Abstract
Background
The incidence of myocarditis after RNA-based vaccines for coronavirus has gained social and medical interest.
Methods
We performed an intention-to-treat meta-analysis, following the PRISMA statement. After a systematic search, without language restriction, 9 publications were selected. Two were excluded (one was only in subjects with age 12–17 and other might had included subjects from a larger publication). We followed the PRISMA guidelines for abstracting data and assessing data quality and validity. Data was verified by 2 investigators.
Results
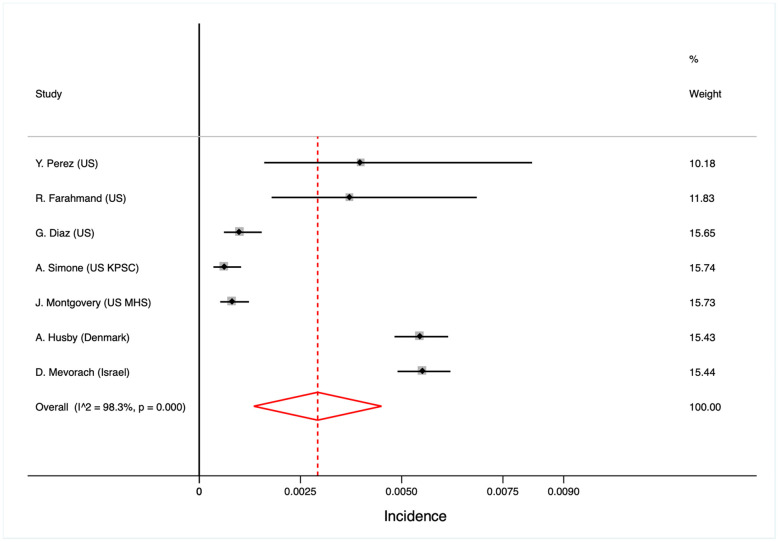
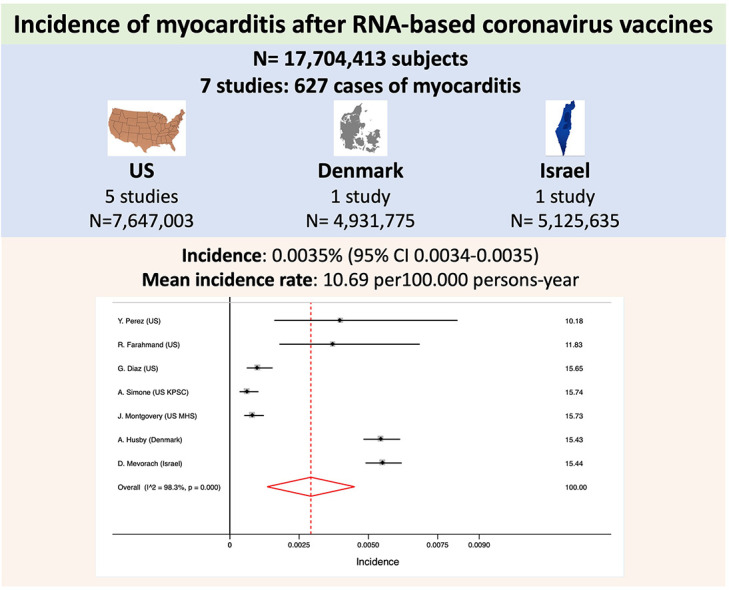
We analyzed 17,704,413 subjects, from 7 studies, that included 627 cases of confirmed myocarditis). The incidence of myocarditis was 0.0035% (95% CI 0.0034–0.0035). Mean incidence rate was 10.69 per 100.000 persons-year. Cases reported from Israel represented 45.14% from total (283 out of the 627). Only 1 case of fatal myocarditis or death was reported. There was significant heterogeneity between results. The meta-regression analysis excluded mean age, region, number of cases or number of people included as sources of heterogeneity. No small-study effect was observed (p = 0.19).
Conclusions and relevance
Myocarditis incidence after RNA vaccines is very rare (0.0035%) and has a very favorable clinical course.
Keywords: Myocarditis, Covid vaccine, RNA vaccine
Graphical abstract
1. Introduction
Coronavirus vaccines have demonstrated to reduce very effectively the incidence of severe covid-19 and mortality [1]. Nonetheless, the incidence of myocarditis after RNA-based vaccines for coronavirus has gained social and medical interest [[2], [3], [4]]. Several reports have provided very low rates of myocarditis after the second dose of RNA-based vaccines [5,6]. Moreover, some studies have suggested that the myocarditis incidence has increased since the introduction of this type of vaccines [7,8] meanwhile other reported similar rates than expected based on previous time-periods rates [9].
2. Methods
We performed an intention-to-treat meta-analysis in line with recommendations from the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [10]. We performed a systematic search (using PUBMED, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar), without language restriction, for publications using the Medical Subject Headings terms “myocarditis”, “vaccine”, “coronavirus” and “RNA vaccine”. A total of 9 reports were identified [[5], [6], [7], [8], [9],[11], [12], [13], [14]]. The report from Witberg et al. [6], evaluated patients who were enrolled in Clalit Health Services, the largest health insurance company in Israel, and the one by Mevorach et al. [5] was based on medical records obtained from the Ministry of Health database from Israel; therefore, patients could be have been included in both reports and we excluded data from Witberg et al. [6]. The report by Foltran et al. [14]only adolescents with age between 12 and 17and, therefore, it was not included. So finally, 7 reports were analyzed [5,[7], [8], [9],[11], [12], [13]].
Numeric rates of confirmed cases of myocarditis were extracted from each report. The percentage of variability across studies attributable to heterogeneity beyond chance was estimated using the I2 statistic. A random effect model was applied because significant heterogeneity was detected. Sensitivity analysis included the identification of potential sources of heterogeneity between trials, tested by meta-regression analyses, and the assessment of the small-study effects, by the Egger test [10]. All analyses were performed using STATA 14.3 (StataCorp. 2009. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
3. Results
We included the results of 7 reports with 17,704,413 subjects (Table 1 ). The report from the Israel Ministry of Health database represented 28.95% of the subjects, the report from the Denmark 27.86% and the 5 reports from the United States represented the resting 43.19% for the population included in the analyses. A total of 627 cases of confirmed myocarditis were reported what represented an incidence of 0.0035% (95% CI 0.0034–0.0035). Mean incidence rate was 10.69 per 100.000 persons-year. Cases reported from Israel represented 45.14% (283 out of the 627). Only 1 case of fatal myocarditis or death was reported.
Table 1.
Characteristics of the reports included in the metanalysis.
| First author | Country | Type of study | Fully vaccinated people | Confirmed myocarditis | Rate/100.000 person-years | Deaths | Diagnostic criteria | Pfizer | Moderna |
|---|---|---|---|---|---|---|---|---|---|
| Y. Perez | US | Rochester Epidemiology Project | 175,472 | 7 | 55.35 | 0 | ICD-10 codes | ||
| R. Farahmand | US | Massachusetts Immunization Information System | 268,320 | 10 | 0.08 | 0 | ICD-10 codes | 54% | 41% |
| G. Diaz | US | 40 hospital records | 2,000,287 | 20 | 1.00 | 0 | Hospital diagnosis | 62.2% | 32.4% |
| A. Simone | US | Kaiser Permanente Southern California | 2,392,924 | 15 | 0.58 | 0 | Hospital diagnosis and adjudication by 2 cardiologist | 50% | |
| J. Montgovery | US | US Military Health Service | 2,810,000 | 23 | 23.00 | 0 | CDC criteria | ||
| A. Husby | Denmark | Population based cohort study using the Danish Vaccination Register | 4,931,775 | 269 | 1.7 | 0 | Hospital-based and ICD-10 codes | 83.8% | 12% |
| D. Mevorach | Israel | Retrospective study using the Ministry of Health database | 5,125,635 | 283 | 1.76 | 1 | ICD-9 and CDC criteria | 100% | |
CDC: Centers for Disease Control and Prevention; ICD: international classification of the diseases.
As shown in Fig. 1 , there was significant heterogeneity between results. The meta-regression analysis excluded mean age, region, number of cases, number of people included or diagnostic criteria as sources of heterogeneity. The Egger test excluded the small-study effect (p = 0.19).
Fig. 1.
Forest plot for the incidence of myocarditis.
4. Discusion
This metanalysis with >17 millions of subjects vaccinated with the 2 doses of RNA vaccines for coronavirus demonstrates the low rate of myocarditis and mortality. Myocarditis are a well described side effect of vaccines [2] and this metanalysis demonstrates that it also occur after RNA vaccines for coronavirus and have an excellent prognosis.
The Vaccine Adverse Event Reporting System (VAERS) reported and incidence of myocarditis of 0.1% (708 confirmed cases from 620,195 subjects) between 1990 and 2018 [2]. The analysis also revealed that despite the introduction of new vaccines over the years, myopericarditis had very low incidence in the United States. RNA coronavirus vaccines have been widely applied since January 2020 and it is estimated that by the end of 2021 more than 3500 millions of people in the world will be vaccinated with 2 doses of any of the vaccines available [15]. We believe that our results, as well as other reports that exclude cardiovascular complications [16], should reinforce the wide generalization of coronavirus vaccines that have demonstrated to reduce, dramatically, the incidence of severe covid-19 and mortality [1,17].
Clinical course of myocarditis was benign in all cases but one. The VAERS reported that 69% of the cases diagnosed after vaccines were classified as serious [2] although some reports have highlighted the rare presentation of fulminant myocarditis leading to multiorgan failure and death [3,4]. These reports reinforce the need of wider studies as our metanalysis. A part from this, the clinical presentation revealed that myocarditis is more incident in men, symptom onset is <2 weeks postvaccination and the highest rates is observed in subjects aged 25 to 44 years is similar to the other forms of vaccine-related myocarditis [2]. Myocarditis have been also reported in many patients with severe covid-19 [18] although mortality and myocarditis incidence had a very different pattern in patients with covid-19, since both increased exponentially with age [19]. Finally, there is scarce evidence on the optimal therapy for this kind of RNA vaccine-related myocarditis.
Some analyses concluded that coronavirus vaccination has led to a particular increase in the diagnosis of myopericarditis in men [7,8,11] meanwhile some other concluded that expected and observed rates of myocarditis in their areas or population remained unchanged [9]. Such differences could be attributed to diagnostic criteria, active search or, even, random or uncontrolled effects. We also found different diagnostic criteria that might highlight the clinical challenge of the definite diagnosis of myocarditis. Nonetheless, all evidence concludes that whatever the incidence of myopericarditis might be, it is always much lower than the morbidity seen in patients who got infected or developed covid-19 disease [5,7,8,11,13].
Our study might be limited by data source. We included >17 millions of people that represents a low percentage of the population vaccinated world-wide but, in contrast, these data source have been accurately collected. The most solid evidence might be derived from randomized controlled trials although real-life records provide evidence in less homogenous and non-selected population. We only included definite cases of people that received the 2 doses of the vaccines because incidence after the first dose was reported in some studies [5,7,8,11] but not in others Perez [8,9,13]; however, the study from the Ministry of Health database of Israel reported that the risk difference between the first and second doses was 1.76 per 100,000 persons [5]. Finally, some authors have highlighted that establishing a causal link between a RNA-based vaccine and a case of myocarditis could have a limited scientific value, especially without a cardiac biopsy [20].
In conclusion, myocarditis incidence after RNA vaccines is very rare (0.0035%) and has favorable clinical course. National or regional reports on side effects of coronavirus vaccines provide a great tool for the reinforcement of global vaccination.
Declaration of Competing Interest
-
-
Alberto Cordero reports a) honoraria for lectures from AstraZeneca, Bristol-Myers Squibb, Ferrer, Boehringer Ingelheim, MSD, and Bristol-Myers Squibb and AMGEN; b) consulting fees from AstraZeneca, Ferrer and AMGEN.
-
-
María Amparo Quintanilla reports a) honoraria for lectures from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, and Rovi; b) consulting fees from Boehringer Ingelheim, Eli Lilly and AstraZeneca
-
-
Vicente Bertomeu-González reports a) honoraria for lectures from Daiichi Sankyo, Boehringer Ingelheim, Bayer, Pfizer-BMS, LivaNova, Ferrer, Cardiome, MSD; b) consulting fees none; c) research grants from Medtronic Iberica.
Acknowledgements
Investigators received the support of the Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV) Spain, the National Network for Biomedical Research in Cardiovascular Disease.
References
- 1.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su J.R., McNeil M.M., Welsh K.J., Marquez P.L., Ng C., Yan M., et al. Myopericarditis after vaccination, vaccine adverse event reporting system (VAERS), 1990–2018. Vaccine. 2021;39(5):839–845. doi: 10.1016/j.vaccine.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 3.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbate A., Gavin J., Madanchi N., Kim C., Shah P.R., Klein K., et al. Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients. Int. J. Cardiol. 2021;340:119–121. doi: 10.1016/j.ijcard.2021.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mevorach D., Anis E., Cedar N., Bromberg M., Haas E.J., Nadir E., et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N. Engl. J. Med. 2021;385(23):2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., et al. Myocarditis after Covid-19 vaccination in a large health care organization. N. Engl. J. Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson I.V., Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326(12):1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez Y., Levy E.R., Joshi A.Y., Virk A., Rodriguez-Porcel M., Johnson M., et al. Myocarditis following COVID-19 mRNA vaccine: a case series and incidence rate determination. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montgomery J., Ryan M., Engler R., Hoffman D., McClenathan B., Collins L., et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Cook D.J., Eastwood S., Olkin I., Rennie D., Stroup D.F. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354(9193):1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 11.Farahmand R., Trottier C.A., Kannam J.P., Ho K.K.L. Incidence of myopericarditis and myocardial injury in coronavirus disease 2019 vaccinated subjects. Am. J. Cardiol. 2022;164:123–130. doi: 10.1016/j.amjcard.2021.10.022. (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simone A., Herald J., Chen A., Gulati N., Shen A.Y.-J., Lewin B., et al. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern. Med. 2021;181(12):1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husby A., Hansen J.V., Fosbøl E., Thiesson E.M., Madsen M., Thomsen R.W., et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375 doi: 10.1136/bmj-2021-068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foltran D., Delmas C., Flumian C., De Paoli P., Salvo F., Gautier S., et al. Myocarditis and pericarditis in adolescents after first and second doses of mRNA COVID-19 vaccines. Eur. Heart J. - Quality of Care and Clinical Outcomes. 2021 doi: 10.1093/ehjqcco/qcab090. qcab090. (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO https://covid19whoint/
- 16.Jabagi M.J., Botton J., Bertrand M., Weill A., Farrington P., Zureik M., et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA. 2022;327(1):80–82. doi: 10.1001/jama.2021.21699. (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbel R., Hammerman A., Sergienko R., Friger M., Peretz A., Netzer D., et al. BNT162b2 vaccine booster and mortality due to Covid-19. N. Engl. J. Med. 2021;385(26):2413–2420. doi: 10.1056/NEJMoa2115624. (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng Q., Hu B., Zhang Y., Wang H., Zhou X., Hu W., et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonanad C., García-Blas S., Tarazona-Santabalbina F., Sanchis J., Bertomeu-González V., Fácila L., et al. The effect of age on mortality in patients with COVID-19: a Meta-analysis with 611,583 subjects. J. Am. Med. Dir. Assoc. 2020;21(7):915–918. doi: 10.1016/j.jamda.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cereda A., Conca C., Barbieri L., Ferrante G., Tumminello G., Lucreziotti S., et al. Acute myocarditis after the second dose of SARS-CoV-2 vaccine: serendipity or atypical causal relationship? Anatol. J. Cardiol. 2021;25(7):522–523. doi: 10.5152/AnatolJCardiol.2021.99. [DOI] [PMC free article] [PubMed] [Google Scholar]