Abstract
Aging is associated with gradual changes in liver structure and physiological/pathological functions in hepatic cells including hepatocytes, cholangiocytes, Kupffer cells, hepatic stellate cells (HSCs), and liver sinusoidal endothelial cells (LSECs). LSECs are specialized hepatic endothelial cells that regulate liver homeostasis. These cells actively impact the hepatic microenvironment as they have fenestrations and a thin morphology to allow substance exchange between circulating blood and the liver tissue. As aging occurs, LSECs have a reduction in both the number and size of fenestrations, which is referred to as pseudocapillarization. This along with the aging of the liver leads to increased oxidative stress, decreased availability of nitric oxide, decreased hepatic blood flow, and increased inflammatory cytokines in LSECs. Vascular aging can also lead to hepatic hypoxia, HSC activation, and liver fibrosis. In this review, we described the basic structure of LSECs, and the effect of LSECs on hepatic inflammation and fibrosis during aging process. We briefly summarized the changes of hepatic microcirculation during liver inflammation, the effect of aging on the clearance function of LSECs, the interactions between LSECs and immunity, hepatocytes or other hepatic nonparenchymal cells, and the therapeutic intervention of liver diseases by targeting LSECs and vascular system. Since LSECs play an important role in the development of liver fibrosis and the changes of LSEC phenotype occur in the early stage of liver fibrosis, the study of LSECs in the fibrotic liver is valuable for the detection of early liver fibrosis and the early intervention of fibrotic response.
Keywords: aging, chronic liver injury, endothelial dysfunction, inflammation, liver fibrosis
Abbreviations
- AGEs
advanced glycation end products
- AMPK
adenosine monophosphate‐activated protein kinase
- APCs
antigen‐presenting cells
- CR
caloric restriction
- CXC
C‐X‐C motif chemokine
- CXCR4
CXC chemokine receptor 4
- ECM
extracellular matrix
- EIIIA
extra domain A
- eNOS
endothelial nitric oxide synthase
- FVIIIRag
factor VIII‐related antigen
- HBV
hepatitis B virus
- HGF
hepatocyte growth factor
- HIF
hypoxia‐inducible transcription factor
- HO‐1
heme oxygenase‐1
- HSCs
hepatic stellate cells
- ICAM‐1
intercellular adhesion molecule‐1
- IL‐1
interleukin‐1
- IL‐6
interleukin‐6
- IL‐8
interleukin‐8
- KLF2
kruppel‐like factor 2
- LSECs
liver sinusoidal endothelial cells
- MET
mesenchymal‐epithelial transition
- MHC‐I
major histocompatibility complex‐I
- MHV3
mouse hepatitis virus 3
- NAFLD
non‐alcoholic fatty liver disease
- PD‐1
programed cell death protein 1
- PDGF
platelet‐derived growth factor
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- SDF‐1
stromal cell‐derived factor‐1
- SIRT1
Sirtuin 1
- TGF‐β1
transforming growth factor β1
- TLR2
toll‐like receptor 2
- TNF‐α
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- ɑ‐SMA
α‐smooth muscle actin
1. BACKGROUND
Aging is generally considered to be the gradual deterioration of function in most organisms that lead to senescence or dysfunction, and a decline of the organism's ability to adapt to metabolic and oxidative stress. Because the rate of aging varies among individuals of the same species, a single marker of aging does not accurately reflect the aging process of an organism. Changes known to regulate aging include telomere shortening, inactivation of inhibitory proteases, mitochondrial dysfunction, epigenetic changes, and stem cell depletion. 1
Liver sinusoidal endothelial cells (LSECs) are specialized endothelial cells that make up approximately 3% of the total liver volume. 2 LSECs are phenotypically different from vascular endothelial cells. 3 LSECs perform multiple functions including endocytosis and regulation of the liver microenvironment (Figure 1). These cells have an incredibly high endocytic capacity and lysosomal activity, which allow them to clear lipoproteins and waste products from the blood. 2 LSECs have multiple types of scavenger receptors that allow them to clear a large amount of macromolecular waste. 4 The liver is the main site to remove advanced glycation end products (AGEs) from circulation, while AGEs are only cleared by LSECs and Kupffer cells through scavenger receptors. 5 These scavenger receptors allow LSECs to bind many different types of viruses, resulting in the clearance of some blood‐borne viruses. 6 Vesicles in LSEC can efficiently remove macromolecular wastes such as immune complexes and lysosomes. 7 This is only one part of LSECs role in liver immunity; they also recruit immune cells to the liver and maintain hepatic stellate cell (HSC) quiescence. 6
FIGURE 1.
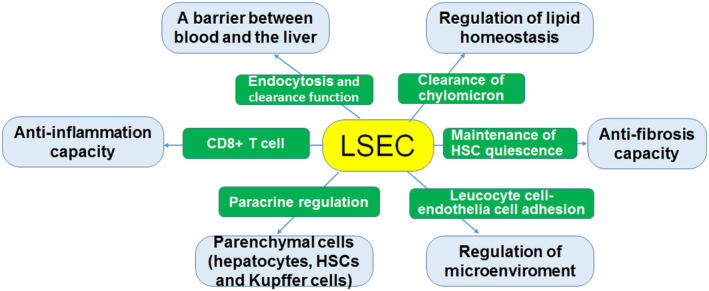
Relationship between normal LSECs and liver function. Healthy LSECs are involved in the regulation of liver function in several ways. The structural characteristics of LSECs ensure the bidirectional transport of metabolites and solutes between the blood and liver parenchyma. In addition, healthy LSECs can regulate lipid homeostasis as well as liver microenvironment. Further, LSECs have anti‐inflammation, anti‐fibrosis, and paracrine regulation capacities
LSECs can effectively regulate the liver microenvironment partly due to their morphology. 8 Some of the unique morphological features of LSECs include the absence of a basement membrane and the presence of a window in the endothelium. 3 These pores aggregate in the cytoplasm to form dynamic sieve plates that facilitate the spatial transport of material from lumen to disk and into the parenchyma. 9 , 10 The maintenance of high metabolic levels in the liver requires a complex vascular system within the organ. LSECs line up to form the capillary network of the liver. The permeable barrier of LSECs allows for the bidirectional movement of molecules between circulating blood and liver cells and allows for the regulation of the liver microenvironment. 2 , 11 LSECs sit on a minimal basal lamia, and there is very little collagen in the perisinusoidal area. 8 This perisinusoidal area, the space between LSECs and the hepatocytes, is referred to as the space of Disse. The minimal amount of extracellular matrix (ECM) in the space of Disse minimizes transport barriers between the liver tissue and blood. 8 Furthermore, the fenestrations in LSECs facilitate this transport effect. 2 Fenestrations are pores that are between 50 and 250 nm in diameter, which collectively form sieve plates. 8 Fenestrations allow small molecules to easily pass from the circulating blood to the space of Disse. 2 While larger molecules pass through LSECs using permselectivity. 2 Both the size and number of fenestrations change due to both physiological changes, and pathological conditions, for example, a variety of liver injury, toxins and disease. 2 , 9 , 10
Studies have shown that significant structural or biochemical changes rarely occur in the aging liver. 12 , 13 However, it has been reported that there are significant age‐related ultrastructural changes in LSECs and space of Disse in the livers of many species, including human, rat, mouse, and the nonhuman primate, 14 , 15 , 16 , 17 which are called pseudocapillarization. 16 Psedocapillarization of sinusoidal endothelial cells occurred in 27‐month–old mice. These changes include a reduction about 14%–50% in porosity and number of fenestrations (that is defenestration) and increased thickness of endothelium. Meanwhile, the diameter of fenestration is narrowed and there are maybe swollen endothelial cells and increased expression of endothelial marker von Willebrand factor and intercellular adhesion molecule‐1 (ICAM‐1), and reduced expression of caveolin‐1. 18 These changes are related to perisinusoidal basal lamina formation and increased deposition of ECM in the space of Disse. 19 Peseudocaplillarization may impede the movement of lipoproteins from blood to hepatocytes and lead to hyperlipidemia or induce aged–related vascular disease. 19 , 20 Pseudocapillarization and narrowing of the sinusoids caused by dysfunction in the elderly reduce sinusoidal blood flow. 15 Liver aging is associated with a decrease in liver mass and a 30%–40% decrease in blood flow. 21 Along with liver aging, dysfunction of LSECs is involved in hepatic inflammation and fibrosis.
2. THE IMMUNOLOGICAL FUNCTION AND PARACRINE EFFECTS OF NORMAL LSECs
2.1. Normal LSECs and immune function
The liver is a unique immunological organ and LSECs are involved in hepatic immune regulation and immune monitoring processes in at least two approaches. First, LSECs can restrict the entry of immune complexes and leucocytes into liver tissue. LSECs can form a barrier between the blood and the rest of the liver tissue. Under physiological conditions, LSECs have the uptake capacity and clear most blood‐borne antigens and small immune complexes. 22
Second, LSECs can act as antigen‐presenting cells (APCs) and regulate lymphocyte action. In the liver sinusoid, except the dendritic cells, all resident cells including the Kupffer cell and LSECs also have been reported to have the capability to engage in antigen presentation. 23 LSECs can act as APCs because they constitutively express MHC class I and II, CD54 (ICAM‐1), CD4, CD11, CD106 (VCAM‐1) molecules as well as costimulatory molecules CD40, CD80, and CD86, which are necessary for the antigen presentation to T cells. 24 In physiological conditions, antigen presentation from LSECs leads to immune tolerance in CD8+ cells. 25 Some data suggest that the MHC II molecules expressed by LSECs may lead to immune tolerance. 26 , 27 LSECs participate in antigen‐specific down‐regulation of immune response by inducing differentiation of T cells into regulatory T cells (Tregs). 28 , 29 An increased number of Tregs can play a preventive and therapeutic role in some autoimmune animal models. 30 Plus, LSECs exert anti‐inflammatory effects by increasing IL‐10 expression in Th1 cells through the Notch pathway. 31
In addition, LSECs lead to immune tolerance of antigenic T cells by presenting MHC class molecules CD8+ T cells in some cross‐presented immune responses. 25 Apoptotic tumor cells present antigens to LSECs and CD8+ cells through cross‐presentation, leading to immune tolerance. 32 Vascular adhesion protein 1 mediates the adhesion of T cells to the hepatic sinus epithelium so that the normal liver can serve as a conduit for activated T cells. 24 The cross‐presentation of major hiscompatibility complex‐1 (MHC‐I) in LSECs promotes liver CD8+ T cell immunity, which may be an important mechanism of liver immunity. 33 In addition, LSECs play a positive role in immune regulation in the liver, including bacterial processing, leukocyte adhesion and virus clearance. 34 , 35 , 36
In response to liver injury, naïve CD8+ T cells can differentiate into effector cells. 37 When LSEC is exposed to high levels of antigen, the immune tolerance response of naïve CD8+ T cells mediated by LSEC's antigen presentation to CD8 T cells disappears. 6 , 25 , 37 In human hepatitis, the number of LSECs windows changes, the size of the windows decreases, and the basement membrane are discontinuous. These features become apparent with the progression of hepatitis. 38 In addition, the clearance of LSECs plays an important role in the initial absorption of viral pathogens into the liver. 38 It has been reported that molecules larger than 12 nm in diameter cannot reach liver cells via LSECs. 39 Hepatitis B virus (HBV)‐coated particles are larger than 50 nm and cannot directly enter the window hole of LSECs. Data support the active transport of the virus to liver cells through hepatic endothelial cells. In addition, hepatitis virus clearance occurs only in liver cells, so viruses in LSECs can serve as sources for endogenous reinfection. 40 LSECs, as one unique kind of APCs, exhibited antigen‐specific immune tolerance in CD4+ and CD8+ cells. Thus, they can help the virus escape surveillance from the immune system. 38
Under physiological conditions, LSECs mediate the immune tolerance of the liver to its own or foreign antigens by expressing and secreting anti‐inflammatory mediators, but they achieve pro‐inflammatory function during viral infection. 41 Studies have shown that the low tendency of the weak mouse hepatitis virus 3 (MHV3) variant to LSECs is associated with less replication of the specific virus in the liver and less damage caused by it. In vitro, the attenuated MHV3 strain‐induced small amounts of fibrinogen 2, prothrombin that promotes vascular thrombosis and hepatitis, are expressed by LSECs. These results suggest that the MHV3 strain can promote the release of pro‐inflammatory cytokines. In addition, MHV3‐specific activation of toll‐like receptor 2 (TLR2) signaling is associated with high replication and pro‐inflammatory activation of LSECs during acute hepatitis. 42 However, so far, there is no direct evidence showing that the relationship between aging LSECs and hepatitis, and LSECs modification in the setting of aged HBV and HCV infections is currently poorly investigated; therefore, more research is needed.
2.2. Paracrine effects of LSECs on hepatocytes and hepatic nonparenchymal cells
Under homeostatic conditions, LSECs are not only a fenestrated endothelium but also exhibit vasodilatory, anti‐inflammatory, anti‐thrombotic, and anti‐fibrotic effects 2 (Figure 1). Communication between LSECs and parenchyma of the liver is crucial in the normal liver as well as in the progression of liver fibrosis.
LSECs promote hepatocyte regeneration through paracrine action of hepatocyte growth factor (HGF). 43 Studies have shown that the expression of angiopoietin‐2 in LSECs fluctuates after hepatectomy. 44 Angiopoietin‐2 expression in LSECs is down‐regulated in early liver regeneration to ensure hepatocyte proliferation, whereas angiopoietin‐2 expression in LSECs is up‐regulated in late liver regeneration and vascular endothelial growth factor receptor 2 (VEGFR2) is regulated to achieve vascular proliferation. 44 , 45
Exosomes are vesicle structures that can be taken up by cells independently of receptors, and their use as a mediator for transfer between different liver cells has made them of great interest. It has been found that exosomes released by LSECs in patients with cirrhosis contain small molecules that regulate the function of liver cells. 46
In normal livers, healthy LSECs prevent HSCs activation partly through vascular endothelial growth factor (VEFG)‐induced nitric oxide (NO) production. 47 Furthermore, healthy LSECs can reverse activated HSCs to quiescence, 48 although the involved paracrine factor(s) has not been identified. LSECs (decapillarized form) isolated from normal rat livers can suppress HSC activation, indicated by reduced alpha‐smooth muscle actin (α‐SMA) expression, but capillarized LSECs isolated from the rat livers with thioacetamide‐induced cirrhosis promoted HSC activation. 47 The use of a soluble guanosine cyclase activator can inactivate HSCs and down‐regulate the expression of fibrin in cirrhotic rats. 48 At the same time, the VEGF secreted from hepatocytes and quiescent HSCs have been identified to mediate fenestrations of LSECs via both NO‐dependent and NO‐independent manners. 48
Kupffer cells, also called as stellate macrophages and Kupffer–Browicz cells, are the resident macrophages in the liver and play an important role in scavenging foreign materials that enter the portal circulation. 49 They are the innate immune cells localized in the liver within the lumen of the liver sinusoids and are adhesive to LSECs which make up the blood vessel walls. The cross‐talk between LSECs and Kupffer cells remains largely unknown. In the fibrosis model, the interaction between LSECs and Kupffer cells has been shown to result in loss of fenestration and increased CD31 expression. 50
Capillarized LSECs secrete fibroconnectin extra domain A (EIIIA) that activates HSCs. 51 LSECs play a key role in activating transforming growth factor beta 1 (TGF‐β1) through plasminolytic enzyme and thereby activating HSCs. 52 Similarly, platelet‐derived growth factor (PDGF) also mediates activation of HSCs through paracrine and autocrine pathways.
3. AGING‐RELATED LSECs AND LIVER INFLAMMATION
Aging LSECs manifest changes in endocytosis, clearance, secretion, vasodilation, inflammation, and anti‐fibrotic capacities. Aging LSECs can reduce the uptake of insulin and lipoproteins, thereby leading to insulin resistance and hyperlipidemia that may cause cardiometabolic problems such as atherosclerosis. 8 Additionally, aging‐related pseudocapillarization also results in decreased endocytotic activity in LSECs leading to the decreased clearance of macromolecule such as antibodies and collagen degradation products. 8 Some data suggest that the ability to phagocyte in the liver is decreased, especially in the central lobular region, indicating impaired scavenger function during aging and decreased age‐related scavenging. 15
Aging and accumulation of oxidative stress contribute to the progression of non‐alcoholic fatty liver disease (NAFLD), steatohepatitis, and liver cancer. 53 Impaired regulation of nitric oxide synthase (NOS) and impaired LSEC function caused by the loss of window openings may contribute to the progression of NAFLD. 54 , 55 , 56 These results suggest that targeting LSEC may prevent the progression of NAFLD as well as its conversion to steatohepatitis.
3.1. Expression changes of angiosecretin, adhesion molecules and pro‐inflammatory cytokines in aging LSECs
Endothelial angiosecretin molecules such as HGF, Wnt2, and Hamp as well as anti‐oxidant molecules like stablinlin‐2 and HO‐1 seem to be reduced and the expression of various pro‐inflammatory cytokines such as tumor necrosis factor alpha (TNF‐α), interleukin 1(IL‐1) and interleukin 1(IL‐6) is up‐regulated in elderly LSECs. 57 Changes in LSEC porosity affect the thickness of the LSEC cross‐section. 1 These morphologic changes affect the expression of many vascular proteins, such as ICAM‐1, laminin, and various collagens. 18
Aging leads to a moderate pro‐inflammatory state in LSECs and increased cell adhesion marker expression. 2 The increase of inflammatory cells in the aging liver is related to the increase of ICAM‐1 expression in the hepatic sinusoidal wall. One study shows that liver microcirculation dysfunction occurs in the old mice liver. 15 Age‐related changes in liver microcirculation include sinus blood flow impairment associated with leucocyte‐cell‐endothelial cell interactions and macrophage aggregation, suggestive of the hepatic microvascular inflammatory response. 15 The accumulation of white blood cells in the livers of elderly mice is at least in part due to the increased expression of ICAM‐1 in the sinus, indicating an age‐related inflammatory response (Figure 2). Kupffer cells can adhere to these markers increasing the number of Kupffer cells in the liver. 8 The higher number of Kupffer cells in the liver then leads to an increase in the release of IL‐6 and IL‐1, which further contributes to liver inflammation. 8 The pro‐inflammatory state of the liver also leads to increased numbers of neutrophils and CD68+ macrophages in the sinusoidal area, 57 while CD163+ cells are decreased. 3 This is consistent with reports of the increased age‐related inflammatory response. 58 , 59 Reduced sinusoid width and swollen LSECs in elderly mice also lead to white blood cell capture and sinus obstruction, leading to impaired sinus perfusion. 15 Meanwhile, early capillarization may be stimulated by age‐related inflammation. 58
FIGURE 2.
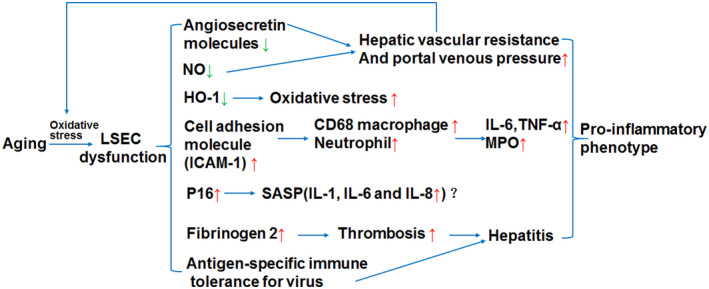
The effects of aging LSEC in liver inflammation. Aging leads to LSEC dysfunction, which increases the expression of cell adhesion molecules (e.g., ICAM‐1) so that there is an accumulation of neutrophils and CD68 macrophages in the liver. The expression of IL‐6 secreted by Kupffer cells and macrophages is up‐regulated and promotes liver inflammation. Aging LSECs lead to down‐regulation of nitric oxide (NO), which, together with down‐regulation of angiosecretin molecules induced by aging, leads to increased hepatic vascular resistance and portal venous pressure. The down‐regulation of HO‐1 expression caused by the aging of LSEC will lead to the increase of oxidative stress response, in which reactive oxygen species (ROS) will further lead to the aging of LSECs. Aging LSECs may have senescence‐associated secretion phenotype (SASP), producing the cytokines such as IL‐1, IL‐6 and IL‐8, contributing to inflammation. Besides, LSECs dysfunction is also involved in pro‐inflammatory reaction in virus hepatitis
The increased oxidative stress that occurs as a result of aging is especially detrimental to LSECs because they are very sensitive to oxidative stress 11 (Figure 2). This is because reactive oxygen species (ROS) cause damage to LSECs and alter their function. 11 Additionally, there is a reduced expression of heme oxygenase‐1 (HO‐1), an anti‐oxidant enzyme, in LSECs due to aging. 57 This further contributes to the oxidative stress in LSECs. 57 In LSECs, oxidative stress can cause an increase of pro‐inflammatory cytokine and chemokine expression, as well as angiogenesis. 11 When exposed to oxidative stress, LSECs attempt to maintain homeostasis through autophagy. 60 Autophagy is an intracellular recycling system that is used to regulate the cell response to stress to maintain homeostasis. 60 Loss of autophagy in LSECs results in increased endothelial dysfunction, activation of HSCs, and decreased NO levels. 60
3.2. Decreased NO production in LSECs and inflammation
As aging occurs, LSECs have a decreased availability of NO. 58 NO is a vasodilation agent, and LSECs are a major source of NO in the liver. 2 LSECs produce NO when stimulated by shear stress or other stimuli such as VEGF. 61 Shear stress upregulates kruppel‐like factor 2 (KLF2) which in turn upregulates endothelial nitric oxide synthase (eNOS). 2 NO inhibits the activation of inflammatory Kupffer cells and protects against pathological conditions in the liver. 61 The reduced availability of NO due to aging is believed to be partly due to reduced eNOS activity. 57 eNOS is mainly expressed in LSECs and other endothelial cells in the liver. eNOS‐derived NO is helpful to maintain liver homeostasis, for example, the regulation of hepatic perfusion. In contrast, inducible nitric oxide synthase (iNOS) is induced in various liver cells, such as LSECs, hepatocytes, HSCs, Kupffer cells, cholangiocytes, and other immune cells. 62 Under pathological conditions, iNOS‐derived NO is a major source of reactive nitrogen species (RNS). Specially, peroxynitrite (ONOO−) can damage various cellular molecules such as DNA, proteins and lipids, and can also facilitate protein nitration in acute and chronic liver diseases. 63
In addition, other study showed that significant up‐regulation of iNOS might promote the inflammatory process in fulminant hepatic failure. 64 Recently, it was reported that LPS induced LSEC dysfunction, which included a decreased vasodilation response to acetylcholine, decreased eNOS phosphorylation at Ser1176 and increased nitrooxidative stress induced by iNOS. Furthermore, iNOS inhibition alleviated liver endothelial dysfunction, reduced nitrooxidative stress and delayed the development of liver injury. 65 Another latest study reported that statins may restore a healthy LSEC and HSC by inhibiting the activation of HSC through modulating the expression of iNOS and eNOS. 66 But in fact, there are few studies about the changes of iNOS in aging LSECs and the subsequent effects in chronic liver diseases during aging. So, further studies are necessary to explore this aspect.
NO is not only important for inflammatory inhibition but also for prevention of stenosis, hepatic metabolism, and regulation of hepatic blood flow. LSECs help regulate hepatic blood flow by releasing NO and angiocrine molecules. 8 The angiocrine molecules that LSECs produce help to keep the liver balanced between fibrosis and liver regeneration. 2 However, aging results in reduced production of angiocrine molecules such as stabilin‐2, CD32b, and VEGFR2. 57 Additionally, there is reduced expression of vasodilator genes in LSECs. 57 This, along with the decreased NO availability, results in increased hepatic vascular resistance and portal pressure. 8 As a result, there is decreased hepatic blood flow resulting in further oxidative stress 2 (Figure 2).
Interestingly, increased expression of senescent markers p16 was observed in LSECs isolated from old rat livers. 3 Senescent LSECs may exhibit senescence‐associated secretory phenotype (SASP), along with secreting some inflammatory cytokines such as IL‐6, IL‐1 and IL‐8, and so on. But further studies are necessary to verify the role of SASPs in LESCs during chronic liver injuries.
4. AGING‐RELATED LSECs AND LIVER FIBROSIS
4.1. Alteration in LSECs phenotype and liver fibrosis
The occurrence of liver fibrosis is a complex process involving molecules, cells, and multiple signaling pathways. Changes in LSEC phenotypes play an important role in liver fibrosis (Figure 3). Alterations in the LSECs phenotype include lack of fenestration, capillarization, 67 and tissue basement membrane formation, which precede fibrosis and promote HSC activation.
FIGURE 3.
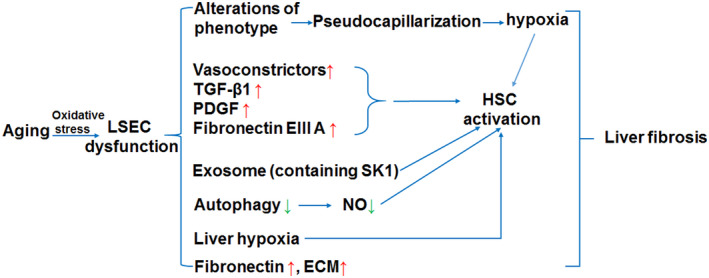
The influence of aging LSEC on liver fibrosis. Pseudocapillarization of LSECs facilitate the occurrence of liver fibrosis. Increased expression of vasoconstrictors together with TGF‐β, PDGF and fibronectin EIIIA can promote activation of HSCs. Loss of autophagy, exosome release and liver hypoxia due to aging LSEC dysfunction are also involved in HSC activation. In addition, aging LSECs may produce extracellular matrix (ECM) and fibronectin. Taken together, Aging LSECs may contribute to the occurrence and progression of liver fibrosis
The alteration of the LSEC phenotype is a key step in liver fibrosis. 68 LSEC phenotype and functions are lost during liver fibrosis by processes known as capillarization and LSEC dysfunction. Changes in the LSECs window can increase chylomicron deposition in the vascular bed and further aggravate liver injury. 69 When capillarization occurs, LSEC becomes continuous endothelial cells, and ECM and interstitial collagen deposition in the Disse space also promote LSECs window closure, 70 which leads to liver cell damage and progression to liver fibrosis. 71
The severity of liver fibrosis can be influenced by the interaction between LSECs, HSCs, and Kupffer cells with cellular interstitial components. 72 , 73 Loss of the LSEC window occurs in the early stages of liver fibrosis. 68 The reversal of LSECs from early environmental tolerance to promoting inflammation and immunogenicity also leads to increased liver inflammation and altered liver immunity. 74 , 75 At the same time, the inactivation of eNOS and the up‐regulation of endothelin‐1 expression also lead to the intensification of liver fibrosis. 76 In addition, capillarized LSECs promote the accumulation of intrahepatic and extrahepatic matrix for fibrotic responses in the form of collagen and fibronectin synthesis.
It has been reported that there are occasional perinusional fibers detected by reticulin staining in older mice. Thickening of LSEC and up‐regulation of endothelial antigen expression are also observed in aging livers. 18 This phenomenon is associated with mild hepatic sinus fibrosis, but not other liver lesions, which may be related to the pathogenesis of endothelial cells. 18
4.2. LSEC dysfunction and HSC activation
In addition to causing hepatic hypoxia, the development of pseudocapillarization leads to LSECs being unable to maintain HSC quiescence, resulting in fibrogenesis. 6 HSC activation is regulated by the LSEC production of vasodilators and vasoconstrictors. 11 The reduced production of NO and other vasodilators in LSECs as a result of aging, therefore, leads to the activation of HSCs 8 (Figure 3). Typically, when activated HSCs lose their fat‐filled vesicles, but in aging mice, this does not occur. 8 The activated HSCs contribute to the increased ECM deposition in the space of Disse which further contributes to LSECs dysfunction. 11 LSECs promote fibrosis by activating HSC in human and mouse liver. First, LSECs affect HSC activity by regulating vasodilator and vasoconstrictor. Secondly, LSECs also increase the activity of HSCs by secreting TGF‐β and PDGF. 51 , 52 Finally, LSECs increase the activity of HSC by activating the Wnt‐ β‐catenin pathway via paracrine or autocrine fashions. 77 , 78 , 79 , 80
The role of exosomes in liver fibrosis is not well understood. Exosomes are known to communicate between cells through protein and lipid exchange. 81 They are released by all cells in order to communicate with surrounding cells. The HSC‐LSEC cross‐talk of LSEC dysfunction caused by exosome signaling in liver fibrosis is bidirectional. In addition, it has been shown that exosomes from the dysfunctional LSECs activate HSC and thus promote the progression of liver fibrosis. 82
4.3. Effect of aging LSECs on extracellular matrix and fibrotic molecules
The role of LSECs in liver fibrosis does not end with the activation of HSCs. The decrease in NO production during aging leads to decreased suppression of pro‐fibrotic pathways. 8 Additionally, pseudocapillarization leads to the release of other pro‐fibrotic factors from LSECs. 60
It has been demonstrated that changes in LSEC phenotype and the feneolation are virtually irreversible once a substantial basement membrane of liver endothelium appears. 67 In the case of liver fibrosis, LSECs may be active contributors to excessive ECM 83 , 84 , 85 (Figure 3). The specific effect of LSECs on fibrotic ECM has been confirmed by several animal and clinical studies. Studies have confirmed that mRNA levels of collagen type I in LSECs increase after liver injury. 86 Injured LSECs can synthesize more type IV collagen. 87 Animal studies have also shown that LSECs can synthesize fibronectin, an essential structural component of ECM 88 (Figure 3). LSECs show a higher expression of fibroconnectin of the EIIIA fragment, which acts as an active biooligomeric and triggers the wound healing response of the organ. 51 The phenotype of LSECs is directly and indirectly affected by the excessive accumulation and structural changes of ECM in the fibrotic liver. The presence of interstitial collagen fibers leads to the uncoating of LSECs. 70 Uncoating of LSECs further reduces the ability of molecular exchange between parenchyma and lumen. Compared with the acellular ECM of other organs, LSECs cultured on the acellular ECM of the liver maintain an open‐cell phenotype for a longer period. 89 ECM remodeling during the development of liver fibrosis also leads to changes in cell‐matrix adhesion molecules.
In the advanced stage of liver fibrosis, LSECs transform into vascular endothelium with the expression of phenotypic marker factor VIII ‐related antigen (FVIIIRag) of vascular endothelial cells 90 (Figure 3). FVIIIRag is a reliable marker for the detection of the sinus to vascular endothelial transformation in advanced liver fibrosis in the elderly. 90 In the FVIIIRag positive endothelial cells, fine particles with a diameter of less than 1 µm were found in the immune sediments, which were located in the perikaryotic cytoplasm and cellular processes. In the parenchymal area of focal perisinusional fibrosis, FVIIIRag immunoreactive endothelial cells are seen overlaying the discoid collagen fiber bundles and altered cell behavior, suggesting a morphological link between vascular endothelial tissue formation and discoid fibrous formation. 90
VEGF increases the fenestration rate by 2–4 times and the down‐regulation of VEGF‐FR2 results in exfoliation of the endothelium, rupture of the sinus cavity, and reduction of the number of fenestration. Therefore, the age‐related decline in the production of VEGF by hepatocytes may play an important role in the pseudocapillarization. 91 The results reveal that aging is associated with the up‐regulation of VEGFR2 in LSECs. 91 Age‐related increased VEGFR2 expression may not contribute to the pathogenesis of pseudocapillarization. On the other hand, the increase of VEGFR2 may be a compensatory response, possibly in response to pseudocapillarization by increasing puncture. 91 The increase in VEGFR2 may reflect an age‐related response to pseudocapillarization. 91
The outcome of liver repair and regeneration or progression to fibrosis or even cirrhosis is determined by the signal generated by LSECs triggered by liver injury. 45 , 92 Stromal cell‐derived factor‐1 (SDF‐1) receptor, a chemokine of the C‐X‐C motif chemokine (CXC) subfamily, is increased during chronic liver injury. The receptors for SDF‐1 include CXC chemokine receptor 7 (CXCR7) and CXCR4, which produce different signaling pathways leading to different outcomes. When there is sustained chronic liver injury, LSEC vascular growth factor receptor together with vascular growth factor 2, actives the MAPK pathways, induces CXCR4 expression, and inhibits CXCR7, which leads to HSC activation and ECM production.
4.4. Loss of LSEC autophagy and liver fibrosis
Autophagy is important for LSEC homeostasis. Autophagy dysregulation as a result of oxidative stress brought on by aging also increases fibrosis 60 (Figure 3). In mouse and rat models, fibrosis‐induced loss of endothelial autophagy reduces intrahepatic NO and impairs the response of LSECs and surrounding cells to oxidative stress. 8 Release of NO from LSECs in physiological and pathological conditions is critical for regulating liver metabolism because it affects liver blood flow, glucose tolerance and fat content, maintenance of stellate cell immobility, inhibition of pro‐fibrosis pathways, and prevention of stenosis. 8 In aging models, LSECs have increased oxidative stress, decreased availability of NO, decreased vasodilation, and increased inflammation. 57
Autophagy of LSEC maintains its special phenotype. 93 When hemodynamics is changed, the expression of Krupple like factor KLF2, which has a protective effect on blood vessels, is up‐regulated, which enhances the autophagy of LSEC and alleviates the injury. When autophagy diminishes, the function of LSECs is impaired, the ability to resist oxygen stress is decreased, and the production of NO is reduced. However, NO can keep HSC in a static state and reduce the production of ECM, so the impairment of LSECs autophagy aggravates liver fibrosis. 60 Down‐regulation of endothelial autophagy is a change that increases oxidative stress in vitro. Selective loss of endothelial autophagy during chronic liver injury in vivo leads to endothelial dysfunction, decreased intrahepatic NO, impaired oxidative stress management, and aggravated liver fibrosis. In the early stage of liver injury, selectively enhancing autophagy of LSECs may effectively prevent the progression of fibrosis; autophagy helps maintain cellular phenotypes and protect LSECs from oxidative stress. But in the late stage of chronic liver injury, autophagy may not be sufficient to restore the damage. Dysregulation of endothelial autophagy activates HSC and worsens the fibrosis in acute liver injury. 60
4.5. Other aging‐related LSEC dysfunctions involved in liver fibrosis
Additionally, age‐related changes result in liver hypoxia, activation of HSC, and fibrosis. 11 The decreased hepatic blood flow can cause hepatic hypoxia, which is a trigger of HSC activation and promotes the induction and progression of liver fibrosis 11 (Figure 3). In addition to the reduced NO and angiocrine molecules, the loss of fenestrations leads to reduced oxygen supply in the liver resulting in hypoxia. 11 The hypoxia leads to increased hypoxia‐inducible transcription factor which in turn increases angiogenic growth factors leading to angiogenesis. 11 Angiogenesis and fibrogenesis are closely linked and the increase of one leads to the increase of the other. 2
Cirrhotic cells, including hepatocytes and LSECs, may lose their restorative function when exposed to a normal hepatic microenvironment. 94 , 95 Microvascular remodeling in the fibrotic liver results in increased capillary resistance and increased blood‐brain barrier shear stress. The expression of NO and NOS in LSECs is significantly reduced in a rodent liver fibrosis model. Maintenance of LSEC differentiated phenotypes is regulated by the NO signaling pathway, suggesting that autocrine NO of LSECs can control the VEGF‐mediated pathways. 96 Studies have shown that HSC activation during the capillarization of LSECs plays a role in the paracrine of LSECs, and HSC mechanical signals also have an indirect effect on LSECs. 96 In a high stiffness microenvironment, LSECs lose the window hole and exhibit increased stress fiber formation. 50
5. THERAPEUTIC INTERVENTIONS TARGETING AGING LSECs FOR LIVER DISEASES
5.1. NOS or Notch1 as a potential target for the treatment of liver fibrosis
Phenotypic restoration may be a potential therapeutic approach to promote fibrosis regression. LSECs have mannose receptors and can rapidly endocytosis of denatured collagen‐α chains in the blood. 67 In rodent models, fibrosis resolution and window‐opening maintenance of LSECs have been achieved through VEGF‐mediated pathways. 97 , 98 NOS synthesized by LSECs plays a regulatory role in portal vein hemodynamics and is an important target for fibrosis mitigation. Interruptions in Notch1 signaling lead to hepatic vascular remodeling, and complete signaling ensures the maintenance of the highly differentiated phenotype of LSECs, thus suggesting the potential of Notch1 as an anti‐angiogenic target for liver fibrosis. 99 But another study has suggested that Notch activation inhibits eNOS‐sGC signaling, resulting in increased LSEC dedifferentiation, HSCactivation and liver fibrosis as well as reduced expression of Wnt2a/9b. 77 Therefore, additional studies are needed for a better understanding of the Notch signaling in the progression and treatment of liver fibrosis.
5.2. Targeting LSECs with resveratrol or metformin
Age‐related windowless behavior can be prevented by the use of agents acting on nutrient‐sensing pathways or more generally by delaying senescence; and cells that act on specific pathways that appear to directly regulate windowing. 57 So far, two anti‐aging drugs have been tested: resveratrol and metformin. 57 Resveratrol acting on the sirtuin nutrient‐sensing pathway increases the hepatic sinusoidal window in mice with Werner syndrome, a model of premature aging. 57 Metformin acts on adenosine monophosphate‐activated protein kinase (AMPK) energy‐sensing pathway, which weakens the window hole of hepatic sinusoids and improves HOMA‐IR and insulin sensitivity as well as endothelial dysfunction in elderly mice. 57 Metformin appears to prevent age‐related window loss with chronic treatment and reverse age‐related window loss with acute treatment in LSECs of young and old mice. Restoration of the healthy LSEC phenotype and endothelial function has been found to prevent fibrosis progression and promote fibrosis regression. 100 This suggests that the effect of metformin on LSECs may be combined with the beneficial effect of AMPK agonists in the treatment of liver fibrosis. 100
5.3. Caloric restriction
In old ages, the thickness of the sinusoidal endothelium increases, and the window rate of endothelium decreases which leads to endothelial dysfunction. 101 The area of perforated endothelial cells decreases by 30%–50%. This is associated with increased intramedullary endothelial cell thickness and ECM, including collagen and basal lamina. Caloric restriction (CR) has a significant effect on sinusoidal endothelial cells, suggesting that the aging process involves pseudocapillarization. 101 CR also improves the age‐related increase in sinusoidal mucosa skin cell thickness and ECM. Caveolin‐1 is found to be strongly expressed parasinusally in young, healthy livers. The expression of caveolin‐1 decreases with age, which is associated with the reduction of the sinus window. These associations support the conclusion that the fossae are structurally related to the fenestration and that changes in caveolin‐1 expression are important during aging. 101 CR alleviates the decrease in age‐related caveolin‐1 parasinus‐like expression. 101 In conclusion, senescence is related to the changes of hepatic sinusoidal endothelial cells and endothelial dysfunction. The known role of CR in age‐related pseudocapillarization suggests that CR may be a new therapeutic target for the control of age‐related dyslipidemia. 101
5.4. Novel measures targeting LSEC and endothelial function
A major challenge for pharmacotherapies is the delivery of the active agent to the specific cell type or tissue. LSECs have unique properties which can be utilized as a drug target. LSECs are the most efficient endocytic cells in the body. 83 They have plenty of clathrin‐coated vesicles and numerous endocytic receptors such as mannose receptors, Fc gamma‐receptor IIb2 and stabilin receptors. 4 Therefore, LSECs have also been exploited as a target for the uptake of nanoparticles, especially for those with a diameter of 5–20 nm. 102 , 103 Thus, nanoparticles have been suggested as an efficient way of drug delivery to LSECs and other liver cells, 104 , 105 decreasing dosages, adverse drug reactions, and off‐target effects. This kind of drug delivery technology may be promising for therapeutic agents that regulate fenestrations in the LSEC. In addition, statins may improve liver endothelial dysfunction and help to alleviate portal hypertension. Statins may act as vasoprotective drugs by maintaining LSEC differentiated phenotypes and improve LSEC autophagy. 66
6. CONCLUSION
LSECs' unusually thin morphology along with their fenestrations allows them to manage the liver microenvironment through both their high endocytic capacity and their high permeability. 2 When aging occurs, there is a reduction in both size and number of fenestrations resulting in reduced movement of molecules, namely, pseucaplillarition which can lead to insulin resistance and hyperlipidemia. 8 In addition, aging leads to oxidative stress, decreased availability of NO, decreased hepatic blood flow, and increased inflammation in LSECs. 57 LSECs are uniquely sensitive to oxidative stress, and as a result of this stress can have reduced NO. 11 , 60 Decreased NO affects the regulation of hepatic blood flow, the prevention of stenosis, and inflammatory inhibition. 8 Decreased hepatic blood flow leads to hepatocyte hypoxia, which in turn leads to HSC activation and liver fibrosis. 11 In addition, LSEC pro‐inflammatory state along with decreased angiocrine molecules results in fibrogenesis. 6 , 11 Since phenotypic changes in the LSEC phenotype occur in the early stages of liver fibrosis, studying the molecules of LSECs in the context of the progression of liver fibrosis will prove valuable for the early detection and subsequent early intervention of liver fibrosis (Figure 4). Meanwhile, pathologic changes of LESCs also contribute to the development of liver fibrosis. Currently, most of the current research is carried out at the level of animal and in vitro experiments, which is very different from the complex, delicate and dynamic environment of the human body. Therefore, in‐depth analysis of the physiological and pathological processes of LSECs and clarification of its mechanism of action associated with aged endothelial dysfunction will surely bring breakthroughs and progress in the targeted treatment of liver fibrosis with LSECs.
FIGURE 4.
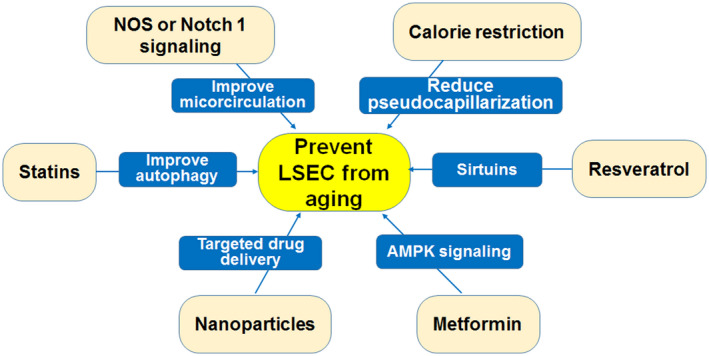
Measures to prevent LSEC aging. Nanoparticle targeted drug delivery can be used for the regulation of fenestrations in LSECs. Resveratrol and metformin act on sirtuin and AMPK nutrient‐sensitive pathways respectively to regulate the aging of LSECs. Calorie restriction can reduce the occurrence of pseudocapillarization and thus affect the aging of LSECs. Statins reduce the occurrence of LSEC aging by increasing the autophagy of LSECs
DISCLOSURES
The authors have declared that no conflict of interest exists.
AUTHOR CONTRIBUTIONS
Ying Wan, Elise Slevin, Xuedong Li, X. Charlie Dong, and Fanyin Meng contributed to conceptualization, resources, supervision, project administration, and funding acquisition. Xuedong Li, Yudian Zhang, Kelly Harrison, Chaodong Wu, Ashok K. Shetty, and James E. Klaunig contributed to writing—original draft preparation. Ying Wan, Elise Slevin, Tian Li, and Fanyin Meng contributed to writing—review and editing. Ying Wan, Xuedong Li, Elise Slevin, and Fanyin Meng prepared figures. Ying Wan, Xuedong Li, and Fanyin Meng drafted the manuscript. X. Charlie Dong and Fanyin Meng finalized the manuscript.
Wan Y, Li X, Slevin E, et al. Endothelial dysfunction in pathological processes of chronic liver disease during aging. FASEB J. 2022;36:e22125. doi: 10.1096/fj.202101426R
Ying Wan, Xuedong Li, and Elise Slevin contributed equally to the manuscript.
Funding information
The work was supported by the VA Merit Review Award to Dr. Meng (1I01BX001724) from the United States Department of Veteran's Affairs Biomedical Laboratory Research and Development Service, U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grants DK054811, DK076898 and DK107310 to Dr. Meng, NIH National Institute on Alcohol Abuse and Alcoholism Grants AA025997 and AA025157 to Dr. Meng, and a grant from Nature Science Foundation of China (No. 81873563) to YW; The project described was supported by the Indiana University Health—Indiana University School of Medicine Strategic Research Initiative. Dr. Meng acknowledges the support from PSC Partners Seeking a Cure. This material is the result of work supported by resources at Richard L. Roudebush VA Medical Center, Indianapolis, IN. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. This work was also partly supported by the National Institute of Diabetes and Digestive and Kidney Diseases awards DK120689 and DK121925 to Dr. Dong
Contributor Information
X. Charlie Dong, Email: xcdong@iu.edu.
Fanyin Meng, Email: mengf@iu.edu.
REFERENCES
- 1. Hunt NJ, McCourt PAG, Le Couteur DG, Cogger VC. Novel targets for delaying aging: the importance of the liver and advances in drug delivery. Adv Drug Deliv Rev. 2018;135:39‐49. [DOI] [PubMed] [Google Scholar]
- 2. Poisson J, Lemoinne S, Boulanger C, et al. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol. 2017;66:212‐227. [DOI] [PubMed] [Google Scholar]
- 3. Li H, You H, Fan X, Jia J. Hepatic macrophages in liver fibrosis: pathogenesis and potential therapeutic targets. BMJ Open Gastroenterol. 2016;3:e000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pandey E, Nour AS, Harris EN. Prominent receptors of liver sinusoidal endothelial cells in liver homeostasis and disease. Front Physiol. 2020;11:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smedsrod B, Melkko J, Araki N, Sano H, Horiuchi S. Advanced glycation end products are eliminated by scavenger‐receptor‐mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochem J. 1997;322(pt 2):567‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shetty S, Lalor PF, Adams DH. Liver sinusoidal endothelial cells—gatekeepers of hepatic immunity. Nat Rev Gastroenterol Hepatol. 2018;15:555‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGary CT, Raja RH, Weigel PH. Endocytosis of hyaluronic acid by rat liver endothelial cells. Evidence for receptor recycling. Biochem J. 1989;257:875‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hunt NJ, Kang SWS, Lockwood GP, Le Couteur DG, Cogger VC. Hallmarks of aging in the liver. Comput Struct Biotechnol J. 2019;17:1151‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ray K. Liver: hepatic stellate cells hold the key to liver fibrosis. Nat Rev Gastroenterol Hepatol. 2014;11:74. [DOI] [PubMed] [Google Scholar]
- 10. Kocabayoglu P, Friedman SL. Cellular basis of hepatic fibrosis and its role in inflammation and cancer. Front Biosci (Schol Ed). 2013;5:217‐230. [DOI] [PubMed] [Google Scholar]
- 11. Lafoz E, Ruart M, Anton A, Oncins A, Hernandez‐Gea V. The endothelium as a driver of liver fibrosis and regeneration. Cells. 2020;9:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jansen PL. Liver disease in the elderly. Best Pract Res Clin Gastroenterol. 2002;16:149‐158. [DOI] [PubMed] [Google Scholar]
- 13. Popper H. Aging and the liver. Prog Liver Dis. 1986;8:659‐683. [PubMed] [Google Scholar]
- 14. Cogger VC, Warren A, Fraser R, Ngu M, McLean AJ, Le Couteur DG. Hepatic sinusoidal pseudocapillarization with aging in the non‐human primate. Exp Gerontol. 2003;38:1101‐1107. [DOI] [PubMed] [Google Scholar]
- 15. Ito Y, Sorensen KK, Bethea NW, et al. Age‐related changes in the hepatic microcirculation in mice. Exp Gerontol. 2007;42:789‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Couteur DG, Cogger VC, Markus AM, et al. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology. 2001;33:537‐543. [DOI] [PubMed] [Google Scholar]
- 17. McLean AJ, Cogger VC, Chong GC, et al. Age‐related pseudocapillarization of the human liver. J Pathol. 2003;200:112‐117. [DOI] [PubMed] [Google Scholar]
- 18. Le Couteur DG, Warren A, Cogger VC, et al. Old age and the hepatic sinusoid. Anat Rec (Hoboken). 2008;291:672‐683. [DOI] [PubMed] [Google Scholar]
- 19. Le Couteur DG, Fraser R, Cogger VC, McLean AJ. Hepatic pseudocapillarisation and atherosclerosis in ageing. Lancet. 2002;359:1612‐1615. [DOI] [PubMed] [Google Scholar]
- 20. Le couteur DG, Cogger VC, Mccuskey RS, et al. Age‐related changes in the liver sinusoidal endothelium: a mechanism for dyslipidemia. Ann N Y Acad Sci. 2007;1114:79‐87. [DOI] [PubMed] [Google Scholar]
- 21. McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56:163‐184. [DOI] [PubMed] [Google Scholar]
- 22. Ganesan LP, Kim J, Wu Y, et al. FcgammaRIIb on liver sinusoidal endothelium clears small immune complexes. J Immunol. 2012;189:4981‐4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147‐163. [DOI] [PubMed] [Google Scholar]
- 24. Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol. 2001;161:III‐XIII, 1‐151. [DOI] [PubMed] [Google Scholar]
- 25. Limmer A, Ohl J, Kurts C, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen‐specific T‐cell tolerance. Nat Med. 2000;6:1348‐1354. [DOI] [PubMed] [Google Scholar]
- 26. Crispe IN. Liver antigen‐presenting cells. J Hepatol. 2011;54:357‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomson AW, Knolle PA. Antigen‐presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753‐766. [DOI] [PubMed] [Google Scholar]
- 28. Knolle PA, Schmitt E, Jin S, et al. Induction of cytokine production in naive CD4(+) T cells by antigen‐presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 1999;116:1428‐1440. [DOI] [PubMed] [Google Scholar]
- 29. Carambia A, Freund B, Schwinge D, et al. TGF‐beta‐dependent induction of CD4(+)CD25(+)Foxp3(+) Tregs by liver sinusoidal endothelial cells. J Hepatol. 2014;61:594‐599. [DOI] [PubMed] [Google Scholar]
- 30. Carambia A, Freund B, Schwinge D, et al. Nanoparticle‐based autoantigen delivery to Treg‐inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice. J Hepatol. 2015;62:1349‐1356. [DOI] [PubMed] [Google Scholar]
- 31. Neumann K, Rudolph C, Neumann C, Janke M, Amsen D, Scheffold A. Liver sinusoidal endothelial cells induce immunosuppressive IL‐10‐producing Th1 cells via the Notch pathway. Eur J Immunol. 2015;45:2008‐2016. [DOI] [PubMed] [Google Scholar]
- 32. Berg M, Wingender G, Djandji D, et al. Cross‐presentation of antigens from apoptotic tumor cells by liver sinusoidal endothelial cells leads to tumor‐specific CD8+ T cell tolerance. Eur J Immunol. 2006;36:2960‐2970. [DOI] [PubMed] [Google Scholar]
- 33. Scholzel K, Schildberg FA, Welz M, et al. Transfer of MHC‐class‐I molecules among liver sinusoidal cells facilitates hepatic immune surveillance. J Hepatol. 2014;61:600‐608. [DOI] [PubMed] [Google Scholar]
- 34. Steinhoff G, Behrend M, Schrader B, Duijvestijn AM, Wonigeit K. Expression patterns of leukocyte adhesion ligand molecules on human liver endothelia. Lack of ELAM‐1 and CD62 inducibility on sinusoidal endothelia and distinct distribution of VCAM‐1, ICAM‐1, ICAM‐2, and LFA‐3. Am J Pathol. 1993;142:481‐488. [PMC free article] [PubMed] [Google Scholar]
- 35. Warren A, Bertolino P, Benseler V, Fraser R, McCaughan GW, Le Couteur DG. Marked changes of the hepatic sinusoid in a transgenic mouse model of acute immune‐mediated hepatitis. J Hepatol. 2007;46:239‐246. [DOI] [PubMed] [Google Scholar]
- 36. Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54‐S62. [DOI] [PubMed] [Google Scholar]
- 37. Schurich A, Berg M, Stabenow D, et al. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J Immunol. 2010;184:4107‐4114. [DOI] [PubMed] [Google Scholar]
- 38. Breiner KM, Schaller H, Knolle PA. Endothelial cell‐mediated uptake of a hepatitis B virus: a new concept of liver targeting of hepatotropic microorganisms. Hepatology. 2001;34:803‐808. [DOI] [PubMed] [Google Scholar]
- 39. Limmer A, Sacher T, Alferink J, et al. Failure to induce organ‐specific autoimmunity by breaking of tolerance: importance of the microenvironment. Eur J Immunol. 1998;28:2395‐2406. [DOI] [PubMed] [Google Scholar]
- 40. Guidotti LG, Borrow P, Brown A, McClary H, Koch R, Chisari FV. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J Exp Med. 1999;189:1555‐1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamada Y, Sato M, Kida S, et al. N‐Acetylglucosaminyltransferase V exacerbates concanavalin A‐induced hepatitis in mice. Mol Med Rep. 2015;11:3573‐3584. [DOI] [PubMed] [Google Scholar]
- 42. Ni Y, Li JM, Liu MK, et al. Pathological process of liver sinusoidal endothelial cells in liver diseases. World J Gastroenterol. 2017;23:7666‐7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ding BS, Nolan DJ, Butler JM, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310‐U240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu J, Srivastava K, Wieland M, et al. Endothelial cell‐derived angiopoietin‐2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343:416‐419. [DOI] [PubMed] [Google Scholar]
- 45. Ding BS, Cao Z, Lis R, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rautou PE, Bresson J, Sainte‐Marie Y, et al. Abnormal plasma microparticles impair vasoconstrictor responses in patients with cirrhosis. Gastroenterology. 2012;143:166‐176 e166. [DOI] [PubMed] [Google Scholar]
- 47. Deleve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48:920‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xie G, Wang X, Wang L, et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012;142:918‐927 e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shan Z, Ju C. Hepatic macrophages in liver injury. Front Immunol. 2020;11:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ford AJ, Jain G, Rajagopalan P. Designing a fibrotic microenvironment to investigate changes in human liver sinusoidal endothelial cell function. Acta Biomater. 2015;24:220‐227. [DOI] [PubMed] [Google Scholar]
- 51. Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037‐2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol. 2003;38(suppl 1):S38‐S53. [DOI] [PubMed] [Google Scholar]
- 53. Sheedfar F, Di Biase S, Koonen D, Vinciguerra M. Liver diseases and aging: friends or foes? Aging Cell. 2013;12:950‐954. [DOI] [PubMed] [Google Scholar]
- 54. Maslak E, Gregorius A, Chlopicki S. Liver sinusoidal endothelial cells (LSECs) function and NAFLD; NO‐based therapy targeted to the liver. Pharmacol Rep. 2015;67:689‐694. [DOI] [PubMed] [Google Scholar]
- 55. Pasarin M, La Mura V, Gracia‐Sancho J, et al. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS One. 2012;7:e32785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miyao M, Kotani H, Ishida T, et al. Pivotal role of liver sinusoidal endothelial cells in NAFLD/NASH progression. Lab Invest. 2015;95:1130‐1144. [DOI] [PubMed] [Google Scholar]
- 57. Maeso‐Diaz R, Ortega‐Ribera M, Fernandez‐Iglesias A, et al. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell. 2018;17:e12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Licastro F, Candore G, Lio D, et al. Innate immunity and inflammation in ageing: a key for understanding age‐related diseases. Immun Ageing. 2005;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miyachi Y, Tsuchiya K, Komiya C, et al. Roles for cell‐cell adhesion and contact in obesity‐induced hepatic myeloid cell accumulation and glucose intolerance. Cell Rep. 2017;18:2766‐2779. [DOI] [PubMed] [Google Scholar]
- 60. Ruart M, Chavarria L, Camprecios G, et al. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J Hepatol. 2019;70:458‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Iwakiri Y, Kim MY. Nitric oxide in liver diseases. Trends Pharmacol Sci. 2015;36:524‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carnovale CE, Ronco MT. Role of nitric oxide in liver regeneration. Ann Hepatol. 2012;11:636‐647. [PubMed] [Google Scholar]
- 63. Abdelmegeed MA, Song BJ. Functional roles of protein nitration in acute and chronic liver diseases. Oxid Med Cell Longev. 2014;2014:149627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Leifeld L, Fielenbach M, Dumoulin FL, Speidel N, Sauerbruch T, Spengler U. Inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) expression in fulminant hepatic failure. J Hepatol. 2002;37:613‐619. [DOI] [PubMed] [Google Scholar]
- 65. La Mura V, Pasarin M, Rodriguez‐Vilarrupla A, Garcia‐Pagan JC, Bosch J, Abraldes JG. Liver sinusoidal endothelial dysfunction after LPS administration: a role for inducible‐nitric oxide synthase. J Hepatol. 2014;61:1321‐1327. [DOI] [PubMed] [Google Scholar]
- 66. Ahsan F, Oliveri F, Goud HK, et al. Pleiotropic effects of statins in the light of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis. Cureus. 2020;12:e10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Natarajan V, Harris EN, Kidambi S. SECs (sinusoidal endothelial cells), liver microenvironment, and fibrosis. Biomed Res Int. 2017;2017:4097205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2015;61:1740‐1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fraser R, Dobbs BR, Rogers GW. Lipoproteins and the liver sieve: the role of the fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology. 1995;21:863‐874. [PubMed] [Google Scholar]
- 70. McGuire RF, Bissell DM, Boyles J, Roll FJ. Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology. 1992;15:989‐997. [DOI] [PubMed] [Google Scholar]
- 71. Babbs C, Haboubi NY, Mellor JM, Smith A, Rowan BP, Warnes TW. Endothelial cell transformation in primary biliary cirrhosis: a morphological and biochemical study. Hepatology. 1990;11:723‐729. [DOI] [PubMed] [Google Scholar]
- 72. Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kitano M, Bloomston PM. Hepatic stellate cells and microRNAs in pathogenesis of liver fibrosis. J Clin Med. 2016;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Connolly MK, Bedrosian AS, Malhotra A, et al. In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity. J Immunol. 2010;185:2200‐2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McMahan RH, Porsche CE, Edwards MG, Rosen HR. Free fatty acids differentially downregulate chemokines in liver sinusoidal endothelial cells: insights into non‐alcoholic fatty liver disease. PLoS One. 2016;11:e0159217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Duan JL, Ruan B, Yan XC, et al. Endothelial Notch activation reshapes the angiocrine of sinusoidal endothelia to aggravate liver fibrosis and blunt regeneration in mice. Hepatology. 2018;68:677‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Glinska‐Suchocka K, Orlowska A, Spuzak J, Jankowski M, Kubiak K. Suitability of using serum hialuronic acid concentrations in the diagnosis of canine liver fibrosis. Pol J Vet Sci. 2015;18:873‐878. [DOI] [PubMed] [Google Scholar]
- 79. Rocha AS, Vidal V, Mertz M, et al. The angiocrine factor Rspondin3 is a key determinant of liver zonation. Cell Rep. 2015;13:1757‐1764. [DOI] [PubMed] [Google Scholar]
- 80. Sakata K, Eda S, Lee ES, Hara M, Imoto M, Kojima S. Neovessel formation promotes liver fibrosis via providing latent transforming growth factor‐beta. Biochem Biophys Res Commun. 2014;443:950‐956. [DOI] [PubMed] [Google Scholar]
- 81. Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30:3–22. [DOI] [PubMed] [Google Scholar]
- 82. Wang R, Ding Q, Yaqoob U, et al. Exosome adherence and internalization by hepatic stellate cells triggers sphingosine 1‐phosphate‐dependent migration. J Biol Chem. 2015;290:30684‐30696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sorensen KK, Simon‐Santamaria J, McCuskey RS, Smedsrod B. Liver sinusoidal endothelial cells. Compr Physiol. 2015;5:1751‐1774. [DOI] [PubMed] [Google Scholar]
- 84. Knolle PA, Wohlleber D. Immunological functions of liver sinusoidal endothelial cells. Cell Mol Immunol. 2016;13:347‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Marrone G, Shah VH, Gracia‐Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol. 2016;65:608‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990;86:1641‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Neubauer K, Kruger M, Quondamatteo F, Knittel T, Saile B, Ramadori G. Transforming growth factor‐beta1 stimulates the synthesis of basement membrane proteins laminin, collagen type IV and entactin in rat liver sinusoidal endothelial cells. J Hepatol. 1999;31:692‐702. [DOI] [PubMed] [Google Scholar]
- 88. Rieder H, Ramadori G, Dienes HP, Meyer zum Buschenfelde KH. Sinusoidal endothelial cells from guinea pig liver synthesize and secrete cellular fibronectin in vitro. Hepatology. 1987;7:856‐864. [DOI] [PubMed] [Google Scholar]
- 89. Sellaro TL, Ravindra AK, Stolz DB, Badylak SF. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ‐specific extracellular matrix scaffolds. Tissue Eng. 2007;13:2301‐2310. [DOI] [PubMed] [Google Scholar]
- 90. Mak KM, Sehgal P, Harris CK. Factor VIII‐related antigen detects phenotypic change of sinusoidal to vascular endothelium in hepatic fibrosis of elderly cadavers. Int Sch Res Notices. 2014;2014:839560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cheluvappa R, Hilmer SN, Kwun SY, et al. The effect of old age on liver oxygenation and the hepatic expression of VEGF and VEGFR2. Exp Gerontol. 2007;42:1012‐1019. [DOI] [PubMed] [Google Scholar]
- 92. Rafii S, Butler JM, Ding BS. Angiocrine functions of organ‐specific endothelial cells. Nature. 2016;529:316‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weiskirchen R, Tacke F. Relevance of autophagy in parenchymal and non‐parenchymal liver cells for health and disease. Cells. 2019;8:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest. 2013;123:1861‐1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu L, Yannam GR, Nishikawa T, et al. The microenvironment in hepatocyte regeneration and function in rats with advanced cirrhosis. Hepatology. 2012;55:1529‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G757‐G763. [DOI] [PubMed] [Google Scholar]
- 97. Yang L, Kwon J, Popov Y, et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146:1339‐1350 e1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. May D, Djonov V, Zamir G, et al. A transgenic model for conditional induction and rescue of portal hypertension reveals a role of VEGF‐mediated regulation of sinusoidal fenestrations. PLoS One. 2011;6:e21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dill MT, Rothweiler S, Djonov V, et al. Disruption of Notch1 induces vascular remodeling, intussusceptive angiogenesis, and angiosarcomas in livers of mice. Gastroenterology. 2012;142:967‐977 e962. [DOI] [PubMed] [Google Scholar]
- 100. Hunt NJ, Lockwood GP, Kang SW, et al. The effects of metformin on age‐related changes in the liver sinusoidal endothelial cell. J Gerontol a‐Biol. 2020;75:278‐285. [DOI] [PubMed] [Google Scholar]
- 101. Jamieson HA, Hilmer SN, Cogger VC, et al. Caloric restriction reduces age‐related pseudocapillarization of the hepatic sinusoid. Exp Gerontol. 2007;42:374‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Alidori S, Bowman RL, Yarilin D, et al. Deconvoluting hepatic processing of carbon nanotubes. Nat Commun. 2016;7:12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sato Y, Hatakeyama H, Hyodo M, Harashima H. Relationship between the physicochemical properties of lipid nanoparticles and the quality of siRNA delivery to liver cells. Mol Ther. 2016;24:788‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bartneck M, Warzecha KT, Tacke F. Therapeutic targeting of liver inflammation and fibrosis by nanomedicine. Hepatobiliary Surg Nutr. 2014;3:364‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kang JH, Toita R, Murata M. Liver cell‐targeted delivery of therapeutic molecules. Crit Rev Biotechnol. 2016;36:132‐143. [DOI] [PubMed] [Google Scholar]


