Abstract
Zinc has potent immunoregulatory and antiviral effects that are critical for growth, immunity, and neurologic development. The aim of this study was to determine the clinical significance of serum zinc levels in pediatric patients with COVID-19 and to demonstrate its association with disease severity. This prospective observational study was conducted between August 3 and November 15, 2020, in pediatric patients aged 1 month to 18 years with confirmed COVID-19 using reverse transcription-polymerase chain reaction. We defined a control group whose serum zinc levels were determined 1 year ago at the same time as those of patients with COVID-19. We used 70 μg/dL as the cut-off zinc value to define zinc deficiency. Statistical analyses were performed using the SPSS for Windows statistics package program. One hundred children with confirmed COVID-19 and 269 children in the control group participated in the study. The median age was 13.3 (IQR: 8–15.4) years in patients with confirmed COVID-19, 11 patients had low serum zinc levels, and 89 patients had normal serum zinc levels. Patients in the group with low zinc levels had a significantly higher hospitalization rate than the group with normal zinc levels (5 (45.5%) and 10 patients (11.2%), respectively) (p = 0.011). The median serum zinc level in patients with COVID-19 was 88.5 mcg/dL (IQR 77.2–100), which was significantly lower than the median level in the control group, which was 98 mcg/dL (IQR 84–111) (p = 0.001). There was no association between the severity of COVID-19 and the serum zinc levels of the children.
Conclusion: Serum zinc levels may be influenced by many factors such as fasting status, diurnal variation, exercise, and sex, and may give an impression of the zinc status of the population rather than reflecting the individual. The fact that the incidence of hospitalization was significantly higher in patients with both COVID-19 and low serum zinc levels suggests that these patients require a detailed assessment of their living environment.
|
What is Known: • Serum zinc levels have been found to be low in adult patients diagnosed with COVID-19. • There was a correlation between the severity of COVID-19 and serum zinc levels in adults. | |
|
What is New: • Children with low serum zinc levels were found to have a higher number of hospitalizations. • No association was found between the severity of COVID-19 disease and serum zinc levels in children. |
Keywords: Children, COVID-19, Zinc, SARS-CoV-2
Introduction
Since December 2019, the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has spread rapidly worldwide. Currently, there is no satisfactory treatment for SARS-CoV-2 infection. Research into possible protective and therapeutic antiviral strategies is urgently needed. Zinc is speculated as one of the micronutrients that can reduce the intensity of COVID-19 infection due to its antiviral properties [1]. Zinc is the second most abundant trace element with potent immunoregulatory and antiviral properties and is essential for growth, immunity, and neurologic development [2]. Zinc is largely localized intracellularly, particularly in the liver and muscle, so small differences in zinc turnover in peripheral organs can greatly affect serum zinc concentrations. In addition, changes in metabolic status can lead to changes in serum zinc levels. For example, fasting status paradoxically causes an increase in serum zinc, whereas satiety causes a decrease in serum concentrations. Time of day, age, sex, hypoalbuminemia, vigorous exercise, infection, and organ failure can also affect serum zinc concentrations [3–5]. For this reason, plasma zinc concentrations are not an indicator of zinc stores in a persons’ body, but plasma levels of zinc are an indicator of the risk of zinc deficiency in the population [3]. Serum zinc concentrations decrease in serum only when zinc intake is greatly reduced, resulting in a short-term decrease in zinc concentrations, or when deficiency persists too long [6, 7]. Despite many shortcomings, the determination of serum or plasma zinc levels is the most useful clinical test for zinc deficiency [6, 7].
Zinc homeostasis in immune signaling pathways is complex because zinc is involved in both pro-inflammatory and anti-inflammatory signaling pathways. Zinc regulates the immune response, and its deficiency increases susceptibility to inflammatory and infectious diseases, including pneumonia [8]. There is also evidence that unregulated zinc homeostasis in macrophages impairs phagocytosis and leads to abnormal inflammatory responses [9]. Nevertheless, it seems clear that low and high zinc status can lead to dysfunction of the adaptive and innate immune systems [10]. In addition, studies have shown that zinc oxide and zinc salts inhibit the replication of SARS-CoV, hepatitis C virus, and H1N1 influenza [11–13]. The antiviral activities of zinc have not been clearly elucidated. However, proposed mechanisms of zinc’s antiviral property include inhibition of RNA synthesis, topoisomerase, viral replication, and viral binding to the mucosal membranes [13, 14]. The exact role of zinc in SARS-CoV-2 infection is also not fully understood. It is speculated that it decreases the activity of angiotensin-converting enzyme 2 [14]. In addition, a recent study has shown that zinc salts can inhibit hepatitis E virus replication by inhibiting RNA-dependent-RNA-polymerase [15]. This enzyme also plays a key role in the replication phase of the coronavirus. These important findings suggest that zinc could be considered as a special antiviral agent in COVID-19 treatment.
There are many studies on zinc deficiency in children with various infectious diseases [16, 17]. However, there are insufficient studies on serum zinc levels in children with COVID-19. The aim of this study was to determine the clinical significance of serum zinc levels in pediatric patients with COVID-19 and to demonstrate its association with disease severity. In addition, serum zinc levels were compared with those of a healthy control group.
Material and method
Study design
This prospective observational study was designed for pediatric patients who underwent reverse transcription-polymerase chain reaction testing (RT-PCR) because of suspected COVID-19 infection. The patients with positive RT-PCR test results who agreed to participate were enrolled in the study. Patients’ clinical data were obtained from medical records, including age, sex, comorbidity, length of hospital stay, and medication. We used the World Health Organization (WHO) definition criteria to represent the clinical stages of COVID-19 pneumonia in children. We divided patients into five groups according to the clinical severity of COVID-19: asymptomatic, mild, moderate, severe, and critical. We also divided patients according to whether they had asymptomatic or symptomatic COVID-19.
Blood sample used to detect serum zinc levels were taken from the patients in the morning or afternoon of the same or next day if there was a positive result for SARS-CoV-2. The patients with confirmed COVID-19 were divided into two groups according to whether they had low or normal serum zinc concentrations. There is no globally accepted cut-off value for low serum zinc concentrations. The International Zinc Nutrition Consultative Group (IZiNCG) has defined 70 μg/dL (10.7 μmol/L) as the cut-off value for low serum zinc concentrations [18]. We used the IZiNCG recommendation to define low serum zinc levels and 70 μg/dL as the cut-off value for zinc concentrations.
Study population
This study was conducted in patients aged 1 month to 18 years. Of the patients who presented to pediatric outpatient clinics with the suspected COVID-19, those who were positive for RT-PCR were included in the study. Patients who had been taking vitamin supplements and those who were reluctant to participate in the study were excluded. The control group was defined as children aged 1 month to 18 years who had presented to the pediatric outpatient clinic of Tepecik Training and Research Hospital in Izmir, 1 year ago (in the same months as the COVID-19 patients) before COVID-19 became known to the world. Patients whose serum zinc levels had been studied 1 year ago were included in the control group, except seven patients who had received vitamin supplementation, three patients who were diagnosed as having chronic malnutrition, and one patient with acrodermatitis enteropathica, who were all excluded from the study. The parents of the children provided written informed consent, and written informed assent was obtained from the children. Ethics committee approval was obtained from Tepecik Training and Research Hospital (No.: 24/07/2020/01).
Study period and study area
This study was conducted between August 3 and November 15, 2020, at Tepecik Training and Research Hospital, Izmir, Turkey. Tepecik Training and Research Hospital is a pandemic center for COVID-19, receiving 77,827 hospitalizations in 2019 before the COVID-19 pandemic, and has 910 inpatient beds.
Testing of zinc
Blood samples were drawn from the antecubital vein and collected in gel-containing clot activator tubes (BD Vacutainer SST II Advance, USA) according to standard hospital guidelines for venipuncture and specimen collection. Samples from the separation tubes were allowed to clot and then centrifuged at 3000 g for 10 min to separate the serum. Serum zinc measurements were performed using an AU 5800 chemistry analyzer (Beckman Coulter, High Wycombe, UK). Zinc for the Architect system Monoreagent kit was purchased from Archem Diagnostics Industry. The intra-assay and inter-assay coefficients of variation (CV) for the zinc assay were less than 1.9% and 2.7%, respectively.
RT-PCR assay
Combined nasopharyngeal and oropharyngeal swab specimens were collected from children with suspected COVID-19 and sent to medical microbiology laboratory. SARS-CoV-2 was detected using RT-PCR (Bio-Speedy SARS CoV-2 double Gene RT-qPCR Kit). Specifically, two target genes, including open reading frame 1ab (ORF1ab) and nucleocapsid protein (N), were tested during the RT-PCR assay.
Statistics
The median, first quartile, and third quartile were used to express continuous variables that were not normally distributed. Differences between two groups were analyzed using the Mann–Whitney U test. Categorical variables were compared using the chi-square test or Fisher’s exact test. P < 0.05 was considered significant. Spearman correlation coefficient was used to test bivariate associations between serum zinc levels and laboratory results. Comparative analysis was performed between patients with COVID-19 and healthy subjects. Logistic regression analyses were performed to examine the effects of age, sex, comorbidity, presence of asymptomatic or symptomatic COVID-19, and the number of hospitalizations as a function of serum zinc level (low serum zinc levels were defined as below 70 μg/dL; normal serum zinc levels were defined as above and equal to 70 μg/dL) in patients with COVID-19. Statistical analyses were performed using SPSS for Windows version 25 software (IBM, USA).
Results
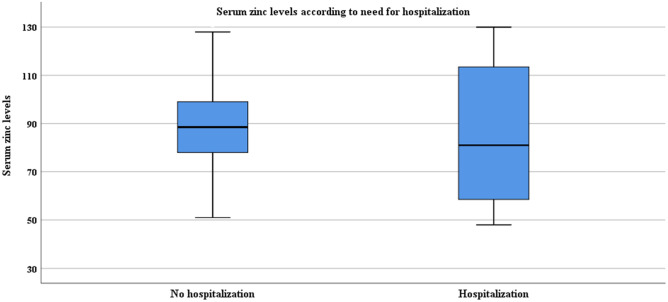
This prospective study was conducted on 100 children aged 1 month to 18 years with confirmed COVID-19. Two hundred sixty-nine healthy children were included in the study as a control group. The characteristics of the patients with confirmed COVID-19 are shown in Table 1. The median age was 13.3 (interquartile range [IQR] 8–15.4) years; 56 were girls. Patients with confirmed COVID-19 were divided into two groups according to serum zinc levels; low serum zinc levels were found in 11 patients and normal zinc levels in 89 patients. Hospitalization was required in 5 (45.5%) patients in the low serum zinc group and 10 (11.2%) patients in the normal serum zinc group (Fig. 1). The low serum zinc group had a significantly higher hospitalization rate than the normal serum zinc group (p = 0.011). The patients with low serum zinc levels did not have longer hospitalization than the group with normal serum zinc levels (4 ± 1.8 days vs. 5.4 ± 2.9 days, p = 0.360). Spearman correlation was performed to determine the relationship between serum zinc level and laboratory values. Serum zinc levels were correlated positively with serum lactate dehydrogenase (Spearman’s rho, r = + 0.253; p = 0.013), D-dimer (r = + 0.236; p = 0.021), and creatinine kinase level (r = + 0.233; p = 0.028). A logistic regression model was performed to find an association between low serum zinc levels, sex, age, presence of comorbidities, and the presence of asymptomatic or symptomatic COVID-19, and no significant association was found between these parameters, except for the need for hospitalization, which was significantly associated with low serum zinc levels (odds ratio (OR) 6.694 95%CI (1.270–35.288); p = 0.025).
Table 1.
Characteristics of patients with COVID-19 according to serum zinc levels
| Total number of patients | Zinc levels < 70 μg/dL | Zinc levels 70 ≥ μg/dL | p value | |
|---|---|---|---|---|
| Patient number, n (%) | 100 (100) | 11 (100) | 89 (100) | - |
| Age, years (IOR) | 13.3 (16.3–15.4) | 14.1 (4.8–16.3) | 13.3 (8.1–15.2) | 0.573 |
| Sex (%) | 0.338* | |||
| Girl | 56 (56) | 8 (72.7) | 48 (53.9) | |
| Boy | 44 (44) | 3 (27.3) | 41 (46.1) | |
| Number of people living in the same house, median (IQR) | 4 (4–5) | 4 (3–5) | 4 (4–5) | 0.154 |
| Number of people in the house diagnosed with COVID-19 | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.698 |
| Underlying medical condition (%) | 8 (8) | 2 (18.2) | 6 (6.7) | 0.213* |
| Neurological disease (%) | 3 (3) | 1 (9.1) | 2 (2.2) | - |
| Asthma (%) | 3 (3) | 1(9.1) | 2 (2.2) | - |
| Diabetes (%) | 1 (1) | - | 1 (1.1) | - |
| Hipotroidi (%) | 1 (1) | - | 1 (1.1.) | - |
| Hospitalization, n (%) | 15 (15) | 5 (45.5) | 10 (11.2) | 0.011* |
| Duration of hospitalization, days, mean (SD) | 4.9 (± 2.6) | 4 (± 1.8) | 5.4 (± 2.9) | 0.360 |
| Clinical features at presentation | ||||
| Fever, n (%) | 39 (39) | 6 (54.5) | 33 (37.1) | 0.331* |
| Dry cough (%) | 25 (25) | 4 (35.4) | 21 (23.6) | 0.460* |
| Runny nose, n (%) | 10 (10) | 3 (27.3) | 7 (7.9) | 0.078* |
| Sore throat (%) | 16 (16) | 2 (18.2) | 14 (15.7) | 1.000* |
| Muscle ache (%) | 12 (12) | 2 (18.2) | 10 (11.2) | 0.618* |
| Abdominal pain (%) | 10 (10) | 1 (9.1) | 9 (10.1) | 1.000* |
| Diarrhea (%) | 11 (11) | 2 (18.2) | 9 (10.1) | 0.347* |
| Shortness of breath | 5 (5) | 2 (18.2) | 3 (3.4) | 0.092* |
| Laboratory values | ||||
| Total white blood cell count cells × 103/μL median (IQR) | 5.7 (4.80–7.57) | 4.8 (3.80–6.80) | 5.8 (4.95–7.60) | 0.227 |
| Neutrophil count cells × 103/μL median (IQR) | 3.1 (2.15–4.00) | 3.1 (2.20–4.05) | 3.1 (2.20–4.05) | 0.941 |
| Lymphocyte count cells × 103/μL median (IQR) | 1.8 (1.3–2.6) | 1.5 (0.9–2.7) | 1.9 (1.3–2.6) | 0.227 |
| Platelet count cells × 103/μL median (IQR) | 247 (195–192) | 206 (176–288) | 249 (199– 294.5) | 0.570 |
| Hemoglobin g/dL mean (IQR) | 13.1 (12.4–14) | 13 (12.40–13.90) | 13.2 (12.3–14.15) | 1.000 |
| CRP mg/L. median (IQR) | 2.4 (0.80–6.12) | 2.6 (0.80–8.80) | 2.2 (0.80–5.50) | 1.000 |
| Ferritin µg/L median (IQR) | 31.4 (24–52.9) | 33 (12.2–46.7) | 31 (24–56.9) | 1.000 |
| LDH U/L median (IQR) | 218 (184–268) | 195 (181–252) | 220 (185–270) | 1.000 |
| Creatinine Kinase U/L median (IQR) | 91 (65–113.5) | 71 (61–104) | 95.5 (68–113.7) | 0.170 |
| D-dimer µg/L. median (IQR) | 330 (200–470) | 240 (190–380) | 340 (200–512.5) | 0.546 |
| Severity of COVID-19 (%) | 0.153* | |||
| Asymptomatic (%) | 43 (43) | 3 (27.3) | 40 (44.9) | |
| Mild (%) | 44 (44) | 5 (45.5) | 39 (43.8) | |
| Moderate (%) | 12 (12) | 3 (27.3) | 9 (10.1) | |
| Severe (%) | - | - | - | |
| Critical (%) | 1 (1) | - | 1 (1.1.) | |
| Serum zinc levels, median(IQR) | 88.5 (77.2–100) | 60 (51–66) | 90 (79–100) | - |
*Fisher's exact probability test was used for cross-classification tables
Fig. 1.
Serum zinc levels according to need for hospitalization
When comparing serum zinc levels were between patients with COVID-19 and the control group, the median serum zinc level in patients with COVID-19 was 88.5 (IQR 77.2–100) mcg/dL, which was significantly lower than the median level in the control group, which was 98 (IQR 84–111) mcg/dL (p = 0.001) (Table 2). When comparing the age between the two groups, the control group was statistically younger than the patients with COVID-19 (13.3 years vs. 10.1 years, p = 0.006). When comparing serum zinc levels between patients with COVID-19 and the control group, significantly lower serum zinc levels were found in patients with COVID-19 (OR 0.977, 95% CI: [0.963–0.991]; p = 0.001).
Table 2.
Characteristics and serum levels of zinc among patients with confirmed COVID-19 and control group
| Patients with confirmed COVID-19 | Control group | p value | |
|---|---|---|---|
| Patient number, n (%) | 100 (100) | 269 (100) | - |
| Age, years (IQR) | 13.3 (8–15.4) | 10.1 (5.1–14.5) | 0.006 |
| Sex, n (%) | 0.982 | ||
| Girl | 56 (56) | 151 (56.1) | |
| Boy | 44 (44) | 118 (43.9) | |
| Serum zinc, median (IQR) (ng/mL) | 88.5 (77.2–100) | 98 (84–111) | 0.001 |
| Patient numbers according to serum levels of zinc, n (%) | 0.623 | ||
| Serum levels of zinc < 70 | 11 (11) | 25 (9.3) | |
| Serum levels of zinc > 70 | 89 (89) | 244 (90.7) | |
| Patient number according to months, n (%) | <0.001 | ||
| August | 35 (35) | 119 (44.2) | |
| September | 31 (31) | 61 (22.7) | |
| October | 21 (21) | 88 (32.7) | |
| November | 13 (13) | 1 (0.4) |
The mean standard deviation score (SDS) of height and weight of hospitalized patients with normal serum zinc levels was 0.24 (± 1.28) and 0.54 (± 1.56), respectively. In the low serum zinc level group, the SDS of height and weight were −0.07 (± 0.62) and −0.54 (± 1.03), respectively. There was no statistical difference between hospitalized patients in both groups for SDS of height and weight (p = 0.608, p = 0.187).
Discussion
The present study focused on the serum zinc status of patients with COVID-19. Our data suggest that patients with COVID-19 had approximately 10% lower serum zinc concentrations than the healthy control group, similar to a study conducted by Thurnham et al. in adult patients, who observed that serum zinc concentrations decreased by almost 10% in patients with inflammation [19]. As a consequence of natural “nutritional immunity,” increased cytokine levels during infections and fever reduce intracellular free zinc and circulating zinc levels to inhibit pathogen growth [20, 21]. However, we found that median serum zinc level was normal in both patients with COVID-19 and control group. Therefore, it was noted that the ages of the two groups were statistically different (13 years vs. 10 years, respectively, p = 0.006) and that the patients in both groups consisted of school-aged children.
Although the median serum zinc levels of patients with COVID-19 were normal, patients with low serum zinc concentrations were significantly more likely to be hospitalized compared with patients with normal serum zinc concentrations. When zinc intake decreases, homeostatic mechanisms initially maintain plasma concentrations in the reference range, but concentrations decrease if deficiency is severe or prolonged. However, although plasma zinc concentrations are moderately correlated with habitual intake, the test also has limited specificity because zinc concentrations may be influenced by inflammatory status, fasting status, recent physical activity, age, sex, and time of day of testing [2, 3, 22, 23]. For this reason, a low serum zinc concentration is more indicative of the zinc status of the population than of the zinc status of the individual [3].
There are some studies on serum zinc levels in adult patients with COVID-19. Jothimani et al. showed that of 47 adult patients hospitalized for COVID-19, 27 (57.4%) had low serum zinc levels [24]. In a study by Yasui et al. in an adult cohort of 29 patients with COVID-19, 9 (31%) patients were found to have low serum zinc levels [25]. In a more recent study of an adult cohort of 33 patients with COVID-19, low serum zinc levels were found in all patients. The authors speculated that the reason for the low serum zinc levels in patients with COVID-19 might be a response to SARS-CoV-2 in the acute phase [26]. Compared with these studies in adults, we found that low serum zinc levels occurred in approximately 10% of patients with COVID-19. This was speculated to be due to the milder clinical course of COVID-19 disease in children compared with adults. In addition, most of the studies conducted with adult patients were hospitalized patients, so it was hypothesized that a higher number of patients with a severe form of COVID-19 and a greater amount of low serum zinc levels were found in these studies.
There are some studies in children that show an association between low serum zinc levels and pneumonia. In a study by Kumar et al., 80% of children with severe pneumonia were found to have low serum zinc levels [27]. Another study showed that low serum zinc levels were found in children with pneumonia complicated by sepsis, mechanical ventilation, and death [28]. In a study that examined serum zinc levels and the prevalence of lower respiratory tract infections, the authors recorded a prevalence of 98.3% for low serum zinc levels in children with acute lower respiratory tract infections compared with 64.2% in the control population [29]. Some studies showed an association between low serum zinc levels and respiratory syncytial virus infection. In particular, it was shown that whole blood zinc levels were significantly lower in children with RSV pneumonia [16].
In developing countries, zinc supplementation reduced pneumonia morbidity, but did not significantly reduce pneumonia-specific mortality [30]. Yao et al. studied the effect of zinc supplementation in hospitalized adult patients with SARS-CoV-2 infection [31]. They found that there was no causal relationship between zinc supplementation and survival in hospitalized adult patients with COVID-19. Their subgroup analyses, stratified by severity of COVID-19, also found no significant causal association [31]. In our study, we found no association between the severity of COVID-19 and serum zinc levels in children. Therefore, we did not administer zinc supplementation to any of our patients.
Low serum zinc levels have been associated with a greater risk of acute respiratory distress syndrome, increased levels of proinflammatory mediators, prolonged hospitalization, and increased mortality in adult patients [24]. In one study using an experimental human model of zinc deficiency, a decrease in CD8 lymphocyte counts was observed in subjects receiving a low zinc diet [32]. In another study, low serum zinc levels were associated with the severity of COVID-19 [25]. Vogel-Gonzalez et al. indicated that serum zinc levels below 50 mcg/dL on hospital admission were correlated with a worse clinical presentation, longer time to stabilization, and higher mortality rates in adults [33]. In a recent study, 35 patients with confirmed COVID-19 had lower serum zinc levels, and non-survivors had significantly lower serum zinc levels compared with survivors [34]. We thought that we would not find an association between serum zinc levels, severity of COVID-19, and longer hospitalization of patients because of the milder clinical course of COVID-19 in children. However, we found higher hospitalization rates (45.5% vs. 11.2%) in patients with low serum zinc levels.
Zinc has several antiviral effects, which are achieved by triggering both innate and acquired immune responses. Although the antiviral modulation of zinc in humans has not been elucidated, zinc has been shown to have antiviral properties by inhibiting RNA synthesis, viral replication, DNA polymerase, reverse transcriptase, and viral proteases [35–37]. A meta-analysis showed that zinc supplementation significantly reduced the incidence of acute lower respiratory tract infections in children aged younger than 5 years [38]. In a randomized trial, the authors showed that zinc supplementation reduced hospitalizations in children with acute lower respiratory tract infections [39]. In a study by Acevedo-Murillo et al., zinc status was assessed in children with pneumonia. Zinc supplementation improved clinical status, respiratory rate, and oxygen saturation in a shorter time compared with the placebo group. They also observed that interferon-gamma and interleukin-2 increased after treatment in the zinc replacement group [40]. In contrast to the protective effects of zinc, some studies have showed that zinc supplementation does not significantly improve survival, reduce inflammation, or decrease the risk of cardiovascular disease [41].
There are publications with much speculation about zinc supplementation in COVID-19 [42, 43]. Unfortunately, in vitro studies have shown that the antiviral activity of zinc is often above physiologic concentrations. Serum and intracellular zinc concentrations are different, and intracellular zinc is up to 100 times higher than serum concentrations [35, 44]. Nevertheless, it is bound to a considerable extent by zinc-binding proteins, so that free zinc concentrations are very low. Zinc is usually present in the cell as a body defense substance bound to metallothionein. Metallothioneins are small proteins that can bind divalent cations such as zinc and copper and store and transfer zinc ions in response to the invasion of the cell by a pathogen. The infected cell reduces the flow of zinc in the cell and prevents the pathogen from gaining access to the zinc it needs. Interferons stimulate the influx of zinc in the virus-infected cell, which in turn promotes the expression of metallothionein [45, 46]. The expression of metallothionein is attributed to the influx or redistribution of zinc from cells [45]. Interventions that alter zinc levels in serum or at local sites in the body could be beneficial, neutral, or even harmful, depending on the mechanistic basis of pathogenesis and the pathogen involved. In a study by Singh et al. from Europe, zinc sufficiency status was consistently positively associated with mortality from COVID-19 in the general population, contrary to expectations [47]. They speculated that the increased mortality observed in populations with high zinc sufficiency might be due to the existing underlying conditions of altered genetic composition, physiology, and response to zinc supplementation in certain parts of the population.
In our study, the median serum zinc concentration was found to be normal in both groups. In a randomized trial of the effect of zinc supplementation in children with chronic kidney disease, the authors divided patients into two groups (receiving 15 mg/day and 30 mg/day), although mean serum zinc levels were normal before treatment. Although serum albumin, zinc, and C-reactive protein (CRP) levels did not change significantly with zinc replacement, there was a small but positive and significant change in normalization of body mass score and body mass index Z-score, hypoalbuminemia, hypozincemia, and high CRP, especially in the group receiving 30 mg/day zinc replacement [48]. In contrast, a study examining the effect of zinc supplementation in zinc-sufficient children showed that normal serum zinc levels did not significantly alter the standard deviation of height or weight during 6–12 months of follow-up, although serum alkaline phosphatase, osteocalcin, and insulin-like growth factor-1 were increased [49]. In another randomized trial of normal serum zinc concentrations, no significant improvement in serum zinc levels was observed in children receiving 10 mg of zinc sulfate daily for 6 months [50].
Some studies have focused on laboratory findings and serum zinc status in patients with COVID-19 [33, 51]. In particular, they found that CRP was inversely proportional to serum zinc levels. In our study, we found no correlation between CRP and serum zinc levels. However, there was a weak positive correlation between serum zinc levels and serum lactate dehydrogenase, D-dimer, and creatinine kinase.
Limitation of the study
The best indicator of an individual’s zinc status is to assess the individual’s zinc intake and absorption. However, these methods are very difficult to apply in practice. One of the limitations of the study is that serum zinc levels were not determined at a standard time during the day, but only in the morning and afternoon. Other factors that may influence serum zinc levels, such as living conditions, income distribution, patients’ hunger/satiety status, exercise status, and patients’ nutritional status were not studied.
Conclusion
Serum zinc levels may be influenced by many factors such as fasting status, diurnal variation, physical activity, and sex, and can be more indicative of the zinc status of the population than of the individual. The fact that the frequency of hospitalization was significantly higher in patients with low serum zinc levels led us to the conclusion that these patients need a detailed assessment of their living environment. The influence of living conditions on the severity of COVID-19 and serum zinc levels remains to be determined in future research. We found no association between the severity of COVID-19 and serum zinc levels. Further studies are needed to demonstrate this association in children.
Recommendation
As serum zinc levels reflect the population in which the assessment is made, the living conditions of individuals in this population should be subject to a very detailed assessment.
Acknowledgements
We want to express our gratitude to all the technicians working in our hospital who work in Department of Biochemistry.
Abbreviations
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- COVID-19
Coronavirus Disease-19
- RT-PCR
Reverse transcription-polymerase chain reaction
- IZiNCG
International Zinc Nutrition Consultative Group
- WHO
World Health Organization
- IQR
Interquartile range
- OR
Odds ratio
Authors’ contributions
Drs Yilmaz-Ciftdogan and Ekemen-Keles conceptualized and designed the study, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. Drs Ustundag, Sahin, Yilmaz, Colak, and Kara Aksay designed the data collection instruments, collected data, critically reviewed the manuscript for important intellectual content, and revised the manuscript. Drs Yilmaz-Ciftdogan and Ekemen-Keles conceptualized and designed the study, collected data drafted the initial manuscript, reviewed, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content.
Availability of data and material
All data and document information is available to give to the European Journal of Pediatrics.
Declarations
Ethics approval
Ethics committee approval was obtained.
Consent to participate
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Consent for publication
All authors have given consent for the study to be published in European Journal of Pediatrics.
Conflict of interest
The authors declare no competing interests.
Footnotes
Key message
Pediatric COVID-19 populations with low serum zinc levels may have an increased incidence of hospitalization, and physicians are advised to exercise caution in this regard.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yıldız Ekemen Keleş, Email: kutupylz@hotmail.com.
Dilek Yılmaz Çiftdoğan, Email: drdilekyilmaz@hotmail.com.
Ayfer Çolak, Email: ayfercolak@gmail.com.
Ahu Kara Aksay, Email: ahukara01@hotmail.com.
Gülnihan Üstündag, Email: gulnihanulker@yahoo.com.
Aslıhan Şahin, Email: aslhansahn@gmail.com.
Nisel Yılmaz, Email: niseloz@yahoo.com.
References
- 1.Samad N, Sodunke TE, Abubakar AR, Jahan I, Sharma P, Islam S, Dutta S, Haque M. The implications of zinc therapy in combating the COVID-19 global pandemic. J inflamm Res. 2021;14:527–550. doi: 10.2147/JIR.S295377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Zinc Nutrition Consultative Group (IZiNCG), Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lönnerdal B, Ruel MT, Sandtröm B, Wasantwisut E, Hotz C (2004) International Zinc Nutrition Consultative Group (IZiNCG) technical document# 1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 25(03):99–203 [PubMed]
- 3.Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull. 2007;28:S403–429. doi: 10.1177/15648265070283S303. [DOI] [PubMed] [Google Scholar]
- 4.Brown KH. Effect of infections on plasma zinc concentration and implications for zinc status assessment in low-income countries. Am J Clin Nutr. 1998;68:425–529. doi: 10.1093/ajcn/68.2.425S. [DOI] [PubMed] [Google Scholar]
- 5.Briggs MH, Briggs M, Austin J. Effects of steroid pharmaceuticals on plasma zinc. Nature. 1971;232(5311):480–481. doi: 10.1038/232480a0. [DOI] [PubMed] [Google Scholar]
- 6.Livingstone C. Zinc: physiology, deficiency, and parenteral nutrition. Nutr Clin Pract. 2015;30(3):371–382. doi: 10.1177/0884533615570376. [DOI] [PubMed] [Google Scholar]
- 7.Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am J Clin Nutr. 2009;89(6):2040–2051. doi: 10.3945/ajcn.2009.27230G. [DOI] [PubMed] [Google Scholar]
- 8.Haase H, Rink L. Multiple impacts of zinc on immune function. Metallomics. 2014;6(7):1175–1180. doi: 10.1039/c3mt00353a. [DOI] [PubMed] [Google Scholar]
- 9.Gao H, Dai W, Zhao L, Min J, Wang F. The role of zinc and zinc homeostasis in macrophage function. J Immunol Res. 2018;6:6872621. doi: 10.1155/2018/6872621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9(12):1286. doi: 10.3390/nu9121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suara RO, Crowe JE. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Chemother. 2004;48(3):783–790. doi: 10.1128/AAC.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butterworth BE, Korant BD. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J Virol. 1974;14(2):282–291. doi: 10.1128/JVI.14.2.282-291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razzaque MS. COVID-19 pandemic: can maintaining optimal zinc balance enhance host resistance? Tohoku J Exp Med. 2020;251(3):175–181. doi: 10.1620/tjem.251.175. [DOI] [PubMed] [Google Scholar]
- 14.Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos DA, Aaseth J, Tsatsakis A, et al. Zinc and respiratory tract infections: perspectives for COVID-19. Int J Mol Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaushik N, Subramani C, Anang S, Muthumohan R, Nayak B, Ranjith-Kumar CT, Surjit M. Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J Virol. 2017;91(21):e00754–e817. doi: 10.1128/JVI.00754-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Che Z, Sun J. Investigation on relationship between whole blood zinc and Fe elements with children pneumonia caused by respiratory syncytial virus. Int J Lab Med. 2016;37(17):2401–2402. doi: 10.3969/j.issn.1673-4130.2016.17.017. [DOI] [Google Scholar]
- 17.Yuan X, Qian S-Y, Li Z, Zhang Z-Z. Effect of zinc supplementation on infants with severe pneumonia. World Journal Pediatr. 2016;12(2):166–169. doi: 10.1007/s12519-015-0072-9. [DOI] [PubMed] [Google Scholar]
- 18.Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976–1980) Am J Clin Nutr. 2003;78(4):756–764. doi: 10.1093/ajcn/78.4.756. [DOI] [PubMed] [Google Scholar]
- 19.Thurnham DI, Mburu AS, Mwaniki DL, De Wagt A. Micronutrients in childhood and the influence of subclinical inflammation. Proc Nutr Soc. 2005;64(4):502–509. doi: 10.1079/pns2005468. [DOI] [PubMed] [Google Scholar]
- 20.Bonaventura P, Benedetti G, Albarède F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14(4):277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Lonergan ZR, Skaar EP. Nutrient zinc at the host–pathogen interface. Trends Biochem Sci. 2019;44(12):1041–1056. doi: 10.1016/j.tibs.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King JC, Cousins RJ (2014) Zinc. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR (ed) Modern Nutrition in Health and Disease, 11rd edn. PA: Lippincott Williams and Wilkins, Philadelphia, p 189–192
- 23.Hambidge KM, Goodall MJ, Stall C, Pritts J. Post-prandial and daily changes in plasma zinc. J Trace Elem Electrolytes Health Dis. 1989;3(1):55–57. [PubMed] [Google Scholar]
- 24.Jothimani D, Kailasam E, Danielraj S, Nallathambi B, Ramachandran H, Sekar P, Manoharan S, Ramani V, Narasimhan G, Kaliamoorthy I, et al. COVID-19: poor outcomes in patients with zinc deficiency. Int J Infect Dis. 2020;100(11):343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasui Y, Yasui H, Suzuki K, Saitou T, Yamamoto Y, Ishizaka T, Nishida K, Yoshihara S, Gohma I, Ogawa Y. Analysis of the predictive factors for a critical illness of COVID-19 during treatment-relationship between serum zinc level and critical illness of COVID-19. Int J of Infect Dis. 2020;100(11):230–236. doi: 10.1016/j.ijid.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel O, Chinni V, El-Khoury J, Perera M, Neto AS, McDonald C, See E, Jones D, Bolton D, Bellomo R, Trubiano J, Ischia J. A pilot double-blind safety and feasibility randomized controlled trial of high-dose intravenous zinc in hospitalized COVID-19 patients. J Medl Virol. 2021;93(5):3261–3267. doi: 10.1002/jmv.26895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar N, Jayaprakash S, Kavitha D (2017) Low serum zinc level-a possible marker of severe pneumonia. JMScR 5:21554–21570. 10.18535/jmscr/v5i5.53
- 28.Saleh NY, Abo El Fotoh WMM. Low serum zinc level: the relationship with severe pneumonia and survival in critically ill children. Int J Clin Pract. 2018;72(6):e13211. doi: 10.1111/ijcp.13211. [DOI] [PubMed] [Google Scholar]
- 29.Ibraheem RM, Johnson AB, Abdulkarim AA, Biliaminu SA. Serum zinc levels in hospitalized children with acute lower respiratory infections in the north-central region of Nigeria. Afr Health Sci. 2014;14(1):136–142. doi: 10.4314/ahs.v14i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yakoob MY, Theodoratou E, Jabeen A, Imdad A, Eisele TP, Ferguson J, Jhass A, Rudan I, Campbell H, Black RE, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health. 2011;11(3):1–10. doi: 10.1186/1471-2458-11-S3-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao JS, Paguio JA, Dee EC, Tan HC, Moulick A, Milazzo C, Jurado J, Della Penna N, Celi LA. The minimal effect of zinc on the survival of hospitalized patients with Covid-19: an observational study. Chest. 2021;159(1):108–111. doi: 10.1016/j.chest.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck FW, Kaplan J, Fine N, Handschu W, Prasad AS. Decreased expression of CD73 (ecto-5'-nucleotidase) in the CD8+ subset is associated with zinc deficiency in human patients. J Lab Clin Med. 1997;130(2):147–156. doi: 10.1016/s0022-2143(97)90091-3. [DOI] [PubMed] [Google Scholar]
- 33.Vogel-González M, Talló-Parra M, Herrera-Fernández V, Pérez-Vilaró G, Chillón M, Nogués X, Gómez-Zorrilla S, López-Montesinos I, Arnau-Barrés I, Sorli-Redó ML, et al. Low zinc levels at admission associates with poor clinical outcomes in SARS-CoV-2 infection. Nutrients. 2021;13(2):562. doi: 10.3390/nu13020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heller RA, Sun Q, Hackler J, Seelig J, Seibert L, Cherkezov A, Minich WB, Seemann P, Diegmann J, Pilz M, et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021;38(1):101764. doi: 10.1016/j.redox.2020.101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko YL, Morihara D, Shibata K, Yamauchi R, Fukuda H, Kunimoto H, Takata K, Tanaka T, Inomata S, Yokoyama K, et al. Factors attenuating zinc deficiency improvement in direct-acting antiviral agent-treated chronic hepatitis C virus infection. Nutrients. 2018;10(11):1620. doi: 10.3390/nu10111620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fridlender B, Chejanovsky N, Becker Y. Selective inhibition of herpes simplex virus type 1 DNA polymerase by zinc ions. Virology. 1978;84(2):551–554. doi: 10.1016/0042-6822(78)90274-x. [DOI] [PubMed] [Google Scholar]
- 38.Roth DE, Richard SA, Black RE. Zinc supplementation for the prevention of acute lower respiratory infection in children in developing countries: meta-analysis and meta-regression of randomized trials. Int J Epidemiol. 2010;39(3):795–808. doi: 10.1093/ije/dyp391. [DOI] [PubMed] [Google Scholar]
- 39.Rerksuppaphol S, Rerksuppaphol L. A randomized controlled trial of zinc supplementation in the treatment of acute respiratory tract infection in Thai children. Pediatr Rep. 2019;11(2):15–20. doi: 10.4081/pr.2019.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acevedo-Murillo JA, García León ML, Firo-Reyes V, Santiago-Cordova JL, Gonzalez-Rodriguez AP, Wong-Chew RM (2019) Zinc supplementation promotes a Th1 response and improves clinical symptoms in fewer hours in children with pneumonia younger than 5 years old. A randomized controlled clinical trial. Front Pediatr 14(7):431. 10.3389/fped.2019.00431 [DOI] [PMC free article] [PubMed]
- 41.Freiberg MS, Cheng DM, Gnatienko N, Blokhina E, Coleman SM, Doyle MF, Yaroslavtseva T, Bridden C, So-Armah K, Tracy R, et al. Effect of zinc supplementation vs placebo on mortality risk and HIV disease progression among HIV-positive adults with heavy alcohol use: a randomized clinical trial. JAMA Netw Open. 2020;3(5):e204330. doi: 10.1001/jamanetworkopen.2020.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar A, Kubota Y, Chernov M, Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med hypotheses. 2020;144:109848. doi: 10.1016/j.mehy.2020.109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlucci PM, Ahuja T, Petrilli C, Rajagopalan H, Jones S, Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020;69(10):1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rükgauer M, Klein J, Kruse-Jarres J. Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J Trace Elem Med Biol. 1997;11(2):92–98. doi: 10.1016/S0946-672X(97)80032-6. [DOI] [PubMed] [Google Scholar]
- 45.Subramanian Vignesh K, Deepe GS., Jr Metallothioneins: emerging modulators in immunity and infection. Int J Mol Sci. 2017;18(10):2197. doi: 10.3390/ijms18102197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lonergan ZR, Skaar EP. Nutrient zinc at the host-pathogen interface. Trends Biochem Sci. 2019;44(12):1041–1056. doi: 10.1016/j.tibs.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh S, Diwaker A, Singh BP, Singh RK. Nutritional immunity, zinc sufficiency, and COVID-19 mortality in socially similar European populations. Front Immunol. 2021;12(9):699389. doi: 10.3389/fimmu.2021.699389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escobedo-Monge MF, Ayala-Macedo G, Sakihara G, Peralta S, Almaraz-Gómez A, Barrado E, Marugán-Miguelsanz JM. Effects of zinc supplementation on nutritional status in children with chronic kidney disease: a randomized trial. Nutrients. 2019;11(11):2671. doi: 10.3390/nu11112671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imamoğlu S, Bereket A, Turan S, Taga Y, Haklar G. Effect of zinc supplementation on growth hormone secretion, IGF-I, IGFBP-3, somatomedin generation, alkaline phosphatase, osteocalcin and growth in prepubertal children with idiopathic short stature. J Pediatr Endocrinol Metab. 2005;18(1):69–74. doi: 10.1515/jpem.2005.18.1.69. [DOI] [PubMed] [Google Scholar]
- 50.Mandlik R, Mughal Z, Khadilkar A, Chiplonkar S, Ekbote V, Kajale N, Patwardhan V, Padidela R, Khadilkar V. Occurrence of infections in schoolchildren subsequent to supplementation with vitamin D-calcium or zinc: a randomized, double-blind, placebo-controlled trial. Nutr Res Pract. 2020;14(2):117–126. doi: 10.4162/nrp.2020.14.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDonald CM, Suchdev PS, Krebs NF, Hess SY, Wessells KR, Ismaily S, Rahman S, Wieringa FT, Williams AM, Brown KH, et al. Adjusting plasma or serum zinc concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2020;111(4):927–937. doi: 10.1093/ajcn/nqz304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and document information is available to give to the European Journal of Pediatrics.