
Keywords: coordination, motor module/synergy, postural adjustment, reach, trunk muscles
Abstract
Postural muscle activity precedes voluntary movements of the upper limbs. The traditional view of this activity is that it anticipates perturbations to balance caused by the movement of a limb. However, findings from reach-based paradigms have shown that postural adjustments can initiate center of mass displacement for mobility rather than minimize its displacement for stability. Within this context, altering reaching distance beyond the base of support would place increasing constraints on equilibrium during stance. If the underlying composition of anticipatory postural activity is linked to stability, coordination between muscles (i.e., motor modules) may evolve differently as equilibrium constraints increase. We analyzed the composition of motor modules in functional trunk muscles as participants performed multidirectional reaching movements to targets within and beyond the arm’s length. Bilateral trunk and reaching arm muscle activity were recorded. Despite different trunk requirements necessary for successful movement, and the changing biomechanical (i.e., postural) constraints that accompany alterations in reach distance, nonnegative matrix factorization identified functional motor modules derived from preparatory trunk muscle activity that shared common features. Relative similarity in modular weightings (i.e., composition) and spatial activation profiles that reflect movement goals across tasks necessitating differing levels of trunk involvement provides evidence that preparatory postural adjustments are linked to the same task priorities (i.e., movement generation rather than stability).
NEW & NOTEWORTHY Reaching within and beyond arm’s length places different task constraints upon the required trunk motion necessary for successful movement execution. The identification of constant modular features, including functional muscle weightings and spatial tuning, lend support to the notion that preparatory postural adjustments of the trunk are tied to the same task priorities driving mobility, regardless of the future postural constraints.
INTRODUCTION
When executing goal-directed arm movements during stance, humans must coordinate the voluntary component of the action (i.e., the reaching arm movement and focal arm muscle activity) with the accompanying postural adjustments in the trunk and lower limbs. It is known that the central nervous system (CNS) does this by activating postural muscles in advance of the movement. Traditionally, this postural activity has been attributed to counteracting the self-generated disturbance to the balance caused by the upcoming movement (1–3). In contrast, other studies have argued that the spatiotemporal patterns of muscle activity in both the upper and lower limbs prepare and assist the desired voluntary action (4–8). Often the experimental paradigms used to produce these opposing interpretations of preparatory mechanisms have incorporated different involvement of the trunk segment for task execution (i.e., arm-raise vs. whole-body reaching). Despite the trunk’s role as a linkage between focal and postural segments, the preparatory activity of trunk musculature has received less attention. This is surprising considering the inertial properties of the trunk and the consequences of trunk motion to postural stability.
Recently, we have shown that for reaching during standing, preparatory muscle activity specifically of the trunk muscles is directionally “tuned” to movements of the center of mass (CoM) and trunk in the direction of the target (9) rather than limiting its displacement. This would suggest that the explicit task requirements determine the involvement of muscles deemed “postural” for their efficient completion. In fact, modularity within the CNS has been shown to drive CoM displacement in nonstepping and stepping perturbations that brake and push the CoM, respectively, and shared modules are evident across reactive balance and gait tasks (10, 11). The constancy of motor modules across seemingly dichotomous functional outcomes (i.e., opposing CoM displacement goals) reiterates the potential lack of distinction between stability and movement often considered in traditional control theories. One way to explore this concept is to examine whether the characteristics of preparatory motor activity before voluntary movement changes as a function of the postural demands of the task.
Motor modularity or muscle synergies have been used to interpret the structure and coordination of activation patterns across multiple muscles during a range of complex voluntary and automatic postural tasks (e.g., reaching: 12–15, locomotion: 10, 16, balance perturbations: 17–20; for review, see Refs. 21 and 22). The origins of modularity have been linked to a “wired” organization of the neuromuscular system, whereby a group of muscles can be called upon with their level of activation being modulated together to produce various behavioral tasks (23, 24). The identification of motor modules can follow certain movement parameters of reaching movements, for example, direction and speed (12, 14, 15).
Within the context of changing balance constraints, motor modularity may highlight the strategies the CNS uses to ensure equilibrium while a voluntary movement is executed. For instance, if the motor activity (both focal arm activity and postural trunk activity during reaching) occurring before movement onset is tied to the maintenance of equilibrium, the expression of modularity may follow changes in equilibrium demands. Figure 1 provides a schema of the predictions that may be made to the composition of module weightings (Wi) and their spatial tuning (Ci) as the contribution of the trunk segment to the reach movement increases (e.g., from a target reach distance of 70% to 130%).
Figure 1.
A: schema of predicted module weightings (Wi) and module tuning (Ci) as a function of mobility, stability, or changing task demands as trunk involvement increases across reach distance. In the current study, targets necessitated reaching movements to 70%, 100%, or 130% of total arm length (see methods) to produce movements within and beyond arm’s length. For movement to targets requiring right rotation (e.g., 0°–30° represented by the gray bar in Ci), increases in weightings for muscles driving movement (i.e., greater contributions in Wi of muscles producing right rotation, Rr, see solid color) would show greater module tuning in the same direction as the movement goals. If reflective of a postural control strategy for mobility (i.e., pPA = Movement Goals), extracted module weightings and coefficient tuning would remain similar irrespective of reach distance, such that Wi 70% ∼ Wi 130% and Ci 70% ∼ Ci 130%. B: schema of experimental setup for reach paradigm. Ant., anterior; Lr, muscles producing leftward (counterclockwise) rotation; Post., posterior; pPA, preparatory postural adjustment; Pred., prediction; rot., rotation; Rr, muscles producing rightward (clockwise) rotation.
Given that we have shown that 1) the spatial organization of preparatory trunk muscle activity is tied to voluntary movement direction (9) and 2) global preparatory postural activity remains similar under postural challenges that constrain CoM displacement (6), the expression of modularity across muscles may also follow the movement-related component of the action (Fig. 1, Movement Goals). As such, postural and focal muscles would act as one functional unit to ensure smooth posture and movement coordination (25–27), promoting the notion of mobility as the purpose of postural control (for review, see Ref. 28) and express similar weightings and coefficients with increasing trunk contributions. Alternatively, similar module weightings may be used, with their spatial tuning opposing the action of target motion (and underlying the increased stability requirements irrespective of trunk involvement—see Fig. 1, Stability Goals) for the traditional purposes of CoM minimization. Finally, task demands may be reflected by altered module weightings or spatial tuning that represent a shift from stability-driven to goal-driven motion as trunk involvement becomes a necessary component of task execution (Fig. 1, Task Demands).
Therefore, we examined these predictions within the structure of preparatory motor activity as trunk involvement for task attainment was increased when reaching moves from within to beyond arm’s length. If preparatory activity reflects movement goals that remain similar across tasks and are prioritized above balance requirements, we expect the composition of motor modules to display shared features across reaching distances linked to driving CoM displacement. Alternatively, if a preparatory activity indicates changing needs from postural stability to movement generation (as reaching distance progresses beyond arm’s length), motor module composition and muscle directional tuning would reflect opposing CoM displacement requirements.
METHODS
Participants
Five (3 males, 2 females) healthy right-hand dominant participants, without any known neurological, visual, or orthopedic impairments, were recruited from the university student population (mean age: 28.4 ± 8.8 yr; mean height: 1.76 ± 0.06 m; mean weight: 71.6 ± 10.1 kg). Participants gave written informed consent for all experimental procedures and local institutional ethical approval (University of Wollongong Human Research Ethics Committee: HE13/188) was granted in accordance with the Declaration of Helsinki (1975).
Experimental Apparatus and Setup
The experimental setup has been described in detail previously (4, 9). Briefly, participants stood within the center of a fully adjustable, custom-built semicircular array consisting of 13 evenly spaced light targets (diameter: 25 mm; spacing: 15° increments). Muscle activity for 14 muscles across the trunk and focal (arm) segment were recorded using two Bagnoli 8-channel surface electromyography (sEMG) systems (Delsys, Boston, MA.) sampling at 1,000 Hz. The following trunk muscles were recorded bilaterally using guidelines from SENIAM (29) and prior muscle architecture work (9): rectus abdominis [left (RAl), right (RAr)], external oblique [left (EOl), right (EOr)], combined internal oblique and transversus abdominis [left (IOTrAl), right (IOTrAr)], lumbar erector spinae [left (LumESl), right (LumESr)], multifidus [left (Multl), right (Multr)], and latissimus dorsi [left (Latl), right (Latr)]. The anterior (ADelr) and posterior heads (PDelr) of the deltoid muscles for the reaching arm were also recorded. Three-dimensional whole body kinematics were recorded using a 10-camera Bonita motion capture system (Vicon, Oxford, UK) sampling at 200 Hz. Retroreflective markers were positioned according to the Vicon PluginGait model. Kinematic data and analog sEMG signals were captured through a Vicon Giganet controller (Vicon, Oxford, UK) and synchronized with a customized LabVIEW program (v. 2013, National Instruments, Austin, TX) controlling target light illumination using a single trigger switch.
Experimental Procedures
Before each trial, participants stood quietly at their preferred mediolateral stance width and centered in the array. The index finger of the right hand was placed at the base of the sternum, and shoulders were aligned perpendicular to the anterior and centrally located 90° target. Array height was centered at the level of the right acromion process and individual targets were adjusted to one of three predetermined distances from the body; at each participant’s total reach length (100% total reach length), measured from the sternum to the tip of the right index finger with the shoulder in neutral scapular retraction and arm extended, within (70% total reach length) and beyond arm reach (130% total reach length). These distances were chosen as 1) the bounds for trunk involvement in a visually guided reaching task have previously been reported to occur at 80%–90% of total reach length (30, 31), 2) many prior studies of preparatory muscle activity during arm movements have occurred at 100% of total reach length (i.e., generally during an arm-raising task requiring a single planar shoulder movement; 32, 33), and 3) the 130% reach distance is known to elicit preparatory postural adjustments in the trunk and lower limb without causing imbalance (4, 6, 9).
Reaching trials to each distance were performed in a blocked format with a randomized time delay preceding target illumination (500–1,000 ms). Upon illumination, participants reached out and depressed the target, maintaining this final position until the end of data collection (total collection period = 3,000 ms). No other instructions were given regarding movement execution. For familiarization, two reaching trials for each direction and distance were performed before data collection (n = 2 trials × 13 directions × 3 distances). In total, data collection consisted of 585 reaching trials (i.e., n = 15 trials × 13 directions × 3 distances). For each distance, an additional 15 trials were conducted (in which no target illuminated) to ensure movement initiation was not preemptive or preceded the stimulus (i.e., light onset). To counteract any fatiguing effects of the protocol, participants received 5-min rest periods between blocks of 50 trials and between each block of reaching distances.
Data Preprocessing
Analyses were completed offline using customized MATLAB scripts (v. 2013b; The MathWorks, Natick, MA). Kinematics were low-pass filtered using a second-order Butterworth algorithm at 20 Hz. Raw analog sEMG signals were high-pass filtered at 35 Hz (to remove motion artifact), demeaned, rectified, and low-pass filtered at 100 Hz (2nd-order Butterworth) for visualization. Movement onset of the reaching task was determined using the bell-shaped tangential velocity profile of the right index finger. A threshold of 3% of the peak velocity was chosen, with movement initiation being the first value exceeding, and movement termination the first value reducing, after the peak (34). Peak changes in trunk flexion angle and center of mass excursion in the anteroposterior and mediolateral planes, as well as tangential center of mass velocity and acceleration, were calculated over the movement time. For muscle activity, a conservative period 250 ms preceding movement onset (termed the preparatory postural adjustment period, or pPA period) was chosen and divided into five 50-ms epochs (i.e., pPA1–pPA5) to assess the spatial and temporal organization of trunk musculature in preparing movement initiation.
Mean muscle activity was calculated to produce a single value for every trial combination within each of the three reaching distances. This resulted in a m × s matrix consisting of 14 muscles (m) and 975 samples (s), a combination of all time epochs and reaching directions for every trial (i.e., 975 samples = 5 epochs × 13 directions × 15 trials). Due to the variation in gain among participants for muscles, sEMG values were normalized based on the maximum sEMG elicited across all epochs, directions, and trials, such that all values lay between 0 and 1. Activations were also normalized to unit variance to ensure that future synergy extraction was not biased by muscles exhibiting high variance, such that the sum of squares for each row (i.e., muscle) was equal to one (35). To allow for future comparisons of similarity between motor modules across the different distances of reach, this unit variance was removed after synergy extraction and restored to its original scaling (17). Pooled values could then be visually represented as muscle tuning curves over the time epochs to depict the evolution of preparatory postural adjustments. To characterize the directional bias present in muscle activity, data for the final preparatory phase (i.e., pPA5) was pooled for all participants and compared using principal component analysis. The first principal component (and respective total percentage of variability accounted for, VAF) acted as a measure of similarity in tuning across participants (6).
Motor Module Extraction Using Nonnegative Matrix Factorization
Although a number of techniques are available to reduce high-dimensional data sets recorded during complex motor behaviors, nonnegative matrix factorization (NMF) is a particularly appealing method for the analysis of muscle activity. From a physiological perspective, the “nonnegative” subspace in which the extracted modules are derived better reflect the interaction between motoneuronal firing and muscle activation (35). NMF has previously been used in a number of tasks to extract motor modules (for recent and comprehensive reviews, refer to Refs. 13, 23, 24, 36, and 37). Based on methods adapted from the study by Lee and Seung (38), when a recorded sample of muscle activity (ms) is analyzed, it may be represented by the linear combination of a number of fixed motor modules (W) recruited by module recruitment coefficients [Ci(t)]. If reflective of a mechanism adopted by the CNS in simplifying motor control, it is expected that the predicted summation of muscle activity (mpred) should be able to approximate the original pattern of muscle activity across time (e.g., the pPA period) and condition (reaching distance and direction) such that
where, for each motor module (i), a coactivated group of muscles (Wi) are recruited through a relative activation coefficient (Ci) that determines the contribution of each component to the overall motor module. Across time and conditions, this coefficient is understood to be related to the change in neural command modifying the identified motor module (20).
As the number of motor modules (Nmod) must be specified before extraction, established criteria were used to determine the fewest selection able to accurately characterize the data set (20, 39–41). After extracting 1–14 motor modules for each participant and reaching distance, the goodness-of-fit of each module reconstruction was quantified using a measure of the variability accounted for (or VAF) within the original data set, defined as 100 × uncentered Pearson correlation coefficient, which requires the regression to pass through the origin (42). Similar to prior work (11, 39, 41), one global criterion (VAFoverall) and two local criteria (VAFmuscle, VAFcondition) were used to estimate the necessary number of motor modules. VAFoverall was required to exceed 90% to ensure that this was indicative of relevant features across the entire data set (i.e., reaching direction, pPA period, and trial), with local criteria requiring the VAF for each muscle (VAFmuscle), as well as all muscles within each pPA period (VAFcondition) to exceed 75% VAF. To validate this selection, original VAF values were compared with VAF values identified from module reconstructions undertaken on a shuffled data set. This shuffled data set retained the salient features of the original data set (e.g., values, ranges, and variances), with only the relationships between muscles being removed (17, 35). Data sets were then resampled 500 times using a bootstrapping with replacement procedure where the VAF was recalculated after each iteration to produce a distribution of VAF values. Estimations of the 95% confidence intervals (CIs) for the original and shuffled data sets were then compared. As a means of cross validating the extracted modules from individual reaching distances, these methods were also applied to the pooled data set of all reach distances combined for an individual. Further validation of the reconstruction of individual muscle activity from motor modules extracted from pooled data showed consistency across all participants in goodness-of-fit measures representing both the magnitude (VAF) and shape (r2) of the tuning profiles (mean r2 = 0.66 ± 0.16; mean VAF = 99.35 ± 0.56). Although the predictive power of extracted motor modules was weaker for individual motor modules, these were also able to reconstruct muscle activity across epochs on a single trial basis. Future interpretations were based on modules extracted from the individual distance-based reach conditions.
Module order was first determined based on module comparisons with the strongest relationships between weightings (Wi). Correlation coefficients were calculated between each extracted muscle synergy weighting (W) within participants across each reaching distance and across participants for a particular reaching distance. Motor modules were deemed to be similar when correlation coefficients exceeded the critical value of r > 0.661 (corresponding to a P = 0.01 for 14 muscles), with modules paired with only one other module for an individual. For module pairs that did not exceed the critical value for similarity, module recruitment profiles displaying similar spatial tuning were grouped. As the number of motor modules extracted for each participant can differ, the number of shared motor modules was calculated using the following formula: 100 × [nsimilar/(nmod70% + nmod100% + nmod130% − nsimilar)], where the number of similar modules (nsimilar) is divided by the number of extracted modules (nmod) within each of the 70%, 100%, and 130% reach distance subtasks (11, 43).
Other Statistical Analyses
Changes in trunk excursion, CoM displacement, and peak tangential CoM velocity across conditions of reach distance were assessed using separate two-way repeated-measures ANOVA (Distance × Direction) in the SPSS statistical package (v. 21, IBM, Oregon). Bonferroni–Holm adjustments were applied to main ANOVA results to reduce the family-wise error rate before determining significance across related measures. This was achieved by altering the initial level of significance (alpha = 0.05) with respect to the total number of tests performed (e.g., 14 muscles × 3 main effects/interaction) to produce a more conservative significance level (alpha = 0.0011). Greenhouse–Geisser adjustments were made in cases where violations of sphericity were observed. When applicable, post hoc analyses were conducted with Bonferroni adjustment for multiple pairwise comparisons.
RESULTS
Trunk Excursion and Center of Mass Displacement Change as a Function of Reach Distance
The contribution of the trunk to final reach posture, CoM displacement, and peak tangential CoM velocity were altered across reaching distances (Fig. 2, A–C). When compared across each reaching distance, interaction effects were observed for trunk excursion in the anteroposterior (AP) plane (Distance × Direction: F24,96 = 16.294, P < 0.001, = 0.803), and mediolateral (ML) plane (Distance × Direction: F24,96 = 8.742, P < 0.001, = 0.686). Specifically, AP excursion was greater in the 130% reach distance (i.e., 100% reach vs. 130% reach, 15°–180°, P < 0.023), with only the 90° target showing significant differences between within arm-reaching distances (70% vs. 100% reach, P = 0.036). For ML excursion, differences were more prominent when reaching beyond arm’s length to ipsilateral targets (0°–30°, P < 0.017) and when initially crossing the midline (90°–120°, P < 0.047). In contrast, contralateral targets tended to show significant changes between within-reach distances (70% reach vs. 100% reach: 105°–150°, P < 0.005). Changes were also seen in CoM excursion (AP: Distance × Direction, F24,96 = 88.805, P < 0.001, = 0.957; ML: Distance × Direction, F24,96 = 21.604, P < 0.001, = 0.844). Ipsilateral targets (0°–60°) showed differences as reach progression moved beyond arm’s reach (i.e., 100% vs. 130% reach, P < 0.026), whereas central and contralateral targets (105°–180°) showed increases in AP excursion across all reach distances (i.e., 70% reach vs. 100% reach and 100% reach vs. 130% reach, P < 0.016). ML excursion increased only once reaching moved beyond arm’s length (100% reach vs. 130% reach, 0°–150°, P < 0.045). Peak tangential CoM velocity (F24,96 = 3.136, P < 0.001, = 0.440) increased across centrally located targets (100% vs. 130% reach, 60°–135°, P < 0.042), with central/contralateral targets (75°–180°) showing changes between within arm-reaching distances (70% vs. 100% reach, P < 0.046). Changes were not reflected in peak tangential CoM acceleration measures (F24,96 = 1.004, P = 0.470, = 0.201); however, these occurred at different phases of the reaching movement (Fig. 2D).
Figure 2.

Mean trunk excursion (A) and center of mass (CoM) metrics (B–D) for all participants across reaching direction and reaching distance. As reaching distance moved beyond arm’s length, trunk contribution to movement increased in both the anteroposterior (AP) and mediolateral (ML) planes and was associated with similar changes in CoM excursion. Tangential CoM velocity and acceleration profiles for each direction were aligned to movement onset and offset (see methods). See legend for direction color scheme and representation of significant differences in AP and ML measures. Acc, acceleration; sig. diff, significant difference; Vel., velocity.
Similarity in Muscle Activation Profiles across Reaching Distance
A consistent pattern of arm and trunk muscle activity was found to precede movement onset across various directions and distances of reach. This is highlighted for a typical participant in Fig. 3, where mean muscle activity is shown for five directions of movement and for each reaching distance. As reaching moved from within to beyond arm’s length (Fig. 3A), the amplitude of mean muscle activity generally increased. Preparatory muscle activity was evident for a number of trunk muscles in the period 250 ms before movement onset (gray bar, Fig. 3A).
Figure 3.

Mean surface electromyography (sEMG) activity (A) and representative muscle tuning curves (B) for 2 arm and 12 trunk muscles of a typical participant (S03) over 5 directions (0°, 45°, 90°, 135°, and 180°) for reaching to targets across reach distances (70%, 100%, and 130% total reach distance). A: traces show a period of 500-ms preceding and proceeding movement onset. The shaded area represents the preparatory postural adjustment period, occurring before finger movement onset (black solid line), where data pertaining to the motor module analysis is derived. B: tuning curves highlighting the evolution of activation for muscles of the arm and trunk across the final 3 epochs of the preparatory postural adjustment period (pPA, 3–5) for reaching to 70% (light gray), 100% (dark gray), and 130% (black) of total reaching distance. Epochs consist of mean muscle activity + SD, for 15 trials, recorded over a 50-ms window for each muscle (filled circles). ADelr, right anterior deltoid muscle; EOl, left external oblique muscle; EOr, right external oblique muscle; IOTrAl, left combined internal oblique and transversus abdominis muscle; IOTrAr, right combined internal oblique and transversus abdominis muscle; Latl, left latissimus dorsi muscle; Latr, right latissimus dorsi muscle; LumESl, left lumbar erector spinae muscle; LumESr, right lumbar erector spinae muscle; Multl, left multifidus muscle; Multr, right multifidus muscle; PDelr, right posterior deltoid muscle; RAl, left rectus abdominis muscle; RAr, right rectus abdominis muscle.
The earliest phases of the preparatory period (i.e., pPA1, pPA2) often showed little directional tuning for any muscle. As such, Fig. 3B shows the final three epochs (pPA3, pPA4, and pPA5) relating to the 150 ms preceding movement onset for each distance of reach. For the majority of muscles active during the pPA period, intertrial variability (denoted by the standard deviation at each reaching direction) allowed a rich data set from which NMF extraction could occur. With respect to variation in the spatial tuning of muscle activity across different reaching distances, the most prominent changes were the preparatory period in which activation initiated, with activity beginning slightly earlier as reaching distance increased (e.g., Latr, 70% vs. 100%, pPA4 vs. 5; IOTrAr, 130% vs. 70%/100%, pPA3 vs. 4; IOTrAl, 130% vs. 70%/100%, pPA4 vs. 5; Fig. 3B). Qualitative tuning could be identified in a number of muscles before movement onset (e.g., pPA5 IOTrAl and LumESl; see Fig. 3B). This was corroborated when tuning curve data for the final preparatory phase (i.e., pPA5), pooled for all participants, were compared using principal component analysis (Fig. 4). Across all analyzed muscles, the first principal component was able to account on average for ∼87% (SD: 12%; range: 57%–99%) of the VAF within the reconstruction of muscle activity.
Figure 4.
Comparison of similarity in mean muscle activation tuning curves across reaching distance data for the final epoch before movement onset (i.e., pPA5) using principal component analysis. The contribution of a single muscle tuning curve from each reaching distance (see color scheme in legend) to the primary extracted principal component (PC1, in black) is reflected by the variance accounted for (i.e., VAF). Greater values of VAF reflect higher similarity in activation coefficients across within (70%), at (100%), and beyond (130%) arm-reaching distances. ADelr, right anterior deltoid muscle; EO, external oblique muscle; IOTrA, internal oblique and transversus abdominis muscle; Lat, latissimus dorsi muscle; LumES, lumbar erector spinae muscle; Mult, multifidus muscle; PDelr, right posterior deltoid muscle; pPA, preparatory postural adjustment; RA, rectus abdominis muscle.
A Robust Set of Motor Modules Can Reproduce Preparatory Muscle Activity for Reaching
For each participant, motor modules identified through NMF could accurately reproduce the spatiotemporal regularities of muscle activity recorded over a number of directions and movement distances. Common motor modules were identified across reaching distances between each participant and within participants across reaching distances.
The final determination of motor modules required enabled us to recreate muscle activation patterns, accounting for >90% VAF regardless of muscle, reaching distance, or epoch across all trials (VAFoverall, 95.53 ± 1.67; VAFmuscle, 92.3 ± 1.53; VAFcondition, 96.20 ± 1.40) when both conservative global and local criteria were applied to individual and combined (i.e., “POOLED”) data sets. The varying goodness-of-fit measures (i.e., VAF) are highlighted in Fig. 5A for a single participant across individual reaching distances. When estimated, confidence intervals for the originally determined number of motor modules were much higher than respective values for the shuffled data set (see Fig. 5A, red vs. blue). On average, the lower bound of the original VAF confidence interval was 4.89 (±2.91) above that of the shuffled VAF but often required the addition of extra motor modules to satisfy the local criteria (75% VAF) for each muscle and condition (e.g., Fig. 5B, ADelr, VAFmuscle).
Figure 5.
Validation of the number of motor modules using both global (A) and local (B) criteria of the variability accounted for (VAF) during reconstruction of S02, and total number of motor modules (C) and percentage of shared motor modules (D) extracted across reaching distances. A: 95% confidence limits were estimated for the reconstruction of the original data set and compared with limits calculated with a shuffled data (see methods). The gray bar shows the number of motor modules identified by all VAF criteria. B: local criteria relating to the VAF for each respective muscle over all trials and under each time condition (i.e. epoch). Additional modules were added until a minimum threshold of 75% VAF was satisfied for both local conditions. C: the mean number of extracted motor modules (red bar) did not change as movements shifted from within to beyond arm’s length. Connected circles (gray) represent the number of motor modules for an individual participant. D: for each participant (gray circle), the percentage of shared motor modules was similar. Although the percentage shared was reduced when all conditions were considered (i.e., All), when subdivided, much greater similarity was seen. Graphs consist of mean + SD (purple bar). ADelr, right anterior deltoid muscle; EOr, right external oblique muscle; IOTrAl, left combined internal oblique and transversus abdominis muscle; IOTrAr, right combined internal oblique and transversus abdominis muscle; Latl, left latissimus dorsi muscle; Latr, right latissimus dorsi muscle; LumESl, left lumbar erector spinae muscle; LumESr, right lumbar erector spinae muscle; Multl, left multifidus muscle; Multr, right multifidus muscle; PDelr, right posterior deltoid muscle; pPA, preparatory postural adjustment; RAl, left rectus abdominis muscle; RAr, right rectus abdominis muscle.
When using the individual reaching distance data sets, an average of five motor modules (range: 3–7) were found that could adequately explain the pattern of muscle activity across all trials, distances, and time epochs (Fig. 5C). Considering each participant separately, changes across reaching distance often included either the addition or removal of a single motor module. When these motor modules were expressed in terms of their similarity, approximately one-third of all motor modules were shared across all reaching distances (28.15 ± 11.47%, Fig. 5D). Comparisons made for targets always within arm’s reach (i.e., Fig. 5D, 70%–100% reach) showed good agreement between motor modules (86% shared), which dropped to 57% when compared with the beyond arm length condition (i.e., Fig. 5D, 100%–130%). Distance-specific motor modules were present but varied across participants and was often limited to the addition of a single module (13.44 ± 6.31%; Fig. 5D, Specific).
Recruitment of Similar Motor Modules Present across All Reach Distances
Patterns of module weightings with similar module tuning (i.e., module recruitment coefficients) were recruited across reaching distance and participants (Fig. 6) despite differences in the number of extracted motor modules (Wi). Muscles were represented in multiple modules, varying in their level of activity and often divided along structurally based anatomical regions. For example, motor module W2 included contralateral muscles (e.g., RAl, IOTrAl, and EOl) to a much greater extent than ipsilateral muscles (with the exception of the EOr), whereas W3 presented with reciprocal weightings. Comparisons of module weightings across reaching distances for each participant (i.e., within-participant) were better represented than those between-participants; however, this influence was primarily attributable to a single participant (S05) responsible for ∼77% of all dissimilar comparisons (24/31 dissimilar comparisons out of 115 total comparisons).
Figure 6.
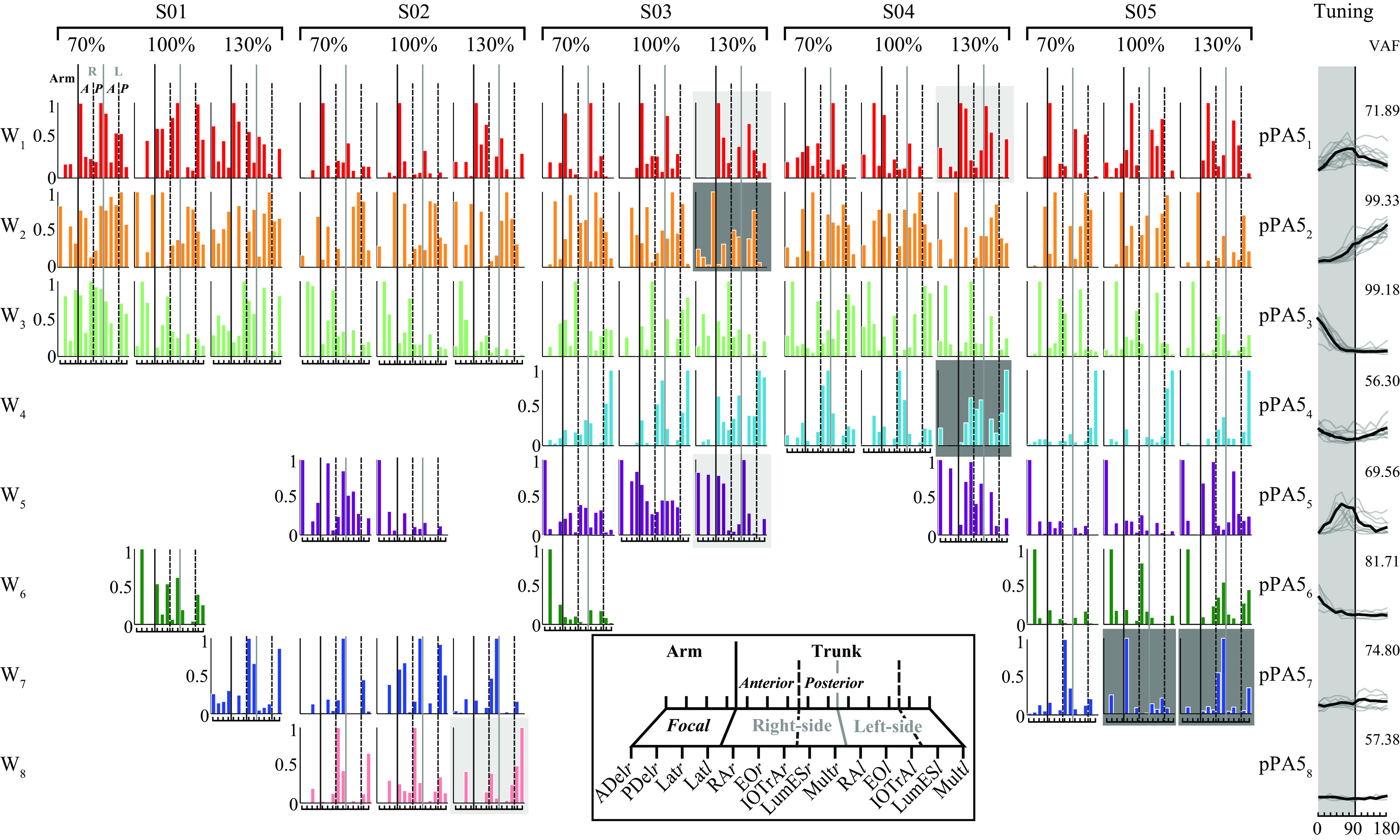
Motor modules extracted from all participants (n = 5) and across each reaching distance (70%, 100%, 130%). Modules are color coded based on their similarity in module weightings (i.e., composition) or module coefficients (see methods for criteria). Individual bars represent the relative weighting of a single muscle to the motor module. Modules that did not meet the criteria for similarity across all reach distance conditions were then grouped based on the spatial tuning reflected in the module tuning for the final epoch (pPA5) using PCA. Such modules are highlighted in light gray for dissimilar weightings comparisons for 70% vs. 130% and dark gray for dissimilar comparisons between 70% and 100% vs. 130%. ADelr, right anterior deltoid muscle; EOr, right external oblique muscle; IOTrAl, left combined internal oblique and transversus abdominis muscle; IOTrAr, right combined internal oblique and transversus abdominis muscle; Latl, left latissimus dorsi muscle; Latr, right latissimus dorsi muscle; LumESl, left lumbar erector spinae muscle; LumESr, right lumbar erector spinae muscle; Multl, left multifidus muscle; Multr, right multifidus muscle; PDelr, right posterior deltoid muscle; PCA, principal component analysis; pPA, preparatory postural adjustment; RAl, left rectus abdominis muscle; RAr, right rectus abdominis muscle; VAF, variability accounted for.
Modules with relatively similar weightings also showed spatiotemporal similarities in module tuning (Fig. 6, Tuning) that aligned with experimentally recorded muscle activity (Figs. 3 and 4). This included similar evolution of module tuning for preparatory muscle activity beginning in the final 150–100 ms preceding movement initiation (i.e., from pPA3–pPA4) and showing peak directional tuning just before movement initiation (i.e., pPA5, seen in Fig. 6, Ci). Motor modules tended to bias either central (W1, W5), ipsilateral (W3, W6), or contralateral (W2, W7) targets in a similar fashion. As distance increased, ipsilateral and contralateral motor modules showed a greater difference in activation as movements neared the centrally located 90° target (e.g., see W2 and W3). This was confirmed when module tuning across reaching distances were pooled across participants using principal component analysis, with the primary principal component showing good agreement (VAF: 76.26% + 16.51%), especially across W1 (VAF: 71.89), W2 (VAF: 99.33), and W3 (VAF: 99.18), modules that were shared across all participants and reaching distances.
DISCUSSION
We examined the structure of motor activity of the trunk muscles before the initiation of reaching to investigate if changes in the expression of modularity were consistent with the constraints of the task in terms of reach distance. Based on our initial predictions (Fig. 1), if the nature of the task dictated the production of preparatory postural adjustments, we expected the structure of extracted motor modules to remain similar (i.e., module weightings, Wi) with their spatial recruitment (i.e., module tuning) altered to satisfy requirements reflecting a shift of priorities from movement to balance as reach distance is altered. Similarity in both the weightings (Wi) and spatial tuning of motor modules in the current study suggests that preparatory postural adjustments are tied to the same task priority of mobility regardless of reach distance. Although this supports our first prediction that movement goals are prioritized over balance during the preparatory period, the change in the number of modules extracted across conditions highlights that certain task demands, not simply related to stability (and CoM minimization), may also be reflected in movement preparation. Therefore, although we provide interpretations of our results within the context of consistency in modularity, additional arguments are provided that consider underlying control mechanisms that may relate to task demands within the current experiment.
Common Modular Features Are Present across Reach Distances
Within each participant, a similar number of modules were required for accurate reconstruction of preparatory trunk muscle activity across reaching distances. This was despite differences in the timing of muscle activation during the epochs preceding reach onset within each reaching condition. For example, motor modules were seen with greater activation in the earlier phases of movement preparation (pPA3 and pPA4) for the furthest target distance that aligned with raw muscle activity changes, supporting earlier studies demonstrating changes in muscle onsets with reach distance (44). Although not shown in the current manuscript, changes in module tuning across time epochs showed evidence of an evolution of spatial tuning as reach distance increased. For example, motor modules within the 70% and 100% reaching distances sometimes showed small relative activations with flat tuning, but when these modules were assessed for the 130% reaching distance, a clear translation to directionally biased activity persisted. When similarity in modules were compared between reaching distances (i.e., 70% vs. 100% and 100% vs. 130%), comparisons of modules between within arm-reaching distances showed strong agreement (mean: 86%, Fig. 5D: 70%–100%), which persisted to a lesser degree for beyond arm reaching (mean: 57%, Fig. 5D: 100%–130%). When compared across individuals, participants showed differences in the total number of motor modules required for accurate reconstruction of preparatory trunk muscle activity, which was reflected in a reduced percentage of total shared modules (mean: 28%, Fig. 5D: All). However, dissimilar modules were not limited to a single reaching distance and most likely reflect subtle changes to modules occurring on a continuum (as reach distance increased), rather than a complete alteration in their composition. Across participants, modules extracted for S05 can explain ∼77% of dissimilar between-participant comparisons. Whether such differences are due to the greater total number of modules extracted, such that modules combined in other participants were recruited separately in S05, are unclear but makes it difficult to attribute this solely to changes in strategy across reach distances.
An addition of motor modules across reach distance may have represented an increase in the neuromuscular complexity required for that particular reaching distance (43) or the necessity for task-specific motor modules to be added for successful EMG reconstruction (20). In fact, the identification of subject-specific motor modules is not new and thought to highlight the capability of a motor module to account for the internal dynamics of the system in creating a unique motor strategy (45, 46). This may also align with the addition of task-specific modules as seen when tasks bias stability. For example, while a number of similar modules are present between perturbed balance and gait tasks, accurate reconstruction of stabilizing automatic postural responses (or APRs) was only possible with the addition of a task-specific motor module related to stability and allowing posterior movement of the CoM (11). Alternatively, such changes in module number and variability within module composition (i.e., across the continuum of within to beyond arm reaching and represented by the reduced value of shared modules, i.e., Fig. 5D) may reflect the combination of behavioral goals with local needs to fully account for task demands to drive CoM displacement. Evidence from single limb studies investigating contributions of muscle activity toward task-level goals (i.e., joint motion) and local joint-level biomechanical features (e.g., joint stresses to passive structures) have shown that covariation in activations are higher for muscle pairs necessitating control of joint stresses when compared with those optimizing task performance (47). However, adaptations of the CNS to perturbation of the system (via nerve lesions or mechanical alterations of muscle action) have shown that a compromise between task performance and regulating internal biomechanical joint stresses prevails (48, 49). Also, the subjective control of reaching, where either movement or balance efficiency are prioritized, can occur on a participant-specific basis (50). This could explain the lack of uniformity across participants in the addition of motor modules catering for a specific distance and function (i.e., stability or movement).
What Do Common Modular Features across Reach Distances Suggest about the Control of Posture and Movement?
Due to the robust nature of motor modules previously shown to be shared across a range of static, balance, and dynamic movement tasks (10, 17–20, 22, 51), it was possible that the same motor modules would be extracted regardless of the need for movement or stability (Fig. 1). Activation coefficients could then alter in their spatial recruitment, such that reach distances that required stability might show reciprocal tuning to those that assist movement. The initial findings of common modular features add to the evidence that motor modules may represent the building blocks of movement that are organized based on the biomechanical considerations of the task (46).
We can attempt to interpret the structure of motor modules derived from preparatory muscle activity within previously defined models of coordination between posture and movement (3, 52). First, if similarly composed motor modules were extracted across all reaching distances it would lend further support to a hierarchical mode of control (53, see Fig. 11 in Ref. 52). Module recruitment would then be contingent on the biomechanical consequences of the task. Within the context of the current study, the similarity of motor modules across reaching distance supports global coordination of focal and postural goals. Considering that module tuning remained spatially robust in their recruitment of a particular motor module, regardless of reaching distance, this suggests that the biomechanical consequences (e.g., minimizing CoM displacement as reach distance increased) are not prioritized. Indirectly, the constancy of modular organization, coupled with the consistency in their recruitment would support the view that the CNS organizes preparatory activity for movement rather than balance (4, 6, 7, 9).
For each participant, the constancy in motor module number across distances aligns with the conservation of motor modules seen for a variety of paradigms altering postural configurations and biomechanical contexts (10, 11, 17, 35, 43, 51). Within these contexts, motor modules have been associated with rectifying task-level errors, such as the deviation of the CoM (54, 55) or control of the center of pressure (CoP) (56). If the same task-level goals are considered during the programming of preparatory postural adjustments, the presence of motor modules in the current study, composed of similar muscle weightings and spatial recruitment patterns irrespective of reach distance, would suggest that feedback and feedforward modes of postural control may use similar modular mechanisms for controlling task-level displacement of the CoM within the base of support (6, 7, 9, 57). Considering the functional anatomy of muscles weighted highly for each spatially defined module, the preparation of movement goals seem to outweigh potential challenges to stability despite the fact that within arm-reaching distances could successfully be achieved without trunk involvement. Changes to the number of modules extracted, and their reduced directional specificity, may also reflect changes in task demands required to tune muscle stiffness in preparation of the upcoming movement. Recent work has shown that proprioceptive afferent firing is tuned to task goals by preemptively modulating feedback gains, thus altering compliance of the muscle in response to stretch (58). This aligns with changes expected in state estimation that underlie internal models associated with optimal feedback control theories and which are known to be influenced by reach distance (59). Considering that reaching under postural challenge also retains similar spatiotemporal features of preparatory activity, which produce similar initial task-level outcomes (i.e., CoM displacement), yet overlay changes in muscle coactivation (potentially associated with tuning mechanical stiffness) lend support to such features underlying task demands ultimately necessary to generate appropriate movement (6).
If modularity is a hard-wired component of the CNS, its presence within feedforward responses may lend further support to the notion that it may be subcortical or spinal in nature (36, 40). In our previous study (9), we suggested that the diffuse connections of the reticulospinal tract may provide the neuroanatomical basis for the execution of postural adjustments. Furthering this line of inquiry, the implication of the pontomedullary reticular formation (PMRF) in both feedforward-based anticipatory postural adjustments (APAs) (60, 61) and feedback-based APRs (62), coupled with the conservation of motor modules in both scenarios, provides a basis for the reticulospinal tract in using modular control. Cortical and subcortical structures may then be responsible for the modulation and task-specific recruitment of such motor modules (36). For preparatory postural adjustments, the supplementary motor area may provide this higher-order control, as experimentally derived and pathological alterations are known to alter their timing and magnitude (63–65).
Limitations
As numerous methods are available for the extraction, and assessment of motor modularity, the modules identified in the current study reflect one representation of the coordination present during reaching to targets requiring varying levels of trunk involvement. Therefore, interparticipant variability may have been due to a combination of methodological decisions. First, our limited sample and decision to prioritize a number of conservative methodological procedures to identify modules likely added to the interparticipant variability. For example, using a less conservative approach that has also been used widely throughout the literature (i.e., VAFoverall > 90%) would have yielded much fewer modules extracted and often resulted with a merging of module weightings that presented with similar coefficient tuning (e.g., W1 with W5, W2 with W6, and W4 with W7). Therefore, it is not known whether interparticipant differences were simply due to the greater number of modules extracted for S05, or if S05 adopted a different strategy to produce similar spatial tuning outcomes. Future work might also leverage new techniques beyond arbitrary VAF thresholds (e.g., single-trial task decoding: 66, 67). In addition, it cannot be discounted that our results highlight the ability for module tunings to be reconstructed by weightings that are only relatively similar at best based on similarity criteria. Greater consensus regarding best practices to applying modular decomposition analyses to motor activity may provide potential solutions to this factor in the future.
Conclusions
Considering the spatial recruitment of motor modules in the preparation of reaching and their generalization across reach distance despite varying degrees of trunk involvement, the current results support our previous findings that trunk muscle activity for reaching favors the initiation of movement, rather than the strict maintenance of balance.
GRANTS
A.S. was supported by an Australian Government Postgraduate Award. P.J.S. was supported by the National Stroke Foundation of Australia. L.H.T. was supported by NIH R01 HD46922.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S. and P.J.S. conceived and designed research; A.S. performed experiments; A.S. analyzed data; A.S., L.H.T., and P.J.S. interpreted results of experiments; A.S. prepared figures; A.S. and P.J.S. drafted manuscript; A.S., L.H.T., and P.J.S. edited and revised manuscript; A.S., L.H.T., and P.J.S. approved final version of manuscript.
REFERENCES
- 1.Bouisset S, Zattara M. A sequence of postural movements precedes voluntary movement. Neurosci Lett 22: 263–270, 1981. doi: 10.1016/0304-3940(81)90117-8. [DOI] [Google Scholar]
- 2.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 35, Suppl 2: ii7–ii11, 2006. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 3.Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol 38: 35–56, 1992. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 4.Leonard JA, Brown RH, Stapley PJ. Reaching to multiple targets when standing: the spatial organization of feedforward postural adjustments. J Neurophysiol 101: 2120–2133, 2009. doi: 10.1152/jn.91135.2008. [DOI] [PubMed] [Google Scholar]
- 5.Pozzo T, Ouamer M, Gentil C. Simulating mechanical consequences of voluntary movement upon whole-body equilibrium: the arm-raising paradigm revisited. Biol Cybern 85: 39–49, 2001. doi: 10.1007/PL00007995. [DOI] [PubMed] [Google Scholar]
- 6.Stamenkovic A, Hollands MA, Stapley PJ. Constancy of preparatory postural adjustments for reaching to virtual targets across different postural configurations. Neuroscience 455: 223–239, 2021. doi: 10.1016/j.neuroscience.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Stapley PJ, Pozzo T, Cheron G, Grishin A. Does the coordination between posture and movement during human whole-body reaching ensure center of mass stabilization? Exp Brain Res 129: 134–146, 1999. doi: 10.1007/s002210050944. [DOI] [PubMed] [Google Scholar]
- 8.Stapley P, Pozzo T, Grishin A. The role of anticipatory postural adjustments during forward whole body reaching movements. NeuroReport 9: 395–401, 1998. doi: 10.1097/00001756-199802160-00007. [DOI] [PubMed] [Google Scholar]
- 9.Stamenkovic A, Stapley PJ. Trunk muscles contribute as functional groups to directionality of reaching during stance. Exp Brain Res 234: 1119–1132, 2016. doi: 10.1007/s00221-015-4536-x. [DOI] [PubMed] [Google Scholar]
- 10.Chvatal SA, Ting LH. Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. J Neurosci 32: 12237–12250, 2012. doi: 10.1523/JNEUROSCI.6344-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chvatal SA, Ting LH. Common muscle synergies for balance and walking. Front Comp Neurosci 7: 48, 2013. doi: 10.3389/fncom.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.d'Avella A, Fernandez L, Portone A, Lacquaniti F. Modulation of phasic and tonic muscle synergies with reaching direction and speed. J Neurophysiol 100: 1433–1454, 2008. doi: 10.1152/jn.01377.2007. [DOI] [PubMed] [Google Scholar]
- 13.d'Avella A, Lacquaniti F. Control of reaching movements by muscle synergy combinations. Front Comput Neurosci 7: 42, 2013. doi: 10.3389/fncom.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.d'Avella A, Portone A, Fernandez L, Lacquaniti F. Control of fast-reaching movements by muscle synergy combinations. J Neurosci 26: 7791–7810, 2006. doi: 10.1523/JNEUROSCI.0830-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muceli S, Boye AT, d'Avella A, Farina D. Identifying representative synergy matrices for describing muscular activation patterns during multidirectional reaching in the horizontal plane. J Neurophysiol 103: 1532–1542, 2010. doi: 10.1152/jn.00559.2009. [DOI] [PubMed] [Google Scholar]
- 16.Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol 556: 267–282, 2004. doi: 10.1113/jphysiol.2003.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH. Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. J Neurophysiol 106: 999–1015, 2011. doi: 10.1152/jn.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol 96: 1530–1546, 2006. doi: 10.1152/jn.00810.2005. [DOI] [PubMed] [Google Scholar]
- 19.Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol 98: 2144–2156, 2007. doi: 10.1152/jn.01360.2006. [DOI] [PubMed] [Google Scholar]
- 20.Torres-Oviedo G, Ting LH. Subject-specific muscle synergies in human balance control are consistent across different biomechanical contexts. J Neurophysiol 103: 3084–3098, 2010. doi: 10.1152/jn.00960.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruton M, O’Dwyer N. Synergies in coordination: a comprehensive overview of neural, computational, and behavioral approaches. J Neurophysiol 120: 2761–2774, 2018. doi: 10.1152/jn.00052.2018. [DOI] [PubMed] [Google Scholar]
- 22.Ting LH. Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res 165: 299–321, 2007. doi: 10.1016/S0079-6123(06)65019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giszter SF. Motor primitives – new data and future questions. Curr Opin Neurobiol 33: 156–165, 2015. doi: 10.1016/j.conb.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ting LH, Chiel HJ, Trumbower RD, Allen JL, Mckay JL, Hackney ME, Kesar TM. Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron 86: 38–54, 2015. doi: 10.1016/j.neuron.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berret B, Bonnetblanc F, Papaxanthis C, Pozzo T. Modular control of pointing beyond arm’s length. J Neurosci 29: 191–205, 2009. doi: 10.1523/JNEUROSCI.3426-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminski TR. The coupling between upper and lower extremity synergies during whole body reaching. Gait Posture 26: 256–262, 2007. doi: 10.1016/j.gaitpost.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Kaminski TR, Simpkins S. The effects of stance configuration and target distance on reaching. I. Movement preparation. Exp Brain Res 136: 439–446, 2001. doi: 10.1007/s002210000604. [DOI] [PubMed] [Google Scholar]
- 28.Le Mouel C, Brette R. Mobility as the purpose of postural control. Front Comput Neurosci 11: 67, 2017. doi: 10.3389/fncom.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for sEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 30.Mark LS, Nemeth K, Gardner D, Dainoff MJ, Paasche J, Duffy M, Grandt K. Postural dynamics and the preferred critical boundary for visually guided reaching. J Exp Psychol Hum Percept Perform 23: 1365–1379, 1997. doi: 10.1037/0096-1523.23.5.1365. [DOI] [PubMed] [Google Scholar]
- 31.Robertson JVG, Roby-Brami A. The trunk as a part of the kinematic chain for reaching movements in healthy subjects and hemiparetic patients. Brain Res 1382: 137–146, 2011. doi: 10.1016/j.brainres.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 32.Hodges PW, Cresswell AG, Daggfeldt K, Thorstensson A. Three dimensional preparatory trunk motion precedes asymmetrical upper limb movement. Gait Posture 11: 92–101, 2000. doi: 10.1016/s0966-6362(99)00055-7. [DOI] [PubMed] [Google Scholar]
- 33.Hodges P, Cresswell A, Thorstensson A. Preparatory trunk motion accompanies rapid upper limb movement. Exp Brain Res 124: 69–79, 1999. doi: 10.1007/s002210050601. [DOI] [PubMed] [Google Scholar]
- 34.Shabbott BA, Sainburg RL. On-line corrections for visuomotor errors. Exp Brain Res 195: 59–72, 2009. doi: 10.1007/s00221-009-1749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ting LH, Chvatal SA. Decomposing muscle activity in motor tasks: methods and interpretation. In: Motor Control: Theories, Experiments, and Applications, edited by Danion F, Latash ML.. New York: Oxford University Press, 2010. [Google Scholar]
- 36.Bizzi E, Cheung VCK. The neural origin of muscle synergies. Front Comput Neurosci 7: 51, 2013. doi: 10.3389/fncom.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latash ML. Motor synergies and the equilibrium-point hypothesis. Motor Control 14: 294–322, 2010. doi: 10.1123/mcj.14.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature 401: 788–791, 1999. doi: 10.1038/44565. [DOI] [PubMed] [Google Scholar]
- 39.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol 103: 844–857, 2010. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roh J, Cheung VCK, Bizzi E. Modules in the brain stem and spinal cord underlying motor behaviors. J Neurophysiol 106: 1363–1378, 2011. doi: 10.1152/jn.00842.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roh J, Rymer WZ, Beer RF. Robustness of muscle synergies underlying three-dimensional force generation at the hand in healthy humans. J Neurophysiol 107: 2123–2142, 2012. doi: 10.1152/jn.00173.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zar JH. Biostatistical Analysis (4th ed.). Upper Saddle River, NJ: Prentice Hall, 1999. [Google Scholar]
- 43.Allen JL, McKay JL, Sawers A, Hackney ME, Ting LH. Increased neuromuscular consistency in gait and balance after partnered, dance-based rehabilitation in Parkinson’s disease. J Neurophysiol 118: 363–373, 2017. doi: 10.1152/jn.00813.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyler AE, Karst GM. Timing of muscle activity during reaching while standing: systematic changes with target distance. Gait Posture 20: 126–133, 2004. doi: 10.1016/j.gaitpost.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Berniker M, Jarc A, Bizzi E, Tresch MC. Simplified and effective motor control based on muscle synergies to exploit musculoskeletal dynamics. Proc Natl Acad Sci USA 106: 7601–7606, 2009. doi: 10.1073/pnas.0901512106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKay JL, Ting LH. Functional muscle synergies constrain force production during postural tasks. J Biomech 41: 299–306, 2008. doi: 10.1016/j.jbiomech.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alessandro C, Barroso FO, Prashara A, Tentler DP, Yeh HY, Tresch MC. Coordination amongst quadriceps muscles suggests neural regulation of internal joint stresses, not simplification of task performance. Proc Natl Acad Sci USA 117: 8135–8142, 2020. doi: 10.1073/pnas.1916578117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alessandro C, Rellinger BA, Barroso FO, Tresch MC. Adaptation after vastus lateralis denervation in rats demonstrates neural regulation of joint stresses and strains. eLife 7: e38215, 2018. doi: 10.7554/eLife.38215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barroso FO, Alessandro C, Tresch MC. Adaptation of muscle activation after patellar loading demonstrates neural control of joint variables. Sci Rep 9: 20370, 2019. doi: 10.1038/s41598-019-56888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hilt PM, Berret B, Papaxanthis C, Stapley PJ, Pozzo T. Evidence for subjective values guiding posture and movement coordination in a free-endpoint whole-body reaching task. Sci Rep 6: 23868, 2016. doi: 10.1038/srep23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawers A, Allen JL, Ting LH. Long-term training modifies the modular structure and organization of walking balance control. J Neurophysiol 114: 3359–3373, 2015. doi: 10.1152/jn.00758.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yakovenko S, Drew T. A motor cortical contribution to the anticipatory postural adjustments that precede reaching in the cat. J Neurophysiol 102: 853–874, 2009. doi: 10.1152/jn.00042.2009. [DOI] [PubMed] [Google Scholar]
- 53.Schepens B, Drew T. Strategies for the integration of posture and movement during reaching in the cat. J Neurophysiol 90: 3066–3086, 2003. doi: 10.1152/jn.00339.2003. [DOI] [PubMed] [Google Scholar]
- 54.Safavynia SA, Ting LH. Sensorimotor feedback based on task-relevant error robustly predicts temporal recruitment and multidirectional tuning of muscle synergies. J Neurophysiol 109: 31–45, 2013. doi: 10.1152/jn.00684.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safavynia SA, Ting LH. Long-latency muscle activity reflects continuous, delayed sensorimotor feedback of task-level and not joint-level error. J Neurophysiol 110: 1278–1290, 2013. doi: 10.1152/jn.00609.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klous M, Mikulic P, Latash ML. Two aspects of feedforward postural control: anticipatory postural adjustments and anticipatory synergy adjustments. J Neurophysiol 105: 2275–2288, 2011. doi: 10.1152/jn.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Commissaris DA, Toussaint HM, Hirschfeld H. Anticipatory postural adjustments in a bimanual, whole-body lifting task seem not only aimed at minimising anterior–posterior centre of mass displacements. Gait Posture 14: 44–55, 2001. doi: 10.1016/S0966-6362(01)00098-4. [DOI] [PubMed] [Google Scholar]
- 58.Papaioannou S, Dimitriou M. Goal-dependent tuning of muscle spindle receptors during movement preparation. Sci Adv 7: eabe0401, 2021. doi: 10.1126/sciadv.abe0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu D, Todorov E. Evidence for the flexible sensorimotor strategies predicted by optimal feedback control. J Neurosci 27: 9354–9368, 2007. doi: 10.1523/JNEUROSCI.1110-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol 92: 2217–2238, 2004. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- 61.Schepens B, Stapley P, Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol 100: 2235–2253, 2008. doi: 10.1152/jn.01381.2007. [DOI] [PubMed] [Google Scholar]
- 62.Stapley PJ, Drew T. The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J Neurophysiol 101: 1334–1350, 2009. doi: 10.1152/jn.91013.2008. [DOI] [PubMed] [Google Scholar]
- 63.Bolzoni F, Bruttini C, Esposti R, Castellani C, Cavallari P. Transcranial direct current stimulation of SMA modulates anticipatory postural adjustments without affecting the primary movement. Behav Brain Res 291: 407–413, 2015. doi: 10.1016/j.bbr.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 64.Jacobs JV, Lou JS, Kraakevik JA, Horak FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience 164: 877–885, 2009. doi: 10.1016/j.neuroscience.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richard A, Van Hamme A, Drevelle X, Golmard J-L, Meunier S, Welter M-L. Contribution of the supplementary motor area and the cerebellum to the anticipatory postural adjustments and execution phases of human gait initiation. Neuroscience 358: 181–189, 2017. doi: 10.1016/j.neuroscience.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 66.Delis I, Berret B, Pozzo T, Panzeri S. Quantitative evaluation of muscle synergy models: a single-trial task decoding approach. Front Comput Neurosci 7: 8, 2013. doi: 10.3389/fncom.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hilt PM, Delis I, Pozzo T, Berret B. Space-by-time modular decomposition effectively describes whole-body muscle activity during upright reaching in various directions. Front Comput Neurosci 12: 20, 2018. doi: 10.3389/fncom.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]





