Abstract
Understanding the molecular mechanisms underlying pituitary organogenesis and function is essential for improving therapeutics and molecular diagnoses for hypopituitarism. We previously found that deletion of the forkhead factor, Foxo1, in the pituitary gland early in development delays somatotrope differentiation. While these mice grow normally, they have reduced growth hormone expression and free serum insulin-like growth factor-1 (IGF1) levels, suggesting a defect in somatotrope function. FOXO factors show functional redundancy in other tissues, so we deleted both Foxo1 and its closely related family member, Foxo3, from the primordial pituitary. We find that this results in a significant reduction in growth. Consistent with this, male and female mice in which both genes have been deleted in the pituitary gland (dKO) exhibit reduced pituitary growth hormone expression and serum IGF1 levels. Expression of the somatotrope differentiation factor, Neurod4, is reduced in these mice. This suggests a mechanism underlying proper somatotrope function is the regulation of Neurod4 expression by FOXO factors. Additionally, dKO mice have reduced Lhb expression and females also have reduced Fshb and Prl expression. These studies reveal FOXO transcription factors as important regulators of pituitary gland function.
Keywords: pituitary, forkhead, growth hormone, FOXO1, FOXO3
The process by which growth hormone (GH) producing somatotropes differentiate is dependent upon highly regulated signaling cascades and gene expression hierarchies. In the pituitary gland, 5 unique hormone-producing cell types can potentially emerge from PROP paired-like homeobox 1 (PROP1)-positive precursors as they migrate through the primordial adenohypophysis (1). Further specification occurs under the control of POU class 1 homeobox 1 (POU1F1), previously called GHF-1 or PIT-1, which is essential for differentiation of thyrotrope, lactotrope, and somatotrope cells (2). Finally, as shown using a knockout mouse model, somatotropes will not be fully functional unless neuronal differentiation 4 (NEUROD4) is also present, although specific targets of this protein are unknown (3, 4). Additional genes that contribute to the function of somatotropes include the growth hormone releasing hormone receptor, Ghrhr, and the growth hormone secretagogue receptor, Ghsr (5, 6). Elucidating the role of each factor in the transcriptional and signaling milieu, including the discovery of yet unidentified molecules, is key to understanding pituitary gland development and function.
Forkhead transcription factors are important regulators of cellular differentiation and function primarily through transcriptional control (7, 8). Previously, we found that premature expression of Foxo1 in the pituitary primordium impaired the ability of cells to adopt a pituitary fate (9). For these studies we employed the Foxg1-cre model because it causes recombination very early in pituitary gland development (10, 11). Interestingly, conditional deletion of Foxo1 (Foxo1Δpit) using the same cre driver, resulted in delayed somatotrope differentiation (12). These mice present with reduced transcripts of Neurod4, Gh1, and Ghrhr at various developmental and postnatal stages, although no growth deficit is observed postnatally. However, it is unknown whether these genes are direct targets of FOXO1 transcriptional regulation.
Based on the fact that FOXO transcription factors cooperatively regulate several processes (13, 14), we considered the possibility that deletion of both Foxo1 and Foxo3 may have a more significant effect on somatotrope function than deletion of Foxo1 alone. For consistency with previous deletion models, we used the Foxg1-cre driver for these studies (10). Here we show that loss of both alleles of Foxo1 and Foxo3 leads to impaired growth. In accordance with the growth phenotype, Foxo-deficient mice have reduced expression of important somatotrope genes including Neurod4, Pou1f1, Ghrhr, and Ghsr.
Materials and Methods
Animals and Genotyping
To obtain Foxo1Δpit mice Foxo1+/- mice (15) were mated to Foxg1+/cre mice (10) to produce Foxo1+/-;Foxg1+/cre mice. These were then mated to Foxo1fl/fl mice (13) to obtain Foxo1fl/-;Foxg1+/cre (Foxo1Δpit) mice. Experimental Foxo1fl/fl;Foxo3fl/fl;Foxg1+/cre (dKO) animals were generated by crossing Foxo1fl/fl;Foxo3fl/fl females with Foxo1+/fl;Foxo3fl/fl; Foxg1+/cre males. Foxg1+/cre mice were purchased from Jackson Laboratories, Bar Harbor, ME, USA (stock no. 004337) and were maintained on a 129SvJ (stock no. 000691) background (10, 11). Foxo1fl/fl mice (Jackson Laboratories, stock no. 024756) which have loxP sites flanking exon 2 of the Foxo1 gene (15) were a generous gift from Drs. Accili and Pajvani, with permission from Dr. DePinho. Foxo1+/- mice (15) were provided by Drs. Accili and Pajvani. Foxo3fl/fl mice were purchased from Jackson Laboratories (stock no. 024668) (16). Genotyping was performed using specific primers for Foxo1-null (LacZ fwd and rev), Foxo1-flox (FK1ckA-C), Foxg1-cre (cre fwd and rev), and Foxo3-flox (ofk2ck1-3). A list of primers used can be found in Table 1.
Table 1.
Oligonucleotide sequences
| Name | Sequence (5′ to 3′) |
|---|---|
| LacZ fwd | TTCACTGGCCGTCGTTTTACAAGC TCGTGA |
| LacZ rev | ATGTGAGCGAGTAACAACCCGTCG GATTCT |
| FK1ckA | GCTTAGAGCAGAGATGTTCTCACATT |
| FK1ckB | CCAGAGTCTTTGTATCAGGCAAATAA |
| FK1ckC | CAAGTCCATTAATTCAGCACATTGA |
| cre fwd | GCGGTCTGGCAGTAAAAACTATC |
| cre rev | GTGAAACAGCATTGCTGTCACTT |
| ofk2ck3 | CATGCAGTCCGAGAGATTTG |
| ofk2ck2 | AGTGTCTGATACCGAAGAGC |
| ofk2ck1 | AACAACCTCACACATGTGCC |
| mPolr2b RTqPCR fwd | AGATGTATGACGCCGACGAG |
| mPolr2b RTqPCR rev | GTAAGAACTGATCACGATCCAGCA |
| mTfrc RTqPCR fwd | GCAAGATGTAAAGCATCCAGTTG ATGG |
| mTfrc RTqPCR rev | GCATATTCTGGAATCCCAGCAG |
| mGh1 RTqPCR fwd | TGGCTGCTGACACCTACAAAGAGT |
| mGh1 RTqPCR rev | GCGAGAAGCGAAGCAATTCCAT |
| mFoxo1 RTqPCR fwd | AGGATAAGGGCGACAGCAAC |
| mFoxo1 RTqPCR rev | CCGCTCTTGCCTCCCTC |
| mFoxo3 RTqPCR fwd | GGGCGACAGCAACAGCT |
| mFoxo3 RTqPCR rev | CCCGCTCTTTCCCCCATC |
| mFoxo4 RTqPCR fwd | TCGGCAGGATGGAAGAACTCC |
| mFoxo4 RTqPCR rev | GCCTCGTTGTGAACCTTGATG |
| mFoxo6 RTqPCR fwd | GAAAGCGAAGAGCTCCCGAC |
| mFoxo6 RTqPCR rev | GTGCCGAATGGAGTTCTTCCAG |
| mGhrh RTqPCR fwd | CTCCCACAACATCACAGAGTCC |
| mGhrh RTqPCR rev | TGCATCCTGAAGGGAGGTGA |
| Pou1f1 RTqPCR fwd | CTGACGCTTCTGCTGCCC |
| Pou1f1 RTqPCR rev | ATGAAGTCCTGTCGCTGTGG |
| mNeurod4 RTqPCR fwd | TTTAACATGGACGACTGAAAGGG |
| mNeurod4 RTqPCR rev | GCTCAGACCTTTGTCCATCCA |
| mGhrhr RTqPCR fwd | ACCCGTATCCTCTGCTTGCT |
| mGhrhr RTqPCR rev | AGGTGTTGTTGGTCCCCTCT |
| mGhsr RTqPCR fwd | GGACCAGAACCACAAACAGACA |
| mGhsr RTqPCR rev | CAGCAGAGGATGAAAGCAAACA |
| Lhb RTqPCR fwd | ATCACCTTCACCACCAGCATCTGT |
| Lhb RTqPCR rev | TGA GGG CTA CAG GAA AGG AGA CTA |
| Fshb RTqPCR fwd | ACAAGGTTAGGCTGCTCAACTCCT |
| Fshb RTqPCR rev | TCCTGTCTGCCTGGAACAATCCAT |
| Pomc RTqPCR fwd | AAGAGCAGTGACTAAGAGAGGCCA |
| Pomc RTqPCR rev | TGCAGAGGCAAACAGATTGGAGG |
| Prl RTqPCR fwd | AGGCCTATCCTGAAGCCAAAGGAA |
| Prl RTqPCR rev | TTGGCACCTCAGGACCTTGAGAAA |
| Tshb RTqPCR fwd | ATTCTCCTTCCCTGTCGCCGTAA |
| Tshb RTqPCR rev | TGCAGTAGTTGGTTCTGACAGCCT |
Abbreviations: fwd, forward; rev, reverse; RTqPCR, quantitative reverse transcription–polymerase chain reaction.
All mice were housed in a 12-hour light/dark cycle with feed (Formulab Diet 5008; Purina Mills, Gray Summit, MO, USA) and water ad libitum. Animals were weighed once per week starting at postnatal day 7. Mice were euthanized using CO2 inhalation. Mouse length was measured post-euthanization by measuring from nose to rump. All procedures were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guidelines for the Care and Use of Experimental Animals and in accordance with Southern Illinois University Carbondale policies.
Immunohistochemistry
Pituitary gland tissue was collected post-euthanization and fixed in 10% formalin in phosphate-buffered saline (PBS) then dehydrated in graded ethanol solutions (50% then 80%). Tissue was then embedded in paraffin blocks and cut into 5 μm sections and mounted on positively charged slides. All immunohistochemistry (IHC) was begun by deparaffinization and rehydration of tissue sections using xylene (twice for 5 minutes each), 100% ethanol (twice for 3 minutes each), 95% ethanol (twice for 3 minutes each), then PBS. For immunofluorescent detection where antibody signal was amplified (Table 2), slides were then incubated in 1.5% H2O2 for 20 minutes. Antigen retrieval for nuclear proteins was performed in boiling 10 mM citric acid buffer for 10 to 12 minutes. All tissue sections were blocked for 60 minutes using the Tyramide Signal Amplification (TSA) Kit Blocking Solution (TSB; Perkin Elmer, Waltham, MA, USA), which was also used as the diluent for all antibody solutions. Primary antibodies were incubated overnight at 4 °C but all other steps were performed at room temperature. After primary antibody incubation, tissue sections were washed 3 times for 3 minutes each in PBS-TWEEN 20 (PBS-T, 0.05%). For hormones, fluorophore-conjugated secondary antibody was then incubated on tissue sections for 60 minutes. In the case of transcription factors, secondary antibody was biotin-conjugated to allow for signal amplification using streptavidin-horseradish peroxidase and either fluorescein isothiocyanate (FITC) or tetramethylrhodamine isothiocyanate (TRITC) as provided in the TSA kit. These steps were performed sequentially with 3 PBS-T washes for 3 minutes each in between. For co-staining, FOXO3 was incubated overnight, washed, secondary antibody and amplification reagents applied, then the anti-hormone antibody was added for a second overnight incubation. Nuclei were stained using 4′,6′-diamidino-2-phenylindole (DAPI). Sections were then mounted using immunofluorescent mount (0.5mM polyvinyl alcohol, 0.12M Tris pH 8.0, 0.3% w/v glycerol, 2.5% w/v 1,4-diazabicyclo[2.2.2]octane) and glass coverslips. See Table 2 for antibody-specific details.
Table 2.
Antibody information
| Name | ID | Antigen | Citation | Host | Company | Cat. No. | Dilution |
|---|---|---|---|---|---|---|---|
| Rabbit Anti-Mouse GH antibody | AB_2721132 | mouse GH1 | (A.F. Parlow National Hormone and Peptide Program Cat# AFP5672099, RRID:AB_2721132) | rabbit | National Hormone and Pituitary Program | AFP5672099 | wb 1:50 000 |
| IF 1:10 000 | |||||||
| FoxO1 (C29H4) Rabbit mAb antibody | AB_2106495 | FOXO1 (C29H4) | (Cell Signaling Technology Cat# 2880, RRID:AB_2106495) | rabbit | Cell Signaling Technology | 2880 | wb 1:1000 |
| IF 1:50 | |||||||
| Goat Anti-Actin, beta Polyclonal Antibody, Unconjugated | AB_306374 | ACTB | (Abcam Cat# ab8229, RRID:AB_306374) | goat | Abcam | ab8229 | wb 1:1000 |
| FoxO3a (D19A7) Rabbit mAb antibody | AB_2636990 | human FoxO3a | (Cell Signaling Technology Cat# 12829, RRID:AB_2636990) | rabbit | Cell Signaling Technology | 12829 | IF 1:400 |
| Guinea Pig anti-Rat LHβ antibody | AB_2665565 | rat LHB | (A.F. Parlow National Hormone and Peptide Program Cat# r-gp-LHb, RRID:AB_2665565) | guinea pig | A.F. Parlow National Hormone and Peptide Program | r-gp-LHb also AFP- 22238790GPOLHB | IF 1:625 |
| Rabbit anti-Rat Follicule Stimulating Hormone Antiserum antibody | AB_2687903 | FSHB | (A.F. Parlow National Hormone and Peptide Program Cat# AFP- C0972881, RRID:AB_2687903) | rabbit | A.F. Parlow National Hormone and Peptide Program | AFP-C0972881 also rFSH | IF 1:10 000 |
Abbreviations: IF, immunofluorescence; wb, Western blot
Imaging of FOXO1 and FOXO3-hormone co-stains was performed using a Retiga 2000R digital camera attached to a Leica DM 5000B fluorescent microscope (Leica Biosystems, St. Louis, MO, USA). Individual captures of FITC, TRITC, and DAPI channels were merged using Adobe Photoshop CS3. Some images were brightened for illustrative purposes; however, the exact alterations were duplicated in both control and experimental images to maintain the ability to compare results. Counting of FOXO3 and hormone-stained sections was performed using the count tool on Adobe Photoshop CS3. Imaging of GH-only sections was performed using confocal microscope system Leica SPE with 4 solid state lasers at 100× magnification. Three animals per genotype were analyzed for these studies.
Quantitative Reverse Transcription–Polymerase Chain Reaction
Tissue collected for mRNA analysis was stored in RNA Later (AM7021, Invitrogen, Carlsbad, CA, USA) until use. Whole pituitary glands were lysed, and RNA was extracted and purified using the RNAqueous Micro Kit (AM1931) according to the kit protocol. The resultant mRNA was reversed transcribed to cDNA using the Promega M-MLV kit according to included instructions (M5313, Promega, Madison, WI, USA). Ten ng of cDNA were used for mRNA analysis. All samples were run in duplicate and a sample processed with no reverse transcriptase enzyme was included as a negative control. Results were calculated using the ΔΔCt method by first normalizing to RNA-polymerase subunit II b (Polr2b) as an internal control then calculated relative to transferrin receptor (Tfrc) to compare between groups. Both genes have been found to be expressed at consistent levels between genotypes. A list of primers used can be found in Table 1. At least 5 mice per genotype were used in these studies.
Western Blot
Pituitary glands were removed from animals and immediately flash frozen in liquid nitrogen. At time of use, tissues were homogenized using a pestle in RIPA buffer (150 mM NaCl, 0.5 M EDTA, 1 M TRIS, 1% Igepal, 10% deoxycholic acid, 10% SDS) with protease and phosphatase inhibitors (1862209, Thermo Fisher Scientific, Waltham, MA, USA). Samples were sonicated and the lysate was assayed for total protein concentration using the Thermo Fisher BCA Assay kit (#23228) according to included instructions. Ten μg of protein from each sample was then loaded onto a 12.5% SDS-PAGE gel and electrophoresed for 2.5 hours at 100 V. Samples were then transferred to a PVDF membrane and after blocking for 60 minutes were probed using anti-growth hormone antibody (AFP5672099, A. F. Parlow, National Hormone and Peptide Program, Santa Cruz, CA, USA) at a 1:500 dilution and anti-beta-actin (ab8229, Abcam, Cambridge, UK) at 1:1000. Membranes were washed 4 times for 5 minutes in 0.1% PBS-T. Bound primary antibody was detected by incubating membranes with Li-Cor IRDye antibodies then imaging using the Odyssey CLx (Li-Cor, Lincoln, NE, USA). Image Studio 3.1 software was used to quantify the signal from each band and data were presented as fold change compared with wild-type (WT) control. Three animals per genotype were used in these studies.
Serum IGF1
In order to assess the IGF1 content in serum of WT and experimental mice, blood was collected via cardiac puncture (dKOs) or post-decapitation (Foxo1Δpit) at the time of euthanization and serum stored at −20 °C until use. For all samples IGF1 was measured using the Mouse IGF1 ELISA Kit (ab100695, Abcam, Cambridge, UK) according to the manufacturer’s instructions. At least 8 animals per genotype were used in these studies.
Statistical Analysis
All data were analyzed using Student’s t test unless otherwise noted, where (*) indicates P < 0.05, (**) indicates P < 0.01, (***) indicates P < 0.001, and (****) indicates P < 0.0001. Error bars indicate standard error of the mean (SEM). Mouse weights were compared using a 2-way analysis of variance (ANOVA) and Dunnett’s post hoc test.
Results
FOXO1 Is Required for Normal Gh1 Expression Postnatally
Previously, we demonstrated that conditional deletion of Foxo1 in the pituitary gland using Foxg1-cre (Foxo1Δpit) results in delayed differentiation of somatotropes during embryogenesis (12). We chose Foxg1 as the cre driver because it results in recombination early in development (10). To determine if the growth axis is affected postnatally, we evaluated Gh1 expression and serum IGF1 levels in Foxo1Δpit animals at postnatal day (P)21. At P21 Foxo1Δpit animals have persistently reduced Gh1 transcript in the pituitary gland and low circulating IGF1 (Fig. 1A, 1B). However, no decrease in weight was evident, indicating that IGF1 levels are not reduced sufficiently to affect growth (12). We previously found that expression of Neurod4, a transcription factor that is required for somatotrope differentiation, is reduced in Foxo1Δpit mice embryonically (12). However, Neurod4 expression is not different between Foxo1Δpit and wild-type mice at P21 (Fig. 1C).
Figure 1.
Growth hormone expression is reduced postnatally in the absence of Foxo1. (a) Quantitative reverse transcription–polymerase chain reaction (RTqPCR) was performed for Gh1 using whole pituitary glands from mixed-sex 21-day-old mice from either WT or Foxo1Δpit animals. (b) Unbound IGF1 protein in the serum of WT and Foxo1Δpit mice was analyzed at postnatal day (P)21 using a sandwich ELISA. Eight animals of mixed sex were analyzed per genotype. Significance was measured using Student’s t test. Significant differences are represented by asterisks, *P < 0.05. (c) Neurod4 expression in whole pituitary glands from mixed-sex 21-day-old mice was measured using RTqPCR. (d) Relative expression of the FOXO family of transcription factors was assessed in wild-type (WT) pituitary gland tissue at embryonic day (e)16.5 (green), P10 (blue), and P21 (purple). Expression was normalized to Tfrc, a gene shown to not differ between genotypes, in order to compare different ages. Analysis was of 4-5 animals per age group of mixed sexes. (e) Foxo3 expression in whole pituitary glands from mixed-sex 21-day-old mice was measured using RTqPCR. (a, c, e) Five animals were compared from each genotype and analyzed using Student’s t test. Expression is relative to WT.
The FOXO family of forkhead factors has several members in addition to Foxo1, namely Foxo3, Foxo4, and Foxo6 (17). Deletion of 2 FOXO family members can have a more severe phenotype than deletion of any 1 member (13, 14, 18). To determine if other FOXO family members are expressed in the pituitary gland, we assayed pituitary gland mRNA from wild-type animals at embryonic day (e)16.5, P10, and P21, for expression of Foxo1, Foxo3, Foxo4, and Foxo6. All FOXO family members are expressed in the pituitary gland both embryonically and postnatally (Fig. 1D). We hypothesized that deletion of additional Foxo alleles may significantly reduce growth. Because Foxo3 is most closely related to Foxo1 (19), we investigated if deletion of both Foxo1 and Foxo3 in the pituitary gland would lead to a growth deficit. Foxo3 expression is not altered in Foxo1Δpit mice (Fig. 1E).
FOXO3 Is Detected in Embryonic Pituitary and in the Hormone-Producing Cell Types of the Anterior Lobe
We first sought to characterize FOXO3 expression. FOXO3 is detected in the mouse primordial pituitary gland as early as e10.5 and appears nuclear in the rostral region of the invagination of oral ectoderm (Fig. 2A). By adulthood, the anterior pituitary is replete with FOXO3 staining (Fig. 2A). Adult animals were analyzed for the proportion of each cell type demonstrating nuclear FOXO3 by performing co-immunohistochemistry with FOXO3 and either GH, prolactin (PRL), luteinizing hormone beta (LHB), adrenocorticotropic hormone (ACTH), or thyroid stimulating hormone beta (TSHB) (Fig. 2B). Quantification indicated approximately 60% to 80% of each hormone-producing cell type is positive for FOXO3 (Fig. 2C).
Figure 2.
FOXO3 is present in all hormone-producing pituitary cell types. (a) Immunohistochemistry was performed on at least 3 wild-type animals of varying sexes at progressive time points to identify FOXO3 protein and localization during development. FOXO3 (green) appears nuclear (identified in blue via DAPI) in the invaginating oral ectoderm as early as e10.5. Scale bars indicate 100 µm. (b) Whole pituitary gland tissue was collected from female and male WT mice at 6 weeks of age and analyzed for co-expression of FOXO3 (green) and various hormones (red) by immunohistochemistry. Three animals were analyzed from each sex with representative images shown. Scale bars indicate 100 µm. (c) The sections were analyzed for relative populations of hormone-FOXO3 co-stained cells by manual counting. The graph shows percent cells that were both hormone and FOXO3 positive where female data is in pink and male data in blue. Data are represented by mean ± SEM. (d) Analysis of scRNAseq data from 7-week-old male mice (Cheung et al, accession: GSE120410) reveals that the majority of FOXO-containing pituitary cells express either Foxo1 (green) or Foxo3 (blue) and they are co-expressed (red) only in a small percentage of cells.
To determine the overlap of Foxo1 and Foxo3 expression within each cell type, we analyzed single cell RNA sequencing (scRNAseq) data from 7-week-old male pituitary glands (Cheung et al, accession: GSE120410) (20). Counts were normalized to transcripts per 10 000 transcripts and cutoff values were determined using Otsu’s optimal threshold (21, 22). While the percentage of cells expressing Foxo3 is approximately 10% to 15%, much lower than determined by IHC, the proportions are similar, with the exception of thyrotropes (Fig. 2D). We found that only a small percentage of pituitary cells express both Foxo1 and Foxo3 (Fig. 2D). A considerably larger percentage of pituitary cells express either one or the other of these FOXOs.
Foxo1/Foxo3 Knockout Mice Exhibit Reduced Somatotrope Function and Resulting Effects on the Growth Axis
In order to ascertain whether deletion of both Foxo1 and Foxo3 has a significant effect on pituitary gland function, we generated a conditional double knockout mouse model (dKO). We again used the Foxg1-cre driver to maintain consistency with the Foxo1Δpit model and to delete Foxo1 and Foxo3 early in development. Pituitary gland samples from male and female dKO (Foxo1fl/fl;Foxo3fl/fl;Foxg1+/cre) and WT (Foxo1fl/fl;Foxo3fl/fl;Foxg1+/+) mice as well as intermediate genotypes where 3 Foxo alleles were deleted (O1Δ/+O3∆/∆ or O1∆/∆O3Δ/+) were evaluated at 6 weeks of age for FOXO1 and FOXO3 protein using IHC, as well as mRNA expression of Foxo1 and Foxo3. Both FOXO1 and FOXO3 are significantly reduced in dKO mice, demonstrating successful cre-mediated recombination of the floxed alleles in both females and males (Fig. 3A-3D). These data indicate the dKO mouse model is a useful tool for investigating FOXO1 and FOXO3 activity in pituitary gland development and function. Interestingly, when 2 alleles of Foxo1 and one allele of Foxo3 are deleted, Foxo3 transcripts are significantly reduced.
Figure 3.
Foxo1 and Foxo3 are effectively excised in dKO mice. Whole pituitary gland tissue was collected from WT and dKO animals at 6 weeks of age and either fixed for immunohistochemistry or processed for mRNA analysis in females (a, b) and males (c, d). Pituitary tissue sections were assayed for FOXO1 and FOXO3 protein (green) via immunohistochemistry. Cell nuclei are blue. Dark green coloration most evident in the male FOXO3-dKO panel is autofluorescence of red blood cells. At least 3 animals from each genotype and each sex were analyzed, representative images are shown. Scale bars indicate 100 µm. Expression was normalized to Tfrc. Testing was performed on 6-7 animals from each group and comparisons used Student’s t test (****P < 0.0001).
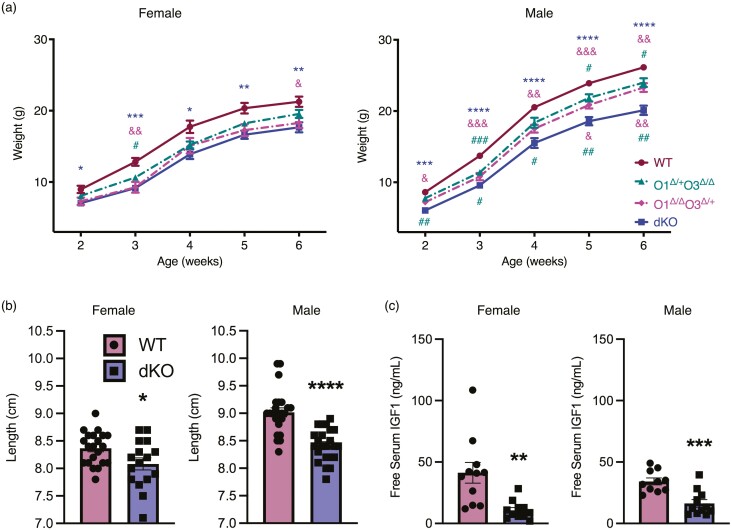
To assess the functional consequences of deleting Foxo1 and Foxo3 from the pituitary gland, we evaluated weights of dKO, WT, and mice with intermediate genotypes. Unlike deletion of Foxo1 or Foxo3 alone (12, 16), deletion of both Foxo1 and Foxo3 results in reduced body weight of female and male animals from 2 to 6 weeks of age compared with WT mice (Fig. 4A). Female mice with 1 functional allele of Foxo1 exhibit reduced body weight at 3 weeks and those with 1 functional Foxo3 allele show reduced body weight relative to WT at 3 and 6 weeks. Interestingly, male mice with 1 functional allele of either Foxo1 or Foxo3 are significantly smaller than WT and significantly larger than dKO (Fig. 4A). Length from nose to rump is notably reduced along with a corresponding decrease in circulating IGF1 in dKO animals (Fig. 4B, 4C).
Figure 4.
Growth is impaired in dKO mice. (a) WT (maroon) and dKO (dark blue) mice, as well as mice of intermediate genotypes (pink and teal dotted lines), were weighed weekly. Data were collected from 12 animals and statistics performed using 2-way ANOVA with repeated measures and Tukey’s post hoc test for multiple comparisons. Asterisks indicate significance when comparing WT with dKO. Ampersands indicate significance when comparing O1∆/∆O3∆/+ to WT (above lines) and dKO (below lines), and number signs for O1∆/+O3∆/∆ vs WT (above lines) and dKO (below lines). (b) Mice were measured for length from nose-to-rump at 6 weeks. Results were compared using Student’s t test with 13-20 animals analyzed per genotype and sex. (c) At 6 weeks of age, serum IGF1 was assayed using an ELISA. Data were analyzed with Student’s t test. Eleven samples were analyzed per genotype per sex. Significance for all figures is shown with an asterisk, ampersand, or number sign (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
In light of the fact that male O1∆/+O3∆/∆ and O1∆/∆O3∆/+ animals exhibited an intermediate phenotype, we sought to determine if they also exhibited intermediate expression of Gh1 and of Pou1f1, an important regulator of Gh1 expression. Transcripts for Gh1 and Pou1f1 are significantly reduced in O1∆/+O3∆/∆, O1∆/∆O3∆/+, and dKO females (Fig. 5A). In male animals, Gh1 and Pou1f1 are significantly reduced in O1∆/+O3∆/∆ and O1∆/∆O3∆/+ animals. In dKO males, Gh1 is significantly reduced while Pou1f1 is more variable and not significantly different (Fig. 5B). Contrary to the weight phenotypes, expression of Gh1 and Pou1f1 is not significantly reduced in dKO animals as compared with O1∆/+O3∆/∆ and O1∆/∆O3∆/+ animals, suggesting there is not a dosage-dependent effect of Foxo deletion at the mRNA level.
Figure 5.
FOXO deletion does not exert a dosage-dependent effect at the mRNA level. Whole pituitary glands were collected from WT (maroon), intermediate genotypes (teal, pink) and dKO (blue) mice at 6 weeks of age. Abundance of mRNA was evaluated for Gh1 and Pou1f1 in females (a) and males (b). Expression was normalized to Tfrc. This gene does differ between sexes, therefore relative expression between male and female groups cannot be compared here. The data represent 7-8 animals for each genotype and sex and were analyzed using 1-way ANOVA with Tukey’s post hoc test (**P < 0.01, ***P < 0.001, ****P < 0.0001).
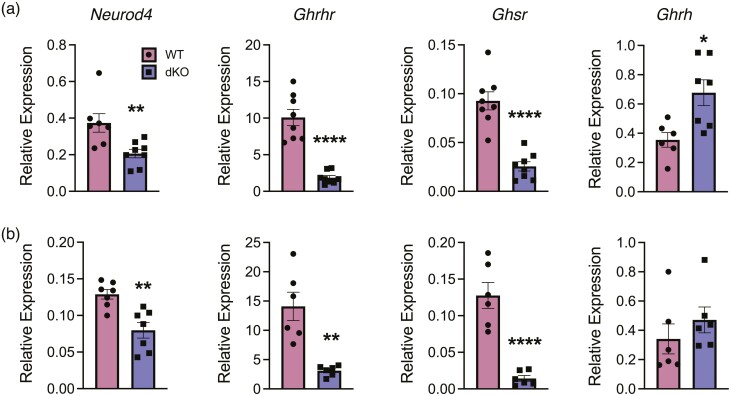
Pituitary glands from dKO animals exhibit altered mRNA for additional somatotrope-important genes, which helps establish Foxo1 and Foxo3 in the hierarchy of functional signaling. Transcripts for Neurod4, Ghrhr, and Ghsr, are all significantly reduced in dKO pituitary glands (Fig. 6A, 6B). However, Prop1 mRNA is unaffected in both males and females (Supplemental Figure 1) (23). Pou1f1 and Neurod4 must both be present during early development for somatotrope differentiation, while Ghsr and Ghrhr expression indicate terminal maturation. Consistent with reduced somatotrope function, hypothalamic Ghrh mRNA was increased in females, which would be expected to rise in the absence of negative feedback from IGF1 (Fig. 4C). Male hypothalamic Ghrh mRNA is not significantly different in dKO animals. There is no change in Sst mRNA in the hypothalamus in either sex, indicating impaired somatotrope function is likely due to loss of FOXO factors in the pituitary gland rather than hypothalamic changes (Supplemental Figure 1) (23). Overall, these data show that FOXO1 and FOXO3 are critical for normal somatotrope gene expression and are likely independent of Prop1.
Figure 6.
Expression of genes in the growth axis is altered in dKO mice. Whole pituitary glands were collected from WT (maroon) and dKO (blue) mice at 6 weeks of age. RNA was isolated and cDNA generated in order to evaluate mRNA abundance for Neurod4, Ghrhr, and Ghsr in females (a) and males (b). Hypothalamus was collected to evaluate expression of Ghrh. Expression was normalized to Tfrc. The data represent 7-8 animals for each genotype and sex and were analyzed using Student’s t test (*P < 0.05, **P < 0.01, ****P < 0.0001).
Immunohistochemical analysis of pituitary gland sections reveals no apparent difference in the localization of somatotropes between WT and dKO animals (Fig. 7A). Western blot analysis of pituitary glands reveals that GH protein content is reduced by approximately half in female and male dKO animals (Fig. 7A, 7B). This is interesting considering Gh1 transcript levels are reduced 5-fold (Fig. 5A, 5B) and serum IGF1 is also significantly reduced (Fig. 4C).
Figure 7.
Pituitary GH protein content is only reduced by half in dKO mice. (a) GH protein was visualized in sections of pituitary glands from 6-week-old male and female mice via immunohistochemistry. GH (red) is detected in the cytoplasm of pituitary gland cells surrounding the nucleus (blue, DAPI). Scale bars indicate 100 µm. (b) Western blot analysis was performed to quantify pituitary GH (red) protein content. ACTB (green) was used as a loading control. (c) Quantification of western blot signal comparing WT (maroon) and dKO (blue). The data represent 3 animals for each genotype and sex, are presented as fold change relative to WT, and were analyzed using Student’s t test (**P < 0.01).
Fshb Expression Is Reduced in Female dKO Mice
As Foxo1 and Foxo3 are expressed in all of the hormone-secreting cell types, we sought to evaluate the expression levels of the other pituitary hormones. Consistent with the Foxo1Δpit mice, Lhb expression is significantly reduced in both male and female dKO mice (Fig. 8). Interestingly, Fshb and Prl expression is reduced only in female dKO mice. The number of FSHB-positive cells is trending downward but is not statistically significant (Fig. 9A, 9B). Immunohistochemical analysis shows no apparent difference in the distribution of any of the cell types (Supplemental Figures 2 and 3) (23) and no significant change in the number of LHB-positive cells in females (Fig. 9C).
Figure 8.
Gonadotropin expression is reduced in dKO mice. RTqPCR analysis of WT and dKO mouse pituitary glands reveals a reduction in Lhb expression in males and females. Females also exhibit reduced Fshb and Prl expression. Expression was normalized to Tfrc. The data represent 7-8 animals for each genotype and sex. Data were analyzed by Student’s t test (*P < 0.05, **P < 0.001).
Figure 9.
The number of FSHB-positive cells is trending down in dKO female mice. (a) Immunohistochemistry for FSHB (red) was performed on pituitary sections from 3 WT and 3 dKO female mice. Two sections each for WT and dKO are shown to demonstrate the range of the phenotype. Scale bars represent 100 μm. The number of (b) FSHB and (c) LHB-positive cells in wild-type (maroon) and dKO (blue) mouse pituitary glands were counted and normalized for anterior lobe area. Data were analyzed by Student’s t test.
Discussion
While several genes have been identified that cause congenital hypopituitarism, the etiology for 80% of cases remains unknown (24). Identifying genes that regulate pituitary gland development and function is crucial for advancing diagnosis and treatment of these conditions. We previously identified Foxo1 as a candidate gene for congenital hypopituitarism (12). In our current study we show that although the number of somatotropes appears normal in Foxo1Δpit mice, Gh1 expression and IGF1 levels are reduced, suggesting that Foxo1 is required for normal somatotrope function. The fact that somatotrope function is not reduced sufficiently to affect growth in Foxo1Δpit or Foxo3 null mice (12, 16), together with the cooperative roles FOXO factors show in other tissues (13, 14), led us to hypothesize that deletion of both Foxo1 and Foxo3 from the pituitary gland would be sufficient to functionally disrupt the pituitary-growth axis.
We find that Foxo3 is expressed in each hormone-producing cell type in similar proportions, with the exception of thyrotropes, which are underrepresented in the scRNAseq data (20). While co-immunohistochemistry identified FOXO3 in approximately 60% of each cell type, scRNAseq showed Foxo3 in 10% to 15% of each cell type, with only 4% in thyrotropes. These differences are likely due to the fact that only 10% to 20% of transcripts typically undergo reverse transcription in single cell studies and thus low-expressing transcripts can be missed in some cells (25, 26). Foxo1 and Foxo3 are expressed at low levels and may not be detected by scRNAseq in all cells that have detectable protein via IHC.
The scRNAseq data suggests that Foxo1 is present in the largest percentage of somatotropes, similar to our findings by co-immunohistochemistry (27). However, the scRNAseq data shows more co-localization of Foxo1 in gonadotropes than predicted by co-immunohistochemistry. While Majumdar et al detected FOXO1 largely in the nucleus, Arriola et al found FOXO1 to be primarily in the cytoplasm (27, 28). Thus, it is possible that FOXO1 protein was missed in some gonadotropes (25, 26).
Foxo3 expression is reduced considerably in O1Δ/ΔO3Δ/+ mice. One possible explanation for this is that FOXO1 promotes Foxo3 expression. However, the fact that very few cells express both Foxo1 and Foxo3 makes this explanation less plausible. Also, Foxo3 expression is not altered in Foxo1Δpit mice. More likely, FOXO3 participates in a positive feedback loop, and deletion of 1 Foxo3 allele causes its expression to decrease more than expected. Consistent with this hypothesis, FOXO3 participates in positive feedback loops with CASC9 and CASC11 in non-small cell lung cancer (29, 30). Additionally, expression of FOXO3 target genes, Myod and Dio2, are similar between Foxo3 heterozygotes and null mice (31, 32).
The first evidence for the contribution of FOXO factors to growth is the reduced weight of both dKO and intermediate genotypes of mice as early as 2 weeks of age. The low weight persists through the cessation of the study at 6 weeks of age. Mice with deletion of only Foxo1 did not weigh less than littermate controls despite low IGF1 at 3 weeks of age, and Foxo3 null mice studied by other groups did not exhibit decreased weight (12, 16). Taken together, these data indicate that deletion of 2 of the 4 alleles for Foxo1 and Foxo3 does not affect size in mice. In male dKOs, weight loss was more pronounced depending on the number of FOXO alleles deleted, suggesting that FOXO factors exert a dose-dependent effect on growth. This is consistent with studies of mouse models with FOXO deletion in other tissues (13, 14). Interestingly, this is observed at the gross physiological level for weight, but not at the level of pituitary mRNA.
Pituitary-derived GH is necessary for multiple physiological processes including linear growth. Circulating IGF1 was used as a biomarker for GH and was significantly reduced in both male and female animals. We posit that the reduction in serum IGF1 is sufficient to contribute to the decrease in length and weight of dKO mice. The somatomedin hypothesis attributes longitudinal growth to the expression of Igf1 as provoked by GH, especially during puberty (33-35). Although Foxo1 knockout mice also had reduced serum IGF1 at 3 weeks of age, it was not reduced to the same extent observed in the dKO mice. While it is important to keep in mind these measurements were taken at different ages, the data suggest that deletion of 4 FOXO alleles may exert a greater effect on IGF1 levels than deletion of 2. Overall, deletion of both Foxo1 and Foxo3 from the pituitary gland resulted in a substantial loss of free serum IGF1.
One of the primary findings from the dKO mouse model is the significant reduction in somatotrope-important gene transcripts in the adenohypophysis. At 6 weeks of age, both male and female mice had fewer Neurod4, Gh1, Ghrhr, and Ghsr transcripts, and females also had less Pou1f1 mRNA. These data indicate that FOXO factors are necessary for the transcriptional regulation of these paramount molecules, either directly or indirectly. Interestingly, Pou1f1 expression is significantly higher in dKO females than O1Δ/ΔO3Δ/+ females. This may indicate that a Pou1f1-negative cell type is decreased in dKO mice as compared with O1Δ/ΔO3Δ/+ mice leading to an increase in the proportion of Pou1f1-positive cells in dKO mice. This could cause there to be a larger proportion of Pou1f1 mRNA in the dKO samples. A similar trend is seen in the males, but it is not significant. This Pou1f1-negative cell type may be nonendocrine, as no apparent change in the number of hormone-producing cell types was observed. We do not observe this pattern of expression for Gh1, which is expressed in Pou1f1-positive cells.
Prop1 is not affected in dKO mice, suggesting that FOXO factors are not upstream of Prop1. FOXO1 is present in Ames dwarf mice, which have a loss-of-function mutation in Prop1, suggesting that Foxo1 is not downstream of Prop1 (27). Together these data indicate that Foxo1 and Foxo3 are independent of Prop1 in the genetic hierarchy of pituitary gland function.
We find that Ghrhr expression is drastically reduced in dKO mice. GHRH signaling is critical for Gh1 expression. This occurs, in part, through activation of CREB leading to increased expression of Pou1f1, a key regulator of Gh1 expression (36-38). Others have shown that a single amino acid substitution in GHRHR inhibits signaling, resulting in GH deficiency, pituitary gland hypoplasia, and postnatal growth insufficiency (39, 40). Thus, the reduced expression of Ghrhr could contribute to the reduced Gh1 expression and reduced postnatal growth in dKO mice. We do not observe a reduction in the number of somatotropes, which occurs with complete loss of GHRHR function. This may be due to the fact that Ghrhr expression is not completely absent and thus, some GHRH signaling likely still occurs in dKO mice.
Confocal images of GH IHC did not demonstrate any readily visible differences in somatotrope distribution in the anterior lobe. Consistent with this, pituitary GH protein is reduced by approximately half, but that may not reflect somatotrope functionality. In fact, Gh1 expression and IGF1 levels are reduced several fold in dKO mice. These data indicate GH protein may be trapped in somatotropes. The low level of Ghrhr expression in dKO mice may result in sufficient receptors to provide enough signaling to obtain normal somatotrope numbers, but not enough for normal GH secretion, resulting in an accumulation of GH protein in cells lacking Foxo1 and Foxo3.
We found that while dKO males were smaller than WT males, they did not exhibit increased hypothalamic Ghrh expression, as was observed in dKO females. This suggests males may be less sensitive to the loss of negative feedback by IGF1. This was not unexpected, as there are a number of sex-specific differences in the hypothalamic-pituitary-growth axis. In the adult pituitary gland, somatotropes are more abundant and tightly clustered in males than in females (41). This is thought to contribute to the pulsatility of GH secretion observed in males, compared with the more continuous GH secretion in females, despite stimulation from the same ligand (42). Consistent with our findings, in the Lewis (dwarf) rat, long-term IGF1 infusion into the cerebrospinal fluid reduces GHRH to a greater extent in females than males, suggesting that females may be more sensitive to negative feedback by IGF1 than males (43).
Analysis of dKO mice revealed reduced Lhb expression in males and females as well as reduced Fshb expression in females only. Consistent with this, we previously found that Lhb expression is reduced in Foxo1Δpit mice at e18.5, and we did not analyze Fshb expression (12). Studies in LβT2 and primary rat pituitary cells show repression of Fshb and Lhb by FOXO1 (28, 44-46). The differences in the effect of FOXO1 on gonadotropin genes may reflect a role of FOXO1 during development, while we deleted Foxo1 and Foxo3 early in development. These early developmental effects may have repercussions for pituitary gland function in adulthood. Alternatively, the differences may be due to the fact that our studies were performed in an intact animal whereas in vitro studies lack the normal hormonal milieu. Foxo3 null mice have elevated LH and FSH serum levels, suggesting that in the absence of FOXO3, gonadotropes are capable of producing LH and FSH and that FOXO1 may be more important for gonadotrope function than FOXO3 (16).
In this study we find that deletion of Foxo1 and Foxo3 impairs both the somatotopic and gonadotropic axes. This may be due to direct effects of FOXOs on each of these lineages or to interaction between the somatotropic and gonadotropic axes. The decreased gonadotropin expression we observe may lead to reduced estradiol and testosterone production, which could exacerbate the reduction in growth observed in dKO animals. There is a significant amount of evidence that the somatotropic and gonadotropic axes interact. For example, sex steroids induce GH secretion and increase IGF1 levels (47) while adequate serum GH levels are required for sexual maturation to occur normally, potentially through IGF1 stimulation of hypothalamic Gnrh expression (48-50).
Recombination events in tissues other than the pituitary gland cannot be entirely ruled out as contributory to this phenotype. The cre driver for this model, Foxg1, is expressed in the primordial pituitary gland, ventral telencephalon, and foregut during early embryonic development (10). In fact, there was a modest reduction of Foxo1 expression in both male and female liver and hypothalamus in dKO mice, and Foxo3 expression in female hypothalamus (Supplemental Fig 4) (23). In the case of the liver, this could possibly have a minor effect on metabolism. However, other groups have shown that liver-specific Foxo1 knockout animals do not weigh less than controls despite metabolic defects (51). A role for forkhead factors is less straightforward in the hypothalamus but appears to be anabolic. In an acute model, Kim et al performed injections of either constitutively active Foxo1 or Foxo1 siRNA directly into the arcuate nucleus of mice (52). Inhibition of Foxo1 in the arcuate nucleus caused an abrupt decrease in food intake and body weight, which they attributed to FOXO1 regulation of the appetite regulatory genes Npy and Pomc. A study using Nkx2.1 as the cre driver to delete Foxo1 from neurons resulted in transient decreases in both weight and length of mice. They did not look at IGF1 levels and these changes did not occur until the animals were 6 weeks of age, much later than our model (53). These data provide the best examples of FOXO1 manipulation in the whole hypothalamus. Therefore, it is possible the loss of Foxo1 in the hypothalamus of the Foxo1/Foxo3 dKO mice may also contribute, in part, to the decreased weight observed.
Overall, the dKO mouse model provides evidence for the importance of Foxo1 and Foxo3 in the hypothalamic-pituitary-growth axis. Loss of both of these genes results in reduced weight, lower circulating IGF1, and fewer transcripts of genes important for somatotrope differentiation and function. Reduced growth in dKO mice is consistent with diminished expression of Neurod4, Pou1f1, and Gh1 (Fig. 10). Detailed studies to identify direct targets of FOXO3 will add crucial information about the extent of functional overlap between FOXO1 and FOXO3 in somatotrope function. Including FOXO1 and FOXO3 in sequencing panels for patients with congenital hypopituitarism would be an important next step toward understanding the possible contribution of FOXO factors to this disease.
Figure 10.

FOXO factors are members of a transcriptional somatotrope feedforward loop. Our studies indicate that FOXO transcription factors are important for expression of Pou1f1, Neurod4, and Gh1. POU1F1 directly binds multiple regulatory sites for Neurod4 and Gh1 as well as autoregulating itself (3, 54-56). Loss of NEUROD4 leads to loss of Gh1 expression; however, direct regulation has not been tested (3). Thus, FOXO transcription factors appear to participate in a transcriptional feedforward loop to promote somatotrope function.
Acknowledgments
Foxo1 fl/fl mice were a generous gift from Drs. Domenico Accili and Uptal B. Pajvani of Columbia University and Dr. Ronald A. DePinho, M.D. Anderson Cancer Center. We thank Dr. A. F. Parlow and the National Hormone and Pituitary Program, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development for providing antibodies for PRL, TSHB, GH, LHB, FSHB and ACTH. We are grateful to Dr. Philip Jensik, Southern Illinois University, for his insightful suggestions and careful reading of this manuscript. We thank Dr. Leonard Cheung, University of Michigan, for his invaluable help with analysis of scRNAseq data.
This work was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant no. R15HD078885 (B.S.E.), and Southern Illinois School of Medicine Research Seed Grant (B.S.E.).
Glossary
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- dKO
double knockout of Foxo1 and Foxo3
- e#
embryonic day #
- GH
growth hormone
- Ghrhr
growth hormone releasing hormone receptor
- Ghsr
growth hormone secretagogue receptor
- IGF1
insulin-like growth factor-1
- IHC
immunohistochemistry
- LHB
luteinizing hormone beta
- NEUROD4
neuronal differentiation 4
- P#
postnatal day #
- PBS
phosphate-buffered saline
- PBS-T
PBS-TWEEN 20
- POU1F1
POU class 1 homeobox 1
- PRL
prolactin
- PROP1
PROP paired-like homeobox 1
- RTqPCR
quantitative reverse transcription–polymerase chain reaction
- scRNAseq
single cell RNA sequencing
- TSHB
thyroid stimulating hormone beta
- WT
wild-type
Disclosures
The authors have nothing to disclose.
Current Affiliations
Caitlin E. Stallings is currently at Promega Corporation, Madison, WI, USA; Jyoti Kapali is currently at Breast Center, Baylor College of Medicine, Houston, TX, USA
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References (23).
References
- 1. Davis SW, Keisler JL, Perez-Millan MI, Schade V, Camper SA. All hormone-producing cell types of the pituitary intermediate and anterior lobes derive from Prop1-expressing progenitors. Endocrinology. 2016;157(4):1385-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li S, Crenshaw EB 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347(6293):528-533. [DOI] [PubMed] [Google Scholar]
- 3. Zhu X, Zhang J, Tollkuhn J, et al. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006;20(19):2739-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ando M, Goto M, Hojo M, et al. The proneural bHLH genes Mash1, Math3 and NeuroD are required for pituitary development. J Mol Endocrinol. 2018;61(3):127-138. [DOI] [PubMed] [Google Scholar]
- 5. Mayo KE, Miller T, DeAlmeida V, Godfrey P, Zheng J, Cunha SR. Regulation of the pituitary somatotroph cell by GHRH and its receptor. Recent Prog Horm Res. 2000;55:237-266; discussion 266-237. [PubMed] [Google Scholar]
- 6. Inoue H, Kangawa N, Kinouchi A, et al. Identification and functional analysis of novel human growth hormone secretagogue receptor (GHSR) gene mutations in Japanese subjects with short stature. J Clin Endocrinol Metab. 2011;96(2):E373-E378. [DOI] [PubMed] [Google Scholar]
- 7. Hoekman MF, Jacobs FM, Smidt MP, Burbach JP. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns. 2006;6(2):134-140. [DOI] [PubMed] [Google Scholar]
- 8. Adachi M, Osawa Y, Uchinami H, Kitamura T, Accili D, Brenner DA. The forkhead transcription factor FoxO1 regulates proliferation and transdifferentiation of hepatic stellate cells. Gastroenterology. 2007;132(4):1434-1446. [DOI] [PubMed] [Google Scholar]
- 9. Stallings CE, Ellsworth BS. Premature expression of FOXO1 in developing mouse pituitary results in anterior lobe hypoplasia. Endocrinology. 2018;159(8):2891-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222(2):296-306. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Martin JF, Bai CB. Direct and indirect requirements of Shh/Gli signaling in early pituitary development. Dev Biol. 2010;348(2):199-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kapali J, Kabat BE, Schmidt KL, et al. Foxo1 is required for normal somatotrope differentiation. Endocrinology. 2016;157(11): 4351-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paik JH, Kollipara R, Chu G, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128(2):309-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paik JH, Ding Z, Narurkar R, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):540-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakae J, Biggs WH III, Kitamura T, et al. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32(2):245-253. [DOI] [PubMed] [Google Scholar]
- 16. Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301(5630):215-218. [DOI] [PubMed] [Google Scholar]
- 17. Link W. Introduction to FOXO biology. Methods Mol Biol. 2019;1890:1-9. [DOI] [PubMed] [Google Scholar]
- 18. Zhang K, Li L, Qi Y, et al. Hepatic suppression of Foxo1 and Foxo3 causes hypoglycemia and hyperlipidemia in mice. Endocrinology. 2012;153(2):631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7(11):847-859. [DOI] [PubMed] [Google Scholar]
- 20. Cheung LYM, George AS, McGee SR, et al. Single-cell RNA sequencing reveals novel markers of male pituitary stem cells and hormone-producing cell types. Endocrinology. 2018;159(12):3910-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Otsu N. Threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 1979;9(1):62-66. [Google Scholar]
- 22. Fletcher PA, Smiljanic K, Maso Previde R, et al. Cell type- and sex-dependent transcriptome profiles of rat anterior pituitary cells. Front Endocrinol (Lausanne). 2019;10:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stallings CE, Evans BW, Ellsworth BS. Data from: FOXO transcription factors are required for normal somatotrope function and growth. Zenodo. Deposited December 5,2021. 10.5281/zenodo.5759531 [DOI]
- 24. Fang Q, George AS, Brinkmeier ML, et al. Genetics of combined pituitary hormone deficiency: roadmap into the genome era. Endocr Rev. 2016;37(6):636-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Islam S, Zeisel A, Joost S, et al. Quantitative single-cell RNA-seq with unique molecular identifiers. Nat Methods. 2014;11(2):163-166. [DOI] [PubMed] [Google Scholar]
- 26. Hwang B, Lee JH, Bang D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med. 2018;50(8):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Majumdar S, Farris CL, Kabat BE, Jung DO, Ellsworth BS. Forkhead Box O1 is present in quiescent pituitary cells during development and is increased in the absence of p27 Kip1. PLoS One. 2012;7(12):e52136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arriola DJ, Mayo SL, Skarra DV, Benson CA, Thackray VG. FOXO1 inhibits transcription of luteinizing hormone beta in pituitary gonadotrope cells. J Biol Chem. 2012;287(40):33424-33435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan R, Jiang Y, Lai B, Lin Y, Wen J. The positive feedback loop FOXO3/CASC11/miR-498 promotes the tumorigenesis of non-small cell lung cancer. Biochem Biophys Res Commun. 2019;519(3): 518-524. [DOI] [PubMed] [Google Scholar]
- 30. Bing Z, Han J, Zheng Z, Liang N. FOXO3-induced oncogenic lncRNA CASC9 enhances gefitinib resistance of non-small-cell lung cancer through feedback loop. Life Sci. 2021;287:120012. [DOI] [PubMed] [Google Scholar]
- 31. Hu P, Geles KG, Paik JH, DePinho RA, Tjian R. Codependent activators direct myoblast-specific MyoD transcription. Dev Cell. 2008;15(4):534-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dentice M, Marsili A, Ambrosio R, et al. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J Clin Invest. 2010;120(11):4021-4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Domene HM, Martinez AS, Frystyk J, et al. Normal growth spurt and final height despite low levels of all forms of circulating insulin-like growth factor-I in a patient with acid-labile subunit deficiency. Horm Res. 2007;67(5):243-249. [DOI] [PubMed] [Google Scholar]
- 34. Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22(1):53-74. [DOI] [PubMed] [Google Scholar]
- 35. Ohlsson C, Mohan S, Sjogren K, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30(5):494-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bodner M, Castrillo JL, Theill LE, Deerinck T, Ellisman M, Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988;55(3):505-518. [DOI] [PubMed] [Google Scholar]
- 37. Ingraham HA, Chen RP, Mangalam HJ, et al. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988;55(3):519-529. [DOI] [PubMed] [Google Scholar]
- 38. McCormick A, Brady H, Theill LE, Karin M. Regulation of the pituitary-specific homeobox gene GHF1 by cell-autonomous and environmental cues. Nature. 1990;345(6278):829-832. [DOI] [PubMed] [Google Scholar]
- 39. Lin SC, Lin CR, Gukovsky I, Lusis AJ, Sawchenko PE, Rosenfeld MG. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature. 1993;364(6434):208-213. [DOI] [PubMed] [Google Scholar]
- 40. Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo KE. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993;4(3):227-232. [DOI] [PubMed] [Google Scholar]
- 41. Sanchez-Cardenas C, Fontanaud P, He Z, et al. Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood. Proc Natl Acad Sci USA. 2010;107(50):21878-21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jansson JO, Eden S, Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985;6(2):128-150. [DOI] [PubMed] [Google Scholar]
- 43. Sato M, Frohman LA. Differential effects of central and peripheral administration of growth hormone (GH) and insulin-like growth factor on hypothalamic GH-releasing hormone and somatostatin gene expression in GH-deficient dwarf rats. Endocrinology. 1993;133(2):793-799. [DOI] [PubMed] [Google Scholar]
- 44. Skarra DV, Arriola DJ, Benson CA, Thackray VG. Forkhead box O1 is a repressor of basal and GnRH-induced Fshb transcription in gonadotropes. Mol Endocrinol. 2013;27(11):1825-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi YS, Lee HJ, Ku CR, et al. FoxO1 is a negative regulator of FSH beta gene expression in basal and GnRH-stimulated conditions in female. Endocrinology. 2014;155(6):2277-2286. [DOI] [PubMed] [Google Scholar]
- 46. Lannes J, L’Hote D, Garrel G, Laverriere JN, Cohen-Tannoudji J, Querat B. Rapid communication: A microRNA-132/212 pathway mediates GnRH activation of FSH expression. Mol Endocrinol. 2015;29(3):364-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Devesa J, Lima L, Tresguerres JA. Neuroendocrine control of growth hormone secretion in humans. Trends Endocrinol Metab. 1992;3(5):175-183. [DOI] [PubMed] [Google Scholar]
- 48. Eden S. Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology. 1979;105(2):555-560. [DOI] [PubMed] [Google Scholar]
- 49. Steyn FJ, Tolle V, Chen C, Epelbaum J. Neuroendocrine regulation of growth hormone secretion. Compr Physiol. 2016;6(2): 687-735. [DOI] [PubMed] [Google Scholar]
- 50. Zhen S, Zakaria M, Wolfe A, Radovick S. Regulation of gonadotropin-releasing hormone (GnRH) gene expression by insulin-like growth factor I in a cultured GnRH-expressing neuronal cell line. Mol Endocrinol. 1997;11(8):1145-1155. [DOI] [PubMed] [Google Scholar]
- 51. Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6(3):208-216. [DOI] [PubMed] [Google Scholar]
- 52. Kim MS, Pak YK, Jang PG, et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9(7):901-906. [DOI] [PubMed] [Google Scholar]
- 53. Heinrich G, Meece K, Wardlaw SL, Accili D. Preserved energy balance in mice lacking FoxO1 in neurons of Nkx2.1 lineage reveals functional heterogeneity of FoxO1 signaling within the hypothalamus.. Diabetes. 2014;63(5):1572-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rhodes SJ, Chen R, DiMattia GE, et al. A tissue-specific enhancer confers Pit-1-dependent morphogen inducibility and autoregulation on the pit-1 gene. Genes Dev. 1993;7(6):913-932. [DOI] [PubMed] [Google Scholar]
- 55. Mangalam HJ, Albert VR, Ingraham HA, et al. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989;3(7):946-958. [DOI] [PubMed] [Google Scholar]
- 56. Jones BK, Monks BR, Liebhaber SA, Cooke NE. The human growth hormone gene is regulated by a multicomponent locus control region. Mol Cell Biol. 1995;15(12):7010-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References (23).