Abstract
This is a multicenter prospective observational study, aimed to evaluate the relations between Fear of COVID-19 and postpartum depression (PPD) symptom, that included a cohort of women who delivered during COVID-19 lockdown between 03 and 05/2020. Participants were approached after delivery and asked to complete an online questionnaire. Data was verified with each center's perinatal database. The validated Fear of COVID-19 Scale was in use. PPD was evaluated using the EPDS questionnaire as a categorical (≥13) and as a continuous scale. Pre-existing maternal disability was defined as any prior physiological/psychological chronic health condition. Continuous medical supervision or stress contributing complications at birth included pregnancy and labor related complications. Regression analysis and ROC statistics were utilized to evaluate associations and control for confounders. Overall, 421 women completed the questionnaires. Of them, 53(12.6%) had a high EPDS score. Fear of COVID-19 was positively correlated with PPD symptoms (r = 0.35,p = 0.000), ROC-AUC 0.73, 95% CI 0.65–0.81, p = 0.000. Following adjustment to confounders (maternal age, nulliparity, ethnicity, marital status, financial difficulties, maternal disability, accessibility to medical services, and continuous medical supervision (, the most important factor that correlated with depression symptoms was maternal disability (aOR 4.61,95% CI 1.96–10.82) followed by Fear of COVID-19 (aOR 1.11,95% CI 1.05–1.17). High accessibility to medical services during pregnancy (aOR 0.62, 95%CI 0.45–0.84) was protective for PPD symptoms. To conclude, during the COVID-19 pandemic, maternal disability and Fear of COVID-19 are positively associated with a high EPDS score. High medical accessibility during pregnancy was found as a protective factor for PPD.
Keywords: COVID-19, Postnatal depression (PPD), Delivery, Edinburgh postnatal depression scale (EPDS)
1. Introduction
Since its first outbreak on December 2019 in Wuhan, China (Phan, 2020), COVID-19 has been responsible for an unprecedented global crisis. As of October 2020, the total number of infected individuals worldwide is 34.8 million, with over one million deaths (WHO Organization, 2020). According to the World Health Organization's (WHO) report from October 4th, 2020, over 255,000 were diagnosed in Israel (WHO Organization, 2020). With the outbreak of the pandemic in Israel in March and until May 2020, the population was subject to restrictions in order to decrease the virus' spread. Restrictions included social distancing and an obligation to wear facial masks in public, and later on, a general quarantine.
Although the majority of research on COVID-19 has focused on its physiological effects, the pandemic has substantial psychological consequences (Pfefferbaum and North, 2020). The fear of COVID-19 as well as the economic recession, social distancing and isolation have all had a negative impact on population mental health and wellbeing (Hamel et al., 2020).
One of the vulnerable groups who the pandemic has impacted are women during their pregnancy, birth, and the postpartum period. After birth, both familial and hormonal changes have a substantial impact on women's mental health, which may aggravate existing psychopathologies or cause the development of a specific psychopathology such as postpartum depression (PPD) (Hendrick et al., 1998). With a baseline prevalence of 6.5%–12.9% in the western population (Stewart and Vigod, 2016), PPD is one of the most common medical complications after birth. In addition to a significant impact on the quality of life of mothers, partners and children (Field, 2010), PPD has a direct influence on maternal-infant emotional attachment and can lead to a decrease in infant's social and emotional development (Feldman et al., 2009).
Recent studies suggested an increased risk for PPD during COVID-19 pandemic (Berthelot et al., 2020; Durankuş and Aksu, 2020; Lebel et al., 2020; Matsushima and Horiguchi, 2020; Oskovi-Kaplan et al., 2020; Zanardo et al., 2020). However, since the pandemic has made sizable changes to all aspects of parturients' life, including the antenatal care, labor and birth experience as well as postpartum maternal domestic and social support (Vieira et al., 2020), it is difficult to define to what extent each stressor has contributed to the prevalence of PPD and how much of it is attributed to the fear of getting infected by COVID-19.
Thus, in this study we aimed to further investigate the psychological effects of the pandemic on the mental well-being of postpartum women focusing on fear of COVID-19 as a contributing factor to PPD.
2. Materials and methods
This was a multicenter, prospective, observational study that was conducted at three university affiliated medical centers in Israel between March 10th, 2020 and May 9th, 2020. Centers included were Hillel Yaffe Medical center (HYMC), Meir Medical Center (MMC) and Wolfson Medical Center (WMC). All of which are university affiliated medical centers. During study period Israel was subjected, as was the rest of the world, to increase in the number of COVID-19 infections. 1st COVID-19 patient was diagnosed in Israel in February 27th, 2020 and first COVID-19 related death was in March 20th, 2020. The time of the study correlated to the beginning of the first lockdown (with ∼5.7% positive tests and overall 5358 active carriers during March 2020). Restrictions included closure of all non-vital shops, restaurants, schools and universities. Public transportation was limited to daily hours and people were forced to stay home. Gradually, numbers of infections decreased (to less than 1% positive tests during May 2020), lockdown was relaxed, and people resumed their daily routines with a common belief that "COVID-19 was over". Schools reopened on May 10th, 2020 and almost all restrictions were cancelled. Overtime, the spread of COVID-19 increased again followed by reinstatement of restrictive regulations up to a second complete lockdown that began on September 18th, 2020. The study was approved by each medical center Institutional Review Board (HYMC-20-0079, MMC-0169-20, WMC-143-20, NIH NCT04609501). Our primary aim was to evaluate fear of COVID-19 as a risk factor for the development of PPD symptoms and PPD diagnosis, utilizing the Edinburgh Postnatal Depression Scale (EPDS).
2.1. Study population
Women were eligible to participate following liveborn delivery in one of the three participating medical centers during the mentioned period. To note, this was the period of first lockdown enforced by the government in Israel to prevent the spread of coronavirus infection. Parturient younger than 18 years or who delivered earlier than 34 gestational weeks were excluded. A comprehensive team of physicians and medical students, both Hebrew and Arabic speaking, approached all women by phone approximately 10 weeks after delivery – the custom time interval from birth that is used to assess PPD symptoms or other birth related psychopathologies (Moraes et al., 2017). Women were given a brief explanation and were asked to participate in the study. Following consent, a text message was sent to each of them, that contained a link to an online questionnaire, either in Hebrew or in Arabic, according to the participant's preference. Questionnaires were sent using the online Qualtrics survey platform.
2.2. Data collection
Maternal demographics, obstetric history, pregnancy surveillance, labor and delivery data as well as short term maternal and neonatal outcome (until home discharge) were all retrieved from the comprehensive computerized perinatal databases at each medical center. To note, at all centers, data was collected prospectively at time of admission to labor ward, during labor and delivery and at postpartum admission. Maternal data included medical background as age, pre-gestational weight and height, weight gain during pregnancy, any known cardiovascular or metabolic illness, medications including any psychiatric medications, smoking, etc. Obstetric characteristics included parity, previous obstetric history and current pregnancy follow up (1st and 2nd trimester genetic screening, anatomy scan, glucose status, any hypertensive disorders). Parameters regarding the course of labor were also included – gestational age at delivery, need for induction of labor, anesthesia, mode of delivery and any birth complications (fever during/after labor, post-partum hemorrhage, obstetric anal sphincter injury, post delivery operations etc). Birth outcomes included newborn's weight, Apgar scores and umbilical blood pH. In addition, variables concerning the postpartum course of both the mother and the newborn were collected: the number of hospitalization days, maternal or neonatal need for intensive care unit, breastfeeding, and more.
2.3. Online questionnaires and definitions
Using the Qualtrics survey platform, women were asked to provide demographic, socioeconomic and obstetrical information and reply to an assembly of mental health status questionnaires.
Demographic and socioeconomic details included questions regarding ethnicity, religious tendencies, education, family status, work status, etc. To complete the medical data retrieved from each medical file, participants were asked to report if they have any disability (i.e. maternal disability), defined as any prior physiological or psychological co-morbidity (per maternal view).
Three specific COVID-19-oriented questionnaires were included: (1) maternal perception of accessibility to medical services during the pandemic on a scale from 1 to 5 (1- “none” to 5- “always available”); (2) COVID-19 exposure questionnaire. This questionnaire was compiled in order to detect any COVID-19-related life events. It included 14 items (for example “I was in contact with someone who was infected by the Coronavirus”). Each participant was asked to indicate whether she experienced such an event, and the number of events was summed up (score 0–14); and (3) “Fear of COVID-19 Scale”, a novel validated questionnaire that was designed to assess different aspects of the fear of the pandemic, and was found to be associated with anxiety, stress and depression in general population (Ahorsu et al., 2020). The scale consists of seven items such as “I am afraid of losing my life because of the Coronavirus” generated by interviews of over 700 Iranian participants, expert evaluations, and after a thorough review of various valid fear scales. To note, this questionnaire was also validated for the Hebrew language)Bitan et al., 2020). Participants were asked to rate their degree of agreement with the statements on a five-point scale (total score 7–35).
PPD diagnosis and symptoms were evaluated using the validated EPDS questionnaire (Cox et al., 1987). This questionnaire is composed of 10 items, scored by using a four-point scale (0–3) with sensitivity of 86% and specificity of 78% for detection of PPD. Depression was evaluated as a continuous variable to evaluate PPD symptoms (Green, 1998) and as a categorical variable (Cox and Holden, 2003), with a score of ≥13 considered as possible depression diagnosis (Cox et al., 1987; Gibson et al., 2009; Schaper et al., 1994). EPDS was utilized not only to evaluate depression symptomology, but also for the evaluation of anxiety and anhedonia (Chiu et al., 2017). Accordingly, three subscales of the EPDS were evaluated separately: anhedonia (items 1 and 2); anxiety (items 3–6); and depression (items 7–10)(Tuohy and McVey, 2008).
Ultimately, all collected data was verified with each center perinatal and postnatal database. To evaluate stress and anxiety that may have originated from objective potential events during pregnancy and delivery, we further defined two variables: Continuous medical supervision, reflecting the need for a high risk clinic follow-up, and was defined as the presence of one of the following: gestational diabetes, any hypertensive disorder, fetal growth restriction or major risk of prematurity defined as need for cerclage; and Stress-contributing complications during delivery, reflecting unexpected negative outcomes during delivery and included one of the following: need for an urgent cesarean delivery, explorative relaparotomy or unplanned hysterectomy, need for a blood transfusion, any anal sphincter injury or need for maternal or neonatal intensive care unit admission.
For the Arabic version, validated previously translated questionnaires were used, or else, questionnaires were double translated by native Arabic speakers, as accepted. A full form of questionnaires is available as supplementary file (Appendix S1).
2.4. Data and statistical analysis
The final analysis included solely women who answered at least 70% of the questionnaire. Data analysis was performed with the SPSS v23.0 package (Chicago, IL).
Maternal characteristics, obstetrics data and COVID-19 related questionnaires were stratified by the EPDS, evaluated both as a categorical value (cutoff ≥13 for definition of possible PPD (Cox et al., 1987)) and as a continuous variable on a scale of 0–30. Correlations between EPDS scores and Fear of COVID-19 scale were evaluated using Pearson correlation test. Results were further analyzed using ROC statistics to evaluate performance of the Fear of COVID-19 scale for prediction of PPD symptoms and using a logistic regression analysis to adjust outcomes for potential confounders. Variables with previously known clinical impact or variables that were found significant in the univariate analysis entered the regression. Continuous variables were compared using Student's t-test and Mann-Whitney U test. The chi-square and Fisher's exact tests were used for categorical variables, as appropriate. Differences were considered significant when p-value was less than 0.05.
3. Results
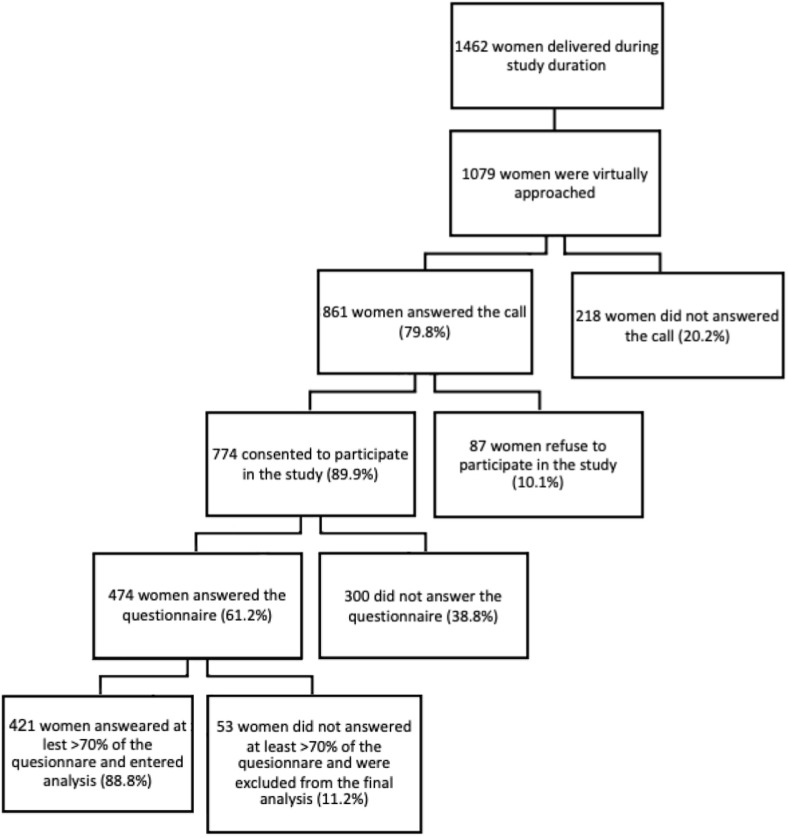
Overall, 1462 women delivered during study duration at the three medical centers. Of them, 1079 (74%) were virtually approached by phone and asked to be included in the study. In total, 774 (53%) consented to answering the questionnaires, and 421 (29%) answered over 70% of questionnaire, thus entering the final analysis (Fig. 1 ).
Fig. 1.
Study cohort.
For the entire cohort, mean maternal age was 31.5 ± 5.3 years. 78.1% were Jewish and 22.9% were Arabic. Overall, 96.1% of the participants were married or in a relationship and for 71.7% this was their first delivery. The majority of the participants delivered at term. The mean gestational age at delivery was 39.5 ± 1.2 weeks and the mean neonatal birthweight was 3271 ± 407 g. Three hundred and forty-two women (81.2%) delivered vaginally and the rest (79, 18.8%) by a cesarean delivery. In 41/421 (9.7%) women, cesarean section was performed urgently.
Possible postnatal depression (defined as EPDS ≥13) was found in 53/421 women (12.6%), and mean EPDS score for the entire cohort was 6.1 ± 5.5 (4.5 ± 3.6 vs. 16.8 ± 3.8 for EPDS < and ≥13, respectively, mean ± SD, p < 0.001). To note, a total of 8 women reported on taking psychiatric mediations upon admission to the hospital, mostly SSRI's. For all eight women, EPDS score was lower than 13.
Demographic, socioeconomic and obstetric data of the study cohort stratified by EPDS score are presented in Table 1 and Table 2 . Compared to postpartum women with EPDS lower than 13, postpartum women with possible PPD had higher prevalence of financial difficulties during the COVID-19 pandemic (52.8%
Table 1.
Maternal characteristics and clinical measurements stratified by subsequent development of postnatal depression (EPDS≥13).
| Characteristic | EPDS <13 N = 368 |
EPDS≥13 N = 53 |
P value |
|---|---|---|---|
| Maternal age, years | 31.5 ± 5.2 | 30.4 ± 5.7 | 0.183 |
| Nulliparity | 100 (27.2%) | 19 (35.8%) | 0.190 |
| Chronic hypertension | 9 (2.4%) | 1 (1.9%) | 0.803 |
| Pregestational diabetes | 6 (1.6%) | 0 (0.0%) | 0.349 |
| Ethnicity: | |||
| Jews | 290 (78.8%) | 39 (73.6%) | |
| Arabic | 78 (21.2%) | 14 (26.4%) | 0.390 |
| Marital Status | |||
| Married | 335 (91.0%) | 45 (84.9%) | |
| In relationship | 21 (5.7%) | 4 (7.5%) | |
| Separated/single | 12 (3.3%) | 4 (7.5%) | 0.260 |
| Religious level (scale 0–4; 0-secular to 4- very religious) | 1.8 ± 0.8 | 1.7 ± 0.8 | 0.369 |
| Education: | |||
| Elementary | 6 (1.6%) | 0 (0.0%) | |
| High school | 139 (37.8%) | 25 (47.2%) | |
| 1st degree | 155 (42.1%) | 20 (37.7%) | |
| 2nd degree | 60 (16.3%) | 8 (15.1%) | |
| 3rd degree | 8 (2.2%) | 0 (0.0%) | 0.505 |
| Financial difficulties due to COVID19 pandemic | 137 (37.2%) | 28 (52.8%) | 0.030 |
| Maternal disability* | 22 (6.0%) | 12 (22.6%) | 0.000 |
| Accessibility to medical services (scale 1–5; 1-none to 5- always available) | 4.5 ± 0.8 | 3.8 ± 1.1 | 0.000 |
| Exposure to COVID19 events (number of exposures) | 3.5 ± 1.8 | 3.9 ± 2.0 | 0.183 |
| EPDS (scale 0–30) | 4.5 ± 3.6 | 16.8 ± 3.8 | 0.000 |
| Fear of COVID19(scale 7–35) | 17.2 ± 5.4 | 21.4 ± 6.3 | 0.000 |
| Delivery to questionnaire interval, weeks | 11.0 ± 1.7 | 11.4 ± 1.6 | 0.214 |
For categorical variables results are presented as n(%) and for continuous variables as mean ± standard deviation (SD). Significant p values (<0.05) are in bold.
EPDS - Edinburgh Postnatal Depression Scale; * Maternal disability - defined as any prior physiological or psychological co-morbidity (per maternal view).
Table 2.
Birth outcome stratified by subsequent development of postnatal depression (EPDS≥13).
| Characteristic | EPDS <13 N = 367 |
EPDS≥13 N = 53 |
P value |
|---|---|---|---|
| Gestational week at delivery | 39.46 ± 1.2 | 39.41 ± 1.5 | 0.727 |
| Birth weight, grams | 3276.1 ± 398.9 | 3235 ± 463.8 | 0.692 |
| Gestational diabetes | 33 (9.0%) | 2 (3.7%) | 0.767 |
| Hypertensive disorder | 22 (5.9%) | 2 (3.7%) | 0.825 |
| Mode of delivery: | |||
| Vaginal delivery | 269 (73.1%) | 40 (75.5%) | |
| Vacuum delivery | 28 (7.6%) | 5 (9.4%) | |
| Elective cesarean | 33 (9.0%) | 5 (9.4%) | |
| Urgent cesarean | 38 (10.3%) | 3 (5.7%) | 0.734 |
| Maternal ICU admission | 5 (1.4%) | 1 (1.9%) | 0.762 |
| Neonatal ICU admission | 12 (3.3%) | 2 (3.8%) | 0.834 |
| OASIS | 1 (0.3%) | 0 (0.0%) | 0.704 |
| Blood transfusion | 9 (2.4%) | 0 (0.0%) | 0.250 |
| Continuousmedical supervisiona | 50 (13.6%) | 3 (5.7%) | 0.104 |
| Stress contributing complications -delivery** | 57 (15.5%) | 8 (15.1%) | 0.941 |
| Maternal hospitalization length, days | 3.4 ± 1.1 | 3.2 ± 0.8 | 0.495 |
| Neonatal hospitalization length, days | 3.2 ± 1.4 | 4.7 ± 12.5 | 0.968 |
| Breastfeeding | 274 (77.6%) | 41 (82.0%) | 0.483 |
For categorical variables results are presented as n(%) and for continuous variables as mean ± standard deviation (SD). Significant p values (<0.05) are in bold.
ICU – intensive care unit; OASIS – obstetrical anal sphincter injury.
Continuous medical supervision including any gestational diabetes, hypertensive disorder, fetal growth restriction or major risk of prematurity defined as need for cerclage. **Stress contributing complications -delivery including any need for urgent cesarean delivery, relaparotomy or unplanned hysterectomy, need for blood transfusion, any anal sphincter injury or need for maternal or neonatal intensive care unit admission.
vs. 37.2%, p = 0.03), and higher prevalence of maternal disability, as defined above, (22.6% vs. 6%, p = 0.000). Also, groups differed by maternal perception of accessibility to medical services: women with EPDS <13 reported more access to medical services (4.5 ± 0.8 vs. 3.8 ± 1.1, p = 0.000). No differences were found between the groups in terms of parity, ethnicity, education, marital status and religious level. Lastly, there was no difference between the groups in the number of exposures to COVID-19 events. In terms of pregnancy outcomes, there was no difference in stress-contributing complications during delivery nor other separate differences were noted between groups.
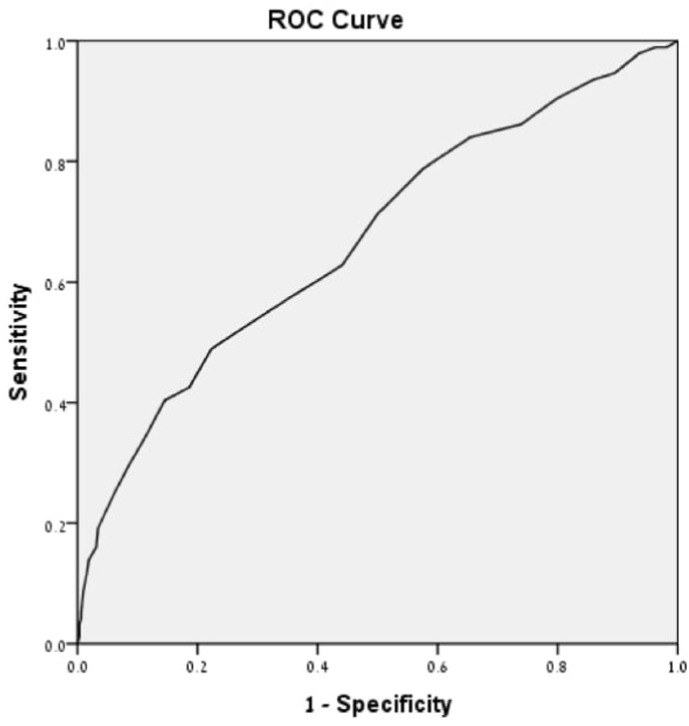
As for the primary outcome – when examined by correlation analysis, Fear of COVID-19 was significantly associated with PPD symptoms, evaluated both as a categorical value (21.4 ± 6.3 vs. 17.2 ± 5.4, EPDS≥13 vs. EPDS<13, respectively, p = 0.000) and as a continuous value (r = 0.35, p = 0.000). As a sole indicator, Fear of COVID-19 predicted 73% of possible PPD cases (Area under the ROC curve 0.73, 95% CI 0.65–0.81, p = 0.000 (Fig. 2) ).
Fig. 2.
Prediction performance of Fear of COVID-19 scale for subsequent development of postnatal depression (EPDS≥13). Prediction performance of Fear of COVID19 scale for subsequent development of postnatal depression (EPDS≥13). Area under the curve is 0.73 (95% confidence interval 0.65–0.81).
Following adjustment to confounders (maternal age, nulliparity, ethnicity, marital status, financial difficulties, maternal disability, accessibility to medical services, and continuous medical supervision (, the most important factor that correlated with possible PPD was prior maternal disability (aOR 4.61, 95% CI 1.96–10.82) followed by Fear of COVID-19 (aOR 1.11, 95% CI 1.05–1.17). High accessibility to medical services (aOR 0.62,95% CI 0.45–0.84) was protective for postnatal maternal possible PPD (Table 3 ).
Table 3.
Variables affecting postnatal depression (EPDS≥13).
| Characteristic | Unadjusted OR | Adjusted OR | 95% CI for aOR | p value |
|---|---|---|---|---|
| Maternal disabilitya | 4.60 | 4.61 | 1.96–10.82 | 0.000 |
| Accessibility to medical services | 0.51 | 0.62 | 0.45–0.84 | 0.002 |
| Fear of COVID-19 | 1.13 | 1.11 | 1.05–1.17 | 0.000 |
Maternal disability – any prior physiological or psychological chronic health condition (per maternal view).
All three EPDS subscales, namely the anhedonia (EPDS question 1–2), anxiety (EPDS question 3–5) and depression (EPDS questions 6–10) subscales demonstrated positive correlation of all three of them: anxiety (r = 0.175, p = 000); depression (r = 169, p = 0.001); and anhedonia (r = 0.130, p = 0.005).
4. Discussion
This was a prospective analysis that aimed to determine the relations between fear of COVID-19 and potential development of PPD symptoms.
Our study results demonstrated that the prevalence of PPD symptoms in our cohort was in the upper limit as compared to historically cited prevalence (12.6% vs. cited prevalence 6.5%–12.9% (Stewart and Vigod, 2016)). This finding, in concordance with previous studies, suggests that postpartum women during the current pandemic are more prone to developing PPD (Berthelot et al., 2020; Durankuş and Aksu, 2020; Lebel et al., 2020). Although not significant, we found that women in our cohort with possible PPD were younger than their counterparts, as was also shown in previous studies (Katon et al., 2014).
Moreover, our results suggest that the prevalence of PPD symptoms during the pandemic among women was similar across the entire cohort, regardless of its ethnic diversity; there were no differences in PPD symptoms among women from different ethnicity, education, marital status or religious believes. Women with possible PPD had more financial problems during the pandemic and reported higher rates of previous physical or mental disability, a well known contributor to development of PPD (Beck, 2001). Interestingly, revising the participants electronic medical files, this difference between groups was not noted. This may be attributed to lack of report, or different perception of chronic illness by the admitting physicians compared to maternal self report. Surprisingly, no difference was found between the groups in terms of absolute exposure to COVID-19 events. This probably emphasizes the impact of maternal fear of COVID-19 pandemic, as contrary to her personal exposure, on maternal well-being and mental health.
Further more, our study demonstrated that Fear of COVID-19 was positively correlated with PPD symptoms and predicted 73% of possible PPD cases (defined as EPDS ≥13 (Cox et al., 1987)). Following adjustment to confounders, the most important factors that correlated with PPD symptoms were prior maternal disability followed by Fear of COVID-19. High accessibility to medical services was protective for developing PPD symptoms. We suggest a possible explanation for these findings: women who experienced more medical encounters and required a more frequent antenatal care, usually were associated with high-risk pregnancy clinics. At the beginning of the pandemic in Israel, there was general anxiety and fear among the public causing people to refrain from visiting medical clinics or hospitals. This was especially true among expecting women, as in that time, the impact of the virus on the course of pregnancy and on the postnatal outcomes was unclear. Thus, many antenatal clinics reduced their activities or turned to virtual encounters. As a result, many women with low risk pregnancy avoided medical appointments. On the contrary to routine care, throughout the pandemic, high risk clinics remained open, and women with high risk pregnancies were encouraged to attend their visits regularly, consequently allowing them a more supportive medical environment. As our results suggest, the frequent medical encounters during the COVID-19 pandemic gave the patients a sense of safety, and as a result, these women might have experienced less fear of COVID-19 and later developed less PPD symptoms. These results are supported by a study conducted by Sade et al. (2020) in Israel. Their study demonstrated that even under the current pandemic, which is known to increase the risk of PPD (Berthelot et al., 2020; Durankuş and Aksu, 2020; Lebel et al., 2020), women who were hospitalized in high-risk pregnancy unit during the COVID-19 pandemic and were under closer medical follow-up did not develop more PPD as compared to a historical cohort of women who were hospitalized prior the outbreak of the pandemic.
To the best of our knowledge, our study is the first to evaluate the relations between Fear of COVID and the prevalence of PPD symptoms. It benefits from being a multicenter cohort study, which allows us to reach a diverse population in terms of ethnicity, religion or socioeconomic status. Exclusion of women delivered before 34 gestational weeks, minimized the bias of the development of PPD symptoms due to prematurity (Vigod et al., 2010). Also, unlike other studies that recruited participants through social media (Berthelot et al., 2020; Lebel et al., 2020; Matsushima and Horiguchi, 2020), participants in our study were personally approached and recruited following delivery in one of the study centers, thus decreasing the bias associated with involvement with social networks. In addition, at least some of the maternal reports on demographics, obstetric and delivery data were validated against their medical records. Lastly, our study benefits from the time elapsed from delivery to maternal questioning. Unlike previous studies that examined pregnant women or women immediately after labor (Berthelot et al., 2020; Durankuş and Aksu, 2020; Lebel et al., 2020; Matsushima and Horiguchi, 2020; Oskovi-Kaplan et al., 2020; Zanardo et al., 2020), we approached the participants approximately ten weeks after giving birth, which is the ideal time to assess any initial PPD symptoms (Moraes et al., 2017).
Nevertheless, our study is not free of limitations. A substantial number of women did not answer the phone call, which is an obvious bias, and data about reason for rejection or their demographic and obstetric data is lacking. In addition, we had no data on type of medical encounters, whether virtual or frontal. As women in our study delivered during the first lockdown, the fear of COVID was so intense that both providers and women avoided frontal sessions, nevertheless, at that time healthcare system only begun its adjustment and virtual encounters were not common. Moreover, it is important to remember that while some of the data regarding birth course was calculated as objective events, it may not reflect women's subjective experience. Lastly, we did not evaluate a cause and effect relationship between Fear of COVID-19 and PPD symptoms, as this may represent the chicken and egg dilemma. To note, in our study Fear of COVID-19 only increased the risk for possible PPD by 11%. As PPD has multifactorial etiology, this may be explained by the above limitations as well as other maternal, social and genetics variables that could have contributed to PPD symptoms and were not evaluated in this study.
Our study has shown an independent association between maternal fear of COVID-19 and the development of PPD symptoms. Postpartum women are a particularly vulnerable population for the risk of depression, especially during the current worldwide crisis which evoked unparalleled challenges. Accessibility to medical services is not only important to prevent physical morbidity but also to maintain maternal mental well-being. Our study results support international guidelines encouraging women to continue their routine antenatal care and imply that ongoing access to medical services and the ongoing maternal-healthcare provider relationship, are essential in supporting women's mental health during the pandemic and the prevention of PPD. Women who have any risk factors, especially if self-considered as a disability, should be in the healthcare providers' focus, as these women are more inclined to develop PPD symptoms during the pandemic. Considering these implications, public health actions are important to minimize PPD during the COVID-19 pandemic.
Disclosure of interest
The authors declare no conflicts of interests.
Contribution to authorship
H. Gluska - Conception and design of the study and manuscript, critical revision of the manuscript for important intellectual content.
N. Shiffman- Conception and design of the study, critical revision of the manuscript for important intellectual content.
Y. Mayer - Conception and design of the study, critical revision of the manuscript for important intellectual content.
L. Elyasyan- Acquisition of data.
N. Elia- Acquisition of data.
R. Daher- Acquisition of data.
M. Sharon Weiner - Acquisition of data.
H. Miremberg- Acquisition of data.
M. Kovo – Critical revision of the manuscript for important intellectual content, Supervision.
T. Biron-Shental – Critical revision of the manuscript for important intellectual content, Supervision.
R. Gabbay-Benziv - Conception and design of the study and manuscript, statistical analysis, critical revision of the manuscript for important intellectual content.
Ethics approval
This is a multicenter study, that was approved byeach medical center the Institutional Review Board (HYMC-20-0079, MMC-0169-20, WMC-143-20, NIH NCT04609501).
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2022.01.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahorsu D.K., Lin C.-Y., Imani V., Saffari M., Griffiths M.D., Pakpour A.H. The fear of COVID-19 scale: development and initial validation. Int. J. Ment. Health Addiction. 2020:1–9. doi: 10.1007/s11469-020-00270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C.T. Predictors of postpartum depression: an update. Nurs. Res. 2001;50:275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Berthelot N., Lemieux R., Garon-Bissonnette J., Drouin-Maziade C., Martel É., Maziade M. Uptrend in distress and psychiatric symptomatology in pregnant women during the COVID-19 pandemic. Acta Obstet. Gynecol. Scand. 2020 doi: 10.1111/aogs.13925. [DOI] [PubMed] [Google Scholar]
- Bitan D.T., Grossman-Giron A., Bloch Y., Mayer Y., Shiffman N., Mendlovic S. Fear of COVID-19 scale: psychometric characteristics, reliability and validity in the Israeli population. Psychiatr. Res. 2020:113100. doi: 10.1016/j.psychres.2020.113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y.-H.M., Sheffield P.E., Hsu H.-H.L., Goldstein J., Curtin P.C., Wright R.J. Subconstructs of the Edinburgh postnatal depression scale in a multi-ethnic inner-city population in the US. Arch. Womens. Ment. Health. 2017;20:803–810. doi: 10.1007/s00737-017-0765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Holden J. Perinatal mental health: a guide to the Edinburgh postnatal depression scale (EPDS) Royal College Psychiatr. 2003 [Google Scholar]
- Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. Br. J. psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Durankuş F., Aksu E. Effects of the COVID-19 pandemic on anxiety and depressive symptoms in pregnant women: a preliminary study. J. Matern. Neonatal Med. 2020:1–7. doi: 10.1080/14767058.2020.1763946. [DOI] [PubMed] [Google Scholar]
- Feldman R., Granat A., Pariente C., Kanety H., Kuint J., Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Field T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav. Dev. 2010;33(1):1–6. doi: 10.1016/j.infbeh.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J., McKenzie-McHarg K., Shakespeare J., Price J., Gray R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr. Scand. 2009;119:350–364. doi: 10.1111/j.1600-0447.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- Green J.M. Postnatal depression or perinatal dysphoria? Findings from a longitudinal community-based study using the Edinburgh Postnatal Depression Scale. J. Reprod. Infant Psychol. 1998;16:143–155. [Google Scholar]
- Hamel L., Kearney A., Kirzinger A., Lopes L., Muñana C., Brodi M. 2020. 2020. KFF health tracking poll. June. [Google Scholar]
- Hendrick V., Altshuler L.L., Suri R. Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics. 1998;39:93–101. doi: 10.1016/S0033-3182(98)71355-6. [DOI] [PubMed] [Google Scholar]
- Katon W., Russo J., Gavin A. Predictors of postpartum depression. J. women’s Heal. 2014;23:753–759. doi: 10.1089/jwh.2014.4824. [DOI] [PubMed] [Google Scholar]
- Lebel C., MacKinnon A., Bagshawe M., Tomfohr-Madsen L., Giesbrecht G. 2020. Elevated depression and anxiety among pregnant individuals during the COVID-19 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima M., Horiguchi H. The COVID-19 pandemic and mental well-being of pregnant women in Japan: need for Economic and Social Policy interventions. Disaster Med. Public Health Prep. 2020:1–11. doi: 10.1017/dmp.2020.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes G.P., de A., Lorenzo L., Pontes G.A.R., Montenegro M.C., Cantilino A. Screening and diagnosing postpartum depression: when and how? Trends Psychiatry Psychother. 2017;39:54–61. doi: 10.1590/2237-6089-2016-0034. [DOI] [PubMed] [Google Scholar]
- Organization W.H. 2020. Coronavirus disease (COVID-19) [Google Scholar]
- Oskovi-Kaplan Z.A., Buyuk G.N., Ozgu-Erdinc A.S., Keskin H.L., Ozbas A., Tekin O.M. The effect of COVID-19 pandemic and social restrictions on depression rates and maternal attachment in immediate postpartum women: a preliminary study. Psychiatr. Q. 2020:1–8. doi: 10.1007/s11126-020-09843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum B., North C.S. Mental health and the covid-19 pandemic. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- Phan T. Novel coronavirus: from discovery to clinical diagnostics. Infect. Genet. Evol. 2020;79:104211. doi: 10.1016/j.meegid.2020.104211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade S., Sheiner E., Wainstock T., Hermon N., Yaniv Salem S., Kosef T., Lanxner Battat T., Oron S., Pariente G. Risk for depressive symptoms among hospitalized women in high-risk pregnancy units during the COVID-19 Pandemic. J. Clin. Med. 2020;9:2449. doi: 10.3390/jcm9082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper A.M., Rooney B.L., Kay N.R., Silva P.D. Use of the Edinburgh Postnatal Depression Scale to identify postpartum depression in a clinical setting. J. Reprod. Med. 1994;39:620–624. [PubMed] [Google Scholar]
- Stewart D.E., Vigod S. Postpartum depression. N. Engl. J. Med. 2016;375:2177–2186. doi: 10.1056/NEJMcp1607649. [DOI] [PubMed] [Google Scholar]
- Tuohy A., McVey C. Subscales measuring symptoms of non-specific depression, anhedonia, and anxiety in the Edinburgh Postnatal Depression Scale. Br. J. Clin. Psychol. 2008;47:153–169. doi: 10.1111/j.2044-8260.2008.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Vieira L.G., Camargo E.L.Si, Schneider G., da Silva G.P.R., Thomazini M., Possani M.A., Matioli M.R., de Sousa Ibiapina A.R. A Systematic Review. medRxiv; 2020. Repercussions of the Covid-19 Pandemic on the Mental Health of Pregnant and Puerperal Women. [Google Scholar]
- Vigod S.N., Villegas L., Dennis C., Ross L.E. Prevalence and risk factors for postpartum depression among women with preterm and low-birth-weight infants: a systematic review. BJOG an Int. J. Obstet. Gynaecol. 2010;117:540–550. doi: 10.1111/j.1471-0528.2009.02493.x. [DOI] [PubMed] [Google Scholar]
- Zanardo V., Manghina V., Giliberti L., Vettore M., Severino L., Straface G. Psychological impact of COVID-19 quarantine measures in northeastern Italy on mothers in the immediate postpartum period. Int. J. Gynecol. Obstet. 2020;150(2):184–188. doi: 10.1002/ijgo.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.