Abstract
The prevalence of unconventional oil and gas (UOG) operations raises concerns regarding the potential for adverse health outcomes following exposure to water tainted by mixtures of UOG associated chemicals. The potential effects that exposure to complex chemical mixtures has on the immune system has yet to be fully evaluated. In this study, effects on the immune system of adult mice exposed to a mixture of 23 chemicals that have been associated with water near active UOG operations were investigated. Female and male mice were exposed to the mixture via their drinking water for at least 8 weeks. At the end of the exposure, cellularity of primary and secondary immune organs, as well as immune system function, were assessed using three different models of disease, i.e., house dust mite (HDM)-induced allergic airway disease, influenza A virus infection, and experimental autoimmune encephalomyelitis (EAE). The results indicated exposures resulted in different impacts on T-cell populations in each disease model. Furthermore, the consequences of exposure differed between female and male mice. Notably, exposure to the chemical mixture significantly increased EAE disease severity in female, but not in male, mice. These findings indicated that direct exposure to this mixture leads to multiple alterations in T-cell subsets and that these alterations differ between sexes. This suggested to us that direct exposure to UOG-associated chemicals may alter the adult immune system, leading to dysregulation in immune cellularity and function.
Keywords: Water pollutants, immunotoxicity, hydraulic fracturing, influenza, autoimmune, allergy
Introduction
The utilization of unconventional oil and gas (UOG) extraction methods has made available oil and gas reserves that were previously unreachable. These methods involve the injection of undisclosed mixtures of chemicals underground at high pressures in order to break up layers of coal or shale to release trapped gas and oil reserves Vengosh et al. (2014). Multiple studies have found that wastewater from UOG operations, as well as surface and groundwater in areas where UOG drilling occurs, contains measurable amounts of a number of the chemicals associated with this process (Vengosh et al. 2014; Webb et al. 2014; Elsner and Hoelzer 2016; Maloney et al. 2017; United States Environmental Protection Agency 2021). Information about potential health effects from exposure to water contaminated with mixtures of chemicals remains limited, yet at least 25 separate reports have found associations between enhanced use of this technology and adverse impacts on health (Deziel et al. 2020). For example, several studies have reported negative health outcomes associated with proximity to UOG operations, including preterm birth, low birth weight, neurological symptoms and increased hospitalizations (McKenzie et al. 2014; McKenzie et al. 2017; Jemielita et al. 2015; Tustin et al. 2017; Elliott et al. 2018). Proximity to UOG operations has also been associated with increased reports of upper respiratory symptoms and asthma exacerbations (Rabinowitz et al. 2015; Rasmussen et al. 2016; Elliott et al. 2018).
In addition to epidemiological studies, some of the chemicals that have been detected in water sources near active UOG production sites have been used in experimental studies to further examine pathophysiological consequences of exposure (Nagel et al. 2020). In particular, 23 chemicals that were identified in water samples exhibited antagonism or agonism of the estrogen, androgen, progesterone, glucocorticoid, and thyroid receptors (Kassotis et al. 2014, 2015). Developmental exposure of mice to this 23-chemical mixture negatively-impacted development of male and female reproductive systems and modulated reproductive parameters in the offspring (Kassotis et al. 2015, 2016; Boule et al. 2018). Developmental exposure also altered the immune system of the mice, inducing changes to the proportions of T-cell sub-populations in the lymph nodes, thymus, and spleen, and exacerbating immune-mediated disease severity in female, but not male, offspring (Boule et al. 2018). Early-life exposure to this mixture of 23 compounds also had long-lasting effects on immune homeostasis and anti-viral defenses in frogs (Robert et al. 2018, 2019). While these prior studies indicate that exposure to this complex mixture during development has durable effects on offspring, little is known about the effects of direct exposure to UOG-associated chemicals on the function of a fully-formed mature immune system.
The immune system is necessary to maintain defenses against pathogens, and also self-regulates to protect against immune-mediated damage to healthy tissues. Imbalances in the function of the immune system can lead to poorer responses to infectious agents or an increase in incidence\severity of allergic reactions and autoimmune diseases. To investigate the effect of direct exposure to UOG-associated chemicals on the adult immune system, the study reported here utilized the same chemical mixture previously-used in reproductive and developmental exposure studies (Kassotis et al. 2014, 2016; Boule et al. 2018). Specifically, the current study examined the effect of exposures to this mixture on immune cells in primary and secondary immune organs of immunologically-naïve mice. Given that developmental exposure affected T-cells, whether direct exposure of adult mice to this mixture resulted in altered T-cell populations was also investigated in the context of three different T-cell-dependent disease model systems: viral infection (influenza A virus, or IAV), autoimmune disease (experimental autoimmune encephalomyelitis, or EAE), and allergic airway disease, induced using extracts of house dust mites (HDM).
Materials and Methods:
Chemical mixture
A mixture of all 23 chemicals was prepared by first preparing a 10,000X master stock (1 mg/ml) of each chemical in 100% ethanol (Table 1). Each week, 100 μl of each chemical from these stocks was combined in 1 L purified water, and immediately portioned out into individual water bottles. All chemicals were purchased from Sigma-Aldrich (St. Louis, Missouri).
Table 1.
The 23 chemicals present in equimass proportions in the drinking water.
| Chemical | CAS No | Catalog number |
|---|---|---|
| 1,2,4-trimethylbenzene | 95-63-6 | 47324 |
| 2-ethylhexanol | 104-76-7 | 04050-250ML |
| Acrylamide | 79-06-1 | A9099-25G |
| Benzene | 71-43-2 | 270709-100ML |
| Bronopol | 52-51-7 | 32053-250MG |
| Cumene | 98-82-8 | 36698-1G |
| Diethanolamine | 111-42-2 | 31589-500G |
| Diethylene glycol methyl ether | 111-77-3 | 579548-1L |
| Dimethylformamide | 68-12-2 | 227056-100ML |
| Ethoxylated nonylphenol | 9016-45-9 | 74385-1L |
| Ethoxylated octylphenol | 9002-93-1 | T8787-100ML |
| Ethylbenzene | 100-41-4 | 296848-100ML |
| Ethylene glycol | 107-21-1 | 324558-100ML |
| Ethylene glycol butyl ether | 111-76-2 | 256366-1L |
| Methyl-isothiazolin | 2682-20-4 | 73569-1G |
| Naphthalene | 91-20-3 | PHR1275-1G |
| Phenol | 108-95-2 | PHR1047-1G |
| Propylene glycol | 57-55-6 | P4347-500ML |
| Sodium tetraborate decahydrate | 1303-96-4 | S9640-25G |
| Styrene | 100-42-5 | 45993-250MG |
| Toluene | 108-88-3 | 244511-100ML |
| Triethylene glycol | 112-27-6 | 95126-100ML |
| Xylenes | 1330-20-7 | 214736-1L |
All chemicals are at final concentration of 0.1 μ/ml. All chemicals purchased from Sigma
Mice
Adult (6–8 wk-of-age) male and female C57Bl/6 mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were housed in pre-washed polysulfone microisolator cages under specific pathogen-free conditions in a facility maintained at 22°C [±2°C] with a 30–50% relative humidity and a 12-hr light/dark cycle. Mice received a standard chow diet (LabDiet 5010, Purina, St. Louis, MO). Water was provided using glass water bottles; all water was purified using reverse osmosis. For the study, mice of each sex were randomly divided into one of two treatment groups, i.e., control or mixture. Specifically, for mixture-exposed mice, the drinking water was spiked with an equimass mixture such that the final concentration for each constituent chemical was 0.1 μg/ml. For mice receiving control water, the water contained a final concentration of 0.2% ethanol. This concentration of chemicals in the drinking water was chosen as it mirrors levels detected in ground and surface water proximal to UOG production area (Gross et al. 2013; DiGiulio and Jackson 2016; Cozzarelli et al. 2017; Orem et al. 2017). Female and male mice remained on water containing the mixture or the vehicle control for 8–10 wk prior to immunological assessment. This range in treatment lengths was employed because it was not possible to initiate all three disease models simultaneously. Water consumption was measured two times/wk for the duration of the exposures. There were no differences in volume of water consumed across sexes and treatment groups (Figure S7). Water and water bottles were changed weekly with freshly-prepared dilutions. The female mice used in these experiments were from a group of previously-pregnant dams that had been exposed to this chemical mixture or vehicle during pregnancy (Boule et al. 2018). To maintain consistency, male mice were exposed to the same mixture for the same length of time as the females. For all experiments, administration of antigens and collection of organs and cells was initiated in the morning.
All animal treatments were conducted with prior approval of Institutional Animal Care and Use Committee and Institutional Biosafety Committee of the University of Rochester. The University has accreditation through the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Mice were treated humanely and with due consideration to alleviation of any distress and discomfort. All guidelines from the U.S. Public Health Service Policy on Human Care and Use of Laboratory Animals were followed in handling of vertebrate animals.
Influenza A virus (IAV) infection
Following 8 weeks of exposure to the chemical mixture or water containing the vehicle control, mice were anesthetized by intraperitoneal (IP) injection of avertin (2% 2,2,2-tribromoethanol; Sigma). Anesthetized mice were infected intranasally (IN) with 120 hemagglutinating units (HAU) of IAV (HKx31; H3N2). The virus stock was initially obtained from M. Coppola (Argonex, Charlottesville, VA), propagated in embryonated specific-pathogen free chicken eggs (Charles River, Wilmington. MA), and titered using a hemagglutination assay (Barrett and Ingles 1985). For infection, IAV was diluted in sterile endotoxin-tested phosphate-buffered saline (PBS, pH 7.4). Mice were maintained on drinking water containing the chemical mixture or vehicle control following infection. Morbidity and mortality were monitored daily, starting on the day of infection. Using flow cytometry, T-cells in the mediastinal lymph nodes (MLN) were examined on the peak day of host response to IAV infection, which is Day 8 in male and Day 9 in female mice (Boule et al. 2014; Lawrence et al. 2000, 2006). All work with infectious agents was conducted with prior approval of the Institutional Biosafety Committee of the University of Rochester, following guidelines of the NIH/CDC.
Experimental autoimmune encephalomyelitis (EAE) induction
After 8 weeks on water containing the mixture or control, mice were immunized by subcutaneous injection of an emulsion of myelin oligodendrocyte glycoprotein (MOG35–55; 200 μg/mouse; AnaSpec, Freemont, CA) and complete Freund’s adjuvant (4 mg/ml; M. Tuberculosis; Becton Dickinson, Franklin Lakes, NJ) (Stromnes and Goverman 2006). This is considered Day 0 for triggering EAE. Two IP doses of pertussis toxin (400 ng/mouse; List Biologicals, Campbell, CA), were also administered; one contemporaneously with MOG peptide and the other 2 days later. Mice were maintained on drinking water containing the chemical mixture or vehicle control following immunization and for the duration of the disease. After administration of MOG peptide, disease progression was monitored and scored every other day for 42 days, using the following scoring system: 0 = normal mouse, 1 = limp tail, 2 = limp tail and hind limb weakness, 3 = partial hind limb paralysis, 4 = complete hind limp paralysis, 5 = moribund (Robinson et al. 2014). The percentage and number of T-cell subsets in the cervical lymph nodes were determined on Day 42 after immunization.
House dust mite (HDM)-induced allergic airway disease
HDM (Dermatophagoides pteronyssinus) extract (lot #262538, Greer Laboratories, Lenoir, NC) was diluted in sterile endotoxin-tested PBS. After 10 weeks of exposure to the chemical mixture or vehicle control, mice were sensitized and challenged for 10 days by daily oropharyngeal administration of 3 μg HDM (in volume of 30 μl) which induces CD4+ T-cell-dependent allergic airway disease (Knowlden et al. 2016). Mice continued to receive water containing either the chemical mixture or vehicle control throughout HDM treatments. Forty-eight hours after the final HDM dosing, the mice were euthanized by a lethal overdose of anesthetic (0.1 ml Euthasol; Virbac, Greeley, CO). Bronchoalveolar lavages (BAL) was performed by instilling 0.75 ml PBS twice into the lungs via a Teflon cannula to permit examination of leukocytes in the airways by differential cell counting. Differential counts of the BAL cells were performed after cytocentrifugation onto coded slides, and staining with Hema3 Staining Set (Fisher Scientific, Waltham, MA). The lung-draining mediastinal lymph nodes (MLN) were removed for examination of T-cells using flow cytometry. BAL and MLN cells were collected from the same mice (BAL cells obtained first).
Tissue collection and cell processing
For the termination of studies using immunologically-naïve mice, and from mice used in the HDM, IAV, or EAE models, animals were sacrificed with a lethal dose of anesthetic (Avertin > 0.5 ml or 0.1 ml Euthasol), followed by a secondary method (e.g., cervical dislocation or exsanguination). Tissue collection was started between 8:30 and 9:30 AM. The mediastinal, cervical, inguinal, axillary, and brachial lymph nodes, as well as the thymus, spleen, lung airways, and/or bone marrow were collected at necropsy and processed into single cell suspensions (Vorderstrasse et al. 2006; Bauer et al, 2012, Reilly et al. 2015). Cells from individual mice were re-suspended in cold Hank Balanced Salt Solution (HBSS) containing 2.5% FBS (HBSS/FBS; Hyclone, Logan, UT). To remove red blood cells, pelleted cells were incubated in a solution containing 0.15 M NH4Cl, 10 mM NaHCO3, and 1 mM EDTA for 5 min at room temperature. Erythrocyte lysis was terminated by adding a 10-fold volume of cold HBSS/FBS. After centrifugation (200 × g; 10 min), cells were re-suspended in cold HBSS/FBS for enumeration using a TC10 automated cell counter (BioRad, Hercules, CA), or a hemocytometer and Trypan blue exclusion. Cells were used immediately for analysis by flow cytometry or centrifuged onto microscope slides.
Analytical flow cytometry
Before incubation with fluorochrome-conjugated reagents, single cell suspensions containing 2 × 106 cells were incubated with anti-mouse CD16/32 mAb (clone 93; eBioscience, San Diego, CA) for 15 min at 4°C to prevent non-specific binding. Cells were then incubated for 20 min at 4°C with fluorochrome-conjugated antibodies directed against cell surface antigens. To identify virus-specific CD8+ T-cells in IAV-infected mice, allophycocyanin (APC)-labeled Major Histocompatibility (MHC) Class I tetramers containing an immunodomi-nant peptide epitope of the viral nucleoprotein (DbNP366–375) were used (Lawrence et al. 2006; Boule et al. 2018). After cell surface labeling, cells were fixed using 2% formaldehyde in PBS for 20 min, and analyzed directly by flow cytometry, or permeabilized using a FoxP3 Staining Kit (eBioscience, San Diego, CA). Specifically, intracellular staining was used to identify CD4+ T-cell subsets in immune-challenged mice, using fluorochrome-conjugated antibodies against transcription factors unique to TH1, TH2, TH17, or Treg cells (Boule et al. 2018). Table S1 lists all antibodies used. Antibodies were purchased from eBioscience (San Diego, CA), Biolegend (San Diego, CA), or BD Biosciences (San Jose, CA). All staining procedures were performed as previously described (Lawrence et al. 2006; Bauer et al. 2012; Boule et al. 2018). Fluorescence minus one (FMO) controls were used to determine non-specific fluorescence and define gating parameters. Cellular viability was assessed using a fluorescent viability dye (fixable viability dye eFlour506, Invitrogen/e-Bioscience). Data were obtained using an LSRII flow cytometer (BD Biosciences, San Jose, CA), and analyzed using FlowJo software (TreeStar, Ashland, OR). For all samples, a minimum of 500,000 events/sample was acquired.
Statistical analysis
All data were analyzed using JMP software (SAS, Cary, NC). Differences between exposure groups and sex were evaluated using a two-way analysis of variance (ANOVA). Analyses included comparisons within sex between the two exposure groups, and across sex and exposure groups, using Tukey post-hoc tests. Comparisons at a single point in time and within sex were analyzed using a Student’s t-test. Onset of symptoms in mice with EAE was analyzed using a Kaplan-Meier curve, and comparisons between treatment groups were performed using a Wilcoxon test. Differences were considered statistically significant when p-values were < 0.05. Error bars on all graphs represent the standard error of the mean (SEM).
Results
Effect of exposure to 23-chemical mixture on the immune system at homeostasis
To determine whether this mixture of 23 chemicals affects immune cell populations, the cellularity of primary and secondary lymphoid organs in female and male mice exposed to the mixture and control water were compared. In female mice, there were no statistically-significant differences in the percentage (Table 2) or number (Table 3) of hematopoietic stem and progenitor populations, such as HSC, MPP, LSK, or CLP cells, in bone marrow cells from mixture-exposed females, compared to females in the control group (Tables 2 and 3). There were also no significant differences in the total number of cells recovered from bone marrow or spleen in female mice exposed to the mixture compared to control water (Table 3). Yet, there were several significant differences in percentage and number of distinct immune cell populations from the thymus, spleen, and PLN (Tables 2 and 3). For example, in the thymus, the number of double-negative, double-positive, and single-positive thymocytes was ≈ 2-fold higher in mixture-exposed female mice compared to in control female mice (Table 3). Similarly, there were 1.8-times more peripheral lymph node (PLN) cells in these hosts as compared to in female mice that received water containing the vehicle (Table 3). In the spleen, the percentage of CD19+ cells was about 10% lower in the mixture group (Table 2). However, the majority of cell types examined were not significantly different between the two treatment groups of female mice.
Table 2.
Primary and secondary immune organ cell populations from immunologically-naïve female and male mice.
| Cell type | Female Control | Female 0.1 μg/ml | p-valuea | Male Control | Male 0.1 μg/ml | p-valuea | Female vs. Male p-valueb |
|---|---|---|---|---|---|---|---|
| Bone Marrow | |||||||
| HSC | 0.008 ± 0.001 | 0.008 ± 0.001 | 0.99 | 0.007 ± 0.001 | 0.01 ± 0.001 | 0.07 | 0.34 |
| LSK | 0.95 ± 0.001 | 0.10 ± 0.01 | 0.65 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.25 | 0.28 |
| LK | 1.62 ± 0.09 | 1.76 ± 0.06 | 0.13 | 2.03 ± 0.06 | 1.99 ± 0.02 | 0.70 | 0.09 |
| MPP1 | 0.009 ± 0.0004 | 0.01 ± 0.002 | 0.83 | 0.006 ± 0.002 | 0.008 ± 0.001 | 0.46 | 0.57 |
| MPP2 | 0.0027 ± 0.0003 | 0.003 ± 0.0001 | 0.09 | 0.0016 ± 0.0003 | 0.002 ± 0.0003 | 0.25 | 0.01 |
| MPP3 | 0.013 ± 0.001 | 0.016 ± 0.003 | 0.42 | 0.014 ± 0.002 | 0.015 ± 0.002 | 0.71 | 0.99 |
| MPP4 | 0.026 ± 0.002 | 0.029 ± 0.004 | 0.54 | 0.025 ± 0.002 | 0.033 ± 0.003 | 0.09 | 0.79 |
| Lineage - | 11.68 ± 0.48 | 11.74 ± 0.46 | 0.93 | 11.18 ± 0.31 | 11.25 ± 0.44 | 0.91 | 0.85 |
| CLP | 0.01 ± 0.001 | 0.01 ± 0.004 | 0.24 | 0.02 ± 0.002 | 0.01 ± 0.001 | 0.67 | 0.99 |
| GMP | 0.42 ± 0.03 | 0.45 ± 0.01 | 0.38 | 0.61 ± 0.04 | 0.67 ± 0.01 | 0.07 | < 0.0001 |
| pre-GM | 0.35 ± 0.01 | 0.37 ± 0.02 | 0.35 | 0.57 ± 0.02 | 0.48 ± 0.02 | 0.01 | 0.004 |
| pre-MegE | 0.122 ± 0.006 | 0.128 ± 0.007 | 0.41 | 0.13 ± 0.002 | 0.15 ± 0.01 | 0.04 | 0.13 |
| Thymus | |||||||
| DN1 | 0.58 ± 0.02 | 0.56 ± 0.03 | 0.61 | 0.91 ± 0.12 | 1.37 ± 0.32 | 0.24 | 0.02 |
| DN2 | 0.16 ± 0.01 | 0.15 ± 0.02 | 0.45 | 0.17 ± 0.03 | 0.17 ± 0.01 | 0.79 | 0.62 |
| DN3 | 0.72 ± 0.03 | 0.63 ± 0.06 | 0.22 | 0.91 ± 0.08 | 0.97 ± 0.05 | 0.54 | 0.002 |
| DN4 | 7.88 ± 0.30 | 6.89 ± 0.37 | 0.07 | 6.24 ± 0.54 | 6.21 ± 0.41 | 0.96 | 0.23 |
| DP | 53.37 ± 1.45 | 59.05 ± 1.48 | 0.02 | 49.92 ± 2.01 | 51.10 ± 3.52 | 0.79 | 0.09 |
| DP CD3+ | 0.71 ± 0.07 | 0.74 ± 0.09 | 0.81 | 0.72 ± 0.14 | 1.09 ± 0.35 | 0.39 | 0.60 |
| TCRβ+ | 7.97 ± 0.29 | 8.94 ± 0.24 | 0.24 | 8.11 ± 1.07 | 9.83 ± 0.58 | 0.06 | 0.69 |
| CD4+ | 4.44 ± 0.29 | 4.30 ± 0.09 | 0.84 | 4.51 ± 0.54 | 6.66 ± 0.78 | 0.008 | 0.01 |
| CD8+ | 1.65 ± 0.05 | 1.86 ± 0.11 | 0.32 | 2.67 ± 0.26 | 2.91 ± 0.13 | 0.28 | 0.0003 |
| TCRγδ+ | 0.295 ± 0.01 | 0.297 ± 0.01 | 0.93 | 0.37 ± 0.02 | 0.33 ± 0.02 | 0.04 | 0.39 |
| Treg | 0.10 ± 0.004 | 0.11 ± 0.01 | 0.21 | 0.09 ± 0.01 | 0.15 ± 0.02 | 0.01 | 0.08 |
| Spleen | |||||||
| CD19+ | 31.27 ± 0.69 | 28.32 ± 1.10 | 0.04 | 53.66 ± 0.82 | 49.18 ± 1.25 | 0.02* | < 0.0001 |
| CD4+ | 12.80 ± 0.44 | 12.12 ± 0.07 | 0.16 | 11.61 ± 0.79 | 12.45 ± 0.53 | 0.39 | 0.96 |
| CD8+ | 8.09 ± 0.42 | 7.30 ± 0.24 | 0.13 | 6.29 ± 0.35 | 6.21 ± 0.24 | 0.85 | 0.09 |
| NK1.1+ | 2.82 ± 0.07 | 2.47 ± 0.17 | 0.08 | 1.39 ± 0.15 | 1.50 ± 0.14 | 0.58 | 0.0003 |
| TCRγδ+ | 2.49 ± 0.09 | 2.65 ± 0.15 | 0.41 | 2.11 ± 0.14 | 2.24 ± 0.13 | 0.55 | 0.14 |
| Treg | 1.04 ± 0.05 | 0.99 ± 0.05 | 0.47 | 1.24 ± 0.13 | 1.30 ± 0.11 | 0.72 | 0.08 |
| F4/80+ | 5.05 ± 0.41 | 6.61 ± 0.23 | 0.01 | 5.28 ± 0.32 | 5.81 ± 0.62 | 0.50 | 0.55 |
| Gr-1+ | 3.37 ± 0.14 | 3.31 ± 0.17 | 0.78 | 5.97 ± 0.25 | 6.66 ± 0.83 | 0.48 | 0.0002 |
| Gr-1+CD11b+ | 0.96 ± 0.09 | 1.08 ± 0.05 | 0.32 | 4.29 ± 0.21 | 5.01 ± 0.67 | 0.37 | < 0.0001 |
| CD11c+MHCIIhi DC | 1.87 ± 0.15 | 1.76 ± 0.18 | 0.66 | 8.89 ± 0.15 | 8.65 ± 0.46 | 0.66 | < 0.0001 |
| CD11b+ DC | 0.11 ± 0.01 | 0.09 ± 0.01 | 0.43 | 0.41 ± 0.03 | 0.37 ± 0.04 | 0.39 | < 0.0001 |
| CD103+ DC | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.94 | 0.69 ± 0.04 | 0.70 ± 0.10 | 0.97 | < 0.0001 |
| Peripheral Lymph nodes | |||||||
| NK1.1+ | 1.27 ± 0.15 | 0.80 ± 0.07 | 0.02 | 0.60 ± 0.19 | 0.47 ± 0.06 | 0.49 | 0.24 |
| CD19+ | 20.65 ± 1.43 | 18.27 ± 0.82 | 0.18 | 31.06 ± 2.73 | 32.60 ± 1.08 | 0.59 | < 0.0001 |
| TCRγδ+ | 1.31 ± 0.05 | 1.25 ± 0.04 | 0.36 | 1.42 ± 0.13 | 1.21 ± 0.09 | 0.20 | 0.95 |
| CD4+ | 21.17 ± 0.81 | 21.27 ± 0.66 | 0.93 | 21.10 ± 0.82 | 20.07 ± 0.43 | 0.27 | 0.59 |
| CD8+ | 18.62 ± 1.12 | 16.60 ± 1.02 | 0.21 | 17.42 ± 1.02 | 14.07 ± 1.25 | 0.07 | 0.38 |
| Treg | 2.36 ± 0.12 | 2.44 ± 0.19 | 0.71 | 2.59 ± 0.19 | 2.79 ± 0.12 | 0.38 | 0.38 |
| F4/80+ | 1.26 ± 0.08 | 1.37 ± 0.10 | 0.39 | 1.46 ± 0.25 | 1.39 ± 0.29 | 0.87 | 0.99 |
| Gr-1+ | 1.84 ± 0.19 | 1.68 ± 0.19 | 0.60 | 6.18 ± 0.51 | 4.92 ± 0.72 | 0.20 | 0.0004 |
| Gr-1+CD11b+ | 0.19 ± 0.04 | 0.22 ± 0.02 | 0.53 | 2.68 ± 0.68 | 2.69 ± 0.55 | 0.98 | 0.002 |
| CD11c+MHCIIhi DC | 1.16 ± 0.07 | 1.16 ± 0.08 | 1.00 | 4.82 ± 0.59 | 4.38 ± 0.43 | 0.56 | < 0.0001 |
| CD11b+ DC | 0.26 ± 0.03 | 0.26 ± 0.03 | 0.90 | 1.12 ± 0.28 | 0.92 ± 0.19 | 0.55 | 0.03 |
| CD103+ DC | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.45 | 0.40 ± 0.03 | 0.36 ± 0.04 | 0.45 | < 0.0001 |
Values shown are mean percentages (± SEM) of indicated cell population. For the analysis, 6 mice of each sex from mixture and 6 of each sex from control exposure groups were used. Bone marrow, thymus, spleen, and peripheral lymph nodes (mediastinal, cervical, inguinal, axillary, and brachial lymph nodes) were isolated. Cell populations within each organ were identified using analytical flow cytometry. Lineage-denotes cells that do not express the following cell surface markers: CD3ε, CD11b, CD45R, Ly6C/Ly6G, TER-119.
p-Value of mixture-exposed vs. control group within indicated sex.
p-Value of females vs. males within mixture-exposed group. Bold font indicate p-values that were < 0.05 (two-way ANOVA; Tukey post-hoc test).
Table 3.
Primary and secondary immune organ cell numbers in immunologically-naive female and male mice.
| Number of cells | Female Control vs. 0.1μg/ml | Male Control vs. 0.1μg/ml | Female vs. Male | ||||
|---|---|---|---|---|---|---|---|
| Cell type | Control | 0.1 μg/ml | p-valuea | Control | 0.1μg/ml | p-valuea | p-valueb |
| Bone Marrow | |||||||
| Cell Count | 74150000 ± 2083386 | 71275000 ± 3669213 | 0.51 | 103890000 ± 3209649 | 78625000 ± 5603179 | 0.01 | 0.54 |
| HSC | 5755 ± 383 | 5657 ± 653 | 0.93 | 7721 ± 771 | 7800 ± 1192 | 0.95 | 0.26 |
| LSK | 70697 ± 2522 | 70655 ± 4413 | 0.99 | 110532 ± 5507 | 93233 ± 7901 | 0.04 | 0.03 |
| LK | 1203702 ± 89143 | 1252875 ± 64443 | 0.71 | 2112735 ± 91858 | 1571912 ± 118362 | 0.001 | 0.09 |
| MPP1 | 6958 ± 403 | 6853 ± 513 | 0.95 | 6850 ± 1978 | 5991 ± 1113 | 0.59 | 0.94 |
| MPP2 | 1990 ± 193 | 2405 ± 143 | 0.16 | 1721 ± 299 | 1619 ± 184 | 0.73 | 0.05 |
| MPP3 | 9796 ± 976 | 10729 ± 1402 | 0.66 | 14543 ± 2246 | 11562 ± 1396 | 0.19 | 0.98 |
| MPP4 | 19602 ± 1867 | 20148 ± 1831 | 0.84 | 26134 ± 2008 | 24909 ± 1857 | 0.66 | 0.29 |
| Lineage - | 8671300 ± 465033 | 8423180 ± 681084 | 0.99 | 11643240 ± 626662 | 8882450 ± 779163 | 0.04 | 0.96 |
| CLP | 8898 ± 525 | 10067 ± 927 | 0.46 | 15664 ± 1517 | 11298 ± 1383 | 0.01 | 0.86 |
| GMP | 315805 ± 30077 | 322645 ± 15641 | 0.89 | 632088 ± 52107 | 529802 ± 39834 | 0.06 | 0.002 |
| pre-GM | 255960 ± 12376 | 263497 ± 17263 | 0.79 | 593370 ± 10167 | 379342 ± 30872 | < 0.0001 | 0.003 |
| Pre-MegE | 90120 ± 4822 | 91537 ± 6929 | 0.88 | 133101 ± 538320 | 115155 ± 893994 | 0.09 | 0.09 |
| Thymus | |||||||
| Cell Count | 39566667 ± 2742039 | 77800000 ± 7206802 | 0.001 | 57460000 ± 5236921 | 48966667 ± 2524898 | 0.16 | 0.002 |
| DN1 | 228771 ± 18231 | 430871 ± 33136 | 0.0003 | 528002 ± 77197 | 684348 ± 183745 | 0.49 | 0.32 |
| DN2 | 63719 ± 4147 | 116658 ± 20146 | 0.03 | 91274 ± 8771 | 85060 ± 6435 | 0.57 | 0.25 |
| DN3 | 283368 ± 22236 | 500511 ± 73589 | 0.02 | 528325 ± 69737 | 470680 ± 21930 | 0.42 | 0.97 |
| DN4 | 3098698 ± 193127 | 5253866 ± 348030 | 0.0003 | 3516655 ± 297171 | 3032900 ± 244715 | 0.24 | < 0.0001 |
| DP | 21185867 ± 1671590 | 46455433 ± 5250584 | 0.001 | 28684210 ± 2695319 | 24983000 ± 2176632 | 0.31 | 0.001 |
| DP CD3+ | 279687 ± 34674 | 593170 ± 98337 | 0.01 | 417740 ± 80852 | 554356 ± 196147 | 0.57 | 0.99 |
| TCRβ+ | 3139156 ± 201484 | 7018648 ± 759410 | 0.0001 | 4637615 ± 719438 | 4868273 ± 496447 | 0.79 | 0.06 |
| CD4+ | 1746048 ± 152463 | 3325491 ± 281110 | 0.003 | 2643763 ± 460394 | 3269340 ± 413500 | 0.22 | 0.99 |
| CD8+ | 655323 ± 54490 | 1414560 ± 101941 | 0.0002 | 1552499 ± 206125 | 1424421 ± 93797 | 0.46 | 0.99 |
| TCRγδ+ | 115893 ± 7104 | 230278 ± 20420 | 0.0001 | 213010 ± 26574 | 160718 ± 12934 | 0.05 | 0.04 |
| Treg | 4f)f)4S ± 9979 | 8fS4fSfS ± QD7S | 0.001 | 4QQS7 ± 7fSS8 | 777S8 ± 10974 | 0 11 | 0 67 |
| Spleen | |||||||
| Cell Count | 176533333 ± 36985508 | 129650000 ± 14447370 | 0.27 | 16022000 ± 21355758 | 173333333 ± 1739428 | 0.64 | 1.00 |
| CD19+ | 55585900 ± 12170861 | 36471167 ± 3959027 | 0.17 | 86368300 ± 12115852 | 86007167 ± 9978949 | 0.98 | 0.01 |
| CD4+ | 22665567 ± 48237488 | 15738667 ± 1796156 | 0.21 | 18199320 ± 2043785 | 21461333 ± 2261007 | 0.32 | 0.55 |
| CD8+ | 14919797 ± 4166458 | 9447475 ± 1065301 | 0.23 | 9845764 ± 1066520 | 10766700 ± 1192964 | 0.59 | 0.98 |
| NK1.1+ | 5078700 ± 1202146 | 3232180 ± 450399 | 0.18 | 2274898 ± 489308 | 2621591 ± 377426 | 0.58 | 0.93 |
| TCRγδ+ | 4475020 ± 1006481 | 3437493 ± 432072 | 0.37 | 3369216 ± 521134 | 3949666 ± 544420 | 0.47 | 0.95 |
| Treg | 1909856 ± 488900 | 1303140 ± 200698 | 0.28 | 1950326 ± 294570 | 2294933 ± 360439 | 0.49 | 0.22 |
| F4/80+ | 9350560 ± 2407468 | 8627746 ± 1037805 | 0.79 | 8612104 ± 1566687 | 10093300 ± 1446602 | 0.51 | 0.92 |
| Gr-1+ | 5856606 ± 1089238 | 4280960 ± 484647 | 0.22 | 9549944 ± 1368336 | 11569333 ± 1566339 | 0.37 | 0.002 |
| Gr-1+CD11b+ | 1858551 ± 600020 | 1378273 ± 137053 | 0.45 | 6864606 ± 1029306 | 8674733 ± 1205152 | 0.29 | < 0.0001 |
| CD11c+MHCIIhi DC | 3434646 ± 880749 | 2264778 ± 268622 | 0.23 | 14311818 ± 2084904 | 15027983 ± 1783199 | 0.79 | < 0.0001 |
| CD11b+ DC | 208771 ± 6652 | 123497 ± 15246 | 0.24 | 647576 ± 78515 | 647766 ± 104352 | 0.99 | 0.0003 |
| CD103+DC | 230090 ± 51103 | 171223 ± 24508 | 0.32 | 1128714 ± 180879 | 1216000 ± 187835 | 0.75 | < 0.0001 |
| Peripheral Lymph nodes | |||||||
| Cell Count | 562268 ± 65949 | 1066666 ± 157504 | 0.01 | 597411 ± 85114 | 966372 ± 251508 | 0.23 | 0.97 |
| NK1.1+ | 7566 ± 1627 | 8540 ± 1481 | 0.67 | 3182 ± 576 | 4554 ± 1332 | 0.40 | 0.19 |
| CD19+ | 119767 ± 21336 | 198588 ± 33740 | 0.08 | 187883 ± 32821 | 313563 ± 83910 | 0.23 | 0.38 |
| TCRγδ+ | 7278 ± 747 | 13415 ± 2106 | 0.02 | 8333 ± 1050 | 11779 ± 3380 | 0.39 | 0.59 |
| CD4+ | 118682 ± 13678 | 225766 ± 33925 | 0.02 | 125021 ± 16974 | 194704 ± 53532 | 0.28 | 0.92 |
| CD8+ | 104086 ± 12381 | 174908 ± 25993 | 0.03 | 106010 ± 19157 | 134585 ± 41627 | 0.57 | 0.72 |
| Treg | 13324 ± 1842 | 25999 ± 4607 | 0.03 | 15512 ± 2391 | 27737 ± 8245 | 0.22 | 0.99 |
| F4/80+ | 6843 ± 549 | 14158 ± 1778 | 0.003 | 8172 ± 1052 | 13296 ± 5104 | 0.39 | 0.99 |
| Gr-1+ | 10712 ± 2130 | 18925 ± 4412 | 0.12 | 37287 ± 6740 | 45554 ± 14635 | 0.64 | 0.15 |
| Gr-1+CD11b+ | 1032 ± 176 | 2456 ± 508 | 0.02 | 15543 ± 3834 | 23395 ± 7883 | 0.42 | 0.01 |
| CD11c+ MHCIIhi DC | 6564 ± 918 | 12433 ± 2111 | 0.03 | 28224 ± 4293 | 41545 ± 12896 | 0.39 | 0.04 |
| CD11b+ DC | 1482 ± 267 | 2773 ± 524 | 0.05 | 6310 ± 1369 | 8867 ± 3601 | 0.55 | 0.16 |
| CD103+DC | 518 ± 70 | 1215 ± 275 | 0.03 | 2379 ± 351 | 3226 ± 907 | 0.44 | 0.05 |
Table presents mean numbers (± SEM) of indicated cell populations in primary and secondary immune organs from immunologically-naïve female and male mice. For this analysis, 6 female and 6 male mice were treated with either the 23-chemical mixture or vehicle control for 8–10 weeks. Bone marrow, thymus, spleen, and peripheral lymph nodes (mediastinal, cervical, inguinal, axillary, and brachial lymph nodes) were isolated and analytical flow cytometry was used to identify immune cell populations. Lineage- denotes cells that do not express the following cell surface markers: CD3ε, CD11b, CD45R, Ly6C/Ly6G, TER-119.
p-Value of mixture-exposed vs. control group within indicated sex.
p-Value of females vs. males within mixture-exposed group. Bold font indicate p-values that were < 0.05 (two-way ANOVA; Tukey post-hoc test).
In males, there were also several statistically-significant differences in cell populations in the bone marrow, thymus, and spleen due to exposures to the chemical mixture (Table 2). These altered populations included those for bone marrow pre-GM and pre-MegE cells, thymic CD4+ cells, regulatory T (Treg) cells, and TCRγδ+ cells, and CD19+ cells in the spleen. In the male mixture group, there was also a significant decrease compared to control males in the total number of bone marrow cells; however, no differences in total cell number were observed in the thymus, spleen, or PLN due to treatment (Table 3). In contrast to the female mixture-exposed mice, there were no significant differences in the number of lineage-committed immune cell populations in the thymus, spleen, or PLN of the exposed males. However, there were significant differences in several immune progenitor populations in the bone marrow of mixture-exposed males compared to bone marrow cells from control exposed males. Along with observing differences between mixture and control groups within sex, there were also some differences in cellularity between males and females from all lymphoid organs examined (Tables 2 and 3).
Influenza A virus infection
To determine if exposure to the chemical mixture altered immune responses during mild acute respiratory viral infection, mice here were infected with influenza A virus (IAV). Mice were weighed daily after infection to assess morbidity. There were no significant differences in morbidity between mixture and vehicle control-treated mice of either sex (Figures 1A and 1C). CD8+ cytotoxic T-lymphocytes (CTL) are a central means to fight acute primary virus infection (Tscharke et al. 2015). Using flow cytometry, numbers of CD8+ T-cells in the MLN of IAV-infected mice exposed to the mixture or vehicle control were compared. There were no significant differences in number (Figure 1B) or percentage (Table S3) of CD8+ T-cells in mixture-exposed female mice compared to in counterpart controls. There was also no significant difference in number (Figure 1E) or percentage (Table S3) of IAV nucleoprotein (NP)-specific CD8+ T-cells or CTL among the female mice (Figure 1F and Table S3). In contrast, there were significantly fewer CD8+ T-cells, CTL, and virus NP-specific CD8+ T-cells in the male mice that had been exposed to the chemical mixture (compared to infected control male mice) (Figures 1D, 1G, and 1H, and Tables S2 and S3). Lymph nodes harvested from IAV-infected male mice in the mixture-exposed group also had a significantly lower number of cells compared to control males. Comparatively, there was not a significant difference in total cellularity of lymph nodes from the female mice (Tables S2 and S3).
Figure 1. Effects of exposure to 23-chemical mixture on CD8+ T-cells and morbidity after viral infection.
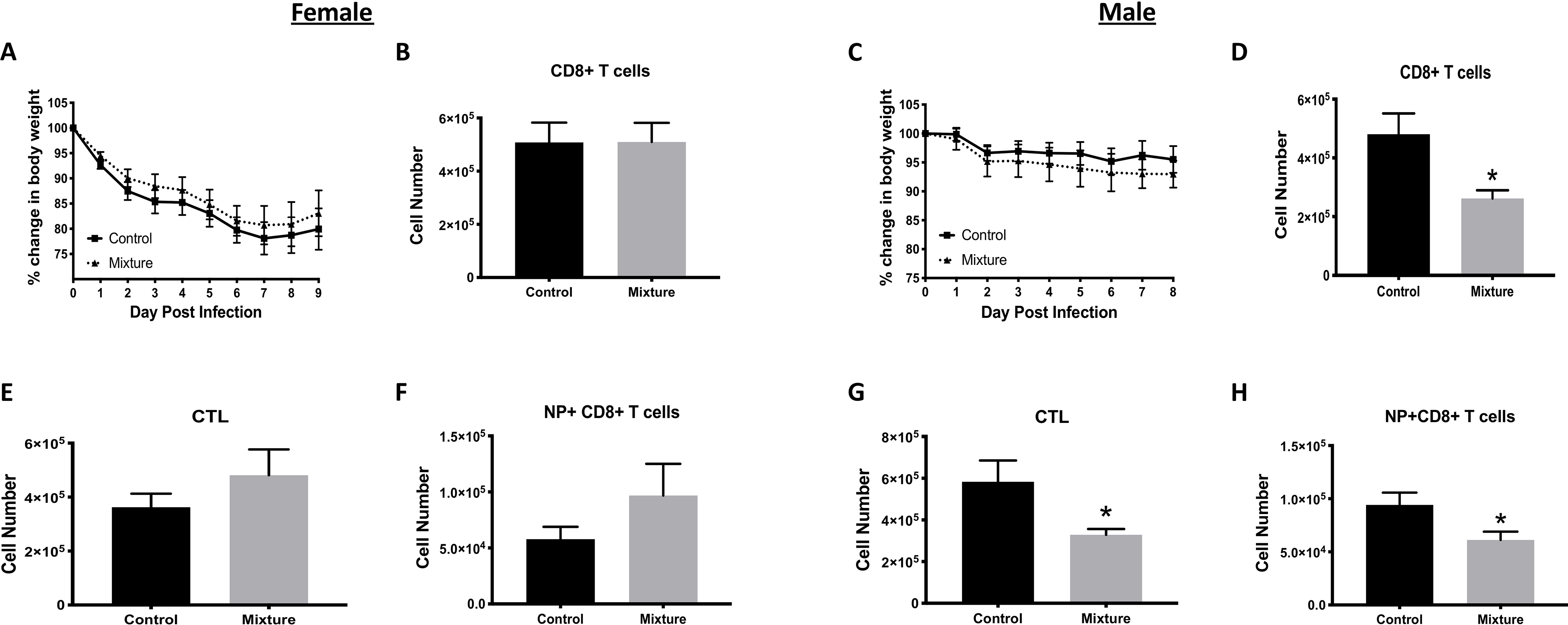
Starting at 6 wk-of-age, C57Bl/6 mice were placed on drinking water containing an equimass mixture of 23 chemicals (Table 1) or vehicle. The final concentration of each chemical in the water was 0.1 μg/ml; control water contained 0.2% ethanol. Mice were maintained on these regimens for at least 8 wk prior to infection. After 8 weeks of exposure, 10 female and 10 male mice from each exposure group were infected intranasally (IN) with IAV (H3N2). Mice were maintained on their respective water treatment regimens throughout infection. (A,C) Mean change in body weight of (A) female and (C) male mice following infection. (B,D-H) CD8+ T-cells were examined 9 days post-infection in female mice, and 8 days after infection male mice, using flow cytometry. Mean number of CD8+ T-cells in mediastinal lymph nodes (MLN) of infected (B) female and (D) male mice. Mean number of cytotoxic T-lymphocytes (CTL; CD8+CD44hiCD62Llo cells) in IAV-infected (E) female and (G) male mice from each group. Mean number of IAV NP-specific CD8+ T-cells (DbNP366–375+CD8+ T-cells) in infected (F) female and (H) male mice. *Significantly different compared to same-sex control mice (p < 0.05; Student’s t-test). Data presented as means ± SEM. Numerical values that correspond to graphs, as well as p-values for each comparison, are listed in Tables S2 and S3.
CD4+ T-cells also play an important role in the immune response to IAV infection. Two CD4+ T-cell sub-populations particularly important during acute primary IAV infection are T-helper (TH)-1, T-follicular helper (Tfh), TH17, and T-regulatory (Treg) cells (Swain et al. 2012; Strutt et al. 2013). Flow cytometric analyses here did not reveal any significant differences in the CD4+ T-cell numbers or in the numbers of TH1, TH17, Tfh, or Treg cell subsets in the MLN of female mice as a result of their exposure paradigms (Figures 2A–2E). There were also no significant differences between the females with respect to percentages of these subsets or in the Treg:TH1, Treg:Tfh and Treg:TH17 cell ratios (Table S3). In contrast, in male mice, the number of all CD4+ T-cell subsets, except for TH17 cells, were significantly lower in the mixture-treated group compared to in the vehicle controls (Figures 2F–2J). Also, in the infected males exposed to the mixture, the percentage of TH17 cells was 33% greater than in the controls, and the Treg:TH17 ratio was significantly lower compared to infected control mice (Table S3).
Figure 2. Consequences of mixture exposure on CD4+ T-cells during viral infection.
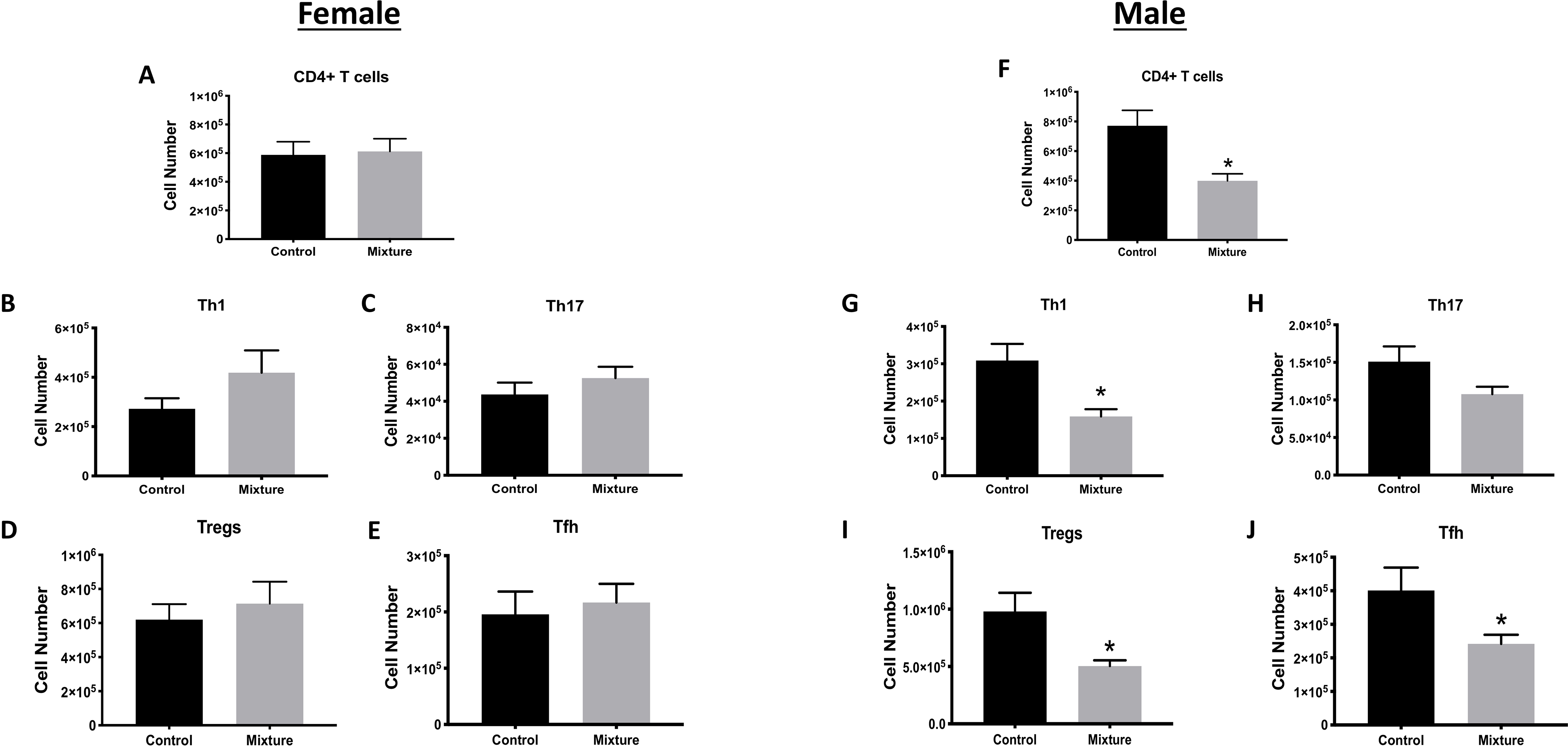
Starting at 6 wk-of-age, C57Bl/6 mice were placed on drinking water containing a mixture of 23 chemicals or containing vehicle control, and 10 female and 10 male mice from each exposure group were infected with IAV at least 8 wk later (see Figure 1). CD4+ T-cell responses were measured 9 days and 8 days after infection in, respectively, the female and male mice. Cell suspensions of MLN cells were prepared and stained for flow cytometry. CD4+ T-cells were defined as CD3+CD4+; CD4+ T-cell subsets were further defined using the following markers: TBet+ (TH1 cells), RORγt+ (TH17 cells), PD1+CXCR5+ (Tfh cells), and Foxp3+CD25+ (Treg cells). Bar graphs shows numbers of (A,F) CD4+ T, (B,G) TH1, (C,H) TH17 (C,H), (D,I) Treg, and (E,J) Tfh cells in, respectively, infected female and male mice. *Significantly different compared to same sex control mice (p < 0.05; Student’s t-test). Data presented as means ± SEM. Numerical values that correspond to graphs as well as p-values for each comparison are listed in Tables S2 and S3.
Experimental Autoimmune Encephalomyelitis (EAE)
To determine if exposure to the chemical mixture affected disease progression in a model of autoimmune disease, experimental autoimmune encephalomyelitis (EAE), which induces a pathology similar to multiple sclerosis (Mendel et al. 1995) was induced. Female mice exposed to the mixture exhibited a higher severity of disease compared to their control counterparts (Figure 3A). However, neither the percentage of mice that develop disease (Figure 3B) nor the mean day of disease onset (Figure 3C) significantly-differed between the mixture and control females. In contrast to females, mixture-exposed male mice showed no significant differences in disease severity, frequency, or time of onset compared to their control male counterparts (Figures 3D–3F).
Figure 3. EAE disease symptom onset and severity.
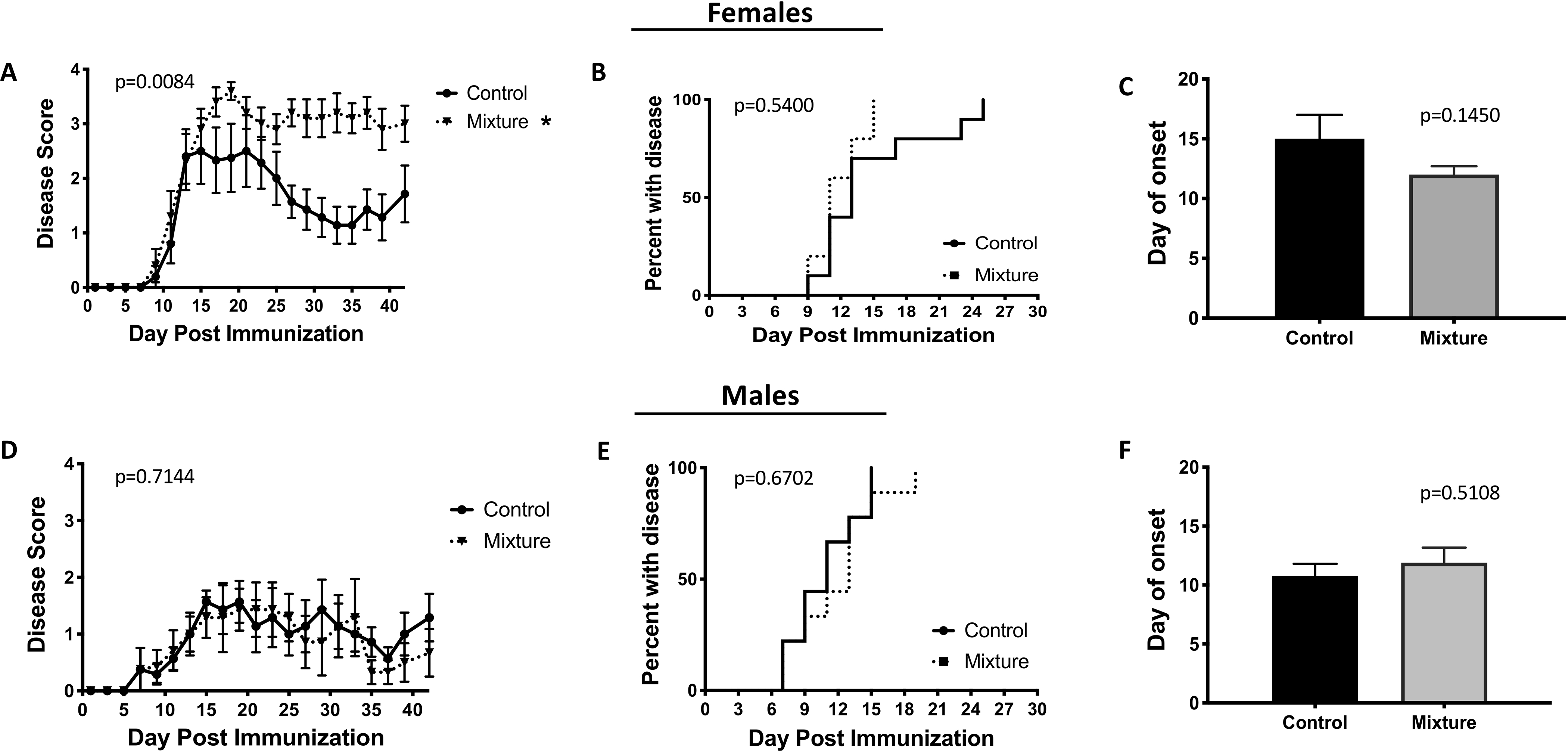
Following 8 wk of exposure to chemical mixture or vehicle control, 10 female and 10 male mice from each exposure group were immunized with CFA/MOG35–55 emulsion. Disease progression was monitored and scored every other day for 42 days. Average disease score for each treatment group over time was determined in (A) female, and (D) male mice. Graphs also depict the day of disease onset (disease score ≥ 1) in (B) female, (E) male, and the average day of onset in (C) female and (F) male mice. Disease scores were 0 = normal mouse, 1 = limp tail, 2 = limp tail and hind limb weakness, 3 = partial hind limb paralysis, 4 = complete hind limp paralysis, 5 = moribund. *Significantly different compared to control mice (p < 0.05; ANOVA). Data presented as means ± SEM.
EAE is a T-cell-mediated disease, and the primary CD4+ T-cell subsets that drive EAE are TH and TH17 cells; conversely, Treg cells dampen this immunopathology (Fletcher et al. 2010). In mixture-exposed female mice, there were no significant differences in the number or percentage of TH1 (Figures 4A and 4B) or TH17 (Figures 4C and 4D) cells compared to in female controls. However, there was a significant decrease in the number of Treg cells in the exposed females (Figure 4I). In contrast, there were no significant differences in percentages of Treg cells (Table S4) and the Treg:TH1 and Treg:TH17 cell ratios due to exposure (Figures 4J and 4K). Male mice that were exposed to the chemical mixture had a significantly lower number (Figure 4E) and percentage (Figure 4F) of TH1 cells, but no difference in TH17 cells (Figures 4G and 4H), compared to values seen in vehicle control males. In contrast to in the females, there was no significant difference in number of Treg cells between the mixture and control males (Figure 2L, Table S5). However, Treg:TH1 cell ratios were significantly higher, while Treg:TH17 ratios did not change in the mixture males, indicating a higher proportion of Treg cells compared to TH1 cells in these hosts (Figures 2M and 2N).
Figure 4. CD4+ T-cell subsets in EAE disease.
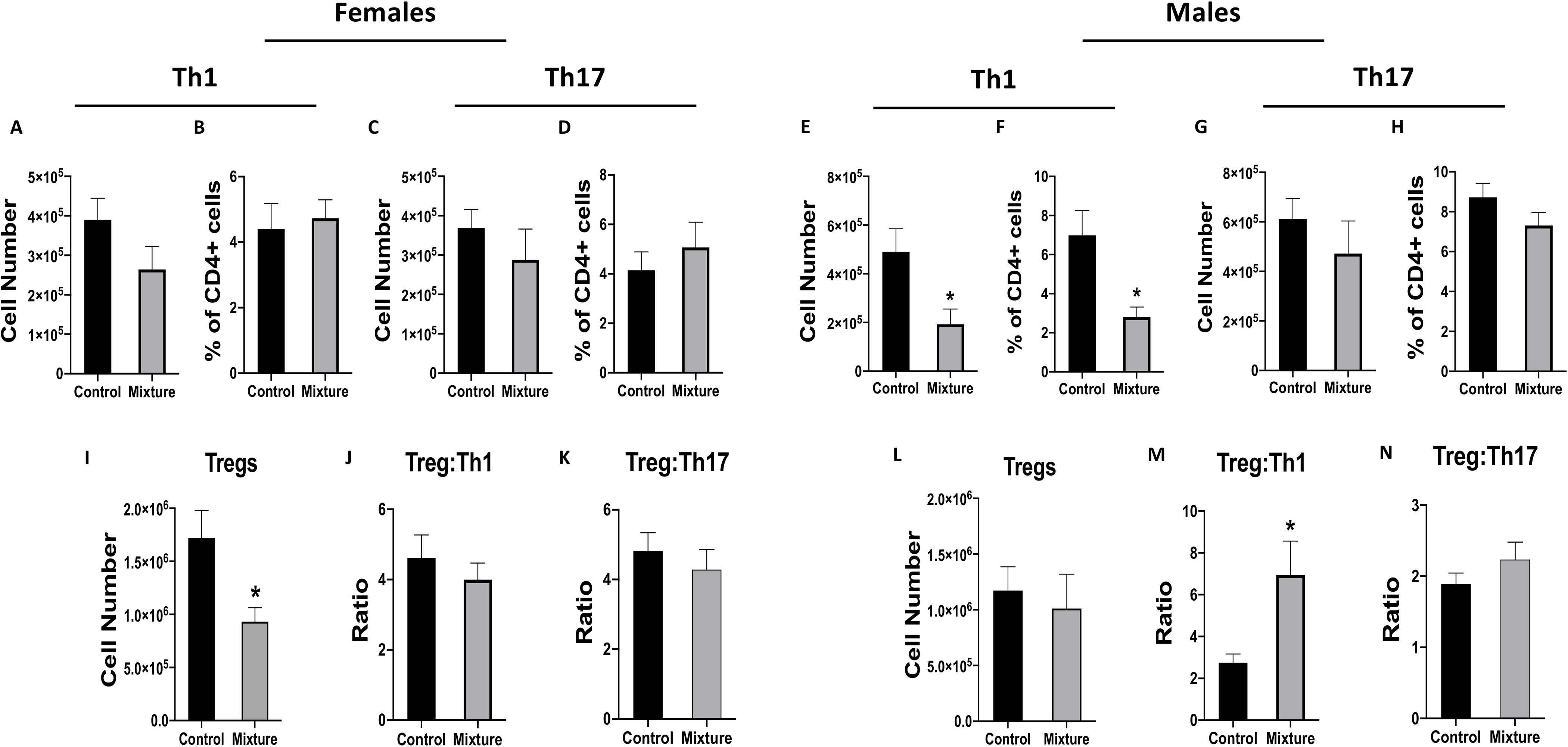
Following 8 wk of exposure to the mixture of 23 chemicals or water containing the vehicle control, 10 female and 10 male mice in each exposure group were immunized with CFA/MOG35–55 emulsion to induce EAE (see Figure 3). All mice were euthanized 42 days after immunization, and cervical lymph nodes then obtained. (A,C,E,G) Mean number and (B,D,F,H) percentage of TH1 (TBet+CD4+CD3+ cells) and TH17 (RORγt+CD4+CD3+) cells based on flow cytometry. (I,L) Mean number of Treg cells (Foxp3+CD25+ CD4+ CD3+) in (I) female and (L) male mice. (J,M) Mean Treg:TH1 ratios in (J) female and (M) male mice. (K,N) Mean Treg:TH17 ratios in (K) female and (N) male mice. *Significantly different compared to control mice (p < 0.05; Student’s t-test). Data presented as means ± SEM. Numerical values that corre-spond to graphs as well as p-values for each comparison are listed in Tables S4 and S5.
House Dust Mite (HDM)-Induced Allergic Airway Disease
Male and female mice from both exposure groups were sensitized and challenged with HDM, which induces many hallmarks of allergic airway disease (Knowlden et al. 2016). The primary CD4+ T-cell subsets that drive the immune-mediated pathology observed in this case are TH2 and TH17 cells (Vroman et al. 2015; Hirahara and Nakayama 2016). Again, Treg cells play a role in dampening the response (Langier et al. 2012). Female mice that were exposed to this 23-chemical mixture mice had a 2.4-fold higher number (Figure 5A) and 1.8-times greater percentage (Figure 5B) of TH2 cells in their mediastinal lymph nodes as compared to in the control females. Further, the ratio of Treg:TH2 cells was significantly lower in the female mixture-exposed mice. While the number of Treg cells did not differ between the two groups, there was a significantly lower percentage of Treg cells in the mediastinal lymph nodes of mixture-exposed female mice compared to in control females (Table S6). In contrast, there were no significant differences in number (Figure 5G) or percentage (Figure 5H) of TH17 cells nor in the ratio of Treg:TH17 cells (Figure 5I) due to mixture exposure in the females. In the lungs, exposure to this chemical mixture did not change the number of lavageable leukocytes (Table S7); however, it altered the proportion of leukocytes from the airways of the exposed female mice compared to from the control females (Figure 5M). Specifically, the percentage of eosinophils was 1.5-fold higher, while those of macrophages and lymphocytes were 2.1- and 1.6-fold lower, respectively, in these females when compared to in controls (Figure 5M, and Table S6).
Figure 5. Effects of exposure to chemical mixture on immune response in allergic airway disease model.
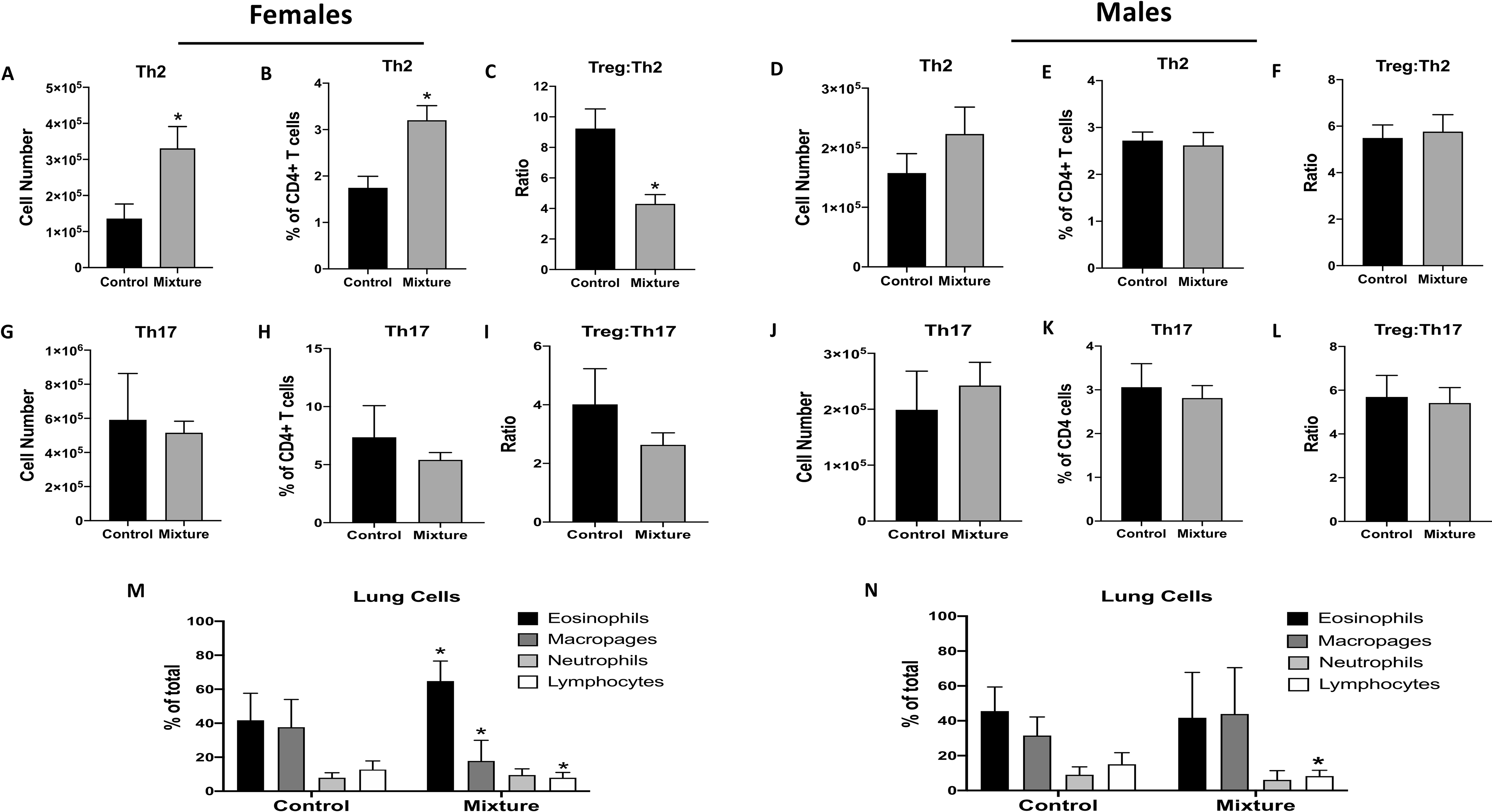
Following 10 wk of exposure to to the mixture or vehicle control, 10 female and 8 male mice in each exposure group were sensitized and challenged with HDM. To assess CD4+ T-cell subsets in their MLN, mice were euthanized 10 days after HDM administration. MLN of female mice: mean (A,G) number, (B,H) percentage, and (C,I) ratios of indicated CD4+ T-cell types based on flow cytometry. MLN of male mice: mean (D,J) number, (E,K) percentage, and (F,L) ratios of indicated CD4+ T-cell types. Airway leukocytes were examined via bronchoalveolar lavage (BAL); mean percentage neutrophil, lymphocyte, macrophage, and eosinophil in BAL from (M) female and (N) male mice. *Significantly different compared to control mice (p < 0.05; Student’s t-test). Data presented as means ± SEM. Numerical values that correspond to graphs as well as p-values for each comparison are listed in Tables S6 and S7.
In contrast to what was observed in the females after HDM sensitization\challenge, mixture exposure had no significant impact on the number or percentage of TH2 (Figures 5D and 5E), TH17 (Figures 5J and 5K) or Treg (Tables S6 and S7) cells of male mice. There were also no differences between Treg:TH2 or Treg:TH17 ratios of the two groups (Figures 5F and 5L). Unlike the females, male mice exposed to the mixture did not exhibit any increase in percentage of airway eosinophils or macrophages (Figure 5N); however, compared to control males, they had a significantly higher number of airway macrophages (Tables S6 and S7). These males also had a significantly lower percentage (but not number) of airway lymphocytes compared to control males (Figure 5N, Tables S6 and S7). There were no differences in number or percentage of eosinophils or neutrophils between the two male groups (Figure 5N, Tables S6 and S7).
Discussion
Water contamination is a major global health concern (WHO 2016). However, investigating links between environmental chemical contaminants in water and adverse health outcomes can be difficult. Multiple reasons underlie these challenges, including lack of comprehensive knowledge of anthropogenic chemicals in water supplies and a delayed nature in which many health effects from such exposures occur. Another limitation is that research often examines the consequences of exposure to environmental contaminants one at a time. Thus, there is limited knowledge of specific physiologic functions affected by exposure to mixtures of chemical contaminants, including what cell types are affected. This further complicates the ability to assess causality between water contaminants and disease.
In the present study, links between exposure to a mixture of chemicals associated with water tainted by UOG operations and changes in the cellular repertoire\function of an adult mammalian immune system was investigated. This study established that exposure to a 23-chemical mixture led to multiple changes in the composition of immune cell populations at homeostasis, as well as alterations in functionally distinct T-cell subsets in the context of three different disease models. Interestingly, the extent of the effects observed varied between sexes. These results indicated to us that exposure to this 23-chemical mixture imparted modulatory effects on the immune system, and that these effects manifest differently between sexes.
Overall, the study of immunotoxicity has largely focused on examining immune modulation by single chemical exposures. This is a valuable and important approach, particularly to understanding mechanisms of toxicity. For the mixture used in the current study, there is minimal, and in some cases no, extant data on possible immunomodulatory effects of many of the chemical constituents. However, there is some evidence that exposure to several components of this mixture, such as naphthalene and 2-ethyl-hexanol disturbs aspects of immune cell functions (Kawabata and White 1990; Yoshida et al. 2009; McGuire et al. 2021). Benzene and styrene are also known carcinogens and immunotoxicants in mammals (Veraldi et al. 2006; McHale et al. 2012). Other studies have shown that direct exposure to volatile organics, including ethylbenzene, styrene, and benzene, was associated with lymphopenia in an all-female study population (Baines et al. 2004). Similar to the current findings, another study that found that inhalation exposure of male mice to formaldehyde, benzene, toluene, and xylene decreased the number of T-cells in peripheral immune organs (Wang et al. 2016).
There is also considerable research into health effects of exposure to a mixture of benzene, toluene, ethylbenzene, and xylenes (BTEX; Bahadar et al. 2014; Bolden et al. 2015). Most BTEX research to-date has focused on cancer; consequently, non-carcinogenic effects are less well-studied. Nonetheless, there is some evidence that the mammalian immune system is a target of the BTEX (Bolden et al. 2015; Wen et al. 2016; Li et al. 2018); although the majority of studies investigated effects of exposure to each component individually rather than combined. Additionally, most studies of BTEX immunotoxicity focused on the developing immune system in children (Webb et al. 2014) rather than direct exposure of a fully mature adult immune system. While collectively these studies indicated some components of the 23-chemical mixture impart immunomodulatory effects, research into effects of these chemicals as a mixture remains limited. Lastly, in many of the cited studies, the individual components were used at higher concentrations than those used in the present study.
Another interesting aspect of findings from the current study was the apparent sex-biased nature of effects on immune cell populations and function observed among the mixture-exposed mice. The most notable example of this was in the EAE model. Exposure to the 23-chemical mixture significantly increased disease severity and decreased Treg cell numbers in female mice, but not in male mice. The Treg cells play a central regulatory role in EAE by dampening the immunopathology (Fletcher et al. 2010). This suggested to us that modulating Treg cell numbers is a means by which exposure to this mixture could potentially heighten disease severity in female hosts. Consistent with this idea is that mixture-exposed males exhibited disease severities similar to in control males. Mixture males also had significantly fewer TH1 cells and an increase in Treg:TH1 cell ratios compared to values in counterpart controls. Although a subtle distinction, this is important because it indicates the male immune system was not unaffected by exposure to this mixture; rather, in the context of this disease model, the consequences from this level of exposure did not exacerbate disease.
In addition to modulating Treg cell numbers, attenuating TH1 cell activation and proliferation could reduce disease severity in the EAE model (Xie et al. 2018). Repeated host exposure to this chemical mixture might have impacted disease severity via multiple changes in T-cells. This would align with observations here. Specifically, exposure to the 23-chemical mixture affected responses of some T-cell subsets, but did not uniformly affect the same T-cell subsets across all immune challenges used. This indicated that exposure to this mixture may affect pathways involved in regulating T-cell responses to specific immune challenge.
Alterations in the number or frequency of T-cell subsets can affect relative proportions of regulatory and effector T-cells. Such changes have been shown to impact on the progression and severity of infections and allergic airway disease (Swain et al 2012; Strut et al 2013; Chapman and Georas 2014). Evidence of the impact that these alterations could potentially have were observed in the current work. For example, an increase in disease severity coupled with a significant decrease in Treg cell numbers in female mixture-exposed mice, and conversely a lack of difference in disease severity coupled with a significant decrease in TH1 cell levels in the cervical lymph nodes of male-exposed mice after EAE induction, were observed. Further investigations into T-cell responses to various immune challenges, as well as an examination of pathways involved in these responses in affected populations, will further illuminate the potential impact on the immune system from exposure to water contaminants, such as those associated with UOG operations.
Numerous studies have shown that the immune response during many diseases, including autoimmune diseases, asthma, and respiratory infections, differ in males vs. females (McClelland and Smith 2011; Ngo et al. 2014; Klein and Flanagan 2016; Peckham et al. 2020). For instance, there are differences in the frequency and timing of T-cell responses in female and male mice infected with IAV (Oertelt-Prigione 2012; Gabriel and Arck 2014; Fink et al. 2018). In addition, it is known that the endocrine system affects the immune system (Oertelt-Prigione 2012; Gabriel and Arck 2014; Klein and Flanagan 2016). It is unclear whether sex differences contribute to distinct toxicokinetic properties of chemicals in this mixture. Assessment of non-occupational BTEX exposure did not demonstrate sex-based differences in a human cohort study (Tsangari et al 2017), which suggests that this is probably not a sufficient explanation. On the other hand, the compounds used in this study are generally considered endocrine-disrupting chemicals (EDC). In particular, when examined singly or in combination, these chemicals demonstrated evidence of agonism or antagonism of the estrogen, androgen, progesterone, glucocorticoid, and thyroid receptors (Kassotis et al. 2014; Kassotis et al. 2015). Also, some constituents of the mixture used in this current work contain chemicals that have carcinogenic properties (Marina 2014). Therefore, there may be intersections between immune modulation, EDC exposures and cancers, providing opportunities to further explore causal underpinnings of a range of disease processes.
While exogenous chemicals that interfere with normal actions of hormones are defined as EDC, endocrine-active chemicals have also been shown to affect the immune system (Robert et al. 2019). For example, the well-studied EDC bisphenol A has numerous reported immunomodulatory effects (Bauer et al. 2012; Malaise et al. 2020). Moreover, developmental exposure to the same 23-chemical mixture used in the current study altered immune function in adult offspring (Boule et al. 2018; Nagel et al. 2020). Similar to the present study, there were differences in the effect of developmental exposure to this mixture based on sex, with female offspring generally showing a broader range of immunomodulatory consequences (Boule et al. 2018). Thus, it is possible endocrine-disrupting activity of components in this mixture contributed to the alterations in immune function reported here. Moreover, in considering the impact of direct exposure to EDC in the water, immunomodulatory effects may present differently between the sexes.
The present study demonstrated that direct exposure to a 23-chemical mixture significantly altered immune cell populations and immune responses to antigenic challenge. Nevertheless, the study had some limitations. One was that only a single dose was used. Dose-response studies may reveal additional differences related to exposure. Another was that the female mice used were not nulliparous, though they had all given birth at least 6 wk prior to assessments of immune cellularity and immune function. It is possible their immune system was altered by prior pregnancy. The female immune system post-pregnancy compared to that of non-pregnant females is not well-characterized. Some studies suggest changes to immune cell populations and function that occur during pregnancy revert to pre-pregnancy states during the post-partum period (Lima et al. 2016; Brann et al. 2019). One study investigating the impact of pregnancy on the immune system in allergic asthma, found that many of the changes to regulatory T- and B-cell subsets observed during pregnancy reverted to non-pregnant levels within 6 wk after delivery (Martins et al. 2017). Based on those reports, it is unlikely prior pregnancy affected the results of the present study in a substantive manner.
Another potential limitation is that a strain and dose of IAV that causes mild infection was intentionally used (Lawrence et al. 2000, 2006; Boule et al. 2014). This approach was chosen to ensure host survival, so that T-cell responses to infection, which reach their peak 8–10 days after infection (Boule et al. 2014), could be examined. Assessment using strains of IAV with a higher pathogenicity, as well as other types of pathogens, will further characterize how T-cells are impacted by exposure to this mixture. Also, while the present study chose to focus on T-cells, there are many other types of immune cells that play important roles during disease, and these cell types could also have been affected by exposure to the complex mixture. Studies of the effects from individual components of this 23-chemical mixture have yielded diverse impacts on a variety of immune cells (Kawabata and White 1990; Zabrodskii et al. 2002; Yoshida et al. 2009). This then implies that the effects of the mixture exposure may include other immune cell populations as well as the T-cell subsets investigated here.
Conclusions
This study broadly and comprehensively examined the immune effects of direct exposure to a mixture of 23 chemicals that have been associated with water contaminated by UOG operations. The study employed three categories of disease models, i.e., infection, autoimmune, and allergic. The primary finding was that exposure to the mixture altered the immune system, but the consequences in male and female mice were not the same. The alterations observed in the exposed mice ranged from subtle (such as differences in number/percentage of cells) to more robust (such as increase in disease severity). Overall, these findings indicate that direct exposure to mixtures of chemicals in drinking water can alter the immune system in adult mammals in a manner that subsequently contributes to the potential onset/increase in severity of a range of diseases.
Supplementary Material
Acknowledgements
The authors thank Dr. Susan Nagel (University of Missouri) for input on the initial concept of this work, and guidance on preparation of the 23-chemical mixture. Appreciation is also extended the outstanding teams at the URMC Flow Cytometry Core and Department of Comparative Medicine for their on-going support of research.
Funding support
The this work was supported by research and training grants from the National Institutes of Health (R01ES23260, R01ES030300, R01AI073772, R21ES030690, T32ES007026, T32HL066988, P30ES001247, and R24AI059830), and funds from a University of Rochester Provost’s Research Award.
Footnotes
Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
Data availability
Data will be provided to interested parties based on reasonable request to the corresponding author (BPL).
References
- Bahadar H, Mostafalou S, Abdollahi M. 2014. Current understandings and perspectives on non-cancer health effects of benzene: A global concern. Toxicol. Appl. Pharmacol. 276:83–94. [DOI] [PubMed] [Google Scholar]
- Baines C, McKeown-Eyssen G, Riley N, Cole D, Marshall L, Loescher B, Jazmaji V. 2004. Case-control study of multiple chemical sensitivity, comparing hematology, biochemistry, vitamins and serum volatile organic compound measures. Occup. Med. (London) 54:408–418. [DOI] [PubMed] [Google Scholar]
- Barrett T, Ingles SC. 1985. Growth, purification and titration of influenza viruses. In: Virology: A Practical Approach (Mahy B, ed.). Washington, DC, IRL Press, pp. 119–150. [Google Scholar]
- Bauer S, Roy A, Emo J, Chapman T, Georas S, Lawrence BP. 2012. Effects of maternal exposure to bisphenol A on allergic lung inflammation into adulthood. Toxicol. Sci. 130:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden A, Kwiatkowski C, Colborn T. 2015. New look at BTEX: Are ambient levels a problem? Environ. Sci. Technol. 49:5261–5276. [DOI] [PubMed] [Google Scholar]
- Boule LA, Winans B, Lawrence BP. 2014. Effects of developmental activation of AHR on CD4+ T-cell responses to influenza virus infection in adult mice. Environ. Health Perspect. 122:1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boule LA, Chapman T, Hillman S, Kassotis C, O’Dell C, Robert J, Georas S, Nagel S, Lawrence BP. 2018. Developmental exposure to a mixture of 23 chemicals associated with unconventional oil and gas operations alters the immune system of mice. Toxicol. Sci. 163:639–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann E, Edvinsson A, Rostedt Punga A, Sundstrom-Poromaa I, Skalkidou A. 2019. Inflammatory and anti-inflammatory markers in plasma: From late pregnancy to early post-partum. Sci. Rep. 9:1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, and Georas S. 2014. Regulatory tone and mucosal immunity in asthma. Intl. Immunopharmacol. 23:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli I, Skalak K, Kent D, Engle M, Benthem A, Mumford A. 2017. Environmental signatures and effects of an oil and gas wastewater spill in the Williston Basin, North Dakota. Sci. Total Environ. 579:1781–1793. [DOI] [PubMed] [Google Scholar]
- Deziel N, Brokovich E, Grotto I, Clark C, Barnett-Itzhaki Z, Broday D, Shay K. 2020. Unconventional oil and gas development and health outcomes: A scoping review of the epidemiological research. Environ. Res. 182:109124. [DOI] [PubMed] [Google Scholar]
- DiGiulio D, and Jackson R. 2016. Impact to underground sources of drinking water and domestic wells from production well stimulation and completion practices in the Pavillion, Wyoming, field. Environ. Sci. Technol. 50:4524–4536. [DOI] [PubMed] [Google Scholar]
- Elliott E, Ma X, Leaderer B, McKay L, Pedersen C, Wang C, Gerber C, Wright T, Sumner A, Brennan M, et al. 2018. A community-based evaluation of proximity to unconventional oil and gas wells, drinking water contaminants, and health symptoms in Ohio. Environ. Res. 167:550–557. [DOI] [PubMed] [Google Scholar]
- Elsner M, and Hoelzer K. 2016. Quantitative survey and structural classification of hydraulic fracturing chemicals reported in unconventional gas production. Environ. Sci. Technol. 50:3290–3314. [DOI] [PubMed] [Google Scholar]
- Fink A, Engle K, Ursin R, Tang W, Klein S. 2018. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc. Natl. Acad. Sci. USA 115:12477–12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J, Lalor S, Sweeney C, Tubridy N, Mills K. 2010. T-Cells in Multiple Sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 162:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel G, and Arck P. 2014. Sex, immunity, and influenza. J. Infect. Dis. 209(S3):S93–99. [DOI] [PubMed] [Google Scholar]
- Gross S, Avens H, Banducci A, Sahmel J, Panko J, Tvermoes B. 2013. Analysis of BTEX groundwater concentrations from surface spills associated with hydraulic fracturing operations. J. Air Waste Manag. Assoc. 63:424–432. [DOI] [PubMed] [Google Scholar]
- Hirahara K, and Nakayama T. 2016. Cd4+ T-cell subsets in inflammatory diseases: Beyond the TH1/TH2 paradigm. Intl. Immunol. 28:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemielita T, Gerton G, Neidell M, Chillrud S, Yan B, Stute M, Howarth M, Saberi P, Fausti N, Penning T, et al. 2015. Unconventional gas and oil drilling is associated with increased hospital utilization rates. PLOS One 10:e1031093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis C, Tillitt D, Davis J, Hormann A, Nagel S. 2014. Estrogen and androgen receptor activities of hydraulic fracturing chemicals and surface and ground water in a drilling-dense region. Endocrinology 155:897–907. [DOI] [PubMed] [Google Scholar]
- Kassotis C, Klemp K, Vu D, Lin C, Meng C, Besch-Williford C, Pinatti L, Zoeller R, Drobnis E, Balise V, et al. 2015. Endocrine-disrupting activity of hydraulic fracturing chemicals and adverse health outcomes after prenatal exposure in male mice. Endocrinology 156:4458–4473. [DOI] [PubMed] [Google Scholar]
- Kassotis C, Bromfield J, Klemp K, Meng C, Wolfe A, Zoeller R, Balise V, Isiguzo C, Tillitt D, Nagel S. 2016. Adverse reproductive and developmental health outcomes following prenatal exposure to a hydraulic fracturing chemical mixture in female C57Bl/6 mice. Endocrinology 157:3469–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata T, and White K, Jr. 1990. Effects of naphthalene and naphthalene metabolites on in vitro humoral immune response. J. Toxicol. Environ. Health 30:53–67. [DOI] [PubMed] [Google Scholar]
- Klein S, and Flanagan K. 2016. Sex differences in immune responses. Nat. Rev. Immunol. 16:626–638. [DOI] [PubMed] [Google Scholar]
- Knowlden S, Hillman S, Chapman T, Patil R, Miller D, Tigyi G, Georas S. 2016. Novel inhibitory effect of a lysophosphatidic acid 2 agonist on allergen-driven airway inflammation. Am. J. Respir. Cell Mol. Biol. 54:402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langier S, Sade K, Kivity S. 2012. Regulatory T-cells in allergic asthma. Isr. Med. Assoc. J. 14:180–183. [PubMed] [Google Scholar]
- Lawrence BP, Warren T, Luong H. 2000. Fewer T-lymphocytes and decreased pulmonary influenza virus burden in mice exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). J. Toxicol. Environ. Health 61:39–53. [DOI] [PubMed] [Google Scholar]
- Lawrence BP, Roberts A, Neumiller J, Cundiff J, Woodland D. 2006. Aryl hydrocarbon receptor activation impairs the priming but not the recall of influenza virus-specific CD8+ T-cells in the lung. J. Immunol. 177:5819–5828. [DOI] [PubMed] [Google Scholar]
- Li H, Li D, He Z, Fan J, Li Q, Liu X, Guo P, Zhang H, Chen S, Li Q, et al. 2018. Effects of Nrf2 knockout on regulation of benzene-induced mouse hematotoxicity. Toxicol. Appl. Pharmacol. 358:56–67. [DOI] [PubMed] [Google Scholar]
- Lima J, Martins C, Leandro M, Nunes G, Sousa M, Branco J, Borrego L. 2016. Characterization of b cells in healthy pregnant women from late pregnancy to post-partum: A prospective observational study. BMC Pregnancy Childbirth 16:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaise Y, Lencina C, Cartier C, Olier M, Menard S, Guzylack-Piriou L. 2020. Perinatal oral exposure to low doses of bisphenol A, S, or F impairs immune functions at intestinal and systemic levels in female offspring mice. Environ. Health 19:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney K, Baruch-Mordo S, Patterson L, Nicot J, Entrekin S, Fargione J, Kiesecker J, Konschnik K, Ryan J, Trainor A, et al. 2017. Unconventional oil and gas spills: Materials, volumes, and risks to surface waters in four U.S. states. Sci. Total Environ. 581–582:369–377. [DOI] [PubMed] [Google Scholar]
- Marina M 2014. Xenoestrogens challenge 17β-estradiol protective effects in colon cancer. World J Gastrointest Oncol. Mar 15;6(3):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins C, Lima J, Nunes G, Borrego L. 2017. Regulatory T- and B-cells in asthmatic women: Variations from pregnancy to post-partum. J. Invest. Allergol. Clin. Immunol. 27:46–57. [DOI] [PubMed] [Google Scholar]
- McClelland E, and Smith J. 2011. Gender specific differences in the immune response to infection. Arch. Immunol. Ther. Exp. (Warszaw) 59:203–213. [DOI] [PubMed] [Google Scholar]
- McGuire C, Lawrence BP, Robert J. 2021. Thyroid disrupting chemicals in mixture perturb thymocyte differentiation in Xenopus laevis tadpoles. Toxicol. Sci. 10.1093/toxsci/kfab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale C, Zhang L, Smith M. 2012. Current understanding of the mechanism of benzene-induced leukemia in humans: Implications for risk assessment. Carcinogenesis 33:240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie L, Guo R, Witter R, Savitz D, Newman L, Adgate J. 2014. Birth outcomes and maternal residential proximity to natural gas development in rural Colorado. Environ. Health Perspect. 122:412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie L, Allshouse W, Byers T, Bedrick E, Serdar B, Adgate J. 2017. Childhood hematologic cancer and residential proximity to oil and gas development. PLoS One 12:e0180423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel I, Kerlero de Rosbo N, Ben-Nun A. 1995. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: Fine specificity and T-cell receptor Vβ expression of encephalitogenic T-cells. Eur. J. Immunol. 25:1951–1959. [DOI] [PubMed] [Google Scholar]
- Nagel S, Kassotis C, Vandenberg L, Lawrence BP, Robert J, Balise V. 2020. Developmental exposure to a mixture of unconventional oil and gas chemicals: A review of experimental effects on adult health, behavior, and disease. Mol. Cell. Endocrinol. 513:110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo S, Steyn F, McCombe P. 2014. Gender differences in autoimmune disease. Front. Neuroendocrinol. 35:347–369. [DOI] [PubMed] [Google Scholar]
- Oertelt-Prigione S 2012. The influence of sex and gender on the immune response. Autoimmun. Rev. 11:A479–485. [DOI] [PubMed] [Google Scholar]
- Orem W, Varonka M, Crosby L, Haase K, Loftin K, Hladik M,. 2017. Organic geochemistry and toxicology of a stream impacted by unconventional oil and gas wastewater disposal operations. Appl. Geochem. 80:155–167. [Google Scholar]
- Peckham H, de Gruijter N, Raine C, Radziszewska A, Ciurtin C, Wedderburn L, Rosser E, Webb K, Deakin C. 2020. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ICU admission. Nat. Commun. 11:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz P, Slizovskiy I, Lamers V, Trufan S, Holford T, Dziura J, Peduzzi P, Kane M, Reif J, Weiss T, et al. 2015. Proximity to natural gas wells and reported health status: Results of a household survey in Washington County, Pennsylvania. Environ. Health Perspect. 123:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S, Ogburn E, McCormack M, Casey J, Bandeen-Roche K, Mercer D, Schwartz B. 2016. Association between unconventional natural gas development in the Marcellus shale and asthma exacerbations. JAMA Intern. Med. 176:1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly E, Martin K, Jin G, Yee M, O’Reilly M, Lawrence BP. 2015. Neonatal hyperoxia leads to persistent alterations in nk responses to influenza A virus infection. Am. J. Physiol. 308:L76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, McGuire C, Kim F, Nagel S, Price S, Lawrence BP, de Jesus Andino F. 2018. Water contaminants associated with unconventional oil and gas extraction cause immunotoxicity to amphibian tadpoles. Toxicol. Sci. 166:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, McGuire C, Nagel S, Lawrence BP, de Jesus Andino F. 2019. Developmental exposure to chemicals associated with unconventional oil and gas extraction alters immune homeostasis and viral immunity of the amphibian xenopus. Sci. Total Environ. 671:644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A, Harp C, Noronha A, Miller S. 2014. Experimental autoimmune encephalomyelitis (EAE) model of MS: Utility for understanding disease pathophysiology and treatment. Handbook Clin. Neurol. 122:173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes I, and Goverman J. 2006. Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 1:1810–1819. [DOI] [PubMed] [Google Scholar]
- Strutt T, McKinstry K, Marshall N, Vong A, Dutton R, Swain S. 2013. Multi-pronged CD4+ T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunol. Rev. 255:149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S, McKinstry K, Strutt T. 2012. Expanding roles for CD4+ T-cells in immunity to viruses. Nat. Rev. Immunol. 12:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsangari X, Andrianou XD, Agapiou A, Mochalski P, Makris KC. 2017. Spatial characteristics of urinary BTEX concentrations in the general population. Chemosphere. 173: 261–266. [DOI] [PubMed] [Google Scholar]
- Tscharke D, Croft N, Doherty P, la Gruta N. 2015. Sizing up the key determinants of the CD8+ T-cell response. Nat. Rev. Immunol. 15:705–716. [DOI] [PubMed] [Google Scholar]
- Tustin A, Hirsch A, Rasmussen S, Casey J, Bandeen-Roche K, Schwartz B. 2017. Associations between unconventional natural gas development and nasal and sinus, migraine headache, and fatigue symptoms in Pennsylvania. Environ. Health Perspect. 125:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (USEPA). 2021. Hydraulic fracturing for oil and gas: Impacts from the hydraulic fracturing water cycle on drinking water resources in the United States (Final Report). https://cfpub.epa.gov/ncea/hfstudy/recordisplay.cfm?deid=332990 (last accessed 07/06/21)
- Vengosh A, Jackson R, Warner N, Darrah T, Kondash A. 2014. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the united states. Environ. Sci. Technol. 48:8334–8348. [DOI] [PubMed] [Google Scholar]
- Veraldi A, Costantini A, Bolejack V, Miligi L, Vineis P, van Loveren H. 2006. Immunotoxic effects of chemicals: A matrix for occupational and environmental epidemiological studies. Am. J. Ind. Med. 49:1046–1055. [DOI] [PubMed] [Google Scholar]
- Vorderstrasse B, Cundiff J, Lawrence BP. 2006. A dose-response study of the effects of prenatal and lactational exposure to TCDD on the immune response to influenza A virus. J. Toxicol. Environ. Health 69:445–463. [DOI] [PubMed] [Google Scholar]
- Vroman H, van den Blink B, Kool M. 2015. Mode of dendritic cell activation: The decisive hand in TH2/TH17 cell differentiation. Implications in asthma severity? Immunobiology 220:254–261. [DOI] [PubMed] [Google Scholar]
- Wang F, Liu F, Liu H, Chen W, Si X, Ma X. 2016. Effects of immunological and hematological parameter in mice exposed to mixture of volatile organic compounds. Inhal. Toxicol. 28:164–169. [DOI] [PubMed] [Google Scholar]
- Warren T, Mitchell K, Lawrence BP. 2000. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses the humoral and cell-mediated immune responses to influenza a virus without affecting cytolytic activity in the lung. Toxicol. Sci. 56:114–123. [DOI] [PubMed] [Google Scholar]
- Webb E, Bushkin-Bedient S, Cheng A, Kassotis C, Balise V, Nagel S. 2014. Developmental and reproductive effects of chemicals associated with unconventional oil and natural gas operations. Rev. Environ. Health 29:307–318. [DOI] [PubMed] [Google Scholar]
- Wen H, Yuan L, Wei C, Zhao Y, Qian Y, Ma P, Ding S, Yang X, Wang X. 2016. Effects of combined exposure to formaldehyde and benzene on immune cells in the blood and spleen in Balb/c mice. Environ. Toxicol. Pharmacol. 45:265–273. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). 2016. Protecting Surface Water for Health. Geneva: World Health Organization Report. ISBN: 978 92 4 151055 4. [Google Scholar]
- Xie L, Gong W, Chen J, Xie H, Wang M, Yin X, Wu W. 2018. The flavonoid kurarinone inhibits clinical progression of EAE through inhibiting TH1 and TH17 cell differentiation and proliferation. Intl. Immunopharmacol. 62:227–236. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Liu J, Sugiura T, Ishidao T, Ueno S, Yanagita H, et al. 2009. The indoor air pollutant 2-ethyl-hexanol activates CD4 cells. Chem.-Biol. Interact. 177:137–141. [DOI] [PubMed] [Google Scholar]
- Zabrodskii P, Kirichuk V, Osipov O. 2002. In vitro effects of alcohols and their metabolites on antibody-forming activity of T- and B-lymphocytes. Exp. Biol. Med. 133:258–260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be provided to interested parties based on reasonable request to the corresponding author (BPL).


