Abstract
Purpose of the review:
Decades of research in ARDS have led to few interventions that impact clinical outcomes. The pandemic of patients with ARDS due to the novel SARS-CoV-2 infection has stressed the need for more effective therapies in ARDS. Phenotyping may enable successful trials and precision therapeutics in this patient population.
Recent findings:
Clinical phenotypes that group patients by shared etiology, time-course, or radiographic presentation are of prognostic value but their use is limited by misclassification. Physiological phenotypes including the P/F ratio, ventilatory ratio, and dead space fraction predict poor outcomes but can rapidly change, making them unstable over time. Biologic phenotypes have prognostic value with composite clinical and biomarker sub-phenotypes additionally impacting treatment response but are yet to be prospectively validated.
Summary:
Though much progress has been made in ARDS phenotyping, implementation of precision medicine practices will depend on conducting phenotype-aware trials using rapid point of care assays or machine learning algorithms. Omics studies will enhance our understanding of biologic determinants of clinical outcomes in ARDS sub-phenotypes. Whether biologic ARDS sub-phenotypes are specific to this syndrome or rather more broadly identify endotypes of critical illness remains to be determined.
Keywords: Phenotyping, ARDS, precision medicine, critical care
Introduction:
Acute Respiratory Distress Syndrome (ARDS) has long suffered from a paucity of practice-changing discoveries despite rigorous research, owing to its wide range of triggers(1), broad definition(2), and variable outcomes. The surge of patients with ARDS beginning in 2020 from the novel SARS-CoV-2 infection overwhelmed healthcare systems and highlighted the need for more effective therapies in ARDS. Phenotyping this heterogeneous syndrome into more homogeneous subgroups may lead to more success in identifying effective therapies.
Since ARDS was defined in 1967, researchers have endeavored to understand its heterogeneity, fueled of late by advancements in phenotyping in other fields. In oncology, molecular phenotyping of melanoma led to the introduction of checkpoint inhibitors and therapies targeting BRAF V600 mutation that have significantly prolonged survival(3). Breast cancer treatment approaches and outcomes are vastly different based on hormone receptor and gene mutation phenotyping(4). In pulmonology, biomarker-based phenotyping has led to targeted therapies for patients with Th2 dependent inflammation and eosinophilic asthma(5–9). These successes have spurred the search for treatable phenotypes in syndromes of critical illness, namely sepsis and ARDS.
The COVID-19 pandemic led to large numbers of patients with a uniform trigger for ARDS. Clinical trials in patients with COVID-19-related ARDS (CARDS) met with more success in identifying effective therapies than decades of large, well-designed randomized trials had in “classical” ARDS. For example, numerous studies on the role of steroids in ARDS arrived at varying conclusions(10–12), yet steroids in patients with CARDS have more consistently demonstrated a mortality benefit(13, 14). The contrast between these findings in COVID-19 and classical ARDS studies suggest that a steroid-responsive subgroup likely exists within groups of patients with classical ARDS and needs characterization. Even within CARDS, randomized trials studying the same drugs have met with mixed results, likely in part due to heterogeneous biologic response to ARDS and/or differing management strategies for patients with complex critical illness(14–23). Nonetheless, CARDS trials demonstrate that we are more likely to find successful ARDS therapies by selecting sub-groups of patients more likely to respond to a given treatment (predictive enrichment) and those at higher risk of poor outcomes (prognostic enrichment).
In this paper, we aim to briefly review current concepts in phenotyping ARDS, highlight some inherent challenges to phenotyping, and identify key directions toward which the field is headed in the coming decade.
Phenotyping: The Present
Clinical Phenotyping:
Clinical phenotyping in ARDS subdivides patients based on either a shared etiology, time-course, or radiographic presentation of ARDS (Table 1). Evidence suggests that etiologic sub-phenotypes carry different prognoses and, in some cases, different treatment responses. The most prominent recent example of phenotyping based on a shared etiology is CARDS. Outside of CARDS, however, ascertaining an ARDS trigger can be difficult, and sometimes multiple etiologies are at play. Nevertheless, data suggests that prognosis and attributable mortality of ARDS differ based on etiology(24, 25). For example, ARDS induced by trauma carries a lower mortality rate than non-trauma related ARDS(26). ARDS resulting from direct injury to the lungs portends a better prognosis than ARDS due to indirect insults (e.g. non-pulmonary sepsis)(27). As of yet, aside from COVID-19, there is no compelling evidence that ARDS due to different etiologies responds differently to therapies.
Table 1.
Overview of selected ARDS phenotypes, clinical utility, and main limitations.
| Phenotypes | Sub-phenotypes | Established clinical use | Main limitations | |
|---|---|---|---|---|
| Prognostic | Therapeutic | |||
| Clinical | ||||
| X | X (COVID-19 only) | High risk of misclassification | ||
| Sepsis | ||||
| Direct lung injury | ||||
| Time course | X | |||
| Rapidly resolving | ||||
| Radiographic | X | |||
| Focal vs non-focal | ||||
| Physiological | ||||
| P/F ratio | X | X | Rapidly changing variables | |
| Dead space fraction | X | |||
| Ventilatory ratio | X | |||
| Driving pressure | X | |||
| Biological | ||||
| Protein biomarkers | X | Identifying interventions is complicated by partial understanding of complex biology | ||
| Endothelial injury | ||||
| Reactive vs uninflamed | ||||
| Composite clinical/protein | X | X | ||
| Hypoinflammatory | ||||
| Metabolomics | X | |||
The time-course of ARDS also has prognostic value. Studies have shown that late onset of ARDS (more than 48 hours after ICU admission) is associated with higher mortality rates than earlier onset(28, 29). Among those who present with ARDS, one study found that 63% with moderate to severe disease based on P/F ratio paradoxically have a rapidly resolving phenotype and a better prognosis(30). Thus, trials recruiting patients with moderate to severe ARDS upon hospital presentation may inadvertently be enriched with this rapidly resolving clinical sub-phenotype.
Radiographic findings provide another means for prognostic and predictive trial enrichment in ARDS. The RALE score, which systematically quantifies the extent and density of alveolar infiltrates on plain films, predicts 28-day mortality with an AUC of 0.82(31). Similarly, the Murray Lung Score, which incorporates radiographic findings, was used as one approach to prognostic enrichment in the CESAR trial for ECMO(32, 33). Radiographic findings were used for predictive enrichment in the LIVE trial, an innovative study across 20 ICUs in France(34). The investigators randomized patients to receive either standard lung-protective ventilation, or a personalized mechanical ventilation strategy based on the presence of focal or non-focal radiographic findings. In the personalized arm, the patients with focal ARDS received higher tidal volumes (8 mL/kg) and low PEEP, while those with non-focal disease received a lower tidal volume (6 mL/kg), recruitment maneuvers, and high PEEP. The trial found no significant differences in 90-day mortality, its primary outcome of interest. However, a post-hoc review led to the discovery that 21% of patients were radiographically misclassified. Accounting for this misclassification, the investigators found that a ventilator strategy misaligned with radiographic findings significantly increased 90-day mortality. The LIVE trial highlights both the perils of “one size fits all” therapies in ARDS and misclassification inherent in clinical phenotyping.
Physiological phenotyping:
Physiologic phenotyping separates groups of patients based on severity of lung impairment (Table 1). The Berlin Criteria introduced the most commonly applied physiologic sub-phenotypes of mild, moderate and severe ARDS, defined using the ratio of partial pressure of oxygen in arterial blood (PaO2) to the fraction of inspired oxygen (FiO2), the P/F ratio. These three categories of disease severity were associated with escalating mortality rates, and many recent trials have used P/F sub-phenotypes for prognostic enrichment by enrolling only those with moderate to severe ARDS (P/F <150). Despite the prevalence of its use, the P/F ratio is a mediocre predictor of mortality with an AUC of only 0.577 in one analysis(2). Other physiologic sub-phenotypes that predict poor outcomes include dead space fraction, ventilatory ratio, and driving pressure(35–37). The main limitation of physiological phenotyping is that variables can rapidly change, creating unstable sub-phenotypes that in some cases may be challenging to study in trial settings.
Biological phenotyping:
Drugs targeting biologic processes to reverse lung injury or enhance lung repair in ARDS have not lowered mortality, likely in part due to heterogeneity of the host response to ARDS. Biological phenotyping seeks to identify subgroups with a similar host response to ARDS to elucidate its pathophysiology and allow for prognostic and predictive trial enrichment (Table 1).
The largest body of work in this arena involves analyses of plasma protein biomarkers, several of which have demonstrated diagnostic and prognostic value in ARDS. These include biomarkers of alveolar epithelial injury such as soluble receptor for advanced glycation end products (sRAGE) and surfactant protein-D (SP-D)(38–47); endothelial injury such as angiopoietin-2 (Ang-2), von Willebrand factor (vWF), and intercellular adhesion molecule (CAM)-1(48–51); proinflammatory cytokines such as soluble tumor necrosis factor receptor I (sTNFr-1), interleukin (IL)-6, and IL-8(49, 50, 52–56); and disordered coagulation such as plasminogen activator inhibitor-1 (PAI-1) and protein C(57). Combinations of these biomarkers perform better in diagnosing and risk stratifying ARDS than each biomarker alone(44, 52, 58, 59).
Using latent class analysis (LCA), a retrospective study of clinical and protein biomarker data from the landmark ARMA and ALVEOLI trials of ARDS identified two sub-phenotypes, designated as “hyperinflammatory” and “hypoinflammatory”, associated with distinct clinical outcomes(60–62). Retrospective analyses of data from five ARDS trials (ARMA(61), ALVEOLI(62), FACTT(63), SAILS(64), HARP-2(65)) as well as analyses of ARDS patients from two prospective observational cohorts, altogether comprising over 4000 patients, consistently demonstrate that patients with the “hyperinflammatory” sub-phenotype experience higher mortality rates than those with the “hypoinflammatory” sub-phenotype(66–69). Beyond prognostic utility, these sub-phenotypes additionally seem to have different responses to therapies such as PEEP, fluid strategy, and simvastatin(60, 66, 67) in secondary analyses of completed trials. A separate study using cluster analysis of protein biomarker data from a large cohort of patients across two ICUs in the Netherlands identified the presence of two molecular sub-phenotypes of ARDS designated as “reactive” and “uninflamed”(70). Specifically, the “reactive” sub-phenotype had higher levels of IL-6, Ang-1 and 2, PAI-1, and interferon-gamma levels and experienced worse outcomes than the “uninflamed” sub-phenotype.
Other types of biomarkers including RNA, metabolites, lipids, and extracellular vesicles hold potential for further untangling the complex biological phenotypes within ARDS. Transcriptomic analyses of peripheral blood leukocytes from the Netherland ICU cohort of patients identified upregulation of pathways of oxidative phosphorylation and mitochondrial dysfunction in the “reactive” sub-phenotype of ARDS(71). More recently in patients with CARDS, transcriptomic analyses of tracheal aspirates demonstrated reduced pro-inflammatory gene expression compared to classical ARDS(72). The dysregulated host response in patients with CARDS was characterized by expression of genes associated with non-canonical roles in inflammation, potentially explaining why these patients benefit from steroids. Using metabolomic analyses, a subgroup of patients from a small cohort with ARDS were found to have a distinct metabolic profile in pulmonary edema fluid samples associated with higher mortality(73).
Phenotyping: The Challenges
While phenotyping holds great promise for future research trials in ARDS, the field faces several considerable challenges that have limited clinical implementation. One noteworthy challenge is disagreement over what constitutes sufficient data to recommend a change in clinical practice(74). Here, the COVID-19 pandemic serves as an illustrative example. Early in the pandemic, researchers published observations of two apparent sub-phenotypes of CARDS with divergent lung compliance, radiographic, and physiological features(75). The “L” sub-phenotype was defined by having low lung elastance (high compliance) and was postulated to predominate in CARDS. The less common “H” sub-phenotype was defined by having high lung elastance and dense airspace filling on CT. The authors theorized that the high mortality rates early on in CARDS may have been partially related to inappropriate use of lung protective ventilation, one of the two interventions ever demonstrated to have a mortality benefit in ARDS(61), and recommended that the L sub-phenotype be treated with a different ventilator management strategy. Published in a prominent journal and cited over 600 times, the article reached a wide audience. However, numerous subsequent studies failed to identify evidence in support of the “L” and “H” sub-phenotype model, instead indicating that lung compliance in CARDS follows a normal distribution and is generally low, similar to its non-COVID counterpart(76, 77). Ideally, phenotyping should be data-driven and externally validated before clinical use.
Even phenotypes derived from rigorous, data-rich, and large studies still have limitations and must be interpreted with caution. For instance, as mentioned with the LIVE trial, clinical phenotypes suffer from high rates of misclassification(34). Despite retrospective reproducibility across multiple cohorts of ARDS, latent biologic phenotypes still require prospective validation before they can be of clinical utility. Lastly, detecting which phenotypes amongst the many that have and will be identified are clinically relevant and impact treatment response remains a major challenge. Adding to this complexity is our limited ability to identify successful interventions for complex biologic phenotypes. For instance, a retrospective subgroup analysis of a randomized controlled trial on the use of recombinant IL-1 receptor antagonist in patients with sepsis showed a paradoxical treatment benefit in the subset of patients with higher baseline levels of IL-1 receptor antagonists(78). This study illustrates that our understanding of pathobiology in critical illness remains rudimentary and stresses the need for more studies in preclinical models.
Phenotyping: The Potential
Advances in phenotyping and the COVID-19 pandemic have escalated the pace of phenotyping in ARDS. Implementation of precision medicine practices in ARDS will depend upon the research community conducting phenotype-aware trials, elucidating pathophysiologic pathways of lung injury in various forms of ARDS, and translating such discoveries to personalized therapies (Figure 1).
Figure 1.
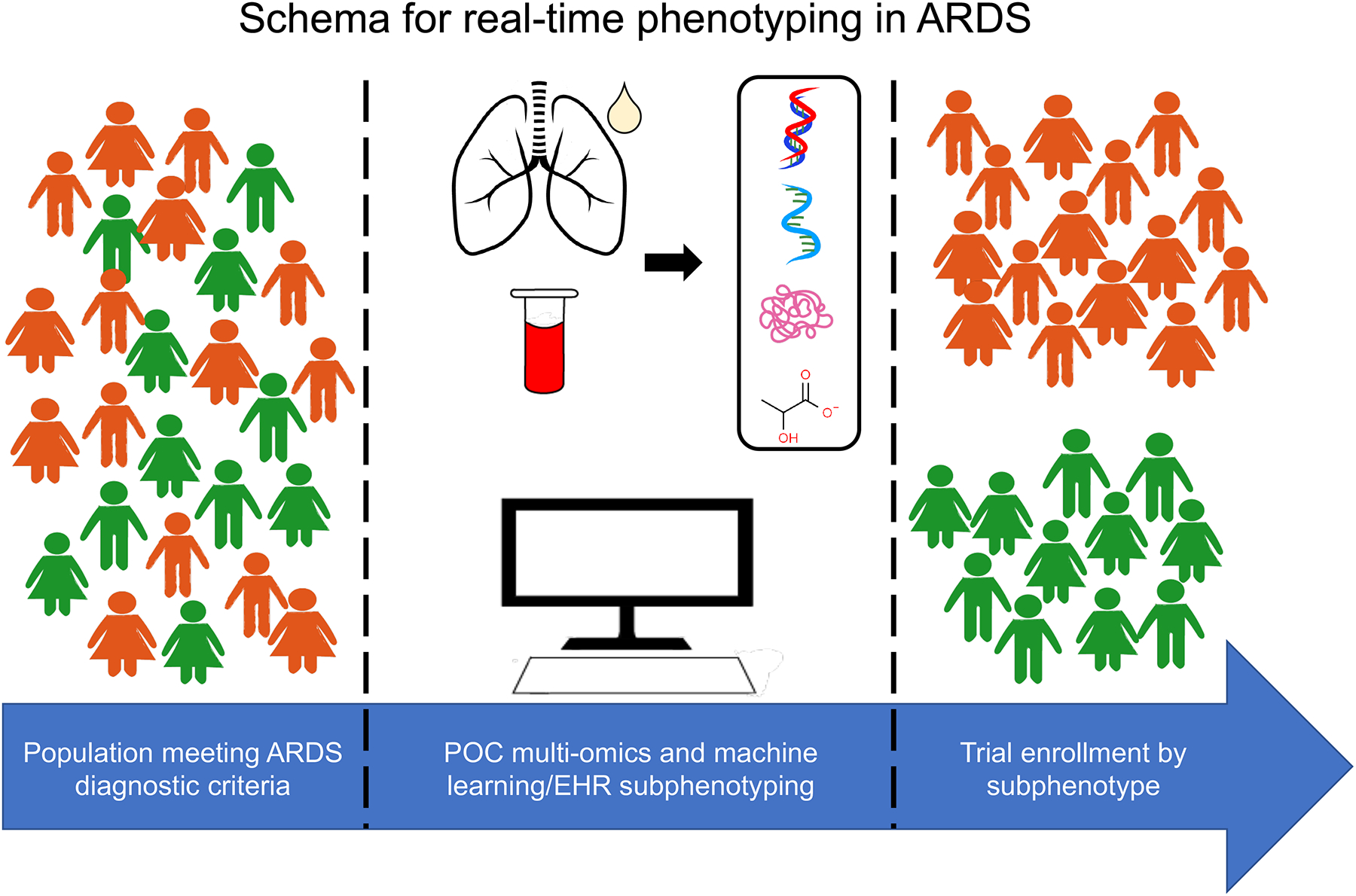
Schema for real-time phenotyping in ARDS.
EHR: electronic health record. POC: point of care.
Phenotype-aware trials and cohort studies enrolling patients with predictive and prognostic enrichment in mind are necessary to prospectively validate sub-phenotypes of ARDS. However, lack of point of care assays for rapid biologic phenotyping presents a significant barrier to conducting such studies. To circumvent this issue, Sinha and colleagues proposed a machine learning algorithm tool that uses readily available laboratory and clinical data to phenotype patients into “hyperinflammatory” and “hypoinflammatory” ARDS on admission to the ICU and correlates well with the gold standard LCA based biomarker sub-phenotypes(79). Furthermore, this algorithm might be incorporated into existing electronic health records for ease of use. Investigators have also found that a parsimonious model using three plasma biomarkers can classify patients with ARDS into the two inflammatory sub-phenotypes with high accuracy(80). Based on these findings, a point of care assay for rapid analysis of plasma IL-6 and soluble TNFr1 levels has been developed and is being studied in the PHIND trial, the first prospective cohort study of ARDS patients undergoing biologic phenotyping upon study entry(81). The PHIND trial also aims to prospectively validate these biologic sub-phenotypes and test their stability over time. The future holds promise that “real time” phenotyping in patients with ARDS is imminent.
More studies on genomic, transcriptomic, and metabolomic phenotyping in ARDS are underway and will enhance our understanding of the biologic determinants of clinical outcomes in sub-phenotypes of ARDS. A recent multi-omics study by Overmyer and colleagues in patients admitted with moderate to severe respiratory issues with and without COVID-19 found 219 molecules strongly associated with COVID-19 status and severity and pointed to dysregulation of biologic processes involving lipid transport, coagulation, endotheliopathy, and neutrophil degranulation(82). Studies on the local versus systemic host response in ARDS using tracheal aspirates, bronchoalveolar lavage fluid, and fluid from heat-moisture exchange filters will advance our understanding of ARDS pathophysiology and offer novel therapeutic targets. Genome wide association studies hold the promise of identifying novel ARDS biology, though are challenged by the syndromic definition of ARDS and the difficulties of identifying genetic control groups. Ultimately, the field of phenotyping in ARDS is moving towards deep phenotyping wherein multiple types of data using a variety of technologies lead to whole-body physiological profiling, furthering our understanding of host response mechanisms, and enabling personalized therapies in ARDS(83, 84).
The question remains as to whether ARDS phenotypes are specific to this syndrome or rather more broadly identify endotypes of critical illness. A recent study applied classifiers for cluster and LCA-derived biologic sub-phenotypes of ARDS to a population of mechanically ventilated patients without ARDS and found that the “reactive” and “hyperinflammatory” sub-phenotypes were associated with higher probability of mortality even in patients without ARDS(85). Another study sub-phenotyping patients at risk for developing ARDS found that a distinct LCA defined baseline “hyperinflammatory” sub-phenotype was associated with higher mortality and prolonged mechanical ventilation(86). Similar studies on ICU patients with and without ARDS are necessary to determine sub-phenotype specificity. One can imagine a future in which our approach to treatment of critically ill patients revolves around biologically identified treatable traits rather than the current syndrome-based paradigm.
Conclusions:
Phenotyping ARDS has identified subgroups of patients with distinct outcomes. The composite LCA defined “hyperinflammatory” and “hypoinflammatory” sub-phenotypes have additionally demonstrated differential responses to ARDS therapies, albeit in post-hoc analyses. Progress in the field is challenged by insufficient data, lack of prospective validation, difficulties identifying clinically relevant sub-phenotypes that impact treatment outcomes, and translating discoveries into effective therapies. Real-time sub-phenotyping using novel assays and machine learning algorithms will enable phenotype-aware trials that hold the promise of identifying successful ARDS therapies. Phenotyping ARDS and critical illness more broadly may lead to a paradigm shift away from syndrome-based definitions towards treatable traits.
Key points:
Phenotyping ARDS using clinical, physiologic, and biologic data has identified subgroups of patients with distinct clinical outcomes with the hyperinflammatory and hypoinflammatory biologic subphenotypes demonstrating differential treatment response in retrospective analyses of randomized controlled trials and cohort studies.
Researchers and clinicians must recognize the limitations of current phenotypes, including phenotypes derived from insufficient data, misclassification of clinical phenotypes, instability of physiologic phenotypes over time, and lack of prospective validation of biologic phenotypes.
Precision medicine practices in ARDS depends upon the research community conducting phenotype-aware trials, elucidating pathophysiologic pathways of lung injury in various forms of ARDS, and translating such discoveries to personalized therapies.
Acknowledgements:
Financial support and sponsorship: NIH R35HL140026 (CSC), NIH 5T32HL007185-44 (NA)
Conflicts of interest: Dr. Calfee has funding from the NIH related to precision medicine in ARDS; in addition, she has funding for observational studies of ARDS from Roche-Genentech, for clinical trials in ARDS from the Department of Defense, and for clinical trials in COVID-19 from Quantum Leap Healthcare Collaborative. She has had prior funding from Bayer for an observational study of ARDS. She has served as a consultant for Vasomune, Quark, and Gen1e Life Sciences.
References:
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. [DOI] [PubMed] [Google Scholar]
- 2.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. [DOI] [PubMed] [Google Scholar]
- 3.Helgadottir H, Rocha Trocoli Drakensjo I, Girnita A. Personalized Medicine in Malignant Melanoma: Towards Patient Tailored Treatment. Front Oncol. 2018;8:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green AR, Powe DG, Rakha EA, Soria D, Lemetre C, Nolan CC, et al. Identification of key clinical phenotypes of breast cancer using a reduced panel of protein biomarkers. Br J Cancer. 2013;109(7):1886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–207. [DOI] [PubMed] [Google Scholar]
- 6.Pavord ID, Menzies-Gow A, Buhl R, Chanez P, Dransfield M, Lugogo N, et al. Clinical Development of Mepolizumab for the Treatment of Severe Eosinophilic Asthma: On the Path to Personalized Medicine. J Allergy Clin Immunol Pract. 2021;9(3):1121–32 e7. [DOI] [PubMed] [Google Scholar]
- 7.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–41. [DOI] [PubMed] [Google Scholar]
- 8.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhakta NR, Woodruff PG. Human asthma phenotypes: from the clinic, to cytokines, and back again. Immunol Rev. 2011;242(1):220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brun-Buisson C, Richard J-CM, Mercat A, Thiébaut ACM, Brochard L. Early Corticosteroids in Severe Influenza A/H1N1 Pneumonia and Acute Respiratory Distress Syndrome. https://doiorg/101164/rccm201101-0135OC. 2012.
- 11.Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler J, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. The Lancet Respiratory medicine. 2020;8(3). [DOI] [PubMed] [Google Scholar]; * This trial is one of the few that demonstrated a benefit to using steroids in classical ARDS. Together with data from COVID-19 related ARDS, this study suggests that there may be a subphenotype of patients with classical ARDS who benefit from steroids and need to be clearly identified.
- 13.Group TWREAfC-TW. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324(13):1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. https://doiorg/101056/NEJMoa2028700. 2021. [DOI] [PMC free article] [PubMed]
- 16.Veiga VC, Prats JAGG, Farias DLC, Rosa RG, Dourado LK, Zampieri FG, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. 2021. [DOI] [PMC free article] [PubMed]
- 17.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. https://doiorg/101056/NEJMoa2028836. 2020. [DOI] [PMC free article] [PubMed]
- 18.Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial - The Lancet Respiratory Medicine. 2021. [DOI] [PMC free article] [PubMed]
- 19.Soin AS, Kumar K, Choudhary NS, Sharma P, Mehta Y, Kataria S, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial. The Lancet Respiratory medicine. 2021;9(5):511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Investigators R-C, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384(16):1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;384(1):20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, et al. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. JAMA Intern Med. 2021;181(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern Med. 2021;181(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auriemma CL, Zhuo H, Delucchi K, Deiss T, Liu T, Jauregui A, et al. Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med. 2020;46(6):1222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah CV, Localio AR, Lanken PN, Kahn JM, Bellamy S, Gallop R, et al. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Crit Care Med. 2008;36(8):2309–15. [DOI] [PubMed] [Google Scholar]
- 26.Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, Wheeler AP, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35(10):2243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, et al. Clinical Predictors of Hospital Mortality Differ Between Direct and Indirect ARDS. Chest. 2017;151(4):755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao KM, Chen CW, Hsiue TR, Lin WC. Timing of acute respiratory distress syndrome onset is related to patient outcome. J Formos Med Assoc. 2009;108(9):694–703. [DOI] [PubMed] [Google Scholar]
- 29.Zhang R, Wang Z, Tejera P, Frank AJ, Wei Y, Su L, et al. Late-onset moderate to severe acute respiratory distress syndrome is associated with shorter survival and higher mortality: a two-stage association study. Intensive Care Med. 2017;43(3):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schenck EJ, Oromendia C, Torres LK, Berlin DA, Choi AMK, Siempos II. Rapidly Improving ARDS in Therapeutic Randomized Controlled Trials. Chest. 2019;155(3):474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren MA, Zhao Z, Koyama T, Bastarache JA, Shaver CM, Semler MW, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73(9):840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. The American review of respiratory disease. 1988;138(3):720–3. [DOI] [PubMed] [Google Scholar]
- 33.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–63. [DOI] [PubMed] [Google Scholar]
- 34.Constantin JM, Jabaudon M, Lefrant JY, Jaber S, Quenot JP, Langeron O, et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. The Lancet Respiratory medicine. 2019;7(10):870–80. [DOI] [PubMed] [Google Scholar]; ** This trial was a great attempt at real-time clinical phenotyping to study a targeted therapy. The future of the field will require similar studies to enable more precision therapies.
- 35.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346(17):1281–6. [DOI] [PubMed] [Google Scholar]
- 36.Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, et al. Physiologic Analysis and Clinical Performance of the Ventilatory Ratio in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2019;199(3):333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–55. [DOI] [PubMed] [Google Scholar]
- 38.Jabaudon M, Berthelin P, Pranal T, Roszyk L, Godet T, Faure JS, et al. Receptor for advanced glycation end-products and ARDS prediction: a multicentre observational study. Sci Rep. 2018;8(1):2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jabaudon M, Blondonnet R, Pereira B, Cartin-Ceba R, Lichtenstern C, Mauri T, et al. Plasma sRAGE is independently associated with increased mortality in ARDS: a meta-analysis of individual patient data. Intensive Care Med. 2018;44(9):1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jabaudon M, Blondonnet R, Roszyk L, Bouvier D, Audard J, Clairefond G, et al. Soluble Receptor for Advanced Glycation End-Products Predicts Impaired Alveolar Fluid Clearance in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2015;192(2):191–9. [DOI] [PubMed] [Google Scholar]
- 41.Jabaudon M, Futier E, Roszyk L, Chalus E, Guerin R, Petit A, et al. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med. 2011;39(3):480–8. [DOI] [PubMed] [Google Scholar]
- 42.Jones TK, Feng R, Kerchberger VE, Reilly JP, Anderson BJ, Shashaty MGS, et al. Plasma sRAGE Acts as a Genetically Regulated Causal Intermediate in Sepsis-associated Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020;201(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173(9):1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ware LB, Koyama T, Zhao Z, Janz DR, Wickersham N, Bernard GR, et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013;17(5):R253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park J, Pabon M, Choi AMK, Siempos II, Fredenburgh LE, Baron RM, et al. Plasma surfactant protein-D as a diagnostic biomarker for acute respiratory distress syndrome: validation in US and Korean cohorts. BMC Pulm Med. 2017;17(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58(11):983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008;63(12):1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VW, Groeneveld AB. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008;63(10):903–9. [DOI] [PubMed] [Google Scholar]
- 49.Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, et al. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187(7):736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170(7):766–72. [DOI] [PubMed] [Google Scholar]
- 51.Calfee CS, Eisner MD, Parsons PE, Thompson BT, Conner ER Jr., Matthay MA, et al. Soluble intercellular adhesion molecule-1 and clinical outcomes in patients with acute lung injury. Intensive Care Med. 2009;35(2):248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fremont RD, Koyama T, Calfee CS, Wu W, Dossett LA, Bossert FR, et al. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma. 2010;68(5):1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terpstra ML, Aman J, van Nieuw Amerongen GP, Groeneveld AB. Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis*. Crit Care Med. 2014;42(3):691–700. [DOI] [PubMed] [Google Scholar]
- 54.Parsons PE, Matthay MA, Ware LB, Eisner MD. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L426–31. [DOI] [PubMed] [Google Scholar]
- 55.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147(6):1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.García-Laorden MI, Lorente JA, Flores C, Slutsky AS, Villar J. Biomarkers for the acute respiratory distress syndrome: how to make the diagnosis more precise. Ann Transl Med. 2017;5(14):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35(8):1821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137(2):288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Z, Wickersham N, Kangelaris KN, May AK, Bernard GR, Matthay MA, et al. External validation of a biomarker and clinical prediction model for hospital mortality in acute respiratory distress syndrome. Intensive Care Med. 2017;43(8):1123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. The Lancet Respiratory medicine. 2014;2(8):611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acute Respiratory Distress Syndrome N, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8. [DOI] [PubMed] [Google Scholar]
- 62.The National Heart L, and Blood Institute ARDS Clinical Trials Network. Higher versus Lower Positive End-Expiratory Pressures in Patients with the Acute Respiratory Distress Syndrome. http://dxdoiorg/101056/NEJMoa032193. 2009.
- 63.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75. [DOI] [PubMed] [Google Scholar]
- 64.The National Heart L, and Blood Institute ARDS Clinical Trials Network. Rosuvastatin for Sepsis-Associated Acute Respiratory Distress Syndrome. http://dxdoiorg/101056/NEJMoa1401520. 2014. [DOI] [PMC free article] [PubMed]
- 65.McAuley DF, Laffey JG, O’Kane CM, Perkins GD, Mullan B, Trinder TJ, et al. Simvastatin in the Acute Respiratory Distress Syndrome. http://dxdoiorg/101056/NEJMoa1403285. 2014. [DOI] [PubMed]
- 66.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med. 2017;195(3):331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. The Lancet Respiratory medicine. 2018;6(9):691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sinha P, Delucchi KL, Thompson BT, McAuley DF, Matthay MA, Calfee CS, et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018;44(11):1859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sinha P, Delucchi KL, Chen Y, Zhuo H, Abbott J, Wang C, et al. Latent class analysis-derived subphenotypes are generalisable to observational cohorts of acute respiratory distress syndrome: a prospective study. 2021. [DOI] [PMC free article] [PubMed]; ** This study is the first time latent class analysis (LCA) derived biologic subphenotypes were studied in an observational cohort. The LCA subphenotypes previously had only been shown to impact treatment outcomes in patients enrolled in randomized controlled trials.
- 70.Bos LD, Schouten LR, van Vught LA, Wiewel MA, Ong DSY, Cremer O, et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax. 2017;72(10):876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bos LDJ, Scicluna BP, Ong DSY, Cremer O, van der Poll T, Schultz MJ. Understanding Heterogeneity in Biologic Phenotypes of Acute Respiratory Distress Syndrome by Leukocyte Expression Profiles. Am J Respir Crit Care Med. 2019;200(1):42–50. [DOI] [PubMed] [Google Scholar]
- 72.Sarma A, Christenson S, Mick E, Deiss T, DeVoe C, Pisco A, et al. COVID-19 ARDS is characterized by a dysregulated host response that differs from cytokine storm and is modified by dexamethasone. Research square. 2021. [Google Scholar]
- 73.Rogers AJ, Contrepois K, Wu M, Zheng M, Peltz G, Ware LB, et al. Profiling of ARDS pulmonary edema fluid identifies a metabolically distinct subset. Am J Physiol Lung Cell Mol Physiol. 2017;312(5):L703–l9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bos LDJ, Sinha P, Dickson RP. The perils of premature phenotyping in COVID-19: a call for caution. Eur Respir J. 2020;56(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. Jama. 2020;323(22):2329–30. [DOI] [PubMed] [Google Scholar]
- 76.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The first case series on outcomes of critically ill patients with COVID-19 pneumonia that demonstrated lung compliance in COVID-19 ARDS likely follows a normal distribution and is similar to non-COVID ARDS.
- 77.Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study. Am J Respir Crit Care Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyer NJ, Reilly JP, Anderson BJ, Palakshappa JA, Jones TK, Dunn TG, et al. Mortality Benefit of Recombinant Human Interleukin-1 Receptor Antagonist for Sepsis Varies by Initial Interleukin-1 Receptor Antagonist Plasma Concentration. Crit Care Med. 2018;46(1):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sinha P, Churpek MM, Calfee CS. Machine Learning Classifier Models Can Identify Acute Respiratory Distress Syndrome Phenotypes Using Readily Available Clinical Data. Am J Respir Crit Care Med. 2020;202(7):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is one of the few studies on potential methods to make real-time phenotyping of patients with ARDS possible.
- 80.Sinha P, Delucchi KL, McAuley DF, O’Kane CM, Matthay MA, Calfee CS. Development and validation of parsimonious algorithms to classify acute respiratory distress syndrome phenotypes: a secondary analysis of randomised controlled trials. The Lancet Respiratory medicine. 2020;8(3):247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study discovered that a few key biomarkers can accurately subphenotype patients into hyper and hypoinflammatory groups. These biomarkers may be used to develop simple point of care assays for rapid phenotyping.
- 81.Clinical Evaluation of a Point of Care (POC) Assay to Identify Phenotypes in the Acute Respiratory Distress Syndrome - Full Text View - ClinicalTrials.gov 2021. [Available from: https://clinicaltrials.gov/ct2/show/NCT04009330.; ** The results from this study, when it is completed, will be integral to the field as it is the first study on a point of care assay to enable real time biologic phenotyping of ARDS prior to treatment assignment.
- 82.Overmyer KA, Shishkova E, Miller IJ, Balnis J, Bernstein MN, Peters-Clarke TM, et al. Large-Scale Multi-omic Analysis of COVID-19 Severity. Cell Syst. 2021;12(1):23–40.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Multi-omics approaches, as used by the authors in this study, will be important to better characterize biologic heterogeneity in patients with ARDS and identify potential targets for therapies.
- 83.Yurkovich JT, Tian Q, Price ND, Hood L. A systems approach to clinical oncology uses deep phenotyping to deliver personalized care. Nature Reviews Clinical Oncology. 2020;17(3):183–94. [DOI] [PubMed] [Google Scholar]
- 84.Swan M. The Quantified Self: Fundamental Disruption in Big Data Science and Biological Discovery. Big Data. 2013;1(2):85–99. [DOI] [PubMed] [Google Scholar]
- 85.Heijnen NFL, Hagens LA, Smit MR, Cremer OL, Ong DSY, van der Poll T, et al. Biological Subphenotypes of ARDS Show Prognostic Enrichment in Mechanically Ventilated Patients Without ARDS. Am J Respir Crit Care Med. 2021. [DOI] [PubMed] [Google Scholar]; ** This study demonstrates that biologic supphenotypes of ARDS may not be specific to this syndrome but rather describing patients with critical illness.
- 86.Kitsios GD, Yang L, Manatakis DV, Nouraie M, Evankovich J, Bain W, et al. Host-Response Subphenotypes Offer Prognostic Enrichment in Patients With or at Risk for Acute Respiratory Distress Syndrome. Critical care medicine. 2019;47(12). [DOI] [PMC free article] [PubMed] [Google Scholar]


