Introduction
Testosterone deficiency (TD), or hypogonadism, is defined by the combination of a low serum testosterone laboratory value and associated symptoms such as decreased libido, fatigue, depression, and lean body mass, erectile dysfunction1. The prevalence of TD in the United States ranges from 6 to 39% depending on definitions used and populations studied2. TD is readily treatable with testosterone therapy (TTh) and its use has increased more than six-fold between 2002 and 2013, from 0.52% of men to 3.2% in the United States3. Despites its popularity and efficacy4,5, a concern surrounding the link between testosterone and prostate cancer gives some clinicians pause in prescribing TTh to patients with a history of prostate cancer (CaP). Indeed, the FDA requires labels on testosterone products warning of “a potential risk of prostate cancer and increase in prostatic specific antigen (PSA)6.” This may lead to some men failing to receive treatment that could otherwise benefit them.
In 1941, Drs. Huggins and Hodges established the androgen-dependent model of CaP by demonstrating regression of metastatic CaP in men who had been castrated7. The corollary to this model led to a fear that administering testosterone to men will likewise fuel CaP growth. However, evidence has emerged in the interim that have provided a more contemporary understanding of the relationship between androgen receptor function, prostate specific antigen (PSA) levels, and CaP growth which suggest TTh does not increase the risk of CaP recurrence. Indeed, groups have reported treating men with testosterone after the diagnosis and treatment of prostate cancer8,9. The American Urological Association guidelines as well as numerous reviews and meta-analyses have reinforced the safety of TTh in men with TD and treated prostate cancer10–13. Given the contradictions in product warning labels and current clinical evidence, we sought to characterize the demographics and usage patterns of TTh in men with a history of prostate cancer in the U.S.
Materials and Methods
The present study utilized the Optum’s De-identified Clinformatics® Data Mart database, which is a commercial and Medicare Advantage claims database that includes patient enrollment, physician, facility data, laboratory results and pharmacy claims for 77 million individuals in the United States. To assess TTh prescribing patterns, associated diagnoses and laboratory values, we examined the cohort from 2003 to 2018.
International Classification of Diseases 9th revision and 10th revision, Clinical Modification (ICD-9-CM, ICD-10-CM) codes, Current Procedural Terminology (CPT) codes were used to identify men 40 years and over who had a diagnosis of CaP and who had undergone subsequent treatment with either surgery or radiation (See Supplementary Table 1). Logical Observation Identifiers Names and Codes (LOINC®) were used to query testosterone and PSA laboratory test values which were available for a subset of individuals before and after TTh initiation. Pharmacy and procedural claims data using prescription names, Healthcare Common Procedure Coding System (HCPCS), and CPT codes were used to identify specific testosterone formulations administered. If testosterone or PSA laboratory values were available, they must have occurred within 1 year before initiation of TTh after prostate cancer treatment. PSA and testosterone laboratory values were converted to pg/mL and ng/mL respectively. We excluded men who had less than 6 months of enrollment time before or after CaP treatment. This study was deemed exempt by the Institutional Review Board of Stanford University. Mean and standard deviation of age was reported and compared by Student’s t-test between men with TTh after CaP and those without. Frequencies and percentages were reported for categorical variables and compared by Chi-square tests. Medians and interquartile ranges were reported for PSA, testosterone lab tests due to non-normal data distribution. Due to data-use agreements regarding minimum cell size numbers, we can only provide approximation when any individual cell would be <11. Rather than exclude all numbers, we provide approximations when necessary. All analyses were performed by SAS (version 9.4; SAS Institute Inc., Cary, NC).
Results
Over the duration of the study period, a total of 126,374 men completed treatment for CaP (42,515 surgery, 75,186 radiation, 8,673 both). Of these, 3,074 men (2.4%) received testosterone after CaP treatment. Compared to those who did not receive TTh after Cap treatment, most men who went on to receive testosterone were younger (63.8 vs 67.3), white, college educated, and higher earning, and living in the South. They were also more likely to have erectile dysfunction and depressive disorder, and less likely to have a pre-treatment PSA of >10 (Table 1). Asians were the least likely ethnic group to undergo TTh.
Table 1:
Demographics of men receiving and not receiving testosterone therapy (TTh)after prostate cancer treatment. “Other” testosterone routes include subcutaneous pellets, buccal, and intranasal formulations.
| Men without TTh after CaP treatment Mean (STD) | Men with TTh after CaP treatment Mean (STD) | p-value | ||
|---|---|---|---|---|
| n= | 123,300 | 3,074 | ||
| Mean Age (STD), years | 67.3 (8.9) | 63.8 (9.3) | <.0001 | |
| Age | <55 | 10332 (8.38) | 524 (17.05) | <.0001 |
| 55–65 | 39023 (31.65) | 1198 (38.97) | ||
| 65+ | 73945 (59.97) | 1352 (43.98) | ||
| Follow-Up | <1 year | 19189 (15.56) | 149 (4.85) | <.0001 |
| 1–2 | 27032 (21.92) | 355 (11.55) | ||
| 2+ | 77079 (62.51) | 2570 (83.6) | ||
| Year | 2003–6 | 15830 (12.84) | 429 (13.96) | <.0001 |
| 2007–10 | 32411 (26.29) | 1186 (38.58) | ||
| 2011–2014 | 32222 (26.13) | 912 (29.67) | ||
| 2015+ | 42837 (34.74) | 547 (17.79) | ||
| Race | Asian | 2458 (1.99) | 27 (0.88) | <.0001 |
| Black | 14107 (11.44) | 269 (8.75) | ||
| Hispanic | 9282 (7.53) | 209 (6.8) | ||
| White | 86517 (70.17) | 2335 (75.96) | ||
| Unknown | 10936 (8.87) | 234 (7.61) | ||
| Education | Less than 12th grade | 602 (0.49) | <11 | <.0001 |
| High School Diploma | 30342 (24.61) | 632 (20.56) | ||
| Less than Bachelor degree | 63352 (51.38) | 1602 (52.11) | ||
| Bachelor Degree or plus | 20910 (16.96) | 684 (22.25) | ||
| Unknown | 8094 (6.56) | 140+ | ||
| Annual Household Income | <50K | 26766 (21.71) | 539 (17.53) | <.0001 |
| 50–100K | 38606 (31.31) | 949 (30.87) | ||
| 100K+ | 33354 (27.05) | 1111 (36.14) | ||
| Unknown | 24574 (19.93) | 475 (15.45) | ||
| Region | Midwest | 29279 (23.75) | 370+ | <.0001 |
| Northeast | 13282 (10.77) | 190 (6.18) | ||
| South | 53346 (43.27) | 1781 (57.94) | ||
| West | 27225 (22.08) | 725 (23.58) | ||
| Unknown | 168 (0.14) | <11 | ||
| Testosterone Therapy Route: | Injection | 1412 (46.60) | ||
| Topical | 1029 (33.96) | |||
| Other | 589 (19.44) | |||
| Comorbidities: | % With Diabetes | 30330 (24.60) | 760 (24.72) | 0.8738 |
| % with Erectile Dysfunction | 20801 (16.87) | 863 (28.07) | <.0001 | |
| % with Depressive Disorder | 4593 (3.73) | 184 (5.99) | <.0001 | |
| % with Hypertension | 78550 (63.71) | 1994 (64.87) | 0.1863 | |
| Pretreatment PSA (pg/dL) | 0–4 | 10863 (26.88) | 386 (36.18) | <.0001 |
| 4–10 | 21316 (52.75) | 560 (52.48) | ||
| 10+ | 8232 (20.37) | 121 (11.34) |
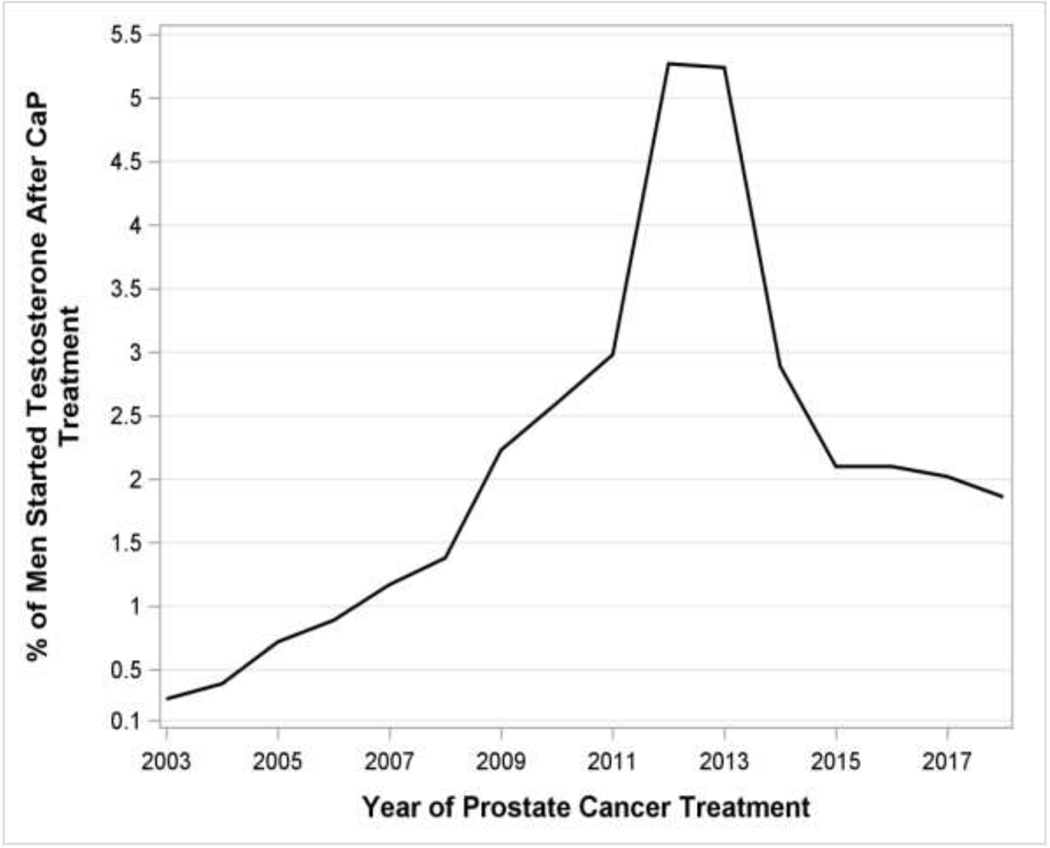
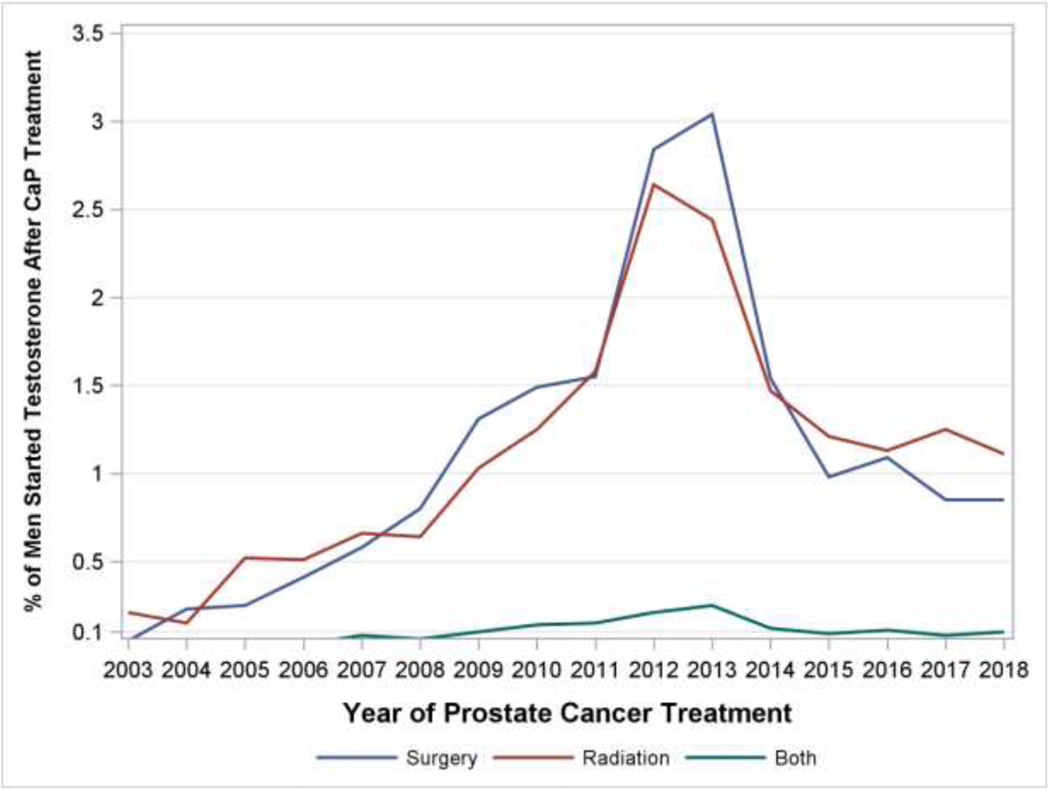
Temporally, we observed a rise in the use of testosterone after CaP from the beginning of the study period until it peaked in 2013, going from 0.21% of men to 4.9% of men representing a more than 20-fold increase over 10 years. This trend was seen after separating men by prostate cancer treatment modality (radiation versus surgery versus combination). After 2013, rates decreased annually until a plateau of approximately 1.79% of men (Figure 1A).
Figure 1A:
Percentage of men started on Testosterone after prostate cancer treatment. B: Percentage of men started on Testosterone after prostate cancer treatment, separated by treatment modality
There were differences in the cohorts of men receiving testosterone depending on whether their prostate cancer treatment was surgery or radiation. The median (interquartile range) pre-treatment PSA in the post-surgery group was 0 (0 –0), 0.2 (0 –0.8) in the post-radiation group, and 0 (0 –0.5) in those that received both radiation and surgery for prostate cancer treatment. Median (Interquartile Range) of Testosterone levels before initiation of T therapy was 254 (188 – 343), 214 (98 – 303), and 172 (14 – 335) ng/dL respectively in those groups. The median time to initiation of TTh from prostate cancer treatment was 78.4 weeks in the post-surgical cohort and 100.4 weeks in the post-radiation cohort and 137.4 weeks in the combination cohort, and the median duration of TTh in years was 1.37, 0.83, and 0.94 in those cohorts respectively (Table 2). The cohort receiving testosterone after surgery was comprised of men who were younger, more likely to be White, more likely to have a Bachelor Degree or higher, and more likely to earn 100k+ per year when compared to the radiation cohort (Table 3). 34.0 to 37.4% of men did not have a testosterone lab value checked before initiation of TTh and the most popular route of TTh was through injection, and secondarily, topical.
Table 2:
Characteristics of Men Receiving Testosterone Therapy after Prostate Cancer Treatment by Prostate Cancer Treatment Modality.
| T after Surgery | T after Radiation | T after Radiation and Surgery | |
|---|---|---|---|
| n= | 1587 | 1631 | 144 |
| Median (IQR) Testosterone Level before starting testosterone therapy (ng/dL) | 254 (188 – 343) | 214 (98 – 303) | 172 (14 – 335) |
| Percent of Men without a testosterone lab level before starting testosterone therapy | 37.4% | 35.7% | 34.0% |
| Median (IQR) Time between prostate cancer treatment and starting testosterone (Weeks) | 78.4 (33.3 – 171.4) | 100.4 (41.4 - 189.9) | 137.4 (62.5 - 261.4) |
| Median (IQR) Post-Treatment PSA before starting testosterone therapy (ng/mL) | 0 (0 – 0) | 0.2 (0 – 0.8) | 0 (0 – 0.5) |
| Duration of TTh (years), median (IQR) | 1.37 (0.27 – 4.82) | 0.83 (0.18 – 3.45) | 0.94 (0.17 – 3.41) |
Table 3:
Demographic Differences in Men Receiving Testosterone Therapy (TTh) after Prostate Cancer Treatment by Prostate Cancer Treatment Modality. “Other” testosterone routes include subcutaneous pellets, buccal, and intranasal formulations.
| TTh after Surgery | TTh after Radiation | TTh after Radiation and Surgery | ||
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| n= | 1587 | 1631 | 144 | |
| Mean Age (STD), years | 59.8 (7.8) | 67.5 (9.0) | 61.7 (7.9) | |
| Age | <55 | 410 (25.83) | 145 (8.89) | 31 (21.53) |
| 55–65 | 775 (48.83) | 493 (30.23) | 70 (48.61) | |
| 65+ | 402 (25.33) | 993 (60.88) | 43 (29.86) | |
| Education | Less than 12th Grade | <11 | <11 | 0 (0) |
| High School Diploma | 309 (19.47) | 352 (21.58) | 20+ | |
| Less than Bachelor Degree | 810 (51.04) | 861 (52.79) | 69 (47.92) | |
| Bachelor Degree Plus | 395 (24.89) | 328 (20.11) | 39 (27.08) | |
| Unknown | 70+ | 80+ | <11 | |
| Race | Asian | 14 (0.88) | 13 (0.8) | 0 (0) |
| Black | 120 (7.56) | 164 (10.06) | 11+ | |
| Hispanic | 91 (5.73) | 124 (7.6) | <11 | |
| White | 1247 (78.58) | 1200 (73.57) | 112 (77.78) | |
| Unknown | 115 (7.25) | 130 (7.97) | 11 (7.64) | |
| Region | Midwest | 221 (13.93) | 171 (10.48) | 11+ |
| Northeast | 80+ | 100+ | <11 | |
| South | 945 (59.55) | 922 (56.53) | 86 (59.72) | |
| West | 332 (20.92) | 427 (26.18) | 34 (23.61) | |
| Unknown | <11 | <11 | 0 | |
| Income | <50K | 218 (13.74) | 345 (21.15) | 24 (16.67) |
| 50–100K | 481 (30.31) | 513 (31.45) | 45 (31.25) | |
| 100K+ | 664 (41.84) | 508 (31.15) | 61 (42.36) | |
| Unknown | 224 (14.11) | 265 (16.25) | 14 (9.72) | |
| Comorbidities | % With Diabetes | 338 (21.30) | 459 (28.14) | 37 (25.69) |
| % with Erectile Dysfunction | 495 (31.19) | 404 (24.77) | 36 (25.00) | |
| % with Depressive Disorder | 107 (6.74) | 84 (5.15) | <11 | |
| % with Hypertension | 981 (61.81) | 1106 (67.81) | 93 (64.58) | |
| Testosterone Therapy Route: | Injection | 746 (47.76) | 718 (44.57) | 52 (36.36) |
| Topical | 605 (38.73) | 463 (28.74) | 39 (27.27) | |
| Other | 211 (13.51) | 430 (26.69) | 52 (36.36) |
Discussion
During the study period, we observed an annual increase in men receiving testosterone after prostate cancer treatment until a peak is seen in 2013 followed by subsequent year-over-year declines (Figure 1A and 1B). A higher proportion of men who end up receiving testosterone have pre-treatment PSAs in the 0–4 pg/dL range suggesting men with lower risk disease are more likely to be prescribed testosterone than those higher risk patients with a pre-treatment PSA greater than 10. Demographic differences in race, education and income between those receiving TTh after prostate cancer treatment and those not suggest that socioeconomic factors may play a role in who ends up needing and receiving testosterone. 144 of the 3,074 men (4.7%) had a combination of surgical and radiation treatment of prostate cancer diagnoses and still went on to receive TTh. In addition, among subjects with laboratory data available, TTh was started on men with low or unmeasurable PSA values duration consistent with treated prostate cancer, and with testosterone levels consistent with AUA guideline suggested thresholds of ≤ 300 ng/dL. Significantly higher rates of diagnoses of erectile dysfunction and depression in the group receiving testosterone suggest that men are being treated for symptoms in addition to low laboratory levels. Over one third of men did not have a testosterone level obtained before initiation of TTh, slightly higher than the approximate 25% reported in previous series on the general U.S. population14. TTh was started sooner and was sustained for longer durations after those completing surgical therapy compared to those completing radiation therapy. Starting TTh sooner after surgery may be potentially explained by differences in post-treatment PSA kinetics amongst the two cohorts, with PSAs reaching stable nadirs more expediently after surgery than radiation. Unique post-radiotherapy effects such as PSA bounce, which can occur in approximately a third of patients15, may lead to cessation of testosterone therapy that would otherwise not occur in the surveillance of patients post-surgery.
The current analysis demonstrates that the trends in testosterone prescriptions in patients that had CaP treated mirrored general national trends overall. Indeed, a report by Baillargeon et. al reviewed testosterone prescriptions in commercially insured men from 2002–2016 and demonstrated the same peak and decline seen in the current report. Their hypothesis suggests that publications in the years 2013 and 2014 regarding cardiovascular risks associated with TTh may have contributed to hesitation among providers in starting patients on testosterone in general3. The work of Huggins and Hodges established the testosterone-dependent nature of prostate cancer and postulated a directional association between the two. However, more modern data suggest that the association is complex and likely not simply linear in nature. Studies have shown increases in PSA in hypogonadal men treated with exogenous testosterone, but not in eugonadal subjects16–18 Morgentaler and Traish sought to resolve this paradox when they proposed the saturation theory of prostate cancer in 200919 which holds that prostate tissue, while responsive to changes in serum androgens at low concentrations, are no longer sensitive to them once a saturation point is reached, at approximately the 250ng/dL range20. Numerous studies have demonstrated a lack of increased risk of either biochemical or clinical evidence of prostate cancer recurrence in men receiving clinically-indicated TTh after surgery21–23 or radiation24–27. There is even evidence supporting the safety of TTh in men on active surveillance28.
Despite the growing body of knowledge supporting the safety of TTh in men with treated prostate cancer, the Endocrine Society guidelines recommend against starting TTh in men with a history of prostate cancer. In contrast, the AUA guidelines note an absence of evidence linking TTh to the development of prostate cancer1,29. In 2016, Kaplan et. al published independent recommendations30 after reviewing the literature. In it, they recommend PSA levels be undetectable following radical prostatectomy or stable for a 6-month period after radiation treatment. They also caution starting TTh in men at high risk for recurrence or progression. Several limitations warrant mention. As an analysis of population-based administrative claims database, the validity of the results rely on valid and proper diagnosis and procedural coding. Some men may obtain testosterone that is not reimbursed by insurance, and therefore are not captured in the database. Testosterone prescriptions may have been filled but not actually taken. This methodology of this study allows it to evaluate clinical trends and patterns over time, but it does not reveal causality or etiology of observations. Importantly, relevant clinicopathoogical CaP data such as Gleason Score or cancer stage were unfortunately not available for analysis, and precludes the study’s ability to comment on safety or oncologic outcomes of TTh in treated CaP.
Nevertheless, the current report demonstrates that many men are treated with TTh after prostate cancer therapy with pre-TTh PSA levels attributable to no active disease. The data on testosterone prescriptions are consistent with trends seen in the general population. TTh initiation often occurs in the presence of evidence of clinical and laboratory-confirmed TD. However, the findings that only roughly two-thirds of patients have testosterone levels checked before initiation of TTh suggests room for improvement for guideline-adherent treatment of TD.
Increased provider vigilance in obtaining appropriate laboratory workup in symptomatic patients before TTh is encouraged.
Conclusions
National trends in testosterone prescriptions for men with treated prostate cancer demonstrates that many men are treated with TTh after prostate cancer therapy. TTh is being initiated post-treatment with patterns of indications and monitoring consistent with the general population. More data are necessary and continued monitoring of testosterone prescribing trends will be important as more data become available.
Supplementary Material
Acknowledgments
Study funding/competing interests: National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085). Funding source had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mulhall JP, Trost LW, Brannigan RE, et al. : Evaluation and Management of Testosterone Deficiency: AUA Guideline. J. Urol. 2018; 200: 423–432. Available at: http://www.jurology.com/doi/10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 2.Anaissie J, DeLay KJ, Wang W, et al. : Testosterone deficiency in adults and corresponding treatment patterns across the globe. Transl. Androl. Urol. 2017; 6: 183–191. Available at: http://tau.amegroups.com/article/view/13624/14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baillargeon J, Kuo Y-F, Westra JR, et al. : Testosterone Prescribing in the United States, 2002–2016. JAMA 2018; 320: 200–202. Available at: http://www.ncbi.nlm.nih.gov/pubmed/29998328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storer TW, Woodhouse L, Magliano L, et al. : Changes in Muscle Mass, Muscle Strength, and Power but Not Physical Function Are Related to Testosterone Dose in Healthy Older Men. J. Am. Geriatr. Soc. 2008; 56: 1991–1999. Available at: http://doi.wiley.com/10.1111/j.1532-5415.2008.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CUNNINGHAM GR HIRSHKOWITZ, KORENMAN SG, et al. : Testosterone Replacement Therapy and Sleep-Related Erections in Hypogonadal Men. J. Clin. Endocrinol. Metab. 1990; 70: 792–797. Available at: https://academic.oup.com/jcem/article-lookup/doi/10.1210/jcem-70-3-792. [DOI] [PubMed] [Google Scholar]
- 6.Metzger SO and Burnett AL: Impact of recent FDA ruling on testosterone replacement therapy (TRT). Transl. Androl. Urol. 2016; 5: 921–926. Available at: http://tau.amegroups.com/article/view/12198/13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huggins C and Hodges C V: Studies on Prostatic Cancer. I. The Effect of Castration, of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. Cancer Res. 1941; 1: 293 LP –297. Available at: http://cancerres.aacrjournals.org/content/1/4/293.abstract. [Google Scholar]
- 8.Morgentaler A, Lipshultz LI, Bennett R, et al. : Testosterone Therapy in Men With Untreated Prostate Cancer. J. Urol. 2011; 185: 1256–1261. Available at: http://www.jurology.com/doi/10.1016/j.juro.2010.11.084. [DOI] [PubMed] [Google Scholar]
- 9.Pastuszak AW, Khanna A, Badhiwala N, et al. : Testosterone Therapy after Radiation Therapy for Low, Intermediate and High Risk Prostate Cancer. J. Urol. 2015; 194: 1271–1276. Available at: http://www.jurology.com/doi/10.1016/j.juro.2015.05.084. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez KM, Pastuszak AW and Khera M: The Role of Testosterone Therapy in the Setting of Prostate Cancer. Curr. Urol. Rep. 2018; 19: 1–9. [DOI] [PubMed] [Google Scholar]
- 11.Dupree JM, Langille GM, Khera M, et al. : The safety of testosterone supplementation therapy in prostate cancer. Nat. Rev. Urol. 2014; 11: 526–530. Available at: http://www.nature.com/articles/nrurol.2014.163. [DOI] [PubMed] [Google Scholar]
- 12.Klap J, Schmid M and Loughlin KR: The Relationship between Total Testosterone Levels and Prostate Cancer: A Review of the Continuing Controversy. J. Urol. 2015; 193: 403–414. Available at: http://www.jurology.com/doi/10.1016/j.juro.2014.07.123. [DOI] [PubMed] [Google Scholar]
- 13.Boyle P, Koechlin A, Bota M, et al. : Endogenous and exogenous testosterone and the risk of prostate cancer and increased prostate-specific antigen (PSA) level: a meta-analysis. BJU Int. 2016; 118: 731–741. Available at: http://doi.wiley.com/10.1111/bju.13417. [DOI] [PubMed] [Google Scholar]
- 14.Baillargeon J, Urban RJ, Ottenbacher KJ, et al. : Trends in Androgen Prescribing in the United States, 2001 to 2011. JAMA Intern. Med. 2013; 173: 1465. Available at: http://archinte.jamanetwork.com/article.aspx?doi=10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darwis NDM, Oike T, Kubo N, et al. : Characteristics of PSA Bounce after Radiotherapy for Prostate Cancer: A Meta-Analysis. Cancers (Basel). 2020; 12. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32764448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper CS, Perry PJ, Sparks AET, et al. : EFFECT OF EXOGENOUS TESTOSTERONE ON PROSTATE VOLUME, SERUM AND SEMEN PROSTATE SPECIFIC ANTIGEN LEVELS IN HEALTHY YOUNG MEN. J. Urol. 1998; 159: 441–443. Available at: http://www.jurology.com/doi/10.1016/S0022-5347%2801%2963944-2. [DOI] [PubMed] [Google Scholar]
- 17.Morgentaler A, Benesh JA, Denes BS, et al. : Factors Influencing Prostate-Specific Antigen Response among Men Treated with Testosterone Therapy for 6 Months. J. Sex. Med. 2014; 11: 2818–2825. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1743609515306123. [DOI] [PubMed] [Google Scholar]
- 18.Khera M, Bhattacharya RK, Blick G, et al. : Changes in Prostate Specific Antigen in Hypogonadal Men After 12 Months of Testosterone Replacement Therapy: Support for the Prostate Saturation Theory. J. Urol. 2011; 186: 1005–1011. Available at: http://www.jurology.com/doi/10.1016/j.juro.2011.04.065. [DOI] [PubMed] [Google Scholar]
- 19.Morgentaler A and Traish AM: Shifting the Paradigm of Testosterone and Prostate Cancer: The Saturation Model and the Limits of Androgen-Dependent Growth. Eur. Urol. 2009; 55: 310–321. Available at: https://linkinghub.elsevier.com/retrieve/pii/S030228380801124X. [DOI] [PubMed] [Google Scholar]
- 20.Morgentaler A and Traish AM: Letter to the editor: Questioning the evidence behind the Saturation Model for testosterone replacement therapy in prostate cancer. Investig. Clin. Urol. 2020; 61: 452. Available at: https://icurology.org/DOIx.php?id=10.4111/icu.2020.61.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.KAUFMAN JM and GRAYDON RJ: ANDROGEN REPLACEMENT AFTER CURATIVE RADICAL PROSTATECTOMY FOR PROSTATE CANCER IN HYPOGONADAL MEN. J. Urol. 2004; 172: 920–922. Available at: http://www.jurology.com/doi/10.1097/01.ju.0000136269.10161.32. [DOI] [PubMed] [Google Scholar]
- 22.Khera M, Grober ED, Najari B, et al. : Testosterone Replacement Therapy Following Radical Prostatectomy. J. Sex. Med. 2009; 6: 1165–1170. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1743609515324553. [DOI] [PubMed] [Google Scholar]
- 23.Pastuszak AW, Pearlman AM, Lai WS, et al. : Testosterone Replacement Therapy in Patients with Prostate Cancer After Radical Prostatectomy. J. Urol. 2013; 190: 639–644. Available at: http://www.jurology.com/doi/10.1016/j.juro.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastuszak AW, Pearlman AM, Godoy G, et al. : Testosterone replacement therapy in the setting of prostate cancer treated with radiation. Int. J. Impot. Res. 2013; 25: 24–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22971614. [DOI] [PubMed] [Google Scholar]
- 25.Balbontin FG, Moreno SA, Bley E, et al. : Long-acting testosterone injections for treatment of testosterone deficiency after brachytherapy for prostate cancer. BJU Int. 2014; 114: 125–30. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25101359. [DOI] [PubMed] [Google Scholar]
- 26.Sarosdy MF: Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer 2007; 109: 536–541. Available at: http://doi.wiley.com/10.1002/cncr.22438. [DOI] [PubMed] [Google Scholar]
- 27.Morales A, Black AM and Emerson LE: Testosterone administration to men with testosterone deficiency syndrome after external beam radiotherapy for localized prostate cancer: preliminary observations. BJU Int. 2009; 103: 62–4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18671790. [DOI] [PubMed] [Google Scholar]
- 28.Kacker R, Hult M, San Francisco IF, et al. : Can testosterone therapy be offered to men on active surveillance for prostate cancer? Preliminary results. Asian J. Androl. 18: 16–20. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26306850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhasin S, Brito JP, Cunningham GR, et al. : Testosterone Therapy in Men With Hypogonadism: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018; 103: 1715–1744. Available at: https://academic.oup.com/jcem/article/103/5/1715/4939465. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan AL, Hu JC, Morgentaler A, et al. : Testosterone Therapy in Men With Prostate Cancer. Eur. Urol. 2016; 69: 894–903. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0302283815012130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.