Abstract
BACKGROUND
Metabolic syndrome (MetS) has been reported as a risk factor of atrial fibrillation (AF) recurrence after radiofrequency catheter ablation. This study aimed to investigate the long-term influence of MetS on paroxysmal AF recurrence after a single cryoballoon ablation procedure, which was scarcely investigated yet in Chinese population.
METHODS
In total, 137 paroxysmal AF patients who had successfully completed a single cryoballoon ablation procedure at Fuwai Hospital, Beijing, China from December 2013 to October 2015 were enrolled. Excepting for patients with AF recurrence, all patients were followed up for no less than five years. Independent predictors of AF recurrence were determined by Cox proportional hazards regression analysis.
RESULTS
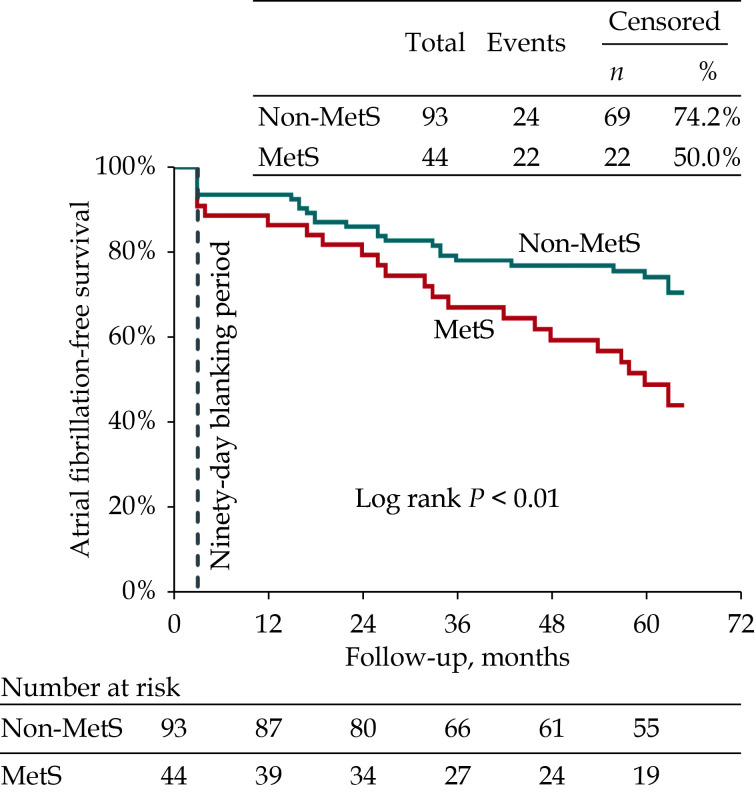
Among 137 paroxysmal AF patients, 91 patients (66.4%) had successfully achieved overall five-year follow-up after a single cryoballoon ablation procedure, and 44 patients (32.1%) had MetS. Patients with MetS had a significant lower incidence of freedom from AF recurrence than those without MetS (50.0% vs. 74.2%, log-rank P < 0.01) during the five-year follow-up. MetS (HR = 1.95, 95% CI: 1.069−3.551, P = 0.030) was an independent predictor of AF recurrence after adjusting for multiple factors. After the second year post cryoballoon ablation procedure, the recurrence rate of AF gradually increased in patients with MetS, in contrast, decreased recurrence rate of AF in patients without MetS.
CONCLUSIONS
MetS is an independent predictor for five-year AF recurrence after a single cryoballoon ablation procedure in paroxysmal AF patients. Combination therapy of AF and MetS may improve the long-term outcomes of AF patients.
Metabolic syndrome (MetS) is a pathological state characterized by a cluster of cardiovascular risk factors including obesity, hypertension, diabetes mellitus and dyslipidemia, which are also associated with the development and pathogenesis of atrial fibrillation (AF).[1,2] The prevalence of MetS and AF is increasing worldwide and significantly contributes to the high morbidity and mortality of cardiovascular diseases. Thus, MetS and AF have become major clinical and public health challenges.[3,4]
Pulmonary vein (PV) isolation (PVI) is the cornerstone for the ablation of AF.[5] PVI via cryoballoon ablation has been proven to be non-inferior to radiofrequency catheter ablation (RFCA) in terms of both efficacy and safety in treating patients with symptomatic drug-refractory paroxysmal AF.[6] However, cryoballoon ablation offers many potential advantages over RFCA, such as short procedure time and learning curve.[7] A few studies have reported that MetS increases risk of AF recurrence after RFCA.[8–11] However, little is known about the influence of MetS on the AF recurrence after cryoballoon ablation, especially the long-term outcomes among Chinese population. Our aim was to investigate the long-term influence of MetS on paroxysmal AF recurrence after a single cryoballoon ablation procedure based on a five-year follow-up study in Chinese patients.
METHODS
Study Population
From December 2013 to October 2015, consecutive patients with documented symptomatic and drug-refractory (failure of ≥ 1 class I or III antiarrhythmic drugs) paroxysmal AF who underwent PVI using the cryoballoon ablation at Fuwai Hospital were included. According to the guideline, the AF episodes terminated spontaneously or cardioverted within seven days were defined as paroxysmal AF.[5] Exclusion criteria: (1) patients with heart valve disease; (2) patients with any contraindications for PVI procedure, including the presence of acute thrombosis or severe bleeding within eight weeks before procedure; (3) patients with previous AF ablation; (4) patients with left atrium diameter (LAD) ≥ 50 mm; and (5) patients aged < 18 years. All enrolled patients had given written consent for data collection and publication. This study was carried out in compliance with the Helsinki Declaration and was approved by the Fuwai Hospital Ethics Committee (No.2015-670).
Definition of MetS
The MetS was assessed at admission using the Chinese Medical Association Diabetes Branch (CMADB) and the National Cholesterol Education Program Third Adult Treatment Panel (NCEP-ATPIII) criteria.[12,13] MetS was diagnosed if at least three of the following characteristics were presented: (1) overweight/obesity: body mass index (BMI) ≥ 25 kg/m2; (2) high blood pressure (BP): BP ≥ 130/85 mmHg or treatment for hypertension; (3) high blood glucose: fasting blood glucose ≥ 5.6 mmol/L or a clinical history of diabetes mellitus; (4) hypertriglyceridemia: fasting triglycerides ≥ 1.69 mmol/L; and (5) low high-density lipoprotein cholesterol (HDL-C): fasting level of HDL-C < 1.03 mmol/L in males and < 1.29 mmol/L in females.
Pre-ablation Management
All patients gave written informed consent for the procedure. Cardiac structure and functions, including LAD and left ventricular ejection fraction (LVEF), were evaluated by routine transthoracic echocardiography examination. Anatomic structure of PV and left atrium were assessed by transesophageal echocardiography and multi-detector computed tomographic scan before cryoballoon ablation procedure. All patients were given anticoagulation treatment by oral administration of vitamin K antagonists for at least four weeks to maintain the international normalized ratio between 2.0 and 3.0, and were substituted with subcutaneous injection of heparin three days prior to cryoballoon ablation procedure till the early morning of the operation day. Antiarrhythmic drugs (AADs) were also discontinued three days before ablation except β-blockers.
Cryoballoon Ablation Procedure
The cryoballoon ablation procedure was performed as our previously reported.[14] Briefly, the procedure was performed under local anesthesia and deep sedation using midazolam and fentanyl. A decapolar diagnostic catheter (C. R. Bard Inc., NY, USA or St. Jude Medical Inc., MN, USA) and a bipolar catheter (St. Jude Medical Inc., MN, USA) were placed into coronary sinus and right ventricular apex, respectively. After a single transseptal puncture by BRK-1 needle and standard 8.5-Fr SL1 long sheath (Synaptic Medical Inc., Beijing, China), heparin (100 IU/kg) was injected through peripheral vein, and then activated clotting time was measured every 30 min to maintain it for 200−300 s by supplementing heparin. The anatomy and caliber of PVs were confirmed by selective PV angiography, and the sizes of cryoballoon (Arctic Front, Medtronic Inc., MN, USA) and circular mapping catheter (Achieve, Medtronic, CA, USA) were selected accordingly. If the maximum diameter of three or all PVs were less than 22 mm, a 23-mm cryoballoon and a 15-mm circumferential mapping catheter (CMC) were selected. Otherwise, a 28-mm cryoballoon and a 20-mm CMC were used. After that, the transseptal sheath was exchanged with a 15-Fr steerable sheath (FlexCath, Medtronic Inc., MN, USA) by a stiff guidewire. The cryoballoon with CMC was advanced into each PV ostium, and PV potentials were recorded by CMC. After achieving complete blockage of PV ostium indicated by the contrast retention in PV, the PVs were frozen for 240 s, additional application of freezing was delivered to achieve PVI if necessary. To prevent phrenic nerve injury during ablation of right-sided PVs, the bipolar catheter was positioned into superior vena cava for constant phrenic nerve pacing (10 mA, 2 ms, 50 beats/min). Ablation procedure was immediately ceased upon weakening of diaphragmatic motion.[15] The procedural endpoint was set as bilateral electric isolation of all PVs. If procedural endpoint could not be achieved with cryoballoon ablation alone, touch up ablations with conventional radiofrequency catheter were performed to achieve bilateral isolation of PVs.
Post-ablation Management and Follow-up
Transthoracic echocardiography was performed within 3 h after the completion of ablation procedure for all patients to rule out pericardial effusion. Oral administration of anticoagulation agent was re-initiated 48–72 h after ablation procedure and continued for three months, and its subsequent need was determined by individual CHA2DS2-VASc score.[5] The AADs were prescribed for all patients throughout the three-month postprocedural blanking period, and thereafter the AADs were terminated if the patient was free of AF recurrence. Patients were scheduled for clinic follow-up at three-month intervals within the first year after the procedure and at six-month intervals afterwards. Electrocardiogram and 24-hour Holter monitor were performed in each follow-up to detect atrial arrhythmia. Patients who had symptoms of AF recurrence were asked to immediately complete an additional outpatient visit. The AF recurrence was defined as any episode of documented atrial tachycardia, atrial flutter, and/or AF lasting at least 30 s after the three-month blanking period.
Statistical Analysis
Continuous variables are presented as mean ± SD or median (interquartile range), and categorical variables are presented as percentages. Recurrence-free survival was estimated by the Kaplan-Meier method, and the statistical difference between groups was compared by log-rank test. Independent predictors of AF recurrence were determined by Cox proportional hazards regression analysis. Two-sided P-value < 0.05 were considered statistically significant. All statistical analyses were performed with statistical software package in R software (version 3.6.0, R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Clinical Characteristics of Patients With or Without MetS
In total, 137 consecutive patients who underwent cryoballoon ablation for paroxysmal AF were included. Forty-four patients (32.1%) had MetS, presenting as overweight/obesity, high BP and high blood glucose, as well as older age. Their BMI, LAD, CHA2DS2-VASc score and HAS-BLED score were higher than those without MetS. The baseline clinical characteristics of patients with or without MetS were shown in Table 1.
Table 1. Baseline clinical characteristics of patients with or without MetS.
| Characteristics | Overall (n = 137) | Non-MetS (n = 93) | MetS (n = 44) | P-value |
| Data are presented as means ± SD or n (%). *Presented as median (interquartile range). MetS: metabolic syndrome. | ||||
| Age, yrs | 56.7 ± 11.1 | 55.3 ± 11.9 | 59.7 ± 8.88 | 0.042 |
| Female | 58 (42.3%) | 35 (37.6%) | 23 (52.3%) | 0.152 |
| History of atrial fibrillation, months | 71.0 (36.0−108)* | 66.0 (32.0−100)* | 87.5 (44.3−116)* | 0.073 |
| Previous transient ischemic attack/stroke | 16 (11.7%) | 11 (11.8%) | 5 (11.4%) | 1.000 |
| Coronary heart diseases | 17 (12.4%) | 8 (8.6%) | 9 (20.5%) | 0.091 |
| Chronic congestive heart failure | 3 (2.2%) | 2 (2.2%) | 1 (2.3%) | 1.000 |
| Peripheral vascular diseases | 32 (23.4%) | 18 (19.4%) | 14 (31.8%) | 0.163 |
| Smoking status | 39 (28.5%) | 24 (25.8%) | 15 (34.1%) | 0.423 |
| Drinking status | 19 (13.9%) | 15 (16.1%) | 4 (9.1%) | 0.396 |
| Fasting blood glucose, mmol/L | 5.54 ± 3.84 | 5.51 ± 4.51 | 5.62 ± 1.77 | 0.026 |
| Triglycerides, mmol/L | 1.80 ± 1.31 | 1.38 ± 0.53 | 2.70 ± 1.91 | < 0.001 |
| High-density lipoprotein cholesterol, mmol/L | 1.11 ± 0.30 | 1.19 ± 0.29 | 0.94 ± 0.24 | < 0.001 |
| Body mass index, kg/m2 | 24.9 ± 3.19 | 24.1 ± 2.87 | 26.6 ± 3.16 | < 0.001 |
| Overweight/obesity | 67 (48.9%) | 30 (32.3%) | 37 (84.1%) | < 0.001 |
| High blood pressure | 60 (43.8%) | 23 (24.7%) | 37 (84.1%) | < 0.001 |
| High blood glucose | 33 (24.1%) | 12 (12.9%) | 21 (47.7%) | < 0.001 |
| Hypertriglyceridemia | 54 (39.4%) | 20 (21.5%) | 34 (77.3%) | < 0.001 |
| Low high-density lipoprotein cholesterol | 42 (30.7%) | 14 (15.1%) | 28 (63.6%) | < 0.001 |
| Left atrium diameter, mm | 36.1 ± 6.34 | 34.8 ± 5.50 | 38.8 ± 7.17 | < 0.001 |
| Left ventricular end-diastolic diameter, mm | 47.8 ± 4.82 | 47.4 ± 4.70 | 48.5 ± 5.02 | 0.235 |
| Left ventricular ejection fraction, % | 65.8 ± 5.19 | 65.7 ± 4.89 | 66.2 ± 5.83 | 0.597 |
| CHA2DS2-VASc score | 1.8 ± 1.47 | 1.4 ± 1.37 | 2.6 ± 1.37 | < 0.001 |
| HAS-BLED score | 1.2 ± 0.98 | 1.0 ± 1.01 | 1.6 ± 0.82 | 0.001 |
Procedural Characteristics of Patients With or Without MetS
The procedural characteristics of patients with or without MetS were summarized in Table 2. There were 543 PVs in 137 patients, of which 8 patients had a left common PV and 9 patients had a right middle PV. Additional touch up ablations with radiofrequency catheter were performed in 25 PVs (4.6%) to achieve PVI for the reason that it was difficult to completely occlude and isolate PVs with critical angulation. There was no significant difference on the procedural characteristics between patients with or without MetS, except that a 23-mm cryoballoon was used more in patients with MetS.
Table 2. Procedural characteristics of patients with or without MetS.
| Characteristics | Overall (n = 137) | Non-MetS (n = 93) | MetS (n = 44) | P-value |
| Data are presented as means ± SD or n (%). *Presented as median (interquartile range). MetS: metabolic syndrome. | ||||
| Number of all pulmonary veins | 4 (4−4)* | 4 (4−4)* | 4 (4−4)* | 0.363 |
| Procedure time, min | 102.0 ± 23.4 | 103.0 ± 25.2 | 102.0 ± 19.4 | 0.740 |
| Fluoroscopy time, min | 36.5 ± 12.1 | 35.9 ± 13.0 | 37.7 ± 10.1 | 0.127 |
| Cryoballoon type | ||||
| 23-mm | 65 (47.4%) | 51 (54.8%) | 14 (31.8%) | 0.047 |
| 28-mm | 69 (50.4%) | 40 (43.0%) | 29 (65.9%) | 0.800 |
| 23-mm + 28-mm | 3 (2.2%) | 2 (2.2%) | 1 (2.3%) | 1.000 |
| Bi-direction isolation | ||||
| Left superior pulmonary vein | 132 (96.4%) | 88 (94.6%) | 44 (100%) | 0.176 |
| Left inferior pulmonary vein | 132 (96.4%) | 88 (94.6%) | 44 (100%) | 0.176 |
| Left common pulmonary vein (n = 8) | 7 (87.5%) | 3 (75.0%) | 4 (100%) | 1.000 |
| Right inferior pulmonary vein | 127 (92.7%) | 86 (92.5%) | 41 (93.2%) | 1.000 |
| Right middle pulmonary vein (n = 9) | 8 (88.9%) | 6 (85.7%) | 2 (100%) | 1.000 |
| Right superior pulmonary vein | 132 (96.4%) | 88 (94.6%) | 44 (100%) | 0.176 |
| Freezing cycles | ||||
| Left superior pulmonary vein | 2.3 ± 0.54 | 2.3 ± 0.53 | 2.3 ± 0.57 | 0.910 |
| Left inferior pulmonary vein | 2.3 ± 0.67 | 2.3 ± 0.72 | 2.3 ± 0.57 | 0.571 |
| Left common pulmonary vein (n = 8) | 3.8 ± 1.28 | 4.0 ± 1.41 | 3.5 ± 1.29 | 0.653 |
| Right inferior pulmonary vein | 2.3 ± 0.67 | 2.3 ± 0.65 | 2.3 ± 0.67 | 0.650 |
| Right middle pulmonary vein (n = 9) | 1.7 ± 0.72 | 1.7 ± 0.76 | 1.5 ± 0.71 | 0.873 |
| Right superior pulmonary vein | 2.2 ± 0.63 | 2.2 ± 0.62 | 2.3 ± 0.65 | 0.728 |
| Nadir temperature, °C | ||||
| Left superior pulmonary vein | 54.5 ± 6.22 | 54.1 ± 6.45 | 55.3 ± 5.67 | 0.392 |
| Left inferior pulmonary vein | 49.0 ± 5.76 | 48.6 ± 5.64 | 50.1 ± 5.95 | 0.158 |
| Left common pulmonary vein (n = 8) | 51.3 ± 3.33 | 49.5 ± 1.73 | 53.0 ± 3.83 | 0.301 |
| Right inferior pulmonary vein | 49.8 ± 7.70 | 49.4 ± 7.61 | 50.8 ± 7.90 | 0.308 |
| Right middle pulmonary vein (n = 9) | 46.2 ± 5.95 | 45.1 ± 6.31 | 50.0 ± 2.83 | 0.461 |
| Right superior pulmonary vein | 55.3 ± 6.07 | 55.2 ± 5.67 | 55.4 ± 6.91 | 0.394 |
| Mean freezing time, s | ||||
| Left superior pulmonary vein | 224 ± 36.8 | 227 ± 35.8 | 218 ± 38.6 | 0.267 |
| Left inferior pulmonary vein | 246 ± 18.0 | 248 ± 17.3 | 243 ± 19.3 | 0.241 |
| Left common pulmonary vein (n = 8) | 244 ± 30.8 | 259 ± 37.5 | 229 ± 14.4 | 0.137 |
| Right inferior pulmonary vein | 237 ± 33.0 | 236 ± 29.2 | 238 ± 40.2 | 0.951 |
| Right middle pulmonary vein (n = 9) | 236 ± 6.9 | 235 ± 7.7 | 239 ± 1.8 | 1.000 |
| Right superior pulmonary vein | 214 ± 47.0 | 217 ± 45.6 | 209 ± 50.0 | 0.341 |
Outcomes and Predictors of AF Recurrence During the Five-year Follow-up
Ninety-one patients (66.4%) had successfully achieved five-year follow-up after PVI by a single cryoballoon ablation procedure, and 46 patients had AF recurrence, including 5 patients (10.9%) of atrial tachycardia, 3 patients (6.5%) of atrial flutter and 38 patients (82.6%) of AF. The incidence of freedom from AF recurrence was significantly lower in patients with MetS compared with those without MetS (50.0% vs. 74.2%, log-rank P < 0.01; Figure 1).
Figure 1.
MetS and five-year follow-up outcomes.
Kaplan-Meier curves for five-year survival of freedom from atrial fibrillation after a single ablation procedure using cryoballoon in paroxysmal atrial fibrillation patients with MetS (red line) and without MetS (blue line). MetS: metabolic syndrome.
As showed in Table 3, univariate Cox regression analysis indicated that MetS (HR = 2.15, 95% CI: 1.207−3.841,P = 0.009), LAD (HR = 1.08, 95% CI: 1.017−1.138,P = 0.011) and LVEF (HR = 1.05, 95% CI: 1.005−1.094,P = 0.030) were significantly associated with the increased risk of AF recurrence. To further confirm the independent prediction of these variables, only variables with P < 0.1 in univariate Cox regression analysis were included for stepwise multivariate analysis. Considering the collinearity between the factors, we did not include the components of MetS into the multivariate Cox regression analysis. Multivariate Cox regression analysis showed that MetS (HR = 1.95, 95% CI: 1.069−3.551, P = 0.030) and LAD (HR = 1.07, 95% CI: 1.011−1.126,P = 0.018) were significantly and independently associated with the increased risk of AF recurrence after adjusting for LVEF and chronic heart failure (Table 3).
Table 3. Univariate and multivariate Cox regression analyse for predictors of atrial fibrillation recurrence.
| Univariate Cox analysis | Multivariate Cox analysis | ||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| CI: confidence interval; HR: hazard ratio. | |||||||
| Age, yrs | 1.02 | 0.993−1.049 | 0.137 | ||||
| Female | 1.48 | 0.831−2.647 | 0.182 | ||||
| Body mass index, kg/m2 | 1.00 | 0.915−1.092 | 0.993 | ||||
| Metabolic syndrome | 2.15 | 1.207−3.841 | 0.009 | 1.95 | 1.069−3.551 | 0.030 | |
| History of atrial fibrillation, months | 1.00 | 0.996−1.010 | 0.348 | ||||
| Previous transient ischemic attack/stroke | 1.15 | 0.513−2.564 | 0.739 | ||||
| Coronary heart diseases | 1.41 | 0.657−3.025 | 0.378 | ||||
| Peripheral vascular diseases | 1.14 | 0.591−2.207 | 0.693 | ||||
| Chronic congestive heart failure | 3.74 | 0.887−15.80 | 0.072 | 3.03 | 0.671−13.72 | 0.150 | |
| Smoking status | 1.28 | 0.690−2.371 | 0.434 | ||||
| Drinking status | 0.54 | 0.194−1.509 | 0.240 | ||||
| Left atrium diameter, mm | 1.08 | 1.017−1.138 | 0.011 | 1.07 | 1.011−1.126 | 0.018 | |
| Left ventricular end-diastolic diameter, mm | 0.96 | 0.902−1.016 | 0.151 | ||||
| Left ventricular ejection fraction, % | 1.05 | 1.005−1.094 | 0.030 | 1.03 | 0.984−1.082 | 0.199 | |
| Left common pulmonary vein | 1.30 | 0.401−4.184 | 0.665 | ||||
| Right middle pulmonary vein | 0.92 | 0.285−2.966 | 0.889 | ||||
| Cryoballoon type | |||||||
| 28-mm vs. 23-mm | 0.56 | 0.278−1.141 | 0.111 | ||||
| 23-mm + 28-mm vs. 23-mm | 0.44 | 0.060–3.258 | 0.423 | ||||
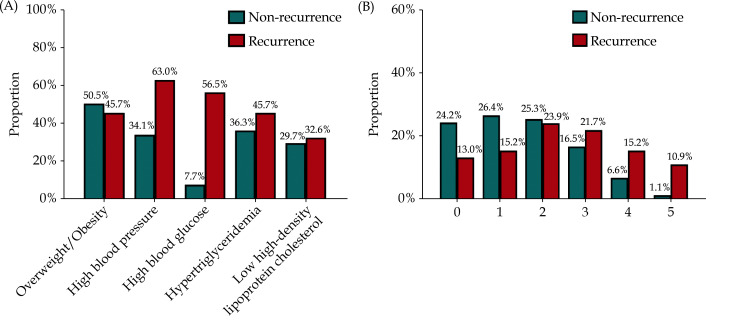
Distribution of MetS Components Among Patients with AF Recurrence Versus Non-recurrence
The distribution of MetS components among patients with AF recurrence versus non-recurrence was shown in Figure 2. The respective rates of overweight/obesity, high BP, high blood glucose, hypertriglyceridemia and low HDL-C were 45.7%, 60.3%, 56.5%, 45.7% and 32.6% in patients with AF recurrence, in contrast to 50.5%, 34.1%, 7.7%, 36.3% and 29.7% in patients without AF recurrence (Figure 2A). The respective rates of 0, 1, 2, 3, 4 and 5 components of MetS were 13.0%, 15.2%, 23.9%, 21.7%, 15.2% and 10.9% in patients with AF recurrence, in contrast to 24.2%, 26.4%, 25.3%, 16.5%, 6.6% and 1.1% in patients without AF recurrence (Figure 2B).
Figure 2.
Distribution of MetS components.
The rate of MetS components among patients with atrial fibrillation recurrence versus non-recurrence (A), and the rate of 0 to 5 MetS components among patients with atrial fibrillation recurrence versus non-recurrence (B). MetS: metabolic syndrome.
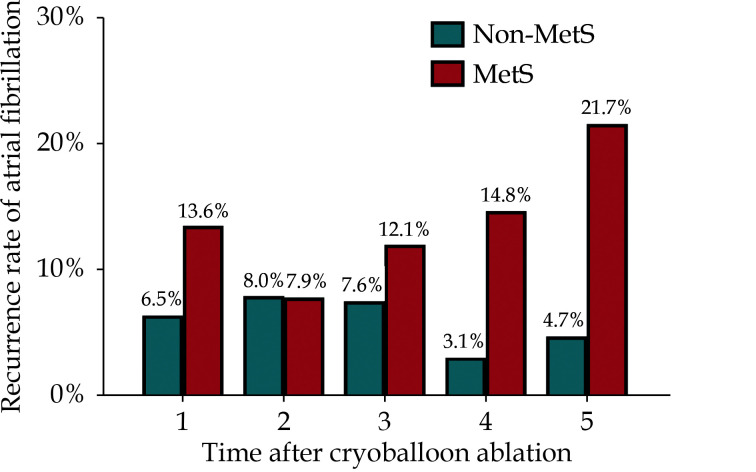
Recurrence Rate of AF at Different Time-point During the Five-year Follow-up
The recurrence rate of AF at different time-point during the five-year follow-up was shown in Figure 3. AF recurrences were evaluated at one-year interval. Patients with MetS had an overall higher recurrence rate of AF than those without MetS at each evaluation time-point. In details, the AF recurrence rate was decreased in the second year in comparison with that in the first year in patients with MetS, however, it was gradually reached the peak of AF recurrence rate (21.7%) in the fifth year. By contrast, the highest recurrences rate of AF was 8.0% in the second year in patients without MetS, and it was decreased into the lowest rate (3.1%) in the fourth year and then slightly increased to 4.8% in the fifth year.
Figure 3.
MetS and time of atrial fibrillation recurrences.
The histogram shows the recurrences rate of atrial fibrillation at one-year interval during the five-year follow-up after a single cryoballoon ablation procedure in paroxysmal atrial fibrillation patients with MetS (red column) and without MetS (blue column). MetS: metabolic syndrome.
DISCUSSION
Main Findings
To our best knowledge, the present study firstly reported a five-year influence of MetS on AF recurrence after a single cryoballoon ablation procedure in a cohort of Chinese patients with paroxysmal AF. The major results were as follows: (1) the prevalence of MetS in enrolled patients was 32.1%; (2) patients with MetS had a significantly lower incidence of freedom from AF recurrence than those without MetS after a single cryoballoon ablation procedure; (3) MetS served as a significant and independent predictor of AF recurrence; and (4) the recurrence rate of AF in patients with MetS was gradually increased after the second year post cryoballoon ablation, while that in patients without MetS was decreased.
Prevalence of MetS in Patients With AF
MetS and AF usually coexist. As patients with MetS predisposes to develop AF, the prevalence of MetS in patients with AF is likely higher than that in general population.[1,2] A few studies have reported the prevalence of MetS in patients with AF was 18.8%−49.9% after RFCA, similar to 32.1% in the present study in patients with Mets after cryoballoon ablation for paroxysmal AF.[8–11] However, data on the prevalence of MetS in patients with AF remains unknown. It should be mentioned that the definition of MetS in present study was a combined criterion of the CMADB and NCEP-ATPIII criteria, which are more applicable to the Chinese population.[16] Although BMI ≥ 25 kg/m2 rather than waist circumference measurement alone was used, the prevalence of MetS in present cohort was comparable with that in studies with similar demographic and clinical profiles.[8–11] This might be attributed to that BMI ≥ 25 kg/m2 has a well correlation with waist circumference measurement for the definition of obesity, which had been validated previously.[17,18]
Influence of MetS on Five-year AF Recurrence
Although several studies have reported the five-year outcomes of AF recurrence after the cryoballoon ablation, the long-term influence of MetS on AF recurrence has not been investigated yet. Disorders included in MetS have been proven to be associated with the development and progression of AF.[1,2] In addition, previous studies also have reported that MetS is an independent predictor of AF recurrence post PVI.[8–11,19,20] Based on the outcome of RFCA in Chinese patients, Tang, et al.[11] have reported that MetS (HR = 1.64, 95% CI: 1.07−2.49,P = 0.022) was an independent predictor of AF recurrence after a mean follow-up of 470 ± 323 days. A meta-analysis of 12,924 AF patients from 23 studies demonstrated that MetS was associated with an increase of AF recurrence after RFCA (pooled RR = 1.63, 95% CI: 1.25−2.12).[20] However, these studies were mainly based on a relatively short follow-up period within one year after RFCA. By a long-term follow-up of five years, we found that the recurrence rate in patients with MetS was nearly twice that in patients without MetS after the cryoballoon ablation of paroxysmal AF.
In present study, AF patients with MetS exhibited a larger LAD than those without MetS (38.8 ± 7.17 mm vs. 34.8 ± 5.50 mm, P < 0.001). Moreover, we found that LAD (HR = 1.07, 95% CI: 1.011−1.126, P = 0.018) was also an independent predictor of AF recurrence during the five-year follow-up, similar to previous reports that different indictors based on LAD were independent predictors for AF recurrence.[21,22] Metabolic and hemodynamic variations associated with MetS can cause structural changes in the atria, manifesting as the enlargement of the left atrium and development of interstitial atrial fibrosis. In addition, components of MetS, such as long-term hypertension, increase the expression of angiotensin II by activating the rennin-angiotensin-aldosterone system, which further affect the functions of the atrial calcium channel and intercellular connection, resulting in atrial electrical remodeling.[23,24] The structure and electrical remodeling of atria could increase the non-uniform anisotropy, promote the conduction disturbance and shorten the refractory period, which potentially produce extra ectopic trigger foci and reentrant circuits.[8,25] Consequently, MetS could promote the increasing rate of AF recurrence by modulating electrophysiological characteristics of the atrial substrate.
Interestingly, we observed a contrary trend of AF recurrence rate after the second year post cryoballoon ablation between patients with or without MetS, it gradually increased in patients with MetS but decreased in patients without MetS. A previous study has showed similar phenomenon that MetS was associated with the delayed AF recurrence two years after RFCA.[8] All components of MetS are closely associated with oxidative stress and inflammation, which persistently affect the cardiovascular system and result in atrial electrical and structural remodeling.[26] In fact, previous studies reported that PV reconnection is the main mechanism for late AF recurrence (less than one year after the ablation),[8,27] however, very late AF recurrence is mainly associated with non-PV foci and atrial remodeling.[8,27,28] As discussed above, MetS promotes AF recurrence by producing extra ectopic trigger foci and reentrant circuits, we thus speculated that the gradually increased incidence of AF recurrence in patients with MetS is attributed to the atrial remodeling by MetS. By contrast, in patients without MetS experienced AF recurrence could be mainly owed to PV reconnection.
Clinical Implications
The present study gave a deeper look at the relationship between MetS and AF recurrence after ablation. MetS was found to be an independent risk factor for AF recurrence within five years after a single cryoballoon ablation procedure. In addition, patients with MetS experienced growing incidence of AF recurrence after the second year post cryoballoon ablation, while those without MetS experienced a contrary trend. Our findings suggested that treatment of metabolic disorders should be included in AF management after a single cryoballoon ablation procedure to improve long-term clinical outcomes. Furthermore, aggressive life-style modification is essential as well to successfully improve the long-term of cryoballoon ablation for AF.[19,29]
LIMITATIONS
There are several mentionable limitations of this retrospective research. Firstly, this study is a single-center investigation and has a relatively small sample, thus the resultant data require further validation in larger population. Secondly, our follow-up was based on intermittent rhythm monitoring via 24-hour Holter monitor or serial electrocardiogram recordings rather than intensive monitoring via continuous transtelephonic monitoring or implantable loop recorder, therefore, some non-sustained or asymptomatic episodes of AF, which could underestimate the recurrence rate, could not be diagnosed.[30] Thirdly, mechanisms linking MetS to the higher AF recurrence rate after a single cryoballoon ablation procedure remains to be investigated. Last but not least, the present study only analyzed the impact of preoperative MetS on AF recurrence after a single cryoballoon ablation procedure. Whether the intervention of MetS improves the long-term outcomes of cryoballoon ablation for AF also need to be further investigated.
CONCLUSIONS
In a Chinese cohort of AF patients, MetS is a risk factor and an independent predictor for the five-year AF recurrence after a single cryoballoon ablation procedure, and it is potentially associated with the increase of AF recurrence after the second year post cryoballoon ablation. Combination therapy of Mets and AF may improve the long-term outcomes of AF patients.
ACKNOWLEDGMENTS
All authors had no conflicts of interest to disclose.
References
- 1.Kwon CH, Kim H, Kim SH, et al The impact of metabolic syndrome on the incidence of atrial fibrillation: a nationwide longitudinal cohort study in South Korea. J Clin Med. 2019;8:1095. doi: 10.3390/jcm8081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choe WS, Choi EK, Han KD, et al Association of metabolic syndrome and chronic kidney disease with atrial fibrillation: a nationwide population-based study in Korea. Diabetes Res Clin Pract. 2019;148:14–22. doi: 10.1016/j.diabres.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Decker JJ, Norby FL, Rooney MR, et al Metabolic syndrome and risk of ischemic stroke in atrial fibrillation: ARIC study. Stroke. 2019;50:3045–3050. doi: 10.1161/STROKEAHA.119.025376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polovina M, Hindricks G, Maggioni A, et al Association of metabolic syndrome with non-thromboembolic adverse cardiac outcomes in patients with atrial fibrillation. Eur Heart J. 2018;39:4030–4039. doi: 10.1093/eurheartj/ehy446. [DOI] [PubMed] [Google Scholar]
- 5.Hindricks G, Potpara T, Dagres N, et al 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 6.Kuck KH, Brugada J, Fürnkranz A, et al Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 7.Reissmann B, Metzner A, Kuck KH Cryoballoon ablation versus radiofrequency ablation for atrial fibrillation. Trends Cardiovasc Med. 2017;27:271–277. doi: 10.1016/j.tcm.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Baek YS, Yang PS, Kim TH, et al Delayed recurrence of atrial fibrillation 2 years after catheter ablation is associated with metabolic syndrome. Int J Cardiol. 2016;223:276–281. doi: 10.1016/j.ijcard.2016.08.222. [DOI] [PubMed] [Google Scholar]
- 9.Mohanty S, Mohanty P, Di Biase L, et al Impact of metabolic syndrome on procedural outcomes in patients with atrial fibrillation undergoing catheter ablation. J Am Coll Cardiol. 2012;59:1295–1301. doi: 10.1016/j.jacc.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 10.Berkowitsch A, Kuniss M, Greiss H, et al Impact of impaired renal function and metabolic syndrome on the recurrence of atrial fibrillation after catheter ablation: a long-term follow-up. Pacing Clin Electrophysiol. 2012;35:532–543. doi: 10.1111/j.1540-8159.2012.03350.x. [DOI] [PubMed] [Google Scholar]
- 11.Tang RB, Dong JZ, Liu XP, et al Metabolic syndrome and risk of recurrence of atrial fibrillation after catheter ablation. Circ J. 2009;73:438–443. doi: 10.1253/circj.CJ-08-0832. [DOI] [PubMed] [Google Scholar]
- 12.Lu YH, Lu JM, Wang SY, et al [Comparison of the diagnostic criteria of metabolic syndrome by International Diabetes Federation and that by Chinese Medical Association Diabetes Branch] Zhonghua Yi Xue Za Zhi. 2006;86:386–389. doi: 10.3760/j:issn:0376-2491.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 13.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. doi: 10.1161/circ.106.25.3143. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Fang P, Liu Z, et al Pulmonary vein anatomy is associated with cryo kinetics during cryoballoon ablation for atrial fibrillation. Arq Bras Cardiol. 2018;110:440–448. doi: 10.5935/abc.20180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mondésert B, Andrade JG, Khairy P, et al Clinical experience with a novel electromyographic approach to preventing phrenic nerve injury during cryoballoon ablation in atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:605–611. doi: 10.1161/CIRCEP.113.001238. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Grundy SM, Wang W, et al Ethnic-specific criteria for the metabolic syndrome: evidence from China. Diabetes Care. 2006;29:1414–1416. doi: 10.2337/dc06-0481. [DOI] [PubMed] [Google Scholar]
- 17.Sundström J, Risérus U, Byberg L, et al Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ. 2006;332:878–882. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen I, Katzmarzyk PT, Ross R Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002;162:2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 19.Mohanty S, Mohanty P, DI Biase L, et al Long-term outcome of catheter ablation in atrial fibrillation patients with coexistent metabolic syndrome and obstructive sleep apnea: impact of repeat procedures versus lifestyle changes. J Cardiovasc Electrophysiol. 2014;25:930–938. doi: 10.1111/jce.12468. [DOI] [PubMed] [Google Scholar]
- 20.Lin KJ, Cho SI, Tiwari N, et al Impact of metabolic syndrome on the risk of atrial fibrillation recurrence after catheter ablation: systematic review and meta-analysis. J Interv Card Electrophysiol. 2014;39:211–223. doi: 10.1007/s10840-013-9863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akkaya E, Berkowitsch A, Zaltsberg S, et al Five-year outcome and predictors of success after second-generation cryoballoon ablation for treatment of symptomatic atrial fibrillation. Int J Cardiol. 2018;266:106–111. doi: 10.1016/j.ijcard.2018.03.069. [DOI] [PubMed] [Google Scholar]
- 22.Neumann T, Wójcik M, Berkowitsch A, et al Cryoballoon ablation of paroxysmal atrial fibrillation: five-year outcome after single procedure and predictors of success. Europace. 2013;15:1143–1149. doi: 10.1093/europace/eut021. [DOI] [PubMed] [Google Scholar]
- 23.Harada M, Tadevosyan A, Qi X, et al Atrial fibrillation activates AMP-dependent protein kinase and its regulation of cellular calcium handling: potential role in metabolic adaptation and prevention of progression. J Am Coll Cardiol. 2015;66:47–58. doi: 10.1016/j.jacc.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 24.Nattel S, Dobrev D The multidimensional role of calcium in atrial fibrillation pathophysiology: mechanistic insights and therapeutic opportunities. Eur Heart J. 2012;33:1870–1877. doi: 10.1093/eurheartj/ehs079. [DOI] [PubMed] [Google Scholar]
- 25.Hakim JB, Murphy MJ, Trayanova NA, et al Arrhythmia dynamics in computational models of the atria following virtual ablation of re-entrant drivers. Europace. 2018;20:iii45–iii54. doi: 10.1093/europace/euy234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell DSH, Goncalves E Atrial fibrillation and type 2 diabetes: prevalence, etiology, pathophysiology and effect of anti-diabetic therapies. Diabetes Obes Metab. 2019;21:210–217. doi: 10.1111/dom.13512. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh MH, Tai CT, Lee SH, et al The different mechanisms between late and very late recurrences of atrial fibrillation in patients undergoing a repeated catheter ablation. J Cardiovasc Electrophysiol. 2006;17:231–235. doi: 10.1111/j.1540-8167.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 28.Sotomi Y, Inoue K, Ito N, et al Incidence and risk factors for very late recurrence of atrial fibrillation after radiofrequency catheter ablation. Europace. 2013;15:1581–1586. doi: 10.1093/europace/eut076. [DOI] [PubMed] [Google Scholar]
- 29.Pathak RK, Middeldorp ME, Meredith M, et al Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY) J Am Coll Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Pieragnoli P, Paoletti Perini A, Ricciardi G, et al Recurrences in the blanking period and 12-month success rate by continuous cardiac monitoring after cryoablation of paroxysmal and non-paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28:625–633. doi: 10.1111/jce.13190. [DOI] [PubMed] [Google Scholar]