Abstract
Optical mapping is an imaging technique that is extensively used in cardiovascular research, wherein parameter-sensitive fluorescent indicators are used to study the electrophysiology and excitation-contraction coupling of cardiac tissues. Despite many benefits of optical mapping, eliminating motion artifacts within the optical signals is a major challenge, as myocardial contraction interferes with the faithful acquisition of action potentials and intracellular calcium transients. As such, excitation-contraction uncoupling agents are frequently used to reduce signal distortion by suppressing contraction. When compared with other uncoupling agents, blebbistatin is the most frequently used, as it offers increased potency with minimal direct effects on cardiac electrophysiology. Nevertheless, blebbistatin may exert secondary effects on electrical activity, metabolism, and coronary flow, and the incorrect administration of blebbistatin to cardiac tissue can prove detrimental, resulting in erroneous interpretation of optical mapping results. In this “Getting It Right” perspective, we briefly review the literature regarding the use of blebbistatin in cardiac optical mapping experiments, highlight potential secondary effects of blebbistatin on cardiac electrical activity and metabolic demand, and conclude with the consensus of the authors on best practices for effectively using blebbistatin in optical mapping studies of cardiac tissue.
Keywords: blebbistatin, cardiac physiology, excitation-contraction uncoupler, optical mapping
INTRODUCTION
Optical mapping is a fluorescence imaging approach used to study physiological processes of cardiac tissue and cell preparations, and it is used extensively in basic cardiac electrophysiology research. The great utility of optical mapping is that it provides unparalleled insight into the spatiotemporal dynamics of electrophysiology and excitation-contraction coupling (ECC), albeit with a few key technical hurdles. The field of cardiac optical mapping sprang from the initial work of Salama and Morad, who first recorded optical action potentials from frog hearts using voltage-sensitive dyes (1). Prior to optical approaches, absolute transmembrane potential was measured from tissue preparations and intact hearts using glass microelectrodes, although this approach is technically challenging and spatial information is limited by the number of electrodes that can be inserted into the tissue (2). Electrical activity can also be mapped using arrays of electrodes placed in contact with the tissue (3, 4). However, electrode array mapping is limited by the number and spacing of electrodes and the electrograms are often contaminated by electrical artifacts that occur during pacing and defibrillation. In contrast, the development of optical mapping offered a new mapping approach with superior spatial resolution (5) that is determined by the image sensor specifications (sensor size, number of pixels, and quantum efficiency) and the optical field of view (6). Additionally, the use of calcium-sensitive probes enabled simultaneous measurements of electrical activity and intracellular calcium cycling, as demonstrated by Choi and Salama at the turn of the century (7) and now provides unprecedented insight into ECC parameters in normal and diseased hearts (8–13).
EXCITATION-CONTRACTION UNCOUPLING
The primary challenge of imaging cardiac tissue lies in the very function of the heart itself, pressure development through contraction. Distortions of optical signals acquired from cardiac tissue that result from unconstrained myocardial contraction are often much larger than the physiological signal transduced by a fluorescent probe. To reduce these distortions, hearts have been mechanically constrained against a glass window or with a nylon mesh (14, 15) while optically mapping the epicardium. A limitation of mechanical constraint is that the contact pressure could reduce coronary flow, causing local ischemia. Any residual motion could still negatively impact the accuracy of measuring action potential duration (APD) or APD restitution due to persistent motion artifact (16–18), especially at the edges of the tissue. More recently, motion tracking and ratiometric imaging have enabled optical mapping of transmembrane potential in unconstrained contracting perfused hearts, including working heart preparations (16, 17, 19–22). Further developments in optics and motion tracking algorithms might one day eliminate the need for motion suppression during optical mapping.
However, fully contracting perfused heart preparations may pose an additional set of challenges, especially in studies of ischemia, hypoxia, and metabolism, in that crystalloid buffers are typically used as a perfusate (23). This is important because the heart’s contractile machinery is responsible for ∼75% of its metabolic demand (24), and as such, crystalloid perfusates may lack adequate oxygen-carrying capacity to meet myocardial oxygen demand during elevated heart rates (20, 23, 25–27). This is likely why coronary flow rate in Langendorff-perfused hearts can be several times greater than what is measured under normal physiological conditions (28, 29). Indeed, perfused heart experiments where the crystalloid perfusate was supplemented with an oxygen carrier, such as erythrocytes or other circulating transporters like perfluorocarbons, have revealed the important impact of myocardial oxygen delivery on the electrophysiology, mitochondrial energetics, and contractile performance of perfused hearts (25, 30–32). Relevant to optical mapping, a limitation of supplementing perfusate with erythrocytes is that hemoglobin absorbs light within visible wavelength bands that overlap with the fluorescence of most voltage- and calcium-sensitive probes, necessitating a mapping approach that prevents the erythrocyte-supplemented perfusate from entering the mapped field of view (33). Furthermore, new near-infrared potentiometric probes that fluoresce beyond the absorption band of hemoglobin have recently been used to optically map in vivo and ex vivo blood-perfused hearts (14, 17, 34, 35). The success of such experiments is an exciting step forward in elevating the physiological relevance of optical mapping.
In most optical mapping studies, motion artifacts are suppressed by administering an excitation-contraction (EC) uncoupling agent to the tissue that prevents myocyte contraction by inhibiting actomyosin cross-bridge cycling. Many early cardiac optical mapping studies used 2,3-butanedione monoxime (BDM), also known as diacetyl monoxime (DAM), and cytochalasin D (CytoD) as EC uncoupling agents. Although the precise mechanism of action of these two compounds is not fully understood, these EC uncouplers generally act as noncompetitive inhibitors of muscle myosin II by inhibiting myosin ATPase, thereby disrupting actin-myosin polymerization to reduce or eliminate contraction (36–38). Since these compounds are nonspecific, relatively high concentrations are often needed to completely inhibit contraction throughout the tissue. The high concentrations and lack of specificity lead to measurable effects on ion channel kinetics, changes to intracellular calcium handling, and altered action potential morphology. As an example, BDM has been shown to decrease L-type calcium current and reduce calcium transients (39–41), flatten the restitution curve, and induce APD shortening (42). Conversely, in mouse hearts, CytoD and BDM have been reported to increase APD and refractory periods and slow conduction velocity (43). Peak systolic and diastolic calcium appears elevated in mouse hearts exposed to CytoD; however, BDM has the opposite effect. Interestingly, the side effects of these uncouplers may be species dependent (40, 41, 43).
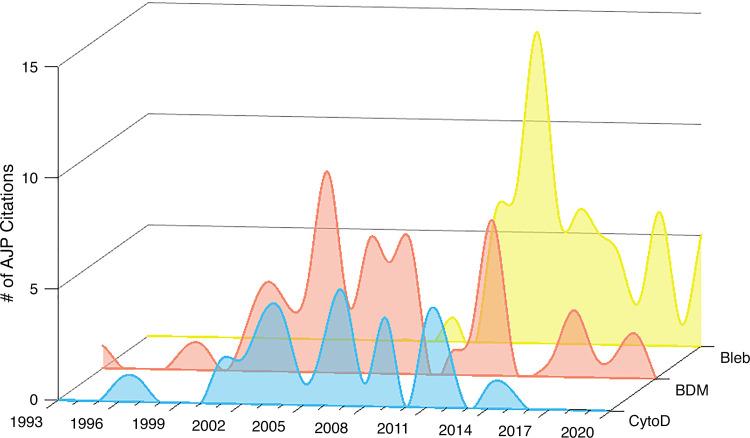
In 2003, blebbistatin (Bleb) was introduced as a more potent and specific myosin II inhibitor (44). It was quickly adopted by many optical mapping laboratories due to its ease of use (it is less toxic than CytoD) and its efficacy in halting cardiac contraction without the apparent side effects observed with other uncoupling agents (45, 46). Named for its ability to disrupt cell motility (44, 47), blebbistatin has proven useful in a range of research areas including cancer research, cell differentiation and migration, cytokinesis, neuroscience, as well as skeletal, smooth, and cardiac muscle research (48). To assess a snapshot of the use of EC uncouplers in cardiac optical mapping research over time, we evaluated articles published in American Journal of Physiology-Heart and Circulatory Physiology (AJP) for the type of EC uncoupling agent used for optical mapping. A total of 164 articles published from 1993 to 2020 were identified from a search of the journal website (accessed on 14 May, 2021 by L.M.S.) using the terms: optical mapping in combination with the following keywords: “2,3-butanedione monoxime” (n = 52) “diacetyl monoxime” (n = 15), “cytochalasin D” (n = 37), and “blebbistatin” (n = 63). The three papers that appeared in both the search for “diacetyl monoxime” and the search for “2,3-butanedione monoxime” were only counted once. As shown in Fig. 1, since its first use for optical mapping in 2007, blebbistatin has all but replaced the other available EC uncouplers. In fact, in the last 3 years alone, blebbistatin was used twice as often (10–13, 49–58) as any other uncoupler for imaging applications (59–65). The use of BDM/DAM and CytoD for optical mapping has essentially ceased; yet due to its lower cost, BDM/DAM may still be a reasonable choice for larger animal (i.e., nonrodent) studies (59, 66–71), which require larger amounts of an EC uncoupler (72). Clearly, blebbistatin is currently the most popular choice in cardiac optical mapping (Fig. 1), as it can be used at much lower concentrations and is less toxic compared with the other EC uncouplers (42, 43, 45, 73).
Figure 1.
Annual number of American Journal of Physiology-Heart publications from 1993 to 2020 that used excitation-contraction uncoupling agents [CytoD, BDM (DAM), Bleb] in cardiac optical mapping studies. BDM, 2,3-butanedione monoxime; Bleb, blebbistatin; CytoD, cytochalasin D; DAM, diacetyl monoxime.
Blebbistatin’s mechanism of action as an EC uncoupler is that it inhibits the ATPases associated primarily with class II myosin isoforms in an actin-detached position, yet it displays little affinity for the rest of the myosin superfamily (47, 74, 75). Importantly, when applied at a concentration that eliminates cardiac tissue contraction, it was reported to have little measurable effect on intracellular calcium handling, action potential morphology, ECG parameters, sinoatrial node activity, and activation patterns (45). Unlike CytoD and BDM/DAM, which must be used at higher concentrations to halt contraction (80 µM to 10 mM), blebbistatin is effective at much lower circulating concentrations (5–15 µM). Notably, even at higher concentrations (25 µM), sinoatrial node cells retain their intrinsic firing rate with no measurable changes in calcium handling, unlike BDM (73). Although blebbistatin has proven to be an incredibly useful compound for cardiac optical mapping studies, it too comes with caveats, including some degree of photoinstability, cytotoxicity, and intrinsic fluorescence (76, 77). There have also been reports of secondary effects on electrical activity, metabolism, and vessel occlusion in excised perfused heart experiments (16, 78).
ELECTROPHYSIOLOGICAL AND METABOLIC CONSIDERATIONS
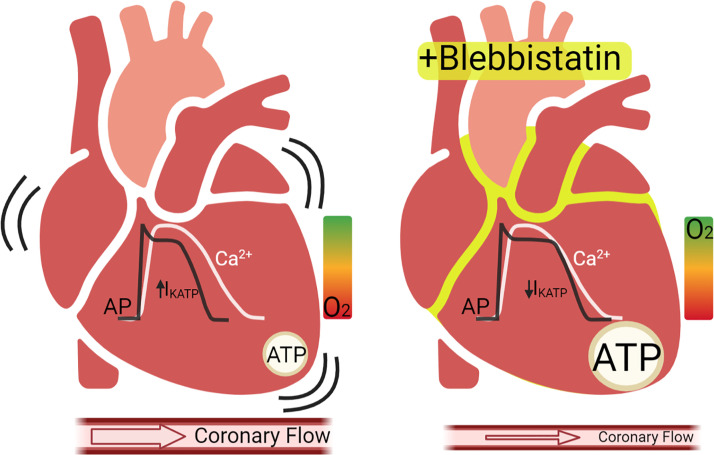
In the foundational 2007 study by Fedorov et al., the effects of blebbistatin on isolated rat cardiomyocytes and excised perfused rabbit hearts were first reported. Blebbistatin was found to inhibit cardiac contraction without measurable effects on cardiac electrophysiology end points, including sinoatrial node activity, epicardial conduction velocity, repolarization, and intracellular calcium cycling (45). Several years later, using monophasic action potential recordings from perfused rabbit hearts, Lou et al. corroborated that BDM flattened the APD restitution curve, but blebbistatin did not; nor did it change monophasic APD (42). Brack et al. later confirmed that atrioventricular and ventricular conduction remained unchanged after administering blebbistatin, but they reported effects on APD restitution, ventricular effective refractory period, and the threshold for ventricular fibrillation in perfused rabbit hearts (78). Recently, Kappadan et al. also reported that blebbistatin prolonged APD in rabbit hearts by 25% (16), and recent in vivo studies found that blebbistatin prolonged APD and slowed conduction velocity in swine hearts (17). It is still unclear whether these observed effects are directly attributed to blebbistatin nonspecifically interacting with ion channels or transporters, or rather, a secondary consequence of the altered metabolic state of the heart during EC uncoupling, as oxygen demand and ATP consumption are reduced (Fig. 2) (26).
Figure 2.
Major metabolic effects of blebbistatin on perfused hearts. Left: beating hearts perfused with crystalloid buffers may have significant IKATP due to low ATP/ADP ratio, which can shorten the action potential duration. Right: blebbistatin treatment reduces metabolic demand, increases ATP/ADP ratio, and may decrease IKATP, resulting in a longer action potential duration. Coronary flow is also typically reduced under these conditions. Notably, intracellular calcium transients are minimally affected by blebbistatin. AP, action potential; Ca2+, intracellular calcium transient; IKATP, ATP-sensitive potassium channel current. Created with BioRender.com, and published with permission.
In particular, several important aspects of using perfused hearts for optical mapping arise from the fact that the heart’s metabolic machinery is intertwined with electrical excitation and mechanical output and feedback mechanisms are dependent on oxygen supply and ATP availability (79). After excitation-contraction is uncoupled, perfused hearts have a fourfold drop in oxygen demand, which dramatically slows cellular metabolic processes throughout the tissue (24, 26). This reduction in myocardial metabolic demand could dramatically impact the sarcolemmal ATP-sensitive potassium channel current (IKATP), essentially reducing the repolarizing current to zero when cellular ATP/ADP ratio is high, such as when there is no cross-bridge cycling during EC uncoupling. Indeed, previous studies in rabbit hearts perfused with fully oxygenated crystalloid perfusate found that IKATP was active during sinus rhythm and this activity increased when heart rate increased (20). Therefore, administering blebbistatin to a perfused heart would then result in a loss of contraction, a large reduction in oxygen demand, an increase in cellular ATP/ADP ratio, a reduction in sarcolemmal IKATP, and a longer APD.
Consideration of this reduced metabolic rate is especially important when using EC uncouplers for research inquiries that have an ischemic component (27, 80). This aspect was highlighted in previous experiments of globally ischemic rabbit hearts, wherein blebbistatin administered before ischemia prolonged (by ∼100%) the time required for mitochondrial membrane potential to collapse as well as the time to asystole (80). This underscores the fact that EC uncoupling dramatically slows the effect of ischemia, so the downstream physiological changes must be considered within this context.
Appropriate oxygenation of perfused cardiac tissue is therefore a critical factor for mimicking in vivo conditions and must be carefully considered during experiments using crystalloid perfusate. Contracting hearts, either retrograde (Langendorff) or anterograde perfused (working heart) with crystalloid perfusate, have an increased risk of myocardial hypoxia compared with EC uncoupled hearts (25–27). This is revealed by action potential triangulation at faster pacing frequencies, which is associated with hypoxia (oxygen demand exceeds oxygen supply) and is linked to KATP channel activation, as explained above. Furthermore, APD restitution curves in contracting rabbit hearts can be achieved down to 100 ms; however, in blebbistatin-treated hearts, electrical alternans are observed at slower rates (16). Altogether, changes in APD, APD restitution, and conduction velocity after administering blebbistatin may therefore be, at least in part, attributed to the dramatic change in metabolic rate. In this way, if a vigorously contracting crystalloid-perfused heart, which may be partially hypoxic with significant IKATP, is uncoupled to a state with increased ATP availability, the reduction in IKATP and subsequent APD prolongation could be substantial.
A related observation is the pronounced effect of blebbistatin on coronary flow rate. In perfused rodent hearts, the administration of blebbistatin results in increased perfusion pressure and decreased flow rate over time. In perfused rabbit hearts, blebbistatin initiates a brief period of increased coronary flow, but as contractions wane and metabolic demand diminishes, vasoconstriction initiates a reduction in the coronary flow rate (78, 81). In summary, it is important to consider that experimental end points measured from EC uncoupled tissue are measured during the condition of a very low rate of cellular metabolism and should therefore be interpreted accordingly.
INTRACELLULAR CALCIUM CYCLING CONSIDERATIONS
In contrast to reports that CytoD is associated with higher diastolic intracellular [Ca2+] and BDM prolongs intracellular Ca2+ transients (43), these parameters appear to be minimally affected by blebbistatin. In the original study by Fedorov et al., blebbistatin was associated with an increase in the fluorescence of an intracellular Ca2+-sensitive probe (Fluo-5F, AM), which the authors attributed to Ca2+ release into the cytoplasm during relaxation and/or the intrinsic fluorescent properties of blebbistatin (45). Using isolated rat cardiac myocytes, Farman et al. reported an elevation in diastolic Ca2+ in the presence of 0.5 µM blebbistatin when Fluo-4, but not Indo-1, was used as the calcium indicator dye (82). This observation may also explain APD prolongation after administering blebbistatin, as increased intracellular [Ca2+] can alter both the APD and intracellular calcium transient amplitude. This phenomenon mirrors the action of CytoD, albeit at higher concentrations (43). In contrast, Jian et al. reported a reduction in the calcium transient amplitude when blebbistatin was applied to cardiomyocytes under strong mechanical load (stiff gel), but the resulting calcium transient amplitude was not different from cells with either no or smaller (soft gel) mechanical load (83). These findings highlight the important role of mechanotransduction in mediating cellular signaling but suggest nominal direct pharmacological effects of blebbistatin per se on intracellular Ca2+ cycling in both isolated cell and perfused hearts.
BEST PRACTICES FOR PREPARING AND USING BLEBBISTATIN
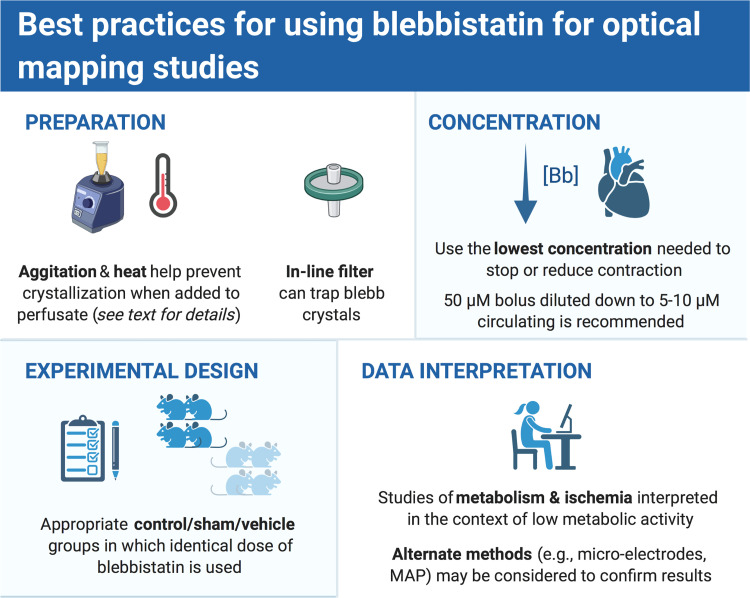
In addition to the physiological effects of EC uncoupling, there are additional technical considerations that could influence optical mapping outcomes and data interpretation when using blebbistatin. First, blebbistatin is reported to undergo photoinactivation that is associated with toxicity in cell culture models (84). Although, to date, this result has not been validated in experiments of perfused cardiac tissue, as constant epicardial illumination for >1 h with UV light did not affect optical action potentials (76). Second, it is important to use an adequate concentration of blebbistatin to halt contraction throughout the tissue to most effectively minimize motion artifacts. The literature commonly reports the use of a circulating blebbistatin concentration of 5–10 µM, although we have found that blebbistatin works best when a higher concentration is administered as a bolus dose to the heart (up to 50 µM), which dilutes in the perfusion system to a 10 µM circulating concentration. If tissue motion returns, an additional bolus dose can be applied. If a very low level of motion persists then the resulting motion artifact can be removed from the mapping data using a nonrigid motion registration algorithm (85), as recently described (58). Depending on desired experimental outcomes, some level of residual motion artifact (or foregoing the use of an uncoupler at all) may be acceptable. For example, activation times, activation maps, conduction velocity, and sites of leading pacemakers or ectopic activity can typically be accurately reconstructed even without EC uncoupling (7, 86), as contraction always occurs after the action potential upstroke. We have found that the amount of acceptable residual motion depends on the experimental design and imaging approach. For instance, motion artifact can be more pronounced at the edges of the tissue and is typically worse at higher magnifications. For each experimental design and hypothesis being tested, pilot experiments are recommended to determine the optimal blebbistatin dose.
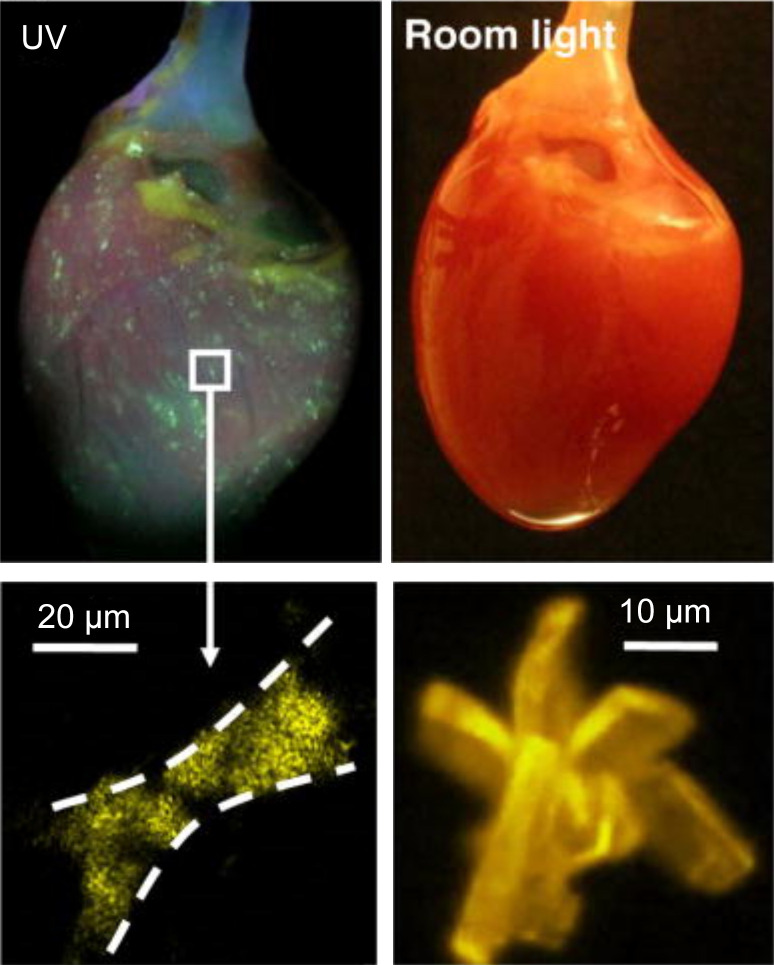
Third, the chemical data sheets for blebbistatin report that it is soluble in DMSO in a range from 12.5 mg/mL (Caymen) up to 58 mg/mL (Selleckchem), but it has limited solubility in aqueous solutions. There is little to no information on the best approach for diluting a concentrated stock solution of blebbistatin dissolved in DMSO. We have found that this is a particularly important procedure, especially when adding a small volume of concentrated DMSO + blebbistatin solution to perfusate media, because the blebbistatin could unexpectedly precipitate out of solution due to spontaneous blebbistatin crystallization (Fig. 3) (76). To prevent this, we have found that vigorous agitation and heating of the perfusate solution (37°C–42°C) are necessary when diluting blebbistatin from DMSO stock. Furthermore, an inline filter (5 µm) is recommended to trap crystals that could occlude the coronary microvessels and capillaries (16, 76). After delivering to the tissue, the bolus dose of DMSO + blebbistatin + perfusate will be diluted by the total volume of the recirculated perfusate, ultimately resulting in a final circulating concentration of ∼10 µM blebbistatin. Perfusate temperature should be maintained at 37°C for the duration of the experiment to reduce the likelihood of blebbistatin crystallization. If the blebbistatin solubility range is exceeded, or the procedure for diluting blebbistatin stock in perfusate is improper, or perfusate temperature is not maintained, then blebbistatin crystals over 20 µm could form in the perfusate and occlude the capillary beds (Fig. 3), leading to local areas of ischemia and increasing incidence of arrhythmias (76).
Figure 3.
Blebbistatin crystallization within a perfused heart—the “disco heart” effect. Top: blebbistatin crystals have accumulated within the vasculature but are not visible unless the tissue is illuminated with UV light (365 ± 5 nm). Bottom: the size of the blebbistatin crystals, both in the vasculature (left) and in solution (right). Reprinted with permission. [Swift 2012, Pflugers Arch (76)].
Fourth, experiments requiring the use of blebbistatin (or any EC uncoupler) should be carefully designed to ensure that appropriate control (or sham/vehicle) groups are studied in which equal doses of blebbistatin and identical methods of administering blebbistatin are used across all experimental groups. In this way, any metabolic effects of EC uncoupling should be similar between groups and any electrophysiological differences observed could be attributed to the hypothesis being tested, rather than secondary effects of EC uncoupling. Finally, for studies related to metabolism, oxygenation, and ATP availability (e.g., ischemia, hypoxia), alternative approaches that do not require EC uncoupling could be considered to validate results, including floating glass microelectrodes (87) or monophasic action potential recordings (22, 88), or isolated cardiomyocyte studies (89).
CONCLUSIONS
Forty-five years after the first optical action potentials were recorded from a frog heart, optical mapping of cardiac tissues continues to inform our understanding of basic cardiac physiology, pathophysiology, and provide insight into the mechanisms of clinical therapies for cardiac disease and arrhythmias. Since 2007, the use of blebbistatin as the EC uncoupler of choice for most experimentalists has no doubt improved the translational relevance of cardiac optical mapping studies due to minimal effects on cardiac electrophysiology and calcium handling, especially when compared with earlier pharmacological agents. Considering the electrophysiological, metabolic, and technical considerations outlined here, the authors have recommended a set of best practices for using blebbistatin as an EC uncoupler (see Fig. 4). Although optical mapping of in vivo, contracting, blood-perfused hearts may be considered the “gold standard,” various hypotheses, experimental designs, animal models, and mapping of human hearts may continue to necessitate in vitro optical mapping studies, and because of its ease of use, blebbistatin is likely to remain a cornerstone of such experiments. To study the orchestrated events that underlie cardiac function, sometimes you need to stop the beat to see the rhythm.
Figure 4.
Authors’ recommendations for use of blebbistatin for optical mapping experiments. MAP, monophasic action potentials. Created with BioRender.com, and published with permission.
GRANTS
L.M.S. and N.G.P. are supported in part by National Heart, Lung, and Blood Institute (NHLBI) Grant HL139472, the Children’s Research Institute, and the Children’s National Heart Institute. C.M.R. is supported, in part, by NHLBI Grant HL111600 and University of California Tobacco Related Disease Research Program Grant T29IP0365C. M.W.K. is supported, in part, by NHLBI Grants HL146169 and HL147279.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.M.S., M.W.K., C.M.R., and N.G.P. conceived and designed research; L.M.S., M.W.K., C.M.R., and N.G.P. interpreted results of experiments; L.M.S. and C.M.R., and N.G.P. prepared figures; L.M.S., M.W.K., C.M.R., and N.G.P. drafted manuscript; L.M.S., M.W.K., C.M.R., and N.G.P. edited and revised manuscript; L.M.S., M.W.K., C.M.R., and N.G.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Devon Guerrelli for assistance in graphing Fig. 1.
REFERENCES
- 1.Salama G, Morad M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science 191: 485–487, 1976. doi: 10.1126/science.191.4226.485. [DOI] [PubMed] [Google Scholar]
- 2.Bleeker WK, Mackaay AJC, Masson-Pévet M, Bouman LN, Becker AE. Functional and morphological organization of the rabbit sinus node. Circ Res 46: 11–22, 1980. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- 3.Frazier DW, Wolf PD, Wharton JM, Tang AS, Smith WM, Ideker RE. Stimulus-induced critical point. Mechanism for electrical initiation of reentry in normal canine myocardium. J Clin Invest 83: 1039–1052, 1989. doi: 10.1172/JCI113945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Rogers JM, Killingsworth CR, Singh KP, Smith WM, Ideker RE. Evolution of activation patterns during long-duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol 286: H1193–H1200, 2004. doi: 10.1152/ajpheart.00773.2003. [DOI] [PubMed] [Google Scholar]
- 5.Nanthakumar K, Jalife J, Massé S, Downar E, Pop M, Asta J, Ross H, Rao V, Mironov S, Sevaptsidis E, Rogers J, Wright G, Dhopeshwarkar R. Optical mapping of Langendorff-perfused human hearts: establishing a model for the study of ventricular fibrillation in humans. Am J Physiol—Heart Circ Physiol 293: H875–H880, 2007. doi: 10.1152/ajpheart.01415.2006. [DOI] [PubMed] [Google Scholar]
- 6.Boukens BJ, Efimov IR. A century of optocardiography. IEEE Rev Biomed Eng 7: 115–125, 2014. doi: 10.1109/RBME.2013.2286296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi BR, Salama G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol 529: 171–188, 2000. doi: 10.1111/j.1469-7793.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaimes R, Walton RD, Pasdois PLCP, Bernus O, Efimov IR, Kay MW. A technical review of optical mapping of intracellular calcium within myocardial tissue. Am J Physiol Heart Circ Physiol 310: H1388–H1401, 2016. doi: 10.1152/ajpheart.00665.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laurita KR, Singal A. Mapping action potentials and calcium transients simultaneously from the intact heart. Am J Physiol Heart Circ Physiol 280: H2053–H2060, 2001. doi: 10.1152/ajpheart.2001.280.5.H2053. [DOI] [PubMed] [Google Scholar]
- 10.Parrish DC, Francis Stuart SD, Olivas A, Wang L, Nykjaer A, Ripplinger CM, Habecker BA. Transient denervation of viable myocardium after myocardial infarction does not alter arrhythmia susceptibility. Am J Physiol Heart Circ Physiol 314: H415–H423, 2018. doi: 10.1152/ajpheart.00300.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swift LM, Burke M, Guerrelli D, Reilly M, Ramadan M, McCullough D, Prudencio T, Mulvany C, Chaluvadi A, Jaimes R, Posnack NG. Age-dependent changes in electrophysiology and calcium handling: implications for pediatric cardiac research. Am J Physiol Circ Physiol 318: H354–H365, 2020. doi: 10.1152/ajpheart.00521.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi M, Yokoshiki H, Mitsuyama H, Watanabe M, Temma T, Kamada R, Hagiwara H, Takahashi Y, Anzai T. SK channel blockade prevents hypoxia-induced ventricular arrhythmias through inhibition of Ca2+/voltage uncoupling in hypertrophied hearts. Am J Physiol Heart Circ Physiol 320: H1456–H1469, 2021. doi: 10.1152/ajpheart.00777.2020. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Olivas A, Francis Stuart SD, Tapa S, Blake MR, Woodward WR, Habecker BA, Ripplinger CM. Cardiac sympathetic nerve transdifferentiation reduces action potential heterogeneity after myocardial infarction. Am J Physiol Heart Circ Physiol 318: H558–H565, 2020. doi: 10.1152/ajpheart.00412.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matiukas A, Mitrea BG, Qin M, Pertsov AM, Shvedko AG, Warren MD, Zaitsev AV, Wuskell JP, Wei M. D, Watras J, Loew LM. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm 4: 1441–1451, 2007. doi: 10.1016/j.hrthm.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salama G, Lombardi R, Elson J. Maps of optical action potentials and NADH fluorescence in intact working hearts. Am J Physiol Heart Circ Physiol 252: H384–H394, 1987. doi: 10.1152/ajpheart.1987.252.2.H384. [DOI] [PubMed] [Google Scholar]
- 16.Kappadan V, Telele S, Uzelac I, Fenton F, Parlitz U, Luther S, Christoph J. High-resolution optical measurement of cardiac restitution, contraction, and fibrillation dynamics in beating vs. blebbistatin-uncoupled isolated rabbit hearts. Front Physiol 11: 464, 2020. doi: 10.3389/fphys.2020.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee P, Quintanilla JG, Alfonso-Almazán JM, Galán-Arriola C, Yan P, Sánchez-González J, Pérez-Castellano N, Pérez-Villacastín J, Ibañez B, Loew LM, Filgueiras-Rama D. In vivo ratiometric optical mapping enables high-resolution cardiac electrophysiology in pig models. Cardiovasc Res 115: 1659–1671, 2019. doi: 10.1093/cvr/cvz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin H, Kay MW, Chattipakorn N, Redden DT, Ideker RE, Rogers JM. Effects of heart isolation, voltage-sensitive dye, and electromechanical uncoupling agents on ventricular fibrillation. Am J Physiol Heart Circ Physiol 284: H1818–H1826, 2003. doi: 10.1152/ajpheart.00923.2002. [DOI] [PubMed] [Google Scholar]
- 19.Christoph J, Chebbok M, Richter CA, Schröder-Schetelig J, Bittihn P, Stein S, Uzelac I, Fenton FH, G H, Gilmour R, Luther S. Electromechanical vortex filaments during cardiac fibrillation. Nature 555: 667–672, 2018. doi: 10.1038/nature26001. [DOI] [PubMed] [Google Scholar]
- 20.Garrott K, Kuzmiak-Glancy S, Wengrowski A, Zhang H, Rogers J, Kay MW. KATP channel inhibition blunts electromechanical decline during hypoxia in left ventricular working rabbit hearts. J Physiol 595: 3799–3813, 2017. doi: 10.1113/JP273873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai DC-S, Caldwell BJ, LeGrice IJ, Hooks DA, Pullan AJ, Smaill BH. Correction of motion artifact in transmembrane voltage-sensitive fluorescent dye emission in hearts. Am J Physiol Heart Circ Physiol 287: H985–H993, 2004. doi: 10.1152/ajpheart.00574.2003. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Iijima K, Huang J, Walcott GP, Rogers JM. Optical mapping of membrane potential and epicardial deformation in beating hearts. Biophys J 111: 438–451, 2016. doi: 10.1016/j.bpj.2016.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz M, Comtois P. The heart in lack of oxygen? A revisited method to improve cardiac performance ex vivo. Am J Physiol Heart Circ Physiol 314: H776–H779, 2018. doi: 10.1152/ajpheart.00699.2017. [DOI] [PubMed] [Google Scholar]
- 24.Schramm M, Klieber HG, Daut J. The energy expenditure of actomyosin-ATPase, Ca(2+)-ATPase and Na+,K(+)-ATPase in guinea-pig cardiac ventricular muscle. J Physiol 481: 647–662, 1994. doi: 10.1113/jphysiol.1994.sp020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuzmiak-Glancy S, Covian R, Femnou AN, Glancy B, Jaimes R, Wengrowski AM, Garrott K, French SA, Balaban RS, Kay MW. Cardiac performance is limited by oxygen delivery to the mitochondria in the crystalloid-perfused working heart. Am J Physiol Heart Circ Physiol 314: H704–H715, 2018. doi: 10.1152/ajpheart.00321.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuzmiak-Glancy S, Jaimes R, Wengrowski AM, Kay MW. Oxygen demand of perfused heart preparations: how electromechanical function and inadequate oxygenation affect physiology and optical measurements. Exp Physiol 100: 603–616, 2015. doi: 10.1113/EP085042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wengrowski AM, Kuzmiak-Glancy S, Jaimes R, Kay MW. NADH changes during hypoxia, ischemia, and increased work differ between isolated heart preparations. Am J Physiol Heart Circ Physiol 306: H529–H537, 2014. doi: 10.1152/ajpheart.00696.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bratkovsky S, Aasum E, Birkeland CH, Riemersma RA, Myhre ESP, Larsen TS. Measurement of coronary flow reserve in isolated hearts from mice. Acta Physiol Scand 181: 167–172, 2004. doi: 10.1111/j.1365-201X.2004.01280.x. [DOI] [PubMed] [Google Scholar]
- 29.Liao R, Podesser BK, Lim CC. The continuing evolution of the Langendorff and ejecting murine heart: new advances in cardiac phenotyping. Am J Physiol Circ Physiol 303: H156–H167, 2012. doi: 10.1152/ajpheart.00333.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chemnitius JM, Burger W, Bing RJ. Crystalloid and perfluorochemical perfusates in an isolated working rabbit heart preparation. Am J Physiol Heart Circ Physiol 249: H285–H292, 1985. doi: 10.1152/ajpheart.1985.249.2.H285. [DOI] [PubMed] [Google Scholar]
- 31.Gillis AM, Kulisz E, Mathison HJ. Cardiac electrophysiological variables in blood-perfused and buffer-perfused, isolated, working rabbit heart. Am J Physiol Heart Circ Physiol 271: H784–H789, 1996. doi: 10.1152/ajpheart.1996.271.2.H784. [DOI] [PubMed] [Google Scholar]
- 32.Hearse DJ, Ferrari R, Sutherland FJ. Cardioprotection: intermittent ventricular fibrillation and rapid pacing can induce preconditioning in the blood-perfused rat heart. J Mol Cell Cardiol 31: 1961–1973, 1999. doi: 10.1006/jmcc.1999.1027. [DOI] [PubMed] [Google Scholar]
- 33.Zaitsev AV, Guha PK, Sarmast F, Kolli A, Berenfeld O, Pertsov AM, de Groot JR, Coronel R, Jalife J. Wavebreak formation during ventricular fibrillation in the isolated, regionally ischemic pig heart. Circ Res 92: 546–553, 2003. doi: 10.1161/01.RES.0000061917.23107.F7. [DOI] [PubMed] [Google Scholar]
- 34.Martišienė I, Karčiauskas D, Navalinskas A, Mačianskienė R, Kučinskas A, Treinys R, Grigalevičiūtė R, Zigmantaitė V, Ralienė L, Benetis R, Jurevičius J. Optical mapping of the pig heart in situ under artificial blood circulation. Sci Rep 10: 8548, 2020. doi: 10.1038/s41598-020-65464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salama G, Choi B-R, Azour G, Lavasani M, Tumbev V, Salzberg BM, Patrick MJ, Ernst LA, Waggoner AS. Properties of new, long wavelength, voltage-sensitive dyes in the heart NIH public access. J Membr Biol 208: 125–140, 2005. doi: 10.1007/s00232-005-0826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Cabo C, Salomonsz R, Delmar M, Davidenko J, Jalife J. Effects of diacetyl monoxime on the electrical properties of sheep and guinea pig ventricular muscle. Cardiovasc Res 27: 1991–1997, 1993. doi: 10.1093/cvr/27.11.1991. [DOI] [PubMed] [Google Scholar]
- 37.Rueckschloss U, Isenberg G. Cytochalasin D reduces Ca2+ currents via cofilin-activated depolymerization of F-actin in guinea-pig cardiomyocytes. J Physiol 537: 363–370, 2001. doi: 10.1111/j.1469-7793.2001.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sellin LC, McArdle JJ. Multiple effects of 2,3‐butanedione monoxime. Pharmacol Toxicol 74: 305–313, 1994. doi: 10.1111/j.1600-0773.1994.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 39.Backx PH, Gao WD, Azan‐Backx MD, Marban E. Mechanism of force inhibition by 2,3‐butanedione monoxime in rat cardiac muscle: roles of [Ca2+]i and cross‐bridge kinetics. J Physiol 476: 487–500, 1994. doi: 10.1113/jphysiol.1994.sp020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira G, Artigas P, Pizarro G, Brum G. Butanedione monoxime promotes voltage-dependent inactivation of L-type calcium channels in heart. Effects on gating currents. J Mol Cell Cardiol 29: 777–787, 1997. doi: 10.1006/jmcc.1996.0321. [DOI] [PubMed] [Google Scholar]
- 41.de Tombe PP, Burkhoff D, Hunter WC. Comparison between the effects of 2-3 dutanedione monoxime (BDM) and calcium chloride on myocardial oxygen consumption. J Mol Cell Cardiol 24: 783–797, 1992. doi: 10.1016/0022-2828(92)91093-K. [DOI] [PubMed] [Google Scholar]
- 42.Lou Q, Li W, Efimov IR. The role of dynamic instability and wavelength in arrhythmia maintenance as revealed by panoramic imaging with blebbistatin vs. 2,3-butanedione monoxime. Am J Physiol Heart Circ Physiol 302: H262–H269, 2012. doi: 10.1152/ajpheart.00711.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker LC, Wolk R, Choi B-RR, Watkins S, Plan P, Shah A, Salama G. Effects of mechanical uncouplers, diacetyl monoxime, and cytochalasin-D on the electrophysiology of perfused mouse hearts. Am J Physiol Heart Circ Physiol 287: H1771–H1779, 2004. doi: 10.1152/ajpheart.00234.2004. [DOI] [PubMed] [Google Scholar]
- 44.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299: 1743–1747, 2003. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 45.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4: 619–626, 2007. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 46.Li D, Nattel S. Pharmacological elimination of motion artifacts during optical imaging of cardiac tissues: is blebbistatin the answer? Heart Rhythm 4: 627–628, 2007. doi: 10.1016/j.hrthm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Kovács M, Tóth J, Hetényi C, Málnási-Csizmadia A, Sellers JR, Seller JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem 279: 35557–35563, 2004. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 48.Rauscher A, Gyimesi M, Kovács M, Málnási-Csizmadia A. Targeting myosin by blebbistatin derivatives: optimization and pharmacological potential. Trends Biochem Sci 43: 700–713, 2018. doi: 10.1016/j.tibs.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Abouassali O, Chang M, Chidipi B, Martinez JL, Reiser M, Kanithi M, Soni R, McDonald TV, Herweg B, Saiz J, Calcul L, Noujaim SF. In vitro and in vivo cardiac toxicity of flavored electronic nicotine delivery systems. Am J Physiol Heart Circ Physiol 320: H133–H143, 2021. doi: 10.1152/ajpheart.00283.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howard T, Greer-Short A, Satroplus T, Patel N, Nassal D, Mohler PJ, Hund TJ. CaMKII-dependent late Na+ current increases electrical dispersion and arrhythmia in ischemia-reperfusion. Am J Physiol Heart Circ Physiol 315: H794–H801, 2018. doi: 10.1152/ajpheart.00197.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ko JS, Guo S, Hassel J, Celestino-Soper P, Lynnes TC, Tisdale JE, Zheng JJ, Taylor SE, Foroud T, Murray MD, Kovacs RJ, Li X, Lin SF, Chen Z, Vatta M, Chen PS, Rubart M. Ondansetron blocks wild-type and p.F503l variant small-conductance Ca2+-activated K+ channels. Am J Physiol Heart Circ Physiol 315: H375–H388, 2018. doi: 10.1152/ajpheart.00479.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Lange WJ, Farrell ET, Kreitzer CR, Jacobs DR, Lang D, Glukhov AV, Carter Ralphe J. Human iPSC-engineered cardiac tissue platform faithfully models important cardiac physiology. Am J Physiol Heart Circ Physiol 320: H1670–H1686, 2021. doi: 10.1152/ajpheart.00941.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mattapally S, Zhu W, Fast VG, Gao L, Worley C, Kannappan R, Borovjagin AV, Zhang J. Spheroids of cardiomyocytes derived from human-induced pluripotent stem cells improve recovery from myocardial injury in mice. Am J Physiol Heart Circ Physiol 315: H327–H339, 2018. [Erratum in Am J Physiol Heart Circ Physiol 315: H731, 2018]. doi: 10.1152/ajpheart.00688.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramlugun GS, Sands GB, Zhao J, LeGrice IJ, Smaill BH. A novel system for mapping regional electrical properties and characterizing arrhythmia in isolated intact rat atria. Am J Physiol Heart Circ Physiol 321: H412–H421, 2021. doi: 10.1152/ajpheart.00185.2021. [DOI] [PubMed] [Google Scholar]
- 55.Shi YP, Pang Z, Venkateshappa R, Gunawan M, Kemp J, Truong E, Chang C, Lin E, Shafaattalab S, Faizi S, Rayani K, Tibbits GF, Claydon VE, Claydon TW. The hERG channel activator, RPR260243, enhances protective IKr current early in the refractory period reducing arrhythmogenicity in zebrafish hearts. Am J Physiol Heart Circ Physiol 319: H251–H261, 2020. doi: 10.1152/ajpheart.00038.2020. [DOI] [PubMed] [Google Scholar]
- 56.Tenma T, Mitsuyama H, Watanabe M, Kakutani N, Otsuka Y, Mizukami K, Kamada R, Takahashi M, Takada S, Sabe H, Tsutsui H, Yokoshiki H. Small-conductance Ca2+-activated K+ channel activation deteriorates hypoxic ventricular arrhythmias via CaMKII in cardiac hypertrophy. Am J Physiol Heart Circ Physiol 315: H262–H272, 2018. doi: 10.1152/ajpheart.00636.2017. [DOI] [PubMed] [Google Scholar]
- 57.Zaitsev AV, Torres NS, Cawley KM, Sabry AD, Warren JS, Warren M. Conduction in the right and left ventricle is differentially regulated by protein kinases and phosphatases: implications for arrhythmogenesis. Am J Physiol—Heart Circ Physiol 316: H1507–H1527, 2019. doi: 10.1152/ajpheart.00660.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zasadny FM, Dyavanapalli J, Dowling NM, Mendelowitz D, Kay MW. Cholinergic stimulation improves electrophysiological rate adaptation during pressure overload-induced heart failure in rats. Am J Physiol Heart Circ Physiol 319: H1358–H1368, 2020. doi: 10.1152/ajpheart.00293.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.George SA, Hoeker G, Calhoun PJ, Entz M, Raisch TB, King DR, Khan M, Baker C, Gourdie RG, Smyth JW, Nielsen MS, Poelzing S. Modulating cardiac conduction during metabolic ischemia with perfusate sodium and calcium in guinea pig hearts. Am J Physiol Heart Circ Physiol 316: H849–H861, 2019. doi: 10.1152/ajpheart.00083.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Graves JM, Vallejo JA, Hamill CS, Wang D, Ahuja R, Patel S, Faul C, Wacker MJ. Fibroblast growth factor 23 (FGF23) induces ventricular arrhythmias and prolongs QTc interval in mice in an FGF receptor 4-dependent manner. Am J Physiol Heart Circ Physiol 320: H2283–H2294, 2021. doi: 10.1152/ajpheart.00798.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jungen C, Scherschel K, Flenner F, Jee H, Rajendran P, De Jong KA, Nikolaev V, Meyer C, Ardell JL, Tompkins JD. Increased arrhythmia susceptibility in type 2 diabetic mice related to dysregulation of ventricular sympathetic innervation. Am J Physiol Heart Circ Physiol 317: H1328–H1341, 2019. doi: 10.1152/ajpheart.00249.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ling S, Jenkins MW, Watanabe M, Ford SM, Rollins AM. Prenatal ethanol exposure impairs the conduction delay at the atrioventricular junction in the looping heart. Am J Physiol Heart Circ Physiol 321: H294–H305, 2021. doi: 10.1152/ajpheart.00107.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oakley CI, Vallejo JA, Wang D, Gray MA, Tiede-Lewis LM, Shawgo T, Daon E, Zorn G, Stubbs JR, Wacker MJ. Trimethylamine-N-oxide acutely increases cardiac muscle contractility. Am J Physiol Heart Circ Physiol 318: H1272–H1282, 2020. doi: 10.1152/ajpheart.00507.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oropeza-Almazán Y, Blatter LA. Mitochondrial calcium uniporter complex activation protects against calcium alternans in atrial myocytes. Am J Physiol Heart Circ Physiol 319: H873–H881, 2020. doi: 10.1152/ajpheart.00375.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomii N, Yamazaki M, Arafune T, Kamiya K, Nakazawa K, Honjo H, Shibata N, Sakuma I. Interaction of phase singularities on the spiral wave tail: reconsideration of capturing the excitable gap. Am J Physiol Heart Circ Physiol 315: H318–H326, 2018. doi: 10.1152/ajpheart.00558.2017. [DOI] [PubMed] [Google Scholar]
- 66.Bourgeois EB, Reeves HD, Walcott GP, Rogers JM. Panoramic optical mapping shows wavebreak at a consistent anatomical site at the onset of ventricular fibrillation. Cardiovasc Res 93: 272–279, 2012. doi: 10.1093/cvr/cvr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garg V, Taylor T, Warren M, Venable P, Sciuto K, Shibayama J, Zaitsev A. β-adrenergic stimulation and rapid pacing mutually promote heterogeneous electrical failure and ventricular fibrillation in the globally ischemic heart. Am J Physiol Heart Circ Physiol 308: H1155–H1170, 2015. doi: 10.1152/ajpheart.00768.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kay MW, Walcott GP, Gladden JD, Melnick SB, Rogers JM. Lifetimes of epicardial rotors in panoramic optical maps of fibrillating swine ventricles. Am J Physiol Heart Circ Physiol 291: H1935–H1941, 2006. doi: 10.1152/ajpheart.00276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mironov SF, Vetter FJ, Pertsov AM. Fluorescence imaging of cardiac propagation: spectral properties and filtering of optical action potentials. Am J Physiol Heart Circ Physiol 291: H327–H335, 2006. doi: 10.1152/ajpheart.01003.2005. [DOI] [PubMed] [Google Scholar]
- 70.Sorrentino A, Signore S, Qanud K, Borghetti G, Meo M, Cannata A, Zhou Y, Wybieralska E, Luciani M, Kannappan R, Zhang E, Matsuda A, Webster A, Cimini M, Kertowidjojo E, D'Alessandro D, Wunimenghe O, Michler R, Royer C, Goichberg P, Leri A, Barrett E, Anversa P, Hintze T, Rota M. Myocyte repolarization modulates myocardial function in aging dogs. Am J Physiol Heart Circ Physiol 310: H873–H890, 2016. doi: 10.1152/ajpheart.00682.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takemoto Y, Takanari H, Honjo H, Ueda N, Harada M, Kato S, Yamazaki M, Sakuma I, Opthof T, Kodama I, Kamiya K. Inhibition of intercellular coupling stabilizes spiral-wave reentry, whereas enhancement of the coupling destabilizes the reentry in favor of early termination. Am J Physiol Heart Circ Physiol 303: H578–H586, 2012. doi: 10.1152/ajpheart.00355.2012. [DOI] [PubMed] [Google Scholar]
- 72.Swift L, Jaimes R, McCullough D, Burke M, Reilly M, Maeda T, Zhang H, Ishibashi N, Rogers J, Posnack NG. Optocardiography and electrophysiology studies of ex vivo Langendorff-perfused hearts. J Vis Exp (153): e60472, 2019. doi: 10.3791/60472.[31762469] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Segal S, Kirschner Peretz N, Arbel-Ganon L, Liang J, Li L, Marbach D, Yang D, Wang S-QQ, Yaniv Y. Eliminating contraction during culture maintains global and local Ca2+ dynamics in cultured rabbit pacemaker cells. Cell Calcium 78: 35–47, 2019. doi: 10.1016/j.ceca.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol 12: 378–379, 2005. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 75.Ramamurthy B, Yengo CM, Straight AF, Mitchison TJ, Sweeney HL. Kinetic mechanism of blebbistatin inhibition of nonmuscle myosin IIb. Biochemistry 43: 14832–14839, 2004. doi: 10.1021/bi0490284. [DOI] [PubMed] [Google Scholar]
- 76.Swift LM, Asfour H, Posnack NG, Arutunyan A, Kay MW, Sarvazyan N. Properties of blebbistatin for cardiac optical mapping and other imaging applications. Pflugers Arch Eur Arch 464: 503–512, 2012. doi: 10.1007/s00424-012-1147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Várkuti BH, Képiró M, Horváth IÁ, Végner L, Ráti S, Zsigmond Á, Hegyi G, Lenkei Z, Varga M, Málnási-Csizmadia A. A highly soluble, non-phototoxic, non-fluorescent blebbistatin derivative. Sci Rep 6: 26141, 2016. doi: 10.1038/srep26141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brack KE, Narang R, Winter J, Ng GA. The mechanical uncoupler blebbistatin is associated with significant electrophysiological effects in the isolated rabbit heart. Exp Physiol 98: 1009–1027, 2013. doi: 10.1113/expphysiol.2012.069369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balaban RS. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim Biophys Acta 1787: 1334–1341, 2009. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Venable PW, Sciuto KJ, Warren M, Taylor TG, Garg V, Shibayama J, Zaitsev AV. Mitochondrial depolarization and asystole in the globally ischemic rabbit heart: coordinated response to interventions affecting energy balance. Am J Physiol Heart Circ Physiol 308: H485–H499, 2015. doi: 10.1152/ajpheart.00257.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eddinger TJ, Meer DP, Miner AS, Meehl J, Rovner AS, Ratz PH. Potent inhibition of arterial smooth muscle tonic contractions by the selective myosin II inhibitor, blebbistatin. J Pharmacol Exp Ther 320: 865–870, 2007. doi: 10.1124/jpet.106.109363. [DOI] [PubMed] [Google Scholar]
- 82.Farman GP, Tachampa K, Mateja R, Cazorla O, Lacampagne A, de Tombe PP. Blebbistatin: use as inhibitor of muscle contraction. Pflugers Arch—Eur J Physiol 455: 995–1005, 2008. doi: 10.1007/s00424-007-0375-3. [DOI] [PubMed] [Google Scholar]
- 83.Jian Z, Han H, Zhang T, Puglisi J, Izu LT, Shaw JA, Onofiok E, Erickson JR, Chen Y-J, Horvath B, Shimkunas R, Xiao W, Li Y, Pan T, Chan J, Banyasz T, Tardiff JC, Chiamvimonvat N, Bers DM, Lam KS, Chen-Izu Y. Mechanochemotransduction during cardiomyocyte contraction is mediated by localized nitric oxide signaling. Sci Signal 7: ra27, 2014. doi: 10.1126/scisignal.2005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kolega J. Phototoxicity and photoinactivation of blebbistatin in UV and visible light. Biochem Biophys Res Commun 320: 1020–1025, 2004. doi: 10.1016/j.bbrc.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 85.Christoph J, Luther S. Marker-free tracking for motion artifact compensation and deformation measurements in optical mapping videos of contracting hearts. Front Physiol 9: 1483, 2018. doi: 10.3389/fphys.2018.01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asfour H, Swift LM, Sarvazyan N, Doroslovački M, Kay MW. Signal decomposition of transmembrane voltage-sensitive dye fluorescence using a multiresolution wavelet analysis. IEEE Trans Biomed Eng 58: 2083–2093, 2011. doi: 10.1109/TBME.2011.2143713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barbic M, Moreno A, Harris TD, Kay MW. Detachable glass microelectrodes for recording action potentials in active moving organs. Am J Physiol Heart Circ Physiol 312: H1248–H1259, 2017. doi: 10.1152/ajpheart.00741.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zingman LV, Zhu Z, Sierra A, Stepniak E, Burnett CM-L, Maksymov G, Anderson ME, Coetzee WA, Hodgson-Zingman DM. Exercise induced expression of cardiac ATP-sensitive potassium channels promotes action potential shortening and energy conservation. J Mol Cell Cardiol 51: 72–81, 2011. doi: 10.1016/j.yjmcc.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O'Rourke B. Effects of 4′-chlorodiazepam on cellular excitation-contraction coupling and ischaemia-reperfusion injury in rabbit heart. Cardiovasc Res 79: 141–149, 2008. doi: 10.1093/cvr/cvn053. [DOI] [PMC free article] [PubMed] [Google Scholar]