Abstract
Background
Post‐traumatic stress disorder (PTSD) is a debilitating mental health disorder that may develop after exposure to traumatic events. Substance use disorder (SUD) is a behavioural disorder in which the use of one or more substances is associated with heightened levels of distress, clinically significant impairment of functioning, or both. PTSD and SUD frequently occur together. The comorbidity is widely recognised as being difficult to treat and is associated with poorer treatment completion and poorer outcomes than for either condition alone. Several psychological therapies have been developed to treat the comorbidity, however there is no consensus about which therapies are most effective.
Objectives
To determine the efficacy of psychological therapies aimed at treating traumatic stress symptoms, substance misuse symptoms, or both in people with comorbid PTSD and SUD in comparison with control conditions (usual care, waiting‐list conditions, and no treatment) and other psychological therapies.
Search methods
We searched the Cochrane Depression, Anxiety and Neurosis Group’s Specialised Register (CCDANCTR) all years to 11 March 2015. This register contains relevant randomised controlled trials from the Cochrane Library (all years), MEDLINE (1950 to date), EMBASE (1974 to date), and PsycINFO (1967 to date). We also searched the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov, contacted experts, searched bibliographies of included studies, and performed citation searches of identified articles.
Selection criteria
Randomised controlled trials of individual or group psychological therapies delivered to individuals with PTSD and comorbid substance use, compared with waiting‐list conditions, usual care, or minimal intervention or to other psychological therapies.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 14 studies with 1506 participants, of which 13 studies were included in the quantitative synthesis. Most studies involved adult populations. Studies were conducted in a variety of settings. We performed four comparisons investigating the effects of psychological therapies with a trauma‐focused component and non‐trauma‐focused interventions against treatment as usual/minimal intervention and other active psychological therapies. Comparisons were stratified for individual‐ or group‐based therapies. All active interventions were based on cognitive behavioural therapy. Our main findings were as follows.
Individual‐based psychological therapies with a trauma‐focused component plus adjunctive SUD intervention was more effective than treatment as usual (TAU)/minimal intervention for PTSD severity post‐treatment (standardised mean difference (SMD) ‐0.41; 95% confidence interval (CI) ‐0.72 to ‐0.10; 4 studies; n = 405; very low‐quality evidence) and at 3 to 4 and 5 to 7 months' follow‐up. There was no evidence of an effect for level of drug/alcohol use post‐treatment (SMD ‐0.13; 95% CI ‐0.41 to 0.15; 3 studies; n = 388; very low‐quality evidence), but there was a small effect in favour of individual psychological therapy at 5 to 7 months (SMD ‐0.28; 95% CI ‐0.48 to ‐0.07; 3 studies; n = 388) when compared against TAU. Fewer participants completed trauma‐focused therapy than TAU (risk ratio (RR) 0.78; 95% CI 0.64 to 0.96; 3 studies; n = 316; low‐quality evidence).
Individual‐based psychological therapy with a trauma‐focused component did not perform better than psychological therapy for SUD only for PTSD severity (mean difference (MD) ‐3.91; 95% CI ‐19.16 to 11.34; 1 study; n = 46; low‐quality evidence) or drug/alcohol use (MD ‐1.27; 95% CI ‐5.76 to 3.22; 1 study; n = 46; low‐quality evidence). Findings were based on one small study. No effects were observed for rates of therapy completion (RR 1.00; 95% CI 0.74 to 1.36; 1 study; n = 62; low‐quality evidence).
Non‐trauma‐focused psychological therapies did not perform better than TAU/minimal intervention for PTSD severity when delivered on an individual (SMD ‐0.22; 95% CI ‐0.83 to 0.39; 1 study; n = 44; low‐quality evidence) or group basis (SMD ‐0.02; 95% CI ‐0.19 to 0.16; 4 studies; n = 513; low‐quality evidence). There were no data on the effects on drug/alcohol use for individual therapy. There was no evidence of an effect on the level of drug/alcohol use for group‐based therapy (SMD ‐0.03; 95% CI ‐0.37 to 0.31; 4 studies; n = 414; very low‐quality evidence). A post‐hoc analysis for full dose of a widely established group therapy called Seeking Safety showed reduced drug/alcohol use post‐treatment (SMD ‐0.67; 95% CI ‐1.14 to ‐0.19; 2 studies; n = 111), but not at subsequent follow‐ups. Data on the number of participants completing therapy were not for individual‐based therapy. No effects were observed for rates of therapy completion for group‐based therapy (RR 1.13; 95% CI 0.88 to 1.45; 2 studies; n = 217; low‐quality evidence).
Non‐trauma‐focused psychological therapy did not perform better than psychological therapy for SUD only for PTSD severity (SMD ‐0.26; 95% CI ‐1.29 to 0.77; 2 studies; n = 128; very low‐quality evidence) or drug/alcohol use (SMD 0.22; 95% CI ‐0.13 to 0.57; 2 studies; n = 128; low‐quality evidence). No effects were observed for rates of therapy completion (RR 0.91; 95% CI 0.68 to 1.20; 2 studies; n = 128; very low‐quality evidence).
Several studies reported on adverse events. There were no differences between rates of such events in any comparison. We rated several studies as being at 'high' or 'unclear' risk of bias in multiple domains, including for detection bias and attrition bias.
Authors' conclusions
We assessed the evidence in this review as mostly low to very low quality. Evidence showed that individual trauma‐focused psychological therapy delivered alongside SUD therapy did better than TAU/minimal intervention in reducing PTSD severity post‐treatment and at long‐term follow‐up, but only reduced SUD at long‐term follow‐up. All effects were small, and follow‐up periods were generally quite short. There was evidence that fewer participants receiving trauma‐focused therapy completed treatment. There was very little evidence to support use of non‐trauma‐focused individual‐ or group‐based integrated therapies. Individuals with more severe and complex presentations (e.g. serious mental illness, individuals with cognitive impairment, and suicidal individuals) were excluded from most studies in this review, and so the findings from this review are not generalisable to such individuals. Some studies suffered from significant methodological problems and some were underpowered, limiting the conclusions that can be drawn. Further research is needed in this area.
Plain language summary
Psychological therapies for post‐traumatic stress disorder and substance use disorder
Who may be interested in this review?
• Individuals with post‐traumatic stress disorder (PTSD) and substance use disorder (SUD) and their families and friends.
• Healthcare providers for individuals with PTSD and SUD.
Why is this review important?
Many people have PTSD or SUD. Both conditions can impact everyday functioning. A number of different psychological therapies are successful at treating PTSD and SUD when they occur separately. However, PTSD and SUD often occur together, and it may be harder to treat individuals with both PTSD and SUD. A number of psychological therapies have been developed to treat people with both PTSD and SUD, but it is not clear how effective these therapies are.
What questions does this review aim to answer?
We sought to find out whether psychological therapies are effective in treating people with PTSD and SUD in comparison to control conditions and other psychological therapies.
Which studies were included in the review?
We searched scientific databases to find all published and unpublished studies of psychological therapies to treat people with PTSD and SUD up to 11 March 2015. We included 14 studies with 1506 participants.
What does the evidence from the review tell us?
The evidence showed that individual trauma‐focused psychological therapy delivered alongside SUD therapies was more effective in reducing PTSD compared to treatment as usual. This result was found both straight after treatment and at long‐term follow‐up. However, SUD severity only declined at long‐term follow‐up. More people dropped out of the trauma‐focused therapy compared with treatment as usual. Overall, the benefits of trauma‐focused treatment were small.
We found little evidence for the benefit of individual‐ or group‐based non‐trauma‐focused psychological therapies. For group‐based therapies, we found that substance use was reduced post‐treatment when participants were offered a full course of 25 sessions of the therapy 'Seeking Safety', which was delivered in a group setting. However, this positive effect did not continue at later follow‐up points. The level of drop‐out was high across all studies.
We graded the quality of evidence as low to very low. This review includes a small number of studies. Some included studies were poorly designed, and most studies were small. There was also considerable variation in the way that the therapies and control therapies were delivered. It is likely that participants in the included studies received a range of other stabilising interventions alongside trauma‐focused treatment, and we found no evidence to support the delivery of trauma‐focused therapies without SUD‐focused therapies. It is therefore possible that our findings will change as further evidence of higher quality is accumulated. Healthcare providers should exercise caution when considering whether to provide therapies described in this review.
Summary of findings
Background
Description of the condition
Post‐traumatic stress disorder (PTSD) is a relatively common and well‐recognised psychiatric disorder that occurs following a major traumatic event (NCCMH 2005). Characteristic symptoms include re‐experiencing phenomena such as nightmares and recurrent distressing thoughts of the event, avoidance and numbing of general responsiveness such as trying not to talk about or be reminded of the traumatic event, experiencing detachment and estrangement from other people, and hyperarousal symptoms including sleep disturbance, increased irritability, and hypervigilance (APA 2013).
Substance use disorder (SUD) is defined as a complex behavioural disorder characterised by preoccupation with obtaining alcohol or other drugs and a narrowing of behavioural repertoire towards excessive consumption and loss of control over consumption. It is usually also accompanied by the development of tolerance to the substances being consumed and withdrawal and impairment in social and occupational functioning (APA 2013). In diagnostic terms, SUD is characterised by maladaptive misuse of substances (such as alcohol, amphetamines, cannabis, cocaine, hallucinogens, opioids, inhalants, phencyclidine, sedatives, hypnotics, and anxiolytics), which leads to clinically significant impairment or distress (APA 2013). Impairment might include increased tolerance, excessive prolonged usage, recurrent failure to meet important responsibilities, recurrent use in situations when this is likely to be physically dangerous, inability to reduce or limit usage, and considerable time spent obtaining substances or recovering from their effects.
Comorbidity between PTSD and SUD is common (Chilcoat 2003; Ford 2007; Reynolds 2005; Schäfer 2007). Epidemiological studies show significantly increased rates of PTSD amongst individuals with SUD (for example Chilcoat 1998a; Chilcoat 1998b; Cottler 1992; Dragan 2007; Driessen 2008; Helzer 1987; Mills 2006; Najavits 1998; Reynolds 2005; Reynolds 2011; Schäfer 2010), with the prevalence of lifetime PTSD ranging from 26% to 52% and prevalence of current PTSD from 15% to 42% (Driessen 2008; Reynolds 2011; Schäfer 2007; Schäfer 2010). In the Australian National Survey of Health and Wellbeing, Mills 2006 found opiates, sedatives, and amphetamines to be the drug groups to have most frequent comorbid PTSD. SUDs have also been found to be prevalent amongst individuals with PTSD (Chilcoat 2003; Jacobsen 2001; Mills 2006). In PTSD‐diagnosed samples, prevalence rates of comorbid substance abuse range from 19% to 35% and comorbid alcohol abuse from 36% to 52% (Breslau 1992; Kessler 1995; Pietrzak 2011), with estimates being even higher in some clinical populations, such as military veterans (Jacobsen 2001; Keane 1990; Kulka 1990; McDevitt‐Murphy 2010; Ruzek 2003). In a large epidemiological study of over 34,000 individuals in a community sample in the USA, Pietrzak 2011 found that 6.4% of the sample met lifetime diagnosis for full PTSD. Comorbidity was common across the PTSD sample (some 2463 individuals), with 46.4% meeting diagnosis for any alcohol or drug use disorder, 41.8% meeting diagnosis for alcohol abuse or dependence, and 22.3% meeting diagnosis for drug use or dependence. In another large epidemiological study, Kulka 1990 found that 73% of Vietnam veterans who met the diagnosis for PTSD qualified for a lifetime diagnosis of alcohol abuse or dependence. The Australian National Survey of Health and Wellbeing found that 34.4% of those with PTSD also had an SUD, most commonly an alcohol use disorder (24.1%) (Mills 2006). A number of other subgroups have been found to have particularly high rates of comorbidity of PTSD and SUD. Such groups include women, adolescents, the homeless, prisoners, gays and lesbians, rescue workers, sex workers, and victims of domestic violence (Najavits 2006).
Individuals with both disorders have also been found to have a more severe clinical profile than those with either disorder alone, lower general functioning, poorer well‐being, and worse outcomes across a variety of measures (Schäfer 2007). Such individuals are also more likely to meet additional criteria for other psychiatric disorders, such as affective disorders, anxiety disorders, and personality disorders (Mills 2006; Schäfer 2007). For these reasons, randomised controlled trials evaluating PTSD treatment therapies routinely exclude individuals with substance misuse‐related problems (Ouimette 2003b). A number of authors have called for greater understanding of the impact of this comorbidity on treatment outcomes and research to determine which therapies are most effective in treating these comorbid conditions (for example Mills 2006; Ouimette 2003a; Ouimette 2003b; Ouimette 2003c).
Description of the intervention
There are a number of established and evidence‐based forms of psychological therapies for both PTSD and SUD (van Dam 2012). Several forms of trauma‐focused cognitive behavioural therapy (TF‐CBT) have been demonstrated to be effective in treating PTSD (Bisson 2013; Bradley 2005). Evidence‐based therapies include prolonged exposure, cognitive processing therapy, brief eclectic psychotherapy, and cognitive therapy. A common component of these trauma‐focused therapies is that they include some form of guided exposure to the traumatic memory. For example, prolonged exposure involves asking the patient to relive the trauma imaginally. This is often conducted by creating a detailed present‐tense account of exactly what happened during the traumatic event, making an audio recording of it, and asking the individual to listen to this over and over again. Other common components of TF‐CBT include in vivo exposure to feared situations and cognitive therapy focused on distorted thinking and beliefs. Variants of these TF‐CBT models have been developed for specific subgroups. For example, narrative exposure therapy was developed for use with refugees and those who have been exposed to war and violent conflict, and skills training in affective and interpersonal regulation and narrative story telling (STAIR/NST) was developed for individuals with a history of childhood trauma. Eye movement desensitisation and reprocessing (EMDR) has also been well established as an intervention for PTSD (Bisson 2013). EMDR involves the PTSD sufferer focusing on a traumatic image, thought, emotion, and a bodily sensation whilst receiving bilateral stimulation most commonly in the form of eye movements. There is also evidence for the efficacy of stress management training in the treatment of PTSD, although treatment effects have not been demonstrated to be as great as for TF‐CBT‐based interventions or EMDR (Bisson 2013). Concerns remain about the applicability of these types of treatments to complex cases (Ruscio 2006). Studies evaluating interventions for PTSD have typically excluded those individuals with certain complexities such as SUD, suicidality, serious self harm, homelessness, and serious mental illness, and a recent meta‐analysis suggests that the benefits of specific interventions are smaller for individuals with more complex clinical problems (Gerger 2014). This study also highlighted the possible benefits gained from non‐specific interventions. A key principle of treatment that is endorsed by many expert clinicians in the trauma field is that treatment for individuals with complex PTSD presentations should be phased (Herman 1992), with an emphasis on interventions aimed at promoting a sense of safety and stabilisation of symptoms through improving self management and emotional regulation prior to the onset of trauma‐focused intervention (Cloitre 2011).
Cognitive behavioural therapies are also considered to be effective for SUD (Knapp 2007; van Dam 2012). A number of interventions based on the principles of CBT or behaviour therapy have been found to be effective for those with drug and alcohol problems. These include coping‐skills training, relapse prevention, contingency management, and behavioural couples therapy. Coping‐skills training and relapse prevention approaches are aimed at strengthening adaptive coping skills and reducing the risk of relapse in high‐risk or challenging situations. Contingency management is based on principles of operant conditioning. It aims to encourage adaptive abstinence‐focused behaviours through means of positive incentives. Contingency management has been found to be effective in the treatment of cocaine and stimulant misuse (Knapp 2007), and there is some evidence of effectiveness with opioid users (Mayet 2004). Behavioural couples therapy (BCT) recognises that interpersonal and relationship factors are often associated with relapse. In common with other cognitive behavioural therapies, BCT seeks to improve behavioural self control and develop new coping skills to facilitate and maintain abstinence. It also seeks to improve general relationship functioning and partners' coping with drinking or drug use‐related situations. BCT has been found to be effective at reducing frequency of usage, reducing negative consequences of use, and increasing relationship satisfaction in a number of studies with alcohol, opiate, and poly‐substance users (Powers 2008). Other popular psychosocial models for treating addiction include motivational interviewing (MI) and 12‐step approaches. MI is a widely used intervention in many addiction services. MI is a semi‐directive method for enhancing intrinsic motivation to change by exploring and resolving ambivalence through Socratic questioning and cognitive behavioural strategies. There is some evidence for the effectiveness of MI in reducing substance use in a number of studies (Smedslund 2011). One of the most widely used intervention programmes for alcohol misuse and dependence is the 12‐step approach, originally developed by Alcoholics Anonymous. The 12‐step approach consists of a brief, structured, manual‐driven approach to facilitating recovery from alcohol abuse, intended to be implemented over 12 to 15 sessions. Some 12‐step approaches include a spiritual approach, some are led by a professional, and others are led by former alcohol dependents. In a Cochrane review of the 12‐step approach, Ferri 2006 concluded that there was no strong evidence for effectiveness in reducing alcohol dependence, although the programme remains popular.
For various reasons, individuals with PTSD and SUD comorbidity are perceived as being more difficult to treat than individuals with either condition alone (Najavits 2002a; Schäfer 2007). This comorbidity is associated with poorer recruitment and retention in treatment programmes (Foa 2010; Najavits 2002a; Schäfer 2007), poorer treatment outcomes (Berenz 2012; Najavits 2002a; Ouimette 2003a; Ouimette 2003b; Reynolds 2005; Schäfer 2007), poorer treatment adherence, and shorter periods of abstinence post‐treatment (Brown 2003). Despite high prevalence levels, adults in treatment for SUD are frequently not assessed for PTSD (Mills 2006), or offered PTSD‐based interventions (Ford 2007; Ouimette 2003b; Reynolds 2005). There is a paucity of evidence for recommendations about treatment interventions for affective or anxiety disorders that are comorbid with SUD (Watkins 2005; Wilson 2008). In practice, a wide range of pharmacological and psychological therapies are used to treat the comorbidity. A concern for many treating clinicians related to intervention with some pharmacological agents such as benzodiazepines, is that patients might abuse these agents. In recognition of the clinical challenges involved in treating individuals with comorbid PSTD and SUD, a number of specialised psychological therapy approaches have been developed over the past 15 years or so. Three different types of treatment approach are identified in the literature (Gulliver 2010; Weiss 1995a): sequential, concurrent, and integrated. In sequential approaches, one comorbidity ‐ usually substance misuse ‐ is treated first, and the other ‐ usually PTSD ‐ afterwards. One sequential model to have received some attention is 'Transcend', a partially inpatient hospital‐based model (Donovan 2001). With concurrent approaches, each condition is treated separately but simultaneously using established evidence‐based interventions for each condition (Brady 2001; Triffleman 1999). One example of a concurrent approach is concurrent treatment of PTSD and substance use disorders using prolonged exposure (COPE) (Back 2001; Mills 2007). COPE uses cognitive behavioural therapy for substance use throughout the duration of the 13 treatment sessions and prolonged exposure for PTSD from around session five (Foa 1998). Integrative approaches treat both conditions together using interventions to address both disorders at the same time. Amongst integrative models, 'Seeking Safety' has probably received the most attention, with a number of randomised and non‐randomised evaluative studies (Najavits 2002b; Najavits 2007). Seeking Safety is a skills‐based therapy that aims to develop adaptive cognitive, behavioural, and interpersonal coping. Seeking Safety can be delivered on an individual basis or via groups.
Treatment interventions for PTSD and comorbid SUD have recently become a topic for review, in Berenz 2012 and Najavits 2013, and systematic review (Torchalla 2012; van Dam 2012). These reviews suggest some positive preliminary findings in relation to integrated and trauma‐focused psychological therapies for comorbidity. Najavits 2013, Torchalla 2012, and van Dam 2012 based their conclusions on evidence from both controlled and non‐controlled trials. All of these reviews identified significant methodological limitations in the studies reviewed. Several recently published controlled trials were not included in any of these reviews.
How the intervention might work
A number of different explanations for the relationship between SUD and PTSD have been proposed (Meyer 1986; Schäfer 2007). The most widely supported explanation is that PTSD influences the development of SUD, through means such as self medication (Schäfer 2007). Other explanations include the possibility that problematic substance use increases the risk of being exposed to trauma and increases psychological vulnerability to the effects of trauma (Meyer 1986; Schäfer 2007).
psychological therapies may therefore effect change in symptoms and functioning in such individuals through a number of different mechanisms. One potential mechanism by which psychological therapies might work is the development of enhanced coping skills which may increase the ability to regulate negative emotions (Busuttil 2009), leading to increased capacity to tolerate traumatic memories and craving urges. Another potential mechanism is the processing of trauma memories (Ehlers 2000; Foa 1998) leading to a decreased need to 'self medicate'. Psychological therapies such as those based on cognitive behavioural therapy (CBT) are also likely to promote changes in thinking and belief systems underlying trauma memories, and beliefs and ideas about substance use (Ehlers 2000; Najavits 2002b). For example, such interventions may facilitate attitudinal change to substance misuse and aid increased understanding of cognitive and situational risk factors associated with patterns of drug taking or problematic drinking, particularly those associated with past trauma. Other change mechanisms might include the development and reinforcement of adaptive coping skills which support constructive coping with both conditions (Brown 2003). It is likely that different interventions will operate though different means of change.
Why it is important to do this review
A number of systematic reviews of interventions for PTSD have been published in the Cochrane Library. As already noted, Bisson 2013 (along with other reviews, for example Bradley 2005) has described fairly robust evidence for trauma‐focused CBT and EMDR as treatments for chronic PTSD, with emerging evidence for some non‐trauma‐focused CBT‐based interventions and trauma‐focused CBT‐based group interventions. Other Cochrane reviews have considered single‐session psychological ’debriefing’ to prevent PTSD (Rose 2002), multiple‐session early psychological therapies for the prevention of PTSD (Roberts 2009), early psychological therapies to treat acute traumatic stress symptoms (Roberts 2010), pharmacological treatments (Stein 2006), combined pharmacotherapy and psychological therapies for PTSD (Hetrick 2010), and psychological therapies for the treatment of PTSD in children and adolescents (Gillies 2012). Over 70 systematic reviews of interventions for SUD have been published in the Cochrane Library. Reviews of psychological therapies have considered psychosocial interventions for cocaine and psychostimulant amphetamines‐related disorders (Knapp 2007), psychosocial interventions for opiate abuse and dependence (Mayet 2004), motivational interviewing for substance abuse (Smedslund 2011), 12‐step programmes for alcohol dependence (Ferri 2006), and psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users (Klimas 2014).
The issue of how best to manage or plan intervention for individuals with comorbid PTSD and SUD is a challenging one for clinicians (Najavits 2002a), and there is no real consensus about best practice. Most diagnosis‐specific guidelines for PTSD and other mental health disorders are silent as to whether the specific treatment recommendation applies to co‐occurring disorders (Watkins 2005). As we have discussed, comorbidity is a frequent problem, and those individuals with comorbidity are more challenging for general mental health services, trauma specialists, and addiction services to treat (Schäfer 2007). In clinical practice, many clinicians still argue the addiction should be treated first (for example Busuttil 2009; Foa 2000; Zayfert 2007), or that abstinence is necessary before diagnosis and a management plan can be made (see Watkins 2005). The reality for many people with comorbidity is that they can frequently get passed between services with little co‐ordination of care (Najavits 2006). Watkins 2005 argues that there has been a broad shift in the literature towards more co‐ordinated treatment plans over recent years, although it is far from clear that there is strong evidence to support this shift or that it has translated into change in routine clinical practice. There is also contention about perceived high risk of adverse effects of psychological evidence‐based treatment therapies, such as eye movement desensitisation and reprocessing and prolonged exposure, with comorbid groups (see Watkins 2005). We hope this review will be able to shed some light on what evidence there is to support these different models and treatment approaches, in order to aid clinician decision making.
Objectives
To determine the efficacy of psychological therapies aimed at treating traumatic stress symptoms, substance misuse symptoms, or both in people with comorbid PTSD and SUD in comparison with control conditions (including usual care, waiting‐list conditions, and no treatment) and other psychological therapies.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised or cluster‐randomised controlled trial that considers one or more defined psychological therapy aimed at reducing traumatic stress symptoms, SUD symptoms, or both. We did not use sample size and publication status to determine whether or not a study should be included. Studies published in all languages were eligible for inclusion.
We were willing to include for consideration studies using a cross‐over design (for example specified intervention aimed at reducing traumatic stress symptoms followed by intervention aimed at reducing substance use and vice versa), as we felt that this addresses issues of clinical debate. However, we identified no such studies.
Types of participants
Participant characteristics
We made no restriction on age, although we anticipated that most studies would focus on adult populations. We did not make decisions about inclusion or exclusion on the basis of gender or ethnicity.
Diagnosis
Any individual suffering from comorbid PTSD and SUD. Treatment studies of individuals with PTSD and associated disorders such as acute stress disorder have sometimes included individuals who met most but not all criteria for the condition. In light of this, a previous Cochrane review of psychological therapies for PTSD, Bisson 2013, specified that at least 70% of participants had to be diagnosed as suffering from PTSD according to the International Classification of Diseases (ICD), WHO 1993, or Diagnostic and Statistical Manual of Mental Disorders (DSM), APA 2013. We believe that the issue of inclusion of some individuals with subthreshold diagnosis is likely to occur in comorbid studies as well. For this review, we decided to set a more conservative limit that at least 80% of participants will have been diagnosed as suffering from PTSD according to DSM or ICD criteria. Similarly, at least 80% of participants met formal diagnostic criterion for a substance misuse disorder according to DSM, APA 2013, or equivalent ICD definitions, WHO 1993, based on codes F10 to F19, excluding F15 (caffeine) and F17 (tobacco). Codes F10 to F19 include mental and behavioural disorders due to use of alcohol (F10), opioids (F11), cannabinoids (F12), sedatives or hypnotics (F13), cocaine (F14), other stimulants (amphetamine) (F15), hallucinogens (F16), volatile solvents (F18), and multiple drug use and use of other psychoactive substances (F19). There was no restriction on the basis or severity of PTSD symptoms, type of traumatic event, or nature of substance use (including alcohol).
Comorbidities
We made no restriction on other comorbidity.
Setting
There was no restriction on the setting in which a study took place.
Subset data
Although we applied an 80% threshold for diagnosis of PTSD and SUD, we also decided that when we identified studies where a significant subset of participants met our inclusion criteria (below the 80% threshold), we would approach the study authors to see if we could obtain outcome data for the subset who met inclusion, if such information was not available in the study report. If we were able to obtain these data and other inclusion criteria were met, we would then include the data in the review. We made the decision to potentially include studies on this basis after the review protocol was published.
Types of interventions
Experimental interventions
We considered any experimental psychological therapy designed to reduce symptoms of PTSD, substance usage, or both.
For the purposes of this review, a psychological therapy included any specified non‐pharmaceutical intervention aimed at reducing traumatic stress symptoms, SUD, or both, offered by one or more health professional or layperson. Potential therapy categories included any of the following.
Trauma‐focused psychological therapy: any psychological therapy including trauma‐focused cognitive behavioural therapy (TF‐CBT) and eye movement desensitisation and reprocessing (EMDR), delivered to individuals with comorbidity. TF‐CBT includes any intervention that uses predominantly trauma‐focused cognitive, behavioural, or cognitive‐behavioural techniques. This category includes individual exposure therapy and specialised treatment packages such as concurrent treatment of PTSD and substance use disorders using prolonged exposure (COPE), which include interventions for SUD (Back 2001; Mills 2007), and group approaches such as 'Transcend' (Donovan 2001). Individual trauma‐focused interventions for PTSD have been found to be more effective than group‐based intervention (Bisson 2013). We therefore made a post hoc decision to present and analyse individual‐ and group‐based trauma‐focused approaches separately.
Non‐trauma‐focused therapy for both PTSD and SUD or PTSD or SUD only: any psychological therapy including CBT aimed at addressing symptoms of PTSD and SUD on a sequential or integrated basis that does not include treatment of PTSD symptoms through a trauma‐focused or exposure‐based therapy. Interventions are likely to be targeted at increasing knowledge through psychoeducation and on improving coping skills. This category includes Seeking Safety (Najavits 2002b), which can be delivered on an individual basis or through groups. Group interventions are generally considered to show weaker effects than individual interventions (Najavits 2014 [personal communication]). We made a post hoc decision to present and analyse individual‐ and group‐based non‐trauma‐focused approaches separately.
Active psychological therapy for SUD only. This includes structured therapeutic programmes based on CBT, 12‐step, contingency management, and reinforcement‐based therapies. It also includes interventions based on motivational interviewing and psychological therapies aimed at management of cravings or to achieve abstinence.
The experimental intervention could be delivered as a monotherapy or as an adjunct to an established treatment that was received (in an identical way) by participants in both the experimental and the comparator group, for example TF‐CBT plus CBT for SUD versus CBT for SUD alone.
Comparator interventions
A control intervention included no intervention or any minimal intervention such as a waiting‐list control, treatment as usual, minimal or placebo condition.
An alternative active psychological therapy as described above.
Types of outcome measures
Primary outcomes
1. Severity of traumatic stress symptoms using a standardised measure such as the Clinician Administered PTSD Symptom Scale (CAPS) (Blake 1995), the Impact of Event Scale (Horowitz 1979), the Davidson Trauma Scale (Davidson 1997), or the Post‐Traumatic Diagnostic Scale (Foa 1997a). In circumstances where an individual study utilised both a clinician‐administered and a self report measure, primacy was given to outcomes using the clinician‐administered measure, as such measures are considered to provide the 'gold standard' in the traumatic stress field (for example Foa 1997b).
2. Reduction in drug use, alcohol use, or both as measured by a standardised measure such as the Addiction Severity Index (ASI) (McLellan 1992), the Substance Use Inventory (Weiss 1995b), the Opiate Treatment Index (Darke 1992), the Severity of Drug Dependence Scale (Gossop 1995), or the Substance Abuse Module (Haro 2006), or biological markers of drug and alcohol use, such as urine, saliva, and hair analysis, or self reported days of substance use/abstinence within a specified period such as the Timeline Followback Interview (Sobell 1995). There is less consensus about gold‐standard outcomes in the addiction field. We prioritised outcomes in the order of standardised instruments, followed by biological markers, followed by self report measures.
3. Treatment completion as measured by number of participants who were identified as treatment completers by study authors. We undertook to interpret drop‐out data with caution, as it is recognised that participants can withdraw from studies for various and complex reasons and reported drop‐out can be influenced by experimental factors related to practice of the research team (Loke 2011).
Secondary outcomes
4. PTSD diagnosis after treatment.
5. SUD diagnosis after treatment.
6. Adverse events reported by number and type.
7. Compliance, as measured by proportion of treatment sessions attended.
8. General functioning, including quality of life measures such as the 36‐Item Short Form Survey (SF‐36) (Ware 2003).
9. Use of health‐related resources (e.g. hospital admission, outpatient contacts, visits to primary care).
Timing of outcome assessment
When information was available primary outcomes were analysed at the following time points.
Immediately post‐treatment
3 to 4 months post‐treatment
5 to 7 months post‐treatment
8 to 11 months post‐treatment
12 months and beyond post‐treatment
Our primary outcome point was immediately post‐treatment. We analysed secondary outcomes only at this time point.
Search methods for identification of studies
The Cochrane Depression, Anxiety and Neurosis Review Group's Specialised Register (CCDANCTR)
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintains two clinical trials registers at their editorial base in Bristol, UK: a references register and a studies‐based register. The CCDANCTR‐References Register contains over 39,500 reports of randomised controlled trials (RCTs) in depression, anxiety, and neurosis. Approximately 60% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies Register, and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual, using a controlled vocabulary; please contact the CCDAN Trials Search Co‐ordinator for further details. Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950‐), EMBASE (1974‐), and PsycINFO (1967‐); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL); and review‐specific searches of additional databases. Reports of trials are also sourced from international trials registers c/o the World Health Organization's trials portal (the International Clinical Trials Registry Platform (ICTRP)), pharmaceutical companies, and the handsearching of key journals, conference proceedings, and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCDAN's generic search strategies (used to identify RCTs) can be found on the Group's website.
Electronic searches
We conducted searches for Condition (PTSD) and Population (patients with comorbid substance abuse) to 11 March 2015.
1. CCDANCTR‐Studies Register
We searched the studies register using the following terms:
Condition = ("post‐traumatic stress disorders") AND Comorbidity = ("alcohol dependence" or "substance related disorders" or "substance abuse")
2. CCDANCTR‐References Register
We searched the references register using a more sensitive set of free‐text terms:
[Condition] 1. (PTSD or post‐trauma* or "post trauma*" or posttrauma* or "stress disorder*" or "combat disorder*" or "war neuros*") 2. (trauma* and (psycho* or stress*)) 3. (stress* and (extreme or disorder*)) 4. DESNOS 5. (1 or 2 or 3 or 4) [Population: comorbid substance abuse] 6. ("substance use disorder*" or SUD) 7. "drug abuse" 8. (abuser* or abusing or addict* or depend* or habit* or misuse or user*) 9. (abuse and not (child* or sex*)) [Common drugs of abuse] 10. (adinazolam or aerosol* or alcohol* or alprazolam or amphetamin* or anthramycin or anxiolytic* or ativan or barbituat* or bentazepam or benzodiazepin* or bromazepan or brotizolam or buprenorphin* or camazepam or cannabi* or chlordiazepoxid* or cinolazepam or clobazam or clonazepam or clorazepam or clotiazepam or cloxazolam or cocaine* or codeine or crack or crystal or cyprazepam or depressant* or diacetylmorphin* or diazepam* or doxefazepam or ecstasy or estazolam or etizolam or fentanyl or flunitrazepam or flurazepam or flutazoram or flutoprazepam or fosazepam or gases or GHB or girisopam or halazepam or hallucinogen* or haloxazepam or heroin* or hydromorphone or hydroquinone or hypnotic* or inhalant* or ketamin* or ketazolam or librium or loflazepate or loprazolam or lorazepam or lormetazepam or LSD or marihuana* or marijuana* or MDMA or meclonazepam or medazepam or meperidine or mephedrone or mescalin* or metaclazepam or methadone or methamphetamin* or methaqualone or mexazolam or midazepam or midazolam or morphine* or narcotic* or nerisopam or nimetazepam or nitrazepam or nitrites or "nitrous oxide" or "n‐methyl‐3,4‐methylenedioxyamphetamine" or nordazepam or opiate* or opiod* or opium or oxazepam or oxazolam or oxazypam or oxycodone or oxzepam or painkiller* or "pain killer*" or PCP or pethidin* or phencyclidin* or pinasepam or prazepam or propazepam or propoxyphene or psilocybin or psychedelic* or psychoactive* or psychostimulant* or quinazolinone or ripazepam or ritalin or sedative* or serazepin* or solvent* or steroid* or stimulant* or substance* or temazepam or tetrazepam or tofisopam or tramadol or triazolam or triflubazam or valium or vicodin) 11. (drug* and (recreational or street)) 12. (6 or 7 or 8 or 9 or 10 or 11) [Condition + Population] 13. (5 and 12)
We performed a further search on 4 December 2015 (prior to publication). We screened results and placed studies of interest in those awaiting classification; we may include or exclude these in a future update to this review (as appropriate).
3. Cochrane Central Register of Controlled Trials (CENTRAL)
We also searched the Cochrane Central Register of Controlled Trials (CENTRAL) to 3 January 2015 (Appendix 1).
4. International trial registries
We searched the World Health Organization's trials portal (ICTRP) and ClinicalTrials.gov to identify additional unpublished or ongoing studies (11 March 2015 and 4 December 2015).
Searching other resources
We also checked reference lists of studies identified in the search, as well as related review articles and management guidelines. We conducted Internet searches of known websites, conference proceedings, and discussion for the following: American Association for the Treatment of Opioid Dependence (http://www.aatod.org/), DrugScope (http://www.drugscope.org.uk/), European Society for Traumatic Stress Studies (https://www.estss.org), International Harm Reduction Association (http://www.ihra.net/), International Society for Traumatic Stress Studies (http://www.istss.org), Society for the Study of Addiction (http://www.addiction‐ssa.org/), and the United Kingdom Psychological Trauma Society (http://www.ukpts.co.uk). We also searched studies included in the Cochrane review 'Psychological therapies for chronic post‐traumatic stress disorder (PTSD) in adults' (Bisson 2013), and reviews of psychological therapies undertaken for the Cochrane Drug and Alcohol Group. We searched studies within these reviews on the basis that a significant subset of participants might warrant inclusion.
Data collection and analysis
Selection of studies
Two review authors (NPR and PAR) independently read the abstracts of all potential trials. If an abstract appeared to represent an RCT, the two review authors independently read the full report to determine if the trial met the inclusion criteria. In case of disagreement, a third review author was consulted (JIB).
Data extraction and management
We used a data extraction sheet to capture data, which we then entered into Review Manager 5 software (RevMan 2011). Information extracted included demographic details of participants, details of the traumatic event, type of substance use, the randomisation process, the interventions used, drop‐out rates, and outcome data. Three review authors (NPR, PAR, and NJ) independently extracted data. In case of disagreement, the fourth review author was consulted (JIB).
Main planned comparisons
Trauma‐focused psychological therapy versus control intervention
Trauma‐focused psychological therapy versus non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only
Trauma‐focused psychological therapy versus active psychological therapy for SUD only
Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only versus control intervention
Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only versus active psychological therapy for SUD only
Active psychological therapy for SUD only versus control intervention
We undertook to present and analyse data for individual‐ and group‐based interventions separately.
Assessment of risk of bias in included studies
We assessed risk of bias using The Cochrane Collaboration’s 'Risk of bias' tool and reported the results in a standard 'Risk of bias' table. We assessed the following domains:
Sequence generation: Was the allocation sequence adequately generated?
Allocation concealment: Was allocation adequately concealed?
Blinding of participants, personnel, and outcome assessors for each main outcome or class of outcomes: Was knowledge of the allocated intervention adequately prevented during the study?
Incomplete outcome data for each main outcome or class of outcomes: Were incomplete outcome data adequately addressed?
Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting?
Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias?
We judged the risk of bias for each domain within and across studies, based on the following three categories:
low risk of bias;
unclear risk of bias;
high risk of bias.
Three review authors (NPR, PAR, and NJ) independently assessed risk of bias for each study. Any disagreements were initially to be discussed between the three rating review authors. Where disagreement persisted, advice was sought from the fourth review author (JIB).
Measures of treatment effect
We analysed continuous outcomes using mean difference when all trials had measured outcome on the same scale. When trials measured outcomes on different scales, we used the standardised mean difference. We used risk ratio as the main categorical outcome measure, as this is more widely used than odds ratio in health‐related practice. We presented all outcomes using 95% confidence intervals.
Unit of analysis issues
Cross‐over trials
We did not identify any cross‐over trials. However, we specified at the protocol stage that if we included such trials, we would include final outcomes from these trials where the study addressed order of intervention for trauma‐related intervention and control or management of SUD symptoms. For trials that had a cross‐over design that did not address these clinical pathway issues, we would only consider results from the first randomisation period. We decided that each stage of analysis would be stratified by treatment type and that further analysis would include follow‐up data where these were available. We would only make comparisons involving follow‐up data when outcome data were available for similar time points.
Studies with multiple treatment groups
We specified that if the trial had three (or more) arms, we would consider undertaking pair‐wise meta‐analysis with each arm, depending upon the nature of the intervention in each arm and its relevance to the review objectives. We aimed to avoid multiple comparisons to limit the risk of false‐positive results. When a study had three or more arms that were relevant to the review, we would consider the appropriateness of combining data from two arms if interventions were sufficiently similar or of using data from the arms of the trial that fit closest to the review objective. Decisions would follow the guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we would report the rationale for any decisions made. In actuality, only one study included in the review had more than two treatment arms, and for reasons described below we only included two arms in comparisons.
Cluster‐randomised trials
We specified that management of cluster‐randomised trials would follow the guidance provided in the Cochrane Handbook. We identified no cluster‐randomised trials.
Dealing with missing data
When intention‐to‐treat (ITT) data were available, we reported this in the results. We attempted to access ITT data wherever possible. For dichotomous outcomes, we conducted ITT analysis by making imputations based on the assumption that all missing participants had a negative outcome. We included completer‐only data when this was the only data source available. In cases where there was inadequate information within a particular paper to undertake analysis, we made attempts to compute missing data from other information available within the paper, using guidance provided by Higgins 2011. For continuous data when only the standard error, t‐statistics, or P values were reported, we calculated standard deviations using the guidance provided by Higgins 2011. When imputation was not possible or when further clarification was required, we attempted to contact the authors to request additional information. In cases where no further useable data was available, we did not include the study in further analysis.
Assessment of heterogeneity
We initially used visual inspection of the forest plots to explore for possible heterogeneity. We also examined heterogeneity between studies by observing the I² statistic and Chi² test (P < 0.10). As suggested in the Cochrane Handbook (Higgins 2011), we took an I² of less than 30% to indicate mild heterogeneity, and we used a fixed‐effect model to synthesise the results. We considered an I² of 30% to 60% to indicate moderate heterogeneity and an I² of 60% to 90% substantial heterogeneity (Higgins 2011). Due to the level of clinical heterogeneity in the included studies, we decided to use a random‐effects model to summarise results including more than one study. We specified that where significant heterogeneity was present, we would attempt to explain the variation.
Assessment of reporting biases
We specified that if sufficient studies (10 or more) were available in a meta‐analysis, we would prepare funnel plots and examine them for signs of asymmetry (Egger 1997). We specified that if asymmetry was identified, we would consider possible reasons for this.
Data synthesis
In recognition of the substantial clinical heterogeneity between included studies, we pooled all data using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We specified that we would explore the following possible causes of clinical heterogeneity if data were sufficient to allow.
Specified treatment intervention model (e.g. Seeking Safety, Transcend, concurrent treatment of PTSD and substance use disorders using prolonged exposure (COPE)).
Specified treatment plans (e.g. sequential versus concurrent versus integrated approaches).
Participant subgroup (e.g. veterans versus victims of sexual, physical, and domestic violence versus childhood trauma versus rescue workers).
Specific substances of misuse (e.g. alcohol versus opioids versus cocaine versus amphetamines).
Intervention objectives (treating symptoms of PTSD versus SUD versus general well‐being/coping).
Sensitivity analysis
We specified that we would consider sensitivity analysis to explore possible causes of methodological heterogeneity if data were sufficient to allow. We would base analyses on the following criteria.
We would exclude trials considered most susceptible to bias based on the following quality assessment criteria:
those judged to be at high risk of bias or unclear risk of bias for allocation concealment;
high levels of postrandomisation losses (more than 40%) or exclusions;
unblinded outcome assessment or blinding of outcome assessment uncertain.
Summary of findings
We evaluated the quality of the available evidence of our findings using the GRADE approach (Guyatt 2011; Langendam 2013). We generated 'Summary of findings' tables using GRADEprofiler software (http://tech.cochrane.org/revman/gradepro) using data imported from Review Manager 5.3 (RevMan 2011). These tables provide outcome‐specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes that were considered. We assessed the quality of evidence using five factors:
Limitations in study design and implementation of available studies;
Indirectness of evidence;
Unexplained heterogeneity or inconsistency of results;
Imprecision of effect estimates;
Potential publication bias.
For each outcome that included pooled data, we classified the quality of evidence for each outcome according to the following categories.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate.
We downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias. We included the primary outcomes of PTSD severity, drug and/or alcohol use, and treatment completion in the 'Summary of findings' tables.
Results
Description of studies
Results of the search
We conducted electronic searches to 11 March 2015 (with results fully incorporated into the review). We also contacted 42 trial investigators; see Appendix 2.
We identified 1099 references, 1057 of which remained after de‐duplication. Two review authors (NPR and PAR) independently screened the titles and abstracts of these records and excluded 885 that did not meet the inclusion criteria. For 3 of the remaining 172 study reports we were only able to obtain conference abstracts. We judged these studies as potentially relevant to the review, but were unable to undertake classification of these abstracts. Twelve references were for ongoing studies. We retrieved and inspected the full‐text papers for the remaining 157 reports, excluding 143 of them as not meeting our inclusion criteria. Thirteen of the remaining studies met the full inclusion criteria and so were included in the review. We also identified a number of studies with a significant subset of individuals who met all inclusion criteria. We were able to obtain data on this subset from the authors of one study (Mueser 2008), resulting in a total of 14 studies being included in the review. Thirteen of these 14 studies contributed to the quantitative synthesis. The study selection process is also detailed in our PRISMA flow diagram (see Figure 1).
1.
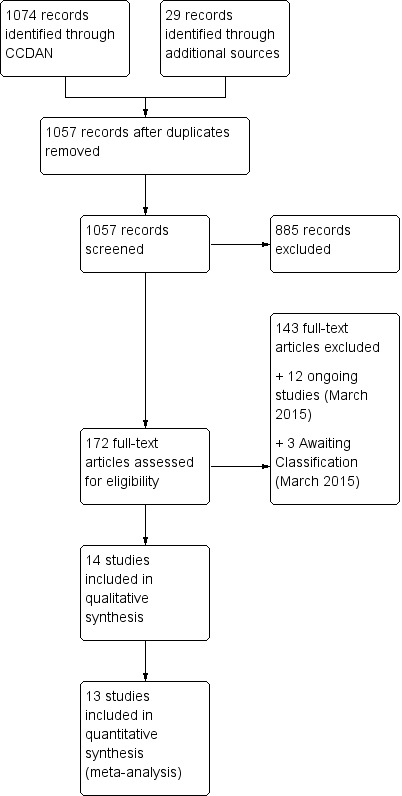
Study flow diagram.
Note: We conducted a further search on 4 December 2015, prior to publication, but did not incorporate results at this time. We screened the abstracts (n = 72) and identified 4 new studies, which we've added to those awaiting classification. Two of these studies meet the eligibility criteria for this review (McGovern 2015; Perez‐Dandieu 2015), and a further two will do so if subset data is available (Barrett 2015; Wolf 2015). The December search also identified Stappenbeck 2015 (NCT00760994), which after contacting the trialists was confirmed to be the same trial as Simpson 2011 (already awaiting classification). We also identified an additional five ongoing study protocols (NCT01211106; NCT01457404; NCT01663337; NCT01849029; NCT02335125).
Included studies
We included 14 studies in this review, with characteristics as follows (see also Characteristics of included studies).
Design
All of the included studies were randomised controlled trials or pilot randomised controlled trials. One study was described as a laboratory‐based study investigating the effects of trauma‐focused intervention on alcohol craving elicited by trauma cues (Coffey 2006). One study had two intervention arms in which allocation was randomised (Hien 2004); a third control arm was added part way through the study, and allocation to this arm was made on a non‐randomised basis. We have not included data from this third arm in the review. Studies were randomised at the participant level and used a parallel‐group design.
Sample sizes
A total of 1506 participants were allocated to groups across the 14 included studies. The number of participants ranged from 29, in Norman unpublished, to 353, in Hien 2009. Three other studies had fewer than 50 participants (Coffey 2006; Najavits 2006a; Zlotnick 2009), with the subsample of 44 from a cohort of 108 in Mueser 2008. Three studies had 50 to 100 participants (Hien 2004 ‐ excluding the arm that was non‐randomised; McGovern 2011; Sannibale 2013), and the remaining six studies included more than 100 participants (Boden 2012; Coffey submitted; Foa 2013; Frisman 2008; Hien 2009; Mills 2012).
Setting
Twelve studies were conducted in the USA; the remaining two studies were carried out in Australia (Mills 2012; Sannibale 2013). The majority of studies recruited individuals from community outpatient substance abuse services. One study recruited from veteran outpatient substance abuse services (Boden 2012). Four studies also made use of advertisements or flyers (Foa 2013; Hien 2004; Mills 2012). Najavits 2006a also recruited from hospitals and schools. Sannibale 2013 recruited from a range of services. Coffey submitted recruited from a residential substance misuse service, and Zlotnick 2009 from the minimum‐security wing of a female prison. Mueser 2008 recruited from community mental health services. All participants were seen on an outpatient basis, apart from those in Zlotnick 2009, who received most of their intervention in prison, with some follow‐up on release.
Participants
All studies were of adults, apart from Najavits 2006a, who investigated intervention for adolescent girls with a mean age of 16.06 years. One study recruited from veteran populations with an all‐male cohort (Boden 2012). Zlotnick 2009 recruited female prisoners. Other studies with a female‐only cohort were Hien 2004, Hien 2009, Najavits 2006a, and Norman unpublished. All other studies were of mixed gender and from community groups. All studies met the minimum threshold of 80% of participants meeting full diagnosis for PTSD. Across all studies, 1387 (92.1%) of participants met full diagnosis for PTSD, with the remaining group being described as having subthreshold PTSD. All participants in all studies met minimum criteria for a substance use disorder. Coffey 2006, Coffey submitted, and Foa 2013 included people with alcohol dependence, and Norman unpublished and Sannibale 2013 included people with alcohol use disorder. The majority of participants in Coffey submitted were also drug dependent. The other 10 studies included people with substance abuse. Substance use in these studies was typically polydrug use, with many participants using multiple drugs. None of the included studies targeted one specific substance other than alcohol. The subsample in Mueser 2008 excluded people with substance dependence. Hien 2004 and Mills 2012 only included people with substance dependence, and the majority (93.9%) of participants in Najavits 2006a were also substance dependent.
Exclusion criteria were not identified in Frisman 2008. Most other studies excluded on the basis of current or acute psychosis, current suicidal/homicidal ideation, and significant cognitive impairment (for example resulting from dementia or brain injury). Hien 2009 also excluded on the grounds of past history of psychosis. Mueser 2008 was a study that was primarily interested in intervention for individuals with severe mental illness, and they only excluded individuals who were in psychiatric hospital. In recruiting participants with alcohol dependence, Foa 2013 excluded people with other substance dependence conditions. Sannibale 2013 excluded people with severe substance dependence. Mills 2012 excluded people who had a history of self harm in the past six months. Coffey 2006 excluded people with combat‐related PTSD. Coffey submitted excluded those who were in an abusive relationship at the time of recruitment, and Norman unpublished only included participants who had been out of an abusive relationship for at least a month. Hien 2004 and Hien 2009 excluded people with advanced‐stage medical diseases. Hien 2009 and McGovern 2011 excluded those involved in ongoing legal disputes. Najavits 2006a also excluded if people were mandated to treatment, or had characteristics that would interfere with treatment completion (mental retardation, homelessness, impending incarceration, or a life‐threatening illness). This was the only study to report on exclusion on the basis of homelessness. However, it is argued that people who are homeless are routinely excluded from these kinds of studies (Najavits 2014 [personal communication]).
Interventions
All of the experimental interventions included in the review were based on some form of cognitive behavioural therapy (CBT). Following van Dam 2012, these interventions can perhaps best be summarised and divided into trauma‐focused approaches ‐ some of which included combined interventions for SUD ‐ and non‐trauma‐focused interventions, which mainly involved integrated treatment of PTSD and SUD.
Trauma‐focused/combined interventions
Individual‐based trauma‐focused/combined interventions
Five studies included trauma‐focused/exposure‐based components as a part of the intervention program, delivered individually. Four studies tested combined coping skills‐focused intervention for SUD with exposure‐based interventions for PTSD as the experimental condition (Coffey submitted; Foa 2013; Mills 2012; Sannibale 2013). Coffey submitted compared 9 to 12 sessions imaginal and in vivo exposure plus treatment as usual against an equivalent health‐related psycho‐education intervention. Foa 2013 was a 2x2 study examining the effects of prolonged exposure and naltrexone. For psychological therapies, prolonged exposure plus supportive counselling was compared with supportive counselling alone. The supportive counselling intervention combined medication management with compliance enhancement techniques based on motivational interviewing. We considered this to be equivalent to a treatment‐as‐usual intervention. For medication, naltrexone was compared against a placebo. The numbers of participants receiving the two psychological therapies were equal in the two medication groups. Mills 2012 compared concurrent treatment of PTSD and substance use disorders using prolonged exposure (COPE) against treatment as usual for substance abuse only. COPE includes motivational enhancement and CBT for substance use; psycho‐education relating to both disorders and their interaction; in vivo exposure; imaginal exposure; and cognitive therapy for PTSD. Finally, Sannibale 2013 evaluated integrated CBT for PTSD and alcohol use disorder against CBT for alcohol use disorder and supportive counselling. The experimental condition in this trial included cognitive behavioural exposure‐based therapy for PTSD, based on a prolonged exposure model with cognitive restructuring, in addition to cognitive therapy for problem drinking. The control intervention had no PTSD components in it. Coffey 2006 tested an exposure‐based intervention that has been established for the treatment of PTSD, but recruited from within alcohol abuse services. They compared six sessions of imaginal exposure with six sessions of imagery‐based relaxation training, with the primary aim of evaluating effects on alcohol‐related craving.
Group‐based trauma‐focused/combined interventions
We identified no studies offering trauma‐focused intervention through groups.
Non‐trauma‐focused intervention
Individual‐based non‐trauma‐focused interventions
Four studies evaluated individual integrated PTSD/SUD intervention. One study compared an integrated PTSD/SUD intervention against treatment as usual for SUD (Najavits 2006a). The active condition in this trial was Seeking Safety plus treatment as usual. One study evaluated individual CBT against participants' usual psychiatric care (Mueser 2008). Treatment components included psycho‐education, cognitive restructuring, and generalisation training. As described previously, this study evaluated treatment of PTSD for people with serious mental illness and did not include a component focusing on SUD. Two studies evaluated an integrated PTSD/SUD intervention delivered on an individual basis against an alternative psychological therapy for SUD alone (Hien 2004; McGovern 2011). Hien 2004 compared Seeking Safety plus treatment as usual to a relapse prevention comparison condition and a non‐randomised treatment‐as‐usual arm, which we have not included in this review. McGovern 2011 compared integrated CBT plus treatment as usual (ICBT) with individual addiction counselling plus treatment as usual (IAC) as the control condition. There was no PTSD component to the IAC, which at 10 to 12 sessions was shorter than the 12‐ to 14‐session ICBT intervention, which included psycho‐education, cognitive restructuring, and generalisation training in relation to PTSD and SUD.
Group‐based non‐trauma‐focused interventions
Five studies evaluated group interventions, four of which included Seeking Safety, Najavits 2002b, plus treatment as usual as the active treatment condition (Boden 2012; Hien 2009; Norman unpublished; Zlotnick 2009). Seeking Safety is a structured cognitive behavioural treatment with both safety/trauma and substance use components integrated into each session. Its primary goal is to reduce both PTSD and SUD by focusing on safe coping skills addressed through cognitive, behavioural, interpersonal, and case management domains over 24 to 25 sessions. In two of these trials (Boden 2012; Zlotnick 2009), treatment as usual was the control condition. The intervention in Hien 2009 provided a partial dose of Seeking Safety with 12 sessions to cover the core components of the model. This study used a female health psycho‐education (Women's Health Education) comparison condition, which was delivered over the same number of sessions with the same level of attention given to participants. Norman unpublished included some components from cognitive trauma therapy for battered women with PTSD (CTT‐BW) (Kubany 2004). The control condition in this study was a minimal‐intervention therapist‐led supportive 12‐step group. The fifth study to evaluate a group‐based integrated program was Frisman 2008. This study compared TARGET, an 8‐ to 9‐week intervention that aims to improve adaptive coping skills, with treatment as usual for SUD only.
A fuller description of interventions can be found in the Characteristics of included studies tables.
Comparisons
The included studies compared:
psychological therapy versus 'control' (as defined in Types of interventions);
psychological therapy versus other psychological therapy (as defined in Types of interventions).
We made the following specific comparisons:
-
Trauma‐focused psychological therapy versus control intervention
Individual‐based therapy: Coffey 2006; Coffey submitted; Foa 2013; Mills 2012.
Group‐based therapy: No studies.
-
Trauma‐focused psychological therapy versus active psychological therapy for SUD only
Individual‐based intervention: Sannibale 2013.
Group‐based intervention: No studies.
-
Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only versus control intervention
Individual‐based intervention: Mueser 2008; Najavits 2006a.
Group‐based intervention: Boden 2012; Frisman 2008; Hien 2009; Norman unpublished; Zlotnick 2009.
-
Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only versus active psychological therapy for SUD only
Individual‐based intervention: Hien 2004; McGovern 2011.
Group‐based intervention: No studies.
Outcomes
PTSD outcomes
Of the 14 studies, 10 used a clinician‐administered measure of PTSD (Coffey submitted; Foa 2013; Hien 2004; Hien 2009; McGovern 2011; Mills 2012; Mueser 2008; Norman unpublished; Sannibale 2013; Zlotnick 2009). In nine cases, this was the Clinician Administered PTSD Symptom Scale (CAPS) (Blake 1995). The Impact of Event Scale ‐ Revised (IES‐R), Weiss 1997, was the primary PTSD outcome measure in two studies (Boden 2012; Coffey 2006). Frisman 2008 used the Post‐Traumatic Cognitions Inventory, which is a measure of trauma‐related beliefs. We considered this to be a reasonable proxy to a PTSD outcome measure.
SUD outcomes
A range of measures were used to assess outcomes for SUD. These included the Timeline Followback Interview (Sobell 1995), which was used in five studies (Coffey submitted; Foa 2013; Norman unpublished; Sannibale 2013; Zlotnick 2009); the Addiction Severity Index (ASI) (McLellan 1992), used in three studies (Boden 2012; McGovern 2011; Zlotnick 2009); the Composite International Diagnostic Interview for DSM–IV (Robins 1989), used in three studies (Hien 2009; Mills 2012; Mueser 2008); and the Substance Use Inventory (Weiss 1995b), used in two studies (Hien 2004; Hien 2009). Toxicology screens were administered in Hien 2009 and McGovern 2011. Some studies used several SUD measures. In meta‐analysis we included outcomes according to the specifications described in Types of outcome measures. Some studies also included outcomes for both alcohol use and drug use. In such cases we included the outcome that was most associated with the treatment condition (that is where alcohol was identified as the key comorbidity, we included the alcohol outcome, and where SUD was identified as the key comorbidity, we included the SUD outcome).
Treatment completion
Many of the included studies recognised high levels of treatment drop‐out as a pervasive problem in the field (for example Hien 2004; Mills 2012). Definitions of the number of sessions attended for a participant to be considered a completer varied across studies. We used the definition of treatment completer provided by each study to undertake analyses of treatment acceptability. Boden 2012, Foa 2013, Mills 2012, Najavits 2006a, and Zlotnick 2009 provided no definition, although Mills 2012 provided data on the number of participants who attended at least one exposure session and the number attending all 13 treatment sessions. Hien 2004 and Norman unpublished identified a "minimum dose" as 25% of sessions attended. Hien 2009, Mueser 2008, and Sannibale 2013 specified a completer as an participants who had attended 50% of sessions. Definitions in the range of 70% to 80% of sessions were described in Coffey submitted, Frisman 2008, and McGovern 2011, and 100% of sessions was described in Coffey 2006.
Secondary outcomes
PTSD diagnostic status was reported in Coffey submitted, McGovern 2011, Mills 2012, Norman unpublished, Sannibale 2013, and Zlotnick 2009. SUD diagnostic status was reported only in Mills 2012. Adverse effects were reported by Boden 2012, Foa 2013, Hien 2009 (see Killeen 2008), Mills 2012, and Norman unpublished. Compliance as measured by the mean number of sessions attended was frequently reported, although some studies only reported attendance for the experimental group (Hien 2004; Najavits 2006a; Zlotnick 2009). General functioning was not evaluated in any study. Use of health‐related resources (other service utilisation) was evaluated in Najavits 2006a, but was not clearly reported.
Timing of outcome assessment
All included trials apart from Frisman 2008 reported PTSD and SUD as a continuous outcome at the end of treatment. Long‐term follow‐up data ranging from 3 to 12 months was reported in all studies except Coffey 2006. Follow‐up data was available at 3 months from the end of treatment in Boden 2012, Coffey submitted, Hien 2004, Hien 2009, McGovern 2011, Mueser 2008, Najavits 2006a, and Zlotnick 2009; at 5 to 6 months post‐treatment in Coffey submitted, Foa 2013, Frisman 2008, Hien 2004, Hien 2009, Mills 2012, Mueser 2008, Sannibale 2013, and Zlotnick 2009; at 9 months in Sannibale 2013; and at 12 months in Frisman 2008 and Hien 2009. We note that Mills 2012 reported their follow‐up points at 6 weeks, 3 months, and 9 months from baseline. As the planned intervention period was 3 months, we took the 3‐month follow‐up point as the end of treatment and the 9‐month follow‐up point as 6‐month follow‐up post‐treatment. However, some participants did continue to receive therapy more than three months after baseline assessment.
Excluded studies
See Characteristics of excluded studies.
We identified 172 studies as being potentially eligible for this review after initial screening, of which 143 were excluded after closer examination. Of these, 21 did not meet study design criteria (that is not RCTs). We excluded 49 on the basis that less that 80% of the sample met diagnosis for PTSD. In some of these studies PTSD was not assessed at all. Five of these 49 studies assessed for traumatic stress symptoms, but a formal and reliable diagnosis of PTSD was not established (for example Ford 2011; Ghee 2009). We excluded 12 studies because less than 80% of the sample met diagnosis for SUD. We excluded seven studies evaluating interventions in trauma‐exposed people with significant SUD history (for example van Dam 2013) on the basis that less than 80% of the sample met diagnosis for PTSD and SUD. Four studies did not provide outcomes that were either PTSD or SUD related, three studies did not evaluate a psychological therapy, and one study evaluated the addition of sertraline to a psychological therapy (Hien 2015). Twenty‐seven studies were companion papers to studies included in this review. The remaining 19 studies were conference abstracts related to other studies that were either included in or excluded from the review.
Ongoing studies
See Characteristics of ongoing studies.
We identified 12 ongoing studies as being of potential relevance to this review (to March 2011) via the World Health Organization's trials portal (ICTRP) and ClinicalTrials.gov and through a published protocol (DRKS00004288; NCT00946322; NCT01029197; NCT01186315; NCT01274741; NCT01338506; NCT01357577; NCT01365247; NCT01597856; NCT01693978; NCT02081417; NTR3084). Interventions currently being evaluated include Seeking Safety plus treatment as usual (TAU) in a Dutch population (planned recruits 130) (NTR3084), a multicentre trial of Seeking Safety versus structured relapse prevention versus TAU (DRKS00004288), a trial of Seeking Safety versus a past‐focused integrated CBT‐based therapy for PTSD and SUD (planned recruits 52) (NCT01274741), a trial of peer‐led versus clinician‐led Seeking Safety (NCT02081417), a trial of CBT in 160 military veterans (NCT01357577), a 3‐arm trial comparing CBT (including prolonged exposure) with relapse prevention and TAU for PTSD and SUD (planned recruits 168) (NCT01365247), a trial of COPE in military veterans (planned recruits 90) (NCT01338506), a trial of prolonged exposure versus prolonged exposure with contingency management for drug users with PTSD (NCT01693978), a trial of prolonged exposure versus prolonged exposure plus virtual reality‐based exposure to addiction cues (NCT01186315), a trial of group and individual CBT and exposure for people with serious mental illness (NCT01029197), a phase 1 trial of couple‐based treatment of PTSD and alcohol use disorder (NCT00946322), and a phase 1 trial evaluating a screening, motivation enhancement, and referral program for US veterans seeking pension and compensation benefits (NCT01597856).
A further search of the trial registries (4 December 2015), prior to publication, identified five additional ongoing studies (NCT01211106; NCT01457404; NCT01663337; NCT01849029; NCT02335125).
Studies awaiting classification
See Characteristics of studies awaiting classification.
We identified these studies through records in clinical trial registries and conference abstracts, but could not identify or access full‐text reports (to 11 March 2015). We have not yet formally evaluated these studies for eligibility, and may include or exclude them in a future update of this review. We initially identified three such studies (Park 2012; Simpson 2011; Skidmore 2013). Park 2012 have evaluated an integrated chronic disease management intervention in a primary care setting for people with SUD and a range of co‐occurring mental disorders. Of the 553 total participants, 204 are reported to have met diagnosis for PTSD at baseline. Simpson 2011 sought to evaluate the feasibility of a methodology focused on deciphering mechanisms of change for decreasing alcohol use and PTSD symptomatology in people with current PTSD and alcohol dependence. Participants were assigned to one of three brief interventions (acceptance, cognitive restructuring, or attention placebo); 84 participants were reported to have been randomised (a report describing the main outcomes of this study has subsequently been published (Stappenbeck 2015)). Skidmore 2013 evaluated integrated cognitive behavioural therapy (ICBT) versus cognitive processing therapy, an established and evidence‐based intervention for PTSD, in a group of 153 participants with co‐occurring alcohol or substance dependence, depression, and trauma exposure. It is unclear whether a subgroup of participants met diagnosis for PTSD.
A further search of the Cochrane Depression, Anxiety and Neurosis Review Group's Specialised Register (4 December 2015), prior to publication, identified four additional studies (added to those awaiting classification). Two met the eligibility criteria for this review (McGovern 2015; Perez‐Dandieu 2015), and a further two will also meet the eligibility criteria if subset data are available (Barrett 2015; Wolf 2015).
Risk of bias in included studies
For details of the 'Risk of bias' judgements for each study, see Characteristics of included studies. Graphical representations of the overall risk of bias in included studies are presented in Figure 2 and Figure 3.
2.
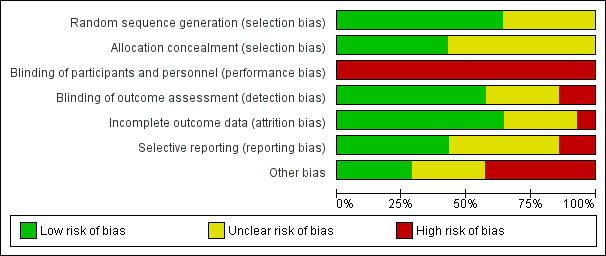
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
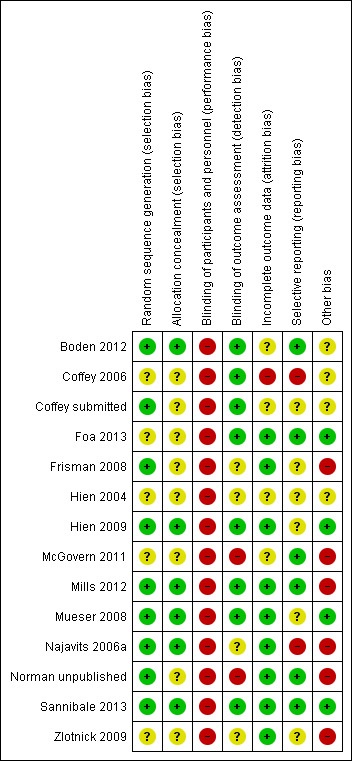
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eight studies specified the method of sequence generation and were judged as being at low risk of bias (Boden 2012; Coffey submitted; Frisman 2008; Hien 2009; Mills 2012; Mueser 2008; Norman unpublished; Sannibale 2013). The method of allocation was unclear in the remaining studies. Of the nine studies that adequately reported method of sequence generation, we judged five as also describing their method of allocation adequately (Boden 2012; Hien 2009; Mills 2012; Mueser 2008; Sannibale 2013). We were unclear about the process of sequence generation and allocation for Najavits 2006a, but received additional information from the lead author that allowed us to judge that these were of low risk. The process of sequence generation and allocation were unclear in the remaining studies.
Blinding
In trials of psychological therapy participants cannot normally be blind to the treatment allocation. We therefore classified all trials as being at high risk of performance bias. Eight trials included blinding of the outcome assessor and so were rated as being at low risk of bias (Boden 2012; Coffey 2006; Coffey submitted; Foa 2013; Hien 2009; Mills 2012; Mueser 2008; Sannibale 2013). Research interviewers were not blinded to treatment assignment at the follow‐up assessments in McGovern 2011 and Norman unpublished, which we therefore rated as high risk. We were unclear about the blinding of assessors in the remaining studies.
Incomplete outcome data
Seven studies reported drop‐out and loss to follow‐up clearly and performed intention‐to‐treat (ITT) analyses on all participants who were randomised, so that missing outcome data were replaced using a recognised statistical method, for example last observation carried forward (Foa 2013; Frisman 2008; Hien 2009; Mills 2012; Mueser 2008; Najavits 2006a; Sannibale 2013). We also judged Zlotnick 2009 as low risk because loss to follow‐up was low. Three studies undertook ITT analysis by including participants in analyses if they had attended at least one treatment session, following randomisation (Coffey submitted; Hien 2004; Norman unpublished). For Norman unpublished, people who did not attend any treatment sessions remained blind to their allocation, and based on guidance provided by Fergusson 2002, we judged this study to be of low risk. We were unclear about whether participants in Coffey submitted and Hien 2004 were aware of their allocation and whether this might have influenced drop‐out; we therefore judged these studies to be of unclear risk. We judged that methods of analysis were at high risk of bias in Coffey 2006.
Selective reporting
We were able to identify protocols with prespecified outcome measures for six trials (Boden 2012; Foa 2013; McGovern 2011; Mills 2012; Norman unpublished; Sannibale 2013). We checked that all prespecified outcomes were reported, and rated these studies as being at low risk of bias. We identified two trials as being at high risk (Coffey 2006; Najavits 2006a). We were able to extract only minimal data from Najavits 2006a because all outcomes were not fully reported and tabular data were mainly reported only for positive outcomes. We were unable to make a judgement about reporting bias for the other six trials.
Other potential sources of bias
We could identify no other potential sources of bias in four studies (Foa 2013; Hien 2009; Mueser 2008; Sannibale 2013). We judged that there was a high risk of bias because of additional factors in six studies (Frisman 2008; McGovern 2011; Mills 2012; Najavits 2006a; Norman unpublished; Zlotnick 2009). We were unable to make informed judgements about the remaining trials (Boden 2012; Coffey 2006; Coffey submitted; Hien 2004), and therefore rated them as unclear.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
for the main comparison.
| Trauma‐focused psychological therapy compared to control intervention | ||||||
| Patient or population: Individuals with post‐traumatic stress disorder and comorbid substance use disorder Settings: Community addiction and mental health services Intervention: Individual‐based psychological therapy including a trauma‐focused component Comparison: Treatment as usual/minimal intervention/placebo intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU/ minimal intervention | Individual‐based psychological therapyincluding a trauma‐focused component | |||||
|
PTSD severity following treatment completion As assessed by the CAPS, PSS‐I, or IES‐R. High scores indicate greater symptom severity |
‐ | The mean PTSD severity following treatment completion in the intervention groups was 0.41 standard deviations lower (0.72 to 0.1 lower) | ‐ | 405 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | SMD ‐0.41 (‐0.72 to ‐0.1) Effect sizes of the range 0.2 to 0.5 indicate a small treatment effect |
|
Drug or alcohol use, or both following treatment completion As assessed by the TLFB or CIDI. High scores indicate greater symptom severity |
‐ | The mean drug/alcohol use following treatment completion in the intervention groups was 0.13 standard deviations lower (0.41 lower to 0.15 higher) | ‐ | 388 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | SMD ‐0.13 (‐0.41 to 0.15) Not significant |
| Treatment completers | Study population | RR 0.80 (0.69 to 0.93) | 316 (3 studies) | ⊕⊕⊝⊝ low1,3 | Indicates higher drop‐out in the intervention group | |
| 761 per 1000 | 609 per 1000 (525 to 708) | |||||
| Moderate | ||||||
| 718 per 1000 | 574 per 1000 (495 to 668) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CAPS: Clinician Administered PTSD Scale; CI: confidence interval; CIDI: Composite International Diagnostic Interview; IES‐R: Impact of Events Scale‐Revised; PSS‐I: PTSD Symptom Scale‐Interview; PTSD: post‐traumatic stress disorder; RR: risk ratio; SMD: standardised mean difference; TAU: treatment as usual; TLFB: Timeline Followback Interview | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Quality of evidence downgraded by one point because the risk of bias in most trials was high or unclear in several domains. 2Quality of evidence downgraded by one point because of a high level of unexplained statistical heterogeneity. 3Quality of evidence downgraded by one point as a result of significant clinical heterogeneity.
SUD based adjunctive therapy was not a formal part of either the experimental or control condition in one study (Coffey 2006). However, participants were recruited through an SUD based service and it is likely that they would have had access to adjunctive SUD‐ based therapy on an informal basis. All other studies in this comparison included formal access SUD‐based adjunctive therapy.
Summary of findings 2. Trauma‐focused psychological intervention compared to active psychological intervention for SUD only.
| Trauma‐focused psychological therapy compared to active psychological therapy for SUD only | ||||||
| Patient or population: Individuals with post‐traumatic stress disorder and comorbid substance use disorder Settings: Community addiction and mental health services Intervention: Individual‐based psychological therapy including a trauma‐focused component Comparison: Active psychological therapy for SUD only | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Active psychological therapyfor SUD only | Individual‐based psychological therapyincluding a trauma‐focused component | |||||
|
PTSD severity following treatment completion As assessed by the CAPS. High scores indicate greater symptom severity |
‐ | The mean PTSD severity following treatment completion in the intervention groups was 3.91 lower (19.16 lower to 11.34 higher) | ‐ | 46 (1 study) | ⊕⊕⊝⊝ low1 | Not significant |
|
Drug or alcohol use, or both following treatment completion As assessed by the TLFB. High scores indicate greater symptom severity |
‐ | The mean drug/alcohol use following treatment completion in the intervention groups was 1.27 lower (5.76 lower to 3.22 higher) | ‐ | 46 (1 study) | ⊕⊕⊝⊝ low1 | Not significant |
| Treatment completers | Study population | RR 1 (0.74 to 1.36) | 62 (1 study) | ⊕⊕⊝⊝ low1 | Not significant | |
| 724 per 1000 | 724 per 1000 (536 to 985) | |||||
| Moderate | ||||||
| 724 per 1000 | 724 per 1000 (536 to 985) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CAPS: Clinician Administered PTSD Scale; CI: confidence interval; PTSD: post‐traumatic stress disorder; RR: risk ratio; SUD: substance use disorder; TLFB: Timeline Followback Interview | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Quality of evidence downgraded by two points because findings were based on outcomes from one study with a small sample size.
SUD based adjunctive therapy was not a formal part of either the experimental or control condition in the study contributing to this comparison.
Summary of findings 3. Non‐trauma‐focused psychological intervention for PTSD and SUD or PTSD only compared to control intervention.
| Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only compared to control intervention | ||||||
| Patient or population: Individuals with post‐traumatic stress disorder and comorbid substance use disorder Settings: Community addiction services and prison service Intervention: Group‐ and individual‐based non‐trauma‐focused psychological therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU/minimal intervention | Group or Indvidual based non‐trauma‐focused psychological therapy | |||||
|
PTSD severity following treatment completion ‐ Individual‐based intervention As assessed by the CAPS. High scores indicate greater symptom severity |
‐ | The mean PTSD severity following treatment completion in the intervention groups was 0.22 standard deviations lower (0.83 lower to 0.39 higher) | ‐ | 44 (1 study) | ⊕⊕⊝⊝ low1 | SMD ‐0.22 (‐0.83 to 0.39) |
|
PTSD severity following treatment completion ‐ Group‐based intervention As assessed by the CAPS or IES‐R. High scores indicate greater symptom severity |
‐ | The mean PTSD severity following treatment completion in the intervention groups was 0.02 standard deviations lower (0.19 lower to 0.16 higher) | ‐ | 513 (4 studies) | ⊕⊕⊝⊝ low2,3 | SMD ‐0.02 (‐0.19 to 0.16) |
| Drug or alcohol use, or both following treatment completion ‐ Individual‐based intervention | ‐ | No data | ‐ | ‐ | ‐ | Not estimable |
|
Drug or alcohol use, or both following treatment completion ‐ Group‐based intervention As assessed by the ASI, TLFB or CIDI. High scores indicate greater symptom severity |
‐ | The mean drug/alcohol use following treatment completion in the intervention groups was 0.41 standard deviations lower (0.97 lower to 0.14 higher) | ‐ | 464 (3 studies) | ⊕⊝⊝⊝ very low2,3,4 | SMD ‐0.41 (‐0.97 to 0.14) Not significant |
| Treatment completers ‐ Individual‐based intervention | ‐ | No data | ‐ | ‐ | ‐ | Not estimable |
| Treatment completers ‐ Group‐based intervention | Study population | RR 1.13 (0.88 to 1.45) | 381 (2 studies) | ⊕⊕⊝⊝ low2,3 | ‐ | |
| 538 per 1000 | 608 per 1000 (473 to 780) | |||||
| Moderate | ||||||
| 493 per 1000 | 557 per 1000 (434 to 715) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASI: Addiction Severity Index; CAPS: Clinician Administered PTSD Scale; CI: confidence interval; CIDI: Composite International Diagnostic Interview; IES‐R: Impact of Events Scale‐Revised; PTSD: post‐traumatic stress disorder; RR: risk ratio; SMD: standardised mean difference; SUD: substance use disorder; TAU: treatment as usual; TLFB: Timeline Followback Interview | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Quality of evidence downgraded by two points because findings were based on outcomes from one study with a small sample size. 2Quality of evidence downgraded by one point because the risk of bias in most trials was high or unclear in several domains. 3Quality of evidence downgraded by one point because of significant clinical heterogeneity. 4Quality of evidence downgraded by one point because of a high level of unexplained statistical heterogeneity.
The individual‐based study (Mueser 2008) in this comparison did not include access to SUD based adjunctive therapy. Participants in all other studies were able to access SUD‐based adjunctive therapy.
Summary of findings 4. Non‐trauma‐focused psychological intervention for PTSD and SUD or PTSD only compared to active psychological intervention for SUD only.
| Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only compared to active psychological therapy for SUD only | ||||||
| Patient or population: Individuals with post‐traumatic stress disorder and comorbid substance use disorder Settings: Community substance abuse treatment programs Intervention: Individual‐based combined non‐trauma‐focused psychological therapy Comparison: Active psychological therapy for SUD only | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Active psychological therapyfor SUD only | Individual‐based combined non‐trauma‐focused psychological therapy | |||||
|
PTSD severity following treatment completion As assessed by the CAPS. High scores indicate greater symptom severity |
‐ | The mean PTSD severity following treatment completion in the intervention groups was 0.26 standard deviations lower (1.29 lower to 0.77 higher) | ‐ | 128 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | SMD ‐0.26 (‐1.29 to 0.77) Not significant |
|
Drug or alcohol use, or both following treatment completion As assessed by the SUI or ASI. High scores indicate greater symptom severity |
‐ | The mean drug/alcohol use following treatment completion in the intervention groups was 0.22 standard deviations higher (0.13 lower to 0.57 higher) | ‐ | 128 (2 studies) | ⊕⊕⊝⊝ low1,3 | SMD 0.22 (‐0.13 to 0.57) Not significant |
| Treatment completers | Study population | RR 0.91 (0.68 to 1.20) | 128 (2 studies) | ⊕⊝⊝⊝ very low1,3 | Not significant | |
| 618 per 1000 | 563 per 1000 (420 to 742) | |||||
| Moderate | ||||||
| 591 per 1000 | 538 per 1000 (402 to 709) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASI: Addiction Severity Index; CAPS: Clinician Administered PTSD Scale; CI: confidence interval; PTSD: post‐traumatic stress disorder; RR: risk ratio; SMD: standardised mean difference; SUD: substance use disorder; SUI: Substance Use Inventory | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Quality of evidence downgraded by one point because the risk of bias in most trials was high or unclear in several domains. 2Quality of evidence downgraded by two points because of a high level of unexplained statistical heterogeneity. 3Quality of evidence downgraded by one point because findings were based on outcomes from two studies with small sample sizes.
Both studies in this comparison involved access to adjunctive SUD‐based therapy.
We have grouped results according to the intervention characteristics identified above in the Interventions section of Included studies.
Comparison 1: Trauma‐focused psychological therapy versus control intervention
Four studies contributed to this comparison. These studies either evaluated trauma‐focused CBT‐based interventions in combination with SUD‐focused interventions, or in the context of a substance abuse setting where SUD interventions were already being received. All studies evaluated interventions delivered individually.
Primary outcomes
1.1 PTSD severity
Four studies including 405 participants reported severity of PTSD symptoms at post‐treatment (Analysis 1.1). We noted a small effect in favour of individual psychological therapy (standardised mean difference (SMD) ‐0.41; 95% confidence interval (CI) ‐0.72 to ‐0.10), although heterogeneity was moderate (I² = 49%). One study with 120 participants reported follow‐up at 3 to 4 months (Analysis 1.2); we again noted a small effect in favour of individual psychological therapy (mean difference (MD) ‐9.83; 95% CI ‐17.11 to ‐2.55). Three studies including 388 participants reported follow‐up at 5 to 7 months (Analysis 1.3). There was a small effect in favour of individual psychological therapy (SMD ‐0.34; 95% CI ‐0.58 to ‐0.10), and we noted no important heterogeneity (I² = 26%).
1.1. Analysis.
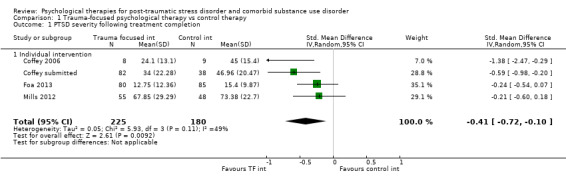
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 1 PTSD severity following treatment completion.
1.2. Analysis.
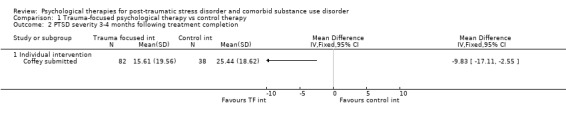
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 2 PTSD severity 3‐4 months following treatment completion.
1.3. Analysis.
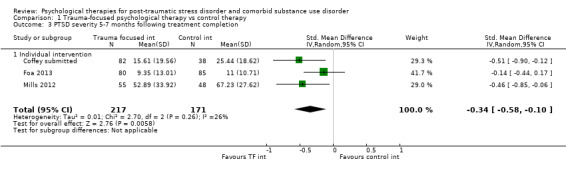
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 3 PTSD severity 5‐7 months following treatment completion.
1.2 Drug or alcohol use, or both
Three studies including 388 participants reported severity of SUD symptoms post‐treatment (Analysis 1.4). We found no significant difference (SMD ‐0.13; 95% CI ‐0.41 to 0.15). We noted a moderate level of heterogeneity (I² = 45%). One study with 120 participants reported SUD symptom severity at 3 to 4 months (Analysis 1.5). No significant difference was found (MD ‐2.33; 95% CI ‐12.87 to 8.21). Three studies including 388 participants reported follow‐up at 5 to 7 months (Analysis 1.6). There was a small effect in favour of individual psychological therapy (SMD ‐0.28; 95% CI ‐0.48 to ‐0.07), and we noted no heterogeneity (I² = 0%).
1.4. Analysis.
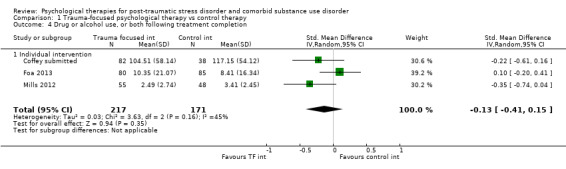
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 4 Drug or alcohol use, or both following treatment completion.
1.5. Analysis.

Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 5 Drug or alcohol use, or both 3‐4 months following treatment completion.
1.6. Analysis.
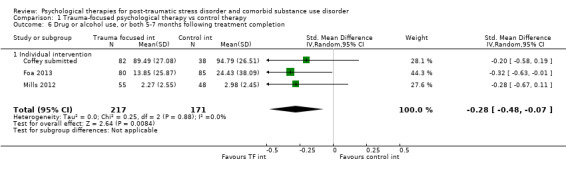
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 6 Drug or alcohol use, or both 5‐7 months following treatment completion.
1.3 Treatment completion
Three studies including 316 participants reported treatment completers (Analysis 1.7). We found a significant difference in favour of the control condition (risk ratio (RR) 0.78; 95% CI 0.64 to 0.96). We noted a moderate level of heterogeneity (I² = 41%).
1.7. Analysis.
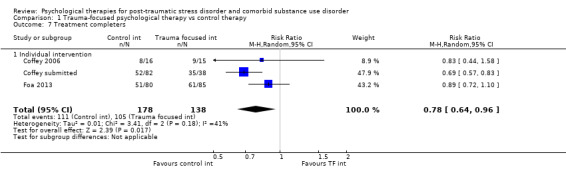
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 7 Treatment completers.
Secondary outcomes
1.4 PTSD diagnosis after treatment
Only one study with 120 participants reported outcomes for PTSD diagnosis (Analysis 1.8). There was a small treatment effect in favour of individual psychological therapy (RR 0.71; 95% CI 0.51 to 1.00).
1.8. Analysis.
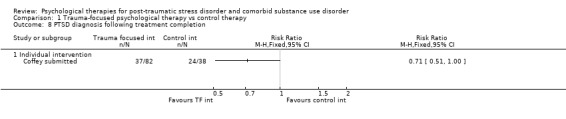
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 8 PTSD diagnosis following treatment completion.
1.5 SUD diagnosis after treatment
No data were available for diagnostic status for substance use.
1.6 Adverse events
Two studies provided data on adverse events (Analysis 1.9). These two studies reported a total of 20 events. None of these events were attributed to treatment interventions provided in the studies. We found no significant differences between the two groups (RR 0.81; 95% CI 0.34 to 1.90), noted no heterogeneity (I² = 0%) (Analysis 1.10).
1.9. Analysis.
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 9 Adverse events.
| Adverse events | |
|---|---|
| Study | |
| Individual intervention | |
| Coffey 2006 | Not reported |
| Coffey submitted | Not reported |
| Foa 2013 | Twelve participants were removed from the study because of serious adverse events (serious suicidal ideation, n = 7; serious medical illness, n = 3; psychotic symptoms, n = 1; death, n = 1; however, none of these events was determined to be related to the study). |
| Mills 2012 | Two participants from the treatment group (3.6%) and 5 participants from the control group (10.4%) attempted suicide during the study (OR, 0.32 [95% CI, 0.06‐1.76]). Although it is possible that these attempts were related to participation in the study, all 7 individuals reported that this was not the case and elected to remain involved with the study. Additionally, 1 participant from the treatment group (1.8%) died as a result of a preexisting medical condition. |
1.10. Analysis.
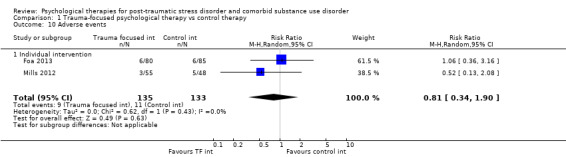
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 10 Adverse events.
1.7 Compliance, as measured by proportion of treatment sessions attended
Three studies reported data on the mean number of treatment sessions attended by participants in the experimental group (Analysis 1.11), with participants attending a mean of 6.89 (standard deviation (SD) = 4.63) sessions. The proportions of available sessions attended per study varied from 35.2% to 68.0%.
1.11. Analysis.
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 11 Mean number of sessions attended for intervention group.
| Mean number of sessions attended for intervention group | |||
|---|---|---|---|
| Study |
Mean number sessions attended by intervention group (& SD) |
Number sessions available | Percentage attended |
| Studies including intervention for SUD | |||
| Coffey submitted | 8.16 (3.26) approximated | 12 | 68.0% |
| Foa 2013 | 6.33 (5.31) | 18 | 35.2% |
| Mills 2012 | 5.83 (4.94) | 13 | 44.9% |
1.8 General functioning
No data were available.
1.9 Use of health‐related resources
No data were available.
Comparison 2: Trauma‐focused psychological therapy versus active psychological therapy for SUD only
One study with 62 participants contributed to the comparison. Intervention in this study was delivered individually.
Primary outcomes
2.1 PTSD severity
One study reported severity of PTSD symptoms. No significant difference was found post‐treatment (MD ‐3.91; 95% CI ‐19.16 to 11.34; n = 46; Analysis 2.1), at 5 to 7 months' follow‐up (MD ‐9.32; 95% CI ‐22.89 to 4.25; n = 45; Analysis 2.2), or at 8 to 10 months' follow‐up (MD 2.11; 95% CI ‐16.10 to 20.32; n = 47; Analysis 2.3). Differences in the numbers of participants contributing to each analysis were a result of the numbers available to follow‐up at each time point.
2.1. Analysis.
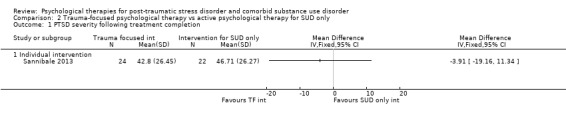
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 1 PTSD severity following treatment completion.
2.2. Analysis.
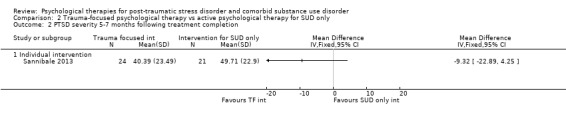
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 2 PTSD severity 5‐7 months following treatment completion.
2.3. Analysis.
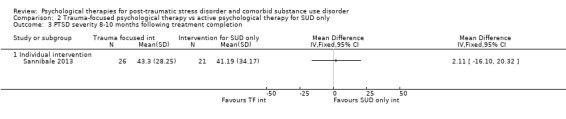
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 3 PTSD severity 8‐10 months following treatment completion.
2.2 Drug or alcohol use, or both
One study reported severity of PTSD symptoms. No significant difference was found post‐treatment (MD ‐1.27; 95% CI ‐5.76 to 3.22; n = 46; Analysis 2.4), at 5 to 7 months' follow‐up (MD 1.90; 95% CI ‐1.65 to 5.45; n = 45; Analysis 2.5), or at 8 to 10 months' follow‐up (MD ‐0.93; 95% CI ‐4.04 to 2.18; n = 47; Analysis 2.6).
2.4. Analysis.
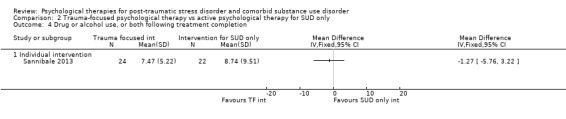
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 4 Drug or alcohol use, or both following treatment completion.
2.5. Analysis.
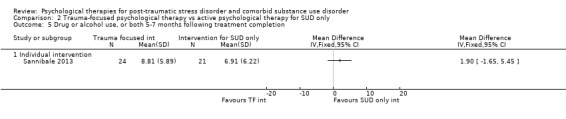
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 5 Drug or alcohol use, or both 5‐7 months following treatment completion.
2.6. Analysis.
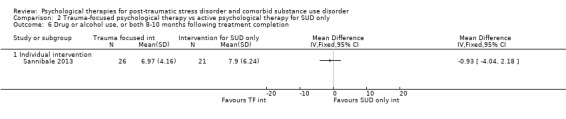
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 6 Drug or alcohol use, or both 8‐10 months following treatment completion.
2.3 Treatment completion
Data on treatment completers were available from one study (Analysis 2.7). No difference was found between the two groups on rate of treatment completion (RR 1.00; 95% CI 0.74 to 1.36).
2.7. Analysis.
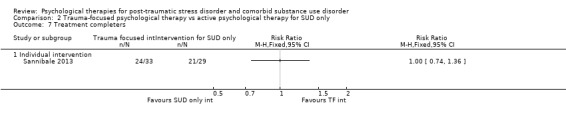
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 7 Treatment completers.
Secondary outcomes
2.4 PTSD diagnosis after treatment
One study reported data on PTSD diagnosis post‐treatment (Analysis 2.8). No difference was found between the two groups (RR 1.04; 95% CI 0.67 to 1.62).
2.8. Analysis.
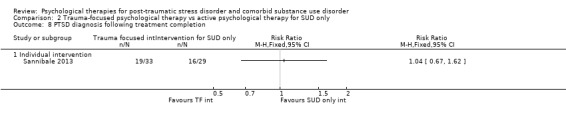
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 8 PTSD diagnosis following treatment completion.
2.5 SUD diagnosis after treatment
One study reported data on SUD diagnosis post‐treatment (Analysis 2.9). No difference was found between the two groups (RR 1.16; 95% CI 0.83 to 1.60).
2.9. Analysis.
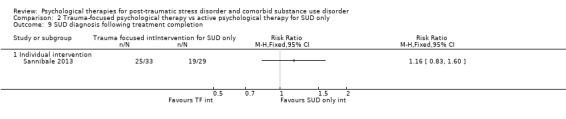
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 9 SUD diagnosis following treatment completion.
2.6 Adverse events
Adverse events were not reported.
2.7 Compliance, as measured by proportion of treatment sessions attended
No data were available for the mean number of treatment sessions attended.
2.8 General functioning
No data were available.
2.9 Use of health‐related resources
No data were available.
Comparison 3: Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only versus control intervention
One study evaluated non‐trauma‐focused psychological therapy delivered individually (Mueser 2008). Five studies evaluated group‐based interventions. Four studies evaluated Seeking Safety as the experimental condition. Frisman 2008 evaluated an alternative group‐based intervention (Frisman 2008 also had a far lower rate of completion than other studies).
Primary outcomes
3.1 PTSD severity
Individual‐based intervention
One study with 44 participants reported severity of PTSD symptoms. No significant difference was found post‐treatment (SMD ‐0.22; 95% CI ‐0.83 to 0.39; Analysis 3.1), at 3 to 4 months' follow‐up (SMD ‐0.25; 95% CI ‐0.86 to 0.36; Analysis 3.2), or at 5 to 7 months' follow‐up (SMD ‐0.20; 95% CI ‐0.81 to 0.41; Analysis 3.3).
3.1. Analysis.
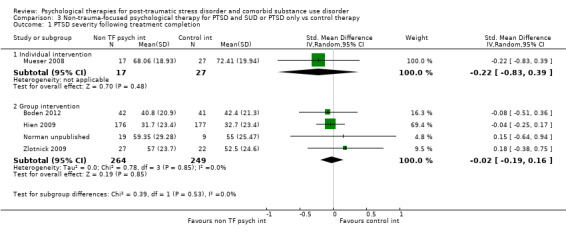
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 1 PTSD severity following treatment completion.
3.2. Analysis.
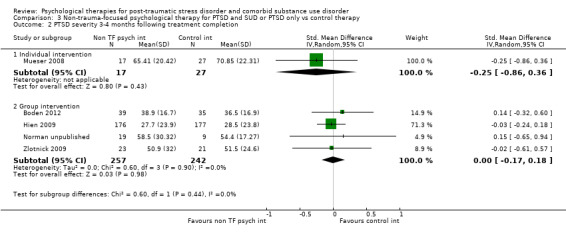
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 2 PTSD severity 3‐4 months following treatment completion.
3.3. Analysis.
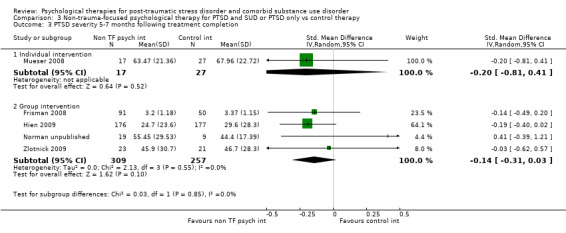
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 3 PTSD severity 5‐7 months following treatment completion.
Group‐based intervention
Post‐treatment data were available from 4 studies including 513 participants. All 4 studies evaluated Seeking Safety or partial‐dose Seeking Safety as the experimental condition. We found no significant difference between the two groups (SMD ‐0.02; 95% CI ‐0.19 to 0.16; Analysis 3.1). There was general consistency in study results (I² = 0%). Data were also available from the same four studies at 3 to 4 months' follow‐up (Analysis 3.2); again, we found no significant differences (SMD 0.00; 95% CI ‐0.17 to 0.18; n = 499) and noted no heterogeneity (I² = 0%). Data at 5 to 7 months' follow‐up were available from 4 studies including 566 participants. We identified no effect (SMD ‐0.14; 95% CI ‐0.31 to 0.03) and noted no heterogeneity (I² = 0%) (Analysis 3.3). Two studies with 518 participants provided data at 12 months' follow‐up (Analysis 3.4). No effect was evident (SMD ‐0.07; 95% CI ‐0.25 to 0.10). There was general consistency in study results (I² = 0%).
3.4. Analysis.
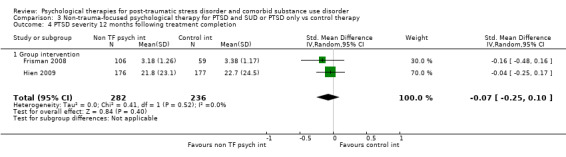
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 4 PTSD severity 12 months following treatment completion.
3.2 Drug or alcohol use, or both
Individual‐based intervention
No data were available.
Group‐based intervention
Post‐treatment data were available from 3 studies including 464 participants (Analysis 3.5). All three studies evaluated Seeking Safety or partial‐dose Seeking Safety as the experimental condition. We found no significant difference between the two conditions (SMD ‐0.41; 95% CI ‐0.97 to 0.14). The degree of heterogeneity was substantial (I² = 79%). It should be noted that there was a significant difference in favour of the Seeking Safety intervention for two studies post‐treatment (Boden 2012; Norman unpublished), but not for the third study (Hien 2009), which was much larger. Hien 2009 differed from Boden 2012 and Norman unpublished in that intervention was based on a partial‐dose programme. This is addressed further under 'Subgroup analyses' below. Data at 3 to 4 months' follow‐up were available from the 4 Seeking Safety or partial‐dose Seeking Safety studies with 499 participants (Analysis 3.6). We found no significant differences (SMD ‐0.08; 95% CI ‐0.40 to 0.23). The degree of heterogeneity post‐treatment was moderate (I² = 48%). Data at 5 to 7 months' follow‐up were available from 4 studies with 572 participants (Analysis 3.7). We found no differences (SMD ‐0.06; 95% CI ‐0.23 to 0.11) and noted no heterogeneity (I² = 0%). Two studies with 528 participants provided data at 12 months (Analysis 3.8). We found no differences (SMD 0.02; 95% CI ‐0.15 to 0.20) and noted no heterogeneity (I² = 0%).
3.5. Analysis.
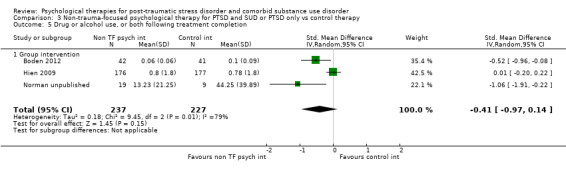
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 5 Drug or alcohol use, or both following treatment completion.
3.6. Analysis.
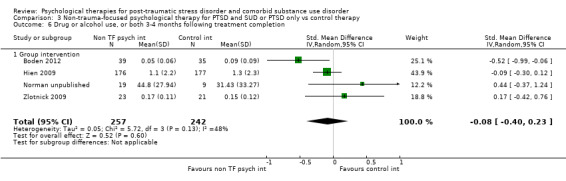
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 6 Drug or alcohol use, or both 3‐4 months following treatment completion.
3.7. Analysis.
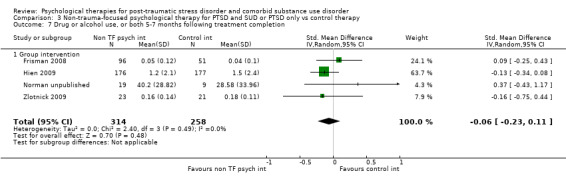
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 7 Drug or alcohol use, or both 5‐7 months following treatment completion.
3.8. Analysis.
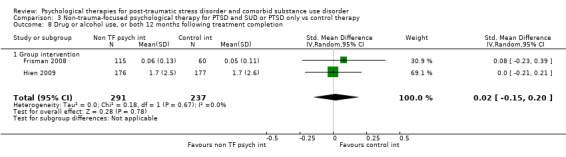
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 8 Drug or alcohol use, or both 12 months following treatment completion.
3.3 Treatment completion
Individual‐based intervention
Data on treatment completion were available from one study only for participants receiving the experimental intervention (Analysis 3.9); 70.6% of participants attended a minimum of 6 of the 16 available treatment sessions and were considered treatment completers; 52.9% attended 12 or more sessions.
3.9. Analysis.
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 9 Treatment completers.
| Treatment completers | |
|---|---|
| Study | |
| Individual intervention | |
| Mueser 2008 | 12/16 (70.6%) |
| Group intervention | |
| Frisman 2008 | 39/141 (28%) |
Group‐based intervention
Two studies including 381 participants reported data on treatment completers for both the experimental and control condition (Analysis 3.10). We found no significant difference between the two conditions (RR 1.13; 95% CI 0.88 to 1.45). There was general consistency in study results (I² = 0%). One study only provided data about the number of participants in the experimental group considered to be completers (Analysis 3.9). At 28%, treatment adherence for the group intervention in this study was very low.
3.10. Analysis.
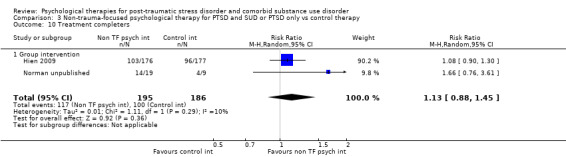
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 10 Treatment completers.
Secondary outcomes
3.4 PTSD diagnosis after treatment
Individual‐based intervention
No data were available.
Group‐based intervention
Two studies with 77 participants provided data on PTSD diagnosis post‐treatment (Analysis 3.11). We found no differences (RR 1.01; 95% CI 0.66 to 1.54) and noted no heterogeneity (I² = 0%). Both studies evaluated full‐dose Seeking Safety interventions as the experimental condition.
3.11. Analysis.
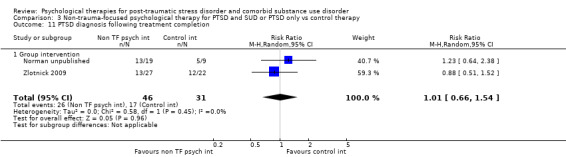
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 11 PTSD diagnosis following treatment completion.
3.5 SUD diagnosis after treatment
No data were available.
3.6 Adverse events
Individual‐based intervention
No data were available.
Group‐based intervention
Three studies reported on adverse events (Analysis 3.12). Two of the studies reported no adverse events. The third study reported 83 study‐related adverse events from 353 participants (Analysis 3.13). We found no differences in the number of events experienced between the two conditions (RR 1.03; 95% CI 0.71 to 1.50).
3.12. Analysis.
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 12 Adverse events.
| Adverse events | |
|---|---|
| Study | |
| Group intervention | |
| Boden 2012 | No harmful or unintended effects were observed during the trial. |
| Frisman 2008 | Not reported |
| Hien 2009 | 83 study related adverse events were identified (Killeen 2008). Of these 61 were rated as moderate to severe: 28 for the experimental condition; 33 for the control condition. |
| Najavits 2006a | Not reported |
| Norman unpublished | No adverse events occurred during the study. |
| Zlotnick 2009 | Not reported |
3.13. Analysis.
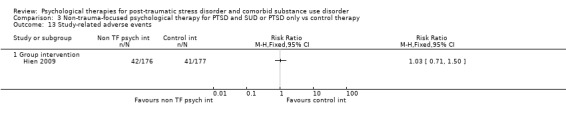
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 13 Study‐related adverse events.
3.7 Compliance, as measured by proportion of treatment sessions attended
Individual‐based intervention
No data were available.
Group‐based intervention
Five studies reported data on the mean number of treatment sessions attended by participants in the experimental group (Analysis 3.14; Analysis 3.15), with participants attending a mean of 6.31 (SD = 5.71) sessions. The proportions of available sessions attended for the experimental group per study ranged from 37.9% to 62.4%.
3.14. Analysis.
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 14 Mean number of sessions attended for intervention group.
| Mean number of sessions attended for intervention group | |||||
|---|---|---|---|---|---|
| Study | Mean number treatment condition sessions attended by intervention group (& SD) | Number sessions available | Percentage active intervention sessions attended | Mean number sessions attended by control group (& SD) | Percentage attended |
| Group intervention | |||||
| Boden 2012 | Not reported | Not reported | |||
| Frisman 2008 | 3.41 (3.38) active intervention sessions + 30.67 (37.38) TAU sessions | 9 active intervention sessions plus TAU sessions | 37.9% | 39.0 (69.62) TAU sessions | |
| Hien 2009 | 6.2 (4.5) | 12 | 51.7% | 6.9 (4.3) | 57.5% |
| Najavits 2006a | 9.67(5.05) active intervention session (11.78 (6.25) active intervention +TAU sessions) | 25 active intervention sessions plus TAU sessions | 38.7% | Not reported | |
| Norman unpublished | 12.5 (8.77) | 24 | 52.1% | 7.78 (5.78) | 32.4% |
| Zlotnick 2009 | 15.6 (6.2) | 25 | 62.4% | Not reported | |
3.15. Analysis.
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 15 Mean number of sessions attended.
3.8 General functioning
No data were available.
3.9 Use of health‐related resources
No data were available.
Comparison 4: Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only versus active psychological therapy for SUD only
Two studies including 128 participants contributed to this comparison. Both studies evaluated interventions delivered individually.
Primary outcomes
4.1 PTSD severity
Two studies including 128 participants reported severity of PTSD symptoms. We found no significant difference post‐treatment (SMD ‐0.26; 95% CI ‐1.29 to 0.77; Analysis 4.1). The level of heterogeneity was considerable (I² = 87%). There was a significant difference in favour of the combined intervention post‐treatment (MD ‐16.52; 95% CI ‐27.99 to ‐5.05) for one of these two studies, McGovern 2011, although it was noted that the two groups in this study were not well matched for PTSD severity at baseline. The same studies including 128 participants reported follow‐up at 3 to 4 months, finding no significant difference between the two conditions (SMD 0.12; 95% CI ‐0.31 to 0.55; Analysis 4.2). We noted a moderate level of heterogeneity (I² = 33%). One study with 75 participants reported severity of PTSD symptoms at 5 to 7 months, finding no significant difference (MD 7.52; 95% CI ‐3.78 to 18.82; Analysis 4.3).
4.1. Analysis.
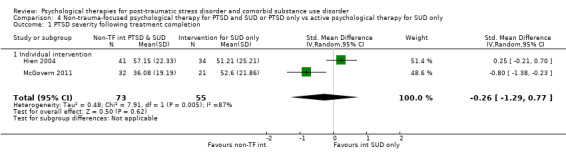
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 1 PTSD severity following treatment completion.
4.2. Analysis.
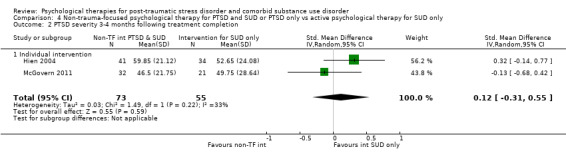
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 2 PTSD severity 3‐4 months following treatment completion.
4.3. Analysis.
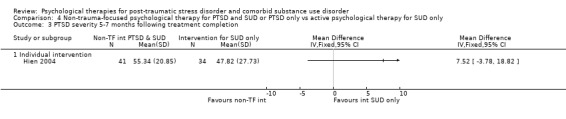
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 3 PTSD severity 5‐7 months following treatment completion.
4.2 Drug or alcohol use, or both
Two studies including 128 participants reported severity of SUD symptoms. We found no significant differences post‐treatment (SMD 0.22; 95% CI ‐0.13 to 0.57; Analysis 4.4), or at 3 to 4 months' follow‐up (SMD 0.18; 95% CI ‐0.18 to 0.53; Analysis 4.5); we noted no heterogeneity at either follow‐up point (I² = 0%). One study with 75 participants reported severity of SUD symptoms at 5 to 7 months' follow‐up (Analysis 4.6); we found no significant difference (MD 0.10; 95% CI ‐0.20 to 0.40).
4.4. Analysis.
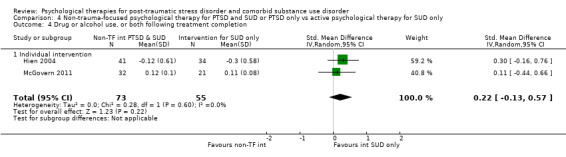
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 4 Drug or alcohol use, or both following treatment completion.
4.5. Analysis.
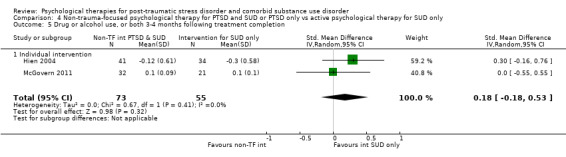
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 5 Drug or alcohol use, or both 3‐4 months following treatment completion.
4.6. Analysis.
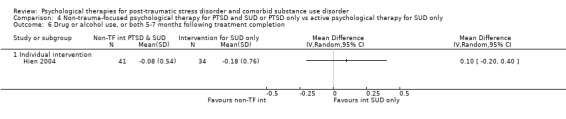
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 6 Drug or alcohol use, or both 5‐7 months following treatment completion.
4.3 Treatment completion
Data on treatment completers were available from 2 studies with 128 participants. We found no difference between the two groups on rate of treatment completion (RR 0.91; 95% CI 0.68 to 1.20; Analysis 4.7).
4.7. Analysis.
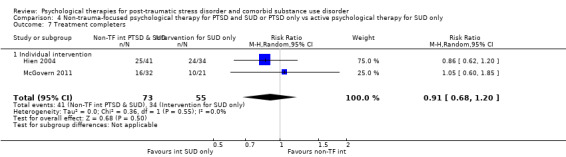
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 7 Treatment completers.
Secondary outcomes
4.4 PTSD diagnosis after treatment
One study reported data on PTSD diagnosis post‐treatment (Analysis 4.8). No difference was found between the two groups (RR 0.94; 95% CI 0.68 to 1.30).
4.8. Analysis.
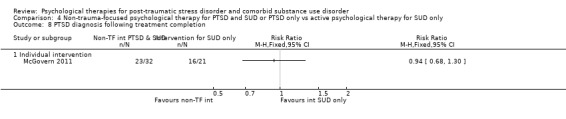
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 8 PTSD diagnosis following treatment completion.
4.5 SUD diagnosis after treatment
No data were available.
4.6 Adverse events
Adverse events were not reported.
4.7 Compliance, as measured by proportion of treatment sessions attended
Data were available from 1 study with 75 participants (Analysis 4.9). There was no difference between the two groups for treatment attendance (MD ‐0.10; 95% CI ‐3.75 to 3.55), with attendance of 48.0% at the experimental group sessions and 48.4% at control group sessions.
4.9. Analysis.

Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 9 Mean number of sessions attended.
4.8 General functioning
No data were available.
4.9 Use of health‐related resources
No data were available.
Reporting bias
Twelve of the 14 studies included in this review were published, and one study completed recently, Coffey submitted, was in the process of seeking publication. We followed up all appropriate trials registered with World Health Organization's trials portal (ICTRP) and ClinicalTrials.gov and made attempts to contact authors known to be active in this field. We identified one unpublished study that we were able to include. The numbers of studies in each comparison were insufficient to allow for meaningful consideration of publication bias using funnel plots (Lau 2006).
Subgroup analyses
Comparison 3: Group‐based combined non‐trauma‐focused psychological therapy for PTSD and SUD versus waiting list/treatment as usual/minimal intervention/placebo intervention
We reported analysis for the effects of group‐based interventions on PTSD severity and drug/alcohol use at initial post‐treatment follow‐up above (Analysis 3.1; Analysis 3.5). Seeking Safety is widely used as a group‐based intervention for treatment of comorbid PTSD and SUD. All studies contributing to these analyses evaluated Seeking Safety. Other analyses in Comparison 3 included one study that did not evaluate Seeking Safety (Frisman 2008). We therefore undertook subgroup analyses of PTSD severity and drug/alcohol use at later follow‐up points excluding Frisman 2008. There was no evidence of an effect of Seeking Safety on PTSD severity at 5 to 7 months (SMD ‐0.12; 95% CI ‐0.34 to 0.10; k = 3; n = 425; Analysis 3.16) or at 12 months (SMD ‐0.04; 95% CI ‐0.25 to 0.17; k = 1; n = 353; Analysis 3.17). There was also no evidence of an effect on drug/alcohol use at either 5 to 7 months (SMD ‐0.11; 95% CI ‐0.30 to 0.08; k = 3; n = 425; Analysis 3.18) or at 12 months (SMD 0.00; 95% CI ‐0.21 to 0.21; k = 1; n = 353; Analysis 3.19).
3.16. Analysis.
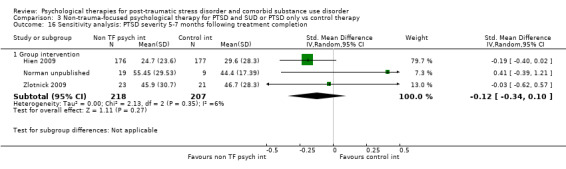
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 16 Sensitivity analysis: PTSD severity 5‐7 months following treatment completion.
3.17. Analysis.
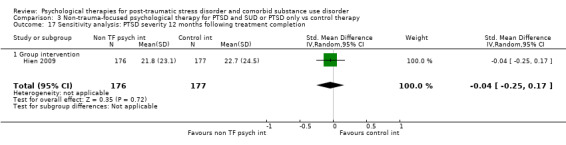
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 17 Sensitivity analysis: PTSD severity 12 months following treatment completion.
3.18. Analysis.
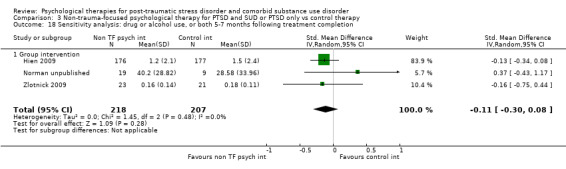
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 18 Sensitivity analysis: drug or alcohol use, or both 5‐7 months following treatment completion.
3.19. Analysis.
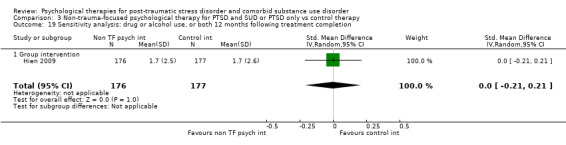
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 19 Sensitivity analysis: drug or alcohol use, or both 12 months following treatment completion.
As we noted, the study by Hien 2009 included a 12‐session partial dose of Seeking Safety. We therefore undertook post hoc analyses including only data from the full‐dose Seeking Safety studies. These analyses showed no effect post‐treatment (SMD ‐0.02; 95% CI ‐0.19 to 0.16; k = 3; n = 160), at 3 to 4 months (SMD 0.09; 95% CI ‐0.24 to 0.42; k = 3; n = 146), or at 5 to 7 months (SMD 0.13; 95% CI ‐0.35 to 0.60; k = 2; n = 72) for PTSD severity. We did find a moderate effect in favour of full‐dose Seeking Safety post‐treatment for drug/alcohol use (SMD ‐0.67; 95% CI ‐1.14 to ‐0.19; k = 2; n = 111). We noted no important heterogeneity (I² = 20%). There was no effect at 3 to 4 months (SMD ‐0.03; 95% CI ‐0.62 to 0.56; k = 3; n = 146) or at 5 to 7 months (SMD 0.03; 95% CI ‐0.45 to 0.51; k = 2; n = 72) for drug/alcohol use.
Sensitivity analyses
Comparison 1: Individual‐based psychological therapy including a trauma‐focused component plus SUD intervention versus waiting list/treatment as usual/minimal intervention/placebo intervention
Coffey 2006 provided outcome data only on the basis of participants who were available for follow‐up, and loss to follow‐up was high. We therefore undertook a sensitivity analysis for PTSD severity (Analysis 1.1) excluding this study. We continued to find a small effect post‐treatment in favour of individual‐based psychological therapy, although this effect was reduced (SMD ‐0.33; 95% CI ‐0.56 to ‐0.10; Analysis 1.12). We noted no important heterogeneity (I² = 19%). Coffey 2006 did not contribute to analysis at other follow‐up points.
1.12. Analysis.
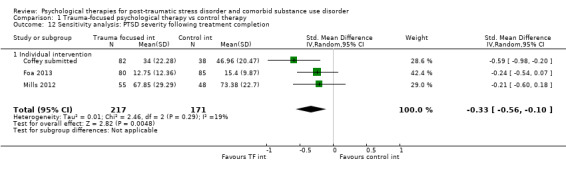
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 12 Sensitivity analysis: PTSD severity following treatment completion.
Discussion
Summary of main results
We included 14 studies including 1506 participants in this review. It was apparent that research attention is mainly focused on two broad types of intervention for PTSD and SUD comorbidity (van Dam 2012). These are CBT‐based interventions delivered on an individual basis consisting of a significant trauma‐focused component; and CBT‐based interventions aimed at developing and enhancing positive coping skills, without a trauma‐focused component. These latter interventions are offered on both an individual and a group basis.
psychological therapy including a trauma‐focused component
We were only able to identify individual‐based studies of psychological therapies including a trauma‐focused component. All trauma‐focused interventions were delivered alongside intervention aimed at treating SUD, or participants were recruited from SUD services. We found evidence of a small benefit for individual‐based psychological therapies that included a trauma‐focused component when compared against usual care or minimal intervention conditions for PTSD at the end of treatment and at 3 to 4 and 5 to 7 months after treatment. We found evidence of a small reduction in drug/alcohol use at 5 to 7 months after treatment, but not at earlier time points. There was evidence of lower rates of treatment completion in those receiving trauma‐focused intervention compared to treatment as usual/minimal intervention, when considered against study‐defined criteria for a treatment completer. There were no differences in PTSD‐ or SUD‐related outcomes when individual‐based trauma‐focused psychological therapy was compared with an active psychological therapy for SUD only. However, data were only available from one trial, which was not well powered.
Non‐trauma‐focused interventions
Individual‐based psychological therapy without a trauma‐focused component
We identified one study that evaluated an individual‐based psychological therapy without a trauma‐focused component against a usual care or minimal intervention condition (Mueser 2008). We obtained outcome data for a small cohort of individuals with SUD; this study did not include any SUD intervention. We identified only one study that evaluated individual‐based psychological therapy and included both PTSD‐ and SUD‐related components (Najavits 2006a). We were not able to use data from this study. We could not identify any benefits for individual‐based psychological therapy that did not offer a trauma‐focused component when compared against a usual care condition or another active psychological therapy. These findings were also based on data from two small trials.
Group‐based psychological therapy without a trauma‐focused component
The majority of the group‐based studies evaluating non‐trauma‐focused interventions were of Seeking Safety (Najavits 2002b). We found no improvement for PTSD severity at any time point when these interventions were compared against usual care/minimal intervention or against another active psychological therapy. We also found no benefits in terms of reduction in drug/alcohol use across studies, but did find a moderate reduction in drug/alcohol use for full‐dose Seeking Safety through post‐hoc analyses, when compared against treatment as usual/minimal intervention. This effect was not sustained at later follow‐up points. Non‐trauma‐focused group‐based interventions maintained a level of retention similar to control conditions for individual‐ and group‐based interventions.
Treatment completion and drop‐out
The level of drop‐out was high across all studies. Study criteria for defining a treatment completer were often modest. Of those studies reporting treatment completers, only one achieved retention of over 70% (Sannibale 2013). Rates of completion in most other studies were between 50% and 70%, with one large study reporting a completion rate of 28% for those in the active intervention group (Frisman 2008). However, studies (including Frisman 2008) were often more successful at retaining participants in follow‐up for research purposes. One study, Hien 2009, did investigate the reasons for drop‐out in a follow‐up study (Pinto 2011). Pinto 2011 found that participants with more education, higher attendance at 12‐step meetings, and strong therapeutic alliance with their therapists had better retention rates. This multisite trial also found some site differences, with retention being highest in a site that offered childcare and had the lowest overall intake.
Adverse events
In contrast to Bisson 2013, 5 of the 14 included studies noted information about adverse events, with 3 studies reporting on adverse events in some detail (Foa 2013; Hien 2009; Mills 2012). There was no evidence of differences in adverse events for these studies (for details see Analysis 1.9 and Analysis 3.12).
Overall completeness and applicability of evidence
The objective of this review was to determine the efficacy of psychological therapies aimed at treating traumatic stress symptoms, substance misuse symptoms, or both in people with comorbid PTSD and SUD. In terms of applicability of findings, the studies included in this review were mainly conducted in veterans groups and amongst groups who had experienced significant abuse and interpersonal violence, and evaluated males only, females only, and both genders together. Studies took place in high‐income, English‐speaking countries, but participants were largely of low income and often living in relatively deprived settings. A strength of this review is that samples were ethnically diverse in the majority of studies. However, the results of this review may not be generalisable to other settings or participant groups. We only identified one study involving adolescents and no studies involving younger children. Most studies did not include people with comorbid psychiatric diagnoses or with cognitive impairment, who are arguably more difficult to treat. Generalising the results to more complex presentations is therefore problematic.
In addition to high drop‐out rates, recruitment appears to have been a significant problem for some studies. Some studies ended before reaching recruitment targets (for example Boden 2012; Mills 2012; Norman unpublished; Sannibale 2013). One study took eight years to reach its recruitment target, with a large number of individuals who met inclusion criteria refusing to participate in the study (Foa 2013).
We outlined earlier that there is little consensus about the process of interventions in terms of treatment pathways. We had hoped this review would help to address this issue. It has to a degree, but in practice, most studies recruited participants from substance misuse services, so many participants had received some level of substance use intervention prior to recruitment, and in most instances it is likely that substance or alcohol use, or both was stabilised to some extent prior to study intervention, often with the aid of pharmacological intervention. It is possible that the efficacy of psychological therapies is stronger than suggested by the data, as in several studies the usual care group were able to access a significant amount of clinician contact and would have been of the understanding that they were being treated, which may have been experienced as therapeutic. The review does include studies that evaluated combined intervention and integrated interventions, but we did not identify any studies where interventions were tested and delivered sequentially. The review also includes studies of some of the most well‐known models for treating comorbidity, such as Seeking Safety, COPE, and programmes based around CBT. Most studies attempted to evaluate follow‐up beyond the immediate completion of treatment, and several of the larger studies reported follow‐up between 6 and 12 months after treatment was completed.
Some studies reported data on medium‐ to long‐term follow‐up. However, follow‐up rates tended to be low, and whilst many studies used intention‐to‐treat (ITT) analyses, true follow‐up effects relied heavily on estimation and may be too conservative or too optimistic of true effects. It should also be noted that whilst the majority of studies undertook ITT analyses, several studies based their ITT sample only on participants who attended at least one treatment session. This can be a legitimate approach when ineligible people are mistakenly randomised into a trial and if a potential participant is prematurely randomised as long as allocation to treatment arm cannot influence the likelihood that people receive the intervention (Fergusson 2002). However, the fact that some studies did this and others did not probably means that some studies included more participants who were likely to be ambivalent about engaging in treatment. We should note further that Coffey 2006, McGovern 2011, and Zlotnick 2009 only reported data for participants who were available to follow‐up, and in some analyses this data was used in conjunction with ITT data from other studies. We undertook several sensitivity analyses excluding these studies. These analyses made no difference to our findings.
Quality of the evidence
We assessed the results for primary outcomes using GRADE protocols; see Table 1; Table 2; Table 3; Table 4. We found the quality of evidence for each comparison to be low or very low.
Risk of bias was a factor in downgrading GRADE judgements in all comparisons. As with the vast majority of trials of psychological therapy, participants and providers in the included studies were unblinded to allocation. We therefore judged all studies as being at high risk in terms of performance bias. More recent studies tended to be rated more favourably. In a number of studies, the information provided by the published report was insufficient to determine the risk of bias associated with key methodological indicators. We judged three studies as being at high risk on at least one other aspect of study design and four studies as at high risk on two other aspects of design. 'Other bias' was the most common aspect of design to be judged as high risk. Negative judgements on this aspect were made for a number of different reasons, and there was no particular common reason for this (see Characteristics of included studies). Lack of blinding of outcome assessors and selective reporting were the other main reasons for judgements of high risk.
We also downgraded GRADE judgements on the basis of unexplained statistical and clinical heterogeneity. Clinical populations were diverse in terms of type of trauma exposure, nature of substance misuse, and severity of substance abuse and dependence, with some studies only including participants with dependence, and one study excluding individuals with substance dependence (Mueser 2008). Mueser 2008 also differed from other studies in that all participants also had comorbidity for a serious mental illness. We attempted to group studies together in a way that was logical and clinically meaningful. It is, however, important for us to acknowledge that there is significant clinical heterogeneity within comparisons. The first of our main comparisons was of individual‐based interventions with a trauma‐focused component versus treatment as usual/minimal intervention (Analysis 1.1 to Analysis 1.12). Whilst all active interventions in this comparison included a trauma‐focused component, the nature of delivery of trauma‐focused intervention and the number of sessions of trauma‐focused intervention differed, as did session length, the number of treatment sessions available, and the nature of additional substance use intervention. There was also significant clinical heterogeneity in the control conditions. The majority of studies included in these comparisons offered what would be considered combined PTSD/ SUD intervention. However, in some studies, SUD intervention was integral to the active intervention (for example through COPE, Mills 2012), whereas in other studies, participants in the active intervention received their SUD intervention from a separate source. Treatment‐as‐usual conditions in the studies in this comparison depended on what was available through local service delivery and therefore varied across studies. Not surprisingly, there was notable statistical heterogeneity in many of the analyses undertaken in this comparison, but the small number of studies limited the additional analyses we were able to undertake. There were also some differences in the interventions in the comparison of non‐trauma‐focused individual‐ and group‐based interventions against treatment as usual/minimal intervention (Analysis 3.1 to Analysis 3.19;Analysis 4.1 to Analysis 4.9). Most group‐based studies evaluated Seeking Safety. One study offered only 12 core sessions, rather than 25 sessions (Hien 2009); another incorporated some other cognitive intervention (Norman unpublished), and in a third participants received intervention whilst in a prison (Zlotnick 2009). Treatment as usual and minimal interventions also varied in this comparison, although statistical heterogeneity tended to be absent or smaller. In attempting to group studies and identify meaningful comparison, we tried to draw a distinction between control interventions evaluating an active psychological therapy from treatment as usual/minimal intervention. However, it is notable that "treatment as usual" interventions were often quite active and in most cases were likely to have contained some form of established or non‐specific psychological therapy. We were only able to undertake limited investigation of factors potentially contributing to statistical heterogeneity because the number of studies included in each comparison was small.
Two of the comparisons used in this review were mostly moderately well powered. We downgraded the GRADE judgements of the other two comparisons (see Table 2; Table 4) for reasons of imprecision of results, as the numbers of participants contributing to these comparisons were small. Evidence was collected by direct means for most outcomes used in meta analysis in this review.
Potential biases in the review process
The review followed guidelines set out by The Cochrane Collaboration (Higgins 2011). Two review authors independently read all the candidate studies and assessed them for inclusion. Three review authors rated all included studies for risk of bias and independently extracted data from them. In case of disagreements we consulted a fourth review author. This will have minimised potential bias.
Several unavoidable issues remain. For example, we were unable to undertake an assessment of publication bias. We searched numerous online databases systematically, scrutinised reference lists, contacted experts in the field, and handsearched relevant additional sources. We were able to identify a number of unpublished studies from conference abstracts and attempted to contact study authors where possible. We were able to include data from one unpublished study (Norman unpublished). It is, of course, possible that there are other unpublished studies that we have missed. We mainly contacted research groups who were known to us, in English‐speaking countries (particularly the USA) and elsewhere in Europe, and a significant number of authors did not respond. We were not able to identify any relevant research groups outside of these areas of the world. We also included data from one study (Mueser 2008) that did not specifically provide interventions to treat PTSD/SUD comorbidity but included a significant minority of participants who met diagnosis. We included this study on the basis that it may provide evidence on how individuals with PTSD/SUD comorbidity respond to such non‐specific intervention. We attempted to identify other similar studies by reviewing studies included in other relevant Cochrane reviews undertaken by the Cochrane Drugs and Alcohol Group and those reported in Bisson 2013. Some of the reviews that we searched were published several years ago, and it is therefore possible that we missed other relevant studies. We did also approach authors of several other studies, but we were not able to obtain subset data.
Although most studies reported data on an ITT basis, several studies reported incomplete data. We contacted authors to obtain missing results where possible. Some comparisons therefore included data from those participants available to follow‐up, alongside ITT data. As we have outlined above, there was a great deal of clinical heterogeneity between trials included within comparisons within the review. There was also considerable statistical heterogeneity evident in many of the comparisons. In circumstances where we thought statistical heterogeneity to be an issue, we used a random‐effects model, and reported this. The majority of studies that we identified compared an active psychological therapy against treatment‐as‐usual or minimal‐intervention conditions. The studies comparing active psychological therapy against other psychological therapy tended to be small; this may mean that we were unable to identify effects that might be more apparent in studies that are better powered.
We reported some significant findings for treatment completion/drop‐out. These findings were based on study definitions of what constituted a treatment completer. These definitions varied greatly across studies. It was not possible to compile data around more unified definitions, but it is possible that our finding in relation to treatment completion may not have occurred if the studies included were more consistent about their definitions of treatment completers.
Agreements and disagreements with other studies or reviews
This is the first systematic review of psychological therapies for PTSD and SUD that we are aware of to be based only on randomised controlled trials. Other reviews have based their conclusions on findings from both controlled and non‐controlled studies (Berenz 2012; Najavits 2013; Torchalla 2012; van Dam 2012). To our knowledge, this is also the first review to undertake a detailed 'Risk of bias' assessment of included studies using the Cochrane 'Risk of bias' criteria (Higgins 2011), although other reviews have also commented upon methodological concerns (for example Najavits 2013; Torchalla 2012; van Dam 2012). In common with Berenz 2012 and van Dam 2012, we found that the most promising outcome data thus far are for psychological therapies that incorporate trauma‐focused intervention alongside intervention for SUD. However, these treatment effects were small. This finding is consistent with the findings from a recent meta‐analysis that compared treatment effects of studies for people with PTSD who had complex versus non‐complex presentations (Gerger 2014). Gerger 2014 also found that the benefits of specific interventions were small in studies with participants with more complex clinical problems. Our findings are not consistent with the conclusions of Najavits 2013, in that the data we included found little evidence to support the use of non‐trauma‐focused interventions, although our findings were somewhat consistent with this review in that PTSD tended to improve (with some interventions) more than SUD. We also found that fewer participants assigned to trauma‐focused intervention completed treatment than participants assigned to the comparable control condition. This finding is consistent with findings from the recently updated Cochrane review of psychological therapies for PTSD (Bisson 2013), suggesting that such interventions may not always be well tolerated.
Authors' conclusions
Implications for practice.
We found evidence to suggest that psychological therapy that includes a trauma‐focused component alongside intervention for SUD can help reduce PTSD symptom severity for people with PTSD and comorbid SUD. There is evidence to suggest that treatment effects may be sustained in the medium term. These results need to be interpreted with caution. Treatment effects were small and mostly for PTSD. We also found evidence to suggest that participants allocated to receive trauma‐focused intervention were less likely to complete treatment than those who did not receive trauma‐focused intervention. This suggests that there may be problems with tolerability of trauma‐focused intervention for some people with this comorbidity. Clinicians and patients may want to consider this when making decisions about treatment. Participants in the studies included in this review are likely to have received a range of other stabilising interventions alongside trauma‐focused treatment, and we found no evidence to support the delivery of trauma‐focused interventions alone.
We found little evidence to support the use of non‐trauma‐focused interventions, delivered individually or through a group, although individual‐based interventions have not been widely evaluated. Treatment drop‐out was high across all included studies, regardless of intervention type; this is clearly a problem area in trying to help individuals with PTSD and comorbid SUD (Foa 2010; Schäfer 2007).
Clinical heterogeneity was a prominent feature of this review, which included studies across a number of populations, using a number of different forms of intervention, which we grouped in terms of similarity. Positive treatment effects for trauma‐focused intervention alongside SUD interventions were small, and clinicians will want to exercise caution in deciding whether to provide the interventions identified in this review (Cloitre 2011; Najavits 2013). People with more severe and complex presentations (such as those with other serious mental illness, with cognitive impairment, and who are suicidal) were excluded from most studies in this review, and it would be inappropriate to generalise our findings to such individuals. Our conclusions are compromised by methodological issues evident in some studies and the high level of heterogeneity we found. Some studies were underpowered, and there were limited follow‐up data, which limit conclusions regarding the long‐term effects of psychological therapy. The follow‐up period for most studies was short, limiting the conclusions that can be drawn about long‐term effects. Only Frisman 2008 and Hien 2009 followed participants up to 12 months. PTSD and SUD are both cyclical and relapsing disorders, and longer‐term follow‐ups are important to demonstrating whether change is sustained over time (Bradley 2005). We assessed most of the evidence for each of the comparisons made in this review as being of low to very low quality, and findings may be liable to change as further evidence is accumulated.
Implications for research.
We found few well‐controlled studies of psychological therapies for PTSD and comorbid substance use. Further well‐designed trials are clearly required, incorporating appropriate methods of randomisation, blinding of assessors, long‐term follow‐up, and appropriate training of therapists and monitoring of treatment adherence. The results of this review suggest that there are small benefits from interventions including a trauma‐focused component alongside or following intervention treating symptoms of SUD, when compared against treatment as usual/minimal intervention. However, there is a need to replicate these findings over longer follow‐up periods and to identify optimal modes of intervention delivery and new interventions that are more effective and can successfully retain participants in treatment. Future studies could investigate mediators and moderators of treatment compliance by evaluating factors that interfere with treatment retention (Ouimette 2003c; Pinto 2011). Non‐trauma‐focused interventions delivered on an individual basis have not been widely studied and should be evaluated further. Further comparison studies of one type of psychological therapy against another are required. As evidence accumulates, there will also be a need to differentiate effective interventions with different subgroups and different complexities of clinical presentation. Most of the studies in this review recruited participants from substance misuse services and/or provided supplementary SUD‐related intervention, which was equivalent across treatment arms. There is little evidence about treatment effects when SUD‐related intervention is minimal. Future studies should examine whether this is an essential component of treatment. There is some evidence to suggest that outcomes may be improved when psychological therapy is delivered in combination with pharmacological intervention for this patient group (Foa 2013); this should also be examined further. Finally, people with PTSD and comorbid SUD often require interventions from different services, and there is a need to evaluate different intervention combinations in order to identify optimal treatment pathways. Although non‐trauma‐focused interventions such as Seeking Safety have not shown significant evidence of effectiveness in this review, it may be that such programs, or components of them, have a role in enabling those seeking treatment to develop coping skills that may help them to tolerate trauma‐focused intervention more easily. This should be investigated further.
Acknowledgements
With thanks to the editorial team of the Cochrane Depression, Anxiety and Neurosis Group. Thanks also to Delyth Lloyd, David Forbes, Rosemarie Wolfe, Kim Mueser, Scott Coffey, Catherine Mills, Denise Hien, Sonya Norman, and Ursula Myers for additional data. We would like to thank Lisa Najavits for feedback on an earlier draft of the review.
CRG funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Depression, Anxiety and Neurosis Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL)
We will search CENTRAL using the following terms:
[Condition]
#1 MeSH descriptor STRESS DISORDERS, TRAUMATIC, explode all trees
#2 (trauma* NEXT stress*)
#3 (psycho* NEXT trauma*)
#4 (stress* NEXT (extreme or disorder*))
#5 DESNOS [Disorders of Extreme Stress]
#6 (posttrauma* or post‐trauma* or (post NEXT trauma*) or PTSD)
#7 (#1 or #2 or #3 or #4 or #5 or #6)
[Population: comorbid substance abuse]
#8 MeSH descriptor SUBSTANCE‐RELATED DISORDERS explode all trees
#9 (substance use disorder or SUD)
#10 drug NEXT abuse
#11 (abuser* or abusing or addict* or depend* or habit* or misuse or user*)
#12 ((abuse) not (child* or sex*))
[Common drugs of abuse]
#13 (adinazolam or aerosol* or alcohol* or alprazolam or amphetamin* or anthramycin or anxiolytic* or ativan or barbituat* or bentazepam or benzodiazepin* or bromazepan or brotizolam or buprenorphin* or camazepam or cannabi* or chlordiazepoxid* or cinolazepam or clobazam or clonazepam or clorazepam or clotiazepam or cloxazolam or cocaine* or codeine or crack or crystal or cyprazepam or depressant* or diacetylmorphin* or diazepam* or doxefazepam or ecstasy or estazolam or etizolam or fentanyl or flunitrazepam or flurazepam or flutazoram or flutoprazepam or fosazepam or gases or GHB or girisopam or halazepam or hallucinogen* or haloxazepam or heroin* or hydromorphone or hydroquinone or hypnotic* or inhalant* or ketamin* or ketazolam or librium or loflazepate or loprazolam or lorazepam or lormetazepam or LSD or marihuana* or marijuana* or MDMA or meclonazepam or medazepam or meperidine or mephedrone or mescalin* or metaclazepam or methadone or methamphetamin* or methaqualone or mexazolam or midazepam or midazolam or morphine* or narcotic* or nerisopam or nimetazepam or nitrazepam or nitrites or (nitrous NEXT oxide) or n‐methyl‐3,4‐methylenedioxyamphetamine or nordazepam or opiate* or opiod* or opium or oxazepam or oxazolam or oxazypam or oxycodone or oxzepam or painkiller* or (pain NEXT killer*) or PCP or pethidin* or phencyclidin* or pinasepam or prazepam or propazepam or propoxyphene or psilocybin or psychedelic* or psychoactive* or psychostimulant* or quinazolinone or ripazepam or ritalin or sedative* or serazepin* or solvent* or steroid* or stimulant* or substance* or temazepam or tetrazepam or tofisopam or tramadol or triazolam or triflubazam or valium or vicodin)
#14 (drug* NEAR (recreational or street))
#15 (#8 or #9 or #10 or #11 or #12 or #13 or #14)
[Condition + Population]
#16 (#7 and #15)
Appendix 2. Researchers contacted through the search process
Sudie Back, Matthew Boden, Marcel Bonn Miller, Kathleen Brady, Pamela Brown, Karen Cusack, Marylene Cloitre, Scott Coffey, Mark Creamer, Colin Drummond, Edna Foa, David Forbes, Julian Ford, Elizabeth Forshay, Anna Cash Ghee, Benjamin Goldstein, Michael Hase, Denise Hien, Debra Kaysen, Daniel Kivlahan, Karen Krinsley, Mark McGovern, Sarah Meshberg‐Cohen, Nena Messina, Katherine Mills, Kim Mueser, Lisa Najavits, Sonya Norman, Paige Ouimette, Tae Woo Park, Barbera Rothbaum, Josef Ruzek, Elizabeth Santa Ana, Ingo Schäfer, Paula Schnurr, Jodie Trafton, Elisa Triffleman, Debora van Dam, Richard Velleman, Anka Vujanovic, Doug Zatzick, Caron Zlotnick.
Of these, Scott Coffey, David Forbes, Julian Ford, Denise Hien, Debra Kaysen, Katherine Mills, Kim Mueser, Lisa Najavits, Sonya Norman and Elizabeth Santa Ana responded with additional data.
Data and analyses
Comparison 1. Trauma‐focused psychological therapy vs control therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PTSD severity following treatment completion | 4 | 405 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.72, ‐0.10] |
| 1.1 Individual intervention | 4 | 405 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.72, ‐0.10] |
| 2 PTSD severity 3‐4 months following treatment completion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PTSD severity 5‐7 months following treatment completion | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.58, ‐0.10] |
| 3.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.58, ‐0.10] |
| 4 Drug or alcohol use, or both following treatment completion | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 4.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 5 Drug or alcohol use, or both 3‐4 months following treatment completion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Drug or alcohol use, or both 5‐7 months following treatment completion | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.48, ‐0.07] |
| 6.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.48, ‐0.07] |
| 7 Treatment completers | 3 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.64, 0.96] |
| 7.1 Individual intervention | 3 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.64, 0.96] |
| 8 PTSD diagnosis following treatment completion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adverse events | Other data | No numeric data | ||
| 9.1 Individual intervention | Other data | No numeric data | ||
| 10 Adverse events | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.34, 1.90] |
| 10.1 Individual intervention | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.34, 1.90] |
| 11 Mean number of sessions attended for intervention group | Other data | No numeric data | ||
| 11.1 Studies including intervention for SUD | Other data | No numeric data | ||
| 12 Sensitivity analysis: PTSD severity following treatment completion | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.56, ‐0.10] |
| 12.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.56, ‐0.10] |
Comparison 2. Trauma‐focused psychological therapy vs active psychological therapy for SUD only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PTSD severity following treatment completion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PTSD severity 5‐7 months following treatment completion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PTSD severity 8‐10 months following treatment completion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Drug or alcohol use, or both following treatment completion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Drug or alcohol use, or both 5‐7 months following treatment completion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Drug or alcohol use, or both 8‐10 months following treatment completion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Treatment completers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 PTSD diagnosis following treatment completion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 SUD diagnosis following treatment completion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PTSD severity following treatment completion | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Individual intervention | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.83, 0.39] |
| 1.2 Group intervention | 4 | 513 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.19, 0.16] |
| 2 PTSD severity 3‐4 months following treatment completion | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Individual intervention | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.86, 0.36] |
| 2.2 Group intervention | 4 | 499 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.17, 0.18] |
| 3 PTSD severity 5‐7 months following treatment completion | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Individual intervention | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.81, 0.41] |
| 3.2 Group intervention | 4 | 566 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.31, 0.03] |
| 4 PTSD severity 12 months following treatment completion | 2 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.25, 0.10] |
| 4.1 Group intervention | 2 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.25, 0.10] |
| 5 Drug or alcohol use, or both following treatment completion | 3 | 464 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.97, 0.14] |
| 5.1 Group intervention | 3 | 464 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.97, 0.14] |
| 6 Drug or alcohol use, or both 3‐4 months following treatment completion | 4 | 499 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.40, 0.23] |
| 6.1 Group intervention | 4 | 499 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.40, 0.23] |
| 7 Drug or alcohol use, or both 5‐7 months following treatment completion | 4 | 572 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.11] |
| 7.1 Group intervention | 4 | 572 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.11] |
| 8 Drug or alcohol use, or both 12 months following treatment completion | 2 | 528 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.15, 0.20] |
| 8.1 Group intervention | 2 | 528 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.15, 0.20] |
| 9 Treatment completers | Other data | No numeric data | ||
| 9.1 Individual intervention | Other data | No numeric data | ||
| 9.2 Group intervention | Other data | No numeric data | ||
| 10 Treatment completers | 2 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.88, 1.45] |
| 10.1 Group intervention | 2 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.88, 1.45] |
| 11 PTSD diagnosis following treatment completion | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.54] |
| 11.1 Group intervention | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.54] |
| 12 Adverse events | Other data | No numeric data | ||
| 12.1 Group intervention | Other data | No numeric data | ||
| 13 Study‐related adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 Group intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Mean number of sessions attended for intervention group | Other data | No numeric data | ||
| 14.1 Group intervention | Other data | No numeric data | ||
| 15 Mean number of sessions attended | 2 | 381 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.59, 0.79] |
| 15.1 Group intervention | 2 | 381 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.59, 0.79] |
| 16 Sensitivity analysis: PTSD severity 5‐7 months following treatment completion | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Group intervention | 3 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.34, 0.10] |
| 17 Sensitivity analysis: PTSD severity 12 months following treatment completion | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.25, 0.17] |
| 17.1 Group intervention | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.25, 0.17] |
| 18 Sensitivity analysis: drug or alcohol use, or both 5‐7 months following treatment completion | 3 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.08] |
| 18.1 Group intervention | 3 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.08] |
| 19 Sensitivity analysis: drug or alcohol use, or both 12 months following treatment completion | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.21, 0.21] |
| 19.1 Group intervention | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.21, 0.21] |
Comparison 4. Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PTSD severity following treatment completion | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.29, 0.77] |
| 1.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.29, 0.77] |
| 2 PTSD severity 3‐4 months following treatment completion | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.31, 0.55] |
| 2.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.31, 0.55] |
| 3 PTSD severity 5‐7 months following treatment completion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Drug or alcohol use, or both following treatment completion | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.13, 0.57] |
| 4.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.13, 0.57] |
| 5 Drug or alcohol use, or both 3‐4 months following treatment completion | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.18, 0.53] |
| 5.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.18, 0.53] |
| 6 Drug or alcohol use, or both 5‐7 months following treatment completion | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Individual intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Treatment completers | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.20] |
| 7.1 Individual intervention | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.20] |
| 8 PTSD diagnosis following treatment completion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Mean number of sessions attended | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boden 2012.
| Methods | Design: Randomised effectiveness trial | |
| Participants |
Setting: Participants were military veterans recruited from a VA outpatient substance use disorder clinic. Treatment was delivered on an outpatient basis. Inclusion criteria: male veteran status and VA health care eligibility; a diagnosis of any current alcohol or drug use disorder; completion of an intake for outpatient SUD treatment at the participating mental health clinic; and meeting partial (i.e. defined as meeting criteria for 2 out of 3 PTSD symptom clusters, or at least 1 symptom in each symptom cluster) or full PTSD in clinical evaluation using CAPS. Exclusion criteria: current participation in other day or inpatient mental health treatment; any contraindications communicated by the person’s primary clinician; and acute psychosis, mania, dementia, or suicidal intent. Sample size: 125 individuals were assessed for eligibility; 117 were randomised; 8 participants were withdrawn after randomisation because they were found to meet 1 or more exclusion criteria; 98 attended at least 1 treatment session and were included in the analyses. PTSD diagnosis: 90.8% participants met full diagnosis for PTSD as measured by the CAPS; 9.8% met subthreshold diagnosis for PTSD. SUD type and diagnosis: All participants met diagnosis for SUD. Participants were polydrug users. Mean age: Seeking safety 55.1 (SD = 9.2) years; treatment as usual 52.9 (SD = 10.0) Gender: 100% male Ethnicity: 60.2% African American; 19.4% white; 7.1% Hispanic; 2% Native American; 5.1% other. Country: USA |
|
| Interventions |
Group 1: Group‐based Seeking Safety plus treatment as usual: n = 54. Seeking Safety is a present‐focused, manualised, cognitive behavioural integrated treatment for PTSD and SUD, designed for both genders. Its primary goal is to reduce both PTSD and SUD by focusing on safe coping skills addressed through cognitive, behavioural, interpersonal, and case management domains. Participants were also able to access the treatment‐as‐usual interventions (described below). However, participants in this arm substituted SS groups and case management for the clinic’s core substance use‐focused group therapy and case management sessions. Groups were held twice weekly. Case management was based on the SS manual. Group 2: Group‐based treatment as usual: n = 55. Treatment as usual involved participants entering twice‐weekly "recovery" groups, focusing on building abstinence and, after approximately 90 days of therapy, on maintaining abstinence. Participants attended additional groups on smoking cessation, sobriety support, cocaine recovery, alcohol recovery, dual‐diagnosis recovery, family therapy, anger management, cognitive behavioural therapy, fitness, relaxation, health education, hepatitis education, and developing outside activities as needed. All participants were assigned a case manager, and case management and individual therapy were available as deemed appropriate. Participants made use of clinic services as indicated by their treating clinician or as desired. Experimental intervention modality: Integrated |
|
| Outcomes |
PTSD: IES‐R SUD: ASI drug and alcohol composite scores for the previous 30 days. Treatment acceptability: Data are reported for the mean number of treatment sessions attended; participant satisfaction at 3‐month assessment based on the Client Satisfaction Questionnaire. Other: Coping Responses Inventory Follow‐up: End of treatment and at 3 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation sequences were generated by the study statistician and implemented by use of sequentially numbered containers |
| Allocation concealment (selection bias) | Low risk | Random allocation sequences were generated by the study statistician and implemented by use of sequentially numbered containers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff who enrolled participants and conducted outcomes assessment were blind to treatment assignment. To maintain blinding, staff conducting outcomes assessment were password‐restricted from accessing data with information regarding treatment assignment, and participants were warned not to divulge information that might compromise blinding during interviews |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Treatment dropouts and withdrawals were clearly reported. Analysis of treatment outcomes was based on those participants available to follow‐up |
| Selective reporting (reporting bias) | Low risk | Primary outcomes are specified in the protocol registered with ClinicalTrials.gov |
| Other bias | Unclear risk | Insufficient information available to be able to assess |
Coffey 2006.
| Methods | Design: RCT ‐ described as a laboratory‐based experiment. The authors tested the hypothesis that alcohol craving elicited by a trauma cue might be attenuated if trauma‐elicited negative emotion was reduced following trauma‐focused imaginal exposure | |
| Participants |
Setting: Participants were recruited from 2 outpatient substance use treatment programmes. Inclusion criteria: Participants needed to meet current diagnosis for alcohol dependence and PTSD. All participants were involved in alcohol treatment. Exclusion criteria: Individuals were excluded if they met current diagnostic criteria for a psychotic disorder or were currently experiencing a manic episode. Although current major depression was not an exclusion criterion, severe major depression was. Individuals were also excluded if their PTSD diagnosis stemmed from combat or if they were currently or had ever engaged in exposure‐based PTSD treatment. Sample size: The number of individuals assessed for eligibility is not reported. 43 individuals were invited to take part in the study and were randomised and 31 (who attended at least 1 treatment session) were included in the analyses. PTSD diagnosis: 43 (100%) of participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: 43 (100%) of participants met diagnosis for alcohol dependence. Mean age: 37.5 (SD = 8.0) years Gender: 29 (67%) female Ethnicity: 65% African American; 28% white; 5% Native American; 2% other Country: USA |
|
| Interventions |
Group 1: Individual imaginal exposure: n= 16. Participants assigned to the exposure condition took part in six 60‐min sessions of imaginal exposure targeting an index traumatic event. Participants were instructed to tell the story of their trauma by describing the event in the present tense from the first‐person perspective. Participants were encouraged to include emotions and cognitions in their verbal description of the event. Participants described their trauma repeatedly and continuously over the course of the six 60‐min clinical sessions. SUDS ratings were collected approximately every 5 min during each session. Each session was audiotaped, and participants were instructed to listen to the tape daily. Group 2: Imagery‐based relaxation: n= 15. Participants assigned to the relaxation condition listened to an imagery‐based relaxation audiotape for the 60‐min session. As in the exposure condition, SUDS ratings were collected approximately every 5 min during each session. Participants were instructed to listen to the relaxation tape daily. Experimental intervention modality: Concurrent |
|
| Outcomes |
PTSD: IES‐R SUD: A cue reactivity paradigm was used to assess alcohol craving prior to, and after completion of, the 6 clinical sessions. Treatment acceptability: Attendance of 1, at least 4, and all 6 clinical sessions is described. Other: The PANAS; emotional distress as measured by SUDS Follow‐up: End of treatment. |
|
| Notes | 43 participants were assessed and randomised. Outcome data is reported for 17 responders of 31 participants who attended 1 or more intervention session | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research assistant who conducted the assessment session and both laboratory sessions was unaware of the experimental condition to which participants had been randomly assigned. The research staff involved in the clinical sessions did not participate in the assessment session or either laboratory session |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data is reported for 17 responders of 31 participants who attended 1 or more intervention session |
| Selective reporting (reporting bias) | High risk | Data related to craving response was based on a subset of 12 participants who reported a craving response to the cues at the first or the second laboratory session. This appears to have been based on a post‐hoc decision |
| Other bias | Unclear risk | High attrition rates |
Coffey submitted.
| Methods | Design: RCT | |
| Participants |
Setting: Participants were recruited from an unlocked residential SUD treatment facility. Inclusion criteria: DSM‐IV diagnosis of both PTSD related to a non‐combat trauma and alcohol dependence; 1 heavy drinking day in the past 60 days, as defined by consumption of 4 standard drinks for women and 5 standard drinks for men; and age between 18 and 60. Exclusion criteria: The presence of an acute psychotic disorder, bipolar disorder with an active manic episode (but not the presence of bipolar disorder, per se), imminent risk for suicide, prescription of craving‐reducing medications (e.g. naltrexone) or medications to reduce alcohol use (e.g. disulphiram), self reported use, or urine drug screen indicating use, of a benzodiazapine, judged to have a medical condition that might limit co‐operation or compromise the integrity of the data (e.g. organic brain syndrome, dementia, head injury, neuropathy, etc.), illiteracy in English, and being in an ongoing abusive relationship that resulted in a PTSD Criterion A event (but not a history of intimate partner violence, per se). Sample size: 222 individuals were assessed for eligibility; 148 were randomised, but 28 were subsequently excluded. Reasons for exclusion included cognitive impairment, psychosis, medical issues, drug screening, moved away, refusal to participate, and in one case for unknown reasons. The remaining 120 participants attended at least 1 treatment session and were included in analyses. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: All participants met diagnosis for alcohol dependence and 98.3% met criteria for other drug dependence, as measured by the CDIS‐IV. Mean age: 33.72 (SD = 10.25) years Gender: 64 (53.3%) male; 56 (46.7%) female Ethnicity: 18.3% African American; 80.0% white; 0.8% other Country: USA |
|
| Interventions |
Group 1: Trauma‐focused exposure therapy (EXP) + TAU: n = 82. EXP is a well‐described cognitive behavioural therapy that utilises imaginal and in vivo exposure techniques, either singly or in combination, to reduce the symptoms of PTSD resulting from a range of traumas. In addition to imaginal and in vivo exposure techniques, in the current study participants were provided psycho‐education about PTSD, a rationale for EXP, and were taught breathing retraining as a method to manage arousal associated with PTSD. The imaginal exposures were audiotaped, and participants were instructed to listen to the tapes daily. 9 sessions of exposure were offered initially; if PTSD symptom severity did not decrease by at least 70%, an additional 3 sessions of EXP were offered. A number of adaptations were made to conventional exposure. Traditionally, exposure sessions are 90 minutes. The current study utilised 60‐min EXP sessions. Additionally, protocol contained added psycho‐education about the relationship between trauma and SUD symptoms and weekly check‐ins about SUD treatment progress. Finally, the protocol provides integration of care at the team level, rather than the individual provider level. All participants received standard TAU for substance abuse. TAU consisted of daily group therapy for approximately 3 hours each day, daily recreation therapy, AA and NA meetings, individual drug counselling sessions, and completion of drug counselling homework. The 6‐week TAU was provided by drug and alcohol counsellors unaffiliated with the current study. Group 2: Healthy Lifestyles Sessions (HLS) + TAU: n = 38. HLS is a structured 9‐ to 12‐session intervention that provides education about a variety of health‐related topics. HLS was designed to involve a similar amount of therapist contact and between‐session homework as exposure. Topics covered included an introduction to treatment; sleep hygiene; progressive muscle relaxation; starting/maintaining an exercise programme; personal role identification; healthy eating and nutrition (2 sessions); diabetes (prevention or diabetes treatment adherence, depending on diabetes status); monitoring goals and values; cancer (a focus on breast cancer for women and colon cancer for men); HIV (reducing HIV risk or adhering to HIV treatment, depending on HIV status); and a final review session. Sessions included the provision of information, discussing participants’ understanding of information, and answering questions about the information provided. Experimental intervention modality: Combined |
|
| Outcomes |
PTSD: CAPS; IES‐R SUD: TLFB, the primary outcome was per cent days abstinent; ACQ‐Now Treatment acceptability: Number completing at least 8 treatment sessions Other: BDI; BAI Follow‐up: End of treatment; 3 and 6 months post‐treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was undertaken by urn randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Treatment dropouts and withdrawals were clearly reported. An ITT approach was used to analyse data based on participants who attended at least 1 treatment session. A number of other participants were also excluded from the study postrandomisation, and it was unclear if some of these exclusions might have been due to intervention‐related factors |
| Selective reporting (reporting bias) | Unclear risk | All outcomes that were described were reported, but we were not able to identify a previously published protocol |
| Other bias | Unclear risk | 28 individuals who were randomised were removed from the study. It is unclear whether this may have caused additional bias. Approximately half of the participants receiving the experimental intervention were also provided a session of motivational enhancement therapy for PTSD prior to beginning intervention. The other half of the experimental group and all of the HLS participants were provided a 60‐minute relaxation session prior to the first scheduled treatment session. No significant differences were found between the 2 experimental groups, and they were therefore collapsed into a single experimental condition |
Foa 2013.
| Methods | Design: RCT | |
| Participants |
Setting: Treatment‐seeking individuals were recruited through advertisements and professional referrals and treated on an outpatient basis. Inclusion criteria: Current PTSD and alcohol dependence according to DSM‐IV; clinically significant trauma‐related symptoms, as indicated by a score of at least 15 on the PSS‐I; and heavy drinking in the past 30 days, defined as an average of more than 12 standard alcohol drinks per week with at least 1 day of 4 or more drinks determined by the TFBI. Exclusion criteria: Current substance dependence other than nicotine or cannabis; current psychotic disorder (e.g. schizophrenia, bipolar disorder); clinically significant suicidal or homicidal ideation; opiate use in the month prior to study entry; medical illnesses that could interfere with treatment (e.g. AIDS, active hepatitis); or pregnancy or nursing. Sample size: 657 individuals were assessed for eligibility; 165 were randomised, and all were included in the analyses. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: All participants met full diagnosis for alcohol dependence. Mean age: prolonged exposure + naltrexone 40.1 (95% CI 36.7 to 43.5); prolonged exposure + placebo 44.7 (95% CI 41.8 to 47.7); supportive counselling + naltrexone 44.9 (95% CI 41.8 to 47.9); supportive counselling + placebo 41.2 (95% CI 38.6 to 43.9) Gender: 108 (65.5%) male; 57 (34.5%) female Ethnicity: 63% African American; 30% white; 4.2% Hispanic; 0.6% other Country: USA |
|
| Interventions | Before randomisation to treatment, participants completed outpatient medical detoxification (at least 3 consecutive days of alcohol abstinence) with oxazepam as required to manage alcohol withdrawal symptoms. Group 1: Prolonged exposure + naltrexone + supportive counselling: n = 40. Prolonged exposure therapy consisted of 12 weekly 90‐minute sessions followed by 6 biweekly sessions and included repeated imaginal exposure (i.e. revisiting and recounting traumatic memories) and processing the memory (i.e. discussing thoughts and feelings related to revisiting the memory). The target dose of naltrexone was 100 mg/d, starting with 50 mg/d for a minimum of 3 days and titrating up within 1 week. Supportive counselling was available as described below. Group 2: Prolonged exposure + placebo + supportive counselling: n = 40. Participants received PE as described above. Supportive counselling was available as described below. Group 3: Supportive counselling + naltrexone: n = 42. Supportive counselling was based on the BRENDA model, which combines medication management with compliance enhancement techniques based on motivational interviewing. Supportive counselling sessions were administered by a study nurse and lasted 30 to 45 minutes. Input included dispensing medication, monitoring compliance, assessing and providing education about alcoholism, and offering support and advice concerning drinking. Visits were weekly during the first 3 months and biweekly during the remaining 3 months. Group 4: Supportive counselling + placebo: n = 43. Supportive counselling was as described above. Experimental intervention modality: Combined |
|
| Outcomes |
PTSD: PSS‐I SUD: TLFB; PACS Treatment acceptability: Reported in terms of the mean number of sessions attended for PE. Other: ‐ Follow‐up: End of treatment and 6 months' post‐treatment |
|
| Notes | For the purpose of the review, we were interested in the comparison between prolonged exposure plus supportive counselling and supportive counselling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not be blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluators were blind to treatment group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Treatment dropouts and withdrawals were clearly reported. An ITT approach was employed using hierarchical linear and non‐linear modelling, which took into account dropouts and missing data |
| Selective reporting (reporting bias) | Low risk | Key outcomes are as specified in the protocol registered with ClinicalTrials.gov |
| Other bias | Low risk | There was no significant difference between the treatment groups on any demographic or baseline diagnostic characteristics. We identified no other potential biases |
Frisman 2008.
| Methods | Design: RCT | |
| Participants |
Setting: Participants were recruited from amongst outpatients at 3 participating substance use disorder clinics. Inclusion criteria: (a) a history of trauma that fulfilled the conditions for DSM‐IV PTSD criterion A, (b) a substance use disorder, and (c) DSM criteria for one of the following: PTSD, DESNOS plus at least 1 or more DSM‐IV Axis I disorders, or a diagnosis of major depressive disorder, dysthymic disorder, or dissociative disorder. Exclusion criteria: Not specified. Sample size: 274 individuals were assessed for eligibility; 213 were randomised PTSD diagnosis: 202 (94.8%) of participants met full diagnosis for PTSD as measured by the CAPS. Of these, 72 (33.8%) met criteria for PTSD with DESNOS. 7 (3.3%) met criteria for DESNOS without PTSD, and 4 (1.9%) met criteria for other disorders, such as dissociative disorder and major depression. SUD type and diagnosis: Participants met diagnosis for substance abuse and substance dependence. Participants were polydrug users. Mean age: Intervention group: 37.84 (SD = 8.42) years; control group: 36.85 (SD = 8.44) years Gender: 130 (61%) female Ethnicity: 24.4% African American; 56.3% white; 10.3% Hispanic; 8.92% other Country: USA |
|
| Interventions |
Group 1: Group‐based trauma‐sensitive usual care plus Trauma Adaptive Recovery Group Education and Therapy (TARGET): n = 141 Participants randomised to TARGET treatment were offered 8 or 9 weeks of manualised group treatment. TARGET provided psycho‐education about the impact of traumatic exposure and PTSD on the body’s stress response system and the brain using the strength‐based concept of an adaptive psychobiological “alarm reaction”. Participants were taught 7 core skills: focusing, recognising stress triggers, emotion identification, evaluating cognitions, defining personal goals, making choices with options grounded in personal strengths, and making a contribution to restore a sense of hope, faith, and purpose in the wake of trauma and PTSD. Experiential exercises were used to teach, model, role‐play, and integrate skills and to use them to develop a coherent memory narrative of the client’s life that incorporates a range of experiences including but not limited to traumatic stress. To enhance retention in the groups, small incentives that also reinforced aspects of the TARGET model (e.g. pens, key chains) were handed out on 3 occasions during the group. Group 2: Trauma‐sensitive usual care: n = 72 Participants received regular substance abuse treatment sessions. Counsellors providing this intervention received training on trauma‐sensitive care. Training workshops included information about the effect of traumatic events and disorders that trauma may cause or exacerbate. The counsellors also learned about the typical problems of trauma survivors and some of the ways in which past trauma can interfere with substance abuse recovery. Counsellors received literature about trauma, post‐traumatic stress, and substance abuse recovery that could be shared with clients. Experimental intervention modality: Integrated |
|
| Outcomes |
PTSD: GAIN‐traumatic stress symptoms; PTCI SUD: GAIN subscales for substance use frequency, per cent drinking to intoxication, per cent using any drugs, and per cent abusing drugs or alcohol were used to assess changes in substance use and abuse. Treatment acceptability: Mean number of sessions attended for the active intervention group and mean number of standard‐care sessions attended for both groups. Other: GAIN for depressive symptoms, anxiety symptoms, and self efficacy Follow‐up: 6 and 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By cohort using a random number program |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to be able to assess |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | A trained research assistant conducted face‐to‐face interviews. No further information was available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and drop‐out are well described. Hierarchical linear modelling was used to enable estimation where data were unavailable |
| Selective reporting (reporting bias) | Unclear risk | Traumatic stress symptoms as indexed by GAIN were described in the methodology as a primary outcome, along with a number of other outcomes. However, this outcome is not reported, although other prespecified outcomes are reported |
| Other bias | High risk | The process of allocation was modified partway through the study, as delays in starting groups early on "meant that many participants received an insufficient dose". It was unclear as to why the number of participants randomised to the active intervention group was nearly double the number randomised to the control group. There was a high drop‐out rate. Counsellors to participants in the control condition could not be prevented from formally referring to the FREEDOM steps or using the handouts or other tangible materials from TARGET in non‐TARGET groups. This led study authors to conclude that there was "contamination of the comparison group treatment with TARGET principles and techniques". |
Hien 2004.
| Methods | Design: Partial RCT described as a "quasi‐experimental clinical trial" | |
| Participants |
Setting: Participants were recruited through advertisements and referred through substance use treatment programmes. Participants were treated on an outpatient basis. Inclusion criteria: Current or subthreshold PTSD (defined as DSM‐IV criteria A, B, and E and the presence of either C or D) and current DSM‐IV substance dependence; if they reported using substances at least 3 times a week on the Substance Use Inventory; Mini‐Mental State Examination score greater than 21; age 18 to 55 years; female; and English‐speaking. Exclusion criteria: Advanced‐stage medical disease (e.g. AIDS, tuberculosis) as indicated by global physical deterioration and incapacitation, organic mental syndrome (associated with chronic drug abuse), and psychiatric exclusions (current active suicidality; current Axis I diagnoses other than atypical bipolar, depressive, or anxiety disorders; and history of psychosis). Sample size: 207 individuals were assessed for eligibility; 128 met full study eligibility criteria, 115 agreed to participate, and 96 of these were randomised. 32 of the 128 women became a non‐randomised community care comparison group. 75 of the 96 women who were randomised attended at least 1 treatment session and were included in the ITT analyses. PTSD diagnosis: 88% of women met full diagnosis for PTSD as measured by the CAPS. The other 12% met criteria for subthreshold PTSD. SUD type and diagnosis: Women were included on the basis that they met criteria for substance dependence. Women were polydrug users. Mean age: 38.2 (SD = 9.1) for Seeking Safety; 33.8 (SD = 8.3) for relapse prevention. The difference in age was statistically significant. Gender: 128 (100%) female Ethnicity: 42.7% African American; 36% white; 20% Hispanic; 13.3% multiracial; 1.3% other Country: USA |
|
| Interventions |
Group 1: Individual‐based Seeking Safety plus standard care: n = 41. The intervention is not fully described but is introduced as a short‐term, manualised cognitive behavioural treatment that simultaneously addresses trauma and substance abuse. Women were offered two 1‐hour treatment sessions weekly over 12 weeks. Group 2: Individual‐based relapse prevention plus standard care: n = 34. The intervention is not fully described but is introduced as an empirically validated cognitive behavioural therapy focusing on the identification of triggers and coping strategies for managing substance cravings and relapse. Group 3: Standard community treatment: n = 32. The intervention is not described. Women in this arm were not randomised. Experimental intervention modality: Integrated |
|
| Outcomes |
PTSD: The primary PTSD outcome was a composite score from scores on the CAPS, IES‐R, and CGI Scale. SUD: The primary SUD outcome was a composite score from scores on the Substance Use Inventory and CGI Scale. Treatment acceptability: Data are reported for number of participants attending at least 25% of treatment sessions. Other: Items from CGI Scale were used to assess global severity of psychiatric symptoms; Global Assessment Scale and the HDRS were also used. Follow‐up: 3, 6, and 9 months postbaseline |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to be able to assess |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to be able to assess |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to be able to assess |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Treatment dropouts and withdrawals were clearly reported. An ITT approach was used, using last observation carried forward. However, ITT was based on participants attending at least 1 treatment session. It was unclear if those who were randomised but who did not attend any sessions were aware of their allocation (Fergusson 2002) |
| Selective reporting (reporting bias) | Unclear risk | Composite outcome scores for both PTSD and SUD were used as primary outcomes. The authors state that this was done to reduce the possibility of Type I error. It is unclear how composite scores were generated. Raw scores are reported for PTSD measures but not for SUD measures |
| Other bias | Unclear risk | Insufficient information to be able to assess |
Hien 2009.
| Methods | Design: RCT | |
| Participants |
Setting: Participants were enrolled in 7 community‐based substance abuse treatment programs (CTPs) across the USA. Inclusion criteria: To be eligible, individuals needed to have had at least 1 traumatic event in their lifetime and to meet DSM–IV–TR criteria for either full or subthreshold PTSD (where they did not meet criteria for either category C (avoidance and numbing symptoms) or category D (symptoms of increased arousal) but met all other criteria). Other inclusion criteria were between 18 and 65 years of age, had used alcohol or an illicit substance within the past 6 months, had a current diagnosis of drug or alcohol abuse or dependence, and were capable of giving informed consent. Exclusion criteria: Individuals were excluded if they had an advanced stage medical disease as indicated by global physical deterioration, impaired cognition, significant risk of suicidal/homicidal intent or behaviour, a history of schizophrenia‐spectrum diagnosis, a history of active (past 2 months) psychosis, or involvement in litigation related to PTSD. Individuals were also excluded if they did not speak English or if they refused to be video‐ or audiotaped. Sample size: 1963 individuals were assessed for eligibility; 353 were randomised, and all were included in the analyses. PTSD diagnosis: 80.4% of women met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: Women met diagnosis for substance abuse and substance dependence. Women were polydrug users. Mean age: 39.2 (SD = 9.2) years Gender: 353 (100%) female Ethnicity: 34% African American; 45.6% white; 6.5% Latina; 13.3% multiracial; 0.6% other Country: USA |
|
| Interventions |
Group 1: Group‐based Seeking Safety plus treatment as usual: n = 176. Seeking Safety is a structured cognitive behavioural treatment with both safety/trauma and substance use components integrated into each session. All sessions have the same structure: (a) check‐in, including reports of any unsafe behaviours and use of coping skills, (b) session quotation, a brief point of inspiration to affectively engage women and link to the session topic, (c) relating the material to the women’s lives, in which handouts are used to facilitate discussion and structured skill practice, and (d) check‐out, including a commitment to specific between‐session skills practice. Each session covered a different topic as follows: safety, taking back power from PTSD, when substances are in control, honesty, setting boundaries in relationships, compassion, healing from anger, creating meaning, integrating the split self, taking good care of oneself, red and green flags, and detaching from emotional pain (grounding). Seeking Safety treatment was abbreviated from 25 to 12 core sessions (75 to 90 minutes) delivered over 6 weeks to fit within a feasible time frame for community‐based outpatient treatment programs. However, because 2 women needed to be present to conduct the group, many women took longer than 6 weeks to complete the interventions. All study participants were enrolled in 1 of the participating community treatment programs and were asked to attend treatment as usual at the program during the 6‐week treatment phase of the study. Treatment as usual was not kept constant across sites but was allowed to vary. Outpatient treatment differed across sites in frequency and length of sessions per week, although most offered intensive outpatient services of 3 days per week or more. The treatment orientation of the programs also varied, but none of the programs provided trauma‐focused treatment to women during the study. Group 2: Group‐based Women's Health Education plus treatment as usual: n = 177. Women’s Health Education (WHE) was intended to control for therapeutic time and attention. WHE is a psycho‐educational, manualised health curriculum focused on topics such as understanding the female body, human sexual behaviour, pregnancy and childbirth, sexually transmitted diseases, HIV, and AIDS. WHE was designed to provide equivalent therapeutic attention, expectancy of benefit, and an issue‐oriented focus, but without theory‐driven techniques (i.e. those of Seeking Safety) or any explicit focus on or psycho‐education specific to substance abuse or trauma. All WHE sessions followed a common format: (a) introduction of topic, (b) review of group rules and between‐session assignment, (c) topic presentation, (d) a video, storytelling, and/or text readings, and (e) topic exercises in a variety of formats to facilitate group discussion and application of session materials, and (f) setting between‐session goals. Treatment as usual for the WHE group was as described above. Experimental intervention modality: Integrated |
|
| Outcomes |
PTSD: CAPS total score; PSS‐SR total score SUD: Substance use diagnosis as measured by the Composite International Diagnostic Interview for DSM–IV; quantity and frequency of substance use as measured by the Substance Use Inventory; biologically confirmed abstinence from drugs of abuse was obtained by use of the SureStep urine drug screen card; recent alcohol use was tested with the Alco Screen‐Saliva Alcohol Test. Treatment acceptability: Data are reported for number of women attending at least 1 group treatment session and number attending at least 6 treatment sessions. Other: None Follow‐up: 1 week, 3, 6, and 12 months. The PSS‐SR and SUI were administered weekly during the treatment phase as well as at all other time points. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician generated 1 blocked randomisation list (block size known only to the statistician) for the entire study |
| Allocation concealment (selection bias) | Low risk | Each participating community‐based substance abuse treatment program received sets of 60 sealed, tamper evident security envelopes, containing 1 randomisation number and the corresponding treatment assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent assessors who remained unaware of randomisation assignment performed all baseline and post‐treatment assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Treatment dropouts and withdrawals were clearly reported. An ITT approach was employed using generalised estimating equations |
| Selective reporting (reporting bias) | Unclear risk | Follow‐up outcomes were obtained at 1 week and 3, 6, and 12 months. The summary of outcomes table reports average outcome scores for the follow‐up period of 3 to 12 months. Use of averaged outcome scores was not specified in the methodology. It is unclear how data from these time points were used in analyses. Some analyses are reported for the 12‐month follow‐up point. It is unclear if data were reported in this way at the request of the publishing journal or by decision of the research group |
| Other bias | Low risk | There was no significant difference between the 2 treatment groups on any demographic or baseline diagnostic characteristics. We identified no other potential biases |
McGovern 2011.
| Methods | Design: RCT | |
| Participants |
Setting: Participants were new admissions to a community addiction treatment program and recruited from 1 of 7 participating community intensive outpatient or methadone maintenance programs. Inclusion criteria: Participants were at least 18 years of age, were actively enrolled in outpatient addiction services, and met criteria for any substance use disorder. Participants were also required to have a diagnosis of PTSD verified by the CAPS with total symptom score equal to or greater than 44. Exclusion criteria: Acute psychotic symptoms (people with a psychotic disorder were eligible if their symptoms were stable and they were receiving appropriate mental health services); psychiatric hospitalisation or suicide attempt in the past month, unless the hospitalisation or attempt was directly related to substance intoxication or detoxification and the person was currently stable; or unstable medical and legal situations such that ability to participate in the full duration of the study seemed unlikely. Sample size: 77 individuals were assessed for eligibility; 53 were randomised, and 36 attended at least 1 treatment session. 53 were included in the analyses. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: All participants met diagnosis for a substance use disorder. Type of substance use and the number meeting substance dependence were not specified. Mean age: Integrated CBT plus standard care group: 39.09 (SD = 11.32) years; individual addiction counselling plus standard care group: 35.48 (SD = 9.44) years Gender: 23 (43.4%) male; 30 (56.6%) female Ethnicity: 90.6% white; other ethnicities were not described Country: USA |
|
| Interventions |
Group 1: Integrated cognitive behavioural therapy (ICBT) plus standard care: n = 32. ICBT is a manual‐guided individual‐based therapy focusing on PTSD symptoms and substance use. It was designed for integration into routine community addiction treatment programming. Participants were required to be active in either intensive outpatient or methadone maintenance services. ICBT consisted of 8 modules: introduction to treatment, crisis and relapse prevention planning, breathing retraining, psycho‐education about PTSD primary symptoms, psycho‐education about additional associated symptoms, two cognitive restructuring modules, and generalisation training. ICBT was delivered in an individual format, within a weekly 45‐ to 50‐minute session, over approximately 12 to 14 sessions. A client workbook was to be used in conjunction with the therapist manual with practice handout items for homework in between treatment sessions. Standard care occurred in either methadone maintenance or intensive outpatient clinics. 2 of the 7 recruiting programs were methadone maintenance, and 5 were intensive outpatient programs. Group 2: Individual addiction counselling (IAC) plus standard care: n = 21. IAC is a manual‐guided individual‐based therapy designed to be integrated into an addiction treatment or methadone maintenance program. IAC targeted substance use only and was considered complementary to a typical community addiction treatment program. IAC consisted of 5 modules: treatment initiation, early abstinence, maintaining abstinence, recovery, and termination. IAC was delivered in 10 to 12 weekly sessions. As with ICBT, individual addiction counselling had participant practice handouts for homework in between treatment sessions. Standard care was as described above. Experimental intervention modality: Integrated |
|
| Outcomes |
PTSD: CAPS SUD: ASI alcohol and drug composites; toxicology: recent alcohol intake and drug metabolites for amphetamine, benzodiazepines, cannabis, cocaine, methamphetamine, and opiates were screened for using urine and breath samples gathered at each assessment period. Treatment acceptability: This was described in terms of initiation (number of participants attending at least 1 treatment session), engagement (number completing at least 2 sessions), and completion (number attending at least 75% of sessions). Other: BDI Follow‐up: 3 and 6 months postbaseline |
|
| Notes | It is unclear as to why there is such sizeable difference between the numbers of participants randomised to the 2 conditions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported. It is reported that research interviewers were blinded to treatment assignment at randomisation, but other information about the randomisation process is not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research interviewers were not blinded to treatment assignment at the follow‐up assessments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analysis was undertaken using generalised estimating equations analyses, which allowed analyses without excluding participants based on missing data points or drop‐out. However, the study only achieved a follow‐up rate of 53%, and as the authors acknowledge, this "reduces the power and the ability to detect differences between treatment conditions". |
| Selective reporting (reporting bias) | Low risk | Primary outcomes are specified in the protocol registered with ClinicalTrials.gov |
| Other bias | High risk | There were a number of minor differences between the 2 groups at baseline (e.g. PTSD severity). The effects of these differences are unclear. The number of treatment sessions provided to the intervention (12 to 14) was longer than that provided to the control intervention (10 to 12) |
Mills 2012.
| Methods | Design: RCT | |
| Participants |
Setting: Participants were seen on an outpatient basis and were recruited from substance use treatment services, media advertisements, and practitioner referrals. Inclusion criteria: Past‐month DSM‐IV‐TR diagnoses of PTSD and substance dependence, age 18 years or older, and fluency in English. Exclusion criteria: Individuals were excluded from participating if they were currently suicidal (expressed suicidal ideation accompanied by a plan and intent), had a recent history of self harm (past 6 months), had current active symptoms of psychosis, or experienced cognitive impairment severe enough to impede treatment. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. Sample size: 334 individuals were assessed for eligibility; 103 were randomised, and all were included in the analyses. SUD type and diagnosis: All participants were reported to be substance dependent. Participants were polydrug users and had used a mean of 4 drug classes in the previous month. Mean age: 33.7 (SD = 7.9) years Gender: 64 (62.1%) female Ethnicity: Australian born: 87 (84.5%); 6 (5.8%) Aboriginal or Torres Strait Islander Country: Australia |
|
| Interventions |
Group 1: Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE): n = 55. COPE is a modified version of Concurrent Treatment of PTSD and Cocaine Dependence. The model represents an integration of existing evidence‐based manualised CBT interventions for PTSD and substance dependence. Intervention consists of 13 individual‐based 90‐minute sessions (i.e. 19.5 hours) delivered by a clinical psychologist. Although designed to be delivered weekly, flexibility was permitted. Treatment components include motivational enhancement and CBT for substance use; psycho‐education relating to both disorders and their interaction; in vivo exposure; imaginal exposure; and cognitive therapy for PTSD. The final session was dedicated to providing a review of the treatment, devising an aftercare plan, and termination of therapy. Group 2: Usual treatment: n = 48. Both the treatment and the control group were able to engage in usual treatment for substance dependence. As such, participants could access any type of substance use treatment currently available in the community, including outpatient counselling, inpatient or outpatient detoxification, residential rehabilitation, and pharmacotherapies (e.g. methadone, buprenorphine, buprenorphine plus naloxone, naltrexone). Experimental intervention modality: Combined |
|
| Outcomes |
PTSD: CAPS SUD: CIDI Treatment acceptability: Data are reported for number of participants attending at least 1 treatment session; number attending at least 1 imaginal exposure session; and number attending all sessions. Other: BDI, STAI, IPDE Follow‐up: 6 weeks, 3 and 9 months postbaseline |
|
| Notes | The study concluded with a lower sample size than planned due to a low recruitment rate. It is unclear why there is such sizeable difference between the numbers of participants randomised to the 2 conditions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomization was conducted in groups of 10, stratified according to sex ... " |
| Allocation concealment (selection bias) | Low risk | " ... by a person independent of the research." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviews were administered by 2 trained research officers blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were analysed on an ITT basis. Missing data were imputed using multiple imputation |
| Selective reporting (reporting bias) | Low risk | Key outcomes are as specified in the protocol registered with the World Health Organization's trials portal |
| Other bias | High risk | Participants in the control group were more likely to have reported a history of childhood sexual abuse. This was controlled for in analyses. There was considerable variability in the time taken to complete treatment, with some participants continuing treatment well beyond the planned treatment period of 13 weeks and at least 1 receiving treatment around the final follow‐up point |
Mueser 2008.
| Methods | Design: RCT | |
| Participants |
Setting: Individuals with severe mental illness were recruited from community mental health centres. Inclusion criteria: Minimum age 18 years; designation by the states of New Hampshire or Vermont as having a severe mental illness, defined as a DSM–IV Axis I disorder and persistent impairment in the areas of work, school, or ability to care for oneself; DSM–IV diagnosis of major depression, bipolar disorder, schizoaffective disorder, or schizophrenia; current DSM–IV diagnosis of PTSD; and legal ability and willingness to provide informed consent to participate in the study. Exclusion criteria: Psychiatric hospitalisation or suicide attempt within the past 3 months; current DSM–IV substance dependence. Sample size: 270 individuals were assessed for eligibility; 108 were randomised, and all were included in the analyses. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: Nature of substance abuse was not specified. 44 (40.7%) of participants met diagnosis for substance use disorder. Outcome data are available for this subgroup. Mean age: 44.21 (SD = 10.64) years Gender: 35/44 (79.5%) female Ethnicity: 38 (86.4%) white; (4.5%) African American; (4.5%) American Indian/Alaska Native; (2.3%) Hispanic; (2.3%) Asian‐Pacific Islander Country: USA |
|
| Interventions |
Group 1: Individual CBT for PTSD: n = 17. Sessions included an introduction to the programme; crisis plan review; psycho‐education on core and associated symptoms of PTSD; cognitive restructuring; generalisation training; and termination. Participants were offered 12 to 16 sessions over 4 to 6 months. Group 2: Treatment as usual: n = 27. Participants assigned to TAU continued to receive the usual services they had been receiving before enrolment in the program. None of the mental health centres offered either cognitive restructuring or exposure therapy treatments for PTSD, although supportive counselling for trauma‐related problems was available. Experimental intervention modality: Treatment of PTSD only |
|
| Outcomes |
PTSD: CAPS SUD: ‐ Treatment acceptability: Data are reported for number of participants in the experimental condition attending at least 6 treatment sessions. Other: PTCI; PTSD Knowledge Test; Brief Psychiatric Rating Scale; BDI‐II; BAI; 12‐Item Short Form Health Survey; client version of the Working Alliance Inventory Follow‐up: End of treatment and at 3 and 6 months' post‐treatment |
|
| Notes | This study did not specifically aim to treat individuals meeting diagnosis for SUD. A subset of participants met SUD diagnosis, and study authors provided data for these individuals. These participants only met criteria for substance abuse; individuals meeting diagnosis for substance dependence were excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted by a computer‐based randomisation program |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted at a central location in a research centre. Assignments were not known in advance by either clinical or research staff |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were conducted by blinded interviewers at all assessment points. Participants were instructed at the beginning of interviews not to talk about any treatments for trauma‐related problems they may have received. Interviewers were requested to inform the project co‐ordinator if the client broke the blind during an interview. Interviewers were not asked to guess clients’ treatment assignments, to avoid directly encouraging them to formulate hypotheses about how treatment may have affected clients’ symptoms, which could have influenced subsequent ratings. No specific instances of blind breaking were noted in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals are thoroughly described. ITT analysis was conducted to determine the effects of primary outcomes |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were reported as specified in the methodology section, but we were not able to identify a previously published protocol |
| Other bias | Low risk | There were no differences between the groups on any demographic, diagnostic, or baseline measures or in the rates of follow‐up assessments |
Najavits 2006a.
| Methods | Design: RCT | |
| Participants |
Setting: Participants were adolescent girls who were treated on an outpatient basis and were recruited through posted fliers and active recruitment from local clinics, hospitals, schools, and clinicians. Inclusion criteria: Participants met current DSM‐IV criteria for both PTSD and SUD. They also had to report active substance use within the past 60 days. Exclusion criteria: Potential participants were excluded if they had a history of bipolar I disorder (mania), psychotic disorder, were mandated to treatment, or had characteristics that would interfere with treatment completion (mental retardation, homelessness, impending incarceration, or a life‐threatening illness). Sample size: The number of individuals assessed for eligibility was not specified; 33 were randomised, and all were included in the analyses. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: Most participants (n = 31, 93.9%) met diagnosis for substance dependence. Participants were polydrug users. Mean age: 16.06 (1.22) Gender: 100% female Ethnicity: 78.8% white; 12.8% Asian‐Pacific Islander; 3% Hispanic; 3% African American; 3% multiethnic Country: USA |
|
| Interventions |
Group 1: Individual‐adapted Seeking Safety plus treatment as usual: n = 18. This coping skills therapy targets current PTSD and SUD. The treatment manual has 25 topics representing cognitive, behavioural, and interpersonal domains. Each topic offers a safe coping skill relevant to both disorders, such as Asking for Help, Compassion, Setting Boundaries in Relationships, and Honesty. The treatment has five principles: (1) safety as the priority; (2) integrated treatment of both disorders; (3) a focus on ideals; (4) four content areas: cognitive, behavioural, interpersonal, and case management; and (5) attention to therapist processes. The original manual was modified to take account of the developmental level of adolescents. Participants were offered 25 50‐minute sessions over 3 months. Group 2: Treatment as usual: n = 15. All participants were allowed to attend any concurrent treatments they naturalistically sought (e.g. Alcoholics Anonymous, psychotropic medication, and other individual and group psychotherapies). Experimental intervention modality: Integrated |
|
| Outcomes |
PTSD: Trauma Symptom Checklist for Children SUD: Personal Experiences Inventory Treatment acceptability: For the Seeking Safety group, the mean number of all treatment sessions attended and the number of Seeking Safety sessions attended were reported. Data were also reported for client satisfaction (see below). Other: Beliefs About Substance Use; World Assumptions Scale; Adolescent Psychopathology Scale; CSQ; HAQ; Teen Treatment Services Review interview Follow‐up: End of treatment and at 3 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information about sequence generation and allocation concealment were provided by the lead study author. A statistician independent of the study generated the randomisation list prior to the first randomisation using a random number generator |
| Allocation concealment (selection bias) | Low risk | The list was administered "lock‐step", and the principal investigator and therapists were unable to influence randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The process of outcome assessment was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were well reported. Authors employed a full intent‐to‐treat analysis using a random effects model to analyse data |
| Selective reporting (reporting bias) | High risk | The Personal Experiences Inventory was identified as the primary measure. This measure was described as having 2 subsections (chemical involvement problem severity and psychosocial problems), each with multiple subscales. More specific information about whether a total score, subsection score, or subscale score was the primary outcome was not given. Other outcome measures also had multiple subscales. Outcomes were not clearly reported. A table describing outcomes at intake, end of treatment, and 3 months' follow‐up only shows data for outcomes that were significant |
| Other bias | High risk | The TAU group had a higher level of psychopathology as measured at baseline. There was no attempt to control for this. The study describes a number of outcome measures, with a large number of subscales. We estimated 40 outcomes. It appears there were no attempts to correct for the use of multiple statistical testing |
Norman unpublished.
| Methods | Design: RCT | |
| Participants |
Setting: Participants were female victims of interpersonal violence. They were recruited through flyers posted in community agencies that serve IPV victims and in primary care and psychiatry clinics. Inclusion criteria: Female interpersonal violence victims over the age of 18, with at least 1 month out of the abusive relationship, met DSM‐IV criteria for PTSD and an alcohol use disorder, literate in English, had not changed psychotropic medications or dosages within the previous 2 months and agreed not to during the active phase (first 12 weeks) of the intervention, and had an identified primary care physician. Exclusion criteria: Moderate or severe cognitive impairment as measured by a Mini‐Mental State Examination score less than or equal to 18, history of psychosis (women with histories of psychosis or mania were only included if their symptoms had been well managed by pharmacotherapy for the most recent 6‐month period). Sample size: 78 individuals were assessed for eligibility, 35 were randomised, and 29 received at least 1 session of treatment or remained in contact with the research group and were included in analysis. PTSD diagnosis: 25 (86.2%) of women met full diagnosis for PTSD as measured by the CAPS; other women met subthreshold diagnosis for PTSD. SUD type and diagnosis: All women met diagnosis for alcohol use disorder. Mean age: Seeking Safety: 45.27 (SD = 8.44); facilitated 12‐step: 37.38 (SD = 9.13) Gender: 100% female Ethnicity: 65.5% white; 6.9% African American; 24.1% Hispanic; 3.4% American Indian Country: USA |
|
| Interventions |
Group 1: Adapted group‐based Seeking Safety plus treatment as usual: n = 20. Seeking Safety plus Cognitive Trauma Therapy for Battered Women with PTSD (CTT‐BW) (Kubany 2004).The intervention was a 24‐session group treatment protocol delivered over 12 weeks, incorporating the following interventions: psycho‐education regarding PTSD and alcohol use disorders, skills to reduce self harm behaviours, behavioural activation, exposure to trauma, identifying and managing triggers, building social support, coping skills, assertive communication, managing affect, problem solving, grounding, and cognitive restructuring. Group 2: 12‐step supportive group: n = 9. Therapist‐led supportive group using a 12‐step model where women were encouraged to discuss issues related to domestic violence and abstinence from alcohol. Experimental intervention modality: Integrated |
|
| Outcomes |
PTSD: CAPS, PCL‐C SUD: TLFB, Conceptual Cues/Coping Questionnaire Treatment acceptability: ‐ Other: Adult Attachment Interview, Motivation/Self Esteem Scale, Anxiety Sensitivity Index, BDI, CD‐RISC‐10, Negative Mood Regulation Expectancies Scale, Self Compassion Scale, Coping Skills Follow‐up: End of treatment and at 3 and 6 months' post‐treatment |
|
| Notes | This study had originally intended to recruit 100 individuals but were unable to achieve this within the period that the study was funded. Investigators had great difficulty gathering data after the initial follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated |
| Allocation concealment (selection bias) | Unclear risk | Allocation was handled by the study co‐ordinator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blind to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was performed on women who attended at least 1 treatment session. Women who were randomised but did not attend any intervention sessions were unaware of their allocation (Fergusson 2002) |
| Selective reporting (reporting bias) | Low risk | Key outcomes are as specified in the protocol registered with ClinicalTrials.gov |
| Other bias | High risk | There was a significant difference in the average age of the two groups. It is unclear why there is such sizeable difference in the numbers of women randomised to the 2 conditions. There was also a sizeable, though non‐significant, difference in alcohol consumption at baseline, and notable differences in ethnic makeup between the 2 groups |
Sannibale 2013.
| Methods | Design: RCT | |
| Participants |
Setting: Participants were recruited from a range of services in metropolitan Sydney, Australia and seen on an outpatient basis. Inclusion criteria: Individuals were eligible if they were 18 years of age or older, consumed alcohol at hazardous levels (men 29 or more and women 15 or more 10 g ethanol drinks per week) and met DSM‐IV diagnostic criteria for PTSD, determined by the CAPS. AUD diagnosis was determined by the Structured Clinical Interview for DSM‐IV. Individuals on stable doses (for 2 months or longer) of pharmacotherapy for depression or alcohol dependence were eligible, as were individuals who needed and completed alcohol withdrawal. Exclusion criteria: People were excluded if they were 17 years or younger, had current psychosis, severe suicide risk, significant cognitive impairment, limited English comprehension, or severe substance dependence. Sample size: 154 individuals were screened and 90 assessed for eligibility; 62 were randomised, and all were included in the analyses. PTSD diagnosis: 58 (94%) of participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: All participants met criteria for AUD. Mean age: 41.18 (SD = 11.91) years Gender: 33 (53%) female Ethnicity: Not reported Country: Australia |
|
| Interventions |
Group 1: Integrated CBT for PTSD and AUD: n = 33. Participants in both conditions received the same treatment targeting AUD. This consisted of motivational interviewing, intervention focused on coping with cravings, cognitive intervention related to drinking and management of negative moods. Participants in this arm also received cognitive behavioural intervention for PTSD, based on a prolonged exposure model with cognitive restructuring. Treatment in both the experimental and control condition was manualised, and consisted of 12, once‐weekly 90‐minute individual sessions with structured daily homework tasks. Group 2: CBT for AUD and supportive counselling: n = 29. In addition to the shared components described above, this group also received supportive counselling. Treatment in this arm targeted AUD symptoms only, not PTSD symptoms. Experimental intervention modality: Combined |
|
| Outcomes |
PTSD: CAPS, PDS SUD: TLFB, SADQ Treatment acceptability: Median sessions attended, number attending 1 or more sessions, 6 or more sessions, 9 or more sessions. Other: Short Inventory of Problems, BDI, STAI Follow‐up: Post‐treatment and 5 and 9 months' post‐treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was conducted according to a random number system by a person independent of the study ... " |
| Allocation concealment (selection bias) | Low risk | " ... and treatment was concealed." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up assessments were conducted by independent clinicians who were unaware of the participants’ treatment condition and did not have access to participant clinical or supervision notes or treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses were based on intent‐to‐treat, including all participants who entered the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified in the methodology section |
| Other bias | Low risk | There were no differences between the groups on any demographic, diagnostic, or baseline measures or in the rates of follow‐up assessments |
Zlotnick 2009.
| Methods | Design: RCT | |
| Participants |
Setting: Treatment was conducted on the minimum‐security wing of a female prison. Participants were recruited from a voluntary residential substance abuse treatment program for women requesting intensive substance abuse treatment. Inclusion criteria: DSM‐IV criteria for current PTSD or subthreshold PTSD (i.e. had at least 1 symptom from all 3 clusters that were associated with impairment/distress) within the previous month as determined by the CAPS; and DSM‐IV criteria for substance dependence 1 month prior to entering prison as determined by the Structured Clinical Interview for DSM‐IV. Exclusion criteria: Women were excluded if they were actively psychotic at the time of recruitment, did not know English well enough to be able to understand the consent form or measures, or were diagnosed with organic brain impairment. Sample size: 92 women were assessed for eligibility; 49 were randomised, and 44 were included in the analyses. PTSD diagnosis: 83.5% of women met full diagnosis for PTSD as measured by the CAPS, and 16.5% met the subthreshold definition. SUD type and diagnosis: Women were polydrug users. 87.8% met criteria for alcohol dependence prior to imprisonment, with another 4.1% meeting criteria for lifetime alcohol abuse. The percentages of women who had ever used a single substance at a level typically indicating dependence (10 or more times in 1 month) were 93.9% for cocaine, 75.5% for cannabis, 59.2% for heroin or other opioids, 38.8% for sedatives/hypnotics/anxiolytics, 30.6% for hallucinogens/PCP, and 26.5% for stimulants. Mean age: 34.6 (SD = 7.4) years Gender: 100% female Ethnicity: 23 (46.9%) white; 16 (32.7%) African American; 7 (14.2%) Hispanic; and 3 (6.1%) other races/ethnicities Country: USA |
|
| Interventions |
Group 1: Group‐based Seeking Safety plus treatment as usual: n = 27. Seeking Safety is a present‐focused therapy to help people attain safety from trauma/PTSD and substance abuse. The treatment was designed for flexible use. SS is based on a number of key principles: safety, integrated treatment of both PTSD and substance abuse at the same time, a focus on ideals, and attention to clinician processes. Interventions are in the domain of cognitive, behavioural, interpersonal, and case management. Seeking Safety consists of 25 topics that can be conducted in any order: Introduction/Case Management, Safety, PTSD: Taking Back Your Power, When Substances Control You, Honesty, Asking for Help, Setting Boundaries in Relationships, Getting Others to Support Your Recovery, Healthy Relationships, Community Resources, Compassion, Creating Meaning, Discovery, Integrating the Split Self, Recovery Thinking, Taking Good Care of Yourself, Commitment, Respecting Your Time, Coping with Triggers, Self‐Nurturing, Red and Green Flags, Detaching from Emotional Pain (Grounding), Life Choices, and Termination. SS was conducted in group modality for 90 min, typically 3 times a week for 6 to 8 weeks while the women were in prison, with 3 to 5 women per group. After release from prison, each woman in SS was offered weekly individual 60‐min “booster” sessions for 12 weeks to reinforce material from the group sessions. Group 2: Treatment as usual: n = 22. All women in this study were enrolled in a 28‐bed residential substance use treatment program in the minimum‐security wing (approximately 30 hours per week). Women typically attend this program for 3 to 6 months, depending on the length of their sentences. Substance use treatment was abstinence‐oriented, focused on the 12‐step model, and took place in a psycho‐educational large‐group format, with weekly individual case management and drug counselling. Psycho‐educational groups included attention to women's health, domestic violence, affect management, relapse prevention, career exploration, anger management, and parenting, conducted by the same clinicians who conducted the SS treatment. This program did not offer any treatment specifically for trauma. Prior to prison release, the women received case management services, although this discontinued once the women were released from prison. All women leaving prison were referred for further substance use treatment. Experimental intervention modality: Integrated |
|
| Outcomes |
PTSD: CAPS; Trauma Symptom Checklist 40 SUD: ASI; TLFB Treatment acceptability: Treatment utilisation; CSQ; mean number of sessions attended Other: Brief Symptom Inventory; legal composite score of the ASI (for criminal activity) Follow‐up: 12 weeks after the start of the program and 3 and 6 months following release from prison |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study included data only from those who were available to follow‐up. The number of women who did not provide data at follow‐up was fairly small but slightly disproportionate to the SS group. We did not feel this difference was sufficient to have a clinically relevant impact on observed effect sizes |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were reported as specified in the methodology section, but we were not able to identify a previously published protocol |
| Other bias | High risk | The authors acknowledge potential contamination of treatment and control conditions in the closed communal setting of a prison wing. Postrelease follow‐up dose was not equivalent. Women in the SS group were offered up to 12 booster sessions on release from prison. Women in the control group were referred for further substance use treatment |
AA: Alcoholics Anonymous ACQ‐Now: Alcohol Craving Questionnaire‐Now ASI: Addiction Severity Index AUD: alcohol use disorder BAI: Beck Anxiety Inventory BDI: Beck Depression Inventory CAPS: Clinician Administered PTSD Scale CBT: cognitive behavioural therapy CDIS‐IV: Computerized Diagnostic Interview Schedule CD‐RISC‐10: 10‐item Connor‐Davidson Resilience Scale CGI: Clinical Global Impressions CI: confidence interval CIDI: Composite International Diagnostic Interview CSQ: Client Satisfaction Questionnaire DESNOS: Disorders of Extreme Stress Not Otherwise Specified DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Text Revision FREEDOM: self‐regulation via Focusing (SOS: Slow down, Orient, Self‐check); processing current traumatic stress reactions via Recognizing current triggers, Emotions, and cognitive Evaluations, and, strength‐based reintegration by Defining core goals, identifying currently effective responses (Options), and affirming core values by Making positive contributions GAIN: Global Appraisal of Individual Needs HAQ: Helping Alliance Questionnaire HDRS: Hamilton Depression Rating Scale HLS: Healthy Lifestyles Sessions IES‐R: Impact of Events Scale‐Revised IPDE: International Personality Disorder Examination IPV: interpersonal violence ITT: intention‐to‐treat HIV: human immunodeficiency virus NA: Narcotics Anonymous PACS: Penn Alcohol Craving Scale PANAS: Positive and Negative Affect Schedule PCL‐C: PTSD Checklist‐Civilian Version PDS: Post‐traumatic Stress Diagnostic Scale PE: prolonged exposure PSS‐I: PTSD Symptom Scale‐Interview PSS‐SR: PTSD Symptom Scale–Self‐Report PTCI: Post‐traumatic Cognitions Inventory PTSD: post‐traumatic stress disorder RCT: randomised controlled trial SADQ: Severity of Alcohol Dependence Questionnaire SD: standard deviation SS: Seeking Safety STAI: State‐Trait Anxiety Inventory SUD: substance use disorder SUDS: Subjective Units of Distress SUI: Substance Use Inventory TAU: treatment as usual TLFB: Timeline Followback Interview VA: Veterans Affairs
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brief 2013 | Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument |
| Cucciare 2013 | Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument |
| Forbes 2012 | Types of participants: Less than 80% had SUD at baseline. We did obtain outcome data for a subgroup of participants in this study but decided that we could not include the study as participants who were identified as having an alcohol use disorder were not diagnosed through a clinician‐administered assessment |
| Ford 2007 | Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument |
| Ford 2011 | Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument |
| Ghee 2009 | Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument |
| Glasner‐Edwards 2013 | Types of assessment: PTSD was established in a subset of participants at 3 years' follow‐up. PTSD was not established at baseline |
| Hien 2015 | Type of intervention: The experimental intervention was pharmacological |
| Lynch 2012 | Types of studies: Not a randomised controlled trial |
| McDevitt‐Murphy 2014 | Types of participants: Participants were included on the basis of screening for hazardous drinking. Alcohol use disorder was not diagnosed through a clinician‐administered assessment |
| Meshberg‐Cohen 2010 | Types of participants: Less than 80% had PTSD at baseline. We were unable to obtain outcome data for the subset who did have PTSD |
| Perez‐Dandieu 2014 | Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument |
| Rosen 2013 | Type of intervention: Not a psychological therapy |
| Saladin 1995 | Types of studies: Not a randomised controlled trial |
| Triffleman 2000 | Types of participants: Less than 80% had PTSD at baseline. We were unable to obtain outcome data for the subset who did have PTSD |
| Triffleman 2001 | A full report of this study is not yet available |
| van Dam 2013 | Types of participants: Less than 80% of participants were diagnosed as having PTSD/SUD at baseline, and we were unable to obtain subset data |
PTSD: post‐traumatic stress disorder SUD: substance use disorder
Characteristics of studies awaiting assessment [ordered by study ID]
Barrett 2015.
| Methods | Randomised controlled trial |
| Participants | Male prisoners with PTSD and comorbid substance use |
| Interventions | (Seeking Safety + TAU) vs TAU (alone) |
| Outcomes | SUDs, PTSD (acceptability, feasibility, and preliminary efficacy of Seeking Safety among male Australian prisoners) |
| Notes | Does not meet 80% PTSD threshold but does meet other criteria. I will approach the author for subset data |
McGovern 2015.
| Methods | Randomised controlled trial |
| Participants | Outpatients in addiction services with PTSD and SUDs |
| Interventions | Cognitive behavioural therapy vs individual addiction counselling vs TAU |
| Outcomes | Primary outcomes: PTSD symptom severity (CAPS score); Drug and alcohol symptom severity (ASI‐Self Administered); Frequency of substance use (TLFB Interview); Positive toxicology screens (urine drug screen and breathalyser). |
| Notes | Study reports not retrieved in March 2015 search results (first added to CCDANCTR 29 July 2015, via an OVID PsycINFO alert) |
Park 2012.
| Methods | Randomised controlled trial |
| Participants | A subset of 204 participants with PTSD were included in a cohort of 553 randomised participants with co‐occurring addiction and mental disorder |
| Interventions | Integrated chronic disease management vs primary care intervention |
| Outcomes | Abstinence, depression, anxiety |
| Notes | Conference abstract only (full study report not retrieved in March 2015 search results, added to CCDANCTR 13 April 2015, via PsycINFO OVID alert) |
Perez‐Dandieu 2015.
| Methods | Randomised controlled trial |
| Participants | Treatment of SUD in 7 women with SUD and PTSD comorbidity |
| Interventions | Effects of EMDR associated with ST 3‐phase protocol: 1. Eight EMDR sessions focused on reprocessing traumatic memory; 2. Eight EMDR sessions (traumatic memory) associated with ST (traumatic attachment) 3. Eight EMDR sessions (addictive memory) associated with ST |
| Outcomes | PTSD, SUDs, and attachment disorder |
| Notes | Conference abstract only, full study report not yet available |
Simpson 2011.
| Methods | Randomised controlled trial |
| Participants | 84 individuals with current PTSD and alcohol dependence were randomised |
| Interventions | Experiential acceptance vs cognitive restructuring vs attention placebo |
| Outcomes | 71 participants completed the study (84.5%); 13 of the 84 were lost to follow‐up. PTSD and alcohol‐related outcomes were not reported in the conference abstract. |
| Notes | Report of primary outcomes identified later (Stappenbeck 2015), added to CCDANCTR 13 April 2015, via a PsycINFO OVID alert dated 25 March 2015) Contact with trialists Tracey Simpson and Cindy Stappenbeck, confirmed this was the same trial as NCT00760994 |
Skidmore 2013.
| Methods | Randomised controlled trial |
| Participants | 145 individuals with co‐occurring alcohol or substance dependence, depression, and trauma exposure were randomised |
| Interventions | Integrated cognitive behavioural therapy (ICBT) for co‐occurring depression and addiction plus cognitive processing therapy vs ICBT for co‐occurring depression and addiction plus continuation of ICBT |
| Outcomes | Group assignment (ICBT vs CPT) was not related to attendance. PTSD and alcohol‐related outcomes were not reported in abstract |
| Notes | Conference abstract, full study report not yet available |
Wolf 2015.
| Methods | Randomised and non‐randomised participants |
| Participants | Incarcerated men with PTSD and SUDs, housed at a high‐security prison operated by the Pennsylvania Department of Corrections |
| Interventions | Integrated group therapy: Seeking Safety vs Men’s Trauma Recovery and Empowerment Model vs wait‐list control |
| Outcomes | Primary outcomes: PTSD Checklist‐Civilian Version, CAPS, Global Severity Index, Brief Symptom Inventory |
| Notes | This study meets most of our inclusion criteria. However, it includes both randomised and non‐randomised participants. We would need to determine whether we could obtain data for randomised participants only |
ASI: Addiction Severity Index CAPS: Clinician Administered PTSD Scale EMDR: eye movement desensitisation and reprocessing PTSD: post‐traumatic stress disorder ST: schema therapy SUD: substance use disorder TAU: treatment as usual TLFB: Timeline Followback Interview
Characteristics of ongoing studies [ordered by study ID]
DRKS00004288.
| Trial name or title | Cognitive‐behavioral treatment for female patients with PTSD and SUD |
| Methods | Multicentre randomised controlled trial |
| Participants | 342 females with PTSD and SUD |
| Interventions | Seeking Safety (14 sessions) vs structured relapse prevention vs TAU |
| Outcomes | PTSD symptoms, substance use |
| Starting date | October 2012 |
| Contact information | Ingo Schäfer: ischafer@uke.de |
| Notes |
NCT00946322.
| Trial name or title | Couple‐based treatment for alcohol use disorders and post‐traumatic stress disorder (CTAP) |
| Methods | Randomised controlled trial |
| Participants | Veterans meeting current DSM‐IV diagnosis for alcohol abuse or dependence and PTSD |
| Interventions | TAU vs couple‐based treatment for alcohol use disorders and PTSD |
| Outcomes | Number of days drinking or using drugs; problems related to drinking or using drugs; PTSD; couple relationship adjustment; number of days of heavy drinking or using drugs (outcome measures not specified) |
| Starting date | August 2010 |
| Contact information | Jeremiah A Schumm: Jeremiah.Schumm@va.gov |
| Notes |
NCT01029197.
| Trial name or title | Multicomponent cognitive behavioral therapy (CBT) for posttraumatic stress disorder (PTSD) and substance abuse (PTSD/SUD) |
| Methods | Pilot randomised controlled trial |
| Participants | Participants will be 50 volunteer adults with PTSD, SUD, and serious mental illness who are receiving services at the Freedom House Recovery Center, served through the Orange Person Chatham Area Program |
| Interventions | Group and individual CBT and exposure therapy for PTSD |
| Outcomes | Clinician Administered PTSD Scale, Addiction Severity Index |
| Starting date | August 2009 |
| Contact information | Karen Cusack: Karen.cusack@va.gov |
| Notes |
NCT01186315.
| Trial name or title | Post‐traumatic stress disorder (PTSD), addiction, and virtual reality |
| Methods | Randomised controlled trial |
| Participants | Military veterans, National Guardsmen, and reservists with PTSD and problems with addiction |
| Interventions | Prolonged exposure vs prolonged exposure plus virtual reality‐based exposure to cues for marijuana, cocaine, heroin, cigarette, and/or alcohol use, and phone‐based reminders of learning (extinction reminders) to virtual reality exposure |
| Outcomes | Acceptibility, change in PTSD symptoms, change in substance use |
| Starting date | December 2008 |
| Contact information | Zachary Rosenthal, Duke University |
| Notes |
NCT01211106.
| Trial name or title | Integrated vs sequential treatment for PTSD and addiction among OEF/OIF veterans |
| Methods | Randomised controlled trial |
| Participants | Male or female Persian Gulf‐era veterans (18 to 65 years old). Current diagnosis of PTSD (symptom duration > 3 months) with clinically significant trauma‐related symptoms, as indicated by a score of at least 50 on the PTSD Checklist. Current abuse or dependence on alcohol, stimulants such as cocaine, opioids, including prescription opioids or benzodiazepines. |
| Interventions | Prolonged exposure vs motivational enhancement therapy |
| Outcomes | Substance use and PTSD symptoms |
| Starting date | February 2011 |
| Contact information | David W. Oslin: oslin@upenn.edu |
| Notes |
NCT01274741.
| Trial name or title | Pilot study of an integrated exposure‐based model for posttraumatic stress disorder and substance use disorder |
| Methods | Randomised controlled trial |
| Participants | Individuals meeting DSM‐IV criteria for current PTSD and current SUD |
| Interventions | Integrated psychotherapy for PTSD/SUD ("Creating Change") vs modified TAU |
| Outcomes | Change in substance use from baseline to 3 months' post‐treatment measured via urine drug screens and the Addiction Severity Index composite scores; change in PTSD symptoms from baseline to 3 months' post‐treatment assessed using the PTSD Checklist and the Clinician Administered PTSD Scale |
| Starting date | January 2011 |
| Contact information | Lisa Najavits: Lnajavits@hms.harvard.edu |
| Notes |
NCT01338506.
| Trial name or title | Integrated treatment of Operation Enduring Freedom/Operation Iraqi Freedom veterans with post‐traumatic stress disorder and substance use disorders |
| Methods | Randomised controlled trial |
| Participants | Adult male and female active‐duty Operation Iraqi Freedom (OIF)/Operation Enduring Freedom (OEF) military personnel and separated OIF/OEF veterans aged 18 to 65 years |
| Interventions | Concurrent treatment of PTSD and substance use disorders using prolonged exposure (COPE) vs TAU |
| Outcomes | Clinician Administered PTSD Scale; reduction of substance use or abstinence |
| Starting date | April 2011 |
| Contact information | Sudie E. Back: backs@musc.edu |
| Notes |
NCT01357577.
| Trial name or title | Cognitive behavioral therapy (CBT) for PTSD in veterans with co‐occurring SUDs |
| Methods | Randomised controlled trial |
| Participants | Veterans with a current SUD diagnosis, scoring at least 45 on the Clinician Administered PTSD Scale |
| Interventions | CBT plus TAU vs TAU |
| Outcomes | PTSD symptom severity will be measured by the Clinician Administered PTSD Scale. Other measures not provided |
| Starting date | October 2012 |
| Contact information | Liz Forshay: elizabeth.forshay@va.gov |
| Notes |
NCT01365247.
| Trial name or title | Concurrent treatment for substance dependent individuals with post‐traumatic stress disorder (PTSD) |
| Methods | Randomised controlled trial |
| Participants | Participants must meet DSM‐IV criteria for current or past substance dependence and current PTSD |
| Interventions | Concurrent treatment of PTSD and substance use disorders using prolonged exposure (COPE) and relapse prevention therapy vs a delayed‐treatment control group |
| Outcomes | PTSD symptom severity, substance use severity, global psychiatric symptom severity, treatment retention and compliance |
| Starting date | September 2008 |
| Contact information | Teresa Lopez‐Castro: lopezcastro.phd.ccny@gmail.com; Lesia Ruglass: lmr2146@columbia.edu |
| Notes |
NCT01457404.
| Trial name or title | Integrated cognitive behavioral therapy for co‐occurring PTSD and substance use disorders |
| Methods | Randomised controlled trial |
| Participants | OEF/OIF/OND Veteran status with a diagnosis of PTSD (confirmed by the Clinician Administered PTSD Scale with a total symptom score of 44 or more) and a diagnosis of a SUD (abuse or dependence) (confirmed by the Structured Clinical Interview for DSM‐IV Section E) |
| Interventions | Integrated cognitive behavioural therapy vs TAU |
| Outcomes | Decrease from baseline in Clinician Administered PTSD Scale score (PTSD symptom severity) at 3 and 6 months. Reduction from baseline in substance use severity (Addiction Severity Index) at 3 and 6 months. |
| Starting date | February 2011 |
| Contact information | Mark McGovern: mark.p.mcgovern@dartmouth.edu |
| Notes |
NCT01597856.
| Trial name or title | Evaluation and treatment of substance abuse in veterans with PTSD disability claims |
| Methods | Randomised controlled trial |
| Participants | Veteran of OEF or OIF enrolling for compensation and pension for PTSD |
| Interventions | Screening, brief intervention, and referral to treatment (SBIRT) vs no additional treatment |
| Outcomes | Treatment attendance, substance use, days of alcohol use, PTSD |
| Starting date | March 2013 |
| Contact information | Marc Rosen: marc.rosen@va.gov |
| Notes |
NCT01663337.
| Trial name or title | Sequence of symptom change during AUD (alcohol use or dependence) or PTSD (posttraumatic stress disorder) treatment for comorbid PTSD/AUD |
| Methods | Randomised controlled trial |
| Participants | Adults ≥ 18 years of age with a current DSM‐V diagnosis of alcohol abuse/dependence and PTSD |
| Interventions | Cognitive processing therapy vs relapse prevention therapy vs assessment only |
| Outcomes | Primary outcomes: Reduction in PTSD symptom severity (Clinician Administered PTSD Scale) Reduction in alcohol consumption (Form 90 (Alcohol Consumption)) |
| Starting date | March 2013 |
| Contact information | Debra Kaysen: dkaysen@u.washington.edu |
| Notes |
NCT01693978.
| Trial name or title | Contingency outcomes in prolonged exposure (COPE) |
| Methods | Randomised controlled trial |
| Participants | Participants must meet DSM‐IV criteria for SUD and current PTSD |
| Interventions | Prolonged exposure with contingency management vs prolonged exposure |
| Outcomes | Prolonged exposure attendance, PTSD symptoms, drug use |
| Starting date | September 2012 |
| Contact information | Jessica Peirce, Johns Hopkins University |
| Notes |
NCT01849029.
| Trial name or title | Cognitive processing intervention for HIV/STI and substance use among native women |
| Methods | Randomised controlled trial |
| Participants | Sexually active women with current substance use and PTSD (score 30 or hire on the PTSD Checklist) |
| Interventions | Cognitive processing therapy vs wait‐list control |
| Outcomes | Primary outcome: PTSD Symptom Scale‐Interview |
| Starting date | October 2013 |
| Contact information | Cynthia Pearson: pearsonc@u.washington.edu |
| Notes |
NCT02081417.
| Trial name or title | Patient‐centered trauma treatment (PaCTT) |
| Methods | Randomised controlled trial |
| Participants | Meet DSM‐IV diagnostic criteria for lifetime and current full or subthreshold PTSD and DSM‐IV diagnostic criteria for current substance abuse or dependence |
| Interventions | Peer‐led Seeking Safety group vs clinician‐led Seeking Safety group |
| Outcomes | PTSD severity as indexed by the PTSD Checklist, change in substance use as indexed by the Addiction Severity Index |
| Starting date | October 2013 |
| Contact information | Annette Crisanti: acrsanti@salud.unm.edu |
| Notes |
NCT02335125.
| Trial name or title | A policy relevant US trauma care system pragmatic trial for PTSD and comorbidity pilot (TSOS 6) |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: Inpatient/emergency admission for traumatic injury (The goal of this pilot study is to develop and implement a larger‐scale, multisite, stepped collaborative care trial that targets injured patients with presentations of PTSD and related comorbidity) |
| Interventions | The intervention aims to prevent the development of chronic PTSD and depressive symptoms, alcohol use problems, and enduring physical disability in survivors of both traumatic brain and non‐traumatic brain injuries |
| Outcomes | Primary outcomes: PTSD Checklist‐Civilian Version at 1 month; Alcohol Use Disorders Identification Test at 1 month; Patient Health Questionnaire 9‐item Depression Scale at 1 month |
| Starting date | February 2015 |
| Contact information | Douglas Zatzick: dzatzick@u.washington.edu |
| Notes |
NTR3084.
| Trial name or title | A study on the effectiveness of the cognitive behavioral therapy "Seeking Safety" in reducing trauma and addiction related symptoms in a Dutch substance‐use disorder population |
| Methods | Randomised controlled trial |
| Participants | Outpatients from substance misuse services with active symptoms of PTSD in the past 6 months |
| Interventions | Seeking Safety vs TAU |
| Outcomes | The primary outcome measure will be substance use severity. Secondary outcome measures are PTSD and trauma symptoms, coping skills, functioning, and cognitions |
| Starting date | January 2012 |
| Contact information | Tim Kok: t.kok@tactus.nl |
| Notes |
AUD: alcohol use disorder CBT: cognitive behavioural therapy DSM: Diagnostic and Statistical Manual of Mental Disorders OEF/OIF/OND: Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn PTSD: post‐traumatic stress disorder SUD: substance use disorder TAU: treatment as usual
Differences between protocol and review
We added a section explaining how we would approach meta‐analysis under the heading 'Main planned comparisons' in Data extraction and management. We decided to include studies where less than 80% of participants met our diagnostic inclusion criteria if we were able to obtain study data on the subset that met diagnosis.
In the protocol we framed the section 'Experimental interventions' as a 'catch‐all' list of the types of intervention that we thought might potentially have been investigated in this patient group. It was not our intention to necessarily group our comparisons on this basis. We have therefore revised this section in order to provide a more meaningful structure to the review. Specific treatment models such as COPE and Seeking Safety were subsumed into other types of approaches, as they provided specific examples of these approaches. The distinction between trauma‐focused and non‐trauma‐focused approaches was consistent with that of the review undertaken by van Dam 2012. We also recognised that we needed to articulate that we would undertake separate analysis for group‐ and individual‐based interventions. Group‐based interventions are generally considered to show weaker effects than individual‐based interventions (Najavits 2014 [personal communication]). This finding has been specifically in relation to PTSD (Bisson 2013). We have removed categories for other psychological approaches, stepped care and interventions aimed at enhancing positive well‐being through physiotherapy, occupational therapy, or guided self help. We can say with hindsight that it is highly unlikely we would have found evaluations of these types of interventions in this specific population.
We included a fourth review author, NJ, in order to provide balance to the review author group's expertise in the fields of treatment of PTSD and SUD. NJ is an expert in the treatment of SUDs.
As a post hoc addition, we defined the time points of interest in the Types of outcome measures section. As a further post hoc addition, we described our approach to summarising comparison findings under the heading 'Summary of findings'.
Contributions of authors
NPR drafted the review.
PAR undertook screening of papers, evaluation of risk of bias, data extraction, and commented on the protocol and write‐up of the review.
NJ undertook evaluation of risk of bias, data extraction, and commented on the write‐up of the review.
JIB undertook supervision of the review, arbitrated over issues of contention, and commented on the protocol and write‐up of the review.
Declarations of interest
NPR: None declared.
PAR: None declared.
NJ: None declared.
JIB: None declared.
New
References
References to studies included in this review
Boden 2012 {published data only}
- Boden MT, Kimerling R, Jacobs‐Lentz J, Bowman D, Weaver C, Carney D, et al. Seeking Safety treatment for male veterans with a substance use disorder and post‐traumatic stress disorder symptomatology. Addiction 2012;107(3):578‐86. [DOI] [PubMed] [Google Scholar]
- Boden MT, Kimerling R, Kulkarni M, Bonn‐Miller MO, Weaver C, Trafton J. Coping among military veterans with PTSD in substance use disorder treatment. Journal of Substance Abuse Treatment 2014;47(2):160‐7. [DOI] [PubMed] [Google Scholar]
Coffey 2006 {published and unpublished data}
- Coffey SF, Stasiewicz PR, Hughes PM, Brimo ML. Trauma‐focused imaginal exposure for individuals with comorbid posttraumatic stress disorder and alcohol dependence: revealing mechanisms of alcohol craving in a cue reactivity paradigm. Psychology of Addictive Behaviors 2006;20(4):425‐35. [DOI] [PubMed] [Google Scholar]
Coffey submitted {unpublished data only}
- Coffey SF, Schumacher JA, Nosen E. Trauma‐focused exposure therapy for chronic posttraumatic stress disorder in alcohol and drug dependent patients: a randomized clinical trial (as supplied 3 September 2013). Study manuscript and data on file. [DOI] [PMC free article] [PubMed]
- Nosen E, Littlefield AK, Schumacher JA, Stasiewicz PR, Coffey SF. Treatment of co‐occurring PTSD‐AUD: Effects of exposure‐based and non‐trauma focused psychotherapy on alcohol and trauma cue‐reactivity. Behaviour Research and Therapy 2014;61:35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foa 2013 {published data only}
- Avny SB. Long‐term outcomes of prolonged exposure and naltrexone for patients with co‐morbid posttraumatic stress disorder and alcohol dependence. Dissertation Abstracts International: Section B: the Sciences and Engineering 2015;76(2‐BE):No Pagination Specified. [Google Scholar]
- Foa EB, McLean CP, Yusko D. Therapy for posttraumatic stress and alcohol dependence ‐ reply comment. JAMA 2013;310:2458‐9. [DOI] [PubMed] [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Oslin D, et al. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial. JAMA 2013;310(5):488‐95. [DOI] [PubMed] [Google Scholar]
- McLean CP, Su Y‐J, Foa EB. Mechanisms of symptom reduction in a combined treatment for comorbid posttraumatic stress disorder and alcohol dependence. Journal of Consulting and Clinical Psychology 2015;83(3):655‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Su YJ, Foa EB. Posttraumatic stress disorder and alcohol dependence: Does order of onset make a difference?. Journal of Anxiety Disorders 2014;28:894‐901. [DOI] [PubMed] [Google Scholar]
- Powers MB, Gillihan SJ, Rosenfield D, Jerud AB, Foa EB. Reliability and validity of the PDS and PSS‐I among participants with PTSD and alcohol dependence. Journal of Anxiety Disorders 2012;26(5):617‐23. [DOI] [PubMed] [Google Scholar]
- Zandberg LJ, Rosenfield D, McLean CP, Powers MB, Asnaani A, Foa EB. Concurrent treatment of posttraumatic stress disorder and alcohol dependence: predictors and moderators of outcome. Journal of Consulting and Clinical Psychology 2015;84(1):43‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frisman 2008 {published data only}
- Ford J, Frisman L. Outcome of trauma treatment with comorbid substance abuse [symposium presentation 96D]. Proceedings of the 156th Annual Meeting of the American Psychiatric Association, May 17‐22, San Francisco, CA. 2003:170‐1.
- Frisman L, Ford J, Lin H‐J, Mallon S, Chang R. Outcomes of trauma treatment using the TARGET model. Journal of Groups in Addiction & Recovery 2008;3(3‐4):285‐303. [Google Scholar]
Hien 2004 {published data only}
- Caldeira NA. Dissociation and treatment outcome in urban women with comorbid PTSD and substance use disorders. Dissertation Abstracts International 2004;65(3‐B):1540. [Google Scholar]
- Hartl CG. The course of cravings in women with co‐morbid disorder undergoing cognitive behavioral treatments. Dissertation Abstracts International 2005;65(12‐B):6653. [Google Scholar]
- Hien D. Trauma and PTSD: Issues in the treatment of drug‐dependent women [symposium presentation 85C]. Proceedings of the 157th Annual Meeting of the American Psychiatric Association; 1‐6 May; New York, NY. 2004:221.
- Hien DA, Cohen LR, Miele GM, Litt LC, Capstick C. Promising treatments for women with comorbid PTSD and substance use disorders. American Journal of Psychiatry 2004;161(8):1426‐32. [DOI] [PubMed] [Google Scholar]
- Ruglass LM. Ethnocultural differences in therapeutic alliance and outcome for women with comorbid posttraumatic stress disorder and substance use disorder. Dissertation Abstracts International 2006;66(8‐B):4499. [Google Scholar]
- Stiffler CL. PTSD symptom reductions following Seeking Safety and relapse prevention treatments. Dissertation Abstracts International 2006;66(9B):5107. [Google Scholar]
Hien 2009 {published and unpublished data}
- Anderson ML, Najavits LM. Does Seeking Safety reduce PTSD symptoms in women receiving physical disability compensation?. Rehabilitation Psychology 2014;59:349‐53. [DOI] [PubMed] [Google Scholar]
- Cohen LR, Field C, Campbell ANC, Hien DA. Intimate partner violence outcomes in women with PTSD and substance use: A secondary analysis of NIDA Clinical Trials Network "Women and Trauma" Multi‐site Study. Addictive Behaviors 2013;38(7):2325‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LR, Greenfield SF, Gordon S, Killeen T, Jiang H, Zhang Y, et al. Survey of eating disorder symptoms among women in treatment for substance abuse. The American Journal on Addictions 2010;19(3):245‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Campbell AN, Killeen T, Hu MC, Hansen C, Jiang H, et al. The impact of trauma‐focused group therapy upon HIV sexual risk behaviors in the NIDA Clinical Trials Network 'Women and trauma' multi‐site study. AIDS and Behavior 2010;14(2):421‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Campbell ANC, Ruglass LM, Hu MC, Killeen T. The role of alcohol misuse in PTSD outcomes for women in community treatment: A secondary analysis of NIDA's Women and Trauma Study. Drug and Alcohol Dependence 2010;111(1‐2):114‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Campbell ANC, Ruglass LM, Saavedra L, Mathews AG, Kiriakos G, et al. Maximizing effectiveness trials in PTSD and SUD through secondary analysis: Benefits and limitations using the National Institute on Drug Abuse Clinical Trials Network "Women and Trauma" Study as a case example. Journal of Substance Abuse Treatment 2015;56:23‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Jiang H, Campbell AN, Hu MC, Miele GM, Cohen LR. Do treatment improvements in PTSD severity affect substance use outcomes? A secondary analysis from a randomized clinical trial in NIDA's Clinical Trials Network. American Journal of Psychiatry 2010;167(1):95‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Morgan‐Lopez AA, Campbell AN, Saavedra LM, Wu E, Cohen L, et al. Attendance and substance use outcomes for the Seeking Safety program: sometimes less is more. Journal of Consulting and Clinical Psychology 2012;80(1):29‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Wells EA, Jiang H, Suarez‐Morales L, Campbell ANC, Cohen LR, et al. Multisite randomized trial of behavioral interventions for women with co‐occurring PTSD and substance use disorders. Journal of Consulting and Clinical Psychology 2009;77(4):607‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JL. Understanding the arrest experiences of women with co‐occurring substance abuse and posttraumatic stress disorder: An application of general strain theory [thesis]. Dissertation Abstracts International Section A: Humanities and Social Sciences 2015;75(8‐A(E)):No pagination specified. [Google Scholar]
- Killeen T, Hien D, Campbell A, Brown C, Hansen C, Jiang H, et al. Adverse events in an integrated trauma‐focused intervention for women in community substance abuse treatment. Journal of Substance Abuse Treatment 2008;35(3):304‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Castro T, Hu MC, Papini S, Ruglass LM, Hien DA. Pathways to change: Use trajectories following trauma‐informed treatment of women with co‐occurring posttraumatic stress disorder and substance use disorders. Drug and Alcohol Review 2015;34(3):242‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Hu M‐C, Campbell ANC, Hilario EY, Weiss RD, Hien DA. Changes in sleep disruption in the treatment of co‐occurring posttraumatic stress disorder and substance use disorders. Journal of Traumatic Stress 2014;27:82‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan‐Lopez AA, Saavedra LM, Hien DA, Campbell AN, Wu E, Ruglass L. Synergy between Seeking Safety and twelve‐step affiliation on substance use outcomes for women. Journal of Substance Abuse Treatment 2013;45(2):179‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan‐Lopez AA, Saavedra LM, Hien DA, Campbell AN, Wu E, Ruglass L, et al. Indirect effects of 12‐session Seeking Safety on substance use outcomes: overall and attendance class‐specific effects. The American Journal on Addictions 2014;23(3):218‐25. [DOI] [PubMed] [Google Scholar]
- Pinto RM, Campbell ANC, Hien DA, Yu G. Retention in the National Institute on Drug Abuse Clinical Trials. American Journal of Orthopsychiatry 2011;81(2):211‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resko SM, Mendoza NS. Early attrition from treatment among women with co‐occurring substance use disorders and PTSD. Journal of Social Work Practice in the Addictions 2012;12(4):348‐69. [Google Scholar]
- Ruglass LM, Hien DA, Hu M‐C, Campbell ANC. Associations between post‐traumatic stress symptoms, stimulant use, and treatment outcomes: A secondary analysis of NIDA's Women and Trauma Study. The American Journal on Addictions 2014;23(1):90‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruglass LM, Hien DA, Hu M‐C, Campbell ANC, Caldeira NA, Miele GM, et al. Racial/ethnic match and treatment outcomes for women with PTSD and substance use disorders receiving community‐based treatment. Community Mental Health Journal 2014;50:811‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruglass LM, Miele GM, Hien DA, Campbell AN, Hu MC, Caldeira N, et al. Helping alliance, retention, and treatment outcomes: A secondary analysis from the NIDA Clinical Trials Network Women and Trauma Study. Substance Use and Misuse 2012;47(6):695‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya JA. An investigation of early response as a mediator in group psychotherapy for women with post‐traumatic stress disorder and substance use disorders. Dissertation Abstracts International: Section B: the Sciences and Engineering 2014;75. [Google Scholar]
- Smith S. Examining the influence of peritraumatic dissociation on treatment outcomes and symptom severity among women substance users. Dissertation Abstracts International: Section B: the Sciences and Engineering 2015;75(10‐B(E)):No pagination specified. [Google Scholar]
- Winhusen T, Winstanley EL, Somoza E, Brigham G. The potential impact of recruitment method on sample characteristics and treatment outcomes in a psychosocial trial for women with co‐occurring substance use disorder and PTSD. Drug and Alcohol Dependence 2012; Vol. 120:225‐8. [DOI] [PMC free article] [PubMed]
McGovern 2011 {published data only}
- McGovern MP, Lambert‐Harris C, Alterman AI, Xie H, Meier A. A randomized controlled trial comparing integrated cognitive behavioral therapy versus individual addiction counseling for co‐occurring substance use and posttraumatic stress disorders. Journal of Dual Diagnosis 2011;7(4):207‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2012 {published and unpublished data}
- Barrett EL, Mills KL, Teesson M. Hurt people who hurt people: Violence amongst individuals with comorbid substance use disorder and post traumatic stress disorder. Addictive Behaviors 2011;36(7):721‐8. [DOI] [PubMed] [Google Scholar]
- Farrugia PL, Mills KL, Barrett E, Back SE, Teesson M, Baker A, et al. Childhood trauma among individuals with co‐morbid substance use and post‐traumatic stress disorder. Mental Health and Substance Use 2011;4(4):314‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Teeson M, Back SE, Brady KT, Baker AL, Hopwood S, et al. Integrated exposure based therapy for co‐occuring posttraumatic stress disorder and substance dependence. JAMA 2012;308(7):690‐9. [DOI] [PubMed] [Google Scholar]
- Mills KL, Teesson M, Back S, Baker A, Hopwood S, Brady K, et al. A randomized controlled trial of an integrated treatment for substance use and PTSD incorporating exposure therapy: preliminary findings. Proceedings of the 70th Annual Scientific Meeting of the College on Problems of Drug Dependence; Jun 14‐19; San Juan, Puerto Rico, USA. 2008:131.
- Mills KL, Teesson M, Barrett E, Merz S, Rosenfeld J, Farrugia P, et al. Is exposure therapy for posttraumatic stress disorder efficacious among people with substance use disorders? results from a randomised controlled trial. Proceedings of the 72th Annual Scientific Meeting of the College on Problems of Drug Dependence; Jun 12‐17; Scottsdale, Arizona. USA. 2010:115.
Mueser 2008 {published and unpublished data}
- Mueser KT, Rosenburg SD, Xie H, Jankowski MK, Bolton EE, Lu W, et al. A randomized controlled trial of cognitive–behavioral treatment for posttraumatic stress disorder in severe mental illness. Journal of Consulting and Clinical Psychology 2008;76(2):259‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Najavits 2006a {published data only}
- Najavits LM, Gallop RJ, Weiss RD. Seeking Safety therapy for adolescent girls with PTSD and substance use disorder: A randomized controlled trial. Journal of Behavioral Health Services and Research 2006;33(4):453‐63. [DOI] [PubMed] [Google Scholar]
Norman unpublished {unpublished data only}
- Norman S. Alcohol use disorders (AUDs) and post‐traumatic stress disorder (PTSD) treatment for victims of partner violence. http://clinicaltrials.gov/ct2/show/NCT00607412 2007 (accessed 3 January 2014). [Google Scholar]
Sannibale 2013 {published data only}
- Sannibale C, Teesson M, Creamer M, Sitharthan T, Bryant RA, Sutherland K, et al. Randomized controlled trial of cognitive behaviour therapy for comorbid post‐traumatic stress disorder and alcohol use disorders. Addiction 2013;108(8):1397‐410. [DOI] [PubMed] [Google Scholar]
Zlotnick 2009 {published data only}
- Zlotnick C, Johnson J, Najavits LM. Randomized controlled pilot study of cognitive‐behavioral therapy in a sample of incarcerated women with substance use disorder and PTSD. Behavior Therapy 2009;40(4):325‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Brief 2013 {published data only}
- Brief DJ, Rubin A, Keane TM, Enggasser JL, Roy M, Helmuth E, et al. Web intervention for OEF/OIF veterans with problem drinking and PTSD symptoms: A randomized clinical trial. Journal of Consulting and Clinical Psychology 2013;81(5):890‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cucciare 2013 {published data only}
- Cucciare MA, Boden MT, Weingardt KR. Brief alcohol counseling improves mental health functioning in veterans with alcohol misuse: Results from a randomized trial. Journal of Affective Disorders 2013;147(1‐3):312‐7. [DOI] [PubMed] [Google Scholar]
- Cucciare MA, Weingardt KR, Ghaus S, Boden MT, Frayne SM. A randomized controlled trial of a web‐delivered brief alcohol intervention in Veterans Affairs primary care. Journal of Studies on Alcohol and Drugs 2013;74(3):428‐36. [DOI] [PubMed] [Google Scholar]
- Mason AE, Boden MT, Cucciare MA. Prospective associations among approach coping, alcohol misuse and psychiatric symptoms among veterans receiving a brief alcohol intervention. Journal of Substance Abuse Treatment 2014;46(5):553‐60. [DOI] [PubMed] [Google Scholar]
Forbes 2012 {published and unpublished data}
- Forbes D, Lloyd D, Nixon RD, Elliott P, Varker T, Perry D, et al. A multisite randomized controlled effectiveness trial of cognitive processing therapy for military‐related posttraumatic stress disorder. Journal of Anxiety Disorders 2012;26(3):442‐52. [DOI] [PubMed] [Google Scholar]
Ford 2007 {published data only}
- Ford JD, Hawke J, Alessi S, Ledgerwood D, Petry N. Psychological trauma and PTSD symptoms as predictors of substance dependence treatment outcomes. Behaviour Research and Therapy 2007;45(10):2417‐31. [DOI] [PubMed] [Google Scholar]
Ford 2011 {published data only}
- Ford JD, Steinberg KL, Zhang W. A randomized clinical trial comparing affect regulation and social problem‐solving psychotherapies for mothers with victimization‐related PTSD. Behavior Therapy 2011;42(4):560‐78. [DOI] [PubMed] [Google Scholar]
Ghee 2009 {published data only (unpublished sought but not used)}
- Ghee AC, Bolling LC, Johnson CS. The efficacy of a condensed Seeking Safety intervention for women in residential chemical dependence treatment at 30 days posttreatment. Journal of Child Sexual Abuse 2009;18(5):475‐88. [DOI] [PubMed] [Google Scholar]
- Ghee AC, Johnson CS, Burlew AK, Boiling LC. Enhancing retention through a condensed trauma‐integrated intervention for women with chemical dependence. North American Journal of Psychology 2009;11(1):157‐72. [Google Scholar]
Glasner‐Edwards 2013 {published data only}
- Glasner‐Edwards S, Mooney LJ, Ang A, Hillhouse M, Rawson R. Does posttraumatic stress disorder affect post‐treatment methamphetamine use?. Journal of Dual Diagnosis 2013;9(2):123‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hien 2015 {published data only}
- Hien DA, Levin FR, Ruglass L, Lopez‐Castro T. Enhancing the effects of cognitive behavioral therapy for PTSD and alcohol use disorders with antidepressant medication: A randomized clinical trial. Drug and Alcohol Dependence [abstracts of the Annual Meeting of the College on Problems of Drug Dependence; 2014 Jun 14‐19; San Juan, Puerto Rico] 2015:e142. [Google Scholar]
- Hien DA, Levin FR, Ruglass LM, López‐Castro T, Papini S, Hu MC, et al. Combining Seeking Safety with Sertraline for PTSD and alcohol use disorders: A randomized controlled trial. Journal of Consulting and Clinical Psychology 2015;83(2):359‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lynch 2012 {published data only}
- Lynch SM, Heath NM, Matthews KC, Cepeda GJ. Seeking Safety: An intervention for trauma exposed incarcerated women?. Journal of Trauma and Dissociation 2012;13:88‐101. [DOI] [PubMed] [Google Scholar]
McDevitt‐Murphy 2014 {published data only}
- McDevitt‐Murphy ME, Murphy JG, Williams JL, Monahan CJ, Bracken‐Minor KL, Fields JA. Randomized controlled trial of two brief alcohol interventions for OEF/OIF veterans. Journal of Consulting and Clinical Psychology 2014;82(4):562‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meshberg‐Cohen 2010 {published data only (unpublished sought but not used)}
- Meshberg‐Cohen S. Expressive writing as a therapeutic process for drug dependent women. Dissertation Abstracts International: Section B: the Sciences and Engineering 2010;70(12‐B):7860. [Google Scholar]
Perez‐Dandieu 2014 {published data only}
- Perez‐Dandieu B, Tapia G. Treating Trauma in Addiction with EMDR: A Pilot Study. Journal of Psychoactive Drugs 2014;46(4):303‐9. [DOI] [PubMed] [Google Scholar]
Rosen 2013 {published data only}
- Rosen CS, Tiet QQ, Harris AH, Julian TF, McKay JR, Moore WM, et al. Telephone monitoring and support after discharge from residential PTSD treatment: A randomized controlled trial. Psychiatric Services 2013;64(1):13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saladin 1995 {published data only}
- Saladin ME, Brady KT, Dansky BS, Kilpatrick DG. Understanding comorbidity between PTSD and substance use disorders: two preliminary investigations. Addictive Behaviors 1995;20(5):643‐55. [DOI] [PubMed] [Google Scholar]
Triffleman 2000 {published data only (unpublished sought but not used)}
- Triffleman E. Gender differences in a controlled pilot study of psychosocial treatments in substance dependent patients with post‐traumatic stress disorder: Design considerations and outcomes. Alcoholism Treatment Quarterly 2000;18(3):113‐26. [Google Scholar]
Triffleman 2001 {published data only (unpublished sought but not used)}
- Triffleman E. SDPT vs CBCST: a randomized controlled trial among ptsd + opiate dependent subjects. Drug and Alcohol Dependence 2001;63(1):159. [Google Scholar]
van Dam 2013 {published data only}
- Dam D, Ehring T, Vedel E, Emmelkamp PMG. Trauma‐focused treatment for posttraumatic stress disorder combined with CBT for severe substance use disorder: a randomized controlled trial. BMC Psychiatry 2013;13(172):202‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Barrett 2015 {published data only}
- Barrett EL, Indig D, Sunjic S, Sannibale C, Sindicich N, Rosenfeld J, et al. Treating comorbid substance use and traumatic stress among male prisoners: A pilot study of the acceptability, feasibility, and preliminary efficacy of Seeking Safety. The International Journal of Forensic Mental Health 2015;14(1):45‐55. [Google Scholar]
McGovern 2015 {published data only}
- McGovern MP, Lambert‐Harris C, Xie H, Meier A, McLeman B, Saunders E. A randomized controlled trial of treatments for co‐occurring substance use disorders and post‐traumatic stress disorder. Addiction 2015;110(7):1194‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A, McGovern MP, Lambert‐Harris C, McLeman B, Franklin A, Saunders EC, et al. Adherence and competence in two manual‐guided therapies for co‐occurring substance use and posttraumatic stress disorders: Clinician factors and patient outcomes. American Journal of Drug and Alcohol Abuse 2015;41(6):527‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders EC, McGovern MP, Lambert‐Harris C, Meier A, McLeman B, Xie H. The impact of addiction medications on treatment outcomes for persons with co‐occurring PTSD and opioid use disorders. The American Journal on Addictions 2015;24(8):722‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2012 {published data only}
- Park TW, Cheng DM, Samet JH, Winter M, Saitz R. Effectiveness of chronic disease management for co‐occurring substance and mental health disorders (abstract). Alcoholism: Clinical and Experimental Research [abstracts of the 2012 International Society for Biomedical Research on Alcoholism World Congress, ISBRA; 2012 Sep 9‐12; Sapporo, Japan] 2012;36:17A. [Google Scholar]
- Park TW, Cheng DM, Samet JH, Winter MR, Saitz R. Chronic care management for substance dependence in primary care among patients with co‐occurring disorders. Psychiatric Services 2015;66(1):72‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TW, Cheng DM, Samet JH, Winter MR, Saitz R. Chronic care management for substance dependence in primary care among patients with co‐occurring disorders. Psychiatric Services Hospital & Community Psychiatry 2015;66(1):72‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Dandieu 2015 {published data only}
- Perez‐Dandieu B, Lenoir H, Othily E, Tapia G, Cassen M, Delile J‐M. The impact of eye movement desensitization and reprocessing and schema therapy on addiction severity among a sample of French women suffering from PTSD and SUD [abstract]. Drug and Alcohol Dependence 2015:e68‐9. [Google Scholar]
Simpson 2011 {published data only}
- Simpson T, Rosenthal C, Gurrad B, Luterek J, Kaysen D. A pilot study evaluating mechanisms of change among patients with comorbid PTSD and alcohol dependence: Methods and feasibility. Alcoholism: Clinical and Experimental Research [abstracts from the 34th Annual Scientific Meeting of the Research Society on Alcoholism, RSA. 25‐29 Jun 2011; Atlanta, GA United States] 2011. [Google Scholar]
- Simpson TL, Stappenbeck CA, Luterek JA, Rosenthal CF, Gurrad B, Kaysen D. Outcomes of an RCT comparing two coping skills among dually diagnosed individuals with alcohol dependence and PTSD. Alcoholism, Clinical and Experimental Research [abstracts of the 38th Annual Scientific Meeting of the Research Society on Alcoholism; 2015 Jun 20‐24; San Antonio, TX United States] 2015:299A. [Google Scholar]
- Stappenbeck CA, Luterek JA, Kaysen D, Rosenthal CF, Gurrad B, Simpson TL. A controlled examination of two coping skills for daily alcohol use and PTSD symptom severity among dually diagnosed individuals. Behaviour Research and Therapy 2015;66:8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skidmore 2013 {published data only}
- Skidmore JR, Goldsteinholm K, Trim RS, Tate SR. Predictors of treatment attendance among veterans receiving treatment for co‐occurring substance dependence, depression, and trauma. Alcoholism, Clinical and Experimental Research [abstracts from the 36th Annual Scientific Meeting of the Research Society on Alcoholism, RSA; 2013 Jun 22‐26; Orlando, FL United States] 2013;37(suppl 2):135A. [Google Scholar]
Wolf 2015 {published data only}
- Wolff N, Huening J, Shi J, Frueh BC, Hoover DR, McHugo G. Implementation and effectiveness of integrated trauma and addiction treatment for incarcerated men. Journal of Anxiety Disorders 2015;30:66‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
DRKS00004288 {published data only}
- DRKS00004288. Cognitive‐behavioral treatment for female patients with PTSD and SUD ‐ CANSAS 2.A. http://www.drks.de/DRKS00004288 (accessed 25 January 2015).
NCT00946322 {published data only}
- NCT00946322. Couple‐Based Treatment for Alcohol Use Disorders and Post‐Traumatic Stress Disorder (CTAP). http://clinicaltrials.gov/show/NCT00946322 (accessed 25 January 2015).
NCT01029197 {published data only}
- NCT01029197. Multicomponent Cognitive Behavioral Therapy for Posttraumatic Stress Disorder and Substance Abuse: A Pilot Study. http://clinicaltrials.gov/show/NCT01029197 (accessed 25 January 2015).
NCT01186315 {published data only}
- NCT01186315. Developing a computer‐based intervention to enhance behavioral treatments for PTSD and addiction. http://clinicaltrials.gov/show/NCT01186315 (accessed 25 January 2015).
NCT01211106 {published data only}
- NCT01211106. Integrated vs sequential treatment for PTSD and addiction among OEF/OIF veterans. https://clinicaltrials.gov/ct2/show/NCT01211106 (accessed 4 December 2015). [Google Scholar]
- Oslin DW, Polusny M, Kehle‐Forbes S, Horn D, Yusko D, et al. Integrated versus sequential treatment for comorbid PTSD/addiction among veterans [abstract]. Alcoholism, Clinical and Experimental Research 2015:142A. [Google Scholar]
NCT01274741 {published data only (unpublished sought but not used)}
- NCT01274741. Study of treatment for posttraumatic stress disorder and substance use. https://clinicaltrials.gov/show/NCT01274741 (accessed 25 January 2015).
NCT01338506 {published data only}
- Jobe‐Shields L, Flanagan JC, Killeen T, Back SE. Family composition and symptom severity among Veterans with comorbid PTSD and substance use disorders. Addictive Behaviors 2015;50:117‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano BE, Gros DF, Killeen T, Jaconis M, Beylotte FM, Boyd S, et al. To reduce or abstain? Substance use goals in the treatment of veterans with substance use disorders and comorbid PTSD. The American Journal on Addictions 2015;24(7):578‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01338506. Integrated treatment of OEF/OIF veterans with PTSD & substance use disorders (COPE). https://clinicaltrials.gov/show/NCT01338506 (accessed 25 January 2015). [Google Scholar]
NCT01357577 {published data only}
- Forshay E. Cognitive behavioral therapy (CBT) for PTSD in veterans with co‐occurring SUDs. ClinicalTrials.gov (accessed 25 January 2015).
NCT01365247 {published data only}
- Hien D. Concurrent treatment for substance dependent individuals with post‐traumatic stress disorder (PTSD). ClinicalTrials.gov (accessed 25 January 2015).
NCT01457404 {published data only}
- NCT01457404. Integrated cognitive behavioral therapy for co‐occurring PTSD and substance use disorders. https://clinicaltrials.gov/ct2/show/NCT01457404 (accessed 25 January 2015).
NCT01597856 {published data only (unpublished sought but not used)}
- NCT01597856. Evaluation and treatment of substance use in veterans with PTSD disability claims. ClinicalTrials.gov (accessed 25 January 2015).
NCT01663337 {published data only}
- NCT01663337. Sequence of Symptom Change During AUD or PTSD Treatment for Comorbid PTSD/AUD. https://clinicaltrials.gov/ct2/show/NCT01663337 (Accessed 13 March 2016).
NCT01693978 {published data only}
- NCT01693978. Incentivizing adherence to prolonged exposure with substance users. http://clinicaltrials.gov/show/NCT01693978 (accessed 25 January 2015). [Google Scholar]
NCT01849029 {published data only}
- NCT01849029. Cognitive Processing Intervention for Trauma, HIV/STI Risks, and Substance Use Among Native Women. https://clinicaltrials.gov/ct2/show/NCT01849029 (Accessed 13 March 2016).
NCT02081417 {published data only}
- NCT02081417. Patient‐centered trauma treatment for PTSD and substance abuse: Is it an effective treatment option?. http://clinicaltrials.gov/show/NCT02081417 (accessed 25 January 2015). [Google Scholar]
NCT02335125 {published and unpublished data}
- NCT02335125. A Policy Relevant US Trauma Care System Pragmatic Trial for PTSD and Comorbidity Pilot (TSOS 6). https://clinicaltrials.gov/ct2/show/NCT02335125 (Accessed 13 March 2016). [Google Scholar]
NTR3084 {published data only}
- Kok T, Haan HA, Meer M, Najavits LM, DeJong CAJ. Efficacy of "seeking safety" in a Dutch population of traumatized substance‐use disorder outpatients: Study protocol of a randomized controlled trial. BMC Psychiatry 2013;13:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Back 2001
- Back SE, Dansky BS, Carroll KM, Foa EB, Brady KT. Exposure therapy in the treatment of PTSD among cocaine‐dependent individuals: description of procedures. Journal of Substance Abuse Treatment 2001;21:35‐45. [DOI] [PubMed] [Google Scholar]
Berenz 2012
- Berenz EC, Coffey SF. Treatment of co‐occurring posttraumatic stress disorder and substance use disorders. Current Psychiatry Reports 2012;14(5):469‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisson 2013
- Bisson JI, Roberts NP, Andrew M, Cooper R, Lewis C. Psychological therapies for chronic post‐traumatic stress disorder (PTSD) in adults. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD003388.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blake 1995
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician administered PTSD scale. Journal of Traumatic Stress 1995;8:75‐90. [DOI] [PubMed] [Google Scholar]
Bradley 2005
- Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta‐analysis of psychotherapy for PTSD. American Journal of Psychiatry 2005;162:214‐27. [DOI] [PubMed] [Google Scholar]
Brady 2001
- Brady KT, Dansky BS, Back SE, Foa EB, Carroll KM. Exposure therapy in the treatment of PTSD among cocaine‐dependent individuals: preliminary findings. Journal of Substance Abuse Treatment 2001;21:47‐54. [DOI] [PubMed] [Google Scholar]
Breslau 1992
- Breslau N, Davis GC. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. American Journal of Psychiatry 1992;149:671‐5. [DOI] [PubMed] [Google Scholar]
Brown 2003
- Brown PJ, Read JP, Kahler CW. Comorbid posttraumatic stress disorder and substance use disorders: treatment outcomes and the role of coping. In: Ouimette P, Brown PJ editor(s). Trauma and Substance Abuse. Causes, Consequences and Treatment of Comorbid Disorders. Washington, DC: American Psychological Association, 2003:171‐88. [Google Scholar]
Busuttil 2009
- Busuttil W. Complex PTSD: a useful diagnostic frame work?. Psychiatry 2009;8(8):310‐4. [Google Scholar]
Chilcoat 1998a
- Chilcoat H, Breslau N. Investigations of causal pathways between PTSD and drug use disorders. Addictive Behaviors 1998;23:827‐40. [DOI] [PubMed] [Google Scholar]
Chilcoat 1998b
- Chilcoat HD, Breslau N. Posttraumatic stress disorder and drug disorders: testing causal pathways. Archives of General Psychiatry 1998;55:913‐7. [DOI] [PubMed] [Google Scholar]
Chilcoat 2003
- Chilcoat H, Menard C. Epidemiological investigations: comorbidity of posttraumatic stress disorder and substance use disorder. In: Ouimette P, Brown PJ editor(s). Trauma and Substance Abuse: Causes, Consequences and Treatment of Comorbid Disorders. Washington, DC: American Psychological Publishing, 2003:9‐28. [Google Scholar]
Cloitre 2011
- Cloitre M, Courtois CA, Charuvastra A, Carapezza R, Stolbach BC, Green BL. Treatment of complex PTSD: results of the ISTSS expert clinician survey on best practices. Journal of Traumatic Stress 2011;24(6):615‐27. [DOI] [PubMed] [Google Scholar]
Cottler 1992
- Cottler LB, Compton WM, Mager D, Spitznagel EL, Janca A. Posttraumatic stress disorder among substance users from the general population. American Journal of Psychiatry 1992;149:664–70. [DOI] [PubMed] [Google Scholar]
Darke 1992
- Darke S, Hall W, Wodak A, Heather N, Ward J. Development and validation of a multi‐dimensional instrument for assessing outcome of treatment among opiate users: the Opiate Treatment Index. British Journal of Addiction 1992;87:733‐42. [DOI] [PubMed] [Google Scholar]
Davidson 1997
- Davidson JR, Book SW, Colket L, Tupler LA, Roth S, David D, et al. Assessment of a new self‐rating scale for posttraumatic stress disorder. Psychological Medicine 1997;27:153‐60. [DOI] [PubMed] [Google Scholar]
Donovan 2001
- Donovan B, Padin‐Rivera E, Kowaliw S. "Transcend": initial outcomes from a posttraumatic stress disorder/substance abuse treatment program. Journal of Traumatic Stress 2001;14:757‐72. [DOI] [PubMed] [Google Scholar]
Dragan 2007
- Dragan M, Lis‐Turlejska M. Prevalence of posttraumatic stress disorder in alcohol dependent patients in Poland. Addictive Behaviors 2007;32:902‐11. [DOI] [PubMed] [Google Scholar]
Driessen 2008
- Driessen M, Schulte S, Luedecke C, Schaefer I, Sutmann F, Ohlmeier M. Trauma and PTSD in patients with alcohol, drug, or dual dependence: a multi‐center study. Alcoholism: Clinical and Experimental Research 2008;32(3):481‐8. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehlers 2000
- Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy 2000;38(4):319‐45. [DOI] [PubMed] [Google Scholar]
Fergusson 2002
- Fergusson D, Aaron SD, Guyatt G, Hébert P. Postrandomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325:652‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferri 2006
- Ferri M, Amato L, Davoli M. Alcoholics Anonymous and other 12‐step programmes for alcohol dependence. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005032.pub2] [DOI] [PubMed] [Google Scholar]
Foa 1997a
- Foa E, Cashman L, Jaycox L, Perry K. The validation of a self‐report measure of PTSD: the Posttraumatic Diagnostic Scale. Psychological Assessment 1997;9:445‐51. [Google Scholar]
Foa 1997b
- Foa EB, Meadows EA. Psychosocial treatments for posttraumatic stress disorder: a critical review. Annual Review of Psychology 1997;48:449‐80. [DOI] [PubMed] [Google Scholar]
Foa 1998
- Foa EB, Rothbaum BO. Treating the Trauma of Rape: Cognitive Behavioral Therapy for PTSD. New York: Guilford Press, 1998. [Google Scholar]
Foa 2000
- Foa EB, Keane TM, Friedman MJ. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. New York: Guilford Press, 2000. [Google Scholar]
Foa 2010
- Foa EB, Williams MT. Methodology of a randomized double‐blind clinical trial for comorbid posttraumatic stress disorder and alcohol dependence. Mental Health and Substance Use 2010;3(2):131‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerger 2014
- Gerger H, Munder T, Barth J. Specific and nonspecific psychological interventions for PTSD symptoms: A meta‐analysis with problem complexity as a moderator. Journal of Clinical Psychology 2014;70(7):601‐15. [DOI] [PubMed] [Google Scholar]
Gillies 2012
- Gillies D, Taylor F, Gray C, O’Brien L, D’Abrew N. Psychological therapies for the treatment of post‐traumatic stress disorder in children and adolescents. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD006726.pub2] [DOI] [PubMed] [Google Scholar]
Gossop 1995
- Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W, et al. The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 1995;90(5):607‐14. [DOI] [PubMed] [Google Scholar]
Gulliver 2010
- Gulliver SB, Steffen LE. Towards integrated treatments for PTSD and substance use disorders. PTSD Research Quarterly 2010;21(2):1‐7. [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [DOI] [PubMed] [Google Scholar]
Haro 2006
- Haro JM, Arbabzadeh‐Bouchez S, Brugha TS, Girolamo G, Guyer ME, Jin R, et al. Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health Surveys. International Journal of Methods in Psychiatric Research 2006;15(4):167‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Helzer 1987
- Helzer JE, Robins LN, McEvoy L. Post‐traumatic stress disorder in the general population. The New England Journal of Medicine 1987;317:1630‐4. [DOI] [PubMed] [Google Scholar]
Herman 1992
- Herman J. Trauma and Recovery: The Aftermath of Violence ‐ from Domestic Abuse to Political Terror. New York: Guilford Press, 1992. [Google Scholar]
Hetrick 2010
- Hetrick SE, Purcell R, Garner B, Parslow R. Combined pharmacotherapy and psychological therapies for posttraumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD007316.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horowitz 1979
- Horowitz MJ, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic Medicine 1979;41:209–18. [DOI] [PubMed] [Google Scholar]
Jacobsen 2001
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. American Journal of Psychiatry 2001;158(8):1184–90. [DOI] [PubMed] [Google Scholar]
Keane 1990
- Keane TM, Wolfe J. Comorbidity in post‐traumatic stress disorder: an analysis of community and clinical studies. Journal of Applied Social Psychology 1990;20:1776‐88. [Google Scholar]
Kessler 1995
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry 1995;52(12):1048‐60. [DOI] [PubMed] [Google Scholar]
Klimas 2014
- Klimas J, Tobin H, Field CA, O’Gorman CSM, Glynn LG, Keenan E, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD009269.pub3] [DOI] [PubMed] [Google Scholar]
Knapp 2007
- Knapp WP, Soares B, Farrell M, Silva de Lima M. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003023.pub2] [DOI] [PubMed] [Google Scholar]
Kubany 2004
- Kubany ES, Hill EE, Owens JA, Iannce‐Spencer C, McCaig MA, Tremayne KJ, Williams PL. Cognitive trauma therapy for battered women with PTSD (CTT‐BW). Journal of Consulting and Clinical Psychology 2004;72(1):3‐18. [DOI] [PubMed] [Google Scholar]
Kulka 1990
- Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, et al. Trauma and the Vietnam War Generation: Report of Findings from the National Vietnam Veterans Readjustment Study. New York: Bruner/Mazel, 1990. [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;23(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loke 2011
- Loke YK, Price D, Herxheimer A on behalf of the Cochrane Adverse Effects Methods Group. Chapter 14: Adverse effects. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Mayet 2004
- Mayet S, Farrell M, Ferri M, Amato L, Davoli M. Psychosocial treatment for opiate abuse and dependence. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004330.pub2] [DOI] [PubMed] [Google Scholar]
McDevitt‐Murphy 2010
- McDevitt‐Murphy ME, Williams JL, Bracken KL, Fields JA, Monahan CJ, Murphy JG. PTSD symptoms, hazardous drinking, and health functioning among U.S. OEF and OIF veterans presenting to primary care. Journal of Traumatic Stress 2010;23(1):108‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLellan 1992
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grisson, et al. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment 1992;9:199‐213. [DOI] [PubMed] [Google Scholar]
Meyer 1986
- Meyer RE. How to understand the relationship between psychopathology and addictive disorders: Another example of the chicken and the egg. In: Meyer RE editor(s). Psychopathology and Addictive Disorders. New York: Guilford Press, 1986. [Google Scholar]
Mills 2006
- Mills KL, Teeson M, Ross J, Peters L. Trauma, PTSD, and substance use disorders: findings from the Australian National Survey of Mental Health and Well‐Being. American Journal of Psychiatry 2006;163(4):651‐8. [DOI] [PubMed] [Google Scholar]
Mills 2007
- Mills KL, Teeson M, Ross J, Darke S. The impact of post‐traumatic stress disorder on treatment outcomes for heroin dependence. Addiction 2007;102(3):447‐54. [DOI] [PubMed] [Google Scholar]
Najavits 1998
- Najavits LM, Weiss RD, Shaw SR, Muenz LR. “Seeking Safety”: outcome of a new cognitive‐behavioral psychotherapy for women with posttraumatic stress disorder and substance dependence. Journal of Traumatic Stress 1998;11(3):437‐56. [DOI] [PubMed] [Google Scholar]
Najavits 2002a
- Najavits LM. Clinicians’ views on treating posttraumatic stress disorder and substance use disorder. Journal of Substance Abuse Treatment 2002;22:79‐85. [DOI] [PubMed] [Google Scholar]
Najavits 2002b
- Najavits LM. Seeking Safety: A Treatment Manual for PTSD and Substance Abuse. New York: Guilford Press, 2002. [Google Scholar]
Najavits 2006
- Najavits LM. Seeking Safety. In: Follette V, Ruzek JL editor(s). Cognitive‐Behavioral Therapies for Trauma. 2nd Edition. New York: Guilford Press, 2006:228‐57. [Google Scholar]
Najavits 2007
- Najavits LM. Psychosocial treatments for posttraumatic stress disorder. In: Nathan PE, Gorman JM editor(s). A Guide to Treatments that Work. 3rd Edition. New York: Oxford University Press, 2007:513‐29. [Google Scholar]
Najavits 2013
- Najavits LM, Hien D. Helping vulnerable populations: a comprehensive review of the treatment outcome literature on substance use disorder and PTSD. Journal of Clinical Psychology 2013;69(5):433‐79. [DOI] [PubMed] [Google Scholar]
Najavits 2014 [personal communication]
- Najavits L. Attached comments re Cochrane Review [personal communication]. Email to: N Roberts 24 February 2014.
NCCMH 2005
- National Collaborating Centre for Mental Health (NCCMH). Post‐traumatic Stress Disorder: the Management of PTSD in Adults and Children in Primary and Secondary Care [Full Guideline]. Leicester and London: The British Psychological Society and the Royal College of Psychiatrists, 2005. [Google Scholar]
Ouimette 2003a
- Ouimette P, Moos RH, Finney JW. PTSD treatment and 5‐year remission among patients with substance use and posttraumatic stress disorders. Journal of Consulting and Clinical Psychology 2003;71(2):410‐4. [DOI] [PubMed] [Google Scholar]
Ouimette 2003b
- Ouimette P, Moos RH, Brown PJ. Substance use disorder‐posttraumatic stress disorder comorbidity: a survey of treatments and proposed practice guidelines. In: Ouimette P, Brown PJ editor(s). Trauma and Substance Abuse: Causes, Consequences, and Treatment of Comorbid Disorders. Washington, DC: American Psychological Association, 2003:91‐10. [Google Scholar]
Ouimette 2003c
- Ouimette P, Brown PJ. Epilogue: future directions. In: Ouimette P, Brown PJ editor(s). Trauma and Substance Abuse: Causes, Consequences, and Treatment of Comorbid Disorders. 1st Edition. Washington, DC: American Psychological Association, 2003:243‐5. [Google Scholar]
Pietrzak 2011
- Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Anxiety Disorders 2011;25(3):456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinto 2011
- Pinto RM, Campbell ANC, Hien DA, Yu G. Retention in the National Institute on Drug Abuse clinical trials. American Journal of Orthopsychiatry 2011;81(2):211‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Powers 2008
- Powers MB, Vedel E, Emmelkamp PMG. Behavioral couples therapy (BCT) for alcohol and drug use disorders: a meta‐analysis. Clinical Psychology Review 2008;28:952‐62. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Reynolds 2005
- Reynolds M, Mezey G, Chapman M, Wheeler M, Drummond C, Baldacchino A. Co‐morbid post‐traumatic stress disorder in a substance misusing clinical population. Drug and Alcohol Dependence 2005;77:251‐8. [DOI] [PubMed] [Google Scholar]
Reynolds 2011
- Reynolds M, Hinchliffe K, Asamoah V, Kouimtsidis C. Trauma and post‐traumatic stress disorder in a drug treatment community service. The Psychiatrist 2011;35:256‐60. [Google Scholar]
Roberts 2009
- Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. Multiple session early psychological interventions for the prevention of post‐traumatic stress disorder. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006869.pub2] [DOI] [PubMed] [Google Scholar]
Roberts 2010
- Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. Early psychological interventions to treat acute traumatic stress symptoms. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD007944.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robins 1989
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The Composite International Diagnostic Interview: An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry 1989;45:1069–77. [DOI] [PubMed] [Google Scholar]
Rose 2002
- Rose S, Bisson J, Wessely S. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000560] [DOI] [PubMed] [Google Scholar]
Ruscio 2006
- Ruscio AM, Holohan DR. Applying empirically supported treatments to complex cases: Ethical, empirical, and practical considerations. Clinical Psychology: Science and Practice 2006;13(2):146‐62. [Google Scholar]
Ruzek 2003
- Ruzek JI. Concurrent posttraumatic stress disorder and substance use disorder among veterans: evidence and treatment issues. In: Ouimette P, Brown PJ editor(s). Trauma and Substance Abuse: Causes, Consequences, and Treatment of Comorbid Disorders. Washington, DC: American Psychological Association, 2003:191‐207. [Google Scholar]
Schäfer 2007
- Schäfer I, Najavits LM. Clinical challenges in the treatment of patients with posttraumatic stress disorder and substance abuse. Current Opinion in Psychiatry 2007;20:614‐8. [DOI] [PubMed] [Google Scholar]
Schäfer 2010
- Schäfer I, Langeland W, Hissbach J, Luedecke C, Ohlmeier MD, Chodzinski C, et al. Childhood trauma and dissociation in patients with alcohol dependence, drug dependence, or both ‐ a multi‐center study. Drug and Alcohol Dependence 2010;109:84‐9. [DOI] [PubMed] [Google Scholar]
Smedslund 2011
- Smedslund G, Berg RC, Hammerstrøm KT, Steiro A, Leiknes KA, Dahl HM, et al. Motivational interviewing for substance abuse. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD008063.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sobell 1995
- Sobell L, Sobell MB. Alcohol Timeline Followback Users’ Manual. Toronto: Addiction Research Foundation, 1995. [Google Scholar]
Stappenbeck 2015
- Stappenbeck CA, Luterek JA, Kaysen D, Rosenthal CF, Gurrad B, Simpson TL. A controlled examination of two coping skills for daily alcohol use and PTSD symptom severity among dually diagnosed individuals. Behaviour Research and Therapy 2015;66:8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2006
- Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD002795.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torchalla 2012
- Torchalla I, Nosen L, Rostam H, Allen P. Integrated treatment programs for individuals with concurrent substance use disorders and trauma experiences: A systematic review and meta‐analysis. Journal of Substance Abuse Treatment 2012;42(1):65‐77. [DOI] [PubMed] [Google Scholar]
Triffleman 1999
- Triffleman E, Carroll K, Kellogg S. Substance dependence posttraumatic stress disorder therapy: an integrated cognitive‐behavioral approach. Journal of Substance Abuse Treatment 1999;17:3‐14. [DOI] [PubMed] [Google Scholar]
van Dam 2012
- Dam D, Vedel E, Ehring T, Emmelkamp PMG. Psychological treatments for concurrent posttraumatic stress disorder and substance use disorder: a systematic review. Clinical Psychology Review 2012;32:202‐14. [DOI] [PubMed] [Google Scholar]
Ware 2003
- Ware JE, Kosinski, M, Dewey JE. Version 2 of the SF36 Health Survey. Lincoln, RI: Quality Metric, 2003. [Google Scholar]
Watkins 2005
- Watkins KE, Hunter SB, Burnam MA, Pincus HA, Nicholson G. Review of treatment recommendations for persons with a co‐occurring affective or anxiety and substance use disorder. Psychiatric Services 2005;56(8):913‐26. [DOI] [PubMed] [Google Scholar]
Weiss 1995a
- Weiss RD, Greenfield SF, Najavits LM. Integrating psychological and pharmacological treatment of dually diagnosed patients. NIDA Research Monograph 1995;150:110‐28. [PubMed] [Google Scholar]
Weiss 1995b
- Weiss RD, Hufford C, Najavits LM, Shaw SR. Weekly Substance Use Inventory. Boston: Harvard University Medical School, Unpublished. [Google Scholar]
Weiss 1997
- Weiss DS, Marmar CR. The Impact of Event Scale‐Revised. In: JP Wilson, TM Keane editor(s). Assessing Psychological Trauma and PTSD. New York: Guilford Press, 1997:399‐411. [Google Scholar]
WHO 1993
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders. Diagnostic Criteria for Research. Geneva: WHO, 1993. [Google Scholar]
Wilson 2008
- Wilson D, Ipser JC, Stein DJ. Pharmacotherapy for anxiety disorders and comorbid alcohol dependency. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007505] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zayfert 2007
- Zayfert C, Becker CB. Cognitive‐Behavioral Therapy for PTSD: A Case Formulation Approach. New York: Guilford Press, 2007. [Google Scholar]


