Abstract
Foodborne diseases caused by pathogenic bacteria pose a serious threat to human health. Early and rapid detection of foodborne pathogens is an urgent task for preventing disease outbreaks. Microfluidic devices are simple, automatic, and portable miniaturized systems. Compared with traditional techniques, microfluidic devices have attracted much attention because of their high efficiency and convenience in the concentration and detection of foodborne pathogens. This article firstly reviews the bio-recognition elements integrated on microfluidic chips in recent years and the progress of microfluidic chip development for pathogen pretreatment. Furthermore, the research progress of microfluidic technology based on optical and electrochemical sensors for the detection of foodborne pathogenic bacteria is summarized and discussed. Finally, the future prospects for the application and challenges of microfluidic chips based on biosensors are presented.
Graphical abstract
Keywords: Foodborne pathogenic bacteria, Microfluidic chip, Bio-recognition element, Biosensors
Introduction
Foodborne diseases caused by the consumption of contaminated food and water are a major public health burden, seriously threatening people's health and life, and hindering economic development worldwide [1]. Pathogenic infections can lead to recurrent intestinal inflammation, chronic kidney disease, diarrhea, reactive arthritis, blindness, and even death [2, 3]. Therefore, to address this global challenge and reduce the impact on human health, effective and rapid identification of foodborne pathogens is essential. To date, much effort has been devoted to the rapid detection of foodborne pathogens in food samples, and various detection methods have been developed, such as conventional bacterial culture methods, high-performance liquid chromatography (HPLC), nucleic acid-based PCR, immune-based methods, and optical and electrochemical biosensors [4, 5]. In general, plate culture is considered the gold standard method for sensitive and accurate detection of bacteria, but this method requires 3 to 7 days of bacterial culture and is not suitable for rapid onsite detection of pathogens [6]. In addition, PCR is sometimes prone to false-positive results due to DNA contamination [7, 8]. Immunological detection methods are characterized by high specificity due to the specific interaction between antibodies and antigens on the surface of the target bacteria. However, the high cost, poor stability, and limited sources of antibodies hinder the further application of immunoassay techniques [9]. Therefore, it is necessary to establish a rapid, simple, sensitive, and reproducible field detection method for evaluating food safety.
In recent years, microfluidics technology has provided powerful tools for detection applications with its portability, miniaturization, automation, multichannel sample detection, minimal treatment of harmful substances, and cost savings [10–12]. The greatest advantage of microfluidics over traditional methods is the creation of a controlled microenvironment that precisely drives and controls microfluidic flow in microchannels, improving detection sensitivity [13]. In addition, all analytical processes, including sample preparation, reaction, separation, and detection, are integrated into a single microfluidic chip for field testing applications [11]. Biosensors using various technologies combined with microfluidic chips for the detection of foodborne pathogens have been widely reported [14, 15]. A number of emerging microfluidic chips have been successfully developed for the detecting foodborne pathogens. For example, smartphone-based microfluidic platforms [16] for rapid and multiplex detection of foodborne pathogens, microfluidic platforms based on immunomagnetic nanoparticles combined with urease and impedance measurement [17], and paper-based [18] or plastic-based [19] microfluidic platforms have been proposed. Li et al. [20] reported an adaptable microfluidic system for rapid pathogen classification and antimicrobial susceptibility testing (AST) at the single-cell level. By incorporating tuned microfluidic valves and real-time optical detection, pathogen classification can be performed by capturing and classifying bacteria based on their physical shape and size. The antimicrobial susceptibility of the bacteria can be determined within 30 min by monitoring their growth in the presence of antibiotics at the single-cell level. The microfluidic system can quickly determine the presence of bacteria, classify major categories of bacteria, detect multi-microbial samples, and identify antimicrobial susceptibility directly from clinical samples at the single-cell level. However, microfluidic-based food bacterial pathogen detection methods remain challenging [21]. For example, small-volume samples in microfluidic channels may encounter obstacles to achieving the sensitivity, selectivity, and stability required by microfluidic sensors. Furthermore, the integration of effective bio-recognition molecules on microfluidic chips is challenging [22, 23]. Several strategies, including gravity, electricity, magnetism, and affinity chemistry, have been developed to address unresolved problems related to rapid separation, enrichment, and detection of foodborne pathogens by microfluidic devices [24, 25].
This article reviews the latest research progress regarding bio-recognition elements in microfluidic chip-integrated biosensors for food sample detection, summarizes the shortcomings and advantages of microfluidic chip-based biosensor applications in food detection (Table 1), and discusses strategies for more rapid and accurate detection of foodborne pathogens using microfluidic chip biosensors.
Table 1.
Application of biosensor-based microfluidic technology for the detection of foodborne pathogenic bacteria
| Biosensor type | Receptor | Target pathogen | Advantages | Disadvantages | Improvement measures | Limit of detection |
|---|---|---|---|---|---|---|
| Colorimetric biosensor | Enzyme | Cronobacter spp. | Simple, fast, low-cost, and visual detection |
Low sensitivity, low multiplexing capacity, and quantitative detection limitation |
Enrichment of bacterial cells by magnetic beads; replacement of colloidal gold by quantum dots; use of chemiluminescence (CL) substrates and porous substrates |
10 CFU/cm2 [26] |
| Enzyme |
Escherichia coli (E. coli) O157: H7, Salmonella typhimurium (S. Typhimurium), Listeria monocytogenes (L. monocytogenes) |
10 CFU/cm2 [27] | ||||
| Fluorescence biosensor | Quantum dots (QDs) | S. typhimurium | High sensitivity, high speed, and non-contact detection | Weak fluorescence signal, large interference background, and the detection device is highly required |
Use of new fluorescent materials such as metal nanoclusters, carbon dots, QDs, graphene, combined with immunomagnetic separation |
3.3 × 102 CFU/mL [28] |
| Antibody | Salmonella enterica (S. enterica) | 5.0 × 104 cells /mL[29] | ||||
| CL biosensor | Antibody | E. coli, Enterobacter jejuni (E. jejuni) | Convenient and fast, high sensitivity, low detection limit, convenient automation, and excellent selectivity | The labeling process is tedious, complex, and difficult to automate | Nanotechnology is modified on the surface of the electrode; CL reagents are immobilized | 5.0 × 105 cells/mL; 1.0 × 105 cells/mL[30] |
| Antibody | E. coli | Single-cell level [31] | ||||
| SPR biosensor | Antibody | E. coli and Staphylococcus aureus (S. aureus) |
Specificity, multiplexing, and unlabeled |
The detection of intact bacterial cells is limited and complex |
Optimizes the attenuation length; use long-distance SPR; surface to prepare nanostructures |
105 CFU/mL[32] |
|
SERS biosensor |
Unlabeled |
S. aureus, Pseudomonas aeruginosa (P. aeruginosa), E. coli |
High sensitivity, multiplexing, and unlabeled | Poor stability and molecular difficulty in molecular fingerprint spectroscopy |
Use of stable substrate; synthesis of controllable nanoparticles; use of pathogen database |
3.0 × 103 CFU/mL, 5.0 × 103 CFU/mL, 1.0 × 104 CFU/mL [33] |
| Electrochemical biosensors | Antibody | Salmonella | Low resistance, high signal-to-noise ratio, good stability, fast response and high sensitivity | Easy to be disturbed by other ions in the solution and high requirements for the reaction system |
Select bio-recognition elements with high specificity; broadens the types of electrode modification materials; improves the surface microstructure of electrodes; uses composite materials and nanomaterials; expands the development of other diverse technologies such as fusion medium electrophoresis and electroporation |
300 cells/mL [34] |
| Antibody | S. typhimurium | 3.0 × 103 CFU/mL [35] |
Types of bio-recognition elements integrated with microfluidic techniques for foodborne pathogenic bacteria detection
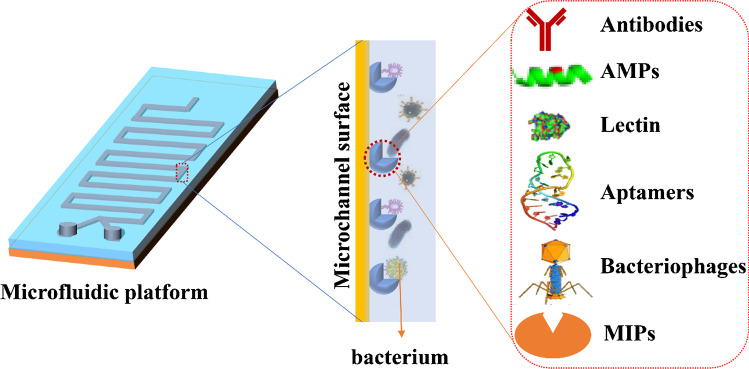
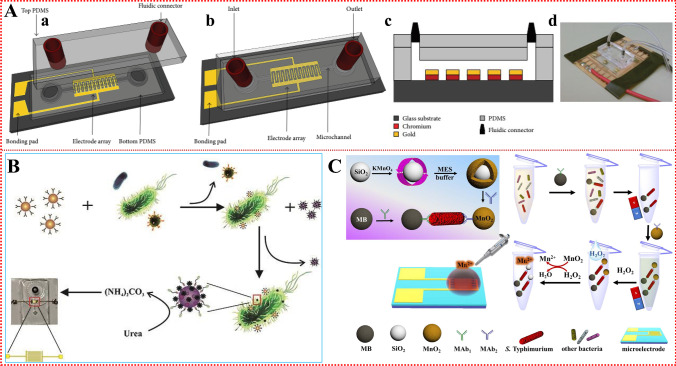
Microfluidic technology has great potential in the detection of foodborne pathogens because it can integrate all analytical processes. However, microfluidics-based methods for the detection of pathogenic bacteria in food are still challenging. This is because of the complex matrix of food samples and the limitations of integration with different key steps (e.g. sample pretreatment, detection operation, and detection on a single microfluidic chip) [36]. One strategy to solve the problem lies in the highly specific interactions between surface antigen biomarkers of pathogenic bacteria and bio-recognition elements. Several different types of bio-recognition elements, including antibodies, aptamers, phages, antimicrobial peptides, lectins, cells, and enzymes [37], have been employed to capture pathogens efficiently, and these bio-recognition elements can be used as capture agents for enriching or concentrating foodborne pathogens on microfluidic devices (Fig. 1).
Fig. 1.
Overview of types of bio-recognition elements integrated with microfluidic platforms
Antibodies
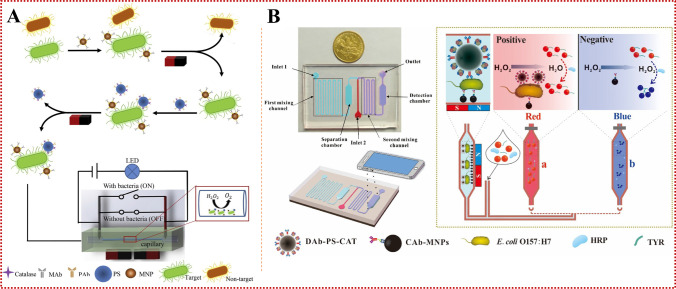
Antibodies serve as host proteins that are produced by the immune system of eukaryotes to identify and eliminate pathogens. Because of their ease of use and high affinity for target antigens, antibodies are considered the preferred ligand for bacterial biosensor detection, and are one of the most widely used bio-recognition elements in microfluidic devices. Antibodies are typically fixed on the surface of electrode arrays and combined with microfluidic biosensors for detecting bacteria. Magnetic nanoparticles (MNPs) have the advantages of large specific surface area, detecting small steric hindrance, and conducting uniform distribution [35]. Therefore, microfluidic biosensors are often combined with immunomagnetic separation (IMS) for specific isolation and efficient concentration of foodborne pathogens in complex matrices [38]. To pre-concentrate bacterial cells in large-volume samples, the microfluidic device should be large enough to handle a large number of samples in a few hours. Park et al. [39] reported a three-dimensional (3D) microfluidic magnetic pre-concentrator made of plastic which requires no assembly, and can selectively pre-concentrate E. coli O157:H7 to 100 mL in a 700-fold ratio within 1 h, separated and enriched with antibody-conjugated MNPs. Combined with an ATP photometer, the pre-concentrator can detect E. coli O157:H7 at concentrations as low as 10 CFU/mL in blood. The 3D microfluidic magnetic pre-concentrator can be used in combination with various types of detection systems, such as PCR and ATP photometers, and can effectively achieve a pre-concentration of E. coli O157:H7 in large samples to a sub-milliliter level, greatly improving the detection limit of pathogenic bacteria. Lin et al. [40] first used immunomagnetic nanoparticles to isolate S. typhimurium from a complex background of food samples to form magnetic bacteria, which were reacted with polystyrene microspheres (PSMs) modified with anti-Salmonella polyclonal antibodies and catalases to form enzymatic bacteria. Finally, hydrogen peroxide was injected into a capillary tube to produce an oxygen gap under the catalysis of catalase, which led to a change in the electrical signal to detect S. typhimurium (Fig. 2a). The biosensor has the advantages of simplicity, short detection time, low cost and small size. It can be easily extended to the detection of other foodborne pathogens by changing antibodies, and universality of pathogen detection can be achieved. The biosensor was able to detect Salmonella concentrations as low as 33 CFU/mL within 2 h, and the change in electrical voltage was found to have a good linear relationship with the concentration of Salmonella from 3.7 × 101 ~ 3.7 × 106 CFU/mL. On this basis, a new biosensor was developed by combining a smartphone, microfluidic chip, and immunoassay technology, and MNPs modified by a capture antibody and a polystyrene microsphere (PSM) modified by a detection antibody and catalase were simultaneously reacted with the target bacteria in the first mixed channel of the microfluidic chip. Gold nanoparticles (AuNPs) were used to indicate different concentrations of E. coli O157:H7, and a smartphone imaging application was developed to monitor the color change of AuNPs to determine the concentration of target bacteria (Fig. 2b). The sensor demonstrated good specificity and sensitivity for E. coli O157:H7 in chicken samples, with a detection limit of 50 CFU/mL [16]. The detection system has advantages such as data processing equipment, high integration of sample and reagent processing, low manufacturing cost of microfluidic chips, and small field application volume. We expect that the detection system will be combined with continuous-flow IMS technology to further improve the detection sensitivity in order to meet the 1 CFU/mL requirements of the lower limit of food safety detection.
Fig. 2.
(a) The principle of the S. typhimurium biosensor based on IMS, enzyme catalysis and electrical signal for the detection of S. typhimurium. Reproduced from [40] with permission of Elsevier. (b) The principle of the colorimetric biosensor for rapid detection of E. coli O157:H7 based on AuNPs aggregation and smartphone imaging. Reproduced from [16] with permission of Elsevier
Although the use of antibodies as bio-recognition elements in microfluidic devices has seen many advances in the concentration and detection of foodborne pathogens, there are still some shortcomings that limit their application. For example, cross-reactions may occur between different targets, with high costs. In addition, antibodies are susceptible to physical, chemical, and enzymatic damage, and poor stability is considered to hinder their further application in biosensors. However, the combination of microfluidic technology with nanoparticles and smartphones will successfully overcome the difficulties, and it will be more widely used in the future, especially in the application of point-of-care testing (POCT).
Antimicrobial peptides (AMPs)
AMPs are short peptide fragments (mostly containing less than 40 amino acid residues) existing in many organisms. They are a part of the innate immune system to protect the host from pathogens [41]. AMPs have the advantages of simple synthesis, low cost, high stability in extreme environments, and broad activity against various microorganisms, which can compensate for the shortcomings of current pathogen detection systems such as poor stability and low sensitivity [42]. In addition, compared with antibodies and aptamers, AMPs have a wide range of sources, and it is easy to select an ideal AMP molecule from existing databases for application in biological detection devices. It has been reported that all AMPs are amphiphilic and have an affinity for negatively charged bacterial membranes. There are additional hydrophobic interactions between cell membranes and hydrophobic chambers of peptides [43]. Magainin I is a naturally occurring polypeptide on the skin of African Rana unguiculata, which has antimicrobial and antiparasitic activities and can selectively bind to E. coli O157:H7. Furthermore, this peptide can semi-selectively bind to the cell surface of Gram-negative bacteria [44]. Thuy et al. [45] combined a Magainin I microfluidic platform with a recombinase polymerase amplification (RPA) sensor for the detection of low pathogenic bacteria levels in complex samples. In addition, the enrichment platform is based on unlabeled real-time amplification and detection of pathogenic bacterial DNA that can detect pathogens in urine within 60 min, with a detection limit of 5 CFU/mL for Salmonella and 10 CFU/mL for Brucella. Compared with conventional methods, the combined system for various bacteria has improved the detection limit. The detection limits of Salmonella and Brucella in 10 mL urine were 20 times and 10 times higher than those of nonselective enrichment real-time PCR, respectively. Therefore, this enrichment platform has the potential to improve the detection limit of pathogen DNA by improving the capture efficiency of pathogens in large samples for better diagnosis of pathogens.
However, most of the current AMP-mediated microfluidic platforms use microscopy, which makes them less sensitive and more complicated to operate. To solve these problems, researchers need to combine simple and sensitive biosensing methods with microfluidic systems and collect data easily using simple tools such as smartphones. To meet the need of highly specific recognition, specific peptide fragments can be synthesized to capture and detect target pathogens. Therefore, researchers need to further explore how to integrate specific peptide fragments into microfluidic devices to improve the specific identification of pathogenic bacteria.
Aptamers
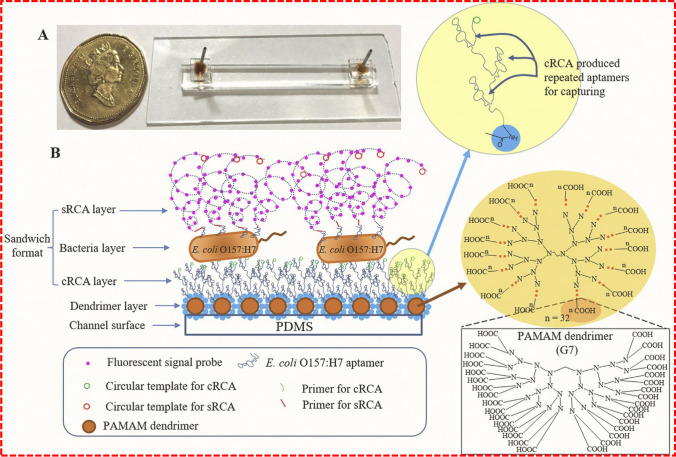
Aptamers are in vitro-synthesized DNA or RNA probes and are obtained by a selection method called systematic evolution of bio-recognition elements by exponential enrichment (SELEX) [46]. Compared with antibodies, aptamers (commonly known as artificial antibodies) have many advantages, including high stability in complex environments, non-toxicity, ease of production, ease of modification, long shelf life, and low cost. In addition, aptamers can be synthesized by chemical methods, are accurate and inexpensive, and importantly, do not rely on the immune response of animals [47, 48]. Specifically, as a potential bio-recognition element, aptamers are integrated into microfluidic sensors to recognize and detect target antigens in complex food samples. Jiang et al. [49] immobilized poly(amido amine) (PAMAM) dendrimers onto poly(dimethyl siloxane) (PDMS) microchannels and developed a fluorescence intensity-based microfluidic detection platform for the detection of E. coli O157:H7 cells by modifying microchannels with DNA aptamers. The platform enables rolling circle amplification (RCA) to enhance the detection signal 50-fold, and the detection limit is as low as 102 CFU/mL. Here, the microchannels modified with polyaminoamine dendrimers are free of contamination, providing multiple binding sites for subsequent aptamer capture of E. coli O157:H7. In order to improve the detection sensitivity of the microfluidic platform and realize whole-cell sensitive detection in microfluidic devices, a new method of dual-RCA detection has been developed by Jiang's research group after improving the above devices (Fig. 3). This dual-RCA approach consists of a capture RCA (cRCA) designed to modify the surface of the microfluidic channel with a polyaptamer to significantly enhance the capture of E. coli O157:H7 cells, and signal RCA (sRCA) to further enhance the detection signal. The integration of these two detection signals can lead to a significant increase by about 250 times. The results showed that the detection limit of E. coli O157:H7 in different food matrices was 80 cells/mL [50]. Thus, this newly developed dual-RCA whole-cell assay is a promising method for rapid and sensitive detection of foodborne bacteria using microfluidic devices.
Fig. 3.
Schematic drawing of a dual-RCA E. coli O157:H7 whole-cell detection system. (a) Photograph of a completely packaged microfluidic detection device. (b) Schematic illustration of sandwich detection format, in which the surface of the microfluidic channel is modified with PAMAM dendrimers, followed by cRCA in situ to produce repeated aptamers to capture target cells. Reproduced from [50] with permission of Elsevier
Aptamers have been tested for their multiplexing ability to simultaneously identify a variety of bacteria in real food samples, indicating their potential application in microfluidic systems. Wang et al. [46] first screened bacteria-specific aptamers with SELEX and integrated them into microfluidic systems. First, a biotin-labeled aptamer was bound to the nitrocellulose membrane in the chip, followed by incubation with bacteria, and finally, tetramethylbenzidine-streptavidin (blue) chromogenic reaction was utilized to simultaneously identify three target bacteria. This new microfluidic chip has advantages such as fast detection time (35 min), small size, high specificity, and simultaneous detection of multiple pathogens. Based on the development of nucleic acid aptamer microfluidic detection of pathogenic bacteria mentioned above, nucleic acid aptamer has some limitations, including fast nuclease degradation and short action time. In many cases, the sensitivity and selectivity of aptamer assays are affected by sample conditions, such as interfering components (i.e. proteins, lipids, carbohydrates), pH, and ionic strength. In addition, nonspecific binding of aptamers to sample components may lead to false-positive results. Therefore, the samples must be pretreated before detection. A common method is magnetic separation using magnetic beads or magnetic nanoparticles, which can significantly improve the sensitivity. These limitations affect the development of efficient and sensitive microfluidic detection devices and require further research.
Carbohydrate-binding protein
Compared with other bio-recognition elements, lectins are the most commonly used due to their broad spectrum of pathogen capture capability. Lectins can detect carbohydrates on bacterial surfaces. Interestingly, because of the adhesion mechanism between lectins and bacteria, carbohydrates can also recognize lectins contained in bacterial structures [51]. Mannose-binding lectins (MBL) are considered the first defense mechanism of the host and are expressed on the surface of more than 90 different viruses, bacteria, and fungi by binding to terminal mannose and fucose residues [52]. This feature has inspired researchers to combine MBL on microfluidic devices to capture foodborne pathogens. Cooper et al. [53] developed a microfluidic device coated with modified MBL on magnetic beads for the detection of various pathogens. The device can detect Candida albicans cells in human blood at a concentration of 1 cell/mL within 3 h. Similar methods are employed to capture S. aureus from plasma and blood with a detection limit of 102 CFU/mL [54]. Concanavalin A (ConA) is also a typical lectin commonly used to enrich pathogenic bacteria. A highly sensitive nanobiosensor based on isothermal amplification and microfluidic enrichment was developed using a microfluidic chip immobilized with ConA as an enrichment probe to enrich pathogenic bacteria and extract genomic DNA from isolated bacteria. Under optimal conditions, S. typhimurium at a concentration of 5 CFU/mL could be detected in 10 mL urine samples in real time by a label-free method. In addition, the whole experiment was completed within 100 min [55]. This sensor is a simple, rapid, and sensitive diagnostic platform. Importantly, this method can be used for the detection of urinary tract infection caused by Salmonella or other pathogenic bacteria.
Overall, although lectins have rarely been used in microfluidic platforms and have been less studied for foodborne pathogen detection, the above examples demonstrate their potential in this regard. Furthermore, due to the unique nature of the interaction between lectins and glycoproteins, the resulting vectors can be easily regenerated. Lectins may be used in combination with other simple bio-recognition elements in the future.
Phages
Bacteriophages have attracted extensive attention due to their specificity and sensitivity to targeted microorganisms. They can be turned into a potential foodborne pathogen bio-recognition element that can remain active under various extreme conditions (such as high temperature, organic solvents), and are a potential candidate for the development of biosensors for bacterial detection [56]. Phages usually consist of two distinct parts, the head containing phage DNA and the tail responsible for bacterial recognition. Since there are many different types of phages, with many shapes and characteristics, the designed biosensor can detect almost all bacterial strains [57]. Unlike other bio-recognition elements, phages can be easily and inexpensively produced in large quantities. Generally, a large number of progeny phages can be obtained by simply infecting bacterial solutions. These advantages make phages a popular bio-recognition element in biosensors [58]. A novel label-free electrochemiluminescence (ECL) biosensor for the detection of P. aeruginosa was prepared by immobilizing PaP1 phages on carboxyl graphene with a detection limit as low as 56 CFU/mL [59].
Despite the many advantages of phages, however, they lose activity if they are fixed to a sensor surface and have dried out. In addition, because of the relatively large size of the phage, using whole phages as the recognition element leads to a decrease in the measurement signal. Therefore, instead of using whole phages, it is better to only use phage-derived proteins for detection. Endolysin is one of these proteins and is produced at the end of phage lysis. Endolysin is immobilized on the cell wall by carbohydrate-binding domains (CBD), particularly targeting the bacterial peptidoglycan layer [60]. Because CBD is smaller in size than antibodies and there are more CBD binding sites in the bacterial cell walls, CBD can be used as a bacterial capture recognition element in microfluidic devices. Yi et al. [61] isolated S. aureus cells from sample matrix with lysine CBD-functionalized beads, and used fluorescence detection for rapid (50 min), specific, and sensitive quantitative analysis of S. aureus in real samples. In phosphate-buffered saline (PBS), the detection limit was as low as 78 CFU/mL for S. aureus in a concentration range from 1.0 × 102 to 1.0 × 107 CFU/mL. However, CBD was unable to recognize and adhere to Gram-negative bacteria, mainly because the outer membrane of these bacteria shields peptidoglycans. However, the above reports show the potential for further exploring the integration of bacteriophage CBD into microfluidic chips to develop a sensitive detection device for foodborne pathogenic bacteria.
Molecularly imprinted polymers (MIPs)
The biometric elements listed above are natural biomolecules, such as proteins, peptides, and sugars. Although the applications of these bio-recognition elements in biosensors are relatively mature, their high cost, poor stability, complex preparation, and non-reusability have limited their further development in biosensors. MIPs are a kind of artificially prepared recognition materials; the preparation process is simple and easy [62]. Compared with natural bio-recognition elements, the outstanding advantages of MIPs include on-demand preparation and reversible adsorption/release of target molecules [38]. In addition, MIPs can recognize not only small molecules, but also various macromolecular targets, including proteins [63], viruses [64], and microorganisms [65]. For example, S. aureus was isolated from complex food samples by molecular imprinting. Dopamine was used as a functional monomer, magnetic MIPs by imprinting on the surface of magnetic particles, and fluorescence microscopy was used for detection. Subsequently, MIPs were used to extract S. aureus from milk and rice, and bacteria in milk could be detected at a concentration of 1 × 103 CFU/mL [66].
To further improve the detection sensitivity of low-abundance targets, in recent years MIPs have been immobilized on sensors for enrichment, separation, and detection of low-abundance target molecules. Bezdekova et al. [67] combined fluorescent QDs with MIPs on a 3D origami paper-based microfluidic device for specific recognition and sensitive fluorescence detection of phycocyanin. QDs are typically small fluorescent nanocrystals that have been widely used for sensor development and enable ultrasensitive detection of target molecules due to their unique optical and photochemical properties. Furthermore, MIPs were used in this study because of their reusability and good specificity and selectivity. Importantly, the device can provide quantitative information very conveniently, and can be further expanded and applied to the detection of other proteins or biomarkers in environmental and food safety studies. Although there are few examples of the use of MIPs in microfluidic devices for the capture and detection of foodborne pathogens, the facile process and broad-spectrum potential will be illustrated in other future examples.
Application of microfluidic chips in sample pretreatment of foodborne pathogens
It is difficult to detect pathogens found in untreated food samples without proper sample pretreatment steps. The concentration of many pathogens in food samples is very low. Additionally, the variety and content of other substances in complex food matrices may interfere with detection and reduce its accuracy. Therefore, enrichment and separation of target analytes are important for detection purposes. Accordingly, many microfluidic platforms have been adapted to the complexity of sample preparation and enrichment [68]. Typically, the duration of food analysis work is 2–5 days, while microfluidic technology can reduce the analysis time to several hours. For example, a modular integrated microfluidic chip can be utilized to rapidly and efficiently prepare foodborne pathogen screening samples. It employs an integrated microheater for thermal cleavage and DNA amplification on the chip, reaching the temperatures required for DNA amplification (65 °C) and lysis steps (95 °C) in 25 s and 60 s, respectively [69]. The proposed bacterial sample preparation LOC platform has all the required performance criteria and holds promise for its wide applicability to bacterial identification in food, clinical, and water samples.
When microfluidic chips are used directly to detect pathogens in complex matrices, the reproducibility of the method is poor because the concentration of pathogenic bacteria to be detected is too low. Therefore, it is necessary to isolate and enrich analytes from the food matrix to improve the efficiency of target detection. Because of the high specific surface area of magnetic particles, they are widely used in microfluidic devices to increase pathogen bio-recognition. Pathogen-specific RNA of the Phalaenopsis orchid was pretreated with magnetic beads, followed by reverse transcription loop-mediated isothermal amplification (RT-LAMP) to amplify pathogen RNA. The results demonstrated that the system could complete detection in 65 min with a detection limit of 25 fg [70]. To carry out the continuous pretreatment operation of biological detection reagents, a microfluidic bioseparation chip integrating mixer, heater, and soft magnet were proposed for nucleic acid detection and magnetic bead extraction of target substances [71]. Recently, Guan et al. [72] successfully prepared a novel magnetically driven droplet microfluidic immunosensor without a complex operating system for the enrichment of cyanobacterial toxins in aquatic products. Compared with widely used microfluidic systems, the proposed sensor has two significant advantages: simple and effective operation by magnetic force alone rather than inconvenient actuation devices, and high-throughput parallel detection on a 15-channel chip.
IMS can be used to concentrate bacterial cells at lower concentrations, but it is only suitable for small-volume samples (1 mL), which limits the enrichment of large-volume samples. To solve this problem, Ngamsom et al. [73] combined magnetic electrophoresis and IMS for the rapid enrichment, sorting, and detection of live S. typhimurium and E. coli O157:H7 in food samples. The platform utilizes two different types of specific magnetic beads. According to the different size and number of magnetic beads, they can specifically capture and concentrate live S. typhimurium and E. coli O157:H7 by applying a nonuniform magnetic field perpendicular to the flow direction, and the beads deviate from the flow direction in the chip to different degrees. Thus, the combination of magnetic electrophoresis and IMS may be the future direction for generating high-throughput concentrations of target pathogens from complex food samples.
In addition to microfluidic devices integrated with magnetic beads, filters and membranes are another direct and cost-effective alternative for rapid concentration of target pathogens, especially in large-volume samples. Li et al. [74] used a polysulfone hollow fiber membrane module to isolate and concentrate bacterial cells from chicken homogenates in cross-flow microfiltration. The special microfluidic system can effectively recover 70% of the analytes in the mixture within 30–45 min. This greatly improves the concentration of analytes and shortens the experimental time. In addition, special microfluidic injection channels are used to increase the concentration of analytes [75].
Typically, most biosensors use a small number (tens of microliters) of samples to detect bacteria [76]. However, achieving significant results requires at least milliliters of samples to detect bacteria. Therefore, the bacterial concentration process is essential for the rapid and accurate detection of bacteria. Most bacterial concentration devices have low throughput (200 μL/h ~ 3.6 mL/h), and take several hours to process a few milliliters of samples [77]. To significantly increase the throughput, Jung et al. [78] developed a magnetic electrophoresis-based bacterial concentration device using commercial polyethylene tubes. This 3D device is made by winding a tube on a magnet, and its channel length can be easily adjusted by using different tube lengths. Therefore, particles can be concentrated even at high flow rates. The performance of the proposed device was evaluated using two different food samples (milk and homogeneous cabbage) incorporating bacteria. At a flow rate of 40 mL/h, the separation efficiency and concentration factor were higher than 92% and 110 times, respectively. These results confirm that the device has considerable throughput and can achieve continuous and rapid concentration of foodborne bacteria (S. aureus, S. typhimurium, and L. monocytogenes). Kwon et al. [79] developed a high-throughput sample pretreatment microfluidic chip that can enrich microorganisms in food with magnetic particles and extract DNA from the photothermal effect of the magnetic particles. Magnetic particles modified with ConA can capture a variety of pathogens in samples. When magnetic particles and bacteria are injected into the microfluidic chip at a high flow rate, they actively bind in the mixed channel. After passing through the mixed channel, the magnetic particle complexes bound to bacteria are captured and enriched by magnetic force in the chamber. Combining different techniques similar to those described above helps achieve higher sensitivity in microfluidic devices. Generally, it is very beneficial to integrate the magnetic electrophoresis technology into the sensing device because magnetic beads are considered powerful tools for sample concentration because of their large surface-to-volume ratio and flexible functionalization. They offer the possibility of skipping the tedious washing process and purification of captured pathogens.
Biosensor-based microfluidics for the detection of foodborne pathogenic bacteria
The development of biosensors integrates knowledge from biology, chemistry, physics, medicine, and electronics. Biosensors are sensitive to biological substances and can convert signals such as the concentration and activity of an analyte into electrical and optical signals for rapid detection [80, 81]. Microfluidic technology can reduce pretreatment steps because of its specificity, sensitivity, rapidity, simplicity of equipment, and low skill requirements for operators [82, 83]. Combining sample pretreatment and analysis of foodborne pathogens into a microfluidic chip can help achieve high efficiency, high speed, and automation [84].
Microfluidic chip with optical biosensor detection methods
The optical biosensor has strong anti-interference ability, miniaturized equipment, convenient operation, telemetry, real-time, on-line and dynamic monitoring, fast response, and high sensitivity [85]. The detection system integrated with an optical biosensor and microfluidic chip can add, separate, prepare, and analyze samples on a single device. It has the advantages of low cost, short detection time, multi-throughput, automation, portability, versatility, and low sample quantity [64].
Colorimetric biosensor
The colorimetric biosensor is a very attractive optical biosensor system because one can easily observe the pathogenic microorganisms in the sample with the naked eye by changing the color of the reaction solution without any analytical instrument. The combination of colorimetric analysis and microfluidic chip technology has been widely used in the detection of pathogenic bacteria [26, 86]. Among the wide range of microfluidic chip detection technologies, paper-based material has unique advantages, as the high specific surface area and volume increase the detection limit of colorimetry. The material is suitable for developing diagnostic tools that are reasonably priced, portable, disposable, and easy to detect and handle.[87] Sun et al. [26] developed a paper-based analytical device (μ-PAD) by combining PVC pad with filter paper. The detection is achieved by measuring the color changes from colorless to indigo for the specific species of enzymes associated with Cronobacter spp. The substance of interest reacts with the chromogenic substrate. Because of the optimization of the specific enrichment process, living bacteria measured as low as 10 CFU/cm2 on the inoculated surface of the sample can be detected. This paper-based analysis device can also be combined with an IMS phase to detect S. typhimurium. The lowest detection limit for S. typhimurium in the culture medium is 10 CFU/mL. This detection system was applied to starling bird fecal samples and whole milk with detection limits of 10 CFU/g and 10 CFU/mL, without any pre-enrichment and with high reproducibility [88]. The μPAD-colorimetric detection system developed by Jokerst et al. [27] uses filter paper wax printing technology that is realized by measuring the color change of the enzyme associated with the pathogen of interest when it reacts with the chromogenic substrate. When combined with the enrichment process, the method allows for enrichment time of 12 h or less, and the concentration in inoculated ready-to-eat meat can be detected at levels as low as 10 CFU/mL. The main drawbacks of colorimetric detection are low selectivity, low sensitivity, and long detection time. Combining the paper microdot test with the colorimetric method can provide a simple, economical, and less time-consuming method for detecting pathogens.
Fluorescence biosensor
As an important branch of optical biosensors, fluorescence biosensors have advantages including high sensitivity and easy readability [88–90]. Fluorescence detection is more sensitive than colorimetric detection. New methods and techniques for quantitative detection of bacteria chips are constructed by combining a microfluidic chip analysis system with fluorescence detection technology [91], which has become a potentially powerful tool and research hotspot in the field of bacterial analysis and detection due to its high efficiency and specificity in detecting bacteria [92]. Wang and colleagues used CdSe/ZnS@SiO2-NH2 composite nanoparticles (FNPs) as a fluorescent label for the detection of S. typhimurium. Hydrophobic CdSe/ZnS QDs were introduced into SiO2 microspheres by self-modified inverse microemulsion technology. FNPs were coupled with bacteria by a glutaraldehyde two-step method. The detection limit was 3.3 × 102 CFU/mL. To further reduce the detection limit and obtain better visualization, an integrated dielectrophoresis (DEP) microfluidic chip and related microsystem were created. Positive DEP was used to enrich FNP-labeled bacteria along the edge of the microchannel cross microelectrode by positive electrophoresis and counted under a fluorescence microscope [28]. Zhang et al. [93] designed a simple, rapid, fixed-point magnetic immunofluorescence detection method for avian influenza virus (AIV), enabling the integration of immunomagnetic target capture, concentration, and fluorescence detection in the microfluidic chip. By optimizing the flow rate and incubation time, the detection limit was 3.7 × 104 copy/μL, the injection volume was 2 μL, and the total detection time was less than 55 min. The method is characterized by good portability, fast analysis, high specificity, high precision, and good reproducibility. This microfluidic system can provide a powerful platform for rapid detection of AIV and may be expanded to detect other viruses and pathogens (Fig. 4A). This convenient experimental device enables the development of a real-time diagnostic system while maintaining appropriate sensitivity. Kubol et al. [29] developed a method for detecting intestinal Salmonella by PCR using fluorescent probes on microfluidic disc devices. The cells of Salmonella enterica were isolated in the microchamber of the apparatus and then thermally lysed, and the polymerase chain reaction targeting the invA gene specific to S. enterica was observed by measuring the fluorescence signal generated by gene amplification. This method can detect living cells without inhibition by any egg component. Salmonella enterica was detected in egg yolks at 5.0 × 104 cells/mL or higher concentrations within 6 h including sampling time. The use of functionalized MNPs to capture and enrich bacteria has aroused the interest of many researchers in the field. Microfluidic and MNPs combined with effective capture antibody immobilization, as well as a fluorescence detection system, enabled the detection of L. monocytogenes at a detection limit of 10 CFU/mL [94]. The system integration can better show the design and application concept of lab-on-a-chip (LOC), indicating the direction of research and development of microfluidic microchip bacterial analysis systems and instruments.
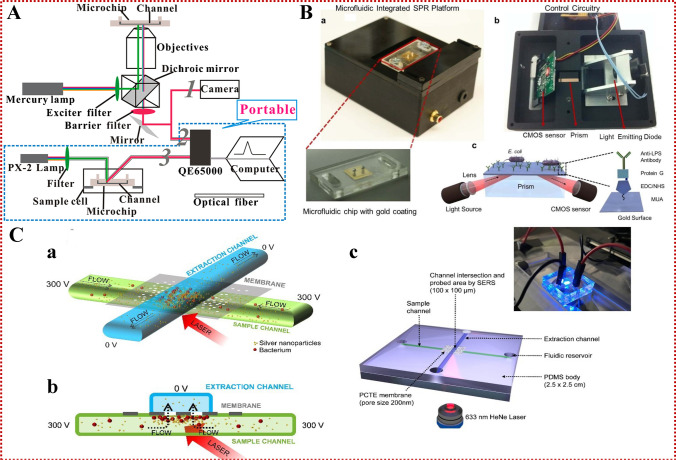
Fig. 4.
(A) Schematic diagram of the combination of fluorescence and microfluidic detection device. Reproduced from [93] with permission of the American Chemical Society. (B) Schematic of a quantitative portable plasma platform integrated with microfluidic for pathogen detection and quantification. (a) The surface activated disposable microfluidic chips were mounted on the top side of the device. (b) The electronic setup of the device is represented from bottom. (c) Schematics of the microfluidic integrated SPR platform. Reproduced from [32] with permission of Springer Nature. (C) The working principle of the microfluidic device for bacteria enrichment and SERS analysis. (a) Side view, (b) cross-section, (c) the overall appearance of the LOC device for the concentration of bacteria followed by SERS detection. Reproduced from [118] with permission of John Wiley and Sons
CL biosensor
The CL technique has several favorable features including fast reaction and high sensitivity, as well as perfect measurement instruments and good supply of reagents. CL is widely used for the determination of hormones and IgG, and has received increasing attention in microbiological research [95]. Generally, the CL method is used for determining the number of microorganisms and has been widely applied in the detection of bacteria [96, 97]. Dong et al. [30] developed an organic hybrid heterojunction photodiode (OPD) array integrated with a multi-cavity microfluidic biosensor for the detection of multiple pathogens. The composite microfluidic biosensor made of polymethylenoate (PMMA) was integrated into the OPD array for the CL detection of pathogens. The CL detection limit of E. coli is 5 × 105 cells/mL and E. jejuni is 1 × 105 cells/mL. The detection system uses simple spin coating and injection molding to manufacture the OPD array and PMMA chip, and thus can realize the mass production of an integrated optical biosensor platform. When analyzing and detecting bacteria in real samples, microfiltration and/or IMS is usually incorporated into the integrated optical biosensor platform to realize the micro total analysis system. While the full analysis system has advantages, pathogen pre-concentration on the chip will lead to longer analysis time and higher chip cost. On the contrary, the design and manufacture of the CL platform will encourage its use as a disposable system. After the large-scale production of OPD arrays and microfluidic chips, the analysis cost of the platform is greatly reduced.
Electrochemiluminescence (ECL) is a method for initiating and controlling CL reactions by applying electrochemical potentials, and has become increasingly important in analytical chemistry in recent years. Delaney et al. [98] first developed a method to combine paper-based microfluidic technology with ECL detection. Inkjet printing is used to produce paper microfluidic substrates. The substrate was combined with screen-printed electrodes (SPEs) to form a simple, cheap, and disposable sensor that could be read without a traditional photodetector. 2-(Dibutylamino)-ethanol (DBAE) and nicotinamide adenine dinucleotide (NADH) could be detected to levels of 0.9 μM and 72 μM, respectively. It is worth noting that mobile camera phones can be used to detect the glow emitted by sensors. A calibration curve was constructed by analyzing the intensity of red pixels in the digital images of ECL emission, showing that DBAE could be detected to levels of 250 μM using the mobile phone. Park and Yoon [31] used paper microfluidic technology to successfully detect E. coli in field water samples with smartphones. They designed a three-channel paper with a negative control channel preloaded with bovine serum albumin (BSA)-conjugated beads and two E. coli detection channels preloaded with anti-E. coli-conjugated beads for low- or high-concentration detection. This paper-based microfluidic device combines the smartphone with its internal gyroscope to allow users to position the smartphone at the best scattering detection angle. The lower limit is single-cell level, and the total detection time is 90 s. The device does not require any external hardware except paper chips, smartphones with built-in gyro sensors, and installed software applications. It provides a good example of rapid detection of pathogenic bacteria. Researchers not only improve the convenience of microfluidic in intelligence, but also reduce the cost of biosensor material selections to develop simpler and cheaper biosensors. Shang et al. [99] proposed a cloth-based CL biosensor for the first time to detect the hlyA gene of L. monocytogenes with high sensitivity. The CL biosensor has good analytical performance, such as high detection ability and specificity, wide linear range, and acceptable reproducibility and stability. Finally, the proposed biosensor has been proven to successfully detect target DNA prepared from milk samples supplemented with L. monocytogenes. Therefore, it is considered to have application potential in food safety and environmental monitoring.
Surface plasmon resonance (SPR) biosensor
SPR is generated by normal-mode light excitation of electron density fluctuations at the interface between two different media with opposite relative tolerance, for example, at the interface of a metal and a dielectric material. SPR-based microfluidic technology has advantages including automation, small size, and fast processing speed [100]. It is possible to combine methods by proper design and thereby improve sensing efficiency. Because samples react simultaneously with multiple bio-recognition elements immobilized on a single chip, the microfluidic can help increase the throughput of a single chip to provide better sample stream delivery [101]. Commercial SPR sensors have been widely employed to detect E. coli, S. enterica, S. typhimurium, and L. monocytogenes in food samples [102]. Lee et al. [100] used optical fiber SPR sensors and PCR chips integrated into a compact form for the production and detection of Salmonella DNA amplification products. The SPR optical fiber sensor was made of multimode optical fiber with bimetallic SPR coating, while the PCR chip was composed of PMMA substrates in the form of microchannels. Although the integrated device does not need fluorescence labeling-related parameters, including background signal and sample size, it can detect the refractive index change around the sensor surface caused by the injected DNA amplifier, as long as the DNA sample is close to the sensor surface. The unlabeled characteristic of the device can ensure its reproducibility. At present, the device is being further miniaturized to facilitate POCT [103]. Demirci et al. [32] prepared a portable, multichannel, and inexpensive microfluidic-integrated SPR platform which can quickly detect and quantify bacteria, namely E. coli and S. aureus. Specifically in PBS and peritoneal dialysis (PD) solutions, the platform provides reliable E. coli capture and detection capabilities when the E. coli concentration is in the range of 105 ~ 3.2 × 107 CFU/mL. The reusability and specificity of the platform have also been well verified by testing S. aureus samples (Fig. 4B). Thus, the proposed detection platform may be suitable for capturing and detecting other pathogens in POCT and primary care settings. By using disposable and easy-to-manufacture SPR technology on the unlabeled microfluidic platform, the detection platform meets the POCT requirements of portability, low cost, small sample size, and low practical operation requirements. Using modern micromachining methods, SPR sensors are compared in a matrix layout to enable parallel detection and simultaneous screening of multiple species or concentrations. With SPR, the entire sensor surface can be imaged (termed SPRI), and surfaces can be divided into "spots" of any size, with surface functionalization for detecting specific positions [104]. The SPRI system is easily automated and compatible with real food samples. For example, Boulade et al. recently used resolution-optimized prism-based surface plasmon resonance imaging (RO-SPRI) and data processing to detect L. monocytogenes and Listeria innocua (L. innocua) of foodborne origin. The system could detect two kinds of L. monocytogenes with an initial concentration of 2 × 102 CFU/mL in less than 7 h. The bacteria surface density at the positive sites was 15 ± 4 bacteria/mm2. This method provides great potential for developing a rapid and specific detection system based on affinity monitoring [105]. Morlay et al. [106] developed an approach involving the detection of live bacteria during their growth phase via the use of an immuno-chip SPR imaging process. The sensor can detect very small amounts of L. monocytogenes in food samples in a day. This method for real-time detection of L. monocytogenes showed remarkable performance in terms of sensitivity and specificity. A gold biochip was functionalized with seven different polyclonal antibodies. All the antibodies could successfully bind to bacterial cells. This method can detect L. monocytogenes within 30 min. Compared with the current methods, the method reduces the time of processing and producing results, but it still takes 24 h of enrichment steps. Recently, a custom SPRI sensor was connected to a smartphone for antibody detection. Guner and colleagues developed a low-cost grating coupled SPR sensor chip using off-the-shelf optical storage discs [107]. The transmission surface plasmon resonance (TSPR) measurement offers outstanding advantages over traditional SPR techniques. Since the TSPR optical signal does not have optical noise derived from the reflected light, the unique TSPR peak and the main bright spots on the dark background indicate transmitted light enhancement and improved signal-to-noise ratio. A strong and narrow TSPR excitation peak covering the visible near-infrared region (900 nm) was obtained on the gold-plated grating substrate [108]. This work makes use of special transmission signals from the plasma grating structure, which has a microfluidic system called microfluidic transmission surface plasmon resonance (MTSPR). The technology has benefits including simple operation, strong flexibility, good repeatability, and great possibility for further commercialization. Lertvachirapaiboon and colleagues applied MTSPR fabricated by a gold-plated grating substrate and microchannel to the biosensor for the detection of glucose. The detection limit of the developed system for glucose was 2.31 mM. This practical platform represents high potential for the further development of several biomolecules, multiple systems, and POC analysis for practical biosensor applications [109]. Although this MTSPR platform is not designed for bacterial detection, its miniaturized design is suitable for POC applications that require disposable SPR sensors. This typical sensor chip provides a simple program and practical system for the application of biosensors. In addition, the possibility of further developing sensor chips for multiple detection, POC detection, and portable biosensor devices is high. SPR sensors are based on localized surface plasmon resonance (LSPR) of metal nanostructures with surface plasmon modes at the structural interface. The LSPR sensor with various nanostructures is a miniature version of the SPR sensor, and requires neither a prism nor a complex optical system, but a simple transmittance measurement [110]. Therefore, the SPR-microfluidic chip analysis platform will appear at the forefront of POCT, promote new ideas and changes to modern laboratory medicine, and highlight the characteristics of simplicity and speed.
Surface-enhanced Raman spectroscopy (SERS) biosensor
SERS is a technique that strongly amplifies the Raman scattering effect of analyzed molecules. These molecules are located in the "hot spots" of metal nanostructures and are characterized by strong local field enhancement caused by SPR [111]. SERS technology is also known for its high sensitivity and specificity, which can be used to detect, identify and characterize various compounds or biomaterials. As a result, specific and unique fingerprints of the compounds/materials studied can be obtained. SERS technology has a wide range of practical application prospects because of its high sensitivity, high selectivity and the possibility of rapid, unmarked and non-destructive analysis [112]. Since the 1990s, the field of SERS analysis of microorganisms has attracted special attention of researchers [113]. Tycova and others have reviewed the microfluidic devices based on SERS for the detection of bacteria in drinking water [114]. Cheng et al. [33] designed a new microfluidic platform, which uses hybrid electric matrix to enrich bacteria to the stagnation area on a rough electrode with SERS activity in the center of the concentric circular electrode. The results showed that the isolation and enrichment of bacteria were completed in 3 min, and the enhancement factor increased by about 1000 times in the local area of about 5000 μm2 with a low bacterial concentration of 5 × 103 CFU/mL. Escherichia coli, S. aureus and P. aeruginosa can be successfully identified in 1 min without using antibodies/chemical reagents during fixation and reaction. The detection limits are 1 × 104, 3 × 103, 5 × 103 CFU/mL, respectively. Pu et al. [115] investigated the latest application of SERS microfluidic platforms in the detection of food contaminants, including pesticides, antibiotic residues and foodborne pathogens. At present, this technology has been widely used in pathogen detection, including various food substrates. Since the preparation of SERS substrate generally requires gold or silver nanoparticles as the enhanced substrate, the selection of target molecules is greatly limited in the current study of microfluidic SERS sensors with immobilized metal nanostructures [116]. Li et al. [117] designed a microfluidic SERS sensor for rapid, label-free detection of biomolecules. This is the first report of the integration of nanoporous gold core-related materials into the microfluidic chip to increase the total surface area of available nanostructures, thus providing more adsorption sites for biomolecules. Nanoporous gold disk (NPGD) array, an efficient SERS substrate, was monolithically integrated into a microfluidic chip. The device employs Rhodamine 6G to quantify the spatial uniformity and determine the shortest detection time to verify the performance of the sensor. Next, the sensor can detect dopamine and urea biomolecules. The sensor has unprecedented detection limits and speed compared to other microfluidic SERS sensors. In the past few decades, centrifugal microfluidic technology has made great progress, showing the rapid and automatic implementation of complex analysis. Krafft et al. [118] developed a feasible, low-cost chip laboratory device, equipped with nanoporous films to connect two stacked vertical microfluidic channels. One of the channels provides the sample, while the second channel attracts the sample through the potential energy driving force. Silver nanoparticles were added to the sample by the SERS effect. The pores of the membrane act as filters to trap microbiomes and nanoparticle clusters, creating suitable conditions for sensitive SERS detection. In this study, the optimized experimental parameters were used to analyze the common pathogenic bacteria (E. coli DH5VLB120 and Pseudomonas Huangshan VLB120) in tap water after adding water. The system can construct a one-time optical detection platform to realize the coupling of electrokinetic concentration of pathogens on integrated nanoporous membranes and SERS detection (Fig. 4C). The device offers fast and simple operation, shows small dimensions, is durable and can be used as a disposable device. Therefore, it has high potential in rapid POC analysis of bacterial samples, especially used in combination with portable Raman instruments. Keys et al. [119] developed a new, rugged, low-cost cavity array SERS substrate fabrication technology and integrated it into microfluidic devices. The manufacturing depends on the two-photon 3D printing of the main template and can mass-produce simple modifications of the reproducible array and cavity structure with submicron resolution. By introducing a nanostructure-plasma “hot spot” and directly integrating into microfluidic devices, this method achieves Raman signal enhancement up to 6.7 × 107. The proposed manufacturing method for microfluidic stents and SERS platforms is highly repetitive, and the microstructure of 3D printing or a PDMS master sheet observed after dozens of applications will not deteriorate. Therefore, the reported method allows many times the number of replicas to be made from a single parent microstructure, so it is very economical. In addition, the process is relatively simple and does not require skilled personnel or expensive manufacturing equipment. Therefore, it is hoped that this kind of equipment can be used as a reference in bacterial detection, and more stable enhanced substrates and portable devices can be developed as soon as possible to cope with the challenges brought by the application of POCT, and can gradually improve the ability to integrate concentrated bacteria [118, 120].
Microfluidic chip with electrochemical biosensor detection methods
An impedance biosensor is a typical electrochemical biosensor, which has the advantages of compact design, fast detection speed, relatively low cost, and easy integration, so it has become an ideal choice for biosensor applications. In general, impedance biosensors require simpler devices, are easily integrated with electronic reading devices, and are less vulnerable to environmental impacts and pollution than other analytical technologies [121]. Some impedance biosensor-based microfluidic technology that has been developed for the detection of foodborne pathogenic bacteria can provide high specific surface area, small volume, strong sample treatment ability, short detection time, automatic operation, and no cross contamination. Dastider et al. [35] proposed a low-cost, easy-to-manufacture biosensor that can detect S. typhimurium quickly and accurately. This study also compares the advantages of microfluidic biosensors with non-microfluidic biosensors. The biosensor can provide qualitative and quantitative results within 3 h without any enrichment steps. The detection limit of the microfluidic biosensor is 3 × 103 CFU/mL compared to the 3 × 104 CFU/mL for the non-microfluidic biosensor, which indicates that the sensitivity of the microfluidic biosensor is increased tenfold (Fig. 5A). In addition, the samples used for bacteria detection in microfluidic biosensors are very small, usually at the microliter or nanoliter level. Even if biosensors can detect bacteria as low as one cell, that is, the detection limit is 103 CFU/mL or 106 CFU/mL, they are still not sensitive enough to detect foodborne pathogens because most bacteria should not be found in food samples. Yao et al. [17] developed a microfluidic impedance biosensor for rapid, sensitive continuous-flow detection of E. coli O157:H7. After coupling of MNPs modified by streptavidin and biotin-tagged polyclonal antibodies (PABS) to form immune MNPs, the target bacteria were first isolated from the background using MNPs to form an MNP-bacterial complex. Then, the AuNPs modified by urease and an adaptor against E. coli O157:H7 were conjugated with MNP-bacteria to form a MNP-bacteria-AuNPs-urease complex. Finally, the complex was used to catalyze the hydrolysis of urea to produce ammonium carbonate, resulting in reduced impedance. The impedance was measured online with the biosensor, and the concentration of E. coli O157:H7 was determined by impedance normalization analysis. There was a good linear relationship between the relative change rate of impedance and the concentration of bacteria and the detection limit was 12 CFU/mL (Fig. 5B). A MNP-Listeria-AuNPs-urease sandwich complex was prepared by adding AuNPs. The capture rate of Listeria cells could reach 93%, and the capture time could be shortened to 30 min. The detection limit of Listeria cells was as low as 1.6 × 102 CFU/mL. The detection time was shortened from 2 h to 1 h, and the standard recovery rate of lettuce samples was 82.1–89.6% [14]. The detection method captures the complex in the separation chip by applying a high-gradient magnetic field and uses active magnetic mixing, which leads to a rapid immune reaction and greatly improves the capture efficiency of L. monocytogenes in the separation chip. Within 30 min, the capture efficiency reached ~ 93%. Research shows that a microfluidic impedance biosensor has the potential to isolate and detect bacteria online, automatically and sensitively. To reduce the steps of bacterial screening and proliferation, Jiang et al. [122] proposed a method based on pathogen concentration and Mn2+ impedance analysis to reduce SiO2@MnO2 nanocomposites for rapid quantification of S. typhimurium in food samples. In this biosensor, SiO2@MnO2 nanocomposites were reduced to Mn2+ by H2O2, magnetic bead-modified monoclonal antibody for capturing S. typhimurium was used for IMS, and the enrichment process was about 45 min. The biosensor has a detection limit of 21 CFU/mL for S. typhimurium in added milk samples (Fig. 5C). This method can effectively avoid the interference of food matrix in the detection of foodborne S. typhimurium. The crossed array microelectrode can be used to determine the change of ionic strength and can be reused more than 100 times. Joint detection technology can be further integrated into the immunosensor part of the chip laboratory to track foodborne pathogens in real time, and can be extended to the detection and quantification of other biological macromolecules and harmful compounds. In the future, the development of a variety of hybrid systems coupled with a microfluidic device, as well as the application of intelligent detection equipment and electrochemical integration technology, such as smartphones and microelectronic devices, will provide the basic guarantees for the rapid development of microfluidic technology.
Fig. 5.
(A) 3D schematic of the impedance biosensor showing the electrode array embedded under a microchannel with (a) inlet and (b) outlet. (c) Cross-sectional profile demonstrating various layers of the impedance biosensor. (d) Fabricated and packaged device. Reproduced from [35] with permission of Hindawi. (B) The principle of the microfluidic impedance biosensor based on immunomagnetic separation and urease catalysis for continuous-flow detection of E. coli O157:H7. Reproduced from [17] with permission of Elsevier. (C) Schematic diagram of impedance biosensor for detection of S. typhimurium with a cross-finger microelectrode array based on immunomagnetic separation. Reproduced from [122] with permission of Elsevier
Conclusions and prospects
Because of their inherent advantages such as high integration, good accuracy, low reagent consumption, and fast reaction speed, microfluidic chips are becoming a hot research topic in food testing. There are benefits from the rapid development of micro-processing technology, which is expected to provide integrated complex detection and diagnosis functions. In the future, the main development directions of microfluidics are as follows:
Generally speaking, it is difficult for biosensors to detect low levels of bacteria, partly because of the size of the sample, the long incubation time for bacteria, the many washing and drying steps required to process the sample, and the insufficient sensitivity caused by the interference of the food matrix. These are the challenges in developing sensor systems. The automatic flow control delivery on the microfluidic chip is a necessary condition for the realization of the POCT application of pathogen bacteria detection, which can minimize sample processing of unskilled operators. Therefore, the combination of a microfluidic chip and more powerful smartphone application software for field analysis, while providing fast transmission and data storage for tracking and recording, is a future research direction.
Although microfluidic technology shows high potential in the detection of pathogenic bacteria, it is still greatly limited when used commercially. When a microfluidic sensor is designed in an environment with limited resources, other factors must be considered. In particular, the efficient capture of multiple pathogenic bacteria from complex samples for high-throughput multiplex analysis is still a key problem to be solved. This is mainly due to the lack of a "bio-recognition element" with high binding affinity, although some bio-recognition elements used for specific binding of pathogenic bacteria are described in detail in this paper. However, most biosensors still use antibodies, so it is necessary to further develop microfluidic chip technology to improve the specific recognition ability of pathogenic bacteria. In order to realize the portability, ease of use, multiplex reusability and sensitivity of pathogenic bacteria detection, enhance the adaptability of the instrument to the environment and improve the reliability of the instrument is the key to the commercialization of microfluidics.
Isolating the target bacteria from the sample background is an important part of bacteria detection. MNPs feature large specific surface area, small steric hindrance, and uniform distribution. They are usually modified with antibodies against the target bacteria, react with the bacteria, and then magnetically isolate them with a strong magnetic field to remove the background, concentrating bacteria in a volume of buffer. So far, because the IMS usually uses manual operations, it is not suitable for separation from large-volume (10 mL or more) target bacterial samples to improve sensitivity. Although high-gradient magnetic separation (HGMS) has been investigated as a method for the separation of target bacteria in large-volume samples, the blocking of separation channels by large particles in actual samples and the nonspecific binding of proteins and other residues have greatly restricted its use in complex food samples. It is very important to develop effective methods for the isolation and enrichment of pathogenic bacteria.
Metal organic skeleton nanoparticles (MOFNP) can be used not only as an electrode interface, but also as an oxidative beacon, which combines microfluidics and biosensor technology to detect foodborne pathogens to a great extent. The existence of sources of pathogenic bacteria can also aid in establishing an early diagnostic system for foodborne diseases to reduce the incidence and associated mortality.
The μPAD has become a promising tool and can provide a variety of analytical applications for the analysis of pathogenic bacteria in POCT. Compared with traditional microfluidic devices, the μPAD has significant advantages: it is economical, easy to manufacture, disposable, and portable, so researchers can integrate methods to control fluid transport in paper channels, such as layer assembly, the use of wax valves, or the patterning of hydrophobic and hydrophilic substrates in the channel. When fluid transmission is programmed, complex detection schemes can be completed automatically without the need for an external control system. Fluid flow can be better managed programmatically, and multi-target analytes can be detected with high sensitivity by using one-step or multistep analysis schemes. The μPAD is most suitable for onsite health screening with high-throughput function. This plays an important role in early diagnosis (if isolation is necessary) and treatment to eliminate the spread of infectious or deadly diseases throughout the community, especially in the event of an epidemic or pandemic.
In summary, there are many promising applications in the field of rapid and automated detection methods for foodborne pathogens. In view of the wide applicability and great potential of these methods, there exist great development opportunities in the near future. At present, smart microfluidics technology can realize autonomous, precise operation and control of trace liquids, and has been applied to cell screening, drug delivery, real-time detection, and organ chips.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22064016), Tianshan Innovation Team Plan of Xinjiang Uygur Autonomous Region (2021D14017), Natural Science Foundation of Xinjiang Uygur Autonomous Region (2019D01A69, 2019D01B36), the Xinjiang Uygur Autonomous Region University Scientific Research Program Key Project (XJEDU2019I019), the Scientific Research and Development Project of Xinjiang Normal University (XJNUZX202003), and the Doctoral Research and Innovation Program of Xinjiang Normal University (XJ107622007).
Abbreviations
- PCR
Polymerase chain reaction
- ELISA
Enzyme-linked immunosorbent assay
- LAMP
Loop-mediated isothermal amplification
- POCT
Point-of-care testing
- HPLC
High-performance liquid chromatography
- AST
Antimicrobial susceptibility testing
- QDs
Quantum dots
- E. coli
Escherichia coli
- S. typhimurium
Salmonella typhimurium
- L. monocytogenes
Listeria monocytogenes
- S. enterica
Salmonella enterica
- E. jejuni
Enterobacter jejuni
- S. aureus
Staphylococcus aureus
- P. aeruginosa
Pseudomonas aeruginosa
- CL
Chemiluminescence
- MNPs
Magnetic nanoparticles
- IMS
Immunomagnetic separation
- 3D
Three-dimensional
- PSMs
Polystyrene microspheres
- PSM
Polystyrene microsphere
- AuNPs
Gold nanoparticles
- AMPs
Antimicrobial peptides
- RPA
Recombinase polymerase amplification
- SELEX
Systematic evolution of bio-recognition elements by exponential enrichment
- PAMAM
Poly(amido amine)
- PDMS
Poly(dimethyl siloxane)
- RCA
Rolling circle amplification
- cRCA
Capture RCA
- sRCA
Signal RCA
- MBL
Mannose-binding lectins
- ConA
Concanavalin A
- ECL
Electrochemiluminescence
- CBD
Carbohydrate-binding domains
- PBS
Phosphate-buffered saline
- MIPs
Molecularly imprinted polymers
- RT-LAMP
Reverse transcription loop-mediated isothermal amplification
- μ-PAD
Paper-based analytical device
- FNPs
CdSe/ZnS@SiO2-NH2 composite nanoparticles
- DEP
Dielectrophoresis
- AIV
Avian influenza virus
- LOC
Lab-on-a-chip
- OPD
Organic hybrid heterojunction photodiode
- PMMA
Polymethylenoate
- ECL
Electrochemiluminescence
- SPEs
Screen-printed electrodes
- DBAE
2-(Dibutylamino)-ethanol
- NADH
Nicotinamide adenine dinucleotide
- BSA
Bovine serum albumin
- SPR
Surface plasmon resonance biosensor
- PD
Peritoneal dialysis
- RO-SPRI
Prism-optimized surface plasmon resonance imaging
- L. innocua
Listeria innocua
- TSPR
Transmission surface plasmon resonance
- MTSPR
Microfluidic transmission surface plasmon resonance
- LSPR
Localized surface plasmon resonance
- SERS
Surface enhanced Raman spectroscopy
- NPGD
Nanoporous gold disk
- HGMS
High-gradient magnetic separation
- MOFNP
Metal organic skeleton nanoparticles
Declarations
Conflicts of interest
The authors declare they have no financial or nonfinancial conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lv M, Liu Y, Geng J, Kou X, Xin Z, Yang D. Engineering nanomaterials-based biosensors for food safety detection. Biosens Bioelectron. 2018;106:122–128. doi: 10.1016/j.bios.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 2.Yeni F, Yavaş S, Alpas H, Soyer Y. Most common foodborne pathogens and mycotoxins on fresh produce: a review of recent outbreaks. Crit Rev Food Sci. 2016;56(9):1532–1544. doi: 10.1080/10408398.2013.777021. [DOI] [PubMed] [Google Scholar]
- 3.Bolton DJ, Robertson LJ. Mental health disorders associated with foodborne pathogens. J Food Protect. 2016;79(11):2005–2017. doi: 10.4315/0362-028X.JFP-15-587. [DOI] [PubMed] [Google Scholar]
- 4.Law WF, Ab Mutalib NS, Chan KG, Lee LH. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol. 2015;5:770. doi: 10.3389/fmicb.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umesha S, Manukumar HM. Advanced molecular diagnostic techniques for detection of food-borne pathogens: current applications and future challenges. Crit Rev Food Sci. 2018;58(1):84–104. doi: 10.1080/10408398.2015.1126701. [DOI] [PubMed] [Google Scholar]
- 6.Kumar N, Hu Y, Singh S, Mizaikoff B. Emerging biosensor platforms for the assessment of water-borne pathogens. Analyst. 2018;143(2):359–373. doi: 10.1039/C7AN00983F. [DOI] [PubMed] [Google Scholar]
- 7.Min LS, Mi SK. The effects of violence coping program based on middle-range theory of resilience on emergency room nurses’ resilience, violence coping, nursing competency and burnout. J Korean Acad Nurs. 2017;47(3):332. doi: 10.4040/jkan.2017.47.3.332. [DOI] [PubMed] [Google Scholar]
- 8.Lee KJ, Lee WS, Hwang A, Moon J, Kang T, Park K, Jeong J. Simple and rapid detection of bacteria using a nuclease-responsive DNA probe. Analyst. 2017;143. [DOI] [PubMed]
- 9.Chen J, Andler SM, Goddard JM, Nugen SR, Rotello VM. Integrating recognition elements with nanomaterials for bacteria sensing. Chem Soc Rev. 2017;46(5):1272–1283. doi: 10.1039/C6CS00313C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Y, Pan X, Xu S, Liu Z, Wu S. Fluorescence-enhanced microfluidic sensor for highly sensitive in-situ detection of copper ions in lubricating oil. Mater Design. 2020;191:108693. doi: 10.1016/j.matdes.2020.108693. [DOI] [Google Scholar]
- 11.Zhao X, Li M, Liu Y. Microfluidic-based approaches for foodborne pathogen detection. Microorganisms. 2019;7(10):381. doi: 10.3390/microorganisms7100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou L, Chen Y, Fang X, Liu Y, Du M, Lu X, Li Q, Sun Y, Ma J, Lan T. Microfluidic-RT-LAMP chip for the point-of-care detection of emerging and re-emerging enteric coronaviruses in swine. Anal Chim Acta. 2020;1125:57–65. doi: 10.1016/j.aca.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasseri B, Soleimani N, Rabiee N, Kalbasi A, Karimi M, Hamblin MR. Point-of-care microfluidic devices for pathogen detection. Biosens Bioelectron. 2018;117:112–128. doi: 10.1016/j.bios.2018.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q, Wang D, Cai G, Xiong Y, Li Y, Wang M, Huo H, Lin J. Fast and sensitive detection of foodborne pathogen using electrochemical impedance analysis, urease catalysis and microfluidics. Biosens Bioelectron. 2016;86:770–776. doi: 10.1016/j.bios.2016.07.071. [DOI] [PubMed] [Google Scholar]
- 15.Ma Ková T, Hárendar Íková L, Petr J. Determination of Escherichia coli in urine using a low-cost foil-based microfluidic device. Talanta. 2017;170:36–40. doi: 10.1016/j.talanta.2017.03.089. [DOI] [PubMed] [Google Scholar]
- 16.Zheng L, Cai G, Wang S, Liao M, Li Y, Lin J. A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157:H7 using gold nanoparticle aggregation and smart phone imaging. Biosens Bioelectron. 2019;124–125:143–149. doi: 10.1016/j.bios.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Yao L, Wang L, Huang F, Cai G, Xi X, Lin J. A microfluidic impedance biosensor based on immunomagnetic separation and urease catalysis for continuous-flow detection of E. coli O157:H7. Sensor Actuat B-Chem. 2018;259:1013–1021. doi: 10.1016/j.snb.2017.12.110. [DOI] [Google Scholar]
- 18.Magro L, Escadafal C, Garneret P, Jacquelin B, Kwasiborski A, Manuguerra J, Monti F, Sakuntabhai A, Vanhomwegen J, Lafaye P and others. Paper microfluidics for nucleic acid amplification testing (NAAT) of infectious diseases. Lab Chip. 2017;17(14):2347–2371. [DOI] [PubMed]
- 19.Trinh KTL, Zhang Y, Lee NY. One-step DNA purification and amplification on an integrated plastic microdevice for on-site identification of foodborne pathogens. Anal Chim Acta. 2018;1040:63–73. doi: 10.1016/j.aca.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Torab P, Mach KE, Surrette C, England MR, Craft DW, Thomas NJ, Liao JC, Puleo C, Wong PK. Adaptable microfluidic system for single-cell pathogen classification and antimicrobial susceptibility testing. Proc Natl Acad Sci. 2019;116(21):10270–10279. doi: 10.1073/pnas.1819569116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang D, Bi H, Liu B, Qiao L. Detection of pathogenic microorganisms by microfluidics based analytical methods. Anal Chem. 2018;90:5512–5520. doi: 10.1021/acs.analchem.8b00399. [DOI] [PubMed] [Google Scholar]
- 22.Foudeh AM, Didar TF, Veres T, Tabrizian M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip. 2012;12(18):3249–3266. doi: 10.1039/c2lc40630f. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Zou S, Cao X. Rapid and ultra-sensitive detection of foodborne pathogens by using miniaturized microfluidic devices: a review. Anal Methods-Uk. 2016;8(37):6668–6681. doi: 10.1039/C6AY01512C. [DOI] [Google Scholar]
- 24.Chen L, Chen C, Wang P, Chen C, Wu L, Song T. A compound magnetic field generating system for targeted killing of Staphylococcus aureus by magnetotactic bacteria in a microfluidic chip. J Magn Magn Mater. 2017;427:90–94. doi: 10.1016/j.jmmm.2016.10.109. [DOI] [Google Scholar]
- 25.Park C, Kong M, Lee J, Ryu S, Park S. Detection of bacillus cereus using bioluminescence assay with cell wall-binding domain conjugated magnetic nanoparticles. Biochip J. 2018;12(4):287–293. doi: 10.1007/s13206-018-2408-8. [DOI] [Google Scholar]
- 26.Sun L, Jiang Y, Pan R, Li M, Wang R, Chen S, Fu S, Man C. A novel, simple and low-cost paper-based analytical device for colorimetric detection of Cronobacter spp. Anal Chim Acta. 2018;1036:80–88. doi: 10.1016/j.aca.2018.05.061. [DOI] [PubMed] [Google Scholar]
- 27.Jokerst JC, Adkins JA, Bisha B, Mentele MM, Goodridge LD, Henry CS. Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. Anal Chem. 2012;84(6):2900–2907. doi: 10.1021/ac203466y. [DOI] [PubMed] [Google Scholar]
- 28.Wang R, Xu Y, Jiang Y, Chuan N, Su X, Ji J. Sensitive quantification and visual detection of bacteria using CdSe/ZnS@SiO2 nanoparticles as fluorescent probes. Anal Methods-Uk. 2014;6(17):6802–6808. doi: 10.1039/C4AY01257G. [DOI] [Google Scholar]
- 29.Kubo I, Kajiya M, Aramaki N, Furutani S. Detection of Salmonella enterica in egg yolk by PCR on a microfluidic disc device using immunomagnetic beads. Sensors-Basel. 2020;20(4):1060. doi: 10.3390/s20041060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matos Pires N, Dong T. Microfluidic biosensor array with integrated Poly(2,7-Carbazole)/fullerene-based photodiodes for rapid multiplexed detection of pathogens. Sensors-Basel. 2013;13(12):15898–15911. doi: 10.3390/s131215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park TS, Yoon JY. Smartphone detection of escherichia coli from field water samples on paper microfluidics. IEEE Sens J. 2015;15(3):1902–1907. doi: 10.1109/JSEN.2014.2367039. [DOI] [Google Scholar]
- 32.Tokel O, Yildiz UH, Inci F, Durmus NG, Ekiz OO, Turker B, Cetin C, Rao S, Sridhar K, Natarajan N and others. Portable microfluidic integrated plasmonic platform for pathogen detection. Sci Rep-Uk. 2015;5(1):9152. [DOI] [PMC free article] [PubMed]
- 33.I-Fang C, Chang H, Chen T, Hu C, Yang F. Rapid (<5 min) Identification of Pathogen in Human Blood by Electrokinetic Concentration and Surface-Enhanced Raman Spectroscopy. Sci Rep-Uk. 2013;3(1):2365. [DOI] [PMC free article] [PubMed]
- 34.Liu J, Jasim I, Shen Z, Zhao L, Dweik M, Zhang S, Almasri M. A microfluidic based biosensor for rapid detection of Salmonella in food products. Plos One. 2019;14(5):e0216873. doi: 10.1371/journal.pone.0216873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh Dastider S, Barizuddin S, Yuksek NS, Dweik M, Almasri MF, Evoy S. Efficient and rapid detection of Salmonella using microfluidic impedance based sensing. J Sensors. 2015;2015:293461. doi: 10.1155/2015/293461. [DOI] [Google Scholar]
- 36.Kim TH, Park J, Kim CJ, Cho YK. Fully integrated lab-on-a-disc for nucleic acid analysis of food-borne pathogens. Anal Chem. 2014;86(8):3841–3848. doi: 10.1021/ac403971h. [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Hinkley T, Chen J, Talbert JN, Nugen SR. Phage based electrochemical detection of Escherichia coli in drinking water using affinity reporter probes. Analyst. 2019;144(4):1345–1352. doi: 10.1039/C8AN01850B. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Hao L, Qi W, Huo X, Xue L, Liu Y, Zhang Q, Lin J. A sensitive Salmonella biosensor using platinum nanoparticle loaded manganese dioxide nanoflowers and thin-film pressure detector. Sensor Actuat B-Chem. 2020;321:128616. doi: 10.1016/j.snb.2020.128616. [DOI] [Google Scholar]
- 39.Park C, Lee J, Kim Y, Kim J, Lee J, Park S. 3D-printed microfluidic magnetic preconcentrator for the detection of bacterial pathogen using an ATP luminometer and antibody-conjugated magnetic nanoparticles. J Microbiol Meth. 2017;132:128–133. doi: 10.1016/j.mimet.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Hou Y, Cai G, Zheng L, Lin J. A microfluidic signal-off biosensor for rapid and sensitive detection of Salmonella using magnetic separation and enzymatic catalysis. Food Control. 2019;103:186–193. doi: 10.1016/j.foodcont.2019.04.008. [DOI] [Google Scholar]
- 41.Zhan J, Wang L, Zhu Y, Gao H, Chen Y, Chen J, Jia Y, He J, Fang Z, Zhu Y and others. Temperature-Controlled Reversible Exposure and Hiding of Antimicrobial Peptides on an Implant for Killing Bacteria at Room Temperature and Improving Biocompatibility in Vivo. Acs Appl Mater Inter. 2018;10(42):35830–35837. [DOI] [PMC free article] [PubMed]
- 42.Yuan K, Mei Q, Guo X, Xu Y, Yang D, Sánchez BJ, Sheng B, Liu C, Hu Z, Yu G. Antimicrobial peptide based magnetic recognition elements and Au@Ag-GO SERS tags with stable internal standards: a three in one biosensor for isolation, discrimination and killing of multiple bacteria in whole blood. Chem Sci. 2018;9(47):8781–8795. doi: 10.1039/C8SC04637A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Gao M, Wang L, Li S, He J, Qin A, Ren L, Wang Y, Tang BZ. Aggregation-induced emission probe for study of the bactericidal mechanism of antimicrobial peptides. Acs Appl Mater Inter. 2018;10(14):11436–11442. doi: 10.1021/acsami.7b18221. [DOI] [PubMed] [Google Scholar]
- 44.Cardoso MH, de Almeida KC, Cândido EDS, Murad AM, Dias SC, Franco OL. Comparative NanoUPLC-MSE analysis between magainin I-susceptible and -resistant Escherichia coli strains. Sci Rep-Uk. 2017;7(1):4197. doi: 10.1038/s41598-017-04181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dao TNT, Lee EY, Koo B, Jin CE, Lee TY, Shin Y. A microfluidic enrichment platform with a recombinase polymerase amplification sensor for pathogen diagnosis. Anal Biochem. 2018;544:87–92. doi: 10.1016/j.ab.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 46.Wang C, Wu J, Lee G. Screening of highly-specific aptamers and their applications in paper-based microfluidic chips for rapid diagnosis of multiple bacteria. Sensor Actuat B-Chem. 2019;284:395–402. doi: 10.1016/j.snb.2018.12.112. [DOI] [Google Scholar]
- 47.Chen J, Li H, Xie H, Xu D. A novel method combining aptamer-Ag10NPs based microfluidic biochip with bright field imaging for detection of KPC-2-expressing bacteria. Anal Chim Acta. 2020;1132:20–27. doi: 10.1016/j.aca.2020.07.061. [DOI] [PubMed] [Google Scholar]
- 48.Majdinasab M, Hayat A, Marty JL. Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. TrAC Trend Anal Chem. 2018;107:60–77. doi: 10.1016/j.trac.2018.07.016. [DOI] [Google Scholar]
- 49.Jiang Y, Zou S, Cao X. A simple dendrimer-aptamer based microfluidic platform for E. coli O157:H7 detection and signal intensification by rolling circle amplification. Sensor Actuat B-Chem. 2017;251:976–984. doi: 10.1016/j.snb.2017.05.146. [DOI] [Google Scholar]
- 50.Jiang Y, Qiu Z, Le T, Zou S, Cao X. Developing a dual-RCA microfluidic platform for sensitive E. coli O157:H7 whole-cell detections. Anal Chim Acta. 2020;1127:79–88. doi: 10.1016/j.aca.2020.06.046. [DOI] [PubMed] [Google Scholar]
- 51.Mystkowska AA, Robb C, Vidal-Melgosa S, Vanni C, Fernandez-Guerra A, Höhne M, Hehemann J. Molecular recognition of the beta-glucans laminarin and pustulan by a SusD-like glycan-binding protein of a marine Bacteroidetes. FEBS J. 2018;285(23):4465–4481. doi: 10.1111/febs.14674. [DOI] [PubMed] [Google Scholar]
- 52.Mu L, Yin X, Liu J, Wu L, Bian X, Wang Y, Ye J. Identification and characterization of a mannose-binding lectin from Nile tilapia (Oreochromis niloticus) Fish Shellfish Immun. 2017;67:244–253. doi: 10.1016/j.fsi.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 53.Cooper RM, Leslie DC, Domansky K, Jain A, Yung C, Cho M, Workman S, Super M, Ingber DE. A microdevice for rapid optical detection of magnetically captured rare blood pathogens. Lab Chip. 2013;14(1):182–188. doi: 10.1039/C3LC50935D. [DOI] [PubMed] [Google Scholar]
- 54.Kang JH, Um E, Diaz A, Driscoll H, Rodas MJ, Domansky K, Watters AL, Super M, Stone HA, Ingber DE. Optimization of pathogen capture in flowing fluids with magnetic nanoparticles. Small. 2015;11(42):5657–5666. doi: 10.1002/smll.201501820. [DOI] [PubMed] [Google Scholar]
- 55.Dao TNT, Yoon J, Jin CE, Koo B, Han K, Shin Y, Lee TY. Rapid and sensitive detection of Salmonella based on microfluidic enrichment with a label-free nanobiosensing platform. Sensor Actuat B-Chem. 2018;262:588–594. doi: 10.1016/j.snb.2017.12.190. [DOI] [Google Scholar]
- 56.Koskella B, Meaden S. Understanding bacteriophage specificity in natural microbial communities. Viruses. 2013;5:806. doi: 10.3390/v5030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richter Ł, Janczuk-Richter M, Niedziółka-Jönsson J, Paczesny J, Hołyst R. Recent advances in bacteriophage-based methods for bacteria detection. Drug Discov Today. 2018;23(2):448–455. doi: 10.1016/j.drudis.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Singh S, Dhanjal DS, Sonali, Thotapalli S, Kumar V, Datta S, Kumar V, Kumar M, Singh J. An insight in bacteriophage based biosensors with focus on their detection methods and recent advancements. Environ Technol Innov. 2020;20:101081. doi: 10.1016/j.eti.2020.101081. [DOI] [Google Scholar]
- 59.Yue H, He Y, Fan E, Wang L, Lu S, Fu Z. Label-free electrochemiluminescent biosensor for rapid and sensitive detection of pseudomonas aeruginosa using phage as highly specific recognition agent. Biosens Bioelectron. 2017;94:429. doi: 10.1016/j.bios.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 60.Kong M, Sim J, Kang T, Nguyen HH, Park HK, Chung BH, Ryu S. A novel and highly specific phage endolysin cell wall binding domain for detection of Bacillus cereus. Eur Biophys J Biophy. 2015;44(6):437–446. doi: 10.1007/s00249-015-1044-7. [DOI] [PubMed] [Google Scholar]
- 61.Yi Z, Wang S, Meng X, Wu A, Li Q, Song Y, Zhao R, Qiao J. Lysin cell-binding domain-functionalized magnetic beads for detection of Staphylococcus aureus via inhibition of fluorescence of Amplex Red/hydrogen peroxide assay by intracellular catalase. Anal Bioanal Chem. 2019;411(27):7177–7185. doi: 10.1007/s00216-019-02099-0. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Z, Liu J. Intracellular delivery of a molecularly imprinted peroxidase mimicking DNAzyme for selective oxidation. Mater Horiz. 2018;5(4):738–744. doi: 10.1039/C8MH00453F. [DOI] [Google Scholar]
- 63.Chunta S, Suedee R, Lieberzeit PA. Low-density lipoprotein sensor based on molecularly imprinted polymer. Anal Chem. 2016;88(2):1419–1425. doi: 10.1021/acs.analchem.5b04091. [DOI] [PubMed] [Google Scholar]
- 64.Zhang F, Luo L, Gong H, Chen C, Cai C. A magnetic molecularly imprinted optical chemical sensor for specific recognition of trace quantities of virus. Rsc Adv. 2018;8(56):32262–32268. doi: 10.1039/C8RA06204H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poller A, Spieker E, Lieberzeit PA, Preininger C. Surface imprints: advantageous application of ready2use materials for bacterial quartz-crystal microbalance sensors. Acs Appl Mater Inter. 2017;9(1):1129–1135. doi: 10.1021/acsami.6b13888. [DOI] [PubMed] [Google Scholar]
- 66.Bezdekova J, Zemankova K, Hutarova J, Kociova S, Smerkova K, Adam V, Vaculovicova M. Magnetic molecularly imprinted polymers used for selective isolation and detection of Staphylococcus aureus. Food Chem. 2020;321:126673. doi: 10.1016/j.foodchem.2020.126673. [DOI] [PubMed] [Google Scholar]
- 67.Li B, Zhang Z, Qi J, Zhou N, Qin S, Choo J, Chen L. Quantum dot-based molecularly imprinted polymers on three-dimensional origami paper microfluidic chip for fluorescence detection of phycocyanin. ACS Sensors. 2017;2(2):243–250. doi: 10.1021/acssensors.6b00664. [DOI] [PubMed] [Google Scholar]
- 68.Yang S, Ouyang H, Su X, Gao H, Kong W, Wang M, Shu Q, Fu Z. Dual-recognition detection of Staphylococcus aureus using vancomycin-functionalized magnetic beads as concentration carriers. Biosens Bioelectron. 2016;78:174–180. doi: 10.1016/j.bios.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 69.Tsougeni K, Kastania AS, Kaprou GD, Eck M, Jobst G, Petrou PS, Kakabakos SE, Mastellos D, Gogolides E, Tserepi A. A modular integrated lab-on-a-chip platform for fast and highly efficient sample preparation for foodborne pathogen screening. Sensor Actuat B-Chem. 2019;288:171–179. doi: 10.1016/j.snb.2019.02.070. [DOI] [Google Scholar]
- 70.Lin C, Chang W, Wang C, Lee C, Chen T, Jan F, Lee G. A microfluidic system integrated with buried optical fibers for detection of Phalaenopsis orchid pathogens. Biosens Bioelectron. 2015;63:572–579. doi: 10.1016/j.bios.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Y, Zhang B, Gu J, Li S. Magnetic beads separation characteristics of a microfluidic bioseparation chip based on magnetophoresis with lattice-distributed soft magnets. J Magn Magn Mater. 2020;501:166485. doi: 10.1016/j.jmmm.2020.166485. [DOI] [Google Scholar]
- 72.Guan T, Liu D, Li X, Shu B, Shen X. Magnet-actuated droplet microfluidic immunosensor coupled with gel imager for detection of microcystin-LR in aquatic products. Talanta. 2020;219:121329. doi: 10.1016/j.talanta.2020.121329. [DOI] [PubMed] [Google Scholar]
- 73.Ngamsom B, Esfahani MMN, Phurimsak C, Lopez-Martinez MJ, Raymond J, Broyer P, Patel P, Pamme N. Multiplex sorting of foodborne pathogens by on-chip free-flow magnetophoresis. Anal Chim Acta. 2016;918:69–76. doi: 10.1016/j.aca.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 74.Li X, Ximenes E, Amalaradjou MAR, Vibbert HB, Foster K, Jones J, Liu X, Bhunia AK, Ladisch MR. Rapid sample processing for detection of food-borne pathogens via cross-flow microfiltration. Appl Environ Microb. 2013;79(22):7048–7054. doi: 10.1128/AEM.02587-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shu B, Zhang C, Xing D. Segmented continuous-flow multiplex polymerase chain reaction microfluidics for high-throughput and rapid foodborne pathogen detection. Anal Chim Acta. 2014;826:51–60. doi: 10.1016/j.aca.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 76.Abbaspour A, Norouz-Sarvestani F, Noori A, Soltani N. Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of staphylococcus aureus. Biosens Bioelectron. 2015;68:149–155. doi: 10.1016/j.bios.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 77.Wang D, Wang Z, Chen J, Kinchla AJ, Nugen SR. Rapid detection of Salmonella using a redox cycling-based electrochemical method. Food Control. 2016;62:81–88. doi: 10.1016/j.foodcont.2015.10.021. [DOI] [Google Scholar]
- 78.Jung T, Jung Y, Ahn J, Yang S. Continuous, rapid concentration of foodborne bacteria (Staphylococcus aureus, Salmonella typhimurium, and Listeria monocytogenes) using magnetophoresis-based microfluidic device. Food Control. 2020;114:107229. doi: 10.1016/j.foodcont.2020.107229. [DOI] [Google Scholar]
- 79.Kwon K, Gwak H, Hyun K, Kwak B, Jung H. High-throughput microfluidic chip for magnetic enrichment and photothermal DNA extraction of foodborne bacteria. Sensor Actuat B-Chem. 2019;294:62–68. doi: 10.1016/j.snb.2019.05.007. [DOI] [Google Scholar]
- 80.Lee H, Xu L, Koh D, Nyayapathi N, Oh K. Various on-chip sensors with microfluidics for biological applications. Sensors-Basel. 2014;14(9):17008–17036. doi: 10.3390/s140917008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yasaki H, Yasui T, Yanagida T, Kaji N, Kanai M, Nagashima K, Kawai T, Baba Y. A real-time simultaneous measurement on a microfluidic device for individual bacteria discrimination. Sensor Actuat B-Chem. 2018;260:746–752. doi: 10.1016/j.snb.2018.01.079. [DOI] [Google Scholar]
- 82.Nilghaz A, Bagherbaigi S, Lam CL, Mousavi SM, Corcoles EP, Wicaksono DHB. Multiple semi-quantitative colorimetric assays in compact embeddable microfluidic cloth-based analytical device (μCAD) for effective point-of-care diagnostic. Microfluid Nanofluid. 2015;19(2):317–333. doi: 10.1007/s10404-015-1545-9. [DOI] [Google Scholar]
- 83.Nilghaz A, Guan L, Tan W, Shen W. advancement of paper-based microfluidics for diagnostics – the original motivation and current status. Acs Sensors. 2016;1(12):1382–1393. doi: 10.1021/acssensors.6b00578. [DOI] [Google Scholar]
- 84.Jing W, Zhao W, Liu S, Li L, Tsai CT, Fan X, Wu W, Li J, Yang X, Sui G. Microfluidic device for efficient airborne bacteria capture and enrichment. Anal Chem. 2013;85(10):5255–5262. doi: 10.1021/ac400590c. [DOI] [PubMed] [Google Scholar]
- 85.Huang F, Guo R, Xue L, Cai G, Wang S, Li Y, Liao M, Wang M, Lin J. An Acid-Responsive Microfluidic Salmonella Biosensor Using Curcumin as Signal Reporter and ZnO-Capped Mesoporous Silica Nanoparticles for Signal Amplification. Sensor Actuat B-Chem. 2020;312:127958. doi: 10.1016/j.snb.2020.127958. [DOI] [Google Scholar]
- 86.Yu J, Wu H, He L, Tan L, Jia Z, Gan N. The universal dual-mode aptasensor for simultaneous determination of different bacteria based on naked eyes and microfluidic-chip together with magnetic DNA encoded probes. Talanta. 2021;225:122062. doi: 10.1016/j.talanta.2020.122062. [DOI] [PubMed] [Google Scholar]
- 87.Tan S, Agarwal T, Kar S, Borrelli MR, Maiti TK, Makvandi P. 23 - A progressive review on paper-based bacterial colorimetric detection and antimicrobial susceptibility testing. In: Pal K, Banerjee I, Sarkar P, Bit A, Kim D, Anis A, Maji S, editors. Food, Medical, and Environmental Applications of Polysaccharides: Elsevier; 2021. p 687–718.
- 88.Srisa-Art M, Boehle KE, Geiss BJ, Henry CS. Highly sensitive detection of salmonella typhimurium using a colorimetric paper-based analytical device coupled with immunomagnetic separation. Anal Chem. 2018;90(1):1035. doi: 10.1021/acs.analchem.7b04628. [DOI] [PubMed] [Google Scholar]
- 89.Yu S, Tang Y, Yan M, Aguilar ZP, Lai W, Xu H. A fluorescent cascade amplification method for sensitive detection of Salmonella based on magnetic Fe3O4 nanoparticles and hybridization chain reaction. Sensor Actuat B-Chem. 2019;279:31–37. doi: 10.1016/j.snb.2018.09.091. [DOI] [Google Scholar]
- 90.Xue L, Zheng L, Zhang H, Jin X, Lin J. An ultrasensitive fluorescent biosensor using high gradient magnetic separation and quantum dots for fast detection of foodborne pathogenic bacteria. Sensor Actuat B-Chem. 2018;265:318–325. doi: 10.1016/j.snb.2018.03.014. [DOI] [Google Scholar]
- 91.Choi JR, Yong KW, Choi JY, Cowie AC. Emerging point-of-care technologies for food safety analysis. Sensors-Basel. 2019;19(4):817. doi: 10.3390/s19040817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shu Z, Kemper F, Beckert E, Eberhardt R, Tünnermann A. Highly sensitive on-chip fluorescence sensor with integrated fully solution processed organic light sources and detectors. Rsc Adv. 2017;7(7):26384. doi: 10.1039/C7RA03841K. [DOI] [Google Scholar]
- 93.Zhang RQ, Liu SL, Zhao W, Zhang WP, Yu X, Li Y, Li AJ, Pang DW, Zhang ZL. A simple point-of-care microfluidic immunomagnetic fluorescence assay for pathogens. Anal Chem. 2013;85(5):2645–2651. doi: 10.1021/ac302903p. [DOI] [PubMed] [Google Scholar]
- 94.Malic L, Zhang X, Brassard D, Clime L, Daoud J, Luebbert C, Barrere V, Boutin A, Bidawid S, Farber J and others. Polymer-based microfluidic chip for rapid and efficient immunomagnetic capture and release of Listeria monocytogenes. Lab Chip. 2015;15(20):3994–4007. [DOI] [PubMed]
- 95.Tan X, David A, Day J, Tang H, Dixon ER, Zhu H, Chen Y-C, Khaing Oo MK, Shikanov A, Fan X. Rapid mouse follicle stimulating hormone quantification and estrus cycle analysis using an automated microfluidic chemiluminescent ELISA system. ACS Sensors. 2018;3(11):2327–2334. doi: 10.1021/acssensors.8b00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song Q, Sun J, Mu Y, Xu Y, Zhu Q, Jin Q. A new method for polydimethylsiloxane (PDMS) microfluidic chips to maintain vacuum-driven power using Parylene C. Sensor Actuat B-Chem. 2018;256:1122–1130. doi: 10.1016/j.snb.2017.10.006. [DOI] [Google Scholar]
- 97.Fan E, Peng J, He Y, Wu Y, Ouyang H, Xu Z, Fu Z. Chemiluminescent analysis of Staphylococcus aureus utilizing phe11-protonectin against Gram-positive bacteria. Sensor Actuat B-Chem. 2019;285:271–276. doi: 10.1016/j.snb.2019.01.034. [DOI] [Google Scholar]
- 98.Delaney JL, Hogan CF, Tian J, Shen W. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal Chem. 2011;83(4):1300–1306. doi: 10.1021/ac102392t. [DOI] [PubMed] [Google Scholar]
- 99.Shang Q, Su Y, Liang Y, Lai W, Jiang J, Wu H, Zhang C. Ultrasensitive cloth-based microfluidic chemiluminescence detection of Listeria monocytogenes hlyA gene by hemin/G-quadruplex DNAzyme and hybridization chain reaction signal amplification. Anal Bioanal Chem. 2020;412(15):3787–3797. doi: 10.1007/s00216-020-02633-5. [DOI] [PubMed] [Google Scholar]
- 100.Nguyen TT, Trinh KTL, Yoon WJ, Lee NY, Ju H. Integration of a microfluidic polymerase chain reaction device and surface plasmon resonance fiber sensor into an inline all-in-one platform for pathogenic bacteria detection. Sensor Actuat B-Chem. 2017;242:1–8. doi: 10.1016/j.snb.2016.10.137. [DOI] [Google Scholar]
- 101.Sonato A, Agostini M, Ruffato G, Gazzola E, Liuni D, Greco G, Travagliati M, Cecchini M, Romanato F. A surface acoustic wave (SAW)-enhanced grating-coupling phase-interrogation surface plasmon resonance (SPR) microfluidic biosensor. Lab Chip. 2016;16(7):1224–1233. doi: 10.1039/C6LC00057F. [DOI] [PubMed] [Google Scholar]
- 102.Stephen Inbaraj B, Chen BH. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J Food Drug Anal. 2016;24(1):15–28. doi: 10.1016/j.jfda.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang S, Xie J, Jiang M, Chang K, Chen R, Ma L, Zhu J, Guo Q, Sun H, Hu J. The development of a portable SPR bioanalyzer for sensitive detection of escherichia coli O157:H7. Sensors-Basel. 2016;16(11):1856. doi: 10.3390/s16111856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bouguelia S, Roupioz Y, Slimani S, Mondani L, Casabona MG, Durmort C, Vernet T, Calemczuk R, Livache T. On-chip microbial culture for the specific detection of very low levels of bacteria. Lab Chip. 2013;13(20):4024. doi: 10.1039/c3lc50473e. [DOI] [PubMed] [Google Scholar]
- 105.Boulade M, Morlay A, Piat F, Roupioz Y, Livache T, Charette PG, Canva M, Leroy L. Early detection of bacteria using SPR imaging and event counting: experiments with Listeria monocytogenes and Listeria innocua. Rsc Adv. 2019;9(27):15554–15560. doi: 10.1039/C9RA01466G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morlay A, Duquenoy A, Piat F, Calemczuk R, Mercey T, Livache T, Roupioz Y. Label-free immuno-sensors for the fast detection of Listeria in food. Measurement. 2017;98:305–310. doi: 10.1016/j.measurement.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 107.Guner H, Ozgur E, Kokturk G, Celik M, Esen E, Topal AE, Ayas S, Uludag Y, Elbuken C, Dana A. A smartphone based surface plasmon resonance imaging (SPRi) platform for on-site biodetection. Sensor Actuat B-Chem. 2017;239:571–577. doi: 10.1016/j.snb.2016.08.061. [DOI] [Google Scholar]
- 108.Lertvachirapaiboon C, Baba A, Ekgasit S, Thammacharoen C, Shinbo K, Kato K, Kaneko F. Distance-dependent surface plasmon resonance coupling between a gold grating surface and silver nanoparticles. Plasmonics. 2014;9(4):899–905. doi: 10.1007/s11468-014-9695-2. [DOI] [Google Scholar]
- 109.Lertvachirapaiboon C, Baba A, Ekgasit S, Shinbo K, Kato K, Kaneko F. Microfluidic transmission surface plasmon resonance enhancement for biosensor applications. Jpn J Appl Phys. 2017;56(1):017002. doi: 10.7567/JJAP.56.017002. [DOI] [Google Scholar]
- 110.Wang DS, Fan SK. Microfluidic surface plasmon resonance sensors: from principles to point-of-care applications. Sensors-Basel. 2016;16(8):1175. doi: 10.3390/s16081175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haynes CL, McFarland AD, Van Duyne RP. Surface-Enhanced Raman Spectroscopy. Anal Chem. 2005;77(17):338 A–346 A. doi: 10.1021/ac053456d. [DOI] [Google Scholar]
- 112.Mosier-Boss P. Review of SERS substrates for chemical sensing. Nanomaterials-Basel. 2017;7(6):142. doi: 10.3390/nano7060142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Díaz-Amaya S, Lin L, Deering AJ, Stanciu LA. Aptamer-based SERS biosensor for whole cell analytical detection of E. coli O157:H7. Anal Chim Acta. 2019;1081:146–156. doi: 10.1016/j.aca.2019.07.028. [DOI] [PubMed] [Google Scholar]
- 114.Tycova A, Prikryl J, Foret F. Recent strategies toward microfluidic-based surface-enhanced Raman spectroscopy. Electrophoresis. 2017;38(16):1977–1987. doi: 10.1002/elps.201700046. [DOI] [PubMed] [Google Scholar]
- 115.Pu H, Xiao W, Sun D. SERS-microfluidic systems: a potential platform for rapid analysis of food contaminants. Trends Food Sci Tech. 2017;70:114–126. doi: 10.1016/j.tifs.2017.10.001. [DOI] [Google Scholar]
- 116.Wu HY, Choi CJ, Cunningham BT. Plasmonic nanogap-enhanced Raman scattering using a resonant nanodome array. Small. 2012;8(18):2878–2885. doi: 10.1002/smll.201200712. [DOI] [PubMed] [Google Scholar]
- 117.Li M, Zhao F, Zeng J, Qi J, Lu J, Shih WC. Microfluidic surface-enhanced Raman scattering sensor with monolithically integrated nanoporous gold disk arrays for rapid and label-free biomolecular detection. J Biomed Opt. 2014;19(11):111611. doi: 10.1117/1.JBO.19.11.111611. [DOI] [PubMed] [Google Scholar]
- 118.Krafft B, Tycova A, Urban RD, Dusny C, Belder D. Microfluidic device for concentration and SERS-based detection of bacteria in drinking water. Electrophoresis. 2021;42(1–2):86–94. doi: 10.1002/elps.202000048. [DOI] [PubMed] [Google Scholar]
- 119.Šakalys R, Kho KW, Keyes TE. A reproducible, low cost microfluidic microcavity array SERS platform prepared by soft lithography from a 2 photon 3D printed template. Sensor Actuat B-Chem. 2021;340:129970. doi: 10.1016/j.snb.2021.129970. [DOI] [Google Scholar]
- 120.Chattopadhyay S, Sabharwal PK, Jain S, Kaur A, Singh H. Functionalized polymeric magnetic nanoparticle assisted SERS immunosensor for the sensitive detection of S. typhimurium. Anal Chim Acta. 2019;1067:98–106. doi: 10.1016/j.aca.2019.03.050. [DOI] [PubMed] [Google Scholar]
- 121.Tan X, David A, Day J, Tang H, Dixon ER, Zhu H, Chen Y, Khaing Oo MK, Shikanov A, Fan X. Rapid mouse follicle stimulating hormone quantification and estrus cycle analysis using an automated microfluidic chemiluminescent ELISA system. Acs Sensors. 2018;3(11):2327–2334. doi: 10.1021/acssensors.8b00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang S, Peng T, Meng Q, Zhu X, Guo L, Yao K, Wang Z, Zheng P, Ren Z, He Z and others. Rapid and ultrasensitive detection of Salmonella typhimurium using a novel impedance biosensor based on SiO2@MnO2 nanocomposites and interdigitated array microelectrodes. Sensor Actuat B-Chem. 2020;324:128654.