SUMMARY
Background
As countries move towards the UNAIDS’s 95–95-95 targets and with the strong evidence of “Undetectable=untransmittable,” it is increasingly important to assess whether those receiving antiretroviral therapy (ART) achieve viral suppression. We estimated the proportions of viral suppression among children/adolescents and adults at 1, 2 and 3 years after initiating ART.
Methods
Seven regional cohorts from the International epidemiology Databases to Evaluate AIDS (IeDEA) consortium contributed data from individuals initiating ART between 2010 and 2019 at 148 sites in 31 countries with annual viral load (VL) monitoring. Data up to March 2020 were analyzed. We estimated the proportions of children/adolescents and adults with viral suppression (VL<1000 copies/mL) using an intention-to-treat (ITT)-like approach and an adjusted approach which accounted for missing VL measurements.
Findings
A total of 21,594 children/adolescents from 106 sites in 22 countries (55% female) and 255,662 adults from 143 sites in 30 countries (64% female) were included. Among those who were in follow-up and had VL testing, 4287 (76%) of 5641 children/adolescents and 57,970 (90%) of 64,487 adults were virally suppressed 3 years after ART initiation. In the ITT analyses, 4287 (24%) of 17,589 children/adolescents and 57,970 (29%) of 201,124 adults were virally suppressed 3 years after ART initiation. After adjusting for missing VL measurements among those who transferred, were lost to follow-up, or in follow-up without VL testing, 9177 (59%) of 15,667 children/adolescents and 115,260 (65%) of 178,458 adults were virally suppressed at 3 years following ART initiation. These estimates varied widely across IeDEA regions from 37% to 83% in children/adolescents and from 21% to 90% in adults.
Interpretation
While adults with HIV are approaching the global target of 95% viral suppression, progress among children/adolescents is much slower. Substantial efforts are still needed for reaching the viral suppression target for children/adolescents.
Funding
US National Institutes of Health
Keywords: HIV, viral suppression, children, adults, viral load
Introduction
In 2020, an estimated 26 million people, or nearly 70% of all people living with HIV (PWH) worldwide, were receiving antiretroviral therapy (ART).1 With increasing access to ART, the number of PWH receiving treatment is expected to continue to increase. Consistent adherence to an effective ART regimen suppresses viral load (VL) to undetectable levels, limits transmission, and improves health outcomes and associated health care costs.2–4
In 2013, the World Health Organization (WHO) recommended routine VL testing as the preferred way to improve monitoring and earlier identification of treatment failure.5 The Joint United Nations Program on HIV/AIDS (UNAIDS) established the 95–95-95 targets with the goal of achieving viral suppression in 95% of all people taking ART by 2030.6 To track progress, the goal has placed increased emphasis on the need for short- and long-term data on virologic outcomes. In 2016, WHO recommended to conduct VL testing at 6 and 12 months after ART initiation and then every 12 months thereafter if the person is stable on ART.5 To support “Undetectable=untransmittable (U=U)” and treatment-as-prevention strategies, accurate estimation of the third 95 target is critical. While routine VL testing is the standard of care in high-income countries, VL testing has been slow to expand in low- and middle-income countries (LMICs).7 Consequently, in settings that have only recently implemented VL testing for routine monitoring of PWH on ART, data on viral suppression are limited.8–12
The International epidemiology Databases to Evaluate AIDS (IeDEA) (https://www.iedea.org) is a global research network established in 2006 by the US National Institutes of Health. IeDEA merges and analyzes routinely collected data from large and diverse populations of PWH across seven international regions: Central Africa; East Africa; Southern Africa; West Africa; Asia-Pacific; Caribbean, Central and South America (CCASAnet); and North America (NA-ACCORD). In the present study, we analyzed data from IeDEA treatment sites that provided at least annual routine VL monitoring to estimate the proportions of children/adolescents and adults who achieved viral suppression at 1, 2, and 3 years after initiating ART.
Methods
Study population
Children/adolescents and adults with HIV who initiated ART between 2010 and 2019 at an IeDEA site with routine VL monitoring were eligible for inclusion in the analysis. Clinical management, selection of initial ART regimen, laboratory tests, or interventions were performed according to local guidelines. Routine VL monitoring was defined as at least 1 annual VL test per person as reported by each participating region. Only PWH who started ART after the time a site started routine VL monitoring were included. If no information was provided regarding VL testing frequency and its start date, we calculated the number of tests for each patient at each calendar year after ART start, and obtained the median number of tests per site, per year. Sites were considered as having routine VL from the year when a median of at least one annual VL test per person was observed. Data from the subsequent calendar years were included irrespective of their median values. We excluded from the analyses PWH who were not ART-naïve at clinic enrolment, PWH who had less than 6 months of follow-up after the first visit, and those who did not have a known date of ART initiation. The final analysis database included data available up to March 2020. The date of database closure differed for each of the participating sites ranging between September 2012 and March 2020 (10% before 2017, 22% between 2017 and 2018, 68% between 2019 and 2020).
Ethics review
Primary data collection by all participating sites and the pooling of the data in collaborative analyses were approved by their respective ethics committees or institutional review boards. Each participating IeDEA region had separate ethics approvals to contribute data to this analysis. Consent requirements and procedures were determined by the local regulatory bodies, and adherence to those standards was the responsibility of each site.
Children/adolescents were defined as individuals <18 years of age and adults as those ≥18 years at ART initiation. The main endpoints analyzed were the unadjusted and adjusted proportions of children/adolescents and adults with viral suppression (i.e, VL <1000 copies/mL) at 1, 2, and 3 years after ART initiation. For VL, we selected the single closest value reported during a window of ±6 months from the specified time point, and then classified this measurement as suppressed or not suppressed. PWH were considered active at each time point if they had a clinic visit on the specified time point or later. Only active PWH were included in the numerator to examine the proportions with and without VL testing at year 1, year 2 and year 3. PWH without evidence of contact with the clinic for more than 6 months before site-specific closure dates were classified as lost to follow-up (LTFU), with their follow-up period ending at the date of last clinic visit.
For laboratory and clinical measurements at ART initiation, we used the measurement closest to ART start within a window of 6 months before and 1 week after ART start, with the pre-ART measurement used in the case of two measurements with the same number of days before and after the ART date. Severe HIV-associated immunodeficiency was defined according to WHO criteria as CD4% <25% (age <1 year), <20% (age 1 to <3 years), <15% (age 3 to 5 years), and <15% or <200 cells/mm3 (age ≥5 years).13 We calculated height-for-age z-score using the WHO 2006/2007 child growth standards14 and weight-for-age z-score using the WHO 1977 standards15.
Statistical methods
We conducted separate analyses for children/adolescents and adults. We used descriptive statistics to summarize patient characteristics at ART initiation, stratified by IeDEA region. We first used the intention-to-treat (ITT)-like approach to include all PWH started who ART with or without VL outcomes. Quantifying the viral suppression rates among all PWH who have started ART, including those with missing VL measurements due to LTFU or transfers, is an important indicator in HIV care programs, especially in the era of treatment-as-prevention or “U=U”. Therefore, in our analysis, we calculated the proportions of PWH with VL <1000 copies/mL, VL ≥1000 copies/mL, no VL testing, and those who died, transferred, and LTFU. Proportions were plotted for each duration of ART (i.e., 1, 2, or 3 years after ART initiation). The proportions of PWH without VL testing were calculated by including in the numerator only those who were in follow-up but did not have a VL measurement. In this ITT analysis, PWH who died, transferred, or were classified as LTFU and those who were presumed to be in care but did not reach a specified time point, were censored at the time of last clinic visit. An inverse variance weighted meta-analysis of the proportions was conducted across regions to account for the differences between the sizes of the cohorts. Second, we determined the proportions of PWH with VL <1000 copies/mL only among those alive, in follow-up, and with VL assessed, for each duration of ART.
Third, we conducted an adjusted analysis to provide estimates of the overall proportions of PWH still alive (including those in follow-up without VL testing, transfers, and LTFU) and virally suppressed at 1, 2, and 3 years after ART initiation. For this analysis, we considered PWH in follow-up with no VL testing, and those transferred (after excluding the estimated deaths among transfers) as having equal proportions of virally suppressed individuals as those in care with VL testing. For plausible ranges, we applied to our data the lower and upper bounds for the proportion of viral suppression using the literature reporting on viral outcomes among PWH in care (supplementary Box 1; appendix pp 1–2). The common estimated plausible ranges of deaths among transfers, and viral suppression among transfers and those without VL testing, were used for all four African regions.
For LTFU, the proportions of PWH who died, and of PWH who were still alive and reconnected to care were extracted from the tracing studies reporting on LTFU outcomes,16–18 and we applied these proportions to our LTFU population. We then estimated the proportion of PWH who were virally suppressed (including plausible ranges) among those alive and reconnected to care (i.e., unofficial transfers) among LTFU using the data reported from a study assessing viral suppression in a large population of LTFU PWH in Zambia.18 The same estimated viral suppression proportion (and plausible ranges) were applied to both children/adolescents and adults in all regions. In this way, we estimated the overall proportions of PWH alive and virally suppressed at 1, 2, and 3 years after ART initiation. The steps taken in the adjusted analysis are provided in supplementary box 1, appendix pp 1–2. Sensitivity analyses varying the common estimate of viral suppression among PWH re-connected to care are provided in the supplementary figures S1 and S2 (appendix pp 19–20).
Multiregional data were managed and analyzed by the Kirby Institute, UNSW Sydney, using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata (StataCorp, STATA 14.0 for Windows, College Station, TX, USA).
Role of the Funding Source
The study funders had no role in design of the study, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to the study data and final responsibility for the decision to submit for publication.
Results
A total of 21,594 children/adolescents (55% female) were included from 106 sites in 22 countries across six IeDEA regions: Asia-Pacific (n=1500, 6.9%; 14 sites in 5 countries), CCASAnet (n=245, 1.1%; 9 sites in 5 countries), Central Africa (n=182, 0.8%; 10 sites in 1 country), East Africa (n=6032, 28%; 51 sites in 3 countries), Southern Africa (n=11,455, 53%; 14 sites in 3 countries), and West Africa (n=2180, 10%; 8 sites in 5 countries); 69% of sites were urban or semiurban clinics and 40% were located within regional, provincial, or university hospitals. The median age of children/adolescents at ART initiation was 6.2 (interquartile range [IQR], 1.7–11.8) years (table 1). Overall, 4777 (22%) started ART at age <1.5 years; this varied by region from 627 (10%) in East Africa to 3433 (30%) in Southern Africa. Adolescents (10–17 years at ART start) represented 7050 (33%) of the children/adolescent group, ranging from 383 (26%) in Asia-Pacific to 105 (58%) in Central Africa. Among 11,863 (55%) of children/adolescents with available CD4 count (if age ≥5 years) or CD4 percentage data, 4732 (40%) had severe immunosuppression. Among 13,968 (65%) with measurements, 3533 (25%) were severely underweight (weight-for-age z-score <−3) and 2538 (22%) were severely stunted (height-for-age score <−3). West Africa had the highest proportions of severely underweight (834, 42%) and severely stunted (454, 24%) children/adolescents. 8020 (37%) of children/adolescents were from lower middle-income and 11,981 (55%) were from upper middle-income countries.
Table 1:
Characteristics of children and adolescents at ART initiation, by IeDEA region
| Asia-Pacific (n=1500) | Caribbean, Central America, and South America (n=245) | Central Africa (n=182) | East Africa (n=6032) | Southern Africa (n=11 455) | West Africa (n=2180) | Total (n=21 594) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sex | |||||||
| Female | 726 (48%) | 121 (49%) | 102 (56%) | 3414 (57%) | 6400 (56%) | 1049 (48%) | 11812 (55%) |
| Male | 774 (52%) | 124 (51%) | 80 (44%) | 2618 (43%) | 5055 (44%) | 1131 (52%) | 9782 (45%) |
| Age at ART initiation, years | |||||||
| <1·5 | 290 (19%) | 42 (17%) | 20 (11%) | 627 (10%) | 3433 (30%) | 365 (17%) | 4777 (22%) |
| 1·5 to <5 | 364 (24%) | 42 (17%) | 28 (15%) | 1419 (24%) | 2191 (19%) | 686 (31%) | 4730 (22%) |
| 5 to <10 | 463 (31%) | 38 (16%) | 29 (16%) | 1634 (27%) | 2258 (20%) | 615 (28%) | 5037 (23%) |
| 10 to <15 | 306 (20%) | 35 (14%) | 54 (30%) | 1284 (21%) | 1952 (17%) | 455 (21%) | 4086 (19%) |
| 15 to 17 | 77 (5%) | 88 (36%) | 51 (28%) | 1068 (18%) | 1621 (14%) | 59 (3%) | 2964 (14%) |
| Median | 6·1 (2·1 to 10.0) | 10·6 (2·7 to 16·4) | 11·7 (4·7 to 15·6) | 7·9 (3·4 to 13·1) | 5·1 (1·0 to 11·6) | 5·3 (2·0 to 9·6) | 6·2 (1·7 to 11·8) |
| Proportion of CD4 cells | |||||||
| Available data | 1060 (71%) | 126 (51%) | 2 (1%) | 762 (13%) | 5066 (44%) | 1095 (50%) | 8111 (38%) |
| Overall median | 15 (7 to 22) | 21 (14 to 27) | 24 (17 to 31) | 19 (11 to 27) | 17 (10 to 25) | 16 (8 to 23) | 16 (9 to 25) |
| Number aged <5 years; median (IQR) | 437; 17 (10 to 25) | 44; 26 (12 to 32) | ·· | 191; 22 (14 to 30) | 3020; 19 (13 to 28) | 542; 17 (11 to 26) | 4234; 19 (12 to 28) |
| CD4 count, cells per μL | |||||||
| Available data | 1170 (78%) | 209 (85%) | 101 (55%) | 2271 (38%) | 7680 (67%) | 1619 (74%) | 13050 (60%) |
| Overall median | 356 (130 to 725) | 424 (249 to 752) | 667 (494 to 889) | 526 (282 to 909) | 414 (210 to 833) | 475 (191 to 882) | 440 (215 to 842) |
| Number aged ≥5 years; median (IQR) | 681; 256 (71 to 443) | 143; 351 (182 to 529) | 87; 625 (469 to 829) | 1655; 436 (234 to 725) | 4211; 291 (142 to 460) | 852; 287 (59 to 538) | 7629; 316 (146 to 526) |
| HIV RNA, log10 copies per mL | |||||||
| Available data | 456 (30%) | 203 (83%) | 7 (4%) | 380 (6%) | 4490 (39%) | 559 (26%) | 6095 (28%) |
| Median | 5·3 (4·4 to 5·9) | 4·6 (3·6 to 5·3) | 2·7 (1·3 to 5·5) | 4·3 (2·0 to 5·2) | 5·3 (4·4 to 5·9) | 5·0 (4·0 to 5·8) | 5·2 (4·3 to 5·9) |
| Severe HIV-associated immunodeficiency* | 536/1118 (48%) | 55/187 (29%) | 4/87 (5%) | 420/1846 (23%) | 3089/7231 (43%) | 628/1394 (45%) | 4732/11 863 (40%) |
| Weight-for-age z-score | |||||||
| Available data | 1389 (93%) | 208 (85%) | 167 (92%) | 5875 (97%) | 4323 (38%) | 2006 (92%) | 13 968 (65%) |
| Median | −2·0 (−3·2 to −0·9) | −1·1 (−2·4 to −0·2) | −1·2 (−2·5 to −0·2) | −1·4 (−2·6 to −0·3) | −1·8 (−3·0 to −0·7) | −2·5 (−4·2 to −1·2) | −1·7 (−3·0 to −0·6) |
| z-score less than −3 | 398 (29%) | 35 (17%) | 34 (20%) | 1136 (19%) | 1096 (25%) | 834 (42%) | 3533 (25%) |
| Height-for-age z-score | |||||||
| Available data | 1287 (86%) | 185 (76%) | 124 (68%) | 5159 (86%) | 2972 (26%) | 1874 (86%) | 11 601 (54%) |
| Median | −2·0 (−2·9 to −1·0) | −1·5 (−2·6 to −0·7) | −1·5 (−2·5 to −0·6) | −1·5 (−2·5 to −0·4) | −2·2 (−3·2 to −1·2) | −1·9 (−3·0 to −0·8) | −1·8 (−2·8 to −0·7) |
| z-score less than −3 | 289 (22%) | 28 (15%) | 25 (20%) | 874 (17%) | 868 (29%) | 454 (24%) | 2538 (22%) |
| Calendar year of ART initiation | |||||||
| 2010–12 | 452 (30%) | 97 (40%) | 0 | 0 | 5583 (49%) | 540 (25%) | 6672 (31%) |
| 2013–15 | 495 (33%) | 91 (37%) | 0 | 3440 (57%) | 3603 (31%) | 958 (44%) | 8587 (40%) |
| 2016–19 | 553 (37%) | 57 (23%) | 182 (100%) | 2592 (43%) | 2269 (20%) | 682 (31%) | 6335 (29%) |
| World Bank country income group | |||||||
| High income | 0 | 6 (2%) | 0 | 0 | 0 | 0 | 6 (<1%) |
| Upper-middle income | 574 (38%) | 188 (77%) | 0 | 0 | 11 219 (98%) | 0 | 11 981 (55%) |
| Lower-middle income | 926 (62%) | 51 (21%) | 0 | 5396 (89%) | 236 (2%) | 1411 (65%) | 8020 (37%) |
| Low income | 0 | 0 | 182 (100%) | 636 (11%) | 0 | 769 (35%) | 1587 (7%) |
| Initial ART regimen | |||||||
| NNRTI-based | 1176 (78%) | 164 (67%) | 147 (81%) | 5201 (86%) | 6903 (60%) | 1576 (72%) | 15 167 (70%) |
| Protease inhibitor-based | 274 (18%) | 69 (28%) | 34 (19%) | 818 (14%) | 4513 (39%) | 569 (26%) | 6277 (29%) |
| INSTI-based | 26 (2%) | 9 (4%) | 1 (1%) | 9 (<1%) | 4 (<1%) | 0 | 49 (<1%) |
| Others† | 24 (2%) | 3 (1%) | 0 | 4 (<1%) | 35 (<1%) | 35 (2%) | 101 (1%) |
Data are n (%), median (IQR), n; median (IQR), or n/N (%). ART consists of the combination of at least three antiretrovirals. ART=antiretroviral therapy. IeDEA=International epidemiology Databases to Evaluate AIDS. INSTI=integrase strand transfer inhibitors. NNRTI-non-nucleoside reverse transcriptase inhibitors.
Severe HIV-associated immunodeficiency was defined according to WHO criteria as proportion of CD4 cells <25% (age <1 year), <20% (age 1 to <3 years), <15% (age 3 to 5 years), and <15% or <200 cells/mm2 (age ≥5 years); denominators are the total number of individuals with available CD4 data.
Others category in initial ART regimen included dual therapy, entry inhibitors, and other atypical ART regimen.
Using the ITT approach, the overall proportions of children/adolescents with viral suppression were 36% (7303 of 20,478) at 1 year, 30% (5709 of 19,135) at 2 years, and 24% (4287 of 17,589) at 3 years after ART initiation (figure 1). The proportions presented in figure 1 and the estimated weighted averages are provided in table S1 (appendix pp 3–4). When the analysis was limited to only children/adolescents in ‘follow-up with an available VL measurement’ (i.e., year 1: n=9902; year 2: n=7649; year 3: n=5641), the proportions with viral suppression increased to 74% at 1 year, 75% at 2 years, and 76% at 3 years. Overall, among 17,589 children/adolescents by the end of year 3, 735 (4.2%) had died, 3076 (17%) had transferred out, and 5819 (33%) had been classified as LTFU, with percentages varying between regions (figure S3, appendix p 21).
Figure 1.
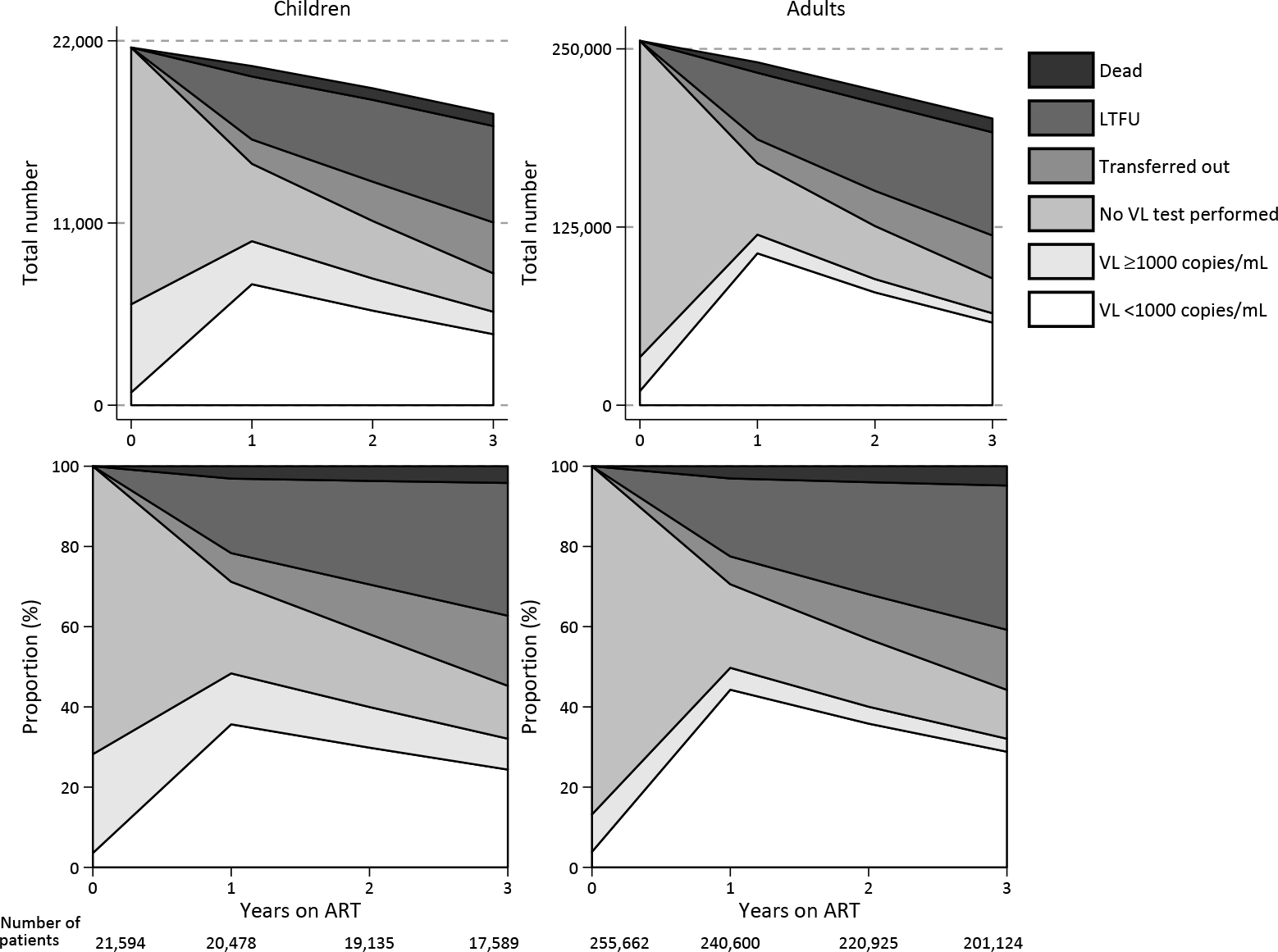
HIV viral suppression and treatment outcomes for children/adolescents and adults with HIV in the IeDEA global consortium by region at years 1, 2, and 3 following ART initiation (ITT analysis).
The proportions presented in this Figure and the estimated weighted averages are provided in table S1 (appendix pp 3–4).
In the adjusted analysis that accounted for the proportions assumed to have suppressed VL among those who were in follow-up but did not have a VL test, and among those who transferred out or were classified as LTFU, the estimated proportions suppressed 1 year after ART initiation ranged from 48% (836 of 1736) in West Africa to 84% (1192 of 1426) in the Asia-Pacific region (table 3; table S2, appendix pp 5–9); estimates ranged from 44% (720 of 1640) in West Africa to 84% (1123 of 1332) in Asia-Pacific at 2 years and 37% (572 of 1529) in West Africa to 83% (995 of 1194) in Asia-Pacific at 3 years after ART initiation (table S2, appendix pp 5–9).
Table 3:
Characteristics of adults at ART initiation, by IeDEA region
| Asia-Pacific (n=2270) | Caribbean, Central America, and South America (n=9898) | Central Africa (n=2545) | East Africa (n=61 413) | Southern Africa (n=162 856) | West Africa (n=6087) | North America (n=10 593) | Total (n=255 662) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Sex | ||||||||
| Female | 855 (38%) | 1977 (20%) | 1487 (58%) | 39 724 (65%) | 113 396 (70%) | 4162 (68%) | 2230 (21%) | 163 831 (64%) |
| Male | 1415 (62%) | 7921 (80%) | 1058 (42%) | 21 689 (35%) | 49 460 (30%) | 1925 (32%) | 8363 (79%) | 91 831 (36%) |
| Age, years | ||||||||
| <24 | 265 (12%) | 1414 (14%) | 297 (12%) | 7199 (12%) | 16 808 (10%) | 230 (4%) | 594 (6%) | 26 807 (10%) |
| 24 to 50 | 1723 (76%) | 7519 (76%) | 2030 (80%) | 46 889 (76%) | 129 853 (80%) | 4809 (79%) | 6687 (63%) | 199 510 (78%) |
| >50 | 282 (12%) | 965 (10%) | 218 (9%) | 7325 (12%) | 16 195 (10%) | 1048 (17%) | 3312 (31%) | 29 345 (11%) |
| Median | 35 (29–44) | 33 (27–41) | 34 (28–41) | 34 (28–43) | 34 (28–42) | 39 (33–47) | 43 (33–52) | 34 (28–43) |
| CD4 count, cells per μL | ||||||||
| <200 | 628 (28%) | 3565 (36%) | 275 (11%) | 9940 (16%) | 64 040 (39%) | 2121 (35%) | 2786 (26%) | 83 354 (33%) |
| 200–349 | 312 (14%) | 2276 (23%) | 308 (12%) | 7327 (12%) | 43 695 (27%) | 1477 (24%) | 2133 (20%) | 57 527 (23%) |
| 350–499 | 157 (7%) | 1396 (14%) | 312 (12%) | 7052 (11%) | 18 366 (11%) | 641 (11%) | 2001 (19%) | 29 923 (12%) |
| ≥500 | 152 (7%) | 1211 (12%) | 748 (29%) | 6035 (10%) | 14 761 (9%) | 649 (11%) | 2554 (24%) | 26 110 (10%) |
| Missing data | 1021 (45%) | 1450 (15%) | 902 (35%) | 31 059 (51%) | 21 994 (14%) | 1199 (20%) | 1119 (11%) | 58 748 (23%) |
| Median | 196 (43–348) | 243 (94–388) | 463 (263–678) | 309 (148–464) | 219 (113–341) | 231 (104–360) | 336 (162–520) | 237 (118–377) |
| HIV RNA, log10 copies per mL | ||||||||
| Available data | 987 (43%) | 7813 (79%) | 117 (5%) | 2208 (4%) | 12 432 (8%) | 964 (16%) | 9217 (87%) | 33 738 (13%) |
| Median | 4·9 (4·3–5·5) | 4·9 (4·2–5·4) | 2·3 (1·3–4·1) | 2·7 (0·0–4·5) | 3·9 (2·6–4·9) | 4·0 (0·0–5·4) | 4·3 (2·7–5·0) | 4·4 (2·6–5·2) |
| Calendar year of ART initiation | ||||||||
| 2010–12 | 861 (38%) | 2517 (25%) | 0 | 0 | 56 309 (35%) | 1802 (30%) | 6731 (64%) | 68 220 (27%) |
| 2013–15 | 777 (34%) | 4021 (41%) | 0 | 29 180 (48%) | 57 232 (35%) | 2256 (37%) | 3373 (32%) | 96 839 (38%) |
| 2016–19 | 632 (28%) | 3360 (34%) | 2545 (100%) | 32 233 (52%) | 49 315 (30%) | 2029 (33%) | 489 (5%) | 90 603 (35%) |
| World Bank country income group | ||||||||
| High income | 170 (7%) | 1828 (18%) | 0 | 0 | 0 | 0 | 10 593 (100%) | 12591 (5%) |
| Upper-middle income | 975 (43%) | 7721 (78%) | 0 | 0 | 161 615 (99%) | 0 | 0 | 170 311 (67%) |
| Lower-middle income | 1125 (50%) | 349 (4%) | 0 | 49 722 (81%) | 0 | 3137 (52%) | 0 | 55 574 (22%) |
| Low income | 0 | 0 | 2545 (100%) | 11 691 (19%) | 1241 (1%) | 2950 (48%) | 0 | 17 186 (7%) |
| Initial ART regimen | ||||||||
| NNRTI-based | 2099 (92%) | 7499 (76%) | 2507 (99%) | 60 206 (98%) | 160 949 (99%) | 5389 (89%) | 1117 (11%) | 239 766 (94%) |
| Protease inhibitor-based | 158 (7%) | 1557 (16%) | 14 (1%) | 533 (1%) | 1557 (1%) | 608 (10%) | 6148 (58%) | 10 575 (4%) |
| INSTI-based | 3 (<1%) | 746 (8%) | 19 (1%) | 657 (1%) | 22 (<1%) | 21 (<1%) | 2290 (22%) | 3758 (1%) |
| Others* | 10 (<1%) | 96 (1%) | 5 (<1%) | 17 (<1%) | 244 (<1%) | 69 (1%) | 1038 (10%) | 1563 (1%) |
Data are n (%) or median (IQR). ART consists of the combination of at least three antiretrovirals. ART=antiretroviral therapy. IeDEA=International epidemiology Databases to Evaluate AIDS. INSTI=integrase strand transfer inhibitors. NNRTI=non-nucleoside reverse transcriptase inhibitors.
Others category in initial ART regimen included dual therapy, entry inhibitors, and other atypical ART regimen.
A total of 255,662 adults (64% female) were included from 143 sites in 30 countries across seven IeDEA regions: Asia-Pacific (n=2270, 0.9%; 17 sites in 11 countries), CCASAnet (n=9898, 3.9%; 10 sites in 6 countries), NA-ACCORD (n=10,593, 4.1%; 17 sites in 2 countries), Central Africa (n=2545, 1.0%; 9 sites in 1 country), East Africa (n=61,413, 24%; 68 sites in 2 countries), Southern Africa (n=162,856, 64%; 16 sites in 4 countries), and West Africa (n=6087, 2.4%; 6 sites in 4 countries). Seventy percent of sites were urban or semiurban clinics and 43% were located within regional, provincial, or university hospitals. The median age at ART initiation was 34 (28–43) years (table 2). At ART initiation, 196,914 (77%) had CD4 testing; the median CD4 count at ART initiation was 237 (IQR, 118–377) cells/μL; 83,354 (33%) had a CD4 count <200cells/μL. 55,504 (22%) were from lower middle-income and 17,0311 (66%) were from upper middle-income countries.
Table 2:
Estimated total numbers and proportions of children and adolescents still alive with HIV viral load less than 1000 copies per mL by years on ART, stratified by IeDEA region (adjusted analysis)
| Year 1 |
Year 2 |
Year 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated number with viral load <1000 copies per mL (plausible range) | Total non-deaths | Proportion with viral load <1000 copies per mL (plausible range) | Estimated number with viral load <1000 copies per mL (plausible range) | Total non-deaths | Proportion with viral load <1000 copies per mL (plausible range) | Estimated number with viral load <1000 copies per mL (plausible range) | Total non-deaths | Proportion with viral load <1000 copies per mL (plausible range) | |
|
| |||||||||
| Asia-Pacific | 1192 (961–1259) | 1426 | 84% (68–89) | 1123 (887–1165) | 1332 | 84% (67–87) | 995 (778–1026) | 1194 | 83% (65–86) |
| Caribbean, Central America, and South America | 174 (125–201) | 238 | 73% (53–84) | 151 (110–180) | 226 | 67% (49–80) | 129 (91–150) | 210 | 61% (43–71) |
| Central Africa | 94 (55–109) | 156 | 60% (35–70) | 65 (36–74) | 142 | 46% (25–52) | NA | NA | NA |
| East Africa | 3293 (2115–4115) | 5085 | 65% (42–81) | 2598 (1671–3279) | 4312 | 60% (39–76) | 1980 (1238–2454) | 3478 | 57% (36–71) |
| Southern Africa | 6459 (4311–8375) | 10 194 | 63% (42–82) | 6139 (3960–7740) | 9901 | 62% (40–78) | 5501 (3481–6855) | 9256 | 59% (38–74) |
| West Africa | 836 (586–1169) | 1736 | 48% (34–67) | 720 (492–999) | 1640 | 44% (30–61) | 572 (390–815) | 1529 | 37% (26–53) |
| Overall | 12 048 (8153–15 228) | 18 835 | 64% (43–81) | 10 796 (7156–13 437) | 17 553 | 62% (41–77) | 9177 (597–14 300) | 15 667 | 59% (38–91) |
ART consists of the combination of at least three antiretrovirals. Plausible ranges were generated by applying data for lower and upper bounds for the proportion of viral suppression using the literature reporting on viral outcomes among people with HIV in care (appendix pp 1–2). IeDEA=International epidemiology Databases to Evaluate AIDS. NA=not available.
Using the ITT approach, the proportion of adults with viral suppression was 44% (106,541 of 240,600) at 1 year, 36% (79,141 of 220,925) at 2 years, and 29% (57,970 of 201,124) at 3 years after ART initiation (figure 1; table S1, appendix pp 3–4). When the analysis was limited to only adults in ‘follow-up with available VL measurements’ (i.e., year 1: n=119,699; year 2: n=88,463; year 3: n=64,487), the proportions who were virally suppressed increased to 89% at 1 year, 89% at 2 years, and 90% at 3 years. Overall, among 201,124 adults by the end of year 3, 30,130 (5%) had died, and 72,337 (36%) were classified as LTFU, while 30,130 (15%) had transferred out, with percentages varying by regions (figure S4, appendix p 22).
After adjusting the estimates to account for proportions assumed to have viral suppression among those who were in follow-up without a VL test, and among those who transferred or were LTFU, the overall estimated proportions with suppressed VL 1 year after ART initiation ranged from 62% (2994 of 4814) in West Africa to 90% (1851 of 2050) in the Asia-Pacific region (table 4; table S3, appendix pp 10–14). Estimates of suppression ranged from 42% (807 of 1941) in Central Africa to 90% in Asia-Pacific at 2 years after ART initiation. At 3 years after ART initiation, the estimates ranged from 21% in Central Africa (339 of 1641) to 87% (1539 of 1761) in Asia-Pacific (table S3, appendix pp 10–15).
Table 4:
Estimated total numbers and proportions of adults still alive with HIV viral load less than 1000 copies per mL by years on ART, stratified by IeDEA region (adjusted analysis)
| Year 1 |
Year 2 |
Year 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated number with viral load <1000 copies per mL (plausible range) | Total non-deaths | Proportion with viral load <1000 copies per mL (plausible range) | Estimated number with viral load <1000 copies per mL (plausible range) | Total non-deaths | Proportion with viral load <1000 copies (plausible range) | Estimated number with viral load <1000 copies per mL (plausible range) | Total non-deaths | Proportion with viral load <1000 copies per mL (plausible range) | |
|
| |||||||||
| Asia-Pacific | 1851 (1568–1898) | 2050 | 90% (77–93) | 1691 (1385–1705) | 1886 | 90% (73–90) | 1539 (124–1557) | 1761 | 87% (71–88) |
| Caribbean, Central America, and South America | 7611 (4356–7784) | 9067 | 84% (48–86) | 6327 (3641–6563) | 8111 | 78% (45–81) | 5161 (297–5426) | 7255 | 71% (41–75) |
| North America | 7603 (6257–7790) | 9516 | 80% (66–82) | 6119 (4960–6294) | 8232 | 74% (60–76) | 4991 (397–5161) | 7219 | 69% (55–71) |
| Central Africa | 1373 (871–1520) | 2231 | 62% (39–68) | 807 (504–955) | 1941 | 42% (26–49) | 339 (188–470) | 1641 | 21% (11–29) |
| East Africa | 37 824 (25 039–41 583) | 50 525 | 75% (50–82) | 30 354 (20 158–32 589) | 41 749 | 68% (44–75) | 19 505 (12 544–21 759) | 32 799 | 59% (38–66) |
| Southern Africa | 117 708 (79 580–116 654) | 147 215 | 78% (52–86) | 97 793 (65 164–108 645) | 134 739 | 73% (48–81) | 81 832 (53 831–86 679) | 123 456 | 66% (44–70) |
| West Africa | 2994 (1933–3327) | 4814 | 62% (40–69) | 2461 (1576–2794) | 4580 | 54% (34–61) | 1893 (1182–2208) | 4327 | 44% (27–51) |
| Overall | 176 964 (119 604–180 556) | 225 418 | 79% (53–80) | 145 552 (97 388–159 545) | 201 238 | 72% (48–79) | 115 260 (75 933–123 260) | 178 458 | 65% (43–69) |
ART consists of the combination of at least three antiretrovirals. Plausible ranges were generated by applying data for lower and upper bounds for the proportion of viral suppression using the literature reporting on viral outcomes among people with HIV in care (appendix pp 1–2). ART=antiretroviral therapy. IeDEA=International epidemiology Databases to Evaluate AIDS.
The estimated viral suppression using ITT, adjusted analysis, and estimated proportions among children/adolescents and adults with VL measurements are presented in figure 2 for the overall population and in table S4 and table S5 (appendix pp 15–17) by regions.
Figure 2.
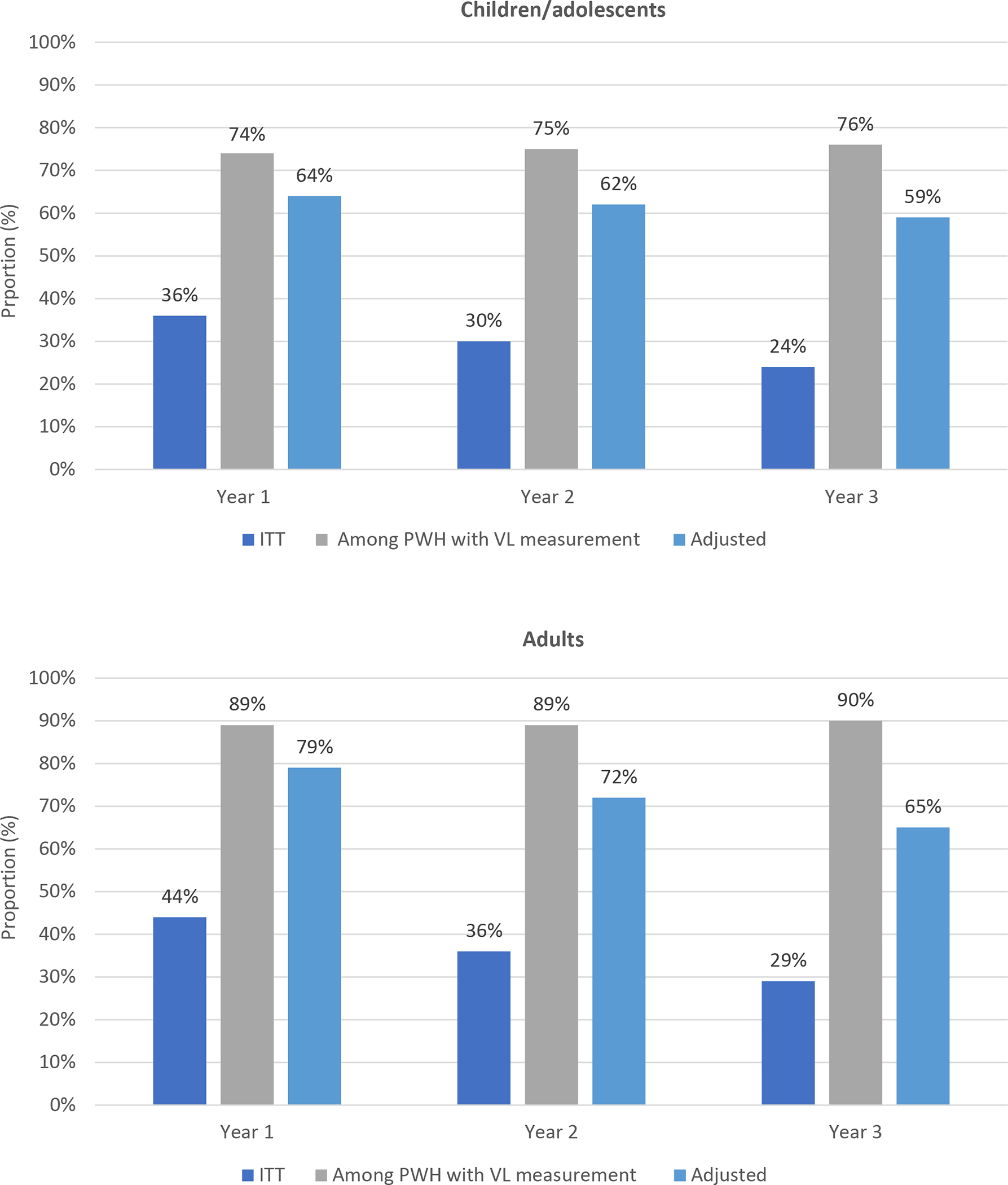
HIV viral suppression proportions using ITT, children/adolescents and adults with viral load measurements and adjusted analyses in the IeDEA global consortium at years 1, 2, and 3 following ART initiation
Discussion
This study described viral suppression among children/adolescents and adults starting ART between 2010 and 2019 at 148 IeDEA sites in 31 countries. The proportion of people with HIV who were virologically suppressed was low in the unadjusted ITT-like analysis. However, when we adjusted estimates for missing VL among PWH in care but without VL testing, transferred, and LTFU, the proportion virally suppressed increased considerably. The unadjusted analyses, with a denominator that includes a large number of individuals who were LTFU, did not give a plausible approximation of the proportion of people who were virally suppressed, and those results consequently should be interpreted with caution. As countries move towards the third 95 of UNAIDS’s targets, a more accurate approximation of the proportion of PWH on ART who are virally suppressed can be achieved by better routine VL testing and maximizing retention or reconnecting PWH to care.
Overall, only 24% of children/adolescents and 29% of adults (unadjusted ITT-like analysis) were virally suppressed after 3 years of ART initiation. Although comparisons with other studies are difficult owing mainly to variations in the populations included in the denominators of the ITT analyses, our estimates of viral suppression are similar to or lower than those reported in other published studies,19–21 and reports from the Population-based HIV Impact Assessment (PHIA) surveys of multiple African countries and UNAIDS modeling studies.22,1 A large meta-analysis study conducted after 2010 reported ITT results for children/adolescents aged <18 years living in LMICs reported in which 63% were virally suppressed after 12 months on ART.19 This study considered all children with missing VL results as having viral failure but did not adequately describe the population included in the analysis. A recent systematic review, including participants from a wide range of settings, showed substantial variation (27% to 89%) in the proportion of children/adolescents achieving viral suppression after 12 months of ART.20 The proportions of suppressed adults in our ITT-like analysis were lower than the ITT results reported in two meta-analyses, which found that viral suppression in individual studies ranged from 69% to 87% after 12 months of ART.21,23 However, those studies included PWH only from LMICs or those who started with triple ART regimens, in contrast to our study which included PWH from more diverse populations regardless of the ART regimen used.
Within 3 years after ART initiation, over half of children/adolescents and adults were LTFU or had transferred out. This partially explains the low proportions of viral suppression observed in our ITT-like analyses. The common risk factors responsible for LTFU such as patient-related factors, site-level factors, tracking systems and lack of access to HIV services were reported in the IeDEA regions.24 In studies that traced LTFU PWH, a substantial proportion of individuals were found to be alive, connected to care at a different clinic, and still taking ART.25,26 A recent tracing study of children/adolescents and adults who started ART and were LTFU found that undocumented transfers increased, and mortality decreased over time after the scale-up of ART and decentralization of ART care.27 Transferred PWH have also been reported to have comparable outcomes to those retained in care, beyond the 3 months following transfer.28,29 In this study, when we accounted for the estimated percentages of PWH with viral suppression among those who transferred or were classified as LTFU, the overall proportions of PWH with viral suppression increased considerably; however, the proportions with viral suppression at 3 years after ART initiation – after accounting for those LTFU, transferred out, and in care with no VL testing – remained low in regions with large proportions of LTFU (e.g., Central, East and West Africa regions).
In the second analysis, in which we calculated viral suppression among PWH who were alive, in follow-up and had VL testing, proportions remained above 74% in children/adolescents and above 89% in adults. These results were roughly comparable to the previous estimates reported for PWH undergoing routine VL monitoring. Data from the scale-up of Kenya’s national HIV program from 2012 to 2016 showed a lower proportion (64%) of children/adolescents virally suppressed after at least 6 months on treatment, but a similar proportion (86%) of adults virally suppressed.30 Our findings were somewhat higher than an earlier large study of treatment programs in Southern, East, and West Africa, in which 80% of PWH (age ≥16 years) achieved viral suppression by 12 months.19,31 The earlier studies on HIV viral suppression from settings which performed targeted testing or were still transitioning to routine VL testing may have underestimated viral suppression when missing VL measurements were not taken into account in reporting the suppressed proportions.
Our analysis has several limitations. Despite having a relatively large overall sample size, only one country within Central Africa had annual VL testing across the 2010–2019 period, limiting generalizability to this region. Furthermore, treatment programs included in the IeDEA consortium may not be fully representative of their countries or sub-populations. Data on ethnicity was not available. It is possible that some clinically stable PWH may have been exempted from VL testing within sites offering routine VL testing We also cannot rule out the possibility that mortality was underestimated because of the misclassification of deaths as LTFU. For the adjusted analyses of those LTFU, the common estimate for unascertained mortality we applied to all regions was derived from the three tracing studies conducted in East Africa.16–18 We did not have access to national-level surveillance databases to more thoroughly assess rates of reconnection to care across the consortium. In addition, with substantial site-level variations in clinical and program management, choices of treatment regimens/switches, and assays used for VL measurements, reasons for not returning to clinic and outcomes after transferring or becoming LTFU are likely to have varied across settings.32, 33 These differences may have influenced or biased the results of viral suppression. Heterogeneity in country-specific treatment programs limit generalizability when analyzing large collaborative datasets.
In conclusion, in this analysis of children/adolescents and adults receiving care at IeDEA sites, results from the ITT-like analyses showed that low proportions of children/adolescents and adults had attained viral suppression after 3 years on ART. When the estimates were adjusted to account for missing VL measurements, regardless of whether PWH remained in care, the estimated overall proportions with viral suppression increased substantially. Estimates of viral suppression that do not account for the sizeable proportion of LTFU PWH who are connected to care elsewhere and still receiving ART or estimates that do not account for PWH in care who are not tested are unlikely to reflect the actual proportion virally suppressed among those who are accessing care. Although adults with HIV are approaching the 95% target, progress among children/adolescents is slower and estimates are still behind the UNAIDS targets.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
To support “Undetectable=untransmittable (U=U)” and treatment-as-prevention strategies, accurate estimation of the third 95 target of UNAIDS’s 95–95-95 is critical. While routine viral load (VL) testing is the standard of care in high-income countries, VL testing has been slow to expand in low- and middle-income countries (LMICs). We searched PubMed database on March 18, 2020, for studies published in English after December 31, 1999, using the terms “HIV”, “viral suppression”, “loss to follow-up”, “tracing”, “viral load”, and “reconnected to care”. We identified only one tracing study which integrated the viral suppression proportion among a random sample who were lost to follow-up (LFTU) from Zambia. The study found that the HIV viremia, defined by VL ≥1,000 copies/mL, was present in 18.1% (95% CI 14.0%−22.3%) of people living with HIV (PWH) in care, and 71.3% (95% CI 58.2%−84.4%) of individuals lost to follow up. After incorporating tracing outcomes and VL results among those who were LTFU and traced into the cohort, the study provided an overall prevalence of HIV viremia of 24.7% (95% CI 21.0%–29.3%).
Added value of this study
To our knowledge, this is the first study using multiregional HIV cohort databases to estimate the viral suppression proportions accounting for missing VL measurements of PWH both in and out of care. We found that 76% of children/adolescents and 90% of adults, who were in follow-up and had VL measurements were suppressed at 3 years after ART initiation. After adjusting for missing VL measurements among those who transferred, were lost to follow-up, or were in follow-up but with no VL testing, 9177 (59%) of 15,667 children/adolescents and 115,260 (65%) of 178,458 adults were virally suppressed at 3 years after ART initiation. The estimated proportions varied widely across regions from 37% to 83% in children/adolescents and from 21% to 90% in adults.
Implications of all the available evidence
Reports on viral suppression proportions that do not account VL for the sizeable proportion of LTFU PWH who are connected to care elsewhere and still receiving ART or VL estimates that do not account for PWH in care who are not tested are unlikely to reflect the actual proportion of virally suppressed among the PWH population who are accessing care. In the era of “U=U”, strategies and increased efforts for better retention in care and more systematic routine VL testing could be helpful in estimating the actual suppressed population. Although adults with HIV are approaching the UNAID 95% target, progress among children/adolescents is slower and estimates are still behind the target.
Acknowledgements
We thank the children, adolescents, adults, caregivers, and staff at our participating clinics who inspire and support our work. Additional appreciation goes to the IeDEA Data Harmonization Working Group, Strategic Data Working Group, Pediatric Working Group, pediatric and adult investigators, regional data managers, and the IeDEA-WHO collaboration. This work was supported by the US National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, and the National Institute on Drug Abuse: U01AI069907 (Asia-Pacific); U01AIQI096299 (Central Africa); U01AI069911 (East Africa); U01AI069924 (Southern Africa); U01AI069919 (West Africa); U01AI069923 (CCASAnet); and for NA-ACCORD: U01AI069918, F31DA037788, G12MD007583, K01AI093197, K23EY013707, K24DA000432, K24AI065298, KL2TR000421, M01RR000052, N02CP055504, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01CA165937, R01DA004334, R01DA011602, R01DA012568, R24AI067039, U01AA013566, U01AA020790, U01AI031834, U01AI034989, U01AI034993, U01AI034994, U01AI035004, U01AI035039, U01AI035040, U01AI035041, U01AI035042, U01AI037613, U01AI037984, U01AI038855, U01AI038858, U01AI042590, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01AI103390, U01AI103397, U01AI103401, U01AI103408, U01DA036935, U01HD032632, U10EY008057, U10EY008052, U10EY008067, U24AA020794,U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR000454, UM1AI035043, Z01CP010214 and Z01CP010176; contracts CDC-200–2006-18797 and CDC-200–2015-63931 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; grants CBR-86906, CBR-94036, HCP-97105 and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided to NA-ACCORD by the Intramural Research Program of the National Cancer Institute. Informatics resources are supported by the Harmonist project, R24AI124872. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above. Complete investigator lists and regional acknowledgements are in the Appendix.
Declarations of interests
The authors have read the journal’s policy and declare the following competing interests: AHS reports grants support to her institution for activities unrelated to this work. ML reports unrestricted grants from Gilead Sciences, Janssen-Cilag, and ViiV HealthCare. Other authors have no conflicts to report.
Footnotes
Data sharing
All study data were stored at the IeDEA Asia-Pacific Regional Data Centre at the Kirby Institute, University of New South Wales, Sydney, Australia. Each site retains ownership of their original data. External users with a formal analysis plan can request access to the data through a formal process detailed at https://www.iedea.org/.
References
- 1.Joint United Nations Programme on HIV/AIDS. Global HIV & AIDS statistics — 2020 fact sheet 2021. [Available from: http://www.unaids.org/en/resources/fact-sheet.
- 2.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. Aids. 2009;23(11):1397–404. [DOI] [PubMed] [Google Scholar]
- 3.Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. New England Journal of Medicine. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Zhou J, He G, Luo Y, Li X, Yang A, et al. Consistent ART adherence is associated with improved quality of Life, CD4 counts, and reduced hospital costs in central China. AIDS research and human retroviruses. 2009;25(8):757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection—Recommendations for a public health approach. 2016. [Available from: https://www.who.int/hiv/pub/guidelines/arv2013/download/en/. [PubMed]
- 6.Joint United Nations Programme on HIV /AIDS. Ambitious treatment targets: writing the final chapter of the AIDS epidemic. [Available from: http://www.unaids.org/sites/default/files/media_asset/JC2670_UNAIDS_Treatment_Targets_en.pdf.
- 7.Lecher S, Ellenberger D, Kim AA, Fonjungo PN, Agolory S, Borget MY, et al. Scale-up of HIV Viral Load Monitoring--Seven Sub-Saharan African Countries. MMWR Morbidity and mortality weekly report. 2015;64(46):1287–90. [DOI] [PubMed] [Google Scholar]
- 8.. Arpadi SM, Shiau S, De Gusmao EP, Violari A. Routine viral load monitoring in HIV-infected infants and children in low- and middle-income countries: challenges and opportunities. J Int AIDS Soc. 2017;20 Suppl 7(Suppl 7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas AD, Keiser O, Balestre E, Brown S, Bissagnene E, Chimbetete C, et al. Monitoring and switching of first-line antiretroviral therapy in adult treatment cohorts in sub-Saharan Africa: collaborative analysis. Lancet HIV. 2015;2(7):e271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiamsakul A, Kariminia A, Althoff KN, Cesar C, Cortes CP, Davies MA, et al. HIV Viral Load Suppression in Adults and Children Receiving Antiretroviral Therapy-Results From the IeDEA Collaboration. J Acquir Immune Defic Syndr. 2017;76(3):319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, et al. Progress with Scale-Up of HIV Viral Load Monitoring - Seven Sub-Saharan African Countries, January 2015-June 2016. MMWR Morb Mortal Wkly Rep. 2016;65(47):1332–5. [DOI] [PubMed] [Google Scholar]
- 12.Euvrard J, Schulz T, Hilderbrand K, Bosland M, Osler M, Boulle A, et al. How accurately do routinely reported HIV viral load suppression proportions reflect progress towards the 90–90-90 target in the population on antiretroviral treatment in Khayelitsha, South Africa? S Afr Med J. 2019;109(3):174–7. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Antiretroviral Therapy of HIV Infection in Infants and Children: Towards Universal Access. Recommendations for a Public Health Approach. [Available from: http://www.who.int/hiv/pub/guidelines/paediatric020907.pdf?ua=1. [PubMed] [Google Scholar]
- 14.World Health Organization. Child growth standards [Available from: https://www.who.int/tools/child-growth-standards.
- 15.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000(314):1–27. [PubMed] [Google Scholar]
- 16.Geng EH, Odeny TA, Lyamuya RE, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Estimation of mortality among HIV-infected people on antiretroviral treatment in East Africa: a sampling based approach in an observational, multisite, cohort study. The lancet HIV. 2015;2(3):e107–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rachlis B, Ochieng D, Geng E, Rotich E, Ochieng V, Maritim B, et al. Implementation and operational research: evaluating outcomes of patients lost to follow-up in a large comprehensive care treatment program in western Kenya. J Acquir Immune Defic Syndr. 2015;68(4):e46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sikazwe I, Eshun-Wilson I, Sikombe K, Czaicki N, Somwe P, Mody A, et al. Retention and viral suppression in a cohort of HIV patients on antiretroviral therapy in Zambia: Regionally representative estimates using a multistage-sampling-based approach. PLoS Med. 2019;16(5):e1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boerma RS, Boender TS, Bussink AP, Calis JC, Bertagnolio S, Rinke de Wit TF, et al. Suboptimal Viral Suppression Rates Among HIV-Infected Children in Low- and Middle-Income Countries: A Meta-analysis. Clin Infect Dis. 2016;63(12):1645–54. [DOI] [PubMed] [Google Scholar]
- 20.Ferrand RA, Briggs D, Ferguson J, Penazzato M, Armstrong A, MacPherson P, et al. Viral suppression in adolescents on antiretroviral treatment: review of the literature and critical appraisal of methodological challenges. Trop Med Int Health. 2016;21(3):325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon JH, Elliott JH, Bertagnolio S, Kubiak R, Jordan MR. Viral suppression after 12 months of antiretroviral therapy in low- and middle-income countries: a systematic review. Bull World Health Organ. 2013;91(5):377–85e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farahani M, Radin E, Saito S, Sachathep KK, Hladik W, Voetsch AC, et al. Population Viral Load, Viremia, and Recent HIV-1 Infections: Findings From Population-Based HIV Impact Assessments (PHIAs) in Zimbabwe, Malawi, and Zambia. Journal of acquired immune deficiency syndromes (1999). 2021;87(Suppl 1):S81–s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartlett JA, Fath MJ, Demasi R, Hermes A, Quinn J, Mondou E, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. Aids. 2006;20(16):2051–64. [DOI] [PubMed] [Google Scholar]
- 24.Kariminia A, Law M, Davies MA, Vinikoor M, Wools-Kaloustian K, Leroy V, et al. Mortality and losses to follow-up among adolescents living with HIV in the IeDEA global cohort collaboration. J Int AIDS Soc. 2018;21(12):e25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geng EH, Odeny TA, Lyamuya R, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Retention in Care and Patient-Reported Reasons for Undocumented Transfer or Stopping Care Among HIV-Infected Patients on Antiretroviral Therapy in Eastern Africa: Application of a Sampling-Based Approach. Clin Infect Dis. 2016;62(7):935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: systematic review and meta-analysis. Trop Med Int Health. 2015;20(3):365–79. [DOI] [PubMed] [Google Scholar]
- 27.Zürcher K, Mooser A, Anderegg N, Tymejczyk O, Couvillon MJ, Nash D, et al. Outcomes of HIV-positive patients lost to follow-up in African treatment programmes. Trop Med Int Health. 2017;22(4):375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornell M, Lessells R, Fox MP, Garone DB, Giddy J, Fenner L, et al. Mortality among adults transferred and lost to follow-up from antiretroviral therapy programmes in South Africa: a multicenter cohort study. J Acquir Immune Defic Syndr. 2014;67(2):e67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies M-A, Tsondai P, Tiffin N, Eley B, Rabie H, Euvrard J, et al. Where do HIV-infected adolescents go after transfer? - Tracking transition/transfer of HIV-infected adolescents using linkage of cohort data to a health information system platform. Journal of the International AIDS Society. 2017;20(Suppl 3):21668-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mwau M, Syeunda CA, Adhiambo M, Bwana P, Kithinji L, Mwende J, et al. Scale-up of Kenya’s national HIV viral load program: Findings and lessons learned. PLoS One. 2018;13(1):e0190659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulle A, Schomaker M, May MT, Hogg RS, Shepherd BE, Monge S, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014;11(9):e1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geng EH, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, Yiannoutsos CT, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53(3):405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. Jama. 2008;300(5):506–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


