Abstract
Objectives
The aim of the study was to analyze the mortality and characteristics of deceased patients with COVID-19 during the first year of the pandemic.
Methods
All admissions owing to COVID-19 at a tertiary hospital in Madrid were analyzed. Three waves were considered: March 2020 to June 2020, July 2020 to November 2020, and December 2020 to April 2021.
Results
A total of 3,676 patients were identified. Among inpatients, no differences regarding age, sex, length of admission, or mortality were found between the 3 waves (p >0.05). The overall mortality rate was 12.9%. Among deceased patients, the median age was 82 years and the median Charlson Comorbidity Index was 6. Considering the main predictors for mortality by COVID-19 (age, sex, and concomitant comorbidities), only patients with previous lung disease were more prevalent in the third period (p <0.01). Finally, higher intensive care unit admission rates, a lower rate of patients coming from nursing homes, and a lower rate of patients with dementia were noted in the third period (p <0.05) among deceased patients.
Conclusion
One year after the onset of the pandemic, the mortality rate of hospitalized patients and the profile of non-survivors have not changed significantly. In the absence of vaccine benefits, advanced age and multiple pathologies are uniform characteristics of non-survivors.
Keywords: COVID-19, mortality rate, Spain, comorbidities
Abbreviations
- ARDS
Acute respiratory distress syndrome.
- CCI
Charlson Comorbidity Index.
- ICU
Intensive care unit.
- SD
Standard deviation
Introduction
SARS-CoV-2 infection is currently the largest health problem worldwide, which led to more than 3 million deaths all over the globe during the first year of the pandemic (WHO, 2021). From the beginning, population studies regarding mortality and susceptible population highlighted that older patients and those who suffered from chronic conditions such as hypertension, diabetes, heart failure, lung disease, or immunosuppression, among others, were at a higher risk of death [Wu et al., 2020; Chen et al., 2020]. Similarly, with a 13.8% mortality rate, we confirmed that mortality was strongly related to age and comorbidities during the first wave of COVID-19 in a referral center in Madrid [Moreno-Torres et al., 2021].
Since the pandemic outbreak, different strategies have been implemented to reduce the transmission of the virus and assess its impact on health. In the hospital setting, several approaches were tried to treat the disease in those patients with acute respiratory distress syndrome (ARDS). Among others, dexamethasone, tocilizumab, and anakinra have been shown to be effective in treating severe COVID-19 [Horby et al., 2021; Stone et al., 2020; Kyriazopoulou et al., 2021]. In addition, the measures introduced, including wearing of facial masks, social distancing, and mass lockdowns, resulted in fewer hospital admissions. Finally, the rapid development of vaccines led to wide vaccination programs all over the world at the beginning of 2021 [Privor-Dumm et al., 2021].
All these factors contributed to the different waves of the disease that occurred throughout 2020 and have affected variations in the number and profile of affected individuals. However, there are no data to confirm that the profile of patients with severe COVID-19 and related mortality has changed during the first year of the pandemic. Our study analyzed the COVID-19 mortality rate at a reference hospital in Madrid for a year since the start of the pandemic (March 2020 to April 2021) and before the impact of vaccination programs.
Material and methods
Study design and patients
This retrospective study included all admitted patients with COVID-19 pneumonia at the Puerta de Hierro-Majadahonda University Hospital from March 1st, 2020, to April 30th, 2021. This is a large tertiary university hospital with 613 beds, whose catchment population approaches 375,000 people. The hospital review board approved the study (PI134-20), and a waiver for informed consent was granted.
Data collection and outcomes
We reviewed electronic medical records for all hospital-admitted patients with confirmed COVID-19. The main demographics (sex and age), admission to intensive care unit (ICU), and diagnosis discharge (including death) were recorded for each one. For all deceased patients with COVID-19, origin (home, nursing home, or referral from other institutions) and the presence of comorbidities were recorded, including neurocognitive impairment (Alzheimer's disease, other dementias, and/or mental disorders), arterial hypertension, diabetes, dyslipidemia, heart disease, cerebrovascular disease, peripheral arterial disease, chronic kidney disease, liver disease, lung disease, cancer, autoimmune diseases, and other immunosuppression conditions. The baseline Charlson Comorbidity Index (CCI) was used to estimate the risk of mortality, taking into consideration that the overall 10-year survival rate is 98.3% in patients with a CCI of 0, which decreases to 77.48% in those with a CCI of 3 and decreases further to 21.36% in those with a CCI of 5 or more [Charlson et al., 1987].
Study periods
In order to examine the course of the pandemic and changes in the patient profile during the first year after the onset of the pandemic, 3 different periods were considered: March 1st 2020 to June 7th, 2020 (first wave), June 8th, 2020, to December 5th, 2020 (second wave), and December 6th, 2020, to April 30th, 2021 (third wave). These dates were selected according to the national restrictions and the defined waves seen corresponding to spring 2020, after summer 2020, and from Christmas up to April 2021.
Statistical analysis
Continuous variables were expressed as mean and SDs and medians with percentiles (P25-P75). Categorical variables were summarized as counts and percentages. The Kolmogorov test was used to evaluate data distribution and the Student t-test or Mann-Whitney U-test was performed consequently to assess differences between groups. Levene´s test was used for the homogeneity of variance test, and the chi-square test (with the 2-sided Fisher's exact test) was used to compare categorical variables. For all analyses, significance was defined as a P value of less than 0.05. Statistical analysis was performed using the SPSS version 26.0 software statistical package (SPSS Inc., IBM, Armonk, New York).
Results
Between March 1st, 2020, and April 30th, 2021, a total of 3,676 patients were hospitalized with COVID-19 at the Hospital Puerta de Hierro-Majadahonda (table 1 ). Overall, 60.1% of admitted patients were male and the average age was 65 years (54-77). The median length of admission was 8 days (4-14). During the whole study period, 474 patients died (12.9% mortality rate).
Table 1.
Main characteristics of inpatients with COVID-19 according to the study period
| First period (1st March- 7th June 2020) | Second period (8th June-5th Dec 2020) | Third period (6th Dec 2020-30th April 2021) | Total | |
|---|---|---|---|---|
| N (% of admissions) | 1,788 (48.6) | 926 (25.2) | 962 (26.2) | 3,676 |
| Age (years) (Median, P25-P75) | 65 (55-76) | 66 (54-77) | 66 (54-77) | 65 (54-77) |
| Male (N,%) | 1,109 (62) | 537 (58) | 564 (58.7) | 2,210 (60.1) |
| Length of admission (days) (Median, P25-P75) | 7 (4-12,8) | 9 (5-15) | 9 (5-15) | 8 (4-14) |
| Deaths (N) | 246 | 104 | 124 | 474 |
| Mortality rate (%) | 13.8 | 11.2 | 12.9 | 12.9 |
SD = standard deviation.
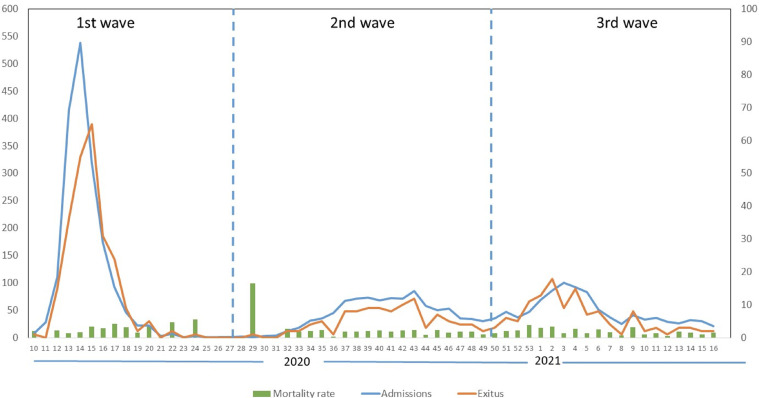
The hospital admissions during this year were evaluated taking into consideration the 3 waves (figure 1 , table 1 ). Overall, almost 50% of individuals were included in the first wave (1,788, 48.6%), followed by the second and third wave, with a similar number of individuals in each (926, 25.2% and 962, 26.2%, respectively). When compared, no differences regarding age, sex, length of admission, or mortality rates were found.
Figure 1.
Weekly hospital admissions and exitus with COVID-19 from March 2020 to April 2021 at Puerta de Hierro-Majadahonda Hospital in Madrid.
In order to evaluate the differences between the 3 waves of the pandemic, as well as the clinical impact of the measures adopted, we compared the characteristics of the deceased patients. As expected, the mean age of the deceased patients was significantly higher than the age of the survivors (62.8 vs 79.4 years, p < 0.001), but no significant differences were noted in the median age of deceased individuals when the 3 periods were compared (82 years (72-88)). In addition, no differences were seen regarding sex, length of admission, or previous comorbidities, measured as CCI (median 6) (table 2 ). For previous conditions, only patients with lung disease were more prevalent in the third wave (17.5%, 5.1%, and 37.1% in the first, second, and third waves, respectively, p <0.01). In contrast, non-survivors in the third period presented higher ICU admission rates (12.6% in the first wave, 14.4% in the second wave, and 25.8% in the third wave, p <0.03), a lower rate of patients coming from nursing homes (35%, 31.7%, and 12.2% in the first, second, and third waves, respectively, p <0.001), and a lower rate of patients with dementia (35%, 42.3%, and 23.4% in the first, second, and third waves, respectively, p <0.02). Similarly, dependence rates were higher in the second period (39.4%, 56.7%, 31.5% in the first, second, and third waves, respectively, p <0.01). To note, only 2 patients (1.6%) who died in the third period had received the complete vaccination cycle.
Table 2.
Main characteristics of deceased patients with COVID-19 according to the study period
| First period | Second period | Third period | |
|---|---|---|---|
| N (mortality rate) | 246 (13.8) | 104 (11.2) | 124 (12.9) |
| Age (years) (Median, P25-P75) | 81 (72-87) | 83 (76-89) | 82 (72-88) |
| Male, N (%) | 149 (60.6) | 62 (59.6) | 79 (63.7) |
| ICU admission, N (%) | 31 (12.6) | 15 (14.4) | 32 (25.8)* |
| Length of admission (d) (Median, P25-P75) | 6 (3-14) | 9 (6-18) | 11 (6-20) |
| PriorPrevious living at nursing homes, N (%) | 86 (35) | 33 (31.7) | 15 (12.2)* |
| Dementia, N (%) | 86 (35) | 44 (42.3) | 29 (23.4)* |
| Dependence, N (%) | 97 (39.4) | 59 (56.7)* | 39 (31.5) |
| CCI (median, P25-P75) | 6 (4-8) | 6 (5-8) | 6 (4-8) |
| Hypertension, N (%) | 169 (68.7) | 80 (76.9) | 90 (72.6) |
| Diabetes, N (%) | 76 (30.9) | 36 (34.6) | 39 (31.5) |
| Dyslipidemia, N (%) | 107 (43.5) | 49 (47.1) | 51 (41.1) |
| Heart disease, N (%) | 84 (34.1) | 43 (41.3) | 40 (32.3) |
| Cerebrovascular disease, N (%) | 54 (22) | 21 (20.2) | 25 (20.2) |
| Peripheral arterial disease, N (%) | 13 (5.4) | 13 (12.5) | 11 (8.9) |
| Chronic kidney disease, N (%) | 44 (17.9) | 25 (24) | 27 (21.8) |
| Liver disease, N (%) | 11 (4.5) | 4 (3.8) | 2 (1.6) |
| Lung disease, N (%) | 43 (17.5) | 24 (5.1) | 46 (37.1)* |
| Cancer, N (%) | 41 (16.7) | 13 (12.5) | 20 (16.1) |
| Autoimmune disease, N (%) | 19 (7.7) | 11 (10.6) | 7 (5.6) |
| Immunosuppression, N (%) | 41 (1.7) | 16 (15.4) | 26 (21) |
ICU: Intensive Care Unit. CCI: Charlson Comorbidity Index
p<0.05 (marked with bold): when compared with the other periods.
Discussion
The aim of the current study was to analyze the course and the different waves during the first year of the COVID-19 pandemic in our hospital, focusing on the epidemiology and characteristics of the deceased patients. After the abrupt start of COVID-19, many cohorts worldwide analyzed the mortality rates and the severe disease risk factors, uniformly concluding that frail and comorbid patients were at a higher risk (Wu et al., 2020; Chen et al., 2020; Moreno-Torres et al., 2021). In addition, once the pandemic stabilized during the summer in 2020, several studies showed that mortality rates significantly improved after the first wave probably because of a better understanding of the disease; a greater availability of infrastructures, staff, or equipment; and the wide use of corticosteroids, among other therapies [Vahidy et al., 2020; Kurtz et al., 2021; Armstrong et al., 2021; Roth et al., 2021; Jones et al., 2021]. However, there is conflicting evidence about the association between these improvements and the changes in the characteristics of the admitted patients. Finally, there are no data about whether the mortality rate and profile of deceased patients have changed in the last months.
Our study reflects that admissions dramatically decreased from the first wave because of the national lockdown and population prophylactic measures adopted in Spain. In addition, it is noticeable that each wave presented a peak that was attributed to the greater flexibility and poorer compliance of restrictions during holiday periods such as summer, December national holidays, and Christmas (Soriano et al., 2021a).
Despite these clearly identifiable dynamics regarding the number of new cases and hospital-admitted patients, no differences in the deceased patients, by age or sex, were found between the 3 waves. Moreover, the mortality rate remained constant throughout the study period, and the profile of non-survivor individuals remained unchanged. Thus, these results confirm that age and frailty are still by far the strongest risk factors, despite greater knowledge of the disease and better therapeutic management of patients with severe COVID-19.
These findings and the discrepancies with other studies could be partially explained for the following reasons. First, broad corticosteroid and tocilizumab treatment was promptly considered in our institution's protocols, leaving less room for improvement in subsequent waves [Fernández-Cruz et al., 2020, Ruiz-Antorán et al., 2021]. Second, early planning and the possibility to double the number of beds per room delayed the early overload of wards and ICUs, contributing to attenuating the mortality rate in comparison with other Spanish hospital cohorts [Moreno-Torres et al., 2021, Yehia et al., 2020, Casas-Rojo et al., 2020]. Third, poor knowledge of the natural course of COVID-19 during the first wave resulted in a higher number of hospital admissions for patients who would have benefited from adequate home care. This fact was translated into lower mortality rates at the beginning [Moreno-Torres et al., 2021]. At the same time, the higher number of ICU admissions during the third wave could also be a consequence of the greater severity of COVID-19 observed in hospitalized patients during this period. Finally, it should be considered that most studies carried out during the first wave were based on the diagnosis made in the hospital context [Preskorn, 2020, Soriano et al., 2021b]. In the following waves, the greater availability of diagnostic tests led to the inclusion of information related to asymptomatic or less severe diagnoses, thus reducing the overall mortality due to the infection. Consequently, overall population reported mortality rates could have been decreased because a higher number of non-severe patients have been identified, although not necessarily because the disease prognosis has improved. However, our study is based exclusively on the diagnoses that required hospital admission and on the mortality observed in this setting during the whole study period. Overall and despite these possible explanations, the fact that mortality rates and the profile of deaths have not changed in 1 year at our center, yields 2 possible interpretations. On the one hand, with the therapeutic tools at our disposal, we have managed the disease in an acceptable way from the beginning, and on the other hand, these findings emphasize the need for specific antivirals against SARS-CoV-2 [Beigel et al., 2020; Jayk-Bernal et al., 2021; Owen et al., 2021].
Finally, it should be mentioned that mass vaccination programs, which began in Madrid at the beginning of 2021, have clearly modified the course of severe COVID-19 [Barandalla et al., 2021]. In our study, the vaccine impact was intentionally not yet fully noted because in April only a small percentage of the population have been fully vaccinated. However, it is observed that during the third wave, the number of dependent and patients with dementia was significantly lower than in the previous waves, which can be explained by the impact of the vaccine in these groups. Besides, it is noteworthy that we only identified 2 deaths in this period who have received the complete vaccination schedule. Altogether, previous data show that in the absence of effective treatment, a vaccine is an essential tool to reduce mortality from COVID-19 and to prevent the current wave of infections (November 2021) such as the one that is occurring in some Central European countries among unvaccinated people.
Our analysis has several limitations. First, it is a single-center, observational, retrospective study. Second, we have not analyzed the different therapeutic options and the possible associated benefits. Finally, we have limited ourselves to analyzing the baseline characteristics of the deceased patients, not being able to rule out differences in the total number of hospitalized patients.
1 year after the onset of the pandemic, the mortality rate of in-hospital patients and the profile of deceased patients have not changed, with older age and pluripathology being constant features. Despite the absence of solid improvements in mortality rates, recent trends might indicate the expected benefits of the vaccine.
Acknowledgments
Competing Interests
The authors declare no conflicts of interest.
Funding source
This work has been supported by a grant from Instituto de Salud Carlos III (Expedient number CM19/00223).
Ethical Approval Statement
The hospital review board approved the study (PI134-20), and a waiver for informed consent was granted.
Contributions
Victor Moreno-Torres: Study concept and design, statistical analysis, interpretation of results, drafting of the manuscript, critical revision of the manuscript, and approval of the final version of the manuscript.
Carmen de Mendoza: Study concept and design, interpretation of results, drafting of the manuscript, critical revision of the manuscript, and approval of the final version of the manuscript.
All other authors: Interpretation of results, critical revision of the manuscript, and approved the final version of the manuscript.
References
- Armstrong RA, Kane AD, Kursumovic E, et al. Mortality in patients admitted to intensive care with COVID-19: an updated systematic review and meta-analysis of observational studies. Anaesthesia. 2021;76:537–548. doi: 10.1111/anae.15425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barandalla I, Alvarez C, Barreiro P, et al. Impact of scaling up SARS-CoV-2 vaccination on COVID-19 hospitalizations in Spain. Int J Infect Dis. 2021;112:81–88. doi: 10.1016/j.ijid.2021.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas-Rojo JM, Antón-Santos JM, Millán-Núñez-Cortés J, et al. en nombre del Grupo SEMI-COVID-19 Network. Clinical characteristics of patients hospitalized with COVID-19 in Spain: Results from the SEMI-COVID-19 Registry. Rev Clin Esp (Barc) 2020;220:480–494. doi: 10.1016/j.rceng.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Cruz A, Ruiz-Antorán B, Muñoz-Gómez A, et al. A Retrospective Controlled Cohort Study of the Impact of Glucocorticoid Treatment in SARS-CoV-2 Infection Mortality. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01168-20. e01168-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. MOVe-OUT Study Group. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2021 doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Mason N, Palser T, et al. Trends in Risk-Adjusted 28-Day Mortality Rates for Patients Hospitalized with COVID-19 in England. J Hosp Med. 2021;16:290–293. doi: 10.12788/jhm.3599. [DOI] [PubMed] [Google Scholar]
- Kurtz P, Bastos LSL, Dantas LF, et al. Evolving changes in mortality of 13,301 critically ill adult patients with COVID-19 over 8 months. Intensive Care Med. 2021;47:538–548. doi: 10.1007/s00134-021-06388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1850. doi: 10.1038/s41591-021-01569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Torres V, de la Fuente S, Mills P, et al. Major determinants of death in patients hospitalized with COVID-19 during the first epidemic wave in Madrid. Spain. Medicine (Baltimore) 2021;100:e25634. doi: 10.1097/MD.0000000000025634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- Privor-Dumm LA, Poland GA, Barratt J, et al. International Council on Adult Immunization. A global agenda for older adult immunization in the COVID-19 era: A roadmap for action. Vaccine. 2021;39:5240–5250. doi: 10.1016/j.vaccine.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH. COVID-19: Why Has the Mortality Rate Declined? J Psychiatr Pract. 2020;26:394–399. doi: 10.1097/PRA.0000000000000494. [DOI] [PubMed] [Google Scholar]
- Roth GA, Emmons-Bell S, Alger HM, et al. Trends in Patient Characteristics and COVID-19 In-Hospital Mortality in the United States During the COVID-19 Pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Antorán B, Sancho-López A, Torres F. TOCICOV-study group. Combination of Tocilizumab and Steroids to Improve Mortality in Patients with Severe COVID-19 Infection: A Spanish, Multicenter, Cohort Study. Infect Dis Ther. 2021;10:347–362. doi: 10.1007/s40121-020-00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano V, Ganado-Pinilla P, Sanchez-Santos M, et al. Main differences between the first and second waves of COVID-19 in Madrid, Spain. Int J Infect Dis. 2021;105:374–376. doi: 10.1016/j.ijid.2021.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano V, de Mendoza C, Gómez-Gallego F, et al. Third wave of COVID-19 in Madrid, Spain. Int J Infect Dis. 2021;107:212–214. doi: 10.1016/j.ijid.2021.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahidy FS, Drews AL, Masud FN, Schwartz RL, et al. Characteristics and Outcomes of COVID-19 Patients During Initial Peak and Resurgence in the Houston Metropolitan Area. JAMA. 2020;324:998–1000. doi: 10.1001/jama.2020.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Weekly epidemiological update on COVID-19-27 April 2021. www.who.int
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia BR, Winegar A, Fogel R, et al. Association of Race With Mortality Among Patients Hospitalized With Coronavirus Disease 2019 (COVID-19) at 92 US Hospitals. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]