Abstract
A prominent health issue nowadays is the COVID-19 pandemic, which poses acute risks to human health. However, the long-term health consequences are largely unknown and cannot be neglected. An especially vulnerable period for infection is pregnancy, when infections could have long-term health effect on the child. Evidence suggests that maternal immune activation (MIA) induced by either bacteria or viruses presents various effects on the offspring, leading to adverse phenotypes in many organ systems. This review compares the mechanisms of bacterial and viral MIA and the possible long-term outcomes for the offspring by summarizing the outcome in animal LPS and Poly I:C models. Both models are activated immune responses mediated by Toll-like receptors. The outcomes for MIA offspring include neurodevelopment, immune response, circulation, metabolism, and reproduction. Some of these changes continue to exist until later life. Besides different doses and batches of LPS and Poly I:C, the injection day, administration route, and also different animal species influence the outcomes. Here, we specifically aim to support colleagues when choosing their animal models for future studies.
Keywords: fetal programming, lipopolysaccharide, long-term outcome, maternal infection, polyinosinic:polycytidylic acid
INTRODUCTION
The respiratory syndrome COVID-19 pandemic, caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a prominent health issue nowadays. The acute effects of infection can be severe whereas the long-term health consequences are largely unknown and cannot be neglected. In SARS-CoV-2-related pregnancies, immunoglobulins have been found in the neonates of positive diagnosed mothers, whereas the offspring tested negative (1). Higher levels of IgM and IgG together with increased levels of IL-6 were observed in the infants (1–3). Furthermore, there are reports on fetal distress, admission to the intensive care unit (4, 5), and stillbirth (6) for mother with COVID-19 during pregnancy. Shortness of breath, fever, abnormal liver function, and increased heart rate were observed in neonates as immediate outcomes (5).
Potentially, there could be long-term health effects on the fetus when the mother gets infected with SARS-CoV-2 during pregnancy. Adaption to adverse circumstances, like infection, during the developmental period of the fetuses is known to have adverse health effects in their later life. This adaptation is known as fetal programming or the developmental origins of health and disease paradigm (DOHaD) (7, 8). However, the long-term health consequences of SARS-CoV-2 infection during pregnancy for the child are currently unknown because this pandemic is too recent for long-term studies yet. This makes it necessary to include data from other virus infections to predict the outcome. However, for the similar Middle Eastern respiratory syndrome (MERS) and SARS infections, we could not identify studies on the long-term outcome probably because of low case numbers.
Importantly, long-term effects can be initiated even when there is no direct infection of the fetal-placental unit, but only of the mother. Most likely, activation of the maternal immune system by the intruder is a mediator of long-term effects in the fetus. These long-term health effects on the children can be studied with the use of a maternal immune activation (MIA) model. In this model, the maternal immune system is triggered by a stimulus that simulates an infection. The triggering of the immune system results in cytokine release and immunological alterations that also affect the fetus (9). Both epidemiological and experimental evidence showed that gestational infections have a significant impact on preterm birth (10). Besides, many studies have shed light on the adverse effect of MIA to the health of infants. This not only includes retarded fetal growth leading to low birthweight of the infant (7), but also contains abnormal organ development and neurodevelopment, and metabolic-related diseases in later life (8, 11).
MIA is induced by bacteria or viruses in different ways. The bacterial endotoxin lipopolysaccharide (LPS) and the synthetic viral RNA polyinosinic:polycytidylic acid (Poly I:C) are often used in rodents to mimic the pathological process caused by bacteria and viruses, respectively. LPS composes the major part of the outer membrane of gram-negative bacteria and binds to Toll-like receptor 4 (TLR4). Poly I:C is a synthetic analogue of double-stranded RNA typically found in some viruses and activates Toll-like receptor 3 (TLR3) (12). Noteworthy, SARS-CoV-2 is a single-stranded RNA virus; however, its replication cycle involves a phase of double-stranded RNA as well. Whether Poly I:C is a good model for SARS-CoV-2 infection, hence, needs more research. LPS and Poly I:C are both strong innate immune inducers leading to cytokine production, inflammation, and fever. This enhances the synthesis of pro- and anti-inflammatory cytokines, causing a cascade that can lead to disorders in both mother and fetus (13). For the long-term effect of MIA, prenatal inflammation induced by LPS is associated with diverse adverse neurobehavioral outcomes (14) and induces reproductive disorders (15), renal dysfunctions (16), and intestinal injury in the offspring (17). Prenatal exposure to Poly I:C as a MIA model mainly increased the risk for neurodevelopmental disorders. For example, impaired cognitive flexibility is detected in offspring, which is similar to that exhibited in autism spectrum disorder (ASD) in humans (18–20).
Here, we explore the effects of the LPS and Poly I:C models and present possible offspring outcomes to provide information for optimal use of these models in future studies.
MECHANISMS OF THE TWO MODELS INDUCING MIA
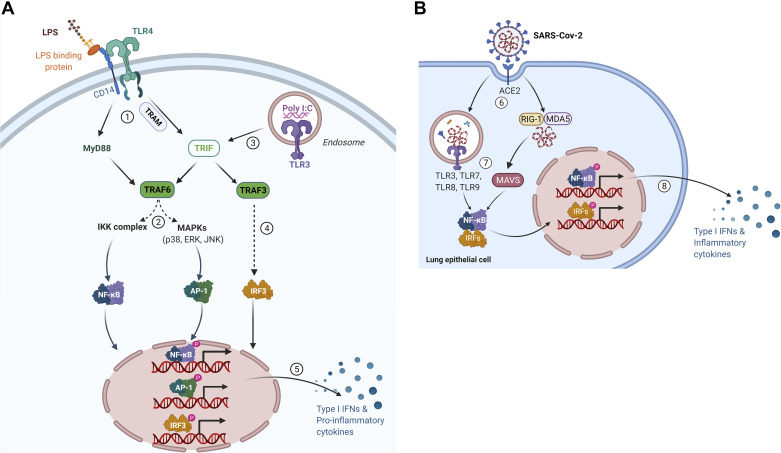
LPS and Poly I:C both induce activation of the innate immune response through TLR signaling pathways (Fig. 1A). The TLR signaling contains two pathways: the MyD88-dependent and the Toll-IL-1 receptor domain-containing adapter-inducing interferon β (TRIF)-dependent pathway. MyD88 and TRIF are recruited to TLRs to initiate the pathways. This causes the activation of NF-κB, activator protein 1 (AP-1), and interferon regulatory factor 3 (IRF3) that induce the expression of inflammatory cytokine genes and type 1 interferon (IFN) (26). In short, LPS activate TLR4 by binding to LPS-binding proteins with the assistance of CD14, initiating both MyD88-dependent pathway and TRIF-dependent pathway. Eventually, the expression of several inflammatory genes, such as Il1b, Il6, and Tnfa (21) and type 1 IFN genes such as Ifna and Ifnb are induced (23). Poly I:C is internalized and subsequently recognized by TLR3 resulting in proinflammatory cytokines and type 1 IFNs gene expression by NF-κB, AP-1, and IRF3 (22, 23).
Figure 1.
A: activation of innate immune response by lipopolysaccharide (LPS) and polyinosinic:polycytidylic acid (Poly I:C) through Toll-like receptor (TLR) signaling pathway. LPS combines with LPS binding protein (LBP) and CD14 and delivers LPS-LBP to activate TLR4, initiating both MyD88-dependent pathway and Toll-IL-1 receptor domain-containing adapter-inducing interferon β (TRIF)-dependent pathway (21). 1) MyD88 activates TNF receptor associated factor-6 (TRAF6). In addition, TLR4 recruits TRIF-related adaptor molecule (TRAM) leading to TRAF6 activation. 2) TRAF6 drives the activation of transforming growth factor-β-activated kinase 1 (TAK1), which activates IκB kinase (IKK) complex and MAPK family members leading to the formation of NF-κB dimers and activator protein 1 (AP-1) transcription factor complex (21). NF-κB and AP-1 translocate into the nucleus to induce proinflammatory gene expression. 3) TLR3 is activated by Poly I:C, initiating TRIF-dependent pathway (22). TRIF directly interacts with TLR3 and dissociation leads to activation of TRAF3 and TRAF6 (23). 4) TRAF3 recruits IKK-related kinases TANK-binding kinase 1 (TBK1), IKKi, and IKKγ for Interferon regulatory factor 3 (IRF3) phosphorylation and dimer formation (23). 5) After translocation into the nucleus, NF-κB, AP-1, and IRF3 induce proinflammatory gene expression. NF-κB and AP-1 induce the expression of proinflammatory genes such as Il1b, Il6, and Tnfa (22). IRF3 induces the gene expression of type 1 IFN genes, such as Ifna and Ifnb (23). Retrieved from https://app.biorender.com/biorender-templates. B: immune response to corona virus through TLR signaling pathway. 6) Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) virus binds its cellular receptor ACE2 to enter airway epithelial cells (24). 7) TLR3, -7, -8, and -9 sense the virus in cells. Retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) recognize viral RNA and interact with mitochondrial antiviral-signaling protein (MAVS) (25). The activation of the TLRs and MAVS leads to the formation of NF-κB and IRFs dimers. The NF-κB and IRFs dimers translocate into the nucleus. 8) Gene expression of type 1 IFN genes and other proinflammatory genes are induced by NF-κB and IRF3 (25). ACE2, angiotensin converting enzyme 2. Retrieved from https://app.biorender.com/biorender-templates.
Including TRL3 and -4, there are 10 functional TLRs (TLR1–TLR10) identified in human and 12 functional TLRs (TLR1–TLR9, TLR11–TLR13) are found in mice (23). Among these TLRs, TLR2 along with TLR1 can recognize triacylated lipopeptides from gram-negative bacteria. In addition to binding gram-negative bacteria, TLR2 along with TLR6 can recognize diacylated lipopeptides from gram-positive bacteria (27). As we mentioned earlier, TLR3 recognizes viral double-stranded RNA (dsRNA) and TLR4 mainly binds gram-negative bacteria. Bacterial flagellum proteins can be detected by TLR5. Single-stranded RNA (ssRNA) from viruses is recognized by TLR7. TLR8 responds to viral and bacterial RNA and TLR9 binds 5′–C–phosphate–G–3 (CpG)-DNA motifs from viruses and bacteria (28, 29). In addition to TLRs, there are many other classes of pattern-recognition receptors (PRRs) such as AIM2-like receptors (ALRs), C-type lectin receptors (CLRs), NOD-like receptors (NLRs), and retinoic acid-inducible gene (RIG)-like helicase receptors (RLRs) (28).
As for the SARS-CoV-2 virus (Fig. 1B), it binds its cellular receptor angiotensin-converting enzyme 2 (ACE2) to enter airway epithelial cells and then provokes a relatively weak IFN response (24). Two pathways are activated when the viruses enter the cells. Viral RNA is recognized by TLRs including TLR3, -7, -8, and -9. Furthermore, members of the retinoic acid-inducible gene I (RIG-I)-like receptor (RLR) family and melanoma differentiation-associated gene 5 (MDA5) can also recognize viral RNA and interact with mitochondrial antiviral-signaling protein (MAVS) (30, 31). Both pathways ultimately initiate type 1 IFNs gene expression by activating NF-κB and IRF3 as downstream factors (25). Finally, the uncontrolled immune response to SARS-Cov-2 triggers a “cytokine storm,” which can be featured by the overexpression of IL-6 and NF-κB (32).
OUTCOMES IN THE OFFSPRING OF MIA INDUCED BY LPS AND POLY I:C
A comprehensive overview of animal studies applying LPS (Table 1) or Poly I:C (Table 2) to induce MIA, including characterization of the offspring, is presented in the tables. These MIA animal models are widely used to study the long-term health effect of the offspring and possible related mechanisms. They mimic bacterial or viral infections, respectively, without the need to use a pathogen. LPS and Poly I:C activate shared inflammatory pathways as demonstrated in Fig. 1. Both LPS and Poly I:C play a prominent role in stimulating the production and secretion of TNF-α and type 1 interferons. Different immune responses and developmental consequences triggered by LPS and Poly I:C can be observed. The dose and batch of LPS and Poly I:C, timing of the injection, administration route, and also different species may influence the outcome. For instance, a high dose of LPS can directly induce intrauterine fetal death (71). For the long-term outcome, we hence focus on low-dose LPS experiments. LPS is used from Escherichia coli in most of studies while there are batch differences for the Poly I:C studies and differences in batches of Poly I:C influence the maternal physiology, immune response, and pregnancy outcomes in MIA models (70, 72). Some specific findings are discussed here.
Table 1.
Outcomes of offspring after inducing MIA by LPS
| Animals | Dose and Injection Day | Outcomes | Notes | Refs. |
|---|---|---|---|---|
| BALB/c mice | 0.26 mg/kg GD15 |
– Fetal brain damage – Microglial/macrophage activation – ↑ Il1b, iNos, nNos gene expression |
Melatonin administration prevents: fetal brain damage, long-term neurodevelopmental impairments |
(10) |
| C57BL6/J mice | 120 μg/kg GD15–17 |
– Fetal brain: ↓ Astrocytic marker: glial fibrillary acidic protein (GFAP) ↓ Neuronal marker: NeuN |
MIA induced by Poly I:C in parallel (see Table 2) |
(33) |
| C57 mice | 75 μg/ kg GD11 |
– Abnormal levels fat development, blood lipids, and glucose metabolism – ↑ Adipocyte differentiation markers: CEBPA, CEBPB, PPARG, and activator protein 2 (AP2) |
MAPK pathway | (34) |
| C57BL6/J mice | 100 μg/kg (total) GD15–17 |
– Anxiety-like behaviors – ↓ Cerebral serotonin (5-HT) – ↓ Tph2 and Slc6a4 gene expression |
(35) | |
| C57BL6/J mice | 100 μg/kg (total) GD15–17 |
– Social deficits – Cerebral expression changes immune, developmental- and neuronal structural-related genes |
Reinjection, 40 mg/kg LPS, 8 wk offspring: ↑ Cerebral cytokines, chemokines, and CAMs |
(36) |
| C57BL6/J mice | 0.12 μg/g GD17 |
– Memory deficits – Alteration fatty acid composition – ↑ IL-6 cytokine fetal brain |
Deleterious effects n-3 PUFA deficiency on brain: Lipid composition, inflammation, memory performances |
(37) |
| C57BL6/J mice | 100 μg/kg GD15.5 |
Intestinal injury | (38) | |
| C57BL/6J mice | 20 μL GD0–16 |
♂ Offspring: – Anxiety-related behaviors – ↑ Corticotropin-releasing hormone (CRH) protein expression – ↑ c-Fos-positive cells |
HPA axis signaling | (39) |
| C57BL6/J mice | 100 µg/kg GD15.5 |
– Intestinal injury – ↓ Goblet and Paneth cells – ↑ Serum levels of IL-6, TNF, KC/GRO, IL-10, and IL-1β |
(17) | |
| C57BL6/J mice | 20 mg/kg GD16 |
♂ Offspring: – ↑ Adipose tissue – ↓ Muscle mass – ↑ Plasma leptin |
TLR4 signaling Naloxone suppressed cytokine expression |
(40) |
| CD-1 mice | 50 μg/dam GD15 |
Alterations fetal brain: – ↑ Lipid metabolism – ↑ Amino acid metabolism – ↑ Purine metabolism |
(14) | |
| CD-1 mice | 20 μg/kg GD7.5–17.5 |
– ↓ Body weight – ↑ Cox2 expression and related inflammatory factors |
NF-κB pathway Aspirin attenuated Cox2 and inflammatory factor expression, rescued trophoblast invasion |
(41) |
| CD-1 mice | 25 μg in 100 μL PBS GD17 |
– Fetal vessel resistance – Fetal brain: ↑ Iba1 |
(42) | |
| ICR mice | 2 mg/kg GD16.5 |
Morphological changes fetal brain | SIRT1/Nrf2 signaling pathway Melatonin pretreatment prevents: Preterm birth, acute fetal, neuroinflammation, neuromotor, and perinatal brain injury |
(43) |
| Sprague-Dawley rats | 1 mg/kg GD14 |
– ↑ Fetal resorption rates – ↓ Fetal weight |
(44) | |
| Sprague-Dawley rats | 0.79 mg/kg GD8, 10, 12 |
– Hypertension – Renal: ↑ Il6, Fli1, Tnfa, Dnmt1 and Dnmt3b gene expression ↑ DNA methylation |
IL-6/Fli-1 pathway Prenatal PDTC intervention reverses outcomes |
(45) |
| Sprague-Dawley rats | 0.79 mg/kg GD8, 10, 12 |
– ↓ Body weight – Dyslipidemia – Serum and hepatic levels: ↑ Total cholesterol, triglycerides, low-density lipoprotein cholesterol ↑ Aspartate amino transferase and alanine aminotransferase – Gene expression hepatic lipid metabolism ↑ Vldlr ↓ Tm7sf2 |
High-fat diet: – ↑ Body weight – Shift lipid profile – Liver dysfunction – Mitochondrial disorders |
(46) |
| Sprague-Dawley rats | 100 µg/kg GD15, 17, 19 |
– ↓ Motor activity – ↑ Hippocampal expression of SERT protein |
Age-dependent long lasting impact neur genesis ♀ offspring |
(47) |
| Sprague-Dawley rats | 1.5 mg/kg GD12 |
– ↑ Anxiety-like behaviors – ↓ Social behaviors – Brain: ↑ Lipid peroxidation ↓ Total antioxidant content ↑ Inflammatory genes: Tnfa, Il6, Il1b ↑ Apoptotic genes: Bax, Cas3, Cas9 ↓ Neuroprotective genes: Bdnf, Bcl2 |
MIA induced by Poly I:C in parallel (see Table 2) |
(48) |
| Wistar rats | 0.25 mg/kg GD15 |
– Behavioral impairment – Fetal brain: ↑ Cytokine levels ↑ Oxidative stress parameters ↑ Matrix metalloproteinase (MMP)-2 and MMP-9 |
Ketamine injection PND54: – Memory impairment – Deficit in pre-pulse inhibition test |
(49) |
| Wistar rats | 0.5 mg/kg GD13, 15, 17, 19 |
– Endothelial dysfunction – Renal hemodynamic changes |
Angiotensin II/NADPH oxidase pathway | (16) |
| Wistar rats | 50 mg/kg GD12 |
– ↓ Body weight – ↓ Testis weight – ↓ Testosterone level – ↓ Seminiferous tubule diameter – ↓ Number Sertoli and spermatid cells – ♂ Offspring: Development of sexual disorders |
Fulvestrant treatment restored outcomes | (15) |
| Wistar rats | 50 μg/kg GD12 |
– Delayed reproductive maturity – ↓ Body weight – ↓ Sex steroids ♀ offspring |
Postnatal estradiol and testosteroneantagonist treatment – ♀ offspring: ↑ healthy follicles ↑ endogenous steroid production |
(50) |
| Wistar rats | 500 μg/kg GD9.5 |
– Microbiome abundance: ↑ Alistipes, Fusobacterium, and Ruminococcus ↓ Coprococcus, Erysipelotrichaies, and Actinobacteria – ♂ Offspring: ↓ Social behaviors ↑ Anxiety-like and repetitive behavior ↓ Hypomyelination in the prefrontal cortex and thalamic nucleus |
Abnormal brain-gut-microbiota | (51) |
GD, gestational days; LPS, lipopolysaccharide; MIA, maternal immune activation; Poly I:C, polyinosinic:polycytidylic acid; PUFA, polyunsaturated fatty acid; TLR4, Toll-like receptor 4 ↑, increase; ↓, decrease; ♂, male; ♀, female.
Table 2.
Outcomes of offspring after inducing MIA by Poly I:C
| Animals | Dose and Injection Day | Outcomes | Notes | Refs. |
|---|---|---|---|---|
| BALB/c mice | 20 mg/kg GD9 |
– Differences in species richness microbiome – ♂ Offspring: ↑ Abundance of four families of Firmicutes phylum – ♀ Offspring: ↑ Abundance of Lactobacillaeles ↓ Abundance of Prevotellaceae and Porpyromonadaceae |
Differential bacterial findings P30 correspond with activation immune system, strong increase in microglial cells |
(52) |
| C57BL/6 mice | 20 mg/kg GD12 |
Preferential to Th17 cell differentiation of lymphocytes | Adaptive immune system | (53) |
| C57BL/6J mice | 20 mg/kg GD12.5 |
– Behavioral impairments – Adult splenocytes: ↓ Mitochondrial ATP production |
Lower complex I activity Long-lasting effects bioenergetics splenocytes |
(54) |
| C57BL/6J mice | 5 mg/kg GD15–17 |
– ↓ Growth and sensorimotor development – Fetal brain: ↑ IL-2, IL-5, and IL-6 cytokines ↑ Metabotropic receptor 5: mGluR5 |
MIA induced by LPS in parallel (see Table 1) | (33) |
| C57BL6/J mice | 5 mg/kg GD9 |
– Juvenile cortex: Hypoacetylation of histone H3 and H4 ↓ Promotor-specific histone acetylation (Gria1, Slc17a7) – Juvenile hippocampus: ↑ Disc1 and Ntrk3 genes – Adult offspring: Behavioral abnormalities No changes in histone acetylation ↓ Promotor-specific histone acetylation (Gria1, Slc17a7) |
Early epigenetic changes contribute to delayed behavioral abnormalities in adult offspring |
(55) |
| C57BL/6J mice | 5 mg/kg GD9.5 |
Adult frontal cortex: – Activated innate immune receptor TLR3 signaling pathway – Oxidative/nitrosative stress – Accumulation of proinflammatory mediators (Nfkb and iNOS) |
Chronic paliperidone: – Blocked neuroinflammatory response – Stimulate M2 polarization microglia – ↑ Spatial working memory |
(56) |
| C57BL/6J mice | 5 mg/kg GD9 or GD17 |
– Neuroanatomical alterations – Behavioral alterations – ↓ Brain volume – ↓ Glucose preferences |
(57) | |
| C57BL/6N mice | 5 mg/kg GD9 |
Hypomethylation of adult brain | n-3 PUFA intervention reduces outcomes |
(58) |
| C57BL/6J mice | 20 mg/kg GD12.5 |
– Activation of local circuit interneurons adult brain – ↑ Synaptic strength adult brain |
Alterations in flow of signals within the mPFC to basolateral amygdala pathway |
(59) |
| C57BL/6J mice | 20 mg/kg GD12 |
– Neonate immune organs and brain: – ↑ Cytokine levels of TNFα and IL-18 – Adult: Alteration in behavioral responses |
(19) | |
| C57BL/6J mice | 20 mg/kg GD12.5 |
– ♂ Offspring: ↓ mRNA and protein levels of TNFα/iNOS, IL-6/IL-1B, anti-inflammatory factors – ♀ Offspring: ↑ mRNA and protein levels of TNFα/iNOS, IL-6/IL-1β, anti-inflammatory factors |
(60) | |
| C57BL/6J mice | 20 mg/kg GD12.5 |
Fetal and placental sex influenced: – Responses of immune genes to metabolic and inflammatory stress. |
Gene pathways: ♂ P53 feedback and Wnt signaling ♀ TGF-β and Insulin/IGF/MAPK |
(61) |
| C57BL/6 mice | 20 mg/kg GD12.5 |
– ↑ CCL5 and CXCL10 fetal brain – ↑ Cytokines/chemokines in Map2k7 Hz mice |
JNK signaling | (62) |
| C57BL/6J mice | 20 mg/kg GD12 |
– Neonate immune organs and brain: ↑ Cytokine levels of TNFα and IL-18 – Adult: Alteration in behavioral responses |
(19) | |
| C57BL/6J mice | 5 mg/kg/mL GD9 |
Fetal brains: – Dysregulation in brain development-related gene pathways – ↑ RNA-editing |
Gene pathways: – Brain and neuronal development – Energy and metabolism – Immunological signatures |
(63) |
| CD-1 mice | 5 mg/kg GD12.5, 17.5 |
– ♂ Offspring: Alteration in social interaction ↑ Nrg1 and Erbb4 gene expression – Adult: Behavioral changes |
NRG-ErbB signaling pathway Behavioral changes are time-related and sex-specific |
(64) |
| Sprague-Dawley rats | 10 mg/kg GD9 |
– Age-related behavioral and neuro-inflammatory changes – Activation of microglia Astrocytes activated at PND60 |
(65) | |
| Sprague-Dawley rats | 10 mg/kg GD14 |
Fetal brains: – ↑ Amino acid transporters – ↓ Snat5, Eaat1, and Glyt gene expression |
(66) | |
| Sprague-Dawley rats | 10 mg/kg GD17 |
– Depressive-like behavior – Dendrite development obstruction – ↑ Isg15 expression brain |
NEDD4/Rap2A signaling | (67) |
| Sprague-Dawley rats | 20 mg/kg GD12 |
– ↑ Anxiety-like behaviors – ↓ Social behaviors – Brain: ↑ Lipid peroxidation ↓ Total antioxidant content ↑ Inflammatory genes: Tnfa, Il6, Il1b ↑ Apoptotic genes: Bax, Cas3, Cas9 ↓ Neuroprotective genes: Bdnf, Bcl2 |
MIA induced by LPS in parallel (see Table 1) |
(48) |
| Wistar rats | 4 mg/kg Early = GD10 Late = GD19 |
♂ Offspring: – Sensorimotor gating deficits – ↑ D1r gene expression in nucleus accumbens |
Schizophrenia-like phenotypes |
(68) |
| Wistar rats | 5 mg/kg GD10 and GD9 |
Fetal brains: – Dysregulation in brain development-related gene pathways – ↑ RNA editing Neonates: – ↑ lymphoid aggregates – Altered intestinal inflammatory profile – Disruption in GI barrier tight junction protein Adults: – ↑ Anxiety-like behavior |
Gene pathways: – Brain and neuronal development – Energy and metabolism |
(69) |
| Wistar rats | 10 mg/kg GD15 |
– ↓ Litter size depending on Poly I:C supplier – ↓ Placenta weight ♂ Offspring: – ↓ Fetal brain weight |
Outcome depending on Poly I:C supplier and endotoxin concentration. |
(70) |
GD, gestational days; LPS, lipopolysaccharide; MIA, maternal immune activation; Poly I:C, polyinosinic:polycytidylic acid; PUFA, polyunsaturated fatty acid; TLR3, Toll-like receptor 3. HPA, hypothalamic-pituitary-adrenal; PDTC, pyrrolidine dithiocarbamate; mPFC, medial prefrontal cortex; GI, Gastrointestinal; CAMs, cell adhesion molecules; PND, postnatal day; CCL5, chemokine (C-C motif) ligand 5; CXCL10, C-X-C motif chemokine ligand 10; ↑, increase; ↓, decrease; ♂, male; ♀, female.
Placenta and Body Weight
Fetal development is often related to the status of the placenta. In a mouse study injecting LPS, MRI results revealed that the volume of the placenta was reduced, Doppler ultrasound showed impaired blood flow, and immunohistochemistry confirmed endothelial damage of the placenta (42). LPS, as inducer of MIA, causes developmental changes in the placenta and the placenta develops resistance to cytokine challenges (44, 73). MIA provoked an inflammatory response in the placenta and alterations in immune-related genes have been reported in the Poly I:C murine model (43, 61). Other changes reported for the placenta after exposure to MIA include a decrease in placenta weight (70). Furthermore, upregulation of certain amino acids transporters was reported (66), potentially leading to nutritional changes of the fetus. Damage or irreversible changes to the placenta caused by MIA could affect outcomes in the offspring. LPS-induced inflammation directly increased fetal resorption and decreased fetal weight both in CD1 mice and Sprague-Dawley rats. However, on the long run it increased body weight in the 8- and 12-wk C57 mouse and Sprague-Dawley rat offspring from LPS groups (34, 41, 44, 45). The long-term effects of MIA on body weight might be related to specific MIA effects on metabolism, as discussed in Metabolism and Microbiome.
Nervous System
The LPS model.
Histological evidence showed some brain damage among BALB/c and ICR mice offspring from LPS MIA models and the activation of microglia and macrophages on gestational day (GD) 15 and 16 (10, 43). In the BALB/c fetal brain, an increased anti-inflammatory gene expression was found after MIA induction with LPS (10). During neonatal development, the C57BL6/J mice offspring of the MIA LPS model showed aberrant social behaviors. In the offspring brain also changes in gene expression, such as immune and neuronal system-related genes, were found. At 5 wk, expression of adaptive immune response genes and developmental signaling genes in the offspring brain were altered (36). In adulthood, at 8 wk, gene expression of serotonergic and dopaminergic neuron-related and synaptic structural genes was altered (36). The abnormal neuroimmune interactions, which are associated with the inflammatory response in the fetal brains, could ultimately lead to the aberrant behaviors of the offspring. Anxiety-like behaviors in C57BL/6 offspring aged from 5 to 8 wk have been described after inducing MIA with low-dose LPS (35, 39, 47). Elevated pro- and anti-inflammatory cytokines were detected after MIA induction with LPS in the Wistar fetal brain along with behavioral impairment at 8 wk old (49). Furthermore, Labrousse et al. (37) showed induction of spatial memory deficits in 12-wk-old C57BL6/J offspring in LPS-treated dams. The studies with LPS models suggest several plausible mechanisms causing neurodevelopment disorders in the offspring. Increased expression of neuronal Nos, inducible Nos and Il1b, and enhanced histone H3 hyperacetylation and histone acetyltransferase activity have been shown together with fetal brain damage and microglial and macrophage activity (10). Also decreased immune cell migration such as patrolling T cells, monocytes or macrophages in the brain has been proposed which might be related to the social behavioral deficits (36). Others speculated a direct link between modulation of memory abilities and signaling of IL-6 family members (37).
Early effects in the Poly I:C model
Offspring exposed to Poly I:C in a MIA model demonstrate an altered cytokine profile. The dysregulated cytokine and chemokine profile, including IL-17a, IL-6, chemokine (C-C motif) ligand 5 (CCL5), and C-X-C motif chemokine ligand 10 (CXCL10), is presented in the fetal brain of Poly I:C mice C57BL/6J model (19, 62, 74). The increased cytokine and chemokines present in the fetal brain compromises the GABAergic neurons (62). Besides cytokine profile changes, differences in gene expression in the CD1 fetal brain have also been reported, with an increased expression for neuregulin, its receptor Erbb4 and dopamine D2 receptor (64). Increased RNA editing levels and dysregulation in brain development-related gene pathways in the fetal brains were reported for C57BL6/J MIA offspring (63). Furthermore, in Sprague-Dawley rat offspring exposed to Poly I:C during gestation, an increase in gene expression for several amino acid transporters was observed in the fetal brain. In contrast to the gene expression, a decreased protein expression of specific amino acid transporters was seen in the fetal brain and also in the placenta. This resulted in differences in concentration of free amino acids in the offspring fetal brain (66).
Consequences of Poly I:C for adult life
Changes in the offspring brain due to Poly I:C are as with LPS not limited to the fetus. Epigenetic changes, hypoacetylation of histone H3 and H4, in the cortex, together with gene expression alterations associated with neuronal development, synaptic transmission, and immune signaling have been reported in 3-wk-old MIA C57BL6/J mouse offspring. However, the epigenetic changes did not last into adulthood (55). At 4 wk of age, upregulation of interferon-stimulated gene 15 was reported, resulting into abnormal dendrite development in prenatal Poly I:C exposed Sprague-Dawley offspring (67). In the hippocampus and prefrontal cortex of MIA exposed Sprague-Dawley offspring at the age of 6 and 8 wk, an increased proinflammatory cytokine profile was reported together with an increased number of microglia cells and microglia activation (65). At the age of 8 wk, a decrease in parvalbumin-positive (PV+) neurons and neurotransmitter GABA has been reported in the prefrontal cortex (74). Activation of the TLR-3 signaling pathway, increased oxidative and nitrosative stress, and accumulation of proinflammatory mediators were found in the frontal cortex of 8- to 12-wk-old MIA-exposed C57BL/6J offspring (56). A decrease in global DNA methylation in the hypothalamus was observed together with 320 differentially methylated CpG sites for 12-wk-old C57BL/6N offspring. The differentially methylated genes are related to nervous system development and function and cellular development (58). Long-term gene expression changes, at 27 wk of age, due to the prenatal Poly I:C challenge are reported in the frontal cortex of C57BL/6J mice, such as changes in glutamatergic neurotransmission and potassium ion channel activity (18). In adulthood of C57BL/6J mice, alterations in neurotransmission from the prefrontal cortex to the basolateral amygdala have been reported. An increased efficacy in signal transduction of glutamatergic synaptic transmission was described, resulting in a shift in balance between excitation and inhibition (59). Furthermore, a decreased brain volume in 1-yr-old C57BL/6J offspring exposed to early or late gestation Poly I:C-induced MIA has been reported together with an increased locomotor activity and reduced glucose preference (57).
The reported changes in the C57BL/6J offspring brain, like the cytokine profile, correlate with behavioral changes present in adulthood (19). The impact of Poly I:C-induced MIA is related to mental health disorders such as schizophrenia and ASD. The behavioral alterations found in juvenile and adult offspring include anxiety-like (19, 57, 60, 69, 74, 75), depression-like behavior (67, 68, 74), and reduced social interaction (18–20, 60, 74). Sex-specific differences have been found for the offspring of Poly I:C-treated dams. C57BL/6J male offspring demonstrated a reduction in social interaction and motor development and showed coordination deficits, whereas this was not the case for female offspring. These alterations, found at the age of 4 and 13 wk, were accompanied with alterations in inflammatory gene expression (20). At 13 wk, also proinflammatory gene and protein expression were increased for both C57BL/6J male and female offspring. However, anti-inflammatory gene and protein expression were decreased in male offspring, whereas they increased in female offspring (60). Dabbah-Assadi et al. (64) reported sex-specific and time-related behavioral changes for Poly I:C MIA-exposed offspring. Exposure to MIA in late gestation resulted in a lack of preference to novel objects in female adult offspring, whereas alterations in working memory were observed in male adult offspring (64). There are many possible pathways to explain the effect on the offspring of Poly I:C model. For example, the schizophrenia-associated neurodevelopmental abnormalities were correlated with c-Jun NH2-terminal kinase (JNK) signaling. Altered cytokine and chemokine profiles, resulting into compromised development of GABAergic interneurons, were found in Poly I:C-exposed offspring through mediation of JNK signaling (62). The Poly I:C studies also focus on brain inflammation, reduced numbers of Purkinje cells and neurons, and NRG1-ErbB or NEDD4/Rap2A signaling pathway (20, 64, 67).
Comparison of the LPS and the Poly I:C model.
Many studies focused on exploring the association between MIA and neurodevelopmental disorders in the offspring (11, 18, 22). Hanamsagar and Bilbo (76) noted that maternal infection triggers the immune response in fetal brain, disrupting the normal development of microglia and dysregulating microglial function, which may contribute to the increased risk of neurodevelopmental disorders. Animal models were used to further investigate possible mechanisms. Animal models using LPS or Poly I:C showed the relationship between MIA and brain cell disruption, memory deficiency, and behavioral impairment related to neurological disorders in the offspring (35, 67). Arsenault et al. found that LPS- and Poly I:C-induced MIA have different effect on the development of offspring. An initial inflammatory response is seen in both models at cellular level. However, some brain cell markers were found to be decreased in LPS models, whereas no changes were found in the Poly I:C model. For the Poly I:C model, an inhibited growth in body weight and changes in sensor motor development in the offspring were shown (33). However, Talukdar et al. showed same outcomes in rat offspring from LPS- and Poly I:C-treated dams. Increased signs of lipid peroxidation and a decrease in total antioxidant content were reported in the hippocampus of adult Sprague-Dawley rats exposed to MIA. Expression of inflammatory genes Tnfa, Il6, Il1b and apoptotic Bax, Cas3, Cas9 genes were increased. Expression of neuroprotective genes Bdnf and Bcl2 were decreased (48). In addition, the observed changes in the offspring include elevated anxiety, impaired social interaction, deficits in cognitive flexibility and memory, reduction in neurogenesis, and morphological changes in the fetal brain (18, 39, 43, 64). The study further explained that the behavioral deficits in offspring were probably caused by lower neuroprotective defenses, increased oxidative stress toxicity and inflammatory, and apoptotic pathways (48). Meanwhile, the studies showed that social behavioral deficits are related to the disturbance of microbiome in the guts of offspring from both LPS- and Poly I:C-induced MIA (51, 52).
Immune System
As discussed in the previous paragraph, profound changes in immune cells of the offspring brain have been found following MIA. Examples are the reported alteration in cytokine and chemokine profile, the increased numbers of microglia cells, and the activation of microglia. Not surprisingly, alterations in cytokine profiles in spleen and liver were also reported in C57BL/6J fetal offspring exposed to Poly I:C (19). This shows that MIA influences the immune system of the offspring not only in the brain but also other target organs. Besides, the adaptive immune system of C57B/6 offspring was affected by MIA, resulting into Th17 development of lymphocytes of offspring from Poly I:C-injected immune pregnant dams (53). Splenocytes from Poly I:C-induced MIA C57BL/6J adult offspring showed a decrease in mitochondrial ATP production. This caused a long-lasting effect of MIA on the bioenergetics of the splenocytes (54). Furthermore, an increase in lymphoid aggregates was reported in the intestine of Wistar rats (69).
Metabolism and Microbiome
Metabolomics profile changes of the fetal and postnatal brain have been reported (14). A significant increase in amino acids and purine metabolites was present in the fetal brain of CD1 mice 6 h after LPS exposure of the dam. Alterations in lipid metabolites, particularly fatty acids, were found in neonatal brains of the LPS-exposed offspring and they further suggested that xanthine/hypoxanthine may be a biomarker of fetal brain injury (14). At 8 wk of age, an increased susceptibility to LPS-induced intestinal injury in C57BL6/J mice has been demonstrated (17, 38). According to these studies, the injury in fetal intestine is caused by chorioamnionitis that is induced when fetuses are exposed to inflammatory cytokine.
There are a few reports demonstrating an effect of Poly I:C-induced MIA on the offspring gastrointestinal tract. The intestinal muscle thickness of MIA-exposed offspring was decreased and there was a decrease in gastrointestinal tight junction proteins markers in Sprague-Dawley rats. Furthermore, an increase in inflammatory response of the gut was described and the gut microbiota composition was altered (75). Hollins et al. (69) reported also alterations in inflammatory profile and tight junction protein gene expression in the intestine of MIA-exposed Wistar rats. Whole body metabolic issues appeared in the C57 offspring of LPS-treated dams, including abnormal levels of blood lipids, glucose metabolism, and fat development where pentraxin 3 (PTX3) through mitogen-activated protein kinase (MAPK) pathway plays an important role (34). Dyslipidemia was found in Sprague-Dawley male offspring at the age of 4 and 8 wk and is related to abnormal mitochondria and disturbed cholesterol metabolism (46).
Gut microbiota is important to regulate metabolism. Wistar rat offspring from LPS-induced MIA showed a reduced abundance of Actinobacteria, Coprococcus, and Erysipelotrichaies and an increased abundance of Alistipes, Fusobacterium, and Ruminococcus (51). Alterations in gut microbiota compositions were reported for offspring exposed to Poly I:C-induced MIA (75). Sex-specific differences in species richness of the microbiome between Poly I:C-induced MIA BALB/c offspring and controls have been demonstrated at the age of 30 days. Female offspring exposed to MIA had a reduced abundance of Prevotellacea and Porphyromonadaceae and an increased abundance of Lactobacillales, whereas male offspring have an increased abundance of four families of the Firmicutes (52).
Cardiovascular System and Renal System
Hypertension was reported in 12-wk-old Sprague-Dawley offspring exposed to LPS-induced MIA (45). An increased expression of DNA methyltransferase (DNMT) was reported in the renal cortex of offspring from LPS-treated dams. This resulted in an increased global DNA methylation in the renal cortex. The increased expression of DNMTs, as a result of MIA, is regulated by IL-6 through transcription factor Friend leukemia virus integration 1 (FLI-1). The epigenetic modifications in the renal cortex could ultimately lead to hypertension in adulthood (45). Vieira et al. demonstrated that oxidative stress plays a role in the MIA-induced hypertension in the Wistar offspring. The hypertension reported in adult offspring of LPS-treated dams was maintained by vascular and renal dysfunction that is regulated by angiotensin II and NADPH oxidase signaling pathway (16).
Reproductive System
Abnormal reproductive parameters have been reported for offspring of LPS-treated dams. Changes in sex steroid levels have been found for both Wistar male and female offspring at the age of 11 wk (15, 50). Furthermore, a decreased testis weight, seminiferous tubule diameter, and number of spermatid cells in 11-wk-old male offspring have been reported (15). The immunological stress from LPS caused delayed reproductive maturity during offspring development. However, the reproductive field is largely unexplored with regards to long-term effects of MIA and clearly deserves more attention in future.
PREVENTIVE STRATEGIES
A critical inflammation regulator in MIA is NF-κB, which increases the production of proinflammatory cytokines and chemokines to promote inflammation (44). Upregulation of Il6 and Tnfa expression is NF-κB-dependent and is a result of MIA induction by LPS (45). Studies applied pharmacologic and dietary interventions targeting the signaling pathways to attenuate the effect on the offspring. First of all, the TLR4 antagonist (+)-naloxone protected fetal developmental programming in mice from maternal inflammatory mediator. The results showed that the drug suppressed the inflammatory cytokines induced by LPS (40). Furthermore, chronic treatment with antipsychotic paliperidone for 21 days blocked the neuroinflammatory response and stimulated the polarization of microglia to anti-inflammatory M2 profile. The treatment with paliperidone also improved the spatial working memory of the adult offspring (56). Diet-related reduction in n-3 polyunsaturated fatty acid (n-3 PUFAs) aggravated maternal and fetal inflammatory response after exposure to LPS during gestation and revealed spatial memory deficits in adult offspring (37). At the same time, Basil et al. (58) reported that n-3 PUFAs diet tend to attenuate MIA-induced changes on certain genes and further reduce the effect on neurodevelopmental conditions of offspring in Poly I:C model. The LPS and Poly I:C models all indicate that PUFAs are involved in modulating the immune responses and inhibit deleterious effect on offspring. The outcomes for the offspring have some overlaps in both models, not only for triggering the inflammation, but also for the neurological outcomes. Besides, the n-3 PUFAs diet intervention, oral probiotic administration to pregnant dams with MIA prevented the increase of proinflammatory cytokines in the fetal brain and also prevented the social deficits, depression-like and anxiety-like behavior, which have been reported in 8-wk-old offspring exposed to Poly I:C-induced MIA. Furthermore, the decrease of PV+ neurons and neurotransmitter GABA in the prefrontal cortex of adult MIA-exposed offspring was also prevented with intervention with probiotics during pregnancy (74).
FUTURE DIRECTIONS
Numerous studies have examined the effect of MIA on the offspring. Most of these studies induce MIA in rodents, especially in mice or rats, by administration of LPS or Poly I:C on specific gestational days (GD9-17). Administration of LPS or Poly I:C results in a discrete inflammatory response in the dam. Various outcomes were reported in the literature, which could be partially explained by the use of different strains of mice or rats, dose used, and route of application, as summarized in the Tables 1 and 2. The two models, bacterial versus viral mimetics, present similarities and differences in the evaluation of their long-term effect in the offspring. In summary, the LPS MIA model is widely used to explore the effects of MIA on the different systems in the offspring, whereas Poly I:C MIA models are mostly applied to study neurodevelopmental disorders. However, both of them share interaction with neuroimmune pathways (Fig. 1A), which might have influence on outcome of offspring as we described in Fig. 2. Therefore, the difference in observed outcomes might be related to a bias in the experimenter’s choice rather than in biological mechanisms. Therefore, systematic comparisons within the same laboratory facility are necessary.
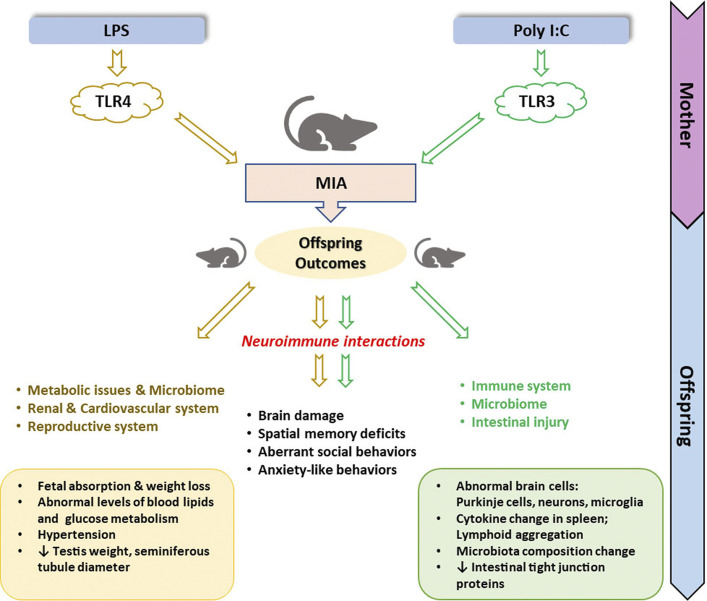
Figure 2.
Overview of offspring outcomes from lipopolysaccharide (LPS) and polyinosinic:polycytidylic acid (Poly I:C) maternal immune activation (MIA) dams.
Research into the effect of MIA on the development of the fetus and the long-term health outcomes is important since maternal inflammation is a common complication during pregnancy. Maternal inflammation can originate from a common cold, influenza, or a bacterial infection like cystitis. LPS and Poly I:C animal models might provide us with possibilities to explain these long-term effects on the offspring. This also applies to SARS-CoV-2, the cause of the current COVID-19 pandemic. However, SARS-CoV-2 is a single-stranded RNA virus with only a short phase of double-stranded RNA in its life cycle. Therefore, further research is needed to evaluate whether Poly I:C is indeed a good model for SARS-CoV-2 infection.
Stimulation of the maternal immune system by a real-world virus not only activates the innate immune system, but also triggers acquired immune response (77). However, whether the vertical transmission exists between the SARS-CoV-2-infected mothers and fetuses is still on debate, not to mention long-term effect on children (2). Some studies report SARS-CoV-2 infection in placenta (78, 79). Meanwhile, evidence in Poly I:C models shows that the changes of the placenta cause abnormal vascular flow and following neural inflammation in rat offspring (66). This leaves the possibility that children from SARS-CoV-2 infected mothers may have neurologic involvement as well.
PERSPECTIVES AND SIGNIFICANCE
The comparative use of different models that mimic bacterial or viral infection or the use of real bacteria and viruses to induce MIA can help elucidate these pathways involved in fetal programming. This, in turn, is of importance for putative future therapies for abnormal organ development, adverse neurological outcomes, and metabolic-related diseases in later life. The LPS and Poly I:C animal models of MIA provide many possibilities for exploring the fetal programming phenomenon and the early development of complex diseases of adulthood. Animal models are valuable experimental models for studying these long-term health consequences.
GRANTS
The China Scholarship Council provided financial support to M. Bao. This research is funded within the Partnership between Nederlandse Organisatie voor Wetenschappelijk (NOW) domain Applied and Engineered Sciences and Danone Nutricia Research (to N. Hofsink) and with additional financial support from Topsector Agri and Food.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.P. conceived and designed research; M.B. and N.H. prepared figures; M.B., N.H., and T.P. drafted manuscript; M.B., N.H., and T.P. edited and revised manuscript; M.B., N.H., and T.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Prof. Dr. Sicco A. Scherjon for critical reading the manuscript and valuable comments.
REFERENCES
- 1.Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA 323: 1848–1849, 2020. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riedel C, Rivera JC, Canedo-Marroquín G, Kalergis AM, Opazo MC. Respiratory viral infections during pregnancy: effects of SARS-CoV-2 and other related viruses over the offspring. J Dev Orig Health Dis. In press, 2021. doi: 10.1017/S2040174420001373. [DOI] [PubMed] [Google Scholar]
- 3.Dong L, Tian J, He S, Zhu C, Wang J, Liu C, Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 323: 1846–1848, 2020. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet (London, England) 395: 809–815, 2020. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, Xia S, Zhou W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr 9: 51–60, 2020. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baud D, Greub G, Favre G, Gengler C, Jaton K, Dubruc E, Pomar L. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 323: 2198–2200, 2020. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandy M, Nyirenda M. Developmental origins of health and disease: the relevance to developing nations. Int Health 10: 66–70, 2018. doi: 10.1093/inthealth/ihy006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simeoni U, Armengaud JB, Siddeek B, Tolsa JF. Perinatal origins of adult disease. Neonatology 113: 393–399, 2018. doi: 10.1159/000487618. [DOI] [PubMed] [Google Scholar]
- 9.Minakova E, Warner BB. Maternal immune activation, central nervous system development and behavioral phenotypes. Birth defects Res 110: 1539–1550, 2018. doi: 10.1002/bdr2.1416. [DOI] [PubMed] [Google Scholar]
- 10.Domínguez Rubio AP, Correa F, Aisemberg J, Dorfman D, Bariani MV, Rosenstein RE, Zorrilla Zubilete M, Franchi AM. Maternal administration of melatonin exerts short- and long-term neuroprotective effects on the offspring from lipopolysaccharide-treated mice. J Pineal Res 63: e12439, 2017. doi: 10.1111/jpi.12439. [DOI] [PubMed] [Google Scholar]
- 11.Solek CM, Farooqi N, Verly M, Lim TK, Ruthazer ES. Maternal immune activation in neurodevelopmental disorders. Dev Dyn 247: 588–619, 2018. doi: 10.1002/dvdy.24612. [DOI] [PubMed] [Google Scholar]
- 12.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1β maturation by caspase-8. J Exp Med 205: 1967–1973, 2008. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sens J, Schneider E, Mauch J, Schaffstein A, Mohamed S, Fasoli K, Saurine J, Britzolaki A, Thelen C, Pitychoutis PM. Lipopolysaccharide administration induces sex-dependent behavioural and serotonergic neurochemical signatures in mice. Pharmacol Biochem Behav 153: 168–181, 2017. doi: 10.1016/j.pbb.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Brown AG, Tulina NM, Barila GO, Hester MS, Elovitz MA. Exposure to intrauterine inflammation alters metabolomic profiles in the amniotic fluid, fetal and neonatal brain in the mouse. PLoS One 12: e0186656, 2017. doi: 10.1371/journal.pone.0186656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izvolskaia MS, Sharova VS, Ignatiuk VM, Voronova SN, Zakharova LA. Abolition of prenatal lipopolysaccharide-induced reproductive disorders in rat male offspring by fulvestrant. Andrologia 51: e13204, 2019. doi: 10.1111/and.13204. [DOI] [PubMed] [Google Scholar]
- 16.Vieira LD, Farias JS, de Queiroz DB, Cabral EV, Lima-Filho MM, Sant’Helena BRM, Aires RS, Ribeiro VS, Santos-Rocha J, Xavier FE, Paixão AD. Oxidative stress induced by prenatal LPS leads to endothelial dysfunction and renal haemodynamic changes through angiotensin II/NADPH oxidase pathway: prevention by early treatment with α-tocopherol. Biochim Biophys acta Mol basis Dis 1864: 3577–3587, 2018. doi: 10.1016/j.bbadis.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Elgin TG, Fricke EM, Gong H, Reese J, Mills DA, Kalantera KM, Underwood MA, McElroy SJ. Fetal exposure to maternal inflammation interrupts murine intestinal development and increases susceptibility to neonatal intestinal injury. Dis Model Mech 12: dmm040808, 2019. doi: 10.1242/dmm.040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amodeo DA, Lai CY, Hassan O, Mukamel EA, Behrens MM, Powell SB. Maternal immune activation impairs cognitive flexibility and alters transcription in frontal cortex. Neurobiol Dis 125: 211–218, 2019. doi: 10.1016/j.nbd.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Valtanen P, van Diermen BA, Lakhan N, Lousberg EL, Robertson SA, Hayball JD, Diener KR. Maternal host responses to Poly(I:C) during pregnancy leads to both dysfunctional immune profiles and altered behaviour in the offspring. Am J Reprod Immunol 84: e13260, 2020. doi: 10.1111/aji.13260. [DOI] [PubMed] [Google Scholar]
- 20.Haida O, Al Sagheer T, Balbous A, Francheteau M, Matas E, Soria F, Fernagut PO, Jaber M. Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Transl Psychiatry 9: 124, 2019. doi: 10.1038/s41398-019-0457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 26: 203–234, 2012. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection—maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol 299: 241–251, 2018. doi: 10.1016/j.expneurol.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol 5: 461, 2014. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussman JP. Cellular and molecular pathways of COVID-19 and potential points of therapeutic intervention. Front Pharmacol 11: 1169, 2020. doi: 10.3389/fphar.2020.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frieman M, Heise M, Baric R. SARS coronavirus and innate immunity. Virus Res 133: 101–112, 2008. doi: 10.1016/j.virusres.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeley JJ, Ghosh S. Molecular mechanisms of innate memory and tolerance to LPS. J Leukoc Biol 101: 107–119, 2017. doi: 10.1189/jlb.3MR0316-118RR. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384, 2010. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 28.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun 388: 621–625, 2009. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 29.Moresco EMY, LaVine D, Beutler B. Toll-like receptors. Curr Biol 21: R488–R493, 2011. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 30.Ren Z, Ding T, Zuo Z, Xu Z, Deng J, Wei Z. Regulation of MAVS expression and signaling function in the antiviral innate immune response. Front Immunol 11: 1030, 2020. doi: 10.3389/fimmu.2020.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol 20: 537–551, 2020. doi: 10.1038/s41577-020-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol 93: 250–256, 2021. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arsenault D, St-Amour I, Cisbani G, Rousseau LS, Cicchetti F. The different effects of LPS and Poly I:C prenatal immune challenges on the behavior, development and inflammatory responses in pregnant mice and their offspring. Brain Behav Immun 38: 77–90, 2014. doi: 10.1016/j.bbi.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Qin S, Chen X, Gao M, Zhou J, Li X. Prenatal exposure to lipopolysaccharide induces PTX3 expression and results in obesity in mouse offspring. Inflammation 40: 1847–1861, 2017. doi: 10.1007/s10753-017-0626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsueh PT, Wang HH, Liu CL, Ni WF, Chen YL, Liu JK. Expression of cerebral serotonin related to anxiety-like behaviors in C57BL/6 offspring induced by repeated subcutaneous prenatal exposure to low-dose lipopolysaccharide. PLoS One 12: e0179970, 2017. doi: 10.1371/journal.pone.0179970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsueh PT, Lin HH, Wang HH, Liu CL, Ni WF, Liu JK, Chang HH, Sun DS, Chen YS, Chen YL. Immune imbalance of global gene expression, and cytokine, chemokine and selectin levels in the brains of offspring with social deficits via maternal immune activation. Genes Brain Behav 17: e12479, 2018. doi: 10.1111/gbb.12479. [DOI] [PubMed] [Google Scholar]
- 37.Labrousse VF, Leyrolle Q, Amadieu C, Aubert A, Sere A, Coutureau E, Grégoire S, Bretillon L, Pallet V, Gressens P, Joffre C, Nadjar A, Layé S. Dietary omega-3 deficiency exacerbates inflammation and reveals spatial memory deficits in mice exposed to lipopolysaccharide during gestation. Brain Behav Immun 73: 427–440, 2018. doi: 10.1016/j.bbi.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Fricke EM, Elgin TG, Gong H, Reese J, Gibson-Corley KN, Weiss RM, Zimmerman K, Bowdler NC, Kalantera KM, Mills DA, Underwood MA, McElroy SJ. Lipopolysaccharide-induced maternal inflammation induces direct placental injury without alteration in placental blood flow and induces a secondary fetal intestinal injury that persists into adulthood. Am J Reprod Immunol 79: e12816, 2018. doi: 10.1111/aji.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Pei D, Yang R, Wan C, Ye Y, Peng S, Zeng Q, Yu Y. Prenatal maternal vaginal inflammation increases anxiety and alters HPA axis signalling in adult male mice. Int J Dev Neurosci 75: 27–35, 2019. doi: 10.1016/j.ijdevneu.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Chin PY, Dorian C, Sharkey DJ, Hutchinson MR, Rice KC, Moldenhauer LM, Robertson SA. Toll-Like receptor-4 antagonist (+)-naloxone confers sexually dimorphic protection from inflammation-induced fetal programming in mice. Endocrinology 160: 2646–2662, 2019. doi: 10.1210/en.2019-00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li G, Ma L, Lin L, Wang Y, Yang H. The intervention effect of aspirin on a lipopolysaccharide-induced preeclampsia-like mouse model by inhibiting the nuclear factor-κB pathway. Biol Reprod 99: 422–432, 2018. doi: 10.1093/biolre/ioy025. [DOI] [PubMed] [Google Scholar]
- 42.Eloundou SN, Lee JY, Wu D, Lei J, Feller MC, Ozen M, Zhu Y, Hwang M, Jia B, Xie H, Clemens JL, McLane MW, AlSaggaf S, Nair N, Wills-Karp M, Wang X, Graham EM, Baschat A, Burd I. Placental malperfusion in response to intrauterine inflammation and its connection to fetal sequelae. PLoS One 14: e0214951, 2019. doi: 10.1371/journal.pone.0214951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JY, Song H, Dash O, Park M, Shin NE, McLane MW, Lei J, Hwang JY, Burd I. Administration of melatonin for prevention of preterm birth and fetal brain injury associated with premature birth in a mouse model. Am J Reprod Immunol 82: e13151, 2019. doi: 10.1111/aji.13151. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Yang J, Bao J, Li X, Ye A, Zhang G, Liu H. Activation of the cholinergic anti-inflammatory pathway by nicotine ameliorates lipopolysaccharide-induced preeclampsia-like symptoms in pregnant rats. Placenta 49: 23–32, 2017. doi: 10.1016/j.placenta.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Cui J, Chen R, Deng Y, Liao X, Wei Y, Li X, Su M, Yu J, Yi P. Prenatal exposure to lipopolysaccharide alters renal DNA methyltransferase expression in rat offspring. PLoS One 12: e0169206, 2017. doi: 10.1371/journal.pone.0169206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu S, Wen Y, Li J, Zhang H, Liu Y. Prenatal lipopolysaccharide exposure promotes dyslipidemia in the male offspring rats. Front Physiol 9: 542, 2018. doi: 10.3389/fphys.2018.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mouihate A, Kalakh S, AlMutairi R, Alashqar A. Prenatal inflammation dampens neurogenesis and enhances serotonin transporter expression in the hippocampus of adult female rats. Med Princ Pract 28: 352–360, 2019. doi: 10.1159/000499658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talukdar PM, Abdul F, Maes M, Binu VS, Venkatasubramanian G, Kutty BM, Debnath M. Maternal immune activation causes schizophrenia-like behaviors in the offspring through activation of immune-inflammatory, oxidative and apoptotic pathways, and lowered antioxidant defenses and neuroprotection. Mol Neurobiol 57: 4345–4361, 2020. doi: 10.1007/s12035-020-02028-8. [DOI] [PubMed] [Google Scholar]
- 49.Simões LR, Sangiogo G, Tashiro MH, Generoso JS, Faller CJ, Dominguini D, Mastella GA, Scaini G, Giridharan VV, Michels M, Florentino D, Petronilho F, Réus GZ, Dal-Pizzol F, Zugno AI, Barichello T. Maternal immune activation induced by lipopolysaccharide triggers immune response in pregnant mother and fetus, and induces behavioral impairment in adult rats. J Psychiatr Res 100: 71–83, 2018. doi: 10.1016/j.jpsychires.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Ignatiuk VM, Izvolskaya MS, Sharova VS, Voronova SN, Zakharova LA. Disruptions in the reproductive system of female rats after prenatal lipopolysaccharide-induced immunological stress: role of sex steroids. Stress 22: 133–141, 2019. doi: 10.1080/10253890.2018.1508440. [DOI] [PubMed] [Google Scholar]
- 51.Lee GA, Lin YK, Lai JH, Lo YC, Yang YC, Ye SY, Lee CJ, Wang CC, Chiang YH, Tseng SH. Maternal immune activation causes social behavior deficits and hypomyelination in male rat offspring with an autism-like microbiota profile. Brain Sci 11: 1085, 2021. doi: 10.3390/brainsci11081085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juckel G, Manitz M-P, Freund N, Gatermann S. Impact of Poly I:C induced maternal immune activation on offspring’s gut microbiome diversity—implications for schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 110: 110306, 2021. doi: 10.1016/j.pnpbp.2021.110306. [DOI] [PubMed] [Google Scholar]
- 53.Mandal M, Marzouk AC, Donnelly R, Ponzio NM. Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain Behav Immun 25: 863–871, 2011. doi: 10.1016/j.bbi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Giulivi C, Napoli E, Schwartzer J, Careaga M, Ashwood P. Gestational exposure to a viral mimetic Poly(I:C) results in long-lasting changes in mitochondrial function by leucocytes in the adult offspring. Mediators Inflamm 2013: 609602, 2013. doi: 10.1155/2013/609602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang B, Jia H, Kast RJ, Thomas EA. Epigenetic changes at gene promoters in response to immune activation in utero. Brain Behav Immun 30: 168–175, 2013. doi: 10.1016/j.bbi.2013.01.086. [DOI] [PubMed] [Google Scholar]
- 56.MacDowell KS, Munarriz-Cuezva E, Caso JR, Madrigal JLM, Zabala A, Meana JJ, García-Bueno B, Leza JC. Paliperidone reverts Toll-like receptor 3 signaling pathway activation and cognitive deficits in a maternal immune activation mouse model of schizophrenia. Neuropharmacology 116: 196–207, 2017. doi: 10.1016/j.neuropharm.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 57.da Silveira VT, Medeiros D de C, Ropke J, Guidine PA, Rezende GH, Moraes MFD, Mendes EMAM, Macedo D, Moreira FA, de Oliveira ACP. Effects of early or late prenatal immune activation in mice on behavioral and neuroanatomical abnormalities relevant to schizophrenia in the adulthood. Int J Dev Neurosci 58: 1–8, 2017. doi: 10.1016/j.ijdevneu.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Basil P, Li Q, Gui H, Hui TCK, Ling VHM, Wong CCY, Mill J, McAlonan GM, Sham PC. Prenatal immune activation alters the adult neural epigenome but can be partly stabilised by a n − 3 polyunsaturated fatty acid diet. Transl Psychiatry 8: 125, 2018. doi: 10.1038/s41398-018-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Missig G, Finger BC, Landino SM, Alexander AJ, Mokler EL, Robbins JO, Manasian Y, Kim W, Kim KS, McDougle CJ, Carlezon WA, Bolshakov VY. Maternal and early postnatal immune activation produce dissociable effects on neurotransmission in mPFC–amygdala circuits. J Neurosci 38: 3358–3372, 2018. doi: 10.1523/JNEUROSCI.3642-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carlezon WA, Kim W, Missig G, Finger BC, Landino SM, Alexander AJ, Mokler EL, Robbins JO, Li Y, Bolshakov VY, McDougle CJ, Kim K-S, Kim K-S. Maternal and early postnatal immune activation produce sex-specific effects on autism-like behaviors and neuroimmune function in mice. Sci Rep 9: 16928, 2019. doi: 10.1038/s41598-019-53294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barke TL, Money KM, Du L, Serezani A, Gannon M, Mirnics K, Aronoff DM. Sex modifies placental gene expression in response to metabolic and inflammatory stress. Placenta 78: 1–9, 2019. doi: 10.1016/j.placenta.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Openshaw RL, Kwon J, McColl A, Penninger JM, Cavanagh J, Pratt JA, Morris BJ. JNK signalling mediates aspects of maternal immune activation: importance of maternal genotype in relation to schizophrenia risk. J Neuroinflammation 16: 18, 2019. doi: 10.1186/s12974-019-1408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsivion-Visbord H, Kopel E, Feiglin A, Sofer T, Barzilay R, Ben-Zur T, Yaron O, Offen D, Levanon EY. Increased RNA editing in maternal immune activation model of neurodevelopmental disease. Nat Commun 11: 5236, 2020. doi: 10.1038/s41467-020-19048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dabbah-Assadi F, Alon D, Golani I, Doron R, Kremer I, Beloosesky R, Shamir A. The influence of immune activation at early vs late gestation on fetal NRG1-ErbB4 expression and behavior in juvenile and adult mice offspring. Brain Behav Immun 79: 207–215, 2019. doi: 10.1016/j.bbi.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Ding S, Hu Y, Luo B, Cai Y, Hao K, Yang Y, Zhang Y, Wang X, Ding M, Zhang H, Li W, Lv L. Age-related changes in neuroinflammation and prepulse inhibition in offspring of rats treated with Poly I:C in early gestation. Behav Brain Funct 15: 3, 2019. doi: 10.1186/s12993-019-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McColl ER, Piquette-Miller M. Poly(I:C) alters placental and fetal brain amino acid transport in a rat model of maternal immune activation. Am J Reprod Immunol 81: e13115, 2019. doi: 10.1111/aji.13115. [DOI] [PubMed] [Google Scholar]
- 67.Hu Y, Hong XY, Yang XF, Ma RH, Wang X, Zhang JF, Feng Q, Li XG, Sun DS, Li X, Wan HL, Li T, Wang Q, Ke D, Wang JZ, Liu GP. Inflammation-dependent ISG15 upregulation mediates MIA-induced dendrite damages and depression by disrupting NEDD4/Rap2A signaling. Biochim Biophys Acta Mol Basis Dis 1865: 1477–1489, 2019. doi: 10.1016/j.bbadis.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 68.Meehan C, Harms L, Frost JD, Barreto R, Todd J, Schall U, Shannon Weickert C, Zavitsanou K, Michie PT, Hodgson DM. Effects of immune activation during early or late gestation on schizophrenia-related behaviour in adult rat offspring. Brain Behav Immun 63: 8–20, 2017. doi: 10.1016/j.bbi.2016.07.144. [DOI] [PubMed] [Google Scholar]
- 69.Hollins SL, Brock L, Barreto R, Harms L, Dunn A, Garcia-Sobrinho P, Bruce J, Dickson PW, Walker MM, Keely S, Hodgson DM. A rodent model of anxiety: the effect of perinatal immune challenges on gastrointestinal inflammation and integrity. Neuroimmunomodulation 25: 163–175, 2018. doi: 10.1159/000493320. [DOI] [PubMed] [Google Scholar]
- 70.Kowash HM, Potter HG, Edye ME, Prinssen EP, Bandinelli S, Neill JC, Hager R, Glazier JD. Poly(I:C) source, molecular weight and endotoxin contamination affect dam and prenatal outcomes, implications for models of maternal immune activation. Brain Behav Immun 82: 160–166, 2019. doi: 10.1016/j.bbi.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Xu D-X, Wang H, Zhao L, Ning H, Chen Y-H, Zhang C. Effects of low-dose lipopolysaccharide (LPS) pretreatment on LPS-induced intra-uterine fetal death and preterm labor. Toxicology 234: 167–175, 2007. doi: 10.1016/j.tox.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 72.Mueller FS, Richetto J, Hayes LN, Zambon A, Pollak DD, Sawa A, Meyer U, Weber-Stadlbauer U. Influence of Poly(I:C) variability on thermoregulation, immune responses and pregnancy outcomes in mouse models of maternal immune activation. Brain Behav Immun 80: 406–418, 2019. doi: 10.1016/J.BBI.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 73.Tsukada T, Shimada H, Sakata-Haga H, Iizuka H, Hatta T. Molecular mechanisms underlying the models of neurodevelopmental disorders in maternal immune activation relevant to the placenta. Congenit Anom (Kyoto) 59: 81–87, 2019. doi: 10.1111/cga.12323. [DOI] [PubMed] [Google Scholar]
- 74.Wang X, Yang J, Zhang H, Yu J, Yao Z. Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism Res 12: 576–588, 2019. doi: 10.1002/aur.2079. [DOI] [PubMed] [Google Scholar]
- 75.Li W, Chen M, Feng X, Song M, Shao M, Yang Y, Zhang L, Liu Q, Lv L, Su X. Maternal immune activation alters adult behavior, intestinal integrity, gut microbiota and the gut inflammation. Brain Behav 11: e02133, 2021. doi: 10.1002/brb3.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanamsagar R, Bilbo SD. Environment matters: microglia function and dysfunction in a changing world. Curr Opin Neurobiol 47: 146–155, 2017. doi: 10.1016/j.conb.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reisinger S, Khan D, Kong E, Berger A, Pollak A, Pollak DD. The Poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther 149: 213–226, 2015. doi: 10.1016/j.pharmthera.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol 154: 23–32, 2020. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baergen RN, Heller DS. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol 23: 177–180, 2020. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]