Keywords: atherogenesis, abdominal adiposity, hyperandrogenism, polycystic ovary syndrome, saturated fat
Abstract
Inflammation and dyslipidemia are often present in polycystic ovary syndrome (PCOS). We determined the effect of saturated fat ingestion on circulating heat shock protein-70 (HSP-70) and mononuclear cell (MNC) toll-like receptor-2 (TLR2) gene expression, activator protein-1 (AP-1) activation, and matrix matalloproteinase-2 (MMP-2) protein in women with PCOS. Twenty reproductive-age women with PCOS (10 lean, 10 with obesity) and 20 ovulatory controls (10 lean, 10 with obesity) participated in the study. HSP-70 was measured in serum and TLR2 mRNA and protein, AP-1 activation, and MMP-2 protein were quantified in MNC from blood drawn while fasting and 2, 3, and 5 h after saturated fat ingestion. Insulin sensitivity was derived from an oral glucose tolerance test (ISOGTT). Androgen secretion was assessed from blood drawn while fasting and 24, 48, and 72 h after human chorionic gonadotropin (HCG) administration. In response to saturated fat ingestion, serum HSP-70, TLR2 gene expression, activated AP-1, and MMP-2 protein were greater in lean women with PCOS compared with lean controls and in women with PCOS and obesity compared with controls with obesity. Both PCOS groups exhibited lower ISOGTT and greater HCG-stimulated androgen secretion compared with control subjects of their respective weight classes. Lipid-stimulated proatherogenic inflammation marker responses were negatively correlated with ISOGTT and positively correlated with abdominal adiposity and HCG-stimulated androgen secretion. In PCOS, saturated fat ingestion stimulates proatherogenic inflammation independent of obesity. This effect is greater when PCOS is combined with obesity compared with obesity alone. Abdominal adiposity and hyperandrogenism may perpetuate proatherogenic inflammation.
NEW & NOTEWORTHY This paper demonstrates that in polycystic ovary syndrome (PCOS), ingestion of saturated fat triggers a molecular pathway of inflammation known to drive atherogenesis. This effect is independent of obesity as it occurs in lean women with PCOS and not in lean ovulatory control subjects. Furthermore, the combined effects of PCOS and obesity are greater compared with obesity alone.
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a highly prevalent endocrine disorder impacting 15%–18% of premenopausal women (1, 2). Diagnostic features of PCOS include hyperandrogenism, ovarian dysfunction, and polycystic ovarian morphology (2). Metabolic abnormalities associated with accelerated atherogenesis such as obesity, insulin resistance, and dyslipidemia are present in 60%–70% of women with the disorder (3–6). A proatherogenic predisposition in PCOS is reflected by a high prevalence of endothelial dysfunction and coronary artery calcification (7, 8). Chronic low-grade inflammation in PCOS has been implicated in the development of accelerated atherogenesis (9–11).
Our previous studies have demonstrated that peripheral blood mononuclear cells (MNC) of women with PCOS generate oxidative stress and inflammation following nutrient ingestion even in the absence of obesity (12–16). Indeed, there is increased reactive oxygen species (ROS) generation from MNC of lean women with PCOS in response to glucose and saturated fat ingestion (15, 17, 18). ROS-induced oxidative stress upregulates the gene transcription of heat shock protein-70 (HSP-70), a molecular chaperone that facilitates restorative renaturation of oxidized proteins to favor cell survival (19, 20). However, HSP-70 can also bind to toll-like receptor-2 (TLR-2), a pathogen pattern recognition receptor present on MNC and MNC-derived macrophages, thereby culminating in activator protein-1 (AP-1) activation (21). AP-1 is a proinflammatory transcription factor heterodimer predominantly encoded by the fos and jun gene families with the cJun protein subunit being the most potent transcriptional activator (22, 23). AP-1 regulates the transcription of matrix metalloproteinase-2 (MMP-2) that is involved in extracellular matrix remodeling within the blood vessel wall (23, 24). Extensive degradation of the extracellular matrix by MMP-2 produced by MNC-derived foamy macrophages and activated vascular smooth muscle cells within atherosclerotic plaque leads to plaque instability and susceptibility to rupture that ultimately contributes to vascular occlusion (25). Indeed, circulating levels of HSP-70 and MMP-2 are elevated in PCOS (26, 27). We have shown that glucose ingestion stimulates MNC-derived AP-1 activation and MMP-2 protein content and fails to suppress circulating MMP-2 levels in lean women with PCOS (10, 11). Saturated fat ingestion is also capable of stimulating AP-1 activation, as well as TLR-2 and MMP-2 expression in metabolically aberrant conditions (28–30). These findings implicate inflammation triggered by nutrient intake as a driver of atherogenesis in PCOS.
To expand our previous observations implicating nutrient-induced oxidative stress and inflammation in the development of a proatherogenic state in PCOS, we evaluated the effect of saturated fat ingestion on circulating HSP-70 levels and on the mRNA and protein content of TLR-2 from MNC in women with PCOS. We also examined this effect on MNC-derived AP-1 activation and MMP-2 protein content. We hypothesized that in response to saturated fat ingestion, circulating HSP-70 levels, TLR-2 mRNA and protein content, activated AP-1, and MMP-2 protein content are increased in women with PCOS compared with ovulatory controls of similar age and body mass index (BMI), and that these markers of proatherogenic inflammation are linked to adiposity, insulin sensitivity, levels of fasting lipids, and ovarian androgen secretion. Lean women with PCOS representing the authentic syndrome were evaluated separately from women with PCOS and obesity representing the superimposed effects of obesity on this disorder.
MATERIALS AND METHODS
Participants
Twenty women with PCOS (10 lean and 10 with obesity) 18–35 yr of age and 20 control subjects (10 lean and 10 with obesity) 19–40 yr of age with a similar BMI volunteered to participate in the study. Thirty-three of these subjects were involved in our previous work on lipid-induced inflammation and insulin resistance in PCOS via NF-κB-TNFα signaling (16). Lean subjects had a BMI between 18 and 25 kg/m2. Obesity was defined as a BMI between 30 and 40 kg/m2. The women with PCOS were diagnosed on the basis of oligo-amenorrhea and hyperandrogenemia after excluding nonclassic congenital adrenal hyperplasia, Cushing syndrome, hyperprolactinemia, and thyroid disease. The subjects with PCOS also exhibited polycystic ovaries on ultrasound. All control subjects had regular menses every 25 to 35 days with evidence of ovulation based on a luteal range serum progesterone level (>5 ng/mL). All control subjects had normal circulating androgen levels, no androgen excess manifestations and no signs of polycystic ovaries on ultrasound.
Diabetes and inflammatory illnesses were excluded in all subjects. However, five women with PCOS (1 lean and 4 with obesity) had impaired glucose tolerance based on WHO criteria, and four of these subjects (1 lean and 3 with obesity) also had metabolic syndrome based on modified ATP III criteria (31, 32). Data from these five volunteers were included in the analysis based on our previous studies (16, 18, 33), since there is no appreciable stimulatory effect on inflammation measurements from the transient, less pronounced hyperglycemia present in these individuals beyond that observed from saturated fat ingestion. All control subjects had normal glucose tolerance and none of them had metabolic syndrome. Fourteen women with PCOS (4 lean and 10 with obesity) and 13 control subjects (5 lean and 8 with obesity) had a family history of type 2 diabetes. None of the subjects smoked tobacco or used medications that would impact carbohydrate metabolism or immune function for at least 6 wk before entering the study. All subjects were weight stable within 5 pounds and were either sedentary or lightly active during the 6 mo before study participation. The extent of physical activity was similar among study groups. This research protocol involving human subject studies was reviewed and approved by the Indiana University Institutional Review Board before starting the study, and all subjects provided written informed consent. The clinical trial registration number is ClinicalTrials.gov NCT01489319 (registered 9 December 2011).
Study Design
A cream challenge test (CCT) was performed on all study subjects between days 5 and 8 after the onset of menses. In four amenorrheic subjects with PCOS (2 lean and 2 with obesity), menses was induced with a 5-day course of micronized progesterone. An oral glucose tolerance test (OGTT) was performed the very next day. All subjects fasted overnight for ∼12 h before undergoing both tests. All subjects were given a healthy diet consisting of 50% carbohydrate, 35% fat, and 15% protein for 3 consecutive days before the CCT and on the day preceding the OGTT once they completed the CCT. Body composition was assessed on the same day as the CCT. All subjects then underwent a human chorionic gonadotropin stimulation test (HCG-ST) over 4 days beginning on the day of the OGTT.
Cream Challenge Test
As adapted from Deopurkar et al. (34), all subjects consumed 100 mL of dairy cream (gourmet heavy whipping cream; Land O Lakes Inc., Arden Hills, MN) composed in volume of 70% saturated fat, 28% unsaturated fat, <2% protein, and 0% glucose. Blood sampling was performed in the fasting state and 2, 3, and 5 h after cream ingestion to quantify molecular markers of proatherogenic inflammation from MNC isolated as previously described (11). Serum and plasma were isolated from these same blood samples and stored at −80°C until assayed for HSP-70 and fasting lipids, respectively.
Oral Glucose Tolerance Test
A 75 g glucose beverage was administered to all subjects. Blood samples were drawn in the fasting state and at 30, 60, 90, 120, and 180 min after glucose ingestion to measure glucose and insulin. Plasma glucose levels were measured immediately, and insulin was measured later from plasma stored at −80°C. Insulin sensitivity was derived from the OGTT (ISOGTT) using the Matsuda index formula (35).
HCG Stimulation Test
As described by Koivunen et al. (36), an intramuscular injection of 5,000 IU of HCG (Pregnyl; Merck & Co., Whitehouse Station, NJ) was administered after obtaining a baseline blood sample at 8:00 AM after an overnight fast of ∼12 h. Fasting blood samples were subsequently obtained at 24, 48, and 96 h after the HCG injection. Serum was isolated from these samples and stored at −80°C until assayed for testosterone, androstenedione, and 17-hydroxyprogesterone (17-OHP). The trapezoidal rule was used to calculate the area under the curve (AUC) for androgens and 17-OHP (37).
Body Composition Assessment
Height without shoes was measured to the nearest 1.0 cm. Body weight was measured to the nearest 0.1 kg. Dual-energy X-ray absorptiometry was performed in all subjects to assess percent total body fat, percent truncal fat, and R1 central abdominal fat using a QDR 4500 Elite model scanner (Hologic Inc., Waltham, MA) as previously described (38, 39).
Real-Time PCR
An RNAeasy kit (Quiagen, Germantown, MD) was used to isolate total RNA from MNC that were previously stabilized in RNAlater (Sigma-Aldrich, St Louis, MO). Real-time PCR was used to quantify the mRNA content of TLR-2 as previously described (40). However, an ABI Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) was used for the current study. PRIMER EXPRESS software (PE Biosystems, Foster City, CA) was used to select the primer sequences for TLR-2 (NM_003264, forward primer 5′- GGCCAGCAAATTACCTGTGT-3′, reverse primer 5′- ATACCACAGGCCATGGAAAC-3′), and for the housekeeping gene ribosomal protein L13a (NM_000977.3, forward primer 5′- AACAAGTTGAAGTACCTGGCTTTC-3′, reverse primer 5′- TGGTTTTGTGGGGCAGCATA-3′). The rRNA signal for L13a was used to normalize against differences in RNA isolation and degradation and in reverse transcription and PCR efficiencies using the comparative cycle threshold method.
Western Blotting
Total protein concentrations from MNC lysates were determined using the Bradford protein assay (Bio-Rad Laboratories, Inc., Hercules, CA). The protein content of TLR-2, MMP-2, and actin from MNC was quantified by Western blotting (WB) as previously described (41) using a monoclonal antibody against TLR-2 (Novus Biologicals, LLC, Centennial, CO) at a dilution of 1:400, a polyclonal antibody against MMP-2 (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:400, and a monoclonal antibody against actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a dilution of 1:1,000. Densitometry was performed on the scanned films of Western blots using Carestream molecular imaging software version 5.0.2.30 (Carestream Health, Rochester, NY), and all values for TLR-2 and MMP-2 were corrected for loading using those obtained for actin.
Oligonucleotide-Based Enzyme-Linked Immunosorbent Assay
Nuclear extracts of DNA-binding protein from MNC were prepared using a method described by Andrews et al. (42). Nuclear extract total protein concentrations were determined using the Bradford protein assay (Bio-Rad Laboratories, Inc., Hercules, CA). The AP-1 c-Jun protein in nuclear extracts reflecting intranuclear activated AP-1 was quantified using an oligonucleotide-based enzyme-linked immunosorbent assay (ELISA; Active Motif, Carlsbad, CA) as previously described (11).
Serum and Plasma Measurements
Serum HSP-70 was measured by an ultrahigh sensitivity ELISA [Enzo Life Sciences, Inc., Farmingdale, NY; sensitivity, 7 pg/mL; intraassay coefficient of variation (CV) 9.6%; interassay CV 8.8%]. Plasma levels of glucose, insulin, total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol and serum levels of luteinizing hormone (LH), androstenedione, dehydroepiandrosterone-sulfate (DHEA-S), and 17-OHP were measured as previously described (33). Serum testosterone levels were measured by a radioimmunoassay (Siemens, Los Angeles, CA; sensitivity, 5 ng/dL, 0.1 ng/mL; intraassay CV 6.8%; interassay CV 11.2%) that demonstrates good correlation with commercial liquid chromatography tandem mass spectrometry (43). All samples from each subject were measured in duplicate in the same assay at the end of the study.
Statistics
The StatView software package (SAS Institute, Cary, NC) was used to perform the statistical analysis. All values were initially examined graphically for departure from normality. The presence or absence of normality was subsequently confirmed using the Shapiro–Wilk test. The natural logarithm transformation was applied to total cholesterol and LH before the analysis, since these values were not normally distributed, and the Shapiro–Wilk test was repeated on the log-transformed data to confirm a normal distribution. Treatment effects of saturated fat on HSP-70 were determined by calculating the absolute change from baseline for each participant. However, treatment effects on MNC-derived inflammation markers were determined by calculating the percent change from baseline due to intersubject variability. The incremental AUC (iAUC) for each inflammation marker was also calculated using the trapezoidal rule (37). Two-way ANOVA revealed no significant interaction between the main effects of weight class (obese vs. lean) and PCOS status (PCOS vs. control). Consequently, the data were further analyzed by one-way ANOVA for comparisons across groups (lean PCOS vs. lean control vs. obese PCOS vs. obese control), since our prior work suggests that obesity increases inflammation and reduces insulin sensitivity in PCOS (11, 13, 15, 16, 33). Post hoc analyses of significant ANOVA comparisons used the Tukey’s honestly significant difference test to identify the source of significance. Differences across groups in the response of inflammation markers over time during the CCT were analyzed using repeated-measures ANOVA followed by post hoc analyses. Pearson product moment correlation coefficients were calculated initially for correlation analyses. For the combined group analyses, variables were assessed by weight class (lean and obese) with or without PCOS and by PCOS status (lean and obese). Partial Pearson correlations with inflammation markers were subsequently calculated that separately adjusted for each measure of adiposity due to collinearity. Data are presented as means ± SE, and results with a two-tailed α-level of 0.05 were considered to be significant. However, Pearson correlation results required an α-level of 0.02 to be considered significant after correcting for multiple comparisons using the Benjamini–Hochberg approach to determine the false discovery rate (44).
RESULTS
Age, Body Composition, and Blood Pressure
Age, height, systolic and diastolic blood pressures were similar in all four groups. (Table 1). Weight, BMI, percent total body fat, percent truncal fat, and R1 fat were significantly (P < 0.05) higher in subjects with obesity compared with lean subjects whether or not they had PCOS. However, these measures of body composition were similar when women with PCOS were compared with control subjects of similar weight class. Percent truncal fat and R1 fat were significantly (P < 0.05) greater in lean women with PCOS compared with lean control subjects.
Table 1.
Age, body composition, endocrine, and metabolic parameters of subjects
| Lean Controls | Obese Controls | Lean PCOS | Obese PCOS | P Value | |
|---|---|---|---|---|---|
| n, subjects | 10 | 10 | 10 | 10 | |
| Age, yr | 30 ± 2 | 29 ± 2 | 26 ± 1 | 29 ± 1 | 0.41 |
| Height, cm | 164.5 ± 1.5 | 163.3 ± 2.5 | 163.5 ± 1.8 | 162.8 ± 3.0 | 0.96 |
| Body weight, kg | 62.6 ± 2.1 | 93.4 ± 3.6a | 60.0 ± 2.2b | 91.0 ± 4.1a,c | <0.0001 |
| Body mass index, kg/m2 | 23.1 ± 0.7 | 35.0 ± 0.8a | 22.4 ± 0.6b | 34.2 ± 0.9a,c | <0.0001 |
| Total body fat, % | 29.4 ± 1.5 | 42.3 ± 0.8a | 32.4 ± 2.2b | 44.1 ± 1.2a,c | <0.0001 |
| Truncal fat, % | 23.8 ± 1.9 | 41.7 ± 1.3a | 29.0 ± 2.2a,b | 42.6 ± 1.1a,c | <0.0001 |
| Central fat (R1), g | 748 ± 81 | 2,095 ± 129a | 982 ± 108a,b | 2,102 ± 108a,c | <0.0001 |
| Systolic blood pressure, mmHg | 109 ± 6 | 124 ± 3 | 112 ± 6 | 120 ± 5 | 0.18 |
| Diastolic blood pressure, mmHg | 69 ± 2 | 73 ± 2 | 67 ± 3 | 76 ± 2 | 0.09 |
| HSP-70, pg/mL | 201 ± 37 | 330 ± 41a | 349 ± 49a | 380 ± 52a | <0.05 |
| Fasting glucose, mg/dL | 89 ± 2 | 88 ± 2 | 86 ± 2 | 92 ± 2 | 0.13 |
| 2 h glucose, mg/dL | 95 ± 5 | 93 ± 9 | 108 ± 11 | 135 ± 8a,b,c | <0.007 |
| Fasting insulin, µU/mL | 5.3 ± 1.3 | 16.2 ± 2.6a | 8.6 ± 1.7b | 18.0 ± 3.9a,c | <0.004 |
| ISOGTT | 12.2 ± 2.2 | 4.7 ± 0.8a | 7.7 ± 1.3a | 3.3 ± 0.4a | <0.0004 |
| Total cholesterol, mg/dL# | 138 ± 5 | 149 ± 10 | 186 ± 14a,b | 188 ± 9a,b | <0.002 |
| Triglycerides, mg/dL | 57 ± 6 | 90 ± 11 | 78 ± 12 | 147 ± 22a,b,c | <0.0006 |
| HDL cholesterol, mg/dL | 53 ± 3 | 49 ± 3 | 52 ± 3 | 43 ± 1a,c | <0.03 |
| LDL cholesterol, mg/dL | 74 ± 6 | 82 ± 8 | 118 ± 13a,b | 113 ± 9a,b | <0.003 |
| LH, mIU/mL# | 6.4 ± 0.6 | 5.0 ± 0.7 | 13.1 ± 2.0a,b | 12.9 ± 2.0a,b | <0.0003 |
| Testosterone, ng/dL | 29.6 ± 6.0 | 21.4 ± 3.8 | 55.9 ± 6.3a,b | 69.7 ± 7.8a,b | <0.0001 |
| Androstendione, ng/mL | 1.9 ± 0.2 | 2.0 ± 0.2 | 3.9 ± 0.3a,b | 3.8 ± 0.2a,b | <0.0001 |
| DHEA-S, µg/dL | 212 ± 20 | 153 ± 21 | 260 ± 35b | 197 ± 22 | <0.04 |
| Testosterone AUC | 3,202 ± 487 | 3,607 ± 202 | 5,739 ± 635a,b | 7,626 ± 1,140a,b | <0.0003 |
| Androstendione AUC | 302 ± 25 | 337 ± 34 | 494 ± 22a,b | 572 ± 47a,b | <0.0001 |
| 17-OH-progesterone AUC | 10,508 ± 1211 | 10,605 ± 932 | 20,637 ± 2,801a,b | 21,669 ± 3,027a,b | <0.0005 |
Data are expressed as means ± SE. P value represents analysis of variance (ANOVA). Conversion factors to SI units: testosterone ×3.467 (nmol/L), androstenedione ×3.492 (nmol/L), DHEA-S ×0.002714 (µmol/L), glucose ×0.0551 (mmol/L), insulin ×7.175 (pmol/L). AUC, HCG-stimulated area under the curve; DHEA-S, dehydroepiandrosterone-sulphate; HDL, high-density lipoprotein; HSP-70, heat shock protein-70; ISOGTT, insulin sensitivity derived from an oral glucose tolerance test; LDL, low-density lipoprotein; LH, luteinizing hormone; PCOS, polycystic ovary syndrome.
#Data log-transformed for statistical analysis. Comparison across groups (one-way ANOVA and post hoc Tukey’s Honestly Significant Difference test).
aSignificantly different compared with lean controls (P < 0.05).
bSignificantly different compared with controls with obesity (P < 0.05).
cSignificantly different compared with lean women with PCOS (P < 0.05).
Proatherogenic Inflammation Markers in Serum and MNC
Basal serum HSP-70 levels were significantly (P < 0.05) higher in women with PCOS regardless of weight class and in control subjects with obesity compared with lean control subjects (Table 1).
In response to saturated fat ingestion, the change from baseline in serum HSP-70, TLR-2 protein and mRNA content, activated AP-1, and MMP-2 protein content decreased in lean control subjects and was significantly (P < 0.003) different compared with the increase observed in women with PCOS regardless of weight class and control subjects with obesity after 2 and 3 h (Figs. 1 and 2). In all four groups, the maximum response of all five lipid-stimulated proatherogenic inflammation markers was achieved at 2 h and sustained through 3 h.
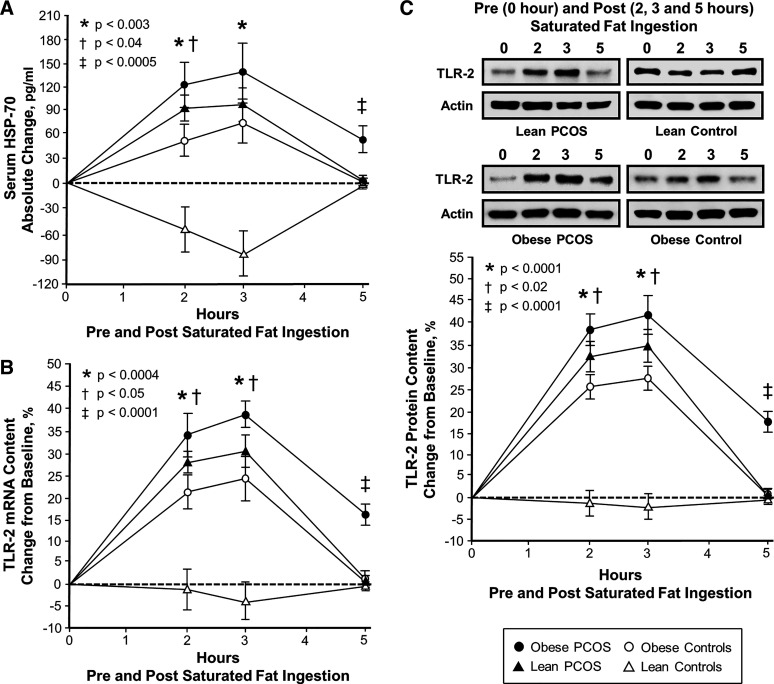
Figure 1.
Comparison of the 4 study groups (n = 10 subjects per group) of the change from baseline (%) in serum heat shock protein-70 (HSP-70; A) and mononuclear cell (MNC)-derived toll-like receptor-2 (TLR-2) mRNA content (B) and protein content (C) from blood samples collected while fasting and 2, 3, and 5 h after saturated fat ingestion. Representative Western blots (C) show the change in quantity of TLR-2 and actin in MNC homogenates in samples collected before and after saturated fat ingestion. The samples used to quantify TLR-2 and actin protein content by densitometry were run on the same gel. Data are presented as means ± SE. Differences across groups (repeated-measures ANOVA). *Response in women with polycystic ovary syndrome (PCOS) and obesity, lean women with PCOS and control subjects with obesity was significantly different compared with lean control subjects; P < 0.003 (A), P < 0.0004 (B), and P < 0.001 (C). †Response in women with PCOS and obesity was significantly different compared with control subjects with obesity; P < 0.04 (A), P < 0.05 (B), and P < 0.02 (C). ‡Residual response in women with PCOS and obesity was significantly different compared with the other 3 groups; P < 0.0005 (A) and P < 0.0001 (B and C).
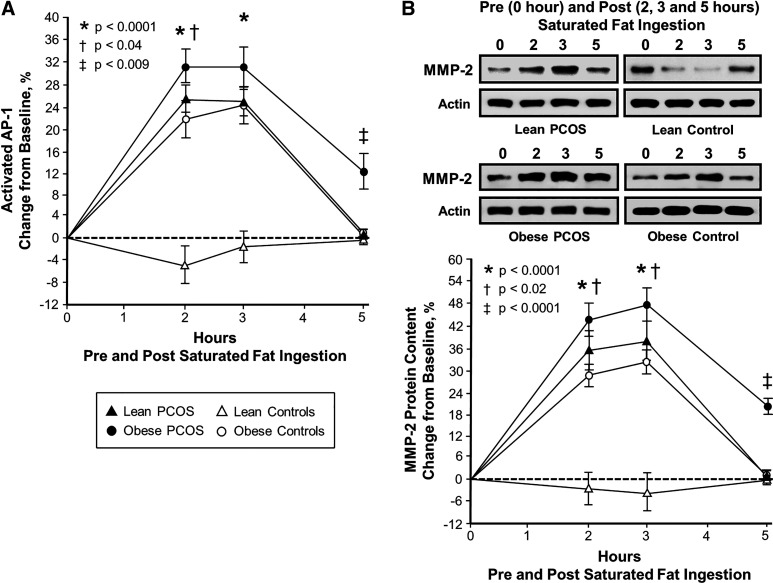
Figure 2.
Comparison of the four study groups (n = 10 subjects per group) of the change from baseline (%) in mononuclear cell (MNC)-derived activated activator protein-1 (AP-1; A) and matrix metalloproteinase-2 (MMP-2; B) from blood samples collected while fasting and 2, 3, and 5 h after saturated fat ingestion. Representative Western blots (B) show the change in quantity of MMP-2 and actin in MNC homogenates in samples collected before and after saturated fat ingestion. The samples used to quantify MMP-2 protein content by densitometry were run on the same gel. Data are presented as means ± SE. Differences across groups (repeated measures ANOVA). *Response in women with polycystic ovary syndrome (PCOS) and obesity, lean women with PCOS and control subjects with obesity was significantly different compared with lean control subjects; P < 0.0001 (A and B). †Response in women with PCOS and obesity was significantly different compared with control subjects with obesity; P < 0.04 (A) and P < 0.02 (B). ‡Residual response in women with PCOS and obesity was significantly different compared with the other 3 groups; P < 0.009 (A) and P < 0.0001 (B).
Compared with control subjects with obesity, women with PCOS and obesity exhibited significantly (P < 0.05) greater serum HSP-70 and activated AP-1 responses after 2 h and TLR-2 protein and mRNA content and MMP-2 protein content after 2 and 3 h. All five proatherogenic inflammation markers returned to baseline in both lean groups and in control subjects with obesity after 5 h. In contrast, women with PCOS and obesity exhibited significantly (P < 0.009) greater residual responses in all five inflammation markers compared with the other three groups after 5 h.
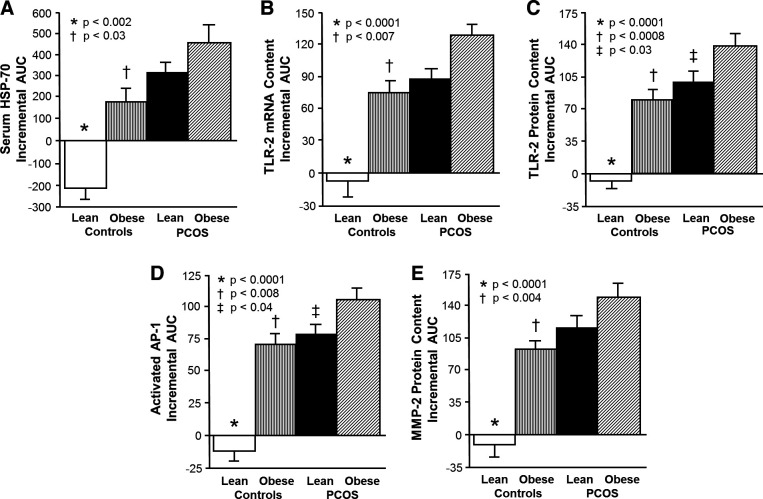
The iAUC for serum HSP-70, TLR-2 protein and mRNA content, activated AP-1, and MMP-2 protein content decreased in lean control subjects and was significantly (P < 0.002) different compared with the increase observed in women with PCOS regardless of weight class and in control subjects with obesity (Fig. 3). Women with PCOS and obesity exhibited a significantly (P < 0.04) greater iAUC for all five proatherogenic inflammation markers compared with control subjects with obesity and for TLR-2 protein content and activated AP-1 compared with lean women with PCOS.
Figure 3.
Comparison of the 4 study groups (n = 10 subjects per group) of the incremental area under the curve (iAUC) in response to saturated fat ingestion for serum heat shock protein-70 (HSP-70; A) and mononuclear cell (MNC)-derived toll-like receptor-2 (TLR-2) mRNA content (B) and protein content (C) as well as MNC-derived activated activator protein-1 (AP-1; D) and matrix metalloproteinase-2 (MMP-2) protein content (E). Data are presented as means ± SE. Comparison across groups (one-way ANOVA and post hoc Tukey’s Honestly Significant Difference test). *The iAUC in lean control subjects was significantly different compared with the other 3 groups; P < 0.002 (A) and P < 0.0001 (B and C). †The iAUC in control subjects with obesity was significantly different compared with women with polycystic ovary syndrome (PCOS) and obesity; P < 0.03 (A), P < 0.007 (B), P < 0.0008 (C), P < 0.008 (D), and P < 0.004 (E). ‡The iAUC in lean women with PCOS was significantly different compared with women with PCOS and obesity; P <0.03 (C) and P < 0.04 (D).
Insulin Sensitivity and Fasting Lipids
ISOGTT was significantly lower (P < 0.05) in subjects with obesity whether or not they had PCOS compared with lean control subjects and in lean women with PCOS compared with lean control subjects (Table 1). Plasma cholesterol and LDL were significantly (P < 0.05) higher in women with PCOS compared with control subjects regardless of body composition status. Plasma triglycerides was significantly (P < 0.05) higher in women with PCOS and obesity compared with those of the other three groups. Plasma HDL was significantly (P < 0.04) lower in women with PCOS and obesity compared with lean subjects whether or not they had PCOS. Based on modified ATP III criteria (32), the mean HDL level was clinically decreased in both obese groups, and the mean LDL level was clinically elevated in both PCOS groups.
Basal Hormone Levels and HCG-Stimulated Androgen and 17-OHP Responses
Serum levels of LH, testosterone, and androstenedione were significantly (P < 0.05) higher in women with PCOS compared with control subjects regardless of body composition status. Serum DHEA-S levels were significantly (P < 0.05) higher in lean women with PCOS compared with control subjects with obesity, although mean DHEA-S levels in all four groups were clinically normal based on age-related assay cutoffs (Table 1).
The AUC for the HCG-stimulated responses of testosterone, androstenedione, and 17-OHP were significantly (P < 0.05) higher in women with PCOS compared with control subjects regardless of weight class.
Correlations
Adiposity versus proatherogenic inflammation.
BMI, percent total body fat, percent truncal fat, and R1 fat were positively correlated with basal serum HSP-70 levels in control subjects (Table 2). All body composition measurements were also positively correlated with the iAUC for TLR-2 mRNA content, activated AP-1, and MMP-2 protein content for the combined groups and separately in control subjects. Percent truncal fat and R1 fat were positively correlated with the iAUC for serum HSP-70 and TLR-2 protein content for the combined groups and separately in control subjects.
Table 2.
Pearson correlations of proatherogenic inflammation markers iAUC during the cream challenge test with measures of adiposity and insulin sensitivity for the combined groups and in control subjects
| Basal Serum HSP-70 | Serum HSP-70 iAUC | TLR-2 mRNA Content iAUC | TLR-2 Protein Content iAUC | Activated AP-1 iAUC | MMP-2 Protein Content iAUC | |
|---|---|---|---|---|---|---|
| Combined groups | ||||||
| BMI, kg/m2 | ||||||
| r | 0.156 | 0.346 | 0.387 | 0.389 | 0.447 | 0.404 |
| P | 0.336 | 0.031 | 0.014* | 0.011* | 0.004* | 0.009* |
| Total body fat, % | ||||||
| r | 0.366 | 0.430 | 0.392 | 0.457 | 0.549 | 0.535 |
| P | 0.028 | 0.006* | 0.012* | 0.003* | 0.0002* | 0.0004* |
| Truncal fat, % | ||||||
| r | 0.164 | 0.430 | 0.389 | 0.544 | 0.513 | 0.505 |
| P | 0.312 | 0.006* | 0.013* | 0.0003* | 0.0007* | 0.001* |
| Central fat (R1), g | ||||||
| r | 0.164 | 0.430 | 0.389 | 0.544 | 0.513 | 0.505 |
| P | 0.312 | 0.006* | 0.013* | 0.0003* | 0.0007* | 0.001* |
| ISOGTT | ||||||
| r | −0.548 | −0.413 | −0.433 | −0.534 | −0.596 | −0.520 |
| P | 0.0004* | 0.008* | 0.006* | 0.0004 | 0.0001* | 0.0007* |
| Controls | ||||||
| BMI, kg/m2 | ||||||
| r | 0.568 | 0.511 | 0.604 | 0.767 | 0.784 | 0.729 |
| P | 0.017* | 0.006* | 0.005* | 0.0001* | 0.0001* | 0.0003* |
| Total body fat, % | ||||||
| r | 0.598 | 0.589 | 0.641 | 0.715 | 0.801 | 0.631 |
| P | 0.011* | 0.006* | 0.004* | 0.0004* | 0.0001* | 0.003* |
| Truncal fat, % | ||||||
| r | 0.572 | 0.668 | 0.632 | 0.758 | 0.779 | 0.728 |
| P | 0.013* | 0.001* | 0.005* | 0.0001* | 0.0001* | 0.0003* |
| Central fat (R1), g | ||||||
| r | 0.572 | 0.668 | 0.632 | 0.758 | 0.779 | 0.728 |
| P | 0.013* | 0.001* | 0.005* | 0.0001* | 0.0001* | 0.0003* |
| ISOGTT | ||||||
| r | −0.904 | −0.529 | −0.511 | −0.688 | −0.720 | −0.695 |
| P | 0.0008* | 0.029 | 0.036 | 0.0008* | 0.0003* | 0.001* |
>AP-1, activator protein-1; BMI, body mass index; HDL, high-density lipoprotein; HSP-70, heat shock protein-70; iAUC, incremental area under the curve; ISOGTT, insulin sensitivity index derived from an oral glucose tolerance test; LDL low-density lipoprotein; MMP-2, matrix metalloprotease-2; mRNA, messenger ribonucleic acid; TLR-2, toll-like receptor-2.
Correlation analyses (Pearson product moment correlation coefficient calculations).*P < 0.02.
In women with PCOS, the iAUC for activated AP-1 was positively correlated with BMI (r = 0.59, P < 0.02), percent total body fat (r = 0.56, P < 0.02), and R1 fat (r = 0.59, P < 0.02), and the iAUC for MMP-2 protein content was positively correlated with BMI (r = 0.61, P < 0.01) and R1 fat (r = 0.58, P < 0.02).
Insulin sensitivity versus proatherogenic inflammation.
ISOGTT was negatively correlated with BMI (r = −0.49, P < 0.002), percent total body fat (r = 0.52, P < 0.0007), percent truncal fat (r = −0.60, P < 0.0001), and R1 fat (r = −0.57, P < 0.0002) for the combined groups. ISOGTT was also negatively correlated with basal serum HSP-70 and the iAUC for serum HSP-70, TLR-2 protein and mRNA content, activated AP-1, and MMP-2 protein content for the combined groups, with basal serum HSP-70 and the iAUC for TLR-2 mRNA content, activated AP-1, and MMP-2 protein content in control subjects (Table 2), and with the iAUC for serum HSP-70 (r = 0.59, P < 0.02) in women with PCOS.
Fasting lipids versus proatherogenic inflammation.
For the combined groups, plasma total cholesterol and triglycerides were positively correlated with basal serum HSP-70 and the iAUC for TLR-2 protein and mRNA content, activated AP-1, and MMP-2 protein content (Table 3). Plasma HDL was negatively correlated with the iAUC for activated AP-1, and plasma LDL was positively correlated with basal serum HSP-70 and the iAUC for activated AP-1 and MMP-2 protein content.
Table 3.
Pearson correlations of proatherogenic inflammation markers iAUC during the cream challenge test with circulating lipids for the combined groups
| Basal Serum HSP-70 | Serum HSP-70 iAUC | TLR-2 mRNA Content iAUC | TLR-2 Protein Content iAUC | Activated AP-1 iAUC | MMP-2 Protein Content iAUC | |
|---|---|---|---|---|---|---|
| Total cholesterol, mg/dL | ||||||
| r | 0.499 | 0.329 | 0.505 | 0.425 | 0.498 | 0.430 |
| P | 0.001* | 0.041 | 0.0009* | 0.006* | 0.001* | 0.006* |
| Triglycerides, mg/dL | ||||||
| r | 0.589 | 0.405 | 0.524 | 0.551 | 0.520 | 0.457 |
| P | 0.0001* | 0.012* | 0.0005* | 0.0002* | 0.001* | 0.003* |
| HDL cholesterol, mg/dL | ||||||
| r | −0.079 | −0.333 | −0.338 | −0.149 | −0.391 | −0.228 |
| P | 0.627 | 0.044 | 0.036 | 0.358 | 0.017* | 0.158 |
| LDL cholesterol, mg/dL | ||||||
| r | 0.382 | 0.347 | 0.396 | 0.355 | 0.448 | 0.400 |
| P | 0.019* | 0.035 | 0.011* | 0.029 | 0.004* | 0.012* |
AP-1, activator protein-1; HDL, high-density lipoprotein; HSP-70, heat shock protein-70; iAUC, incremental area under the curve; LDL, low-density lipoprotein; MMP-2, matric metalloprotease-2; mRNA, messenger ribonucleic acid; TLR-2, toll-like receptor-2.
Correlation analyses (Pearson product moment correlation coefficient calculations). *P < 0.02.
In women with PCOS, plasma triglycerides were positively correlated with basal serum HSP-70 (r = 0.72, P < 0.002) and the iAUC for serum HSP-70 (r = 0.64, P < 0.007) and TLR-2 protein and mRNA content (r = 0.70, P < 0.002; r = 0.63, P < 0.006). Plasma HDL was negatively correlated with the iAUC for TLR-2 mRNA content (r = 0.58, P < 0.02).
LH and androgens versus proatherogenic inflammation.
For the combined groups, basal LH levels were positively correlated with the iAUC for TLR-2 protein content, activated AP-1, and MMP-2 protein content, and basal testosterone levels and the HCG-stimulated AUC for testosterone were positively correlated with the iAUC for serum HSP-70, TLR-2 protein and mRNA content, and MMP-2 protein content (Table 4). Basal androstenedione levels and the HCG-stimulated AUC for androstenedione and 17-OHP were positively correlated with the entire panel of proatherogenic inflammation markers.
Table 4.
Pearson correlations of proatherogenic inflammation markers iAUC during the cream challenge test with circulating LH and androgens for the combined groups
| Basal Serum HSP-70 | Serum HSP-70 iAUC | TLR-2 mRNA Content iAUC | TLR-2 Protein Content iAUC | Activated AP-1 iAUC | MMP-2 Protein Content iAUC | |
|---|---|---|---|---|---|---|
| LH, IU/mL | ||||||
| r | 0.155 | 0.171 | 0.357 | 0.460 | 0.372 | 0.382 |
| P | 0.338 | 0.291 | 0.024 | 0.003* | 0.018* | 0.005* |
| Testosterone, ng/dL | ||||||
| r | 0.018 | 0.476 | 0.479 | 0.377 | 0.353 | 0.445 |
| P | 0.912 | 0.002* | 0.002* | 0.016* | 0.025 | 0.005* |
| Androstenedione, ng/mL | ||||||
| r | 0.471 | 0.475 | 0.519 | 0.632 | 0.549 | 0.568 |
| P | 0.003* | 0.002* | 0.0006* | 0.0001* | 0.0002* | 0.0003* |
| DHEA-S, µg/dL | ||||||
| r | 0.011 | 0.217 | 0.163 | 0.008 | 0.007 | 0.076 |
| P | 0.945 | 0.179 | 0.314 | 0.961 | 0.964 | 0.640 |
| Testosterone AUC | ||||||
| r | 0.077 | 0.496 | 0.486 | 0.490 | 0.421 | 0.490 |
| P | 0.638 | 0.001* | 0.002* | 0.002* | 0.008* | 0.002* |
| Androstenedione AUC | ||||||
| r | 0.468 | 0.483 | 0.529 | 0.541 | 0.429 | 0.562 |
| P | 0.003* | 0.002* | 0.0004* | 0.0003* | 0.006* | 0.0002* |
| 17-OH-progesterone AUC | ||||||
| r | 0.474 | 0.450 | 0.509 | 0.388 | 0.538 | 0.446 |
| P | 0.003* | 0.001* | 0.0008* | 0.013* | 0.0005* | 0.003* |
AP-1, activator protein-1; AUC, HCG-stimulated area under the curve; DHEA-S, dehydroepiandrosterone-sulphate; HSP-70, heat shock protein-70; iAUC, incremental area under the curve; LH, luteinized hormone; MMP-2, matric metalloprotease-2; mRNA, messenger ribonucleic acid; TLR-2, toll-like receptor-2.
Correlation analyses (Pearson product moment correlation coefficient calculations). *P < 0.02.
In women with PCOS, basal testosterone levels was positively correlated with the iAUC for serum HSP-70 (r = 0.57, P < 0.02) and activated AP-1 (r = 0.68, P < 0.004). The iAUC for TLR-2 mRNA content was positively correlated with basal levels of androstenedione (r = 0.60, P < 0.01) and DHEA-S (r = 0.57, P < 0.02) and the AUC for androstenedione (r = 0.57, P < 0.02) and 17-OHP (r = 0.60, P < 0.02).
None of the proatherogenic inflammation markers were correlated with fasting lipids and hormones in control subjects (data not shown). Likewise, correlations of proatherogenic inflammation markers with measures of adiposity, insulin sensitivity, fasting lipids, and hormones in women with PCOS that were not significant are not reported. The relationships of proatherogenic inflammation markers with insulin sensitivity, fasting lipids, and hormones were maintained after adjusting for adiposity (data not shown).
DISCUSSION
Our data clearly show for the first time that in PCOS, saturated fat ingestion is capable of stimulating a proatherogenic inflammatory response even in the absence of obesity. However, this inflammatory response is more pronounced with the combination of PCOS and obesity compared with obesity alone. In response to saturated fat ingestion, lean women with PCOS exhibit increases in serum HSP-70, TLR-2 gene expression, AP-1 activation, and MMP-2 protein content compared with lean control subjects. Women with PCOS and obesity also exhibit lipid-induced increases in these proatherogenic inflammation markers and higher triglycerides levels compared with control subjects with obesity, along with higher levels of fasting insulin, total cholesterol and triglycerides, and lower HDL levels compared with both lean groups. The inverse relationship between insulin sensitivity and lipid-stimulated serum HSP-70, TLR-2 gene expression, AP-1 activation, and MMP-2 protein content provides added support for this concept. In addition, lean women with PCOS have a greater amount of abdominal fat compared with lean control subjects, and multiple proatherogenic inflammation markers are directly associated with abdominal adiposity and basal and HCG-stimulated androgen secretion. These findings implicate abdominal adiposity and hyperandrogenism as distinct risk factors in PCOS for promoting proatherogenic inflammation that may predispose to a cardiovascular event.
Lean women with PCOS may be at increased risk for accelerated atherogenesis. The subjects in this group have insulin resistance, a strong predictor of atherosclerotic cardiovascular disease (45), along with higher basal HSP-70 levels and greater lipid-induced responses in serum HSP-70, TLR-2 gene expression, AP-1 activation, and MMP-2 protein content compared with lean control subjects. In fact, all of these proatherogenic inflammation markers are inversely associated with insulin sensitivity. In corroboration, our previous studies showed that lean women with PCOS exhibit similar increases in AP-1 activation and MMP-2 protein content in addition to failed suppression of circulating MMP-2 levels in response to glucose ingestion (10, 11). This heightened proatherogenic inflammatory response has been shown to contribute to atherosclerotic plaque instability and predispose to plaque rupture (25). Previous studies have shown that lean women with PCOS also have elevated circulating levels of C-reactive protein (CRP) which is another well-established predictor of atherosclerotic cardiovascular disease (11, 16, 46). Although CRP elevations in lean women with PCOS tend to be milder (1–3 mg/L) than what is observed in obesity (>3 mg/L), they are nonetheless associated with an intermediate risk of incurring a cardiovascular event (47). We have previously reported glucose and/or lipid-induced increases in MNC-derived nuclear factor κB (NF-κB) activation and circulating CRP along with elevated circulating monocyte chemotactic protein-1 (MCP-1) levels in lean women with PCOS (10, 13, 16). NF-κB is a proinflammatory transcription factor that regulates the gene transcription of CRP and MCP-1, both of which participate in atherogenesis. Whereas MCP-1 facilitates MNC migration into the vascular interstitium, CRP promotes the subsequent uptake of lipids into MNC-derived foamy macrophages within atherosclerotic plaques (48, 49). In contrast, lean controls exhibit suppression of serum HSP-70, TLR-2 gene expression, AP-1 activation, and MMP-2 protein content, suggesting that this may be the normal in vivo response to saturated fat ingestion to preserve blood vessel integrity. This is corroborated by our previous studies showing a similar response pattern in glucose-stimulated activation of MNC-derived AP-1 and NF-κB and MMP-2 protein content in lean healthy young women (11, 13, 16). Thus, women with PCOS have a proinflammatory risk profile for atherogenesis incited by saturated fat ingestion that is independent of obesity.
Women with PCOS and obesity may be at even greater risk for accelerated atherogenesis. This group exhibits greater insulin resistance and lipid-induced increases in serum HSP-70, TLR-2 gene expression, AP-1 activation, and MMP-2 protein content compared with control subjects with obesity and lean control subjects. We have previously reported marked elevations in both circulating interleukin-6 (IL-6) and CRP (>3 mg/L) in women with PCOS and obesity beyond those observed in lean women with PCOS and are most likely of adipose tissue origin given their similarity to those of women with obesity who do not have PCOS (11, 16, 50). In fact, IL-6 stimulates CRP synthesis in the liver and in adipose tissue of individuals with obesity (51). We have also previously demonstrated circulating and molecular alterations that promote vascular thrombosis in women with PCOS and obesity such as glucose-stimulated increases in the protein content of early growth response-1 (EGR-1) and tissue factor (TF), as well as elevated circulating plasminogen activator inhibitor (PAI-1) levels (10, 11). EGR-1 is the transcription factor that regulates the gene expression of TF, the receptor for coagulation factor VII that induces thrombin generation to promote fibrin formation and platelet activation (52). Exposure of TF to the circulating blood following atherosclerotic plaque rupture triggers thrombosis culminating in vascular occlusion. This process is perpetuated by PAI-1 that inhibits fibrinolysis, thereby retarding thrombus resolution (53). Thus, the combination of PCOS and obesity may lead to a more profound proinflammatory milieu that confers a significant risk for both atherogenesis and thrombosis compared with PCOS or obesity alone.
In PCOS, proatherogenic inflammation induced by saturated fat ingestion is linked to adiposity. Lipid-stimulated proatherogenic inflammation markers are directly associated with measures of adiposity for the combined groups and in women with PCOS. The iAUC for lipid-induced AP-1 activation in particular is directly associated with BMI, total body fat, and abdominal fat in women with PCOS. Indeed, migration of MNC into the stromal-vascular compartment of excess adipose tissue in response to hypoxia-related cell death incites oxidative stress during phagocytosis and a subsequent inflammatory response by MNC-derived macrophages serving as a proinflammatory paracrine stimulus to adipocytes (54). In fact, approximately half of the IL-6 emanating from the expanded adipose mass of individuals with obesity is produced by MNC-derived macrophages (53). Lean women with PCOS also have excess abdominal fat as described in previous studies (38, 55, 56). Thus, the prooxidant, proinflammatory environment in excess adipose tissue of women with PCOS, especially in the abdominal region, may be an additional driver of circulating and molecular mediators of atherogenesis. These data are striking because they implicate abdominal adiposity as a contributor to early and possibly premature onset of atherogenesis.
In PCOS, proatherogenic inflammation induced by saturated fat ingestion is linked to dyslipidemia. In the current study, total cholesterol and LDL are directly associated with the iAUC for basal serum HSP-70, activated AP-1, and MMP-2 protein content for the combined groups. Triglycerides are directly associated with basal serum HSP-70 and the iAUC for TLR-2 protein and mRNA content and MMP-2 protein content in the combined groups and in women with PCOS. HDL is inversely associated with the iAUC for activated AP-1 for the combined groups and with iAUC for TLR-2 mRNA content in women with PCOS. Both NF-κB and AP-1 from MNC and MNC-derived macrophages are capable of regulating the production of cytokines that promote dyslipidemia in response to saturated fat ingestion (16, 28). Moreover, IL-6 and TNFα promote adipose tissue lipolysis, fatty acid transport to the liver, and de novo hepatic fatty acid synthesis to provide substrates for hepatic triglyceride and triglyceride-rich VLDL production (57). The rise in VLDL concentration drives the transfer of triglycerides from VLDL to LDL, the latter of which is hydrolyzed by hepatic lipase to small dense LDL. The highly atherogenic small dense LDL molecule is easily oxidized and taken up by foamy macrophages present in atherosclerotic plaques (58). Cytokines can also reduce the amount of HDL in the bloodstream and alter the structure of HDL to decrease HDL-mediated cholesterol efflux from foamy macrophages (58, 60). Indeed, cholesterol efflux capacity is reduced in women with PCOS (61). In the current study, cholesterol and LDL are higher in women with PCOS regardless of weight class, whereas triglycerides are higher and HDL is lower in women with PCOS and obesity. Thus, lipid-induced inflammation may serve as a potent inciter of dyslipidemia in PCOS especially when obesity is present, such that the dual effects of inflammation and lipoprotein abnormalities may culminate in accelerated atherogenesis.
In PCOS, proatherogenic inflammation induced by saturated fat ingestion is linked to hyperandrogenism. In the current study, basal LH and androgen levels along with the HCG-stimulated androgen secretion are directly associated with the cadre of lipid-stimulated inflammation markers for the combined groups and in women with PCOS. This corroborates similar associations between androgens and glucose- or lipid-stimulated measures of inflammation in our past reports (11, 13, 15, 16, 18, 33). Although the link with LH suggests a central impact of androgen secretion on proatherogenic inflammation, local androgen effects have been reported. Androgen exposure in vitro promotes MNC adhesion to vascular endothelium and LDL oxidation by MNC-derived macrophages (62, 63). Induction of hyperandrogenism in cholesterol-fed female cynomolgus monkeys leads to the development of atherosclerosis (64). Most importantly, induction of hyperandrogenism in normal reproductive-age women activates MNC and increases MNC sensitivity to glucose ingestion (65). Thus, hyperandrogenism in PCOS may potentiate the action of proatherogenic mediators of inflammation from lipid-activated MNC, thereby raising the risk of atherogenesis.
This study extends our previous findings on nutrient-induced inflammation in PCOS showing that besides glucose ingestion (10, 11), intake of saturated fat also contributes to atherogenesis by triggering a proinflammatory mechanism involving HSP-70 signaling through TLR-2 not previously reported in the disorder. Lean women with PCOS exhibit increases in baseline serum HSP-70 and lipid-stimulated serum HSP-70, TLR-2 gene expression, activated AP-1, and MMP-2 protein content that is independent of obesity. Women with PCOS and obesity exhibit similar findings that are more pronounced than in simple obesity, in addition to a greater degree of insulin resistance and more profound dyslipidemia. Thus, both PCOS and obesity contribute to a proatherogenic environment. The association of proatherogenic inflammation markers with abdominal adiposity and basal and HCG-stimulated androgen secretion also suggests that in PCOS, excess abdominal fat and hyperandrogenism may serve as distinct perpetuators of atherogenesis.
GRANTS
This research was supported by grant R01 DK107605 to F.G. from the National Institutes of Health; the Indiana Clinical and Translational Sciences Institute Clinical Research Center which is funded in part by grant UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award; and the Indiana University Center for Diabetes and Metabolic Diseases funded by grant P30 DK097512 from the National Institutes of Health.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.G. conceived and designed research; F.G., O.A.A., J.X., and A.J.A. performed experiments; F.G. analyzed data; F.G. and R.V.C. interpreted results of experiments; F.G. prepared figures; F.G. drafted manuscript; F.G., R.V.C., O.A.A., J.X., and A.J.A. edited and revised manuscript; F.G., R.V.C., O.A.A., J.X., and A.J.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the nursing staff of the Indiana Clinical and Translational Sciences Institute Clinical Research Center for supporting the implementation of the study and assisting with data collection. We gratefully acknowledge Merck Sharp & Dohme for generously donating the Pregnyl used in this study.
This paper was presented at the 70th meeting of the American Society for Reproductive Medicine, Honolulu, HI, on October 18–22, 2014.
REFERENCES
- 1.March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 25: 544–551, 2010. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JSE, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 97: 28–38.e25, 2012. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 18: 618–637, 2012. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 4.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33: 981–1030, 2012. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 111: 607–613, 2001. doi: 10.1016/S0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 6.Lechner K, McKenzie AL, Kränkel N, Von Schacky C, Worm N, Nixdorff U, Lechner B, Scherr J, Weingärtner O, Krauss RM. High-risk atherosclerosis and metabolic phenotype: the roles of ectopic adiposity, atherogenic dyslipidemia, and inflammation. Metab Syndr Relat Disord 18: 176–185, 2020. doi: 10.1089/met.2019.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, Baron AD. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 103: 1410–1415, 2001. doi: 10.1161/01.CIR.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 8.Talbott EO, Zborowski JV, Rager JR, Boudreaux MY, Edmundowicz DA, Guzick DS. Evidence for an association between metabolic cardiovascular syndrome and coronary and aortic calcification among women with polycystic ovary syndrome. J Clin Endocrinol Metab 89: 5454–5461, 2004. doi: 10.1210/jc.2003-032237. [DOI] [PubMed] [Google Scholar]
- 9.Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JMC, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 86: 2453–2455, 2001. doi: 10.1210/jcem.86.6.7580. [DOI] [PubMed] [Google Scholar]
- 10.González F, Rote NS, Minium J, Kirwan JP. Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metabolism 58: 954–962, 2009. doi: 10.1016/j.metabol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González F, Rote NS, Kirwan JP, Minium J. Glucose ingestion stimulates atherothrombotic inflammation in polycystic ovary syndrome. Am J Physiol Endocrinol Metab 304: E375–E383, 2013. doi: 10.1152/ajpendo.00491.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab 91: 336–340, 2006. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- 13.González F, Rote NS, Minium J, Kirwan JP. Increased activation of nuclear factor κB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab 91: 1508–1512, 2006. doi: 10.1210/jc.2005-2327. [DOI] [PubMed] [Google Scholar]
- 14.González F, Sia CL, Shepard MK, Rote NS, Minium J. Inflammation in response to glucose ingestion is independent of excess abdominal adiposity in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab 97: 4071–4079, 2012. doi: 10.1210/jc.2012-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González F, Sia CL, Shepard MK, Rote NS, Minium J. The altered mononuclear cell-derived cytokine response to glucose ingestion is not regulated by excess adiposity in polycystic ovary syndrome. J Clin Endocrinol Metab 99: E2244–E2251, 2014. doi: 10.1210/jc.2014-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González F, Considine RV, Abdelhadi OA, Acton AJ. Inflammation triggered by saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab 105: e2152–e2167, 2020. doi: 10.1210/clinem/dgaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González F, Sia CL, Shepard MK, Rote NS, Minium J. Hyperglycemia-induced oxidative stress is independent of excess abdominal adiposity in normal-weight women with polycystic ovary syndrome. Hum Reprod 27: 3560–3568, 2012. doi: 10.1093/humrep/des320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González F, Considine RV, Abdelhadi OA, Acton AJ. Oxidative stress in response to saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab 104: 5360–5370, 2019. doi: 10.1210/jc.2019-00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall L, Martinus RD. Hyperglycaemia and oxidative stress upregulate HSP60 & HSP70 expression in HeLa cells. SpringerPlus 2: 431, 2013. doi: 10.1186/2193-1801-2-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Zvi A, De Los Rios P, Dietler G, Goloubinoff P. Active solubilization and refolding of stable protein aggregates by cooperative unfolding action of individual hsp70 chaperones. J Biol Chem 279: 37298–37303, 2004. doi: 10.1074/jbc.M405627200. [DOI] [PubMed] [Google Scholar]
- 21.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277: 15028–15034, 2002. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 22.Ryseck RP, Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene 6: 533–542, 1991. [PubMed] [Google Scholar]
- 23.Radler-Pohl A, Gebel S, Sachsenmaier C, König H, Krämer M, Oehler T, Streile M, Ponta H, Rapp U, Rahmsdorf HJ, Cato ACB, Angel P, Herrlich P. The activation and activity control of AP-1 (Fos/Jun). Ann N Y Acad Sci 684: 127–148, 1993. doi: 10.1111/j.1749-6632.1993.tb32277.x. [DOI] [PubMed] [Google Scholar]
- 24.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 90: 251–262, 2002. doi: 10.1161/res.90.3.251. [DOI] [PubMed] [Google Scholar]
- 25.Heo SH, Cho CH, Kim HO, Jo YH, Yoon KS, Lee JH, Park JC, Park KC, Ahn TB, Chung KC, Yoon SS, Chang DI. Plaque rupture is a determinant of vascular events in carotid artery atherosclerotic disease: involvement of matrix metalloproteinases 2 and 9. J Clin Neurol 7: 69–76, 2011. doi: 10.3988/jcn.2011.7.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao H, Meng J, Xu M, Zhang S, Ghose B, Liu J, Yao P, Yan H, Wang D, Liu L. Serum heat shock protein 70 concentration in relation to polycystic ovary syndrome in a non-obese Chinese population. PLoS One 8: e67727, 2013. doi: 10.1371/journal.pone.0067727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewandowski KC, Komorowski J, O’Callaghan CJ, Tan BK, Chen J, Prelevic GM, Randeva HS. Increased circulating levels of matrix metalloproteinase-2 and -9 in women with the polycystic ovary syndrome. J Clin Endocrinol Metab 91: 1173–1177, 2006. doi: 10.1210/jc.2005-0648. [DOI] [PubMed] [Google Scholar]
- 28.Hu X, Zhou J, Song SS, Kong W, Shi YC, Chen LL, Zeng TS. TLR4/AP-1-targeted anti-inflammatory intervention attenuates insulin sensitivity and liver steatosis. Mediators Inflamm 2020: 2960517, 2020. doi: 10.1155/2020/2960517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snodgrass RG, Huang S, Choi IW, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J Immunol 191: 4337–4347, 2013. doi: 10.4049/jimmunol.1300298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grewal N, Thornton GM, Behzad H, Sharma A, Lu A, Zhang P, Reid WD, Granville Alex Scott DJ. Accumulation of oxidized LDL in the tendon tissues of C57BL/6 or apolipoprotein E knock-out mice that consume a high fat diet: potential impact on tendon health. PLoS One 9: e114214, 2014. [Erratum in PLoS One 10: e0123280, 2015]. doi: 10.1371/journal.pone.0114214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modan M, Harris MI, Halkin H. Evaluation of WHO and NDDG criteria for impaired glucose tolerance. Results from two national samples. Diabetes 38: 1630–1635, 1989. doi: 10.2337/diab.38.12.1630. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112: 2735–2752, 2005. [Erratum in Circulation 112: e297, e298, 2005]. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 33.González F, Considine RV, Abdelhadi OA, Acton AJ. Saturated fat ingestion promotes lipopolysaccharide-mediated inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab 104: 934–946, 2019. doi: 10.1210/jc.2018-01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deopurkar R, Ghanim H, Friedman J, Abuaysheh S, Sia CL, Mohanty P, Viswanathan P, Chaudhuri A, Dandona P. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 33: 991–997, 2010. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 36.Koivunen RM, Morin-Papunen LC, Ruokonen A, Tapanainen JS, Martikainen HK. Ovarian steroidogenic response to human chorionic gonadotrophin in obese women with polycystic ovary syndrome: effect of metformin. Hum Reprod 16: 2546–2551, 2001. doi: 10.1093/humrep/16.12.2546. [DOI] [PubMed] [Google Scholar]
- 37.Yeh ST. Using a trapezoidal rule for the area under a curve calculation – SAS advanced tutorial. Proceedings of the 27th Annual Conference of SAS Users Group International. Orlando, FL, April 14–17, 2002, Abstract 229. [Google Scholar]
- 38.González F, Minium J, Rote NS, Kirwan JP. Hyperglycemia alters tumor necrosis factor-α release from mononuclear cells in women with polycystic ovary syndrome. J Clin Endocrinol Metab 90: 5336–5342, 2005. doi: 10.1210/jc.2005-0694. [DOI] [PubMed] [Google Scholar]
- 39.Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, Di Fede G, Rini G. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab 92: 2500–2505, 2007. doi: 10.1210/jc.2006-2725. [DOI] [PubMed] [Google Scholar]
- 40.Bell LN, Cai L, Johnstone BH, Traktuev DO, March KL, Considine RV. A central role for hepatocyte growth factor in adipose tissue angiogenesis. Am J Physiol Endocrinol Metab 294: E336–E344, 2008. doi: 10.1152/ajpendo.00272.2007. [DOI] [PubMed] [Google Scholar]
- 41.Aljada A, Ghanim H, Dandona P. Translocation of p47phox and activation of NADPH oxidase in mononuclear cells. Methods Mol Biol 196: 99–103, 2002. doi: 10.1385/1-59259-274-0:99. [DOI] [PubMed] [Google Scholar]
- 42.Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res 19: 2499, 1991. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Legro RS, Schlaff WD, Diamond MP, Coutifaris C, Casson PR, Brzyski RG, Christman GM, Trussell JC, Krawetz SA, Snyder PJ, Ohl D, Carson SA, Steinkampf MP, Carr BR, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Myers ER, Santoro N, Eisenberg E, Zhang M, Zhang H; Reproductive Medicine Network. Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab 95: 5305–5313, 2010. doi: 10.1210/jc.2010-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 45.Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev 40: 1447–1467, 2019. doi: 10.1210/er.2018-00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14,719 initially healthy American women. Circulation 107: 391–397, 2003. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 47.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 49: 2129–2138, 2007. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 48.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation 103: 1194–1197, 2001. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 49.Libby P. Inflammation in atherosclerosis. Nature 420: 868–874, 2002. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 50.Peng Z, Sun Y, Lv X, Zhang H, Liu C, Dai S. Interleukin-6 levels in women with polycystic ovary syndrome: a systematic review and meta-analysis. PloS One 11: e0148531, 2016. doi: 10.1371/journal.pone.0148531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation 107: 671–674, 2003. doi: 10.1161/01.CIR.0000055188.83694.B3. [DOI] [PubMed] [Google Scholar]
- 52.Cui MZ, Parry GC, Oeth P, Larson H, Smith M, Huang RP, Adamson ED, Mackman N. Transcriptional regulation of the tissue factor gene in human epithelial cells is mediated by Sp1 and EGR-1. J Biol Chem 271: 2731–2739, 1996. doi: 10.1074/jbc.271.5.2731. [DOI] [PubMed] [Google Scholar]
- 53.Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther 28: e72–e91, 2010. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr.. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ibañez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod 19: 1725–1727, 2004. doi: 10.1093/humrep/deh329. [DOI] [PubMed] [Google Scholar]
- 56.Yildirim B, Sabir N, Kaleli B. Relation of intra-abdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertil Steril 79: 1358–1364, 2003. doi: 10.1016/S0015-0282(03)00265-6. [DOI] [PubMed] [Google Scholar]
- 57.Brewer HB Jr. Hypertriglyceridemia: changes in the plasma lipoproteins associated with an increased risk of cardiovascular disease. Am J Cardiol 83: 3F–12F, 1999. doi: 10.1016/s0002-9149(99)00308-2. [DOI] [PubMed] [Google Scholar]
- 58.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 45: 1169–1196, 2004. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Feingold KR, Hardardottir I, Memon R, Krul EJ, Moser AH, Taylor JM, Grunfeld C. Effect of endotoxin on cholesterol biosynthesis and distribution in serum lipoproteins in Syrian hamsters. J Lipid Res 34: 2147–2158, 1993. doi: 10.1016/S0022-2275(20)35355-4. [DOI] [PubMed] [Google Scholar]
- 60.Khovidhunkit W, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin down-regulates ABCG5 and ABCG8 in mouse liver and ABCA1 and ABCG1 in J774 murine macrophages: differential role of LXR. J Lipid Res 44: 1728–1736, 2003. doi: 10.1194/jlr.M300100-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Roe A, Hillman J, Butts S, Smith M, Rader D, Playford M, Mehta NN, Dokras A. Decreased cholesterol efflux capacity and atherogenic lipid profile in young women with PCOS. J Clin Endocrinol Metab 99: E841–E847, 2014. doi: 10.1210/jc.2013-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCrohon JA, Jessup W, Handelsman DJ, Celermajer DS. Androgen exposure increases human monocyte adhesion to vascular endothelium and endothelial cell expression of vascular cell adhesion molecule-1. Circulation 99: 2317–2322, 1999. doi: 10.1161/01.CIR.99.17.2317. [DOI] [PubMed] [Google Scholar]
- 63.Zhu XD, Bonet B, Knopp RH. 17β-Estradiol, progesterone, and testosterone inversely modulate low-density lipoprotein oxidation and cytotoxicity in cultured placental trophoblast and macrophages. Am J Obstet Gynecol 177: 196–209, 1997. doi: 10.1016/S0002-9378(97)70462-9. [DOI] [PubMed] [Google Scholar]
- 64.Adams MR, Williams JK, Kaplan JR. Effects of androgens on coronary artery atherosclerosis and atherosclerosis-related impairment of vascular responsiveness. Arterioscler Thromb Vasc Biol 15: 562–570, 1995. doi: 10.1161/01.ATV.15.5.562. [DOI] [PubMed] [Google Scholar]
- 65.González F, Nair KS, Daniels JK, Basal E, Schimke JM. Hyperandrogenism sensitizes mononuclear cells to promote glucose-induced inflammation in lean reproductive-age women. Am J Physiol Endocrinol Metab 302: E297–E306, 2012. doi: 10.1152/ajpendo.00416.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]