | low due to serious risk of bias and serious imprecision |
| difference: 2.0 days more (95% CI 0.13 more to 3.87 more) | ||||
| Weaning of HFNC (time to event within 28 days) | difference of means 0.9 days 1121 patients from 1 study |
6.0 days | 6.9 days | moderate due to serious risk of bias |
| difference: 0.9 days more (95% CI 0.35 more to 1.45 more) | ||||
| Skin lesions within 28 days | RR 0.5 (95% CI 0.16–1.56) 1196 patients from 2 studies |
32 per 1000 |
16 per 1000 |
very low due to serious risk of bias and very serious imprecision |
| difference: 16 less per 1000 (95% CI 27 less to 18 more) | ||||
4. Discussion
This review aimed to assess the efficacy and safety of different non-invasive strategies of respiratory support for the treatment of COVID-19.
So far, substantial recommendations on the use of non-invasive respiratory techniques in COVID-19 acute respiratory failure could not be made due to a lack of evidence. Nevertheless, previous studies in non-COVID-19 acute respiratory failure have shown beneficial outcomes for patients treated with HFNC or NIV [30]. Similarly, prone positioning has been a key part of non-COVID-19 ARDS treatment strategies for years [31]. Considering the urgent need for effective and efficient treatment strategies in the face of an ongoing pandemic, HFNC/NIV and APP could benefit both patients and healthcare systems alike. Obviously, the dynamic of the pandemic with changes in standard of care, vaccination as well as virus variants leaves us with the problem of transferability to present and future patients.
Regarding the question whether HFNC is superior to NIV as non-invasive respiratory support in COVID-19 patients suffering acute respiratory failure, we identified three RCTs (Grieco 2021, Nair 2021, Perkins 2021). These had been conducted in three different countries, including two high-income (United Kingdom, Italy) and one lower-middle-income country (India) [32]. The largest study, Perkins, included a considerable share (about 15%) of patients without laboratory-confirmed COVID-19.
The available evidence on APP is based on two RCTs, of which Ehrmann et al. was designed and conducted as a prospective meta-analysis/multi-national meta-RCT [28] and contributed more than 90% of the patients included in this review. Both trials recruited patients during comparable time periods and relevant accompanying treatments as part of SoC were similar (e.g., administration of glucocorticoids in a large proportion of all patients). The included trials reported difficulties applying the intended intervention in a standardized manner: Ehrmann et al. aimed to apply awake prone positioning for as long as possible each day. However, the absolute durations of actual APP varied widely between the involved countries (1.7 ± 1.2 h in Spain vs. 9.0 ± 3.2 h in Mexico; overall trial: 5.6 ± 4.4 h). Longer APP durations were associated with greater treatment success. APP in the SoC control group was little to none, which was an issue the trial by Rosén was facing. There, the authors described increasing durations of APP in patients from the control group, which eventually also led to the termination of the trial.
The five RCTs on non-invasive respiratory strategies took place during the second half of 2020 and first half of 2021, hence presumably different variants of SARS-CoV-2 were prevalent and vaccination programs, not yet rolled out at full scale or at all. Although basic pathophysiology might not, pharmacologic and non-pharmacologic treatment recommendations and practice in COVID-19 have changed over time. We cannot quantify possible indirectness but consider it to be of minor influence.
The certainty of the evidence regarding our predefined outcomes was downgraded for all outcomes, mostly due to serious risk of bias, serious imprecision and/or serious indirectness.
In our analysis of the study data on benefits and harms of HFNC compared to NIV, there remains uncertainty whether HFNC slightly increases or decreases mortality (different mortality outcomes, i.e., in-hospital mortality, mortality up to day 30 with different effect directions). Nonetheless, NIV may reduce the need for intubation up to day 30 and the composite endpoint “need for intubation or death”. Regarding patient safety, both HFNC and NIV resulted in numerous adverse events. However, we are uncertain whether either one resulted in more adverse events due to very low certainty of the evidence. NIV resulted in considerably more serious adverse events than HFNC (7/380 vs. 0/417, Perkins), yet again with very low certainty of evidence. In summary, based on our analyses there is no evidence for a substantial benefit in mortality for either non-invasive respiratory therapy technique. However, NIV may result in a decreased need for endotracheal intubation, which certainly must be considered an outcome relevant to the patient.
Considering the question of benefits and harms of awake prone positioning as an additional strategy of non-invasive respiratory support for COVID-19 patients, with low certainty APP seems to have little or no effect on mortality, while there is evidence of moderate certainty that APP reduces the need for endotracheal intubation.
To make use of the presented evidence, national guideline committees could use our analyses for evidence-based decision making in the context of their local resources and requirements. Based on the patient-centered outcomes identified in this work, we consider NIV accompanied by APP to be the method of choice for respiratory support in the non-intubated COVID-19 patient with hypoxemic respiratory failure.
There is still lack of evidence with regards to overall mortality, the extent of adverse events, and for the treatment of distinct groups of patients (e.g., obesity) or pathophysiological conditions (e.g., prominent hypercapnic respiratory failure). Further high-quality trials will need to address these open questions. As it has strong implications for clinical research, we would like to stress that immanent bias in open-label studies should be countered as best as possible by increased rigor in planning, conducting, and reporting of the trials to generate useful evidence ready for meta-analysis.
5. Conclusions
There was no certain evidence that one of the three different non-invasive respiratory support techniques (HFNC, NIV and APP) increases or decreases mortality in patients with COVID-19 acute respiratory failure. With moderate certainty our analysis revealed that NIV compared to HFNC and APP compared to SoC decrease the need for endotracheal intubation. Given the uncertain safety profiles of NIV and HFNC, critically ill patients should be closely monitored. Irrespective of the kind of respiratory support, APP seems to be an advantageous supportive measure for non-intubated patients in respiratory failure due to COVID-19.
Acknowledgments
We would like to thank Kathrin Grummich for her peer review of the search strategy.
Appendix A. Search Strategy
Appendix A.1. Awake Prone Positioning (APP)
Appendix A.1.1. Cochrane COVID-19 Study Register
“prone position” OR “prone positioning” OR proning OR pronation OR suping OR “lateral recumbent position” OR “lateral recumbent positioning” OR “supine position” OR “supine positioning” OR supping
Filtered by: “Intervention assignment”: Randomised OR Unclear
Appendix A.1.2. WHO COVID-19 Global Literature on Coronavirus Disease
Title, Abstract, Subject: “prone position*” OR proning OR pronation OR suping OR “lateral recumbent position*” OR “supine position*”
Excluded databases: PubMed, MEDLINE, ICTRP, EMBASE, PMC
Appendix A.1.3. Web of Science (Science Citation Index Expanded and Emerging Citation Index)
(AB=((COVID OR COVID19) OR (“SARS-CoV-2” OR “SARS-CoV2” OR SARSCoV2 OR “SARSCoV-2” OR “SARS coronavirus 2”) OR (“2019 nCoV” OR “2019nCoV” OR “2019-novel CoV” OR “nCov 2019” OR “nCov 19”) OR (“severe acute respiratory syndrome coronavirus 2” OR “novel coronavirus disease” OR “novel corona virus disease” OR “corona virus disease 2019” OR “coronavirus disease 2019” OR “novel coronavirus pneumonia” OR “novel corona virus pneumonia”) OR (“severe acute respiratory syndrome coronavirus 2”)) OR TI=((COVID OR COVID19) OR (“SARS-CoV-2” OR “SARS-CoV2” OR SARSCoV2 OR “SARSCoV-2” OR “SARS coronavirus 2”) OR (“2019 nCoV” OR 2019nCoV OR “2019-novel CoV” OR “nCov 2019” OR “nCov 19”) OR (“severe acute respiratory syndrome coronavirus 2” OR “novel coronavirus disease” OR “novel corona virus disease” OR “corona virus disease 2019” OR “coronavirus disease 2019” OR “novel coronavirus pneumonia” OR “novel corona virus pneumonia”) OR (“severe acute respiratory syndrome coronavirus 2”))) AND (AB=((pron* position*) OR proning OR pronation OR suping OR “lateral recumbent position” OR (supin* position*))OR TI=((pron* position*) OR proning OR pronation OR suping OR “lateral recumbent position” OR (supin* position*))), Timespan: 2020–2021
Appendix A.1.4. CINAHL (Cumulative Index to Nursing and Allied Health Literature)
((TI (“SARS-CoV-2” OR “SARS-CoV2” OR “SARSCoV-2” OR SARSCoV2 OR “SARS-CoV*” OR SARSCoV* OR “severe acute respiratory syndrome 2” OR “severe acute respiratory syndrome cov*” OR “COVID-19” OR COVID19* OR COVID OR nCoV* OR 2019nCoV* OR 19nCoV* OR “HCoV-19” OR coronavirus* OR “corona virus*”) OR AB (“SARS-CoV-2” OR “SARS-CoV2” OR “SARSCoV-2” OR SARSCoV2 OR “SARS-CoV*” OR SARSCoV* OR “severe acute respiratory syndrome 2” OR “severe acute respiratory syndrome cov*” OR “COVID-19” OR COVID19* OR COVID OR nCoV* OR 2019nCoV* OR 19nCoV* OR “HCoV-19” OR coronavirus* OR “corona virus*”) OR SU (“SARS-CoV-2” OR “SARS-CoV2” OR “SARSCoV-2” OR SARSCoV2 OR “SARS-CoV*” OR SARSCoV* OR “severe acute respiratory syndrome 2” OR “severe acute respiratory syndrome cov*” OR “COVID-19” OR COVID19* OR COVID OR nCoV* OR 2019nCoV* OR 19nCoV* OR “HCoV-19”)) AND (DT 20191117-3000)) AND (MH (“Prone Position” OR “Supine Position”) OR TI ((pron* position*) OR proning OR proned OR pronation OR suping OR “lateral recumbent position” OR (supin* position*)) OR AB ((pron* position*) OR proning OR proned OR pronation OR suping OR “lateral recumbent position” OR (supin* position*)))
Appendix A.2. High-Flow Nasal Oxygen or Non-Invasive Ventilation (NIV)
Appendix A.2.1. Cochrane COVID-19 Study Register
“noninvasive mechanical ventilation” OR “noninvasive ventilation” OR “non-invasive ventilation” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR NIV OR NIPPV OR NPPV OR “high-flow oxygen” OR “oxygen insufflation” OR “oxygen inhalation” OR “inhaled oxygen” OR “high-flow nasal” OR “nasal canula” OR “nasal oxygen” OR HFNC OR “oxygen mask” OR “oxygen therapy” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR “positive pressure” OR “positive airway pressure” OR “end-expiratory pressure” OR “endexpiratory pressure” OR CPAP OR NCPAP OR PEEP OR autoPEEP OR iPEEP OR BiPAP OR helmet OR “bidirectional oxygenation” OR “face mask ventilation” OR “oxygenation strategy” OR “oxygenation strategies” OR “oxygen strategy” OR “oxygen strategies” OR “respiratory support” OR “respiratory strategies” OR “respiratory strategy”
Filtered by: “Intervention assignment”: Randomised OR Unclear
Appendix A.2.2. WHO COVID-19 Global Literature on Coronavirus Disease
Title, abstract, subject: (“noninvasive mechanical ventilation” OR “noninvasive ventilation” OR “non-invasive ventilation” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR NIV OR NIPPV OR NPPV OR “high-flow oxygen” OR “oxygen insufflation” OR “oxygen inhalation” OR “inhaled oxygen” OR “high-flow nasal” OR “nasal canula” OR “nasal oxygen” OR HFNC OR “oxygen mask*” OR “oxygen therapy” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR “positive pressure” OR “positive airway pressure” OR “end-expiratory pressure” OR “endexpiratory pressure” OR CPAP OR NCPAP OR PEEP OR autoPEEP OR iPEEP OR BiPAP OR helmet OR “bidirectional oxygenation” OR “face mask ventilation” OR “oxygenation strategy” OR “oxygenation strategies” OR “oxygen strategy” OR “oxygen strategies” OR “respiratory support” OR “respiratory strateg*”) AND (random* OR placebo OR trial OR groups OR “phase 3” or “phase3” or p3 or “pIII”)
Excluded databases: PubMed, MEDLINE, ICTRP, EMBASE, PMC
Appendix A.2.3. Web of Science (Science Citation Index Expanded and Emerging Citation Index)
#1 TI=((COVID OR COVID19) OR (“SARS-CoV-2” OR “SARS-CoV2” OR SARSCoV2 OR”SARSCoV-2” OR “SARS coronavirus 2”) OR (“2019 nCoV” OR “2019nCoV” OR “2019-novel CoV” OR “nCov 2019” OR “nCov 19”) OR (“severe acute respiratory syndrome coronavirus 2” OR “novel coronavirus disease” OR “novel corona virus disease” OR “corona virus disease 2019” OR “coronavirus disease 2019” OR “novel coronavirus pneumonia” OR “novel corona virus pneumonia”) OR (“severe acute respiratory syndrome coronavirus 2”))
#2 AB=((COVID OR COVID19) OR (“SARS-CoV-2” OR “SARS-CoV2” OR SARSCoV2 OR “SARSCoV-2” OR “SARS coronavirus 2”) OR (“2019 nCoV” OR “2019nCoV” OR “2019-novel CoV” OR “nCov 2019” OR “nCov 19”) OR (“severe acute respiratory syndrome coronavirus 2” OR “novel coronavirus disease” OR “novel corona virus disease” OR “corona virus disease 2019” OR “coronavirus disease 2019” OR “novel coronavirus pneumonia” OR “novel corona virus pneumonia”) OR (“severe acute respiratory syndrome coronavirus 2”))
#3 #1 OR #2
#4 TI=(“noninvasive mechanical ventilation” OR “noninvasive ventilation” OR “non-invasive ventilation” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR NIV OR NIPPV OR NPPV OR “high-flow oxygen” OR “oxygen insufflation” OR “oxygen inhalation” OR “inhaled oxygen” OR “high-flow nasal” OR “nasal canula” OR “nasal oxygen” OR HFNC OR “oxygen mask*” OR “oxygen therapy” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR “positive pressure” OR “positive airway pressure” OR “end-expiratory pressure” OR “endexpiratory pressure” OR CPAP OR NCPAP OR PEEP OR autoPEEP OR iPEEP OR BiPAP OR helmet OR “bidirectional oxygenation” OR “face mask ventilation” OR “oxygenation strategy” OR “oxygenation strategies” OR “oxygen strategy” OR “oxygen strategies” OR “respiratory support” OR “respiratory strateg*”)
#5 AB=(“noninvasive mechanical ventilation” OR “noninvasive ventilation” OR “non-invasive ventilation” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR NIV OR NIPPV OR NPPV OR “high-flow oxygen” OR “oxygen insufflation” OR “oxygen inhalation” OR “inhaled oxygen” OR “high-flow nasal” OR “nasal canula” OR “nasal oxygen” OR HFNC OR “oxygen mask*” OR “oxygen therapy” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR “positive pressure” OR “positive airway pressure” OR “end-expiratory pressure” OR “endexpiratory pressure” OR CPAP OR NCPAP OR PEEP OR autoPEEP OR iPEEP OR BiPAP OR helmet OR “bidirectional oxygenation” OR “face mask ventilation” OR “oxygenation strategy” OR “oxygenation strategies” OR “oxygen strategy” OR “oxygen strategies” OR “respiratory support” OR “respiratory strateg*”)
#6 #4 OR #5
#7 #3 AND #6, Indexes=SCI-EXPANDED, ESCI Timespan=2020-2021
Appendix A.2.4. CINAHL (Cumulative Index to Nursing and Allied Health Literature)
S1. TI ((COVID OR COVID19) OR (“SARS-CoV-2” OR “SARS-CoV2” OR SARSCoV2 OR”SARSCoV-2” OR “SARS coronavirus 2”) OR (“2019 nCoV” OR “2019nCoV” OR “2019-novel CoV” OR “nCov 2019” OR “nCov 19”) OR (“severe acute respiratory syndrome coronavirus 2” OR “novel coronavirus disease” OR “novel corona virus disease” OR “corona virus disease 2019” OR “coronavirus disease 2019” OR “novel coronavirus pneumonia” OR “novel corona virus pneumonia”) OR (“severe acute respiratory syndrome coronavirus 2”))
S2. AB ((COVID OR COVID19) OR (“SARS-CoV-2” OR “SARS-CoV2” OR SARSCoV2 OR”SARSCoV-2” OR “SARS coronavirus 2”) OR (“2019 nCoV” OR “2019nCoV” OR “2019-novel CoV” OR “nCov 2019” OR “nCov 19”) OR (“severe acute respiratory syndrome coronavirus 2” OR “novel coronavirus disease” OR “novel corona virus disease” OR “corona virus disease 2019” OR “coronavirus disease 2019” OR “novel coronavirus pneumonia” OR “novel corona virus pneumonia”) OR (“severe acute respiratory syndrome coronavirus 2”))
S3. S1 OR S2
S4. TI (“noninvasive mechanical ventilation” OR “noninvasive ventilation” OR “non-invasive ventilation” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR NIV OR NIPPV OR NPPV OR “high-flow oxygen” OR “oxygen insufflation” OR “oxygen inhalation” OR “inhaled oxygen” OR “high-flow nasal” OR “nasal canula” OR “nasal oxygen” OR HFNC OR “oxygen mask” OR “oxygen therapy” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR “positive pressure” OR “positive airway pressure” OR “end-expiratory pressure” OR “endexpiratory pressure” OR CPAP OR NCPAP OR PEEP OR autoPEEP OR iPEEP OR BiPAP OR helmet OR “bidirectional oxygenation” OR “face mask ventilation” OR “oxygenation strategy” OR “oxygenation strategies” OR “oxygen strategy” OR “oxygen strategies” OR “respiratory support” OR “respiratory strategies” OR “respiratory strategy”)
S5. AB (“noninvasive mechanical ventilation” OR “noninvasive ventilation” OR “non-invasive ventilation” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR NIV OR NIPPV OR NPPV OR “high-flow oxygen” OR “oxygen insufflation” OR “oxygen inhalation” OR “inhaled oxygen” OR “high-flow nasal” OR “nasal canula” OR “nasal oxygen” OR HFNC OR “oxygen mask” OR “oxygen therapy” OR “noninvasive oxygenation” OR “non-invasive oxygenation” OR “positive pressure” OR “positive airway pressure” OR “end-expiratory pressure” OR “endexpiratory pressure” OR CPAP OR NCPAP OR PEEP OR autoPEEP OR iPEEP OR BiPAP OR helmet OR “bidirectional oxygenation” OR “face mask ventilation” OR “oxygenation strategy” OR “oxygenation strategies” OR “oxygen strategy” OR “oxygen strategies” OR “respiratory support” OR “respiratory strategies” OR “respiratory strategy”)
S6. S4 OR S5
S7. S3 AND S6
Author Contributions
All authors have made substantial contributions to this systematic review. Conceptualization: S.W., B.S., M.G. and F.F.; methodology: S.W., B.S., A.-L.F., M.G. and M.-I.M.; software: S.W., B.S., A.-L.F., C.S.R., M.G. and M.-I.M.; literature search: M.-I.M., literature screening: C.S.R., M.G., A.-L.F. and B.S.; extraction: A.-L.F., M.G., B.S. and S.W.; RoB assessment: A.-L.F., M.G., B.S. and S.W.; writing—original draft preparation, B.S., M.G., A.-L.F. and F.F.; writing—review and editing: S.W. and B.S.; supervision and project administration: B.S., S.W. and F.F.; funding acquisition: S.W. and F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This review is part of the CEOsys project funded by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Details of the literature search are reported in the Appendix A. Extracted data and details of the risk of bias assessments are available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Word Health Organization WHO Coronavirus (COVID-19) Dashboard. [(accessed on 24 November 2021)]. Available online: https://covid19.who.int.
- 2.Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., Felix S.E.B., Tie Y., Fullerton K.E. Coronavirus Disease 2019 Case Surveillance—United States, 22 January–30 May 2020. Mmwr Morb. Mortal. Wkly. Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Karagiannidis C., Mostert C., Hentschker C., Voshaar T., Malzahn J., Schillinger G., Klauber J., Janssens U., Marx G., Weber-Carstens S., et al. Case Characteristics, Resource Use, and Outcomes of 10 021 Patients with COVID-19 Admitted to 920 German Hospitals: An Observational Study. Lancet Respir. Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 Does not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiumello D., Busana M., Coppola S., Romitti F., Formenti P., Bonifazi M., Pozzi T., Palumbo M.M., Cressoni M., Herrmann P., et al. Physiological and Quantitative CT-Scan Characterization of COVID-19 and Typical ARDS: A Matched Cohort Study. Intensiv. Care Med. 2020;46:2187–2196. doi: 10.1007/s00134-020-06281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrando C., Suarez-Sipmann F., Mellado-Artigas R., Hernández M., Gea A., Arruti E., Aldecoa C., Martínez-Pallí G., Martínez-González M.A., Slutsky A.S., et al. Clinical Features, Ventilatory Management, and Outcome of ARDS Caused by COVID-19 Are Similar to Other Causes of ARDS. Intensiv. Care Med. 2020;46:2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G., Tonetti T., Protti A., Langer T., Girardis M., Bellani G., Laffey J., Carrafiello G., Carsana L., Rizzuto C., et al. Pathophysiology of COVID-19-Associated Acute Respiratory Distress Syndrome: A Multicentre Prospective Observational Study. Lancet Respir. Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botta M., Tsonas A.M., Pillay J., Boers L.S., Algera A.G., Bos L.D.J., Dongelmans D.A., Hollmann M.W., Horn J., Vlaar A.P.J., et al. Ventilation Management and Clinical Outcomes in Invasively Ventilated Patients with COVID-19 (PRoVENT-COVID): A National, Multicentre, Observational Cohort Study. Lancet Respir Med. 2020;9:139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alhazzani W., Evans L., Alshamsi F., Møller M.H., Ostermann M., Prescott H.C., Arabi Y.M., Loeb M., Gong M.N., Fan E., et al. Surviving Sepsis Campaign Guidelines on the Management of Adults with Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit. Care Med. 2021;49:e219–e234. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]
- 11.Rochwerg B., Einav S., Chaudhuri D., Mancebo J., Mauri T., Helviz Y., Goligher E.C., Jaber S., Ricard J.-D., Rittayamai N., et al. The Role for High Flow Nasal Cannula as a Respiratory Support Strategy in Adults: A Clinical Practice Guideline. Intensiv. Care Med. 2020;46:2226–2237. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter C., Aedy H., Notter J. COVID-19 Disease: Non-Invasive Ventilation and High Frequency Nasal Oxygenation. Clin. Integr. Care. 2020;1:100006. doi: 10.1016/j.intcar.2020.100006. [DOI] [Google Scholar]
- 13.Ferreyro B.L., Angriman F., Munshi L., Sorbo L.D., Ferguson N.D., Rochwerg B., Ryu M.J., Saskin R., Wunsch H., da Costa B.R., et al. Association of Noninvasive Oxygenation Strategies with All-Cause Mortality in Adults with Acute Hypoxemic Respiratory Failure. JAMA. 2020;324:57–67. doi: 10.1001/jama.2020.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalmers J.D., Crichton M.L., Goeminne P.C., Cao B., Humbert M., Shteinberg M., Antoniou K.M., Ulrik C.S., Parks H., Wang C., et al. Management of Hospitalised Adults with Coronavirus Disease 2019 (COVID-19): A European Respiratory Society Living Guideline. Eur. Respir. J. 2021;57:2100048. doi: 10.1183/13993003.00048-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albert R.K., Hubmayr R.D. The Prone Position Eliminates Compression of the Lungs by the Heart. Am. J. Respir. Crit. Care. 2000;161:1660–1665. doi: 10.1164/ajrccm.161.5.9901037. [DOI] [PubMed] [Google Scholar]
- 16.Rehder K., Knopp T.J., Sessler A.D. Regional Intrapulmonary Gas Distribution in Awake and Anesthetized-Paralyzed Prone Man. J. Appl. Physiol. 1978;45:528–535. doi: 10.1152/jappl.1978.45.4.528. [DOI] [PubMed] [Google Scholar]
- 17.Hallifax R.J., Porter B.M., Elder P.J., Evans S.B., Turnbull C.D., Hynes G., Lardner R., Archer K., Bettinson H.V., Nickol A.H., et al. Successful Awake Proning Is Associated with Improved Clinical Outcomes in Patients with COVID-19: Single-Centre High-Dependency Unit Experience. BMJ Open Respir. Res. 2020;7:e000678. doi: 10.1136/bmjresp-2020-000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paternoster G., Sartini C., Pennacchio E., Lisanti F., Landoni G., Cabrini L. Awake Pronation with Helmet Continuous Positive Airway Pressure for COVID-19 Acute Respiratory Distress Syndrome Patients Outside the ICU: A Case Series. Med. Intensiv. :2020. doi: 10.1016/j.medin.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winearls S., Swingwood E.L., Hardaker C.L., Smith A.M., Easton F.M., Millington K.J., Hall R.S., Smith A., Curtis K.J. Early Conscious Prone Positioning in Patients with COVID-19 Receiving Continuous Positive Airway Pressure: A Retrospective Analysis. BMJ Open Respir. Res. 2020;7:e000711. doi: 10.1136/bmjresp-2020-000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid B., Grummich K., Romero C.S., Popp M., Meybohm P., Kranke P., Weibel S. Risk and Harm of Prone Positioning vs. Supine or Lateral Positioning in Hospitalised COVID-19 Patients with Severe Respiratory Insufficiency (PROSPERO Protocol) [(accessed on 29 November 2021)]. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021261862.
- 21.Stroehlein J., Griesel M., Popp M., Fichtner F., Skoetz N., Metzendorf M.-I., Wedekind L. Patients with Severe/Critical COVID-19 and Respiratory Failure: Non-Invasive Ventilation Versus Intubation and Invasive Mechanical Ventilation. [(accessed on 1 December 2021)]. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021230825.
- 22.WHO. Marshall J.C., Murthy S., Diaz J., Adhikari N.K., Angus D.C., Arabi Y.M., Baillie K., Bauer M., Berry S., et al. Working Group on the Clinical Characterisation and Management of COVID-19 Infection. A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.-Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 24.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins G.D., Ji C., Connolly B.A., Couper K., Lall R., Baillie J.K., Bradley J.M., Dark P., Dave C., Soyza A.D., et al. An Adaptive Randomized Controlled Trial of Non-Invasive Respiratory Strategies in Acute Respiratory Failure Patients with COVID-19. Medrxiv. 2021:58. doi: 10.1101/2021.08.02.21261379. [DOI] [Google Scholar]
- 26.Grieco D.L., Menga L.S., Raggi V., Bongiovanni F., Anzellotti G.M., Tanzarella E.S., Bocci M.G., Mercurio G., Dell’Anna A.M., Eleuteri D., et al. Physiological Comparison of High-Flow Nasal Cannula and Helmet Noninvasive Ventilation in Acute Hypoxemic Respiratory Failure. Am. J. Respir. Crit. Care. 2019;201:303–312. doi: 10.1164/rccm.201904-0841OC. [DOI] [PubMed] [Google Scholar]
- 27.Nair P.R., Haritha D., Behera S., Kayina C.A., Maitra S., Anand R.K., Ray B.R., Soneja M., Subramaniam R., Baidya D.K. Comparison of High-Flow Nasal Cannula and Noninvasive Ventilation in Acute Hypoxemic Respiratory Failure Due to Severe COVID-19 Pneumonia. Respir. Care. 2021;66:1824–1830. doi: 10.4187/respcare.09130. [DOI] [PubMed] [Google Scholar]
- 28.Ehrmann S., Li J., Ibarra-Estrada M., Perez Y., Pavlov I., McNicholas B., Roca O., Mirza S., Vines D., Garcia-Salcido R., et al. Awake Prone Positioning for COVID-19 Acute Hypoxaemic Respiratory Failure: A Randomised, Controlled, Multinational, Open-Label Meta-Trial. Lancet Respir. Med. 2021;9:1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosén J., von Oelreich E., Fors D., Fagerlund M.J., Taxbro K., Skorup P., Eby L., Jalde F.C., Johansson N., Bergström G., et al. Awake Prone Positioning in Patients with Hypoxemic Respiratory Failure Due to COVID-19: The PROFLO Multicenter Randomized Clinical Trial. Crit. Care. 2021;25:209. doi: 10.1186/s13054-021-03602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochwerg B., Brochard L., Elliott M.W., Hess D., Hill N.S., Nava S., Navalesi P., Antonelli M., Brozek J., Conti G., et al. Official ERS/ATS Clinical Practice Guidelines: Noninvasive Ventilation for Acute Respiratory Failure. Eur. Respir. J. 2017;50:1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 31.Papazian L., Aubron C., Brochard L., Chiche J.-D., Combes A., Dreyfuss D., Forel J.-M., Guérin C., Jaber S., Mekontso-Dessap A., et al. Formal Guidelines: Management of Acute Respiratory Distress Syndrome. Ann. Intensiv. Care. 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The World Bank World Bank Country and Lending Groups. [(accessed on 2 December 2021)]. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Details of the literature search are reported in the Appendix A. Extracted data and details of the risk of bias assessments are available from the authors upon request.