Keywords: nutrient excess, oxidative stress, redox physiology
Abstract
A role for fat overfeeding in metabolic dysfunction in humans is commonly implied in the literature. Comparatively less is known about acute carbohydrate overfeeding (COF). We tested the hypothesis that COF predisposes to oxidative stress by channeling electrons away from antioxidants to support energy storage. In a study of 24 healthy human subjects with and without obesity, COF was simulated by oral administration of excess carbohydrates; a two-step hyperinsulinemic clamp was used to evaluate insulin action. The distribution of electrons between oxidative and reductive pathways was evaluated by the changes in the reduction potentials (Eh) of cytoplasmic (lactate, pyruvate) and mitochondrial (β-hydroxybutyrate, acetoacetate) redox couples. Antioxidant redox was measured by the ratio of reduced to oxidized glutathione. We used cross-correlation analysis to evaluate the relationships between the trajectories of Eh, insulin, glucose, and respiratory exchange during COF. DDIT3 and XBP1s/u mRNA were measured as markers of endoplasmic reticulum stress (ER stress) in adipose tissue before and after COF. Here, we show that acute COF is characterized by net transfer of electrons from mitochondria to cytoplasm. Circulating glutathione is oxidized in a manner that significantly cross-correlates with increasing insulin levels and precedes the decrease in cytoplasmic Eh. This effect is more pronounced in overweight individuals (OW). Markers of ER stress in subcutaneous fat are detectable in OW within 4 h. We conclude that acute COF contributes to metabolic dysfunction through insulin-dependent pathways that promote electron transfer to the cytoplasm and decrease antioxidant capacity. Characterization of redox during overfeeding is important for understanding the pathophysiology of obesity and type 2 diabetes.
NEW & NOTEWORTHY Current principles assume that conversion of thermic energy to metabolically useful energy follows fixed rules. These principles ignore the possibility of variable proton uncoupling in mitochondria. Our study shows that the net balance of electron distribution between mitochondria and cytoplasm is influenced by insulin in a manner that reduces proton leakage during overfeeding. Characterization of the effects of insulin on redox balance is important for understanding obesity and insulin resistance.
INTRODUCTION
Over the past 30 years, research has emphasized the role of dietary fat in the development of obesity. Mechanisms linking excess fat to obesity, insulin resistance, and type 2 diabetes (T2D) are widely accepted (1, 2). It is also known that overfeeding of long-chain triglycerides is associated with increased mitochondrial reactive oxygen species (ROS) production in isolated human muscle fibers (3, 4). Smith et al. (5) have shown that ROS generation is directly proportional to β-oxidation and the rate of electron flux through the mitochondrial electron transport chain (ETC). On the other hand, the pathophysiology relating excess carbohydrates (CHO) to metabolic dysfunction is less clear. Despite evidence from large epidemiological studies linking sugary beverages to metabolic dysfunction (6–8), the public remains complacent about excessive CHO consumption. For example, the casual use of sugary beverages as “refreshments” remains a widely accepted cultural norm in our society. Efforts aiming to draw attention to the harmful effects of sugar-containing soft drinks are frequently countered by industry-sponsored statements claiming lack of sufficient evidence (9).
The immediate impact of carbohydrate overfeeding (COF), defined as the consumption of excess CHO in one meal, on metabolic dysfunction has not been studied in real time. We hypothesize that COF adversely impacts the antioxidant capacity by channeling electrons, in the form of reducing equivalents, to support new fat synthesis. Electrons carried by the pyridine nucleotide NADPH are needed in the chemical reduction steps that add 2-carbon acetate moieties into new fatty acid molecules. As excess carbons entering the TCA cycle during COF are directed to fatty acid synthesis (de novo lipogenesis, DNL), reducing equivalents are simultaneously transported from mitochondria to cytoplasm through the malate and isocitrate shuttle mechanism. However, this mechanism provides only half of the necessary electrons to support DNL, thus leaving the possibility that other sources of electrons are required (10, 11). We postulate that, at least in part, this demand for reducing equivalents is met by channeling NADPH molecules that are otherwise used to maintain antioxidant activity via peroxidases and reductases.
The purpose of the current study was to test this hypothesis in healthy normal weight (NW) and overweight (OW) human subjects. We conducted two experimental protocols during which subjects consumed an oral CHO load up to 3.75 mg/kg body wt (protocol 1) and underwent a modified hyperinsulinemic glucose clamp following an oral load of 100 g glucose (protocol 2). Changes in cytoplasmic and mitochondrial redox couples and reduced/oxidized glutathione were measured and used to determine the balance of oxidative and reductive processes. The primary expected outcome under the premise of our hypothesis of COF is a more reduced cytoplasmic redox state (to support DNL) and a more oxidized glutathione redox state. In addition, we measured markers of endoplasmic reticulum (ER) stress in subcutaneous adipose tissue after 4 h of COF or intravenous glucose in both protocols. The results of this study support our hypothesis and provide a framework for understanding the reductive actions of insulin in relation to overfeeding and bioenergetics.
METHODS
Subjects
Twenty-four subjects (15 females, 9 males; ages 21–54 yr) participated in one of two separate protocols designed to achieve acute carbohydrate overfeeding. Subjects were chosen based on body mass index (BMI, kg/m2) to represent two groups, overweight (OW, n = 12, BMI > 28) and normal weight (NW, n = 12, BMI 21–28). Exclusion criteria included diagnosis of T2D, use of insulin sensitizing agents, hormonal supplements, psychotropic, and anti-inflammatory medications. All subjects had normal blood chemistry profiles, including renal and liver function tests and thyroid stimulating hormone levels. The study protocol was approved by the Institutional Review Board at the Boston University Medical Center; all subjects gave their verbal and written informed consent before participating in the study.
Study Design
The study was designed to increase the likelihood of activating DNL pathways in response to acute COF (Fig. 1). For this purpose, subjects were asked to consume a eucaloric high CHO diet to maximize glycogen storage before the acute CHO overloading procedure. The pretest diet was adjusted to each participant’s energy requirement, which was based on measured resting metabolic rate (RMR) assessed at the screening visit. The diet provided ∼70% energy as CHO, 15% as protein, and 15% as fat, while maintaining stable weight over the 7-day period before the testing procedures.
Figure 1.

Diagram of the study design. Subjects consumed a eucaloric, high-carbohydrate diet for 1 wk, then participated in one of two protocols. In protocol 1, subjects consumed a large carbohydrate load given as breakfast and glucose beverage. In protocol 2, subjects consumed a glucose beverage and then underwent a hyperinsulinemic clamp procedure at 150 mg/dL plasma glucose concentration. Blood and respiratory gas exchange were sampled throughout the 4-h procedures. Subcutaneous abdominal fat was sampled before and at the end of the procedures. AcAc, acetoacetate; BMI, body mass index; βOHB, beta hydroxybutyrate; GSH, reduced glutathione; GSSG, oxidized glutathione; n = number of subjects; NW, normal weight; OW, overweight.
Subjects chose between two protocols, which were conducted at the General Clinical Research Unit of Boston University Medical Campus following a 10–12 h overnight fast.
Protocol 1 (oral).
The objective of this protocol was to activate DNL in response to excess CHO in a single meal. A nonprotein respiratory quotient (RQ) > 1.0 was considered an indicator of net DNL. Overfeeding started after obtaining baseline blood and subcutaneous adipose tissue samples and consisted of a meal of cinnamon raisin bagels followed by a glucose beverage (Trutol). The glucose beverage was administered over a period of 2 h and discontinued when the RQ exceeded 1.0 or when the total CHO load (bagel + beverage) was 3.75 g per kg of body weight. Since some subjects achieved RQ > 1.0 before reaching the maximum CHO load, the actual mean CHO intake was 336 ± 35 g.
Protocol 2 (clamp).
The purpose of this protocol was to evaluate insulin actions under conditions of excess CHO. Following catheter placement and baseline blood and fat sampling, subjects consumed 100 g glucose as a bolus oral beverage (Trutol only). When blood glucose reached 150 mg/dL, a second baseline blood sample was collected and insulin infusion (Humulin R) was started at a rate of 100 mU/m2/min (clamp 1); clamp conditions were maintained at plasma glucose concentration of 150 mg/dL by variable infusion rate of 20% dextrose, adjusted every 5 min. At 2 h, the insulin infusion was increased to 200 mU/m2/min (clamp 2) and the clamp conditions were maintained for another 2 h. The procedures for respiratory gas exchange and blood sampling were identical in the two protocols.
Respiratory Exchange
Respiratory exchanges were measured by indirect calorimetry (TrueOne 2400 Metabolic Measurement System, Parvomedics, Salt Lake City, UT). Gas measurements were obtained in continuous 15-min segments at baseline (fasting) and at 30-min intervals over the 240-min of either procedure. Energy expenditure and RQ were calculated from oxygen consumption and carbon dioxide production averaged over the last 10-min of each 15-min assessment period. RQ measurements were adjusted for protein metabolism assuming a urinary nitrogen excretion of 11.2 g/day (0.008 g/min) based on the reported average dietary protein intake.
Blood and Fat Sampling
Blood was sampled every 30 min for redox couple measurements; subcutaneous abdominal fat was sampled before and after the procedures (total 48 samples, 2 per subject) by needle biopsy at the suprailiac crest. Briefly, a sterile field was prepared, and a 10-cm area was isolated, cleaned, and anesthetized with 1% lidocaine. A liposuction cannula (Unitech 1 or 3-hole cannula, 3 mm × 12 cm) was connected to a 60-mL syringe via a 24 “in.” segment of standard intravenous tubing primed with a small volume of sterile 0.9% normal saline. The cannula was passed through a small cutaneous incision (<3 mm) and ∼1–2 g of adipose tissue was aspirated under negative vacuum pressure in the 60-mL syringe.
Tissue Processing
A portion of adipose tissue was placed in sterile, ambient temperature culture media (Media 199, Invitrogen, Carlsbad, CA) and transported to the laboratory where it was weighed and snap frozen in liquid nitrogen and stored at −80°C until extracted for total RNA with Trizol reagent (Invitrogen/LifeTechnologies). The total RNA was reverse transcribed with random primer (Superscript III, Invitrogen). Taqman format (FAM) was used in the real-time PCR to determine the abundance of target transcripts. A 10-mL mixture containing either target (DDIT3, XBP1s/u variants, PPP1R15A, ASNS, HSPA5) or endogenous control probe (CDKN1A) was run at ABI 7900HT system for 40 cycles (15-s annealing at 93°C and 60-s amplifying-extension at 60°C). All probes were purchased from Invitrogen (Waltham, MA) and the amplicants were examined against their dissociation curves. The relative abundance of each target transcript was expressed according to the 2−ΔΔCT method.
Blood Sample Collection and Biochemical Analysis
Approximately 4 mL of venous blood was collected at baseline and every 30 min for a total of 240 min. Samples for analysis of glucose were analyzed immediately via the glucose oxidase method (YSI 2300 Stat Plus, YSI Incorporated, Yellow Springs, OH). Concentrations of β-hydroxybutyrate, acetoacetate, lactate, and pyruvate were analyzed based on methods of Williamson and Corkey (12). Briefly, 500 μL of whole blood was aliquoted to cryotubes containing 500 μL of perchloric acid (BioVision, Inc., Milpitas, CA) and spun at 13,000 rpm at 4°C. Aliquots were stored in 2-mL cryotubes at −80°C for no longer than 2–4 days before analysis. Prior to the enzymatic assay procedure, the supernatant was neutralized by adding 3 µL of neutralization solution (BioVision, Inc., Milpitas, CA) to 250 µL of sample and then diluted 1:2 in distilled water. Enzymatic assays were performed, and the concentrations of each analyte were measured spectrophotometrically by determining the optical density of NADH at 340 nM in each reaction mixture using an Infinite M1000 microplate reader (Tecan Systems, San Jose, CA). Enzymes and standards were obtained from Sigma-Aldrich (St. Louis, MO) and buffers were prepared using salts from Sigma-Aldrich (St. Louis, MO) and Thermo Fisher Scientific, Inc. (Waltham, MA).
GSH/GSSG Assay
Venous samples were immediately treated with a thiol-scavenging reagent (1-methyl-2-vinylpyridinium trifluoromethanesulfonate). The treated samples were snap frozen in liquid nitrogen and stored at −80°C until analysis. A GSH/GSSG-412 kit (Percipio Biosciences, Portland, OR) was used for the colorimetric determination of reduced and oxidized blood glutathione (GSH/GSSG), according to the manufacturer’s protocol. Plasma GSH was determined with an Infinite M1000 microplate reader (Tecan Systems, San Jose, CA) at 412 nm via reaction with Ellman reagent (5,5′-dithiobis-2-nitrobenzoic acid). Parallel standard curves were included for each assay separately.
Redox Model and Calculations
The basic principle in this model is that minute-to-minute changes in the redox states of the pyridine nucleotides in cytoplasm and mitochondria are the result of electron flow between oxidative and reductive processes. Oxidation of fuel substrates feeds electrons into the electron transport chain with energy released to support ATP synthesis in mitochondria. On the other hand, synthesis of new substrate molecules in the cytoplasm, such as fat in DNL, utilizes reductive chemical processes requiring the acquisition of new electrons. Figure 2 outlines the pathway for electron transport from mitochondria to cytoplasm through the malate shuttle, which represents a “built-in” mechanism to increase cytoplasmic NADPH when substrate flux favors DNL. The key reaction in this process, catalyzed by malic enzyme, generates pyruvate. In the cytoplasm, pyruvate is also a substrate in the redox equilibrium reaction with lactate. Sharing of pyruvate between the two enzymatic reactions ensures that the changes in the redox state of NADPH/NADP in the cytoplasm are communicated to the redox state of the NADH/NAD couple.
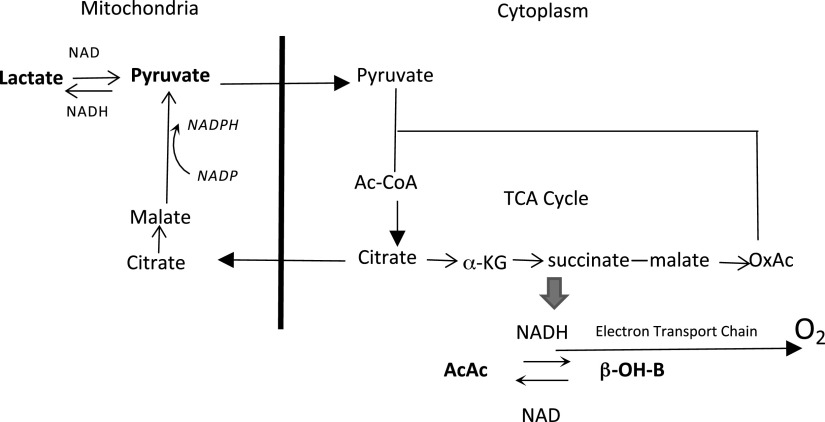
Figure 2.
Model showing substrate flux during feeding/overfeeding and operation of the malate shuttle. During carbohydrate overfeeding, excess carbons are transported to the cytoplasm as citrate. Cytoplasmic citrate generates pyruvate and NADPH through the action of malic enzyme. The NADPH generated through this shuttle mechanism provides half of the reducing equivalents required in de novo lipogenesis (DNL). The two pyridine nucleotide systems share cytoplasmic pyruvate as a common reactant; thus, changes in the NADPH/NADP ratio are reflected in the NADH/NAD ratio. AcAc, acetoacetate; Ac-CoA, acetyl CoA; β-OH-B, beta hydroxybutyrate; OxAc, oxaloacetate.
Cytoplasmic, mitochondrial, and glutathione redox states were estimated from the (lactate/pyruvate), (β-OH-butyrate/acetoacetate), and (GSH/GSSG) ratios, respectively (13, 14). These ratios are converted to reduction potentials (Ehcyto, Ehmito, and EhGSH, respectively) according to the following form of the Nernst equation (15, 16)
where Eo is the standard reduction potential; R is the universal gas constant, 8.314 J/K/mol; T is temperature in degrees kelvin; n is the number of electrons transferred; and F is the Faraday constant, 96,500 C/mol. Eo was obtained from published values for the lactate dehydrogenase (cytoplasm), β-OH butyrate dehydrogenase (mitochondria), and glutathione reductase reactions.
In addition to Ehcyto and Ehmito, we calculated their algebraic difference, EhΔ (Ehmito – Ehcyto). In the appendix, we show that Ehcyto and Ehmito relate to the phosphorylation state of the adenine nucleotides in a reciprocal manner. Thus, EhΔ bears a relationship to the state of cellular energy charge (appendix, Eq. A8) and will be evaluated as a measure of overfeeding in this study.
Clamp Calculations
We calculated total glucose disposal rates from the rates of glucose infusion assuming negligible endogenous glucose production. Oxidative and nonoxidative glucose disposal rates were derived from the total glucose disposal rates and the estimated rates of glucose oxidation derived from the respiratory exchange data. Rates of glucose disposal were normalized per surface area (M values). The increase in total glucose disposal relative to the increase in insulin concentration between the two steps of the clamp procedure was calculated as a measure of insulin sensitivity at hyperglycemic conditions.
Data Analyses and Statistics
Data are summarized as means ± SE. Baseline characteristics were analyzed by ANOVA, with multiple group comparison by Bonferroni. Multivariable repeated measures ANOVA was used to determine difference in substrate concentrations, metabolic attributes, and Eh values within (effect of time) and between subjects (effects of body weight group and protocol as stratification variables). Whenever applicable, we used the general linear model for comparisons of continuous variables between the stratification variables. The correlation of two time series variables was determined contemporaneously and at various time lag intervals by cross-correlation analysis, also known as sliding dot product. This analysis provides a measure of the similarity of two time series variables as a function of the displacement of one variable relative to the other. Cross-correlation coefficients were calculated at each time-lag point and presented graphically within the 95% confidence distribution boundaries. Estimates of the rates of change in Eh (dEh/dt) during the two-step insulin clamp were calculated by the least-squares differentiation method. For both cross-correlation and differentiation analyses, we used mean values of the selected variables separately for OW and NW subjects. Statistical significance was set at a P value of 0.05 or lower. All statistical analyses were performed using the IBM-SPSS statistical package, version 27.
RESULTS
Baseline Characteristics and Fuel Substrates
Characteristics of the subjects participating in this study are summarized in Table 1, separately for each protocol. Differences between the two protocols were not statistically significant except for pyruvate, which was slightly higher in OW subjects of protocol 2 (P = 0.003). Compared with NW (BMI 20.4–28.0 kg/m2), OW (BMI 29.7–36.7 kg/m2) subjects were older (P = 0.041) and more insulin resistant (homeostatic model assessment for insulin resistance, HOMA-IR approximately two times higher, P = 0.051). Fasting glucose and insulin were slightly higher in OW subjects (P not significant); and, except for serum lactate, all the other substrates were similar between the two groups. In OW subjects, fasting lactate was almost twice that in NW subjects (P = 0.003), while pyruvate levels were similar. Thus, OW subjects had a significantly more reduced cytoplasmic redox state (Lac/Pyr ratio) during fasting than NW subjects. In protocol 2, subjects consumed 100 g glucose before the clamp, which led to significant increases in glucose and insulin concentrations. However, all other measured substrates did not change significantly between baseline and start of the clamp. The higher concentration of fasting lactate in OW subjects remained significant (P = 0.016) at the beginning of the clamp.
Table 1.
Baseline characteristics of subjects in both protocols and prior to clamp in protocol 2
| Group | Age, yr | BMI, kg/m2 | Glucose, mg/dL | Insulin, µU/mL | RQ | HOMA-IR | Lactate, µM | Pyruvate, µM | Β-OHB, µM | AcAc, µM | GSH, mM | GSSG, mM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fasting | Protocol 1 | |||||||||||
| OW, n = 6 | 40.9 ± 3.9 | 36.2 ± 3.4 | 101.4 ± 5.7 | 11.7 ± 3.7 | 0.849 ± 0.023 | 2.53 ± 0.83 | 709 ± 90 | 35 ± 4 | 103 ± 34 | 17 ± 6 | 8.443 ± 2.061 | 1.000 ± 0.120 |
| NW, n = 6 | 33.4 ± 4.3* | 21.3 ± 0.2** | 86.7 ± 3.7 | 4.2 ± 2.3 | 0.806 ± 0.027 | 0.52 ± 0.08* | 533 ± 87** | 53 ± 4 | 134 ± 33 | 21 ± 6 | 4.760 ± 2.287 | 1.060 ± 0.183 |
| Fasting | Protocol 2 | |||||||||||
| OW, n = 6 | 39.6 ± 4.8 | 31.9 ± 1.1 | 94.7 ± 3.6 | 15.4 ± 6.3 | 0.800 ± 0.018 | 2.48 ± 0.68 | 1,106 ± 110 | 121 ± 16^^ | 126 ± 53 | 36 ± 11 | 4.033 ± 1.040 | 0.433 ± 0.105 |
| NW, n = 6 | 30.5 ± 3.2* | 25.0 ± 1.4** | 100.2 ± 10.1 | 11.3 ± 4.0 | 0.853 ± 0.035 | 1.82 ± 0.32* | 429 ± 138** | 55 ± 15 | 92 ± 23 | 26 ± 8 | 1.833 ± 1.173 | 0.183 ± 0.113 |
| Pre-clamp | Protocol 2 | |||||||||||
| OW, n = 6 | 39.6 ± 4.8 | 31.9 ± 1.1 | 145.0 ± 5.9## | 60.0 ± 22.3## | 0.805 ± 0.018 | 2.48 ± 0.68 | 1015 ± 96 | 125 ± 8^^ | 111 ± 38 | 41 ± 8 | 5.483 ± 1.348 | 0.633 ± 0.191 |
| NW, n = 6 | 30.5 ± 3.2* | 25.0 ± 1.4** | 150.3 ± 8.8## | 65.7 ± 18.1## | 0.867 ± 0.031 | 1.82 ± 0.32* | 533 ± 138** | 83 ± 28 | 84 ± 14 | 33 ± 7 | 2.467 ± 1.910 | 0.333 ± 0.196 |
Data are means ± SE. AcAc, acetoacetate; BMI, body mass index; βOHB, beta-hydroxybutyrate; GSH, reduced glutathione; GSSG, oxidized glutathione; HOMA-IR, homeostatic model assessment-insulin resistance index; NW, normal weight; OW, overweight; RQ, respiratory quotient.
*P < 0.05 for NW vs. OW;
**P < 0.01 for NW vs. OW;
##P < 0.01 for fasting vs. pre-clamp;
^^P < 0.01 for protocol 2 vs. protocol 1.
Figure 3 shows the changes in glucose, insulin, and nonprotein RQ in the two protocols. The RQ progressively increased during both protocols (P < 0.001, repeated measures ANOVA, effect of time). Compared with NW subjects, OW subjects had lower RQ levels, which were statistically significant in the interval between minutes 90 and 180 of the clamp (P < 0.05). Data summarizing the changes in glucose disposal (total, oxidative, and nonoxidative) are presented in Table 2. The increase in insulin between clamps 1 and 2 was significant in both groups and was associated with increases in all parameters of glucose utilization (P < 0.005, repeated-measures ANOVA) except for oxidative glucose disposal normalized by insulin concentration (Moxid/I), which decreased during clamp 2 (P < 0.05, repeated-measures ANOVA). There were no significant differences in insulin and glucose disposal rates between OW and NW subjects during the transition from clamp 1 to clamp 2 except for Moxid/I (P < 0.05 for Clamp × Group interaction, repeated measures ANOVA). When clamps 1 and 2 are considered separately, OW subjects had significantly lower M/I, Moxid, Moxid/I, and Mnonoxid/I than NW subjects in clamp 2 (P < 0.05, two-way ANOVA). There were no statistically significant differences between OW and NW subjects in clamp 1 for any of these parameters.
Figure 3.
Changes in blood glucose (mg/dL), respiratory quotient (RQ), and plasma insulin (µU/mL) during the two protocols. A: protocol 1 (oral); B: protocol 2 (clamp). Open circles: normal weight (NW subjects); black circles: overweight (OW) subjects. Data are means. Statistical analyses of trajectories were performed using repeated-measures ANOVA and are described in the text.
Table 2.
Summary of glucose disposal rates in protocol 2
| Group | Insulin, µU/mL | M, mg/min/m2 | M/I, mg/min/m2/µU⋅mL | Moxid, mg/min/m2 | Moxid/I, mg/min/m2/µU⋅mL | Mnon-oxid, mg/min/m2 | Mnon-oxid/I, mg/min/m2/µU⋅mL |
|---|---|---|---|---|---|---|---|
| OW | |||||||
| Clamp 1 | 344.2 ± 102.4 | 271.8 ± 68.5 | 1.20 ± 0.37 | 97.9 ± 11.5 | 0.40 ± 0. 10 | 173.9 ± 59.4 | 0.80 ± 0.28 |
| Clamp 2 | 508.7 ± 101.1 | 660.5 ± 65.0 | 1.52 ± 0.23 | 125.5 ± 8.3 | 0.31 ± 0.07 | 535.0 ± 67.3 | 1.21 ± 0.18 |
| NW | |||||||
| Clamp 1 | 185.2 ± 30.3 | 316.6 ± 68.3 | 1.91 ± 0.48 | 122.6 ± 17.1 | 0.79 ± 0.19 | 194.0 ± 57.3 | 1.12 ± 0.40 |
| Clamp 2 | 302.3 ± 48.4 | 769.0 ± 53.3 | 2.99 ± 0.61* | 162.5 ± 12.7* | 0.69 ± 0.11* | 606.5 ± 52.5 | 2.38 ± 0.51* |
| All | |||||||
| Clamp 1 | 264.7 ± 56.2 | 294.2 ± 46.6 | 1.56 ± 0.31 | 110.2 ± 10.5 | 0.60 ± 0.12 | 183.9 ± 39.5 | 0.96 ± 0.24 |
| Clamp 2 | 405.5 ± 61.8^^ | 714.8 ± 43.3^^ | 2.26 ± 0.38^^ | 144.0 ± 9.2^^ | 0.46 ± 0.08^# | 570.8 ± 42.1^^ | 1.79 ± 0.31^^ |
Data are means ± SE. M, total glucose disposal rate; M/I, total glucose per µU/mL insulin; Mnon-oxid, glucose disposal in nonoxidative pathways; Moxid, glucose disposal in oxidative pathway; NW: normal weight; OW: overweight. All glucose disposal rates are normalized per surface area in m2.
Statistical analyses:
Two-way ANOVA (within-clamp Group comparison), *P < 0.05 for NW vs. OW;
Repeated-measures ANOVA (Clamp and Clamp × Group interaction), ^P < 0.05, ^^P < 0.005 for clamp;
#P < 0.05 for Group × Clamp interaction.
Redox Couples
Changes in redox couples from both protocols are summarized in Fig. 4 as reduction potentials (Eh). We initially determined whether either intervention significantly altered redox homeostasis versus the null hypothesis of random changes in Eh signals (white noise). Multivariate tests (repeated measures ANOVA, Pillai’s trace test) taking into account all redox parameters together were significant for the effect of time (P < 0.0001) and time × weight status (P = 0.003) regardless of the experimental protocol (P = 0.34). In the analyses conducted for each redox couple separately, the effect of time was significant in the Eh trajectories for Lac/Pyr (Ehcyto, P = 0.003), BOHB/AcAc (Ehmito, P = 0.03), and GSH/GSSG (EhGSH, P < 0.0001).
Figure 4.
Trajectories of redox couples during protocol 1 (A) and protocol 2 (B). Data are mean values of reduction potentials (mV, millivolts). Reduction potentials (Eh) (GSH) is derived from reduced/oxidized glutathione; Eh(cyto) is derived from lactate/pyruvate; Eh(mito) is derived from acetoacetate/β-OH-butyrate. Open circles: normal weight (NW) subjects; black circles: overweight (OW) subjects. Inset shows detail of the increase in EhGSH, which persisted throughout the clamp procedure in OW subjects, but not in NW subjects. Statistical analyses using repeated measures ANOVA are described in the text.
Ehcyto
After an initial increase in the first hour, Ehcyto started to decrease (more negative) consistently over the next 3 h. The downward trend in Ehcyto is noted in both protocols (linear contrast, P = 0.007) but is more apparent after protocol 1, especially among OW subjects (P = 0.037). On average, Ehcyto was 10–11 mV lower in OW versus NW subjects throughout the 4 h of protocol 1 (P < 0.005 at each time point). Following the oral glucose load in protocol 1, the maximal decease in Ehcyto was −219.1 mV to −224.6 mV in OW subjects and −205.4 mV to −211.4 mV in NW subjects. In protocol 2, the maximal changes in Ehcyto during the clamp were −211.4 mV to −220.0 mV and −210.6 mV to −208.5 mV in OW and NW subjects, respectively.
Ehmito
In general, changes in Ehmito were opposite to those noted in Ehcyto, indicative of more oxidized NADH/NAD couple in response to COF. The effect of time was persistent during continuous glucose infusion in protocol 2 compared with bolus oral administration in protocol 1, where Ehmito returned to baseline after 4 h (P = 0.005). The maximum increase in Ehmito was similar for both protocols (−370.7 mV to −360.5 mV in oral; −366.8 mV to −355.0 mV in clamp). Differences between OW and NW subjects were not significant in either protocol.
EhGSH
The initial effect of glucose on EhGSH was an increase in reduction potential which was observed in both protocols, with a net effect favoring a more oxidized GSH/GSSH ratio (P = 0.001, protocol 1; P = 0.024, protocol 2). Differences between OW and NW subjects were noted in both protocols. Regardless of the mode of glucose administration, EhGSH continued to increase (less negative) in OW subjects but was biphasic and returned to baseline in NW subjects. Maximum increases in protocol 1 were from −133.9 mV to −112.0 mV in OW subjects and from −96.9 mV to −90.2 mV in NW subjects. During the clamp, EhGSH increased from −131.5 mV to −124.6 mV in OW subjects and from −108.9 mV to −98.8 mV in NW subjects. As in protocol 1, the increase in EhGSH was transient in NW subjects returning to baseline (reduced) by the time the second fat biopsy was obtained. Compared with baseline, EhGSH was significantly more oxidized at 240 min in OW subjects in protocol 1 (−115.6 mV ± 4.1 vs. −134.9 mV ± 5.6, P < 0.05) and in protocol 2 (−125.1 mV ± 4.0 vs. −136 mV ± 5.0, P < 0.05, noted in the graphical inset in Fig. 4). In contrast, EhGSH at 240 min was similar to baseline in NW subjects in both protocols (−109.5 mV ± 6.2 vs. −106.1 mV ± 8.5 in protocol 1; and −113.2 mV ± 4.4 vs. −114.0 mV ± 6.0 in protocol 2, P not significant). In the repeated-measures ANOVA, the P value for the interaction of Time × Group was 0.006, whereas the interactions of either Time × Protocol or Time × Group × Protocol were not significant. Thus, at the time of the second fat biopsy, circulating glutathione was oxidized only in OW subjects irrespective of study protocol. The overall mean difference contrast in EhGSH between baseline and 240 min for OW subjects relative to NW subjects was 18.3 mV ± 5.3, P < 0.005.
Cross-Correlations of Redox with Insulin, Glucose, and RQ
To determine how the changes in redox signals relate to each other and to the changes in glucose, insulin, and RQ, we conducted a series of cross-correlation analyses between pairs of time-series variables. The data from this analysis are presented in Fig. 5 with the leading variable on the left y-axis. The lagging variable is shown on the right y-axis. The hypothesis being tested in this analysis is whether the two variable time series are associated contemporaneously and whether changes in one variable are predictive of subsequent changes in the other variable. Positive and negative lag numbers are generated in reference to the leading variable. Figure 5A shows the trajectories of Ehmito and Ehcyto from protocol 1. Based on theoretical considerations of the relationship between redox and cellular energy charge in cytoplasm and mitochondria, we expect that Ehcyto and Ehmito vary in opposite directions (appendix). This case is noted in Fig. 5A′, which shows a negative cross-correlation coefficient (CCF) for the two reduction potentials at lag 2 (CCFlag 2 −0.72 ± 0.35, P = 0.05). Thus, the increase in Ehmito shown in Fig. 5A to occur during the interval between 30 and 120 min is predictive of the subsequent decrease in Ehcyto starting on minute 90 following oral glucose administration.
Figure 5.
Cross-correlation analyses of selected redox trajectories. In each set, the two signals being compared are plotted with the leading variable on the left y-axis (black circles, bolded trajectory) and the “predicted (lagging)” variable on the right y-axis (open circles). Cross-correlation coefficients (CCF) are shown at seven positive and seven negative time lags, together with their 95% confidence range. Wherever a significant lag is detected, small arrows are placed to mark the relative x-axis positions of the cross-correlations between the two signals. A and A′: negative cross-correlation between reduction potentials (Eh)mito and Ehcyto at lag +2. This relationship implies that the rise in Ehmito (which commences at the time indicated by the small arrow) is predictive of the decrease in Ehcyto (also indicated by a small arrow). B and B′: negative cross-correlation between insulin and Ehcyto. The rise in insulin (small arrow) is predictive of the decrease in Ehcyto (CCF is statistically significant at lag +2). C and C′: insulin and Ehmito are contemporaneously cross correlated. D and D′: the increase in respiratory quotient (RQ) (small arrow) predicts the decrease in Ehcyto (CCF is statistically significant at lag 2). E and E′: increase in RQ is contemporaneously associated with the increase in Ehmito.
The cross-correlations of Ehcyto with insulin (Fig. 5, B and B′) and RQ (Fig. 5, D and D′) were negative (CCFlag 2 −0.87 ± 0.35, P < 0.01; and CCFlag 2 −0.85 ± 0.35, P < 0.01, respectively). In both analyses, the decrease in Ehcyto was predicted by the increases in insulin and RQ (lag =2). On the other hand, the cross-correlations of Ehmito with insulin (Fig. 5, C and C′) and RQ (Fig. 5, E and E′) were positive and contemporaneous (CCFlag 0 0.85 ± 0.32, P < 0.01 and CCFlag 0 0.80 ± 0.33, P < 0.05, respectively). Thus, mitochondrial oxidation (NAD/NADH) increases with the rise in insulin and RQ; the subsequent increase in cytoplasmic reduction (NADH/NAD) is predicted by the increases in insulin and CHO oxidation and by mitochondrial redox. Finally, we note that the cross-correlations of Ehcyto and Ehmito with blood glucose levels did not achieve statistical significance (data not shown).
EhΔ
The reciprocal relationship between Ehmito and Ehcyto (appendix) is demonstrated by cross-correlation analysis in Protocol 1 (Fig. 5, A and A′). This is further verified during the clamp procedure in Protocol 2, as shown in Fig. 6. Therefore, defined as the algebraic difference between mitochondrial and cytoplasmic reduction potentials, EhΔ represents the net balance of electron transfer between the two compartments. Consequently, an increase in EhΔ (less negative) implies electron transfer from mitochondria to cytoplasm.
Figure 6.
Detail of the reciprocal changes in cytoplasmic and mitochondrial reduction potentials during the two-step clamp procedure. The transfer of electrons from mitochondria to cytoplasm increases with increasing insulin infusion. Black circles are reduction potentials (Eh)mito; open circles are Ehcyto.
The trajectories of EhΔ are summarized in Fig. 7 for the two protocols. In the repeated-measures ANOVA, the effect of time was significant in both protocols (P = 0.05, protocol 1; P = 0.01, protocol 2). Differences between OW and NW subjects were significant only in protocol 2 (Group × Time interaction, P < 0.05). As noted in Fig. 7A, EhΔ was similar in OW and NW subjects in protocol 1 with biphasic trajectories following the bolus administration of glucose and the biphasic changes in insulin levels. In protocol 1, EhΔ was significantly cross-correlated with insulin (presented in Fig. 9C, CCFlag 0 0.84 ± 0.31, P < 0.01; CCFlag 1 0.78 ± 0.33, P < 0.05). This relationship between insulin and EhΔ is directly demonstrated in protocol 2. As shown in Fig. 7B, EhΔ increased stepwise with the stepwise increase in insulin infusion in NW and OW subjects. Given the relationship between electric current and potential difference, we estimated the rate of increase in EhΔ (dEhΔ/dt) during the two-step clamp procedure. As noted in Fig. 7C, dEhΔ/dt increased stepwise with insulin in both groups. During the transition from clamp 1 to clamp 2, dEhΔ/dt increased from 0.019 mV/min to 0.064 mV/min in NW subjects and from 0.041 mV/min to 0.095 mV/min in OW subjects. As noted in Fig. 7C, there was no overlap between the 95% confidence ranges for the two groups at the higher insulin infusion rate.
Figure 7.
Trajectories of reduction potentials (Eh)Δ during protocol 1 (A) and protocol 2 (B). The time derivative of EhΔ in protocol 2 (dEhΔ/dt), calculated by least-square differentiation, is shown in (C). Open circles: normal weight (NW); Black circles: overweight (OW). The shaded areas represent the 95% confidence ranges for the corresponding trajectories. Estimates of dEhΔ/dt are significantly higher in OW vs NW during Clamp 2.
To determine the functional significance of the change in EhΔ relative to insulin, we calculated the maximal increase in this redox parameter between the two insulin infusion rates in protocol 2 (EhΔmax). Figure 8 shows the correlations between the insulin-induced increases in glucose disposal rates and EhΔmax. For oxidative glucose disposal, the correlation was significant in the combined group analysis (adjusted r2 0.37, slope 0.143 ± 0.053, P = 0.025, Fig. 8A) and in OW subjects (adjusted r2 0.80, slope 0.213 ± 0.046, P = 0.01) but not in NW subjects (adjusted r2 −0.21, slope 0.013 ± 0.10). Differences between OW and NW did not achieve statistical significance (slope × Group interaction P = 0.1, Fig. 8A′). Similarly, the correlation between the increase in nonoxidative glucose disposal and EhΔmax was significant in the combined group analysis (adjusted r2 0.59, slope 0.021 ± 0.005, P < 0.005, Fig. 8B) and in OW subjects (adjusted r2 0.97, slope 0.034 ± 0.003, P < 0.001), but not in NW subjects (adjusted r2 0.33, slope 0.012 ± 007, P = 0.1). Differences between OW and NW subjects were significant (slope × Group interaction P < 0.025) with OW subjects having a steeper slope than NW subjects by 0.022 ± 0.008, Fig. 8B). To further evaluate EhΔ in terms of insulin action, we calculated the insulin-mediated increase in total glucose disposal relative to the increase in insulin levels in the two-step clamp procedure (ΔM/ΔI). It should be noted that this parameter is different from the traditional expression of the insulin sensitivity index which is calculated from euglycemic insulin-induced increase in glucose disposal relative to baseline insulin (17). In addition, the subjects of the current study consumed 100 g of glucose orally before the start of the hyperglycemic clamp. As demonstrated in Fig. 8C, subjects with higher ΔM/ΔI had larger increases in EhΔmax in response to insulin infusion (adjusted r2 0.45, slope 1.256 ± 0.399 P = 0.01). This relationship was particularly strong among OW subjects (OW, adjusted r2 0.93, slope 1.863 ± 0.220, P = 0.001; NW, adjusted r2 −0.24, slope 0.221 ± 1.050, P = 0.84). Differences in ΔM/ΔI between OW and NW subjects did not achieve statistical significance (Group × slope interaction P = 0.44, Fig. 8C′).
Figure 8.

Linear regression analyses of the maximal increase in reduction potentials (Eh)Δ during the transition from clamp 1 to clamp 2 (EhΔmax) with the changes in oxidative glucose disposal (A and A′), nonoxidative glucose diposal (B and B′), and total glucose disposal relative to insulin (ΔM/ΔI; C and C′). Data are shown for all subjects in the top and separately for overweight (OW) and normal weight (NW) in the bottom. Details of the statistical analyses and group comparisons are presented in the text. Differences between OW and NW were significant for nonoxidative glucose disposal (B′), but not for oxidative glucose disposal (A′) or ΔM/ΔI (C′). Black squares: OW + NW; Open circles, NW; Black circles, OW.
Finally, in Fig. 9 we show the trajectories and associations between EhΔ, insulin, and EhGSH in protocol 1. Specifically, the increase in insulin during COF significantly cross-correlated with the increase in glutathione oxidation (Fig. 9B) and with the increase in EhΔ (Fig. 9C). We also note that the increase in EhΔ followed the increase in glutathione oxidation (CCFlag-1 0.73 ± 0.33, P < 0.05, Fig. 9D). Together, this three-way cross-correlation between insulin, EhGSH, and EhΔ supports the hypothesis that glutathione oxidation contributes reducing equivalents to the cytoplasm during COF.
Figure 9.

Relationships between reduction potentials (Eh)Δ, insulin, and glutathione oxidation during carbohydrate overfeeding (COF) (protocol 1). A: trajectories of redox signals (left y-axis) and insulin (right y-axis). Three-way cross-correlations of insulin with EhGSH (B) and with EhΔ (C) and of EhΔ with EhGSH are statistically significant. The cross-correlation of EhGSH with EhΔ is also significant lag at −1, suggesting that glutathione oxidation precedes the increase in EhΔ (D).
ER Stress
In both protocols, we evaluated the change in expression levels in adipose tissue of the proapoptotic factor C/EBP homologous protein (CHOP, DDI3T), which is downstream of PERK, and the spliced variant of XBP-1 (XBP1s), a transcription factor downstream of IRE-1. Data summarizing the 4-h changes in the expression of these signals are shown in Fig. 10 for the two protocols. The increase in DDI3T was significant in protocol 1 for all subjects and separately (not shown) in OW subjects (P < 0.05). After 4 h of COF in protocols 1 and 2, the expression of CHOP mRNA in adipose tissue increased in 9 subjects and that of XBP1s in 16 subjects out of the total of 24 participants. To further characterize the response of ER-stress expression, we stratified all subjects by the combined score of both markers. ER stress was considered positive if the combined score increased by 25% or more; otherwise, it was considered negative. Data summarizing the characteristics of ER stress-positive and ER stress-negative subjects are presented in Table 3. Subjects who had evidence of ER stress activation were older and had higher BMI (P < 0.01). The HOMA-IR and basal insulin levels were higher among ER stress-positive subjects, although these differences did not achieve statistical significance. Finally, subjects who had evidence of ER stress activation were characterized by larger increases in EhΔ and more oxidized circulating glutathione at the time of the second fat sampling (P < 0.05).
Figure 10.
Box and whisker plots of DDI3T and XBPs before and at the end of the procedures in protocols 1 and 2. Units are arbitrary relative to the expression of the control probe (CDKN1A). P < 0.05 comparing pre- and postprocedure.
Table 3.
Subject characteristics stratified by ER stress
| ER Stress | Age, yr | BMI, kg/m2 | Insulin, µU/mL | HOMA-IR | ΔEhGSH, mV | ΔEhΔ, mV |
|---|---|---|---|---|---|---|
| Negative, n = 14 | 31.9 ± 2.6 | 26.3 ± 1.4 | 10.9 ± 3.4 | 1.43 ± 0.9 | 12.1 ± 2.5 | 10.9 ± 2.0 |
| Positive, n = 8 | 43.8 ± 2.6** | 31.9 ± 0.8** | 10.1 ± 1.8 | 2.49 ± 2.0 | 20.1 ± 3.0* | 17.9 ± 2.5* |
Data are means ±SE. Age in year; body mass index (BMI) in kg/m2; insulin in µU/mL. ΔEhGSH, maximal increase in glutathione reduction potential in mVolts; ΔEhΔ, maximal increase in EhΔ in mVolts; ER, endoplasmic reticulum; HOMA-IR, homeostatic model assessment for insulin resistance.
*P < 0.05;
**P < 0.01.
DISCUSSION
We tested the main hypothesis that single-meal COF predisposes to oxidative stress by channeling reducing equivalents away from antioxidants to support DNL. The results presented here are consistent with the hypothesis, as noted by the acute changes in the redox state of circulating glutathione and the increased expression of markers of ER stress in subcutaneous adipose tissue. We also show that as the main hormone-promoting DNL, insulin is associated with net transfer of electrons to the cytoplasm and oxidation of glutathione, through cross-correlation with EhΔ and EhGSH, respectively (Fig. 9). In addition, our study addresses the conceptual relationship between cytoplasmic and mitochondrial redox couples and cellular bioenergetics (appendix) and provides a framework for understanding the role of insulin in the physiology of overfeeding and the pathophysiology of metabolic dysfunction.
COF Promotes Oxidative Stress
In both protocols of this study, circulating glutathione was oxidized in response to excess glucose administered orally and to intravenous infusion of glucose and insulin. The premise that glutathione oxidation contributes new electrons to promote DNL is supported by cross-correlation analysis showing that the increase of EhGSH is predictive of the increase in EhΔ as demonstrated in Fig. 9, A and D. Taken together, these concomitant associations between EhΔ, insulin, and glutathione oxidation (Fig. 9, B–D) highlight the strength of our main hypothesis. Although we have not measured ROS in the current study, the magnitude of change in EhGSH is significant enough to imply increased risk of oxidative stress (14, 18). In addition, the increase in expression of markers of ER stress in subcutaneous adipose tissue further corroborates our hypothesis. It is interesting to note that glutathione was more reduced at baseline in OW compared with NW subjects. Although the significance of this observation is unclear, it could possibly be related to the more reduced fasting cytoplasmic redox in these subjects, which is well documented in this study and in the literature (19, 20).
The demand for reducing equivalents arises from consecutive chemical reduction steps that add 2-carbon units to the lengthening fatty acid molecules (21, 22). Thus, while energy production entails the extraction of electrons through the oxidation of fuel substrates, storage of excess energy requires new electrons for the synthesis of new molecules. Two pathways generate the necessary electrons, which are transported to the cytoplasm as NADPH (reducing equivalents), to support DNL. In the first pathway, NADPH is concomitantly produced when carbons from excess fuel are incorporated into citrate in mitochondria and transported to the cytoplasm (Fig. 2) (23–25). This process yields acetyl CoA in the cytoplasm and returns pyruvate back to mitochondria while generating new NADPH through the action of cytoplasmic malic enzyme. A second possible source of NADPH is the pentose phosphate shunt pathway (PPP) (26). In one human study, PPP contributed 18%–22% at baseline and did not increase in activity despite a two- to threefold increase in the expression of lipogenic enzymes (27). In the current study, we estimate that at least 150-g glucose was channeled into DNL during the 4 h of protocol 1. At this high rate of DNL, we propose that circulating glutathione, which utilizes NADPH to maintain antioxidants in the reduced form, becomes another source of reducing equivalents (28).
For the redox findings in this study to support the etiologic role of DNL, it must be assumed that the lactate/pyruvate ratio, which represents cytoplasmic NADH/NAD, also correlates with the cytoplasmic NADPH/NADP. This assumption is theoretically valid because the redox states of two pyridine nucleotide systems, NAD and NADP, are linked through sharing of pyruvate as a reactant in both of the lactate dehydrogenase (NAD system) and malic enzyme (NADP system) reactions in the cytoplasm, as originally noted by Krebs (29–31) and as shown in Fig. 2. Thus, we expect that a more reduced Ehcyto is also indicative of more reduced NADPH/NADP ratio in the cytoplasm.
Redox Actions of Insulin
Insulin has the unique role of stimulating both energy-producing and energy-storing pathways. This dual role for the same hormone in oxidative and reductive chemical reactions ensures a smooth and seamless transition from normal feeding to overfeeding and energy storage. Although these separate insulin actions are well understood individually, it has been difficult to characterize the dual insulin role in a unified model using conventional methodology. In this study, we used EhΔ as a measure of the redox actions of insulin. The theoretical justification for this new redox parameter is twofold. First, insulin is significantly cross-correlated with each of Ehcyto and Ehmito. Second, these two reduction potentials are related together by a reciprocal relationship which takes into consideration the phosphorylation state of the adenine nucleotides in cytoplasm and mitochondria (appendix).
Our study shows that that EhΔ is highly correlated with insulin-stimulated glucose disposal rates. In particular, EhΔ is correlated best with nonoxidative glucose disposal rates, implying a linear relationship with the reductive effects of insulin in DNL. By analogy to the law of capacitance in physics, the rate of increase in reduction potential (expressed in mV) represents an increase in the flow of electrons, in this case from mitochondria to cytoplasm. The higher estimate of dEhΔ/dt in OW subjects compared with NW subjects, suggests more rapid channeling of reducing equivalents (electrons) to the cytoplasm in response to the glucose-clamped insulin infusion. Therefore, the reductive action of insulin during overfeeding is not diminished in OW subjects despite lower oxidative glucose disposal rates (Table 2) and higher HOMA-IR values. This observation is consistent with the findings of Smith et al. (32) who demonstrated higher rates of hepatic DNL in conjunction with lower hepatic insulin sensitivity and reduced glucose disappearance rates in subjects with obesity.
Brown and Goldstein (33) have previously identified the dilemma of “universal” versus “selective” insulin resistance where insulin actions promoting hepatic fat synthesis are increased together with SRBP-1c expression, whereas insulin-stimulated phosphorylation of FoxO1 is reduced (34). This dichotomy of insulin actions in the liver, affecting glucose production but not fat deposition, poses a challenge to the understanding of the pathophysiology of T2D and obesity. The current study suggests that the redox approach has the potential to distinguish between the oxidative and reductive actions of insulin, thus further clarifying our understanding of metabolic dysfunction in obesity and T2D.
Relationship of Redox to Cellular Bioenergetics and Overfeeding
The reciprocal correlation between Ehcyto and Ehmito is rooted in the relationships of these redox signals to the state of cellular energy charge, as originally described by Krebs (29, 30) and summarized in the appendix. It is interesting to note that this study implies an actual decrease in the ratio of ATP to ADP in response to COF, which is counterintuitive. Although this effect could result from the redistribution of reducing equivalents between mitochondria and cytoplasm, it could also arise from the energy cost of DNL. In addition, the decrease in ATP/ADP could result from inhibition of mitochondrial adenine nucleotide translocator activity when electron donors are in excess. This effect, which reduces ETC flux to counter the increase in mitochondrial transmembrane potential, has been demonstrated following accumulation of long-chain acyl-CoA in the cytoplasm (35–37). Whether a similar mechanism also occurs during CHO excess is worthy of further investigation.
Other mechanisms proposed to explain the regulation of excess mitochondrial ETC flux are based on studies in isolated cells. For example, electrons could “leak” within the mitochondria to generate ROS, which are subsequently scavenged by electrons from NADPH. The enzyme nicotinamide nucleotide transhydrogenase (NNT) utilizes NADH to generate NADPH to maintain the thiol redox state in its reduced form (high GSH). The net effect of this NNT-mediated increase in proton conductance is increased oxygen utilization and energy expenditure. Thus, ROS generation from ETC flux “overflow” fine tunes energy expenditure and modulates energy efficiency to maintain weight stability (5, 38). Our study suggests that the whole organism has the additional ability to reduce excessive ETC flux by diverting electrons to the cytoplasm to support energy storage.
Figure 11A summarizes the conceptual redox role of insulin during overfeeding. The higher rate of electron flux into the mitochondrial ETC increases the positive electric potential across the inner mitochondrial membrane (ΔΨ). As noted earlier, NNT-mediated proton conductance counteracts excessive ΔΨ and could potentially increase energy expenditure (5, 38). However, the actual contribution of this mechanism to energy regulation in humans has not been established. The alternative mechanism that channels excess electrons to the cytoplasm could reduce the need for mitochondrial proton leaks, increase the efficiency of energy utilization, and promote energy storage and obesity, all driven by insulin. In Fig. 11B, we present a graphical analogy of the two mechanisms in a simple electric circuit. Like an in-parallel resistor, which increases the current, proton leaks would increase oxygen consumption and raise the metabolic rate. On the other hand, the reductive effect of insulin would be akin to an in-series resistor, thus potentially reducing oxygen consumption.
Figure 11.
Electron transport model during carbohydrate overfeeding (COF). A: diagram of the chemiosmotic mechanism for generating ATP showing the accumulation of positive charges inside the intermembrane space and coupled proton pumping into the matrix. Excess electrons flux in electron transport chain (ETC) could possibly have two fates. Leakage through the inner mitochondrial membrane, which is associated with reactive oxygen species (ROS) generation, or transport to the cytoplasm support synthesis of new molecules. B: analogy to electric circuit. The “leakage” pathway is akin to an in-parallel resistor, which would increase the current (ic). The alternative pathway of shunting electrons to the cytoplasm (C2) is akin to adding an in-series variable resistor (L2), controlled by a relay (CR). Data from the current study support the view that humans dispose of excess electron flux through the latter pathway. Overfeeding opens the relay to C2 with insulin acting to reduce the resistance at L2.
The possibility that insulin controls ΔΨ during overfeeding conditions is intriguing. Previous observations have shown a reduction in glucose-induced thermogenesis in relationship to insulin resistance in subjects with obesity (39–41). Although the significance of this observation has been questioned (42), we note that it is consistent with our finding of increased reductive effects of insulin among OW subjects. In addition, low-carbohydrate diets, which are associated with lower insulin levels, have been shown to increase energy expenditure as measured by doubly labeled water (43). Therefore, the redox approach presented in the current study could ultimately enhance our understanding of energy regulation in obesity and T2D and provide a novel focus on regulation of energy efficiency.
The observation that OW subjects sustained a greater impact on glutathione oxidation and had increased expression of markers of ER stress in subcutaneous fat corroborates the potential harmful effects of insulin during COF. Thus, hyperinsulinemia is a direct contributor to the pathophysiology of T2D rather than a compensatory response to insulin resistance (44, 45). This conclusion has important clinical implications for the management of T2D and obesity, especially in situations where insulin actions are augmented to achieve tighter glycemic control. The potential for harm from use of certain insulin-sensitizing agents has previously been raised in the literature (46) and could be explained on the basis of increased insulin action during overfeeding, as documented here. Further studies are necessary to fully understand the clinical implications of COF in relation to insulin resistance and T2D treatment.
In conclusion, this study introduces a quantitative redox approach to understanding the effects of acute overfeeding in human subjects. The main strength of this approach is the simplicity of combining basic biochemical principles and analytical tools to evaluate the minute-to-minute balance of oxidative and reductive metabolic processes in human subjects. Limitations of the current study include its small sample size and the fact that the degree of COF is not matched between the oral and intravenous protocols. Differences in glucose metabolism related to mode of administration need to be further considered in future study designs. Although measurement of markers of ER stress adds to the functional relevance of the redox data in the current study, lack of direct measurement of oxidative stress must be considered as a weakness. Despite these limitations, we are able to provide direct support to the hypothesis that acute COF is a contributor to metabolic dysfunction in humans through mechanisms that implicate the reductive actions of excess insulin.
GRANTS
This work was supported by Grants 1-13-IN-23 from the American Diabetes Association and P30DK046200 from the National Institute of Diabetes and Digestive and Kidney Diseases and internal funds from Boston University School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.I. conceived and designed research; N.I., B.H., T.M., and W.A. performed experiments; N.I., B.H., L.Y., and B.E.C. analyzed data; N.I., B.H., C.A., T.M., L.Y., W.A., and B.E.C. interpreted results of experiments; N.I. prepared figures; N.I. drafted manuscript; N.I., B.H., C.A., W.A., and B.E.C. edited and revised manuscript; N.I., B.H., C.A., T.M., L.Y., W.A., and B.E.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Megan Ruth, Ava Port, and Ana Junqueira for assistance in initiating the study; Nathan Burritt for assistance in blood sample analyses; and Ashley McCarthy for contribution to subject recruitment and manuscript editing.
Present addresses: N. Istfan and C. Apovian, Section of Endocrinology, Diabetes and Hypertension, Brigham and Women’s Hospital, 75 Francis Street, and Harvard Medical School, Boston, Massachusetts (email: nistfan@bwh.harvard.edu); B. Hasson, Sage Therapeutics, Cambridge, Massachusetts.
APPENDIX
Relationship between Cytoplasmic and Mitochondrial Redox
In the cytoplasm, two reversible glycolytic reactions establish a link between redox and the phosphorylation state of the adenine nucleotides. These reactions involve the oxidation of d-glyceraldehyde 3-phosphate (GAP) to 1,3 biphosphoglycerate (1,3-PG) and 3-phosphoglycerate (3-PG) as follows:
| (A1) |
| (A2) |
Since these two reactions are at near equilibrium, the following relationship can be written
| (A3) |
where K1 is the product of the equilibrium constants of glyceraldehyde phosphate dehydrogenase and 3-PG kinase.
In mitochondria, the initial step in oxidative phosphorylation is the delivery of electrons from NADH to electron carrier in Complex I through a reversible reaction. Therefore, the expression for energy charge in mitochondria leads to the following expression:
| (A4) |
where K2 is the equilibrium constant of NADH-Q oxidoreductase. Comparison of Eqs. A3 and A4 suggest that redox changes are expected to be opposite in direction between cytoplasm and mitochondria, which is consistent with experimental data in the current study.
Substituting p1 and p2 for pyruvate/lactate and acetoacetate/β-OH butyrate, respectively, in the Nernst equations for mitochondria and cytoplasm, and obtaining their algebraic difference, EhΔ,
| (A5) |
| (A6) |
| (A7) |
| (A8) |
where,
| (A9) |
| (A10) |
and, K1, K2 are as aforementioned; K3 is the equilibrium constant for lactate dehydrogenase.
Relationship between the Pyridine Nucleotides in Cytoplasm
With pyruvate as a common reactant in the malic enzyme and lactate dehydrogenase reactions, the following relationship exists:
The two systems are also linked by the three reactions catalyzed by isocitrate dehydrogenase (NADP), malate dehydrogenase, and glutamate-oxalacetate transaminase (NAD), such that:
where K5, K6, and K7 are the equilibrium constants for isocitrate dehydrogenase, glutamate oxalacetate transaminase, and malate dehydrogenase, respectively.
REFERENCES
- 1.Arenaza L, Medrano M, Oses M, Huybrechts I, Díez I, Henriksson H, Labayen I. Dietary determinants of hepatic fat content and insulin resistance in overweight/obese children: a cross-sectional analysis of the Prevention of Diabetes in Kids (PREDIKID) study. Br J Nutr 121: 1158–1165, 2019. doi: 10.1017/S0007114519000436. [DOI] [PubMed] [Google Scholar]
- 2.Lackey DE, Lazaro RG, Li P, Johnson A, Hernandez-Carretero A, Weber N, Vorobyova I, Tsukomoto H, Osborn O. The role of dietary fat in obesity-induced insulin resistance. Am J Physiol Endocrinol Metab 311: E989–E997, 2016. doi: 10.1152/ajpendo.00323.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 15: 595–605, 2012. doi: 10.1016/j.cmet.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith CD, Schmidt CA, Lin CT, Fisher-Wellman KH, Neufer PD. Flux through mitochondrial redox circuits linked to nicotinamide nucleotide transhydrogenase generates counterbalance changes in energy expenditure. J Biol Chem 295: 16207–16216, 2020. doi: 10.1074/jbc.RA120.013899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeown NM, Meigs JB, Liu S, Rogers G, Yoshida M, Saltzman E, Jacques PF. Dietary carbohydrates and cardiovascular disease risk factors in the Framingham offspring cohort. J Am Coll Nutr 28: 150–158, 2009. doi: 10.1080/07315724.2009.10719766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 27: 538–546, 2004. doi: 10.2337/diacare.27.2.538. [DOI] [PubMed] [Google Scholar]
- 8.Makarem N, Scott M, Quatromoni P, Jacques P, Parekh N. Trends in dietary carbohydrate consumption from 1991 to 2008 in the Framingham Heart Study Offspring Cohort. Br J Nutr 111: 2010–2023, 2014. doi: 10.1017/S0007114513004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schillinger D, Jacobson MF. Science and public health on trial: warning notices on advertisements for sugary drinks. JAMA 316: 1545–1546, 2016. [Erratum in JAMA 316: 1319, 2016]. doi: 10.1001/jama.2016.10516. [DOI] [PubMed] [Google Scholar]
- 10.Zelewski M, Swierczyński J. Comparative studies on lipogenic enzyme activities in the liver of human and some animal species. Comp Biochem Physiol B 95: 469–472, 1990. doi: 10.1016/0305-0491(90)90004-d. [DOI] [PubMed] [Google Scholar]
- 11.Wise EM Jr, Ball EG. Malic enzyme and lipogenesis. Proc Natl Acad Sci USA 52: 1255–1263, 1964. doi: 10.1073/pnas.52.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson JR, Corkey BE. Assay of citric acid cycle intermediates and related compounds—update with tissue metabolite levels and intracellular distribution. Methods Enzymol 55: 200–222, 1979. doi: 10.1016/0076-6879(79)55025-3. [DOI] [PubMed] [Google Scholar]
- 13.Jones DP, Sies H. The redox code. Antioxid Redox Signal 23: 734–746, 2015. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med 28: 625–635, 2000. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 15.Hancock JT, Whiteman M. Equations to support redox experimentation. Methods Mol Biol 1990: 183–195, 2019. doi: 10.1007/978-1-4939-9463-2_15. [DOI] [PubMed] [Google Scholar]
- 16.Martinovich GG, Cherenkevich SN, Sauer H. Intracellular redox state: towards quantitative description. Eur Biophys J 34: 937–942, 2005. doi: 10.1007/s00249-005-0470-3. [DOI] [PubMed] [Google Scholar]
- 17.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294: E15–E26, 2008. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 18.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem 86: 715–748, 2017. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 19.Corkey BE, Deeney JT. The redox communication network as a regulator of metabolism. Front Physiol 11: 567796, 2020. doi: 10.3389/fphys.2020.567796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adeva-Andany M, López-Ojén M, Funcasta-Calderón R, Ameneiros-Rodríguez E, Donapetry-García C, Vila-Altesor M, Rodríguez-Seijas J. Comprehensive review on lactate metabolism in human health. Mitochondrion 17: 76–100, 2014. doi: 10.1016/j.mito.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Kather H, Rivera M, Brand K. Interrelationship and control of glucose metabolism and lipogenesis in isolated fat-cells. Control of pentose phosphate-cycle activity by cellular requirement for reduced nicotinamide adenine dinucleotide phosphate. Biochem J 128: 1097–1102, 1972. doi: 10.1042/bj1281097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballak DB, van Diepen JA, Moschen AR, Jansen HJ, Hijmans A, Groenhof GJ, Leenders F, Bufler P, Boekschoten MV, Müller M, Kersten S, Li S, Kim S, Eini H, Lewis EC, Joosten LA, Tilg H, Netea MG, Tack CJ, Dinarello CA, Stienstra R. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun 5: 4711, 2014. [Erratum in Nat Commun 6: 6039, 2015]. doi: 10.1038/ncomms5711. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Cánovas-Márquez JT, Tang X, Chen H, Chen YQ, Chen W, Garre V, Song Y, Ratledge C. Role of malate transporter in lipid accumulation of oleaginous fungus Mucor circinelloides. Appl Microbiol Biotechnol 100: 1297–1305, 2016. doi: 10.1007/s00253-015-7079-y. [DOI] [PubMed] [Google Scholar]
- 24.Jitrapakdee S, Vidal-Puig A, Wallace JC. Anaplerotic roles of pyruvate carboxylase in mammalian tissues. Cell Mol Life Sci 63: 843–854, 2006. doi: 10.1007/s00018-005-5410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel MS, Jomain-Baum M, Ballard FJ, Hanson RW. Pathway of carbon flow during fatty acid synthesis from lactate and pyruvate in rat adipose tissue. J Lipid Res 12: 179–191, 1971. [PubMed] [Google Scholar]
- 26.Haut MJ, London JW, Garfinkel D. Simulation of the pentose cycle in lactating rat mammary gland. Biochem J 138: 511–524, 1974. doi: 10.1042/bj1380511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minehira K, Bettschart V, Vidal H, Vega N, Di Vetta V, Rey V, Schneiter P, Tappy L. Effect of carbohydrate overfeeding on whole body and adipose tissue metabolism in humans. Obes Res 11: 1096–1103, 2003. doi: 10.1038/oby.2003.150. [DOI] [PubMed] [Google Scholar]
- 28.Méndez I, Vázquez-Martínez O, Hernández-Muñoz R, Valente-Godínez H, Díaz-Muñoz M. Redox regulation and pro-oxidant reactions in the physiology of circadian systems. Biochimie 124: 178–186, 2016. doi: 10.1016/j.biochi.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Krebs HA. Pyridine nucleotides and rate control. Symp Soc Exp Biol 27: 299–318, 1973. [PubMed] [Google Scholar]
- 30.Krebs HA, Veech RL. Equilibrium relations between pyridine nucleotides and adenine nucleotides and their roles in the regulation of metabolic processes. Adv Enzyme Regul 7: 397–413, 1969. doi: 10.1016/0065-2571(69)90030-2. [DOI] [PubMed] [Google Scholar]
- 31.Veech RL, Guynn R, Veloso D. The time-course of the effects of ethanol on the redox and phosphorylation states of rat liver. Biochem J 127: 387–397, 1972. doi: 10.1042/bj1270387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith GI, Shankaran M, Yoshino M, Schweitzer GG, Chondronikola M, Beals JW, Okunade AL, Patterson BW, Nyangau E, Field T, Sirlin CB, Talukdar S, Hellerstein MK, Klein S. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest 130: 1453–1460, 2020. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7: 95–96, 2008. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Brown MS, Goldstein JL, Horton JD. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab 15: 240–246, 2012. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciapaite J, Bakker SJ, Diamant M, van Eikenhorst G, Heine RJ, Westerhoff HV, Krab K. Metabolic control of mitochondrial properties by adenine nucleotide translocator determines palmitoyl-CoA effects. Implications for a mechanism linking obesity and type 2 diabetes. FEBS J 273: 5288–5302, 2006. doi: 10.1111/j.1742-4658.2006.05523.x. [DOI] [PubMed] [Google Scholar]
- 36.Ciapaite J, Van Eikenhorst G, Bakker SJ, Diamant M, Heine RJ, Wagner MJ, Westerhoff HV, Krab K. Modular kinetic analysis of the adenine nucleotide translocator-mediated effects of palmitoyl-CoA on the oxidative phosphorylation in isolated rat liver mitochondria. Diabetes 54: 944–951, 2005. doi: 10.2337/diabetes.54.4.944. [DOI] [PubMed] [Google Scholar]
- 37.Ciapaite J, van Eikenhorst G, Krab K. Application of modular control analysis to inhibition of the adenine nucleotide translocator by palmitoyl-CoA. Mol Biol Rep 29: 13–16, 2002. doi: 10.1023/a:1020385714555. [DOI] [PubMed] [Google Scholar]
- 38.Kaludercic N, Di Lisa F. The energetic cost of NNT-dependent ROS removal. J Biol Chem 295: 16217–16218, 2020. doi: 10.1074/jbc.H120.016368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravussin E, Acheson KJ, Vernet O, Danforth E, Jéquier E. Evidence that insulin resistance is responsible for the decreased thermic effect of glucose in human obesity. J Clin Invest 76: 1268–1273, 1985. doi: 10.1172/JCI112083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravussin E, Zawadzki JK. Thermic effect of glucose in obese subjects with non-insulin-dependent diabetes mellitus. Diabetes 36: 1441–1447, 1987. doi: 10.2337/diab.36.12.1441. [DOI] [PubMed] [Google Scholar]
- 41.Tataranni PA, Larson DE, Snitker S, Ravussin E. Thermic effect of food in humans: methods and results from use of a respiratory chamber. Am J Clin Nutr 61: 1013–1019, 1995. doi: 10.1093/ajcn/61.4.1013. [DOI] [PubMed] [Google Scholar]
- 42.de Jonge L, Bray GA. The thermic effect of food and obesity: a critical review. Obes Res 5: 622–631, 1997. doi: 10.1002/j.1550-8528.1997.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 43.Ebbeling CB, Feldman HA, Klein GL, Wong JMW, Bielak L, Steltz SK, Luoto PK, Wolfe RR, Wong WW, Ludwig DS. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ 363: k4583, 2018. doi: 10.1136/bmj.k4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas DD, Corkey BE, Istfan NW, Apovian CM. Hyperinsulinemia: an early indicator of metabolic dysfunction. J Endocr Soc 3: 1727–1747, 2019. doi: 10.1210/js.2019-00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 61: 4–13, 2012. doi: 10.2337/db11-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drazen JM, Morrissey S, Curfman GD. Rosiglitazone—continued uncertainty about safety. N Engl J Med 357: 63–64, 2007. doi: 10.1056/NEJMe078118. [DOI] [PubMed] [Google Scholar]