Abstract
To the best of our knowledge, aspirin (ASA) is known as a commonly used medication worldwide. Although the cardiovascular aspects of ASA are well-established, recently, it has been identified that ASA can yield multiple extra-cardiovascular therapeutic potencies in facing neurodegenerative disorders, various cancers, inflammatory responses, and the COVID-19 pandemic. In this review, we aimed to highlight the proven role of ASA administration in the variety of non-cardiovascular diseases, particularly in the field of anesthesiology.
Keywords: Aspirin, Clinical Applications, Extra-Cardiovascular Diseases, Off-Label Uses
1. Context
Aspirin (ASA) is one of the first drugs that has come into a routine therapeutic protocol treatment since the willow bark was used as an agent by the ancient Sumerians and Egyptians in folk medicine (1). Due to its low toxicity and inexpensive cost, ASA is mainly touted as a widely used drug worldwide (2). ASA was a principal pharmaceutical achievement in the 20th century and has been prescribed since then for various purposes, particularly for inhibiting cardiovascular and cerebrovascular pathologies (3). However, the prospective role of ASA in decreasing the long-standing risk of numerous cancers has plausibly opened up a novel era (4). Also, mounting evidence has been developed about the importance of ASA consumption in women at high risk of preterm preeclampsia (5). Although the conventional prescription of ASA is due to its analgesic, antipyretic, and anti-inflammatory effects, some neurodegenerative disorders, such as Alzheimer’s disease, are also considered other pathological conditions for ASA medication (6). Herein, we tended to highlight all clinical applications of ASA in dealing with anesthesiologists.
2. Mode of Action
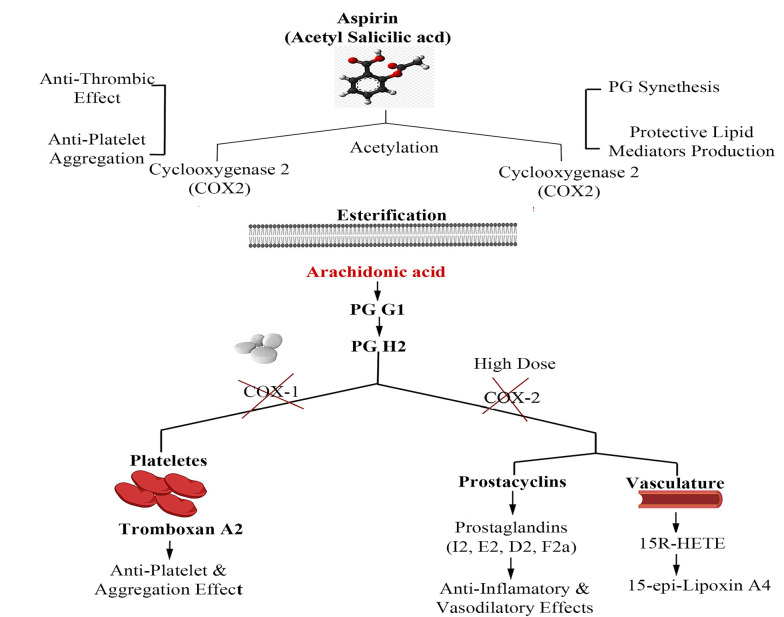
Under pathophysiological conditions, arachidonic acid (AA), as a prostaglandin (PG) precursor, is released from the cell membrane by the activity of an enzyme named phospholipase A2 (7). Various PGs, e.g., PGG2 and PGH2, are synthesized by the cyclooxygenase isoenzymes (COX-1 and -2) involved in inflammatory responses. The essential activity of COX-1 and -2 is diminished in the presence of ASA after the irreversible acetylation of a serine residue in the active site of AA-binding channels (8).
Since platelets have no DNA content, they cannot synthesize new proteins; thus, the inhibition of COX-1 by ASA is associated with the whole platelet lifetime (~ 7 - 10 days) (9). To be specific, ASA can quench the COX-2 activity directly by acetylating the serine residue (Ser 516), which can, in turn, promote 15 (R)-HETE synthesis (10). It should also be noted that the low-dose administration of ASA is partially effective on COX2/PGI2-mediated vascular modulation. In Figure 1, the mechanism of action of ASA is elucidated in detail.
Figure 1. The aspirin mechanism of action. The inhibitory role of aspirin on both COX-1 and -2 enzymes for the emergence of antiplatelet, anti-thrombotic, and anti-inflammatory effects.
3. Cardiovascular Related Beneficial Effects
ASA is well known to decrease cardiovascular events (11), exerting multiple beneficial effects in different dose ranges. For example, it can prevent COX-2 more effectively than COX-1 at high doses (> 1000 mg/day) (12), while low-dose ASA (75 - 100 mg/day) inhibits COX-1 (13). The role of ASA in reducing cardiovascular disease (CVD)-related mortalities and acute myocardial infarction recurrence was shown first in an ISIS-2 clinical trial (14). However, the role of ASA in primary CVD prevention is challenging because of conflicting effects on CVD mortality rates (15). Mounting evidence suggests that the apparent benefits of ASA as a therapy modality for primary CVD prevention outweigh its less significant disadvantages causing the risk of some life-threatening conditions such as bleeding (16-19). A meta-analysis of 20 clinical cases including 3000 patients who received ASA as secondary cardiovascular prevention indicated that “aspirin resistance” in 28% of the patients correlated with a four-fold risk of CVD (20, 21). ASA can be prescribed in numerous cardiovascular issues, including coronary artery bypass surgery (22-26), venous thrombosis, peripheral arterial disease, and cerebrovascular diseases (27-29).
4. Extra-Cardiovascular Applications
4.1. Preeclampsia
Preeclampsia (PE), one of the leading causes of maternal and fetal mortality, is a significant risk factor for preterm birth (30). PE is identified by hypertension, proteinuria, and an extreme maternal systemic inflammatory response (31). ASA is recommended as an effective therapeutic candidate due to its anticoagulant and anti-inflammatory potentials (32). It was also assumed that ASA (50 - 150 mg) could improve the placental blood flow and reduce the risk of placental thrombosis through decreasing TXA2 and PGI2 contents (33). ASA-triggered lipoxins (ATLs) play a critical role in reproductive medicine. In this regard, several studies have revealed that exogenous ATL has the benefits of reversing inflammatory responses observed in PE (34). Also, 150 mg ASA remarkably reduces the prevalence of preterm prelabor rupture of membranes in women at high risk of early-onset PE (35). ASA should be used before week 20 and over at week 34 of pregnancy without any substantial complication to prevent the high risk of perinatal bleeding following its administration (36). The American College of Obstetrics and Gynecology and the Society for Maternal-Fetal Medicine recommended that low-dose ASA (81 mg/day) should be initiated between 12 - 28 weeks of gestation and continued daily for PE prevention until the delivery time and prophylaxis induction in women with a high risk of PE (37). In the ASA for Evidence-Based Preeclampsia Prevention (ASPRE) trial, treatment with ASA (150 mg/day) decreased the incidence of PE in 32, 34, and 37 weeks of gestation by approximately 90, 80, and 60%, respectively, while it had no significant effects on the incidence of term PE (38). According to the results of Combined Multi-Marker Screening and Randomized Patient Treatment with the ASPRE trial, ASA inhibits both preterm and term PE. Also, it can postpone the gestational age by 4.4 weeks (39).
4.2. Analgesic and Antipyretic Effects
ASA is successfully used as a painkiller for mild pains such as headache (40, 41), toothache, and menstrual cramps (dysmenorrhea). Also, it can be used to alleviate colds and "flu-like" symptoms due to its antipyretic property (42). However, ASA has been contraindicated as an antipyretic analgesic in children suffering from flu-like symptoms because of the observed higher risk of Reye’s syndrome (43). The anti-nociception property of ASA mainly exerts through divergent pathways, including the stimulation of adenosine A2 receptors; the suppression of the inhibitor of NF-κB (IkB) kinase β; and the translocation of NF-κB to the nucleus, acid-sensing ion channels (with high doses of ASA), and α, β-methylene ATP-induced nociception. The acetylation of COX-2 causes the production of ASA-triggered lipoxins (15-epi-lipoxin A4) and activity of the ASA-triggered docosahexaenoic acid pathway (e.g., neuroprotection D1/protectin) (44-46). It has also been revealed that ASA contributes to the analgesic effect through the salicylate-induced stimulation of adenosine monophosphate-activated protein kinase (47).
4.3. Inflammatory Diseases
4.3.1. Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disorder described by chronic synovitis, leading to tissue dysfunction and localized damage into the articular cartilage, bone, tendon, and ligament (48). Given that PGs are the primary mediators of pain and inflammatory responses in RA, ASA has been approved for the first-line treatment of RA (49). This drug can reduce pain in the early stage of RA via anti-inflammatory mechanisms without losing articular function (50). Besides inhibiting COXs, ASA can inhibit the synthesis of chondroitin sulfate, an essential component involved in cartilage formation (21). The standard therapeutic regimen for RA contains ASA (3 g) + ibuprofen (3200 mg) daily, which are taken separately (51). Furthermore, ASA affects cell proliferation and apoptosis by reducing cell viability and increasing the proportion of apoptotic and arrested cells in the G0/G1 phase in a dose-dependent manner (52). A recent study has reported that ASA substantially prevents the proliferation of RA-fibroblast‑like synoviocytes via inhibiting JAK/STAT3 and the NF-κB axis (52).
4.3.2. Osteoarthritis
Osteoarthritis is a chronic degeneration syndrome of synovial joints, influencing the cartilage, ligaments, joint lining, and surrounding bones (53-57). Although ASA can positively synergize the analgesic and anti-inflammatory effects of other NSAIDs, following a single-dose consumption of ASA (1g) along with typical OTC drugs, 6.3% of patients display various side effects (58). Moreover, there is a possible contraindication between the anti-thrombotic effect of ASA and the concomitant prescription of other NSAIDs (59), which can be clinically worsened when ASA is required in patients with increased risk of cardiovascular events (60). Also, several studies have revealed that low-dose ASA (< 100 μg/mL) is suitable for maintaining the bone mineral density through activating osteoblastic bone formation and preventing osteoclast activities in a COX-independent manner (61). However, the role of high-dose ASA (150 - 300 μg/mL) is challenging in bone self-regeneration due to its dual effects on osteoclast activities (61).
4.3.3. Systemic Inflammatory Response Syndrome
Systemic inflammatory response syndrome (SIRS) is caused by inappropriate immune responses, either infectious or non-infectious conditions, with occasionally an intense shock and multiple organ failure (33). The clinical manifestations of SIRS include sepsis, multiple organ failure, and acute respiratory distress syndrome (ARDS), which can benefit from early ASA treatment (62, 63). At the antiplatelet dose (81 mg daily), ASA can primarily decrease COX-1-mediated reactions, sepsis, and SIRS-related thrombotic risk (64). The prehospital use of ASA at low-to-medium doses (81 - 325 mg/day) is associated with a low rate of ARDS occurrence (65). On the other hand, ASA (100 mg/day) administration in patients admitted at the intensive care unit (ICU) leads to a significantly lower hospital mortality rate (64). Moreover, it has been reported that ASA at 75 or 1200 mg for seven days before the lipopolysaccharide inhalation decreases the pulmonary neutrophilia and matrix metalloproteinase-8/-9, the bronchoalveolar lavage, the concentrations of tumor necrosis factor-α, and systemic and pulmonary TXB2 (66).
4.4. Kawasaki’s Disease
Kawasaki's disease (KD) is an acute childhood vasculitis diagnosed with high fever, cervical lymphadenopathy, rashes, oral enanthema, conjunctivitis, and erythematous (67). Coronary artery (CA) dilatation and aneurysms may progress in ∼ 15 to 25% of untreated children to promote the upcoming thrombosis and myocardial infarction (68). ASA has been used to treat KD before the intravenous immunoglobulin (IVIG) treatment (69). In the acute phase of the disease, ASA is administered at anti-inflammatory doses (80 to 100 mg/kg per day) with IVIG (70). The anti-inflammatory effect of high-dose ASA can decrease the duration of fever and reduce hemoglobin levels (71). Low-dose ASA due to have a potent anti platelete effect should be continued until 6-8 weeks after the disease onset (72). Despite administrating high-dose ASA for the beginning of treatment, it is not usual to determine Reye’s syndrome in children with KD (73). A meta-analysis on ASA efficacy in KD showed that 20-40% of children developed CA aneurysms after monotherapy with ASA. However, treatment with ASA and high-dose IVIG (a single infusion at 2g/kg) decreases the incidence of CA aneurysms by 9 and 4% in 30 days and 60 days after the disease onset, respectively (74). Nevertheless, there is no randomized controlled trial regarding the use of high-dose ASA in the acute stage of KD.
4.5. Cancers
Previous studies supported the hypothesis that long-term ASA administration may prevent mortality caused by cancers (75, 76). A growing body of evidence has also shown that ASA is a promising chemo-preventive agent for treating numerous cancers, including colorectal, breast, lung, stomach, ovarian, hepatocellular, and prostate cancers (77). The underlying mechanisms responsible for the potential anti-neoplastic action of ASA are not entirely understood. Some possible mechanisms include the antiplatelet effect, the regulation of PGs, the phosphoinositide 3-kinase signaling pathway, and anti-inflammatory effects (78). It has been proposed that the anti-cancer impact of low-dose ASA is pertinent to the inhibition of preliminary neoplastic transformation and the anti-metastatic action, which are directly associated with the antiplatelet effect of low-dose ASA (79). Low-dose ASA instigates the inhibition of platelet activation through its capacity to irreversibly inactivate the COX-1 enzyme and signaling pathways involved in the aberrant expression of epithelial COX-2 in neoplastic lesions (80, 81). It is worth noting that ASA also prevents platelet aggregation by targeting COX-1, which may be necessary for the multi-step process of metastasis (82). Since platelet aggregations promote dynamic tumor cells in the vascular environment, the inhibition of platelet activity can presumably restrict tumor metastasis (83). However, Haykal et al. found that the administration of ASA for the primary prevention of cancer provided a higher risk of bleeding and did not decrease cancer-related mortality (84).
4.6. Neurological Disorders
Multiple progressive neurological disorders, including Alzheimer's disease (AD) and Parkinson's disease (PD), are mainly described by the accumulation of insoluble and misfolded proteins (85). Recently, it has been declared that ASA may mitigate both phosphorylation and aggregation processes through acetylating target proteins (amyloid-beta and tau proteins) and can reduce the risk of AD and PD occurrence (86). A recent study has indicated that ASA decreases superoxide dismutase (SOD-1) aggregation by acetylation in amyotrophic lateral sclerosis (ALS), as a fatal neurodegenerative disease (87). Moreover, it has been shown that ASA can postpone the appearance of the ALS symptoms, including reflex, coordination, and muscle strength deficits, in the experimental model of ALS (88).
4.7. Asthma
Aspirin-induced asthma (AIA) or aspirin-exacerbated respiratory disease (AERD) is mainly characterized by nasal polyposis, eosinophilic rhinosinusitis, hypersensitivity reaction, and asthma (89). The epidemiological reports have declared that up to 20% of asthmatic patients show hypersensitivity to ASA (89). Moreover, several desensitization protocols have been developed to treat AIA, particularly in patients who cannot avoid ASA consumption (90). ASA desensitization upon daily use is a desirable therapeutic option in most patients with AIA, especially in those with recurrent nasal polyposis (91). According to some novel protocols, the initial doses of ASA are considered between 20 - 40 mg or in combination with the intranasal lysine or ketorolac spray. The dose of ASA increases gradually every 1 to 3 h. Once a positive result is achieved, the primary purpose is to repeat the dose and raise it to 325 mg (92). Although 81 mg/day is an optimal dose to sustain ASA tolerance orally, patients with AERD need doses between 325 and 650 mg twice daily to achieve therapeutic goals for ASA desensitization (93).
4.8. HIV
Recent studies have shown the rising incidence of CVD-related morbidity and mortality in HIV-positive patients (94). Interestingly, low-dose ASA (81mg/day) for seven days promotes the reduction of platelet hyperactivity in patients subjected to the antiretroviral treatment and significantly decreases the number of CD4 positive lymphocytes and activated CD14 positive monocytes (95). However, a follow-up study of an ASA regimen for 12 weeks failed to show a therapeutic effect on inflammation biomarkers, monocyte activation, and the function of endothelial cells (96).
4.9. Coronavirus Disease 2019 (COVID-19)
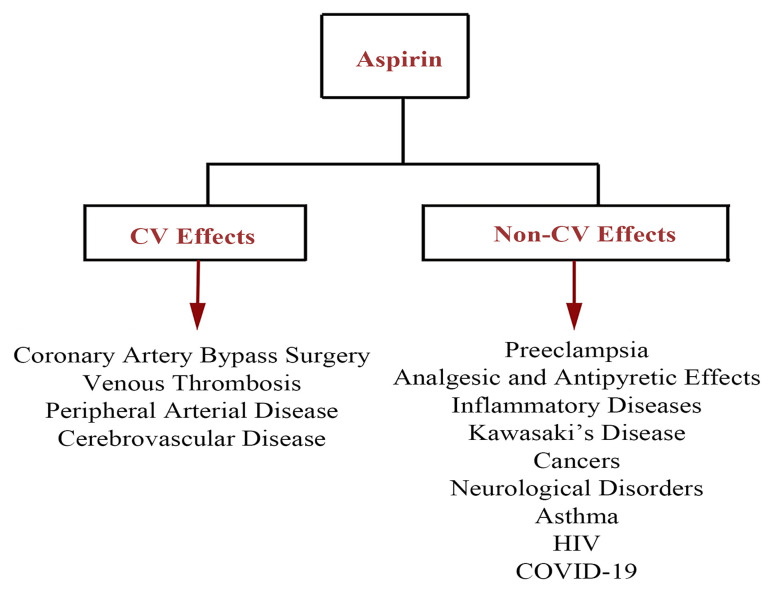
Coronavirus disease 2019 (COVID-19), as a pandemic leading to a public health concern, is caused by severe acute respiratory syndrome coronavirus 2 (97-101). Regarding thromboembolic events following the cytokine storm, it was suggested that antiplatelet agents, such as ASA, could help prevent arterial thromboembolic complications (102-105). Following the previous comprehensive clinical evaluations, it has been revealed that the administration of ASA does not show significant efficacy in COVID-19 patients and may increase the severe bleeding risk in thrombocytopenic COVID-19 patients. In contrast, the recent literature has demonstrated that ASA can be a breakthrough drug to prevent coagulopathy events, particularly in high-risk patients. In this regard, the ongoing randomized evaluation of COVID-19 therapy (RECOVERY) trial in 176 medical centers in the UK has evaluated the possible therapeutic impact of ASA on hospitalized patients at risk of blood clotting complications (106) and assessed whether these benefits outweigh the potential side effects such as the risk of bleeding. In this line, the results of another clinical trial revealed that 44 and 43% of patients receiving low-dose ASA within 24 h of their admission and seven days before hospitalization were less likely to be under the mechanical ventilator and requiring for ICU admission, respectively (107). Similarly, a more recent cohort study has revealed that ASA use might be associated with improved outcomes, including decreased mechanical ventilation, the decreased need for ICU admission, and decreased in-hospital mortality in COVID-19 patients receiving ASA within 24 h of admission or seven days before admission (107). A schematic presentation of the CV and non-CV effects of ASA applications is shown in Figure 2.
Figure 2. The CV and non-CV desirable effects of aspirin on the treatment of various pathological conditions. Abbreviations: COVID-19, coronavirus disease-19; CV, cardiovascular; HIV, human immunodeficiency virus.
4.9.1. Bullet Points in the Field of General and Neuraxial Anesthesia
It is a critical issue to argue preoperative ASA use in both elective and noncardiac surgeries. Given that the cessation of long-term ASA treatment results in adverse cardiac complications in patients at risk, it is necessary to explore factors associated with the decision to stop ASA ingestion (108). In this regard, it has been found that the administration of ASA before operations and during the early postoperative period has no profound effect on the mortality rate or nonfatal myocardial infarction but enhances the risk of severe bleeding (109). Kim et al. attempted to determine the optimal preoperative cessation time of ASA intake. They showed that platelet function could recover when ASA intake was stopped more than seven days before the operation. However, a preoperative platelet function test using the platelet function analyzer is not essential for these patients. Platelet function assay (PFA) testshould be performed in patients who discontinue ASA < 7 days before general anesthesia (110).
According to the recent guidelines regarding ASA safety in subjects undergoing neuraxial anesthesia and analgesia, it has been well-established that a wide variety of ASA doses are safe and do not show contraindication to neuraxial blocks in these patients. Also, the reported spinal hematoma depends on some underlying and predisposing conditions, e.g., a traumatic vascular injury. However, the risk of spinal hematoma can increase following a concurrent administration of multiple anticoagulation agents and antihaemostatic drugs. Also, the recommended safety time of these drugs should be monitored (111). Hence, a proper individualized assessment for ASA administration should be considered before interventions, including neuraxial analgesia or catheter removal, in patients receiving ASA. Moreover, the risk of ASA-induced asthma, as an emergency condition, during anesthesia is regarded as a big challenge for clinicians, highlighting the need for collaboration between anesthesiologists and allergists (112).
5. Conclusion
As the most commonly prescribed medication, ASA has a substantial role in preventing first and second recurrent CV events. Surprisingly, the extra-CV therapeutic effects of ASA were frequently evidenced during numerous clinical trials while treating divergent pathological conditions, such as cancers, inflammatory diseases, neurodegenerative disorders, asthma, and HIV, and managing global pandemic COVID-19 complications. During anesthesia, ASA use is not considered a preoperative concerning challenge; however, the individualized assessment of the cessation of long-term ASA should be a priority.
Acknowledgments
We would like to appreciate the cooperation of the Clinical Research Development Unit, Imam Reza General Hospital, Tabriz, Iran in conducting this research.
Footnotes
Authors' Contribution: Study concept, HS; Study design, AR, KS, and AM; Systematic search, MS; Critical reviews, HS, and KS; Writing of the manuscript, AR and MS.
Conflict of Interests: The authors have no perceived competing interest related to this article.
Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Aysa Rezabakhsh, Email: aysapharma.rezabakhsh@gmail.com.
Ata Mahmoodpoor, Email: amahmoodpoor@yahoo.com.
Maryam Soleimanpour, Email: maryamsoleimanpour@gmail.com.
Kavous Shahsavarinia, Email: kavous.shahsavari@yahoo.com.
Hassan Soleimanpour, Email: h_mofid1357@yahoo.com.
References
- 1.Rezabakhsh A, Mahmoodpoor A, Soleimanpour H. Historical perspective of aspirin: A journey from discovery to clinical practice Ancient and modern history. J Cardiovasc Thorac Res. 2021;13(2):179–80. doi: 10.34172/jcvtr.2021.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrono C. Platelets. 4th ed. Cambridge, Massachusetts: Academic Press; 2019. Aspirin. pp. 921–36. [DOI] [Google Scholar]
- 3.Montinari MR, Minelli S, De Caterina R. The first 3500years of aspirin history from its roots - A concise summary. Vascul Pharmacol. 2019;113:1–8. doi: 10.1016/j.vph.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Desborough MJR, Keeling DM. The aspirin story - from willow to wonder drug. Br J Haematol. 2017;177(5):674–83. doi: 10.1111/bjh.14520. [DOI] [PubMed] [Google Scholar]
- 5.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218(3):287–293 e1. doi: 10.1016/j.ajog.2017.11.561. [DOI] [PubMed] [Google Scholar]
- 6.Paez Espinosa EV, Murad JP, Khasawneh FT. Aspirin: pharmacology and clinical applications. Thrombosis. 2012;2012:173124. doi: 10.1155/2012/173124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherwood L. Human physiology: From cells to systems. Boston, USA: Cengage Learning; 2015. [Google Scholar]
- 8.Toth L, Muszbek L, Komaromi I. Mechanism of the irreversible inhibition of human cyclooxygenase-1 by aspirin as predicted by QM/MM calculations. J Mol Graph Model. 2013;40:99–109. doi: 10.1016/j.jmgm.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Giaretta A, Rocca B, Di Camillo B, Toffolo GM, Patrono C. In Silico Modeling of the Antiplatelet Pharmacodynamics of Low-dose Aspirin in Health and Disease. Clin Pharmacol Ther. 2017;102(5):823–31. doi: 10.1002/cpt.694. [DOI] [PubMed] [Google Scholar]
- 10.Fiorucci S, Distrutti E, de Lima OM, Romano M, Mencarelli A, Barbanti M, et al. Relative contribution of acetylated cyclo-oxygenase (COX)-2 and 5-lipooxygenase (LOX) in regulating gastric mucosal integrity and adaptation to aspirin. FASEB J. 2003;17(9):1171–3. doi: 10.1096/fj.02-0777fje. [DOI] [PubMed] [Google Scholar]
- 11.Arif H, Aggarwal S. Salicylic Acid (Aspirin). Treasure Island (FL): StatPearls; 2021. [PubMed] [Google Scholar]
- 12.Abate A, Yang G, Dennery PA, Oberle S, Schroder H. Synergistic inhibition of cyclooxygenase-2 expression by vitamin E and aspirin. Free Radic Biol Med. 2000;29(11):1135–42. doi: 10.1016/s0891-5849(00)00425-1. [DOI] [PubMed] [Google Scholar]
- 13.Carbone LD, Tylavsky FA, Cauley JA, Harris TB, Lang TF, Bauer DC, et al. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J Bone Miner Res. 2003;18(10):1795–802. doi: 10.1359/jbmr.2003.18.10.1795. [DOI] [PubMed] [Google Scholar]
- 14.ISIS-2 (Second International Study of Infarct Survival) Collaborative Group Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2(8607):349–60. [PubMed] [Google Scholar]
- 15.Bell AD, Roussin A, Cartier R, Chan WS, Douketis JD, Gupta A, et al. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2011;27 Suppl A:S1–59. doi: 10.1016/j.cjca.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Berger JS, Lala A, Krantz MJ, Baker GS, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients without clinical cardiovascular disease: A meta-analysis of randomized trials. Am Heart J. 2011;162(1):115–24 e2. doi: 10.1016/j.ahj.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Javaherforooshzadeh F, Monajemzadeh SA, Soltanzadeh M, Janatmakan F, Salari A, Saeed H. A Comparative Study of the Amount of Bleeding and Hemodynamic Changes between Dexmedetomidine Infusion and Remifentanil Infusion for Controlled Hypotensive Anesthesia in Lumbar Discopathy Surgery: A Double-Blind, Randomized, Clinical Trial. Anesth Pain Med. 2018;8(2):e66959. doi: 10.5812/aapm.66959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janatmakan F, Nassajian N, Sarkarian M, Ghandizadeh Dezfuli M, Salari A, Tabatabaei SK, et al. Effect of Local Fibrinogen Administration on Postoperative Bleeding in Open Prostatectomy Surgery. Anesth Pain Med. 2018;8(3):e73983. doi: 10.5812/aapm.73983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janatmakan F, Nesioonpour S, Javaherforoosh Zadeh F, Teimouri A, Vaziri M. Comparing the Effect of Clonidine and Dexmedetomidine on Intraoperative Bleeding in Spine Surgery. Anesth Pain Med. 2019;9(1):e83967. doi: 10.5812/aapm.83967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu JW, Wong CK, Chambers J, Wout JV, Herbison P, Tang EW. Aspirin resistance determined from a bed-side test in patients suspected to have acute coronary syndrome portends a worse 6 months outcome. QJM. 2010;103(6):405–12. doi: 10.1093/qjmed/hcq038. [DOI] [PubMed] [Google Scholar]
- 21.Schrör K. Acetylsalicylic Acid. 2nd ed. Hoboken, New Jersey: John Wiley & Sons, Inc; 2016. [DOI] [Google Scholar]
- 22.Soenarto RF, Mansjoer A, Amir N, Aprianti M, Perdana A. Cardiopulmonary Bypass Alone Does Not Cause Postoperative Cognitive Dysfunction Following Open Heart Surgery. Anesth Pain Med. 2018;8(6):e83610. doi: 10.5812/aapm.83610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narimani M, Ansari Jaberi A, Negahban Bonabi T, Sadeghi T. Effect of Acupressure on Pain Severity in Patients Undergoing Coronary Artery Graft: A Randomized Controlled Trial. Anesth Pain Med. 2018;8(5):e82920. doi: 10.5812/aapm.82920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salehi Derakhtanjani A, Ansari Jaberi A, Haydari S, Negahban Bonabi T. Comparison the Effect of Active Cyclic Breathing Technique and Routine Chest Physiotherapy on Pain and Respiratory Parameters After Coronary Artery Graft Surgery: A Randomized Clinical Trial. Anesth Pain Med. 2019;9(5):e94654. doi: 10.5812/aapm.94654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fathi M, Valaei M, Ghanbari A, Ghasemi R, Yaghubi M. Comparison of Patient's Kidney Function Based on Kidney Disease Improving Global Outcomes (KDIGO) Criteria and Clinical Parameters in Isolated Coronary Artery Bypass Graft (CABG) Surgery in On-Pump and Off-pump Methods in Patients with Low Cardiac Output Syndrome (LCOS) After Surgery. Anesth Pain Med. 2020;10(2):e100517. doi: 10.5812/aapm.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yousefshahi F, Samadi E, Paknejad O, Bastan Hagh E, Aminzadeh S. Effect of Hypoxemia in the Determination of Short-Term Prognosis of Coronary Artery Bypass Graft Patients: A Prospective Study. Anesth Pain Med. 2019;9(1):e81785. doi: 10.5812/aapm.81785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javaherforoosh Zadeh F, Janatmakan F, Soltanzadeh M, Zamankhani M. Investigating the Effect of Fibrinogen Injection on Bleeding in Coronary Artery Bypass Surgery: A Clinical Trial. Anesth Pain Med. 2019;9(4):e92165. doi: 10.5812/aapm.92165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Javaherforoosh Zadeh F, Janatmakan F, Shafaee Tonekaboni M, Soltanzadeh M. The Effect of Fibrinogen on Blood Loss After Lumbar Surgery: A Double-Blind Randomized Clinical Trial. Anesth Pain Med. 2019;9(3):e91199. doi: 10.5812/aapm.91199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janatmakan F, Javaherforooshzadeh F, Khorrami M, Jarirahmadi S, Khademali H. Is Intra-Articular Administration of Fibrinogen Effective in Postoperative Total Knee Arthroplasty Blood Loss? A Randomized Clinical Trial. Anesth Pain Med. 2021;11(1):e107431. doi: 10.5812/aapm.107431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashemi V, Dolati S, Hosseini A, Gharibi T, Danaii S, Yousefi M. Natural killer T cells in Preeclampsia: An updated review. Biomed Pharmacother. 2017;95:412–8. doi: 10.1016/j.biopha.2017.08.077. [DOI] [PubMed] [Google Scholar]
- 31.Eghbal-Fard S, Yousefi M, Heydarlou H, Ahmadi M, Taghavi S, Movasaghpour A, et al. The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J Cell Physiol. 2019;234(4):5106–16. doi: 10.1002/jcp.27315. [DOI] [PubMed] [Google Scholar]
- 32.Park F, Russo K, Williams P, Pelosi M, Puddephatt R, Walter M, et al. Prediction and prevention of early-onset pre-eclampsia: impact of aspirin after first-trimester screening. Ultrasound Obstet Gynecol. 2015;46(4):419–23. doi: 10.1002/uog.14819. [DOI] [PubMed] [Google Scholar]
- 33.Huang WC, Chou RH, Chang CC, Hsu CY, Ku YC, Huang HF, et al. Systemic Inflammatory Response Syndrome is an Independent Predictor of One-Year Mortality in Patients with Acute Myocardial Infarction. Acta Cardiol Sin. 2017;33(5):477–85. doi: 10.6515/acs20170603a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipa M, Bomba-Opon D, Lipa J, Bartnik P, Bartoszewicz Z, Wielgos M. Lipoxin A4 (LXA4) as a potential first trimester biochemical marker of intrauterine growth disorders. J Matern Fetal Neonatal Med. 2017;30(20):2495–7. doi: 10.1080/14767058.2016.1254182. [DOI] [PubMed] [Google Scholar]
- 35.El-Achi V, Park F, O'Brien C, Tooher J, Hyett J. Does low dose aspirin prescribed for risk of early onset preeclampsia reduce the prevalence of preterm prelabor rupture of membranes? J Matern Fetal Neonatal Med. 2021;34(4):618–23. doi: 10.1080/14767058.2019.1611768. [DOI] [PubMed] [Google Scholar]
- 36.Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695–703. doi: 10.7326/M13-2844. [DOI] [PubMed] [Google Scholar]
- 37.Committee on Obstetric Practice. Society for Maternal–Fetal Medicine ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstet Gynecol. 2018;132(1):e44–52. doi: 10.1097/AOG.0000000000002708. [DOI] [PubMed] [Google Scholar]
- 38.Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017;377(7):613–22. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 39.Wright D, Nicolaides KH. Aspirin delays the development of preeclampsia. Am J Obstet Gynecol. 2019;220(6):580 e1–6. doi: 10.1016/j.ajog.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 40.Urits I, Schwartz R, Smoots D, Koop L, Veeravelli S, Orhurhu V, et al. Peripheral Neuromodulation for the Management of Headache. Anesth Pain Med. 2020;10(6):e110515. doi: 10.5812/aapm.110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amighi D, Majedi H, Tafakhori A, Orandi A. The Efficacy of Sphenopalatine Ganglion Block and Radiofrequency Denervation in the Treatment of Cluster Headache: A Case Series. Anesth Pain Med. 2020;10(6):e104466. doi: 10.5812/aapm.104466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis S. Pain relief for minor aches and pains in the pharmacy. SA Pharm Assist. 2019;19(1):6–8. [Google Scholar]
- 43.Chapman J, Arnold JK. Reye Syndrome. Treasure Island (FL): StatPearls; 2021. [PubMed] [Google Scholar]
- 44.Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, et al. Novel proresolving aspirin-triggered DHA pathway. Chem Biol. 2011;18(8):976–87. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Ye SD, Zhou KQ, Wu LM, Huang YN. High doses of salicylate and aspirin are inhibitory on acid-sensing ion channels and protective against acidosis-induced neuronal injury in the rat cortical neuron. J Neurosci Res. 2012;90(1):267–77. doi: 10.1002/jnr.22742. [DOI] [PubMed] [Google Scholar]
- 46.Ulrych T, Bohm A, Polzin A, Daum G, Nusing RM, Geisslinger G, et al. Release of sphingosine-1-phosphate from human platelets is dependent on thromboxane formation. J Thromb Haemost. 2011;9(4):790–8. doi: 10.1111/j.1538-7836.2011.04194.x. [DOI] [PubMed] [Google Scholar]
- 47.Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142(7):1504–15 e3. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolati S, Sadreddini S, Rostamzadeh D, Ahmadi M, Jadidi-Niaragh F, Yousefi M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed Pharmacother. 2016;80:30–41. doi: 10.1016/j.biopha.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Fattahi MJ, Mirshafiey A. Prostaglandins and rheumatoid arthritis. Arthritis. 2012;2012:239310. doi: 10.1155/2012/239310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110(5-6):255–8. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 51.Cates MJ, Beverly A. AB0355 High dose aspirin versus ibuprofen for pain relief in rheumatoid arthritis: A systematic review. Ann Rheum Dis. 2014;72(Suppl 3):A895.3–A896. doi: 10.1136/annrheumdis-2013-eular.2677. [DOI] [Google Scholar]
- 52.Zhang X, Feng H, Du J, Sun J, Li D, Hasegawa T, et al. Aspirin promotes apoptosis and inhibits proliferation by blocking G0/G1 into S phase in rheumatoid arthritis fibroblast-like synoviocytes via downregulation of JAK/STAT3 and NF-kappaB signaling pathway. Int J Mol Med. 2018;42(6):3135–48. doi: 10.3892/ijmm.2018.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fotouhi A, Maleki A, Dolati S, Aghebati-Maleki A, Aghebati-Maleki L. Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis. Biomed Pharmacother. 2018;104:652–60. doi: 10.1016/j.biopha.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 54.Imani F, Patel VB. Therapeutic Challenges for Knee Osteoarthritis. Anesth Pain Med. 2019;9(3):e95377. doi: 10.5812/aapm.95377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rezasoltani Z, Dadarkhah A, Tabatabaee SM, Abdorrazaghi F, Kazempour Mofrad M, Kazempour Mofrad R. Therapeutic Effects of Intra-articular Botulinum Neurotoxin Versus Physical Therapy in Knee Osteoarthritis. Anesth Pain Med. 2021;11(3):e112789. doi: 10.5812/aapm.112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enteshari-Moghaddam A, Isazadehfar K, Habibzadeh A, Hemmati M. Efficacy of Methotrexate on Pain Severity Reduction and Improvement of Quality of Life in Patients with Moderate to Severe Knee Osteoarthritis. Anesth Pain Med. 2019;9(3):e89990. doi: 10.5812/aapm.89990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatefi M, Parvizi R, Borji M, Tarjoman A. Effect of Self-Management Program on Pain and Disability Index in Elderly Men with Osteoarthritis. Anesth Pain Med. 2019;9(4):e92672. doi: 10.5812/aapm.92672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voelker M. Safety and tolerability of aspirin in randomised controlled clinical trials. Drug Saf. 2004;27(12):968. [Google Scholar]
- 59.Hohlfeld T, Saxena A, Schror K. High on treatment platelet reactivity against aspirin by non-steroidal anti-inflammatory drugs--pharmacological mechanisms and clinical relevance. Thromb Haemost. 2013;109(5):825–33. doi: 10.1160/TH12-07-0532. [DOI] [PubMed] [Google Scholar]
- 60.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173–7. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 61.Xie Y, Pan M, Gao Y, Zhang L, Ge W, Tang P. Dose-dependent roles of aspirin and other non-steroidal anti-inflammatory drugs in abnormal bone remodeling and skeletal regeneration. Cell Biosci. 2019;9:103. doi: 10.1186/s13578-019-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eisen DP. Manifold beneficial effects of acetyl salicylic acid and nonsteroidal anti-inflammatory drugs on sepsis. Intensive Care Med. 2012;38(8):1249–57. doi: 10.1007/s00134-012-2570-8. [DOI] [PubMed] [Google Scholar]
- 63.Sossdorf M, Otto GP, Boettel J, Winning J, Losche W. Benefit of low-dose aspirin and non-steroidal anti-inflammatory drugs in septic patients. Crit Care. 2013;17(1):402. doi: 10.1186/cc11886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Otto GP, Sossdorf M, Boettel J, Kabisch B, Breuel H, Winning J, et al. Effects of low-dose acetylsalicylic acid and atherosclerotic vascular diseases on the outcome in patients with severe sepsis or septic shock. Platelets. 2013;24(6):480–5. doi: 10.3109/09537104.2012.724482. [DOI] [PubMed] [Google Scholar]
- 65.Chen W, Janz DR, Bastarache JA, May AK, O'Neal HJ, Bernard GR, et al. Prehospital aspirin use is associated with reduced risk of acute respiratory distress syndrome in critically ill patients: a propensity-adjusted analysis. Crit Care Med. 2015;43(4):801–7. doi: 10.1097/CCM.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamid U, Krasnodembskaya A, Fitzgerald M, Shyamsundar M, Kissenpfennig A, Scott C, et al. Aspirin reduces lipopolysaccharide-induced pulmonary inflammation in human models of ARDS. Thorax. 2017;72(11):971–80. doi: 10.1136/thoraxjnl-2016-208571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Newburger JW, Takahashi M, Burns JC. Kawasaki Disease. J Am Coll Cardiol. 2016;67(14):1738–49. doi: 10.1016/j.jacc.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 68.Dallaire F, Fortier-Morissette Z, Blais S, Dhanrajani A, Basodan D, Renaud C, et al. Aspirin Dose and Prevention of Coronary Abnormalities in Kawasaki Disease. Pediatrics. 2017;139(6) doi: 10.1542/peds.2017-0098. [DOI] [PubMed] [Google Scholar]
- 69.Hsieh KS, Weng KP, Lin CC, Huang TC, Lee CL, Huang SM. Treatment of acute Kawasaki disease: Aspirin's role in the febrile stage revisited. Pediatrics. 2004;114(6):e689–93. doi: 10.1542/peds.2004-1037. [DOI] [PubMed] [Google Scholar]
- 70.Baumer JH, Love SJ, Gupta A, Haines LC, Maconochie I, Dua JS. Salicylate for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev. 2006;(4):CD004175. doi: 10.1002/14651858.CD004175.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuo HC, Guo MM, Lo MH, Hsieh KS, Huang YH. Effectiveness of intravenous immunoglobulin alone and intravenous immunoglobulin combined with high-dose aspirin in the acute stage of Kawasaki disease: study protocol for a randomized controlled trial. BMC Pediatr. 2018;18(1):200. doi: 10.1186/s12887-018-1180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galeotti C, Bayry J, Kone-Paut I, Kaveri SV. Kawasaki disease: aetiopathogenesis and therapeutic utility of intravenous immunoglobulin. Autoimmun Rev. 2010;9(6):441–8. doi: 10.1016/j.autrev.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eleftheriou D, Levin M, Shingadia D, Tulloh R, Klein NJ, Brogan PA. Management of Kawasaki disease. Arch Dis Child. 2014;99:74–83. doi: 10.1136/archdischild-2012-302841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newburger JW, Fulton DR. Kawasaki disease. Curr Treat Options Cardiovasc Med. 2007;9(2):148–58. doi: 10.1007/s11936-007-0008-3. [DOI] [PubMed] [Google Scholar]
- 75.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379(9826):1591–601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 76.Hemati K, Zaman B, Hassani V, Imani F, Dariaie P. Efficacy of fentanyl transdermal patch in the treatment of chronic soft tissue cancer pain. Anesth Pain Med. 2015;5(1):e22900. doi: 10.5812/aapm.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye X, Fu J, Yang Y, Gao Y, Liu L, Chen S. Frequency-risk and duration-risk relationships between aspirin use and gastric cancer: a systematic review and meta-analysis. PLoS One. 2013;8(7):e71522. doi: 10.1371/journal.pone.0071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen WY, Holmes MD. Role of Aspirin in Breast Cancer Survival. Curr Oncol Rep. 2017;19(7):48. doi: 10.1007/s11912-017-0605-6. [DOI] [PubMed] [Google Scholar]
- 79.Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: Long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159(2):77–85. doi: 10.7326/0003-4819-159-2-201307160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dovizio M, Bruno A, Tacconelli S, Patrignani P. Mode of action of aspirin as a chemopreventive agent. Recent Results Cancer Res. 2013;191:39–65. doi: 10.1007/978-3-642-30331-9_3. [DOI] [PubMed] [Google Scholar]
- 81.Patrignani P, Patrono C. Aspirin and Cancer. J Am Coll Cardiol. 2016;68(9):967–76. doi: 10.1016/j.jacc.2016.05.083. [DOI] [PubMed] [Google Scholar]
- 82.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373(9671):1301–9. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 83.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–34. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haykal T, Barbarawi M, Zayed Y, Yelangi A, Dhillon H, Goranta S, et al. Safety and efficacy of aspirin for primary prevention of cancer: A meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol. 2019;145(7):1795–809. doi: 10.1007/s00432-019-02932-0. [DOI] [PubMed] [Google Scholar]
- 85.Erkkinen MG, Kim MO, Geschwind MD. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb Perspect Biol. 2018;10(4) doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ayyadevara S, Balasubramaniam M, Kakraba S, Alla R, Mehta JL, Shmookler Reis RJ. Aspirin-Mediated Acetylation Protects Against Multiple Neurodegenerative Pathologies by Impeding Protein Aggregation. Antioxid Redox Signal. 2017;27(17):1383–96. doi: 10.1089/ars.2016.6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abdolvahabi A, Shi Y, Rhodes NR, Cook NP, Marti AA, Shaw BF. Arresting amyloid with coulomb's law: Acetylation of ALS-linked SOD1 by aspirin impedes aggregation. Biophys J. 2015;108(5):1199–212. doi: 10.1016/j.bpj.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsai CP, Lin FC, Lee JK, Lee CT. Aspirin use associated with amyotrophic lateral sclerosis: A total population-based case-control study. J Epidemiol. 2015;25(2):172–7. doi: 10.2188/jea.JE20140070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakalar EG, Muluk NB, Kar M, Cingi C. Aspirin-exacerbated respiratory disease and current treatment modalities. Eur Arch Otorhinolaryngol. 2017;274(3):1291–300. doi: 10.1007/s00405-016-4273-1. [DOI] [PubMed] [Google Scholar]
- 90.Fajt ML, Petrov AA. Outpatient aspirin desensitization for patients with aspirin hypersensitivity and cardiac disease. Crit Pathw Cardiol. 2011;10(1):17–21. doi: 10.1097/HPC.0b013e318213d5a6. [DOI] [PubMed] [Google Scholar]
- 91.Szczeklik A, Stevenson DD. Aspirin-induced asthma: Advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003;111(5):913–21. doi: 10.1067/mai.2003.1487. quiz 922. [DOI] [PubMed] [Google Scholar]
- 92.Chen JR, Buchmiller BL, Khan DA. An Hourly Dose-Escalation Desensitization Protocol for Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract. 2015;3(6):926–31 e1. doi: 10.1016/j.jaip.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 93.Walgama ES, Hwang PH. Aspirin-Exacerbated Respiratory Disease. Otolaryngol Clin North Am. 2017;50(1):83–94. doi: 10.1016/j.otc.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 94.Triant VA, Grinspoon SK. Epidemiology of ischemic heart disease in HIV. Curr Opin HIV AIDS. 2017;12(6):540–7. doi: 10.1097/COH.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O'Brien M, Montenont E, Hu L, Nardi MA, Valdes V, Merolla M, et al. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: A pilot study. J Acquir Immune Defic Syndr. 2013;63(3):280–8. doi: 10.1097/QAI.0b013e31828a292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Brien MP, Hunt PW, Kitch DW, Klingman K, Stein JH, Funderburg NT, et al. A Randomized Placebo Controlled Trial of Aspirin Effects on Immune Activation in Chronically Human Immunodeficiency Virus-Infected Adults on Virologically Suppressive Antiretroviral Therapy. Open Forum Infect Dis. 2017;4(1):ofw278. doi: 10.1093/ofid/ofw278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pergolizzi JV, Magnusson P, LeQuang JA, Breve F, Paladini A, Rekatsina M, et al. The Current Clinically Relevant Findings on COVID-19 Pandemic. Anesth Pain Med. 2020;10(2):e103819. doi: 10.5812/aapm.103819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mohammed Sheata I, Smith SR, Kamel H, Varrassi G, Imani F, Dayani A, et al. Pulmonary Embolism and Cardiac Tamponade in Critical Care Patients with COVID-19; Telemedicine's Role in Developing Countries: Case Reports and Literature Review. Anesth Pain Med. 2021;11(2):e113752. doi: 10.5812/aapm.113752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mahmoodpoor A, Shadvar K, Ghamari AA, Mohammadzadeh Lameh M, Asghari Ardebili R, Hamidi M, et al. Management of Critically Ill Patients with COVID-19: What We Learned and What We Do. Anesth Pain Med. 2020;10(3):e104900. doi: 10.5812/aapm.104900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Papa A, Salzano AM, Di Dato MT, Lo Bianco G, Tedesco M, Salzano A, et al. COVID-19 Related Acro-Ischemic Neuropathic-like Painful Lesions in Pediatric Patients: A Case Series. Anesth Pain Med. 2021;11(2):e113760. doi: 10.5812/aapm.113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ali H, Ismail AA, Abdalwahab A. Mental Stress in Anesthesia and Intensive Care Physicians During COVID-19 Outbreak. Anesth Pain Med. 2020;10(5):e106623. doi: 10.5812/aapm.106623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Griffin DO, Jensen A, Khan M, Chin J, Chin K, Parnell R, et al. Arterial thromboembolic complications in COVID-19 in low-risk patients despite prophylaxis. Br J Haematol. 2020;190(1):e11–3. doi: 10.1111/bjh.16792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aminnejad R, Hashemi SM, Safari S, Dadkhah P, Bastanhagh E. Impact of COVID-19 on Advanced Cancer Patients' Pain Care: Warning About Chloroquine and Hydroxychloroquine. Anesth Pain Med. 2021;11(1):e111641. doi: 10.5812/aapm.111641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haryalchi K, Heidarzadeh A, Abedinzade M, Olangian-Tehrani S, Ghazanfar Tehran S. The Importance of Happy Hypoxemia in COVID-19. Anesth Pain Med. 2021;11(1):e111872. doi: 10.5812/aapm.111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shahsavarinia K, Ghojazadeh M, Ghabousian A, Hatefnia F, Soleimanpour M, Soleimanpour H. An Umbrella Review of Clinical Efficacy and Adverse Cardiac Events Associated with Hydroxychloroquine or Chloroquine with or Without Azithromycin in Patients with COVID-19. Anesth Pain Med. 2021;11(4):e115827. doi: 10.5812/aapm.115827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alegbeleye BJ, Akpoveso OP, Alegbeleye AJ, Mohammed RK, Esteban-Zubero E. The Novel Aspirin as Breakthrough Drug for COVID-19: A Narrative Review. Iberoamerican Journal of Medicine. 2020;2(4):335–50. doi: 10.53986/ibjm.2020.0058. [DOI] [Google Scholar]
- 107.Chow JH, Khanna AK, Kethireddy S, Yamane D, Levine A, Jackson AM, et al. Aspirin Use Is Associated With Decreased Mechanical Ventilation, Intensive Care Unit Admission, and In-Hospital Mortality in Hospitalized Patients With Coronavirus Disease 2019. Anesth Analg. 2021;132(4):930–41. doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 108.Plumer L, Seiffert M, Punke MA, Kersten JF, Blankenberg S, Zollner C, et al. Aspirin Before Elective Surgery-Stop or Continue? Dtsch Arztebl Int. 2017;114(27-28):473–80. doi: 10.3238/arztebl.2017.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso-Coello P, Kurz A, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494–503. doi: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
- 110.Kim YS, Lee IO, Lim BG, Kim H. Platelet function assay to determine the optimal preoperative cessation period of aspirin. Anesth Pain Med. 2014;9(1):31–5. [Google Scholar]
- 111.Vela Vasquez RS, Pelaez Romero R. Aspirin and spinal haematoma after neuraxial anaesthesia: Myth or reality? Br J Anaesth. 2015;115(5):688–98. doi: 10.1093/bja/aev348. [DOI] [PubMed] [Google Scholar]
- 112.Celiker V, Basgul E. Anaesthesia in aspirin-induced asthma. Allergol Immunopathol (Madr). 2003;31(6):338–41. doi: 10.1016/s0301-0546(03)79208-8. [DOI] [PubMed] [Google Scholar]