Abstract
Competitive Offshore Ocean Sailing is a highly demanding activity in which subjects are exposed to psychophysical stressors for a long time. To better define the physiological adaptations, we investigated the stress response of subjects exposed to 3-days long ocean navigation with disruption of circadian rhythms. 6 male subjects were involved in the study and provided urine and saliva samples before setting sail, during a single day of inshore sailing, during 3-days long ocean navigation, and at the arrival, to measure oxidative stress, cortisol, nitric oxide metabolites (NOx) and metabolic response. Motion Sickness questionnaires were also administered during the navigation. The crew suffered a mean weight loss of 1.58 kg. After the long navigation, a significant increase in ROS production and decrease in total antioxidant capacity and uric acid levels were observed. Lipid peroxidation, NO metabolites, ketones, creatinine, and neopterin levels were also increased. Furthermore, a significant increase in cortisol levels was measured. Finally, we found a correlation between motion sickness questionnaires with the increase of NOx, and no correlation with cortisol levels. Physical and psychological stress response derived from offshore sailing resulted in increased oxidative stress, nitric oxide metabolites, and cortisol levels, unbalanced redox status, transient renal function impairment, and ketosis. A direct correlation between motion sickness symptoms evaluated through questionnaires and NOx levels was also found.
Subject terms: Physiology, Biomarkers, Medical research, Molecular medicine
Introduction
Sailing is a worldwide popular activity that includes various types of boats and disciplines. Offshore Ocean Sailing (OOS) could represent a relaxing leisure activity, but in case of competitions and regattas it is considered one of the most extreme endurance sports, exposing the crew to long-lasting, physically and psychologically demanding efforts1. Competitive OOS usually implies prolonged periods—ranging from days to months—spent at sea, in an extremely harsh environment, in isolation and self-sufficiency, far from safe harbours and with limited access to external aid or rescue2. The boat could represent an extremely uncomfortable, cold, wet, unstable, and enclosed environment without any privacy or comfort. In particular, racing boats are performance-oriented and with little or no comfort onboard. Energy expenditure during offshore sailing is high3, and it is challenging to maintain an adequate nutrient intake onboard, especially during harsh weather conditions4. Negative energy balance often results in weight loss, decreased body fat percentage, and reduced muscle strength, proportionally to the length of the race5,6. Proper sleep management is also essential to maintain adequate performance7. Sailors adopt polyphasic sleep techniques and incur severe sleep restrictions during competition, thus resulting in cognitive performance and alertness decrease8.
The study of adaptations to extreme environments is gaining popularity. Nonetheless, the literature exploring short- and medium-term adaptations to OOS is still insufficient. Seafarers are also exposed to high and prolonged stress levels. Loneliness, circadian rhythms disruption, and fatigue often result in alterations in their physical9 and psychological domains10. Consistently, high-stress levels can induce a modification in normal circadian fluctuations of cortisol, a glucocorticoid whose peak level in normal conditions is recorded after awakening11. During OOS and other highly stressing activities, a flattening of this curve has been recorded, with sustained high cortisol levels throughout the effort12. Due to the constant instability, the maritime environment significantly impacts cognitive and neuromuscular activity13. Moreover, motion sickness often affects people exposed to transportation and visual instability. The underlying cause is still ambiguous, some theories address a possible sensory mismatch mechanism between perceived and expected stimuli14. Others ascribe it to situations in which we are not able to maintain postural stability such as during maritime navigations15. A large percentage of people experience seasickness, with higher work-related risks and detrimental effects on the performance of sailors and seafarers14,16.
During inshore and offshore sailing, physical effort is inconstant, characterized by high intensity and anaerobic bursts, with increases in oxygen consumption and heart rate1,17–19. Such activity often leads to heat loss and dehydration5,20, and produces muscle damage and oxidative stress (OxS). OxS levels have been investigated in other endurance sports, such as triathlon21, ultra-endurance races22, and swimming23, revealing an overproduction of Reactive Oxygen Species (ROS) and a depletion of total antioxidant capacity (TAC). The redox status—namely, the equilibrium between ROS and TAC—deeply affects intracellular function. Maintaining ROS homeostasis is crucial for normal cellular responses, while overproduction is deleterious and can damage cell structures (i.e., proteins, membrane, DNA), leading to progressive organism’s disfunction24,25. Along with ROS production, increased levels of nitrogen metabolites and in particular nitric oxide (NO), a crucial messenger in many tissues such as endothelium and gastrointestinal tissues, can be found under stressful condition26. Nonetheless, a formal assessment in sailing sports and specifically during OOS is still lacking.
This study aimed to investigate oxidative stress variations in sailors involved in OOS. To have a more accurate definition of stress, we also evaluated cortisol levels, biochemical profile, and renal function markers creatinine and neopterin. Motion sickness has been investigated through neurophysiological symptoms questionnaires.
Methods
Experimental design
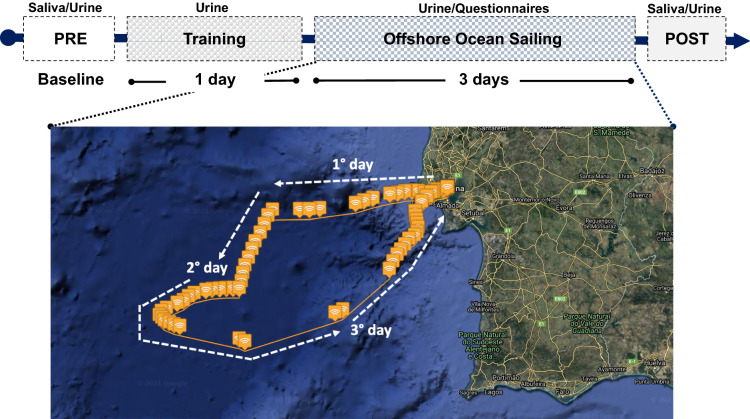
This observational study was carried on in November 2020 during an OOS racing-oriented training that included a theoretical part ashore, a full training day of inshore sailing, and 3 days of non-stop OOS roughly between the latitudes of Gibraltar and Lisbon. The crew sailed in a Class 40 (ITA 84) racing yacht and, during the navigation, was divided into two groups alternating rest and duty shifts every 3 h. Figure 1 depicts the study protocol and samplings. Urine and saliva samples and anthropometric measurements were obtained ashore during the theoretical part (PRE) and approximately two hours after the end of the navigation (POST) to be as accurate as possible. Further, two urine samples were obtained during the single day of inshore navigation (Training) and three times a day during OOS (Sailing).
Figure 1.
Sketch of the experimental protocol and map showing the navigation route. Data were collected before departure (PRE), during the inshore navigation (Training) during each day of Offshore Ocean Sailing (from 1° to 3° day), and at arrival (POST). GPS data and map were obtained with Spot Gen3, SPOT LCC, Globastar, Inc, Covington, Louisiana, USA. Maps by Google, Inst. Geogr. Nacional. Modified geographic map was edited with Microsoft Office PowerPoint 2007, Microsoft Corporation, Washington, USA, version 16.16.27, https://www.microsoft.com/it-it/microsoft-365/powerpoint and at the top was insert the experimental protocol timeline.
Subjects
This study involved six male sailors: the skipper (S.F.)—a professional sailor with experience in ocean solo races—and five recreational sailors with good expertise in seamanship The total number of subjects was determined by the maximum crew that the boat could support and by chance all the participants to this training were healthy male. Their characteristics are reported in Table 1.
Table 1.
Anthropometric parameters of the six sailors.
| Age | Height (cm) | Weight (kg) PRE |
Weight (kg) POST |
BMI (kg m−2) PRE |
BMI (kg m−2) POST |
|
|---|---|---|---|---|---|---|
| 1 | 25 | 170 | 65.2 | 63 | 22.6 | 21.8 |
| 2 | 41 | 180 | 86.2 | 84.5 | 26.6 | 26.0 |
| 3 | 31 | 185 | 93.3 | 90.2 | 27.2 | 26.4 |
| 4 | 51 | 187 | 125.2 | 123.1 | 35.8 | 35.2 |
| 5 | 36 | 184 | 83.6 | 83.5 | 24.6 | 24.6 |
| 6 | 26 | 177 | 84.2 | 83.1 | 26.9 | 26.5 |
| Mean ± SD | 35.16 ± 9.70 | 180.50 ± 6.28 | 89.62 ± 19.76 | 87.90 ± 19.59* | 27.28 ± 4.52 | 26.75 ± 4.50 |
Parameters collected from the sailors at Pre and Post.
BMI Body Mass Index. *p < 0.05.
Navigation
The offshore navigation lasted 3 days, during which the crew sailed into the ocean for a total of 420 miles, with a top speed of 14.89 kn.
During the first day and night, the atmospheric conditions were challenging. Swell of 3/3.5 m Significant Wave Height (SWH) from NW and wind from 5 to 15 kn from S-SE resulted in a boat's inconvenient motion. During the day, the wind increased between 25 and 40 kn in gusts as sailors encountered two significant squalls and had to flee downwind. During the night, sailors were forced to maintain a 70° true wind angle (TWA) sailing upwind to cope with waves, and the wind speed increased up to 45 kn. After the first day, the crew was objectively stressed. These harsh conditions induced major seasickness and vomiting in one crew member, with a total inability to work on deck. This subject started to recover only at the end of the navigation, during which he never ate and vomited many times without being able to drink and rehydrate. Two other people vomited but were not impaired at work. Liquid reintegration started the day after. On the second day, the conditions changed, with waves height reduced to 1–2 m SWH. The boat headed downwind, hoisting a code zero sail, maintaining the boat flat at an average speed of 9–10 kn with 15–25 kn of wind speed. The navigation remained stable until the end of the navigation in Lisbon on the third day.
Motion Sickness Questionnaire
To study motion sickness, previously validated Global Sickness Rating Scale (GSRS)27 and Motion Sickness Questionnaire (MSQ)14 were used. They have been administered once per day during the Offshore Sailing, in the evening, at 9 PM, according to the 3 h shifts.
Saliva and urine collection
Approximately 1 mL of saliva was obtained before and after the training and collected in Salivette devices (Sarstedt, Nümbrecht, Germany) at 8 AM. The subjects were trained on the correct use as previously reported28,29.
Urine samples were collected by voluntary voiding in a sterile container before and after the training and every day during the training and navigation at 9 AM, 3 PM, and 9 PM according to the 3 h shifts. All samples were stored at 4 °C in a portable cooler on board and during the transport back to the laboratory. The specimens were then stored in multiple aliquots at − 20 °C until assayed and thawed only once before analysis.
ROS and TAC
An X-band electron paramagnetic resonance spectroscopy (9.3 GHz) (E-Scan, Bruker Co., MA, USA) was used to detect ROS production and TAC values. Saliva samples were stabilized at 37 °C using a Temperature and Gas Controller “Bio III” unit (Noxigen Science Transfer & Diagnostics GmbH, Germany), interfaced with the E-Scan. ROS production and TAC assessment methods were previously described28,30. Samples were analyzed in triplicate.
Cortisol
The concentration of free cortisol in the saliva was quantitatively determined through ELISA method according to the manufacturer’s protocol (COR(Cortisol) ELISA Kit; FineTest, Wuhan Fine Biotech Co.) as previously described31.
8-Isoprostane
Lipid peroxidation was assessed in urine by competitive immunoassay measuring 8-isoprostane concentration (8-iso-PGF2α) (Cayman Chemical, USA). The method was previously described32.
NO metabolites
NO metabolites (NOx = NO2 + NO3) levels were assessed in urine by a method based on the Griess reaction33, using a commercial kit (Cayman, BertinPharma, Montigny le Bretonneux, France)28,33.
Every assessment was carried out in duplicate and read by a microplate reader spectrophotometer (Infinite M200, Tecan Group Ltd., Männedorf, Switzerland).
Creatinine, neopterin, and uric acid
Urinary creatinine, neopterin, and uric acid concentrations were measured by isocratic high-pressure liquid chromatography. The calibration curves were linear over the range of 0.125–1 μmol/L, 0.625–20 mmol/L, and 1.25–10 mmol/L for neopterin, uric acid, and creatinine levels, respectively. Inter-assay and intra-assay coefficients of variation were < 5%. Methods were previously described28,34.
Urine standard analysis
The Urine Test Strips (Combi screen 11sys PLUS, GIMA, Gessate, Milan, Italy) were used to semi-quantitative determinations of bilirubin, urobilinogen, ketones, proteins, blood, pH, leukocytes, and specific gravity/density in urine. The tests were performed in duplicate.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism package (GraphPad Prism 9.0.1, GraphPad Software Inc., San Diego, CA) and SPSS statistics software (IBM corporation). Data are presented as mean ± SD. Statistical analyses were performed using: non-parametric tests, Wilcoxon matched-pairs signed-rank test for independent samples (ROS and TAC and cortisol in saliva), due to the small sample size for compared pre vs. post and ANOVA repeated measures, with Dunn’s multiple comparison tests to further check the among-groups significance. p < 0.05 was considered statistically significant..dCohen was used for calculating the size effect, and Confidence Interval 95% for dCohen was calculated.
Change Δ% estimation [((post value-pre value)/pre-value) × 100] is also reported in the text. Spearman correlation (r) with 95% confidence intervals. Chi-square (χ2) test and phi coefficient (ϕ) were used to evaluate correlation and association.
Ethics approval
This research study was approved by the Ethical Committee of University of Milan, Italy (Aut. n° 37/17). All procedures conformed to the standards set by the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All authors have read the manuscript and expressed their consent for the publication.
Results
A significant difference (p < 0.05) was observed in weight (kg) between Pre and Post (Table 1). All crew members suffered a loss of weight (Mean weight loss: 1.58 kg).
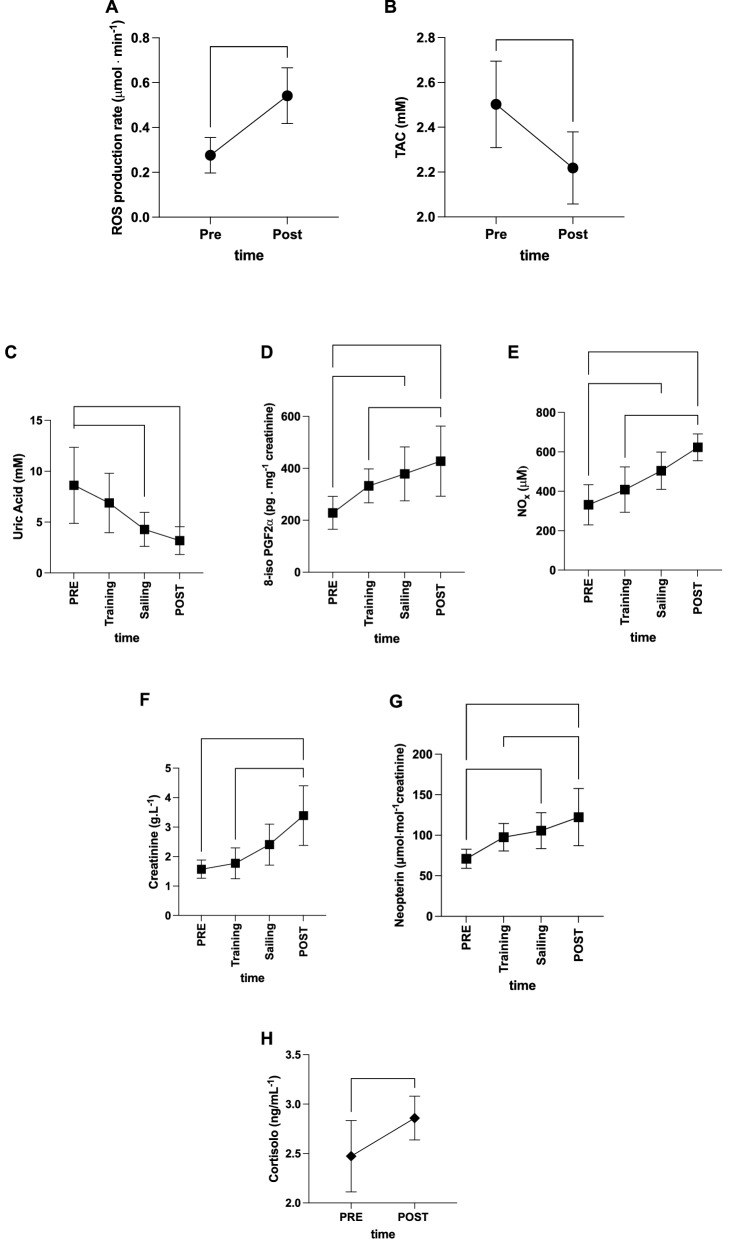
An unbalance of oxidative stress was found. ROS production rate in saliva significantly (p < 0.01) increased at Post OOS (0.27 ± 0.07 vs 0.54 ± 0.16 μmol min−1, dCohen = 0.74; 95% CI 1.171–4.326; Fig. 2A) with a significant decrease (p < 0.05) in antioxidant capacity (TAC 2.50 ± 0.19 vs 2.21 ± 0.16 mM, dCohen = 1.65; 95% CI 2.961–0.341; Fig. 2B). In addition, uric acid measured in urines significantly decreased (range p < 0.05–0.001) during sailing (8.61 ± 3.74 vs. 4.28 ± 1.66 vs. 3.18 ± 1.36 mM at Post, dCohen = 1.49, 95% CI 2.777–0.216; and dCohen = 1.93, 95% CI 3.3–0.56 respectively; Fig. 2C). A significant increase (range p < 0.05–0.001) in lipid peroxidation during OOS (8-isoprostane 228.40 ± 63.1 vs 378.68 ± 103.69 pg mg−1 creatinine; dCohen = 1.75, 95% CI 0.42–3.081) and at Post OOS (8-isoprostane 427.70 ± 134.98 pg mg−1 creatinine, dCohen = 1.89, 95% CI 0.53–3.253; Fig. 2D) was measured; besides NO metabolites significantly (range p < 0.05–0.001) increased during OOS (NOx 331.8 ± 102.2 vs 504.5 ± 94.85 μM; dCohen = 1.75, 95% CI 0.422–3.085) and at Post (NOx 331.8 ± 102.2 vs 623.0 ± 68.24 μM; dCohen = 3.35, 95% CI 1.656–5.218) (Fig. 2E).
Figure 2.
Biomarkers kinetic. Time course of: (A) radical oxygen species (ROS) production rate (μmol·min−1) and (B) total antioxidant capacity (TAC—mM) in saliva assessed by EPR; (C) uric acid (mM); (D) 8-isoprostane (8-iso-PGF2α, pg mg−1 creatinine); (E) nitric oxide metabolites (NOx, μM), (F) creatinine (g L−1), (G) neopterin (μmol mol−1creatinine), concentrations detected in urine. In (H) cortisol levels (ng/mL) measured in saliva. *p < 0.05, **p < 0.01, ***p < 0.001 significantly different. Figure created with: GraphPad Prism, GraphPad Software inc. California, USA, version 9.0.1, https://www.graphpad.com/.
The time course in Fig. 2F,G showed a significant increase post OOS (p < 0.05–0.01) of creatinine (1.57 ± 0.31 vs. 1.77 ± 0.52 vs. 3.38 ± 1.01 g L−1; dCohen = 2.43, 95% CI 0.938–3.922) and neopterin/creatinine (70.92 ± 11.80 vs. 105.603 ± 22.14 vs. 23.33 ± 35.25 μmol mol−1 creatinine: dCohen = 1.99, 95% CI 0.609–3.377, and dCohen = 1.95, 95% CI 0.579–3.33) levels respectively at sailing and post OOS.
Finally, a significant increase (p < 0.05) in cortisol levels was measured Post OOS in saliva (2.47 ± 0.36 vs. 2.85 ± 0.22 ng/mL−1, dCohen = 1.27, 95% CI 0.033–2.515; Fig. 2H).
No significant differences were recorded in GSRS for different items and MSQ during the 3 days of offshore navigation (see Tables 2, 3).
Table 2.
Global Sickness Rating Scale (GSRS), number of subjects and (total value) are reported for each day.
| Sailors (n = 6) | Global Sickness Rating Scale (GSRS) | ||
|---|---|---|---|
| Scores | Day 1 | Day 2 | Day 3 |
| 1: No symptoms | n2 (2) | n2 (2) | n5 (5) |
| 2: Initial symptoms of motion sickness but no nausea | – | n1 (2) | – |
| 3: Mild Nausea | n1 (3) | – | n1 (3) |
| 4: Moderate Nausea | – | n1 (4) | – |
| 5: Severe nausea and/or retching | – | n1 (5) | – |
| 6: Vomiting | n3 (18) | n1 (6) | – |
| Total score | 23 ± 3.8 | 19 ± 2.1 | 8 ± 0.8 |
Total scores ± SD are reported.
Table 3.
Motion Sickness Questionnaire (MSQ).
| Sailors (n = 6) | Motion Sickness Questionnaire (MSQ) | ||
|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |
| General discomfort | 1.0 ± 1.2 | 1.33 ± 1.03 | 0.83 ± 0.72 |
| Fatigue | 1.16 ± 0.98 | 1.16 ± 0.98 | 1.33 ± 0.81 |
| Headache | 0.83 ± 1.16 | 0.66 ± 1.21 | 0.33 ± 0.51 |
| Eye Strain | 0.16 ± 0.40 | 0.16 ± 0.40 | 0.33 ± 0.51 |
| Difficulty focusing | 0.16 ± 0.40 | 0.0 ± 0.0 | 0.16 ± 0.40 |
| Increased salivation | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.16 ± 0.40 |
| Fulness of head | 0.80 ± 1.16 | 0.83 ± 0.51 | 0.33 ± 0.51 |
| Blurred vision | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Dizziness as illusory sense of motion (eyes open) | 0.33 ± 0.51 | 0.33 ± 0.51 | 0.0 ± 0.0 |
| Dizziness as illusory sense of motion (eyes closed) | 0.33 ± 0.81 | 0.66 ± 1. 21 | 0.16 ± 0.40 |
| Vertigo | 0.50 ± 0.83 | 1.33 ± 1.21 | 0.16 ± 0.40 |
| Stomach awareness | 1.50 ± 1.22 | 1.33 ± 1.21 | 0.83 ± 0.75 |
| Burping | 1.50 ± 1.22 | 0.66 ± 0.81 | 0.16 ± 0.40 |
Mean (± SD) values of the investigated variables.
Urine standard parameters are reported in Table 4. A significant increase in urinary ketones levels was detected during the navigation. pH and bilirubin values also increased but did not reach statistical significance.
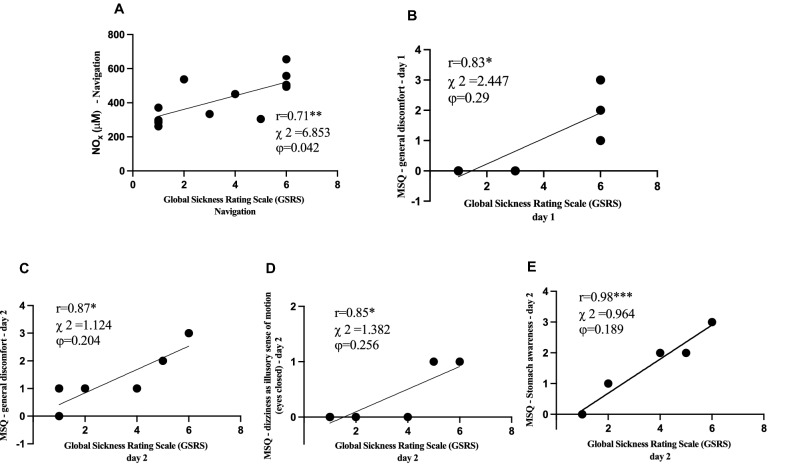
Finally, a positive relationship was found during navigation (training and sailing) between NO metabolites and Global Sickness Rating Scale (GSRS) scores (r = 0.94, p < 0.05) (Fig. 3A); between GSRS score and specific items of Motion Sickness Questionnaire (MSQ), in details: during the 1st day with General discomfort r = 0.83 (p = 0.04) (Fig. 3B), during 2nd day with General discomfort r = 0.87 (p = 0.02) (Fig. 3C), dizziness as illusory sense of motion (eyes open) r = 0.85 (p = 0.03) (Fig. 3D), and stomach awareness r = 0.98 (p = 0.0007) (Fig. 3E); during the 3rd day no correlation was found.
Figure 3.
Relationship panel plot of: (A) NOx and GSRS during navigation; (B) GSRS and general discomfort of MSQ at 1st day; at the 2nd day, the panels show the correlation between GSRS and specific items of MSQ. (C) General discomfort and GSRS. (D) Dizziness as illusory sense of motion (eyes open) and GSRS. (E) Stomach awareness and GSRS. A significant linear relationship (p < 0.05–0.001) between parameters was estimated. The correlation coefficient (r), Chi-Square (χ2) and Phi coefficient (ϕ) are reported. Figure created with: GraphPad Prism, GraphPad Software inc. California, USA, version 9.0.1, https://www.graphpad.com/.
Discussion
To our knowledge, this is the first study to investigate oxidative stress on urine and saliva sampled from non-professional sailors during OOS and possible correlation with motion sickness. This setting is particularly challenging, with rapid changes in terms of environmental conditions and circadian rhythms.
According to the results, subjects exposed to OOS suffer a significant multifactorial increase in oxidative stress and cortisol. A small number of studies considered modifications in cortisol levels in sailors and seafarers. Oldenburg et al. found that cortisol awakening levels were highly dependent on subjective stress perception and work type. Mental work was also associated with higher cortisol levels than physical work35. This is confirmed by cortisol levels found in maritime pilots, increasing their tasks' difficulty36. Some studies’ results reflect that seafarers’ cortisol levels are higher in port stays than at sea, probably because of the break of a working routine found during days at sea35,37. Liberzon et al. found that cortisol response at awakening in the crew increased with navigation time and was not correlated with sleep duration or patterns37. Other confined environments in which the crew suffers a sudden and prolonged change in circadian rhythms and psychosocial stress are, for example, military ships, submarines, or spaceships. In these environments, a flattening of the standard cortisol fluctuation profile has also been recorded under stressful conditions38,39. Similar results were obtained by Gunnarsson et al. on ocean sailors studied for a more extended period during an offshore regatta. They also reported an initial increase in cortisol levels at the beginning of the navigation, with a flattening of the fluctuation when sailors reached the regularization of the shift regimen12. Our study found a significant increase in cortisol levels in sailors after 3-day-long offshore navigation compared to their basal level (+ 15%, see Fig. 2H). These results may be related to the significant stressors that maritime personnel and offshore ocean sailors have to endure, particularly fatigue and poor sleep quality. From a physiological point of view, sleep disturbances induce a decrease in physical and cognitive performance in sailors8 and a disruption of normal cortisol secretion, causing the activation of pro-inflammatory pathways40,41. Physical exercise can also induce a modification in cortisol secretion42. During offshore sailing, a basal level of muscle activation is needed to cope with instability and to aid thermoregulation, but short bursts of anaerobic exercise are required in all the maneuvers1,17–19. It is, therefore, reasonable to think that these aspects also contribute to cortisol levels alterations.
During day one, three subjects suffered from motion sickness and had vomiting episodes. Although cortisol is known to correlate with acute nausea and vomiting43,44, we found no significant correlation between this hormone and the seasickness scale questionnaires administered14,27. Nonetheless, we have been able to measure nitric oxide metabolites (NOx) levels throughout the navigation. Nitric oxide is involved in many gastrointestinal mucosal mechanisms26, and previous studies found a correlation between salivary and serum NO levels with vomiting syndromes and Gastroesophageal Reflux Disease (GERD)43–46. Nausea caused by motion sickness is also characterized by gastric dysrhythmias47. In accordance with these studies, we found a significant linear relationship between NOx levels and GSRS during the first day of navigation, during which the subjects suffered the most intense motion sickness (Fig. 3).
During inshore regattas, short bursts of high-intensity activity are described18,19,48. However, even if data regarding physical effort during offshore sailing seem comparable with inshore activity17, the evidence is scarce, difficult to obtain, and limited to measuring the effects of energy expenditure and physical effort activity after the race. Weight loss, fat percentage decrease, lower limb strength, and muscle mass reduction are often reported1,3,4,6,49. ROS production is enhanced by exercise21–23,29,50. In particular, anaerobic exercise can induce prolonged oxidative stress up to 24 h after the effort51,52, which is then balanced by an enhanced antioxidant response21,23,53. In America’s Cup sailors, oxidative stress markers after the race were higher than their baseline levels, especially in crew members involved in high-intensity physical work54. Our study is the first to analyze oxidative stress markers during OOS. Our results show a significant increase in ROS production after the navigation. The imbalance between the ROS production rate (about + 100%) and the antioxidant scavenging (− 12%, see Fig. 2A–C) reflected the increase in the oxidative stress-related damage to lipids (+ 87%; see Fig. 2D). Oxidative stress is highly involved with inflammation and endothelial dysfunction in developing chronic cardiovascular diseases55,56. Even though more evidence should be produced on ocean sailors, the effects of oxidative stress exposition on seafarers can be a potential cause of their higher cardiovascular risk and mortality rate for coronary heart disease57–59.
Neopterin and creatinine concentration can increase during systemic oxidative stress, as shown in some studies23,28,29,60. Even if a decrease in kidney function can be a hint of organ damage during endurance sports, it is often the result of many physiological responses to stress and physical demands21,22,61. In our study, an increase of evaluated biomarkers concentrations was observed during and post-offshore sailing and was associated with ROS production. In any case, this study did not assess the chronic or long-term effects of offshore sailing. Mainly referred to kidney activity, the subjects manifested a temporary “impairment of renal function” as a likely physiological or adaptive response to dehydration. This could also be linked to significant weight loss (see Table 1) and vomiting, which changes ketones concentration, pH, and specific density (see Table 4). Ketones increase could also hint at how athletes’ metabolism copes with high energy demand and stress. Their production is stimulated by low insulin, high glucagon, and epinephrine concentrations, suggesting a shift to metabolic efficiency and fuel sparing of the organism exposed to endurance exercise and fasting62,63. They are also second messengers for many pathways, such as food intake stimuli64. The ketogenic regimen is also related to the increase of lipid metabolism65 which in the case of OOS is often associated with the decrease of body fat percentage and weight loss4,6,49. Considering that sailors are exposed to harsh environmental conditions and that motion sickness and working rates can influence nutrition habits during a race4, it is of utmost importance to maintain an adequate water intake during navigation to prevent renal damage and to keep proper caloric intake to sustain physical performance.
Table 4.
Urine standard analysis.
| Sailors n = 6 | |||||
|---|---|---|---|---|---|
| PRE | Day 1 | Day 2 | Day 3 | POST | |
| Bilirubin (μmol L−1) | 5.65 ± 8.80 | 6.16 ± 9.55 | 11.66 ± 9.07 | 17.00 ± 1.09 | 11.16 ± 8.75 |
| Urobilinogen (μmol L−1) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Ketones (mmol L−1) | 1.0 ± 2.23 | 1.0 ± 2.0 | 16.16 ± 18.49* | 12.16 ± 14.2* | 9.0 ± 5.47* |
| Protein (mg dL−1) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Blood (Ery μL−1) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| pH | 5.16 ± 0.40 | 6.50 ± 1.25 | 6.12 ± 1.43 | 6.25 ± 1.04 | 5.87 ± 1.18 |
| Leucocytes (Leuko μL−1) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Specific gravity/density | 1.01 ± 0.00 | 1.02 ± 0.00 | 1.03 ± 0.00* | 1.03 ± 0.00* | 1.03 ± 0.00* |
Mean (± SD) values of the investigated variables in the urine test strip in the sailors. Changes in urine standard urinalysis referred to PRE are shown. Statistically significant difference at p < 0.05 (* symbol).
Limitations and conclusions
As for other studies4,6,12,17 that focus on OOS, we found many difficulties in producing reliable data and scientific evidence. The researcher himself, which was part of the crew, had to take part in the strenuous activity schedule, the space for medical devices and samples on board is limited, invasive procedures are complicated to perform because of continuous motion, electronic devices cannot be charged because electrical power is limited and needed for navigation. The complexity of this environment often results in a lack of reliable literature1. Therefore, we chose to obtain urine and saliva samples because of the limited logistic disadvantages of these samples.
A limitation of this study is the lack of data on the quality and duration of sleep. This might have influenced the cortisol level, but Liberzon et al. found no connection between sleep and cortisol levels37, and other OOS experiments show data similar to ours12.
Some previous experiences in literature66 found a correlation between cortisol levels and motion sickness. We speculate that probably due to the low number of investigated subjects and saliva samples, and because we didn’t measure cortisol levels immediately after vomiting episodes, we found no significant correlation between motion sickness questionnaire results and cortisol levels. Therefore, to be more confident on these results our methods could be implemented. Another factor to be considered could be that we investigated only male subjects, and as previously reported by Meissner et al., the cortisol level changes in saliva in male patients could not be significant. Cortisol response in motion sickness, as they suggest, should be corrected for the hour of the day, gender, and basal cortisol levels67. Moreover, high variability was observed in oxidative stress markers, cortisol levels, and motion sickness scales between the same sailors on various days.
Another limitation is that we have not been able to obtain information on sailors’ cardiovascular and metabolic activity during the navigation, even though they have been described in other similar and comparable studies17,18. In the future, we hope we will be able to implement our methods and obtain this data in a similar environment.
However, the present offshore sailing study offers valuable information on the redox state, renal function, and motion sickness response during this high demanding activity. OOS has been shown to induce an increase in oxidative stress biomarkers and NO metabolites. A correlation was found also between the increase in NO metabolites level and motion sickness intensity evaluated through questionnaires and symptoms. In this experiment, a transient reduction in renal function was found. Moreover, salivary cortisol increased in response to physical activity and stress induced by navigation. Future studies are required to investigate the biochemical processes and the clinical correlations consequent to maritime exposure.
Acknowledgements
We thank Extreme Sailing Academy and the skipper S.F. that made this experience possible. A mention should go to the crew that was extremely interested and enthusiastic to take part to the experiment even in those harsh conditions. Thank you S.B., M.W., F.B., A.B. and T.A.G.
Abbreviations
- 8-iso-PGF2α
8-Isoprostane
- EPR
Electron paramagnetic resonance
- GERD
Gastroesophageal reflux disease
- GSRS
Global Sickness Rating Scale
- MSQ
Motion Sickness Questionnaire
- NO
Nitric oxide
- NOx (NO2 + NO3)
Nitric oxide metabolites
- OOS
Offshore ocean sailing
- OxS
Oxidative stress
- ROS
Reactive oxygen species
- SWH
Significant wave height
- TAC
Total antioxidant capacity
- TWA
True wind angle
Author contributions
All experiments were performed at university of Padova (Padova, Italy) and National Research Council (Milano, Italy). T.A.G. contributed to the study design, data collection and drafting of the manuscript; A.V. contributed to the data analysis, interpretation and critical review of the manuscript; C.D. contributed to data analysis. M.P. and D.C. contributed to the study design, and critical review of the manuscript. G.B. contributed to the study design, data interpretation, and critical review of the manuscript. S.M.S. contributed to the study design, data analysis, interpretation and drafting of the manuscript. G.B. and S.M.S. confirm that the study objectives and procedures are honestly disclosed. All the authors approved the final version of the manuscript.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tommaso Antonio Giacon, Email: tommasoantonio.giacon@studenti.unipd.it.
Gerardo Bosco, Email: gerardo.bosco@unipd.it.
References
- 1.Allen JB, De Jong MR. Sailing and sports medicine: A literature review. Commentary. Br. J. Sports Med. 2006;40(7):587–593. doi: 10.1136/bjsm.2002.001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjøgaard G, Inglés E, Narici M. Science in sailing: Interdisciplinary perspectives in optimizing sailing performance. Eur. J. Sport Sci. 2015;15(3):191–194. doi: 10.1080/17461391.2015.1008583. [DOI] [PubMed] [Google Scholar]
- 3.Myers SD, Leamon SM, Nevola VR, Llewellyn MGL. Energy expenditure during a single-handed transatlantic yacht race. Br. J. Sports Med. 2008;42(4):285–288. doi: 10.1136/bjsm.2007.041533. [DOI] [PubMed] [Google Scholar]
- 4.Fearnley D, Sutton L, O’Hara J, Brightmore A, King R, Cooke C. Case study of a female ocean racer: Prerace preparation and nutritional intake during the Vendée Globe 2008. Int. J. Sport Nutr. Exerc. Metab. 2012;22(3):212–219. doi: 10.1123/ijsnem.22.3.212. [DOI] [PubMed] [Google Scholar]
- 5.Bigard A, Guillemot PCJY. Nutrient intake of elite sailors during a solitary long-distance offshore race. Int. J. Sport Nutr. 1998;8:364–376. doi: 10.1123/ijsn.8.4.364. [DOI] [PubMed] [Google Scholar]
- 6.Lafère P, Gatzoff Y, Guerrero F, Provyn S, Balestra C. Field study of anthropomorphic and muscle performance changes among elite skippers following a transoceanic race. Int. Marit. Health. 2020;71(1):20–27. doi: 10.5603/IMH.2020.0007. [DOI] [PubMed] [Google Scholar]
- 7.Léger D, Elbaz M, Raffray T, Metlaine A, Bayon V, Duforez F. Sleep management and the performance of eight sailors in the Tour de France à la voile yacht race. J. Sports Sci. 2008;26(1):21–28. doi: 10.1080/02640410701348636. [DOI] [PubMed] [Google Scholar]
- 8.Hurdiel R, Van Dongen HPA, Aron C, McCauley P, Jacolot L, Theunynck D. Sleep restriction and degraded reaction-time performance in Figaro solo sailing races. J. Sports Sci. 2014;32(2):172–174. doi: 10.1080/02640414.2013.815359. [DOI] [PubMed] [Google Scholar]
- 9.Oldenburg M, Hogan B, Jensen HJ. Systematic review of maritime field studies about stress and strain in seafaring. Int. Arch. Occup. Environ. Health. 2013;86(1):1–15. doi: 10.1007/s00420-012-0801-5. [DOI] [PubMed] [Google Scholar]
- 10.Carotenuto A, Molino I, Fasanaro AM, Amenta F. Psychological stress in seafarers: A review. Int. Marit. Health. 2012;63(4):188–194. [PubMed] [Google Scholar]
- 11.Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, et al. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Gunnarsson LG, Bäck H, Jones I, Olsson T. Stress recovery during an ocean boat race. Stress Health. 2004;20(3):165–171. [Google Scholar]
- 13.Pisula PJ, Lewis CH, Bridger RS. Vessel motion thresholds for maintaining physical and cognitive performance: A study of naval personnel at sea. Ergonomics. 2012;55(6):636–649. doi: 10.1080/00140139.2012.657249. [DOI] [PubMed] [Google Scholar]
- 14.Golding JF. Motion sickness [Internet], Handbook of Clinical Neurology. 1. Elsevier; 2016. pp. 371–390. [DOI] [PubMed] [Google Scholar]
- 15.Stoffregen TA, Chen FC, Varlet M, Alcantara C, Bardy BG. Getting your sea legs. PLoS One. 2013;8:6. doi: 10.1371/journal.pone.0066949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang LL, Wang JQ, Qi RR, Pan LL, Li M, Cai YL. Motion sickness: Current knowledge and recent advance. CNS Neurosci. Ther. 2016;22(1):15–24. doi: 10.1111/cns.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galvani C, Ardigò LP, Alberti M, Daniele F, Capelli C. Physical activity, sleep pattern and energy expenditure in double-handed offshore sailing. J. Sports Med. Phys. Fitness. 2015;55(12):1480–1487. [PubMed] [Google Scholar]
- 18.Neville V, Calefato J, Pérez-Encinas C, Rodilla-Sala E, Rada-Ruiz S, Dorochenko P, et al. America’s Cup yacht racing: Race analysis and physical characteristics of the athletes. J. Sports Sci. 2009;27(9):915–923. doi: 10.1080/02640410902946485. [DOI] [PubMed] [Google Scholar]
- 19.Bernardi M, Quattrini FM, Rodio A, Fontana G, Madaffari A, Brugnoli M, et al. Physiological characteristics of America’s Cup sailors. J. Sports Sci. 2007;25(10):1141–1152. doi: 10.1080/02640410701287172. [DOI] [PubMed] [Google Scholar]
- 20.Neville V, Gant N, Folland JP. Thermoregulatory demands of Elite Professional America’s Cup Yacht Racing. Scand. J. Med. Sci. Sports. 2010;20(3):475–484. doi: 10.1111/j.1600-0838.2009.00952.x. [DOI] [PubMed] [Google Scholar]
- 21.Mrakic-Sposta S, Gussoni M, Vezzoli A, Dellanoce C, Comassi M, Giardini G, et al. Acute effects of triathlon race on oxidative stress biomarkers. Oxid. Med. Cell Longev. 2020;20:20. doi: 10.1155/2020/3062807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vezzoli A, Dellanoce C, Mrakic-Sposta S, Montorsi M, Moretti S, Tonini A, et al. Oxidative stress assessment in response to ultraendurance exercise: Thiols redox status and ROS production according to duration of a competitive race. Oxid. Med. Cell Longev. 2016;20:16. doi: 10.1155/2016/6439037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mrakic-Sposta S, Gussoni M, Porcelli S, Pugliese L, Pavei G, Bellistri G, et al. Training effects on ROS production determined by electron paramagnetic resonance in master swimmers. Oxid. Med. Cell Longev. 2015;20:15. doi: 10.1155/2015/804794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell Longev. 2016;20:16. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-morte D, et al. Oxidative stress and diseases. Oxid. Stress Dis. 2012;20:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott SN, Wallace JL. Nitric oxide: A regulator of mucosal defense and injury. J. Gastroenterol. 1998;33(6):792–803. doi: 10.1007/s005350050178. [DOI] [PubMed] [Google Scholar]
- 27.Golding JF, Bles W, Bos JE, Haynes T, Gresty MA. Motion sickness and tilts of the inertial force environment: Active suspension systems vs active passengers. Aviat Sp. Environ. Med. 2003;74(3):220–227. [PubMed] [Google Scholar]
- 28.Mrakic-Sposta S, Vezzoli A, Rizzato A, Della Noce C, Malacrida S, Montorsi M, et al. Oxidative stress assessment in breath-hold diving. Eur. J. Appl. Physiol. 2019;119(11–12):2449–2456. doi: 10.1007/s00421-019-04224-4. [DOI] [PubMed] [Google Scholar]
- 29.Mrakic-Sposta S, Vezzoli A, D’alessandro F, Paganini M, Dellanoce C, Cialoni D, et al. Change in oxidative stress biomarkers during 30 days in saturation dive: A pilot study. Int. J. Environ. Res. Public Health. 2020;17(19):1–11. doi: 10.3390/ijerph17197118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mrakic-Sposta S, Gussoni M, Montorsi M, Porcelli S, Vezzoli A. Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance. Oxid. Med. Cell Longev. 2012;20:12. doi: 10.1155/2012/973927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorn LD, Lucke JF, Loucks TL, Berga SL. Salivary cortisol reflects serum cortisol: Analysis of circadian profiles. Ann. Clin. Biochem. 2007;44(3):281–284. doi: 10.1258/000456307780480954. [DOI] [PubMed] [Google Scholar]
- 32.Bosco G, Rizzato A, Quartesan S, Camporesi E, Mrakic-Sposta S, Moretti S, et al. Spirometry and oxidative stress after rebreather diving in warm water. Undersea Hyperb. Med. 2018;45(2):191–198. [PubMed] [Google Scholar]
- 33.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 34.Glantzounis G, Tsimoyiannis E, Kappas A, Galaris D. Uric acid and oxidative stress. Curr. Pharm. Des. 2005;11(32):4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 35.Oldenburg M, Jensen HJ. Saliva cortisol level as a strain parameter for crews aboard merchant ships. Chronobiol. Int. 2019;36(7):1005–1012. doi: 10.1080/07420528.2019.1604540. [DOI] [PubMed] [Google Scholar]
- 36.Main LC, Wolkow A, Chambers TP. Quantifying the physiological stress response to simulated maritime pilotage tasks. J. Occup. Environ. Med. 2017;59(11):1078–1083. doi: 10.1097/JOM.0000000000001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberzon J, Abelson JL, King A, Liberzon I. Adaptation Seafaring. 2009;33(7):1023–1026. doi: 10.1016/j.psyneuen.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitson PA, Putcha L, Chen Y-M, Baker E. Melatonin and Cortisol assessment of circadian shifts in astronauts before flight. J. Pineal Res. 1995;18(3):141–147. doi: 10.1111/j.1600-079x.1995.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 39.Hernández LM, Markwald RR, Kviatkovsky SA, Perry LN, Taylor MK. Morning cortisol is associated with stress and sleep in elite military men: A brief report. Mil. Med. 2018;183(9–10):e255–e259. doi: 10.1093/milmed/usy047. [DOI] [PubMed] [Google Scholar]
- 40.Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J. Clin. Endocrinol. Metab. 2000;85(10):3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 41.Abedelmalek S, Chtourou H, Aloui A, Aouichaoui C, Souissi N, Tabka Z. Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. Eur. J. Appl. Physiol. 2013;113(1):241–248. doi: 10.1007/s00421-012-2432-7. [DOI] [PubMed] [Google Scholar]
- 42.Hayes LD, Grace FM, Baker JS, Sculthorpe N. Exercise-induced responses in salivary testosterone, cortisol, and their ratios in men: A meta-analysis. Sport Med. 2015;45(5):713–726. doi: 10.1007/s40279-015-0306-y. [DOI] [PubMed] [Google Scholar]
- 43.Lukina GI, Ivannikova AV, Abramova MY, Kuzmina EM, Lukin AV, Alimova AV, et al. The oral mucosa status and the correlation between the functional parameters and the level of nitric oxide metabolites in saliva among patients with GERD. Int J Dent. 2020;20:20. doi: 10.1155/2020/1273031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beyazit F, Türkön H, Pek E, Ozturk FH, Ünsal M. Elevated circulating nitric oxide levels correlates with enhanced oxidative stress in patients with hyperemesis gravidarum. J. Obstet. Gynaecol. (Lahore) 2018;38(5):668–673. doi: 10.1080/01443615.2017.1383371. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Li J, Yu M, Wang Y, Ma Y. An enhanced expression of hypothalamic neuronal nitric oxide synthase in a rat model of simulated transport stress. BMC Vet. Res. 2019;15(1):1–10. doi: 10.1186/s12917-019-2071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zicari A, Corrado G, Pacchiarotti C, Lucarelli S, Frediani T, Cavaliere M, et al. Cyclic vomiting syndrome: In vitro nitric oxide and interleukin-6 release by esophageal and gastric mucosa. Dig. Dis. Sci. 2001;46(4):831–835. doi: 10.1023/a:1010712703685. [DOI] [PubMed] [Google Scholar]
- 47.Koch KL. Gastric dysrhythmias: A potential objective measure of nausea. Exp. Brain Res. 2014;232(8):2553–2561. doi: 10.1007/s00221-014-4007-9. [DOI] [PubMed] [Google Scholar]
- 48.Philippe K, Paillard T, Dubois R, Maurelli O, Prioux J. Key performance indicators in Tour de France sailing. J. Sports Sci. 2020;00(00):1–11. doi: 10.1080/02640414.2020.1851925. [DOI] [PubMed] [Google Scholar]
- 49.Ghiani G, Magnani S, Doneddu A, Sainas G, Pinna V, Caboi M, et al. Case Study: Physical capacity and nutritional status before and after a single-handed yacht race. Int. J. Sport Nutr. Exerc. Metab. 2018;28(5):558–563. doi: 10.1123/ijsnem.2017-0345. [DOI] [PubMed] [Google Scholar]
- 50.Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: A 30 year history. Dyn. Med. 2009;8(1):1–25. doi: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi M, Wang X, Yamanaka T, Ogita F, Nakatani K, Takeuchi T. Effects of anaerobic exercise and aerobic exercise on biomarkers of oxidative stress. Environ. Health Prev. Med. 2007;12(5):202–208. doi: 10.1265/ehpm.12.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bloomer RJ, Goldfarb AH, Wideman L, McKenzie MJ, Consitt LA. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J. Strength Cond. Res. 2005;19(2):276–285. doi: 10.1519/14823.1. [DOI] [PubMed] [Google Scholar]
- 53.Bloomer RJ, Goldfarb AH. Anaerobic exercise and oxidative stress: A review. Can. J. Appl. Physiol. 2004;29(3):245–263. doi: 10.1139/h04-017. [DOI] [PubMed] [Google Scholar]
- 54.Barrios C, Hadala M, Almansa I, Bosch-Morell F, Palanca JM, Romero FJ. Metabolic muscle damage and oxidative stress markers in an America’s Cup yachting crew. Eur. J. Appl. Physiol. 2011;111(7):1341–1350. doi: 10.1007/s00421-010-1762-6. [DOI] [PubMed] [Google Scholar]
- 55.El Assar M, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013;65:380–401. doi: 10.1016/j.freeradbiomed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascul. Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Oldenburg M. Risk of cardiovascular diseases in seafarers. Int. Marit. Health. 2014;65(2):53–57. doi: 10.5603/IMH.2014.0012. [DOI] [PubMed] [Google Scholar]
- 58.Von Katzler R, Zyriax BC, Jagemann B, Westenhoefer J, Jensen HJ, Harth V, et al. Lifestyle behaviour and prevalence of cardiovascular risk factors—a pilot study comparing Kiribati and European seafarers. BMC Public Health. 2019;19(1):1–9. doi: 10.1186/s12889-019-7186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eriksson HP, Forsell K, Andersson E. Mortality from cardiovascular disease in a cohort of Swedish seafarers. Int. Arch. Occup. Environ Health. 2020;93(3):345–353. doi: 10.1007/s00420-019-01486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr. Drug Metab. 2002;23(3):175–187. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 61.Hodgson L, Walter E, Venn R, Galloway R, Pitsiladis Y, Sardat F, et al. Acute kidney injury associated with endurance events—is it a cause for concern? A systematic review. BMJ Open Sport Exerc. Med. 2017;3:1. doi: 10.1136/bmjsem-2015-000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24(2):256–268. doi: 10.1016/j.cmet.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Sansone M, Sansone A, Borrione P, Romanelli F, Di Luigi L, Sgrò P. Effects of ketone bodies on endurance exercise. Curr. Sports Med. Rep. 2018;17(12):444–453. doi: 10.1249/JSR.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 64.Paoli A, Bosco G, Camporesi EM, Mangar D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015;6(FEB):1–9. doi: 10.3389/fpsyg.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubini A, Bosco G, Lodi A, Cenci L, Parmagnani A, Grimaldi K, et al. Effects of twenty days of the ketogenic diet on metabolic and respiratory parameters in healthy subjects. Lung. 2015;193(6):939–945. doi: 10.1007/s00408-015-9806-7. [DOI] [PubMed] [Google Scholar]
- 66.Otto B, Riepl RL, Klosterhalfen S, Enck P. Endocrine correlates of acute nausea and vomiting. Auton. Neurosci. Basic Clin. 2006;129(1–2):17–21. doi: 10.1016/j.autneu.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 67.Meissner K, Enck P, Muth ER, Kellermann S, Klosterhalfen S. Cortisol levels predict motion sickness tolerance in women but not in men. Physiol. Behav. 2009;97(1):102–106. doi: 10.1016/j.physbeh.2009.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.