Abstract
Background
Drug-related problems cause severe disabilities, premature deaths, and unnecessary costs. Telepharmacy offer easier access to needed medications, preventing DRPs. Adoption has been slow, and it is unclear what aspects of telepharmacy are most important. The COVID-19 pandemic disrupted health services, forcing the rapid adoption of telepharmacy. In Phayao, Thailand, a program was implemented for home delivery of drugs for patients with chronic disease.
Objectives
This study aimed to explore the prevalence and factors associated with DRPs of patients with chronic disease who received home drug-delivery services.
Methods
A cross-sectional study was undertaken in Phayao Province located in North Thailand. Simple random sampling was used to select patients from 6 public hospitals between July and August 2020. Logistic regression was used to analyze multivariate factors that might be related to DRPs.
Results
A total of 246 participants reported at least 1 DRP (49.30%). Most participants were female (58.32%) with elementary education (60.72%). Their mean age was 63.69 (SD = 12.97) years. The 5 most common DRPs were changes of drug packaging or drug brands (18.84%), leftover medications of more than 2 weeks (18.44%), nonadherence (17.43%), having conditions or diseases requiring additional medications (6.81%), and adverse drug reactions (5.21%). Univariate analysis identified number of chronic conditions, diabetes mellitus, dyslipidemia, chronic kidney disease, education level, and drug-delivery channel as predictors of DRPs. In multivariate analysis, predictors of DRPs were the number of drugs used per day (adjusted odds ratio [aOR] 1.11 [95% CI 1.03-1.19], P = 0.004) and dyslipidemia (aOR 1.83 [95% CI 1.18-2.84], P = 0.007). Nonadherence was associated with leftover medicines (aOR 4.22 [95% CI 2.44-7.28], P < 0.001)
Conclusion
The present results indicate that home delivery caused no increase and may have caused a decrease in DRPs, and patients were highly satisfied. These promising results suggest that home delivery should be continued and further investigated even as the COVID-19–induced emergency subsides.
Key Points.
Background
-
•
Drug-related problems (DRPs) are prevalent among patients with chronic disease, leading to wasted resources and increased morbidity and mortality.
-
•
The coronavirus disease 2019 (COVID-19) pandemic disrupted health services. Many countries developed home drug-delivery programs to reduce overcrowding in hospitals. This created a natural experiment in telepharmacy.
-
•
Telepharmacy is a potential platform for decreasing DRPs, but adoption of telepharmacy programs has been slow.
Findings
-
•
Home drug delivery was favorable. Patients were extremely pleased, and several expressed an interest in continuing home delivery.
-
•
Relative to previous findings, patients in the drug-delivery program had lower levels of nonadherence, fewer conditions or diseases requiring additional medications, and fewer adverse drug reactions. These findings suggest that the drug-delivery program may reduce DRPs.
-
•
Telepharmacy programs should be implemented involving home delivery of pharmaceuticals. DRP reduction should be explicitly targeted through better labeling and more clinician follow-up to address common problems.
Introduction
Drug-related problems (DRPs) are undesirable incidents related to medication therapy. They can result in severe disabilities, premature deaths, and billions of dollars in annual economic loss.1 For example, the economic burden arising from drug-related morbidity and mortality in the United States was $177.4 billion.1 Reports from various hospitals have shown that 6.5%-16.2% of all hospital admissions are DRPs.2 , 3 Up to 70% of patients suffer from DRPs.4 , 5
The percentage of patients experiencing DRPs is often near or above 50%. This has been found in many different countries, including Malaysia (50%),6 the Netherlands (53%),7 and Ethiopia (70%).5 DRPs were more common in older patients and especially in people with chronic diseases.4 , 5 In Thailand, studies have estimated that between 39.3% and 52.62% of patients in rural areas have experienced DRPs.8 , 9
In general, a DRP is defined as an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes in patients.10, 11, 12, 13, 14 Common examples of DRPs include nonadherence to prescribed drug regimen, patients lacking understanding of how to take their medication or what dosage to take, patients experiencing unexpected adverse side effects or drug interactions,5 , 8 , 15 changes of drug packaging or drug brands,16 and, in extreme cases, patients experiencing unexpected medical emergencies resulting in hospitalization.1 , 7 , 8 The likelihood of DRPs is heightened in situations involving older patients, suboptimal prescribing (e.g., overuse, inappropriate use, or underuse of medications, and polypharmacy), medication errors (both dispensing and administration problems), and patient medication noncompliance (both intentional and unintentional).17 The study of DRPs has often been clouded by inconsistent definitions. In this study, we used a comprehensive definition combining subtypes of DRPs from several different coding systems (see Methods and Appendix 2). This allowed us to capture the full range of DRPs.
There have been several strategies to reduce DRPs. One promising approach is the implementation of telepharmacy. Telepharmacy is defined as “a method used in pharmacy practice in which pharmacists use telecommunications technology to oversee aspects of pharmacy operations or provide patient-care services.”18 The term telepharmacy acts as an umbrella covering a range of interventions, including home delivery of drugs, phone calls with pharmacists, remote monitoring of patient drug use, text messaging apps, and video consultations.19 Although studies have consistently demonstrated that telepharmacy improves patient outcomes, widespread adoption has been slow. Moreover, because telepharmacy approaches often include several simultaneously applied interventions, it is not clear which aspects of telepharmacy are of most benefit to patients.20
The coronavirus disease 2019 (COVID-19) pandemic has disrupted health services, particularly for patients with chronic disease.21 These disruptions have accelerated the adoption of telepharmacy practices. In South Africa, Qatar, and China, home delivery pharmacy services have been established.22, 23, 24 All 3 of these programs combined home delivery with other aspects of telepharmacy practices (e.g., video consultation). The Qatari study was the only one to evaluate DRPs and found that drug adherence improved for patients in their home delivery program.23
Similar to other countries, Thailand’s health system was disrupted by COVID-19. Telepharmacy was partially implemented to help social distancing and reduce overcrowding of patients in hospitals. Specifically, a home delivery drug refill program (HDDP) was implemented. The HDDP was implemented country-wide through Thailand’s Universal Health Coverage system, which covers more than 75% of the population.25 The initial phase of the HDDP studied here included home delivery without other aspects of telepharmacy.
More specifically, the HDDP was implemented on a patient-by-patient basis following a two-step procedure. First, doctors and hospital pharmacists reviewed chronic patients’ medical records. All patients with chronic disease who did not need to be hospitalized for any condition were enrolled in the HDDP. Second, drug delivery was carried out almost entirely by community volunteers and nurses from local clinics (>95%).
As the world adjusts to a new normal, it is unclear whether effort should be made to improve and make permanent the emergency telepharmacy programs that have been implemented, or whether we should strive for a return to prepandemic standards of care. Today, some hospitals in Thailand have continued the HDDP, but others have reverted to hospital drug distribution. Where it has been continued, Thailand’s drug-delivery system has been augmented with video, text messaging, and mobile application support, but in the early days of the pandemic, this was not so. Thus, this study aimed to explore the prevalence and factors associated with DRPs of patients with chronic disease who received home drug-delivery services during COVID-19 pandemic in isolation from other aspects of telepharmacy.
Methods
Study design and setting
A cross-sectional study was undertaken in Phayao Province located in the northern part of Thailand. Older rural Thai people with chronic diseases were our target population. This study was approved by the Institutional Review Board of Phayao Provincial Public Health Office, Phayao Province, Thailand (No. 27/2563). All procedures performed in the present study involving human participants were in accordance with ethical standard procedures. Consent forms were completed by all participants before their interview.
Study population and study size
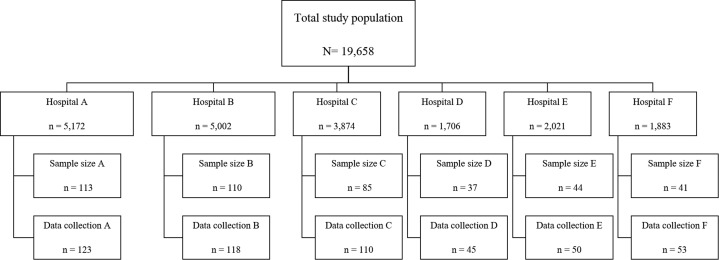
A total of 19,658 patients distributed across 6 regional hospitals in Phayao Province were enrolled in the HDDP and formed our total population of interest. Applying sample size calculation formula26 with N = 19,658 and acceptable sampling error of 0.05 resulted in a target sample size of 392, to which, we added 10% to account for incomplete data sets, yielding a target sample size of 431. To further avoid missing data, the research team collected structured interview data using a purpose-developed questionnaire from 500 patients. Data were collected in proportion to total number of patients enrolled in the HDDP at each hospital (Appendix 1). Within hospitals, patients were assigned deidentified numbers, and then, patients were invited to participate through random number selection until each hospital’s quota had been filled.
All patients in the HDDP suffered from chronic diseases, which were defined broadly as conditions that last 1 year or more and that require ongoing medical attention or that limit activities of daily living or both. Common chronic diseases included heart disease, cancer, and diabetes and are the leading causes of death and disability.27
Data collection
Data were collected between July and August 2020. The period of the HDDP was from March to May 2020. Each interview took about 30 minutes. Six primary care pharmacists were trained before interviewing patients with chronic disease in their charge in each community. DRPs were identified using a validated survey questionnaire.
Questionnaire development
To identify all possible DRPs, we developed a questionnaire that integrated aspects of 5 previously published definitions as well as input from our own expert panel (Appendix 2).10, 11, 12, 13, 14 Our expert panel comprised 2 pharmacy researchers, 6 primary care pharmacists, and 4 policy makers from the Provincial Public Health Office. This resulted in a more inclusive than typical definition of DRPs.
Content validity was assessed using evaluations from 3 chief pharmacists in the Provincial Health Office of Phayao. Using these ratings, we calculated the index of item objective congruence for each item using the mean across evaluators. Items that had scores higher than or equal to 0.5 were reserved.
To enhance reliability of data collection, 6 primary care pharmacists were trained with a 3-hour session before interviewing patients. In addition, a written guide for recording the questionnaires was provided. To quantify reliability, Cronbach’s alpha was calculated to determine interrater reliability among our pharmacists. They all examined the same example questionnaire data to see whether they could detect DRPs.28 This procedure yielded excellent agreement, and interrater reliability was 0.92.
The questionnaire was divided into 3 sections: (1) general information about the informant (Table 1 ), (2) satisfaction with the HDDP (Table 2 ), and (3) drug-related problems (Table 3 ).
Table 1.
Baseline characteristics of the participants
| Characteristics | Total N = 499 n (%) | With DRPs n=246 n (%) | Non-DRPs n=253 n (%) |
P value (DRPs VS non-DRPs) |
|---|---|---|---|---|
| Gender | ||||
| Female | 291 (58.32) | 141 (57.32) | 150 (59.29) | 0.716 |
| Male | 208 (41.68) | 105 (42.68) | 103 (40.71) | |
| Age (years) (Mean ± SD) | 63.69 ± 12.97 | |||
| ≤ 65 | 253 (50.70) | 121 (49.19) | 132 (52.17) | 0.531 |
| ≥ 65 | 246 (49.30) | 125 (50.81) | 121 (47.83) | |
| Number of chronic conditions | ||||
| (Mean ± SD) | 1.9 ± 0.90 | |||
| One | 194 (38.88) | 83 (33.74) | 111 (43.87) | 0.012∗ |
| Two | 190 (38.08) | 95 (38.62) | 95 (37.55) | |
| Three | 91 (18.24) | 49 (19.92) | 42 (16.60) | |
| Four | 18 (3.61) | 14 (5.69) | 4 (1.58) | |
| Five | 6 (1.20) | 5 (2.03) | 1 (0.40) | |
| Chronic conditions | ||||
| Hypertension | ||||
| Yes | 335 (67.13) | 167 (67.89) | 168 (66.40) | 0.775 |
| No | 164 (32.87) | 79 (32.11) | 85 (33.60) | |
| Diabetes mellitus | ||||
| Yes | 166 (33.27) | 94 (38.21) | 72 (28.46) | 0.023a |
| No | 333 (66.73) | 152 (61.79) | 181 (71.54) | |
| Dyslipidemia | ||||
| Yes | 121 (24.25) | 77 (31.30) | 44 (17.39) | <0.0001a |
| No | 378 (75.75) | 169 (68.70) | 209 (82.61) | |
| Chronic kidney disease | ||||
| Yes | 93 (18.64) | 37 (15.04) | 56 (22.13) | 0.05 a |
| No | 406 (81.36) | 209 (84.96) | 197 (77.87) | |
| Asthma | ||||
| Yes | 43 (8.62) | 19 (7.72) | 24 (9.49) | 0.526 |
| No | 456 (91.38) | 227 (92.28) | 229 (90.51) | |
| Polypharmacy | ||||
| No polypharmacy (< 5) | 274 (54.91) | 125 (50.81) | 149 (58.89) | 0.073 |
| Polypharmacy (≥5) | 225 (45.09) | 121 (49.19) | 104 (41.11) | |
| Education level | ||||
| No education | 76 (15.23) | 43 (17.48) | 33 (13.04) | 0.044 a |
| Elementary school | 303 (60.72) | 154 (62.60) | 149 (58.89) | |
| Junior high school | 47 (9.42) | 14 (5.69) | 33 (13.04) | |
| Senior high school | 50 (10.02) | 22 (8.94) | 28 (11.07) | |
| Bachelor’s degree | 20 (4.01) | 12 (4.88) | 8 (3.16) | |
| Postgraduate degree | 1 (0.20) | 0 (0.00) | 1 (0.40) | |
| Others | 2 (0.40) | 1 (0.41) | 1 (0.4) | |
| Occupation | ||||
| No occupation | 243 (48.70) | 127 (51.63) | 116 (45.85) | 0.392 |
| Employee | 71 (14.23) | 27 (10.98) | 44 (17.39) | |
| Agriculturist | 137 (27.45) | 66 (26.83) | 71 (28.06) | |
| Business | 23 (4.61) | 13 (5.28) | 10 (3.95) | |
| Government official | 12 (2.40) | 6 (2.44) | 6 (2.37) | |
| Others | 13 (2.6) | 7 (2.85) | 6 (2.38) | |
| The participants received a comprehensive list of their prescription drugs | 486 (97.39) | 234 (95.12 | 252 (99.60) | 0.2 |
| Drug-delivery channel | ||||
| Primary care pharmacist | 2 (0.40) | 0 (0.00) | 2 (0.79) | 0.013 a |
| Mail carriers | 14 (2.81) | 2 (0.81) | 12 (4.74) | |
| Nurses from local clinics | 162 (32.46) | 89 (36.18) | 73 (28.85) | |
| Community volunteers | 316 (63.33) | 152 (61.79) | 164 (64.82) | |
| Others | 5 (1.0) | 2 (0.81) | 2 (0.79) | |
| If the COVID-19 continues to spread, how would the patients like to receive home drug-delivery channel? | ||||
| Hospital pharmacist (standard before COVID-19) | 82 (16.43) | 34 (13.82) | 48 (18.97) | 0.316 |
| Mail carriers | 10 (2.00) | 4 (1.63) | 6 (2.37) | |
| Nurses from local clinics | 152 (30.46) | 75 (30.49) | 77 (30.43) | |
| Community volunteers | 251 (50.30) | 129 (52.44) | 122 (48.22) | |
| Others | 4 (0.80) | 4 (1.63) | 0 (0.00) | |
Abbreviations used: DRP, drug related problem.
P value < 0.05 was considered statistically significant (DRPs VS non-DRPs).
Table 2.
Satisfaction of home drug-delivery program
| Satisfaction | Satisfaction level |
||
|---|---|---|---|
| Satisfied n (%) | Neutral n (%) | Dissatisfied n (%) | |
| Information received with drug delivery | 322 (64.6) | 114 (22.9) | 63 (12.4) |
| Method of drug delivery | 368 (73.9) | 124 (24.8) | 7 (1.3) |
| Timeliness of drug delivery | 412 (82.5) | 68 (13.7) | 19 (3.8) |
Table 3.
DRPs identified in patients with chronic disease (N total = 427 DRPs)
| Sr. No | DRPs | n (%) |
|---|---|---|
| 1. | Changes of drug packaging or drug brands | 94 (18.84) |
| 2. | Leftover medications more than 2 weeks | 92 (18.44) |
| 3. | Nonadherence | 87 (17.43) |
| 4. | Having conditions or diseases requiring additional medications | 34 (6.81) |
| 5. | Adverse drug reactions | 26 (5.21) |
| 6. | Having an emergency requiring treatment at a hospital before next appointment date | 25 (5.01) |
| 7. | Lack of knowledge about the purpose, administration, and storage of drugs | 23 (4.61) |
| 8. | The quantity of drugs received is not sufficient to cover needs until next follow up | 12 (2.40) |
| 9. | Having abnormal symptoms due to a lack of drugs | 8 (1.60) |
| 10 | The patient did not receive the drug. | 7 (1.40) |
| 11. | Incorrect use of specialized drug administration devices or techniques (e.g., inhalers, injected medications) | 6 (1.20) |
| 12. | Experienced unexpected drug interactions with other drugs, food, or supplements | 5 (1.00) |
| 13. | Patients do not understand the meaning of the printed materials included with drugs | 4 (0.80) |
| 14. | Changes or errors in drug dosage (e.g., dispensing error, brand change/shortage) | 4 (0.80) |
Abbreviations used: DRP, drug related problem.
Definition of DRPs, nonadherence, polypharmacy, and leftover medicine
In this study, a DRP was defined as an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes in the patients,10, 11, 12, 13, 14 including changes of drug packaging or drug brands,16 leftover medications more than 2 weeks,29 and nonadherence and adverse drug reactions. Specific survey questions probed various examples of DRPs (Table 3). Nonadherence was defined as patients not taking medications, adjusting the dose themselves, and/or forgetting to take their medicine.30 Polypharmacy was defined as taking 5 or more medications per day.31 Leftover medicine of more than 2 weeks was defined as prescription drugs that have been oversupplied to patients by more than 2 weeks.32
Primary and secondary outcomes of interest
The primary outcome of interest in this study was the prevalence of DRPs in patients with chronic disease (who were provided with HDDP). The secondary outcomes were factors associated with DRPs, nonadherence, and leftover medicines. Assessment of DRPs was based on the validated questions.
Statistical analysis
Demographic information, types of DRPs, and satisfaction with the drug-delivery system were summarized using descriptive statistics and were described with frequency and percentages. Continuous data were compared using t test, and those results are shown as mean ± SD. Categorical data were compared using chi-square test or Fisher’s exact test based on the criterion that when more than 20% of cells have expected frequencies <5, we need to use Fisher’s exact test.33 The risk of experiencing a DRP as a function of various predictors in the data set were evaluated using univariate logistic regression, and those results are expressed as odds ratios (ORs) and 95% CIs. Both variables with P < 0.05 in univariate analysis and those identified by previous studies4 , 8 , 16 , 34 as predictors of DRPs were submitted to multivariate logistic regression to establish their independent association with DRPs while statistically controlling for other factors.
Results
Baseline characteristics
A total of 499 patients were included in the final analysis. The participants were mostly female (58.32%) with elementary education (60.72%). Their mean age was 63.69 (SD = 12.97) years. The mean number of chronic conditions was 1.90 (SD = 0.90). The most common chronic diseases were hypertension (67.13%), diabetes mellitus (33.27%), dyslipidemia (24.25%), chronic kidney disease (18.64%), and asthma (8.62%) (Table 1. and Appendix 3). Forty-five percent (45.09%) of participants had polypharmacy (used ≥5 drugs daily).31
Drugs were delivered to participants’ homes by community volunteers (63.33 %), nurses from local clinics (32.46 %), mail carriers (2.81%), primary care pharmacists (0.40%), and others (1.0 %). Most of the participants also received a comprehensive list of their prescription drugs (97.39%) (Table 1).
Patients were generally satisfied with the HDDP (Table 2). They reported satisfaction with the information received with HDDP, the method of drug delivery, and the timeliness of delivery at rates of 65%, 74%, and 83%, respectively (Table 2).
Total DRPs in all patients with chronic disease who participated in the study are shown in Table 3 and Appendix 4.
There were 246 participants who reported at least 1 DRP (49.30%). The top 5 DRPs were as follows: changes of drug packaging or drug brands (18.84%), leftover medications of more than 2 weeks (18.44%), nonadherence (17.43%), having conditions or diseases requiring additional medication (6.81%), and ADRs (5.21%).
Association between DRP and patients’ characteristics; univariate analysis
Independent variables were tested for statistically significant association with DRP outcomes. The odds of experiencing a DRP were significantly higher as a function of the number of chronic conditions and for patients with diabetes mellitus, dyslipidemia, or chronic kidney disease. DRP prevalence was also higher for less educated patients and varied across drug-delivery channel (Table 1).
High prevalence DRPs individually predict all of the other DRPs; univariate analysis
For each of these analyses, the association was tested between 1 DRP component and the composite DRP prevalence excluding the 1 tested component. Only the 4 most prevalent DRPs were tested. The prevalence of changes of drug packaging or drug brands, leftover medications more than 2 weeks, nonadherence, and ADRs were all associated with the rate of all other DRPs (all P < 0.001, see Table 4 ).
Table 4.
Univariate analysis in 4 most prevalent DRPs predict all of the other DRPs
| DRPs | OR | 95% CI | P value |
|---|---|---|---|
| Changes of drug packaging or drug brands | 2.31 | 1.43−3.76 | 0.0002 |
| Leftover medications more than 2 weeks | 3.86 | 2.34−6.42 | < 0.001 |
| Nonadherence | 2.39 | 1.46−3.95 | 0.0002 |
| Adverse drug reactions | 3.61 | 1.41−10.33 | 0.0026 |
Abbreviations used: DRP, drug related problem; OR, odds ratio.
Predictors for DRP: multivariate analysis
Probable DRP outcomes were predicted using multivariable logistic regression. Statistically significant association were with the number of drugs used per day (aOR 1.11 [95%CI 1.03-1.19], P = 0.004) and dyslipidemia (aOR 1.83 [95%CI 1.18-2.84], P = 0.007). Non-adherence was associated with leftover medicines (aOR 4.22 [95%CI 2.44-7.28], P < 0.001), as shown in Table 5 .
Table 5.
Multivariate analysis for predictors DRP
| Factors | Adjusted ORa | 95% CI | P value |
|---|---|---|---|
| Associated with DRPa (DRPs VS Non-DRPs) | |||
| Number of drugs used per day | 1.11 | 1.03–1.19 | 0.004 |
| Dyslipidemia | 1.83 | 1.18–2.84 | 0.007 |
| Associated with leftover medicineb (Leftover medicines VS Nonleftover medicines) | |||
| Nonadherence | 4.22 | 2.44–7.28 | <0.001 |
Abbreviations used: DRP, drug related problems; OR, odds ratio.
Adjusted factors: Age, Hypertension, Diabetes Mellitus, Dyslipidemia, Chronic kidney disease, Polypharmacy, Education level, drug-delivery channel
Adjusted factors: Drug-related problems (DRPs) identified in chronic disease patient in Table 3, except Abnormalities due to the ineffectiveness of the drug, The patient did not receive the drug, and Received drugs that interact with drugs, food, or supplements.
Discussion
In this study, the prevalence of DRPs of home drug delivery in rural Northern Thailand was 49.30%. The 5 most common DRPs were changes of drug packaging or drug brands (18.84%), leftover medications of more than 2 weeks (18.44%), nonadherence (17.43%), having conditions or diseases requiring additional medications (6.81%), and ADRs (5.21%). Furthermore, nonadherence was associated with a risk of leftover medications.
Because our program was implemented in an emergency situation, it was not possible to construct a randomized control group, making interpretation difficult. A key question to ask is whether a DRP prevalence of 49.30% is high or low? We believe that this number represents a reduction and points to the general success of the HDDP. Using data from our previous paper, it was possible to compare a subgroup of our patients to a similar group of patients treated at the same hospital under traditional in-clinic drug dispensing conditions (see Appendix 5).9 Although the sample size was small and the data collected were not completely overlapping, the results collected only 3 years apart are promising. When compared with the previous data, patients in the HDDP exhibited lower levels of nonadherence, had fewer conditions or diseases requiring additional medications, fewer ADRs, and experienced less lack of knowledge about the meaning of the labels and stickers on their medications (Appendix 5). These findings suggest that the drug-delivery program may have resulted in a reduction of DRPs.
Comparing specific DRP components, we find that there is modest evidence for a reduction of DRPs in our study relative to past literature. For example, a study by Garin et al35 found that 12% of patients experienced ADRs, but in our sample, only 5% of patients had ADRs. Basheti et al36 found that 46.1% of outpatients with chronic diseases had nonadherence, and Chang et al6 estimated that the pooled prevalence of nonadherence to medication was 60.6%. By contrast, in our study, 17.43% of patients experienced nonadherence. Wilmer7 found that 20.2% of patients had conditions or diseases requiring additional medication, but in our study, the corresponding figure was only 6.81%.
Finally, in thinking about the prevalence of DRPs reported here, it is important to note how inclusive our definition of DRP was. Changes in drug packaging or brand was the most commonly reported DRP in our sample, yet it is not included as a DRP in most studies. If these DRPs are subtracted from our results, then the DRP prevalence in our sample drops to 41.28%, which is below most previous reports, further suggesting that home delivery resulted in a drop in DRP prevalence.
Although DRP prevalence seemed relatively good in the present sample, this is partially because of how sadly common DRPs are. The most common DRP in this study was change of drug packaging or drug brand. Drug packaging changes were associated with an increase in all other DRPs (Table 4), suggesting that packaging changes can lead to confusion that precipitates other, more dangerous DRPs. Previous studies have also shown that changes of drug packaging or drug brands can lead to confusion and are related to low adherence and adverse events.16 , 37 In the population studied here, the main reason for packaging and brand changes was substitution of equivalent drugs necessitated by supply shortages. To mitigate this problem, medical personnel must anticipate shortages in order to plan for continuity whenever possible in order to minimize patient impact.24 When continuity is not possible, it is imperative that brand changes are marked with pharmacist consultation and clear labeling. Specifically, we suggest that brand changes be marked with stickers reading, “Identical type and dose of drugs, but the brand was altered.”
The second most common DRP we observed was leftover medications. Leftover medicine was associated with nonadherence, which was similar to previous reports.32 , 34 , 38 Leftover medications can be caused by several factors, including patients’ discontinuing their drug regimens, adverse effects from drugs, errors of prescription, order or supply problems, over-collection in the past, patients’ being left to self-manage complex regimes, and changes in medical condition.39 , 40 Leftover medication is a common problem, with as many as 74% of outpatients reporting leftover medication. Similar to our observations, it has been reported that antihypertensive drugs represent the highest quantity and value of drug waste, followed by antidiabetic agents.41 Unwanted and unused medications accumulate in patients’ homes and lead to unnecessary costs and harmful effects.29 Community awareness programs and pharmacist collections of unwanted medications could go a long way to alleviating this problem.39
The third most common DRP was nonadherence, which itself can lead to other DRPs. Considering that we observed a relatively low nonadherence prevalence (17.4%) than comparable results in Thailand (42%-45%), home drug delivery may already be a promising avenue for reduction of nonadherence problems.8 , 9 It is likely that augmenting the drug-delivery program with other aspects of telepharmacy (e.g., video and text messaging with pharmacists) will reduce nonadherence even further.24
Although the drug-delivery program described here was born out of the exigency of the COVID-19 crisis, we believe that it has been a positive forward step in the implementation of telepharmacy. Telepharmacy is a health care service growing in popularity. In previous studies, it was found that when telepharmacy is used in health systems, it has been shown to reduce overall costs and help patients access treatment more conveniently.42, 43, 44 Telepharmacy increases satisfaction ratings of both patients and health care providers, including pharmacists, nurses, physicians, and pharmacy technicians.44 , 45 Telepharmacy can also help pharmacists monitor DRPs, improve medication compliance, reduce the number of medication errors, and decrease ADRs, costs, and treatment failures.20 , 46 These gains are likely to be particularly large in rural areas such as the region studied here. This is because telepharmacy makes remote areas more reachable by health professionals.42
Home drug delivery is a central service in many telepharmacy programs, but it is generally not studied in isolation. Programs that implement home drug delivery generally also implement remote consultation services and patient monitoring, but the emergency of COVID-19 prohibited development of these resources during the early days of the pandemic. As a result, our study demonstrates that HDDP might reduce DRPs, and patients had overwhelmingly positive views. This is a particularly interesting result, considering that pharmacists implementing telepharmacy initiatives in the United States have often faced problems with loss of privacy and confidentiality, low digital literacy skills among older patients, lack of affordable and user-friendly platforms to support telepharmacy, and a lack of policy to implement telepharmacy.19 , 47 Our study demonstrates that these challenges need not hinder attempts to implement at least some part of a telepharmacy program, as simply adding a home delivery option may already improve outcomes alone. However, these effects may be lost over a longer period, and the program reported here has now been augmented with remote consultation and monitoring services. Taken together, these results suggest that HDDP is an improvement over traditional pharmacy practices, but our survey also revealed challenges that can be addressed to further improve telepharmacy practices.
Strengths
Strengths of the study were as follows. First, the current COVID-19 situation created a rare opportunity to study the effect of drug delivery on DRPs in isolation from other features of telepharmacy. Second, primary care pharmacists are familiar with their local patients, and this helped them act as effective data collectors, obtaining accurate information. Moreover, most participants were older, so the data collection method we used was appropriate for our samples. Third, the questions posed were assessed by 3 chief pharmacists in the Provincial Health Office to ensure that they were applicable to primary care pharmacy in Phayao, which was the setting of the study.
Limitations
Our study had limitations. First, although well-powered to make inferences about this region, our data are limited to the rural developing region we studied. It is unclear how these results might generalize to other areas. Therefore, multicenter studies are needed to confirm our conclusions. Second, data on over-the-counter medicines may be biased. For example, the participants may not report their use of over-the-counter medicines. This possibility may lead to an underestimation of the level of DRPs in the participants and the number of products used. Third, the HDDP studied here did not specify criteria for screening appropriate patients. Thus, patients may have had more variable disease severity than in other studies, which could have resulted in an increased prevalence of DRPs. However, as this factor would have, if anything, biased our results toward higher prevalence, it is all the more striking that we observed a relatively low prevalence, and the results from this study reflected the reality that occurs in the workflow of drug delivery.
Future studies should make clear whether clinical outcomes of implementing home drug delivery are not worse than clinical outcomes under traditional conditions before widespread adoption of this strategy. Furthermore, appropriate criteria for choosing patients should take into consideration disease severity and other associated factors that might cause DRPs. Although the HDDP effectively helped Thailand navigate the pandemic and seems to improve patient outcomes, delivery costs will likely prohibit its continuation. However, if improvements in DRPs result in savings on future patient care and better outcomes, then delivery costs may be well worth it, and future research should examine long-term financial costs and benefits of drug delivery.
Conclusion
Home drug delivery was implemented during the COVID-19 emergency in rural Thailand, and the results were favorable. Patients were highly satisfied and said that they would like to continue home delivery even after the COVID-19 emergency is over. DRP prevalence was no higher than under normal conditions and may have been reduced. Future telepharmacy practice programs should be implemented involving home delivery of pharmaceuticals. DRP reduction should be explicitly targeted through better labeling and more clinician follow-up to address common problems.
Acknowledgments
The authors thank the management of primary care pharmacists in Phayao Province, Muttana Sombutwuttanawet and Kridtin Anujaree, for their support, and the grant from the School of Pharmaceutical Sciences, University of Phayao, Thailand, via the Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN) [grant number: FF65-UoE005] and Phayao Provincial Public Health Office.
Biographies
Natthaya Chaomuang, PharmD, Lecturer, Division of Clinical Pharmacy, Department of Pharmaceutical Care, School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand; Research Scientist, Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN), School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand; and Research Scientist, Center of Health Outcomes Research and Therapeutic Safety (Cohorts), School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
Adam J.O. Dede, PhD, Research Scientist, Division of Clinical Pharmacy, Department of Pharmaceutical Care, School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand; and Research Scientist, Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN), School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
Surasak Saokaew, PharmD, PhD, BPHCP, FACP, FCPA, Head ,Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN), School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand; Director, Center of Health Outcomes Research and Therapeutic Safety (Cohorts), School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand; andAssociate Professor, Division of Social and Administrative Pharmacy, Department of Pharmaceutical Care, School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
Adinat Umnuaypornlert, PharmD, PhD, Research Scientist, Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN), School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand; Research Scientist, Center of Health Outcomes Research and Therapeutic Safety (Cohorts), School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand; and Assistant Professor, Division of Social and Administrative Pharmacy, Department of Pharmaceutical Care, School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
Footnotes
Disclosure: The authors declare no relevant conflicts of interest or financial relationships.
Funding: This work was supported by the Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN) [grant number: FF65-UoE005], School of Pharmaceutical Sciences, University of Phayao and Phayao Provincial Public Health Office. The funding source had no role in the study design, the collection, analysis, and interpretation of the data.
ORCIDs Natthaya Chaomuang: https://orcid.org/0000-0003-4677-2068
Adam J.O. Dede: https://orcid.org/0000-0002-3619-4631
Surasak Saokaew: https://orcid.org/0000-0002-1382-0660
Adinat Umnuaypornlert: https://orcid.org/0000-0003-1360-5351
Appendix
Appendix 1.
Flow of study population, sample size and data collection
Appendix 2.
Drug-related problems (DRPs) definition from 5 DRPs Guidelines and expert panel.
| Drug-related problems (DRPs) | Expert panel | ASHP | Hepler & Strand | PCNE | Cipolle | Westerlund | |
|---|---|---|---|---|---|---|---|
| 1. | Changes of drug packaging or drug brands | • | |||||
| 2. | Leftover medications more than 2 weeks | • | |||||
| 3. | Non-adherence | • | • | ||||
| 4. | Having conditions or diseases requiring additional medications | • | • | • | |||
| 5. | Adverse drug reactions | • | • | • | • | • | |
| 6. | Having an emergency requiring treatment at a hospital before next appointment date | • | |||||
| 7. | Lack of knowledge about the purpose, administration and storage of drugs | • | |||||
| 8. | The quantity of drugs received is not sufficient to cover needs until next follow up | • | |||||
| 9. | Having abnormal symptoms due to a lack of drugs | • | |||||
| 10 | The patient did not receive the drug. | • | • | ||||
| 11. | Incorrect use of specialized drug administration devices or techniques (e.g. inhalers, injected medications) | • | • | • | • | • | |
| 12. | Experienced unexpected drug interactions with other drugs, food or supplements | • | • | • | • | • | |
| 13. | Patients do not understand the meaning of the printed materials included with drugs | • | • | ||||
| 14. | Changes or errors in drug dosage (e.g. dispensing error, brand change/shortage) | • | • | • | • | • |
Appendix 3.
Baseline chronic disease in this study
| Chronic disease in this study | N (%) | |
|---|---|---|
| 1 | Diabetes | 166 |
| 2 | Hypertension | 335 |
| 3 | Chronic kidney disease | 93 |
| 4 | Dyslipidemia | 121 |
| 5 | Depression | 9 |
| 6 | Asthma | 43 |
| 7 | Rhinitis | 1 |
| 8 | Psychiatrics | 38 |
| 9 | gout | 30 |
| 10 | cataract | 4 |
| 11 | COPD | 32 |
| 12 | HIV infection | 3 |
| 13 | Hepatitis | 3 |
| 14 | Scleroderma | 1 |
| 15 | Rheumatoid Arthritis | 4 |
| 16 | Glaucoma | 3 |
| 17 | Benign Prostatic Hyperplasia | 9 |
Appendix 4.
Drug-related problems identified in chronic disease patients (N total = 427 DRPs) and univariate analysis in DRPs predict all of the other DRPs ( DRPs VS Non-DRPs )
| Drug-related problems (DRPs) | n (%) | Odds ratio | 95%CI | P-value | |
|---|---|---|---|---|---|
| 1. | Changes of drug packaging or drug brands | 94 (18.84) | 2.31 | 1.43-3.76 | 0.0002 |
| 2. | Leftover medications more than 2 weeks | 92 (18.44) | 3.86 | 2.34-6.42 | < 0.001 |
| 3. | Non-adherence | 87 (17.43) | 2.39 | 1.46-3.95 | 0.0002 |
| 4. | Having conditions or diseases requiring additional medications | 34 (6.81) | 0.83 | 0.69-1.01 | 0.058 |
| 5. | Adverse drug reactions | 26 (5.21) | 3.61 | 1.41-10.33 | 0.0026 |
| 6. | Having an emergency requiring treatment at a hospital before next appointment date | 25 (5.01) | 0.87 | 0.72- 1.04 | 0.142 |
| 7. | Lack of knowledge about the purpose, administration and storage of drugs | 23 (4.61) | 0.88 | 0.73- 1.05 | 0.169 |
| 8. | The quantity of drugs received is not sufficient to cover needs until next follow up | 12 (2.40) | 0.92 | 0.77- 1.10 | 0.389 |
| 9. | Having abnormal symptoms due to a lack of drugs | 8 (1.60) | 0.94 | 0.78-1.12 | 0.499 |
| 10 | The patient did not receive the drug. | 7 (1.40) | 0.94 | 0.79 - 1.12 | 0.528 |
| 11. | Incorrect use of specialized drug administration devices or techniques (e.g. inhalers, injected medications) | 6 (1.20) | 0.94 | 0.79- 1.13 | 0.558 |
| 12. | Experienced unexpected drug interactions with other drugs, food or supplements | 5 (1.00) | 0.95 | 0.79- 1.13 | 0.589 |
| 13. | Patients do not understand the meaning of the printed materials included with drugs | 4 (0.80) | 0.95 | 0.81-1.14 | 0.621 |
| 14. | Changes or errors in drug dosage (e.g. dispensing error, brand change/shortage) | 4 (0.80) | 0.95 | 0.80- 1.14 | 0.621 |
Appendix 5.
Subgroup analysis DRPs in Chiangkham district
| Drug-related problems (DRPs) | Total DRP (In this study) N (%) |
Chiangkham district (In this study) N=123 |
Chiangkham district1 (3 years apart) N=95 |
|---|---|---|---|
| Non-adherence | 87 (17.43) | 35 (28.46) | 50 (52.62) |
| Having conditions or diseases requiring additional medications | 34 (6.81) | 4 (3.25) | 3 (3.15) |
| Adverse drug reactions | 26 (5.21) | 11 (8.94) | 14 (14.73) |
| Lack of knowledge about the purpose, administration, and storage of drugs | 23 (4.61) | 18 (14.63) | 85 (89.47) |
| Patients do not understand the meaning of the printed materials included with drugs | 4 (0.80) | 4 (3.25) | 51 (53.68) |
References
- 1.Ernst F.R., Grizzle A.J. Drug-related morbidity and mortality: updating the cost-of-illness model. J. Am. Pharm. Assoc. (Wash) 2001;41(2):192–199. doi: 10.1016/s1086-5802(16)31229-3. [DOI] [PubMed] [Google Scholar]
- 2.Pirmohamed M., James S., Meakin S., et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson K.M., Talbert R.L. Drug-related hospital admissions. Pharmacotherapy. 1996;16(4):701–707. [PubMed] [Google Scholar]
- 4.Al-Azzam S.I., Alzoubi K.H., AbuRuz S., Alefan Q. Drug-related problems in a sample of outpatients with chronic diseases: a cross-sectional study from Jordan. Ther Clin Risk Manag. 2016;12:233–239. doi: 10.2147/TCRM.S98165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adem F., Abdela J., Edessa D., Hagos B., Nigussie A., Mohammed M.A. Drug-related problems and associated factors in Ethiopia: a systematic review and meta-analysis. J Pharm Policy Pract. 2021;14(1):36. doi: 10.1186/s40545-021-00312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang C.T., Ang J.Y., Islam M.A., Chan H.K., Cheah W.K., Gan S.H. Prevalence of drug-related problems and complementary and alternative medicine use in Malaysia: a systematic review and meta-analysis of 37,249 older adults. Pharmaceuticals (Basel) 2021;14(3):187. doi: 10.3390/ph14030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilmer C.M., Huiskes V.J.B., Natsch S., Rennings A.J.M., van den Bemt B.J.F., Bos J.M. Drug-related problems in a clinical setting: a literature review and cross-sectional study evaluating factors to identify patients at risk. Eur J Hosp Pharm. 2015;22(4):229–235. [Google Scholar]
- 8.Kongkaew C., Methaneethorn J., Mongkhon P., Dechanont S., Taburee W. Drug-related problems identified at patients' home: A prospective observational study in a rural area of Thailand. J Patient Saf. 2021;17(1):8–14. doi: 10.1097/PTS.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 9.Umnuaypornlert A., Khamchompoo S. Drug-related problems in elderly people in Ban tung mok, Chiang kam district, Phayao Province. Chiangrai Med J. 2018;10(1):59–70. [Google Scholar]
- 10.ASHP guidelines on a standardized method for pharmaceutical care. American Society of Health-System Pharmacists. Am J Health Syst Pharm. 1996;53(14):1713–1716. doi: 10.1093/ajhp/53.14.1713. [DOI] [PubMed] [Google Scholar]
- 11.Cipolle R.J., Strand L.M., Morley P.C. McGraw-Hill; New York: 1998. Pharmaceutical Care Practice; pp. 78–79. [Google Scholar]
- 12.Hepler C.D., Strand L.M. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47(3):533–543. [PubMed] [Google Scholar]
- 13.Pharmaceutical Care Network Europe. DRP-Classification V9.0. https://www.pcne.org/upload/files/410_PCNE_classification_V9-0m.pdf Available at:
- 14.Westerlund T. Department of Social Medicine, Göteborg University; Göteborg, Sweden: 2002. Drug-Related Problems: Identification, Characteristics and Pharmacy Interventions; pp. 25–26. [dissertation] [Google Scholar]
- 15.Adusumilli P.K., Adepu R. Drug related problems: an over view of various classification systems. Asian J Pharm Clin Res. 2014;7:7–10. [Google Scholar]
- 16.Rigby M. Pharmaceutical packaging can induce confusion. BMJ. 2002;324(7338):679. doi: 10.1136/bmj.324.7338.679/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramanath K., Nedumballi S. Assessment of medication-related problems in geriatric patients of a rural tertiary care hospital. J Young Pharm. 2012;4(4):273–278. doi: 10.4103/0975-1483.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Society of Health-Systems Pharmacists ASHP statement on Telepharmacy. https://www.ashp.org/-/media/assets/pharmacy-informaticist/docs/sopit-bp-telepharmacy-statement.ashx Available at: [DOI] [PubMed]
- 19.Casey M.M., Sorensen T.D., Elias W., Knudson A., Gregg W. Current practices and state regulations regarding telepharmacy in rural hospitals. Am J Health Syst Pharm. 2010;67(13):1085–1092. doi: 10.2146/ajhp090531. [DOI] [PubMed] [Google Scholar]
- 20.Schneider P.J. Evaluating the impact of telepharmacy. Am J Health Syst Pharm. 2013;70(23):2130–2135. doi: 10.2146/ajhp130138. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. Information Note: NCDs and Covid-19. [Google Scholar]
- 22.Brey Z., Mash R., Goliath C., Roman D. Home delivery of medication during coronavirus disease 2019, Cape Town, South Africa: short report. Afr J Prim Health Care Fam Med. 2020;12(1):e1–e4. doi: 10.4102/phcfm.v12i1.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Zaidan M., Mohamed Ibrahim M.I., Al-Kuwari M.G., Mohammed A.M., Nawaz Mohammed M., Al Abdulla S., et al. Qatar’s Primary Health Care Medication Home Delivery Service: A response toward COVID-19. J Multidiscip Healthc. 2021;14:651–657. doi: 10.2147/JMDH.S282079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S., Luo P., Tang M., et al. Providing pharmacy services during the coronavirus pandemic. Int J Clin Pharm. 2020;42(2):299–304. doi: 10.1007/s11096-020-01017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telehealth, telemed, telepharmacy seen as new normal in Universal health Coverage (UHC): NHSO. http://eng.nhso.go.th/view/1/home/Telehealth-telemed-telepharmacy-seen-as-new-normal-in-Universal-health-Coverage-UHC-NHSO/231/EN-US Available at:
- 26.Adam A.M. Sample size determination in survey research. J Sci Res Rep. 2020;26:90–97. [Google Scholar]
- 27.National Center for Chronic Disease Prevention and Health Promotion About chronic diseases. https://www.cdc.gov/chronicdisease/about/index.htm Available at:
- 28.Wadkar S.K., Singh K., Chakravarty R., Argade S.D. Assessing the reliability of attitude scale by Cronbach's alpha. J Glob Commun. 2016;9(2):113. [Google Scholar]
- 29.Schuh J.L., Hewuse A.J. The cost of unused medications. Fed Pract. 2015;32(4):14–18. [PMC free article] [PubMed] [Google Scholar]
- 30.Jimmy B., Jose J. Patient medication adherence: measures in daily practice. Oman Med J. 2011;26(3):155–159. doi: 10.5001/omj.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masnoon N., Shakib S., Kalisch-Ellett L., Caughey G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadoyoo S., Jirapreeya N., Treesak C., Sangjam P. Leftover medications in patients with chronic diseases from home health care visits: a community study in Bangkok. Dial Pharm Health Care Pract. 2014;1(1):1–7. [Google Scholar]
- 33.Kim H.Y. Statistical notes for clinical researchers: chi-squared test and Fisher's exact test. Restor. Dent. Endod. 2017;42(2):152–155. doi: 10.5395/rde.2017.42.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chantapattarankul P., Thongprong S., Thongmee M. Survey of leftover drugs among patients with chronic diseases at Bangsaphan Hospital, Prachuap Kirikhan. Hua Hin Sook Jai Klai Kangwon. 2018;3(1):119–125. [Google Scholar]
- 35.Garin N., Sole N., Lucas B., et al. Drug related problems in clinical practice: a cross-sectional study on their prevalence, risk factors and associated pharmaceutical interventions. Sci Rep. 2021;11(1):883. doi: 10.1038/s41598-020-80560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basheti I.A., Hait S.S.E., Qunaibi E.A., Aburuz S., Bulatova N. Associations between patient factors and medication adherence: a Jordanian experience. Pharm Pract (Granada) 2016;14(1):639. doi: 10.18549/PharmPract.2016.01.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spence C. The multisensory design of pharmaceuticals and their packaging. Food Qual Preference. 2021:91. [Google Scholar]
- 38.Thavornwatthanayong W., Geesitthisomboon W., Jansuriyakul W., Srinuanrod K., Janpen S. Survey of leftover drugs and drugs use behavior among patients with chronic disease in Nonng Pak Long, Muang, Nakhon Pathom. J Health Sci. 2012;21:1140–1148. [Google Scholar]
- 39.Azad A.K., Ansary M., Akhtaruzzaman M., Al-Mamun S., Uddin M., Rahman M. Disposal practice for unused medications among the students of the International Islamic University Malaysia. J Appl Pharm Sci. 2012;02:101–106. [Google Scholar]
- 40.Jesson J., Pocock R., Wilson K. Reducing medicines waste in the community. Prim Health Care Res Dev. 2005;6(2):117–124. [Google Scholar]
- 41.Thammawut W., Luewittawat P. A survey of the quantity and value of leftover medicines in outpatients, Department of Internal Medicine Siriraj Hospital. Siriraj Bull. 2014;7(1):20–25. https://he02.tci-thaijo.org/index.php/simedbull/article/view/81545 Available at: [Google Scholar]
- 42.Baldoni S., Amenta F., Ricci G. Telepharmacy services: present status and future perspectives: a review. Medicina (Kaunas) 2019;55(7):327. doi: 10.3390/medicina55070327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omboni S., Tenti M. Telepharmacy for the management of cardiovascular patients in the community. Trends Cardiovasc Med. 2019;29(2):109–117. doi: 10.1016/j.tcm.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Poudel A., Nissen L.M. Telepharmacy: a pharmacist's perspective on the clinical benefits and challenges. Integr Pharm Res Pract. 2016;5:75–82. doi: 10.2147/IPRP.S101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ameri A., Salmanizadeh F., Keshvardoost S., Bahaadinbeigy K. Investigating pharmacists’ views on Telepharmacy: prioritizing key relationships, barriers, and benefits. J Pharm Technol. 2020;36(5):171–178. doi: 10.1177/8755122520931442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole S.L., Grubbs J.H., Din C., Nesbitt T.S. Rural inpatient telepharmacy consultation demonstration for after-hours medication review. Telemed. J. E Health. 2012;18(7):530–537. doi: 10.1089/tmj.2011.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unni E.J., Patel K., Beazer I.R., Hung M. Telepharmacy during COVID-19: a scoping review. Pharmacy (Basel) 2021;9(4):183. doi: 10.3390/pharmacy9040183. [DOI] [PMC free article] [PubMed] [Google Scholar]