Abstract
Maintenance of Hendra virus (HeV) in pteropid bat populations has been associated with spillover events of zoonotic infections in horses, humans and dogs. Experimental studies have demonstrated infections for several other species including guinea pigs, cats and ferrets. The criteria of a sensitive and specific serological test that is effective for a range of species but which does not require use of live virus, has not been satisfactorily addressed by currently available tests. We have evaluated the use of two HeV neutralizing monoclonal antibodies (mAbs) for their application in a blocking format enzyme-linked immunosorbent assay (bELISA) to detect serum antibody against a recombinant expressed HeV G protein (sol G) in several animal species. The human mAb m102.4 neutralises both HeV and the closely related Nipah virus (NiV); the mouse mAb 1.2 neutralises only HeV. Given these functional differences, we have investigated both antibodies using a bELISA format. Diagnostic sensitivity (DSe) and diagnostic specificity (DSp) were optimized using individual thresholds for mAb 1.2 and m102.4. For mAb 1.2 the positive threshold of >33% inhibition yielded DSe and DSp values of 100% (95% CI 95.3 - 100.0) and 99.5 (95% CI 98.8 - 99.8) respectively; for mAb m102.4 a positive threshold of >49% inhibition gave DSe and DSp values of 100 (95% CI 95.3 - 100.0) and 99.8 (95% CI 99.2 - 100.0) respectively. At these thresholds the DSe was 100% for both tests relative to the virus neutralization test. Importantly, the occurrence of false positive reactions did not overlap across the assays. Therefore, by sequential and selective application of these assays, it is possible to identify false positive reactions and achieve a DSp that approximates 100% in the test population.
1. Introduction
Hendra virus (HeV: Paramyxovirus, Henipavirus genus) is endemic in certain populations of Australian flying-foxes (Chiroptera: Pteropodidae). The virus is recognised for sporadic infectious disease spillovers which have resulted in horse and human fatalities (Young et al., 1996; Halpin et al., 2000). The black and spectacled flying-foxes host species (Pteropus alecto and P. conspicillatus) have been implicated as natural reservoirs of the virus (Halpin et al., 2011; Goldspink et al., 2015; Edson et al., 2015). First identified in 1994 from a fulminant disease outbreak in Brisbane (Queensland), involving both human and equine infections and deaths (Murray et al., 1995a; 1995b; Selvey at al., 1995), the virus has since caused 60 outbreak incidents with associated deaths in more than one hundred horses in Queensland and New South Wales (Australian Veterinary Association, 2017). Over the same period, four of seven human infections had lethal outcomes (Goldspink et al., 2015). In two instances, asymptomatic spillover infections have been acquired by domestic dogs (PromedMail, 2014; Kirkland et al., 2013), but a range of other animals may be experimentally infected (Williamson et al., 1998; Li et al., 2010; Middleton et al., 2017) suggesting a capacity for broad zoonotic potential.
Hendra virus infections are thus a significant emerging zoonosis and present challenges in areas of human and animal health management including policy, epidemiology, control, animal movement and trade. Diagnostic tools including serology and agent detection for identifying infection in exposed animals are key components for effective management. Serology for antibodies to HeV provides an important diagnostic approach for disease investigation, surveillance and certification testing. A range of serology tests have been published, including various neutralisation and solid phase immunoassay formats (Williamson et al., 1998; Crowther, 2001; Daniels et al., 2001; Bossart et al., 2005; Bossart et al., 2007; Li et al., 2010; McNabb et al., 2014). While the HeV neutralisation (VN) test is the serology reference standard, an on-going priority has been the development of alternative assays which use non-infectious reagents for antibody detection and thus make such assays suitable for a much wider range of laboratory settings. Most solid phase assays have been indirect immunoassays such as the Sol G indirect ELISA (iELISA) (Colling et al., 2018) using an expressed truncated and soluble form of the Hendra G protein developed by Bossart et al. (2005). A novel HeV receptor binding microsphere assay was developed (Bossart et al., 2007) to measure serum antibody levels by competing these antibodies with biotinylated ephrin-B2. Monoclonal antibody epitope blocking ELISAs (bELISAs) can be highly effective tests for detection of serum antibodies (Crowther, 2001; OIE, 2014) in an assay format without determinants for immunoglobulin class and species under test. Selection of the mAb will determine the range of permissible competing antibody while limiting other influences, thereby allowing testing samples at low dilution to improve assay sensitivity. Monoclonal antibodies with potential applicability to a competition or bELISA format for HeV – specific antibody detection have been developed in earlier studies (White et al., 2005; Zhu, et al., 2006; Zhu et al., 2008). While a mAb bELISA had been developed in 2004 for the closely related Nipah virus (NiV) (Kashiwazaki et al., 2004), the approach has not been applied to HeV serology. In this study, we report on the evaluation and effective use of two distinct mAbs in a bELISA format for the detection of serum antibody to HeV in horses, dogs, cats and bats.
2. Materials and Methods
2.1. Sera
Sera sourced within Australia from export and disease diagnosis testing included: horses (469 antibody-negative by VN and/or Sol G iELISA, 56 with vaccination record and 16 cases of laboratory-confirmed field acquired HeV infection), dogs (312) and cats (112) evidenced as infection-negative by serology or otherwise grouped as negative based on routine submission history and inferred low likelihood of exposure. Sera collected for surveillance purposes included pteropid bat sera, 99 from Australia and 21 from New Caledonia. Five HeV infected canine sera were derived from experimentally infected dogs (Middleton et al., 2017). Further details are documented in Supplementary Table 1. Other sera for use as assay controls and in determining analytical specificity (paramyxoviruses) and analytical sensitivity were as described in a previous experimental study (McNabb et al., 2014).
2.2. Hendra antibody bELISA
Two similarly formatted bELISAs were performed, differing only in the competing mAb. In brief, Nunc Maxisorp ELISA immuno-plate (Thermo Fisher Scientific™) wells were coated with 50 μl of either 4.4 ng or 5.5 ng Hendra virus soluble G tetramer antigen (Bossart et al., 2005) (supplied by CSIRO MSE, Clayton) diluted in phosphate buffered saline (PBS, pH 7.2). Plates were incubated at 37°C for one hour with shaking. At the end of the incubation time, 50 μl of casein blocking buffer (Sigma Aldrich®) diluted 1/10 in ddH2O (BB) were added to all wells. Plates were incubated at 37°C for 30 minutes with shaking. Plate wells were washed 3 times in PBS with 0.05% v/v Tween 20 (MERK) (PBST) using an automatic plate washer (Skatron Instruments, SkanWasher 300). Fifty microlitres of BB were added to all wells followed by 10 μl of test samples and control sera (negative and positive samples of canine, feline and equine sera). Control sera were included in duplicate wells, test sera were assessed in single well format. Plates were incubated at 37°C for one hour with shaking. Ten microliters of anti-HeV mAb 1.2 (White et al., 2005) diluted 1/100 in BB were added to all samples containing wells, excepting mAb binding control and blank wells on each plate. This was separately repeated for the biotin conjugated anti-HeV mAb m102.4 (Bossart et al., 2005) diluted at 1/10,000 in BB. Plates were incubated at 37°C for 30 minutes with shaking followed by washing. Horseradish peroxidase (HRP) conjugated goat anti-mouse IgG diluted 1/2,000 in BB (detection of mAb 1.2) or HRP conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc.) diluted 1/16,000 in BB (detection of mAb m102.4-biotin) were added to all wells. Plates were incubated at 37°C for 30 minutes with shaking followed by washing and addition to each well of 50 μL of 3,3′5,5′-tetramethylbenzidine (TMB) substrate (Sigma®). Developing reaction was monitored at 640 nm using a Thermo Scientific Multiscan plate reader. The reaction was stopped with 1 M sulphuric acid (Ajax Finechem) when negative serum control wells, or maximum optical density (ODmax) wells exhibited values ranging 0.40-0.50 nm when read at 640 nm. Percent inhibition of mAbs were calculated using the formula: % inhibition = 100 – (100 x (TS - B) / (NCS - B)) where TS = averaged OD values of test serum, NCS = averaged OD values of negative control sera, B = background OD.
2.3. Competitive binding assays
Potential congruence of epitopes recognised by mAb 1.2, mAb m102.4 and Ephrin B2 was evaluated in a procedurally similar bELISA, using a lower coating concentration of HeV Sol G antigen (1.1 ng in 50 μl per well). Monoclonal antibodies and Ephrin B2-biotin (mEphrin-B2/Fc Chimera Biotinylated recombinant mouse Cat no. BT496 (R&D systems) were paired in blocking/detecting combinations (shown in Table 1). The blocking step used near saturating levels (mAb 1.2 diluted 1/100, ephrin B2-biotin diluted 1/200 or mAb m102.4 diluted 1/2000). The detecting reagent was titrated on the blocked antigen and highest level of inhibition relative to unblocked binding was taken to represent the level of competition. Levels of inhibition above 30% were deemed as significant interference.
Table 1.
Competitive inhibition assay results (% inhibition)
| Detection reagent | ||||
|---|---|---|---|---|
| mAb 1.2 | mAb m102.4- biotin |
Ephrin B2-biotin |
||
| Blocking reagent | mAb 1.2 | NT | 34 | 43 |
| mAb m102.4 | 9 | 100 | 85 | |
| Ephrin B2-biotin | 51 | 35 | NT | |
2.4. Comparison immunoassays
Fluorescent liquid suspension array microbead assays (Luminex) and virus neutralisation (VN) serology were conducted as detailed previously (McNabb et al., 2014).
2.5. Sol G iELISA
The Sol G iELISA was performed as previously described (Colling et al., 2018). Briefly, 50 μl of Hendra virus sol G tetramer antigen diluted at a concentration of 0.073 μg/mL in PBS were used to coat wells of ELISA plates. The plates were incubated at 37°C for one hour with shaking. The plates were blocked with 5% skimmed milk powder (SMP) diluted in PBS and incubated at 37°C for 30 minutes. Plates were then washed four times with PBST. All test and control sera were diluted at 1/100 in ELISA diluent and 50 μl added in duplicate wells. The plates were incubated at 37°C for one hour with shaking followed by washing. Anti-equine HRP conjugate (Sigma Aldrich®) diluted 1/5000 in ELISA diluent was added at 50 μl per well. Plates were incubated at 37°C for 30 minutes followed by washing. Plates were developed by adding 50 μl per well of TMB substrate and stopped with 1M sulfuric acid prior reading at 450 nm.
3. Results
3.1. Characterisation of reagents – competitive inhibition
As there is evidence from VN serology for functional differences between mAb 1.2 (neutralises HeV) and mAb m102.4 (neutralises HeV and NiV) (White et al., 2005; Zhu, et al, 2006), competitive binding assays were used to explore whether these differences might also affect their relative performance in the HeV ELISA format. Using greater than 30% as evidence of binding inhibition, results suggested that bound mAb m102.4 did not interfere with binding of the mAb 1.2 (Table 1). However bound mAb 1.2 caused weak interference with mAb m102.4 binding. We concluded that the two mAbs do not have identical epitope binding characteristics for HeV and this may influence relative measurements of competitive blocking by polyclonal sera for each mAb in the antibody bELISA format.
Ephrin B2-biotin was included in the comparison to provide overlap with a reported competitive immunoassay format for HeV antibody detection (Bossart et al., 2007). Binding of ephrin B2-biotin was partially blocked (43% inhibition) by pre-bound mAb 1.2. Similarly, binding of mAb 1.2 was partially blocked by pre-bound ephrin B2-biotin and mAb m102.4 was associated with 85% inhibition of ephrin B2-biotin, a level very close to the 89% reported in the previous study.
3.2. Analytical sensitivity
Analytical sensitivity was estimated by interpolation of curves plotted from bELISA values (PI) for corresponding serum dilutions. Equations generated in Excel from fitting third or fourth order polynomial regression curves with r2 values of 0.99 or greater were solved for the dilution corresponding to 40% binding inhibition.
Using a 40% inhibition threshold in the mAb 1.2 and mAb m102.4 bELISAs, serum titres closely approximated VN titres (average VN/ELISA ratios of 0.74 and 0.51 respectively) (Table 2). One sample (post-infection equine serum 2) showed a fourfold higher titre when using the mAb m102.4, relative to results for mAb 1.2. The iELISA, applied only for equine sera, showed lower analytical sensitivity than the bELISA (average VN/ELISA 1.6). Results suggest that the bELISA format has analytical sensitivity characteristics similar or superior to the VN and superior to the Sol G iELISA. Under similar assessment, a bELISA using ephrin B2 had a lower analytical sensitivity (average VN/ELISA ratio of 1.17) and was not further evaluated.
Table 2.
Relative analytical sensitivity of the HeV bELISAs in comparison with the HeV VN and Sol G iELISA
| Species | Status | Identity | VN | mAb 1.2 |
mAb m102.4 |
Ephrin B2 |
Sol G iELISA |
|---|---|---|---|---|---|---|---|
| Equine | Infected | Sample 1 | 11.01 | 10.92 | 10.6 | 10.0 | 10.4 |
| Sample 2 | 9.0 | 8.3 | 10.4 | 6.6 | 7.8 | ||
| Vaccinated | Sample 1 | 8.0 | 9.3 | 9.3 | 10.2 | 8.6 | |
| Sample 2 | 10.0 | 10.6 | 10.9 | 10.9 | 8.6 | ||
| Canine | Infected | Sample 1 | 7.0 | 8.0 | 8.7 | 6.1 | nt3 |
| Average VN/ELISA Ratio | 0.74 | 0.51 | 1.17 | 1.6 | |||
Titres are expressed as the log2 values.
ELISA titres represent the dilution of sera at threshold levels of reactivity. Dilutions routinely used in the test (i.e. bELISA – 1/6; iELISA – 1/100) were produced without taking into consideration the starting dilution.
nt, not tested.
3.3. Analytical specificity
Analytical specificity provides a limited representation of the potential for cross-reactions which may affect assay specificity, particularly regarding more closely related agents. Fourteen sera, antibody positive to related paramyxoviruses and seven sera positive to Nipah virus were tested in the HeV bELISAs. All fourteen positive paramyxovirus sera were negative with both the mAbs (Table 3). Of the seven Nipah positive antisera, the cat and rabbit sera were positive with both the mAbs. Interestingly, the pig sera showed a negative reaction with both mAbs.
Table 3.
Analytical specificity of the HeV bELISAs using various paramyxovirus antibody-positive sera
| Species | Virus | mAb 1.2 % Inhib1 |
mAb 102.4 % Inhib1 |
Interpretation |
|---|---|---|---|---|
| Cat | Nipah | 72 | 90 | Positive |
| Flying fox | Nipah | 70 | 92 | Positive |
| Rabbit | Nipah | 95 | 103 | Positive |
| Pig 1 | Nipah | −3 | 16 | Negative |
| Pig 2 | Nipah | 32 | 36 | Negative |
| Pig 3 | Nipah | 32 | 22 | Negative |
| Pig 4 | Nipah | 16 | 25 | Negative |
| Ferret | cedar | 13 | 0 | Negative |
| Pig | Menangle | 6 | 13 | Negative |
| Rabbit | Menangle | 20 | 30 | Negative |
| Rabbit | Mossman | 5 | 5 | Negative |
| Horse | Mumps | 9 | 2 | Negative |
| Rabbit | Nariva | −4 | 8 | Negative |
| Rabbit | Newcastle disease | −7 | 4 | Negative |
| Horse | Parainfluenza 2 SV-5 | −5 | 10 | Negative |
| Horse | Parainfluenza 3 C243 | 11 | 14 | Negative |
| Guinea pig | Parainfluenza 4 | −1 | 14 | Negative |
| Guinea pig | Parainfluenza 4B | −8 | 3 | Negative |
| Horse | Parainfluenza sendai | 6 | 13 | Negative |
| Rabbit | Rinderpest | −1 | 8 | Negative |
| Pig | Tioman | −1 | −1 | Negative |
% Inhib = Percent inhibition
3.4. Diagnostic specificity - HeV antibody negative sera
Diagnostic specificity has been assessed by testing sera from animals that have been previously tested with negative results in the HeV VN and additional serum samples from healthy animals tested for movement certification Results of the DSp are reported in Table 4. Median PI values were all below 10% and associated with sufficiently narrow data spread to allow setting of thresholds with potentially useful resolution (Figure 1). Measurements of normal cat sera in the mAb m102.4 bELISA showed greater spread, evident in PI values for the interquartile range and 99 percentile.
Table 4.
Summary statistics for bELISAs’ results (percent inhibition, PI) for Hendra antibody-negative equine, canine and feline sera
| Horse (uninfected) | Dog (uninfected) | Cat (uninfected) | ||||
|---|---|---|---|---|---|---|
| mAb 1.2 |
mAb m102.4 |
mAb 1.2 |
mAb m102.4 |
mAb 1.2 |
mAb m102.4 |
|
| Count | 469 | 469 | 312 | 312 | 112 | 112 |
| Average PI | 4 | −2 | 8 | 2 | 4 | 1 |
| Standard deviation | 12.8 | 15.5 | 10.60 | 14.79 | 13.7 | 21.0 |
| Maximum PI | 32.8 | 38.2 | 49.3 | 38.1 | 42.6 | 71.8 |
| Minimum PI | −49.1 | −73.6 | −18.6 | −62.8 | −30.2 | −61.7 |
| Median PI | 5.6 | −2.1 | 7.3 | 2.9 | 4.9 | 5.9 |
| PI interquartile range | 15.0 | 18.9 | 13.0 | 16.4 | 14.8 | 21.4 |
| PI at percentile 99 | 28.8 | 34.6 | 35.7 | 33.1 | 28.8 | 51.5 |
Figure 1.
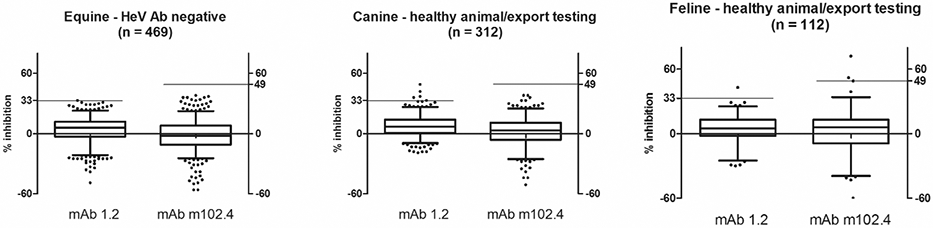
Distribution plots for results evidenced as infection-negative by serology or otherwise presumed HeV antibody negative
Note – the solid bar set at 33 and 49 for mAb 1.2 and mAb m102.4 represent optimized thresholds based on ROC analysis.
3.5. Diagnostic sensitivity - HeV antibody positive sera
Positive sera were represented by post-infection and post-vaccination collections from individual horses, and positive sera by post-infection only from individual dogs; repeat bleeds were excluded from the assessment. For post-infection sera (equine n = 16; canine n = 5) and post-vaccination sera (equine n = 56) classed as true positive (TP – based on positive VN antibody) 72/77 and 70/77 gave bELISA PI values of greater than 80%, indicating good agreement of the majority of positive sera (Table 5a, Table 5b).
Table 5a.
Summary statistics for bELISA results (percent inhibition, PI) for Hendra antibody-positive equine and canine post-infection sera
| Horse (unspecified post-infected) |
Dog (14 to 27-day post-infected) |
|||
|---|---|---|---|---|
| mAb 1.2 |
mAb m102.4 |
mAb 1.2 |
mAb m102.4 |
|
| Count | 16 | 16 | 5 | 5 |
| Average PI | 94 | 94 | 88 | 89 |
| Standard deviation | 17.1 | 16.2 | 7.3 | 7.7 |
| Maximum PI | 100 | 100 | 95 | 99 |
| Minimum PI | 35.4 | 50.5 | 77 | 78 |
| Median PI | 98.5 | 98.7 | 93 | 88 |
| PI at first quartile | 39.5 | 50.9 | 77.2 | 78.2 |
Table 5b.
Summary statistics for bELISA results (percent inhibition, PI) for Hendra antibody-positive equine post-vaccination sera
| Horse (unspecified post-vaccinated) |
||
|---|---|---|
| mAb 1.2 |
mAb m102.4 |
|
| Count | 56 | 56 |
| Average PI | 96 | 94 |
| Standard deviation | 6.2 | 9.6 |
| Maximum PI | 100.0 | 101.9 |
| Minimum PI | 67.9 | 54.8 |
| Median PI | 98.2 | 98.3 |
| PI at percentile 1 | 72.0 | 60.7 |
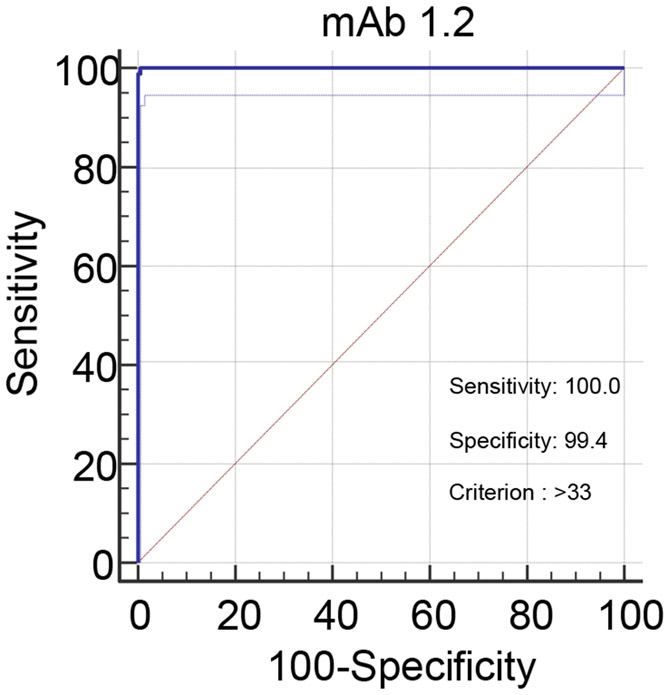
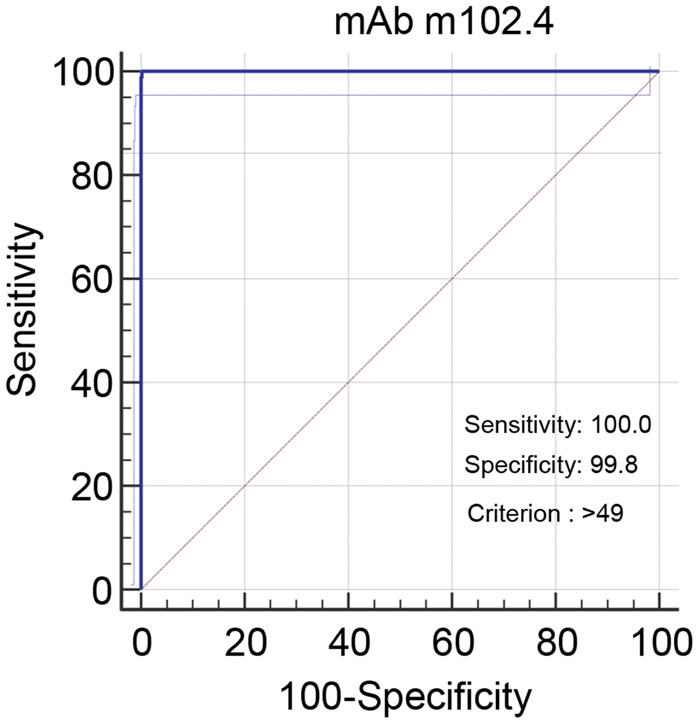
3.6. Setting of thresholds – ROC analysis
Using combined inhibition values for all horse, cat and dog sera, receiver operating characteristics (ROC) graphs plotting sensitivity against (100 – specificity) at incrementally altered positive/negative thresholds were used to optimise selection of thresholds for the two assays. DSe and DSp were optimized when positive thresholds were set for mAb 1.2 at a lower value of >33% inhibition; this gave DSe of 100 (95% CI 95.3 - 100.0) and DSp of 99.5 (95% CI 98.8 - 99.8). For mAb m102.4 the positive threshold was set at >49% inhibition for DSe of 100 (95% CI 95.3 - 100.0) and DSp of 99.8 (95% CI 99.2 - 100.0). Hence, with the availability of a limited panel of positive sera, setting these thresholds gave 100% sensitivity for both tests relative to the VN. However, at these thresholds the DSp values were less than 100%, indicating the occurrence of false positives in both tests. A listing of the results in the normal population sera, that gave results above the assay thresholds, is presented in Table 6. The assay using mAb 1.2 identified five sera above the threshold (4 canine, 1 feline); the assay using mAb m102.4 identified 3 feline sera above the threshold. Importantly, the occurrences of false positive reactions did not overlap. The evidence suggested that parallel or serial application of the assays can improve DSp. For expected negative populations, testing first with the mAb 1.2 HeV antibody bELISA assay and subsequently with the mAb m102.4 bELISA would optimise sample throughput to validate positive reactions or identify false positives.
Table 6.
Results for normal population sera falling above optimised thresholds
| Inhibition % | ||
|---|---|---|
| Species | mAb 1.2 |
mAb m102.4 |
| Canine | 49 | 4 |
| Canine | 42 | −4 |
| Canine | 37 | −16 |
| Canine | 36 | 38 |
| Feline | 43 | 5 |
| Feline | 13 | 72 |
| Feline | 26 | 52 |
| Feline | 15 | 49 |
3.7. Assessing pteropid bat sera
Nineteen bat sera (Grey headed flying fox) randomly collected from the Geelong Botanical Gardens and whose antibody status was presumed negative, were assessed for HeV antibody in both the ELISA formats. All the nineteen sera tested negative under optimised testing conditions with mab 1.2 (Table 7). Eighty-one bat sera (grey headed flying fox) randomly collected in North New South Wales (NSW) were also included in the survey. The HeV antibody status of these sera had previously been assessed by Luminex assay (McNabb et al., 2014). Due to limited sample volume, only 2 μl of serum instead of the 10 μl prescribed for the assay were available for testing. Of these 81 samples however, 33 were positive with mAb 1.2 (40.7%) and 34 were positive with mAb m102.4 (42%). In the Luminex assay, 42 of these samples were designated as positive (52%).
Table 7.
Results for bat sera collected in Victoria and New South Wales
| Bat (Unspecified Geelong, VIC) |
Bat (Unspecified, NSW) | ||||
|---|---|---|---|---|---|
| mAb 1.2 |
mAb m102.4 |
mAb 1.2 |
mAb m102.4 |
Luminex HeV Ab |
|
| Count | 19 | na | 81 | 81 | 81 |
| Number Negative | 19 | na | 48 | 47 | 39 |
| Number Positive | 0 | na | 33 | 34 | 42 |
| Percentage Negative | 100 | na | 59 | 58 | 48 |
| Percentage Positive | 0 | na | 41 | 42 | 52 |
Note: only 2 μL of serum were available for use in the bELISAs for the NSW bats.
Twenty-one off shore (New Caledonia) pteropid bat sera with Luminex and VN results showing evidence for Henipavirus exposure (NiV and HeV antibody positive) were evaluated in the HeV bELISAs (Table 8). Results showed effective detection of antibody in both mAb bELISAs. The mAb 1.2 assay detected 20/21 samples positive and the mAb m102.4 assay detected 18/21 samples positive. While the true exposure status of the bats for one or more particular Henipaviruses is not well defined, limited results for pteropid bats suggest that the HeV bELISAs may also readily detect antibody against NiV or possibly other related but still to be fully characterised viruses.
Table 8.
Serology results for pteropid bat sera originating from off-shore site.
| Sample # | HeV bELISA Result (Percentage Inhibition) |
Luminex Result (Percentage Positive) |
VN Result (Titre) |
|||
|---|---|---|---|---|---|---|
| mAb 1.2 |
mAb m102.4 |
NiV Luminex |
HeV Luminex |
NiV VN |
HeV VN |
|
| bELISA – Negative | ||||||
| 15 | Negative (31) | Negative (16) | Positive (55) | Negative (4) | Positive (5) | N/A |
| bELISA positive / HeV Luminex Negative | ||||||
| 7* | Positive (36) | Negative (30) | Positive (17) | Negative (4) | Positive (20) | N/A |
| 4 | Positive (42) | Negative (37) | Positive (6) | Negative (4) | Positive (10) | Positive (10) |
| 10 | Positive (44) | Positive (62) | Positive (5) | Negative (3) | Positive (10) | N/A |
| 8 | Positive (67) | Positive (80) | Positive (8) | Negative (4) | Positive (10) | N/A |
| 14 | Positive (79) | Positive (92) | Positive (9) | Negative (4) | Positive (10) | N/A |
| bELISA positive / HeV Luminex Positive | ||||||
| 3* | Positive (56) | Positive (68) | Positive (27) | Positive (16) | Positive (20) | Positive (20) |
| 19 | Positive (63) | Positive (78) | Positive (69) | Positive (17) | Positive (20) | N/A |
| 9 | Positive (64) | Positive (80) | Positive (47) | Positive (14) | Positive (5) | N/A |
| 18 | Positive (65) | Positive (84) | Positive (21) | Positive (6) | Positive (20) | N/A |
| 1 | Positive (70) | Positive (92) | Positive (24) | Positive (22) | Positive (20) | Positive (5) |
| 11 | Positive (73) | Positive (88) | Positive (24) | Positive (8) | Positive (10) | N/A |
| 6* | Positive (74) | Positive (54) | Positive (61) | Positive (11) | Positive (80) | Positive (20) |
| 12 | Positive (75) | Positive (80) | Positive (37) | Positive (11) | Positive (10) | N/A |
| 16 | Positive (76) | Positive (84) | Positive (19) | Positive (13) | Positive (10) | N/A |
| 13 | Positive (77) | Positive (80) | Positive (58) | Positive (17) | Positive (20) | N/A |
| 5 | Positive (81) | Positive (64) | Positive (12) | Positive (11) | Positive (10) | Positive (5) |
| 21 | Positive (88) | Positive (78) | Positive (64) | Positive (20) | Positive (10) | N/A |
| 2 | Positive (89) | Positive (95) | Positive (31) | Positive (33) | Positive (>40) | Positive (> 40) |
| 20 | Positive (89) | Positive (90) | Positive (50) | Positive (23) | Positive (10) | N/A |
| 17 | Positive (94) | Positive (95) | Positive (88) | Positive (37) | Positive (20) | N/A |
N/A – not assessed.
Only 2 μL could be used for each mAb instead of the prescribed 10 μL.
Thresholds applied:
bELISA - maB 1.2 PI greater than or equal to 33 is positive; m102.4 PI greater than or equal to 49 is positive
Luminex - percentage positive greater than or equal to 5 is positive
VN - titre greater than or equal to 5 is positive
4. Discussion
We have utilised two previously characterised neutralising monoclonal antibodies (White et al., 2005 and Zhu et al., 2006) to develop a simple bELISA format for HeV specific antibody detection that effectively simulates the sensitivity obtained in VN tests and has the significant advantages of utilization of a non-infectious antigen preparation and an ability to simultaneously test sera from a range of animals in the one assay. Following characterisation of analytical and diagnostic sensitivity and specificity, we have performed a preliminary validation of the assays according to OIE guidelines (OIE, 2014) to detect antibody in equine and canine sera. Additionally, partial validation was possible for feline sera, in absence of antibody positive HeV serum. Notably, this same restriction affects assessment of feline sera in the reference VN assay. A partial assessment was also performed on wildlife sera collected from species of flying fox.
While results suggest very similar outcomes using either mAb, there is also some evidence that a combination of the assays can assist in resolving false positive sera. Within the limitations discussed below, the assays are considered fit for purpose in disease investigation and surveillance, certification/health testing, confirmation of post-vaccination humoral antibody response and may be used to effectively correlate to the VN test for the detection of HeV neutralising antibody.
Previous publications have identified neutralisation differences between the two monoclonal antibodies: mouse-derived mAb 1.2 neutralises only HeV, whereas human antibody m102.4 neutralises both HeV and NiV. In our study, competitive binding provided additional evidence that epitope binding differences exist as cross-interference was either reduced (in pre-binding of mAb 1.2) or absent (in pre-binding of mAb m102.4). We also included biotin labelled ephrin B2, a known ligand receptor for HeV (Bonaparte et al., 2005) and previously reported as useful in Nipah and Hendra antibody neutralisation assay, based on a blocking immunoassay format (Bossart et al., 2007). Binding of biotin labelled ephrin B2 to HeV Sol G was more effectively blocked by pre-binding m102.4 than mAb 1.2, which again may point to differences between the epitopes recognised by the two mAbs. Together, these differences suggested that largely independent antibody populations will differentially interfere with mAb binding and in certain circumstances blocking of one mAb may be more apparent.
Relative analytical sensitivity is a form of limit of detection which provides cross-comparison to a recognised reference test, in this case the HeV VN. While the comparison does not represent diagnostic sensitivity, it is a useful and recommended step in assay validation (OIE, 2014). A small panel of reactive sera (positive for HeV) were titrated in both VN and bELISA formats. The average end-point titre ratios (VN:bELISA) were less than 1.0 for both mAbs 1.2 and m102.4 (0.74 and 0.51), representing an analytical sensitivity of similar order to the VN. By comparison, similar evaluation using biotin labelled ephrin B2 and the iELISA gave average ratios of 1.17 and 1.60 respectively. Limited analytical specificity assessment focused on possible cross-reacting influences by sera with antibody variously positive for different paramyxoviruses. Of these, only antibody against NiV was effective in blocking mAb binding, albeit with some apparent species-related differences. The cross-reaction of NiV was not unexpected as NiV antibodies will cross-react with HeV in the VN, albeit at levels often lower than the homologous NiV neutralisation (Chua et al., 2000). Interestingly, sera collected from VN antibody positive NiV infected pigs did not cross-react in the HeV bELISAs, possibly due to loss of sensitivity in the heterologous format.
Results obtained by testing negative sera from horses, dogs and cats and positive sera from horses and dogs allowed for optimisation of positive and negative thresholds through ROC curve plots (Figure 2a and 2b). From this, we elected for assay-specific thresholds using pooled data from horses, dogs and cats; while this approach may not provide the finer optimisation possible with thresholds set individually for each species, the simplicity of the single threshold for each mAb was considered to be methodologically more robust for diagnostic applications. Using these combined data thresholds, 8 results (4 cats and 4 dogs) from normal population animals were reactive above thresholds in either the mAb m102.4 or mAb 1.2 bELISA. No normal population animals were reactive in both assays, suggesting that a criterion for sera to be reactive in both assays may provide a higher margin of specificity. We have noted that differences between the assay constructions (significant epitope independence, mouse or biotinylated human antibody, conjugate differences) may affect performance characteristics. Additional extrinsic species-related effects may also come into play. It is not possible from our work to more fully account for the occurrence of these asymmetrical reactions involving blocking of only one mAb, however in diagnostic application, any serum reactive in one or both tests would require VN serology. For example, the algorithm at the Australian Animal Health Laboratory (AAHL) requires that only if a positive ELISA results is confirmed by VNT it is called a positive.
Figure 2a.
ROC plot for mAb 1.2 HeV antibody bELISA (dogs, horses and cats)
Figure 2b.
ROC plot for mAb m102.4 HeV antibody bELISA (dogs, horses and cats)
Our assessment of pteropid bat sera, while not commensurate with validation for equine and canine sera, provided further insights into the potential broader applicability of these bELISAs. Using groups of samples collected from unexposed bats (Victorian), potentially HeV exposed (NSW) and potentially Henipavirus exposed (off-shore Oceania), results suggested that in pteropid bats, the assays can effectively identify exposed antibody positive animals. It is considered likely that antibody against NiV is to an undefined extent, cross-reactive in both the HeV bELISAs as also represented in the analytical specificity assessment. Further studies, particularly including complementary HeV and NiV bELISAs are required to refine test validation.
Appropriate use of the HeV bELISA is necessarily governed by intrinsic assay characteristics and extrinsic influences such as sample origin and history. In instances of acute disease, a combination of diagnostic approaches (e.g. agent and antibody detection) is generally more likely to provide useful resolution than the sole application of a single assay. The HeV bELISA properties of high sensitivity relative to the VN combined with ready detection of all competing antibody classes, including early IgM antibody, allows the test to be useful in HeV disease diagnosis. In particular the assessment of late acute stage samples which may not in all cases be positive by molecular or virus isolation techniques. However, the possibility that animals under assessment have been vaccinated against HeV must now be considered when assessing the significance of serology results for antibody against G protein, both in the bELISA and the VN. As the assay target G protein is also used in vaccine preparations, the bELISAs do not differentiate field infection from vaccination. An assay which detects antibody against an alternative target protein, such as the HeV nucleoprotein (N) is now a requirement to differentiate infected from vaccinated animals. The HeV bELISAs are applicable for detection of antibody in in-contact animals, particularly dogs. In animal movement certification serology, the presence of antibody to the HeV G protein in horses with a history of vaccination may be interpreted as confirmation of an immune status (Pallister et al., 2011 and Middleton et al., 2014) in an analogous approach to systems developed for export of rabies vaccinated dogs. The HeV bELISAs are also useful for confirmation of seronegative status in normal horse and dog populations. On the available evidence, the bELISAs are considered likely to provide a useful option for HeV serology in cats. While no HeV positive feline sera were available in this study, we note that analytical specificity assessment confirmed cross-reaction of antibody against NiV in cat serum. On this basis, we can expect that the test will detect HeV antibodies in feline sera, although diagnostic sensitivity has not been estimated for this species.
This work has been undertaken to enable the future introduction of a bELISA format for the detection of HeV-specific neutralizing antibodies that provides a flexible and safer alternative to and more flexible animal species capacity than the VN test. Two monoclonals with different biological characteristics have been shown to effectively function as competing antibodies for detection of HeV-specific antibodies in horses and dogs.
Supplementary Material
Acknowledgements
We are thankful to Ka Yeung Yuen, Student from the School of Veterinary Science from University of Queensland for statistical analysis of data in MedCalc.
Dr. Dimiter S Dimitrov appreciates funding from the University of Pittsburgh Medical Center and Immune Transplant and Therapy Center (UPMC ITTC).
References
- Australian Veterinary Association. Hendra Virus – The story so far. Online available: https://www.vetvoice.com.au/ec/hendra-virus/. Accessed: 05/08/2019.
- Bonaparte MI, Dimitrov AS, Bossart KN, et al. 2005. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc. Natl. Acad. Sci. USA 102, 10652–10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart KN, Crameri G, Dimitrov AS, Mungall BA, Feng YR, Patch JR, Choudhary A, Wang L-F, Eaton BT and Broder CC 2005. Receptor binding, fusion inhibition, and induction of cross-reactive neutralizing antibodies by a soluble g glycoprotein of Hendra virus. J. Virol 79, 6690–6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart KN, McEachern JA, Hickey AC, Choudhry V, Dimitrov DS, Eaton BT, Wang L,F 2007. Neutralization assays for differential henipavirus serology using Bio-Plex protein array systems. J. Virol. Methods 142, 29–40. [DOI] [PubMed] [Google Scholar]
- Burge M, 2017. Gold Coast Hendra virus case ignites renewed call for vaccinations. Online available: http://www.queenslandcountrylife.com.au/story/4693129/hendra-virus-in-gold-coast-hinterland/ . Accessed: 20/06/2017. [Google Scholar]
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, Ksiazek TG, Rollin PE, Zaki SR, Shieh WJ, Goldsmith CS, Gubler DJ, Roehrig JT, Eaton B, Gould AR, Olson J, Field H, Daniels P, Ling AE, Peters CJ, Anderson LJ and Mahy BWJ 2000. Nipah virus: A recently emergent deadly paramyxovirus. Science, 288, 1432–1435. [DOI] [PubMed] [Google Scholar]
- Clayton BA, Wang LF and Marsh GA 2013. Henipaviruses: An Updated Review Focusing on the Pteropid Reservoir and Features of Transmission. Zoonoses and Public Health, 60: 69–83. [DOI] [PubMed] [Google Scholar]
- Colling A, Lunt R, Bergfeld J, McNabb L, Halpin K, Juzva S, Newberry K, Morrissy C, Loomes C, Warner S, Diallo I, Kirkland P, Broder CC, Carlile G, Loh MH, Waugh C, Wright L, Watson J, Eagles D, Zuelke K, McCullough S, Daniels P 2018. A network approach for provisional assay recognition of a Hendra virus antibody ELISA: test validation with low sample numbers from infected horses. J. Vet. Diagn. Invest 1:1040638718760102. doi: 10.1177/1040638718760102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther JR 2001. The ELISA guidebook. In: Methods in Molecular Biology. Humana Press, Totowa, NJ, USA, 1–421. [DOI] [PubMed] [Google Scholar]
- Daniels P, Ksiazek T, Eaton BT, 2001. Laboratory diagnosis of Nipah and Hendra virus infections. Microbes and Infection 3, 289–295. [DOI] [PubMed] [Google Scholar]
- Edson D, Field H, McMichael L, Vidgen M, Goldspink L, Broos A, Melville D, Kristoffersen J, de Jong C, McLaughlin A, Davis R, Kung N, Jordan D, Kirkland P, Smith C 2015. Routes of hendra virus excretion in naturally-infected flying-foxes: implications for viral transmission and spillover risk. PLoS One. 10, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink LK, Edson DW, Vidgen ME, Bingham J, Field HE, Smith CS 2015. Natural Hendra virus infection in flying-foxes - tissue tropism and risk factors. PLoS One. 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin K, Young P, Field HE, Mackenzie J,S 2000. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J. Gen. Virol 81, 1927–32. [DOI] [PubMed] [Google Scholar]
- Halpin K, Hyatt AD, Fogarty R, Middleton D, Bingham J, Epstein JH, Rahman SA, Hughes T, Smith C, Field HE, Daszak P (2011). Henipavirus Ecology Research Group. Pteropod bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg 85, 946–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwazaki Y, Na YN, Tanimura N, Imada T 2004. A solid-phase blocking ELISA for detection of antibodies to Nipah virus. J. Virol. Methods, 121, 259–261. [DOI] [PubMed] [Google Scholar]
- Kirkland PD, Gabor M, Poe I, Neale K, Chaffey K, Finlaison DS, Gu X, Hick PM, Read AJ, Wright T, Middleton D 2015. Hendra virus infection in dog, Australia, 2013. Emerg. Infect. Dis 21(12):2182–5. doi: 10.3201/eid2112.151324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew I, Bonaparte MI, Dimitrov AS, Bossart KN, Crameri G, Mungal BA, Bishop KA, Choudhry V, Dimitrov DS, Wang LF, Eaton BT and Broder CC 2005. Ephrin-B2 ligand is a functional receptor for Hendravirus and Nipah virus. Proc. Natl. Acad. Sci. USA 102, 10652–10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Embury-Hyatt C, Weingartl HM 2010. Experimental inoculation study indicates swine as a potential host for Hendra virus. Vet. Res 41, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb L, Barr J, Crameri G, Juzva S, Riddell S, Colling A, Boyd V, Broder CC, Wang L-F, Lunt R 2014. Henipavirus microsphere immuno-assays for detection of antibodies against Hendra virus. J. Virol. Methods 200, 22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton D 2014. Hendra Virus. Vet. Clin. North Am. Equine Pract 30, 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton D, Pallister J, Klein R Feng YR, Haining J, Arkinstall R, Frazer L, Huang JA, Edwards N, Wareing M, Elhay M, Hashmi Z, Bingham J, Yamada M, Johnson D, White J, Foord A, Heine HG, Marsh GA, Broder CC, and Wang LF Hendra virus vaccine, a One Health approach to protecting horse, human, and environmental health. Emerg. Infect. Dis 20:372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton D, Riddell S, Klein R, Arkinstall R, Haining J, Frazer L, Mottley C, Evans R, Johnson D and Pallister J 2017. Experimental Hendra virus infection of dogs: virus replication, shedding and potential for transmission. Aust. Vet. J 95, 10–18. [DOI] [PubMed] [Google Scholar]
- Murray K, Rogers R, Selvey L, Selleck P, Hyatt A, Gould A, Gleeson L, Hooper P, Westbury H. 1995a. A novel morbillivirus pneumonia of horses and its transmission to humans. Emerg Infect Dis. 1(1):31–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L, Westbury H, Hiley L, Selvey L, Rodwell B, et al. 1995b. A morbillivirus that caused fatal disease in horses and humans. 268(5207):94–7. [DOI] [PubMed] [Google Scholar]
- Pallister J, Middleton D, Wang LF, Klein R, Haining J, Robinson R 2011. A recombinant Hendra virus G glycoprotein–based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine. 29:5623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ProMED-mail, 2014. Hendra virus, equine Australia (18): (Queensland) canine. http://www.promedmail.org. archive no. 20110727.2257.
- Selvey LA, Wells RM, McCormack JG 1995. Infection of humans and horses by a newly described morbillivirus. Med. J Aust 162, 642–5. [DOI] [PubMed] [Google Scholar]
- White JR, Boyd V, Crameri GS, Duch CJ, van Laar RK,, Wang L-F, Eaton BT 2005. Location of, immunogenicity of and relationships between neutralization epitopes on the attachment protein (G) of Hendra virus. J .Gen. Virol 86, 2839–48. [DOI] [PubMed] [Google Scholar]
- Williamson M, Hooper P, Selleck P, Gleeson L, Daniels P, Westbury H and Murray P 1998. Transmission studies of Hendra virus (equine morbillivirus) in fruit bats, horses and cats. Aust. Vet. J 76, 813–818. [DOI] [PubMed] [Google Scholar]
- World Organisation for Animal Health (OIE) 2014. Development and optimisation of antibody detection assays In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Online available: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.6.01_ANTIBODY_DETECT.pdf OIE, Paris, France. Accessed: 20/06/2017. [Google Scholar]
- Young PL, Halpin K, Selleck PW, Field H, Gravel JL, Kelly MA, Mackenzie JS 1996. Serologic evidence for the presence in pteropus bats of a paramyxovirus related to equine morbillivirus. Emerg. Infect. Dis 2, 239–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Dimitrov AS, Bossart KN, Crameri G, Bishop KA, Choudhry V, Mungall BA, Feng YR, Choudhary A, Zhang MY, Feng Y, Wang LF, Xiao X, Eaton BT, Broder CC, Dimitrov DS 2006. Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J Virol. 80, 891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Bossart KN, Bishop KA, Crameri G, Dimitrov AS, McEachern JA, Feng Y, Middleton D, Wang LF, Broder CC, Dimitrov DS 2008. Exceptionally potent cross-reactive neutralization of Nipah and Hendra viruses by a human monoclonal antibody. J. Infect. Dis 197, 846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.