Abstract
Information on neurodevelopmental effects of antenatal exposure to antipsychotics is limited to 10 studies, all examining children up to 5 years of age or less. The paper aimed to investigate the association between in utero exposure to antipsychotics and psychiatric outcomes in children using Danish nationwide registers. In total, 9011 liveborn singletons born 1998–2015 in Denmark whose mothers took antipsychotic medication before pregnancy were identified. Children whose mothers continued to take antipsychotics during pregnancy were compared with children of mothers who discontinued antipsychotics before pregnancy. As a negative control, paternal antipsychotic use in the same window was investigated. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards regression for the primary outcome of psychiatric disorders, as well for subcategories of psychiatric disorders. In total, 9.9% of children in the discontinuation group and 11.0% of children in the continuation group received a psychiatric disorder diagnosis during follow-up. The adjusted HR for psychiatric disorders among offspring in the continuation group compared to the discontinuation group was 1.10 (95% CI 0.93–1.30). For antipsychotic use in the fathers, the HR was 1.05 (95% CI 0.89–1.24). The study does not provide evidence of increased risk of psychiatric disorders among children of women who continue antipsychotic treatment during pregnancy. This was observed after accounting for the underlying risk conferred by maternal psychiatric disorders. This suggests women who need to continue antipsychotic medications during pregnancy can do so without adverse psychiatric outcomes for offspring.
Subject terms: Risk factors, Depression
Introduction
Antipsychotics are widely prescribed for schizophrenia, but also for patients with symptoms of psychosis and agitation, including those with bipolar disorder, depression, and anxiety disorders [1, 2]. Many of these disorders have onset in adolescence, peaking by the third or fourth decades [3]; women of childbearing age are among those who may be prescribed antipsychotics.
In the first decade of the twenty-first century, the number of pregnancies exposed to antipsychotics increased [4, 5]. In the USA, Toh et al. [4] reported that the prevalence of pregnant women on atypical antipsychotic treatment increased from 0.3% to 0.8%; similarly, Park et al. [5] observed an increase from 0.4% to 1.3%. In Europe, prevalence is lower (<0.2%) [6–8], but as in the USA, has risen in recent years [6]. Furthermore, since some typical antipsychotics can be used to treat pregnancy-associated nausea, prescription of these specific medications is relatively high in the first trimester (0.2–1.3% of pregnancies) [7, 8].
Antipsychotics cross the placenta and in some cases the fetus is exposed to a higher concentration than the mother’s serum level [9]. Dopamine receptors are functional in the developing fetus [10], including influencing the proliferation and differentiation of neural progenitor cells [11]. Dysregulation of dopamine has been linked to some psychiatric disorders [12, 13]. Thus it is possible that in utero exposure to antipsychotics could influence neural development and might have long-term psychiatric or neurological effects.
Many women reflect on their medication use pre-conception and during pregnancy, not wishing to expose their fetus to any substances. Information on neurodevelopmental effects of antenatal exposure to antipsychotics is limited to ten studies, all examining only children 5 years of age or less [14]. The most consistent finding is a transient delay in motor development, detectable under 1 year. To our knowledge, there have been no investigations of potential long-term adverse effects of antenatal exposure to antipsychotics beyond 5 years of age. As most mood and psychotic disorders have their onset in adolescence and adulthood, an increased risk would not be detected in studies of young children. Additionally, most studies compared exposed children to children of mothers without history of psychiatric illness, limiting opportunities to control for important confounders such as genetic load, active mental illness in pregnancy, and differences in parenting behavior [15–17].
This study was designed to investigate the incidence of psychiatric disorders in offspring whose mothers were prescribed antipsychotic medication in the 2 years before pregnancy and continued to take these throughout pregnancy. The comparison group were children whose mothers were prescribed antipsychotic medication in the 2 years before pregnancy but discontinued treatment before pregnancy. Additionally, we examined antipsychotic use of the fathers to assess whether observed associations were confounded by shared environmental or genetic factors.
Materials and methods
Study population
We carried out a population-based cohort study using Danish registers. We identified all liveborn singletons during 1998–2015 (n = 1,092,668) from the Danish Medical Birth Registry [18]. We excluded 10,398 children with missing/unlikely gestational age (<154 or >315 days); 2437 with chromosomal abnormalities (ICD-10 [International Classification of Diseases, 10th revision] [19] codes Q90–Q99) identified from the Danish National Patient Register; and 11,760 missing links to their fathers; resulting in 1,068,073 singletons born to 628,479 mothers (Fig. 1). From these children, we identified our study population of 9011 children: 6976 children born to a mother who discontinued antipsychotic medications before pregnancy (0.7%); and 2035 born to a mother who continued to use antipsychotic medications during pregnancy (0.2%). The study was approved by the Danish Data Protection Agency. By Danish law, no informed consent is required for a register based study using anonymised data.
Fig. 1. Identification of the study population.

Flowchart showing identification of the study population and the numbers excluded at each stage.
Exposure: use of antipsychotics
Information on maternal antipsychotic use was obtained from the Danish National Prescription Registry [20]. The anatomical therapeutic chemical (ATC) classification code used to identify antipsychotics was N05A, excluding N05AN (lithium). Start of antipsychotic use was indicated by dispensing date. We defined antipsychotic use during pregnancy as a prescription dispensed from 1 month before pregnancy until delivery. Start of pregnancy was ascertained from gestational age: based on the first- or second-trimester ultrasound scan [18, 21]; or, when ultrasound data were unavailable, the first day of the mother’s last menstrual period. Children were categorized according to dispensing of an antipsychotic to the mother “before pregnancy” (2 years to 1 month before pregnancy) and “during pregnancy” (1 month before until birth): (i) unexposed, with no maternal antipsychotic prescription before or during pregnancy, (ii) discontinuation, with prescription before but not during pregnancy, (iii) continuation, with prescriptions both before and during pregnancy, and (iv) new user, with prescription only during pregnancy. We calculated duration of prescriptions by multiplying number of defined daily doses per package by number of packages dispensed. We calculated total number of days exposed by adding durations of all antipsychotic prescriptions. To provide a negative control, to assess confounded by shared environmental or genetic variables, we repeated analyses using paternal antipsychotic prescriptions during the same window [22]. Our hypothesis was that if any potential effect is due to intrauterine exposure, maternal antipsychotic use during pregnancy should have a greater influence than paternal antipsychotic use in the same period.
Primary and secondary outcomes: any psychiatric disorders and subcategories of psychiatric disorders
The Danish Psychiatric Central Research Register provided information on psychiatric diagnoses [23]. Our primary outcome was first diagnosis of psychiatric disorders (ICD-10 codes F00–F99) in the offspring. We further reported associations for the following subcategories of psychiatric disorders as secondary outcomes, due to sufficient numbers of cases: autism spectrum disorder (F84.0, F84.1, F84.5, F84.8, or F84.9); neurotic, stress related, and somatoform disorder (F40–F48); attention deficit/hyperactivity disorders (F90 or F98.8); and behavioral and emotional disorders other than attention deficit/hyperactivity disorders (F91–F97).
Potential confounders
We identified potential confounders in our analyses using directed acyclic graphs. The majority were maternal characteristics: psychiatric history at delivery, from the Danish Psychiatric Central Research Register (hospitalization, outpatient or emergency visit for ICD-8 codes 290–315 [24] or ICD-10 codes F00–F99; [19] yes/no); age at delivery (<25, 25–34, ≥35 years); primiparity (yes/no); inpatient and outpatient psychiatric treatment from 2 years before pregnancy until delivery (yes/no); prescriptions for other psychotropic drugs (lithium or antidepressants) before or during pregnancy (yes/no); number of non-psychiatric hospital visits during pregnancy (0–1, 2–3, ≥4); smoking during pregnancy (yes/no); marital status (married or cohabiting/single, divorced, widowed); highest education (mandatory school/above mandatory school); and calendar year of delivery (1998–2003, 2004–09, 2010–15). Additionally, we included paternal psychiatric history at delivery. Data on these covariates came from the above-mentioned registers as well as Statistics Denmark’s registers on socioeconomic status [25].
When producing estimates for paternal antipsychotic use as a negative control, we also adjusted for maternal antipsychotic use during pregnancy, paternal age at delivery (continuous variable), and paternal inpatient or outpatient psychiatric treatment from 2 years before pregnancy to delivery (yes/no).
Statistical analysis
Using Stata 15, we followed each child from birth until the first of the following: first psychiatric diagnosis, death, emigration, or 31 December 2016. We used Cox regression models to calculate hazard ratios (HRs) with 95% confidence intervals (CIs), with child’s age as the time scale. To account for dependence between siblings, we used robust estimation for correction of standard errors. For smoking and education, 4.4% and 4.5% of values were missing, respectively; we imputed missing values [26].
Comparisons between antipsychotic continuation and discontinuation groups
To control for the underlying maternal psychiatric disorders, we compared the antipsychotic continuation group with the discontinuation group in our main analysis, adjusting for the above-mentioned covariates. To examine whether associations depended on the timing of exposure, we divided the exposure window into three groups: first trimester only (1 month before pregnancy to 90 days after last menstrual period); second or third trimester only (91–180 days after last menstrual period or 181 days after last menstrual period to childbirth); and more than one trimester. We divided the duration of antipsychotic use during pregnancy into ≤90 days, 91–180 days, and ≥181 days. We also included duration as a continuous variable, per 30-day increase, in the models to see whether duration response existed.
To assess any differences between types of antipsychotics, we carried out the main analysis (comparing the antipsychotic continuation group with the discontinuation group) for each chemical subgroup of antipsychotic (i.e. at the 4th ATC code level: N05AA Phenothiazines with aliphatic side-chain, N05AB Phenothiazines with piperazine structure, N05AC Phenothiazines with piperidine structure, N05AD Butyrophenone derivatives, N05AE Indole derivatives, N05AF Thioxanthene derivatives, N05AG Diphenylbutylpiperidine derivatives, N05AH Diazepines, oxazepines, thiazepines and oxepines, N05AL Benzamides, and N05AX Other antipsychotics).
Recording indications for medication prescription in the data only started in 2004, becoming more complete by 2006. We used indication data from 2006 onward to ascertain reasons for prescriptions of antipsychotic medications in our population. However, as there is still a relatively high proportion of missing/unspecified indication data, this was not included in our analyses.
Sensitivity analyses
We carried out several sensitivity analyses. First, to reduce misclassification of antipsychotic exposure, we restricted analyses to mothers who filled at least two prescriptions for antipsychotics during pregnancy. Second, to reduce misclassification of antipsychotic use discontinuation, we redefined it as maternal use of antipsychotic from 2 years to 3 months before pregnancy but not from 3 months before pregnancy until delivery. Third, as mothers who had inpatient psychiatric treatment may have more severe symptoms, we excluded pregnancies of mothers with inpatient psychiatric treatment from 2 years before pregnancy until delivery. Fourth, we included an interaction term between sex of the child and antipsychotic exposure, to see if there were sex differences. Fifth, we restricted the population to those born before 2002, keeping only children who could reach at least 15 years by the end of follow-up. Sixth, we excluded children of mothers who took other psychotropic medications or benzodiazepines during pregnancy (ATC codes N06A antidepressants, N05AN lithium, N03AE and N05BA benzodiazepine derivatives, N05CF benzodiazepine related drugs, N03AF01 carbamazepine, N03AF02 oxcarbazepine, N03AG01 valproate, N03AX09 lamotrigine), to assess whether the association observed was different in women who did not take these medications. Finally, we restricted the population to children whose mothers did not receive a diagnosis in the National Patient Register during pregnancy for a mental or behavioral disorder due to psychoactive substance use (ICD-10 codes F10-F19).
Results
The characteristics of the mothers of all children born in the study window are shown in Table 1. In the continuation group, a larger proportion of mothers had psychiatric history, received psychiatric treatment before or during pregnancy, and had more non-psychiatric hospital visits. Maximum possible follow-up was 19 years. A psychiatric diagnosis was received by 694 children in the discontinuation group and 224 children in the continuation group before the end of follow-up.
Table 1.
Characteristics of study population according to maternal antipsychotic use before and during pregnancy, n (%).
| Characteristics | Unexposed group (n = 1,058,205) |
Antipsychotic discontinuation group (n = 6976) |
Continuation group (n = 2035) | New user group (n = 857) |
|---|---|---|---|---|
| Maternal age at delivery | ||||
| <25 years | 135,031 (12.8) | 1641 (23.5) | 378 (18.6) | 221 (25.8) |
| 25–34 years | 732,574 (69.2) | 4055 (58.1) | 1121 (55.1) | 470 (54.8) |
| ≥35 years | 190,600 (18.0) | 1280 (18.3) | 536 (26.3) | 166 (19.4) |
| Primiparity | 469,809 (44.4) | 3498 (50.1) | 980 (48.2) | 366 (42.7) |
| Maternal psychiatric history at delivery | 68,466 (6.5) | 4437 (63.6) | 1735 (85.3) | 500 (58.3) |
| Inpatient psychiatric treatment from 2 years before pregnancy to delivery | 3382 (0.3) | 1275 (18.3) | 672 (33.0) | 168 (19.6) |
| Outpatient psychiatric treatment from 2 years before pregnancy to delivery | 20,544 (1.9) | 3109 (44.6) | 1339 (65.8) | 423 (49.4) |
| Dispensing of other psychotropic prescriptions 2 years before pregnancy to delivery | 14,408 (1.4) | 612 (8.8) | 399 (19.6) | 263 (30.7) |
| No of non-psychiatric hospital visits during pregnancy | ||||
| 0–1 | 329,050 (31.1) | 1228 (17.6) | 313 (15.4) | 140 (16.3) |
| 2–3 | 497,373 (47.0) | 2858 (41.0) | 803 (39.5) | 354 (41.3) |
| ≥4 | 231,782 (21.9) | 2890 (41.4) | 919 (45.2) | 363 (42.4) |
| Maternal smoking during pregnancy | ||||
| Yes | 161,169 (15.2) | 2782 (39.9) | 921 (45.3) | 306 (35.7) |
| No | 850,777 (80.4) | 3924 (56.3) | 1031 (50.7) | 502 (58.6) |
| Missing | 46,259 (4.4) | 270 (3.9) | 83 (4.1) | 49 (5.7) |
| Maternal marital status at delivery | ||||
| Married or cohabiting | 924,072 (87.3) | 4932 (70.7) | 1341 (65.9) | 619 (72.2) |
| Single, divorced, or widowed | 134,133 (12.7) | 2044 (29.3) | 694 (34.1) | 238 (27.8) |
| Maternal highest education at delivery | ||||
| Elementary school | 186,361 (17.6) | 3348 (48.0) | 1026 (50.4) | 400 (46.7) |
| Above elementary school | 823,808 (77.8) | 3432 (49.2) | 933 (45.8) | 405 (47.3) |
| Missing | 48,036 (4.5) | 196 (2.8) | 76 (3.7) | 52 (6.1) |
| Calendar year of delivery | ||||
| 1998–2002 | 370,429 (35.0) | 1798 (25.8) | 385 (18.9) | 281 (32.8) |
| 2003–2007 | 361,750 (34.2) | 2157 (30.9) | 707 (34.7) | 280 (32.7) |
| 2008–2015 | 326,026 (30.1) | 3021 (43.3) | 943 (46.3) | 296 (34.5) |
| Paternal psychiatric history at delivery | 44,657 (4.2) | 1093 (15.7) | 454 (22.3) | 122 (14.2) |
| Paternal antipsychotic use | ||||
| No antipsychotic use | 1,046,452 (98.9) | 6423 (92.1) | 1765 (86.7) | 787 (91.8) |
| Use before but not during pregnancy | 5700 (0.5) | 278 (4.0) | 78 (3.8) | 30 (3.5) |
| Use both before and during pregnancy | 3418 (0.3) | 187 (2.7) | 143 (7.0) | 30 (3.5) |
| Use during but not before pregnancy | 2635 (0.2) | 88 (1.3) | 49 (2.4) | 10 (1.2) |
| Sex of child | ||||
| Male | 543,130 (51.3) | 3574 (51.2) | 1072 (52.7) | 420 (49.0) |
| Female | 515,075 (48.7) | 3402 (48.8) | 963 (47.3) | 437 (51.0) |
Indications for antipsychotics in the continuation group
Of 20,073 prescriptions for antipsychotics during pregnancies for children in the continuation group born from 2006 onward, 38.7% were missing indications. The most common recorded indications were “against mental disorders” (41.4%), “sedative” (9.2%) or “against depression” (3.4%). Less than 0.1% were stated to be prescribed “against nausea” or “against vomiting”.
Comparisons between the antipsychotic discontinuation and continuation groups
After adjustment for demographic and psychiatric characteristics of the mother, the risk for psychiatric disorders among offspring in the continuation group was 10% higher than in the discontinuation group; however, the 95% CI included 1 (HR 1.10, 95% CI 0.93, 1.30), as shown in Fig. 2, indicating no statistically significant difference. Similarly, while point estimates were raised when studying timing and duration of antipsychotic use, the estimates do not provide evidence for an increased risk for psychiatric disorders in offspring in the continuation group, due to lack of statistical significance and a modestly raised point estimate. No increase in risk was seen with each 30 day increase in duration of antipsychotic use (HR 1.00, 95% CI 0.98–1.02).
Fig. 2. Maternal antipsychotic use.
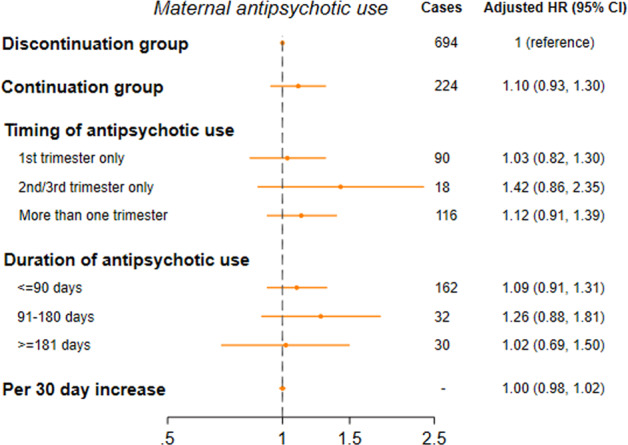
Hazard ratios for psychiatric disorders among children born to mothers with continued antipsychotic use, compared with mothers with discontinued antipsychotic use (N = 9011).
Table 2 shows HRs of continuation compared to discontinuation of types of antipsychotics, for those which we had sufficient numbers for (i.e. meeting Danish regulations for reporting). The most common types to be continued throughout pregnancy were Diazepines, oxazepines, thiazepines and oxepines (n = 826) and Thioxanthene derivatives (n = 599). The HR was highest for those whose mother continued taking Thioxanthene derivatives (1.28, 95% CI 1.01–1.62)
Table 2.
Hazard ratios of psychiatric disorders among children of mothers with continued use of specific types of antipsychotic, compared with mothers with discontinued use.
| Antidepressant use during pregnancya | Cases | Total number | Person years | Crude hazard ratio | Adjusted hazard ratio (95% CI)b |
|---|---|---|---|---|---|
| Prescription of N05AA Phenothiazines with aliphatic side-chain antipsychotic: | |||||
| Discontinuation group | 135 | 1005 | 11,044 | 1 | 1 (ref) |
| Continuation group | 15 | 107 | 1197 | 1.04 | 0.89 (0.50, 1.57) |
| Prescription of N05AB Phenothiazines with piperazine structure antipsychotic: | |||||
| Discontinuation group | 91 | 636 | 7839 | 1 | 1 (ref) |
| Continuation group | 22 | 171 | 1662 | 1.37 | 0.92 (0.54, 1.57) |
| Prescription of N05AF Thioxanthene derivatives antipsychotic: | |||||
| Discontinuation group | 405 | 3720 | 31,591 | 1 | 1 (ref) |
| Continuation group | 97 | 599 | 5251 | 1.40 | 1.28 (1.01, 1.62) |
| Prescription of N05AH Diazepines, oxazepines, thiazepines and oxepines antipsychotic: | |||||
| Discontinuation group | 129 | 2085 | 11,145 | 1 | 1 (ref) |
| Continuation group | 59 | 826 | 4578 | 1.09 | 1.01 (0.72, 1.41) |
| Prescription of N05AX Other antipsychotics: | |||||
| Discontinuation group | 112 | 1169 | 7631 | 1 | 1 (ref) |
| Continuation group | 19 | 238 | 1450 | 0.91 | 0.83 (0.50, 1.40) |
aNumbers for N05AC Phenothiazines with piperidine structure, N05AD Butyrophenone derivatives, N05AE Indole derivatives, N05AG Diphenylbutylpiperidine derivatives and N05AL Benzamides were too small to meet Danish regulations for reporting.
bAdjusted for maternal age at delivery, primiparity, in- and outpatient psychiatric treatment from 2 years before pregnancy until delivery, prescriptions for other psychotropic drugs during pregnancy, prescriptions for antiepileptic drugs during pregnancy, number of non-psychiatric hospital visits during pregnancy, smoking during pregnancy, marital status, highest education, calendar year of delivery and paternal psychiatric history at time of delivery.
Table 3, ST1 and ST2 display HRs and 95% CIs by different types of psychiatric disorders. Point estimates indicated increased risk for emotional disorders other than attention deficit/hyperactivity disorders (HR 1.32, 95% CI 0.99–1.76); neurotic, stress related and somatoform disorders (HR 1.08, 95% CI 0.79–1.50); and other psychiatric disorders (HR 1.07, 95% CI 0.90–1.26). Whereas, point estimates were reduced for autism (HR 0.83, 95% CI 0.53–1.29) and attention deficit/hyperactivity disorders (HR 0.85, 95% CI 0.62–1.15). However, none of the observed associations were statistically significant.
Table 3.
Hazard ratios for types of psychiatric disorders among children of mothers with continued antipsychotic use during pregnancy, compared with mothers with discontinued antipsychotic use.
| Antipsychotic use during pregnancya | Cases | Total number | Person years | Crude hazard ratio | Adjusted hazard ratio (95% CI)b |
|---|---|---|---|---|---|
| Autism spectrum disorder | |||||
| Antipsychotic discontinuation group | 114 | 6976 | 60,436 | 1 (ref) | 1 (ref) |
| Antipsychotic continuation group | 26 | 2035 | 16,141 | 0.92 | 0.83 (0.53, 1.29) |
| Neurotic, stress related and somatoform disorders | |||||
| Antipsychotic discontinuation group | 182 | 6976 | 60,053 | 1 (ref) | 1 (ref) |
| Antipsychotic continuation group | 61 | 2035 | 15,832 | 1.43 | 1.08 (0.79, 1.50) |
| Attention deficit/hyperactivity disorders | |||||
| Antipsychotic discontinuation group | 216 | 6976 | 60,086 | 1 (ref) | 1 (ref) |
| Antipsychotic continuation group | 51 | 2035 | 16,035 | 0.95 | 0.85 (0.62, 1.15) |
| Emotional other than attention deficit/hyperactivity disorders | |||||
| Antipsychotic discontinuation group | 183 | 6976 | 59,953 | 1 (ref) | 1 (ref) |
| Antipsychotic continuation group | 80 | 2035 | 15,776 | 1.71 | 1.32 (0.99, 1.76) |
aNote that these outcomes are not mutually exclusive as a child may receive a diagnoses within more than one disorder category.
bAdjusted for maternal age at delivery, primiparity, in- and outpatient psychiatric treatment from 2 years before pregnancy until delivery, prescriptions for other psychotropic drugs during pregnancy, number of non-psychiatric hospital visits during pregnancy, smoking during pregnancy, marital status, highest education, calendar year of delivery and paternal psychiatric history at time of delivery.
Paternal antipsychotic use (negative control analyses)
When considering antipsychotic use in the fathers, 6086 children were born to fathers who discontinued antipsychotic medication use in the 2 years before pregnancy and 3778 to fathers who continued antipsychotics. The HR among children in the paternal continuation group was 1.05 (95% CI 0.89–1.24) (Fig. 3). As for the associations for maternal antipsychotic use, 95% CIs all included 1, indicating no statistically significant differences. Compared to the association observed for maternal antipsychotic use, the point estimate was attenuated.
Fig. 3. Paternal antipsychotic use.

Hazard ratios for psychiatric disorders among children born to mothers with continued antipsychotic use, compared with mothers with discontinued antipsychotic use (N = 9864).
Sensitivity analyses
Among mothers who continued antipsychotic use during pregnancy, 63.6% (n = 1,295) received two or more prescriptions. Redefining antipsychotic continuation as two or more prescriptions gave a HR for psychiatric disorders of 1.12 (95% CI 0.92–1.36) compared to the discontinuation group (ST3). Redefining discontinuation as stopping antipsychotic use at least 3 months prior to pregnancy resulted in a HR for psychiatric disorders of 1.08 (95% CI 0.91–1.28). When excluding children whose mothers received inpatient psychiatric treatment in the two years before pregnancy up until delivery, the HR was 1.14 (95% CI 0.92, 1.41). The association was slightly higher, but not statistically significantly different, for children of mothers without psychiatric history compared to children of mothers with psychiatric history (HR 1.27, 95% CI 0.85, 1.92 compared to 1.07, 95% CI 0.87–1.27, respectively) for children of mothers with psychiatric history. Risk of psychiatric disorders among boys in the continuation group was higher relative to the discontinuation group (HR 1.33, 95% CI 1.08, 1.62) than for girls (HR 0.79, 95% CI 0.60–1.04; p value for interaction <0.05). Restricting to the 1406 children born prior to 2002 resulted in a HR of 0.86 (95% CI 0.62–1.19). Excluding 3752 children whose mothers received other psychotropic medications during pregnancy produced a HR of 1.13 (95% CI 0.85–1.50). Lastly, a HR of 1.08 (95% CI 0.91–1.28) was found when restricting analyses to the 8917 children whose mothers did not receive a diagnosis of a mental or behavioral disorder due to psychoactive substance use during pregnancy.
Discussion
Across our analyses, we observed modestly elevated point estimates, with no statistically significant differences in risk of psychiatric disorders among children whose mothers continued antipsychotic treatment during pregnancy compared to children whose mothers discontinued antipsychotic treatment. Additionally, risks were not statistically significantly higher in the continuation group for any type of psychiatric disorders that we could examine. We also found risks of psychiatric disorders to be similar for the paternal continuation and discontinuation groups. The study does not provide evidence to indicate that exposure to antipsychotics in pregnancy increases risk of mental disorders.
Our findings are in line with those recently reported by Wang et al. [27], who also did not find evidence of an association between prenatal exposure to antipsychotics and offspring neurodevelopmental disorders (specifically attention deficit/hyperactivity disorder or autism spectrum disorder). If further studies can replicate these findings, they indicate women who need to continue antipsychotic medications during pregnancy can do so without adverse psychiatric outcomes in their offspring. A UK study [28] followed children up to 5 years of age and reported a relative risk of 0.83 (95% CI 0.49, 1.39) for neurodevelopmental and behavioral disorders among children whose mothers continued antipsychotic treatment in pregnancy compared to those who discontinued. More studies with longer follow-up time are needed. Additionally, to minimize confounding by indication (i.e. the disorder being treated), studies should avoid comparing children whose mothers needed antipsychotic medication during pregnancy to women who did not (or never) received antipsychotic treatment. Offspring of parents with severe mental illness are at higher risk of mental illness themselves [29]; with studies suggesting that genetic factors influence risk [30, 31] and a high heritability of disorders like schizophrenia [32] and bipolar disorder [33]. We found a modestly elevated risk for boys when sex-specific HRs were calculated. There is no previous literature highlighting effect modification by sex on the association between in utero antipsychotic exposure and psychiatric disorders; however, literature in the field of stress during pregnancy has suggested male fetuses are more vulnerable to in utero stress exposure than females [34, 35]. This finding warrants further investigation.
This study uses Danish register data, which provides nationwide coverage and a large sample. Additionally, the use of the discontinuation group as the comparator, rather than a group of children born to mothers without psychiatric disorders, is a strength. While the influence of the disorders being treated by antipsychotics cannot be eliminated, confounding by indication and genetic factors is reduced in the present study design; all mothers under study have a disorder requiring treatment. We repeated the analyses using paternal antipsychotic use during the same window which further allowed examination of whether associations were confounded by shared environmental or genetic variables.
There are also several limitations. First, although Danish registers provide data on the entire population, minimizing selection bias, we excluded 2.2% of children born in 1998–2015 (with missing/unfeasible gestational age, chromosomal abnormalities, or missing links to their biological father). Although any impact is likely to be small, this may have introduced selection bias. Second, our study aimed to investigate exposure to maternal antipsychotic use. However, registers hold data on redeemed prescriptions, which may not reflect actual use. This may result in misclassification of some unexposed children as exposed, leading to more conservative estimates. Third, there could be various indications for antipsychotic use. As well as treating symptoms of psychosis, antipsychotics can be used (normally at higher doses) [36] to control nausea and vomiting in pregnancy [36, 37]. The available indication data showed <0.1% of children to be exposed due to antipsychotics prescribed to treat nausea or vomiting; however, 39.6% of indications were missing or unspecified. Fourth, although our study is large with long follow-up time, some children may not reach the typical age for psychiatric disorder diagnosis and it may be underpowered. Our study was not able to consider some other specific types of psychiatric outcomes that would be of interest, e.g. intellectual disabilities, mood disorders or schizophrenia. However, our results suggest that if risk of psychiatric disorders is increased among children in the continuation group compared to the discontinuation group, this is likely to be modest (upper 95% CI 1.30), and restricting to the oldest children in the cohort did not produce statistically significantly different results. Due to the low prevalence of exposure, it was not possible to consider the association in more detail, for example dosages. Similar studies should be carried out in populations with more exposed cases and follow-up time, which may allow more detailed consideration of the exposure and inclusion of further types of psychiatric outcomes.
This study does not provide evidence of increased risk of psychiatric disorders among children of women who continue antipsychotic treatment during pregnancy. The ultimate decision whether to discontinue or continue antipsychotics in pregnancy is complex with additional negative outcomes to consider. Other long-term outcomes of particular interest to study could be motor coordination, given the evidence for transient neuromotor deficits in antenatally exposed infants [14], and diabetes or metabolic syndrome, given that atypical antipsychotics confer an increased risk for gestational diabetes [38]. The risk of further mental health issues stemming from discontinuation should also be considered: relapse can be over twice as likely in people who stop antipsychotic medication, compared with those who continue on it [39]. Adverse effects of untreated maternal mental illness on fetal, neonatal, and child development are well documented, including reduced birthweight, reduced gestational term, and increased risk for obstetric complications and behavioral teratogenicity (affecting intellectual and emotional functioning) detectable in neonates to preschoolers [15, 16, 40–45]. Guidelines urge caution but do not give a blanket recommendation that women should discontinue antipsychotics before pregnancy. Guidelines of the American College of Obstetricians and Gynecologists advise against routine use of antipsychotics in pregnancy, but also highlight detrimental effects of untreated disorders, like schizophrenia [46]. Careful evaluation of risks and benefits of antipsychotic use during pregnancy with input from mental health professionals is advisable, in line with guidelines [46, 47].
Supplementary information
Acknowledgments
Author contributions
TMO and VB conceived the study idea. All authors contributed to the design of the study and the interpretation of the results. NCM conducted the analysis and drafted the manuscript. All authors critically revised it and approved the final version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
NCM, TR, XL, TMO, and VB are supported by the National Institute of Mental Health (NIMH) (R01MH122869). XL is also supported by the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 891079. TMO is supported by iPSYCH, the Lundbeck Foundation Initiative for Integrative Psychiatric Research (R155-2014-1724), The Lundbeck Foundation (R313-2019-569), AUFF NOVA (AUFF-E 2016-9-25), and Fabrikant Vilhelm Pedersen og Hustrus Legat. VB has received funding from the Netherlands Organization for Scientific Research (clinical fellow and VENI incentive). The investigators conducted the research independently. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Data availability
No additional data available. Access to individual-level Denmark data is governed by Danish authorities. These include the Danish Data Protection Agency, the Danish Health Data Authority, the Ethical Committee, and Statistics Denmark. Each scientific project must be approved before initiation, and approval is granted to a specific Danish research institution. Researchers at Danish research institutions may obtain the relevant approval and data. International researchers may gain data access if governed by a Danish research institution having needed approval and data access.
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Danish Data Protection Agency. By Danish law, no informed consent is required for a register based study using anonymised data.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Veerle Bergink, Trine Munk-Olsen.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01223-y.
References
- 1.Zuidema SU, Johansson A, Selbaek G, Murray M, Burns A, Ballard C, et al. A consensus guideline for antipsychotic drug use for dementia in care homes. Bridging the gap between scientific evidence and clinical practice. Int Psychogeriatr. 2015;27:1849–59. doi: 10.1017/S1041610215000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christian R, Saavedra L, Gaynes BN, Sheitman B, Wines RCM, Johas DE, et al. Future research needs for first- and second-generation antipsychotics for children and young adults [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012. [PubMed]
- 3.Kessler RC, Angermeyer M, Anthony JC, De Graaf R, Demyttenaere K, Gasquet I, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Toh S, Li Q, Cheetham C, Cooper WO, Davis RL, Dublin S, et al. Prevalence and trends in the use of antipsychotic medications during pregnancy in the U.S., 2001–2007: a population-based study of 585,615 deliveries. Arch Women’s Ment Health. 2013;16:149–57. doi: 10.1007/s00737-013-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park Y, Huybrechts KF, Cohen JM, Bateman BT, Desai RJ, Patorno E, et al. Antipsychotic medication use among publicly insured pregnant women in the United States. Psychiatr Serv. 2017;68:1112–9. doi: 10.1176/appi.ps.201600408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen I, McCrea RL, Osborn DJ, Evans S, Pinfold V, Cowen PJ, et al. Discontinuation of antipsychotic medication in pregnancy: a cohort study. Schizophr Res. 2014;159:218–25. doi: 10.1016/j.schres.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Reis M, Kallen B. Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol. 2008;28:279–88. doi: 10.1097/JCP.0b013e318172b8d5. [DOI] [PubMed] [Google Scholar]
- 8.Margulis AV, Kang EM, Hammad TA. Patterns of prescription of antidepressants and antipsychotics across and within pregnancies in a population-based UK cohort. Matern Child Health J. 2014;18:1742–52. doi: 10.1007/s10995-013-1419-2. [DOI] [PubMed] [Google Scholar]
- 9.Newport DJ, Calamaras MR, DeVane CL, Donovan J, Beach AJ, Winn S, et al. Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. Am J Psychiatry. 2007;164:1214–20. doi: 10.1176/appi.ajp.2007.06111886. [DOI] [PubMed] [Google Scholar]
- 10.Moody CA, Robinson SR, Spear LP, Smotherman WP. Fetal behavior and the dopamine system: activity effects of D1 and D2 receptor manipulations. Pharmacol Biochem Behav. 1993;44:843–50. doi: 10.1016/0091-3057(93)90015-l. [DOI] [PubMed] [Google Scholar]
- 11.Popolo M, McCarthy DM, Bhide PG. Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon. Dev Neurosci. 2004;26:229–44. doi: 10.1159/000082140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry. 2014;5:47. doi: 10.3389/fpsyt.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belujon P, Grace AA. Dopamine system dysregulation in major depressive disorders. Int J Neuropsychopharmacol. 2017;20:1036–46. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poels EMP, Schrijver L, Kamperman AM, Hillegers MHJ, Hoogendijk WJG, Kushner SA, et al. Long-term neurodevelopmental consequences of intrauterine exposure to lithium and antipsychotics: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2018;27:1209–30. doi: 10.1007/s00787-018-1177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boden R, Lundgren M, Brandt L, Reutfors J, Andersen M, Kieler H. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi: 10.1136/bmj.e7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin HC, Chen IJ, Chen YH, Lee HC, Wu FJ. Maternal schizophrenia and pregnancy outcome: does the use of antipsychotics make a difference? Schizophr Res. 2010;116:55–60. doi: 10.1016/j.schres.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Scott S. Parenting quality and children’s mental health: biological mechanisms and psychological interventions. Curr Opin Psychiatry. 2012;25:301–6. doi: 10.1097/YCO.0b013e328354a1c5. [DOI] [PubMed] [Google Scholar]
- 18.Bliddal M, Broe A, Pottegard A, Olsen J, Langhoff-Roos J. The Danish Medical Birth Register. Eur J Epidemiol. 2018;33:27–36. doi: 10.1007/s10654-018-0356-1. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. International classification of diseases and related health problems. 10th revision. Geneva: World Health Organization; 1992.
- 20.Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39:38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen FS. Epidemiological studies of obstetric ultrasound examinations in Denmark 1989-1990 versus 1994-1995. Acta Obstet Gynecol Scand. 1999;78:305–9. doi: 10.1080/j.1600-0412.1999.780406.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith GD. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin Pharmacol Toxicol. 2008;102:245–56. doi: 10.1111/j.1742-7843.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 23.Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39:54–7. doi: 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. International classification of diseases: Manual of the international statistical classification of diseases, injuries and causes of death (ICD-8). Geneva: WHO; 1967.
- 25.Petersson F, Baadsgaard M, Thygesen LC. Danish registers on personal labour market affiliation. Scand J Public Health. 2011;39:95–8. doi: 10.1177/1403494811408483. [DOI] [PubMed] [Google Scholar]
- 26.Royston P, White IR. Multiple Imputation by Chained Equations (MICE): implementation in stata. J Stat Softw. 2011;45:1–20. doi: 10.18637/jss.v045.i04. [DOI] [Google Scholar]
- 27.Wang Z, Chan AYL, Coghill D, Ip P, Lau WCY, Simonoff E, et al. Association between prenatal exposure to antipsychotics and attention-deficit/hyperactivity disorder, autism spectrum disorder, preterm birth, and small for gestational age. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2021.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen I, McCrea RL, Sammon CJ, Osborn DP, Evans SJ, Cowen PJ, et al. Risks and benefits of psychotropic medication in pregnancy: cohort studies based on UK electronic primary care health records. Health Technol Assess. 2016;20:1–176. doi: 10.3310/hta20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasic D, Hajek T, Alda M, Uher R. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophr Bull. 2014;40:28–38. doi: 10.1093/schbul/sbt114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.International Schizophrenia Consortium. Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilker R, Helenius D, Fagerlund B, Skytthe A, Christensen K, Werge TM, et al. Heritability of schizophrenia and schizophrenia spectrum based on the Nationwide Danish Twin Register. Biol Psychiatry. 2018;83:492–8. doi: 10.1016/j.biopsych.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Kieseppa T, Partonen T, Haukka J, Kaprio J, Lonnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004;161:1814–21. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- 34.Fineberg AM, Ellman LM, Schaefer CA, Maxwell SD, Shen L, Chaudhury NH, et al. Fetal exposure to maternal stress and risk for schizophrenia spectrum disorders among offspring: differential influences of fetal sex. Psychiatry Res. 2016;236:91–7. doi: 10.1016/j.psychres.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu P, Hao JH, Tao RX, Huang K, Jiang XM, Zhu YD, et al. Sex-specific and time-dependent effects of prenatal stress on the early behavioral symptoms of ADHD: a longitudinal study in China. Eur Child Adolesc Psychiatry. 2015;24:1139–47. doi: 10.1007/s00787-015-0701-9. [DOI] [PubMed] [Google Scholar]
- 36.Howard L, Webb R, Abel K. Safety of antipsychotic drugs for pregnant and breastfeeding women with non-affective psychosis. BMJ. 2004;329:933–4. doi: 10.1136/bmj.329.7472.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Axelsson P, Futtrup TB, Buchgreitz L, Høltzermann M, Martinsen AW, Odgaard HS, et al. Hyperemesis gravidarum: Godkendt på Obstetrisk guidelinemøde januar 2013. Denmark: DSOG; 2013.
- 38.Uguz F. Antipsychotic use during pregnancy and the risk of gestational diabetes mellitus: a systematic review. J Clin Psychopharmacol. 2019;39:162–7. doi: 10.1097/JCP.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 39.Viguera AC, Whitfield T, Baldessarini RJ, Newport DJ, Stowe Z, Reminick A, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164:1817–24. doi: 10.1176/appi.ajp.2007.06101639. [DOI] [PubMed] [Google Scholar]
- 40.Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74:e321–41. doi: 10.4088/JCP.12r07968. [DOI] [PubMed] [Google Scholar]
- 41.Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophr Bull. 2011;37:1200–8. doi: 10.1093/schbul/sbq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis G, Rice F, Harold GT, Collishaw S, Thapar A. Investigating environmental links between parent depression and child depressive/anxiety symptoms using an assisted conception design. J Am Acad Child Adolesc Psychiatry. 2011;50:451–9 e1. doi: 10.1016/j.jaac.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uguz F, Yuksel G, Onur OS, Karsidag C, Gezginc K, Arpaci N. Neonatal outcomes in pregnant women with untreated and treated panic disorder. Compr Psychiatry. 2018;87:107–11. doi: 10.1016/j.comppsych.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Hunter SK, Mendoza JH, D’Anna K, Zerbe GO, McCarthy L, Hoffman C, et al. Antidepressants may mitigate the effects of prenatal maternal anxiety on infant auditory sensory gating. Am J Psychiatry. 2012;169:616–24. doi: 10.1176/appi.ajp.2012.11091365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkatesh KK, Riley L, Castro VM, Perlis RH, Kaimal AJ. Association of antenatal depression symptoms and antidepressant treatment with preterm birth. Obstet Gynecol. 2016;127:926–33. doi: 10.1097/AOG.0000000000001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 92: use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001–20. doi: 10.1097/AOG.0b013e31816fd910. [DOI] [PubMed] [Google Scholar]
- 47.Royal College of Obstetricians and Gynaecologists. Management of Women with Mental Health Issues during Pregnancy and the Postnatal Period (Good Practice No. 14). London: RCOG; 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No additional data available. Access to individual-level Denmark data is governed by Danish authorities. These include the Danish Data Protection Agency, the Danish Health Data Authority, the Ethical Committee, and Statistics Denmark. Each scientific project must be approved before initiation, and approval is granted to a specific Danish research institution. Researchers at Danish research institutions may obtain the relevant approval and data. International researchers may gain data access if governed by a Danish research institution having needed approval and data access.


