Despite new therapeutic options, patients with heart failure (HF) still progress to advanced stage. In particular, patients who developed a severe left ventricular (LV) systolic and/or diastolic dysfunction with higher degree of NYHA class, recurrent hospitalizations for HF and showing a severe impairment of exercise capacity were defined as “advanced HF”.[1] Levosimendan was recently approved in order to treat patients hospitalized for acute decompensated HF (ADHF) with severe systolic dysfunction. The pharmacological effects of levosimendan consists of inotropy, vasodilatation and cardioprotection increasing of calcium sensitivity.[2] These effects should be monitored with echocardiography.[3] In particular, in advanced HF patients, levosimendan showed positive effects in reducing mortality and three months hospitalization; about quality of life and symptoms improvement, levosimendan showed contrasting effect among the studies on advanced HF patients available.[4]
In this study, we aim to evaluate the acute effect of slow infusion of levosimendan on the non-invasive measurements of cardiac output (CO) and on the speckle tracking and the three-dimensional echocardiography measurements in advanced HF patients admitted for an acute episode of HF.
We enrolled consecutive patients with diagnosis of ADHF who respect the criteria of “advanced HF”.[1] We excluded patients with recent myocardial infarction (less than six months), concomitant respiratory failure, recent pulmonary embolism (less than six months), end-stage renal disease needing of renal replacement therapy, neoplastic disease, heart rate greater than 110 beats/min, and cardiogenic shock with severe hypotension [systolic arterial pressure (SAP) < 90 mmHg]. All patients receiving one complete infusion of 12.5 mg of levosimendan at 0.1 mcg/kg/min (reduced at 0.05 mcg/kg/min in case of SAP < 90 mmHg during infusion). All patients, pre- and post-levosimendan infusion, underwent to blood sample examination, to non-invasive CO and cardiac index collection through impedance cardiography (CardioScreen 2000, Medis GmbH, Germany) and echocardiography (Vivid E80, GE Healthcare, Horten, Norway) examination (performed by P.G., a cardiologist certified EACVI).
Echocardiograms were performed using a 2.5-MHz transducer with a Vivid E80 ultrasound system (GE Healthcare, Horten, Norway). All participants were examined by conventional two-dimensional echocardiography and three-dimensional echocardiography. Data was stored offline and analysed using echocardiographic software (Echopac, GE Medical, Horten, Norway).
Two-dimensional speckle tracking echocardiography measures were obtained in the apical 4-chamber, 3-chamber and 2-chamber views with an average of 65 frames/s. An automated function defined a region of interest at end-systole and if needed, was manually adjusted by the cardiologist the performed the exam. All strain measurements were obtained from one cardiac cycle and averaged over 17 segments, averaging the values of peak global longitudinal strain (GLS).
Three-dimensional echocardiography started with an optimized two-dimensional 4-chamber view with the left ventricle aligning in the middle of the sector (adjusting the corresponding widths in order to render the complete left ventricle visible). To achieve the needed frame rate (> 30 frames/s), multibeat acquisition of four beats was used.
The impedance cardiography (ICG) has been validated in comparison to cardiac magnetic resonance and right heart catheterization for the calculation of CO at rest and during exercise.[5] Patients were followed for 30 days all cause of mortality/HF re-hospitalization. Differences pre- and post-levosimendan infusion were analysed with mean paired changes t-test using SPSS 20.0 software. The correlation between stroke volume (SV) and CO obtained with ICG and echocardiography has been analysed using the Spearman rank correlation coefficient (rho). The study was conducted in accordance with the Declaration of Helsinki and all patients provided their written consent. The study was approved by the Local Ethical Committee.
A total of 11 consecutive older patients were included in this study (Table 1, Figures 1 & 2). Mean age was 73.8 ± 4.7 years. 72.7% of patients were men. 81.9% of patients recognized ischemic heart disease as HF aetiology. At admission, mean systolic arterial pressure was 100 ± 17 mmHg, mean N-terminal pro-B-type natriuretic peptide (NT-proBNP) was 24,445 ± 12,194 pg/mL and mean serum creatinine was 1.55 ± 0.84 mg/dL. Mean LV ejection fraction (LVEF) was 19.7% ± 5.7% and mean tricuspid anular plane systolic excursion (TAPSE) and pulmonary arterial systolic pressure were 12.5 ± 2.7 mm and 48 ± 15 mmHg, respectively. Mean LV GLS was −3.0 ± 1.8. Mean furosemide in-hospital infusion was 306 ± 102 mg/d and mean urine output was 1,436 ± 496 mL. None developed significant ventricular or supraventricular arrhythmias. All patients were treated with beta-blockers during infusion. At 30 days of follow-up, two patients died and one patient was re-hospitalized. Evaluating the differences among our variables, we found that NT-proBNP was significantly reduced after post-levosimendan infusion ( P = 0.01). Among ICG non-invasive measurement, a significant improvement in SV and CO after levosimendan infusion emerged (P = 0.001 for both). Analysing echocardiography variables, we observed a significant improvement of LVEF (P = 0.003), SV (P = 0.03) and three-dimensional LV GLS (P = 0.002); and a significant reduction in LV end-systolic volume after post-levosimendan infusion (P = 0.02). Furthermore, there was a significant reduction in end-diastolic diameter of right ventricle and B-lines count (P = 0.02 and P = 0.002, respectively). Moreover, we observed a significant improvement in TAPSE (P = 0.003) and LV GLS (P = 0.004). Finally, the ICG and the three-dimensional echocardiography seemed to satisfactorily correlate in the analyse of CO and SV before/post the levosimendan infusion (pre-levosimendan infusion: ICG SV vs. three-dimensional echocardiography SV: r = 0.75, P = 0.008; ICG CO vs. three-dimensional echocardiography CO: r = 0.72, P = 0.01; post-levosimendan infusion: ICG SV vs. three-dimensional echocardiography SV: r = 0.63, P = 0.04; ICG CO vs. three-dimensional echocardiography CO: r = 0.65, P = 0.03).
Table 1. Mean paired difference among variables pre- and post-levosimendan infusion.
| Variables | Pre-levosimendan
infusion |
Post-levosimendan
infusion |
Mean differences | P−value |
| Data are presented as means ± SD. NYHA: New York Heart Association. | ||||
| Age, yrs | 73.8 ± 4.7 | − | − | − |
| Height, cm | 174 ± 7 | − | − | − |
| Weight, kg | 68.2 ± 13.3 | 67.9 ± 13.2 | −0.3 ± 0.9 | 0.279 |
| Systolic arterial pressure, mmHg | 100 ± 17 | 99 ± 11 | −1 ± 16 | 0.847 |
| Diastolic arterial pressure, mmHg | 62 ± 9 | 61 ± 9 | −0.6 ± 3.6 | 0.572 |
| Heart rate, beats/min | 75 ± 8 | 76 ± 11 | 0.9 ± 12.3 | 0.813 |
| NYHA class | 3.5 ± 0.5 | 2.7 ± 0.9 | −0.8 ± 0.7 | 0.005 |
| Laboratory assessment | ||||
| Lactate, mmol/L | 1.26 ± 0.31 | 1.00 ± 0.26 | −0.26 ± 0.36 | 0.035 |
| pH | 7.27 ± 0.07 | 7.33 ± 0.08 | 0.06 ± 0.08 | 0.026 |
| N-terminal pro-B-type natriuretic peptide, pg/mL | 24,445 ± 12,194 | 18,481 ± 10,353 | −5,964 ± 6,288 | 0.01 |
| Serum Na+, mEq/L | 134 ± 4 | 136 ± 3 | 1.3 ± 3.2 | 0.196 |
| Serum K+, mEq/L | 4.0 ± 0.5 | 4.2 ± 0.9 | 0.2 ± 0.7 | 0.366 |
| Serum creatinine, mg/dL | 1.55 ± 0.85 | 1.65 ± 0.84 | 0.1 ± 0.28 | 0.268 |
| Impedance cardiography | ||||
| Impedance, Ohm | 37.5 ± 11.1 | 31.5 ± 12.8 | −6.0 ± 7.6 | 0.026 |
| Fluid, L/Ohm | 31 ± 7 | 37 ± 14 | 5.8 ± 12.3 | 0.149 |
| Stroke volume, mL | 42.8 ± 8.2 | 50.8 ± 9.9 | 8.0 ± 5.3 | 0.001 |
| Cardiac output, L/min | 3.0 ± 0.6 | 3.7 ± 0.9 | 0.69 ± 0.46 | 0.001 |
| Cardiac index, L/min per m2 | 1.96 ± 0.52 | 2.23 ± 0.42 | 0.27 ± 0.52 | 0.110 |
| Systemic vascular resistances, dyns/cm5 | 3,042 ± 759 | 2,773 ± 925 | −269 ± 492 | 0.100 |
| Three-dimensional echocardiography | ||||
| End-diastolic volume left ventricle, mL | 243 ± 91 | 238 ± 91 | −5 ± 11 | 0.160 |
| End-systolic volume left ventricle, mL | 199 ± 81 | 184 ± 80 | −15 ± 17 | 0.016 |
| Left ventricular ejection fraction, % | 19.7 ± 5.7 | 25.0 ± 7.8 | 5.3 ± 4.6 | 0.003 |
| Stroke volume, mL | 43.0 ± 11.9 | 50.5 ± 12.7 | 7.5 ± 10.3 | 0.035 |
| Cardiac output, L/min | 3.06 ± 0.84 | 3.46 ± 0.98 | 0.40 ± 0.81 | 0.135 |
| Left ventricular mass, gr | 96 ± 14 | 98 ± 10 | 2.1 ± 5.9 | 0.274 |
| Left ventricular global longitudinal strain, % | −2.6 ± 1.2 | −4.3 ± 1.5 | −1.7 ± 1.4 | 0.002 |
| Two-dimensional echocardiography | ||||
| End-diastolic diameter right ventricle, mm | 53 ± 7 | 48 ± 7 | −5 ± 6 | 0.018 |
| Tricuspid anular plane systolic excursion, mm | 12.5 ± 2.7 | 15.6 ± 3.9 | 3.1 ± 2.6 | 0.003 |
| Pulmonary arterial systolic pressure, mmHg | 48 ± 16 | 37 ± 18 | −11 ± 18 | 0.059 |
| B-lines | 18 ± 5 | 10 ± 6 | −8 ± 6 | 0.002 |
| Mechanical dispersion, ms | 158 ± 72 | 152 ± 66 | −6 ± 16 | 0.247 |
| Left ventricular global longitudinal strain, % | −3.0 ± 1.8 | −4.7 ± 2.1 | −1.8 ± 1.6 | 0.004 |
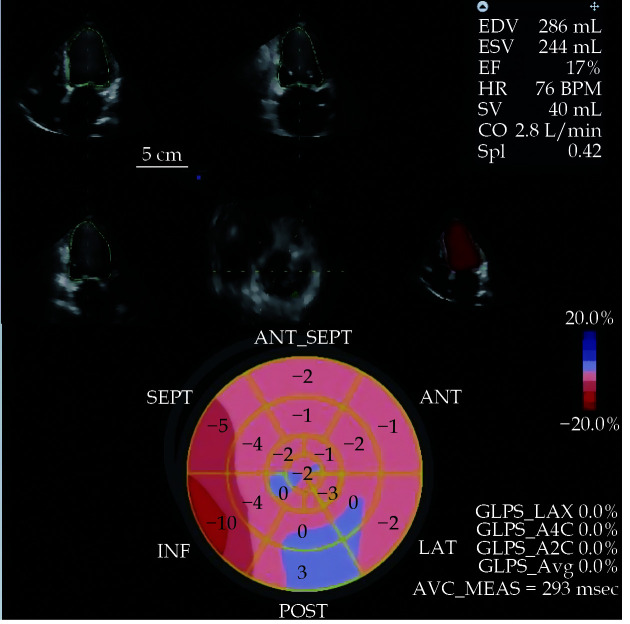
Figure 1.
The baseline two-dimensional echocardiography and three-dimensional echocardiography at rest with the determination of EDV, ESV, EF, SV and CO.
Moreover the analysis of GLS in a polar map has been represented. CO: cardiac output; EDV: end-diastolic volume; EF: ejection fraction; ESV: end-systolic volume; GLS: global longitudinal strain; SV: stroke volume.
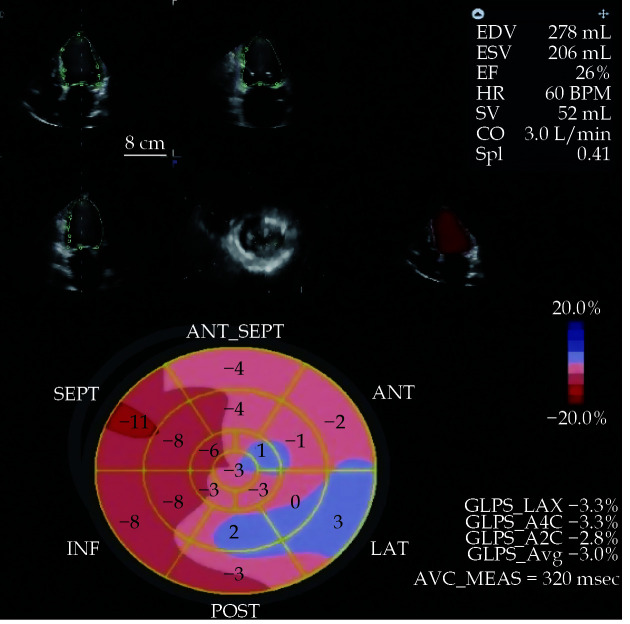
Figure 2.
The baseline two-dimensional echocardiography and three-dimensional echocardiography at rest with the determination of EDV, ESV, EF, SV and CO.
An improvement of EF and in the absolute values of GLS emerged after the levosimendan infusion in the same patient. CO: cardiac output; EDV: end-diastolic volume; EF: ejection fraction; ESV: end-systolic volume; GLS: global longitudinal strain; SV: stroke volume.
To best of our knowledge, although in a limited experience, this study represents the first one evaluating short-term effects of levosimendan infusion through non-invasive measurement of CO, two-dimensional and three-dimensional echocardiography. Findings from REVIVE trials demonstrated that levosimendan infusion in ADHF patients provided a rapid improvement in terms HF symptoms, nevertheless a higher rate of hypotension and arrhythmias was described.[6] In a previous experience,[7] we observed in severe chronic HF patients, the reduction of plasma brain natriuretic peptide and an amelioration of LVEF after a single levosimendan infusion, failing to demonstrate the improvement in CO, SV and right ventricle performance. In advanced HF patients, the strategies might be a definitive intervention (heart transplantation or the installation of a LV assistant device) or a palliative care pathway. In case of a palliative care strategy, the haemodynamic stabilization, the preservation of functional capacity and the possible prevention of HF-related hospitalization seemed to be clinical goals that could be obtained with the levosimendan infusion.[8] In fact, in LevoRep trial,[9] LION-HEART trial,[10] and LAICA trial,[11] the repetitive infusion of levosimendan in advanced HF patients gained a clinical benefit (a trend in reduction in HF readmission and HF mortality) and a significant decrement in plasma NT-proBNP levels without an increase in mortality. In this preliminary report, advanced HF patients admitted for an acute episode of cardiac decompensation, were treated with a single levosimendan infusion, demonstrating to improve SV, CO and LVEF without the development of arrhythmias or severe hypotension. These results could be due to the lack of bolus administration and the lower velocity of levosimendan infusion in our population which allowed the concomitant administration of beta-blockers avoiding arrhythmias and preserving the improvement in cardiac performance. Moreover, all patients included in our study were discharged with a net improvement in cardiac function. Furthermore, we showed the significant reduction in NT-proBNP levels after the infusion as in larger clinical trial.[10]
Finally, the ICG has been tested in the determination of CO in patients with pulmonary hypertension in comparison with thermodilution using a right heart catheterisation.[5,11–13] In all these clinical experiences, the correlation between ICG and invasive determination of CO seemed to be satisfactorily. Moreover, the evidence of a satisfactorily degree of correlation in the analyse of cardiac performance pre/post levosimandan infusion in HF patients suggests that the acute effects of the drug might be accurately follow using non-invasive methods performed by expert operators.
In conclusion, our clinical experience showed that slow levosimendan infusion without bolus could be considered in advanced older ADHF patients to improve cardiac performance. ICG and echocardiography in-hospital evaluation seemed to be necessary to understand treatment success and patients status improvement.
ACKNOWLEDGMENTS
All authors had no conflicts of interest to disclose.
References
- 1.Crespo-Leiro MG, Metra M, Lund LH, et al Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:1505–1535. doi: 10.1002/ejhf.1236. [DOI] [PubMed] [Google Scholar]
- 2.Bouchez S, Fedele F, Giannakoulas G, et al Levosimendan in acute and advanced heart failure: an expert perspective on posology and therapeutic application. Cardiovasc Drugs Ther. 2018;32:617–624. doi: 10.1007/s10557-018-6838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameli M, Incampo E, Navarri R, et al Effects of levosimendan in heart failure: the role of echocardiography. Echocardiography. 2019;36:1566–1572. doi: 10.1111/echo.14419. [DOI] [PubMed] [Google Scholar]
- 4.Farmakis D, Agostoni P, Baholli L, et al A pragmatic approach to the use of inotropes for the management of acute and advanced heart failure: an expert panel consensus. Int J Cardiol. 2019;297:83–90. doi: 10.1016/j.ijcard.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Panagiotou M, Vogiatzis I, Jayasekera G, et al Validation of impedance cardiography in pulmonary arterial hypertension. Clin Physiol Funct Imaging. 2018;38:254–260. doi: 10.1111/cpf.12408. [DOI] [PubMed] [Google Scholar]
- 6.Packer M, Colucci W, Fisher L, et al Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1:103–111. doi: 10.1016/j.jchf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Feola M, Lombardo E, Taglieri C, et al Effects of levosimendan/furosemide infusion on plasma brain natriuretic peptide, echocardiographic parameters and cardiac output in end-stage heart failure patients. Med Sci Monit. 2011;17:P17–13. doi: 10.12659/msm.881433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altenberger J, Gustafsson F, Harjola VP, et al Levosimendan in acute and advanced heart failure: an appraisal of the clinical database and evaluation of its therapeutic applications. J Cardiovasc Pharmacol. 2018;71:129–136. doi: 10.1097/FJC.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altenberger J, Parissis JT, Costard-Jaeckle A, et al Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep ) study: a multicentre randomized trial. Eur J Heart Fail. 2014;16:898–906. doi: 10.1002/ejhf.118. [DOI] [PubMed] [Google Scholar]
- 10.Comín-Colet J, Manito N, Segovia-Cubero J, et al Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentre randomised trial. Eur J Heart Fail. 2018;20:1128–1136. doi: 10.1002/ejhf.1145. [DOI] [PubMed] [Google Scholar]
- 11.García-Gonzáles MJ. Efficacy and security of intermittent repeated levosimendan administration in patients with advanced heart failure: a randomized, doble-blind, placebo controlled multicentre trial: LAICA study. Presented at European Society of Cardiology-HFA Congress, Florence, Italy, May 2016.
- 12.Tonelli AR, Alnuaimat H, Li N, et al Value of impedance cardiography in patients studied for pulmonary hypertension. Lung. 2011;189:369–375. doi: 10.1007/s00408-011-9299-y. [DOI] [PubMed] [Google Scholar]
- 13.Dupuis M, Noel-Savina E, Prévot G, et al Determination of cardiac output in pulmonary hypertension using impedance cardiography. Respiration. 2018;96:500–506. doi: 10.1159/000486423. [DOI] [PubMed] [Google Scholar]