Abstract
Glycation process refers to reactions between reduction sugars and amino acids that can lead to formation of advanced glycation end products (AGEs) which are related to changes in chemical and functional properties of biological structures that accumulate during aging and diseases. The aim of this study was to perform and analyze in vitro glycation by fructose and methylglyoxal (MGO) using salivary fluid, albumin, lysozyme, and salivary α-amylase (sAA). Glycation effect was analyzed by biochemical and spectroscopic methods. The results were obtained by fluorescence analysis, infrared spectroscopy (total attenuated reflection—Fourier transform, ATR-FTIR) followed by multivariate analysis of principal components (PCA), protein profile, immunodetection, enzymatic activity and oxidative damage to proteins. Fluorescence increased in all glycated samples, except in saliva with fructose. The ATR-FTIR spectra and PCA analysis showed structural changes related to the vibrational mode of glycation of albumin, lysozyme, and salivary proteins. Glycation increased the relative molecular mass (Mr) in protein profile of albumin and lysozyme. Saliva showed a decrease in band intensity when glycated. The analysis of sAA immunoblotting indicated a relative reduction in intensity of its correspondent Mr after sAA glycation; and a decrease in its enzymatic activity was observed. Carbonylation levels increased in all glycated samples, except for saliva with fructose. Thiol content decreased only for glycated lysozyme and saliva with MGO. Therefore, glycation of salivary fluid and sAA may have the potential to identify products derived by glycation process. This opens perspectives for further studies on the use of saliva, an easy and non-invasive collection fluid, to monitor glycated proteins in the aging process and evolution of diseases.
Introduction
Glycation process refers to a sequence of non-enzymatic reactions that begin when reducing sugars such as glucose and fructose react with nucleophilic groups of amino acids from proteins, lipids or nucleic acids forming Schiff bases and Amadori products to produce advanced glycation end products (AGEs) [1,2]. In addition to its endogenously formation, AGEs and their precursors are also absorbed from exogenous sources, such as cigarette smoke and through the consumption of highly heated processed foods [3]. Globalization and industrialized methods of processing dramatically alter foods to give desirable properties as longer shelf life, sterility, flavor and color, thus increasing exposure to AGEs [4,5]. AGEs are related to modifying chemical and functional properties of most diverse biological structures and their formation is accelerated in diseases such as diabetes as a result of chronic hyperglycemia and increased oxidative stress [6].
The formation of glycation products or AGEs and their consequences can be elucidated from studies of in vitro glycation of target proteins, such as albumin and lysozyme, using fructose and methylglyoxal (MGO) as glycation agents [7–11]. Fructose is the most common monosaccharide in human diet mostly because of the high-fructose syrup produced by starch, which is usually added to beverages and baked foods; thus, it has specific metabolic characteristics that could potentially contribute to increase body adiposity and insulin resistance [12,13]. In addition to exogenous sources, fructose is endogenously produced as a consequence of activation of the polyol pathway under hyperglycemic conditions and it can be phosphorylated to fructose-6-phosphate, which is broken down into 3-deoxyglucosone; both compounds are powerful glycating agents that enter into the formation of AGEs [14,15]. Reducing sugars not only contribute directly to formation of AGEs, but also lead to the accumulation of dicarbonyl molecules, such as MGO, which is formed from non-enzymatic degradation of endogenous metabolites, mainly glycolytic intermediates. These molecules are very reactive and contribute to formation of AGEs [16,17].
In relation to protein targets for glycation in this study, albumin constitutes about 50% of the proteins present in the plasma of healthy individuals and has a variety of physiological and pharmacological functions [18,19]. Due to its half-life of approximately 21 days and its high concentration, serum albumin is a highly glycation-sensitive plasma protein [20]. It was demonstrated that elevated glycated albumin levels correlated with the severity of coronary artery disease in diabetic patients [21]. Thus, the level of glycated albumin arouses interest in clinical area, related mostly to its use as a short-to-intermediate term biomarker for glycemic control [22]. Lysozymes are important antibacterial defense proteins present at high levels in saliva, nasal secretion, mucus, serum and in the lysosomes of neutrophils and macrophages [23]. This protein affects cell wall peptidoglycans of gram-positive bacteria and catalyzes their degradation [24]. The balance between hydrophilic/hydrophobic surface of lysozyme plays an important role in its catalytic function and glycation process affects this balance decreasing its defense activity [25].
In recent years, saliva has been widely used for diagnosis and monitoring proteins in the body [26,27]. Moreover, studies have analyzed AGEs in saliva of patients, indicating high levels in patients with chronic diseases [28,29]. Younus, Ahmad and Alam [30] verified that amount of AGEs in the saliva of patients with diabetes increases as the history of this disease increases. Therefore, monitoring these AGEs or glycation products can be a powerful tool to delay diseases’ onset and development [31]. However, evidence in the literature around glycated proteins in saliva as biomarkers for diseases may not be so conclusive yet, since according to Khoury et al. [32] salivary fructosamine as biomarker for diabetes may be doubtful. In this way, more studies are needed to elucidate the perspective of glycation products and AGEs in salivary fluid as biomarkers. Regarding biological fluids for the identification of glycation effects and their products, we highlight saliva as an attractive source for its non-invasive, practical and low-cost collection.
Thus, the present study aimed to analyze structural and functional properties of salivary fluid and target proteins albumin and lysozyme after in vitro glycation by fructose and MGO for 21 days incubation, examining fluorescence intensity, ATR-FTIR and protein profiles, as well as protein carbonyl and thiol groups formation. In addition, we investigated whether the activity and protein profile of the main salivary enzyme sAA was affected by fructose and MGO.
Materials and methods
Reagents
Reagents of analytical grade were purchased from Sigma-Aldrich (Sigma, St Louis, MO, USA).
Volunteers
This study was approved by Ethics and Human Research Committee of Federal University of Uberlandia (protocol number: 4.466.521). For this study, saliva samples were collected from twelve healthy individuals, six men and six women, aged between 18 and 40 years. This number of volunteers was determined according to data from the literature involving saliva analysis [33,34]. After fitting inclusion criteria and giving consent, volunteers performed saliva collection. Four individuals performed an extra saliva collection to obtain purified sAA.
Saliva collection and processing
Saliva collection was performed on a single day in the morning (08:00 to 12:00) to guarantee the same conditions for all volunteers. Each volunteer received a tube for collection, which was carried out at Biochemistry and Molecular Biology Laboratory of Institute of Biotechnology of Federal University of Uberlandia. Saliva of 12 volunteers was collected using the method according to Navazesh [35], without mechanical stimulation in plastic conical tubes Falcon (50 mL). The volunteers were instructed not to eat food or any type of drink for at least 30 minutes before collection. Just before collecting, volunteers rinsed their mouths with distilled water to clean cell debris and then spit approximately 30 mL of saliva inside the tube. After collection, samples were centrifuged at 3000 rpm for 15 min at 4°C to obtain the supernatant, and then stored at -80°C. Subsequently, these frozen samples were lyophilized, weighed, and then again stored at -80°C until analysis.
Purification of sAA
This method was performed according to Santos et al. [36]. To obtain purified sAA, saliva samples were collected from four individuals, following the same collection protocol explained in previous topic. The saliva collected from all four volunteers (approximately 80 mL) was reunited and centrifuged at 12000 xg for 12 minutes at 4°C. The supernatant was diluted 1:1 (v/v) in 50 mM Tris-HCl pH 8.0 buffer, containing 10 mM EGTA and 10 mM EDTA and 0.2% sodium azide.
To perform ion exchange chromatography, a glass column was used with 9 cm high x 2 cm in diameter, packed with 63 mL of resin Q-Sepharose fast flow. The column was equilibrated with five volumes of Tris-HCl 25 mM pH 8.0, containing 5 mM EGTA and 5 mM EDTA and 0.2% sodium azide. A volume of 160 mL of the diluted saliva sample was applied in the column. The volume collected was dialyzed in 50 mM ammonium bicarbonate, lyophilized, and stored at -80°C for later analysis. Protein concentration was evaluated by Bradford method [37].
Glycation assay
The glycation assay was performed with all samples diluted in 200 mM phosphate buffer, pH 7.4 containing 0.02% sodium azide and incubated in the dark at 37°C for 21 days with fructose or MGO, according to protocols adapted by Franco et al. [38] and Justino et al. [39]. Samples containing target proteins were incubated with 1.25 M fructose or 53.3 mM MGO (diluted in 200 mM phosphate buffer, pH 7.4 containing 0.02% sodium azide). These concentrations have been used in studies of glycation inhibition by bioactive compounds from natural products, published by our research group [40,41]. We considered maintaining these concentrations in our experiments, even though they are much higher than human physiological levels, to ensure the glycation occurrence in the salivary fluid.
Samples investigated were the following: Bovine serum albumin (BSA) (50 mg/mL), hen egg white lysozyme (10 mg/mL), saliva (50 mg/mL) and sAA (10 mg/mL), all of them, incubated with fructose and MGO (BSA+F, BSA+MGO, LYS+F, LYS+MGO, SAL+F, SAL+MGO, sAA+F and sAA+MGO); These protein concentrations were based on previous studies from our research group [38–41]. Control non-glycated samples without fructose and MGO were incubated under the same conditions, replacing each one with phosphate buffer. After the incubation period, 20% trichloroacetic acid (TCA) was added in the samples and then centrifuged at 10000 xg for 10 minutes. Pellet was resuspended in water. For ATR-FTIR analysis, pellet was lyophilized. Dosage of proteins was measured by Bradford method [37].
Analysis methods
Fluorescence intensity
The fluorescence intensity of samples was measured in a 96-well microplate using a spectrofluorometer with excitation at 350 nm and emission at 420 nm (Perkin-Elmer LS 55, Massachusetts, USA). Measurements of fluorescence intensity in these wavelengths’ values are used to estimate formation of glycation products and AGEs and represent a qualitative measure of damage by glycation [42–44].
Attenuated total reflection—Fourier transform infrared spectroscopy (ATR-FTIR)
The analyzes of infrared spectra were acquired using ATR-FTR spectrophotometer Vertex 70 (Bruker Optics, Reinstetten, Germany) coupled to attenuated total reflectance component (ATR). The crystal material in ATR unit used was a diamond disk as an internal reflection element. The spectra of lyophilized samples were recorded in triplicate. Before each sample analysis the air spectrum was used as a background. The spectra were obtained in a room with a temperature between 22–23°C, 4 cm-1 resolution and 32 scans were performed. In sample processing, the baseline was corrected and normalized by vector before performing analyzes. The 1800–900 cm-1 region of the spectra of all samples was used as input data for multivariate principal component analysis (PCA) technique and vibrational mode areas were calculated from peaks of interest. PCA is a statistical method used to indicate differences between samples. PCA components were analyzed from total variance plot to obtain optimum number of components for datasets. Principal components (PC) scores plots can reveal common clustering of samples in spectra; and loadings plots associated with FTIR spectra provides score plots interpretation. All pre-processing and spectral analysis steps were performed with Origin Pro 9.1 (OriginLab Corporation, Northampton, United States) [45,46].
Measurement of carbonyl and thiol groups attached to proteins
For carbonyl measurement, dinitrophenylhydrazine (DNPH) was added to the samples, which were precipitated with 20% TCA, washed with ethanol-ethyl acetate, and dissolved in 6 mol L-1 of guanidine hydrochloride [40]. The absorbance values were recorded at 370 nm (VersaMax, Molecular Devices, Menlo Park, CA, USA) and carbonyl content was calculated using a molar absorbance of 22,000 mol L-1 cm-1; the results were expressed as nmol ratio of reacted DNPH. The content of free thiols was determined in control and glycated samples according to an established method using 5.5’-dithiobisnitrobenzoic acid (DTNB) [47]. Concentration of free thiol was calculated using 14,150 mol L-1 cm-1 as molar extinction coefficient of NTNB. Reading at 412 nm was performed using a spectrophotometer.
SDS polyacrylamide gel electrophoresis (SDS-PAGE)
The samples after glycation were solubilized in electrophoresis buffer (1:10) (Tris-HCl 31.2 mM, SDS 8.75%, sucrose 20%, β-mercaptoethanol 10%, EGTA-K 11 mM and bromophenol blue 0.25%), and then submitted to electrophoresis in sodium dodecyl sulfate (SDS)—polyacrylamide gel (SDS-PAGE) using 12% polyacrylamide gels for analysis of protein profile [48]. Samples containing 5 μg of proteins [37] were loaded into the wells and electrophoresis occurred at 35 mA (Bio-Rad Laboratories, Hercules, USA). The gels were stained with Coomassie blue R, destained with solution of methanol-acetic acid-water (25:7:68) and photographed by digitalization (Amersham Imager 600 system, GE Healthcare Bio-Sciences AB, Uppsala, Sweden).
Western blotting
Aliquots of saliva samples were solubilized in electrophoresis sample buffer. 5 μg of proteins from each sample were submitted to SDS-PAGE. The samples separated by SDS-PAGE were transferred to a nitrocellulose membrane in Tris-glycine buffer [49]. After transferred, membranes were blocked with 5% skimmed-milk powder in PBS-Tween 20 (PBS-T) and washed 3 times (2 times for 5 minutes and 1 time for 10 minutes) with PBS-T. After being blocked and washed, membranes were incubated overnight with primary anti-amylase antibody [36] followed by washing (twice for 5 minutes and once for 10 minutes) with PBS-T. The membranes were incubated with secondary anti-rabbit antibody (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), and washed with PBS-T. The antibodies bounded to the membranes were visualized by chemiluminescence. Intensity of proteins bands was analyzed by ImageQuantTL software, and results were expressed as densitometry of pixels.
sAA activity
To determine sAA activity after glycation, a colorimetric kinetic assay was used. This method is based on the hydrolysis of substrate 2-chloro-4-nitrophenyl-4-β-D galactopyranosylmaltoside (GalG2CNP), by alpha-amylase, releasing 2-chloro-4-nitrophenyl (CNP). sAA samples were diluted in MES buffer (MES 50 mM, NaCl 300 mM, CaCl2 5 mM, KSCN 140 mM, pH 6.3), followed by addition of GaLG2CNP substrate and reading was performed on a spectrophotometer, for 3 minutes at 37°C, with interval of 1 minute between each reading [50].
Statistical analysis
Statistical and graphical analyzes were performed using GraphPad Prism 6.0 software. All analyzes were performed in duplicate and data were expressed as mean ± standard deviation. The significance of difference was calculated using one-way or two-way ANOVA, and Tukey and Dunnett’s post-tests for multiple comparisons. Values of p <0.05 were considered significant.
Results
Fluorescence intensity
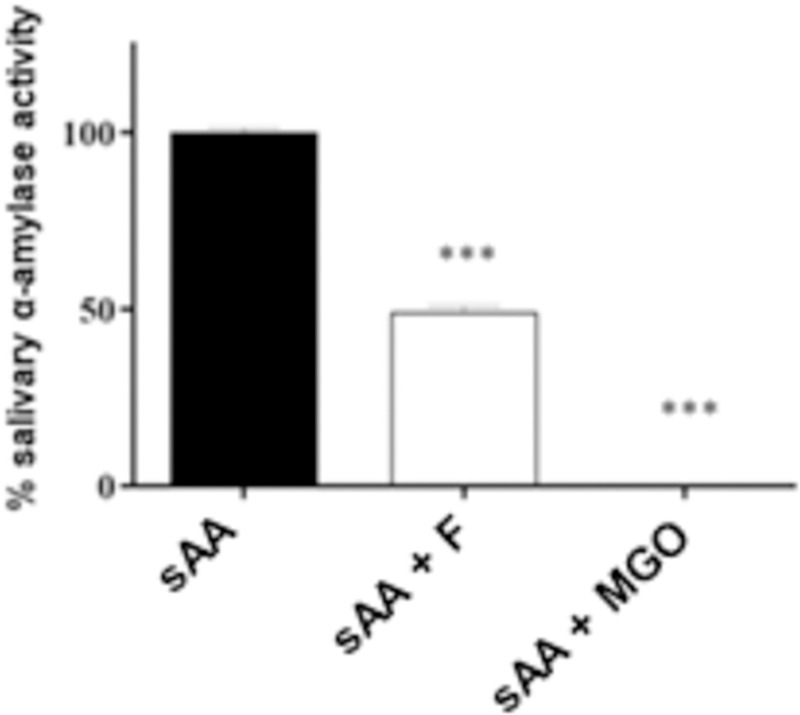
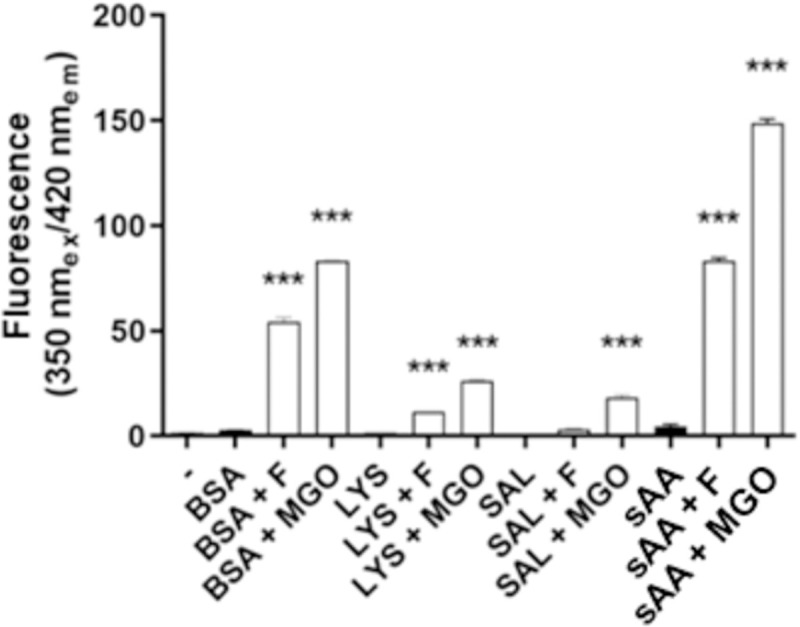
The fluorescence intensity of BSA, lysozyme, saliva and sAA when incubated for 21 days with fructose or MGO is shown in Fig 1. Fluorescence increased in glycated samples by fructose and MGO when compared to non-glycated samples. Values of these increases were as follows: BSA+F (51.6) and BSA+MGO (80.5); LYS+F (9.7), LYS+MGO (24.8); SAL+MGO (17.0); sAA+F (80.0) and sAA+MGO (148.8). However, saliva incubated with fructose showed no difference. Fluorescence intensity of the samples after 7 days of incubation is available as Supporting Information (S1 Fig).
Fig 1. Fluorescence intensity of non-glycated BSA, LYS, SAL and sAA, and glycated by fructose or MGO.
*** p < 0,001.
ATR-FTIR
In order to evaluate spectral differences related to glycation process with fructose and MGO after incubation of 21 days, spectra of samples were submitted to PCA analysis. A clear separation between glycated BSA and lysozyme were observed in the score plots. The percentage of variance explained by the first two PCs showed 99.9% for BSA+F and 100% for BSA+MGO, LYS+F and LYS+MGO (Supporting Information, S2A, S2C, S3A and S3C Figs). Saliva PCA score plot showed an efficient separation of 83.9% by variance explained with the first two PCs of SAL+F, and a moderate distinction of 74.7% of SAL+MGO (Supporting Information S4A and S4C Fig). The graph shows that the first two PCs separate samples data into two clusters.
To evaluate the relation between PCs and original variables, loadings graph shows how the original variables report to PCs (Supporting Information, S2B–S4B and S2D–S4D Figs). In this way, ATR-FTIR data followed by PCA analysis could be used to indicate general changes in glycation process caused by incubation for 21 days with fructose and MGO, without necessarily identifying specific vibrational modes of glycation process. With this, spectral profile of fructose and MGO and samples of BSA, lysozyme and saliva incubated with these glycation agents are represented in Figs 2A, 2B, 3A, 3G, 4A and 4G. From the peak information shown by graphs of PCA loads and spectra of fructose and MGO agents, it was possible to visualize peaks of interest that represented glycation process.
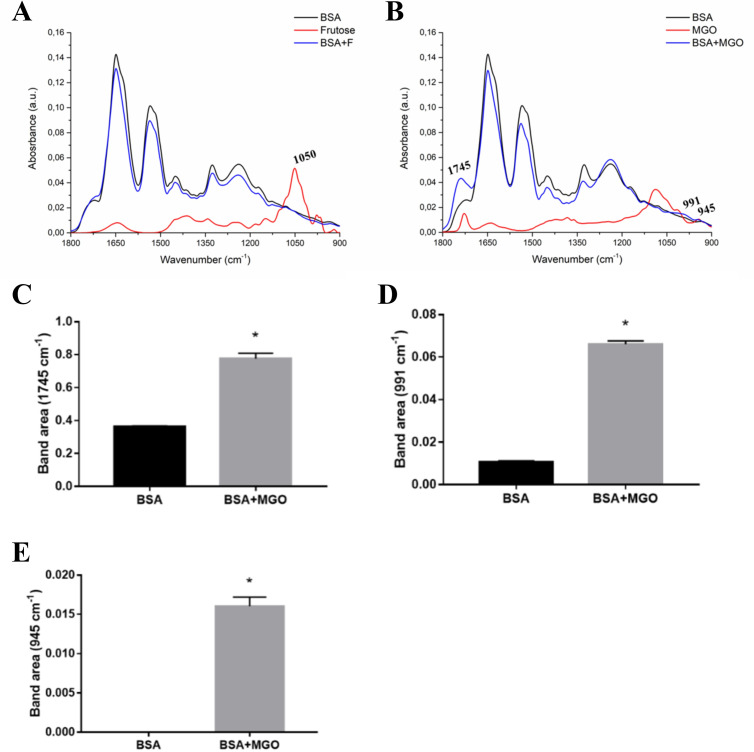
Fig 2. Average ATR-FTIR spectra (1800–900 cm-1) of BSA, fructose, MGO, and BSA incubated with these glycating agents.
(A) BSA and BSA with fructose (BSA+F). (B) BSA and BSA with MGO (BSA+MGO). Peak area of the spectra in vibrational modes in 1745 cm-1 (C), 991 cm-1 (D) and 945 cm-1 (E).
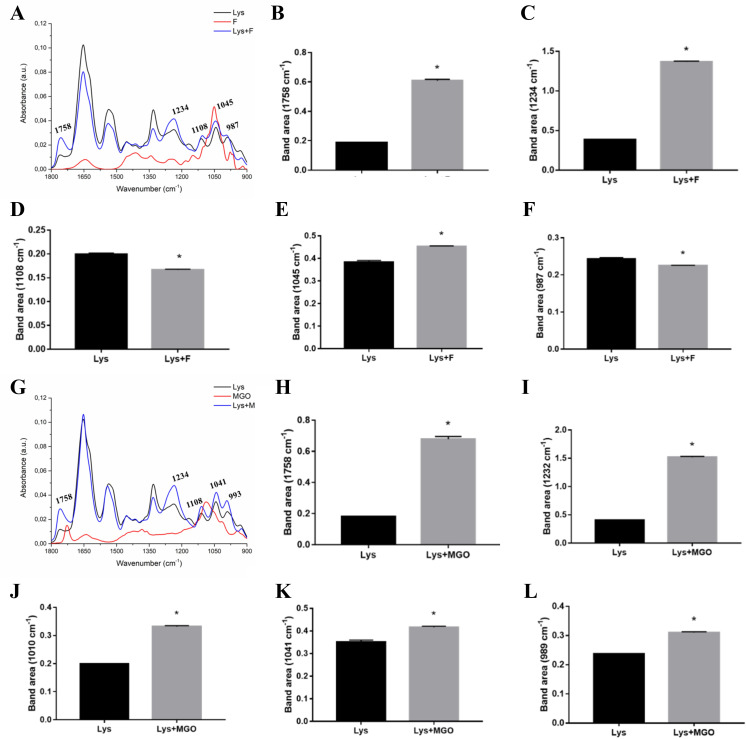
Fig 3. Average ATR-FTIR spectra (1800–900 cm-1) of LYS, fructose, MGO, and LYS incubated with these glycating agents.
(A) LYS and LYS with fructose (LYS+F). Peak area of the spectra in vibrational modes in 1758 cm-1 (B), 1234 cm-1 (C), 1108 cm-1 (D), 1045 cm-1 (E) and 987 cm-1 (F). (G) Average spectra of LYS and LYS with MGO (LYS+MGO). Peak area of the spectra in vibrational modes in 1758 cm-1 (H), 1234 cm-1 (I), 1108 cm-1 (J), 1041 cm-1 (K) and 993 cm-1 (L).
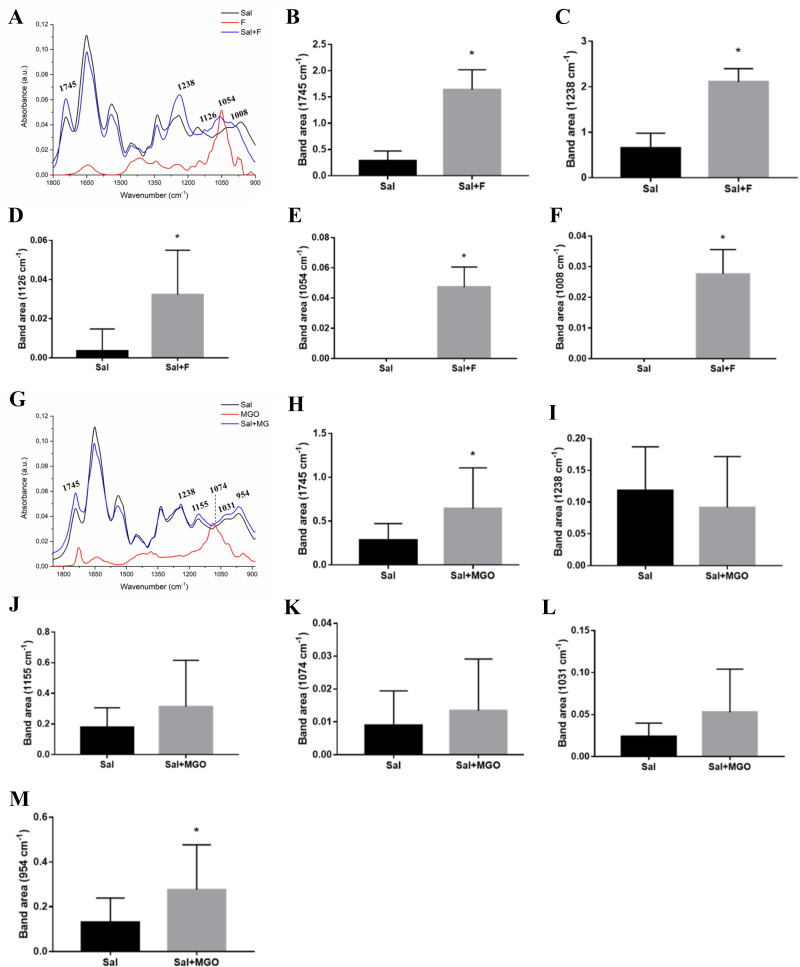
Fig 4. Average ATR-FTIR spectra (1800–900 cm-1) of SAL, fructose, MGO, and SAL incubated with these glycating agents.
(A) SAL and SAL with fructose (SAL+F). Peak area of the spectra in vibrational modes in 1745 cm-1 (B), 1238 cm-1 (C), 1126 cm-1 (D), 1054 cm-1 (E) and 1008 cm-1 (F). (G) Average spectra of SAL and SAL with MGO (SAL+MGO). Peak area of the spectra in vibrational modes in 1745 cm-1 (H), 1238 cm-1 (I), 1155 cm-1 (J), 1074 cm-1 (K), 1031 cm-1 (L) and 954 cm-1 (M).
The main highlight of fructose spectrum was the 1050 cm-1 vibrational mode (OH and CH2). The comparison of BSA spectra did not show any specific differences in glycation process between BSA and BSA+F (Fig 2A). Glycation of BSA with MGO, on the other hand, showed important differences in the spectrum (Fig 2B). An increase in peak areas in vibrational modes were observed at 1745 cm-1 (C = O stretching vibration of proteins and polysaccharides), 991 cm-1 (COC and CO by ring vibrations of carbohydrates; COH bonds and OCH3 of polysaccharides) and 945 cm-1 (COC stretching and OH, COC deformation of carbohydrates) (Fig 2C–2E, respectively).
The spectra of lysozyme, fructose and lysozyme incubated with fructose showed characteristic differences as indicated in Fig 3A, highlighting the wavelengths of different peaks. Analysis of the area of these peaks shows respective area increases in 1758 cm-1 (C = O of the polysaccharides), 1234 cm-1 (components of the CN amide III band of proteins) and 1045 cm-1 (CO elongation frequencies coupled to CO flexion frequencies of the C-OH groups of carbohydrates) (Fig 3B, 3C and 3E). However, there was a decrease in peak areas in 1108 cm-1 (COOH lengthening of the sugar portions) and 987 cm-1 (C-O-C, C-O of carbohydrates and OCH3 of polysaccharides) (Fig 3D and 3F). Regarding the effect of incubation with MGO on glycation of lysozyme, it was possible to observe marked changes in the spectrum (Fig 3G). Analysis of the area of peaks modified by lysozyme glycation shows an increase in 1758 cm-1, 1234 cm-1, 1108 cm-1, 1041 cm-1 (CO elongation frequencies coupled with CO fold frequencies of groups C- OH of carbohydrates) and 993 cm-1 (COC, CO of carbohydrates and OCH3 of polysaccharides) (Fig 3H–3L). As seen in BSA glycation spectrum, these vibrational modes represent structural components mainly related to proteins, polysaccharides and carbohydrates.
Fig 4A represents the average spectra of saliva, fructose, MGO and glycated saliva after incubation with these agents. The main peaks evaluated for fructose-glycated saliva showed several differences in the spectrum with an increase in peak band area at 1745 cm-1 (C = O stretching vibration of proteins and polysaccharides), 1238 cm-1 (CN amide III band components of proteins, CO stretching in carboxylic acid and PO2- stretching), 1126 cm-1 (CO of carbohydrates), 1054 cm-1 (oligosaccharide C-OH bonds) and 1008 cm-1 (CH2OH groups, CO stretching and COH groups bending) (Fig 4B–4F). It is important to highlight that peaks 1054 cm-1 and 1008 cm-1 were exclusive to the incubation of saliva with fructose, therefore did not found in non-glycated saliva. Saliva and MGO spectra is represented in Fig 4G. However, there was observed an increase only in area of peaks bands at 1745 cm-1 and 954 cm-1 (C-O-C, C-O of carbohydrates) of SAL+MGO (Fig 4H and 4M, respectively).
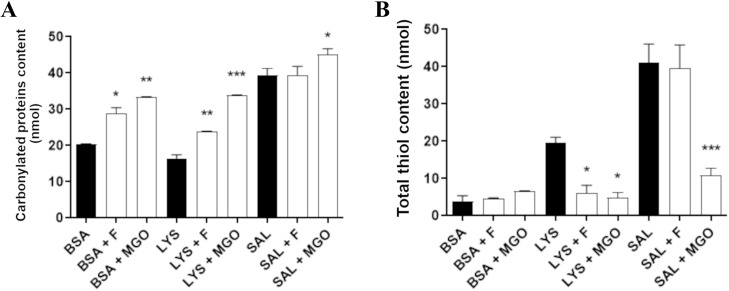
Levels of carbonylated protein and thiol groups
The levels of carbonylated proteins and total thiols in the target proteins and salivary fluid are shown in Fig 5. Protein carbonylation (Fig 5A) was increased in BSA+F, BSA+MGO, LYS+F, LYS+MGO and SAL+MGO, while SAL+F was not different from non-glycated saliva. Total thiols levels in BSA, lysozyme and saliva are shown in Fig 5B. For BSA, there was no significant difference of thiols level among glycated (fructose and MGO) to non-glycated BSA. On the other hand, comparing with non-glycated lysozyme, thiols levels decreased for LYS+F and LYS+MGO, while for saliva, only SAL+MGO showed a reduction in thiols content.
Fig 5.
Carbonylated proteins (A) and total thiol contents (B) in non-glycated BSA, lysozyme and saliva, and glycated by fructose or MGO. * p < 0,05; ** p < 0,01; *** p < 0,001.
Protein profile by SDS-PAGE and immunodetection
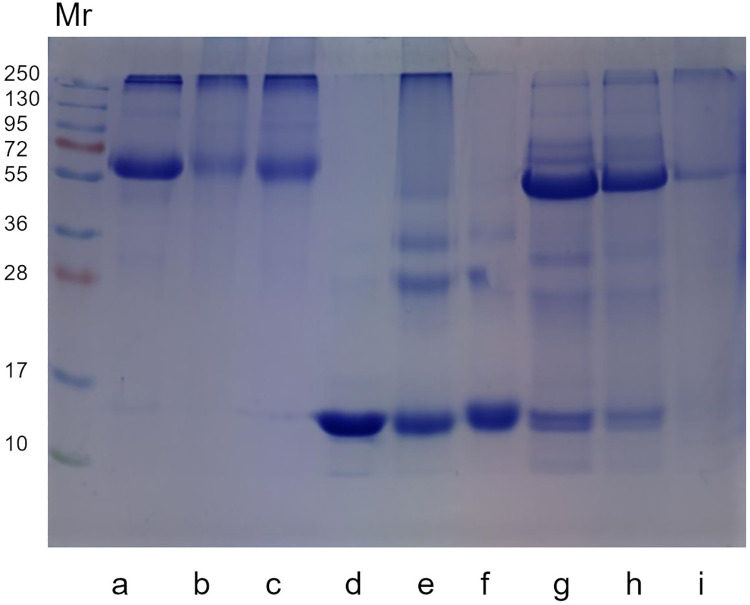
Protein profile in SDS-PAGE (Fig 6) of non-glycated and glycated BSA, LYS and SAL suggest the formation of crosslinks in glycated proteins due to changes in intensity of bands’ staining or appearance of new bands after glycation. BSA (66kDa) reduced Mr 66 band staining when glycated with fructose and MGO compared to non-glycated BSA. In addition, it is possible to detect a possible new band in Mr 95 of glycated BSA. In LYS, (14 kDa) Mr 14 band is intensely colored. However, its intensity reduced in LYS+F and LYS+MGO. Moreover, bands of higher Mr are more observed in LYS+F suggesting the formation of oligomers (28, 36 and 55 kDa). Protein profile of non-glycated saliva reveals two prominent bands with different Mr, we highlight Mr 56 and Mr 14 corresponding to sAA and lysozyme respectively. Glycation of saliva caused a reduction in staining intensity of these bands, also suggesting formation of crosslinks that may made it difficult to enter the polyacrylamide gel. Immunodetection was performed with saliva samples and complements the result by showing the effect of glycation on the expression of Mr 56 band. In SAL+MGO there was a reduction of approximately 74% in pixel intensity of this band (S5A and S5B Fig). In Supporting Information, protein profile of all saliva and sAA samples can be found after 21 days of incubation (S6A–S6C and S7 Figs, respectively), as well as the protein profile of sAA after 7 days of incubation (S8 Fig), and image of all blot (S1 Raw images).
Fig 6. Protein profile (SDS-PAGE) of non-glycated BSA, LYS and saliva and glycated by fructose or MGO.
Each lane was loaded with the following sample: (a) BSA; (b) BSA+F; (c) BSA+MGO; (d) LYS; (e) LYS+F; (f) LYS+MGO; (g) SAL; (h) SAL+F; (i) SAL+MGO. Mr: Relative molecular mass of the protein standard (kDa).
Purified sAA activity
Purified sAA was evaluated for its enzymatic activity, as shown in Fig 7. The result indicates significant decreases of this enzyme activity. sAA+F presented 49% of activity in relation to non-glycated sAA, and sAA+MGO reveled drastic reduction of activity with only 0.2% in relation to non-glycated sAA. sAA activity after 7 days of incubation is shown in Supporting Information (S9 Fig).
Fig 7. Enzymatic activity of non-glycated sAA, and glycated by fructose or MGO. *** p < 0,001.
Discussion
The study of proteins containing early-stage glycation products or AGEs has become of great interest due to evidence of AGEs effects on protein function, tissue damage and oxidative stress in aging and some diseases specially diabetes [50–55]. In this study, salivary fluid and proteins albumin, lysozyme and sAA were used as targets for non-enzymatic glycation by fructose and MGO for 21 days.
Levels of glycation products or AGEs depend on the half-life of proteins. On long-lived proteins they even accumulate over the lifetime of organisms [56]. In general, circulating proteins have a relatively short half-lives compared to structural proteins [57]. Some of the proteins present in saliva have short half-lives such as albumin with approximately 15–20 days [58]; lysozyme with 7 days [59]; immunoglobulin A with 3–6 days, plasmatic amylase has a half-life around 12–24 hours [60], however, in saliva the data seem to be not so clear yet. For having shorts half-lives, these proteins may underestimate the accumulation of AGEs [57] but could represent the glycation status more precisely in the last few days or hours. According to the proteins half-lives above, incubation period of 21 days in our study was enough for proteins to be glycated.
The concentrations of glycation agents used in our incubation were based on previous studies of our research group about antiglycation properties of natural products [40,41]. They are much higher than physiological levels, which range around 35 μM for fructose and 10 μM for MGO. These low values make hard to translate them into a good circulating marker of AGEs [61,62]. Kinetically, fructose may have a very fast conversion of Heyns compounds so that an increased fructose intake from the diet might potentiate the Maillard reaction [63]. Thereby AGEs formed in the intestinal lumen from the diet may contribute to the circulating AGE levels [61]. Although the concentrations of fructose and MGO were high, they might be convenient for modeling the glycation process that occurs in the organism in physiological condition in short time over weeks or months [64]. For ensuring the glycation occurrence in the salivary fluid, we maintained those high concentrations so that it was possible to visualize the effects of glycation, since saliva in vitro glycation is not very approached in literature.
Based on fluorescence properties it was possible to observe an increase in fluorescence intensity of glycated proteins in this study. Analyzing glycated albumin by fructose (BSA+F), we found corresponding results in other studies [65,66]. It has been shown that early and advanced glycation of BSA by fructose has a site specificity, with lysine-524 residue being the main target for BSA modification. Almost every change of lysine in glycated BSA with fructose was attributed to formation of carboxymethylysine (CML) [9]. On the other hand, the effect on albumin exposed to MGO caused a greater increase in fluorescence intensity, which is in line with other results [67,68]. The irreversible changes generated by glycation of MGO in BSA are highly selective for arginine residues, with some modifications of lysine and amino-terminal groups [69,70]. Many studies show that high concentrations of glycated albumin are associated with several diabetic complications, and this has been reported as a powerful indicator of glycemic control because its half-life is shorter than that of HbA1c, better representing glycemic variations [71,72].
Fluorescence intensities in glycation of lysozyme by fructose and MGO were also increased, which coincides with previously found results [11,73]. Glycation process of lysozyme results in reduction of its antibacterial function. Such changes are an important factor in increasing the prevalence of bacterial infections observed in patients with diabetes [23]. MGO is highly reactive and reacts with lysozyme at a higher rate than glucose, resulting in formation of crosslinked AGEs [74].
Glycated saliva by fructose showed no changes on fluorescence intensity. One possible reason for this result is related to saliva antioxidant capacity. It was demonstrated that AGE fluorescence was significantly lower in healthy adults, in response to the most effective antioxidant defense in people aged 25–45 [75]. Moreover, salivary proteins have several functions besides digestive. There are many enzymes with important properties for oral health, regulation of defense and endocrine systems [76]. Thus, it is possible that these enzymatic activities in saliva may also influenced the glycation state indicated by fluorescence. Otherwise, effect of saliva glycation on fluorescence intensity generated a significant increase in SAL+MGO. It is known that MGO forms hydroimidazolone derived from modification of arginine (MG-H1) and also carboxyethylisine (CEL) [53]. Manig et al. [77] suggested that levels of glycation compounds in saliva may be useful biomarkers to assess diabetes, since some AGEs found in saliva, such as CEL, CML and MG-H1, are higher in diabetic individuals. Studies involving the formation of AGEs in a hyperglycemic state have shown that MGO is considered the main precursor to AGEs, which can lead to enzymatic inactivation and protein denaturation [7,78]. A recent study corroborates our data showing high levels of AGEs associated with oxidative stress biomarker of protein oxidation and lipid peroxidation in saliva of patients with chronic heart failure [28].
Results of sAA showed a significant increase in fluorescence intensity due to glycation promoted by incubation with fructose and MGO. This higher fluorescence of glycated sAA may be related to the reduction of its activity as observed in glycated sAA by fructose and MGO. Several studies that investigated levels of sAA in diabetics and healthy individuals, resulted in reduction of their activity and concentration in diabetics, corroborating our result [79–81]. Also, Klimiuk et al. [28] reported a reduction in sAA activity correlated with an increase of AGEs in saliva of patients with progressive chronic heart failure.
As a method used to analyze structural changes in molecules, ATR-FTIR spectroscopy clearly indicated changes in the structure of albumin, lysozyme and saliva caused by glycation by both fructose and MGO. For sAA analysis, methods of sample preparation are still being refined for better readings in the equipment.
In glycation process molecules are attached to albumin at multiple side chains, like lysine, arginine and cysteine residues that induce secondary and tertiary structure changes, contributing to alterations in biological and physiological properties of this protein, such as drug and metabolite binding capacity and free radical scavenging. These post-translational modifications of glycated albumin may be related to long term diabetic complications [20,82,83].
Lysozyme is a protein consisting of ~40% of the α-helical structure and has six lysine residues and eleven arginine residues as potential glycation sites [84,85]. Studies previously showed that glycation alters conformation of lysozyme secondary and tertiary structures, and the loss of α-helix results in reduction in its bactericidal and enzymatic activity, thereby increasing susceptibility to bacterial infections in diabetes [86–88].
In some diseases such as diabetes and hypertension, saliva presents changes in its protein components, and glycation process may contribute to impair their proteins functions [23,89]. Ansari et al. [90] used FTIR technique to verify Amadori’s products and concluded that glycated protein can be a good diagnostic biomarker for early glycation process in diabetes. These possible biomarkers may exhibit specific signatures in infrared spectrum of saliva, as vibration absorbances of side chain spectra associated with glycation appear to be quite prominent in this fluid [91].
Vibrational mode regions can be slip up so that amide I is represented by region around 1600-1800cm−1, amide II around 1400–1500 cm−1, amide III around 1200–1400 cm−1 and at lower wavenumbers, amino acid and sugar molecules between 750–1100 cm−1 [85]. Glycation process in this study contributed to increase intensity of unique vibrational modes. We emphasize the difference in vibrational modes at ~1750 cm-1 and ~1050 cm-1, which are related to carbonyl functional groups and carbohydrate/sugar peak vibrations, respectively. These findings are in line with glycation reaction process, which involves reducing sugars, in polyol pathway with free aldehyde and an amine group, usually in a side chain of lysine and arginine [92,93].
Thus, these data suggest that vibrational modes at 1745 cm-1 and 1758 cm-1 (C = O of carbonyl group) can be used to distinguish the presence of glycation in these proteins. The formation of new molecules detected by ATR-FTIR may be related to aldehyde terminal group in glycation process [94]. Therefore, data presented by ATR-FTIR spectroscopy confirmed changes in glycated proteins both when exposed to fructose or MGO for 21 days. The exception was albumin sample incubated with fructose which it was not possible to detect these changes.
Protein carbonylation can be provoked through covalent modification of proteins with oxidative by-products of reducing sugars, such as reactive dicarbonyl molecules MGO, glyoxal and 3-deoxyglucosone [17,52,95]. Glycation of albumin and lysozyme by fructose and MGO generated a significant increase in carbonyl content, which agrees with results found in previous studies [96–98]. A study that quantified the formation of carbonylated protein in saliva indicated higher levels in diabetics, corroborating our result of an increased level of carbonylation when incubated with MGO in saliva [99]. Carbonyl groups are indirectly introduced into proteins by covalent adduction of reactive carbonyl species to side chains of the nucleophilic amino acids arginine, lysine and cysteine [100]. Interaction of proteins with reactive carbonyls can result in inactivation and modification of essential cellular proteins that can potentially lead to cytotoxicity and contribute to diseases, as indicated in a study that detected elevated amount of protein carbonyls in cardiovascular disease [101].
Dicarbonyl compounds also react rapidly with thiol groups of amino acids, peptides and proteins to give thiol-aldehyde adducts at cysteine residues and this depletion may enhance oxidative damage in proteins [102]. The results of free thiols showed a reduction in their levels for glycated lysozyme by both fructose and MGO. Whereas for saliva only MGO reduced thiol content, indicating association between glycation products and oxidative state of proteins. Studies corroborate these results showing that glycation process provoked a reduction in free thiol group of proteins [83,103,104]. Moreover, Rajeshwari et al. [105] found decreased levels of thiols in saliva of diabetic group in relation to healthy individuals. Thiols are able to scavenge oxidants playing a significant role in protecting biomolecules from oxidative stress [106,107]. Thus, their glycation by carbonyl compounds is highly associated with complications of diseases such as diabetes, in which oxidative and carbonyl stress are elevated leading to decrease in free thiol contents and antioxidant capacity [54,108,109]. sAA represents the most abundant protein in salivary fluid [110], then, we presume that both results of carbonylation and thiol content in glycated saliva would represent sAA as well.
From analysis of SDS-PAGE, it was possible to observe in glycated albumin that a modification of its polypeptide chain of 66 kDa occurs, losing its intensity. Alqahtani et al. [111] also found in their study that MGO had an influence on the electrophoretic pattern of BSA which resulted in lower intensiveness protein band compared to untreated protein. Besides, the appearance of a subtle but distinct band when compared to profile of non-glycated albumin suggested a possible correspondence with a tripolymer that emerged as an indication of crosslinking in glycated albumin. This type of observation has also been described in Zhang et al. [112]. The electrophoretic profile of glycated lysozyme also showed formation of high molecular weight products in the presence of fructose and MGO, characterizing crosslinked lysozyme. These products represent dimers, trimers, and tetramers with molecular weights of approximately 28, 36 and 55 kDa, respectively, induced by glycation process [113]. This corroborates our previous results that showed occurrence of glycation in lysozyme. In electrophoresis of saliva samples, all bands had their intensity decreased with glycation by fructose and MGO, however, formation of probable bands was not observed that would confirm the crosslinking of proteins. One possibility that explains this result is the great heterogeneity of compounds present in salivary fluid, hindering the specific detection of AGEs. There is lack of studies in literature that verify by SDS-PAGE the formation of glycation products or AGEs in glycated saliva, therefore, more researches are needed in this area.
Finally, saliva samples were also subjected to Western blotting, which revealed a significant reduction in expression of sAA, after glycation with MGO. This corroborates previous results in this study, in which saliva showed increased fluorescence intensity and oxidative damage by MGO, as well as difference in vibrational modes in ATR-FTIR, confirming alterations by glycation process. Besides, glycation generated accentuated reduction of sAA activity, especially after MGO incubation. These results indicate that glycation process caused structural alterations on sAA, which contributed for reduction of its enzymatic activity and consequently decrease their band expression. Lower sAA activity has been correlated with oxidation and protein glycation products and several studies showed significant decrease of sAA activity in diabetes [79,80,114,115]. In addition, according to Mekahli et al. [116], hyperglycemia induces perturbation in the intracellular Ca2+ signaling in salivary glands and leads to improper post-translational processing and folding of proteins, consequently decreasing sAA activity in diabetic individuals.
Among all analysis methods performed in this study, we highlight the fluorescence as the most sensible, significative, and practical for using in samples of patients. According to Perrone et al. [31], estimation of serum, urine, and saliva AGEs might be measured by spectroscopic and fluorimetric methods. Pentosidine is one of the fluorescent AGEs and it is a well-accepted marker of cumulative protein damage in aging and a variety of disease states including diabetes [117]. However, the amount of AGEs quantified by this method provides only the quantification of fluorescent AGEs and may be interfered by non-AGE fluorophores [31].
Given all the results obtained in our study, we showed that it is possible to identify glycation products in salivary fluid, and its applicability may contribute to the area of biomarkers in diseases. However, this is a field that still needs to be well investigated, deepened, and should be treated with caution, as studies have also shown that there might not be such a conclusive relation between AGEs, saliva and diagnosis. Yilmaz et al. [118] found a strong correlation between salivary AGEs and HbA1c, otherwise Rao et al. [119] showed weak correlation between salivary protein glycosylation and HbA1c. If salivary fluid is really remarkable to correlate AGEs specifically in diseases, this fluid will be a very promising tool for the diagnosis and monitoring the glycation process, its products and its consequences in various diseases.
The findings of this work promoted the understanding around structural and functional alterations of proteins submitted to glycation by fructose and MGO. In vitro glycations of BSA and hen egg white lysozyme, both homologous to human proteins [120,121], are well established in the literature and could be used as glycation models in order to analyze and improve in vitro glycation assay in saliva, which has become in recent years a promising fluid in clinical area to be used as an indicator of glycation process and salivary protein alterations present in several diseases. We believe that our method configurates a previous vision around glycation products in saliva by biochemical and spectroscopic approach. Adjustments of protein and glycation agents’ concentrations are needed for better representation; or further, analysis of samples in real specific conditions of diseases, until we get a certain conclusion about biomarkers as AGEs or glycation products in salivary fluid for diagnosis.
Conclusion
Overall, in this study glycated samples presented increased florescence intensity, structural changes related to vibrational modes of protein glycation, increased protein carbonylation, decreased thiol groups, protein profile alterations and relative reduction of band corresponding to Mr of sAA and its activity. These results indicate that fructose and/or MGO lead to formation of glycation products, protein alterations and oxidative damage when incubated with saliva, BSA and lysozyme for 21 days. Plus, sAA had reduction of activity and alterations of protein profile as well. We emphasize saliva as a possible biological fluid for glycation analysis. Thus, glycation products and AGEs in saliva may have the potential to contribute to a possible improvement in assessment and monitoring of patients with diseases in an accessible and non-invasive way. In addition, due to scarce studies in literature involving in vitro glycation of saliva and sAA, this study may contribute to direct more research and open perspectives in this area.
Supporting information
** p < 0,01; *** p < 0,001.
(TIF)
(A) Scores scatter plot of PC1 vs PC2 performed on the BSA and BSA+F spectrum. (B) PC1 loadings profile obtained for BSA and BSA+F. (C) Scores scatter plot of PC1 vs PC2 performed on the BSA and BSA+MGO spectrum. (D) PC1 loadings profile obtained for BSA and BSA+MGO.
(TIF)
(A) Scores scatter plot of PC1 vs PC2 performed on the LYS and LYS+F spectrum. (B) PC1 loadings profile obtained for LYS and LYS+F. (C) Scores scatter plot of PC1 vs PC2 performed on the LYS and LYS+MGO spectrum. (D) PC1 loadings profile obtained for LYS and LYS+MGO.
(TIF)
(A) Scores scatter plot of PC1 vs PC2 performed on the SAL and SAL+F spectrum. (B) PC1 loadings profile obtained for SAL and SAL+F. (C) Scores scatter plot of PC1 vs PC2 performed on the SAL and SAL+MGO spectrum. (D) PC1 loadings profile obtained for SAL and SAL+MGO.
(TIF)
(A) Western blotting of sAA expression in SAL (lane a), SAL+F (lane b), and SAL+MGO (lane c). (B) Quantification of immunodetected sAA given in density of pixels.
(TIF)
(A,B and C) Each lane was loaded with de following sample: (a): SAL; (b): SAL+F; (c): SAL+MGO; Mr: relative molecular mass of the protein standard (kDa).
(TIF)
Each lane was loaded with de following sample: (a): sAA (b): sAA+F; (c): sAA +MGO.
(TIF)
Each lane was loaded with de following sample: (a): sAA; (b): sAA+F; (c): sAA+MGO.
(TIF)
** p < 0,01; *** p < 0,001.
(TIF)
(PDF)
Acknowledgments
The authors gratefully acknowledge the Institute of Biotechnology and Graduates Program of the Federal University of Uberlandia (Genetics/Biochemistry and Health Science) for infrastructural support and Renata Roland Teixeira for technical assistance in electrophoresis method.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
M.Y.M and D.C.C. received graduate fellowships from Research Support Foundation of the State of Minas Gerais (FAPEMIG, http://www.fapemig.br/pt/), A.B.J. and J.S.Q. received graduate fellowships from Coordination for the Improvement of Higher Education Personnel (CAPES, https://www.gov.br/capes/pt-br). F.S.E. and R.S.S. received financial support of National Institute of Science and Technology in Theranostic and Nanobiotechnology (INCT-TeraNano, http://www.teranano.ufu.br/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhang Q, Ames JM, Smith RD, Baynes JW, Metz TO. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: probing the pathogenesis of chronic disease. J Proteome Res. 2009;8(2): 754–769. doi: 10.1021/pr800858h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001; 44(2): 129–46. doi: 10.1007/s001250051591 [DOI] [PubMed] [Google Scholar]
- 3.Sharma C, Kaur A, Thind SS, Singh B, Raina S. Advanced glycation end-products (AGEs): An emerging concern for processed food industries. J Food Sci Technol. 2015;52(12): 7561–7576. doi: 10.1007/s13197-015-1851-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: Health implications for the 21st century. Am J Clin Nutr. 2005;81(2): 341–354. doi: 10.1093/ajcn.81.2.341 [DOI] [PubMed] [Google Scholar]
- 5.Henle T. Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids. 2005;29(4): 313–322. doi: 10.1007/s00726-005-0200-2 [DOI] [PubMed] [Google Scholar]
- 6.Jakus V, Rietbrock N. Advanced glycation end products and the progress of diabetic vascular complications. Physiol Research. 2004;53(2): 131–142. [PubMed] [Google Scholar]
- 7.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344(1): 109–116. doi: 10.1042/bj3440109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee S. Methylglyoxal modification reduces the sensitivity of hen egg white lysozyme to stress-induced aggregation: Insight into the anti-amyloidogenic property of α-dicarbonyl compound. J Biomol Struct Dyn. 2020;38(18): 5474–5487. doi: 10.1080/07391102.2019.1702589 [DOI] [PubMed] [Google Scholar]
- 9.Hinton DJ, Ames JM. Site specificity of glycation and carboxymethylation of bovine serum albumin by fructose. Amino Acids. 2006;30(4): 425–434. doi: 10.1007/s00726-006-0269-2 [DOI] [PubMed] [Google Scholar]
- 10.Ahmed A, Shamsi A, Khan MS, Husain FM, Bano B. Methylglyoxal induced glycation and aggregation of human serum albumin: Biochemical and biophysical approach. Int J Biol Macromol. 2018;1(113): 269–276. doi: 10.1016/j.ijbiomac.2018.02.137 [DOI] [PubMed] [Google Scholar]
- 11.Ghosh S, Pandey NK, Singha Roy A, Tripathy DR, Dinda AK, Dasgupta S. Prolonged glycation of hen egg white lysozyme generates non amyloidal structures. PLoS One. 2013;8(9): e74336. doi: 10.1371/journal.pone.0074336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res. 2013;47(Suppl 1): 3–27. doi: 10.3109/10715762.2013.815348 [DOI] [PubMed] [Google Scholar]
- 13.Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94(5): 1562–1569. doi: 10.1210/jc.2008-2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjornstad P, Lanaspa MA, Ishimoto T, Kosugi T, Kume S, Jalal D, et al. Fructose and uric acid in diabetic nephropathy. Diabetologia. 2015;58(9): 1993–2002. doi: 10.1007/s00125-015-3650-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szwergold BS, Kappler F, Brown TR. Identification of fructose 3-phosphate in the lens of diabetic rats. Science. 1990;247(4941): 451–454. doi: 10.1126/science.2300805 [DOI] [PubMed] [Google Scholar]
- 16.Bellier J, Nokin MJ, Lardé E, Karoyan P, Peulen O, Castronovo V, et al. Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res Clin Pract. 2019;148: 200–211. doi: 10.1016/j.diabres.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 17.Allaman I, Bélanger M, Magistretti PJ. Methylglyoxal, the dark side of glycolysis. Front Neurosci. 2015;9(23): 1–12. doi: 10.3389/fnins.2015.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans TW. Review article: albumin as a drug—biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther. 2002;16 Suppl 5: 6–11. doi: 10.1046/j.1365-2036.16.s5.2.x [DOI] [PubMed] [Google Scholar]
- 19.Raoufinia R, Mota A, Keyhanvar N, Safari F, Shamekhi S, Abdolalizadeh J. Overview of Albumin and Its Purification Methods. Adv Pharm Bull. 2016;6(4): 495–507. doi: 10.15171/apb.2016.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rondeau P, Bourdon E. The glycation of albumin: structural and functional impacts. Biochimie. 2011;93(4): 645–658. doi: 10.1016/j.biochi.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 21.Lu L, Pu LJ, Zhang Q, Wang LJ, Kang S, Zhang RY, et al. Increased glycated albumin and decreased esRAGE levels are related to angiographic severity and extent of coronary artery disease in patients with type 2 diabetes. Atherosclerosis. 2009;206(2): 540–5. doi: 10.1016/j.atherosclerosis.2008.12.045 [DOI] [PubMed] [Google Scholar]
- 22.Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, DeBolt E, et al. Review: Glycation of human serum albumin. Clin Chim Acta. 2013;425: 64–76. doi: 10.1016/j.cca.2013.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YM. Glycation ligand binding motif in lactoferrin. Implications in diabetic infection. Adv Exp Med Biol. 1998;443: 57–63. doi: 10.1007/978-1-4757-9068-9_7 [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim HR, Aoki T, Pellegrini A. Strategies for new antimicrobial proteins and peptides: lysozyme and aprotinin as model molecules. Curr Pharm Des. 2002;8(9): 671–93. doi: 10.2174/1381612023395349 [DOI] [PubMed] [Google Scholar]
- 25.Longo MA, Combes D. A novel chemoenzymatic glycosylation strategy: application to lysozyme modification. FEBS Lett. 1995;375(1–2): 63–66. doi: 10.1016/0014-5793(95)01174-d [DOI] [PubMed] [Google Scholar]
- 26.Zhang A, Sun H, Wang P, Wang X. Salivary proteomics in biomedical research. Clin Chim Acta. 2013;415: 261–265. doi: 10.1016/j.cca.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 27.Zhang CZ, Cheng XQ, Li JY, Zhang P, Yi P, Xu X, et al. Saliva in the diagnosis of diseases. Int J Oral Sci. 2016;8: 133–137. doi: 10.1038/ijos.2016.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klimiuk A, Zalewska A, Sawicki R, Knapp M, Maciejczyk M. Salivary Oxidative Stress Increases With the Progression of Chronic Heart Failure. J Clin Med. 2020;9(3): 769. doi: 10.3390/jcm9030769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlík M, Valkovič P, Hančinová V, Krížová L, Tóthová Ľ, Celec P. Markers of oxidative stress in plasma and saliva in patients with multiple sclerosis. Clin Biochem. 2015;48(1–2): 24–8. doi: 10.1016/j.clinbiochem.2014.09.023 [DOI] [PubMed] [Google Scholar]
- 30.Younus H, Ahmad S, Alam MF. Correlation between the Activity of Aldehyde Dehydrogenase and Oxidative Stress Markers in the Saliva of Diabetic Patients. Protein Pept Lett. 2020;27(1): 67–73. doi: 10.2174/0929866526666191002115121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrone A, Giovino A, Benny J, Martinelli F. Advanced glycation end products (AGEs): Biochemistry, signaling, analytical methods, and epigenetic effects. Oxid Med Cell Longev. 2020;2020: 3818196. doi: 10.1155/2020/3818196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoury ZH, Illesca P, Sultan AS. Salivary Fructosamine as a Noninvasive Glycemic Biomarker: A Systematic Review. JDR Clin Trans Res. 2021;6(4): 382–389. doi: 10.1177/2380084420954354 [DOI] [PubMed] [Google Scholar]
- 33.Joubert M, Septier C, Brignot H, Salles C, Panouillé M, Feron G, et al. Chewing bread: impact on alpha‐amylase secretion and oral digestion. Food Funct. 2017; 8(2): 607–614. doi: 10.1039/c6fo00963h [DOI] [PubMed] [Google Scholar]
- 34.Lane AR, Hackney AC. Relationship between salivary and serum testosterone levels in response to different exercise intensities. Hormones. 2015;14(2): 258–264. doi: 10.14310/horm.2002.1561 [DOI] [PubMed] [Google Scholar]
- 35.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694(1): 72–77. doi: 10.1111/j.1749-6632.1993.tb18343.x [DOI] [PubMed] [Google Scholar]
- 36.Santos TVS, Teixeira RR, Franco DL, Madurro JM, Brito-Madurro AG, Espindola FS. Bioelectrode for detection of human salivary amylase. Mater Sci Eng. 2012;32(3): 530–535. doi: 10.1016/j.msec.2011.12.005 [DOI] [Google Scholar]
- 37.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye biding. Anal Biochem. 1976;72(1–2): 248–254. doi: 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 38.Franco RR, da Silva Carvalho D, de Moura FBR, Justino AB, Silva HCG, Peixoto LG, et al. Antioxidant and anti-glycation capacities of some medicinal plants and their potential inhibitory against digestive enzymes related to type 2 diabetes mellitus. J Ethnopharmacol. 2018;215: 140–146. doi: 10.1016/j.jep.2017.12.032 [DOI] [PubMed] [Google Scholar]
- 39.Justino AB, Miranda NC, Franco RR, Martins MM, Silva NMD, Espindola FS. Annona muricata Linn. leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against α-amylase, α-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed Pharmacother. 2018;100: 83–92. doi: 10.1016/j.biopha.2018.01.172 [DOI] [PubMed] [Google Scholar]
- 40.Justino AB, Franco RR, Silva HCG, Saraiva AL, Sousa RMF, Espindola FS. B procyanidins of Annona crassiflora fruit peel inhibited glycation, lipid peroxidation and protein-bound carbonyls, with protective effects on glycated catalase. Sci. Rep. 2019; 9. doi: 10.1038/s41598-019-55779-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franco RR, Zabisky LFR, Lima Júnior JP, Alves VHM, Justino AB, Saraiva AL, et al. Antidiabetic effects of Syzygium cumini leaves: A non-hemolytic plant with potential against process of oxidation, glycation, inflammation and digestive enzymes catalysis. J Ethnopharmacol. 2020;261: 113132. doi: 10.1016/j.jep.2020.113132 [DOI] [PubMed] [Google Scholar]
- 42.Monnier VM, Vishwanath V, Frank KE, Elmets CA, Dauchot P, Kohn RR. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986;314(7): 403–408. doi: 10.1056/NEJM198602133140702 [DOI] [PubMed] [Google Scholar]
- 43.Kalousová M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 2002;51(6): 597–604. [PubMed] [Google Scholar]
- 44.Thornalley PJ, Rabbani N. Detection of oxidized and glycated proteins in clinical samples using mass spectrometry—a user’s perspective. Biochim Biophys Acta. 2014;1840(2): 818–29. doi: 10.1016/j.bbagen.2013.03.025 [DOI] [PubMed] [Google Scholar]
- 45.Jolliffe IT, Cadima J. Principal component analysis: a review and recent developments. Phil Trans R Soc A. 2016;374(2065): 20150202. doi: 10.1098/rsta.2015.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caixeta DC, Aguiar EMG, Cardoso-Sousa L, Coelho LMD, Oliveira SW, Espindola FS, et al. Salivary molecular spectroscopy: A sustainable, rapid and non-invasive monitoring tool for diabetes mellitus during insulin treatment. PLoS ONE. 2020;15(3): e0223461. doi: 10.1371/journal.pone.0223461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1): 70–77. doi: 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 48.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259): 680–685. doi: 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 49.Towbin H, Staehelin T, Gordon J. Eletrophoretic transfer of proteins from polyacrylamide gels to nitrocelulose sheets: procedure and some applications. Proc Natl Acad Sci. 1979;76(9): 4350–4354. doi: 10.1073/pnas.76.9.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiol Behav. 2007;92(4): 583–590. doi: 10.1016/j.physbeh.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 51.Garay-Sevilla ME, Regalado JC, Malacara JM, Nava LE, Wróbel-Zasada K, Castro-Rivas A, et al. Advanced glycosylation end products in skin, serum, saliva and urine and its association with complications of patients with type 2 diabetes mellitus. J Endocrinol Invest. 2005;28(5): 223–230. doi: 10.1007/BF03345377 [DOI] [PubMed] [Google Scholar]
- 52.Nigro C, Leone A, Fiory F, Prevenzano I, Nicolò A, Mirra P, et al. Dicarbonyl stress at the crossroads of healthy and unhealthy aging. Cells. 2019;8(7): 749. doi: 10.3390/cells8070749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maessen DE, Stehouwer CD, Schalkwijk CG. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin Sci. 2015;128(12): 839–861. doi: 10.1042/CS20140683 [DOI] [PubMed] [Google Scholar]
- 54.Gulpamuk B, Tekin K, Sonmez K, Inanc M, Neselioglu S, Erel O, et al. The significance of thiol/disulfide homeostasis and ischemia-modified albumin levels to assess the oxidative stress in patients with different stages of diabetes mellitus. Scand J Clin Lab Invest. 2018;78(1–2): 136–142. doi: 10.1080/00365513.2017.1422540 [DOI] [PubMed] [Google Scholar]
- 55.Bartosz IS, Galiniak S, Bartosz G. Kinetics of glycoxidation of bovine serum albumin by methylglyoxal and glyoxal and its prevention by various compounds. Molecules. 2014;19(4): 4880–4896. doi: 10.3390/molecules19044880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meerwaldt R, Links T, Zeebregts C, Tio R, Hillebrands JL, Smit A. The clinical relevance of assessing advanced glycation endproducts accumulation in diabetes. Cardiovasc Diabetol. 2008;7: 29. doi: 10.1186/1475-2840-7-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med. 2018;24(1): 59. doi: 10.1186/s10020-018-0060-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meurman JH, Rantonen P, Pajukoski H, Sulkava R. Salivary albumin and other constituents and their relation to oral and general health in the elderly. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(4): 432–438. doi: 10.1067/moe.2002.122345 [DOI] [PubMed] [Google Scholar]
- 59.Anastas PT, Rodriguez A, de Winter TM, Coish P, Zimmerman JB. A review A review of immobilization techniques to improve the stability and bioactivity of lysozyme. Green Chem Lett Rev. 2021; 14 (2): 302–338. doi: 10.1080/17518253.2021.1890840 [DOI] [Google Scholar]
- 60.Soo-Quee Koh D, Choon-Huat Koh G. The use of salivary biomarkers in occupational and environmental medicine. Occup Environ Med. 2007;64(3): 202–10. doi: 10.1136/oem.2006.026567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gugliucci A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv Nutr. 2017;8(1): 54–62. doi: 10.3945/an.116.013912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalapos MP. Methylglyoxal in living organisms: chemistry, biochemistry, toxicology and biological implications. Toxicol Lett. 1999;110(3): 145–75. doi: 10.1016/s0378-4274(99)00160-5 [DOI] [PubMed] [Google Scholar]
- 63.Schalkwijk CG, Stehouwer CD, van Hinsbergh VW. Fructose-mediated non-enzymatic glycation: sweet coupling or bad modification. Diabetes Metab Res Rev. 2004;20(5): 369–82. doi: 10.1002/dmrr.488 [DOI] [PubMed] [Google Scholar]
- 64.Sadowska-Bartosz I, Galiniak S, Bartosz G. Kinetics of glycoxidation of bovine serum albumin by methylglyoxal and glyoxal and its prevention by various compounds. Molecules. 2014;19(4): 4880–4896. doi: 10.3390/molecules19044880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oimomi M, Nakamichi T, Ohara T, Sakai M, Igaki N, Hata F, et al. Fructose-related glycation. Diabetes Res Clin Pract. 1989;7(2): 137–179. doi: 10.1016/0168-8227(89)90104-6 [DOI] [PubMed] [Google Scholar]
- 66.Takagi Y, Kashiwagi A, Tanaka Y, Asahina T, Kikkawa R, Shigeta Y. Significance of fructose-induced protein oxidation and formation of advanced glycation end product. J Diabetes Complications. 1995;9(2): 87–91. doi: 10.1016/1056-8727(94)00022-g [DOI] [PubMed] [Google Scholar]
- 67.Ou J, Huang J, Wang M, Ou S. Effect of rosmarinic acid and carnosic acid on AGEs formation in vitro. Food Chem. 2017;221: 1057–1061. doi: 10.1016/j.foodchem.2016.11.056 [DOI] [PubMed] [Google Scholar]
- 68.Liu J, Yang Z, Cheng Y, Wu Q, He Y, Li Q, et al. Eriodictyol and naringenin inhibit the formation of AGEs: An in vitro and molecular interaction study, J Mol Recognit. 2020.33(1): e2814. doi: 10.1002/jmr.2814 [DOI] [PubMed] [Google Scholar]
- 69.Ahmed N, Dobler D, Dean M, Thornalley PJ. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J Biol Chem. 2005;280(7): 5724–5732. doi: 10.1074/jbc.M410973200 [DOI] [PubMed] [Google Scholar]
- 70.Cheung ST, Fonda ML. Reaction of phenylglyoxal with arginine. The effect of buffers and pH. Biochem Biophys Res Commun. 1979;90(30): 940–947. doi: 10.1016/0006-291x(79)91918-1 [DOI] [PubMed] [Google Scholar]
- 71.Hattori Y, Suzuki M, Hattori S, Kasai K. Vascular smooth muscle cell activation by glycated albumin (Amadori adducts). Hypertension. 2002;39(1): 22–28. doi: 10.1161/hy1201.097300 [DOI] [PubMed] [Google Scholar]
- 72.Peacock TP, Shihabi ZK, Bleyer AJ, Dolbare EL, Byers JR, Knovich MA, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73(9): 1062–1068. doi: 10.1038/ki.2008.25 [DOI] [PubMed] [Google Scholar]
- 73.Ahmad MS, Pischetsrieder M, Ahmed N. Aged garlic extract and S-allyl cysteine prevent formation of advanced glycation endproducts. Eur J Pharmacol. 2007;561(1–3): 32–38. doi: 10.1016/j.ejphar.2007.01.041 [DOI] [PubMed] [Google Scholar]
- 74.Aljohi A, Matou-Nasri S, Ahmed N. Antiglycation and antioxidant properties of Momordica charantia. PLoS One. 2016;11(8): e0159985. doi: 10.1371/journal.pone.0159985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maciejczyk M, Zalewska A, Ładny JR. Salivary antioxidant barrier, redox status, and oxidative damage to proteins and lipids in healthy children, adults, and the elderly. Oxid Med Cell Longev. 2019;2019: 4393460. doi: 10.1155/2019/4393460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fábián TK, Hermann P, Beck A, Fejérdy P, Fábián G. Salivary defense proteins: their network and role in innate and acquired oral immunity. Int J Mol Sci. 2012;13(4): 4295–320. doi: 10.3390/ijms13044295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manig F, Hellwig M, Pietz F, Henle T. Quantitation of free glycation compounds in saliva. PLoS One. 2019;14(9): e.02202082019. doi: 10.1371/journal.pone.0220208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, et al. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101(5): 1142–1147. doi: 10.1172/JCI119885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Panchbhai AS, Degwekar SS, Bhowte RR. Estimation of salivary glucose, salivary amylase, salivary total protein and salivary flow rate in diabetics in India. J Oral Sci. 2010;52(3): 359–368. doi: 10.2334/josnusd.52.359 [DOI] [PubMed] [Google Scholar]
- 80.Indira M, Chandrashekar P, Kattappagari KK, Chandra LP, Chitturi RT, Bv RR. Evaluation of salivary glucose, amylase, and total protein in Type 2 diabetes mellitus patients. Indian J Dent Res. 2015;26(3): 271–275. doi: 10.4103/0970-9290.162883 [DOI] [PubMed] [Google Scholar]
- 81.Naseri R, Mozaffari HR, Ramezani M, Sadeghi M. Effect of diabetes mellitus type 2 on salivary glucose, immunoglobulin A, total protein, and amylase levels in adults: A systematic review and meta-analysis of case-control studies. J Res Med Sci. 2018;23: 89. doi: 10.4103/jrms.JRMS_135_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaklai N, Garlick RL, Bunn HF. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984;259(6): 3812–3817. [PubMed] [Google Scholar]
- 83.Neelofar K, Arif Z, Alam K, Ahmad J. Hyperglycemia induced structural and functional changes in human serum albumin of diabetic patients: a physico-chemical study. Mol Biosyst. 2016;12(8): 2481–2489. doi: 10.1039/c6mb00324a [DOI] [PubMed] [Google Scholar]
- 84.Vedantham G, Sparks HG, Sane SU, Tzannis S, Przybycien TM. A holistic approach for protein secondary structure estimation from infrared spectra in H2O solutions. Anal Biochem. 2000;285(1): 33−49. doi: 10.1006/abio.2000.4744 [DOI] [PubMed] [Google Scholar]
- 85.McAvan BS, France AP, Bellina B, Barran PE, Goodacre R, Doig AJ. Quantification of protein glycation using vibrational spectroscopy. Analyst. 2002;145(10): 3686–3696. doi: 10.1039/c9an02318f [DOI] [PubMed] [Google Scholar]
- 86.Bathaie SZ, Nobakht BB, Mirmiranpour H, Jafarnejad A. Effect of chemical chaperones on glucose-induced lysozyme modifications. Protein J. 2011;30(7): 480–489. doi: 10.1007/s10930-011-9353-x [DOI] [PubMed] [Google Scholar]
- 87.Xing H, Yaylayan V. Mechanochemically induced controlled glycation of lysozyme and its effect on enzymatic activity and conformational changes. J Agric Food Chem. 2019;67(11): 3249–3255. doi: 10.1021/acs.jafc.9b00070 [DOI] [PubMed] [Google Scholar]
- 88.Zheng F, Cai W, Mitsuhashi T, Vlassara H. Lysozyme enhances renal excretion of advanced glycation endproducts in vivo and suppresses adverse AGE-mediated cellular effects in vitro: A potential AGE sequestration therapy for diabetic nephropathy? Mol Med. 2001;7(11): 737–747. doi: 10.1007/BF03401963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dodds MW, Yeh CK, Johnson DA. Salivary alterations in type 2 (non-insulin-dependent) diabetes mellitus and hypertension. Community Den Oral Epidemiol. 2000;28(5): 373–381. doi: 10.1034/j.1600-0528.2000.028005373.x [DOI] [PubMed] [Google Scholar]
- 90.Ansari NA, Moinuddin, Alam K, Ali A. Preferential recognition of Amadori-rich lysine residues by serum antibodies in diabetes mellitus: Role of protein glycation in the disease process. Hum Immunol. 2009;70(6): 417–424. doi: 10.1016/j.humimm.2009.03.015 [DOI] [PubMed] [Google Scholar]
- 91.Scott DA, Renaud DE, Krishnasamy S, Meriç P, Buduneli N, Cetinkalp S, et al. Diabetes-related molecular signatures in infrared spectra of human saliva. Diabetol Metab Syndr.2010;2: 48. doi: 10.1186/1758-5996-2-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Horvat S, Jakas A. Peptide and amino acid glycation: new insights into the Maillard reaction. J. Pept. Sci. 2004;10(3): 119–137. doi: 10.1002/psc.519 [DOI] [PubMed] [Google Scholar]
- 93.Takahashi M. Glycation of proteins. In: Endo T, Seeberger PH, Hart GW, Wong CH, Taniguchi N. Glycoscience: Biology and Medicine. Springer. 2014. pp. 1339–1345. [Google Scholar]
- 94.Laroque D, Inisan C, Bergez C, Vouland É, Dufossé L, Guérard F. Kinetic study on the Maillard reaction. Consideration of sugar reactivity. Food Chem. 2008;111(4): 1032–1042. doi: 10.1016/j.foodchem.2008.05.033 [DOI] [Google Scholar]
- 95.Henning C, Glomb MA. Pathways of the Maillard reaction under physiological conditions. Glycoconj J. 2016;33(4): 499–512. doi: 10.1007/s10719-016-9694-y [DOI] [PubMed] [Google Scholar]
- 96.Rai AK, Singh SP, Pandey AR, Ansari A, Ahmad S, Sashidhara KV, et al. Flavonoids from Polyalthia longifolia prevents advanced glycation end products formation and protein oxidation aligned with fructose-induced protein glycation. Nat Prod Res. 2019;35(17): 2921–2925. doi: 10.1080/14786419.2019.1672690 [DOI] [PubMed] [Google Scholar]
- 97.Adisakwattana S, Sompong W, Meeprom A, Ngamukote S, Yibchok-Anun S. Cinnamic acid and its derivatives inhibit fructose-mediated protein glycation. Int J Mol Sci. 2012;13(2): 1778–1789. doi: 10.3390/ijms13021778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tarwadi KV, Agte VV. Effect of micronutrients on methylglyoxal-mediated in vitro glycation of albumin. Biol Trace Elem Res. 2011;143(2): 717–725. doi: 10.1007/s12011-010-8915-7 [DOI] [PubMed] [Google Scholar]
- 99.Su H, Velly AM, Salah MH, Benarroch M, Trifiro M, Schipper HM, et al. Altered redox homeostasis in human diabetes saliva. J Oral Pathol Med. 2012;41(3): 235–241. doi: 10.1111/j.1600-0714.2011.01092.x [DOI] [PubMed] [Google Scholar]
- 100.Hecker M, Wagner AH. Role of protein carbonylation in diabetes. J Inherit Metab Dis. 2018;41(1): 29–38. doi: 10.1007/s10545-017-0104-9 [DOI] [PubMed] [Google Scholar]
- 101.Megson IL, Haw SJ, Newby DE, Pell JP. Association between exposure to environmental tobacco smoke and biomarkers of oxidative stress among patients hospitalised with acute myocardial infarction. PLoS One. 2013;8(12): e81209. doi: 10.1371/journal.pone.0081209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng J, Davies MJ. Evidence for the formation of adducts and S-(carboxymethyl)cysteine on reaction of alpha-dicarbonyl compounds with thiol groups on amino acids, peptides, and proteins. Chem Res Toxicol. 2005;18(8): 1232–1241. doi: 10.1021/tx050074u [DOI] [PubMed] [Google Scholar]
- 103.Krämer AC, Davies MJ. Effect of methylglyoxal-induced glycation on the composition and structure of β-lactoglobulin and α-lactalbumin. J Agric Food Chem. 2019;67(2): 699–710. doi: 10.1021/acs.jafc.8b05809 [DOI] [PubMed] [Google Scholar]
- 104.Ghelani H, Razmovski-Naumovski V, Pragada RR, Nammi S. Attenuation of glucose-induced myoglobin glycation and the formation of advanced glycation end products (ages) by (R)-α-lipoic acid in vitro. Biomolecules. 2018;8(1): 9. doi: 10.3390/biom8010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rajeshwari SG, Choudhry AA, Gururaja A, Prabhu K. Correlation of plasma lipid profile with salivary oxidative stress markers in type II diabetes mellitus patients. J Clin Diagn Res. 2014;8(6): CC08–CC10. doi: 10.7860/JCDR/2014/8233.4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bourdon E, Loreau N, Lagrost L, Blache D. Differential effects of cysteine and methionine residues in the antioxidant activity of human serum albumin. Free Radic Res. 2005;39(1): 15–20. doi: 10.1080/10715760400024935 [DOI] [PubMed] [Google Scholar]
- 107.Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: Emerging roles in cell signaling. Biochem Pharmacol. 2006;71(5): 551–564. doi: 10.1016/j.bcp.2005.10.044 [DOI] [PubMed] [Google Scholar]
- 108.Miyata T, Ishikawa N, van Ypersele de Strihou C. Carbonyl stress and diabetic complications. Clin Chem Lab Med. 2003;41(9): 1150–1158. doi: 10.1515/CCLM.2003.178 [DOI] [PubMed] [Google Scholar]
- 109.Ates I, Kaplan M, Yuksel M, Mese D, Alisik M, Erel O, et al. Determination of thiol/disulphide homeostasis in type 1 diabetes mellitus and the factors associated with thiol oxidation. Endocrine. 2016;51(1): 47–51. doi: 10.1007/s12020-015-0784-6 [DOI] [PubMed] [Google Scholar]
- 110.Scannapieco FA, Torres G, Levine MJ. Salivary alpha-amylase: role in dental plaque and caries formation. Crit Rev Oral Biol Med. 1993;4(3–4): 301–307. doi: 10.1177/10454411930040030701 [DOI] [PubMed] [Google Scholar]
- 111.Alqahtani AS, Li KM, Razmovski-Naumovski V, Kam A, Alam P, Li GQ. Attenuation of methylglyoxal-induced glycation and cellular dysfunction in wound healing by Centella cordifoli. Saudi. J Biol Sci.2020;28 (1): 813–824. doi: 10.1016/j.sjbs.2020.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang L, Lu Y, Ye YH, Yang SH, Tu ZC, Chen J, et al. Insights into the mechanism of quercetin against BSA-fructose glycation by spectroscopy and high-resolution mass spectrometry: Effect on physicochemical properties. J Agric Food Chem. 2019;67(1): 236–246. doi: 10.1021/acs.jafc.8b06075 [DOI] [PubMed] [Google Scholar]
- 113.Perera H, Ranasinghe H. A simple method to detect plant based inhibitors of glycation induced protein cross-linking. Asian J Med Sci. 2014;6(1): 28–33. doi: 10.3126/ajms.v6i1.10181 [DOI] [Google Scholar]
- 114.Ittichaicharoen J, Phrommintikul A, Chattipakorn N, Chattipakorn S. Reduced salivary amylase activity in metabolic syndrome patients with obesity could be improved by treatment with a dipeptidyl peptidase IV inhibitor. Clin Oral Investig. 2018;22(9): 3113–3120. doi: 10.1007/s00784-018-2402-5 [DOI] [PubMed] [Google Scholar]
- 115.Nakajima K. Low serum amylase and obesity, diabetes and metabolic syndrome: A novel interpretation. World J Diabetes. 2016;7(6): 112–121. doi: 10.4239/wjd.v7.i6.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mekahli D, Bultynck G, Parys JB, de Smedt H, Missiaen L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol. 2011;3(6): a004317. doi: 10.1101/cshperspect.a004317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sell DR, Nagaraj RH, Grandhee SK, Odetti P, Lapolla A, Fogarty J, et al. Pentosidine: a molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes Metab Rev. 1991;7(4): 239–51. doi: 10.1002/dmr.5610070404 [DOI] [PubMed] [Google Scholar]
- 118.Yilmaz D, Topcu AO, Akcay EU, Altındis M, Gursoy UK. Salivary human beta-defensins and cathelicidin levels in relation to periodontitis and type 2 diabetes mellitus. Acta Odontol Scand. 2020;78(5): 327–331. doi: 10.1080/00016357.2020.1715471 [DOI] [PubMed] [Google Scholar]
- 119.Rao PV, Laurie A, Bean ES, Roberts CT Jr, Nagalla SR. Salivary protein glycosylation as a noninvasive biomarker for assessment of glycemia. J Diabetes Sci Technol. 2015;9(1): 97–104. doi: 10.1177/1932296814554414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gelamo EL, Tabak M. Spectroscopic studies on the interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants. Spectrochim Acta A Mol Biomol Spectrosc. 2000;56A(11): 2255–2271. doi: 10.1016/s1386-1425(00)00313-9 [DOI] [PubMed] [Google Scholar]
- 121.Sziegat F, Wirmer-Bartoschek J, Schwalbe H. Characteristics of human lysozyme and its disease-related mutants in their unfolded states. Angew Chem Int Ed Engl. 2011;50(24): 5514–5518. doi: 10.1002/anie.201008040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
** p < 0,01; *** p < 0,001.
(TIF)
(A) Scores scatter plot of PC1 vs PC2 performed on the BSA and BSA+F spectrum. (B) PC1 loadings profile obtained for BSA and BSA+F. (C) Scores scatter plot of PC1 vs PC2 performed on the BSA and BSA+MGO spectrum. (D) PC1 loadings profile obtained for BSA and BSA+MGO.
(TIF)
(A) Scores scatter plot of PC1 vs PC2 performed on the LYS and LYS+F spectrum. (B) PC1 loadings profile obtained for LYS and LYS+F. (C) Scores scatter plot of PC1 vs PC2 performed on the LYS and LYS+MGO spectrum. (D) PC1 loadings profile obtained for LYS and LYS+MGO.
(TIF)
(A) Scores scatter plot of PC1 vs PC2 performed on the SAL and SAL+F spectrum. (B) PC1 loadings profile obtained for SAL and SAL+F. (C) Scores scatter plot of PC1 vs PC2 performed on the SAL and SAL+MGO spectrum. (D) PC1 loadings profile obtained for SAL and SAL+MGO.
(TIF)
(A) Western blotting of sAA expression in SAL (lane a), SAL+F (lane b), and SAL+MGO (lane c). (B) Quantification of immunodetected sAA given in density of pixels.
(TIF)
(A,B and C) Each lane was loaded with de following sample: (a): SAL; (b): SAL+F; (c): SAL+MGO; Mr: relative molecular mass of the protein standard (kDa).
(TIF)
Each lane was loaded with de following sample: (a): sAA (b): sAA+F; (c): sAA +MGO.
(TIF)
Each lane was loaded with de following sample: (a): sAA; (b): sAA+F; (c): sAA+MGO.
(TIF)
** p < 0,01; *** p < 0,001.
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.