Abstract
Background:
Monitoring the effects of biologic therapies in skin diseases will benefit from alternative nonivasive skin sampling techniques to examine immune pathways in diseased tissue early and longitudinally.
Objective:
To establish minimally invasive profiling of skin cytokines for diagnosis, therapeutic response monitoring and clinical research in atopic dermatitis (AD) and other skin diseases, particularly in pediatric cohorts.
Methods:
We developed a novel method for cytokine profiling in the epidermis using skin tape strips (STS) in a setting designed to maximize the efficiency of protein extraction from STS. This method was applied to analyze STS protein extracts from lesional skin of AD children (n=41) and normal healthy controls (n=22). Twenty cytokines were probed with ultra sensitive Mesoscale multiplex cytokine assay.
Results:
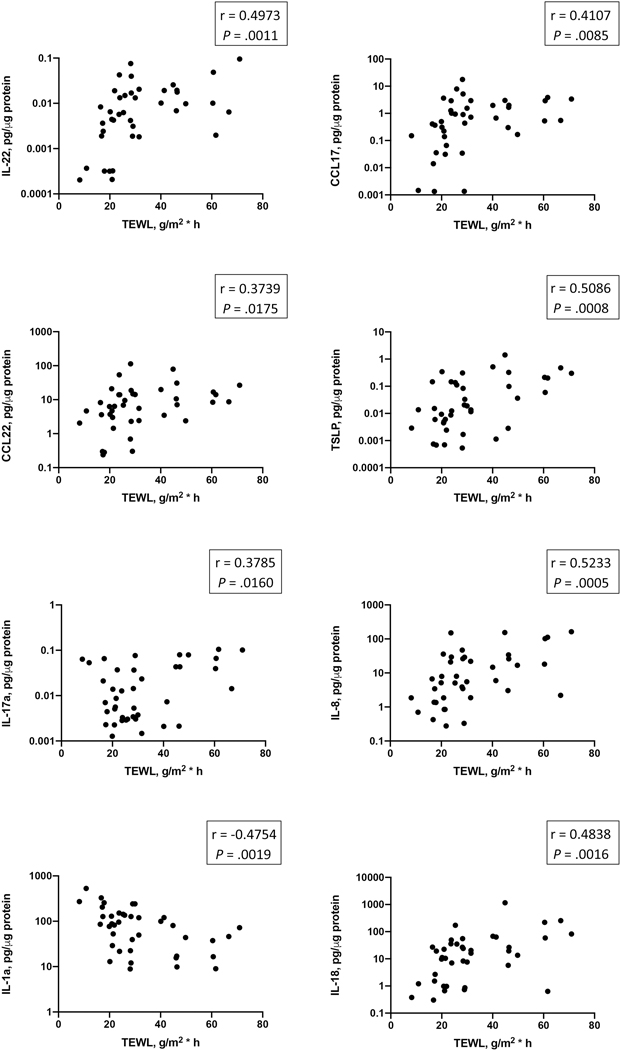
A significant increase in IL-1b, IL-18, IL-8 and a decrease in IL-1a in the stratum corneum of AD lesional skin was found. Concurrently, an increase in markers associated with type 2 inflammatory response was readily detectable in AD lesional skin, including CCL22, CCL17 and TSLP. Levels of IL-1b, IL-18 and TSLP exhibited positive correlations with AD severity index (SCORAD) and skin transepidermal water loss (TEWL), while an inverse correlation between IL-1a and SCORAD, IL-1a and TEWL was found. Levels of CCL17, CCL22, TSLP, IL-22 and IL-17a correlated with skin TEWL measurements.
Conclusion:
Using minimally invasive STS analysis we identified cytokine profiles easily sampled in AD lesional skin. The expression of these markers correlated with disease severity and reflected changes in TEWL in lesional skin. These markers suggest new response assessment targets for AD skin.
Keywords: skin, atopic dermatitis, cytokines
INTRODUCTION
Atopic dermatitis (AD) is the most common pediatric inflammatory skin disease, affecting approximately 20% of children and 5% of adults worldwide.1 It is associated with severe itching, sleep disturbance, and significant impairment of the quality of life.2 There is enormous interest in identifying minimally invasive strategies that allow assessment of skin inflammation following the treatment and monitoring the patient’s adherence to treatment protocols and medications.
Molecular profiling of adult and, more recently, pediatric AD with whole skin biopsies has improved the understanding of the disease pathogenesis and informed new therapeutic strategies. However, biopsies are often difficult to perform in pediatric patients and are not feasible for longitudinal clinical trials that require repetitive skin sampling over long treatment courses. While much less invasive than skin biopsies, analysis of blood samples from AD patients provides only information about systemic inflammation and cannot capture the complexity of localized immune and epithelial interactions in the upper skin layers. Thus, there is an unmet need for non-invasive approaches to accurately capture key immune and barrier biomarkers of lesional and nonlesional AD skin. In addition, the noninvasive skin tape provides samples of only top epidermal layers3 and enables exclusive assessment of epidermal cytokines that might be overlooked in full skin biopsies. This approach will be useful for clinical trials of novel biologics in AD that target epithelial produced cytokines.
A unique skin tape stripping (STS) protocol that allows examination of skin transepidermal water loss (TEWL) and the use of STS for assessment of skin lipids and proteins has been recently developed.4–8 With only two STS, this approach facilitates the assessment of the natural moisturizing factor (NMF) in the skin that is represented by fillagrin (FLG) breakdown products, including cis- and trans-urocanic acid (UCA) and pyroglutamic acid (PCA), as well as comprehensive profiling of major classes of lipids in the skin. In recent studies, this approach demonstrated reduced UCA and PCA levels and altered lipid metabolite levels in lesional and nonlesional STS samples of AD patients.4,6 STS have also been successfully used for the transcriptomic analysis of AD skin.5–8
The current study describes an innovative method for profiling the local skin cytokine environment using STS samples and ultrasensitive Mesoscale multiplex cytokine assay (MSD) and emphasizes the role of epithelial produced cytokines, including IL-1 family members, in AD pathology.
METHODS
Study subjects
A cohort of 62 children from a prospective, clinical mechanistic study registered at ClinicalTrials.gov (identifier: NCT03168113) was used in this study. The details of the study cohort were reported earlier.6 The protocol was approved by the Western Institutional Review Board, protocol number 20170474. Written informed consent was provided by the parent or legal guardian, and written assent was provided by the participant, as applicable, before participation. All AD participants had active skin disease diagnosed using the published criteria9 and were stratified into two groups based on their food allergy (FA) status: 1) AD FA+ (n=21) – these children were allergic to peanut, with a peanut skin prick test wheal ≥ 8 mm, as well as documentation of a previous positive oral food challenge to peanut or convincing history of an immediate allergic reaction to peanut. 2) AD FA- (n=19) – these participants had no personal history of FA and a negative skin prick test (wheal < 3 mm) to peanut, milk, egg, wheat, soy, shellfish mix (clam, crab, oyster, scallops, and shrimp), almond, English walnut, hazelnut, cashew, brazil nut, and sesame seed. 3) NA (n=22) – non-atopic (NA) healthy controls were defined as individuals without a personal history of atopic diseases and negative skin prick tests to the same foods as well as common aeroallergens (cat, dog, dust mite, cockroach, mold mix, tree mix, grass mix, and weeds mix). AD FA+ and AD FA− children were also skin tested for the aeroallergens and the information was recorded. The full inclusion and exclusion criteria for this study and study subject characteristics are detailed in prior publication.6 All participants avoided any treatments, baths, and skin creams or emollients that may potentially affect the skin barrier function before the collection of skin samples.
Skin Disease Severity Assessments
All AD FA+ and AD FA- patients in the study had active AD. None of the study subjects had an overt skin infection. AD severity was evaluated using the SCORing Atopic Dermatitis (SCORAD)10,11 and the Eczema Area and Severity Index (EASI).12 Persistence of AD was evaluated using the Nottingham Eczema Severity Score (NESS),13 a validated index incorporating clinical course over the past 12 months, disease intensity over the past 12 months, and extent of body surface area involvement. Enrollment of AD FA+ and AD FA- participants was actively matched based on AD severity (mild or moderate) as determined by the NESS.
Skin TEWL Assessments
TEWL was evaluated using the AquaFlux AF200 (Biox, London, UK) as previously described.6,9 In this study TEWL measurements from the surface of lesional skin sampled by skin tape stripping for cytokine analysis were used.
STS sample acquisition
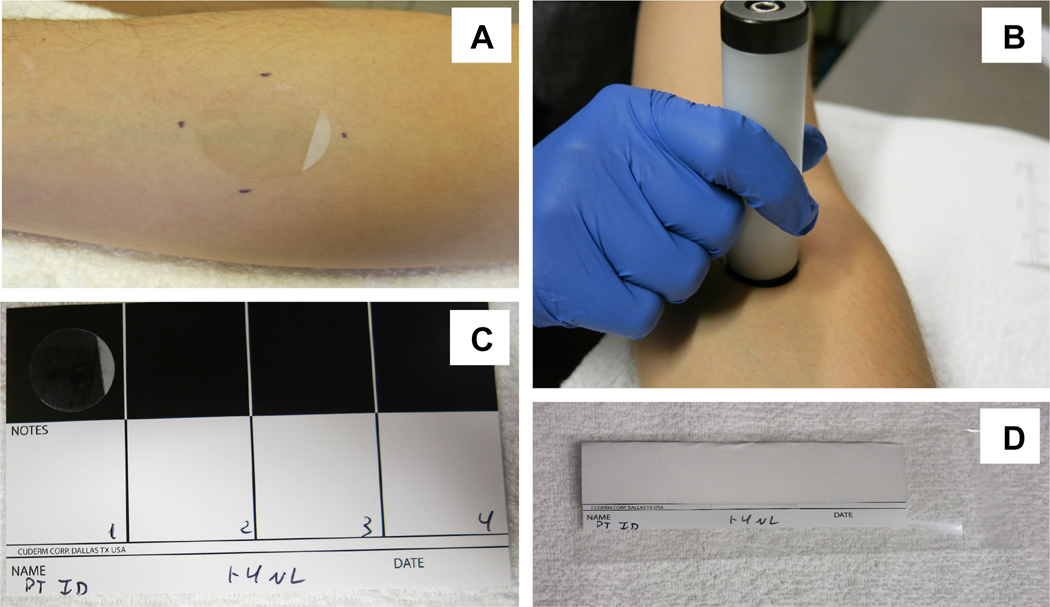
Twenty D-Squame tape strips (CuDerm; Ø22mm) were collected from lesional skin of the upper arm (forearm) of all AD patients using a D-Squame D500 pressure instrument to apply equivalent pressure (225 g/cm2) for STS collection and from similar area of the skin of healthy controls (Fig 1A, B). Collected STS were stored individually adhered to glossy storage cards (CuDerm) to minimize air exposure of the collected material (Fig 1C, D) and kept frozen at - 80°C prior to processing.
Figure 1.
Skin tape strip (STS) sample collection. Adhesive STS (CuDerm; Ø22mm) are applied to the skin area of interest (A) with the standardized pressure device (D-Squame D500; 225 g/cm2) (B). Collected STS are adhered to glossy storage cards (CuDerm) (C, D) to minimize the air exposure of the collected biomaterial and cryopreserved at −80°C.
Protein extraction from STS
Due to the relatively small quantity of biomaterial collected from the skin with adhesive STS, the protocol was optimized to minimize the total volume of extraction buffer for each sample. Extraction chambers containing individual STS (in extraction buffer) were placed in a Cole-Parmer 8891 bath sonicator for 30 minutes at 40kHz at 65W. After the sonication, STS were removed from the buffer, protein extracts were transferred into microtubes and centrifuged to clear cell debris and tape adhesive residue. Samples were cryopreserved for future analysis.
Total protein quantification and normalization of samples variability
Total protein levels in STS extracts were measured with NanoOrange Protein Quantitation Kit (Invitrogen). The MSD assay results for each measured cytokine were normalized to the total protein in each sample.
MSD multiplex assay
Protein extracts were prepared from STS and analyzed using the MSD U-Plex human cytokine multiplex platform. The study aimed to profile the expression of cytokines in lesional samples that were previously reported to be associated with inflammation in AD skin (general inflammation: IL-1a, IL-1b, IL-18, IL-6, and IL-8; Th2 cytokines: CCL17, CCL22, TSLP, IL-33, IL-13, IL-22, IL-31, and eotaxin; Th1/Th17 cytokines: IL-10, IL-17a, IL-17c, IL-21, IL-23, and IP-10). For statistical analysis, cytokine concentrations below the fit curve range (signal below the bottom of the bottom-of-the-curve fit, no concentration given) were assigned the value of the lowest sample concentration detected below the standard curve detection limit to maintain the ranking order.
Statistical analysis
All statistical analyses and figure preparations were done in GraphPad Prizm software. The expression of cytokines in NA and AD lesional STS samples were compared using nonparametric Mann-Whitney test. STS samples of the three study groups was examined using ANOVA. Dunn’s multiple comparisons post-test was used to correct for multiple testing. A p-value <0.05 was considered significant. Expression of individual cytokines in lesional skin samples was correlated with TEWL and SCORAD. Pearson correlation coefficient and p values were reported.
RESULTS
Cytokine analysis of skin tape strip samples collected from AD lesional skin and healthy skin
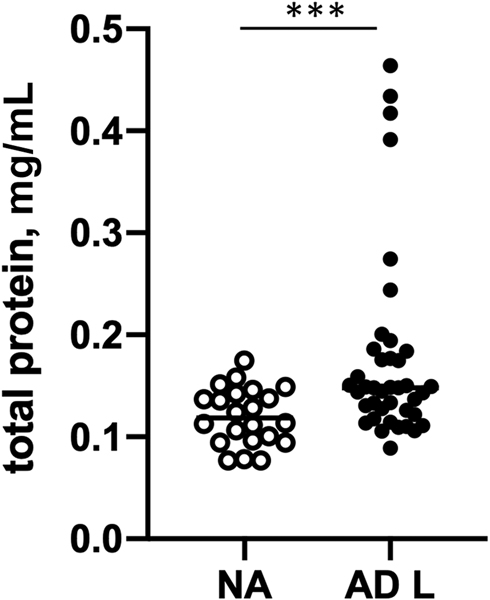
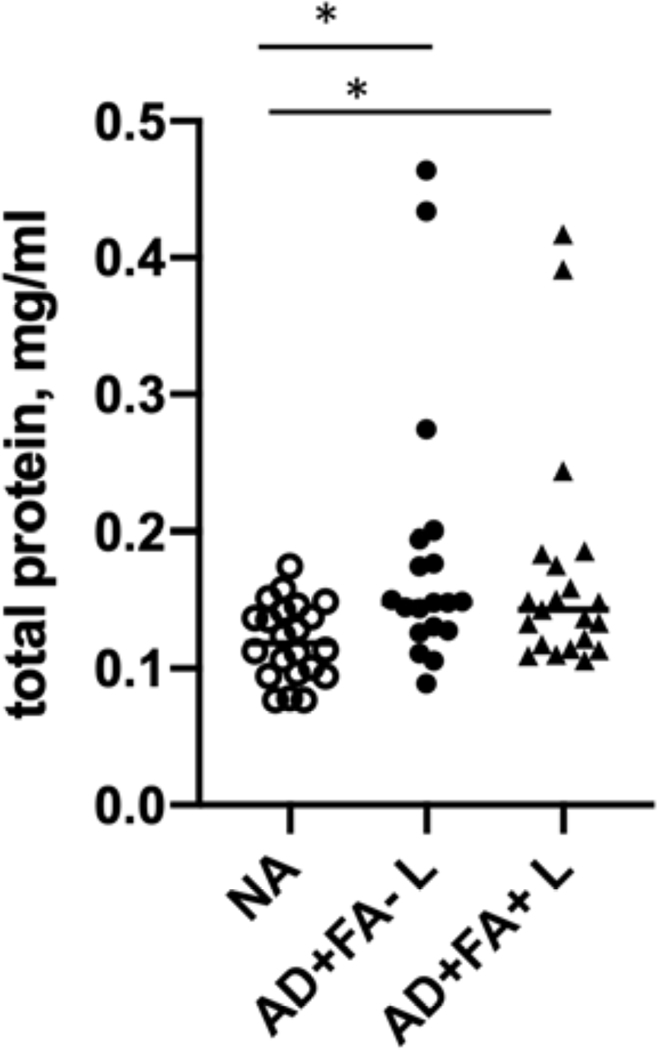
In this study, consecutive STS samples were collected from healthy non-atopic (NA) controls and lesional skin of AD patients (20 STS per patient) with or without FA. Protein extracts were prepared from STS as described in Methods. Total protein amounts extracted from NA STS samples and AD lesional skin STS samples are summarized in Figure 2. Significantly higher protein yields were obtained from STS samples prepared from lesional skin of AD FA+ and AD FA- patients as compared to skin tape strip samples from NA subjects (eFig 1).
Figure 2.
Concentrations of protein extracts prepared from skin tape strip samples from lesional skin of AD patients and NA controls. ***p<0.001.
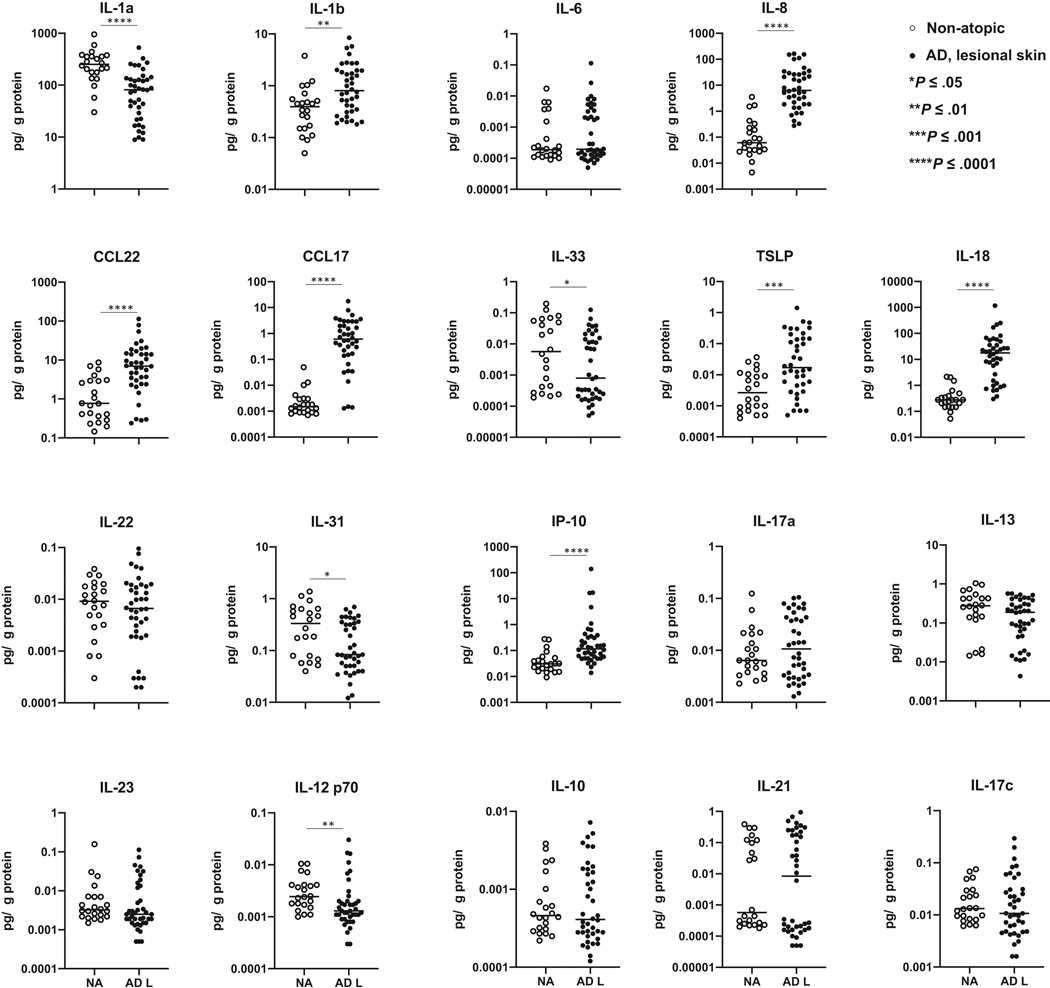
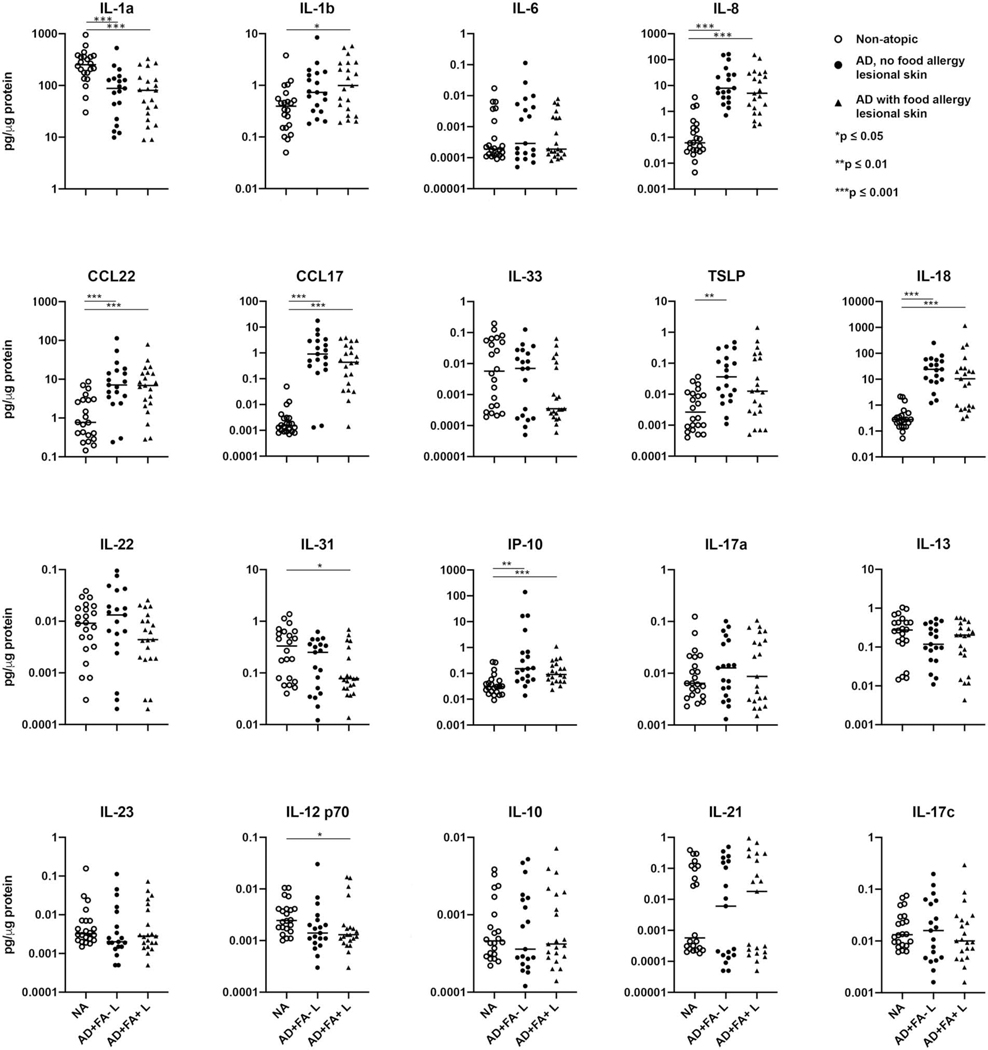
Samples collected from deeper keratinocyte layers (17th and 19th STS sample) were examined for the presence of cytokines with MSD multiplex assay. Forty microliters of protein extracts were designated for analysis and the amounts of cytokines detected were corrected for the total protein concentration in these samples. A broad panel of chemokines and cytokines known to be involved in inflammatory skin reactions associated with AD was screened in this study (Fig 3, eFig 2).
Figure 3.
Cytokine profiles of AD lesional skin determines the changes in the proinflammatory and type 2 cytokine production in these skin samples as compared to skin samples from NA controls, with no significant effect on type 1/type 17 inflammatory pathway. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Significant changes in proinflammatory cytokines were observed in AD lesional skin, with the decrease in preformed IL-1a in stratum corneum of AD lesional STS samples, and a significant increase in IL-8, IL-1b and IL-18 levels in these samples.
Type 2 inflammation is a prominent feature of AD skin pathology. Analysis of AD lesional STS samples revealed significant increases in CCL17 and CCL22, chemokines that attract type 2 cytokine-producing T cells and inflammatory monocytes to the skin14 (Fig 3). Increased TSLP levels were observed in AD lesional STS samples (Fig 3). No significant differences in IL-22, IL-13, or IL-10 were found between the NA and AD lesional STS samples, indicating that these cytokines are not easily detectable in the upper layers of the skin sampled with STS (Fig 3).
Type 1/17 cytokines were also assayed and no significant differences in levels of IL-23, IL-21, IL-17a or IL-17c were observed between the NA and AD lesional skin (Fig 3). A decrease in IL-12 p70 was found in AD lesional skin compared to NA samples (Fig 3). Increased levels of IP-10 were detected in AD lesional samples (Fig 3).
Simiar cytokine trends and no significant differences were detected between AD FA+ and AD FA- STS samples. The details are presented in eFig 1. Of note, a decrease in IL-31 was observed only in AD FA+ lesional skin samples (eFig 1).
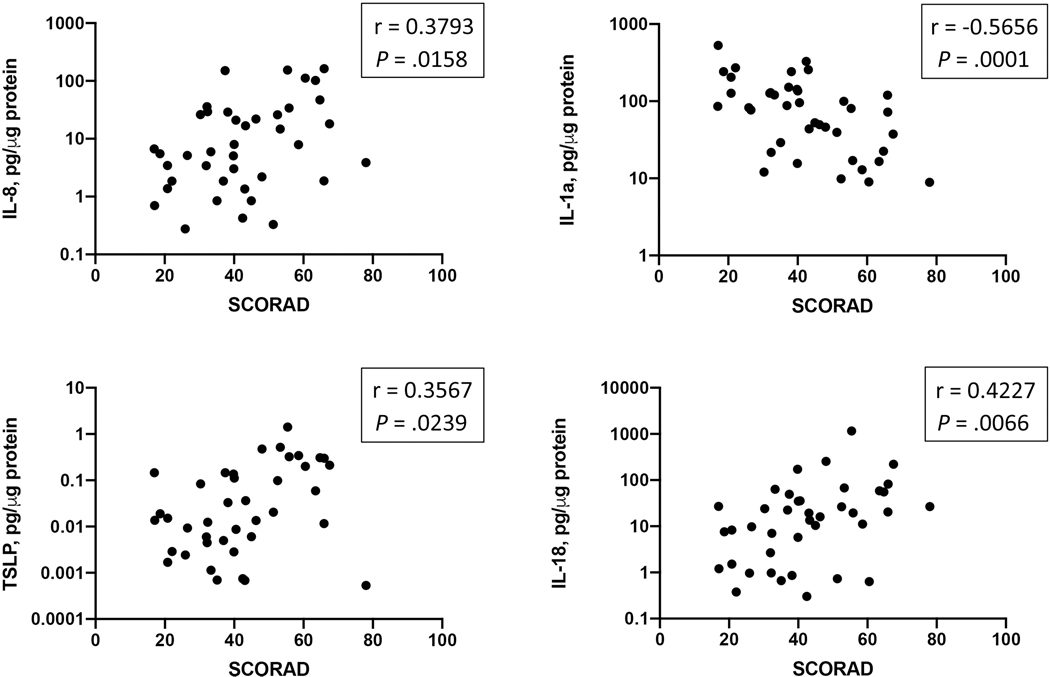
Relationships between cytokine profiles and disease severity and skin barrier function
Several cytokines measured in lesional skin samples demonstrated significant relationships with the AD disease severity index measured in these patients. Significant positive correlations between IL-8, IL-18, TSLP, and AD SCORAD were observed. In addition, the level of IL-1a in AD lesional skin demonstrated an inverse correlation with SCORAD (Fig 4).
Figure 4.
A relationship between inflammatory mediators detected in AD lesional skin and AD disease severity index SCORAD.
TEWL was measured in the same lesional area that was sampled for cytokine analysis in this study. Importantly, significant positive correlations between Th22/Th17 cytokines (IL-22, IL-17a), Th2 cytokines (CCL17, CCL22, TSLP) and lesional TEWL measurements were observed (Fig 5). The levels of IL-8 and IL-18 exhibited significant positive correlations with lesional TEWL levels, while an inverse correlation between lesional IL-1a levels and lesional TEWL measurements was found (Fig 5).
Figure 5.
A correlation analysis reveals a relationship between inflammatory mediators detected in AD lesional skin and TEWL measurements from the sampled skin area.
DISCUSSION
This study established a novel procedure for preparation of protein extracts from skin tape strip samples in a minimal extraction buffer volume, utilizing only 2 STS samples per patient for each multiplex assay. The results demonstrate that collected extracts are suitable for profiling of inflammatory mediators in the skin. A panel of twenty analytes that reflect general inflammatory response (IL-1 family members and IL-8), type 2 and type 17 inflammation in the skin and characterized changes in the expression of these targets in pediatric AD lesional skin was assembled for this study.
Epidermal barrier defects in patients with AD cause skin water loss and result in dry and itchy skin that is further exacerbated by self-inflicted skin injury from scratching.15 There is a strong association between AD and FLG loss-of-function mutations, which is essential for the epidermal barrier function and plays a role in the aggregation of keratin filaments.16 In addition, in AD patients without FLG mutations there is an acquired decrease in FLG expression caused by the activity of Th2 related cytokines (IL-4, IL-13, IL-31, IL-33, TSLP) and IL-22.17 Cytokine profiling results demonstrate presence of type 2 immune activation in upper epithelial layers of AD lesional skin. Increased CCL17 and CCL22 expression by keratinocytes selectively supports the chemotaxis of Th2-producing T-cells into AD lesional skin. In addition, the acquired data revealed increased expression of TSLP in AD lesional skin, a master cytokine involved in dendritic cell programming and promotion of Th2 responses.18
Significant increases in the production of inflammatory mediators (including IL-1b, IL-18, and IL-8) and a decrease in IL-1a in the stratum corneum of AD lesional skin were shown in this study. The expression profile of IL-1 family cytokines (IL-1a, IL-1b, IL-18) exhibited major changes in the AD skin. Other research groups have also investigated IL-1 cytokines in the lesional and nonlesional skin of patients with AD and reported increased levels of IL-1 cytokines in the skin and sweat of these patients.19–23 Keratinocytes release IL-1a and IL-1b after mechanical skin injury, and a recent study demonstrated that FLG-deficient human and mouse keratinocytes increase the expression of IL-1a and IL-1b.24 Skin injury, dysbiosis, and FLG deficiency trigger the release of intracellular IL-1a by keratinocytes, which drives chronic skin inflammation.25 IL-1 family members have been shown to be involved in the regulation of antimicrobial defenses and leukocyte chemotaxis in the skin.26 Notably, a recent study presented at the 2019 American Academy of Dermatology reported that an open label (no placebo control) trial of Bermekimab, an anti-IL-1a monoclonal antibody, showed significant efficacy in reducing skin severity of AD with marked reduction in itch (AAD 2019, unpublished). STS analysis described here provides a unique opportunity to monitor the expression of IL-1 family cytokines in AD skin and is a promising approach to noninvasive assessment of IL-1 expression in AD clinical trials.
Keratin 1 (KRT1) and its heterodimer partner keratin 10 (KRT10) are major constituents of the intermediate filament cytoskeleton in suprabasal epidermis.27 KRT1 is critical for the maintenance of skin integrity and participates in keratinocyte inflammatory networks, as its absence causes prenatal increase in IL-18 and S100A8 and S100A9 proteins, skin barrier defects, and perinatal lethality.28 Of interest, the depletion of IL-18 partially rescued these defects in KRT1-deficient mice.28 STS analysis described here revealed high levels of IL-18 in AD lesional epithelia, suggesting a link between the skin barrier dysfunction and IL-18 production. The results also demonstrate previously unrecognized upregulation of IP-10 (CXCL10) in AD lesional skin. Consistent with findings in this manuscript, it has been reported that IL-18 (high levels of which were found in AD lesional skin) can induce IP-10 production by keratinocytes.29
Of interest, IL-31, a cytokine associated with pruritis in AD skin,30 was decreased in the stratum corneum of AD FA+ patients. Notably, an increase in the visual analogue scale (VAS) itch score in the skin of these patients was previously reported.6 A decrease in IL-31 levels may suggest a release of this cytokine in the skin of these subjects and its utilization by the cells in the deeper layers of the skin, including intraepidermal neuronal endings that respond to IL-31.30
In this study STS samples from lesional skin of AD children with or without FA were examined. Inflammatory cytokine profiles in lesional skin from both groups were very similar.
Associations between proteomics and transcriptomics STS cytokine profiles
The results of cytokine profiling in lesional skin of AD patients in this study were consistent with the transcriptomic analysis of nonlesional and lesional STS samples from this study cohort.6 In the referenced study, the sequencing data were successfully generated only for a subgroup of patients, as RNA recovery from skin strips was not optimally efficient. However, the transcriptomic analysis had sufficient sensitivity to detect type 2 inflammation and dendritic cell activation markers both in the upper layers of lesional skin of all AD patients and in the nonlesional skin of AD FA+ subjects. Both AD groups exhibited increased expression of CCL17, CCL22, IL-13, and IL-7R (a TSLP receptor). However, the transcripts for type 2 immune receptors (i.e., IL-4R, CCR8, and the TSLP receptor CRLF2) were higher in AD FA+ as compared to AD FA- or NA in nonlesional skin. Transcriptomic signatures of the immune activation were equally elevated in lesional skin of both AD FA+ and AD FA- subjects, compared to the NA cohort.6 Similarly, this study revealed increased levels of CCL17 and CCL22 in lesional AD samples. Notably, this study report also demonstrates increased levels of TSLP protein in AD lesional STS samples, which is consistent with the increased expression of TSLP receptors found in the previous study. In contrast to the transcriptomic analysis of the same cohort, no increase in IL-13 protein in AD lesional STS samples was found. Previous analysis of IL-13 expression in skin tapes collected from infants with AD showed a decrease in IL-13 levels as compared to controls.22 Activation of IL-1 and IL-18 pathways was evident in the transcriptomic analysis of AD nonlesional skin, as increased expression of decoy molecules IL-1RN and IL-18BP that are induced in response to IL-1 and IL-18 pathway activation31 was found in AD nonlesional skin samples obtained from the study cohort. This is supported by protein analysis performed in this study: increases in IL-1b and IL-18 and a decrease in preformed IL-1a levels were found in AD lesional STS samples.
Transcriptome analysis of AD nonlesional and lesional samples in this cohort also revealed increased IL-8 levels in STS samples (unpublished). Similar findings were reported by Guttman-Yassky et al. in the PCR analysis of STS transcripts from AD nonlesional and lesional skin.32 IL-8 is involved in neutrophil inflammation and the presence of neutrophils has been previously documented in AD skin,33 although to a lesser degree than in psoriatic skin.34 One of the explanations is that Staphylococcus aureus (S. aureus) skin colonization in AD may interfere with IL-8 signaling as other reports have identified marked defects in neutrophil chemotaxis in response to TLR2 agonists, including S. aureus products, due to internalization and downregulation of CXCR2, one of the IL-8 receptors.35
To validate the relevance of STS cytokine profiling in a clinical setting, the relationship between cytokine levels and SCORAD as well as TEWL were examined. Importantly, four targets in this study demonstrated significant correlations with SCORAD and a number of mediators exhibited significant correlations with lesional skin TEWL measurements. These are critical findings as they highlight the importance of these cytokines/chemokines in the regulation of AD disease severity and skin TEWL. Along with the markers of type 2 inflammation (CCL17, CCL22, TSLP), a relationship between IL-1 family members (IL-1a, IL-1b, IL-18), as well as IL-22, IL-17a and skin TEWL was found, suggesting the involvement of multiple inflammatory mediators in the skin barrier function disruption. Intriguingly, the results of this work also demonstrated a significant correlation between IL-22 and skin TEWL. IL-22 has been documented to inhibit skin barrier function as its expression in the skin of mice caused an AD-like phenotype characterized by chronic pruritic dermatitis associated with Th2-biased local and systemic immune responses, down-regulation of epidermal differentiation complex genes, and enhanced dermatitis after epicutaneous allergen exposure.36
Importantly, multiple clinical trials of both nonspecific and specific agents revealed favorable outcomes in AD, including Dupilumab, JAK inhibitors, antagonists of the TSLP axis, an IL-22 inhibitor, and IL-33 and IL-17 antagonists.37 Of particular relevance is Dupilumab, an IL-4 receptor-blocking antibody which has been a significant development in the management of AD and other atopic conditions. However, only 35%–40% of patients receiving Dupilumab achieve clear or almost clear skin;37 this supports the need for additional treatment options in AD, including the IL-1 pathway. Broad anti-inflammatory inhibition beyond type 2 inflammation has been shown effective in recent trials of JAK inhibitors in AD skin.38 STS cytokine analysis provides a unique opportunity to monitor the expression of pro-inflammatory cytokine targets in AD skin beyond type 2 cytokine pathways and will be useful in future AD trials beyond Dupilumab. The STS cytokine profiling approach outlined in this study allows monitoring of these mediators in the AD skin and can be suitable for longitudinal analysis in clinical trials with various age groups, including children.
Keratinocyte cytokine profile shifts in the lesional stratum corneum of AD patients in this study were consistent with literature reports.22 These observations further validate the experimental setting, sample collection, and protein extraction procedures in this study.
Differentiating between cytokines endogenously produced by keratinocytes vs. resident epidermal lymphocytes/macrophages vs. cytokines that filtered from plasma into the interstitial fluid is beyond the scope of this report. However, cytokines detected in STS protein extracts from the stratum corneum are likely the result of endogenous production by keratinocytes. The majority of cytokines/chemokines examined in this study are endogenous mediators that are not stored as preformed molecules in the producer cells. New synthesis is required for secretion and, once released, their half-life is relatively short39 which hinders the diffusion from deeper epidermal layers or the blood vessel-rich dermis. This is consistent with findings in this manuscript as epithelial-produced cytokines were readily measurable in the skin tape strip analysis in this study, while cytokines produced by the infiltrating immune cells were not considerably different between the study groups. However, IL-1 cytokine family members are an exception as many of them are stored preformed in large quantities in epithelial cells.31,40
In summary, this study developed a new setting for STS protein extraction and demonstrated the utilization of this protein extraction method and ultrasensitive MSD assay for the measurement of inflammatory mediators in AD lesional skin of children. This approach identified a novel group of proinflammatory cytokines involved in lesional AD (IL-1b, IL-8, and IL-18) that extends beyond the type 2 immune response (CCL17, CCL22, and TSLP) and suggests a new therapeutic target in AD. The study provided evidence of relationships between the detected inflammatory markers and AD disease severity and TEWL, proposing that this assay can be used as a minimally invasive approach to monitor skin inflammation.
ACKNOWLEDGEMENT
The authors acknowledge Nicole Meiklejohn for her outstanding editorial assistance in preparing this manuscript.
Funding Source: This work was supported by NIH/NIAID Atopic Dermatitis Research Network U19AI117673 and UM2AI117870.
Abbreviations/Acronyms:
- AD
atopic dermatitis
- EASI
Eczema Area and Severity Index
- FLG
filaggrin
- FA
food allergy
- KRT1
keratin 1
- KRT10
keratin 10
- MSD
Mesoscale multiplex cytokine assay
- NMF
natural moisturizing factor
- NA
nonatopic
- NESS
Nottingham Eczema Severity Score
- PCA
pyroglutamic acid
- SCORAD
SCORing Atopic Dermatitis
- STS
skin tape strip
- S. aureus
Staphylococcus aureus
- TEWL
transepidermal water loss
- UCA
urocanic acid
- VAS
visual analogue scale
APPENDIX
eFigure 1.
Concentrations of protein extracts prepared from skin tape strip samples from lesional skin of ADFA+, AD FA- patients and NA controls. *p<0.05.
eFigure 2.
Cytokine profiles of lesional skin of AD FA+ and AD FA- subjects as compared to NA controls. *p<0.05, **p<0.01, ***p<0.001.
Footnotes
Conflict of Interest: None
Clinical Trials Identifier: NCT03168113.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. [DOI] [PubMed] [Google Scholar]
- 2.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: Shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134(4):769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BE, Goleva E, Kim PS, et al. Side-by-side comparison of skin biopsies and skin tape stripping highlights abnormal stratum corneum in atopic dermatitis. J Invest Dermatol. 2019;139(11):2387–2389.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berdyshev E, Goleva E, Bronova I, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight. 2018;3(4):e98006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyjack N, Goleva E, Rios C, et al. Minimally invasive skin tape strip RNA sequencing identifies novel characteristics of the type 2-high atopic dermatitis disease endotype. J Allergy Clin Immunol. 2018;141(4):1298–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung DYM, Calatroni A, Zaramela LS, et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med. 2019;11(480): eaav2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He H, Bissonnette R, Wu J, Diaz A, Saint-Cyr Proulx E, Maari C, et al. Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. J Allergy Clin Immunol 2020; Jul 9;S0091–6749(20)30824–1. [DOI] [PubMed] [Google Scholar]
- 8.Pavel AB, Renert-Yuval Y, Wu J, Del Duca E, Diaz A, Lefferdink R, et al. Tape strips from early-onset pediatric atopic dermatitis highlight disease abnormalities in nonlesional skin. Allergy 2020; Jul 8. doi: 10.1111/all.14490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck LA, Boguniewicz M, Hata T, et al. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy Clin Immunol. 2009;124(2):260–269.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23–31. [DOI] [PubMed] [Google Scholar]
- 11.Oranje AP. Practical issues on interpretation of scoring atopic dermatitis: SCORAD Index, objective SCORAD, patient-oriented SCORAD and Three-Item Severity score. Curr Probl Dermatol. 2011;41:149–155. [DOI] [PubMed] [Google Scholar]
- 12.Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172(5):1353–1357. [DOI] [PubMed] [Google Scholar]
- 13.Emerson RM, Charman CR, Williams HC. The Nottingham Eczema Severity Score: preliminary refinement of the Rajka and Langeland grading. Br J Dermatol. 2000;142(2):288–297. [DOI] [PubMed] [Google Scholar]
- 14.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–680. [DOI] [PubMed] [Google Scholar]
- 15.Leung DYM, Berdyshev E, Goleva E. Cutaneous barrier dysfunction in allergic diseases. J Allergy Clin Immunol. 2020;145(6):1485–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315–1327. [DOI] [PubMed] [Google Scholar]
- 17.Davidson WF, Leung DYM, Beck LA, et al. Report from the National Institute of Allergy and Infectious Diseases workshop on “Atopic dermatitis and the atopic march: Mechanisms and interventions”. J Allergy Clin Immunol. 2019;143(3):894–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han H, Roan F, Ziegler SF. The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol Rev. 2017;278(1):116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junghans V, Gutgesell C, Jung T, Neumann C. Epidermal cytokines IL-1beta, TNF-alpha, and IL-12 in patients with atopic dermatitis: response to application of house dust mite antigens. J Invest Dermatol. 1998;111(6):1184–1188. [DOI] [PubMed] [Google Scholar]
- 20.Terui T, Hirao T, Sato Y, et al. An increased ratio of interleukin-1 receptor antagonist to interleukin-1alpha in inflammatory skin diseases. Exp Dermatol. 1998;7(6):327–34. [DOI] [PubMed] [Google Scholar]
- 21.Jeong CW, Ahn KS, Rho NK, et al. Differential in vivo cytokine mRNA expression in lesional skin of intrinsic vs. extrinsic atopic dermatitis patients using semiquantitative RT-PCR. Clin Exp Allergy. 2003;33(12):1717–1724. [DOI] [PubMed] [Google Scholar]
- 22.McAleer MA, Jakasa I, Hurault G, et al. Systemic and stratum corneum biomarkers of severity in infant atopic dermatitis include markers of innate and T helper cell-related immunity and angiogenesis. Br J Dermatol. 2019;180(3):586–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai X, Okazaki H, Hanakawa Y, et al. Eccrine sweat contains IL-1alpha, IL-1beta and IL-31 and activates epidermal keratinocytes as a danger signal. PLoS One. 2013;8(7):e67666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kezic S, O’Regan GM, Lutter R, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol. 2012;129(4):1031–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Archer NK, Jo JH, Lee SK, et al. Injury, dysbiosis, and filaggrin deficiency drive skin inflammation through keratinocyte IL-1alpha release. J Allergy Clin Immunol. 2019;143(4):1426–1443.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swindell WR, Beamer MA, Sarkar MK, et al. RNA-Seq analysis of IL-1B and IL-36 responses in epidermal deratinocytes identifies a shared MyD88-dependent gene signature. Front Immunol. 2018;9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haines RL, Lane EB. Keratins and disease at a glance. J Cell Sci. 2012;125(Pt 17):3923–3928. [DOI] [PubMed] [Google Scholar]
- 28.Roth W, Kumar V, Beer HD, et al. Keratin 1 maintains skin integrity and participates in an inflammatory network in skin through interleukin-18. J Cell Sci. 2012;125(Pt 22):5269–5279. [DOI] [PubMed] [Google Scholar]
- 29.Wittmann M, Purwar R, Hartmann C, Gutzmer R, Werfel T. Human keratinocytes respond to interleukin-18: implication for the course of chronic inflammatory skin diseases. J Invest Dermatol. 2005;124(6):1225–1233. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima C, Otsuka A, Kabashima K. Interleukin-31 and interleukin-31 receptor: new therapeutic targets for atopic dermatitis. Exp Dermatol. 2018;27(4):327–331. [DOI] [PubMed] [Google Scholar]
- 31.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 013;39(6):1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guttman-Yassky E, Diaz A, Pavel AB, et al. Use of tape strips to detect immune and barrier abnormalities in the skin of children with early-onset atopic dermatitis. JAMA Dermatol. 2019;155(12):1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choy DF, Hsu DK, Seshasayee D, et al. Comparative transcriptomic analyses of atopic dermatitis and psoriasis reveal shared neutrophilic inflammation. J Allergy Clin Immunol. 2012;130(6):1335–1343.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhingra N, Suarez-Farinas M, Fuentes-Duculan J, et al. Attenuated neutrophil axis in atopic dermatitis compared to psoriasis reflects TH17 pathway differences between these diseases. J Allergy Clin Immunol. 2013;132(2):498–501.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alves-Filho JC, Freitas A, Souto FO, et al. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc Natl Acad Sci U S A. 2009;106(10):4018–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198(7):2543–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124(1):28–35. [DOI] [PubMed] [Google Scholar]
- 38.Pavel AB, Song T, Kim HJ, et al. Oral Janus kinase/SYK inhibition (ASN002) suppresses inflammation and improves epidermal barrier markers in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144(4):1011–1024. [DOI] [PubMed] [Google Scholar]
- 39.Aziz N, Detels R, Quint JJ, Li Q, Gjertson D, Butch AW. Stability of cytokines, chemokines and soluble activation markers in unprocessed blood stored under different conditions. Cytokine. 2016;84:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. [DOI] [PubMed] [Google Scholar]