Abstract
BACKGROUND
Lipoprotein(a) [Lp(a)] has been closely related to coronary atherosclerosis and might affect perivascular inflammation due to its proinflammatory properties. However, there are limited data about Lp(a) and related perivascular inflammation on coronary atheroma progression. Therefore, this study aimed to investigate the associations between Lp(a) and the perivascular fat attenuation index (FAI) with coronary atheroma progression detected by coronary computed tomography angiography (CCTA).
METHODS
Patients who underwent serial CCTA examinations without a history of revascularization and with available data for Lp(a) within one month before or after baseline and follow-up CCTA imaging scans were considered to be included. CCTA quantitative analyses were performed to obtain the total plaque volume (TPV) and the perivascular FAI. Coronary plaque progression (PP) was defined as a ≥ 10% increase in the change of the TPV at the patient level or the presence of new-onset coronary atheroma lesions. The associations between Lp(a) or the perivascular FAI with PP were examined by multivariate logistic regression.
RESULTS
A total of 116 patients were ultimately enrolled in the present study with a mean CCTA interscan interval of 30.80 ± 13.50 months. Among the 116 patients (mean age: 53.49 ± 10.21 years, males: 83.6%), 32 patients presented PP during the follow-up interval. Lp(a) levels were significantly higher among PP patients than those among non-PP patients at both baseline [15.80 (9.09−33.60) mg/dLvs. 10.50 (4.75−19.71) mg/dL,P = 0.029] and follow-up [20.60 (10.45−34.55) mg/dLvs. 8.77 (5.00−18.78) mg/dL,P = 0.004]. However, there were no differences in the perivascular FAI between PP group and non-PP group at either baseline or follow-up. Multivariate logistic regression analysis showed that elevated baseline Lp(a) level (OR = 1.031, 95% CI: 1.005−1.058,P = 0.019) was an independent risk factor for PP after adjustment for other conventional variables.
CONCLUSIONS
Lp(a) was independently associated with coronary atheroma progression beyond low-density lipoprotein cholesterol and other conventional risk factors. Further studies are warranted to identify the inflammation effect exhibited as the perivascular FAI on coronary atheroma progression.
A considerable proportion of patients still suffer from coronary atheroma progression although they have received standard of care (SOC) therapy.[1,2] Therefore, residual risk factors rather than the conventional ones would be associated with the likelihood of concerning alteration. As a special kind of plasma lipoproteins, lipoprotein(a) [Lp(a)] is considered a well-recognized unconventional independent risk factor for atherosclerotic cardiovascular disease (ASCVD).
Lp(a) is a low-density lipoprotein particle with an apolipoprotein(a) moiety covalently bound to its apolipoprotein B component and exerts proatherogenic, prothrombotic and proinflammatory effects.[3] In addition, Lp(a) also preferentially binds oxidized phospholipids (OxPLs) in comparison with other lipoproteins, leading to increased arterial inflammation and promoting its own proatherogenic properties.[4,5] In view of Lp(a) contributing to ASCVD via multiple mechanisms, its importance as a potential residual risk factor could not be ignored.
To monitor the disease evolution process of coronary atheroma, coronary computed tomography angiography (CCTA) allows comprehensive evaluation in both qualitative and quantitative aspects as a widely used noninvasive imaging modality. Furthermore, the perivascular fat attenuation index (FAI) has emerged as a novel imaging biomarker quantified by CCTA one-stop evaluation for coronary arterial inflammation in recent years.[6] However, few previous studies have elucidated the effects of Lp(a) and related arterial inflammation on coronary atheroma progression detected by CCTA.
In this study, we hypothesized that elevated Lp(a) would contribute to coronary atheroma progression independent of low-density lipoprotein cholesterol (LDL-C) and attempted to investigate whether the perivascular FAI might participate in the corresponding process.
METHODS
Study Population
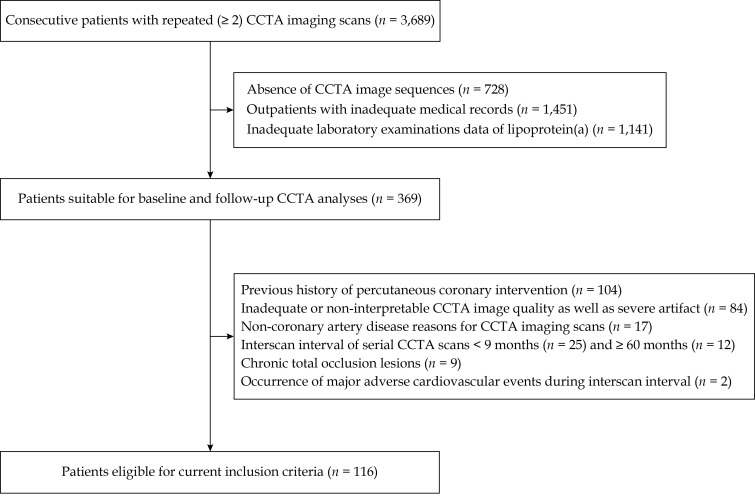
Briefly, this study was a single-center, retrospective, observational study performed at Chinese PLA General Hospital, Beijing, China. We searched the Picture Archiving and Communication System (CV-NET System, Crealife, Beijing, China) for 3,689 subjects who underwent serial CCTA scans from November 2011 to December 2019. In the case of patients who underwent more than three CCTA scans, the first and last examinations were selected. Notably, patients who experienced major adverse cardiovascular events (MACEs) during the interscan interval were not omitted. Finally, 116 patients with available data for demographics, clinical characteristics and laboratory examination profiles [especially for plasma Lp(a)] collected within one month before or after baseline and follow-up CCTA imaging scans were enrolled (Figure 1).
Figure 1.
Study flow diagram.
CCTA: coronary computed tomography angiography.
For this analysis, the exclusion criteria were as follows: (1) absence of clinical data at either baseline or follow-up CCTA imaging scans; (2) a previous history of percutaneous coronary intervention or coronary artery bypass grafting surgery; (3) CCTA scans performed for non-coronary artery disease (CAD) reasons; (4) chronic total occlusion lesions occurring at any segments of the main coronary arteries; (5) inadequate CCTA image quality failed to meet a Likert scale ≥ 3 or severe artifact leaded to nonvaluable qualitative and quantitative analyses at either baseline or follow-up CCTA imaging scans; and (6) an interscan interval for serial CCTA scans < 9 months or ≥ 60 months.
The study was approved by the Ethics Committees of Chinese PLA General Hospital (No.S2020-255-01) and was conducted according to the guidelines of the declaration of Helsinki Declaration. All participants signed written informed consents prior to their CCTA scans.
Definition of ASCVD Risk Stratification and LDL-C Treatment Goals for Lipid Lowering
To assess the ten-year overall ASCVD risk and clarify LDL-C lowering treatment goals, we used a proven risk estimation and stratification chart on the basis of traditional ASCVD risk factors in accordance with the 2016 guidelines for the management of dyslipidaemias in Chinese adults.[7] Patients once diagnosed with ASCVD were stratified as very high risk directly. For other subjects, estimations of their ten-year overall ASCVD risk were classified as high risk (≥ 10%), moderate risk (5%−9%), and low risk (< 5%) separately based on clinical disease status, lipoprotein cholesterol levels and other traditional risk factors. Recommendations about LDL-C treatment goals for lipid lowering varied from ASCVD risk stratification.[7] For patients at very high ASCVD risk, the goal was LDL-C levels < 1.8 mmol/L. For subjects at high ASCVD risk or moderate/low ASCVD risk, the goals were LDL-C levels < 2.6 mmol/L or < 3.4 mmol/L, respectively. It should be emphasized that a 50% LDL-C levels reduction from baseline was also considered an alternative goal.
CCTA Imaging Scan Protocol
Baseline and follow-up CCTA imaging scans were acquired applying the same dual-source CT scanner (Definition Flash, Siemens Healthcare, Forchheim, Germany) for every patient. Data acquisition was performed with a detector collimation of 2 mm × 64 mm × 0.6 mm, z-axis flying focus technique and gantry rotation of 280 ms. Patients with body mass index (BMI) ≥ 25 kg/m2 were examined with a tube voltage of 120 kVp, whereas those with BMI < 25 kg/m 2 were examined with a tube voltage of 100 kVp. Based on heart rate, different scanning protocols, such as prospective electrocardiogram-triggered high-pitch spiral double scans, step-on sequential scans, or retrospective spiral scans, were allocated to different patients as appropriate.[8] Subjective image quality was evaluated using a five-point Likert scale: (1) nondiagnostic with intense noise and artifact; (2) limited diagnostic value with noise and artifact; (3) diagnostic with moderate image quality; (4) diagnostic with good quality with minimal noise and artifact; and (5) diagnostic with excellent image quality.
Qualitative CCTA Analysis
Two cardiologists with at least three years of CCTA assessment experience who were blinded to the enrolled patients’ clinical information majored in qualitative and quantitative CCTA analyses independently. All datasets were sent to an associated workstation (Syngo.via VB10B, Siemens Healthcare, Forchheim, Germany) for generating regular interpretation formats, such as transaxial images, multiplanar reformation, maximum intensity projection, curved multiplanar reformation and volume-rendering technique.[9] Contrast enhanced images were reconstructed with a B26f kernel, slice thickness of 0.75 mm, and an increment of 0.5 mm for visualization of native arteries.[10]
According to the Coronary Artery Disease-Reporting and Data System (CAD-RADS), classification degree scales were defined as follows: (1) no visible stenosis (0%); (2) minimal stenosis (1%−24%); (3) mild stenosis (25%−49%); (4) moderate stenosis (50%−69%); (5) severe stenosis (70%−99%); and (6) occluded (100%).[11] All vessels > 1.5 mm in diameter using a modified 17-segment American Heart Association model were graded for stenosis severity by visual estimation, and CAD-RADS classifications were applied at the patient level for the most clinically relevant stenosis. [9]
Quantitative CCTA Analysis
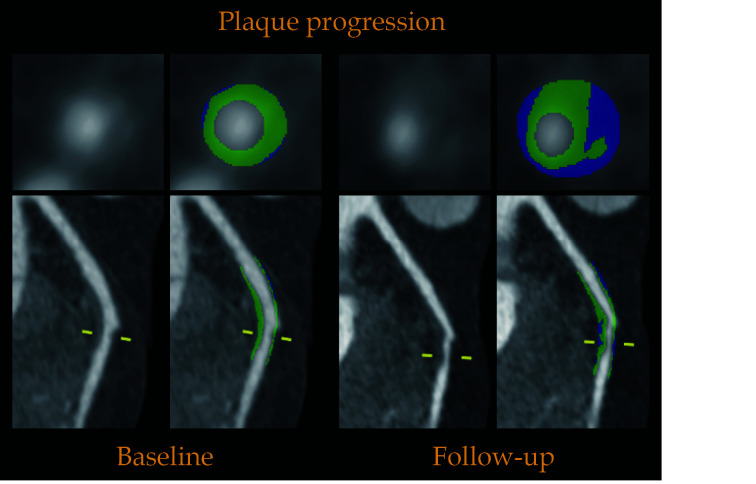
Subsequently, all datasets were transferred to authorized software (Syngo.via Frontier Coronary Plaque Analysis, version 4.2.1, Siemens Healthcare, Forchheim, Germany) for semi-automated plaque quantification analysis. We have accumulated sufficient quantitative analysis experience, as described in our previous article.[12] An isolated coronary plaque was defined as any tissue ≥ 1 mm3 within or adjacent to the lumen that could be discriminated from the surrounding structures and identified in more than two planes.[13] For tracing and comparing longitudinal CCTA images, baseline and follow-up coronary lesions were matched with fiduciary landmarks (e.g., side branches, distance from the ostium) and analyzed side-by-side.[14] Through manual correction of the inner vessel wall and outer vessel wall of definite coronary lesions from the curved multiplanar reformation and cross-sectional view, the main plaque component volumes were accessed accurately within the defined Hounsfield unit (HU) range. The total plaque volume (TPV) was calculated as the sum of all analyzed segments to generate a patient level quantitative analysis volume. Coronary plaque progression (PP) was defined as the follow-up TPV that was increased ≥ 10% compared to the baseline at the patient level or the presence of new-onset coronary atheroma lesions.[15] A typical case for semi-automated coronary plaque quantification analysis is depicted in Figure 2.
Figure 2.
Example for semi-automated coronary plaque quantification analysis on coronary computed tomography angiography.
Above case demonstrates an example for plaque quantification of the same patient in April 2014 and March 2016, respectively. Cross-sectional and curved multiplanar reformation views show a representative non-calcified plaque in the middle left anterior descending artery. The total plaque volume increased from 282.69 mm3 to 396.11 mm3 during interscan interval with an increase of 40.12%.
CCTA Perivascular FAI Analysis
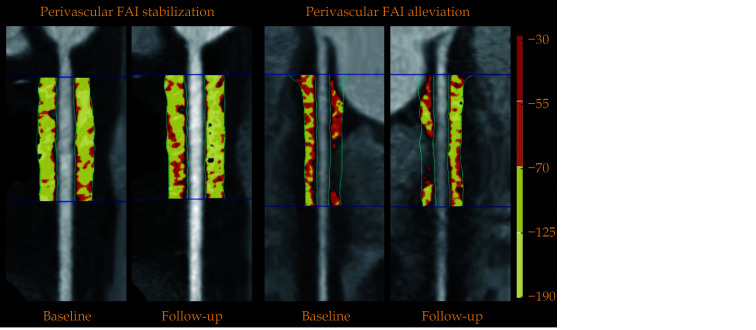
Perivascular FAI analysis was performed utilizing dedicated domestic software (Anythink CT FAI Analysis, version 2.0, Crealife, Beijing, China). The perivascular FAI was distinguished by the weighted mean attenuation of all adipose tissue-containing voxels (−190 HU to −30 HU) located within a radial distance from the outer vessel wall equal to the diameter of the respective vessel.[6,16] We defined the perivascular FAI measured around the right coronary artery (RCA) as a representative metric of global coronary inflammation, due to the absence of major branches and abundance of perivascular fat in the right atrioventricular groove.[16] To avoid any effects of the aortic wall, we analyzed 10 mm to 50 mm of the vessel by excluding the most proximal 10 mm of the RCA, as described previously.[6] A typical case for perivascular FAI analysis is shown in Figure 3.
Figure 3.
Typical example for the perivascular FAI analysis on coronary computed tomography angiography.
Perivascular adipose tissue and the corresponding perivascular FAI analysis around the proximal 10−50 mm of the right coronary artery are shown in curved multiplanar reformation views. In the different two cases, perivascular FAI values were assessed at baseline and follow-up, respectively. FAI values of stabilization situation ranged basically at −90.47 HU and −88.42 HU. FAI values of alleviation situation declined from −67.36 HU to −81.30 HU. FAI: fat attenuation index.
Statistical Analysis
Continuous variables were presented as mean ± SD or medians (interquartile range), while categorical variables were presented as frequencies and percentages. Differences between continuous variables were analyzed using the independent Student’s t-test, the paired-samples t-test, the Mann-Whitney U test or the Kruskal-Wallis H test, as appropriate. Differences between categorical variables were analyzed using the Pearson’s chi-squared test or the Fisher’s exact probability test, as appropriate. Intraobserver and interobserver variability in CCTA plaque quantification and perivascular FAI analysis were assessed by the intraclass correlation coefficient (Table 1). Relationships between continuous variables were assessed using the Pearson’s r-test. Lp(a) concentrations were converted to the logarithmic scale for the Pearson’s r-test. Univariate logistic regression analysis was performed to evaluate the associations between clinical variables and coronary PP. Then, multivariate logistic regression analysis was performed to identify the independent impact of such clinical variables with P-value < 0.10 in the univariate logistic regression analysis on coronary PP. Two-sided P-value < 0.05 was considered statistically significant for all analyses. Statistical analysis was performed using SPSS 23.0 (SPSS Inc., IBM, Chicago, Illinois, USA).
Table 1. Agreement of coronary computed tomography angiography plaque quantification and the perivascular FAI analyses within intraobserver and interobserver.
| Variables | Lesion level | Patient level | |||
| ICC | 95% CI | ICC | 95% CI | ||
| ICC values were classified as excellent (> 0.90), good (0.75−0.90), moderate (0.50−0.75) and poor (< 0.50), respectively. The ICC of intraobserver was executed in 70 lesions of 35 patients, while the ICC of interobserver was executed in 75 lesions of 30 patients. CI: confidence interval; FAI: fat attenuation index; ICC: intraclass correlation coefficient; TPV: total plaque volume. | |||||
| Intraobserver | |||||
| TPV | 0.967 | 0.947−0.979 | 0.949 | 0.885−0.976 | |
| FAI | − | − | 0.994 | 0.987−0.997 | |
| Interobserver | |||||
| TPV | 0.949 | 0.920−0.967 | 0.937 | 0.873−0.970 | |
| FAI | − | − | 0.992 | 0.982−0.996 | |
RESULTS
Study Population and Baseline Characteristics
The baseline demographics and clinical characteristics of all enrolled participants are described in Table 2. The study population consisted of 116 patients (mean age: 53.49 ± 10.21 years, males: 83.6%) with an average CCTA interscan interval of 30.80 ± 13.50 months. At the CCTA baseline examination, ASCVD risk stratification identified patients at very high risk (22.4%), high risk (25.9%) and low to moderate risk (51.7%), respectively. According to definite medical records, 25.9% of patients received concrete statin therapy at baseline but 60.3% of patients achieved LDL-C lowering treatment goals based on different ASCVD risk stratification at follow-up.
Table 2. Baseline demographics and clinical characteristics.
| Variables | Overall (n = 116) | PP (n = 32) | Non-PP (n = 84) | P−value |
| Data are presented as means ± SD or n (%). ACEI: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers; ASCVD: atherosclerotic cardiovascular disease; CAD-RADS: Coronary Artery Disease-Reporting and Data System; CCB: calcium channel blockers; CCTA: coronary computed tomography angiography; PP: plaque progression. | ||||
| Male | 97 (83.6%) | 28 (87.5%) | 69 (82.1%) | 0.584 |
| Age, yrs | 53.49 ± 10.21 | 53.03 ± 9.63 | 53.67 ± 10.47 | 0.766 |
| Body mass index, kg/m2 | 26.46 ± 2.95 | 27.58 ± 3.21 | 26.03 ± 2.75 | < 0.05 |
| Hypertension | 72 (62.1%) | 20 (62.5%) | 52 (61.9%) | 0.953 |
| Diabetes mellitus | 37 (31.9%) | 14 (43.8%) | 23 (27.4%) | 0.091 |
| Dyslipidaemias | 45 (38.8%) | 20 (62.5%) | 51 (60.7%) | 0.860 |
| Current smoking | 35 (30.2%) | 9 (28.1%) | 26 (31.0%) | 0.767 |
| CCTA interscan interval, months | 30.80 ± 13.50 | 33.93 ± 15.37 | 29.60 ± 12.62 | 0.123 |
| ASCVD risk stratification | 0.942 | |||
| Very high risk | 26 (22.4%) | 7 (21.9%) | 19 (22.6%) | |
| High risk | 30 (25.9%) | 9 (28.1%) | 21 (25.0%) | |
| Low and moderate risk | 60 (51.7%) | 16 (50.0%) | 44 (52.4%) | |
| CAD-RADS grade | 0.154 | |||
| 0 (0%) | 26 (22.4%) | 4 (12.5%) | 22 (26.2%) | |
| 1 (1%−24%) | 27 (23.3%) | 9 (28.1%) | 18 (21.4%) | |
| 2 (25%−49%) | 44 (37.9%) | 14 (43.8%) | 30 (35.7%) | |
| 3 (50%−69%) | 11 (9.5%) | 1 (3.1%) | 10 (11.9%) | |
| 4 (70%−99%) | 8 (6.9%) | 4 (12.5%) | 4 (4.8%) | |
| Medication | ||||
| Aspirin | 23 (19.8%) | 5 (15.6%) | 18 (21.4%) | 0.483 |
| Statin | 30 (25.9%) | 7 (21.9%) | 23 (27.4%) | 0.545 |
| Ezetimibe | 1 (0.9%) | 0 | 1 (1.2%) | 1.000 |
| Beta-blockers | 20 (17.2%) | 4 (12.5%) | 16 (19.0%) | 0.583 |
| ACEI or ARB | 27 (23.3%) | 6 (18.8%) | 21 (25.0%) | 0.476 |
| CCB | 29 (25.0%) | 4 (12.5%) | 25 (29.8%) | 0.060 |
Of the enrolled participants, 32 patients (27.6%) and 84 patients (72.4%) were categorized into the PP group and the non-PP group. The patients with PP had a higher BMI (27.58 ± 3.21 kg/m2 vs. 26.03 ± 2.75 kg/m2, P = 0.013), but a similar sex ratio and age. There were no differences observed in traditional clinical risk factors such as hypertension, diabetes mellitus, dyslipidaemias and current smoking, producing comparable distributions of ASCVD risk stratification. Regular qualitative CCTA analysis showed similar CAD-RADS grades between the PP group and the non-PP group.
Comparison of Baseline and Follow-up Main Laboratory Examination Profiles
The baseline and follow-up main laboratory examination profiles are summarized in Table 3. Baseline fasting blood glucose levels were significantly higher in the PP group than those in the non-PP group (6.63 ± 2.56 mmol/L vs. 5.78 ± 1.40 mmol/L, P = 0.024). Although follow-up LDL-C levels were relatively lower in the PP group, the difference between the PP group and the non-PP group was not significant (2.73 ± 0.87 mmol/L vs. 2.41 ± 0.82 mmol/L, P = 0.068). Lp(a) levels were significantly higher in the PP group at both baseline [15.80 (9.09−33.60) mg/dLvs. 10.50 (4.75−19.71) mg/dL,P = 0.029] and follow-up [20.60 (10.45−34.55) mg/dLvs. 8.77 (5.00−18.78) mg/dL,P = 0.004]. There were no differences in total cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL-C), or non-HDL-C at baseline or follow-up between the PP group and the non-PP group. In the non-PP group, the follow-up levels of total cholesterol (4.09 ± 0.99 mmol/L vs. 4.54 ± 1.03 mmol/L, P = 0.005), LDL-C (2.41 ± 0.82 mmol/L vs. 2.81 ± 0.89 mmol/L, P = 0.003) and non-HDL-C (2.96 ± 0.98 mmol/L vs. 3.37 ± 0.95 mmol/L, P = 0.007) demonstrated significant declines compared with those at baseline.
Table 3. Laboratory examination profiles at baseline and follow-up.
| Variables | PP (n = 32) | Non-PP (n = 84) | P−value |
| Data are presented as means ± SD. *Presented as median (interquartile range). #Presented as P < 0.05 (baseline vs. follow-up). PP: plaque progression. | |||
| Fasting blood glucose, mmol/L | |||
| Baseline | 6.63 ± 2.56 | 5.78 ± 1.40 | < 0.05 |
| Follow-up | 6.31 ± 1.90 | 5.82 ± 1.47 | 0.146 |
| Total cholesterol, mmol/L | |||
| Baseline | 4.74 ± 1.31 | 4.54 ± 1.03 | 0.389 |
| Follow-up | 4.31 ± 0.95 | 4.09 ± 0.99# | 0.285 |
| Triglyceride, mmol/L | |||
| Baseline | 1.95 ± 1.18 | 1.92 ± 1.28 | 0.887 |
| Follow-up | 2.01 ± 1.31 | 2.01 ± 1.82 | 0.979 |
| Low-density lipoprotein cholesterol, mmol/L | |||
| Baseline | 2.96 ± 0.99 | 2.81 ± 0.89 | 0.433 |
| Follow-up | 2.73 ± 0.87 | 2.41 ± 0.82# | 0.068 |
| High-density lipoprotein cholesterol, mmol/L | |||
| Baseline | 1.15 ± 0.35 | 1.16 ± 0.25 | 0.827 |
| Follow-up | 1.11 ± 0.32 | 1.13 ± 0.29 | 0.691 |
| Non-high-density lipoprotein cholesterol, mmol/L | |||
| Baseline | 3.58 ± 1.14 | 3.37 ± 0.95 | 0.313 |
| Follow-up | 3.20 ± 0.87 | 2.96 ± 0.98# | 0.223 |
| Lipoprotein(a), mg/dL | |||
| Baseline | 15.80 (9.09−33.60)* | 10.50 (4.75−19.71)* | < 0.05 |
| Follow-up | 20.60 (10.45−34.55)* | 8.77 (5.00−18.78)* | < 0.05 |
Comparison of Baseline and Follow-up Perivascular FAI Analyses
We did not find any differences between the PP group and the non-PP group in the perivascular FAI at either baseline (−76.94 ± 8.55 HUvs. −74.83 ± 7.66 HU, P = 0.201) or follow-up (−77.42 ± 9.55 HUvs. −75.66 ± 7.35 HU, P = 0.292). There were no correlations between log-Lp(a) and the perivascular FAI at either baseline (r = −0.045, P = 0.637) or follow-up (r = −0.028, P = 0.763).
Comparison of Baseline and Follow-up CCTA Quantitative Analyses
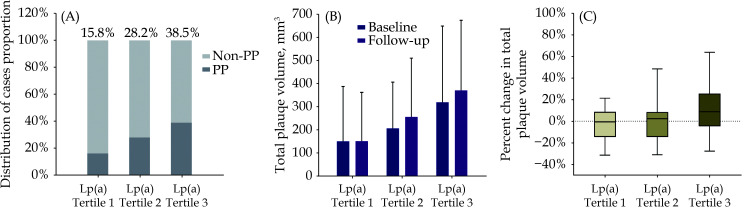
Patients were assigned to three tertiles according to Lp(a) levels, and the corresponding results are exhibited in Figure 4. There were 38 patients, 39 patients, and 39 patients from tertile 1 to tertile 3, and the proportions of PP patients were 15.8% (6 of 38 patients), 28.2% (11 of 39 patients), and 38.5% (15 of 39 patients), respectively. Among the different tertiles, the TPV demonstrated a similar increasing trend as the Lp(a) levels increased at both baseline [149.71 (0.00–387.65) mm3 vs. 206.75 (108.98−406.31) mm3 vs. 318.78 (122.33−648.60) mm3, P = 0.063] and follow-up [150.38 (0.00−361.55) mm3 vs. 255.77 (113.85−509.22) mm3 vs. 369.82 (149.95−673.07) mm3, P = 0.028].
Figure 4.
Proportions of PP and the changes/percent changes of the TPV grouped by Lp(a) level tertile.
The enrolled 116 patients were grouped by Lp(a) level tertile with 38 patients, 39 patients, and 39 patients, respectively. (A): The proportions of PP in each tertile; (B): the absolute changes of the TPV at baseline and follow-up in different tertiles; and (C): the percent changes of the TPV during coronary computed tomography angiography interscan interval in each tertile after excluding 26 patients who were absolutely absent of coronary atheroma at baseline. Lp(a): lipoprotein(a); PP: plaque progression; TPV: total plaque volume.
We recalculated the TPV percent changes after excluding 26 patients who were absolutely absent of coronary atheroma at baseline for mathematical reasons as the denominator is zero. A marginal relative progression trend was observed among the tertiles of Lp(a) levels. The TPV percent changes during the interscan interval were −0.64% (−14.20%−8.15%), 2.27% (−14.23%−7.86%), and 8.72% (−4.34%−25.10%), respectively.
Impact of Lp(a) on PP and Subgroup Analysis
Univariate and multivariate logistic regression analyses to investigate the associations between a series of clinical parameters and PP are shown in Table 4. Elevated Lp(a) levels were significantly related to an increased risk of PP (OR = 1.031, 95% CI: 1.005−1.058,P = 0.019) after adjustment for other confounding variables. In addition, BMI was also an independent risk factor for PP (OR = 1.212, 95% CI: 1.0261−1.433,P = 0.024). However, the perivascular FAI levels were not identified as potential risk factor, as the results for the perivascular FAI were negative in the univariate logistic regression analysis.
Table 4. Univariate and multivariate logistic analyses of CCTA derived parameters and clinical characteristics predicting coronary plaque progression.
| Variables | Univariate analysis | Multivariate analysis | |||||
| OR | 95% CI | P−value | OR | 95% CI | P−value | ||
| CCTA: coronary computed tomography angiography; CI: confidence interval; OR: odds ratio. | |||||||
| Male | 1.522 | 0.464−4.988 | 0.488 | ||||
| Age, yrs | 0.994 | 0.954−1.035 | 0.764 | ||||
| Body mass index, kg/m2 | 1.199 | 1.035−1.389 | < 0.05 | 1.212 | 1.026−1.433 | < 0.05 | |
| Hypertension | 1.026 | 0.443−2.376 | 0.953 | ||||
| Diabetes mellitus | 2.063 | 0.884−4.813 | 0.094 | 1.463 | 0.456−4.697 | 0.522 | |
| Dyslipidaemias | 0.927 | 0.401−2.146 | 0.860 | ||||
| CCTA interscan interval | 1.024 | 0.994−1.055 | 0.125 | ||||
| Statin use | 1.314 | 0.208−8.319 | 0.772 | ||||
| Calcium channel blockers use | 2.841 | 0.638−12.654 | 0.171 | ||||
| Fasting blood glucose, mmol/L | |||||||
| Baseline | 1.271 | 1.011−1.597 | < 0.05 | 1.150 | 0.862−1.535 | 0.343 | |
| Follow-up | 1.192 | 0.937−1.515 | 0.152 | ||||
| Low-density lipoprotein cholesterol, mmol/L | |||||||
| Baseline | 1.200 | 0.763−1.885 | 0.430 | ||||
| Follow-up | 1.569 | 0.962−2.560 | 0.071 | 1.553 | 0.882−2.733 | 0.127 | |
| Lipoprotein(a), mg/dL | |||||||
| Baseline | 1.027 | 1.004−1.051 | < 0.05 | 1.031 | 1.005−1.058 | < 0.05 | |
| Fat attenuation index, HU | |||||||
| Baseline | 0.967 | 0.918−1.018 | 0.201 | ||||
| Follow-up | 0.973 | 0.924−1.024 | 0.291 | ||||
| Total plaque volume, mm3 | |||||||
| Baseline | 1.000 | 0.999−1.001 | 0.800 | ||||
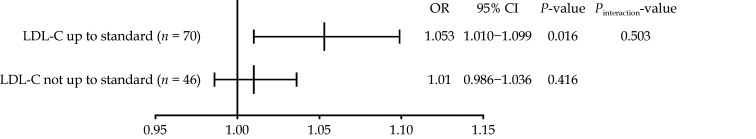
Subgroup analysis of the estimated ORs of Lp(a) for PP is presented in Figure 5. Lp(a) was significantly associated with an increased risk of PP in the subgroup in which LDL-C levels were up to standard (OR = 1.053, 95% CI: 1.010−1.099,P = 0.016), while Lp(a) did not present a significant association with PP if the LDL-C levels were not up to standard (OR = 1.010, 95% CI: 0.986−1.036,P = 0.416). There was no interaction between Lp(a) levels and LDL-C lowering treatment goals (P = 0.503). Following adjustment for hypertension, diabetes mellitus, and dyslipidaemias, Lp(a) remained significantly associated with an increased risk of PP (OR = 1.050, 95% CI: 1.005−1.097,P = 0.028).
Figure 5.
Subgroup analysis for the impact of lipoprotein(a) on coronary plaque progression.
CI: confidence interval; LDL-C: low-density lipoprotein cholesterol; OR: odds ratio.
DISCUSSION
The present study investigated the effects of Lp(a) and related arterial inflammation measured by the perivascular FAI on coronary atheroma progression. The main finding demonstrated the association between elevated Lp(a) levels and coronary PP. After adjustment for conventional risk factors, Lp(a) was recognized as an independent risk factor for PP, especially in the subgroup in which LDL-C levels were up to standard according to ASCVD risk stratification. However, further study should be performed to assure whether the perivascular FAI could be identified as a residual imaging inflammatory biomarker for PP or not.
Recent improvements in CCTA have permitted the serial noninvasive quantitative assessment of ASCVD atheroma changes with excellent intraobserver and interobserver variability. Although academics have established a broad understanding of ASCVD, rare serial visualization monitoring of atheroma evolution in vivo limits our ability to track the disease process over time. In contrast to invasive imaging techniques such as intravascular ultrasound and optical coherence tomography, noninvasive CCTA was emphasized as an alternative but convenient and effective approach in the present study on stable CAD patients.[1,2,14] The advantages of CCTA with excellent sensitivity, specificity, and positive and negative values are universally known, and the accuracy of CCTA plaque quantitative assessment is no less than that of intravascular ultrasound.[17,18] CCTA would contribute to monitoring the natural history of coronary atheroma and understanding the SOC therapeutic effect because CCTA has become a rather mature approach in the one-stop evaluation of qualitative, quantitative, hemodynamic and inflammatory analyses.
Plasma lipoproteins play crucial roles throughout the complex process of atherosclerosis. Routine guidelines suggested clinical practitioners to focus on LDL-C as the primary lipid lowering target to achieve ASCVD risk reduction strategy generally.[19,20] According to definite medical records, we found inconsistencies between proportion of patients with concrete statin therapy at baseline and those achieving LDL-C lowering treatment goals at follow-up. In which condition, it was not ruled out that more patients might receive a strengthened primary or secondary prevention strategy during the interval. Sufficient statin doses to achieve the LDL-C lowering treatment target has been suggested to have significant effectiveness in coronary atheroma regression and plaque stabilization.[21] Nevertheless, a partial study reported that despite receiving intensive medical therapy and achieving very low LDL-C levels, more than 20% of patients with ASCVD still suffer from atheroma progression.[1] Similarly, 27.6% of patients in this study presented coronary atheroma progression, indicating that novel risk factors beyond the conventional ones need to be taken into consideration.
We turned attention to persistent Lp(a) exposure, as a result of that the Lp(a) levels are determined almost entirely genetically and unaffected by SOC therapy.[22] A series of observational and genetic studies have proposed a causal relationship between high plasma concentrations of Lp(a) and increased risks of ASCVD and MACEs, hence the need for including Lp(a) as a potential risk factor for coronary atheroma progression as well.[3,23] We observed that Lp(a) levels were significantly higher among patients with PP than those among non-PP at both baseline and follow-up. Further multivariate logistic regression and subgroup analyses supported that Lp(a) acted as a residual risk factor independent of LDL-C.
It is still controversial whether elevated Lp(a) is a residual risk factor for coronary atheroma progression when LDL-C is controlled. The SATURN study demonstrated that CAD patients prescribed long-term maximally intensive statin therapy achieved low on-treatment LDL-C levels (average levels < 70 mg/dL) and that no significant associations were observed between baseline or on-treatment Lp(a) levels and coronary atheroma progression. [24] However, analyses from the AIM-HIGH study and the JUPITER trial demonstrated that patients with increased Lp(a) concentrations presented a more than 70% higher risk of MACEs even though their LDL-C levels were controlled below 70 mg/dL.[25,26] Our study added to the corresponding meaningful evidence indicating that elevated Lp(a) levels promote coronary atheroma progression with noninvasive CCTA quantitative assessments.
In addition to the proatherogenic mechanism of Lp(a) in coronary atheroma progression, the proinflammatory mechanism associated with OxPLs might play an important role because Lp(a) is the major carrier of OxPLs in the plasma. Van der Valk, et al.[27] reported that Lp(a) derived OxPLs were crucial intermediates in coronary arterial inflammation with elevated Lp(a). With positron emission tomography CT, researchers have proven that elevated Lp(a) levels might contribute to persistent arterial inflammation and that the process is mediated by proinflammatory responses mediated by OxPLs.[5] Therefore, Lp(a) derived coronary arterial inflammation might make sense as a novel biomarker in ASCVD risk stratification or MACEs risk lowering target. Due to the bidirectional proinflammatory mechanisms between perivascular adipose tissue and coronary artery, the perivascular FAI could describe the gradient through the dynamic balance of lipid to aqueous phases detected by CCTA perivascular adipose tissue imaging characteristics and reflect the coronary arterial inflammation as a novel imaging biomarker.[6]
Few studies have discussed coronary arterial inflammation measured by the perivascular FAI in detail since it has been recognized as a novel inflammatory cardiovascular risk factor detected by CCTA imaging in recent years. To our knowledge, this is the first report of the perivascular FAI and serial coronary atheroma changes quantified by CCTA analysis. According to the findings in this study, we considered that chronic low degree inflammation in stable CAD patients was not inclined to promote coronary atheroma progression over a period of time. Our study did not find any correlations between log-Lp(a) and the perivascular FAI or any differences in the perivascular FAI grouped by PP or not. We hypothesized that multiple proinflammatory cytokines together with circulating immune cytokines and inflammatory complexes were involved in a complex inflammatory mechanism besides Lp(a) and related OxPLs.[27,28] The latest update indicated that the extent of arterial inflammation was at a less radical low degree in stable CAD patients and arterial inflammation would be stabilized by SOC therapy.[29,30] Low levels of Lp(a) exposure affect arterial inflammation to a minor extent, and an arterial environment with persistent mild low degree inflammation would not significantly promote PP. In addition, the perivascular FAI might be more effective in enhancing MACEs risk prediction and performing restratification rather than predicting chronic coronary atheroma progression.[16]
STRENGTHS AND LIMITATIONS
Our study investigated both a residual plasma lipoprotein [Lp(a)] risk factor and a novel imaging inflammatory risk characteristic (perivascular FAI) in coronary atheroma progression, relying on a one-stop noninvasive quantitative CCTA assessment, and simultaneously took into account ASCVD risk stratification and LDL-C lowering treatment goals. Briefly, we made a significant and productive attempt to identify the associations of novel residual ASCVD risk factors and coronary atheroma progression.
Despite these promising strengths, our study still had several mentionable limitations. Firstly, the current study was a single center, retrospective, noninterventional analysis with limited individuals. Suitable indications for CCTA examination meant that patients presenting stable angina predominated among the enrolled individuals. Secondly, our study set the target LDL-C values as the only lowering treatment goal, which was insufficient to comprehensively reflect the lipid control condition. Last but not least, our study acquired the perivascular FAI values only in the proximal segments of the RCA and used these values to reflect global coronary background inflammation at the patient level. However, segment or plaque specific measurements of the perivascular FAI are considered more comprehensive local coronary inflammation markers. Therefore, future prospective studies with larger sample sizes and more detailed follow-up information on MACEs are warranted to facilitate further investigation.
CONCLUSIONS
The present study highlighted the value of serial CCTA examinations for assessing the outcome of coronary atheroma progression. Lp(a) was revealed to be an independent residual risk factor for coronary PP, especially in patients with LDL-C levels up to the standard, beyond conventional risk factors. Further study is warranted to identify the effect of chronic low degree arterial wall inflammation measured by the perivascular FAI on coronary atheroma progression.
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Program of China (2016YFC1300304) and the Beijing NOVA Program (Z181100006218055). All authors had no conflicts of interest to disclose.
Contributor Information
Jun-Jie YANG, Email: fearlessyang@126.com.
Yun-Dai CHEN, Email: cyundai@vip.163.com.
References
- 1.Bayturan O, Kapadia S, Nicholls SJ, et al Clinical predictors of plaque progression despite very low levels of low-density lipoprotein cholesterol. J Am Coll Cardiol. 2010;55:2736–2742. doi: 10.1016/j.jacc.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 2.Nozue T, Yamamoto S, Tohyama S, et al Lipoprotein(a) is associated with necrotic core progression of non-culprit coronary lesions in statin-treated patients with angina pectoris. Lipids Health Dis. 2014;13:59. doi: 10.1186/1476-511X-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordestgaard BG, Langsted A Lipoprotein(a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57:1953–1975. doi: 10.1194/jlr.R071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boffa MB, Koschinsky ML Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol. 2019;16:305–318. doi: 10.1038/s41569-018-0153-2. [DOI] [PubMed] [Google Scholar]
- 5.Stiekema LCA, Stroes ESG, Verweij SL, et al Persistent arterial wall inflammation in patients with elevated lipoprotein(a) despite strong low-density lipoprotein cholesterol reduction by proprotein convertase subtilisin/kexin type 9 antibody treatment. Eur Heart J. 2019;40:2775–2781. doi: 10.1093/eurheartj/ehy862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonopoulos AS, Sanna F, Sabharwal N, et al Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9:eaal2658. doi: 10.1126/scitranslmed.aal2658. [DOI] [PubMed] [Google Scholar]
- 7.Chu JR, Gao RL, Zhao SP, et al [2016 guidelines for the management of dyslipidaemias in the Chinese adults] Zhong Guo Xun Huan Za Zhi. 2016;31:937–953. doi: 10.3969/j.issn.1000-3614.2016.10.001. [DOI] [Google Scholar]
- 8.Tan Y, Zhou J, Zhou Y, et al Epicardial adipose tissue is associated with high-risk plaque feature progression in non-culprit lesions. Int J Cardiovasc Imaging. 2017;33:2029–2037. doi: 10.1007/s10554-017-1158-3. [DOI] [PubMed] [Google Scholar]
- 9.Leipsic J, Abbara S, Achenbach S, et al SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:342–358. doi: 10.1016/j.jcct.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Yu Q, Dong W, et al Performance of dual-source CT with high pitch spiral mode for coronary stent patency compared with invasive coronary angiography. J Geriatr Cardiol. 2016;13:817–823. doi: 10.11909/j.issn.1671-5411.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cury RC, Abbara S, Achenbach S, et al CAD-RADSTM Coronary Artery Disease-Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology . J Cardiovasc Comput Tomogr. 2016;10:269–281. doi: 10.1016/j.jcct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Yin P, Dou G, Yang X, et al Noninvasive quantitative plaque analysis identifies hemodynamically significant coronary arteries disease. J Thorac Imaging. 2021;36:102–107. doi: 10.1097/RTI.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 13.Lee SE, Sung JM, Andreini D, et al Differential association between the progression of coronary artery calcium score and coronary plaque volume progression according to statins: the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography Imaging (PARADIGM) study. Eur Heart J Cardiovasc Imaging. 2019;20:1307–1314. doi: 10.1093/ehjci/jez022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smit JM, van Rosendael AR, El Mahdiui M, et al Impact of clinical characteristics and statins on coronary plaque progression by serial computed tomography angiography. Circ Cardiovasc Imaging. 2020;13:e009750. doi: 10.1161/CIRCIMAGING.119.009750. [DOI] [PubMed] [Google Scholar]
- 15.Yu M, Li W, Lu Z, et al Quantitative baseline CT plaque characterization of unrevascularized non-culprit intermediate coronary stenosis predicts lesion volume progression and long-term prognosis: a serial CT follow-up study. Int J Cardiol. 2018;264:181–186. doi: 10.1016/j.ijcard.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Oikonomou EK, Marwan M, Desai MY, et al Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–939. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budoff MJ, Dowe D, Jollis JG, et al Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Dey D, Schepis T, Marwan M, et al Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US. Radiology. 2010;257:516–522. doi: 10.1148/radiol.10100681. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Stone NJ, Bailey AL, et al 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mach F, Baigent C, Catapano AL, et al 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 21.Lee SE, Chang HJ, Sung JM, et al Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475–1484. doi: 10.1016/j.jcmg.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DP, Jacobson TA, Jones PH, et al Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13:374–392. doi: 10.1016/j.jacl.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Clarke R, Peden JF, Hopewell JC, et al Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 24.Puri R, Ballantyne CM, Hoogeveen RC, et al Lipoprotein(a) and coronary atheroma progression rates during long-term high-intensity statin therapy: insights from SATURN. Atherosclerosis. 2017;263:137–144. doi: 10.1016/j.atherosclerosis.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Albers JJ, Slee A, O’Brien KD, et al Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes) J Am Coll Cardiol. 2013;62:1575–1579. doi: 10.1016/j.jacc.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khera AV, Everett BM, Caulfield MP, et al Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) Circulation. 2014;129:635–642. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Valk FM, Bekkering S, Kroon J, et al Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134:611–624. doi: 10.1161/CIRCULATIONAHA.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tmoyan NA, Afanasieva OI, Ezhov MV, et al Lipoprotein(a), immunity, and inflammation in polyvascular atherosclerotic disease. J Cardiovasc Dev Dis. 2021;8:11. doi: 10.3390/jcdd8020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin A, Nerlekar N, Yuvaraj J, et al Pericoronary adipose tissue computed tomography attenuation distinguishes different stages of coronary artery disease: a cross-sectional study. Eur Heart J Cardiovasc Imaging. 2021;22:298–306. doi: 10.1093/ehjci/jeaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai X, Yu L, Lu Z, et al Serial change of perivascular fat attenuation index after statin treatment: insights from a coronary CT angiography follow-up study. Int J Cardiol. 2020;319:144–149. doi: 10.1016/j.ijcard.2020.06.008. [DOI] [PubMed] [Google Scholar]