Abstract
About one-third of the enterotoxigenic Escherichia coli isolates lack any of the known colonization factors. Among this group, those of serogroup O20 are the most frequently isolated in Argentina. By combining analysis of adhesion to Caco-2 cells, random amplified polymorphic DNA, and pulsed-field gel electrophoresis techniques, we were able to identify three sets of closely related strains with different binding properties. Further analysis of the most prevalent group revealed that all these isolates expressed the recently described adhesin CS22.
Enterotoxigenic Escherichia coli (ETEC) is one of the leading causative agents of infantile diarrhea, especially in developing countries (4). Adhesion of the bacteria to the small intestine by means of colonization factors (CFs) precedes the production of heat-labile and/or heat-stable (ST) enterotoxins, which induce diarrhea (2). Several serogroups have been most frequently associated with ETEC isolates, including O6, O78, O8, O127, O153, O159, O20, and others (3, 18).
In Argentina, strains belonging to serogroup O20 have been recovered from children with diarrhea in different epidemiological studies (1, 13, 15). However, in contrast with what has been observed in other regions (18), strains belonging to this serogroup did not produce any of the known CFs (1, 13, 15). In fact, among the CF-negative strains, which constitute between 30 and 40% of the ETEC isolated in this region (1, 13, 15), serogroup O20 was the most frequent. Although CS18 (formerly known as PCFO20) was identified among these isolates, its prevalence was found to be low in this area.
Certain phenotypic characteristics, i.e., O:H type, biotype, toxin, and CF profiles, have been shown to be associated in particular combinations among ETEC isolates (4, 18). These observations lead to the suggestion that ETEC strains sharing these phenotypic traits owe their similarity to recent descent from a common ancestor (8). Moreover, this notion has been confirmed by analysis of electrophoretic patterns generated by randomly amplified polymorphic DNA (RAPD) and by pulsed-field gel electrophoresis (PFGE) techniques (9, 17).
As a strategy to identify prevalent “new” adhesins among a group of O20 CF-negative ETEC strains isolated in Argentina, we determined the genetic relationship among these strains and evaluated the adhesive properties of the isolates belonging to different clusters. By means of a combination of DNA typing approaches and adhesion to Caco-2 cells, we could identify a genetically related, yet not identical, group of strains that bear the recently described adhesin CS22 (10).
Phenotypic traits.
A total of 19 well-characterized ETEC strains belonging to serogroup O20 were selected for this study. They had been isolated from children with diarrhea or from healthy controls from different regions of Argentina (Table 1).
TABLE 1.
Characteristics of the ETEC strains studied
| Strain | Region (city)a | Date of isolation | Serotype | Toxinb | CF profile | Adherence to Caco- 2 cells (no. of bacteria/cell) | RAPD type | PFGE type | Genetic cluster |
|---|---|---|---|---|---|---|---|---|---|
| ARG-3 | NW (Orán) | 1993 | O20:H− | ST | Negative | 8.00 ± 2.60 | 1 | 1 | I |
| ARG-4 | NW (Orán) | 1993 | O20:H− | ST | Negative | 6.75 ± 0.35 | 1 | 1 | I |
| ARG-5 | N (Tucumán) | 1988 | O20:H− | ST | Negative | 5.75 ± 1.75 | 1 | 2 | I |
| ARG-6 | NE (Posadas) | 1988 | O20:H− | ST | Negative | 7.50 ± 3.50 | 1 | 3 | I |
| ARG-7 | NE (Posadas) | 1988 | O20:H− | ST | Negative | 12.50 ± 2.10 | 1 | 16 | I |
| ARG-8 | CE (La Plata) | 1989 | O20:H− | ST | Negative | 5.50 ± 0.70 | 1 | 4 | I |
| ARG-9 | NW (Orán) | 1993 | O20:H− | ST | Negative | 11.75 ± 2.50 | 2 | 5 | |
| ARG-10 | CE (Rosario) | 1989 | O20:H34 | ST | Negative | 22.70 ± 0.25 | 3 | 6 | II |
| ARG-11 | CE (Rosario) | 1989 | O20:H34 | ST | Negative | 35.00 ± 3.15 | 4 | 7 | II |
| ARG-12 | NE (Posadas) | 1989 | O20:H− | ST | Negative | 0.85 ± 0.20 | 5 | 8 | |
| ARG-13 | NE (Posadas) | 1988 | O20:H34 | ST | Negative | 8.25 ± 0.35 | 6 | 9 | |
| ARG-14 | NE (Posadas) | 1989 | O20:H− | ST | Negative | 32.50 ± 3.50 | 7 | 10 | |
| ARG-15 | NE (Posadas) | 1990 | O20:H? | ST | Negative | 6.75 ± 1.05 | 8 | 11 | III |
| ARG-16 | CE (Mar del Plata) | 1991 | O20:H? | ST | Negative | 0.65 ± 0.07 | 8 | 11 | III |
| ARG-17 | NE (Posadas) | 1991 | O20:H− | ST | Negative | 0.58 ± 0.10 | 8 | 11 | III |
| ARG-18 | NE (Posadas) | 1991 | O20:H− | ST | Negative | 15.75 ± 3.15 | 9 | 12 | II |
| ARG-19 | CE (Córdoba) | 1987 | O20:H32 | ST | Negative | 2.75 ± 1.75 | 10 | 13 | |
| ARG-2 | CE (Mar del Plata) | 1988 | O20:H− | LT/ST | CS18 | 3.30 ± 0.35 | 11 | 14 | |
| ARG-20 | NE (Posadas) | 1991 | O20:H− | ST | CS6 | 7.00 ± 0.71 | 12 | 15 |
NW, northwest; N, north; CE, east-central.
LT, heat-labile enterotoxin.
As in other parts of the world, most of the O20 ETEC isolates examined produced ST, as determined by ganglioside GM1-enzyme-linked immunosorbent assay (1), and most of them were nonmotile, except for three H34 strains, one H32 strain, and two strains with undetermined flagellar antigen (H?). Dot blot tests using specific monoclonal antibodies or polyclonal antisera against CFA/I, CS1 to CS8, CS12, CS14, CS15, CS17, CS18, CS19, or CS20 showed that most of the isolates lacked all these CFs, except for one strain that produced CS6 and another one expressing CS18 (Table 1).
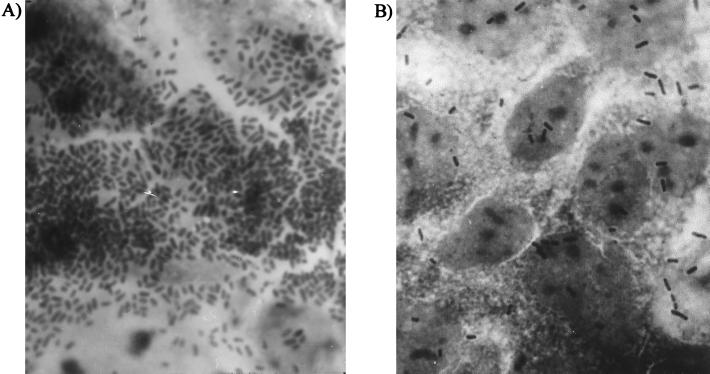
To evaluate whether these strains expressed any unidentified adhesin, the capacity to adhere to intestinal epithelial cell line Caco-2 was evaluated as previously described (10). The assay was performed at least twice for each strain, and the result was expressed as the average number of bacteria/cell ± the standard deviation. A strongly adherent ETEC strain (350C1A, expressing CS12) and its corresponding CF-deficient mutant (350C1B) were included as controls, and their adhesion indexes were 9.25 ± 0.35 bacteria/cell and 0.44 ± 0.08 bacteria/cell, respectively. Most of the O20 ETEC strains bound to the epithelial cells, with adhesion indexes ranging from 2.75 ± 1.75 bacteria/cell to 35.00 ± 3.15 bacteria/cell (Table 1; Fig. 1). However, three of the isolates (ARG-12, ARG-16, and ARG-17) exhibited low adhesion indexes, comparable to those of the negative control.
FIG. 1.
Micrographs showing adhesion of ETEC strains ARG-11 (A) and ARG-19 (B) to intestinal Caco-2 cells.
Genotypic analysis.
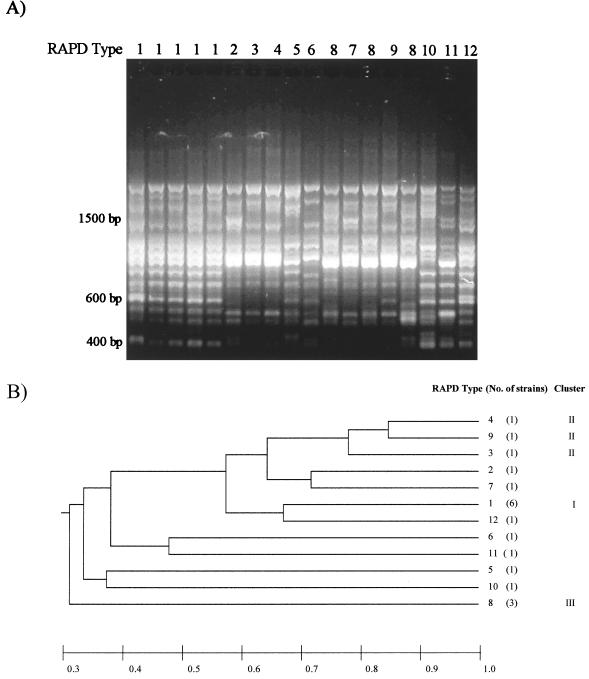
To evaluate the genetic diversity among the O20 ETEC isolates, we used RAPD and PFGE as molecular typing methods. The RAPD assay was carried out as previously described (9) using primer 1254 (CCGCAGCCAA). Relatedness among RAPD or PFGE patterns was estimated by applying the Jaccard coefficient (6); data recording and calculations were performed as described previously (9). Twelve different RAPD types were defined among the strains studied (Table 1; Fig. 2). The degree of similarity among the various RAPD types was calculated, and a phenogram was constructed based on the UPGMA method (Fig. 2). Thus, three main groups of genetically related strains could be defined. The first group (cluster I) included almost 30% of the CF-negative strains studied, which were all nonmotile and produced ST. All of these strains exhibited an identical RAPD pattern, yet the group included isolates from different geographical regions, recovered at different time periods (Table 1). The second group (cluster II) included two O20:H34 strains along with an O20:H− strain. These strains exhibited very similar electrophoretic patterns, sharing more than 78% of the bands. Group III comprised two ST O20:H? strains and one nonmotile strain that shared the same band profile and had been isolated in a one-year period from two different locations. The remaining eight isolates showed different patterns, with less than 72% of the bands in common. The discriminating ability of RAPD was determined to be 0.89 using Simpson's diversity index (DI), as recommended by Hunter and Gaston (5).
FIG. 2.
(A) RAPD patterns exhibited by O20 ETEC strains isolated in Argentina; (B) phenogram representing groups of related ETEC strains. The relatedness among the isolates was estimated based on the proportions of shared bands, which are indicated below the figure.
For PFGE analysis, agarose plugs were cast by mixing equal volumes of bacterial suspensions and 1.6% Pulse Field Certified Agarose (Bio-Rad) and incubated overnight at 50°C in lysis buffer containing 0.5 mg of proteinase K/ml. The plugs were subsequently washed before digestion with 20 U of XbaI. Electrophoresis was performed in a CHEF-DR III chamber (Bio-Rad) for 21 h at 6 V/cm, 14°C, with a linear pulse ramp of 5 to 50 s.
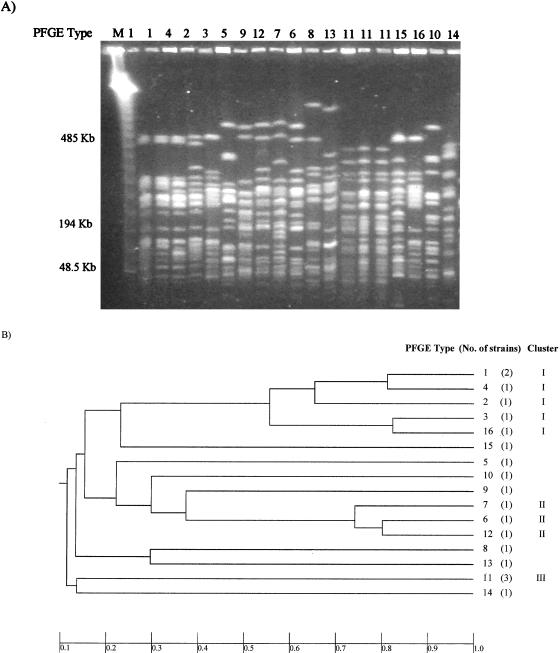
The XbaI-digested chromosomal DNA gave rise to 16 different restriction patterns (Table 1; Fig. 3), with fragments of about 40 to 450 kb. Analysis of the shared bands revealed a higher degree of heterogeneity (DI = 0.976) than that shown by RAPD. However, in accordance with the results of the RAPD analysis, the same groups of closely related isolates could be defined (Fig. 3).
FIG. 3.
(A) PFGE profiles obtained with the XbaI enzyme; (B) phenogram representing groups of related ETEC strains. The relatedness among the isolates was estimated based on the proportions of shared bands, which are indicated below the figure.
The six isolates belonging to group I that were shown to be identical by RAPD gave rise to five electrophoretic patterns in PFGE. Nevertheless, these strains appeared in the same branch of the phenogram, sharing on average 75.5% of the bands (minimum value, 53%; maximum value, 100%). Recent guidelines for interpreting DNA restriction patterns produced by PFGE (12) suggest that two isolates could be considered unrelated if they share less than 50% of the fragments present in their patterns (typically fewer than seven bands). Accordingly, we considered the O20:H− strains included in group I, which shared at least 53% of their bands, to be genetically related.
Strains in group II showed three different patterns, which shared more than 72% of the bands (minimum value, 72.6%; maximum value, 79%), and the isolates included in group III exhibited a common band profile. The rest of the strains exhibited single electrophoretic profiles, with less than 33.4% common bands.
Relation between adhesion capacity and genetic relatedness.
Strains within the same genetic cluster appeared to exhibit similar adhesion indexes, those of group II being the highest (between 15.75 ± 3.15 and 35.00 ± 3.15 bacteria/cell) and those of group III being the lowest ones (0.58 ± 0.10 to 6.75 ± 1.05 bacteria/cell). The isolates comprised in group I showed quite homogeneous adhesion indexes, ranging from 5.50 ± 0.70 to 12.50 ± 2.10 bacteria/cell. Further characterization of this group of strains led us to the identification of the recently reported CS22 adhesin (10). This adhesin, originally identified and characterized in strain ARG-3, was later found in all of the strains belonging to group I, as determined by dot blot and Western blot assays using specific anti-CS22 antisera as described previously (10).
Several studies have shown that O:H serotypes represent good indicators of the clonal relatedness among ETEC strains (7, 9). However, in the case of H-negative types, genotypic methods are necessary to evaluate genetic diversity. In this work, we show that despite sharing several phenotypic traits (toxin profile and serogroup), the Argentinean O20 ETEC isolates studied herein exhibited a high degree of heterogeneity by both RAPD and PFGE. It has been postulated that the nonmotile strains derive from the motile ones by mutational loss of the flagella (11). Since most of the strains analyzed herein are nonmotile, these results suggest that these isolates probably derive from ancestors belonging to different H types. Thus, strain ARG-18, included in group II with two H34 isolates, probably derives from an H34 ancestor. On the other hand, the origin of the strains O20:H− pertaining to the main cluster could not be assessed, since they were not related to isolates expressing the flagellar antigens H32 or H34.
The majority of the O20 ETEC strains tested did not bear any of the known CFs. However, most of them were able to adhere to human intestinal epithelium cells with a certain binding profile (weak, moderate, or strong), suggesting that they might produce previously undescribed adhesins. Although strains from genotype II showed the strongest adhesion indexes, the fact that the group was comprised of only a few isolates makes it not so relevant as a candidate for identification of a new CF. On the other hand, strains from genotype I, which was the most frequently isolated type, presented a moderate adhesion index, and they all were found to express the recently identified CS22 (10). In fact, this new adhesin was found in almost 60% of the CF-negative O20:H− ST-ETEC strains isolated in Argentina. Since this group included strains isolated at different time periods from very distant locations, it is unlikely that they were epidemiologically related.
The fact that adhesion is the first step in ETEC infection makes CFs good candidates for vaccine antigens. However, since the occurrence of the various ETEC adhesins varies significantly within the different geographical areas, it is important to identify the more abundant CFs in regions where ETEC vaccines need to be implemented. We found the approach presented in this work valuable as a start point for identifying new CFs within a population of ETEC strains showing similar phenotypic traits.
Acknowledgments
This work was supported by grants from Centro Argentino-Brasileño de Biotecnología (CABBIO), and Fundación “Alberto J. Roemmers.”
We thank L. C. Souza Ferreira and A. B. F. Pacheco for very helpful discussions; A.-M. Svennerholm for providing monoclonal antibodies against ETEC CFs; A. Darfeuille-Michaud and H. Sommerfelt for reference strains and antisera against CS15, CS19, and CS20; I. Ørskov and F. Ørskov for serotyping the ETEC strains; A. Lewis for culturing the Caco-2 cells; and G. Lafuente Devier, A. Garbini, and N. Martinez for technical assistance.
REFERENCES
- 1.Binsztein N, Jouve M, Viboud G I, Lopez Moral L, Rivas M, Ørskov I, Åhren C, Svennerholm A-M. Colonization factors of enterotoxigenic Escherichia coli isolated from children in Argentina. J Clin Microbiol. 1991;29:1893–1898. doi: 10.1128/jcm.29.9.1893-1898.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black R E. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine. 1993;11:100–106. doi: 10.1016/0264-410x(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 3.Echeverria P, Ørskov F, Ørskov I, Plianbanchang D. Serotypes of enterotoxigenic Escherichia coli in Thailand and the Philippines. Infect Immun. 1982;36:851–856. doi: 10.1128/iai.36.3.851-856.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaastra W, Svennerholm A-M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 5.Hunter P R, Gaston M A. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988;6:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaccard P. Ètude comparative de la distribution florale dans une portion des Alpes et des Jura. Bull Soc Vaudoise Sci Nat. 1901;37:547–549. [Google Scholar]
- 7.Ørskov F, Whittam T S, Cravioto A, Ørskov I. Clonal relationships among classic enteropathogenic Escherichia coli (EPEC) belong to different O groups. J Infect Dis. 1990;162:67–81. doi: 10.1093/infdis/162.1.76. [DOI] [PubMed] [Google Scholar]
- 8.Ørskov I, Ørskov F. Special O:K:H serotypes among enterotoxigenic E. coli strains from diarrhea in adults and children. Occurrence of the CF (colonization factor) antigen and of hemagglutinating abilities. Med Microbiol Immunol. 1977;163:99–110. doi: 10.1007/BF02121825. [DOI] [PubMed] [Google Scholar]
- 9.Pacheco A B F, Guth B E, Soares K C, Nishimura L, Almeida D F, Ferreira L C S. Random amplification of polymorphic DNA reveals serotype-specific clonal clusters among enterotoxigenic Escherichia coli strains isolated from humans. J Clin Microbiol. 1997;35:1521–1525. doi: 10.1128/jcm.35.6.1521-1525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pichel M, Binsztein N, Viboud G I. CS22, a novel human enterotoxigenic Escherichia coli adhesin, is related to CS15. Infect Immun. 2000;68:3280–3285. doi: 10.1128/iai.68.6.3280-3285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues J, Scaletsky I, Campos L, Gomes T, Whittam T, Trabulsi L. Clonal structure and virulence factors in strains of Escherichia coli of the classic serogroup O55. Infect Immun. 1996;64:2680–2686. doi: 10.1128/iai.64.7.2680-2686.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viboud G I, Binsztein N, Svennerholm A-M. Characterization of monoclonal antibodies against putative colonization factors of enterotoxigenic Escherichia coli and their use in an epidemiological study. J Clin Microbiol. 1993;31:558–564. doi: 10.1128/jcm.31.3.558-564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viboud G I, Binsztein N, Svennerholm A-M. A new fimbrial putative colonization factor, PCFO20, in human enterotoxigenic Escherichia coli. Infect Immun. 1993;61:5190–5197. doi: 10.1128/iai.61.12.5190-5197.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viboud G I, Jouve M, Binsztein N, Vergara M, Rivas M, Quiroga M, Svennerholm A-M. Prospective cohort study of enterotoxigenic Escherichia coli infections in Argentinean children. J Clin Microbiol. 1999;37:2829–2833. doi: 10.1128/jcm.37.9.2829-2833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viboud G I, McConnell M M, Helander A, Svennerholm A-M. Binding of enterotoxigenic Escherichia coli expressing different colonization factors to tissue-cultured Caco-2 cells and to isolated human enterocytes. Microb Pathog. 1996;21:139–147. doi: 10.1006/mpat.1996.0049. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Whittam T, Berg C, Berg D E. RAPD (arbitrary primer) PCR is more sensitive than multilocus enzyme electrophoresis for distinguishing related bacterial strains. Nucleic Acids Res. 1993;21:5930–5933. doi: 10.1093/nar/21.25.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf M. Occurrence, distribution, and association of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin Microbiol Rev. 1997;10:569–584. doi: 10.1128/cmr.10.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]