Abstract
Pulmonary fibrosis, a kind of terminal pathological changes in the lung, is caused by aberrant wound healing, deposition of extracellular matrix (ECM), and eventually replacement of lung parenchyma by ECM. Pulmonary fibrosis induced by acute lung injury and some diseases is reversible under treatment. While idiopathic pulmonary fibrosis is persistent and irreversible even after treatment. Currently, the pathogenesis of irreversible pulmonary fibrosis is not fully elucidated. The known factors associated with the development of irreversible fibrosis include apoptosis resistance of (myo)fibroblasts, dysfunction of pulmonary vessel, cell mitochondria and autophagy, aberrant epithelia hyperplasia and lipid metabolism disorder. In this review, other than a brief introduction of reversible pulmonary fibrosis, we focus on the underlying pathogenesis of irreversible pulmonary fibrosis from the above aspects as well as preclinical disease models, and also suggest directions for future studies.
Keywords: pulmonary fibrosis, irreversibility, pathogenesis, lung
Fibrosis is a pathological change manifested as aberrant tissue repair after chronic inflammatory injury, resulting in excessive synthesis of extracellular matrix (ECM) and replacement of normal parenchyma by ECM such as collagen. With continuous accumulation of ECM, organ fibrosis leads to structural damage, dysfunction and eventually organ failure [1]. Fibrosis occurs in many organs, including heart, kidney, liver and lung [2].
Pulmonary fibrosis, a type of terminal pathological change in the lung, is induced by chronic repetitive alveolar injury of various causes (including heredity, infection and environmental exposure, etc.), leading to excessive ECM deposition and accumulation. Some types of pulmonary fibrosis are reversible, while some others are not. Idiopathic pulmonary fibrosis (IPF) is the most common fibrotic lung disease with progressive and irreversible development [3, 4]. In contrast, pulmonary fibrosis induced by drug or acute lung injury seems reversible after systematic treatment [5-9]. The specific concepts of irreversible and reversible pulmonary fibrosis as well as the causes of their (ir)reversibility remain undefined. Thus, exploring the pathophysiological mechanisms of these two different types of pulmonary fibrosis is significant for the treatment. In this article, we review the potential concepts of irreversible pulmonary fibrosis and the underlying pathogenesis. Also, we propose future directions for the preclinical studies of pulmonary fibrosis.
Currently proposed criteria of irreversible pulmonary fibrosis
A number of interstitial lung diseases (ILDs) and rheumatic diseases develop pulmonary fibrosis to a certain extent. These non-IPF fibrotic lung disease phenotypes may be partially stabilized and reversed after treatment [10]. But most pulmonary fibrosis is progressive and cannot be effectively alleviated even after aggressive treatment.
These progressive pulmonary fibrosis phenotypes are generally known as “progressive fibrosing interstitial lung disease” (PF-ILD), that is irreversible or persistent pulmonary fibrosis [11]. Currently, there is no unified standard or definition of irreversible pulmonary fibrosis. Although some inclusion criteria for patients with PF-ILD have been formulated in clinical practice, the detailed index of these criteria is imperfect and not unified (Table 1) [12-18]. Cottin et al. and George et al. considered that patients with progressive pulmonary fibrosis must meet the conditions that the pulmonary function parameters, CT imaging manifestations and clinical symptoms of patients continue to deteriorate even after appropriate management. According to several clinical trials, they proposed the criteria of PF-ILD (Table 2) [11-13, 19, 20]. On the contrary, if the conditions of patients with pulmonary fibrosis are stabilized or even reversed after aggressive treatment, the pulmonary fibrosis phenotype can be considered as reversible.
Table 1.
Inclusion criteria for patients with PF-ILD in clinical practice.
| Project | Inclusion criteria | Ref. |
|---|---|---|
| Nintedanib in PF-ILD | •Relative decline of FVC predicted ≥10% •Relative decline of FVC predicted between 5% to 10% with worsening respiratory symptoms •Relative decline of FVC predicted between 5% to 10% with extending fibrosis on HRCT •Worsening respiratory symptoms with extending fibrosis on HRCT •HRCT features of fibrotic lung diseases and fibrosis extended ≥10% •FVC ≥45% predicted •DLCO ≥30%—<80% of the predicted (HRCT signs must be assessed by experienced thoracic radiologist) |
[12, 13] |
| Pirfenidone in unclassifiable PF-ILD (NCT03099187) | •Absolute decline of FVC >5% predicted or worsening symptoms not due to cardiac, pulmonary, vascular or other causes within the previous 6 months •Extending fibrosis >10% on HRCT •FVC ≥45% predicted, DLCO ≥30% predicted, FEV1/FVC ratio ≥0.7 •6MWD ≥150m (HRCT signs must be assessed by experienced thoracic radiologist) |
[14, 15] |
| Pirfenidone for progressive, non-IPF lung fibrosis (RELIEF; EudraCT 2014-000861-32) | •Absolute annual decline of FVC ≥5% within 6 to 24 months | [16] |
| Antifibrotic drugs in non-IPF PF-ILD | •Relative decline of FVC ≥10% •Relative decline of DLCO ≥15% •Worsening symptoms •Worsening HRCT signs accompanied by a relative decrease of FVC ≥5-<10% (HRCT signs must be assessed by experienced thoracic radiologist) |
[17] |
| Characteristics and outcomes of PF-ILD other than IPF (PROGRESS; NCT03858842) | HRCT shows >10% fibrosis areas and meets the following criteria for 2 years: •Relative decline of FCV ≥10% with or without clinical deterioration •Relative decline of FCV in 5%-10% with deteriorating respiratory symptoms •Relative decline of FVC in 5%-10% associated with extending fibrosis on HRCT •Extending fibrosis on HRCT with worsening respiratory symptoms (HRCT signs must be assessed by experienced thoracic radiologist) |
[18] |
| Pirfenidone for Progressive Fibrotic Sarcoidosis (PirFS) (NCT03260556) | •Pulmonary function testing with CPI score ≥ 40 •HRCT shows the fibrosis signs >20% •Stable prednisone therapy for at least two months and no change in other immunosuppressives in the two months (HRCT signs must be assessed by experienced thoracic radiologist) |
- |
| Nintedanib in Progressive Pneumoconiosis Study (NiPPS) (NCT04161014) | •HRCT shows diffuse fibrosing extent >10% in lung •FVC ≥45% predicted and DLCO >30% predicted (HRCT signs must be assessed by experienced thoracic radiologist) |
- |
| Allogeneic Mesenchymal Stem Cells in Rapidly Progressive Interstitial Lung Disease (NCT02594839) | •Interstitial lung disease is diagnosed based on: ■Clinical symptoms >12 months duration ■Histologically diagnosed or HRCT features of interstitial pneumonia •FVC ≥40% predicted and DLCO ≥20% •Decline of 10% in FVC (L) and DLCO during the last 12 months (HRCT signs must be assessed by experienced thoracic radiologist) |
- |
DLCO: diffusing capacity of the lung for carbon monoxide; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; HRCT: high-resolution CT; PF-ILD: progressive-fibrosing interstitial lung disease; 6WWD: 6 min walk distance.
Table 2.
Suggested criteria or definitions of progressive fibrosis in clinical practice.
| Suggested inclusion criteria 1 for clinical practice | Ref. |
|---|---|
| Patients meeting any of the following criteria within a 24-month period may have PF-ILD: •Relative decline of ≥10% in FVC •Relative decline of ≥15% in DLCO •Worsening symptoms or worsening HRCT signs accompanied by a relative decline of FVC ≥5-<10% (HRCT signs must be assessed by experienced thoracic radiologist) |
[11] |
| Suggested inclusion criteria 2 for clinical practice | Ref. |
| Patients excluded the alternative explanations such as respiratory tract infection and meeting any of the following criteria can be considered to have PF-ILD: •Relative decline of 10% or more in FVC over 24 months despite treatment •Relative decline in FVC of 5% or more with decline in DLCO of 15% or more over 24 months despite treatment •Relative decline in FVC of 5% or more with increased fibrosis on HRCT over 24 months despite treatment •Relative decline in FVC of 5% or more with progressive symptoms over 24 months despite treatment •Progressive symptoms with increased fibrosis on HRCT over 24 months despite treatment (HRCT signs must be assessed by experienced thoracic radiologist) |
[19] |
CPI: Composite Physiologic Index; DLCO: diffusing capacity of the lung for carbon monoxide; FVC: forced vital capacity; HRCT: high-resolution CT; PF-ILD: progressive-fibrosing interstitial lung disease.
Reversibility in pulmonary fibrosis
Cases of reversible pulmonary fibrosis
Other than few clinical cases, pulmonary fibrosis induced by acute injury, viral infection, and some chronic non-IPF ILD (including chronic hypersensitive pneumonia, connective tissue disease-related interstitial lung disease and non-specific interstitial pneumonia) could be alleviated or even be cured, and pulmonary function of these patients was improved after treatment [8, 9, 19]. Besides, drugs like bleomycin (BLM), methotrexate and nitrofurantoin have certain pulmonary toxicity, leading to interstitial pulmonary fibrosis [21-23]. And lung fibrosis caused by drug toxicity is reversible. Drug induced-fibrotic lesions and lung function are substantially improved after drug withdrawal and the following medication [24, 25].
Fundamental mechanisms of pulmonary fibrosis resolution
Most reversible fibrotic lung diseases are secondary to lung injury caused by infection or drug toxicity. Once the hazard is eliminated, lung fibrosis gradually resolves with treatment. This is also the case in the animal disease models. Almost all the animal models of pulmonary fibrosis have the characteristics of spontaneous resolution.
The BLM-induced pulmonary fibrosis animal model is widely used in the studies owing to the simple modeling method, low cost and obvious fibrosis lesion, etc. [26]. BLM causes apoptosis of alveolar epithelia by inducing DNA break and oxidative stress. The cytotoxic effects then result in acute lung injury and excessive inflammation. Finally, fibrosis occurs as a result of inflammation-induced fibroblast activation and ECM deposition [27, 28]. In general, 28 days after BLM injury, the fibrosis lesion gradually subsides and is close to normal at the end [27]. However, the mechanisms underlying spontaneous resolution in the BLM-induced animal model of pulmonary fibrosis are unspecified.
Elimination of matrix-producing cells, clearance of collagen matrix and regeneration of normal tissue are three important criteria necessary for fibrosis resolution [29]. Without these three elements, spontaneous resolution of pulmonary fibrosis in animal models cannot happen.
Activation of lung fibroblasts can be spontaneously reversed in some animal models [29]. Skeletal muscle terminal differentiation regulator MyoD modulates fibroblast (de)differentiation [30]. TGF-β up-regulates MyoD through ALK5 signaling, promoting fibroblast differentiation. While mitogen related factors also promote myofibroblast dedifferentiation by down-regulating MyoD through activating ERK1/2 MAPK and CDKs signaling [31]. The expression of MyoD is age-related. Aging is beneficial to the increase of MyoD, which maintains the phenotype of myofibroblasts. In the young BLM-induced mice, MyoD is significantly increased in fibrosis stage, while down-regulated in resolution stage, promoting the dedifferentiation of myofibroblasts. However, MyoD expression in the aged BLM-induced mice is consistently increased throughout the whole disease course, not showing fluctuant expression as in the young BLM-induced mice [32].
Apolipoprotein E (ApoE) is highly expressed in fibrosis stage of BLM-induced pulmonary fibrosis mice. Nevertheless, ApoE has no effect on the development of fibrosis, but on the regression of fibrosis. It binds to collagen I and mediates collagen phagocytosis via an apolipoprotein E receptor, low-density lipoprotein receptor associated protein 1 (LRP1), promoting resolution of pulmonary fibrosis [33].
In addition, genes related to lung development (such as FGF10) are markedly increased in the resolution stage of BLM-induced pulmonary fibrosis [34, 35]. The temporary up-regulation of FGF10 in resolution stage may play a key role in resolving fibrosis lesions and alveoli regeneration. In contrast, a stable and low expression of FGF10 is found in lung tissue from IPF patients [36].
Gene spectrum data of BLM-induced mouse model at different sampling time points showed that fibrosis-related genes and signaling, such as ECM remodeling, inflammatory response and Wnt signaling pathway, are up-regulated in the inflammatory and fibrosis stages. In the resolution stage, these genes are decreased, while those related to cell cycle and transcriptional regulation are up-regulated [37]. Up-regulation of these genes in resolution stage may inhibit the pro-fibrotic genes, thus inducing fibrosis resolution [38].
Therefore, as long as collagen phagocytosis and lung regeneration regulation are not fundamentally damaged, even though the massive alveoli are impaired and ECM is deposited, the fibrosis lesion will finally resolve.
The mechanism underlying progressive development of irreversible pulmonary fibrosis
IPF, the most common progressive pulmonary fibrosis disease, is recognized as absolutely irreversible [39]. Besides IPF, some above-mentioned non-IPF chronic ILD may lead to irreversible fibrosis due to various unknown reasons [40]. These different irreversible fibrotic lung diseases seem to have similar symptoms and pathogenesis [41, 42]. Owing to the unclear etiology, occult pathogenesis, and lack of relevant animal models, it is difficult to elucidate why and how irreversible pulmonary fibrosis happens.
Recently, researchers found that repetitive intratracheal instillation of BLM in young mice or a single dose of BLM in aged mice shows persistent pulmonary fibrosis without spontaneous resolution [43-45]. These models provide grounds for the pathogenesis studies of persistent pulmonary fibrosis.
In the following paragraphs, we take IPF as the main example of irreversible pulmonary fibrosis to discuss the pathogenesis (Table 3 & Fig. 1).
Table 3.
Mechanisms of persistent pulmonary fibrosis.
| Underlying mechanisms | Model | Ref. | |
|---|---|---|---|
| Apoptosis resistance of lung fibroblasts | Nox4-Nrf2 dysregulation in lung tissue impairs the redox capacity, endowing the myofibroblasts with the senescence and anti-apoptotic phenotype, which causes persistent pulmonary fibrosis. | BLM model of aged mice | [43] |
| Fas signaling dysfunction caused by down-regulation of Fas or overexpression of anti-apoptotic protein induces lung fibroblasts resistant to apoptosis and retain the pro-fibrotic phenotype. | BLM model of Fas deficiency genetic mice | [55] | |
| FLIP induces IPF myofibroblasts to resist apoptosis and evade immune surveillance by activating NF-κB signaling. | IPF primary lung fibroblasts | [56] | |
| Dysregulated expression of miR-34a and FLIP reduces the susceptibility of myofibroblasts to lymphocyte-mediated apoptosis and leads to persistent pulmonary fibrosis. | MiR-34a dominant negative mice, C57BL/6J wild type mice | [57] | |
| HMGB1 released after lung injury induces apoptosis resistance of fibroblasts via activation of TLR4, leading to persistent pulmonary fibrosis. | Pulmonary fibrosis mouse model induced by radiation | [58] | |
| Mitochondrial dysfunction | Mitochondrial dysfunction caused by stable suppression of PGC1α in IPF lung fibroblasts leads to activation of pro-fibrotic phenotype and promotes senescence of adjacent cells through paracrine manner, inducing persistent pulmonary fibrosis. | Aged col1α1-GFP transgenic mice | [62] |
| Reduced expression of PINK1 induces mitochondrial dysfunction and release of profibrotic factors in ATIIs, increasing the susceptibility to lung fibrosis. | Wild type mice and genetic mice inoculated with MHV68; genetic mice treated with BLM | [66] | |
| Pulmonary vascular dysfunction | Deficiency of eNOS caused by loss of endothelial phenotype and pulmonary vascular dysfunction leads to sustained fibroblast activation, resulting in persistent pulmonary fibrosis. | BLM model of aged mice and young eNOS-/- mice | [72] |
| Chronic repeated injury suppresses the CXCR7 expression and promotes macrophage recruitment. The recruited macrophages stimulate PECEs to increase Notch ligand Jagged 1, which then elicits sustained activation of Notch signaling in perivascular fibroblasts, promoting persistent pulmonary fibrosis. | Mouse model of repeated BLM instillation | [73] | |
| Aberrant epithelial hyperplasia | The deficiency of Nedd4-2 enhances MUC5B expression by increasing surface expression and activity of ENaC in airway epithelia cells, inducing progressive pulmonary fibrosis via impaired mucociliary clearance and dysregulation of TGF-β signaling. | Conditional deletion of Nedd4-2 genetic mice | [91] |
| Airway mucociliary dysfunction caused by high concentration of MUC5B in airways may be highly correlated with the persistent development of pulmonary fibrosis. | Mouse model of repeated BLM instillation | [94] | |
| Lipid metabolic disturbance | ApoE binds to collagen I and mediates collagen phagocytosis via low-density lipoprotein receptor associated protein 1 (LRP1), promoting resolution of pulmonary fibrosis. While loss of ApoE leads to dysfunction of collagen phagocytosis, inducing persistent fibrosis. | ApoE-/- mouse model | [33] |
| Autophagy dysfunction | Autophagy dysfunction in IPF lung fibroblasts induces persistent activation of mTOR, which contributes to the apoptosis resistance of lung fibroblasts. | Primary human lung fibroblasts | [107] |
| Loss of autophagy gene ATG7 in endothelial cells induces EndMT and activates the TGF-β signaling pathway, aggravating pulmonary fibrosis. | EC-ATG-/- mice | [108] |
ApoE: Apolipoprotein E; BLM: bleomycin; CXCR7: chemokine (C-X-C motif) receptor 7; eNOS: endothelial nitric oxide synthase; FLIP: FLICE like inhibitory protein; HMGB1: high mobility group box 1 protein; LRP1: lipoprotein receptor associated protein 1; MUC5B: Mucin 5B; mTOR: the mammalian target of rapamycin; NOX4: NADPH oxidase 4; Nrf2: NFE2-related factor 2; PECEs; PGC1α: Peroxisome proliferator activated receptor gamma co-activator 1-alpha; PINK1: PTEN-induced putative kinase 1; TLR4: Toll like receptor 4.
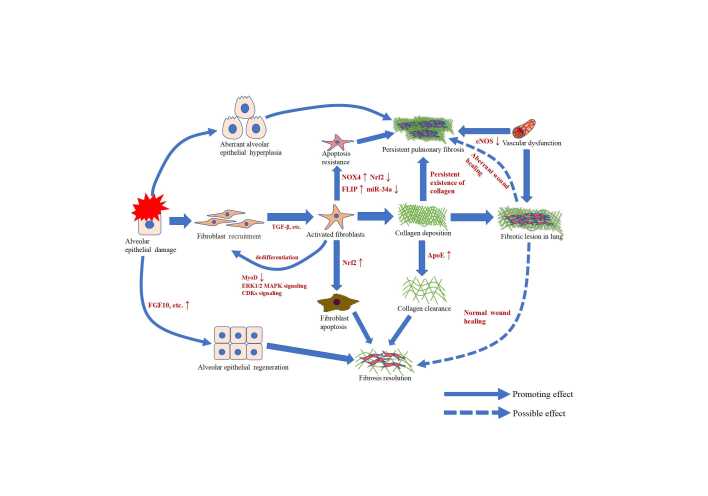
Figure 1.
Schematic representation of the cellular events and basic mechanisms in persistent pulmonary fibrosis and fibrosis resolution. Alveolar epithelial damage causes recruitment of fibroblasts, which is activated by TGF-β, leading to collagen deposition and organ fibrosis. Fibrosis can be persistent, or eventually resolve via wound healing and lung regeneration. With normal collagen clearance and fibroblast apoptosis, fibrosis lesion in the lung is possible to resolve spontaneously. On the contrary, abnormal epithelial hyperplasia, fibroblast apoptosis resistance and collagen clearance failure caused by aberrant wound healing and pulmonary vascular dysfunction may lead to persistent pulmonary fibrosis.
Apoptosis resistance of fibroblasts may be the key of irreversible development in pulmonary fibrosis
(Myo)fibroblasts play a significant role in wound healing. During wound healing, fibroblasts are recruited to the injured area by epithelial damage induced inflammation and differentiate into myofibroblasts induced by TGF-β. Normally, myofibroblasts gradually undergo apoptosis with wound healing. However, in the pathological state, persistent activation of (myo)fibroblasts leads to excessive scar hyperplasia and organ fibrosis [46, 47]. An altered level of apoptosis resistance in IPF (myo)fibroblasts results in their sustained activation and persistent pulmonary fibrosis [48].
Reactive oxygen species (ROS) related factor NADPH oxidase 4 (Nox4) has been evidenced to promote pulmonary fibrosis. Nox4 expression is markedly increased in the lung fibroblasts from IPF patients and BLM-induced pulmonary fibrosis mice [49, 50]. In the fibrotic lung, increased Nox4 induces lung fibroblasts to transform into senescent and apoptosis-resistant phenotype, aggravating pulmonary fibrosis. Generally, increased Nox4 can be neutralized in vivo by antioxidant factors, such as Nrf2, thereby alleviating oxidative stress [51]. But in IPF patients and the BLM-induced persistent fibrosis model of aged mice, Nrf2 expression in fibroblasts is significantly decreased and the antioxidant function is diminished [43, 52]. Nox4-Nrf2 dysregulation in lung tissue impairs the redox capacity, endowing the myofibroblasts with the senescence and anti-apoptotic phenotype, which causes persistent pulmonary fibrosis [43]. Targeting Nox4 and Nox4-related transcription factors restores fibrosis resolution in the aged mouse model [53].
However, Nox4 is not the only molecule that mediates fibroblast apoptosis in pulmonary fibrosis. Death factor Fas also regulates apoptosis of lung fibroblasts [54]. In IPF, dysfunction of Fas signaling caused by down-regulation of Fas or overexpression of anti-apoptotic proteins induces lung fibroblasts resistant to apoptosis. Lung fibroblasts with Fas deficiency retain the pro-fibrotic phenotype and persistently activate COL1A1 and α-SMA promoters [55]. FLICE like inhibitory protein (FLIP), an apoptosis regulator induced by cell death receptor, is highly expressed in myofibroblasts of IPF lungs. FLIP induces IPF myofibroblasts to resist apoptosis and evade immune surveillance by activating NF-κB signaling [56]. Besides, MiR-34a and FLIP negatively regulate each other. In pulmonary fibrosis, miR-34a expression is decreased while FLIP is upregulated, reducing the susceptibility of myofibroblast apoptosis, leading to development of persistent pulmonary fibrosis [57].
In the radiation-induced pulmonary fibrosis, pneumonocytes release high mobility group box 1 protein (HMGB1) after lung injury. HMGB1 may induce apoptosis resistance of fibroblasts via activating Toll like receptor 4 (TLR4) by NF-κB and PI3K/Akt signaling, leading to persistent pulmonary fibrosis [58].
Mitochondrial dysfunction contributes to irreversible pulmonary fibrosis
Mitochondrial dysfunction is an important pathological feature in pulmonary fibrosis [59]. Peroxisome proliferator activated receptor gamma co-activator 1-alpha (PGC1α), a transcription co-activator, regulates mitochondrial biogenesis, oxidative phosphorylation and ROS detoxification [60]. It also mediates the resolution of fibrosis lesion [61]. Therefore, increased PGC1α expression in the late stage of BLM injury is beneficial to fibrosis resolution. In contrast, loss of PGC1α may lead to progressive development of pulmonary fibrosis. The stable suppression of PGC1α in IPF lung fibroblasts leads to decreased mitochondrial mass and function. Mitochondrial dysfunction activates pro-fibrotic fibroblast phenotype and promotes senescence of adjacent cells through paracrine manner, inducing persistent pulmonary fibrosis [62]. Similar as in pulmonary fibrosis, PGC1α expression in renal fibrosis and liver fibrosis is decreased in tubule epithelial cells and hepatocytes, respectively. Overexpressing PGC1α expression restores mitochondrial function and protects from fibrosis [63, 64].
Expression of PTEN-induced putative kinase 1 (PINK1), an aging-associated key regulator of mitochondrial function, is decreased in the aged lung and IPF lung [65]. Reduced PINK1 expression causes mitochondrial dysfunction in type II alveolar cells (ATIIs), leading to endoplasmic reticulum stress and mitophagy dysfunction. Besides, deficient expression of PINK1 in ATII induces release of profibrotic factors. These pathologic processes influence the susceptibility to lung fibrosis and may contribute to irreversible lung fibrosis [66].
Dysfunctional pulmonary vessel offers fundamental environment for progressive development of pulmonary fibrosis
In the mature lung, pulmonary vessel carries blood for gas exchange and nutrient transport. Besides, pulmonary capillary endothelial cells (PCECs) release varieties of cytokines to support the development, regeneration and wound healing in lung [67]. Structure loss, vascular barrier dysfunction, and increased vascular permeability caused by chronic and persistent lung injury continuously recruit and activate fibroblasts, and eventually result in collagen deposition and pulmonary fibrosis [68]. Previous data showed that aberrant vascular remodeling and increased alveolar capillary permeability in fibrotic lungs may result from the imbalanced abundance of pulmonary vascular endothelial cells and progenitors, as well as the imbalance between profibrotic and antifibrotic cytokines. The degree of the increase in vascular permeability is associated with the prognosis of patients with IPF [68-71].
Generally, endothelial cells increase the expression of nitric oxide synthase 3 (NOS3), which synthesizes endothelial nitric oxide synthase (eNOS) following lung injury. ENOS promotes nitric oxide (NO) to bind and activate soluble guanylate cyclase (sGC), inactivating lung fibroblasts and facilitating fibrosis resolution [72]. The aged mouse model of persistent pulmonary fibrosis shows the same pulmonary vascular retrogression as the IPF patients. Degeneration of pulmonary vessel leads to decrease of vascular density, loss of endothelial phenotype and unable to encode Nos3 by endothelial cells, promoting persistent pulmonary fibrosis [72].
PCECs are activated and then increased the expression of chemokine receptor CXCR7 after lung injury. CXCR7 inhibits epithelial-mesenchymal transition (EMT) and pulmonary fibrosis by blocking Jag1-Notch signaling, thereby protecting the alveolar epithelia from injury. Chronic injury induced by repetitive BLM instillation suppresses CXCR7 expression and promotes macrophage recruitment around vessel. The recruited macrophages stimulate PCECs to increase Wnt/β-catenin dependent Notch ligand Jagged 1, which then promotes persistent pulmonary fibrosis by sustained activation of Notch signaling in perivascular fibroblasts [73-75].
Aberrant epithelial hyperplasia and dysfunction may contribute to persistent development of fibrosis
Histologically, the pathological changes of IPF are not just ECM replacement. Atypical epithelial cells usually exist in the fibrosis areas that losing the normal alveolar structure as well as some normal areas. These cells express the markers of ATIIs, proximal airway and submucosal glands in bronchi, resulting in ectopic hyperplasia of alveolar epithelia cells, so-called “epithelial bronchiolization” [76, 77]. It is still unclear where these ectopic epithelial cells come from and what their effects are in persistent pulmonary fibrosis.
A recent study showed that in the pneumonectomy-induced pulmonary fibrosis mouse model, lack of cdc42 causes unsuccessful differentiation of ATII to type I alveolar epithelial cells (ATIs) and promotes formation of transitional cells (TCs) between ATII and ATI, inducing persistent fibrosis [78]. Alveolar TCs are necessary for the differentiation of ATII into ATI during lung regeneration and can be divided into early and late stages [79, 80]. During the early stage, TGF-β is highly activated and increases keratin (KRT)8/KRT18 in TCs; while deactivation of TGF-β promotes early-stage TCs to transdifferentiate into late-stage cells via down-regulating KRT8/KRT18. The late-stage TCs are beneficial to the differentiation of ATII into ATI [80, 81]. A large number of early TCs with high KRT8/KRT18 expression exist in the lungs of IPF patients and BLM-induced pulmonary fibrosis mice [80]. The early TCs gradually transdifferentiate into late TCs in the BLM mouse model, while persistently exist in the IPF lungs [80, 82].
Although the markers of human TCs and mouse TCs differ, these two types of TCs naturally show DNA damage and cell senescence, especially under stimulation of aberrant mechanical stretch [79]. The abnormal biomechanical system in fibrotic lung induces DNA damage and senescence of TCs, promoting vicious development of fibrosis [83].
TCs appear in both persistent and reversible pulmonary fibrosis. They transform to epithelia with resolution of fibrosis, while IPF TCs are stuck in a certain stage and fail to detach, thereby leading to pathological damage and a vicious circle of the organ [84]. In addition, recent single cell RNA sequencing data showed that TCs found in IPF lungs express epithelia cell markers as well as COL1A1 and other pathologic ECM component, suggesting that TCs may promote collagen production and fibrosis progression in IPF [85].
Recently, it has been evidenced that the mutation of MUC5B rs35705950 non-risk alleles is the strongest genetic risk factor of IPF. MUC5B, highly expressed in the IPF lungs, is regulated by ERN2-XBP1S signaling [86-88]. Besides, complement C3, FOXA2 and Nedd4-2 also affect MUC5B expression in the fibrotic lung [89-91]. The E3 ubiquitin-protein ligase Nedd4-2 is involved in epithelial homeostasis. In lung fibrosis, Nedd4-2 deficiency enhances MUC5B expression by increasing surface expression and activity of ENaC on airway epithelia cells [91]. The previous data showed that the overexpressed MUC5B is located in the epithelia cells from honeycomb cysts zone in the IPF lungs, and the ectopic expression of MUC5B may enhance the honeycomb-like cyst formation [92, 93].
In the BLM-induced pulmonary fibrosis mouse model, high concentration of MUC5B in airways causes mucociliary dysfunction and enhances the severity of pulmonary fibrosis [94]. The ectopic expression of MUC5B may lead to more severe and irreversible fibrosis by increasing the sensitivity of ATIIs to BLM and promoting formation of honeycomb structures [93]. Over-secreted MUC5B may not only break the mucosal host defense but also damage ATIIs and interfere with alveolar repair. The damaged alveoli fail to be epithelialized and enhance the collapse and fibrosis of bronchial-alveoli units, eventually leading to idiopathic pulmonary fibrosis [95]. But the direct cytotoxic effect of MUC5B on ATIIs in pulmonary fibrosis remains indistinct. Further investigations need be carried out in the future.
Lipid metabolic disturbance and irreversible pulmonary fibrosis
At present, a growing number of studies have preliminarily demonstrated that lipid metabolism disorder is related with the pathogenesis of pulmonary fibrosis [96]. Previous studies showed decreased levels of lipid metabolism related molecules such as elongation of long-chain fatty acids family member 6 (Elovl6) and stearoyl CoA desaturase 1 (SCD1) in IPF lungs. And fibrosis susceptibility is increased when these genes are suppressed in mice [97, 98]. Besides, a single dose of BLM instillation induces more severe pulmonary fibrosis in the ApoE-/- mice and the fibrosis develops progressively and irreversibly [33].
Sequencing data showed that the genes and signaling pathways related to lipid metabolism are down-regulated in the lungs of IPF patients and the aged mice with BLM injury [99, 100]. The balance of lipid metabolism is important in maintaining structure and function of the alveolar epithelium. Excessive accumulation of cholesterol leads to alveoli collapse and alveolar injury [101]. Based on the above, lipid metabolism disorder may play a key role in persistent pulmonary fibrosis.
Autophagy may contribute to irreversible pulmonary fibrosis
Autophagy, a cytoprotective mechanism, is important to maintain cellular homeostasis and modulate redox equilibrium. Altered autophagy has been observed in pulmonary fibrosis [102]. Studies have shown that decreased autophagy in fibroblasts and alveolar epithelia promotes pulmonary fibrosis [103].
Autophagy dysfunction, mainly induced by aging, is associated with cell apoptosis resistance [104]. Recent studies showed that insufficient autophagy promotes IPF development. Autophagy inhibition in IPF lung fibroblasts and alveolar epithelia induces activation of lung fibroblasts [105, 106]. Autophagy dysfunction in IPF lung fibroblasts induces persistent activation of the mammalian target of rapamycin (mTOR), leading to apoptosis resistance of lung fibroblasts and persistent pulmonary fibrosis [107].
Besides, impaired autophagic flux of lung endothelial cells (ECs) induces change of endothelial structure and affects progression of pulmonary fibrosis [108]. This study also showed that loss of autophagy gene ATG7 in ECs induces endothelial-to-mesenchymal transition (EndMT) and activates the TGF-β signaling pathway in vitro. Comparing with the wild type mice, more serious fibrotic lesions occur in the EC-ATG7-/- mice, indicating that progressive lung fibrosis may be accompanied by the loss of ATG7 [108].
Small airway may be the breeding site for irreversible pulmonary fibrosis
Although IPF has been studied for decades, its onset site is still a mystery. As early as 40 years ago, it was reported that small airway lesion occurs in IPF, which is considered as a disease of small airway and alveoli. This concept was verified by pulmonary function test and histological assessment [109, 110]. Small airway is regarded as a “quiet zone” in lung. The injury and disease may easily accumulate in small airway for years without being noticed [111]. In IPF, pathological change in small airway is an early feature [110].
Club cells are the major epithelial cells in the small airways [112]. In IPF, club cells that express the specific protein SCGB1A1 are absent in the abnormal small airways. Bronchiolization, the characteristic change in IPF lung, precedes the fibrotic process. The density of SCGB1A1+ club cells is negatively correlated with the degree of bronchiectasis and bronchiolization, whereas positively correlated with forced vital capacity (FVC) [113]. Therefore, absence of SCGB1A1+ cells in the pathologic small airway may indicate development of severe fibrosis. Also, an earlier study showed that club cells migrate to the alveolar regions and directly induce alveolar apoptosis in IPF lungs [114]. Therefore, we speculate that the small airway may be the origin site of IPF.
If microdamage initially appears in small airway, the pathological lesion is hard to be noticed. With the accumulation of lesions, disease will rapidly worsen and eventually lead to organ failure. This process is in line with the development of IPF [115]. Thus, pathological change in small airway may be one of the factors for the occult and persistent development of IPF.
Conclusions and Perspective
Here we emphatically discuss the irreversible development of pulmonary fibrosis from aspects of clinical cases, animal models and pathogenesis. The apoptosis resistance of lung fibroblasts as well as dysfunction of mitochondrion, pulmonary vessel and lipid metabolism contribute to development of persistent pulmonary fibrosis. Besides, aberrant epithelial hyperplasia and cell autophagy potentially cause the irreversibility of pulmonary fibrosis. Also, we propose that small airway may be the origin site of persistent pulmonary fibrosis (Fig. 2).
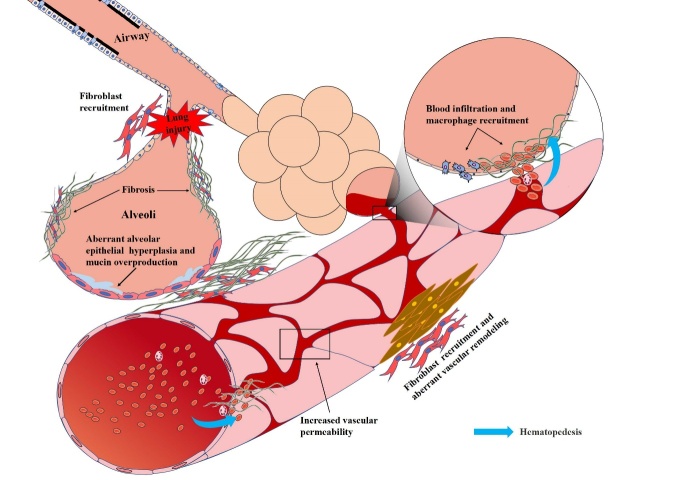
Figure 2.
Possible pathogenesis of persistent pulmonary fibrosis. On the one hand, lung injury induces fibroblast recruitment, leading to collagen deposition and fibrosis. On the other hand, the incomplete differentiation of alveolar epithelia may result in aberrant alveolar epithelial hyperplasia and mucin overproduction, which may destroy the wound healing and aggravate pulmonary fibrosis. Besides, pulmonary vascular dysfunction caused by the loss of endothelia phenotype and high vascular permeability may induce aberrant vascular remodeling, which further enlarges the fibrosis lesion and results in the persistent and progressive development of pulmonary fibrosis.
Several pulmonary fibrosis phenotypes can be cured after treatment, while IPF and some non-IPF PF-ILD are progressive and incurable. Pulmonary fibrosis induced by acute lung injury is curable, probably because of the incomplete injury of epithelial regeneration and fibroblast apoptosis. As long as the normal stem cells exist, as well as wound healing and collagen degradation still work, fibrosis will gradually resolve. However, fibrosis resolution does not happen in PF-ILD, and the pathogenesis of persistent fibrosis remains unclear. As mentioned above, development of persistent pulmonary fibrosis involves a complex network. Therefore, targeting a certain class of cytokines or cell signaling may not be sufficient for disease remission [116].
Small airway damage in pulmonary fibrosis has been noticed for decades, whereas the relationship between the causes of injury in small airway and the initiation as well as sustained progression of pulmonary fibrosis is rarely studied. The main reason may be lack of systematic and continuous monitor methods for small airway damage in patients with PF-ILD. Besides, lack of suitable animal models and tissue models also makes it difficult to carry out studies on the small airway damage in pulmonary fibrosis. Development of relevant models and systematic studies based on small airway may be a key direction to explore the occult onset and persistent development of PF-ILD. Studies on small airway in pulmonary fibrosis may provide not only therapeutic targets, but also strategies for early diagnosis and monitoring of patients with PF-ILD.
Recently, organoids and cell models that modeling the pathological changes of IPF have been developed, including human induced pluripotent stem cell (iPSC)-derived air-liquid interface (ALI) model stimulated by IPF-relevant cocktail (IPF-RC) and the immortalized human small airway basal stem/progenitor cell line [117, 118]. These are expected to be preferred models for studies on small airway damage in pulmonary fibrosis.
Animal models of irreversible and persistent pulmonary fibrosis have been developed, including aged mice with single BLM instillation, young mice with repeated BLM instillation and some genetic mouse models. Nevertheless, these models also have limitations. Genetic mouse models cannot be widely used because of the gene specificity [27, 119]. The single dose of BLM-induced lung fibrosis in the aged mice is irreversible, however, it lacks IPF characteristic lesions such as airway epithelial hyperplasia [44]. Thus, although it takes a long time to establish, the mouse model of repeated BLM instillation may be the most advantageous model of persistent pulmonary fibrosis at present as it not only develops persistent fibrosis, but also represents UIP features, such as airway epithelial hyperplasia seen in IPF. This model is more consistent with the course and lesion of IPF [44, 45, 120].
These animal models of persistent pulmonary fibrosis are generally used for study of pathogenesis. However, few researchers systematically compare different kinds of models which are helpful to find target genes and biomarkers related to persistent pulmonary fibrosis. Furthermore, comparing the clinical symptoms, differentiation of histological features between reversible and irreversible pulmonary fibrosis, as well as exploring the mechanisms underlying persistent pulmonary fibrosis are of great significant for the early diagnosis and treatment of some occult pulmonary fibrosis, such as IPF.
Acknowledgements
This work was supported by the National High-Level Talents Program (XT), the National Natural Science Foundation of China (81770015, XT), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01S155), Open Project of State Key Laboratory of Respiratory Disease (SKLRD-OP-202109), Special Fund for Science and Technology Innovation of Guangdong Province (2020B1111330001), and Guangzhou Institute of Respiratory Health Open Project (Funds provided by China Evergrande Group)-Project No. 2020GIRHHMS16.
Footnotes
Conflict of interest
The authors declare no conflict of interests.
Author contributions
XT conceived and designed the manuscript, provided guidance, and edited the manuscript. Both authors wrote the manuscript and critically revised it.
References
- [1].Henderson NC, Rieder F, Wynn TA (2020). Fibrosis: from mechanisms to medicines. Nature, 587:555-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Katzen J, Beers MF (2020). Contributions of alveolar epithelial cell quality control to pulmonary fibrosis. J Clin Invest, 130:5088-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kolahian S, Fernandez IE, Eickelberg O, Hartl D (2016). Immune Mechanisms in Pulmonary Fibrosis. Am J Respir Cell Mol Biol, 55:309-322. [DOI] [PubMed] [Google Scholar]
- [4].Chioma OS, Drake WP (2017). Role of Microbial Agents in Pulmonary Fibrosis. Yale J Biol Med, 90:219-227. [PMC free article] [PubMed] [Google Scholar]
- [5].O'Neill TJ, Kardinal CG, Tierney LM (1975). Reversible interstitial pneumonitis associated with low dose bleomycin. Chest, 68:265-267. [DOI] [PubMed] [Google Scholar]
- [6].Reynolds BC, Paton JY, Howatson AG, Ramage IJ (2008). Reversible chronic pulmonary fibrosis associated with MMF in a pediatric patient: a case report. Pediatr Transplant, 12:228-231. [DOI] [PubMed] [Google Scholar]
- [7].Mikkelsen LF, Rubak S (2020). Reversible lung fibrosis in a 6-year-old girl after long term nitrofurantoin treatment. BMC Pulm Med, 20:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mittermayer C, Hassenstein J, Riede UN (1978). Is Shock-Induced Lung Fibrosis Reversible? A Report on Recovery from "Shock-Lung". Pathol Res Pract, 162:73-87. [DOI] [PubMed] [Google Scholar]
- [9].Zhang P, Li J, Liu H, Han N, Ju J, Kou Y, et al. (2020). Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res, 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wijsenbeek M, Kreuter M, Olson A, Fischer A, Bendstrup E, Wells CD, et al. (2019). Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin, 35:2015-2024. [DOI] [PubMed] [Google Scholar]
- [11].Cottin V, Hirani NA, Hotchkin DL, Nambiar AM, Ogura T, Otaola M, et al. (2018). Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Flaherty KR, Brown KK, Wells AU, Clerisme-Beaty E, Collard HR, Cottin V, et al. (2017). Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of nintedanib in patients with progressive fibrosing interstitial lung disease. BMJ Open Respir Res, 4:e000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. (2019). Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med, 381:1718-1727. [DOI] [PubMed] [Google Scholar]
- [14].Maher TM, Corte TJ, Fischer A, Kreuter M, Lederer DJ, Molina-Molina M, et al. (2018). Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: design of a double-blind, randomised, placebo-controlled phase II trial. BMJ Open Respir Res, 5:e000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maher TM, Corte TJ, Fischer A, Kreuter M, Lederer DJ, Molina-Molina M, et al. (2020). Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med, 8:147-157. [DOI] [PubMed] [Google Scholar]
- [16].Behr J, Neuser P, Prasse A, Kreuter M, Rabe K, Schade-Brittinger C, et al. (2017). Exploring efficacy and safety of oral Pirfenidone for progressive, non-IPF lung fibrosis (RELIEF) - a randomized, double-blind, placebo-controlled, parallel group, multi-center, phase II trial. BMC Pulm Med, 17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Torrisi SE, Kahn N, Walscher J, Sarmand N, Polke M, Lars K, et al. (2019). Possible value of antifibrotic drugs in patients with progressive fibrosing non-IPF interstitial lung diseases. BMC Pulm Med, 19:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nasser M, Larrieu S, Si-Mohamed S, Ahmad K, Boussel L, Brevet M, et al. (2021). Progressive fibrosing interstitial lung disease: a clinical cohort (the PROGRESS study). Eur Respir J, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].George PM, Spagnolo P, Kreuter M, Altinisik G, Bonifazi M, Martinez FJ, et al. (2020). Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med, 8:925-934. [DOI] [PubMed] [Google Scholar]
- [20].Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. (2011). Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet, 377:1760-1769. [DOI] [PubMed] [Google Scholar]
- [21].O'Sullivan JM, Huddart RA, Norman AR, Nicholls J, Dearnaley DP, Horwich A (2003). Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol, 14:91-96. [DOI] [PubMed] [Google Scholar]
- [22].Imokawa S, Colby TV, Leslie KO, Helmers RA (2000). Methotrexate pneumonitis: review of the literature and histopathological findings in nine patients. Eur Respir J, 15:373-381. [DOI] [PubMed] [Google Scholar]
- [23].Matsuno O (2012). Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res, 13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, et al. (2018). Drug-Induced Interstitial Lung Disease: A Systematic Review. J Clin Med, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Terbuch A, Tiu C, Candilejo IM, Scaranti M, Curcean A, Bar D, et al. (2020). Radiological Patterns of Drug-induced Interstitial Lung Disease (DILD) in Early-phase Oncology Clinical Trials. Clin Cancer Res, 26:4805-4813. [DOI] [PubMed] [Google Scholar]
- [26].Tashiro J, Rubio GA, Limper AH, Williams K, Elliot SJ, Ninou I, et al. (2017). Exploring Animal Models That Resemble Idiopathic Pulmonary Fibrosis. Front Med (Lausanne), 4:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Degryse AL, Lawson WE (2011). Progress toward improving animal models for idiopathic pulmonary fibrosis. Am J Med Sci, 341:444-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mouratis MA, Aidinis V (2011). Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med, 17:355-361. [DOI] [PubMed] [Google Scholar]
- [29].Atabai K, Yang CD, Podolsky MJ (2020). You Say You Want a Resolution (of Fibrosis). Am J Respir Cell Mol Biol, 63:424-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Olson EN (1993). Regulation of muscle transcription by the MyoD family. The heart of the matter. Circ Res, 72:1-6. [DOI] [PubMed] [Google Scholar]
- [31].Hecker L, Jagirdar R, Jin T, Thannickal VJ (2011). Reversible differentiation of myofibroblasts by MyoD. Exp Cell Res, 317:1914-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kato K, Logsdon NJ, Shin Y-J, Palumbo S, Knox A, Irish JD, et al. (2020). Impaired Myofibroblast Dedifferentiation Contributes to Nonresolving Fibrosis in Aging. Am J Respir Cell Mol Biol, 62:633-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cui H, Jiang D, Banerjee S, Xie N, Kulkarni T, Liu RM, et al. (2020). Monocyte-derived alveolar macrophage apolipoprotein E participates in pulmonary fibrosis resolution. JCI Insight, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Farkas L, Farkas D, Gauldie J, Warburton D, Shi W, Kolb M (2011). Transient overexpression of Gremlin results in epithelial activation and reversible fibrosis in rat lungs. Am J Respir Cell Mol Biol, 44:870-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ornitz DM, Itoh N (2015). The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol, 4:215-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu J, Chu X, Chen C, Bellusci S (2018). Role of Fibroblast Growth Factor 10 in Mesenchymal Cell Differentiation During Lung Development and Disease. Front Genet, 9:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cabrera S, Selman M, Lonzano-Bolanos A, Konishi K, Richards TJ, Kaminski N, et al. (2013). Gene expression profiles reveal molecular mechanisms involved in the progression and resolution of bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol, 304:L593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tan Q, Link PA, Meridew JA, Pham TX, Caporarello N, Ligresti G, et al. (2021). Spontaneous Lung Fibrosis Resolution Reveals Novel Antifibrotic Regulators. Am J Respir Cell Mol Biol, 64:453-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Richeldi L, Collard HR, Jones MG (2017). Idiopathic pulmonary fibrosis. Lancet, 389:1941-1952. [DOI] [PubMed] [Google Scholar]
- [40].Cottin V, Wollin L, Fischer A, Quaresma M, Stowasser S, Harari S (2019). Fibrosing interstitial lung diseases: knowns and unknowns. Eur Respir Rev, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brown KK, Martinez FJ, Walsh SLF, Thannickal VJ, Prasse A, Schlenker-Herceg R, et al. (2020). The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kolb M, Vasakova M (2019). The natural history of progressive fibrosing interstitial lung diseases. Respir Res, 20:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, et al. (2014). Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med, 6:231ra247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, et al. (2010). Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol, 299:L442-L452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Redente EF, Black BP, Backos DS, Bahadur AN, Humphries SM, Lynch DA, et al. (2021). Persistent, Progressive Pulmonary Fibrosis and Epithelial Remodeling in Mice. Am J Respir Cell Mol Biol, 64:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Darby IA, Laverdet B, Bonté F, Desmoulière A (2014). Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol, 7:301-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shu DY, Lovicu FJ (2017). Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog Retin Eye Res, 60:44-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bamberg A, Redente EF, Groshong SD, Tuder RM, Cool CD, Keith RC, et al. (2018). Protein Tyrosine Phosphatase-N13 Promotes Myofibroblast Resistance to Apoptosis in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med, 198:914-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J (2010). NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax, 65:733-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li ZM, Xu SY, Feng YZ, Cheng YR, Xiong JB, Zhou Y, et al. (2021). The role of NOX4 in pulmonary diseases. J Cell Physiol, 236:1628-1637. [DOI] [PubMed] [Google Scholar]
- [51].Pendyala S, Moitra J, Kalari S, Kleeberger SR, Zhao Y, Reddy SP, et al. (2011). Nrf2 regulates hyperoxia-induced Nox4 expression in human lung endothelium: identification of functional antioxidant response elements on the Nox4 promoter. Free Radic Biol Med, 50:1749-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mazur W, Lindholm P, Vuorinen K, Myllärniemi M, Salmenkivi K, Kinnula VL (2010). Cell-specific elevation of NRF2 and sulfiredoxin-1 as markers of oxidative stress in the lungs of idiopathic pulmonary fibrosis and non-specific interstitial pneumonia. APMIS, 118:703-712. [DOI] [PubMed] [Google Scholar]
- [53].Sanders YY, Lyv X, Zhou QJ, Xiang Z, Stanford D, Bodduluri S, et al. (2020). Brd4-p300 inhibition downregulates Nox4 and accelerates lung fibrosis resolution in aged mice. JCI Insight, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dodi AE, Ajayi IO, Chang C, Beard M, Ashley SL, Huang SK, et al. (2018). Regulation of fibroblast Fas expression by soluble and mechanical pro-fibrotic stimuli. Respir Res, 19:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Redente EF, Chakraborty S, Sajuthi S, Black BP, Edelman BL, Seibold MA, et al. (2020). Loss of Fas signaling in fibroblasts impairs homeostatic fibrosis resolution and promotes persistent pulmonary fibrosis. JCI Insight, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Golan-Gerstl R, Wallach-Dayan SB, Zisman P, Cardoso WV, Goldstein RH, Breuer R (2012). Cellular FLICE-like inhibitory protein deviates myofibroblast fas-induced apoptosis toward proliferation during lung fibrosis. Am J Respir Cell Mol Biol, 47:271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bulvik R, Biton M, Berkman N, Breuer R, Wallach-Dayan SB (2020). Forefront: MiR-34a-Knockout Mice with Wild Type Hematopoietic Cells, Retain Persistent Fibrosis Following Lung Injury. Int J Mol Sci, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hanson KM, Hernady EB, Reed CK, Johnston CJ, Groves AM, Finkelstein JN (2019). Apoptosis Resistance in Fibroblasts Precedes Progressive Scarring in Pulmonary Fibrosis and Is Partially Mediated by Toll-Like Receptor 4 Activation. Toxicol Sci, 170:489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zank DC, Bueno M, Mora AL, Rojas M (2018). Idiopathic Pulmonary Fibrosis: Aging, Mitochondrial Dysfunction, and Cellular Bioenergetics. Front Med (Lausanne), 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rius-Pérez S, Torres-Cuevas I, Millán I, Ortega ÁL, Pérez S (2020). PGC-1, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid Med Cell Longev, 2020:1452696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ligresti G, Caporarello N, Meridew JA, Jones DL, Tan Q, Choi KM, et al. (2019). CBX5/G9a/H3K9me-mediated gene repression is essential to fibroblast activation during lung fibrosis. JCI Insight, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Caporarello N, Meridew JA, Jones DL, Tan Q, Haak AJ, Choi KM, et al. (2019). PGC1α repression in IPF fibroblasts drives a pathologic metabolic, secretory and fibrogenic state. Thorax, 74:749-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Han SH, Wu M-Y, Nam BY, Park JT, Yoo T-H, Kang S-W, et al. (2017). PGC-1 Protects from Notch-Induced Kidney Fibrosis Development. J Am Soc Nephrol, 28:3312-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang L, Zhang Y, Chang X, Zhang X (2020). Imbalance in mitochondrial dynamics induced by low PGC-1α expression contributes to hepatocyte EMT and liver fibrosis. Cell Death Dis, 11:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang N, Zhu P, Huang R, Wang C, Sun L, Lan B, et al. (2020). PINK1: The guard of mitochondria. Life Sci, 259:118247. [DOI] [PubMed] [Google Scholar]
- [66].Bueno M, Lai Y-C, Romero Y, Brands J, St Croix CM, Kamga C, et al. (2015). PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest, 125:521-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, et al. (2011). Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell, 147:539-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Probst CK, Montesi SB, Medoff BD, Shea BS, Knipe RS (2020). Vascular permeability in the fibrotic lung. Eur Respir J, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].McKeown S, Richter AG, O'Kane C, McAuley DF, Thickett DR (2009). MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J, 33:77-84. [DOI] [PubMed] [Google Scholar]
- [70].Mlika M, Bacha S, Braham E, El Mezni F (2016). The inter-connection between fibrosis and microvascular remodeling in idiopathic pulmonary fibrosis: Reality or just a phenomenon. Respir Med Case Rep, 17:30-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Smadja DM, Mauge L, Nunes H, d'Audigier C, Juvin K, Borie R, et al. (2013). Imbalance of circulating endothelial cells and progenitors in idiopathic pulmonary fibrosis. Angiogenesis, 16:147-157. [DOI] [PubMed] [Google Scholar]
- [72].Caporarello N, Meridew JA, Aravamudhan A, Jones DL, Austin SA, Pham TX, et al. (2020). Vascular dysfunction in aged mice contributes to persistent lung fibrosis. Aging Cell:e13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, et al. (2016). Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med, 22:154-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cully M (2016). Lung disease: CXCR7 activation overrides lung fibrosis. Nat Rev Drug Discov, 15:160. [DOI] [PubMed] [Google Scholar]
- [75].Guan S, Zhou J (2017). CXCR7 attenuates the TGF-β-induced endothelial-to-mesenchymal transition and pulmonary fibrosis. Mol Biosyst, 13:2116-2124. [DOI] [PubMed] [Google Scholar]
- [76].Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, et al. (2016). Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight, 1:e90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wang ZN, Tang XX (2020). New Perspectives on the Aberrant Alveolar Repair of Idiopathic Pulmonary Fibrosis. Front Cell Dev Biol, 8:580026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wu H, Yu Y, Huang H, Hu Y, Fu S, Wang Z, et al. (2020). Progressive Pulmonary Fibrosis Is Caused by Elevated Mechanical Tension on Alveolar Stem Cells. Cell, 180. [DOI] [PubMed] [Google Scholar]
- [79].Kobayashi Y, Tata A, Konkimalla A, Katsura H, Lee RF, Ou J, et al. (2020). Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol, 22:934-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jiang P, Gil de Rubio R, Hrycaj SM, Gurczynski SJ, Riemondy KA, Moore BB, et al. (2020). Ineffectual Type 2-to-Type 1 Alveolar Epithelial Cell Differentiation in Idiopathic Pulmonary Fibrosis: Persistence of the KRT8 Transitional State. Am J Respir Crit Care Med, 201:1443-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Riemondy KA, Jansing NL, Jiang P, Redente EF, Gillen AE, Fu R, et al. (2019). Single cell RNA sequencing identifies TGFβ as a key regenerative cue following LPS-induced lung injury. JCI Insight, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Strunz M, Simon LM, Ansari M, Kathiriya JJ, Angelidis I, Mayr CH, et al. (2020). Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun, 11:3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Suki B, Stamenovic D, Hubmayr R (2011). Lung parenchymal mechanics. Compr Physiol, 1:1317-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Auyeung VC, Sheppard D (2020). Stuck in a Moment: Does Abnormal Persistence of Epithelial Progenitors Drive Pulmonary Fibrosis? Am J Respir Crit Care Med, 203:667-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Habermann AC, Gutierrez AJ, Bui LT, Yahn SL, Winters NI, Calvi CL, et al. (2020). Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv, 6:eaba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. (2011). A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med, 364:1503-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L, et al. (2016). Idiopathic Pulmonary Fibrosis: A Genetic Disease That Involves Mucociliary Dysfunction of the Peripheral Airways. Physiol Rev, 96:1567-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chen G, Ribeiro CMP, Sun L, Okuda K, Kato T, Gilmore RC, et al. (2019). XBP1S Regulates MUC5B in a Promoter Variant-Dependent Pathway in Idiopathic Pulmonary Fibrosis Airway Epithelia. Am J Respir Crit Care Med, 200:220-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Okamoto T, Mathai SK, Hennessy CE, Hancock LA, Walts AD, Stefanski AL, et al. (2018). The relationship between complement C3 expression and the MUC5B genotype in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Helling BA, Gerber AN, Kadiyala V, Sasse SK, Pedersen BS, Sparks L, et al. (2017). Regulation of MUC5B Expression in Idiopathic Pulmonary Fibrosis. Am J Respir Cell Mol Biol, 57:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Duerr J, Leitz DHW, Szczygiel M, Dvornikov D, Fraumann SG, Kreutz C, et al. (2020). Conditional deletion of Nedd4-2 in lung epithelial cells causes progressive pulmonary fibrosis in adult mice. Nat Commun, 11:2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Seibold MA, Smith RW, Urbanek C, Groshong SD, Cosgrove GP, Brown KK, et al. (2013). The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS One, 8:e58658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kurche JS, Dobrinskikh E, Hennessy CE, Huber J, Estrella A, Hancock LA, et al. (2019). Muc5b Enhances Murine Honeycomb-like Cyst Formation. Am J Respir Cell Mol Biol, 61:544-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hancock LA, Hennessy CE, Solomon GM, Dobrinskikh E, Estrella A, Hara N, et al. (2018). Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat Commun, 9:5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang Q, Wang Y, Qu D, Yu J, Yang J (2019). The Possible Pathogenesis of Idiopathic Pulmonary Fibrosis considering. Biomed Res Int, 2019:9712464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mamazhakypov A, Schermuly RT, Schaefer L, Wygrecka M (2019). Lipids - two sides of the same coin in lung fibrosis. Cell Signal, 60:65-80. [DOI] [PubMed] [Google Scholar]
- [97].Sunaga H, Matsui H, Ueno M, Maeno T, Iso T, Syamsunarno MRAA, et al. (2013). Deranged fatty acid composition causes pulmonary fibrosis in Elovl6-deficient mice. Nat Commun, 4:2563. [DOI] [PubMed] [Google Scholar]
- [98].Romero F, Hong X, Shah D, Kallen CB, Rosas I, Guo Z, et al. (2018). Lipid Synthesis Is Required to Resolve Endoplasmic Reticulum Stress and Limit Fibrotic Responses in the Lung. Am J Respir Cell Mol Biol, 59:225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, et al. (2019). Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am J Respir Crit Care Med, 199:1517-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lv T, Jiang K, Wang J, Tang N, Dai H, Wang C (2019). Single-cell RNA sequencing profiling of the effects of aging on alveolar stem cells. Sci China Life Sci, 62:1028-1037. [DOI] [PubMed] [Google Scholar]
- [101].Vockeroth D, Gunasekara L, Amrein M, Possmayer F, Lewis JF, Veldhuizen RAW (2010). Role of cholesterol in the biophysical dysfunction of surfactant in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol, 298:L117-L125. [DOI] [PubMed] [Google Scholar]
- [102].Ornatowski W, Lu Q, Yegambaram M, Garcia AE, Zemskov EA, Maltepe E, et al. (2020). Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol, 36:101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Liao S-X, Sun P-P, Gu Y-H, Rao X-M, Zhang L-Y, Ou-Yang Y (2019). Autophagy and pulmonary disease. Ther Adv Respir Dis, 13:1753466619890538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Rubinsztein DC, Mariño G, Kroemer G (2011). Autophagy and aging. Cell, 146:682-695. [DOI] [PubMed] [Google Scholar]
- [105].Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, et al. (2013). Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol, 304:L56-L69. [DOI] [PubMed] [Google Scholar]
- [106].Hill C, Li J, Liu D, Conforti F, Brereton CJ, Yao L, et al. (2019). Autophagy inhibition-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in pulmonary fibrosis. Cell Death Dis, 10:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Romero Y, Bueno M, Ramirez R, Álvarez D, Sembrat JC, Goncharova EA, et al. (2016). mTORC1 activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell, 15:1103-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Singh KK, Lovren F, Pan Y, Quan A, Ramadan A, Matkar PN, et al. (2015). The essential autophagy gene ATG7 modulates organ fibrosis via regulation of endothelial-to-mesenchymal transition. J Biol Chem, 290:2547-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Fulmer JD, Roberts WC, von Gal ER, Crystal RG (1977). Small airways in idiopathic pulmonary fibrosis. Comparison of morphologic and physiologic observations. J Clin Invest, 60:595-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Verleden SE, Tanabe N, McDonough JE, Vasilescu DM, Xu F, Wuyts WA, et al. (2020). Small airways pathology in idiopathic pulmonary fibrosis: a retrospective cohort study. Lancet Respir Med, 8:573-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Mead J (1970). The lung's "quiet zone". N Engl J Med, 282:1318-1319. [DOI] [PubMed] [Google Scholar]
- [112].Boers JE, Ambergen AW, Thunnissen FB (1999). Number and proliferation of clara cells in normal human airway epithelium. Am J Respir Crit Care Med, 159:1585-1591. [DOI] [PubMed] [Google Scholar]
- [113].Reynaud P, Ahmed E, Serre I, Knabe L, Bommart S, Suehs C, et al. (2021). Club Cell Loss as a Feature of Bronchiolization in ILD. Front Immunol, 12:630096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Akram KM, Lomas NJ, Spiteri MA, Forsyth NR (2013). Club cells inhibit alveolar epithelial wound repair via TRAIL-dependent apoptosis. Eur Respir J, 41:683-694. [DOI] [PubMed] [Google Scholar]
- [115].Bates JH, Davis GS, Majumdar A, Butnor KJ, Suki B (2007). Linking parenchymal disease progression to changes in lung mechanical function by percolation. Am J Respir Crit Care Med, 176:617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].She YX, Yu QY, Tang XX (2021). Role of interleukins in the pathogenesis of pulmonary fibrosis. Cell Death Discov, 7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Schruf E, Schroeder V, Le HQ, Schonberger T, Raedel D, Stewart EL, et al. (2020). Recapitulating idiopathic pulmonary fibrosis related alveolar epithelial dysfunction in a human iPSC-derived air-liquid interface model. FASEB J, 34:7825-7846. [DOI] [PubMed] [Google Scholar]
- [118].Wang G, Lou HH, Salit J, Leopold PL, Driscoll S, Schymeinsky J, et al. (2019). Characterization of an immortalized human small airway basal stem/progenitor cell line with airway region-specific differentiation capacity. Respir Res, 20:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Moore BB, Hogaboam CM (2008). Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol, 294:L152-160. [DOI] [PubMed] [Google Scholar]
- [120].Knudsen L, Ruppert C, Ochs M (2017). Tissue remodelling in pulmonary fibrosis. Cell Tissue Res, 367:607-626. [DOI] [PubMed] [Google Scholar]