Abstract
It has been more than one year since the first case of the coronaviruses was infected by COVID-19 in China. The world witnessed three waves of the corona virus till now, and more upcoming is expected, whereas several challenges are presented. Empirical data displayed that the features of the virus effects do vary between the three periods. The severity of the disease, differences in symptoms, attitudes of the people have been reported, although the comparative characteristics of the three waves still keep essentially indefinite. In contrast, the sense of danger toward the cries gradually decreases in most countries. This may be due to some factors, including the approved vaccines, introducing alternative plans from politicians to control and deal with the epidemic, and decreasing the mortality rates. However, the alarm voice started to rise again with the appearance of new variant strains with several mutations in the virus. Several more questions began to be asked without sufficient answers. Mutations in COVID-19 have introduced an extreme challenge in preventing and treating SARS-COV-2. The essential feature for mutations is producing new variants known by high tensmibility, disturbing the viral fitness, and enhancing the virus replication. One of the variants that has emerged recently is the Delta variant (B.1.617.2), which was firstly detected in India. In November 2021, a more ferocious mutant appeared in South Africa, also called omicron (B.1.1.529). These mutants grabbed world attention because of their higher transmissibility than the progenitor variants and spread rapidly. Several information about the virus are still confusing and remains secret. There are eight approved vaccines in the market; however, the investigation race about their effect against reinfection and their role against the new variants is still under investigation. Furthermore, this is the first time vaccinating against COVID-19, so the question remains: Will we need an annual dose of the corona vaccines, and the side effects don't been observed till now?
Keywords: COVID-19, SARS-COV-2, Mutation, New variant strain, Vaccine, Reinfection
1. Introduction
Since World War II, the COVID-19 pandemic has become the most critical global health emergency in this century and the greatest challenge for the human population [1]. In addition to being a global health calamity, coronaviruses pandemic have several critical effects in all fields of life, including; environmental, economic, social, political, and cultural. The first wave was so dangerous that almost no place on earth was saved from the impact of this epidemic, despite the differences in seasons; the southern hemisphere was affected later, but no less severely [2]. It is worth mentioning that the rate of cases and death in Africa, except in South Africa, was lower. This may be due to the low average age and Ebola disease some years ago, which helped to experience and decrease the problem [3]. The World Health Organization (WHO) is officially announced COVID-19 as a pandemic on 11 March 2020. This is because increasing the cases number dramatically outside China. On April 10, 2020, 1.5 million cases were confirmed in 184 countries and more than 92,000 deaths worldwide [1]. In February of this year, around 115 million cases became infected, and the death rate was estimated at more than 2.5 million worldwide [4]. Most individuals infected by COVID-19 were mild to moderate respiratory illness and recovered without specific treatment. However, older adults with medical complications are at a higher risk of severe prognosis [5], [6].
On the other hand, environmental conditions could affect the present pandemic of COVID-19 [7], [8]. Several studies have investigated the effect of temperature that plays a significant role in virus survival [1], [8]. For instance, Chin and his group are recently reported that SARS-CoV-2 is very stable at 4 °C, but the sensitivity of the virus increased toward heat [9]. The survival time was reduced to 5 min as the incubation temperature rose to 70 °C. Epidemiological investigations have revealed the connection between COVID-19 and meteorological parameters; however, findings are controversial [10], [11]. A published study by Xie and Zhu described that a 1 °C increase was connected with a 4.861% rise in the daily infected cases of COVID-19, when mean temperature (lag 0–14) was lower than 3 °C [11]. A positive relation between diurnal temperature range and daily deaths of COVID-19 and a negative correlation for relative humidity was recently reported [10].
In contrast, Yao and his colleges have reported that the transmission of COVID-19 did not show an association with temperature in different Chinese cities [12]. The viral mutations are also seasonable independent. It is more expected to happen in winter, spring, and autumn, whereas not preferable in summer [13]. The virus's slow spreading in Summer may be related to the higher temperature, as established by a temporal multivariate time series model in study [14].
Preventive methods are the offered approach to avoid cases spreading because an epidemic will increase if we cannot control it very well. Protective strategies focus on careful control against infection and patient isolation, including suitable measures to be accepted through diagnosis and the introduction of effective clinical care to infected patients [15]. The current situation is that the coronavirus is spreading elsewhere while facing significant immune pressure from vaccines and naturally infected and recovered people [4], [16].
2. Symptoms of COVID-19
With increasing numbers of infected cases, there is a simultaneous growing number of recovered patients. Not all people who have recovered from SARS-CoV-2 infection are free of symptoms. The symptoms also become changeable. Several reports have revealed bone and joint pain, continued fatigue, dizziness, insomnia, palpitations, and headaches. Other symptoms were appeared for irreversible pulmonary scarring and dysfunction, particularly in cases with severe pulmonary disease [17]. Several information about the viruses are still scare till now. The long-term investigations remain insufficient, and these patients' outlook is still wholly missing, although several studies have addressed this issue [5]. The long-COVID refers to various symptoms affecting different organs reported by patients following COVID-19 infection [18]. For example, recent research displayed that half of cases (478 patients) after four months of COVID-19 hospitalization get one feature of long-term COVID at least [19]. Another report with 4182 instances of COVID-19 appeared that 13% of respondents self-reported long- COVID features, with some evidence for higher rates in women and older people [20]. The additional study followed 1733 patients hospitalized for COVID-19 for six months and found fatigue or muscle weakness in 63%, sleep difficulties in 26%, anxiety or depression in 23%, and lower rates of myalgia and headache [21]. It is expected that there will be many chronic consequences of COVID-19 beyond that will exceed the first wave of acute infections, and still, the coming time may reveal a lot of secrets. One long-term effect of COVID-19 that is becoming noticeably obvious is its influence on cognitive function and in patients with mild symptoms. Neurological symptoms appeared with one-third of COVID-19 patients, and there have been anecdotal accounts of ‘COVID-19 delirium’, manifesting as agitation, paranoid hallucinations, and confusion with a lot of hospitalized cases (<20%) [22], [23]. Patients over 65 years old are considered one of the groups most susceptible to severe manifestations of COVID-19. Usually, they have mild cognitive impairment; therefore, the risk increased for developing delirium because of the underlying neurocognitive impairment [24], [25]. Inflammation associated with COVID-19 developing blood-brain barrier, silent infarcts, coagulopathy, thrombosis that could increase neurological injury [26]. In addition to poor patient outcomes, the severe agitation associated with delirium in many COVID-19 patients creates difficulties for staff and compounds the stress of caring for these highly sick patients.
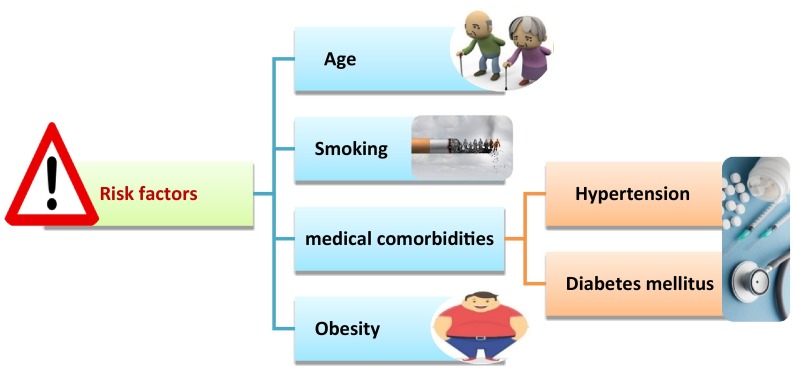
On the other hand, risk factors (Fig. 1 ) for severe SARS-COV-2 infection and increasing mortality rates comprise smoking, advanced age, and medical comorbidities, e.g., diabetes mellitus, hypertension, and obesity, the most common [27], [28]. These risk factors showed a baseline neurocognitive frailty which enhanced the exposure to cognitive complications during inflammation cases [29]. Similar to perioperative neurocognitive disorders associated with surgery and anesthesia. Therefore, the maximum risk persons for intense SARS-COV-2 infection may also display the most fundamentally susceptible people for cognitive decline in the case of SARS-COV-2 inflammation.
Fig. 1.
Risk factors of COVID-19.
3. Development of COVID-19 between three waves
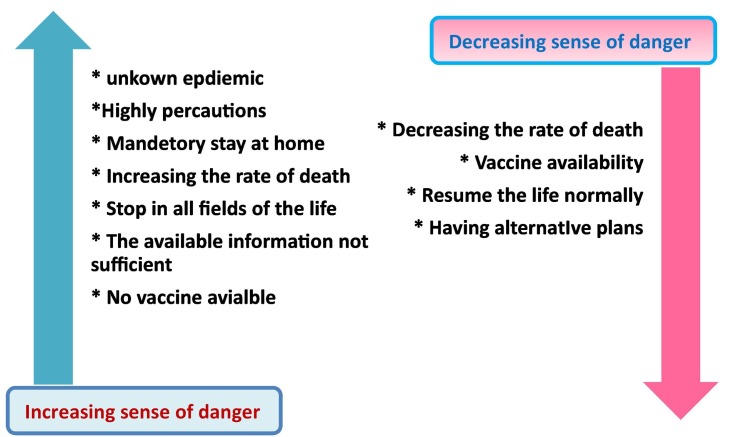
Different countries worldwide have faced a three-wave pattern of reported cases; the first wave occurs in spring, the second period at the end of summer and autumn, and the third at the beginning of 2021. Many changes happened between the three waves of COVID-19 around the world, as tabulated in Table 1 . The people's attitude toward the crisis was variable, and the sense of danger decreased gradually across the globe. This is may be due to some factors such as (i) vaccination availability, (ii) rate of death (iii) alternative plans from governments, as revealed in Fig. 2 . For example, the first wave of infections in the USA started in March 2020, whereas the emergency was announced in the country [5]. The epidemic hit the area for the first time without any previous alarm, and the fighting tools were overwhelmed. Critically ill patients increased over the government's capacity, operating rooms were used as intensive care units, and temporary satellite hospitals were initiated to apply care for non-critical cases [30]. The country succeeded in flattening the curve after extreme mandatory precautions. After some months, different states started returning to their normal lives with ignorance of their stay-at-home restrictions. Hence the number of patients increased at a surprising rate, particularly in western and southern states [31]. Although epidemiologists still debate that the coronaviruses pandemic is far from over, the widespread administration of a COVID-19 vaccine helped remove the high-hearted fear of people [32].
Table 1.
Comparison between the three waves of COVID-19.
| Subject | 1st wave | 2nd wave | 3rd wave |
|---|---|---|---|
| Time | March 2020 | July 2020 | After Christmas 2021 |
| Precautionary measures | Extremely high (Obligatory) | Mandatory | Neglected from some people |
| Changes in symptoms | Fever, cough, sore throat, chest and muscle pain, confusion, dyspnoea, headache, anosmia, and ageusia |
|
Flu symptoms and neurological effect |
| Mortality rate | Horrible rates | Increasing rates | Lower compared to prior waves |
| Economical effect | Completely stopped | Affected | Less affected |
| Social activities | Completely stopped | Decreased | Returned as it is |
| Vaccination | Not available | Under investigation | Available |
| Re-infection | Not appeared | Available | More common |
| Mutations | Not present | Not appeared | Present new variants |
Fig. 2.
Factors affecting the sense of danger toward coronaviruses crisis.
3.1. 1st wave effect
The SARS-COV-2 epidemic was started in Wuhan, China, with the appearance of the first case in December 2019; however, the first wave was internationally detected in March 2020. The main causes of this wave are still secret; it looked like a ghost and fear from the unknown. The first wave was considered a disaster that heavily affected every field in life and represented a significant challenge in public health and disturbed social and economic activities globally [33]. For example, the economy was negatively affected worldwide; good production was almost stopped in all places, and the unemployment rate was highly increased. Another social problem appeared, e.g., violence in the family was increased significantly between children due to the extended stay at home [2].
3.2. 2nd wave effect
Owing to the low numbers of infected cases in the summer season and also in that time mostly the infected young patients were exit from hospitals and became almost empty, some places of society were over thought the pandemic, disregarding the initial announcement for the coming second wave [2]. Like the other viruses, e.g., influenza blaze up seasonally, COVID-19 resumed in autumn as predictable. As several experts predicted, the second wave hit the countries with a much higher force than the first. The leading cause of robust second wave was not a relaxation of intervention, but rather the failure to enforce interventions as occurred in Europe [34].
During the second wave, politics were changed with different priorities than the 1st wave. In Germany, for example, the instructions were very sharp at the beginning of this pandemic, and the government managed very well in the 1st wave, but this position was changed quickly. This is may be due to some different factors and altered instructions from politicians. Now economic life has become the priority in the list, and businesses are open continuously rather than strictly decreasing contacts and thus infections and death rates [2]. In the second wave period, a complicated equation appeared as an alarm. Most countries are almost all filled to achieve the balance between successful medical care with a growing economy. During that wave, most countries were lost a lot of valuable time, and the number of infected cases dramatically increased [2]. However, seroconversions showed a lower effect than the 1st wave. This is because of the high restrictions of outdoor activities, obligatory face masks, and prevention of any people gathering. Such precautions caused hindrance for viral inoculation, whereas high inocula led to more severe SARS-CoV-2 infection [35].
3.3. 3rd wave impact
The third wave of COVID-19 witnessed several changes worldwide and different features observed, such as increasing infected numbers due to home contacts. In addition, significant availability of speedy antigen tests helped in rapid diagnosis and then isolation, whereas severe cases and mortality rates were less than in prior waves. The most notable feature in this wave was the discovery of a new strain (B.1.1.7) that has higher potential transmissibility; hence, re-infections became more common. These several mutations that appeared within the virus may be the main cause of the third wave and the social activities and contacts between people without obligatory precautions. On the other hand, vaccination achieved positive results between patients, health care workers, and the elderly [36].
4. A new strain of COVID-19
Recent studies reported the high affinity of viruses to evade barriers for transmission, mainly when infections are still numerous [36]. Changes in coronaviruses are considered a perfect storm to allow immune escape mutants to emerge. This could initially occur with partial resistance but with possible superior resistance if coronavirus variants develop further [4]. It is worthy of mentioning that the higher infections rates, the higher chances of mutations. This way will support the virus to survive and proliferate [36]. Virus evolutions will not be controlled when we approach herd immunity and do not keep restrictions [4].
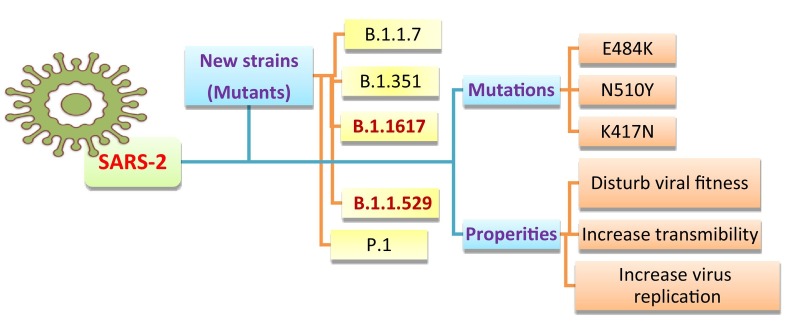
Globally, with virus transmission, many SARS-COV-2 variants have recently appeared [37]. The different levels of genetic changes are basically due to some factors, including; the global absence of immunity against this new pathogen. In addition, mutation rates of the SARS-COV-2 that encode an enzyme with proof-reading function raise the fidelity of replication processes [38]. A recent study investigates gene sequences of COVID-19 to identify mutations. The results showed that 26,844 single mutations were followed in 203,346 human genomes of SARS-CoV-2, while the most common mutations involved S proteins and NSP3 [13]. By the end of December 2020, around 5000 mutations were recognized in the S protein. Virus mutations that lead to the spread of new emerging strains are distributed worldwide, particularly in the United Kingdom, e.g., lineage B.1.1.7 and variant 20I/501Y.V1. This variant is distributed due to several spike (S) protein changes, including deletion 145, N501Y, deletion 69–70, D614G, A570D, T716I, P681H, D1118H, and S982A [13]. The additional new strain discovered in South Africa (lineage B.1.351, variant 20H/501Y.V2) involved eight mutations through S protein; D80A, L18F, R246I, D215G, E484K, K417N, A701V, and N501Y. The variant 20 J/501Y.V3 and lineage P.1 has also spread in Brazil through three S protein mutations, N501Y, E484K, and K417N, in common with 20J/501Y.V2 [13]. The most significant character of all these variants is that they share N501Y mutation concerning the SARS-CoV-2 spike (S) protein which is considered the primary target of most COVID-19 vaccines. Mutations such as E484K, K417N, and N501Y in the S protein could disturb viral fitness and transmissibility (Fig. 3 ). However, the ongoing research on the influence of such variants on COVID-19 vaccines still lacks [4], [13], [39]; hence further and deep investigations are highly recommended.
Fig. 3.
Examples of recent new strains and mutations with the most significant features of COVID-19.
Another study has revealed that global dispersal and the growing frequency of the COVID-19 spike protein variant D614G during the autumn of 2020 was evocative of a selective advantage, basically in increased transmissibility [40]. A blend of mutations in the B.1.1.7 strain makes it exceptionally infectious, while the variant of B.1.351 can evade antibodies owing to E484K [4] as indicated in Table 2 . Lately, the appearance of N501Y mutants associated with super spreading events and outbreaks typically improved transmissibility. Stimulatingly, such variant was initially recognized in Denmark among the infected minks, while emergence in other parts proposes that it might increase independently from such animals [4]. This mutation could support SARS-COV-2 capability for binding with human receptor angiotensin-converting enzyme 2 (ACE2), therefore increasing the spread of the COVID-19 pandemic [41], [42].
Table 2.
Basic characteristics of SARS-COV-2 variants (Data updated from (https://outbreak.info) [58].
| Variants | B.1.1.7 | B.1.351 | P.1 | B.1.1.529 | |
|---|---|---|---|---|---|
| First detection | September 2020 | October 2020 | January 2021 | November 2021 | |
| Detection country | United kingdom | South Africa | Brazil, Japan | South Africa | |
| Mutations | Number | 7 | 9 | 12 | >30 |
| Defined in S protein | P681H, N501Y, A570D, D614G D1118H T716I, S982A | A701V, L18F, N501Y, D80A, R246I, E484K, K417N, D614G, D215G, |
K417T, L18F, R190S, P26S, T20N, D138Y, E484K, D614G, N501Y, V1176F, T1027I, H655Y |
H655Y, N679K, P681H, R203K, G204R, E484, D614G, R93K, G204R | |
| Prospective risk |
|
|
|
|
|
| Countries with reported cases | 82 | 40 | 19 | 52 | |
| Sequenced countries | 64 | 35 | 14 | 87 | |
On the other hand, it is worthy of spotting the light on the new variants that recently appeared in South Africa; Omicron (B.1.1.529) and Delta in India (lineage B.1.1617). The first case of the delta was announced in October 2020, and the variant has an advanced rate of transmission and infection compared with other previously known variants [43]. However, on 9 November 2021, omicron was discovered [44]. With some concern regarding the virus nature, there are no rules for control or ceiling of expectation as the time between the two variants is very short (almost one month), and this didn't happen before. Additionally, omicron was distinguished by remarkable diffusion speed, whereas the transmission rate of this variant is much higher than the pre-existing variants because of the greater number of mutations [45]. The high number of mutations present in the S protein in the omicron variant may increase the virus's ability to evade infection-blocking antibodies and other immune responses such as the T cell response. This observation agrees with preliminary evidence suggesting an increased risk of reinfection with omicron compared to different strains, but the information is still scarce [46]. The initial studies observed that the variant has several spike protein mutations, more than 30 mutations in the virus area, which encodes these proteins responsible for the entrance of the virus to human cells [43]. These mutations that happened in omicron are also identified in the previous strains of COVID-19, including alpha and delta variants [47]. However, several omicron mutations have not been previously appeared, which mandates further investigations to determine it. The number of mutations was twice the delta variant. Ten mutations were found on the RBD of the omicron variant, while there were only two in delta variants [48]. A recent study discussed that mutation group (H655Y, N679K, P681H) in the virus is correlated by increasing entrance ability to the human cell; therefore, this suggests higher transmissibility [49]. In addition, R203K and G204R mutations were previously observed in alpha strain and also seen in omicron, and these mutations enhanced the infection rates [43].
On the other hand, there is currently no information suggesting that omicron symptoms differ from those of other strains [46]. Initially reported infections were among younger people (university students) who tend to have milder disease. Understanding of overall severity of disease associated with omicron will take several days, likely many weeks. A report from the African Medical Association displayed that omicron is seven times more contagious than the delta variant, but the reported cases and deaths in Africa have continued to decline, and the people infected by omicron did not show any serious aggravation in their condition [46].
Many secrets are still present regarding these newly emerging mutants, and most researchers are quite slow and passive in dealing with these potential emergencies. Subsequently, we highly recommended further and profound studies.
5. Vaccination effect against the new variants of COVID-19 and re-infection
Vaccination is considered an effective method applied to prevent several infectious diseases. Vaccines introduced to the body cause an immune response like infection occurs naturally, but there is no disease production [50]. The immunity created through vaccines requires identifying the vaccine-active agent as a foreign substance to the cell, its destruction, and producing an immune memory so that succeeding communication with pathogens. In contrast, the body has an effective and faster defense immunity response. The vaccine immunization aimed to avoid definite infections and hinder their consequences [51].
Currently, vaccination is considered the best way to fight infections of COVID-19. In a surpassing race, COVID-19 vaccines have been amazingly developed to be spread by Christmas 2020, achieving successful results and increasing herd immunity through a couple of months [4]. Many vaccine candidates have been clinically and pre-clinical investigated recently; there are 171 COVID-19 agents tested in pre-clinical trials and 64 COVID-19 agents vaccines in clinical trials [13]. To the best of our knowledge, there are more than eight vaccines accepted for vaccination against COVID-19 among important categories under an Emergency Use Authorization [52]. The currently applied vaccines in the market include Pfizer-BioNTech BNT162b2 [53], Moderna mRNA-1273 [54], Sinopharm, China's CoronaVac™, EpiVacCorona, and Russia's Sputnik vaccines [55], Janssen's Ad26.COV2.S and AstraZeneca's ChAdOx1 novel coronavirus 2019 (nCoV-19) [56]. Despite a massive number of hospitalized and death cases, immunity caused by natural infections had only been estimated by 20–25% of the population by the end of 2020, even in hotspot areas [36], [57]. All the vaccines that have been appeared worldwide are attentive to the spike protein that accumulates great rates of mutations through viral evolution; as proven in the genome sequences from the new emerging COVID-19 variants, it is imperative to study the impact of such mutations on the actual efficacy of COVID-19 vaccines [58]. Currently, most research groups worldwide focused on their investigations about anti-COVID agents but still the most important questions: Are these currently applied vaccines protect against COVID-19 re-infection, especially in the upcoming expected waves?
The protection afforded by COVID-19 infection is still unknown [59]. A number of recent studies have investigated the reinfection with phylogenetically distinct SARS-CoV-2 variants, but these are still rare [60], [61], [62]. Tests on infected patients during the pandemic of SARS in 2003 displayed that antibodies response from infection persists more than two years [63]. In contrast, infection with a mutual seasonal human coronavirus strain does not convene permanent defense against reinfection, while reinfection within six months is not common [64]. The new variants that appeared in Brazil and South Africa increase worrying because these spike mutants decreased neutralizing antibodies, suggesting more re-infections and could render vaccines less effective [4]. In contrast, another study revealed that convalescent patients of COVID-19 and vaccinator persons can neutralize the variant of N501Y, and hence this enhanced protection of the current vaccines against 20B/501Y.V1 strain [37]. This means that vaccines are succeeded in avoiding re-infections. Others have assessed parallel protection of earlier infection. Among the medical sector workers in the UK, the antibodies percent (91%) strongly reduced the risk of symptomatic reinfection in the next six months [65]. In association with the Moderna vaccine report, prior infection estimated 76% protectiveness in the placebo arm. The numbers were minimal (only one case of reinfection), and the confidence intervals were far-reaching [66]. Lately, a preclinical investigation established that the Ad26.COV2.S vaccine improved the presence of neutralizing antibodies and induced defense against the COVID-19 spike variant (G614) through the performance of immune challenge tests in a Syrian hamster model [67]. Furthermore, at the beginning of this year, the new vaccine, Covaxin, displayed significant results in neutralizing the variant in the UK and reducing the capability of immune escape by the mutant virus [68]. Likewise, the neutralizing effect of antibodies promoted by the ChAdOx1 nCoV-19 vaccine was 9-fold lower against the B.1.1.7 variant than canonical non-B. 1.1.7 lineage; however, its efficiency against the B.1.1.7 variant (74.6%) was comparable to that of the vaccine against other lineages (84%) [69]. The vaccine effect against the newly emerged strain B.1.1.529 is currently under investigation. For example, the latest U.K. data confirmed that all COVID-19 vaccines continued to be less effective against symptomatic infection from omicron compared with delta. After two doses of the Pfizer or Moderna vaccines, effectiveness decreased from approximately 65–70% to about 10% 20 weeks after the second dosage. Vaccine effectiveness was around 65–75% two to four weeks after a booster dose, 55–70% five to nine weeks after a booster dose, and 40–50% ten weeks or more after a booster dose [70]. Through Dec. 26, the United Kingdom's Health Security Agency collaborated with Cambridge University's MRC Biostatistics unit to study 528,176 omicron and 573,012 Delta cases. According to the results, three doses of vaccine resulted in a 68% reduction in the probability of being hospitalized with omicron compared to those who were not immunized. A single dose of any vaccine was associated with a 35% reduction in the risk of hospitalization in symptomatic cases carrying the omicron variant, two doses with a 67% reduction up to 24 weeks after the second dose, and a 51% reduction 25 or more weeks after the second dose, when compared to people who had not received a vaccine [70]. In addition, A total of 6314 omicron patients enhanced the eligibility criteria, out of which 6312 were correlated with one delta case at least out of a total number of 8875 depending on gender, age, and onset date. Twenty-one (0.3%) were hospitalized, whereas zero cases were confirmed with death among matched omicron cases. This situation was compared by116 (2.2%) hospitalizations and seven (0.3%) deaths of reported delta cases [71]. The danger of hospitalization or death was 68% lower in patients with omicron infection compared to delta. After applying vaccination, the risk of hospitalization or death was 54% lower in omicron-infected cases. It is worth mentioning that the death rate is less, however increasing the number of infected persons. This may suggest the danger of the new variant in high transformability with increasing infection rate but with no dangerous effect and no more deaths.
On the other hand, a new generation of developed vaccines could be revealed in the future. For example, NVX-CoV2373 announced a new vaccine against SARS-COV-2, which displayed significant protection rates estimated by 95.6% and 85.6% against the original COVID-19 strain and some new variants (UK variant), respectively [72]. However, in South Africa, the same vaccine showed 60% effectiveness (phase II clinical trials) against replication of COVID-19 original strain and 49.4% when applied against the South African variant strain [73]. This is the first vaccine that displayed clinical efficiency against the original strain and the UK and South African [13]. In fact, all vaccines are newly manufactured and have been used quickly to counter the rapid development of the pandemic around the world. Some vaccines have also been developed to counter the genetic changes of the virus. Therefore, research on vaccines' effect and persistence is still few.
It is worthy of mentioning that some reports may overestimate the actual reinfection rates. This phenomenon appeared because some infected cases could still have the virus for months, leading to conflict in distinguishing between existence shedding and reinfection [74]. Protective effects of the currently applied vaccines against the several variants of SARS-CoV-2 have been widely discussed; however, there is still a shortage of in-depth investigations and studies with an extensive sample size [13]. Additionally, the antibodies level decreased over time for all vaccines, and many reports which discussed the immunity to other coronaviruses [75], [76], [77] confirmed that the immunity started to lose within 1–3 years [61]. This is the first time vaccinating against COVID-19, so the question remains: Will we need an annual dose of the corona vaccines? In addition, Will be mandatory for children in the near future, especially with the spreading of the current fourth wave, which started in different countries?.
Moreover, the side effects don't been observed till now. Subsequently, we recommended further and deep investigation, especially after the new variant of concern, omicron, that witnesses an impressive number of mutations cause increasing in disease severity and significant transmission ability. The situation may get worse and get out of hand, especially when the sense of danger decreased and most people neglect precautionary measures with available vaccines.
6. Conclusion
The SARS-COV-2 pandemic is still causing considerable high mortality rates, introducing a significant burden on healthcare services worldwide and having profound economic and social consequences due to the different strategies implemented to control the virus. Three waves hit the world strongly till now, and more coming waves are highly expected. Although there are many recent reports about the new epidemic, there is still a state of mystery about the nature of this virus and its ability to transform. Vaccines are in a continuous race that highly requires controlling COVID-19 and protecting persons at high risk for complications. Vaccination showed significant activity against some new variants and played a positive role against reinfection. However, in the coming days my revealed further questions, especially with several virus mutations and extra waves as expected.
Author contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wu Y., Jing W., Liu J., Ma Q., Yuan J., Wang Y., Du M., Liu M. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Science of the Total Environment. 2020;729:1–7. doi: 10.1016/j.scitotenv.2020.139051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graichen H. What is the difference between the first and the second/third wave of Covid-19? – German perspective. J. Orthop. 2021;24:A1–A3. doi: 10.1016/j.jor.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chersich M.F., Gray G., Fairlie L., Eichbaum Q., Mayhew S., Allwood B., English R., Scorgie F., Luchters S., Simpson G., Haghighi M.M., Pham M.D., Rees H. Covid-19 in Africa: care and protection for frontline healthcare workers. globalHealth. 2020;16:1–6. doi: 10.1186/s12992-020-00574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.F.-M.J. Soriano V New SARS-CoV-2 variants challenge vaccines protection. AIDS Rev. 2021:2–3. doi: 10.24875/AIDSRev.M21000040. [DOI] [PubMed] [Google Scholar]
- 5.Baker H.A., Safavynia S.A., Evered L.A. The ‘third wave’: impending cognitive and functional decline in COVID-19 survivors. Br. J. Anaesth. 2021;126:44–47. doi: 10.1016/j.bja.2020.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lithander F.E., Neumann S., Tenison E., Lloyd K., Welsh T.J., Rodrigues J.C.L., Higgins J.P.T., Scourfield L., Christensen H., Haunton V.J., Henderson E.J. COVID-19 in older people: a rapid clinical review. Age Ageing. 2020;49:501–515. doi: 10.1093/ageing/afaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brassey J., Heneghan C., Mahtani K.R., Aronson J.K. Do weather conditions influence the transmission of the coronavirus (SARS-CoV-2), Oxford COVID-19. Evid. Serv. 2020;5 https://www.cebm.net/do-weather-conditions-influence-the-transmission-of-the-coronavirus-sars-cov-2/ [Google Scholar]
- 8.Chan K.H., Peiris J.S.M., Lam S.Y., Poon L.L.M., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011;2011 doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1 doi: 10.1016/s2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S., Yan J., Niu J. 2020. Effects of Temperature Variation and Humidity on the Death ofCOVID-19 in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie J., Zhu Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y., Pan J., Liu Z., Meng X., Wang W., Kan H., Wang W. No association of COVID-19 transmission with temperature or UV radiation in Chinese cities. 2020. pp. 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia Z., Gong W. Will mutations in the spike protein of SARS-CoV-2 lead to the failure of COVID-19 vaccines? J. Korean Med. Sci. 2021;26:1–11. doi: 10.3346/JKMS.2021.36.E124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rui R., Tian M., Tang M., Ho G.T. Vol. 2020. 2021. Analysis of the Spread of COVID-19 in the USA With a Spatio-Temporal Multivariate Time Series Model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Napoli R.Di. 2020. Features , Evaluation and Treatment Coronavirus ( COVID-19 ) [PubMed] [Google Scholar]
- 16.Agerer B., Immunol S., Agerer B., Koblischke M., Gudipati V., Montaño-gutierrez L.F., Popa A., Genger J., Endler L., Florian D.M., Mühlgrabner V., Graninger M., Aberle S.W., Husa A., Shaw L.E., Lercher A., Torralba-gombau R., Trapin D., Penz T., Barreca D., Fae I., Traugott M., Walder G., Pickl W.F., Thiel V., Allerberger F., Pawelka E., Zoufaly A., Valenta R., Bock C., Paster W. SARS-CoV-2 mutations in MHC-I-restricted epitopes evade CD8 + T cell responses. 2021;6461:17–22. doi: 10.1126/sciimmunol.abg6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bem R.A. Pulmonary fibrosis secondary to COVID-19 : a call to arms ? 2020;8:750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taquet M., Dercon Q., Luciano S., Geddes J.R., Husain M., Harrison P.J. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18:1–22. doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morin L., Savale L., Pham T., Colle R., Figueiredo S., Harrois A., Gasnier M., Lecoq A.L., Meyrignac O., Noel N., Baudry E., Bellin M.F., Beurnier A., Choucha W., Corruble E., Dortet L., Hardy-Leger I., Radiguer F., Sportouch S., Verny C., Wyplosz B., Zaidan M., Becquemont L., Montani D., Monnet X. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA - J. Am. Med. Assoc. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., Molteni E., Modat M., Jorge Cardoso M., May A., Ganesh S., Davies R., Nguyen L.H., Drew D.A., Astley C.M., Joshi A.D., Merino J., Tsereteli N., Fall T., Gomez M.F., Duncan E.L., Menni C., Williams F.M.K., Franks P.W., Chan A.T., Wolf J., Ourselin S., Spector T., Steves C.J. Attributes and predictors of long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., González E., Redondo-Peñas I., Perona-Moratalla A.B., Del Valle-Pérez J.A., Gracia-Gil J., Rojas-Bartolomé L., Feria-Vilar I., Monteagudo M., Palao M., Palazón-García E., Alcahut-Rodríguez C., Sopelana-Garay D., Moreno Y., Ahmad J., Segura T. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helms J., Kremer S., Merdji H., Schenck M., Severac F., Clere-Jehl R., Studer A., Radosavljevic M., Kummerlen C., Monnier A., Boulay C., Fafi-Kremer S., Castelain V., Ohana M., Anheim M., Schneider F., Meziani F. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit. Care. 2020;24:1–11. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safavynia S.A., Arora S., Pryor K.O., García P.S. An update on postoperative delirium: clinical features, neuropathogenesis, and perioperative management. Curr. Anesthesiol. Rep. 2018;8:252–262. doi: 10.1007/s40140-018-0282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safavynia S.A., Goldstein P.A. The role of neuroinflammation in postoperative cognitive dysfunction: moving from hypothesis to treatment. Front. Psychiatry. 2019;9 doi: 10.3389/fpsyt.2018.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girard T.D., Ware L.B., Bernard G.R., Pandharipande P.P., Thompson J.L., Shintani A.K., Jackson J.C., Dittus R.S., Ely E.W. Associations of markers of inflammation and coagulation with delirium during critical illness. Intensive Care Med. 2012;38:1965–1973. doi: 10.1007/s00134-012-2678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de la Peña J.E., Rascón-Pacheco R.A., González-Figueroa E., Fernández-Gárate J.E., Medina-Gómez O.S., Borja-Bustamante P., Santillán-Oropeza J.A., Borja-Aburto V.H., Ascencio-Montiel I.de J. Hypertension, Diabetes and Obesity, Major Risk Factors for Death in Patients with COVID-19 in Mexico. Arch. Med. Res. 2021;52:443–449. doi: 10.1016/j.arcmed.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R., Prill M., Chai S.J., Kirley P.D., Alden N.B., Kawasaki B., Yousey-Hindes K., Niccolai L., Anderson E.J., Openo K.P., Weigel A., Monroe M.L., Ryan P., Henderson J., Kim S., Como-Sabetti K., Lynfield R., Sosin D., Torres S., Muse A., Bennett N.M., Billing L., Sutton M., West N., Schaffner W., Talbot H.K., Aquino C., George A., Budd A., Brammer L., Langley G., Hall A.J., Fry A. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 states, march 1–30. MMWR Morb. Mortal. Wkly Rep. 2020;69(2020):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumgart M., Snyder H.M., Carrillo M.C., Fazio S., Kim H., Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11:718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Jotwani R., Cheung C.A., Hoyler M.M., Lin J.Y., Perlstein M.D., Rubin J.E., Chan J.M., Pryor K.O., Brumberger E.D. Trial under fire: one New York City anaesthesiology residency programme’s redesign for the COVID-19 surge. Br. J. Anaesth. 2020;125:e386–e388. doi: 10.1016/j.bja.2020.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanne J.H. Covid-19 Cases Increase Steeply in US South and West. 2020. pp. 1–2. [DOI] [PubMed] [Google Scholar]
- 32.Hazem Y., Natarajan S., Berikaa E.R. Hasty reduction of COVID-19 lockdown measures leads to the second wave of infection. MedRxiv. 2020 doi: 10.1101/2020.05.23.20111526. [DOI] [Google Scholar]
- 33.Soriano V., Ganado-Pinilla P., Sanchez-Santos M., Gómez-Gallego F., Barreiro P., de Mendoza C., Corral O. Main differences between the first and second waves of COVID-19 in Madrid, Spain. Int. J. Infect. Dis. 2021;105:374–376. doi: 10.1016/j.ijid.2021.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rypdal K., Bianchi F.M., Rypdal M. Intervention fatigue is the primary cause of strong secondary waves in the COVID-19 pandemic. Int. J. Environ. Res. Public Health. 2020;17:1–17. doi: 10.3390/ijerph17249592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guallar M.P., Meiriño R., Donat-Vargas C., Corral O., Jouvé N., Soriano V. Inoculum at the time of SARS-CoV-2 exposure and risk of disease severity. Int. J. Infect. Dis. 2020;97:290–292. doi: 10.1016/j.ijid.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soriano V., de Mendoza C., Gómez-Gallego F., Corral O., Barreiro P. Third wave of COVID-19 in Madrid, Spain. Int. J. Infect. Dis. 2021;107:212–214. doi: 10.1016/j.ijid.2021.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rathnasinghe R., Jangra S., Cupic A., Martínez-Romero C., Mulder L.C.F., Kehrer T., Yildiz S., Choi A., Mena I., De Vrieze J., Aslam S., Stadlbauer D., Meekins D.A., McDowell C.D., Balaraman V., Richt J.A., De Geest B.G., Miorin L., Krammer F., Simon V., García-Sastre A., Schotsaert M. The N501Y mutation in SARS-CoV-2 spike leads to morbidity in obese and aged mice and is neutralized by convalescent and post-vaccination human sera., MedRxiv prepr. Serv. Health Sci. 2021 doi: 10.1101/2021.01.19.21249592. [DOI] [Google Scholar]
- 38.Denison M.R., Graham R.L., Donaldson E.F., Eckerle L.D., Baric R.S., Denison M.R., Graham R.L., Donaldson E.F., Eckerle L.D., Denison M.R., Graham R.L., Donaldson E.F., Eckerle L.D., Baric R.S. a n d e s i o s c i e n c e o n. 2011;6286 doi: 10.4161/rna.8.2.15013. [DOI] [Google Scholar]
- 39.Othman S.I., Nayel M.A., Abdulla M., Fassam H.Al, Abu-Taweel G.M., Altoom N.G., Almalki A.M., Alturki A.M., El-Shabasy R.M., F. A.A.A. Immunology and controlling of coronaviruses; the current enemy for humanity: A review. Int. J. Biol. Macromol. 2021;193:1532–1540. doi: 10.1016/j.ijbiomac.2021.10.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volz E., Hill V., McCrone J.T., Price A., Jorgensen D., O’Toole Á., et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell. 2021;184:64–75.e11. doi: 10.1016/j.cell.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.States U., Galloway S.E., Paul P., Maccannell D.R., Johansson M.A. Emergence of SARS-CoV-2 B . 1 . 1 . 7. Lineage. 2021;70:95–99. doi: 10.15585/mmwr.mm7003e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Najmanovich R. Modelling Conformational State Dynamics and Its Role on Infection for SARS-CoV-2 Spike Protein Variants. 2021. pp. 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lal A., Ahmed N., Maqsood A., Alam M.K. Another Deadly Dilemma. 2021. COVID-19 omicron -another deadly dilemma COVID-19 omicron. [Google Scholar]
- 44.Vol. 2021. 2021. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern [Internet]https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern cited 2021 Dec 3. [Google Scholar]
- 45.Aouissi H.A. 2021. Algeria's Preparedness for Omicron pariant and for the Fourth Wave of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quarleri J., Galvan V., Delpino M.V. Omicron variant of the SARS-CoV-2: a quest to define the consequences of its high mutational load. GeroScience. 2021:4–7. doi: 10.1007/s11357-021-00500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heavily Mutated Omicron Variant Puts Scientists on Alert [Internet]. 2021 [cited 2021 Dec 3]. Available from: https://www.nature.com/articles/d41586-021-03552-w, (n.d.). [DOI] [PubMed]
- 48.Song Y., Masaki F. Preparation for the challenge of heavily mutated omicron variant. Clin. Transl. Med. 2021;11:10–11. doi: 10.1002/ctm2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Explained: What We Know so Far About the Omicron Variant of Covid-19 [Internet]. 2021 [cited 2021 Dec 3]. Available from: https://indianexpress.com/article/explained/covid-variant-south-africa-explained-7642199/, (n.d.).
- 50.Calina D., Sarkar C., Arsene A.L., Salehi B., Docea A.O., Mondal M., Islam M.T., Zali A., Sharifi-rad J. 2020. Recent Advances, Approaches and Challenges in Targeting Pathways for Potential COVID-19 Vaccines Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsatsakis A., Calina D., Falzone L., Petrakis D., Mitrut R., Siokas V., Pennisi M., Lanza G., Libra M., Doukas S.G., Doukas P.G., Kavali L., Bukhari A., Gadiparthi C., Vageli D.P., Kofteridis D.P., Spandidos D.A., Paoliello M.M.B., Aschner M., Docea A.O. SARS-CoV-2 pathophysiology and its clinical implications: an integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem. Toxicol. 2020;146 doi: 10.1016/j.fct.2020.111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klasse P.J., Nixon D.F., Moore J.P. Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans. Sci. Adv. 2021;7:eabe8065. doi: 10.1126/sciadv.abe8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahase E. Covid-19: UK approves Pfizer and BioNTech vaccine with rollout due to start next week. BMJ. 2020;371:1–2. doi: 10.1136/bmj.m4714. [DOI] [PubMed] [Google Scholar]
- 54.Anesi J. The advisory committee on immunization practices’ updated interim recommendation for allocation of COVID-19 vaccine—United States, december 2020. Am. J. Transplant. 2021;21:897. doi: 10.1111/ajt.16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahase E. Covid-19: Russia approves vaccine without large scale testing or published results. BMJ. 2020;370 doi: 10.1136/bmj.m3205. [DOI] [PubMed] [Google Scholar]
- 56.Voysey M., Clemens S.A.Costa, Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., L. V. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sánchez-Romero M., di Lego V., Prskawetz A., Queiroz B.L. An indirect method to monitor the fraction of people ever infected with COVID-19: an application to the United States. PLoS One. 2021;16:1–14. doi: 10.1371/journal.pone.0245845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elena C., Perdiguero B., Esteban M. 2021. Emerging SARS-CoV-2 Variants and Impact in Global Vaccination Programs Against SARS-CoV-2 / COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Megan M.B.R.M.M., Sheehan M., Anita B.S., Reddy J., MBA M.D. NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. 2021. pp. 2–16. [Google Scholar]
- 60.Wu S.L., Mertens A.N., Crider Y.S., Nguyen A., Pokpongkiat N.N., Djajadi S., Seth A., Hsiang M.S., Colford J.M., Reingold A., Arnold B.F., Hubbard A., Benjamin-Chung J. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-18272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tillett R.L., Sevinsky J.R., Hartley P.D., Kerwin H., Crawford N., Gorzalski A., Laverdure C., Verma S.C., Rossetto C.C., Jackson D., Farrell M.J., Van Hooser S., Pandori M. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect. Dis. 2021;21:52–58. doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.A.T. KK-W H. IF-N I. JP C. AW-H C. W-M T. Ar , Review of “COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing” One-Minute Summary PHO Reviewer's Comments, (n.d.) 1–3.
- 63.Wu L.P., Wang N.C., Chang Y.H., Tian X.Y., Na D.Y., Zhang L.Y., Zheng L., Lan T., Wang L.F., Liang G.D. Duration of antibody responses after severe acute respiratory syndrome. Emerg. Infect. Dis. 2007;13:1562–1564. doi: 10.3201/eid1310.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edridge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Loens K., Jebbink M.F., Matser A., Kinsella C.M., Rueda P., Ieven M., Goossens H., Prins M., Sastre P., Deijs M., van der Hoek L. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 65.Lumley S.F., et al. Antibody status and incidence of SARS-CoV-2 infection in. Health Care Workers. 2021:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Lubbe J.E.M., Rosendahl Huber S.K., Vijayan A., Dekking L., van Huizen E., Vreugdenhil J., Choi Y., Baert M.R.M., Feddes-de Boer K., Izquierdo Gil A., van Heerden M., Dalebout T.J., Myeni S.K., Kikkert M., Snijder E.J., de Waal L., Stittelaar K.J., Tolboom J.T.B.M., Serroyen J., Muchene L., van der Fits L., Rutten L., Langedijk J.P.M., Barouch D.H., Schuitemaker H., Zahn R.C., Wegmann F. Ad26.COV2.S protects Syrian hamsters against G614 spike variant SARS-CoV-2 and does not enhance respiratory disease. Npj Vaccines. 2021;6:1–12. doi: 10.1038/s41541-021-00301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sapkal G.N., Yadav P.D., Ella R., Deshpande G.R., Sahay R.R., Gupta N., Mohan K., Abraham P., Panda S., Bhargava B. Neutralization of UK-variant VUI-202012/01 with COVAXIN vaccinated human serum. BioRxiv. 2021 doi: 10.1101/2021.01.26.426986. [DOI] [Google Scholar]
- 69.Emary K.R.W., Golubchik T., Aley P.K., Ariani C.V., Angus B., Bibi S., Blane B., Bonsall D., Cicconi P., Charlton S., Clutterbuck E.A., Collins A.M., Cox T., Darton T.C., Dold C., Douglas A.D., Duncan C.J.A., Ewer K.J., Flaxman A.L., Faust S.N., Ferreira D.M., Feng S., Finn A., Folegatti P.M., Fuskova M., Galiza E., Goodman A.L., Green C.M., Green C.A., Hallis B., Heath P.T., Hay J., Hill H.C., Jenkin D., Kerridge S., Lazarus R., Libri V., Lillie P.J., Ludden C., Marchevsky N.G., Minassian A.M., Mcgregor A.C., Mujadidi Y.F., Phillips D.J., Plested E., Pollock K.M., Robinson H., Smith A., Song R., Snape M.D., Sutherland R.K., Thomson E.C., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Williams C.J., Hill A.V.S., Lambe T., Gilbert S.C., Voysey M., Ramasamy M.N., Pollard A.J. Articles Efficacy of ChAdOx1 nCoV-19 ( AZD1222) vaccine against an exploratory analysis of a randomised controlled trial. 2021;19:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.England Public Health. SARS-CoV-2 Variants of Concern and Variants Under Investigation in England. 01. Sage; 2021. pp. 1–50. [Google Scholar]
- 71.Ulloa A.C., Buchan S.A., Daneman N., Brown K.A., Ontario P.H., Ulloa C., Brown K.A. Early estimates of SARS-CoV-2 omicron variant severity based on a matched cohort study , complete word count ( excluding figures / captions ): 634 corresponding author. 2021;116:1–6. [Google Scholar]
- 72.Callaway E., Mallapaty S. Novavax covid vaccine protects people against variants. Nature. 2021;590:17. doi: 10.1038/d41586-021-00268-9. [DOI] [PubMed] [Google Scholar]
- 73.Shinde V., Bhikha S., Hoosain Z., Archary M., Bhorat Q., Fairlie L., Lalloo U., Masilela M.S.L., Moodley D., Hanley S., Fouche L., Louw C., Tameris M., Singh N., Goga A., Dheda K., Grobbelaar C., Kruger G., Carrim-Ganey N., Baillie V., de Oliveira T., Lombard Koen A., Lombaard J.J., Mngqibisa R., Bhorat A.E., Benadé G., Lalloo N., Pitsi A., Vollgraaff P.-L., Luabeya A., Esmail A., Petrick F.G., Oommen-Jose A., Foulkes S., Ahmed K., Thombrayil A., Fries L., Cloney-Clark S., Zhu M., Bennett C., Albert G., Faust E., Plested J.S., Robertson A., Neal S., Cho I., Glenn G.M., Dubovsky F., Madhi S.A. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N. Engl. J. Med. 2021;384:1899–1909. doi: 10.1056/nejmoa2103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.BS M.M.S., MBA A.J.R.M., MPH M.B.R.M. Reinfection Rates among Patients who Previously Tested Positive for COVID-19: a Retrospective Cohort Study. 2021. pp. 2–16. [Google Scholar]
- 75.Callow K.A., Parry H.F., Sergeant M., Tyrrell D.A.J. The time course of the immune response to experimental coronavirus infection of man. Epidemiol. Infect. 1990;105:435–446. doi: 10.1017/S0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang S.C., Wang J.T., Huang L.M., Chen Y.C., Fang C.T., Sheng W.H., Wang J.L., Yu C.J., Yang P.C. Longitudinal analysis of severe acute respiratory syndrome (SARS) coronavirus-specific antibody in SARS patients. Clin. Diagn. Lab. Immunol. 2005;12:1455–1457. doi: 10.1128/CDLI.12.12.1455-1457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M., Borgert B.A., Moreno C.A., Solomon B.D., Trimmer-Smith L., Etienne V., Rodriguez-Barraquer I., Lessler J., Salje H., Burke D.S., Wesolowski A., Cummings D.A.T. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat. Commun. 2020;11:1–16. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]