Abstract
Background
Radiofrequency (RF) denervation, an invasive treatment for chronic low back pain (CLBP), is used most often for pain suspected to arise from facet joints, sacroiliac (SI) joints or discs. Many (uncontrolled) studies have shown substantial variation in its use between countries and continued uncertainty regarding its effectiveness.
Objectives
The objective of this review is to assess the effectiveness of RF denervation procedures for the treatment of patients with CLBP. The current review is an update of the review conducted in 2003.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, three other databases, two clinical trials registries and the reference lists of included studies from inception to May 2014 for randomised controlled trials (RCTs) fulfilling the inclusion criteria. We updated this search in June 2015, but we have not yet incorporated these results.
Selection criteria
We included RCTs of RF denervation for patients with CLBP who had a positive response to a diagnostic block or discography. We applied no language or date restrictions.
Data collection and analysis
Pairs of review authors independently selected RCTs, extracted data and assessed risk of bias (RoB) and clinical relevance using standardised forms. We performed meta‐analyses with clinically homogeneous studies and assessed the quality of evidence for each outcome using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach.
Main results
In total, we included 23 RCTs (N = 1309), 13 of which (56%) had low RoB. We included both men and women with a mean age of 50.6 years. We assessed the overall quality of the evidence as very low to moderate. Twelve studies examined suspected facet joint pain, five studies disc pain, two studies SI joint pain, two studies radicular CLBP, one study suspected radiating low back pain and one study CLBP with or without suspected radiation. Overall, moderate evidence suggests that facet joint RF denervation has a greater effect on pain compared with placebo over the short term (mean difference (MD) ‐1.47, 95% confidence interval (CI) ‐2.28 to ‐0.67). Low‐quality evidence indicates that facet joint RF denervation is more effective than placebo for function over the short term (MD ‐5.53, 95% CI ‐8.66 to ‐2.40) and over the long term (MD ‐3.70, 95% CI ‐6.94 to ‐0.47). Evidence of very low to low quality shows that facet joint RF denervation is more effective for pain than steroid injections over the short (MD ‐2.23, 95% CI ‐2.38 to ‐2.08), intermediate (MD ‐2.13, 95% CI ‐3.45 to ‐0.81), and long term (MD ‐2.65, 95% CI ‐3.43 to ‐1.88). RF denervation used for disc pain produces conflicting results, with no effects for RF denervation compared with placebo over the short and intermediate term, and small effects for RF denervation over the long term for pain relief (MD ‐1.63, 95% CI ‐2.58 to ‐0.68) and improved function (MD ‐6.75, 95% CI ‐13.42 to ‐0.09). Lack of evidence of short‐term effectiveness undermines the clinical plausibility of intermediate‐term or long‐term effectiveness. When RF denervation is used for SI joint pain, low‐quality evidence reveals no differences from placebo in effects on pain (MD ‐2.12, 95% CI ‐5.45 to 1.21) and function (MD ‐14.06, 95% CI ‐30.42 to 2.30) over the short term, and one study shows a small effect on both pain and function over the intermediate term. RF denervation is an invasive procedure that can cause a variety of complications. The quality and size of original studies were inadequate to permit assessment of how often complications occur.
Authors' conclusions
The review authors found no high‐quality evidence suggesting that RF denervation provides pain relief for patients with CLBP. Similarly, we identified no convincing evidence to show that this treatment improves function. Overall, the current evidence for RF denervation for CLBP is very low to moderate in quality; high‐quality evidence is lacking. High‐quality RCTs with larger patient samples are needed, as are data on long‐term effects.
Plain language summary
Radiofrequency denervation for chronic low back pain
Background Low back pain is a widespread problem that has major social and economic consequences. In all, 85% to 90% of low back pain cases are classified as 'non‐specific'. Most patients with low back pain are treated successfully in primary care, but approximately 10% to 15% develop chronic symptoms (lasting longer than three months). Chronic low back pain can come from any part of the back that has a nerve supply capable of transmitting pain signals. These sources include discs, vertebrae, sacroiliac joints, facet joints, muscles, ligaments and other structures. Pain specialists try to identify the source of low back pain by using nerve blocks. They numb individual spinal nerves with anaesthetic injections to see if this leads to improvement in back symptoms. With substantial pain relief, they attempt to eliminate pain for a longer time by heating the spinal nerves with radiofrequency waves to ensure that the pain stimulus cannot be passed. This invasive procedure is called radiofrequency denervation. At this time, the effectiveness of this approach has not been proven.
Study characteristics The evidence is current to May 2014. This review includes 23 randomised controlled trials with a total of 1309 participants whose chronic low back pain was evaluated with nerve blocks or other diagnostic tests. Both men and women, with a mean age of 50.6 years, were included. Patients with a positive response to a diagnostic block or to discography were given radiofrequency denervation, a placebo or a comparison treatment.
Key results No high‐quality evidence shows that radiofrequency denervation provides pain relief for patients with chronic low back pain. Similarly, no convincing evidence suggests that this treatment improves function. Moderate‐quality evidence suggests that radiofrequency denervation might better relieve facet joint pain and improve function over the short term when compared with placebo. Evidence of very low to low quality shows that radiofrequency denervation might relieve facet joint pain as well as steroid injections. For patients with disc pain, only small long‐term effects on pain relief and improved function are shown. For patients with SI joint pain, radiofrequency denervation had no effect over the short term and a smaller effect (based on one study) one to six months after treatment when compared with placebo. For low back pain suspected to arise from other sources, the results were inconclusive. Radiofrequency denervation is an invasive procedure that can cause a variety of complications.
Quality of the evidence The studies in this review were not of adequate quality and size to document how often complications occur. Given the poor quality of the evidence, large, high‐quality studies are urgently needed to determine whether radiofrequency denervation is safe and effective.
Summary of findings
Background
Description of the condition
A major proportion of the adult population has low back pain at some stage of life. Although most patients are treated successfully with conservative treatment or without treatment, a substantial group of patients develop chronic pain symptoms (lasting longer than three months) (Lambeek 2010). Patients with chronic low back pain (CLBP) account for most reported healthcare and socioeconomic costs (Lambeek 2010). Among low back pain diagnoses, about 85% are defined as non‐specific low back pain, that is, low back pain not attributable to a recognisable, known specific pathology or anatomical structure (e.g. infection, tumour, osteoporosis, fracture) (Koes 2006; Krismer 2007; Waddel 2005). Suspected sources of back pain include lumbar facet (zygapophyseal) joints, sacroiliac (SI) joints and degenerated intervertebral discs (Bogduk 2005; Cohen 2007; Schwarzer 1994; Schwarzer 1995b).
No gold standard is known for diagnosing facet joint, SI joint or disc pain. Such pain cannot be diagnosed clinically (Manchikanti 2000a) or radiologically (Schwarzer 1995a). Little evidence is available for using diagnostic blocks, which locally anaesthetise medial branch nerves that innervate the painful joint (Boswell 2003; Chou 2007; Dreyfuss 1997; Laslett 2003). Despite lack of validity and the chance of false‐positive test results, these diagnostic blocks are used frequently in diagnosing facet joint pain, SI joint pain or disc pain, and in predicting the success of radiofrequency (RF) denervation procedures. However, it should be noted that "nerve blocks" are unvalidated methods of diagnosing the source or sources of CLBP (Chou 2009).
Description of the intervention
Radiofrequency denervation is one of the treatment options for patients with CLBP. In RF denervation, an RF generator produces an alternating current (frequency, 250 to 500 kHz) through an electrode, thereby inducing ionic movements in the tissue directly surrounding the active tip. This leads to molecular friction and heating of the tissue within a limited distance of the electrode (Kline 1996). Since Shealy published his article on RF denervation of the lumbar facet joint in 1976, RF denervation procedures have been modified and now are used frequently for low back pain (Cohen 2007; Dasselaar 1994; Dreyfuss 1997; Dreyfuss 2000; Shealy 1976; Sluijter 1998; Manchikanti 2000b). For example, they are used in the management of SI joint pain and disc pain (Barendse 2001; Ferrante 2001; Rathmell 2001).
How the intervention might work
Radiofrequency denervation is a technique that attempts to modulate neural transmission of nociceptive stimuli to reduce spinal pain. It aims to de‐activate the nerves suspected of contributing to pain by applying an electrical current to coagulate the sensory nerves and prevent conduction of nociceptive impulses (Cosman 2005; Kline 1996). Radiofrequency lesioning is used to produce a partial lesion in the nerves supplying the painful structure.
Why it is important to do this review
The current review will be an update of the review conducted in 2003 (Niemisto 2003; Niemisto 2003a). The original review studied the effects of RF denervation procedures in chronic low back and neck pain. Only four trials evaluating RF denervation procedures in CLBP were selected (one studying discogenic low back pain and three studying facet joint pain). The review produced conflicting evidence on the effectiveness of RF denervation for facet joint pain. Limited evidence suggested that intra discal RF denervation may not be effective in relieving discogenic low back pain. Convincing evidence was lacking. The current review was split into separate reviews for chronic neck pain and chronic low back pain, and the literature search was updated until May 2014. This review focusses on CLBP.
Objectives
The objective of this review is to assess the effectiveness of RF denervation procedures for the treatment of patients with CLBP. The current review is an update of the review conducted in 2003.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs). We imposed no language or date restrictions.
Types of participants
We included patients with CLBP (longer than three months) who had a positive response to diagnostic block or discography. We excluded patients with acute trauma, fracture, malignancy and inflammatory disease.
Types of interventions
Trials had to examine the effects of RF denervation compared with other treatments or placebo. We applied no limits on the temperature used, and we included both continuous and pulsed RF. We included and reported on additional treatments.
Types of outcome measures
Primary outcomes
Primary outcomes considered were pain, functional status (disorder‐specific and generic), global improvement, health‐related quality of life and complications.
Secondary outcomes
Secondary outcomes consisted of ability to work and satisfaction with treatment. We evaluated these outcomes at short‐ (less than one month), intermediate‐ (one to six months) and long‐term (longer than six months) follow‐up.
Search methods for identification of studies
Electronic searches
The search strategy was based on current recommendations of the Cochrane Back and Neck (CBN) Review Group (Furlan 2009) and built on the literature search of the original review (Niemisto 2003).
We searched the following databases from inception to 2014 May 29 and 30.
Cochrane Central Register of Controlled Trials (CENTRAL).
MEDLINE (Ovid SP, 1946 to May Week 3 2014).
MEDLINE In‐Process & Other Non‐Indexed Citiations (Ovid SP, 2014 May 29).
EMBASE (Ovid SP, 1947 to Week 21 2014).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO, from 1981 to 2014 May 30).
PsycINFO (Ovid SP, 1806 to May Week 4 2014).
ClinicalTrials.gov.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP).
For this update, searches were run annually since 2010. Complete search strategies for the eight databases are outlined in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; and Appendix 8. We performed a further search in June 2015 and added one trial report (Hashemi 2014) to 'Studies awaiting classification' and determined that three additional studies (Albareeq 2015; Meckhail 2013; Mekhail 2015) are ongoing. Results of ‘Studies awaiting classification’ and 'Ongoing studies' will be incorporated into the review at the next update.
Searching other resources
We checked the references of identified relevant articles and reviews. Furthermore, we consulted experts in the field of RF denervation treatment to identify potentially relevant studies that might have been missed.
Data collection and analysis
Methods used for this systematic review are based on current recommendations of CBN (Furlan 2009) and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Pairs of review authors independently selected the trials. They reviewed included studies from the original review and titles, keywords and abstracts of identified references initially screened by the trials search co‐ordinator of CBN, to determine whether the study potentially met the inclusion criteria regarding design, participants and interventions. We retrieved full‐text articles on studies that appeared to be relevant and on studies that provided insufficient information to allow a decision. The same pairs of review authors assessed pairwise full‐text articles revealed by this literature search and trials that were included in the original review to make a final decision on which articles should be included in this review. We discussed disagreements, and if consensus could not be reached, we consulted a third review author.
Data extraction and management
Pairs of review authors independently extracted the data, using a standardised form that had been developed by CBN. We extracted the following data: characteristics of study design, population, intervention, control, duration of follow‐up, outcomes and results. We used a consensus method to resolve disagreements, consulting with a third review author if disagreements persisted.
Assessment of risk of bias in included studies
We assessed risk of bias (RoB) of RCTs using the 12 criteria recommended by CBN (Furlan 2009; Higgins 2011) and defined in Appendix 9. In pairs of two, three review authors (MvT, RO, EM) independently assessed RoB. We used a consensus method to resolve disagreements and consulted the third review author if disagreements persisted.
We scored the criteria as 'high risk, 'low risk' or 'unclear risk'. Low RoB was defined as a trial meeting at least six criteria and having no fatal flaws.
Measures of treatment effect
We defined outcome measures from individual trials through meta‐analysis when clinically and methodologically homogeneous. We sent comparisons to an international panel of eight anaesthesiologists, who rated the clinical homogeneity of study populations and interventions within each comparison. The review team assessed homogeneity in comparison treatments, outcomes, measurement instruments and timing of outcomes. An I² value greater than 70% might show considerable heterogeneity between studies. We used fixed effects with an I² value less than 25%, which indicates statistical homogeneity.
We calculated mean differences (MDs) for pain and functional status. We converted all visual analogue scale (VAS) or numerical rating scale (NRS) scores to scales ranging from zero to 10, when necessary. We expressed precision with 95% confidence intervals (95% CIs). If standard deviations (SDs) were not reported, we calculated these using reported values of the CI. If the CI was not available, we used SDs of baseline scores, or estimations of SDs based on other studies with the same population, treatment and score.
Unit of analysis issues
In the study comparing two interventions with a single control group (Tekin 2007), the number of participants in the control group was divided by two to avoid double counting of participants.
Data synthesis
If a meta‐analysis was not possible, we described results from clinically comparable trials in the text.
We assessed the overall quality of evidence for each primary outcome by using an adapted GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (Guyatt 2011), as recommended by CBN (Furlan 2009). The quality of evidence on a specific outcome is based on the following domains and is downgraded by one level for each of the factors encountered.
Limitations in design (> 25% of participants from studies with high RoB).
Inconsistency of results (severe heterogeneity (I² > 50%) or inconsistent findings among studies).
Indirectness in targeted populations, interventions or outcomes that differ from those in which we are interested.
Imprecision of results across all studies that measure that particular outcome (total number of participants < 400 for each outcome).
Publication bias.
We considered comparisons including one RCT with fewer than 400 participants as inconsistent and imprecise and as yielding ‘low‐quality evidence’, which we could further downgrade to ‘very low‐quality evidence’ if we found limitations in design (i.e. high RoB), indirectness or other considerations.
We applied the following grading of evidence (Guyatt 2011).
High quality: Further research is very unlikely to change the quality of evidence that is based on consistent findings from at least two RCTs with low RoB and is generalisable to the population in question. Data are sufficient and include narrow CIs. No reporting biases are known or suspected.
Moderate quality: Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate; one domain is not met.
Low quality: Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change it; two domains are not met.
Very low quality: Great uncertainty surrounds the estimate; three domains are not met.
Assessment of clinical relevance
Assessment of clinical relevance included whether characteristics of participants, interventions and treatment settings were described precisely enough to be comparable with those in practice. Further, we assessed whether clinically relevant outcomes were measured, if their effects were clinically important and if treatment benefits were worth the potential harms (Furlan 2009; Malmivaara 2006; Appendix 10).
Subgroup analysis and investigation of heterogeneity
Subgroups were based on patient selection. We analysed separately participants with pain suspected to originate from the facet joints, SI joints or discs, and those with another type of CLBP. Furthermore, comparisons were based on types of interventions and comparisons, outcomes and timing of outcomes.
We assessed heterogeneity using the Chi2 test, I2 and visual inspection of forest plots. If Chi2 was not statistically significant, if I2 was below 50% and if confidence intervals were overlapping, we considered the data statistically homogeneous.
Sensitivity analysis
We performed sensitivity analyses if uncertainty remained concerning the clinically homogeneity of studies compared.
Results
Description of studies
Results of the search
Database searching yielded 746 individual studies. We included two studies after reference searching and full screening of a total of 36 studies. Five studies were still ongoing and were excluded (Dolin 2010; Maas 2012; Norwegian University 2012; Sarwar 2012; SMART 2012). We assessed the remaining 31 studies by reviewing full‐text articles. We excluded eight studies for various reasons, resulting in a total of 23 included studies (Figure 1). Following the updated search in June 2015, we added one trial report (Hashemi 2014) to Studies awaiting classification and determined that three additional studies (Albareeq 2015; Meckhail 2013; Mekhail 2015) are ongoing. We summarised characteristics of these studies under Characteristics of studies awaiting classification and Characteristics of ongoing studies.
1.
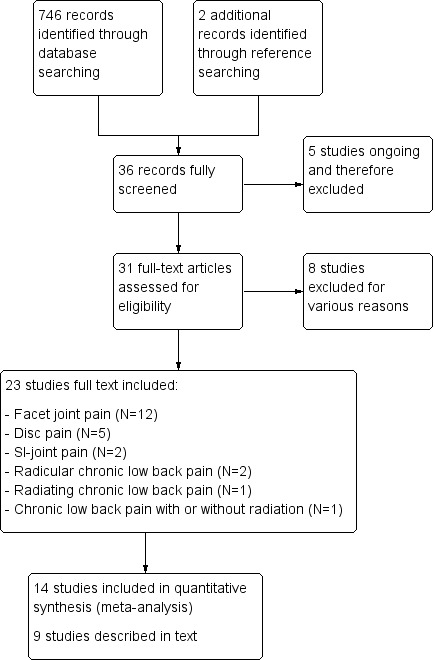
Study flow diagram.
Included studies
The 23 included studies consisted of 1309 participants, and the sample size of each study ranged from 20 to 120 participants. Baseline characteristics of all participants were similar with regard to age, sex and duration of pain for all except two studies, in which these were not described precisely (Gallagher 1994) or were different (Lin Mu‐Lien 2010). Studies included both men and women with a mean age of 50.6 years. Twelve studies examined suspected facet joint pain, five studies disc pain, two studies SI joint pain, two studies radicular CLBP, one study radiating low back pain and one study CLBP with or without radiation. We summarised study characteristics of all included studies under Characteristics of included studies.
Facet joint pain
We included 12 RCTs on suspected chronic facet joint pain (Civelek 2012; Duger 2012; Gallagher 1994; Kroll 2008; Lakemeier 2013; Leclaire 2001; Moon 2013; Nath 2008; Sanders 1999; Tekin 2007; Van Kleef 1999; Van Wijk 2005). These studies included participants with CLBP longer than three months to longer than 12 months. All participants reacted positively to local anaesthetic injections; criteria ranged from a description of ‘good or equivocal response to local anaesthetic injection into and around the appropriate painful joints' to 'at least 80% of pain relief of at least one component of their pain after three separate diagnostic blocks with a local anaesthetic solution'.
For RF denervation, one study used the original Shealy technique (Gallagher 1994). The other studies used modified versions, but all researchers induced an RF lesion at 80°C to 85°C for 60 to 90 seconds.
In five studies, placebo was used for control and electrodes were used in the RF lesion group, but no RF lesion was induced (Gallagher 1994; Leclaire 2001; Nath 2008; Van Kleef 1999; Van Wijk 2005). The study by Kroll et al compared continuous RF (CRF) denervation (80°C, 75 seconds) versus pulsed RF denervation (PRF) (42°C, 120 seconds) (Kroll 2008). The study by Tekin et al compared CRF (80°C, 90 seconds) versus PRF denervation (42°C, 240 seconds) using a sham group (Tekin 2007). Two studies compared different methods of RF denervation; the study of Sanders et al compared intra‐articular versus extra‐articular lumbar facet joint denervation, and the study of Moon et al compared the RF facet denervation distal approach versus the tunnel vision approach (Moon 2013; Sanders 1999). Three studies used steroid injections in the control group (Civelek 2012; Duger 2012; Lakemeier 2013).
Discogenic low back pain
We included five RCTs (Barendse 2001; Ercelen 2003; Kapural 2013; Kvarstein 2009; Oh 2004) on suspected discogenic CLBP, which included participants with duration of low back pain between six months and longer than two years. These trials included only participants with positive response to either analgesic (Barendse 2001; Oh 2004) or provocative discography (Ercelen 2003; Kapural 2013; Kvarstein 2009).
The intervention consisted of percutaneous intra discal RF thermocoagulation (PIRFT) in four studies (Barendse 2001; Ercelen 2003; Kapural 2013; Kvarstein 2009). One study evaluated RF denervation of the ramus communicans nerve (this denervation is performed outside the intervertebral disc) in participants who failed to respond to intra discal electrothermic therapy (IDET) (Oh 2004).
Four studies were placebo‐controlled (Barendse 2001; Kapural 2013; Kvarstein 2009; Oh 2004). One study compared high‐intensity PIRFT versus low‐intensity PIRFT (Ercelen 2003).
Sacroiliac joint pain
We included two RCTs (Cohen 2008; Patel 2012) studying suspected SI joint pain. Both studies included participants with axial low back or buttock pain lasting six months or longer. One study used pain relief of 75% or greater after a single diagnostic SI joint injection as confirmation of SI joint pain (Cohen 2008). The other study performed a dual lateral branch block, in which participants had to have 75% pain relief (Patel 2012).
In the study of Cohen et al, the intervention consisted of RF denervation of 90‐second 80°C RF of L4–L5 primary dorsal rami and S1–S3 lateral branch RF using cooling probe technology (Cohen 2008). The other study applied RF energy for 150 seconds at 60°C on L5, then delivered RF energy for 150 seconds at 60°C on S1, S2 and S3 (Patel 2012). Both studies were placebo controlled.
Spinal dorsal root ganglion (DRG) ‐ lumbosacral radicular pain
We included three RCTs (Geurts 2003; Shanthanna 2014; Simopoulos 2008) performing RF denervation of the dorsal root ganglion (DRG) for suspected lumbosacral radicular pain. Two studies included participants with lumbosacral radicular pain for longer than six months with 75% pain reduction after three separate diagnostic blocks (Geurts 2003), or complete relief of radicular symptoms following low‐volume segmental nerve block (Simopoulos 2008). The other study included participants with a history of chronic lumbar radicular pain for at least four months with clinical features and computed tomography/magnetic resonance imaging findings of lumbosacral radicular pain (Shanthanna 2014).
Two studies used RF denervation as treatment (Geurts 2003; Shanthanna 2014), and one study used pulsed RF denervation as treatment (Simopoulos 2008). Two studies compared treatment versus placebo (Geurts 2003; Shanthanna 2014), and the other study used PRF plus CRF denervation for comparison (Simopoulos 2008).
Low back pain with or without radiation
We included one RCT (Lin Mu‐Lien 2010) on CLBP for longer than six months with or without radiation. This study compared PRF denervation on DRG versus electro‐acupuncture therapy, and versus conservative treatment with medication.
Excluded studies
For this update, we fully screened 36 studies. Five studies were ongoing and were excluded (Dolin 2010; Maas 2012; Norwegian University 2012; Sarwar 2012; SMART 2012). Eight studies were retrieved in full text and were eventually excluded (Buijs 2004; Cohen 2010; Dobrogowski 2005; Fukui 2012; Gautam 2011; Gross 2010; Proschek 2010; Reverberi 2005). Reasons for exclusion included no randomised controlled trial as study design and no direct measurement of the effectiveness of RF denervation. In the Characteristics of excluded studies section, we provide additional details of the excluded studies.
Risk of bias in included studies
Figure 2 shows results of the RoB assessment. Thirteen studies (56%) had low RoB.
2.
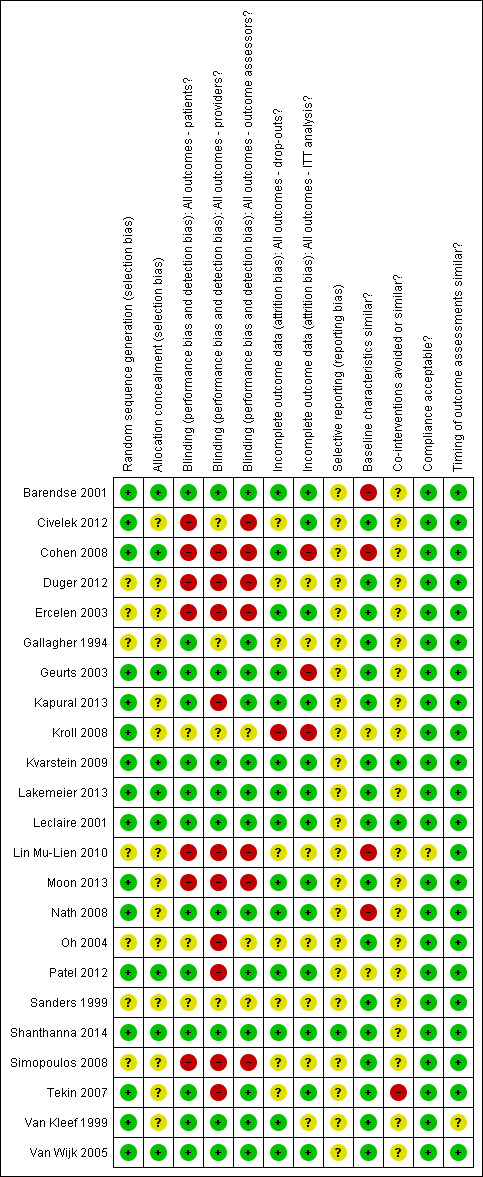
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Nine studies (40%) described an adequate randomisation procedure in combination with adequate concealment of treatment allocation. Method of randomisation remained unclear in seven studies (30%). Treatment allocation remained unclear in 14 studies (61%).
Blinding
Care providers, participants and outcome assessors were blinded in nine studies (47%).
Incomplete outcome data
Fourteen studies (61%) had acceptable dropout rates. Dropout rates were unclear in eight studies, and in one study the dropout rate was high. Three studies did not perform intention‐to‐treat analysis.
Selective reporting
Whether selective reporting occurred remained unclear in all but one study. All studies included core outcomes (pain and function), and we identified no protocols for all but one (Shanthanna 2014) study.
Other potential sources of bias
Groups were similar at baseline in 17 studies (74%) regarding demographic factors and most important prognostic factors. The description of possible co‐interventions was unclear in 20 studies, co‐interventions were avoided in two studies and co‐interventions could have introduced bias in one study. Two studies did not adequately describe compliance, showing unclear risk of bias. Timing of outcome assessments was similar between studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Facet joint: radiofrequency denervation versus placebo.
| Facet joint: radiofrequency denervation versus placebo | ||||||
| Patient or population: patients with chronic low back pain Settings: secondary care Intervention: facet joint radiofrequency denervation Comparision: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Facet joint radiofrequency denervation | |||||
| Pain 1 month post treatment (VAS 0 to 10)a | Mean pain score ranged across control groups from 4.3 to 6 |
Mean pain score in intervention groups was on average 1.5 lower (2.3 to 0.7 lower) | 160 (3 studies) | ⊕⊕⊕⊝ Moderateb | ||
| Pain 1 to 6 months post treatment (VAS 0 to 10)a | Mean pain score ranged across control groups from 4.4 to 4.9 |
Mean pain score in intervention groups was on average 0.7 lower (2.3 lower to 0.8 higher) | 182 (3 studies) | ⊕⊕⊝⊝ Lowb,c | ||
| Pain > 6 months post treatment (VAS 0 to 10) | Mean pain score ranged across control groups from 3.1 to 7.0 |
Mean pain score in intervention groups was on average 0.7 lower (1.5 lower to 0.1 higher) | 140 (3 studies) | ⊕⊕⊕⊝ Moderateb | ||
| Function 1 month post treatment (ODI 0 to 100) | Functional status in control
group was 30.5 |
Mean functioning in intervention groups was on average 5.5 lower (8.7 to 2.4 lower) |
60 (1 study) | ⊕⊕⊝⊝ Lowb,c | ||
| Function > 6 months post treatment (ODI 0 to 100) | Functional status in control
group was 28.9 |
Mean function in intervention groups was on average 3.7 lower (6.9 to 0.5 lower) |
60 (1 study) | ⊕⊕⊝⊝ Lowb,c | ||
| Complications | Not estimable | Not estimable | Not estimable | 0 | No evidence | |
| CI: Confidence interval; VAS: Visual analogue scale. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aResults of the main analyses are presented. bDowngraded when fewer than 400 participants. cI² = 82%, P value = 0.0004, CIs hardly overlap, although the deviating study does not show significant results.
Summary of findings 2. Facet joint: radiofrequency denervation versus steroid injections.
| Facet joint: radiofrequency denervation versus steroid injections | ||||||
| Patient or population: patients with chronic low back pain Settings: secondary care Intervention: facet joint radiofrequency denervation Comparision: steroid injections | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Steroid injections | Facet joint radiofrequency denervation | |||||
| Pain 1 month post treatment (VAS 0 to 10) | Mean pain score ranged across control groups from 4.4 to 5.4 |
Mean pain score in intervention groups was on average 2.2 lower (2.4 to 2.1 lower) | 180 (2 studies) | ⊕⊕⊝⊝ Lowa,b | ||
| Pain 6 months post treatment (VAS 0 to 10) | Mean pain score ranged across control groups from 4.4 to 6.5 |
Mean pain score in intervention groups was on average 2.1 lower (3.5 to 0.8 lower) | 232 (3 studies) | ⊕⊝⊝⊝ Very lowa,b,c | ||
| Pain 12 months post treatment (VAS 0 to 10) | Mean pain score ranged across control groups from 4.9 to 7.0 |
Mean pain score in intervention groups was on average 2.7 lower (3.4 to 1.9 lower) | 180 (2 studies) | ⊕⊝⊝⊝ Very lowa,b,c |
||
| CI: Confidence interval; VAS: Visual Analogue Scale | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aFewer than 6 out of 12 items low risk of bias. bFewer than 400 participants included. cI² higher than 50%.
Summary of findings 3. Discs: radiofrequency denervation versus placebo.
| Discs: radiofrequency denervation versus placebo | ||||||
| Patient or population: patients with chronic low back pain Settings: secondary care Intervention: discs: radiofrequency denervation Comparision: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Radiofrequency denervation | |||||
| Pain 1 month post treatment (VAS 0 to 10) | Pain score in control group was 5.7 |
Pain score in intervention groups was 0.4 lower (1.5 lower to 0.7 higher) | 56 (1 study) | ⊕⊕⊝⊝ Lowa,b | ||
| Pain 1 to 6 months post treatment (VAS 0 to 10) | Mean pain score ranged across control groups from 4.4 to 5.9 |
Mean pain score in intervention groups was on average 0.3 higher (2.3 lower to 2.8 higher) | 84 (2 studies) | ⊕⊕⊝⊝ Lowb,c | ||
| Pain > 6 months post treatment (VAS 0 to 10) | Mean pain score ranged across control groups from 5.3 to 6.6 |
Mean pain score in intervention groups was on average 0.8 lower (1.2 to 0.3 lower) | 75 (2 studies) | ⊕⊕⊕⊝ Moderateb | ||
| Function 1 month post treatment (ODI 0 to 100) | Functional status in control
group was 39.9 |
Mean function in intervention groups was on average 1.0 higher (6.9 lower to 8.9 higher) | 57 (1 study) | ⊕⊕⊝⊝ Lowa,b | ||
| Function 1 to 6 months post treatment (ODI 0 to 100) | Mean functional status ranged across control groups from 36.7 to 40.4 | Mean functioning in intervention groups was on average 0.9 higher (6.4 lower to 8.1 higher) | 85 (2 studies) | ⊕⊕⊕⊝ Moderateb | ||
| Function > 6 months post treatment (ODI 0 to 100) | Mean functional status ranged across control
groups from 28.2 to 41.2 |
Mean functioning in intervention groups was on average 6.8 lower (13.4 to 0.1 lower) | 76 (2 studies) | ⊕⊕⊕⊝ Moderateb | ||
| CI: Confidence interval; VAS: Visual analogue scale. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aSingle study, in any case inconsistent. bFewer than 400 participants included. cI2 > 50%.
Summary of findings 4. SI joint: radiofrequency denervation versus placebo.
| SI joint: radiofrequency denervation versus placebo | ||||||
| Patient or population: patients with chronic low back pain Settings: secondary care Intervention: SI radiofrequency denervation Comparision: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | SI joint radiofrequency denervation | |||||
| Pain 1 month post treatment (VAS 0 to 10) | Mean pain score ranged across control groups from 3.9 to 6.3 |
Mean pain score in intervention groups was on average 2.1 lower (5.5 lower to 1.2 higher) | 79 (2 studies) | ⊕⊝⊝⊝ Very lowa,b,c | ||
| Pain 1 to 6 months post treatment (VAS 0 to 10) | Pain score in control group was 5.0 |
Mean pain score in intervention groups was on average 1.3 lower (2.1 to 0.5 lower) | 51 (1 study) | ⊕⊕⊝⊝ Lowb,d | ||
| Function 1 month post treatment (ODI 0 to 100) | Mean pain score ranged across control groups from 31.0 to 43.6 |
Mean pain score in intervention groups was on average 14.1 lower (30.4 lower to 2.3 higher) | 75 (2 studies) | ⊕⊝⊝⊝ Very lowa,b,c | ||
| Function 1 to 6 months post treatment (ODI 0 to 100) | Pain score in control group was 37.0 |
Mean pain score in intervention groups was on average 11.0 lower (17.9 to 4.1 lower) | 49 (1 study) | ⊕⊕⊝⊝ Lowb,d | ||
| CI: Confidence interval; VAS: Visual analogue scale. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aFewer than 6 out of 12 items low risk of bias. bFewer than 400 participants included. cI2 > 50%. dSingle study, in any case inconsistent.
Feasibility of statistical pooling
We considered statistical pooling only if subgroups of studies were clinically homogeneous, and if study authors provided sufficient information on study characteristics, outcome measures and study results. Review of included study characteristics revealed that four treatment subgroups were sufficiently clinically homogeneous to permit statistical pooling, as shown in the summary of findings tables: Table 1 ‐ Facet joint: RF denervation versus placebo; Table 2 ‐ Facet joint: RF denervation versus steroid injections; Table 3 ‐ Disc: RF denervation versus placebo; and Table 4 ‐ SI joint: RF denervation versus placebo.
Comparisons considering facet joint pain
Facet joint: RF denervation versus placebo
For short‐term outcomes (< one month), three RCTs measured pain on a visual analogue scale (VAS) (Gallagher 1994; Leclaire 2001; Tekin 2007). We considered the studies in this comparison as statistically homogeneous (MD ‐1.47, 95% CI ‐2.28 to ‐0.67) (Analysis 1.1). Moderate‐quality evidence (three RCTs; N = 160; imprecision) suggests that facet joint RF denervation is more effective than placebo for pain relief over the short term.
1.1. Analysis.
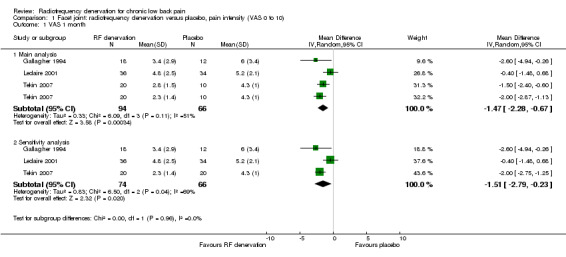
Comparison 1 Facet joint: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10), Outcome 1 VAS 1 month.
For intermediate‐term outcomes (one to six months), three RCTs measured pain on a VAS (Leclaire 2001; Van Kleef 1999; Van Wijk 2005). One study reported outcomes in a different direction from the others. However, because of clinical homogeneity, we performed pooling, with pooled MD of ‐0.71 (95% CI ‐2.25 to 0.84) (Analysis 1.2). Low‐quality evidence (three RCTs; N = 182; inconsistency; imprecision) suggests that facet joint RF denervation is no more effective than placebo for pain relief over the intermediate term.
1.2. Analysis.
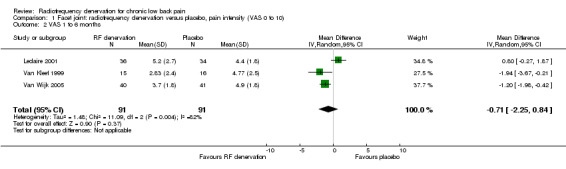
Comparison 1 Facet joint: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10), Outcome 2 VAS 1 to 6 months.
For long‐term outcomes (> six months), three RCTs measured pain on a VAS (Gallagher 1994; Nath 2008; Tekin 2007) and showed statistical homogeneity. The pooled MD was ‐0.70 (95% CI ‐1.48 to 0.08) (Analysis 1.3). Moderate‐quality evidence (three RCTs; N = 130; imprecision) suggests that facet joint RF denervation is no more effective than placebo for pain relief over the long term.
1.3. Analysis.
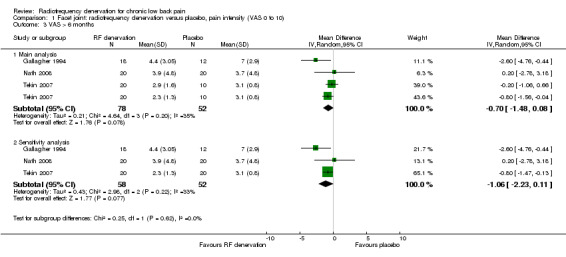
Comparison 1 Facet joint: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10), Outcome 3 VAS > 6 months.
When we removed the comparison of pulsed RF denervation versus placebo in the study of Tekin from the analysis (as recommended by one of the clinicians on the advisory team), the pooled MD for pain intensity was ‐1.51 (95% CI ‐2.79 to ‐0.23) over the short term (Analysis 1.1; sensitivity analysis) and ‐1.06 (95% CI ‐2.23 to 0.11) over the long term (Analysis 1.3; sensitivity analysis). Removal of this study component from the comparisons slightly altered the pooled MD; the long‐term effect became somewhat larger but less precise (moderate quality of evidence).
One RCT with two intervention groups measured functional status on the Oswestry Disability Index (ODI) (zero to 100) over the short term (Tekin 2007). Low‐quality evidence (one RCT; N = 60; inconsistency, imprecision) suggests that facet joint RF denervation is more effective than placebo for functional status over the short term (MD ‐5.53, 95% CI ‐8.66 to ‐2.40) (Analysis 2.1).
2.1. Analysis.
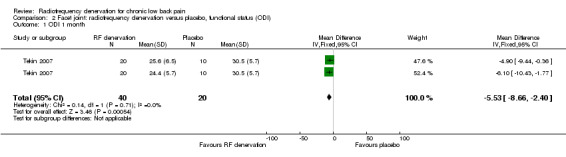
Comparison 2 Facet joint: radiofrequency denervation versus placebo, functional status (ODI), Outcome 1 ODI 1 month.
None of the included studies measured functional status over the intermediate term.
For long‐term outcomes (> six months), one RCT with two intervention groups measured functional status on the ODI (zero to 100) (Tekin 2007). Low‐quality evidence (one RCT; N = 60; inconsistency, imprecision) suggests that facet joint RF denervation is more effective than placebo for functional status over the long term (MD ‐3.70, 95% CI ‐6.94 to ‐0.47) (Analysis 2.2).
2.2. Analysis.
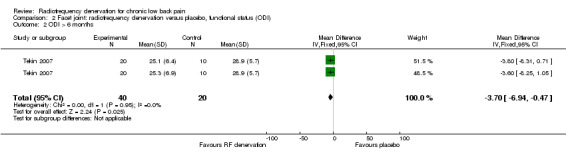
Comparison 2 Facet joint: radiofrequency denervation versus placebo, functional status (ODI), Outcome 2 ODI > 6 months.
One RCT that compared two intervention groups (PRF and CRF) versus placebo measured participant satisfaction on a four‐point scale (Tekin 2007). Low‐quality evidence (one RCT; N = 60; inconsistency, imprecision) suggests that both interventions are more effective than placebo in achieving participant satisfaction. Timing of measurement and MDs between groups were not stated.
Facet joint: continuous RF denervation versus pulsed RF denervation
One RCT compared continuous facet RF denervation versus pulsed RF denervation (Kroll 2008). Investigators reported no significant results for pain three months after treatment. Very low‐quality evidence (one RCT; N = 26; serious RoB; inconsistency, imprecision) suggests that continuous RF denervation is no more effective than pulsed RF denervation (MD 0.07, 95% CI ‐1.82 to 1.96) (Analysis 3.1).
3.1. Analysis.
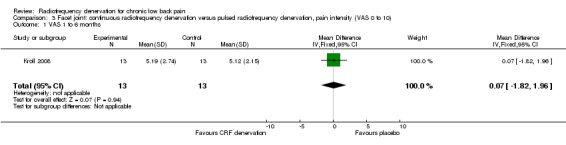
Comparison 3 Facet joint: continuous radiofrequency denervation versus pulsed radiofrequency denervation, pain intensity (VAS 0 to 10), Outcome 1 VAS 1 to 6 months.
Facet joint: percutaneous intra‐articular denervation versus percutaneous extra‐articular denervation
One RCT compared facet percutaneous intra‐articular RF denervation versus percutaneous extra‐articular RF denervation (Sanders 1999). Very low‐quality evidence (one RCT; N = 34; serious RoB; inconsistency, imprecision) suggests that intra‐articular RF denervation is more effective than extra‐articular RF denervation for pain relief three months after the intervention (MD ‐2.20, 95% CI ‐3.69 to ‐0.71) (Analysis 4.1).
4.1. Analysis.
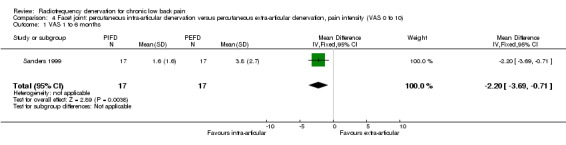
Comparison 4 Facet joint: percutaneous intra‐articular denervation versus percutaneous extra‐articular denervation, pain intensity (VAS 0 to 10), Outcome 1 VAS 1 to 6 months.
Facet joint: RF denervation: distal approach versus tunnel vision approach
One RCT compared the distal approach versus the tunnel vision approach to performing facet joint RF denervation (Moon 2013). Researchers observed no significant results for pain one month (MD ‐0.20, 95% CI ‐1.21 to 0.81) and longer than six months after treatment (MD 0.00, 95% CI ‐1.08 to 1.08). Very low‐quality evidence (one RCT; N = 68; serious RoB; inconsistency, imprecision) suggests that the distal approach is no more effective than the tunnel vision approach (Analysis 5.1; Analysis 5.2).
5.1. Analysis.
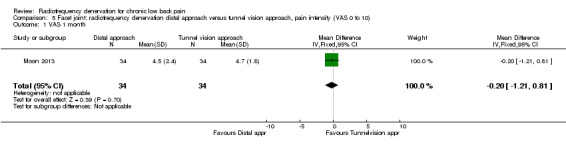
Comparison 5 Facet joint: radiofrequency denervation distal approach versus tunnel vision approach, pain intensity (VAS 0 to 10), Outcome 1 VAS 1 month.
5.2. Analysis.
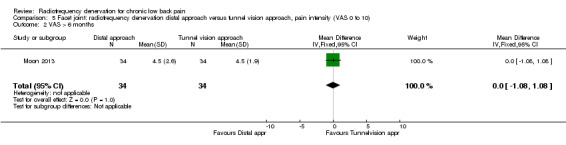
Comparison 5 Facet joint: radiofrequency denervation distal approach versus tunnel vision approach, pain intensity (VAS 0 to 10), Outcome 2 VAS > 6 months.
For functional status, no significant results were found one month (MD 2.20, 95% CI ‐2.28 to 6.68) and longer than six months after treatment (MD 2.90, 95% CI ‐1.71 to 7.51). Very low‐quality evidence (one RCT; N = 68; serious RoB; inconsistency, imprecision) suggests that the distal approach is no more effective than the tunnel vision approach (Analysis 6.1; Analysis 6.2).
6.1. Analysis.
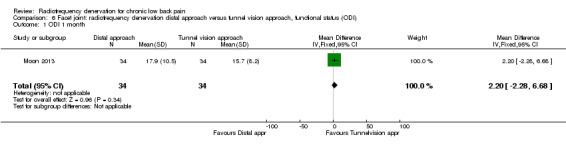
Comparison 6 Facet joint: radiofrequency denervation distal approach versus tunnel vision approach, functional status (ODI), Outcome 1 ODI 1 month.
6.2. Analysis.
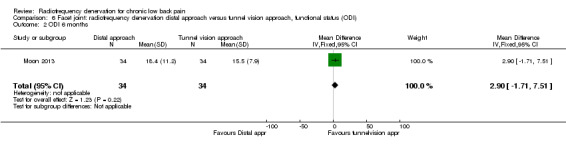
Comparison 6 Facet joint: radiofrequency denervation distal approach versus tunnel vision approach, functional status (ODI), Outcome 2 ODI 6 months.
Facet joint: RF denervation versus steroid injections
For short‐term outcomes (< one month) when RF denervation was compared with steroid injections, two RCTs measured pain on a VAS (Civelek 2012; Duger 2012) and reported statistical homogeneity. When these studies were pooled, the MD was ‐2.23 (95% CI ‐2.38 to ‐2.08) (Analysis 7.1). Low‐quality evidence (two RCTs; N = 180; serious RoB; imprecision) suggests that facet joint RF denervation is more effective than steroid injections for pain relief over the short term.
7.1. Analysis.
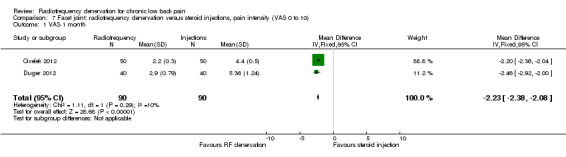
Comparison 7 Facet joint: radiofrequency denervation versus steroid injections, pain intensity (VAS 0 to 10), Outcome 1 VAS 1 month.
For intermediate‐term outcomes (one to six months), three RCTs measured pain on a VAS (Civelek 2012; Duger 2012; Lakemeier 2013). On the basis of Chi2, I2 and confidence intervals, the studies were deemed statistically heterogeneous, in large part because of the small SDs, which were difficult to extract from the study of Civelek (Civelek 2012). Confidence intervals were hardly overlapping, but because of clinical homogeneity, and because all effects were noted to be in the same direction, we decided to pool the results of these studies. The MD was ‐2.13 (95% CI ‐3.45 to ‐0.81) (Analysis 7.2). Very low‐quality evidence (three RCTs; N = 132; serious RoB; imprecision, inconsistency) suggests that facet joint RF denervation is more effective than steroid injection for pain relief over the intermediate term.
7.2. Analysis.
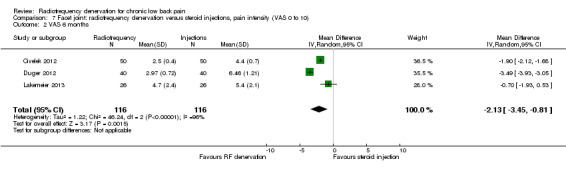
Comparison 7 Facet joint: radiofrequency denervation versus steroid injections, pain intensity (VAS 0 to 10), Outcome 2 VAS 6 months.
For long‐term outcomes (> six months), two RCTs measured pain on a VAS (Civelek 2012; Duger 2012). For the same reason as in Analysis 7.2, statistical pooling was performed unless limitations in this approach were noted. The MD was ‐2.65 (95% CI ‐3.45 to ‐1.88) (Analysis 7.3). Very low‐quality evidence (three RCTs; N = 180; serious RoB; imprecision, inconsistency) suggests that facet joint RF denervation is more effective than steroid injection for pain relief over the intermediate term.
7.3. Analysis.
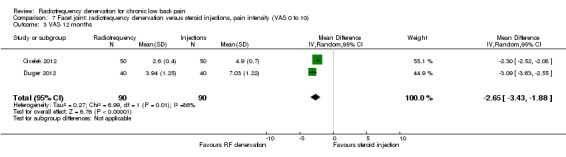
Comparison 7 Facet joint: radiofrequency denervation versus steroid injections, pain intensity (VAS 0 to 10), Outcome 3 VAS 12 months.
One RCT compared RF denervation versus steroid injections and measured function (Lakemeier 2013). Investigators reported no significant results for function six months after treatment (MD ‐5.00, 95% CI ‐15.19 to 5.19). Very low‐quality evidence (one RCT; N = 52; serious RoB; inconsistency, imprecision) suggests that RF denervation is no more effective than steroid injections over the long term (Analysis 8.1).
8.1. Analysis.
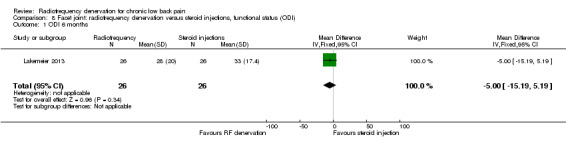
Comparison 8 Facet joint: radiofrequency denervation versus steroid injections, functional status (ODI), Outcome 1 ODI 6 months.
One RCT compared RF denervation versus steroid injections and measured participant satisfaction (Duger 2012). Low‐quality evidence (one RCT; N = 80; serious RoB; inconsistency, imprecision) suggests that facet joint RF denervation is more effective than steroid injection for participant satisfaction over the short, intermediate and long term (Analysis 9.1; Analysis 9.2; Analysis 9.3).
9.1. Analysis.
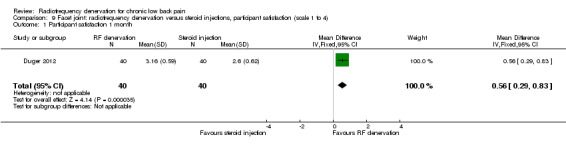
Comparison 9 Facet joint: radiofrequency denervation versus steroid injections, participant satisfaction (scale 1 to 4), Outcome 1 Participant satisfaction 1 month.
9.2. Analysis.
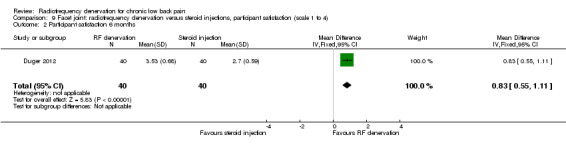
Comparison 9 Facet joint: radiofrequency denervation versus steroid injections, participant satisfaction (scale 1 to 4), Outcome 2 Participant satisfaction 6 months.
9.3. Analysis.
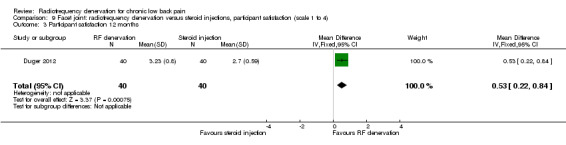
Comparison 9 Facet joint: radiofrequency denervation versus steroid injections, participant satisfaction (scale 1 to 4), Outcome 3 Participant satisfaction 12 months.
Comparisons considering discogenic low back pain
120‐Second disc RF denervation versus 360‐second RF denervation
One study compared 360‐second RF denervation versus 120‐second RF denervation (Ercelen 2003). Researchers found no significant differences in pain and function between groups at any follow‐up assessment. Very low‐quality evidence (one RCT; N = 37; serious RoB; imprecision) suggests that 360‐second RF denervation is no more effective for pain and function than 120‐second RF denervation over the short, intermediate and long term (Analysis 10.1; Analysis 10.2; Analysis 10.3; Analysis 11.1; Analysis 11.2).
10.1. Analysis.
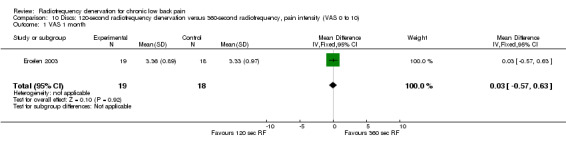
Comparison 10 Discs: 120‐second radiofrequency denervation versus 360‐second radiofrequency, pain intensity (VAS 0 to 10), Outcome 1 VAS 1 month.
10.2. Analysis.
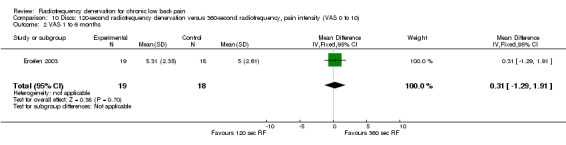
Comparison 10 Discs: 120‐second radiofrequency denervation versus 360‐second radiofrequency, pain intensity (VAS 0 to 10), Outcome 2 VAS 1 to 6 months.
10.3. Analysis.
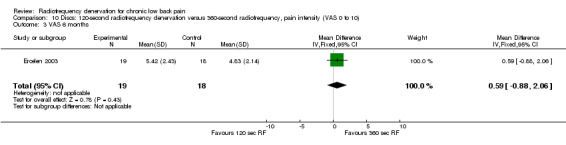
Comparison 10 Discs: 120‐second radiofrequency denervation versus 360‐second radiofrequency, pain intensity (VAS 0 to 10), Outcome 3 VAS 6 months.
11.1. Analysis.
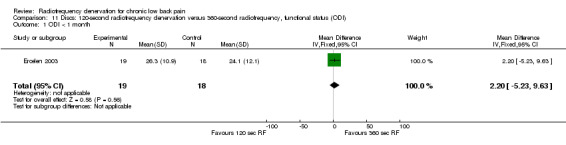
Comparison 11 Discs: 120‐second radiofrequency denervation versus 360‐second radiofrequency, functional status (ODI), Outcome 1 ODI < 1 month.
11.2. Analysis.
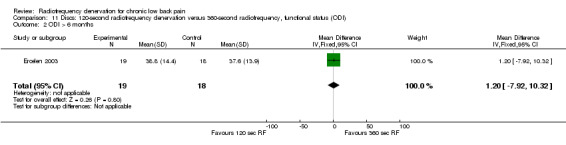
Comparison 11 Discs: 120‐second radiofrequency denervation versus 360‐second radiofrequency, functional status (ODI), Outcome 2 ODI > 6 months.
Disc: RF denervation versus lidocaine
One study compared the effects of RF denervation versus lidocaine in participants with disc pain. Investigators reported no significant results for pain four months after the procedure (Oh 2004). Very low‐quality evidence (one RCT; N = 49; serious RoB; inconsistency, imprecision) suggests that RF denervation is more effective than lidocaine four months post treatment (Analysis 12.1).
12.1. Analysis.
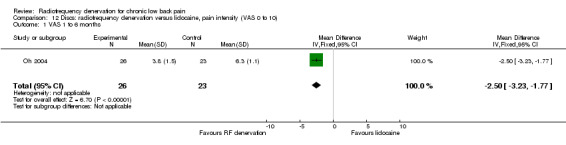
Comparison 12 Discs: radiofrequency denervation versus lidocaine, pain intensity (VAS 0 to 10), Outcome 1 VAS 1 to 6 months.
Disc: RF denervation versus placebo
One study (Kapural 2013) compared RF denervation versus placebo and reported short‐term outcomes. Low‐quality evidence (one RCT; N = 56; inconsistency, imprecision) suggests that RF denervation is no more effective than placebo for pain and function over the short term (Analysis 13.1; Analysis 14.1).
13.1. Analysis.
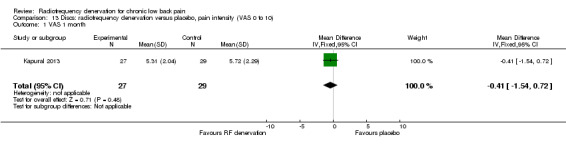
Comparison 13 Discs: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10), Outcome 1 VAS 1 month.
14.1. Analysis.
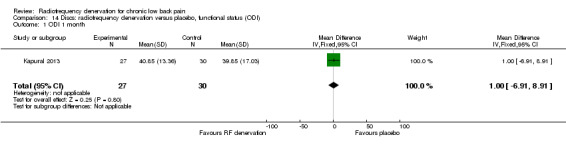
Comparison 14 Discs: radiofrequency denervation versus placebo, functional status (ODI), Outcome 1 ODI 1 month.
Two studies compared RF denervation versus placebo and reported outcome measures for pain and function. Researchers reported no significant results for pain and function one to six months after the intervention (Barendse 2001; Kapural 2013). Low‐quality evidence (two RCTs; N = 84; imprecision, inconsistency) suggests that RF denervation is no more effective than placebo for pain (MD 0.27, 95% CI ‐2.25 to 2.79) and function over the intermediate term (MD 0.86, 95% CI ‐6.37 to 8.10) (Analysis 13.2; Analysis 14.2).
13.2. Analysis.
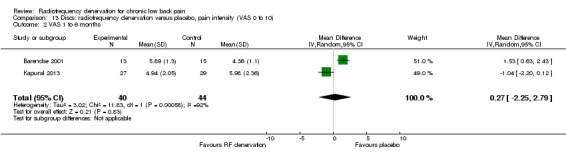
Comparison 13 Discs: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10), Outcome 2 VAS 1 to 6 months.
14.2. Analysis.
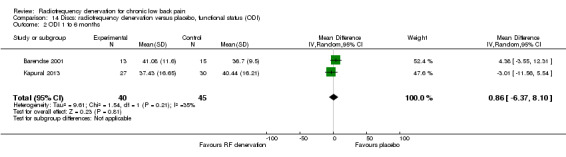
Comparison 14 Discs: radiofrequency denervation versus placebo, functional status (ODI), Outcome 2 ODI 1 to 6 months.
Over the long term, two studies (Kapural 2013; Kvarstein 2009) showed small significant results for pain and function six and 12 months after treatment. Moderate‐quality evidence (two RCTs; N = 75; imprecision) suggests that RF denervation is more effective than placebo for pain (MD ‐1.63, 95% CI ‐2.58 to ‐0.68) and function over the long term (MD ‐6.75, 95% CI ‐13.42 to ‐0.09) (Analysis 13.3; Analysis 14.3).
13.3. Analysis.
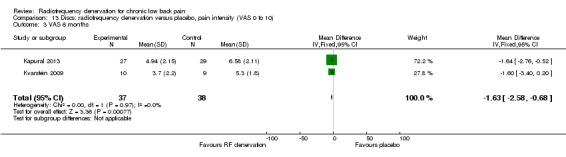
Comparison 13 Discs: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10), Outcome 3 VAS 6 months.
14.3. Analysis.
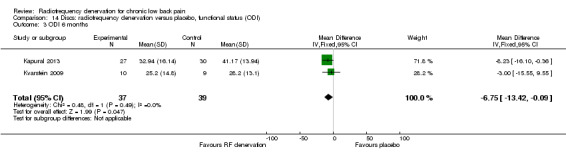
Comparison 14 Discs: radiofrequency denervation versus placebo, functional status (ODI), Outcome 3 ODI 6 months.
Comparison considering SI joint pain
SI joint: RF denervation versus placebo
Two low‐quality studies (N = 79; serious RoB; imprecision, inconsistency) compared RF denervation versus placebo (Cohen 2008; Patel 2012) over the short term. Very low‐quality evidence suggests that RF denervation is no more effective than placebo for pain (MD ‐2.12, 95% CI ‐5.45 to 1.21) and function (MD ‐14.06, 95% CI ‐30.42 to 2.30) one month post treatment (Analysis 15.1; Analysis 16.1).
15.1. Analysis.
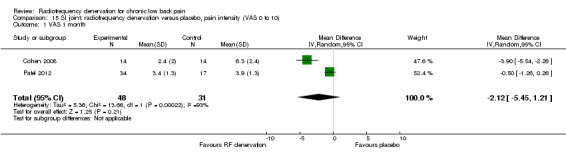
Comparison 15 SI joint: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10), Outcome 1 VAS 1 month.
16.1. Analysis.
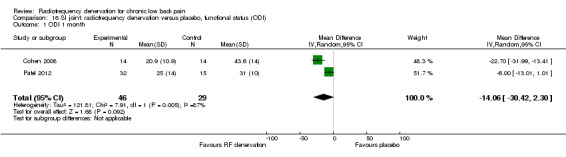
Comparison 16 SI joint: radiofrequency denervation versus placebo, functional status (ODI), Outcome 1 ODI 1 month.
One low‐quality study (Patel 2012) (one RCT; N = 51; inconsistency, imprecision) showed a smaller effect of RF denervation compared with placebo for pain and function one to six months after the intervention (Analysis 15.2; Analysis 16.2).
15.2. Analysis.
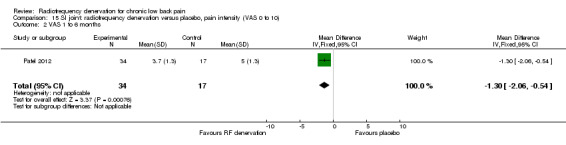
Comparison 15 SI joint: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10), Outcome 2 VAS 1 to 6 months.
16.2. Analysis.
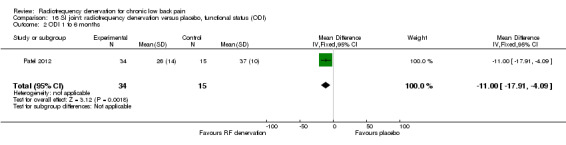
Comparison 16 SI joint: radiofrequency denervation versus placebo, functional status (ODI), Outcome 2 ODI 1 to 6 months.
Comparisons considering the dorsal root ganglion
Radiating low back pain: pulsed RF denervation versus pulsed RF denervation and continuous RF denervation
In one study (N = 76; serious RoB; inconsistency, imprecision), very low‐quality evidence suggests that PRF denervation versus PRF and CRF has no effect three months after treatment on pain relief, functional improvement or health‐related quality of life (Simopoulos 2008); and that PRF is not more or less effective for pain relief than PRF and CRF denervation over the short term (two months). Low‐quality evidence suggests that RF denervation causes no serious complications (Analysis 17.1).
17.1. Analysis.
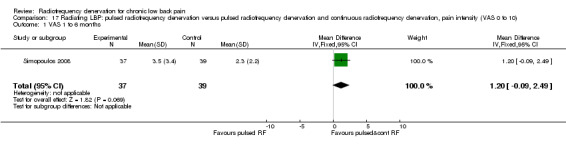
Comparison 17 Radiating LBP: pulsed radiofrequency denervation versus pulsed radiofrequency denervation and continuous radiofrequency denervation, pain intensity (VAS 0 to 10), Outcome 1 VAS 1 to 6 months.
Dorsal root ganglion: RF denervation versus placebo
One study compared RF denervation versus placebo (Geurts 2003). Investigators reported no significant results for pain three months after the procedure. Low‐quality evidence (one RCT; N = 80; imprecision) suggests that RF denervation is no more effective than placebo three months post treatment. Researchers presented no other results for VAS leg, daily physical activities scores, numerical analgesics rating scale scores, global subjective efficacy ratings and Short Form (SF)‐36 scores. Adverse events and complications did not differ between treatments, and no serious complications or side effects occurred in either group (Analysis 18.1).
18.1. Analysis.
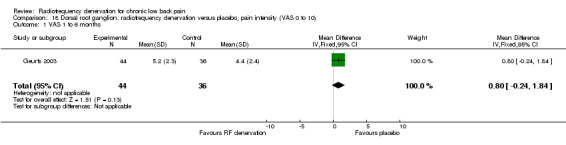
Comparison 18 Dorsal root ganglion: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10), Outcome 1 VAS 1 to 6 months.
Dorsal root ganglion: pulsed RF denervation versus placebo
One study provided low‐quality evidence showing that pulsed RF denervation is no more effective than placebo in the dorsal root ganglion over the short term. Long‐term data or data considering functional status could not be extracted, but the study reports no statistically significant differences in pain and function between PRF and placebo until three months after the intervention (Shanthanna 2014) (Analysis 19.1).
19.1. Analysis.
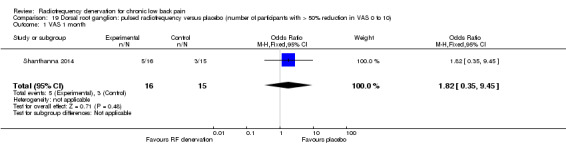
Comparison 19 Dorsal root ganglion: pulsed radiofrequency versus placebo (number of participants with > 50% reduction in VAS 0 to 10), Outcome 1 VAS 1 month.
Low back pain with or without radiation
In one study, very low‐quality evidence (one RCT; N = 100; serious RoB; inconsistency, imprecision) suggests that PRF denervation on dorsal root ganglion compared with either electro‐acupuncture or sham offers better short‐term effects for pain relief and health‐related quality of life but not for functional improvement among individuals suffering from CLBP (Lin Mu‐Lien 2010).
We have summarised additional details in the Characteristics of included studies table.
Clinical relevance of included studies
Table 5 presents clinical relevance scores for each study. Most studies described the study population (91%) and the interventions and settings (87%) well enough for comparison with clinical practice. Seventeen studies (74%) measured clinically relevant outcomes (pain and function). When assessing the clinically important size of the effect, researchers considered 30% reduction on VAS/NRS for pain or 8% to 12% improvement in function on the ODI over the short term as clinically important. Only seven studies (30%) showed clinically relevant effects on one of these outcomes (Cohen 2008; Duger 2012; Gallagher 1994; Oh 2004; Tekin 2007; Van Kleef 1999; Van Wijk 2005). All included studies had small sample sizes, and most poorly described side effects or other complications. Therefore, whether treatment benefits were worth the potential harms remains unclear in all studies.
1. Clinical relevance.
| 1 Patients | 2 Int + setting | 3 Outcomes Pain and function |
4 Effects Pain or function over the short term |
5 Benefits > harms | |
| Facet joint pain | |||||
| Gallagher | + | ‐ | ‐ | + | ? |
| Kroll | + | + | + | ‐ | ? |
| Leclaire | + | ‐ | + | ‐ | ? |
| Nath | + | + | + | ? | ? |
| Tekin | + | + | + | + | ? |
| Van Kleef | + | + | + | + | ? |
| Van Wijk | + | + | + | + | ? |
| Sanders | + | + | + | ‐ | ? |
| Moon | + | + | + | ‐ | ? |
| Civelek | + | + | ‐ | ‐ | ? |
| Duger | + | + | ‐ | + | ? |
| Lakemeier | + | + | + | ‐ | ? |
| Disc pain | |||||
| Barendse | + | + | + | ‐ | ? |
| Kapural | + | + | + | ‐ | ? |
| Ercelen | + | + | + | ‐ | ? |
| Kvarstein | + | + | + | ‐ | ? |
| Oh | ? | + | ‐ | + | ? |
| SI joint pain | |||||
| Cohen | + | + | + | + | ? |
| Patel | + | + | + | ‐ | ? |
| Dorsal root ganglion | |||||
| Geurts | + | + | ‐ | ‐ | ? |
| Shantanna | + | + | + | ‐ | ? |
| Radiating LBP | |||||
| Simopoulos | + | + | ‐ | ‐ | ? |
| LBP with or without radiation | |||||
| Mu‐Lien Lin | ‐ | ? | + | ? | ? |
Discussion
Summary of main results
The objective of this systematic review was to assess the effectiveness of radiofrequency (RF) denervation procedures for treatment of chronic low back pain (CLBP) on the basis of information provided by randomised controlled trials (RCTs). We included 23 RCTs, 13 (56%) of which were considered to have low risk of bias (RoB), even though all had deficiencies, as discussed below. Reviewed studies provided evidence of low to moderate quality suggesting that RF denervation of the facet joint could offer greater pain relief (visual analogue scale (VAS)) (short term) and small improvement in function (Oswestry Disability Index (ODI)) (short and long term) when compared with placebo and steroid injections. For suspected discogenic lumbar pain, evidence of low to very low quality suggests that RF denervation has no effect beyond placebo over the short term, and evidence of moderate quality suggests that RF denervation when compared with placebo has a smaller effect on pain (Numerical Rating Scale (NRS)) and function (ODI) over the long term. For suspected sacroiliac (SI) joint pain, low‐quality evidence shows small effects over the intermediate term and no effects over the short term. For other sources of pain, evidence of low to very low quality shows no effects of RF denervation.
Overall completeness and applicability of evidence
The overall number of participants in all 23 studies ‐ 1309 ‐ makes the number of participants included in each individual trial small. This methodological shortcoming contributes to the overall low quality of the evidence. From a clinical perspective, because of the specialised invasiveness of the technique and exposure to x‐ray, the small number of participants was understandable. However, it should be pointed out that these interventions were tested in highly selected groups that had undergone diagnostic blocks, and the results must therefore be interpreted with care. Furthermore, no reliable data can be found on the diagnostic accuracy or clinical utility of diagnostic facet joint, SI joint or selective nerve root blocks (Chou 2007).
Outcome measures
Five studies did not fulfil the two main clinically relevant outcome measures (Civelek 2012; Duger 2012; Geurts 2003; Oh 2004; Simopoulos 2008): pain and disorder‐specific disability. The studies of Gallagher (Gallagher 1994), Duger (Duger 2012) and Simopoulos (Simopoulos 2008) used pain as the only outcome measure. In this review, we did not consider “ability to work” as an imperative criterion, as it is not always relevant among individuals with CLBP. Only one study assessed treatment‐related costs (Van Wijk 2005).
Follow‐up
Follow‐up time for intention‐to‐treat analysis varied from one month to one year. However, only one study included follow‐up measurement one year after the start of treatment. In six studies, the blinding code was broken in cases of treatment failure, and an escape treatment was offered (Cohen 2008; Geurts 2003; Kvarstein 2009; Leclaire 2001; Patel 2012; Van Wijk 2005). Longer follow‐up periods are needed ‐ not only to prove efficacy in RF denervation, but also to track eventual long‐term adverse effects.
Adverse effects
No adverse effects were reported in 10 studies (Barendse 2001; Duger 2012; Ercelen 2003; Gallagher 1994; Lakemeier 2013; Leclaire 2001; Lin Mu‐Lien 2010; Sanders 1999; Shanthanna 2014; Simopoulos 2008). Two studies (Nath 2008; Patel 2012) reported subsiding pain associated with the procedure. The study of Oh (Oh 2004) reported complaints of mild lower limb weakness that dissipated completely. Cohen (Cohen 2008) reported transient non‐painful paraesthesias that resolved without therapy. Symptoms were more common and lasted longer in the RF denervation group. However, no permanent complications were reported. Two studies found no statistically significant differences between groups (Geurts 2003; Van Wijk 2005). In one study, two actively treated and three sham‐treated participants experienced increased pain (Kvarstein 2009). For ethical reasons, inclusion of new participants was therefore discontinued. Three studies reported complications (change in pain characteristics, exacerbation of pain, small superficial burns after RF denervation) that did not last longer than one month (Civelek 2012; Kapural 2013; Moon 2013). Furthermore, most RCTs were small and were not designed to evaluate adverse events, so no clear conclusion can be drawn regarding risks of RF denervation.
Quality of the evidence
Thirteen studies (56%) had an overall low RoB. The RoB items ‘compliance’ and ‘similar timing of outcome assessment’ were scored best with both 22 studies that complied to these items. However, compliance was in most of the studies irrelevant because it was a single session intervention. Selective reporting and the avoidance or similarity of co‐interventions was scored worst, with respectively one and two studies which complied to this item. Selective reporting was scored unclear if no study protocol was published. Most studies included core outcomes (pain and function), but protocols could not be identified in all but one study (Shanthanna 2014). In most studies it was not reported clearly if co‐interventions were avoided or similar. Especially these RoB items need improvement in future studies. The RoB item 'patient blinding' was scored 'Yes' if the intervention and control groups are indistinguishable for the patients or if the success of blinding was tested among the patients and it was successful (Furlan 2009). However, blinding the RF denervation procedure is very difficult and debatable. For future reviews it can be discussed if this item should be scored 'yes' if blinding was described as indistinguishable for patients but was not tested.
Potential biases in the review process
The primary limitation of this review ‐ lack of studies with low RoB ‐ is encountered in many systematic reviews. Methodologically well‐conducted studies with an appropriate sample size undertaken to examine the effectiveness of RF denervation remain scarce. Also, many included studies had no published protocol and, to our knowledge, had not been registered in any of the trial registries. Another limitation is the possibility of publication bias, which we attempted to minimise by conducting an extensive database search. This search is up‐to‐date until May 2014; the fact that one study is not incorporated may be a source of potential bias. The influence of publication bias on the results was impossible to assess because a small number of studies contributed to each pooled estimate.
Agreements and disagreements with other studies or reviews
Since the original review was published in 2003 (Niemisto 2003), 19 new studies about RF denervation for CLBP have been reported. The original review showed conflicting evidence for the effectiveness of facet joint RF denervation. This evidence remains conflicting; however, we found moderate‐quality evidence for effects favouring RF denervation over placebo for pain (short term), and low‐quality evidence supports RF denervation for functional improvement (over the short, intermediate and long term). In 2003, Niemisto et al (Niemisto 2003) reported limited evidence that intradiscal RF denervation may not be effective for discogenic pain. This review supports these results over the short and intermediate term, but moderate‐quality evidence shows small positive results over the long term. The current review found greater variation among control groups, most of which did not show significant differences. Only low‐quality evidence was found to favour the effects of RF denervation over steroid injections for pain.
In 2010, Henschke et al published a systematic review on injection therapy and denervation procedures for CLBP (Henschke 2010). They concluded that only low‐quality to very low‐quality evidence could support the use of injection and denervation procedures over placebo and other treatments. The only possible beneficial treatment effect reported by these review authors was facet joint RF denervation. The current review supports this conclusion. Henschke et al showed the same limited results for injection therapy, as did the systematic review of Staal et al (Staal 2008), which concluded that evidence was insufficient to support use of injection therapy for subacute and chronic low back pain. This finding was consistent with our results (although based on low‐quality evidence) suggesting that RF denervation is more effective than steroid injections for facet joint pain.
Poetscher et al (Poetscher 2014) concluded that facet joint RF denervation was more effective than placebo for pain control and functional improvement and was possibly more effective than steroid injections for pain control. These results are supported by evidence of low to moderate quality and show similarities with our results. All previously published reviews state that adverse effects were not sufficiently reported.
Authors' conclusions
Implications for practice.
In general, all conclusions concerning the effects of continuous radiofrequency (CRF) or pulsed radiofrequency (PRF) denervation on CLBP are based on evidence of very low, low or moderate quality. Given this overall quality of evidence, it is recommended that practitioners should be careful when making the decision to use RF denervation in routine clinical practice until rigorous, high‐quality studies on effectiveness and cost‐effectiveness have been performed. As the original studies were not of adequate quality and size to permit assessment of how often complications of RF denervation occur, RF denervation for suspected facet joint pain may have smaller effects in reducing pain (short term) and improving function (short term and long term) in comparison with placebo, but valid evidence on harms is lacking. For suspected discogenic pain, evidence of low to moderate quality shows no short‐term and intermediate‐term effects. This undermines the clinical plausibility of moderate evidence for small effects favouring RF denervation over the long term. For suspected SI joint pain, low‐quality evidence suggests that RF denervation may not provide short‐term effects on pain and functional improvement, and may confer small effects over the long term. For other CLBP, the evidence is low in quality and is too sparse to allow any conclusions. Studies listed under Studies awaiting classification and Ongoing studies may alter the conclusions of the review, once assessed.
Implications for research.
Additional high‐quality registered RCTs with larger patient samples, careful pre‐selection of patients with diagnostic blocks, longer follow‐ups and meaningful standardised outcomes are needed, as are trials on indications for which RF denervation is now used without scientific evidence of efficacy.
What's new
| Date | Event | Description |
|---|---|---|
| 19 December 2014 | New citation required but conclusions have not changed | Since the original review in 2003, 19 new studies about radiofrequency (RF) denervation for chronic low back pain have been published. The original review shows conflicting evidence for the effectiveness of facet joint RF denervation. In the current review, evidence remains conflicting; however, moderate evidence supports short‐term effects on pain favouring RF denervation compared with placebo, and low evidence supports effects of RF denervation on function. In 2003, limited evidence showed that intra discal RF denervation may not be effective for discogenic pain. This review supports these results over the short term and over the intermediate term, but evidence of moderate quality shows small effects favouring RF denervation over the long term. The clinical plausibility of evidence of effectiveness only over the long term may be questioned. The current review found greater variation in control groups, most of which do not show significant differences compared with the RF denervation group. Only low‐quality evidence supports effects favouring RF denervation compared with steroid injections for facet joint pain. The inadequate quality and size of the original studies did not allow inferences on the safety of RF denervation |
| 19 December 2014 | New search has been performed | This review is an update of a previous review that focused on both back pain and neck pain. This review incorporated 19 new trials about radiofrequency therapy for chronic low back pain. The search was updated in June 2015. One trial report was added to 'Studies awaiting classification' (Hashemi 2014) and three trial reports were added to ‘Ongoing studies’ (Albareeq 2015; Meckhail 2013; Mekhail 2015) |
| 6 June 2010 | Amended | The original review (Niemisto 2003) (Niemisto L, Kalso EA, Malmivaara A, Seitsalo S, Hurri H. Radiofrequency denervation for neck and back pain. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No.: CD004058. DOI: 10.1002/14651858.CD004058.) was split into separate reviews for neck pain and back pain, and the literature search was updated |
Acknowledgements
The authors are grateful to Mr Wichor Bramer and Mr Johan Juch for advice and assistance in identification of the original trials, and Rachel Couban and Shireen Harbin, Trials Search Co‐ordinator, Cochrane Back and Neck Review Group, for assistance in updating the literature search and screening search results.
Appendices
Appendix 1. CENTRAL search strategy
Last searched May 29, 2014. Lines 29 and 35 were added.
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia
#3 backache
#4 MeSH descriptor: [Low Back Pain] explode all trees
#5 lumbar next pain or coccyx or coccydynia or spondylosis
#6 MeSH descriptor: [Spine] explode all trees
#7 MeSH descriptor: [Spinal Diseases] explode all trees
#8 lumbago OR discitis OR disc near degeneration OR disc near prolapse OR disc near herniation
#9 spinal fusion
#10 facet near joints
#11 MeSH descriptor: [Intervertebral Disk] explode all trees
#12 postlaminectomy
#13 arachnoiditis
#14 failed near back
#15 MeSH descriptor: [Cauda Equina] explode all trees
#16 lumbar near vertebra*
#17 spinal near stenosis
#18 slipped near (disc* or disk*)
#19 degenerat* near (disc* or disk*)
#20 stenosis near (spine or root or spinal)
#21 displace* near (disc* or disk*)
#22 prolap* near (disc* or disk*)
#23 MeSH descriptor: [Sciatic Neuropathy] explode all trees
#24 sciatic*
#25 back disorder*
#26 back near pain
#27 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26
#28 MeSH descriptor: [Radio Waves] explode all trees
#29 MeSH descriptor: [Pulsed Radiofrequency Treatment] explode all trees
#30 radiofrequency
#31 radio frequency or radio‐frequency
#32 MeSH descriptor: [Electrocoagulation] explode all trees
#33 electrocoag*
#34 thermocoag*
#35 neurotom* or neuroly*
#36 #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35
#37 #27 and #36 in Trials
2013 search strategy
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia
#3 backache
#4 MeSH descriptor: [Low Back Pain] explode all trees
#5 lumbar next pain OR coccyx OR coccydynia OR sciatica OR spondylosis
#6 MeSH descriptor: [Spine] explode all trees
#7 MeSH descriptor: [Spinal Diseases] explode all trees
#8 lumbago OR discitis OR disc near degeneration OR disc near prolapse OR disc near herniation
#9 spinal fusion
#10 spinal neoplasms
#11 facet near joints
#12 MeSH descriptor: [Intervertebral Disk] explode all trees
#13 postlaminectomy
#14 arachnoiditis
#15 failed near back
#16 MeSH descriptor: [Cauda Equina] explode all trees
#17 lumbar near vertebra*
#18 spinal near stenosis
#19 slipped near (disc* or disk*)
#20 degenerat* near (disc* or disk*)
#21 stenosis near (spine or root or spinal)
#22 displace* near (disc* or disk*)
#23 prolap* near (disc* or disk*)
#24 MeSH descriptor: [Sciatic Neuropathy] explode all trees
#25 sciatic*
#26 back disorder*
#27 back near pain
#28 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27
#29 MeSH descriptor: [Radio Waves] explode all trees
#30 radiofrequency
#31 radio frequency
#32 MeSH descriptor: [Electrocoagulation] explode all trees
#33 electrocoagul*
#34 thermocoagul$
#35 #29 or #30 or #31 or #32 or #33 or #34
#36 #28 and #35 from 2012 to 2013, in Trials
2010 search strategy
#1 MeSH descriptor Back explode all trees
#2 MeSH descriptor Buttocks, this term only
#3 MeSH descriptor Leg, this term only
#4 MeSH descriptor Back Pain explode tree
#5 MeSH descriptor Back Injuries explode all trees
#6 MeSH descriptor Low Back Pain, this term only
#7 (low next back next pain)
#8 (lbp)
#9 MeSH descriptor Sciatic Neuropathy explode all trees
#10 MeSH descriptor Spine explode all trees
#11 MeSH descriptor Spinal Diseases explode all trees
#12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11)
#13 MeSH descriptor Radio Waves explode all trees
#14 radiofrequency
#15 radio frequency
#16 MeSH descriptor Electrocoagulation explode all trees
#17 electrocoagul*
#18 thermocoagul$
#19 (#13 OR #14 OR #15 OR #16 OR #17 OR #18)
#20 (#12 AND #19)
#21 (#20), from 2009 to 2010
Appendix 2. MEDLINE search strategy
Last searched May 29, 2014. Lines 38 and 44 were added.
randomized controlled trial.pt.
controlled clinical trial.pt.
randomi#ed.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐8
(animals not (humans and animals)).sh.
9 not 10
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatic neuropathy/
spondylosis.ti,ab.
lumbago.ti,ab.
or/12‐21
exp Spine/
discitis.ti,ab.
exp Spinal Diseases/
(disc adj degeneration).ti,ab.
(disc adj prolapse).ti,ab.
(disc adj herniation).ti,ab.
spinal fusion.sh.
(facet adj joints).ti,ab.
intervertebral disc.sh.
postlaminectomy.ti,ab.
arachnoiditis.ti,ab.
(failed adj back).ti,ab.
or/23‐34
22 or 35
exp Radio Waves/
exp Pulsed Radiofrequency Treatment/
radiofrequency.mp.
radio frequency.mp.
exp Electrocoagulation/
electrocoag$.mp.
thermocoag$.mp.
(neurotom$ or neuroly$).mp.
or/37‐44
11 and 36 and 45
2013 strategy. Lines 37 to 39 were removed for the 2014 update.
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐8
(animals not (humans and animals)).sh.
9 not 10
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatic neuropathy/
spondylosis.ti,ab.
lumbago.ti,ab.
or/12‐21
exp Spine/
discitis.ti,ab.
exp Spinal Diseases/
(disc adj degeneration).ti,ab.
(disc adj prolapse).ti,ab.
(disc adj herniation).ti,ab.
spinal fusion.sh.
spinal neoplasms.sh.
(facet adj joints).ti,ab.
intervertebral disc.sh.
postlaminectomy.ti,ab.
arachnoiditis.ti,ab.
(failed adj back).ti,ab.
or/23‐35
Oswestry.tw.
Roland‐Morris.tw.
or/37‐38
22 or 36 or 39
exp Radio Waves/
radiofrequency.mp.
radio frequency.mp.
exp Electrocoagulation/
electrocoag$.mp.
thermocoagulation.mp.
or/41‐46
11 and 40 and 47
limit 48 to yr="2012 ‐ 2013"
limit 48 to ed=20120301‐20130529
49 or 50
Appendix 3. MEDLINE In‐Process & Other Non‐Indexed Citations search strategy
Last searched May 30, 2014.
randomi#ed controlled trial.ti,ab.
controlled clinical trial.ti,ab.
randomi#ed.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐8
dorsalgia.ti,ab.
Back Pain.ti,ab.
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatic$.ti,ab.
spondylosis.ti,ab.
lumbago.ti,ab.
or/10‐18
(spine or sacrum or lumbar vertebrae or intervertebral disc$).ti,ab.
discitis.ti,ab.
(disc adj degeneration).ti,ab.
(disc adj prolapse).ti,ab.
(disc adj herniation).ti,ab.
spinal fusion.ti,ab.
(facet adj joints).ti,ab.
postlaminectomy.ti,ab.
arachnoiditis.ti,ab.
(failed adj back).ti,ab.
or/20‐29
19 or 30
(radiowave$ or radio wave$).ti,ab.
(radiofrequency or radio frequency).ti,ab.
electrocoag$.ti,ab.
thermocoag$.ti,ab.
(neurotom$ or neuroly$).ti,ab.
or/32‐36
9 and 31 and 37
Appendix 4. EMBASE search strategy
Last searched May 29, 2014. The study design filter, disorder and intervention terms were revised.
Clinical Trial/
Controlled clinical trial/
Controlled Study/
Randomized Controlled Trial/
Double Blind Procedure/
Single Blind Procedure/
crossover procedure/
placebo/
allocat$.ti,ab.
assign$.ti,ab.
blind$.ti,ab.
(clinic$ adj25 (study or trial)).ti,ab.
(crossover or cross‐over).ti,ab.
factorial$.ti,ab.
(followup or follow‐up).ti,ab.
prospectiv$.ti,ab.
placebo$.ti,ab.
random$.ti,ab.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
volunteer$.ti,ab.
or/1‐20
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
human/ or normal human/ or human cell/
22 and 23
22 not 24
21 not 25
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
back disorder$.mp.
or/27‐37
exp SPINE/
(discitis or diskitis).mp.
exp Spine Disease/
(disc adj degeneration).mp.
(disc adj prolapse).mp.
(disc adj herniation).mp.
spinal fusion.mp.
(facet adj joints).mp.
(intervertebral disk or intervertebral disc).mp.
postlaminectomy.mp.
arachnoiditis.mp.
(failed adj back).mp.
or/39‐50
38 or 51
exp pulsed radiofrequency treatment/
exp radiofrequency/
exp radiofrequency radiation/
(radiofrequency or radio‐frequency).mp.
exp THERMOCOAGULATION/ or thermocoag$.mp.
exp ELECTROCOAGULATION/ or electrocoag$.mp.
(neurotom$ or neuroly$).mp.
or/53‐59
26 and 52 and 60
2013 strategy.
Clinical Article/
exp Clinical Study/
Clinical Trial/
Controlled Study/
Randomized Controlled Trial/
Major Clinical Study/
Double Blind Procedure/
Multicenter Study/
Single Blind Procedure/
Phase 3 Clinical Trial/
Phase 4 Clinical Trial/
crossover procedure/
placebo/
or/1‐13
allocat$.mp.
assign$.mp.
blind$.mp.
(clinic$ adj25 (study or trial)).mp.
compar$.mp.
control$.mp.
cross?over.mp.
factorial$.mp.
follow?up.mp.
placebo$.mp.
prospectiv$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
trial.mp.
(versus or vs).mp.
or/15‐29
14 and 30
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
human/ or normal human/ or human cell/
32 and 33
32 not 34
31 not 35
neck muscles.mp.
exp NECK/
whiplash injuries.mp.
neck.mp.
or/37‐40
exp SPINE/
discitis.mp.
exp Spine Disease/
(disc adj degeneration).mp.
(disc adj prolapse).mp.
(disc adj herniation).mp.
spinal fusion.mp.
spinal neoplasms.mp.
(facet adj joints).mp.
intervertebral disk.mp.
postlaminectomy.mp.
arachnoiditis.mp.
(failed adj back).mp.
or/42‐54
radiofrequency denervation.mp.
exp RADIOFREQUENCY/
radiofrequency.mp.
denervation.mp. or exp DENERVATION/
57 or 58
59 and 60
exp THERMOCOAGULATION/
exp ELECTROCOAGULATION/
56 or 61 or 62 or 63
41 or 55
36 and 64 and 65
limit 66 to yr="2012 ‐ 2014"
limit 66 to em=201212‐201321
67 or 68
Previous searches used the following animal filter.
31 14 and 30
32 human/
33 Nonhuman/
34 exp ANIMAL/
35 Animal Experiment/
36 33 or 34 or 35
37 32 not 36
38 31 not 36
39 37 and 38
40 38 or 39
Appendix 5. CINAHL search strategy
Last searched May 30, 2014. The intervention terms were revised.
S56 S49 AND S55
S55 S50 OR S51 OR S52 OR S53 OR S54
S54 (MH "Radio Waves")
S53 neurotom* or neuroly*
S52 (MH "Electrocoagulation+") or electrocoag*
S51 thermocoag*
S50 radiofrequency or radio‐frequency
S49 S28 and S48
S48 S35 or S43 or S47
S47 S44 or S45 or S46
S46 "lumbago"
S45 (MH "Spondylolisthesis") OR (MH "Spondylolysis")
S44 (MH "Thoracic Vertebrae")
S43 S36 or S37 or S38 or S39 or S40 or S41 or S42
S42 lumbar N2 vertebra
S41 (MH "Lumbar Vertebrae")
S40 "coccydynia"
S39 "coccyx"
S38 "sciatica"
S37 (MH "Sciatica")
S36 (MH "Coccyx")
S35 S29 or S30 or S31 or S32 or S33 or S34
S34 lumbar N5 pain
S33 lumbar W1 pain
S32 "backache"
S31 (MH "Low Back Pain")
S30 (MH "Back Pain+")
S29 "dorsalgia"
S28 S26 NOT S27
S27 (MH "Animals")
S26 S7 or S12 or S19 or S25
S25 S20 or S21 or S22 or S23 or S24
S24 volunteer*
S23 prospectiv*
S22 control*
S21 followup stud*
S20 follow‐up stud*
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 (MH "Prospective Studies+")
S17 (MH "Evaluation Research+")
S16 (MH "Comparative Studies")
S15 latin square
S14 (MH "Study Design+")
S13 (MH "Random Sample")
S12 S8 or S9 or S10 or S11
S11 random*
S10 placebo*
S9 (MH "Placebos")
S8 (MH "Placebo Effect")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
2011 strategy.
S55 S49 and S53 20100101‐20111231
S54 S49 and S53
S53 S50 or S51 or S52
S52 "electrocoagulation"
S51 thermocoagulation
S50 "radiofrequency denervation"
S49 S28 and S48
S48 S35 or S43 or S47
S47 S44 or S45 or S46
S46 "lumbago"
S45 (MH "Spondylolisthesis") OR (MH "Spondylolysis")
S44 (MH "Thoracic Vertebrae")
S43 S36 or S37 or S38 or S39 or S40 or S41 or S42
S42 lumbar N2 vertebra
S41 (MH "Lumbar Vertebrae")
S40 "coccydynia"
S39 "coccyx"
S38 "sciatica"
S37 (MH "Sciatica")
S36 (MH "Coccyx")
S35 S29 or S30 or S31 or S32 or S33 or S34
S34 lumbar N5 pain
S33 lumbar W1 pain
S32 "backache"
S31 (MH "Low Back Pain")
S30 (MH "Back Pain+")
S29 "dorsalgia"
S28 S26 NOT S27
S27 (MH "Animals")
S26 S7 or S12 or S19 or S25
S25 S20 or S21 or S22 or S23 or S24
S24 volunteer*
S23 prospectiv*
S22 control*
S21 followup stud*
S20 follow‐up stud*
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 (MH "Prospective Studies+")
S17 (MH "Evaluation Research+")
S16 (MH "Comparative Studies")
S15 latin square
S14 (MH "Study Design+")
S13 (MH "Random Sample")
S12 S8 or S9 or S10 or S11
S11 random*
S10 placebo*
S9 (MH "Placebos")
S8 (MH "Placebo Effect")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
2010 strategy. Lines 24 to 29 were removed and the disorder terms were revised in 2011.
S47 S23 and S45 and S46
S46 S30 or S41
S45 S42 or S43 or S44
S44 "electrocoagulation"
S43 thermocoagulation
S42 "radiofrequency denervation"
S41 S40 or S39 or S38 or S37 or S36 or S35 or S34 or S33 or S32 or S31
S40 ""failed W1 back""
S39 (MH "Laminectomy")
S38 ""facet W1 joint""
S37 (MH "Spinal Fusion")
S36 ""disc W5 herniation""
S35 ""disc W5 prolapse""
S34 ""disc W5 degeneration""
S33 (MH "Spinal Diseases+")
S32 (MH "Intervertebral Disk")
S31 (MH "Spine+")
S30 S29 or S28 or S27 or S26 or S25 or S24
S29 (MH "Whiplash Injuries")
S28 (MH "Cervical Vertebrae")
S27 (MH "Neck Pain")
S26 (MH "Neck")
S25 "neck muscles"
S24 (MH "Neck Muscles")
S23 S21 not S22
S22 (MH "Animals+")
S21 S20 or S19 or S18 or S17 or S16 or S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1
S20 "volunteer*"
S19 prospectiv*
S18 "control*"
S17 "follow‐up stud*"
S16 (MH "Prospective Studies+")
S15 (MH "Evaluation Research+")
S14 (MH "Comparative Studies")
S13 "latin square"
S12 (MH "Study Design+")
S11 (MH "Random Sample+")
S10 "random*"
S9 "placebo*"
S8 (MH "Placebos")
S7 (MH "Placebo Effect")
S6 "triple‐blind"
S5 "single‐blind"
S4 "double‐blind"
S3 ""clinical W8 trial""
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
Appendix 6. PsycINFO search strategy
Last searched May 30, 2014. The intervention terms were revised.
clinical trials/
controlled trial.mp.
RCT.mp.
(Random$ adj3 trial).mp.
(clin$ adj3 trial).mp.
(sing$ adj2 blind$).mp.
(doub$ adj2 blind$).mp.
placebo.mp. or exp Placebo/
latin square.mp.
(random$ adj2 assign$).mp.
prospective studies/
(prospective adj stud$).mp.
(comparative adj stud$).mp.
treatment effectiveness evaluation/
(evaluation adj stud$).mp.
exp Posttreatment Followup/
follow?up stud$.mp.
or/1‐17
back pain/
lumbar spinal cord/
(low adj back adj pain).mp.
(back adj pain).mp.
spinal column/
(lumbar adj2 vertebra$).mp.
coccyx.mp.
sciatica.mp.
lumbago.mp.
dorsalgia.mp.
back disorder$.mp.
"back (anatomy)"/
((disc or disk) adj degenerat$).mp.
((disc or disk) adj herniat$).mp.
((disc or disk) adj prolapse$).mp.
(failed adj back).mp.
or/19‐34
(radiofrequency or radio frequency).mp.
thermocoag$.mp.
electrocoag$.mp.
(neurotom$ or neuroly$).mp.
or/36‐39
18 and 35 and 40
2012 strategy.
clinical trials/
controlled trial.mp.
RCT.mp.
(Random* adj3 trial).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
(clin* adj3 trial).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
(sing* adj2 blind*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
(doub* adj2 blind*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
placebo.mp. or exp Placebo/
latin square.mp.
(random* adj2 assign*).mp.
prospective studies/
(prospective adj stud*).mp.
(comparative adj stud*).mp.
treatment effectiveness evaluation/
treatment effectiveness evaluation/
(evaluation adj stud*).mp.
exp Posttreatment Followup/
follow?up stud*.mp.
or/1‐18
back pain/
lumbar spinal cord/
(low adj back adj pain).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
(back adj pain).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
spinal column/
(lumbar adj2 vertebra*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
coccyx.mp.
sciatica.mp.
lumbago.mp.
dorsalgia.mp.
back disorder*.mp.
"back (anatomy)"/
((disc or disk) adj degenerat*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
((disc or disk) adj herniat*).mp.
((disc or disk) adj prolapse*).mp.
(failed adj back).mp.
or/20‐35
radiofrequency denervation.mp.
radiofrequency.mp.
thermocoagulation.mp.
electrocoagulation.mp.
or/37‐40
19 and 36 and 41
limit 42 to yr="2011 ‐ 2012"
2010 strategy, Cambridge Scientific Abstracts (CSA) database
((KW=(Randomi?ed controlled trial*) OR KW=(clinical trial*) OR KW=(clin* near trail*) OR KW= (sing* near blind*) OR KW=(sing* near mask*) OR (doub* near blind*) OR KW=(doubl* NEAR mask*) OR KW=(trebl* near mask*) OR KW=(trebl* near mask*) OR KW=(tripl* near blind*) OR KW=(tripl* near mask*) OR KW=(placebo*) OR KW=(random*) OR DE=(research design) OR KW=(Latin square) OR KW=(comparative stud*) OR KW=(evaluation stud*) OR KW=(follow up stud*) OR DE=(prospective stud*)OR KW=(control*) OR KW=(prospective*) OR KW=(volunteer*)) AND (DE=(back) OR DE=(back pain) OR DE=(neck))) AND ((KW=(radiofrequency denervation)) OR (KW=radiofrequency) OR (KW=thermocoagulation) OR (KW=electrocoagulation))
Date Range: 2010 to 2011
Appendix 7. Clinicaltrials.gov search strategy
Last searched May 29, 2014.
basic search: "back pain" and "radiofrequency"
2011 search.
Condition =back pain
AND
Intervention= radiofrequency OR electrocoagulation OR thermocoagulation
Appendix 8. WHO ICTRP search strategy
Last searched May 29, 2014.
basic search: "back pain" and "radiofrequency"
2011 search.
Condition =back pain
AND
Intervention= radiofrequency OR electrocoagulation OR thermocoagulation
Appendix 9. Assessment of risk of bias
Criteria for a judgement of 'yes' for sources of risk of bias (Furlan 2009).
1. Was the method of randomization adequate? Yes/No/Unsure Random (unpredictable) assignment sequence. Examples of adequate methods include coin toss (for studies with 2 groups), rolling a dice (for studies with 2 or more groups), drawing of balls of different colors, drawing of ballots with study group labels from a dark bag, computer‐generated random sequence, pre‐ordered sealed envelopes, sequentially ordered vials, telephone calls to a central office and pre‐ordered list of treatment assignments. Examples of inadequate methods include alternation, birth date, social insurance/security number, date on which they were invited to participate in the study and hospital registration number.
2. Was the treatment allocation concealed? Yes/No/Unsure Assignment generated by an independent person not responsible for determining eligibility of patients. This person has no information about persons included in the trial and has no influence on assignment sequence nor on the decision about eligibility of the patient.
3. Was the patient blinded to the intervention? Yes/No/Unsure This item should be scored “yes” if index and control groups were indistinguishable for participant, or if the success of blinding was tested among participants, and blinding was found to be successful.
4. Was the care provider blinded to the intervention? Yes/No/Unsure This item should be scored “yes” if index and control groups were indistinguishable for care providers, or if the success of blinding was tested among care providers, and blinding was found to be successful.
5. Was the outcome assessor blinded to the intervention? Yes/No/Unsure
Adequacy of blinding should be assessed for primary outcomes. This item should be scored “yes” if the success of blinding was tested among outcome assessors, and blinding was found to be successful or:
for participant reported outcomes for which the participant was the outcome assessor (e.g. pain, disability): The blinding procedure was adequate for outcome assessors if participant blinding was scored “yes”;
for outcome criteria assessed during scheduled visit and that suppose contact between participants and outcome assessors (e.g. clinical examination): The blinding procedure was adequate if participants were blinded, and if treatment or adverse effects of treatment could not be noticed during clinical examination;
for outcome criteria that do not suppose contact with participants (e.g. radiography, magnetic resonance imaging): The blinding procedure was adequate if treatment or adverse effects of treatment could not be noticed when the main outcome was assessed;
for outcome criteria that were clinical or therapeutic events that would be determined by the interaction between participants and care providers (e.g. co‐interventions, hospitalisation length, treatment failure), for which the care provider was the outcome assessor: The blinding procedure was adequate for outcome assessors if item “4” (caregivers) was scored “yes”; and
for outcome criteria that were assessed from data on medical forms: The blinding procedure was adequate if treatment or adverse effects of treatment could not be noticed from extracted data.
6. Was the dropout rate adequately addressed? Yes/No/Unsure The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and dropouts does not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up, and does not lead to substantial bias, a “yes” is scored. (N.B. these percentages are arbitrary and are not supported by literature).
7. Were all randomly assigned participants analysed in the group to which they were allocated? Yes/No/Unsure All randomly assigned participants were reported/analysed in the groups to which they were allocated by randomisation for the most important moments of effect measurement (minus missing values), irrespective of non‐compliance and co‐interventions.
8. Are reports of the study free of the suggestion of selective outcome reporting? Yes/No/Unsure To assign a “yes”, the review author determines whether all results from all prespecified outcomes have been adequately reported in the published report of the trial. This information can be obtained by comparing the protocol versus the report or, in the absence of the protocol, by assessing that the published report includes enough information to permit this judgement.
9. Were the groups similar at baseline regarding the most important prognostic indicators? Yes/No/Unsure To receive a “yes”, groups have to be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of participants with neurological symptoms and the value of main outcome measure(s).
10. Were co‐interventions avoided or similar? Yes/No/Unsure This item should be scored “yes” if no co‐interventions were provided, or if they were similar between index and control groups.
11. Was compliance acceptable in all groups? Yes/No/Unsure The review author determines whether compliance with interventions is acceptable, based on reported intensity, duration, number and frequency of sessions for both index intervention and control intervention(s). For example, physiotherapy treatment usually is administered over several sessions; therefore, it is necessary to assess how many sessions each participant attended. For single‐session interventions (e.g. surgery), this item is irrelevant.
12. Was the timing of outcome assessment similar in all groups? Yes/No/Unsure Timing of outcome assessment should be identical for all intervention groups and for all important outcome assessments.
Appendix 10. Assessment of clinical relevance
Questions to determine whether results are clinically relevant (Furlan 2009).
Based on the data provided, can you determine whether the results will be clinically relevant?
1. Are participants described in detail, so that you can decide whether they are comparable with those seen in your practice? Yes/No/Unsure
2. Are interventions and treatment settings described well enough that you can provide the same for your patients? Yes/No/Unsure
3. Were all clinically relevant outcomes measured and reported? Yes/No/Unsure
4. Is the size of the effect clinically important? (30% on VAS/NRS; 8% to 12% for function)? Yes/No/Unsure
5. Are likely treatment benefits worth the potential harms? Yes/No/Unsure
Data and analyses
Comparison 1. Facet joint: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 month | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Main analysis | 3 | 160 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐2.28, ‐0.67] |
| 1.2 Sensitivity analysis | 3 | 140 | Mean Difference (IV, Random, 95% CI) | ‐1.51 [‐2.79, ‐0.23] |
| 2 VAS 1 to 6 months | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐2.25, 0.84] |
| 3 VAS > 6 months | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Main analysis | 3 | 130 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.48, 0.08] |
| 3.2 Sensitivity analysis | 3 | 110 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.23, 0.11] |
Comparison 2. Facet joint: radiofrequency denervation versus placebo, functional status (ODI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ODI 1 month | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐5.53 [‐8.66, ‐2.40] |
| 2 ODI > 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐6.94, ‐0.47] |
Comparison 3. Facet joint: continuous radiofrequency denervation versus pulsed radiofrequency denervation, pain intensity (VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 to 6 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐1.82, 1.96] |
Comparison 4. Facet joint: percutaneous intra‐articular denervation versus percutaneous extra‐articular denervation, pain intensity (VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 to 6 months | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐3.69, ‐0.71] |
Comparison 5. Facet joint: radiofrequency denervation distal approach versus tunnel vision approach, pain intensity (VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 month | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.21, 0.81] |
| 2 VAS > 6 months | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.08, 1.08] |
Comparison 6. Facet joint: radiofrequency denervation distal approach versus tunnel vision approach, functional status (ODI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ODI 1 month | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐2.28, 6.68] |
| 2 ODI 6 months | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐1.71, 7.51] |
Comparison 7. Facet joint: radiofrequency denervation versus steroid injections, pain intensity (VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 month | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐2.23 [‐2.38, ‐2.08] |
| 2 VAS 6 months | 3 | 232 | Mean Difference (IV, Random, 95% CI) | ‐2.13 [‐3.45, ‐0.81] |
| 3 VAS 12 months | 2 | 180 | Mean Difference (IV, Random, 95% CI) | ‐2.65 [‐3.43, ‐1.88] |
Comparison 8. Facet joint: radiofrequency denervation versus steroid injections, functional status (ODI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ODI 6 months | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐15.19, 5.19] |
Comparison 9. Facet joint: radiofrequency denervation versus steroid injections, participant satisfaction (scale 1 to 4).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant satisfaction 1 month | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.29, 0.83] |
| 2 Participant satisfaction 6 months | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.55, 1.11] |
| 3 Participant satisfaction 12 months | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [0.22, 0.84] |
Comparison 10. Discs: 120‐second radiofrequency denervation versus 360‐second radiofrequency, pain intensity (VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 month | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.57, 0.63] |
| 2 VAS 1 to 6 months | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐1.29, 1.91] |
| 3 VAS 6 months | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [‐0.88, 2.06] |
Comparison 11. Discs: 120‐second radiofrequency denervation versus 360‐second radiofrequency, functional status (ODI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ODI < 1 month | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐5.23, 9.63] |
| 2 ODI > 6 months | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐7.92, 10.32] |
Comparison 12. Discs: radiofrequency denervation versus lidocaine, pain intensity (VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 to 6 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐3.23, ‐1.77] |
Comparison 13. Discs: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 month | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.54, 0.72] |
| 2 VAS 1 to 6 months | 2 | 84 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐2.25, 2.79] |
| 3 VAS 6 months | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐1.63 [‐2.58, ‐0.68] |
Comparison 14. Discs: radiofrequency denervation versus placebo, functional status (ODI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ODI 1 month | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐6.91, 8.91] |
| 2 ODI 1 to 6 months | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 0.86 [‐6.37, 8.10] |
| 3 ODI 6 months | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐6.75 [‐13.42, ‐0.09] |
Comparison 15. SI joint: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 month | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐5.45, 1.21] |
| 2 VAS 1 to 6 months | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.06, ‐0.54] |
Comparison 16. SI joint: radiofrequency denervation versus placebo, functional status (ODI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ODI 1 month | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐14.06 [‐30.42, 2.30] |
| 2 ODI 1 to 6 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐17.91, ‐4.09] |
Comparison 17. Radiating LBP: pulsed radiofrequency denervation versus pulsed radiofrequency denervation and continuous radiofrequency denervation, pain intensity (VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 to 6 months | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.09, 2.49] |
Comparison 18. Dorsal root ganglion: radiofrequency denervation versus placebo, pain intensity (VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 to 6 months | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.24, 1.84] |
Comparison 19. Dorsal root ganglion: pulsed radiofrequency versus placebo (number of participants with > 50% reduction in VAS 0 to 10).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS 1 month | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.35, 9.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barendse 2001.
| Methods | RCT | |
| Participants | University Hospital Maastricht, The Netherlands (N = 28) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes |
|
|
| Notes | Dropouts: number unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Double‐blind, randomised study |
| Allocation concealment (selection bias) | Low risk | Participants randomly assigned to 2 treatment groups by computer programme through a disinterested third party |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Anaesthesia adequate during the procedure. Participant could not determine whether he or she had received RF or sham treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | Treating physicians left the operating room |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Data obtained by an investigator blinded to allocation of participants |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | One participant lost to follow‐up evaluation |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Intention‐to treat analysis performed |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | High risk | Table 1. Differences in months of pain and VAS scores |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | Measured at baseline and at 3, 6 and 12 months |
Civelek 2012.
| Methods | RCT | |
| Participants | Setting unknown, Turkey (N = 100) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes |
|
|
| Notes | Dropouts: incomplete information on participant flow and follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment to 2 groups performed by random number generation, with balance after every 10 participants |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No stimulation in injection group before treatment; impossible to blind participants |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participant reported outcome measures |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Incomplete information on participant flow and follow‐up |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Data from all participants incorporated into the analysis |
| Selective reporting (reporting bias) | Unclear risk | Remained unclear from text |
| Baseline characteristics similar? | Low risk | Groups comparable on relevant demographic and clinical variables |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant; intervention performed only once |
| Timing of outcome assessments similar? | Low risk | Meaured pre‐procedure, post procedure and at 1, 6 and 12 months post procedure |
Cohen 2008.
| Methods | RCT | |
| Participants | Johns Hopkins Medical Institutions, Maryland & Walter Reed Army Medical Center, Washington, DC, USA (N = 28) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes | Significant changes between groups for pain intensity (VAS) 1 and 3 months after treatment, and for function (ODI) 1 month after treatment
|
|
| Notes | Dropouts: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment in blocks of 4 via pre sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Presealed envelopes |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Blinding evaluated shortly after conclusion of the procedure, when effects of local anaesthetic were still active |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Participants treated with placebo after 1 month |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participant‐reported outcome measures. Participants who did not show adequate symptomatic improvement were unblinded at follow‐up. For those who reported significant relief 1 month after the procedure, unblinding was done 3 months after treatment |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | No dropouts; only 3 placebo participants refused to cross over |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Cross‐over procedure |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | High risk | Meaningful differences in morphine use and function |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | Similar until 3‐month follow‐up |
Duger 2012.
| Methods | RCT; random assignment to 3 groups | |
| Participants | Department of Anesthesiology, Cumhuriyet University School of Medicine, Sivas, Turkey (N = 120) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
RF denervation group
Injection group
RF denervation and injection groups
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Remained unclear from text |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No stimulation in injection group before treatment. Therefore, decision was made that it was impossible to blind participants |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | All procedures performed by the same physician |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participant reported outcome measures |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Incomplete information on participant flow and follow‐up |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Remained unclear from text |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: intervention if performed only once |
| Timing of outcome assessments similar? | Low risk | Table 2. Baseline, day 1, day 2, week 1, week 2, month 1, month 6 and month 12 measures |
Ercelen 2003.
| Methods | RTC | |
| Participants | VKV American Hospital, Pain Management Department, Istanbul, Turkey (N = 39) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group A was treated with a 120‐second 80°C lesion of the intervertebral disc after injection of a mixture of 1 to 2 mL of dye and local anaesthetic (N = 20) Group B was treated with a 360‐second 80°C lesion of the intervertebral disc after injection of a mixture of 1 to 2 mL of dye and local anaesthetic (N = 19) |
|
| Outcomes | No significant changes between groups in pain and function
|
|
| Notes | Dropouts: 2 (5%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly assigned to 2 treatment groups by computer |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Participants not blinded |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Providers not blinded |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participants not blinded and all outcomes participant reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Two participants (5%) dropped out |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Attrition bias unlikely because of design ? |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | Groups comparable on relevant demographic and clinical variables |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | Outcomes measured directly after procedure and at 1 week, 2 weeks, 1 month, 2 months, 3 months and 6 months after procedure |
Gallagher 1994.
| Methods | RCT | |
| Participants | Departments of Pain Relief, Rheumatology and Orthopaedics, Guy's Hospital, London, UK (N = 41) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes | Significant differences in mean pain scores (VAS 0 to 100) between groups A and C at 1 month and at 6 months
|
|
| Notes | Dropouts: number unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Remained unclear from text |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Placebo procedure indistinguishable from intervention |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Participant reported outcome measures by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Remained unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Remained unclear from text |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | No differences between groups in terms of age or duration of pain |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | Participants assessed before any treatment, before denervation, at 1 month and at 6 months |
Geurts 2003.
| Methods | RCT | |
| Participants | University Medical Centre, Utrecht, RIjnstate Hospital, Arnhem, Juliana Hospital, Apeldoorn, Twenteborg Hospital, Almelo (N = 83) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes | No significant changes between groups in pain intensity, global subjective efficacy or any SF‐36 scale
|
|
| Notes | Dropouts: 3 (4%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly allocated by 4 batches of sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Envelope drawn at random by an independent investigator |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Participants masked to allocation |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | Treating doctor masked to allocation |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Clinical outcomes calculated by independent masked doctor |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Dropouts: 3 |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Analyses for secondary outcomes (including pain intensity, physical activity, use of analgesics and quality of life) per protocol |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | Similar during 3 months of follow‐up |
Kapural 2013.
| Methods | RCT | |
| Participants | Department of Pain Management at Cleveland Clinic, Cleveland, Ohio, USA Center for Clinical Research at Carolina's Pain Institute, Winston‐Salem, North Carolina, USA Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes |
|
|
| Notes | Dropouts: 3 (5%) Exclusions before treatment: 5 Breach of eligibility criteria: 1 Participants chose not to receive active treatment: 3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated codes maintained in sequentially numbered opaque envelopes |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Equipment in fluoroscopy suite arranged such that the participant was visually isolated from RF generator |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Randomisation code revealed to treating physician and nurse operating the generator, who was in control of RF delivery to participant |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Instruments completed by blinded participants in both groups at baseline, on the day of the procedure before treatment was given, and at 1 month, 3 months and 6 months post procedure |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | 2 dropouts from 29 participants in experiment group |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | All participants incorporated into the analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single intervention |
| Timing of outcome assessments similar? | Low risk | 1, 3 and 6 months post procedure |
Kroll 2008.
| Methods | RCT | |
| Participants | Henry Ford Hospital IRB, Detroit, Michigan, USA (N = 26) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Continuous RF group
Pulsed RF group
|
|
| Outcomes | No significant differences between groups at 3 months after treatment for pain (zero to 100) and function
|
|
| Notes | Dropouts: 24 (48%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via random numbers generator |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Unclear risk | Unclear whether difference between CRF and PRF could be noticed by participants |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Unclear risk | Remained unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | Dropouts: 24 (48%) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | No ITT analysis. Only 26 of 50 participants completed follow‐up evaluation; their data were analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Unclear risk | Baseline characteristics recorded for only 26 of 50 participants who completed follow‐up evaluation |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | Three months after treatment, VAS and Oswestry scores were measured |
Kvarstein 2009.
| Methods | RCT | |
| Participants | Pain Clinic, The Interventional Centre at Oslo University Hospital, Rikshopitalet (N = 20) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes | No significant changes in pain and function between groups after 6 months and 12 months
|
|
| Notes | No dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Gender‐stratified blocks of 8 by use of random numbers |
| Allocation concealment (selection bias) | Low risk | Block size and randomisation codes not revealed until all measurements had been entered into database after 12‐month observation |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Participants blinded by shutting off sound from the RF generator; blinding tested at 12‐month follow‐up |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | Operator not present during RF treatment procedure |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Participants reported outcome measures and independent assessor not present during RF treatment procedure |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | No dropouts |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Primary endpoint, pain intensity, subjected to both intention‐to‐treat and per‐protocol analyses |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | Table 1. All primary outcomes presented are similar for baseline |
| Co‐interventions avoided or similar? | Low risk | Two participants did not respond to all questionnaires; no additional co‐interventions |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | Baseline, 6 months and 12 months |
Lakemeier 2013.
| Methods | RCT | |
| Participants | Department of Orthopedics, University Hospital Goettingen, Goettingen, Germany (N = 56) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes |
|
|
| Notes | Dropouts: 4 (7%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed randomisation performed through independent institution after participants gave informed consent. Assignments performed using computer‐generated random allocation sequence with permuted blocks, 4 and 6 in size |
| Allocation concealment (selection bias) | Low risk | Concealed randomisation performed through independent institution after participants gave informed consent. Assignments performed using computer‐generated random allocation sequence with permuted blocks, 4 and 6 in size |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Participants unblinded after 6‐month follow‐up examination, or if requested before that time |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | In both groups, all procedures performed by the same experienced spine surgeon. Only unblinded treatment personnel in this study were primary spine surgeon and study nurse assistant. Neither primary spine surgeon nor study nurse assistant were involved in further treatment of participants |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Participants unblinded after 6‐month follow‐up examination, or if requested before that time |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | 4 dropouts among 56 participants |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Citate: For all analyses, intention‐to‐treat principles were used |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single intervention |
| Timing of outcome assessments similar? | Low risk | All outcomes measured at baseline and at 6 months |
Leclaire 2001.
| Methods | RCT | |
| Participants | Center Hospitalier de l'Universite de Montreal, Hopital Notre‐Dame (N = 70) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes | Significant differences were found in RMQ between groups 12 weeks after treatment. No significant differences in VAS (0 to 100) and ODI scores were found
|
|
| Notes | Dropouts: 4 (6%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed in blocks of 4 by opaque prenumbered envelopes |
| Allocation concealment (selection bias) | Low risk | Envelopes containing participant assignments given to physician who performed the technique |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Participants kept blind to treatment group |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | Participants kept blind to treatment group |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Participant reported outcome measures |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Dropouts: 4 (6%) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Primary analysis based on intention‐to‐treat principle |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | No meaningful differences at baseline |
| Co‐interventions avoided or similar? | Low risk | Analysis of co‐interventions showed no significant differences between the 2 groups |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | Baseline, 4 weeks and 12 weeks |
Lin Mu‐Lien 2010.
| Methods | RCT (unclear description of randomisation) | |
| Participants | National Taiwan University, Taipei City, Taiwan (N = 100) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Pulsed RF group
Electro‐acupuncture group
Control group
|
|
| Outcomes |
|
|
| Notes | Dropouts: unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomisation methods |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Treatment methods and frequency of treatment procedures different |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Treatment methods and frequency of treatment procedures different |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participants not blinded and participant reported outcome measures |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Remained unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Remained unclear from text |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | High risk | Baseline characteristics differed statistically for both ODI and VAS scores |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Unclear risk | No data on dropouts, short 1‐month follow‐up |
| Timing of outcome assessments similar? | Low risk | Status measured 1 month after follow‐up |
Moon 2013.
| Methods | RCT | |
| Participants | Department of Anesthesiology and Pain Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea (N = 81) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group (distal approach)
Control group (tunnel vision approach)
|
|
| Outcomes |
|
|
| Notes | Dropouts: 11 (13%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly allocated using envelope method |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Citation from discussion: 'Our investigation could be critiqued for the absence of a control group, lack of blinding and crossover design' |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Citation from discussion: 'Our investigation could be critiqued for the absence of a control group, lack of blinding and crossover design' |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Citation from discussion: 'Our investigation could be critiqued for the absence of a control group, lack of blinding and crossover design' |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | 68 of 82 participants with complete follow‐up |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Figure 1. All participants analysed by group |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | Table 2. Groups comparable on relevant demographic and clinical variables |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single intervention |
| Timing of outcome assessments similar? | Low risk | NRS and ODI measured at 1 month and 6 months after intervention |
Nath 2008.
| Methods | RCT | |
| Participants | Smartkliniken, Umea, Sweden. Department of Hand Surgery, Orebro University Hospital, Orebro, Sweden (N = 40) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes | Significant changes in generalised pain 6 months after treatment. No significant changes in back pain
|
|
| Notes | Dropouts: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedules used |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Participants blinded to their assignment throughout study period |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | RF machine placed behind operator, who was unaware of current level of another operator |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | All outcome measurements performed by same orthopedic surgeon at another institution |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | No dropouts |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Participants analysed by group; no dropouts |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | High risk | Participants in RF treatment group had significantly more generalised pain, low back pain and referred pain to the leg when compared with placebo group. All hip movements were worse in the RF treatment group |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | All participants re‐examined after 6 months |
Oh 2004.
| Methods | RCT | |
| Participants | Clinical Pain Research Center, Sumsang Fine Hospital, Seoul, Korea (N = 49) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes | Pain intensity: change in VAS score at 4 months: ‐3.3 (E), ‐0.7 (C) | |
| Notes | Dropouts: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Remained unclear from text |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Control group received lidocaine injection instead of RF thermocoagulation. Impossible to blind |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Unclear risk | Remained unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Remained unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Remained unclear from text |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | All outcome data evaluated 4 months after procedure |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | All outcome data measured at baseline and after 4 months |
Patel 2012.
| Methods | RCT | |
| Participants | Advanced Pain Management, Green Bay, Wisconsin, USA (N = 51) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes | Pain intensity: change in NRS score at 1 month: ‐2.7 (E), ‐1.7 (C). Not significant Pain intensity: change in NRS score at 3 months: ‐2.4 (E), ‐0.8 (C). Significant Function: change in ODI score at 1 month: ‐12 (E), ‐4 (C). Significant Function: change in ODI score at 3 months: ‐11 (E), 2 (C). Significant |
|
| Notes | Dropouts: 9 (17.6%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned on a 2:1 basis to treatment group or sham group using presealed envelopes |
| Allocation concealment (selection bias) | Low risk | Presealed envelopes given by a nurse not involved in the study |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Participant remained visually isolated from equipment and was exposed to typical equipment noises regardless of treatment group |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Physician blinding not possible |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Both assessors and participants blinded to randomisation at 1‐month and 3‐month follow‐up time points |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Dropouts: 9 out of 51 |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Figure 1. All patients included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Unclear risk | Remained unclear from text |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | All outcomes after 1, 3 and 6 months (cross‐over after 3 months) |
Sanders 1999.
| Methods | RCT | |
| Participants | Spaarne Hospital, Haarlem, The Netherlands (N = 34) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental group
Control group
|
|
| Outcomes | Pain intensity: change in VAS score at 3 months: ‐4.7 (intra‐articular), ‐2.1 (extra‐articular). Significant | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Remained unclear from text |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Unclear risk | Remained unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Remained unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Remained unclear from text |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | Tables 1, 2 and 3. Groups comparable on relevant demographic and clinical variables |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single intervention |
| Timing of outcome assessments similar? | Low risk | Before and 3 months after procured participants were evaluated |
Shanthanna 2014.
| Methods | RCT | |
| Participants | St Joseph's Healthcare Hamilton, Ontario, Canada (N = 31) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes | PRF group achieved 32% (5 out of 16 participants) more than 50% decrease in VAS score (0 to 10) compared with 25% more (3 out of 15 participants) in placebo group | |
| Notes | Dropouts: 2 (6%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was given to the assistant in a sealed opaque envelope to be handed over to the nurse operating the RF machine |
| Allocation concealment (selection bias) | Low risk | Enrolled participants were randomly assigned on the day of the study intervention at a central location by a single research person, who was not involved in any other part of the study |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Participants in control group had low‐intensity sensory stimulation, with no active treatment for the same duration. This was done to ensure participant blinding |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | Allocation was given to the assistant in a sealed opaque envelope to be handed over to the nurse operating the RF machine All other operating room personnel, including the physician performing the intervention and the participant, were blinded to randomisation and treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Assessor (blinded to randomisation code) met with all participants in recovery |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Dropouts: 2 of 31 participants |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | As the study was analysed using the intention‐to‐treat principle, missing outcomes were imputed using 'multiple imputation' |
| Selective reporting (reporting bias) | Low risk | Protocol showed same outcomes |
| Baseline characteristics similar? | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single intervention |
| Timing of outcome assessments similar? | Low risk | 1 month and 3 months of follow‐up |
Simopoulos 2008.
| Methods | RCT | |
| Participants | Beth Israel Deaconess Medical Center, Arnold Pain Management Center, Brookline, Massachusetts, USA (N = 76) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group 1
Experiment group 2
|
|
| Outcomes | No significant difference in VAS change between the 2 groups
|
|
| Notes | Dropouts: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly assigned to 1 of 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Participants not blinded; treatment 60 or 120 seconds |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Treatment 60 or 120 seconds |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participant reported outcome measures |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Dropouts: not reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Remained unclear from text |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | No meaningful differences at baseline |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | Follow‐up until 1 year after procedure |
Tekin 2007.
| Methods | RCT | |
| Participants | Celal Bayar University, Manisa, Turkey (N = 60) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group 1, CRF
Experiment group 2, PRF
Control group
|
|
| Outcomes |
|
|
| Notes | Dropouts: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subsequent randomisation into 3 groups performed by random number generation, with balance after every 8 participants |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Participants not able to state which treatment they were receiving |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | Providers not blinded |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Participant reported outcomes measured and the latest outcome data evaluated by an independent observer |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | It is expected to be unlikely that all participants completed follow‐up |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Participants analysed by group; no dropouts |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | No meaningful differences at baseline |
| Co‐interventions avoided or similar? | High risk | Number of participants using analgesics higher in control group than in PRF group |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | Follow‐up immediately after procedure, at 6 months and at 1 year |
Van Kleef 1999.
| Methods | RCT | |
| Participants | Pain Management and Research Centre, University Hospital Maastricht, The Netherlands (N = 31) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes | Significant differences in mean VAS score and ODI at 8 weeks
|
|
| Notes | Dropouts: 1 (3%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with the help of a computer programme in blocks of 2 |
| Allocation concealment (selection bias) | Unclear risk | Remained unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | Participants not aware of the type of treatment received |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | Treating physician left the operating room after inserting electrodes and injecting local anaesthetic solution |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Participant reported outcome measures |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Only 1 randomly assigned participant was excluded from analyses |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Remained unclear from text |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | Table 1. Groups comparable on relevant demographic and clinical variables |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Unclear risk | Remained unclear from text |
Van Wijk 2005.
| Methods | RCT | |
| Participants | University Medical Center, Utrecht; Rijnstate Hospital, Arnhem, Juliana Hospital, Apeldoorn, Twenteborgh Hospital, Almelo; The Netherlands (N = 81) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experiment group
Control group
|
|
| Outcomes | No significant differences in combined outcome measures, and between changes in VAS back, changes in daily physical activities and use of analgesics. Significant difference in GPE favoured RF treatment 3 months after treatment Pain intensity: change in VAS back score at 3 months: ‐2.1 (E), ‐1.6 (C) |
|
| Notes | No dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed independently and in a separate setting |
| Allocation concealment (selection bias) | Low risk | Envelope drawn at random from appropriate set of envelopes and opened by an independent physician, who instructed RF generator setup by a technician |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | RF generator display turned away from operating table, participant and treating physician could not be informed on the nature of the procedure |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | RF generator display turned away from operating table, participant and treating physician could not be informed on the nature of the procedure |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | Participant reported outcome measures |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | After randomisation and before 3‐month follow‐up, no dropouts occurred |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | No description of ITT analysis but no indication of cross‐overs |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Baseline characteristics similar? | Low risk | Participant characteristics and baseline values showed adequate matching between groups |
| Co‐interventions avoided or similar? | Unclear risk | Remained unclear from text |
| Compliance acceptable? | Low risk | Irrelevant: single‐session intervention |
| Timing of outcome assessments similar? | Low risk | Outcomes measured at 0, 3, 6, 9 and 12 months |
Abbrevations: AIDS= acquired immunodeficiency syndrome; ASA= American Society of Anesthesiologists; C= control; CRF= continuous radiofrequency; CT= computed tomography; E= experiment; GPE= global perceived effect; GSER= global subjective efficacy rating; ITT= intention to treat; LFJ= lumbar facet joints; LBMRFD= lumbar medial branch radiofrequency denervation; MRI= magnetic resonance imaging; NRS= numerical rating scale; ODI= Oswestry disability index; PRF= pulsed radiofrequency; RCT= randomised controlled trial; RF= radiofrequency; RFA= radiofrequency ablation; RMQ= Roland Morris Questionnaire; SF‐36= Short Form 36; VAS= visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Buijs 2004 | Compares temperature‐controlled vs voltage‐controlled mode ‐ not effectiveness of RF denervation |
| Cohen 2010 | Compares diagnostic block treatment paradigms ‐ not effectiveness of RF denervation |
| Dobrogowski 2005 | No control group for RF neurotomy procedure |
| Fukui 2012 | No RCT |
| Gautam 2011 | RF used as additional therapy |
| Gross 2010 | No full‐text article of the study |
| Proschek 2010 | No RCT, no control group |
| Reverberi 2005 | No RCT |
Abbrevations: RCT= randomised controlled trial; RF= radiofrequency;
Characteristics of studies awaiting assessment [ordered by study ID]
Hashemi 2014.
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Group 1: pulsed RF using a 22 G cannula SMK C10 with method described by van Kleef et al applied for a duration of 120 s with 45 V with silent time 480 ms Group 2: steroid injections (1 mL (40 mg) triamcinolone) and 0.5 mL bupivacaine 0.5% |
| Outcomes | Primary outcome: decrease in NRS score > 50% and pain relief defined as up to 6 months Secondary outcome: improvement in functional status (ODI) |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Albareeq 2015.
| Trial name or title | Radiofrequency in sacroiliac arthropathy; bipolar RF 6 points vs monopolar RF at 6 and 3 points (RFSIBIMONO6) |
| Methods | Prospective, single‐centre, double‐blind, controlled randomised trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Visual analogue pain scale after 2 weeks, 1 month, 3 months and 6 months |
| Starting date | September 2014 |
| Contact information | ClinicalTrials.gov identifier: NCT02382289 |
| Notes |
Dolin 2010.
| Trial name or title | Double‐blind, randomised, controlled, cross‐over trial of RF annuloplasty for treatment of low back pain |
| Methods | Double‐blind, randomised, controlled, cross‐over trial |
| Participants |
Inclusion criteria
|
| Interventions | Experimental group: RF annuloplasty Placebo group: same protocol but no lesioning performed ‐ RF generator on test mode |
| Outcomes | Not provided at time of registration |
| Starting date | March 2002 |
| Contact information | Dr Simon Dolin, Pain Service, St Richards Hospital, Spitalfield Lane, Chichester, PO19 4SE, United Kingdom |
| Notes |
Maas 2012.
| Trial name or title | Minimal interventional procedures for chronic mechanical low back pain patients |
| Methods | Three randomised, controlled trials with an economic evaluation |
| Participants |
Inclusion criteria
|
| Interventions | Experimental group: RF therapy and 3‐month physiotherapy programme Control group: 3‐month physiotherapy programme |
| Outcomes | Pain intensity, global perceived effect, functional status, general health, participant satisfaction, pain experience, costs |
| Starting date | Start inclusion January 2013 |
| Contact information | Dutch Trial Register number: NTR3531 |
| Notes |
Meckhail 2013.
| Trial name or title | Comparison of decreased pain in transforaminal epidural steroid injections and pulsed radiofrequency in patients with low back pain |
| Methods | Randomised controlled trial |
| Participants | Main inclusion criteria: persistent low back pain with or without pain radiating to upper leg; age > 18 years; ASA class I to II; Lasek test ≥ 50 degrees; confirmed involvement of nerve roots; vertebral disc protrusion based on clinical examination, CT scan and MRI findings; symptoms of chronic low back pain > 6 months; absence of neurological defects; absence of epidural injection; absence of radicular syndrome; no response to traditional treatments; positive diagnostic block; hyperextension pain; no history of lumbar surgery; contraindication for lumbar surgery; signing consent to participate in the study Main exclusion criteria: patients previously treated with radiofrequency; coagulation disorders; contrast sensitivity radiopaque or local anaesthetic solution; malignancy; psychiatric problems and poor patient co‐operation; speech problems; pregnancy; surgery indication; local skin infection at operative site; spinal deformity; spinal stenosis; discogenic axial pain; degenerative disc herniation; epidural injection of steroids in past 6 months; history of opioid abuse; use of long‐acting opioids; radicular pain over a year ago; patients with history of sensitivity to corticosteroids or contrast material; inflammatory spondylopathy; vertebral fracture, tumour or infection of the spine; no signing of consent to participate in the study |
| Interventions | Experimental group: Participants received pulsed radiofrequency in nerve segment as confirmed by positive diagnostic block Control group: Participants received epidural steroid injection method transforaminal interlaminar under fluoroscopic guidance close to site of pathology |
| Outcomes | Visual analogue scale, Oswestry Disability Index score, success rate, participant request for analgesia |
| Starting date | August 2013 |
| Contact information | Iranian Registry of Clinical Trials number: IRCT201411037984N22 |
| Notes |
Mekhail 2015.
| Trial name or title | Effect of temperature used in thermal radiofrequency ablation on outcomes of lumbar facet medial branch denervation procedures: a randomised, double‐blinded trial |
| Methods | Randomised, double‐blinded trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental group: radiofrequency ablation at 90°C Control group: radiofrequency ablation at 80°C |
| Outcomes | Change in pain relief after 12 months, number of repeats of procedure over 12 months |
| Starting date | May 2014 |
| Contact information | ClinicalTrials.gov Identifier: NCT02148003 |
| Notes |
Norwegian University 2012.
| Trial name or title | The effect of RF treatment on patients with facet joint pain in cervical‐ and lumbar‐columna |
| Methods | Double‐blind, randomised, controlled trial |
| Participants | Patients 20 to 75 years of age with 1‐sided neck pain of at least 1 year's duration |
| Interventions | Experimental intervention: RF neurotomy of medial branch at 80°C needle temperature for 70 seconds, after diagnostic blocks Sham comparator: RF neurotomy of medial branch at 37°C needle temperature for 70 seconds, after diagnostic blocks |
| Outcomes | Reduction in self reported pain intensity |
| Starting date | August 2004 |
| Contact information | ClinicalTrials.gov identifier: NCT00476684 |
| Notes |
Sarwar 2012.
| Trial name or title | A randomised, placebo‐controlled trial of transdiscal RF annuloplasty for treatment of discogenic low‐back pain |
| Methods | Randomised placebo‐controlled trial |
| Participants | 18 + years of age, history of chronic low back pain unresponsive to non‐operative care (including physical therapy and anti‐inflammatory medication) for > 6 months, ≥ 5 on VAS, no surgical interventions within past 3 months, back pain greater than leg pain, which is commonly exacerbated by sitting; pain reproduction present on provocative discography in degenerated disc but not in control discs; disc height ≥ 50% of adjacent control disc; evidence of single‐level degenerative disc disease or 2‐level disease without evidence of additional degenerative changes in other disc spaces on MRI |
| Interventions | Active comparator: Two electrodes are placed on both sides of the posterior annulus fibrosus of the intervertebral disc under x‐ray guidance. RF current flows within the disc between the 2 electrodes, heating tissue in the disc to desired temperature Sham comparator: same procedures as in Active group except no RF current will be applied |
| Outcomes | Effectiveness of intradiscal RF annuloplasty at 1 year |
| Starting date | September 2007 |
| Contact information | ClinicalTrials.gov identifier: NCT00750191 |
| Notes |
SMART 2012.
| Trial name or title | Prospective, randomised, double‐blind, controlled investigation evaluating the intracept intraosseous nerve ablation system for reduction in pain in patients with chronic axial low back pain |
| Methods | Randomised, double‐blind, controlled trial |
| Participants | 25 to 70 years of age, inclusive chronic lower back pain for ≥ 6 months, failure to respond to ≥ 6 months of non‐operative conservative management, Oswestry Disability Index (ODI) at time of evaluation ≥ 30 points, baseline visual analog scale (VAS) score ≥ 4 cm on a 10‐cm scale, with the following test indicating that the vertebral body is the source of pain: MRI showing Type 1 or Type II Modic changes in at least 1 vertebral endplate, at ≥ 1 level from L3 to S1 |
| Interventions | Experimental intervention: percutaneous transpedicular RF ablation of an intraosseous nerve within the lumbar vertebral body to treat chronic axial low back pain. Sham comparator: percutaneous transpedicular access to lumbar vertebra, no RF ablation delivered |
| Outcomes | Oswestry Disability Index at 3 months, participant success at 3 months and Oswestry Disability Index at 6 months |
| Starting date | October 2011 |
| Contact information | ClinicalTrials.gov identifier: NCT01446419 |
| Notes |
Abbrevations: CT= computed tomography; MRI= magnetic resonance imaging; NRS= numerical rating scale; ODI= Oswestry disability index; RF= radiofrequency; RFA= radiofrequency ablation
Differences between protocol and review
In this review, we did not consider “ability to work” ‐ an imperative criterion ‐ as it is not always relevant among patients with CLBP. Only one study assessed treatment‐related costs (Van Wijk 2005).
A description of the plan to analyse types of participants separately was added to the section Subgroup analysis and investigation of heterogeneity.
A description of the plan to perform sensitivity analyses was added to the section Sensitivity analysis.
Contributions of authors
L Niemisto, J Jousimaa, H Hurri and A Malmivaara were the authors of the original review: Radiofrequency denervation for neck and back pain, published in 2003. All participated in interpretation and writing of this update.
ET Maas participated in collection, extraction and analyses of the data; assessment of methodological quality, discussion of core ideas and writing of the paper.
RWJG Ostelo participated in extraction and analyses of the data; assessment of methodological quality; discussion of core ideas and interpretation and writing of the paper.
MW van Tulder participated in extraction of data, discussed core ideas, assessed methodological quality and participated in interpretation and writing of the paper.
Sources of support
Internal sources
Dextra Medical Center, Finland.
External sources
Finnish Office for Health Technology Assessment / Finohta, National Institute for Health and Welfare / THL, Finland.
Declarations of interest
ET Maas: none.
RWJG Ostelo: none.
L Niemisto: none.
J Jousimaa: none.
H Hurri: working for and leading the Rehabilitation Unit and Hospital of Orton, where radiofrequency is applied as well.
A Malmivaara: working as a scientific expert in the Research Unit of Orton.
MW van Tulder: none.
New
References
References to studies included in this review
Barendse 2001 {published data only}
- Barendse GA, Berg SG, Kessels AH, Weber WE, Kleef M. Randomized controlled trial of percutaneous intradiscal radiofrequency thermocoagulation for chronic discogenic back pain: lack of effect from a 90‐second 70 C lesion. Spine 2001;26(3):287‐92. [DOI] [PubMed] [Google Scholar]
Civelek 2012 {published data only}
- Civelek E, Cansever T, Kabatas S, Kircelli A, Yilmaz C, Musluman M, et al. Comparison of effectiveness of facet joint injection and radiofrequency denervation in chronic low back pain. Turkish Neurosurgery 2012;22(2):200‐6. [DOI] [PubMed] [Google Scholar]
Cohen 2008 {published data only}
- Cohen S, Hurley R, Buckenmaier C, Kurihara C, Morlando B, Dragovich A. Randomized placebo‐controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology 2008;109(2):279‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duger 2012 {published data only}
- Duger C, Ozdemir Kol L, Kaygusuz K, Gursoy S, Mimaroglu C. Effects of facet joint nerve block addition to radiofrequency in the treatment of low back pain. Journal of Society for Development of New Net Environment in B&H 2012;6(6):2052‐6. [Google Scholar]
Ercelen 2003 {published data only}
- Ercelen Ö, Bulutcu E, Öktenoglu T, Sasani M, Bozkus H, Saryoglu A, et al. Radiofrequency lesioning using two different time modalities for the treatment of lumbar discogenic pain: a randomized trial. Spine 2003;28:1922‐7. [DOI] [PubMed] [Google Scholar]
Gallagher 1994 {published data only}
- Gallagher J, Petriccione di Vadi PL, Wedley JR. Radiofrequency facet joint denervation in the treatment of low back pain: a prospective controlled double‐blind study to assess its efficacy. The Pain Clinic 1994;7:193‐8. [Google Scholar]
Geurts 2003 {published data only}
- Geurts J, Wijk R, Wynne H, Hammink E, Buskens E, Lousberg R, et al. Radiofrequency lesioning of dorsal root ganglia for chronic lumbosacral radicular pain:a randomized, double‐blind, controlled trial. Lancet 2003;361:21‐6. [DOI] [PubMed] [Google Scholar]
Kapural 2013 {published data only}
- Kapural L, Vrooman B, Sarwar S, Krizanac‐Bengez L, Rauck R, Gilmore C, et al. A randomized, placebo‐controlled trial of transdiscal radiofrequency, biacuplasty for treatment of discogenic lower back pain. Pain Medicine 2013;14(3):362‐73. [DOI] [PubMed] [Google Scholar]
Kroll 2008 {published data only}
- Kroll H, Kim D, Danic M, Sankey S, Gariwala M, Brown M. A randomized, double‐blind, prospective study comparing the efficacy of continuous versus pulsed radiofrequency in the treatment of lumbar facet syndrome. Journal of Clinical Anesthesia 2008;20(7):534‐7. [DOI] [PubMed] [Google Scholar]
Kvarstein 2009 {published data only}
- Kvarstein G, Måwe L, Indahl A, Hol PK, Tennøe B, Digernes R, et al. A randomized double‐blind controlled trial of intra‐annular radiofrequency thermal disc therapy – a 12‐month follow‐up. Pain 2009;145(3):279‐86. [DOI] [PubMed] [Google Scholar]
Lakemeier 2013 {published data only}
- Lakemeier S, Lind M, Schultz W, Fuchs‐Winkelmann S, Timmesfeld N, Foelsch C, et al. A comparison of intraarticular lumbar facet joint steroid injections and lumbar facet joint radiofrequency denervation in the treatment of low back pain: a randomized, controlled, double‐blind trial. Anesthesia & Analgesia 2013;117(1):228‐35. [DOI] [PubMed] [Google Scholar]
Leclaire 2001 {published data only}
- Leclaire R, Fortin L, Lambert R, Bergeron YM, Rossignol M. Radiofrequency facet joint denervation in the treatment of low back pain: a placebo‐controlled clinical trial to assess efficacy. Spine 2001;26(13):1411‐6. [DOI] [PubMed] [Google Scholar]
Lin Mu‐Lien 2010 {published data only (unpublished sought but not used)}
- Mu‐Lien L, Mu‐Hung L, Jun‐Jeng F, Wei‐Tso L, Chii‐Wann L, Po‐Quang C. A comparison between pulsed radiofrequency and electro‐acupuncture for relieving pain in patients with chronic low back pain. Acupuncture & Electro‐therapeutics Research 2010;35(3‐4):133‐46. [DOI] [PubMed] [Google Scholar]
Moon 2013 {published data only}
- Moon JY, Bok Lee P, Chul Kim Y, Pyo Choi S, Seog Sim W. An alternative distal approach for the lumbar medial branch radiofrequency denervation: a prospective randomized comparative study. Anesthesia & Analgesia 2013;116(5):1133‐40. [DOI] [PubMed] [Google Scholar]
Nath 2008 {published data only}
- Nath S, Nath CA, Pettersson K. Percutaneous lumbar zygapophysial (facet) joint neurotomy using radiofrequency current, in the management of chronic low back pain. A randomized double‐blind trial. Spine 2008;33(12):1291‐7. [DOI] [PubMed] [Google Scholar]
Oh 2004 {published data only}
- Oh WS, Shim JC. A randomized controlled trial of radiofrequency denervation of the ramus communicans nerve for chronic discogenic low back pain. Clinical Journal of Pain 2004;20(1):55‐60. [DOI] [PubMed] [Google Scholar]
Patel 2012 {published data only}
- Patel N, Gross A, Brown L, Gekht G. A randomized, placebo‐controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Medicine 2012;13(3):383‐98. [DOI] [PubMed] [Google Scholar]
Sanders 1999 {published data only}
- Sanders M, Zuurmond WWA. Percutaneous intra‐articular lumbar facet joint denervation in the treatment of low back pain: a comparison with percutaneous extra‐articular lumbar facet denervation. The Pain Clinic 1999;11(4):329‐35. [Google Scholar]
Shanthanna 2014 {published data only}
- Shanthanna H, Chan P, McChesney J, Thabane L, Paul J. Pulsed radiofrequency treatment of the lumbar dorsal root ganglion in patients with chronic lumbar radicular pain: a randomized, placebo‐controlled pilot study. Journal of Pain Research 2014;7:47‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simopoulos 2008 {published data only}
- Simopoulos T, Kraemer J, Nagda J, Aner M, Bajwa Z. Response to pulsed and continuous radiofrequency lesioning of the dorsal root ganglion and segmental nerves in patients with chronic lumbar radicular pain. Pain Physician 2008;11(2):137‐44. [PubMed] [Google Scholar]
Tekin 2007 {published data only}
- Tekin I, Mirzai H, Ok G, Erbuyun K, Vatansever D. A comparison of conventional and pulsed radiofrequency denervation in the treatment of chronic facet joint pain. Clinical Journal of Pain 2007;23(6):524‐9. [DOI] [PubMed] [Google Scholar]
Van Kleef 1999 {published data only}
- Kleef M, Barendse GA, Kessels A, et al. Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine 1999;24(18):1937‐42. [DOI] [PubMed] [Google Scholar]
Van Wijk 2005 {published data only}
- Wijk R, Geurts J, Wynne H, Hammink E, Buskens E, Lousberg R, et al. Radiofrequency denervation of lumbar facet joints in the treatment of chronic low back pain. A randomized, double‐blind, sham lesion‐controlled trial. Clinical Journal of Pain 2005;21(4):335‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Buijs 2004 {published data only}
- Buijs E, Wijk R, Geurts J, Weeseman R, Stolker R, Groen G. Radiofrequency lumbar facet denervation: a comparative study of the reproducibility of lesion size after 2 current radiofrequency techniques. Regional Anesthesia and Pain Medicine 2004;29(5):400‐7. [DOI] [PubMed] [Google Scholar]
Cohen 2010 {published data only}
- Cohen SP, Williams KA, Kurihara C, Nguyen C, Shields C, Kim P, et al. Multicenter, randomized, comparative cost‐effectiveness study comparing 0, 1, and 2 diagnostic medial branch (facet joint nerve) block treatment paradigms before lumbar facet radiofrequency denervation. Anesthesiology 2010;113(2):295‐305. [DOI] [PubMed] [Google Scholar]
Dobrogowski 2005 {published data only}
- Dobrogowski J, Wrzosek A, Wordliczek J. Radiofrequency denervation with or without addition of pentoxifylline or methylprednisolone for chronic lumbar zygapophysial joint pain. Pharmacological Reports 2005;57(4):475‐80. [PubMed] [Google Scholar]
Fukui 2012 {published data only}
- Fukui S, Nitta K, Iwashita N, Tomie H, Nosaka S, Rohof O. Results of intradiscal pulsed radiofrequency for lumbar discogenic pain: comparison with intradiscal electrothermal therapy. Korea Journal of Pain 2012;25(3):155‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gautam 2011 {published data only}
- Gautam S, Rastogi V, Jain A, Singh AP. Comparative evaluation of oxygen‐ozone therapy and combined use of oxygen‐ozone therapy with percutaneous intradiscal radiofrequency thermocoagulation for the treatment of lumbar disc herniation. Pain Practice 2011;11(2):160‐6. [DOI] [PubMed] [Google Scholar]
Gross 2010 {published data only}
- Gross A, Patel N. A randomized, placebo controlled study to assess the efficacy of lateral branch denervation for chronic sacroiliac joint pain. Pain Medicine 2010 Conference Publication 2010;11(10):1579. [DOI] [PubMed] [Google Scholar]
Proschek 2010 {published data only}
- Proschek D, Kafchitsas K, Rauschmann M, Kurth A, Vogl T, Geiger F. Reduction of radiation dose during radiofrequency denervation of the lumbar facet joints using the new targeting system SabreSource: a prospective study in 20 patients. Archives of Orthopaedic and Trauma Surgery 2010;130(9):1103‐10. [DOI] [PubMed] [Google Scholar]
Reverberi 2005 {published data only}
- Reverberi C, Bottoli G, Pennini M, Gabba E. Disc coablation and epidural injection of steroids: a comparison of strategies in the treatment of mechanical spinal discogenic pain. Acta Neurochirurgica 2005;92:127‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Hashemi 2014 {published data only}
- Hashemi M, Heshemian M, Mohajerani SA, Sharifi G. Effect of pulsed radiofrequency in treatment of facet‐joint origin back pain in patients with degenerative spondylolisthesis. European Spine Journal 2014;23:1927‐32. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Albareeq 2015 {unpublished data only}
- Radiofrequency in sacroiliac arthropathy; bipolar RF 6 points vs monopolar RF at 6 and 3 points (RFSIBIMONO6). Ongoing study September 2014.
Dolin 2010 {unpublished data only}
- Double‐blind, randomised, controlled, cross‐over trial of RF annuloplasty for treatment of low back pain. Ongoing study March 2002.
Maas 2012 {published data only}
- Maas ET, Juch JNS, Groeneweg JG, Ostelo RWJG, Koes BW, Verhagen AP, et al. Cost‐effectiveness of minimal interventional procedures for chronic mechanical low back pain: design of four randomised controlled trials with an economic evaluation. BMC Musculoskeletal Disorders 2012;13:260‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meckhail 2013 {unpublished data only}
- Comparison of decreased pain in transforaminal epidural steroid injections and pulsed radiofrequency in patients with low back pain. Ongoing study August 2013.
Mekhail 2015 {unpublished data only}
- Effect of temperature used in thermal radiofrequency ablation on outcomes of lumbar facet medial branch denervation procedures: a randomised, double‐blinded trial. Ongoing study May 2014.
Norwegian University 2012 {unpublished data only}
- The effect of RF treatment on patients with facet joint pain in cervical‐ and lumbar‐columna. Ongoing study August 2004.
Sarwar 2012 {unpublished data only}
- A randomised, placebo‐controlled trial of transdiscal RF annuloplasty for treatment of discogenic low‐back pain. Ongoing study September 2007.
SMART 2012 {unpublished data only}
- Prospective, randomised, double‐blind, controlled investigation evaluating the intracept intraosseous nerve ablation system for reduction in pain in patients with chronic axial low back pain. Ongoing study October 2011.
Additional references
Bogduk 2005
- Bogduk N. The zygapophysial joints. Clinical Anatomy of the Lumbar Spine and Sacrum. 3rd Edition. New York: Churchill Livingstone, 2005. [Google Scholar]
Boswell 2003
- Boswell MW, Singh V, Staat PS, Hirsch JA. Accuracy of precision diagnostic blocks in the diagnosis of chronic spinal pain of facet or zygapophysial joint origin: a systematic review. Pain Physician 2003;6(4):449‐56. [PubMed] [Google Scholar]
Chou 2007
- Chou R, Huffman LH. Guideline for the evaluation and management of low back pain. Amerian Pain Society 2007.
Chou 2009
- Chou R, Loeser JD, Owens DK, Rosenquist RW, Atlas SJ, Baisden J, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain. Spine 2009;34(10):1066‐77. [DOI] [PubMed] [Google Scholar]
Cohen 2007
- Cohen S. Epidemics, evolution and sacroiliac joint pain. Regional Anesthesia and Pain Medicine 2007;32(1):3‐6. [DOI] [PubMed] [Google Scholar]
Cosman 2005
- Cosman EJ, Cosman ES. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Medicine 2005;6(6):405‐24. [DOI] [PubMed] [Google Scholar]
Dasselaar 1994
- Dasselaar N, Boersma F, Lange J. Results of the Dutch enquiry on invasive anaesthesiological pain control. Pain Clinic 1994;7(2):145‐54. [Google Scholar]
Dreyfuss 1997
- Dreyfuss P, Schwarzer A, Lau P, Bogduk N. Specificity of lumbar medial branch and L5 dorsal ramus blocks. A computed tomography study. Spine 1997;22:895‐902. [DOI] [PubMed] [Google Scholar]
Dreyfuss 2000
- Dreyfuss P, Halbrook B, Pauza K, Joshi A, McLarty J, Bogduk N. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine 2000;25(10):1270‐7. [DOI] [PubMed] [Google Scholar]
Ferrante 2001
- Ferrante FM, King LF, Roche EA, Kim PS, Aranda M, Delaney LR, et al. Radiofrequency sacroiliac joint denervation for sacroiliac syndrome. Regional Anesthesia and Pain Medicine 2001;26(2):137‐42. [DOI] [PubMed] [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder MW, the Cochrane Back Review Group Editorial Board. 2009 Updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings table. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Henschke 2010
- Henschke N, Kuijpers T, Rubinstein SM, Middelkoop M, Ostelo RWJG, Verhagen A, et al. Injection therapy and denervation procedures for chronic low‐back pain: a systematic review. European Spine Journal 2010;19:1425‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration 2011. www.cochrane‐handbook.org.
Kline 1996
- Kline MT. Radiofrequency techniques in clinical practice. In: Waldman SD, Winnie AP, eds. Interventional Pain Management. Philadelphia, PA: Saunders, 1996. [Google Scholar]
Koes 2006
- Koes BW, Tulder MW, Thomas S. Diagnosis and treatment of low back pain. British Medical Journal 2006;332(7555):1430‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krismer 2007
- Krismer M, Tulder MW. Low back pain (non‐specific). Best Practice and Research Clinical Rheumatology 2007;21(1):77‐91. [DOI] [PubMed] [Google Scholar]
Lambeek 2010
- Lambeek LC, Mechelen W, Knol DL, Loisel P, Anema JR. Randomised controlled trial of integrated care to reduce disability from chronic low back pain in working and private life. British Medical Journal 2010;340:c 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laslett 2003
- Laslett M, Young SB, Aprill CN, McDonald B. Diagnosing painful sacroiliacal joints: a validity study of a McKenzie evaluation and sacroiliac provocation tests. The Australian Journal of Physiotherapy 2003;49(2):89‐97. [DOI] [PubMed] [Google Scholar]
Malmivaara 2006
- Malmivaara A, Koes BW, Bouter LM, Tulder MW. Applicability and clinical relevance of results in randomized controlled trials. Spine 2006;31(13):1405‐9. [DOI] [PubMed] [Google Scholar]
Manchikanti 2000a
- Manchikanti L, Fellows B, Baha A. The inability of the clinical picture to characterize pain from facet joints. Pain Physician 2000;3(2):158‐66. [PubMed] [Google Scholar]
Manchikanti 2000b
- Manchikanti L. The role of radiofrequency in the management of complex regional pain syndrome. Current Review of Pain 2000;4:437‐44. [DOI] [PubMed] [Google Scholar]
Poetscher 2014
- Poetscher AW, Gentil AF, Lenza M, Ferretti M. Radiofrequency denervation for facet joint low back pain. Spine 2014;14:E842‐9. [DOI] [PubMed] [Google Scholar]
Rathmell 2001
- Rathmell JP, Tarver JM, Memon Z. Radiofrequency sacroiliac joint denervation: the devil is in the details. Regional Anesthesia and Pain Medicine 2001;26(6):592‐3. [DOI] [PubMed] [Google Scholar]
Schwarzer 1994
- Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The false‐positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain 1994;58:195‐200. [DOI] [PubMed] [Google Scholar]
Schwarzer 1995a
- Schwarzer AC, Wang SC, O'Driscoll D, Harrington T, Bogduk N, Laurent R. The ability of computed tomography to identify a painful zygapophyseal joint in patients with chronic low back pain. Spine 1995;20(8):907‐12. [DOI] [PubMed] [Google Scholar]
Schwarzer 1995b
- Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine 1995;20(17):1878‐83. [DOI] [PubMed] [Google Scholar]
Shealy 1976
- Shealy CN. Facet denervation in the management of back and sciatic pain. Clinical Orthopaedics and Related Research 1976;115:157‐64. [PubMed] [Google Scholar]
Sluijter 1998
- Sluijter M, Cosman E, Rittman I, Kleef M. The effects of pulsed radiofrequency field applied to the dorsal root ganglion ‐ a preliminary report. Pain Clinic 1998;11:109‐17. [Google Scholar]
Staal 2008
- Staal JB, Bie R, De Vet, HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low‐back pain. Cochrane Database Systematic Reviews 2008;34(2):49‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waddel 2005
- Waddell G. Subgroups within 'nonspecific' low back pain. The Journal of Rheumatology 2005;32(3):395‐6. [PubMed] [Google Scholar]
References to other published versions of this review
Niemisto 2003
- Niemisto L, Kalso EA, Malmivaara A, Seitsalo S, Hurri H. Radiofrequency denervation for neck and back pain. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004058] [DOI] [PubMed] [Google Scholar]
Niemisto 2003a
- Niemisto L, Kalso E, Malmivaara A, Seitsalo S, Hurri H. Radiofrequency denervation for neck and back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine 2003;28(16):1877‐88. [DOI] [PubMed] [Google Scholar]
Niemisto 2010b
- Niemisto L, Jukkapekka J, Hurri H, Kalso EA, Malmivaara A. Radiofrequency denervation for chronic low‐back pain. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD008572] [DOI] [Google Scholar]


