Abstract
Wood ash is a naturally alkaline derived substance containing organic and inorganic constituents. This study investigates the catalytic activity of wood ash as a heterogeneous catalyst for the synthesis of benzochromene derivatives. Several wood ash catalysts, comprising calcium- and potassium-rich carbonates, were prepared from different natural resources under various combustion temperatures. The prepared catalysts were characterized by Fourier transform infrared, scanning electron microscopy, energy dispersive X-ray analysis, transmission electron microscopy, and X-ray diffraction techniques. Catalytic efficiency of the resultant catalysts was tested in the synthesis of benzochromene derivatives. The experimental studies clarified that the catalyst prepared at 850 °C could efficiently expedite the formation of three-component synthesis of benzochromene derivatives in water at 80 °C with high yields. Indeed, alkali, alkaline metal, and metal oxides such as Al2O3, SiO2, MgO, CaO, and Fe2O3, are widely utilized as both catalyst and catalyst support in the heterogeneous catalytic processes. The prepared wood ash catalysts (possessing metal oxides, e.g., CuO, Al2O3, SiO2, and CaO) could effectively prompt the electrophilic activity of the carbonyl groups during the nucleophilic attack intermediate, enhancing the efficiency of the reactions.
Subject terms: Chemistry, Materials science, Nanoscience and technology
Introduction
Wood-ash (WA) is an organic and inorganic residue remaining from the wood or unbleached wood fiber combustion. Its physicochemical features remarkably depend on multiple parameters. In general, hardwoods can produce more ash than softwoods. Subsequently, bark and leaves produce more cash than the interior wood parts of trees1. Various process conditions, e.g., combustion temperature, fuel wood cleanliness, collection site, and the applied procedure significantly impact the ash production in industries. Indeed, WA composition is different according to the geographical location and the applied modification procedure2. WA, produced as a by-product in the wood processing industry, is produced ~ 3 million tons each year only in the United States3. Depending on the type of the burned wood, ~ 0.4% to 2.1% of the utilized weight is produced as ash4.
Several hectares of forests in different countries are burnt yearly by the wildfire producing WA as a waste. It should be noted that WA is considered as a strongly alkaline material which raises environmental issues5. Several studies6,7 indicated that calcium is the most plentiful element in WA, providing the same characteristics as the agricultural lime. In addition, ash has been considered as one of the suitable resources of potassium, phosphorus, and magnesium. Based on a study in the field8, diverse parameters such as various kinds of forest species, combusted plants (e.g., bark, stem, or leaves), climate, combustion conditions, etc., are effective in the composition of forest WA. Therefore, WA is broadly used in agriculture because it is a very good source of lime, potash, and other plant nutrients9.
The high metal content in WA indicates that it has a good potential to be used as catalytic materials for a variety of transformations. Even though several studies have been achieved on WA8–11, none of them have addressed the application of the WA as a heterogeneous catalyst for organic reactions, except the recently limited studies conducted on bio-diesel and esterification11–13.
Green methods are generally employed for various processes to decrease their expense and save resources. In addition, the use of ecological and green solvents instead of toxic solvents and applying moderate conditions and inexpensive reagents are the most important goals to develop simple and benign procedures for the synthesis of organic compounds14. For example, water is an inexpensive and green solvent which accelerates the rate of organic reactions for certain compounds.
Chromenes are important moieties in medicinal and organic chemistry because of their broad spectrum of biological activities including antioxidant, antimicrobial, antimalarial, anticancer, and antibacterial15–19. Among various chromenes, benzochromenes are highly considerable compounds because of their applicability and biological properties in variable applications20. The preparation of benzochromenes has been investigated using different catalysts, e.g., Zn(l-proline)2, 1-butyl-3-methyl imidazolium hydroxide ([bmim]OH) lipase, triethylbenzylammonium chloride (TEBA), etc.21–25. Although there are many novel methods to prepare these compounds, several of them have critical disadvantages such as requirement of toxic solvent, high reaction times, non-reusable catalyst, etc. Consequently, developing efficient and inexpensive catalysts presenting high catalytic activity for the preparation of benzochromenes is highly desirable14. Indeed, the preparation of benzochromenes by multicomponent reactions (MCR) has garnered much attention because of good product yield and their applicability.
In continuation of our studies to explore new preparation procedure for main organic compounds26–32, we introduce a green method for the synthesis of benzochromene derivatives by an efficient three component reaction of 1-(6-hydroxy-2-isopropenyl-1-benzofuran-yl)-1-ethanone or euparin 133, aldehydes 2, alkyl bromides 3 and triphenylphosphine 4 in the presence of water extract WA (WEWA) as a catalyst in water at 70 °C with good yields. In addition, the antioxidant activities of some of the synthesized derivatives were studied by ferric ion reducing power test and DPPH radical scavenging. To the best of our knowledge, the application of WA as a catalyst for MCR reactions has not previously been reported. The aim of this study is to acquire an active and inexpensive catalyst from waste WA for the synthesis of some benzochromene derivatives. For this purpose, several catalysts were prepared from different WA and characterized by the latest analytical techniques.
Results and discussion
Characterization of WA
Basicity
Our initial studies focused on soluble basicity measurement with the aim of finding optimum conditions. It was found that the source of the wood and combustion temperature both have a deep influence on basicity. Therefore, all the WA which prepared at four different burning temperatures are provided for pH measurement and the results of the WA samples are tabulated in Table 1.
Table 1.
Soluble alkalinity of WA catalysts.
| Sample | WA | Burning temp. (°C) | Obtaining ash (%) | pH |
|---|---|---|---|---|
| PiA450 | Pine | 450 | 12.4 | 9.45 ± 0.08 |
| PiA650 | Pine | 650 | 9.1 | 10.04 ± 0.13 |
| PiA850 | Pine | 850 | 5.7 | 11.65 ± 0.09 |
| PiA1050 | Pine | 1050 | 3.9 | 10.94 ± 0.08 |
| ROA450 | R. olive | 450 | 14.3 | 11.67 ± 0.09 |
| ROA650 | R. olive | 650 | 10.4 | 12.33 ± 0.16 |
| ROA850 | R. olive | 850 | 6.4 | 12.79 ± 0.04 |
| ROA1050 | R. olive | 1050 | 4.7 | 12.23 ± 0.12 |
| PoA450 | Poplar | 450 | 12.2 | 9.32 ± 0.11 |
| PoA650 | Poplar | 650 | 9.3 | 10.12 ± 0.06 |
| PoA850 | Poplar | 850 | 5.2 | 11.43 ± 0.09 |
| PoA1050 | Poplar | 1050 | 3.8 | 10.76 ± 0.02 |
It is observed that both source of wood and combustion temperature affect pH or basicity of the ash samples. The results indicated that the pH of Russian olive ash (ROA) is much more than pine ash (PiA) and poplar ash (PoA) and the pH of the ash samples increases with increasing of burning temperature for example for PiA from 9.45 to 11.65 from 450 to 850 °C and decreases to 10.94 with increase of combustion temperature (above 850 °C). The pH of the ROA850 (Russian olive Ash at 850 °C) has the highest value 12.79.
Thermal decomposition of CaCO3 (825 °C) to CaO is perhaps the possible reason for higher basicity with the increase of temperature, which because of higher solubility of CaO in water than CaCO3, an increase in basicity value is resulted34. But the reason that at temperatures above 1000 °C, the pH shows a great decrease is because of the formation of a highly stable silicate phase via the interaction of metal oxides (e.g., SiO2 and CaO). The reason for the decrease in basicity is that the stable silicates are less soluble in the water (Fig. 1)35.
Figure 1.
Thermal decomposition of CaCO3 and formation of CaSiO4.
SEM–EDX analysis
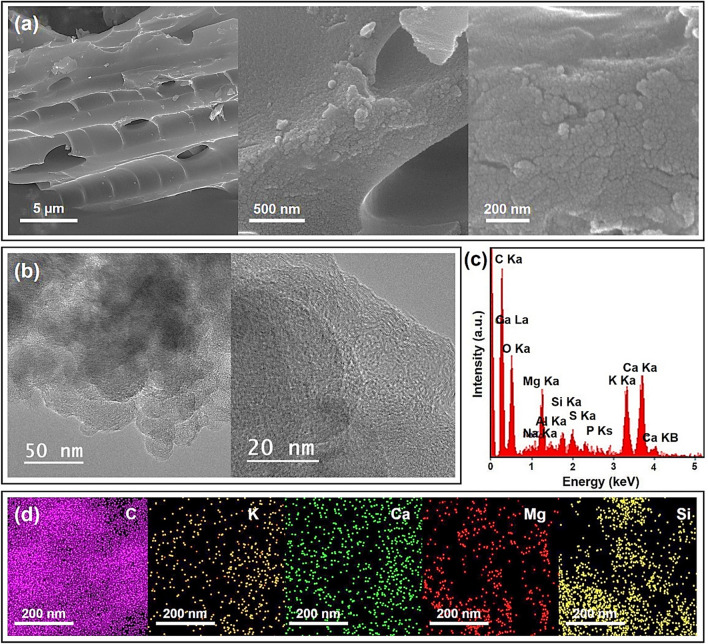
Figure 2a shows the scanning electron microscopy (SEM) analysis of WA from the combustion of the Russian olive wood at 850 °C (ROA850). The SEM image of the ROA850 illustrates the porous and spongy nature with rough surfaces and high surface areas12 in WA particles. Most of the particles characterize sphere-shaped structure. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images show high crystallinity of the prepared wood ash at 850 °C (Fig. 2b). The energy dispersive X-ray (EDX) analysis used to provide the elemental composition and to evaluate a structural vision of the ROA850 sample as shown in Fig. 2c. The elements identified were potassium (K), calcium (Ca), magnesium (Mg), phosphorus (P), oxygen (O), carbon (C), sulfur (S), aluminum (Al), silicon (Si), and sodium (Na). As expected, the main elements of C, and O were consistently dispersed on the surface of the RO WA. Additionally, according to the EDX analysis and elemental mapping, the main elemental compositions of ROA850 were mainly C, K, Ca, Mg, and Si, which indicates the presence of calcium and potassium rich carbonates and oxides on the surface of RO WA (Fig. 2d).
Figure 2.
(a) FE-SEM, (b) TEM and HRTEM images, (c) EDX analysis and (d) EDX elemental mapping of WA prepared at 850 °C.
X-ray diffraction
The X-ray diffraction (XRD) patterns of the Russian olive WA prepared at different combustion temperatures (ROA450, ROA850, and ROA1050) are shown in Fig. 3. The XRD pattern of ROA450 shows the presence of CaCO3, SiO2, Fe2O3, KCl, and MgCO3 components. On burning of Russian olive wood at 850 °C (ROA850), the corresponding XRD pattern indicates the presence of KCl, CaO, Fe2O3, MgO, CaCO3, K2SO4, and Ca2SiO4.0.05Ca3(PO4)2 compounds. After combustion at higher temperature at 1050 °C (ROA1050), XRD pattern indicates that number and intensity of peaks related to Ca2SiO4.0.05Ca3(PO4)2 compound increases and is found as the main component.
Figure 3.
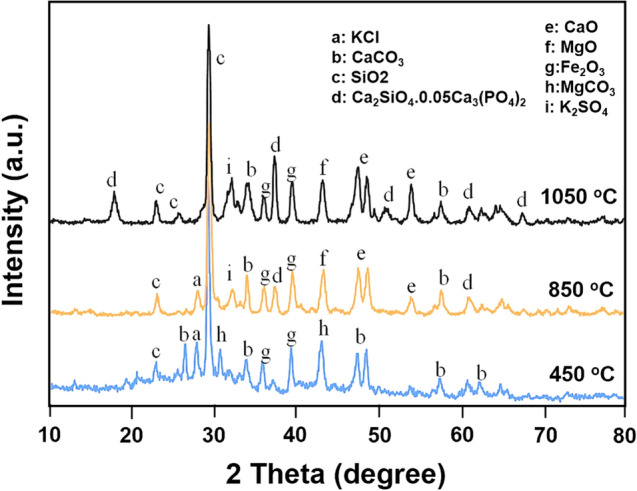
XRD pattern of WA at different combustion temperatures.
X-ray fluorescence
It is not clear by what mechanism mineral compounds are formed as ash through burning of wood although it is acceptable to believe that the exchange depends upon the burning temperature and current atmosphere. Table 2 shows chemical composition analysis of Russian olive WA prepared at different combustion temperatures (ROA450, ROA850, and ROA1050). The minerals in WA determined by X-ray fluorescence (XRF) found to be Na2O, K2O, MgO, BaO, CaO, SiO2, Al2O3, P2O5, Fe2O3, and MnO2. As shown in Table 2, in case of combustion Russian olive WA samples, the changes under the combustion temperature from 450 to 1050 °C causes the modification in composition of the prepared ashes. At 850 °C combustion temperature, the increase in CaO content was observed mainly because of decay of CaCO3 (Eq. 1), also verified by the XRD pattern of ROA850 (Fig. 3). At higher temperature, the percentages of Na2O and MgO decrease due to their carbonates decomposition to oxides and the subsequent volatilization36. The decrease in potassium percentage is predominantly because of vaporization of KCl (Eq. 2). The probable cause for rising CaO content may be because of non-volatility of CaO from the WA36.
Table 2.
Chemical composition of the WA from Russian olive in different combustion temperatures.
| Sample | Na2O | K2O | MgO | BaO | CaO | SrO | CuO | SiO2 | Al2O3 | P2O5 | Fe2O3 | MnO2 | TiO2 | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROA450 | 0.7 | 20.6 | 7.4 | 1.7 | 45.6 | 1.6 | – | 7.2 | 1.6 | 4.3 | 3.6 | 0.3 | 0.2 | 5.2 |
| ROA850 | 0.5 | 14.5 | 9.64 | 0.2 | 61.5 | 2.1 | 0.1 | 2.9 | 0.51 | 2.5 | 0.9 | 0.3 | 0.1 | 4.3 |
| ROA1050 | 0.2 | 4.2 | 3.4 | 1.6 | 64.9 | 3.5 | 1.2 | 3.5 | 3.2 | 2.3 | 4.2 | 1.6 | 0.5 | 5.7 |
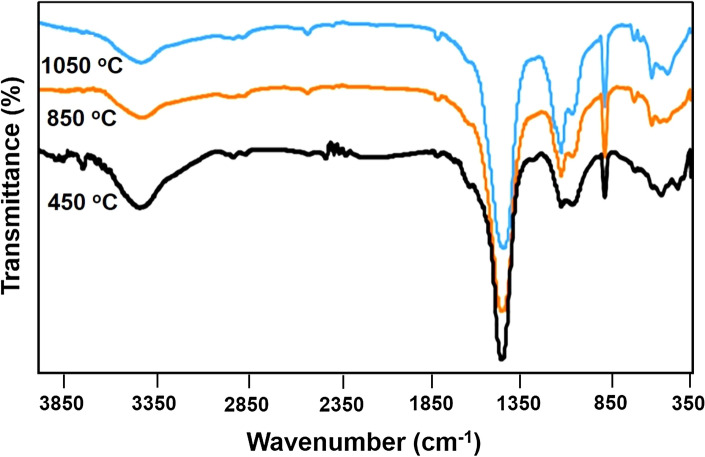
FT-IR analysis
The FT-IR spectra of burned WA samples at 450, 850, and 1050 °C provided in Fig. 4. The broad band at 3453 cm−1 belongs to hydroxyl stretching vibrations in several organic and inorganic constituents37, as can be seen in Fig. 4, the absorbance intensity decreases with increasing temperature due to burning of organic substances. The week absorbance bands at 2922 and 2853 cm−1 from C-H stretching vibrations are corresponded to aliphatic hydrocarbons in the WA and also the absorbance band at 1789 cm−1 and a shoulder peak at 1621 cm−1 belong to carbonyl and C=C groups, respectively38. The absorbance around 1793, 1443, 876, and 712 cm−1 are associated with carbonate (CO32−), and also the characteristic bands at 1111, 1043, and 615 cm−1 for PO43− and SiO2 components, showing metal carbonate like CaCO3, SiO2 and metal phosphate. The FT-IR spectrum of WA burned at higher temperatures (ROA850 and ROA1050) show the absorbance bands at 1413, 1053, 523, and 471 cm−1, indicating presence of Si–O–Al, Si–O–Si and CaO functionalities39.
Figure 4.
FT-IR spectra of RO WA prepared at different combustion temperatures.
Catalytic activity of WEWA in synthesis of benzochromene derivatives
In this research work, we studied a green method for the preparation of some benzochromene derivatives by an efficient three component reaction of euparin 1, aldehydes 2, alkyl bromides 3 and triphenylphosphine 4 in the presence of a catalytic amounts of ROA850 (%5) in water at 80 °C with high yields (Fig. 5).
Figure 5.
Three-component synthesis of benzochromene derivatives of 5 in water.
In the starting step of this work, condensation reaction of euparin 133, 4-methoxy benzaldehyde 2, ethyl bromopyruvate 3 and triphenylphosphine 4 at 80 °C in water was applied as a model reaction to obtain the optimum reaction conditions (Table 3).
Table 3.
Effect of catalyst, its loading, and temperature on the condensation reaction of compound 5a.
| Entry | Catalyst | Temp. (°C) | Time (h) | Yield%a |
|---|---|---|---|---|
| 1 | None | Rt | 15 | – |
| 2 | None | 70 | 15 | Trace |
| 3 | None | 90 | 15 | Trace |
| 4 | ROA850 (%1) | 70 | 2 | 55 |
| 5 | ROA850 (%5) | 70 | 3 | 99 |
| 6 | ROA850 (%10) | 70 | 3 | 99 |
| 7 | ROA850 (%5) | 90 | 3 | 98 |
| 8 | NaOH (%5) | 70 | 5 | 48 |
| 9 | NaOH (%10) | 70 | 5 | 52 |
| 10 | NaOH (%20) | 70 | 5 | 51 |
aIsolated yield
These reactions did not progress without any catalyst even after 15 h (Table 3, entry 1). By increasing the reaction temperature to 70 and 90 °C, a trace amount of 5a generated after 15 h (Table 3, entries 2 and 3). To acquire better results, ROA850 (%1) as a catalyst was added into the reaction mixture. Interestingly, 55% yield of 5a was produced after 2 h (Table 3, entry 4). Then, the reaction was performed in the presence of ROA850 (%5). As it was expected, the yield of product 5a was accomplished in 99% after 3 h under these reaction conditions (Table 3, entry 5). Consequently, various amounts of WEWA catalyst were utilized to discover the optimal catalyst loading. The results displayed that 5% of WEWA (ROA850) are enough for producing an excellent yield of 5a (Table 3, entry 5). To clearly evaluate the catalytic activity of WA as a base catalyst, different percentages of NaOH were used in this reaction. Consequently, these results confirmed the main function of WA as the effective catalyst in this reaction. According to the optimized reaction conditions (Table 3), ROA850 (%5) as the catalyst in water at 70 °C was estimated to be the optimum amount of the catalyst for this reaction.
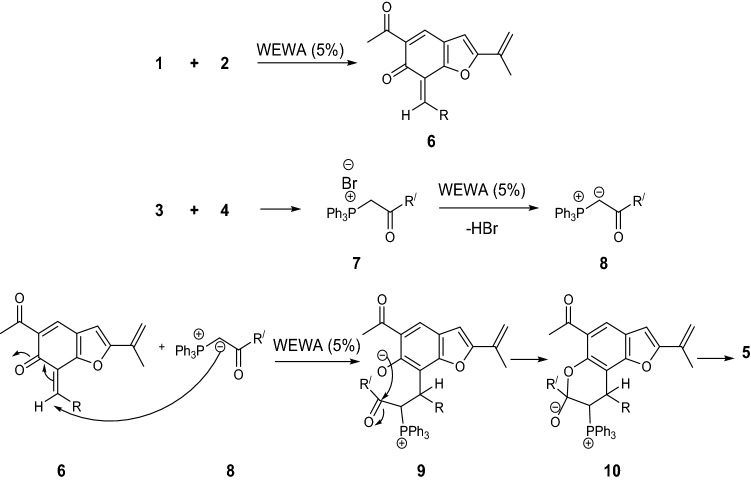
The structures of compounds 5 were verified by FT-IR, 1H NMR, 13C NMR, and mass spectral data (for detain see supporting information). For instance, the 1H NMR spectrum of 5a revealed two singlets at δ = 2.15 and 2.52 ppm for methyl protons, four singlets at 4.58, 5.37, 6.14 and 7.75 ppm for methine proton along with signals for aromatic moiety. In the 13C NMR spectrum, the signals of the carbonyl group of 5a were observed at δ 160.2 and 197.6 ppm. Although there is no exact information to approve the mechanistic details, it can be proposed as shown in Fig. 6.
Figure 6.
Proposed mechanism for the formation of 5.
First, euparin 1 and aldehyde 2 react in the presence of ROA850 (%5) that is generated intermediate 6. In other reaction vials, triphenylphosphine and alkyl bromides reacted in the presence of ROA850 (%5) to produce the intermediate 8 by the elimination of HBr. The intermediate 8 attacks the intermediate 6 and produces intermediate 9. The elimination of triphenylphosphine oxide and Cyclization of intermediate 9 provide compound 5.
Alkali, alkaline metal, and metal oxides (e.g., Al2O3, CaO, MgO, Fe2O3, and SiO2) are widely used as both heterogeneous catalyst and catalyst support40. WA, a rich source of the aforementioned metal oxides, is an appropriate candidate for the reactions requiring basic catalysts. In addition, WA has a good catalytic activity and can be used as a solid base catalyst, and WA due to presence of some metal oxides such as CuO, Al2O3, SiO2, and CaO which increases the electrophilic activity of the carbonyl groups can increase the nucleophilic attack in the reaction media. Therefore, we expect that with the preparation of some benzochromene derivatives, both the basic power and nucleophilic activity will increase by WA.
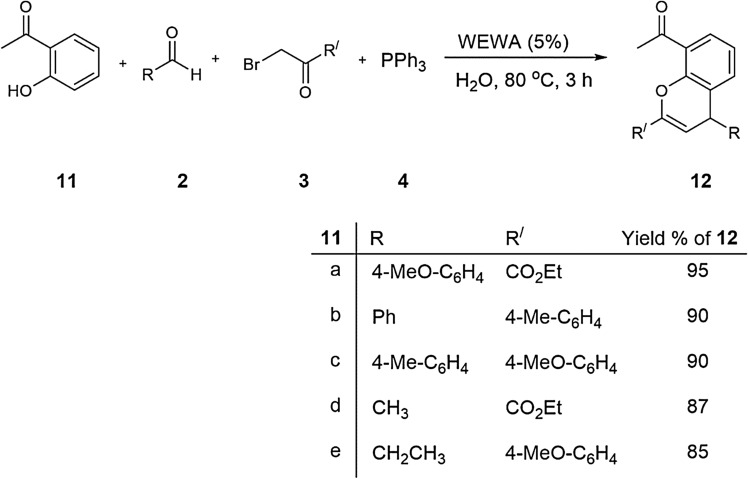
On the other hand, the main advantages of this procedure are green reaction conditions, economical procedure, utilization of small amounts of catalyst, high yield, short reaction times, and easy work-up, which are the required principles of green chemistry41–45. Under similar conditions, we also investigated the reaction between 2-hydroxyacetophenone 11, aldehyde 2, alkyl bromide 3 and triphenylphosphine 4 in the presence of ROA850 (%5) catalyst in water at 80 °C for confirming diversity of these reactions (Fig. 7).
Figure 7.
Three-component reaction for synthesis of benzochromene derivatives of 12 in water.
Experimental
Materials and reagents
Three wood samples used for WA preparation in this study, pine (Pinus alba), poplar (Populus nigra) and Russian olive (Elaeagnus angustifolia), were collected from Mazandaran province, Iran. All the wood samples were dried prior to catalyst preparation. The chemicals and solvents used in this work were obtained from Sigma Aldrich. Euparin was extracted from Petasites hybridus dried roots according to our previous research29,33. All aqueous solutions are freshly prepared using distilled water. FT-IR spectra were recorded using pressed KBr disks, using Perkin–Elmer 781 spectrophotometer. X-ray diffraction (XRD) analyses were carried out with a Philips powder diffractometer type PW 1373 goniometer. The X-ray wavelength (1.5405 Å) and the diffraction patterns were recorded in the 2 h range (10–80°) with scanning rate of 2 °C/min. Fresh WA analyzed for elemental composition using XRF using a Philips 1404 wavelength dispersive spectrometer. All the samples were dried in an oven at 50 °C for 12 h to remove water content prior to the analyses. The morphology and particle dispersion were studied by SEM (Cam scan MV2300). The chemical composition of the prepared WA was confirmed by energy dispersive X-ray spectroscopy (EDS). The elemental analysis was employed to ascertain the resistance of C, H, and N using a Heraeus CHNO-Rapid analyzer. The mass spectra were collected using a FINNIGAN-MAT 8430 spectrometer operating at an ionization potential of 70 eV. FT-IR spectra were recorded on a Shimadzu IR-460 spectrometer. The 1H and 13C NMR spectra were analyzed using a Bruker DRX-500 advance spectrometer at 500.1 MHz and 125.8 MHz, respectively. The 1H and 13C spectra were performed for CDCl3 solutions applying TMS (as the internal standard) or 85 mass% H3PO4 (as external standard); chemical shifts (d) are given as parts per million (ppm).
Preparation of WEWA
The fresh wood samples were washed properly with deionized water, cut into small pieces, and dried under the sunlight at open atmosphere until the constant weight. The dried samples were combusted at the rate of 20 °C/min to reach 450, 650, 850 and 1050 °C in the high temperature laboratory furnace under air condition and kept at the target temperatures for 4 h to form WA, which the amount of obtained ash for 1 kg of the wood samples (pine, poplar, and Russian olive) in different burning temperatures are presented in Table 1. The prepared WA samples indicated as WA450, WA650, WA850 and WA1050, where number 450 shows WA450 for WA burning at 450 °C and 650–1050 denotes the temperature of burning. Then 5 g of WA suspended in 100 mL deionized water in a beaker and stirred for 30 min at room temperature. The mixture filtered through a sintered glass container and the filtrate used as WEWA (5%) as a catalyst for some benzochromene derivatives.
pH determination of WA for basicity measurement
0.5 g of different wood ash samples were suspended in 10 ml of deionized water and agitated for 24 h. The pH of suspension was measured using pH Meter. This measurement was performed for all of WA samples prepared at different temperatures as well as for various types of catalysts prepared from Russian olive ash.
General procedure for preparation of compounds 5a–f
A mixture of 1-(6-hydroxy-2-isopropenyl-1-benzofuran-yl)-1-ethanone 1 (2 mmol), aldehyde 2 (2 mmol) and 4 ml of WEWA catalyst (5%) was added to the magnetically stirred mixture of 4 ml of WEWA catalyst (5%), alkyl bromides 3 (2 mmol), and triphenylphosphine 4 at 80 °C. After completion the reaction by TLC monitoring, the catalyst was isolated by filtration, then the product was dissolved in ethyl acetate and purified by small column chromatography (CC) or paper chromatography (PC) (Hexane: EtOAc = 5:1). During the separation by CC or PC, remaining catalyst and triphenyl phosphine oxide were also removed from the product easily and finally washed with water to afford pure title compound 5.
Ethyl 5-acetyl-2-isopropenyl-9-(4-methoxyphenyl)-9H-furo[2,3-f] chromene-7-carboxylate (5a)
Yellow powder, mp 163–165 °C, Yield: 0.82 g (95%). IR (KBr) (νmax/cm−1): 1724, 1738, 1675, 1585, 1462, 1274 cm−1. 1H NMR (500 MHz, CDCl3): 1.32 (3 H, t, 3J = 7.4 Hz, CH3), 2.15 (3 H, s, Me), 2.52 (3 H, s, Me), 3.75 (3 H, s, MeO), 4.26 (2 H, q, 3J = 7.3 Hz, CH2O), 4.58 (1 H, s, CH), 4.62 (1 H, d, 2J = 3.8 Hz, CH), 5.37 (1 H, s, CH), 5.68 (1 H, d, 2J = 3.8 Hz, CH), 6.14 (1 H, s, CH), 7.12 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.63 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.75 (1 H, s, CH) ppm. 13C NMR (125.7 MHz, CDCl3): 18.7 (Me), 22.6 (Me), 30.2 (Me), 41.2 (CH), 55.7 (MeO), 61.4 (CH2O), 109.3 (CH), 113.8 (2 CH), 114.3 (CH2), 117.4 (C), 117.8 (C), 120.5 (C), 122.6 (CH), 127.5 (CH), 128.6 (C), 131.7 (2 CH), 139.2 (C), 145.3 (C), 151.5 (C), 154.2 (C), 159.2 (C), 159.8 (C), 160.2 (C=O), 197.6 (C=O) ppm. EI-MS: 432 (M+, 15), 389 (86), 43 (100). Anal. Calcd for C26H24O6 (432.46): C 72.21, H 5.59; Found: C 72.36, H 5.72.
Ethyl 5-acetyl-2-isopropenyl-9-(4-methylphenyl)-9H-furo[2,3-f] chromene-7-carboxylate (5b)
Yellow powder, mp 158–160 °C, Yield: 0.82 g (93%). IR (KBr) (νmax/cm−1): 1726, 1742, 1683, 1575, 1472, 1295 cm−1. 1H NMR (500 MHz, CDCl3): 1.34 (3 H, t, 3J = 7.4 Hz, CH3), 2.16 (3 H, s, Me), 2.23 (3 H, s, Me), 2.53 (3 H, s, Me), 4.25 (2 H, q, 3J = 7.4 Hz, CH2O), 4.63 (1 H, s, CH), 4.65 (1 H, d, 2J = 4.2 Hz, CH), 5.45 (1 H, s, CH), 5.73 (1 H, d, 2J = 4.2 Hz, CH), 6.17 (1 H, s, CH), 7.32 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.58 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.78 (1 H, s, CH) ppm. 13C NMR (125.7 MHz, CDCl3): 18.5 (Me), 22.0 (Me), 22.7 (Me), 30.4 (Me), 40.8 (CH), 61.6 (CH2O), 109.5 (CH), 114.2 (CH2), 117.6 (C), 118.3(C), 120.5 (C), 122.8 (CH), 127.3 (CH), 128.5 (2 CH), 130.6 (2 CH), 136.4 (C), 139.2 (C), 145.2 (C), 151.6 (C), 154.5 (C), 159.5 (C), 160.4 (C=O), 197.4 (C=O) ppm. Anal. Calcd for C26H24O5 (416.47): C 74.98, H 5.81; Found: C 75.16, H 5.96.
1-[2-isopropenyl-7-(4-methylphenyl)-9-phenyl-9H-furo[2,3-f] chromene-5-yl]-1-ethanone (5c)
Yellow powder, mp 172–174 °C, Yield: 0.76 g (90%). IR (KBr) (νmax/cm−1): 1725, 1692, 1563, 1485, 1274 cm−1. 1H NMR (500 MHz, CDCl3): 2.15 (3 H, s, Me), 2.35 (3 H, s, Me), 2.54 (3 H, s, Me), 4.72 (1 H, s, CH), 4.74 (1 H, d, 2J = 4.5 Hz, CH), 5.04 (1 H, s, CH), 5.53 (1 H, s, CH), 5.76 (1 H, d, 2J = 4.5 Hz, CH), 6.75 (1 H, t, 3J = 7.5 Hz, CH), 7.12 (2 H, t, 3J = 7.5 Hz, 2 CH), 7.35 (2 H, d, 3J = 7.8 Hz, 2 CH), 7.46 (2 H, d, 3J = 7.5 Hz, 2 CH), 7.73 (2 H, d, 3J = 7.8 Hz, 2 CH), 7.82 (1 H, s, CH) ppm. 13C NMR (125.7 MHz, CDCl3): 18.6 (Me), 21.6 (Me), 30.5 (Me), 42.3 (CH), 102.4 (CH), 109.3 (CH), 114.4 (CH2), 117.8 (C), 118.4 (C), 120.6 (C), 122.8 (CH), 125.6 (2 CH), 126.7 (CH), 127.3 (2 CH), 128.4 (2 CH), 129.4 (2 CH), 133.2 (C), 135.3 (C), 137.5 (C), 139.4 (C), 152.6 (C), 153.3 (C), 153.8 (C), 159.3 (C), 197.5 (C=O) ppm. Anal. Calcd for C29H24O3 (420.49): C 82.83, H 5.75; Found: C 82.96, H 5.92.
1-[2-isopropenyl-7-(4-methoxyphenyl)-9-(4-methylphenyl)-9H-furo[2,3-f] chromene-5-yl]-1-ethanone (5d)
Yellow powder, mp 183–185 °C, Yield: 0.78 g (87%). IR (KBr) (νmax/cm−1): 1726, 1689, 1572, 1486, 1282 cm−1. 1H NMR (500 MHz, CDCl3): 2.12 (3 H, s, Me), 2.17 (3 H, s, Me), 2.56 (3 H, s, Me), 3.87 (3 H, s, MeO), 4.75 (1 H, s, CH), 4.82 (1 H, d, 2J = 3.6 Hz, CH), 5.12 (1 H, s, CH), 5.62 (1 H, s, CH), 5.83 (1 H, d, 2J = 3.6 Hz, CH), 7.32 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.38 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.62 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.75 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.85 (1 H, s, CH) ppm. 13C NMR (125.7 MHz, CDCl3): 18.7 (Me), 21.8 (Me), 30.6 (Me), 42.4 (CH), 55.6 (MeO), 102.3 (CH), 109.5 (CH), 114.5 (CH2), 114.9 (2 CH), 117.6 (C), 118.6 (C), 120.7 (C), 122.7 (CH), 127.6 (2 CH), 128.5 (2 CH), 129.0 (C), 129.5 (2 CH), 130.4 (C), 136.2 (C), 139.3 (C), 152.7 (C), 153.3 (C), 153.8 (C), 159.3 (C), 161.6 (C), 198.2 (C=O) ppm. Anal. Calcd for C30H26O4 (450.53): C 79.98, H 5.82; Found: C 80.16, H 5.97.
Ethyl 5-acetyl-2-isopropenyl-9-methyl-9H-furo[2,3-f] chromene-7-carboxylate (5e)
Yellow powder, mp 131–133 °C, Yield: 0.58 g (85%). IR (KBr) (νmax/cm−1): 1723, 1742, 1684, 1587, 1468, 1275 cm−1. 1H NMR (500 MHz, CDCl3): 1.33 (3 H, t, 3J = 7.4 Hz, CH3), 1.54 (3 H, d, 3J = 7.4 Hz, CH3), 2.16 (3 H, s, Me), 2.54 (3 H, s, Me), 3.94 (1 H, q, 3J = 7.4 Hz, CH), 4.25 (2 H, q, 3J = 7.4 Hz, CH2O), 4.65 (1 H, d, 2J = 4.0 Hz, CH), 5.42 (1 H, s, CH), 5.73 (1 H, d, 2J = 4.0 Hz, CH), 6.05 (1 H, s, CH), 7.83 (1 H, s, CH) ppm. 13C NMR (125.7 MHz, CDCl3): 18.5 (Me), 20.4 (Me), 22.6 (Me), 30.4 (Me), 31.0 (CH), 61.5 (CH2O), 108.6 (CH), 114.2 (CH2), 116.8 (C), 117.6 (C), 120.7 (C), 122.7 (CH), 128.6 (CH), 136.5 (C), 143.8 (C), 151.6 (C), 154.2 (C), 158.6 (C), 160.3 (C=O), 198.3 (C=O) ppm. Anal. Calcd for C20H20O5 (340.37): C 70.57, H 5.92; Found: C 70.68, H 6.04.
1-[9-ethyl-2-isopropenyl-7-(4-methoxyphenyl)-9H-furo [2,3-f] chromene-5-yl]-1-ethanone (5f)
Yellow powder, mp 131–133 °C, Yield: 0.62 g (80%). IR (KBr) (νmax/cm−1): 1727, 1693, 1590, 1485, 1283 cm−1. 1H NMR (500 MHz, CDCl3): 1.12 (3 H, t, 3J = 7.2 Hz, CH3), 1.48–1.57 (1 H, m, CH), 1.65–1.78 (1 H, m, CH), 2.14 (3 H, s, Me), 2.56 (3 H, s, Me), 3.79–3.87 (1 H, m, CH), 3.91(3 H, s, MeO), 4.74 (1 H, d, 2J = 4.2 Hz, CH), 5.48 (1 H, s, CH), 5.75 (1 H, d, 2J = 4.2 Hz, CH), 6.12 (1 H, s, CH), 7.28 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.65 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.87 (1 H, s, CH) ppm. 13C NMR (125.7 MHz, CDCl3): 13.2 (CH3), 18.6 (Me), 29.6 (CH2), 30.5 (Me), 31.2 (CH), 55.6 (MeO), 103.5 (CH), 107.6 (CH), 113.8 (2 CH), 114.5 (CH2), 116.7 (C), 117.5 (C), 119.8 (C), 122.3 (CH), 127.6 (2 CH), 129.6 (C), 135.4 (C), 151.7 (C), 154.3 (C), 155.2 (C), 157.4 (C), 161.9 (C=O), 197.2 (C=O) ppm. EI-MS: 388 (M+, 15), 345 (68), 43 (100). Anal. Calcd for C25H24O4 (388.46): C 77.30, H 6.23; Found: C 77.46, H 6.38.
General procedure for the preparation of compounds 12
A mixture of 2-hydroxyacetophenone 11 (2 mmol), aldehyde 2 (2 mmol) and 4 ml of WEWA catalyst (5%) added to the magnetically stirred mixture of 4 ml of WEWA catalyst (5%), alkyl bromides 3 (2 mmol), triphenylphosphine 4 (2 mmol) at 80 °C. After completion of the reaction by TLC monitoring, the catalyst was isolated by filtration, then the product was dissolved in ethyl acetate and purified by small CC or PC (Hexane: EtOAc = 5:1). During the separation, the remaining catalyst and triphenylphosphine oxide were removed from the product and finally it was washed with water to attain pure title compound 12.
Ethyl 8-acetyl-4-(4-methoxyphenyl)-4H-chromene-2-carboxylate (12a)
Yellow powder, mp 103–105 °C, Yield: 0.65 g (93%). IR (KBr) (νmax/cm−1): 1725, 1743, 1692, 1595, 1484, 1268 cm−1. 1H NMR (500 MHz, CDCl3): 1.28 (3 H, t, 3J = 7.4 Hz, CH3), 2.53 (3 H, s, Me), 3.85 (3 H, s, MeO), 4.25 (2 H, q, 3J = 7.4 Hz, CH2O), 4.46 (1 H, s, CH), 6.34 (1 H, s, CH), 6.95 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.18 (1 H, t, 3J = 7.8 Hz, CH), 7.42 (1 H, d, 3J = 7.8 Hz, CH), 7.52 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.95 (1 H, d, 3J = 7.8 Hz, CH) ppm. 13C NMR (125.7 MHz, CDCl3): 22.5 (Me), 30.6 (Me), 45.8 (CH), 55.6 (MeO), 62.3 (CH2O), 114.3 (2 CH), 124.2 (CH), 126.4 (CH), 129.7 (2 CH), 130.6 (C), 131.2 (CH), 131.8 (CH), 132.3 (CH), 145.2 (C), 154.2 (C), 159.2 (C), 160.3 (C=O), 198.3 (C=O) ppm. Anal. Calcd for C21H20O5 (352.38): C 71.58, H 5.72; Found: C 71.73, H 5.87.
1-[2-(4-methylphenyl)-4-phenyl-4H-chromene-8-yl]-1-ethanone (12b)
Yellow powder, mp 123–125 °C, Yield: 0.59 g (87%). IR (KBr) (νmax/cm−1): 1727, 1687, 1576, 1487, 1282 cm−1. 1H NMR (500 MHz, CDCl3): 2.36 (3 H, s, Me), 2.62 (3 H, s, Me), 4.52 (1 H, s, CH), 5.35 (1 H, s, CH), 6.35 (1 H, t, 3J = 7.5 Hz, CH), 7.04 (2 H, t, 3J = 7.5 Hz, 2 CH), 7.15 (1 H, t, 3J = 7.6 Hz, CH), 7.35 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.42 (1 H, d, 3J = 7.5 Hz, CH), 7.53 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.76 (2 H, d, 3J = 7.6 Hz, 2 CH), 8.03 (1 H, d, 3J = 7.5 Hz, CH) ppm. 13C NMR (125.7 MHz, CDCl3): 21.5 (Me), 30.7 (Me), 47.3 (CH), 101.4 (CH), 124.2 (CH), 124.8 (C), 125.8 (2 CH), 127.3 (CH), 128.0 (2 CH), 128.4 (2 CH), 129.1 (2 CH), 131.2 (CH), 131.8 (CH), 132.2 (C), 135.3 (C), 137.2 (C), 139.7 (C), 153.4 (C), 155.7 (C), 198.6 (C=O) ppm. Anal. Calcd for C24H20O2 (340.41): C 84.68, H 5.92; Found: C 84.82, H 6.12.
1-[2-(4-methoxyphenyl)-4-(4-methylphenyl)-4H-chromene-8-yl]-1-ethanone (12c)
Yellow powder, mp 127–129 °C, Yield: 0.63 g (85%). IR (KBr) (νmax/cm−1): 1728, 1694, 1586, 1485, 1275 cm−1. 1H NMR (500 MHz, CDCl3): 2.14 (3 H, s, Me), 2.58 (3 H, s, Me), 3.85 (3 H, s, MeO), 4.56 (1 H, s, CH), 5.36 (1 H, s, CH), 7.18 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.24 (1 H, t, 3J = 7.5 Hz, CH), 7.32 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.42 (1 H, d, 3J = 7.5 Hz, CH), 7.58 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.75 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.96 (1 H, d, 3J = 7.5 Hz, CH) ppm. 13C NMR (125.7 MHz, CDCl3): 21.7 (Me), 30.8 (Me), 47.4 (CH), 55.6 (MeO), 101.6 (CH), 114.6 (2 CH), 124.3 (CH), 126.4 (2 CH), 127.8 (2 CH), 130.2 (2 CH), 130.6 (C), 131.4 (CH), 131.8 (CH), 132.2 (C), 136.2 (C), 153.2 (C), 155.6 (C), 161.4 (C), 198.5 (C=O) ppm. Anal. Calcd for C25H22O3 (370.44): C 81.06, H 5.99; Found: C 81.21, H 6.14.
Ethyl 8-acetyl-4-methyl-4H-chromene-2-carboxylate (12d)
Yellow powder, mp 100–102 °C, Yield: 0.42 g (80%). IR (KBr) (νmax/cm−1): 1725, 1738, 1687, 1562, 1473, 1295 cm−1. 1H NMR (500 MHz, CDCl3): 1.35 (3 H, t, 3J = 7.4 Hz, CH3), 1.43 (3 H, d, 3J = 7.3 Hz, CH3), 2.58 (3 H, s, Me), 3.92 (1 H, q, 3J = 7.3 Hz, CH), 4.26 (2 H, q, 3J = 7.4 Hz, CH2O), 5.86 (1 H, s, CH), 7.12 (1 H, t, 3J = 7.5 Hz, CH), 7.32 (1 H, d, 3J = 7.6 Hz, CH), 7.93 (1 H, d, 3J = 7.6 Hz, CH) ppm. 13C NMR (125.7 MHz, CDCl3): 19.2 (Me), 22.8 (Me), 30.6 (Me), 35.4 (CH), 61.4 (CH2O), 123.6 (C), 124.2 (CH), 127.6 (CH), 130.5 (C), 131.2 (CH), 131.8 (CH), 143.6 (C), 154.3 (C), 160.5 (C=O), 198.5 (C=O) ppm. Anal. Calcd for C15H16O4 (260.28): C 69.22, H 6.20; Found: C 69.36, H 6.34.
1-[2-ethyl-4-(4-methoxyphenyl)-4H-chromene-8-yl]-1-ethanone (12e)
Yellow powder, mp 118–120 °C, Yield: 0.51 g (83%). IR (KBr) (νmax/cm−1): 1726, 1683, 1587, 1464, 1275 cm−1. 1H NMR (500 MHz, CDCl3): 1.15 (3 H, t, 3J = 7.3 Hz, CH3), 1.45–1.54 (1 H, m, CH), 1.67–1.82 (1 H, m, CH), 2.58 (3 H, s, Me), 3.76–3.84 (1 H, m, CH), 3.87 (3 H, s, MeO), 5.83 (1 H, s, CH), 7.04 (1 H, t, 3J = 7.4 Hz, CH), 7.27 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.56 (1 H, d, 3J = 7.5 Hz, CH), 7.65 (2 H, d, 3J = 7.6 Hz, 2 CH), 7.86 (1 H, d, 3J = 7.5 Hz, CH) ppm. 13C NMR (125.7 MHz, CDCl3): 13.4 (CH3), 29.3 (CH2), 30.2 (Me), 35.6 (CH), 55.7 (MeO), 102.4 (CH), 114.2 (2 CH), 123.7 (CH), 127.4 (2 CH), 129.7 (C), 130.3 (C), 131.0 (CH), 131.6 (CH), 151.8 (C), 156.8 (C), 160.7 (C), 198.6 (C=O) ppm. Anal. Calcd for C20H20O3 (308.37): C 77.90, H 6.54; Found: C 78.06, H 6.68.
Conclusions
We have reported an efficient and facile approach for the synthesis of novel benzochromene derivatives via the multi components reaction of euparin with aldehydes, alkyl bromides, and using triphenylphosphine in the presence of water extracts of wood ash as a catalyst. To the best of our knowledge, this is the first report of this catalyst and its application for the synthesis of these important compounds. Advantages of the presented procedure are high to excellent yields, simple work-up and the lack of need for column chromatography (Supplementary Information).
Supplementary Information
Author contributions
M.K. and D.Z. conceived the experiment; R.R. conducted the experiment; M.K. and R.R. analyzed the data; M.K. and R.R. and D.Z. wrote and reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-05133-x.
References
- 1.Etiégni L, Campbell AG. Physical and chemical characteristics of wood ash. Bioresour. Technol. 1991;37:173. [Google Scholar]
- 2.Olanders B, Steenari B. Characterization of ashes from wood and straw. Biomass Bioenergy. 1995;8:105. [Google Scholar]
- 3.Aprianti SE. A huge number of artificial waste material can be supplementary cementitious material (SCM) for concrete production—A review part II. J. Clean. Prod. 2017;142:4178. [Google Scholar]
- 4.Werkelin J, Skrifvars BJ, Hupa M. Ash-forming elements in four. Scandinavian wood species. Part 1: Summer harvest. Biomass Bioenergy. 2005;29:451. [Google Scholar]
- 5.Erich MS, Ohno T. Titrimetric determination calcium carbonate equivalence of wood ash. Analyst. 1992;117:9. [Google Scholar]
- 6.Ulery L, Graham RC, Amrhein C. Wood-ash composition and soil pH following intense burning. Soil Sci. 1993;156:35. [Google Scholar]
- 7.Gholipour B, Shojaei S, Rostamnia S, Naimi-Jamal MR, Kim D, Kavetskyy T, Nouruzi N, Jang HW, Varma RS, Shokouhimehr M. Metal-free nanostructured catalysts: Sustainable driving forces for organic transformations. Green. Chem. 2021;23:6223. [Google Scholar]
- 8.Al-Rahbi S, Williams PT. Waste ashes as catalysts for the pyrolysis–catalytic steam reforming of biomass for hydrogen-rich gas production. J. Mater. Cycles Waste Manag. 2019;21:1224. [Google Scholar]
- 9.Deyris PA, Adler P, Petit E, Legrand YM, Grison C. Woody species: A new bio-based material for dual Ca/Mg catalysis with remarkable Lewis acidity properties. Green Chem. 2019;21:3133. [Google Scholar]
- 10.Miladinović MR, Zdujić MV, Veljović DN, Krstić JB, Banković-Ilić IB, Veljković VB, Stamenković OS. Valorization of walnut shell ash as a catalyst for biodiesel production. Renew. Energy. 2020;147:1033. [Google Scholar]
- 11.Sharma M, Ali Khan A, Puri SK, Tuli DK. Wood ash as a potential heterogeneous catalyst for biodiesel synthesis. Biomass Bioenergy. 2012;41:94. [Google Scholar]
- 12.Lopinti K, Sharma M, Chakradhar M, Arora AK, Kagdiyal V, Majumdar SK. Ash catalyzed synthesis of long-chain dialkyl carbonates through carbonyl exchange reaction. Catal. Lett. 2020;150:1163. [Google Scholar]
- 13.Basumatary S, Nath B, Kalita P. Application of agro-waste derived materials as heterogeneous base catalysts for biodiesel synthesis. J. Renew. Sustain. Energy. 2018;10:043105. [Google Scholar]
- 14.Shokouhimehr M, Mahmoudi-Gom Yek S, Nasrollahzadeh M, Kim A, Varma RS. Palladium nanocatalysts on hydroxyapatite: Green oxidation of alcohols and reduction of nitroarenes in water. Appl. Sci. 2019;9:4183. [Google Scholar]
- 15.Khafagy MM, Abd El-Wahab AHF, Eid FA, El-Agrody AM. Synthesis of halogen derivatives of benzo[h]chromene and benzo[a]anthracene with promising antimicrobial activities. Farmaco. 2002;57:715. doi: 10.1016/s0014-827x(02)01263-6. [DOI] [PubMed] [Google Scholar]
- 16.Sashidhara KV, Rosaiah JN, Bhatia G, Saxena JK. Novel keto-enamine Schiffs bases from 7-hydroxy-4-methyl-2-oxo-2H-benzo[h] chromene-8,10-dicarbaldehyde as potential antidyslipidemic and antioxidant agents. Eur. J. Med. Chem. 2008;43:2592. doi: 10.1016/j.ejmech.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 17.De Andrade-Neto VF, Goulart MOF, Da Silva Filho JF, Da Silva MJ, Pinto MDCFR, Pinto AV, Zalis MG, Carvalho LH, Krettli AU. Antimalarial activity of phenazines from lapachol, β-lapachone and its derivatives against Plasmodium falciparum in vitro and Plasmodium berghei in vivo. Bioorg. Med. Chem. Lett. 2004;14:1145. doi: 10.1016/j.bmcl.2003.12.069. [DOI] [PubMed] [Google Scholar]
- 18.Kanakaraju S, Prasanna B, Basavoju S, Chandramouli GVP. Ionic liquid catalyzed one-pot multi-component synthesis, characterization and antibacterial activity of novel chromeno [2, 3-d] pyrimidin-8-amine derivatives. J. Mol. Struct. 2012;1017:60. [Google Scholar]
- 19.Qiang Z, Shi JB, Song BA, Liu XH. Novel 2H-chromen derivatives: Design, synthesis and anticancer activity. RSC Adv. 2014;4:5607. [Google Scholar]
- 20.Balou J, Khalilzadeh MA, Zareyee D. An efficient and reusable nano catalyst for the synthesis of benzoxanthene and chromene derivatives. Sci. Rep. 2019;9:3605. doi: 10.1038/s41598-019-40431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang F, Wang H, Jiang L, Yue H, Zhang H, Wang Z, Wang L. A green and one-pot synthesis of benzo[g]chromene derivatives through a multi-component reaction catalyzed by lipase. RSC Adv. 2015;5:5213. [Google Scholar]
- 22.Maleki B, Babaee S, Tayebee R. Zn (l-Proline)2: As a powerful and reusable organomettalic catalyst for the very fast synthesis of 2-amino-4H-benzo[g]chromene derivatives under solvent-free conditions. Appl. Organomet. Chem. 2015;29:408. [Google Scholar]
- 23.Khurana JM, Nand B, Saluja P. DBU: A highly efficient catalyst for one-pot synthesis of substituted 3,4-dihydropyrano[3,2-c]chromenes, dihydropyrano[4,3-b]pyranes, 2-amino-4H-benzo[h]chromenes and 2-amino-4H benzo[g]chromenes in aqueous medium. Tetrahedron. 2010;66:5637. [Google Scholar]
- 24.Yao C, Yu C, Li T, Tu S. An efficient synthesis of 4H-benzo[g]chromene-5,10-dione de-rivatives through triethylbenzylammonium chloride catalyzed multicomponent reaction under solvent-free conditions. Chin. J. Chem. 1989;2009:27. [Google Scholar]
- 25.Yu Y, Guo H, Li X. An improved procedure for the three-component synthesis of benzo[g]chromene derivatives using basic ionic liquid. J. Heterocycl. Chem. 2011;48:1264. [Google Scholar]
- 26.Mirosanloo A, Zareyee D, Khalilzadeh MA. Recyclable cellulose nanocrystal supported Palladium nanoparticles as an efficient heterogeneous catalyst for the solvent-free synthesis of coumarin derivatives via von Pechmann condensation. Appl. Organomet. Chem. 2018;32:e4546. [Google Scholar]
- 27.Rajabi M, Hossaini Z, Khalilzadeh MA, Datta S, Halder M, Mousa SA. Synthesis of a new class of furo [3, 2-c] coumarins and its anticancer activity. J. Photochem. Photobiol. B. 2015;148:66. doi: 10.1016/j.jphotobiol.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Yaghoobi M, Zareyee D, Khalilzadeh MA. A green and chemoselective synthesis of coumarins via Pechmann condensation using recoverable heterogeneous catalyst (Au@pSiO2) Appl. Organomet. Chem. 2020;34:e5913. [Google Scholar]
- 29.Khaleghi F, Bin Din L, Jantan J, Yaacob WA, Khalilzadeh MA. A facile synthesis of novel 1,4-benzoxazepin-2-one derivatives. Tetrahedron Lett. 2011;52:7182. [Google Scholar]
- 30.Khalilzadeh MA, Hossaini Z, Charati FR, Hallajian S, Rajabi M. A mild and efficient method for the synthesis of a new class of furo [3, 2-c] chromenes in aqueous media. Mol. Div. 2011;15:445. doi: 10.1007/s11030-010-9264-3. [DOI] [PubMed] [Google Scholar]
- 31.Charati FR, Hossaini Z, Khalilzadeh MA. Novel isocyanide-based three-component synthesis of substituted 9Hfuro [2, 3-f] chromene-8, 9-dicarboxylates in water. Comb. Chem. High Throughput Screen. 2012;15:433. doi: 10.2174/138620712800194495. [DOI] [PubMed] [Google Scholar]
- 32.Hallajian S, Khalilzadeh MA, Tajbakhsh M, Alipour E, Safaei Z. Nano clinoptilolite: Highly efficient catalyst for the synthesis of chromene derivatives under solvent-free conditions. Comb. Chem. High Throughput Screen. 2015;18:486. doi: 10.2174/1386207318666150424104452. [DOI] [PubMed] [Google Scholar]
- 33.Khaleghi F, Bin Din L, Rostami Charati F, Yaacob WA, Khalilzadeh MA, Skelton B, Makha M. A new bioactive compound from the roots of Petasites hybridus. Phytochem. Lett. 2011;4:254. [Google Scholar]
- 34.Ulery L, Graham RC, Amrhein C. Wood-ash composition and soil pH following intense burning. Soil Sci. 1993;156:358. [Google Scholar]
- 35.Liodakis S, Katsigiannis G, Kakali G. Ash properties of some dominant Greek forest species. Thermochim. Acta. 2005;437:158. [Google Scholar]
- 36.Misra MK, Ragland KW, Baker AJ. Wood ash composition as a function of furnace temperature. Biomass Bioenergy. 1993;4:103. [Google Scholar]
- 37.Gerzabek MH, Antil RS, Kögel-Knabner I, Knicker H, Kirchmann H, Haberhauer G. How are soil use and management reflected by soil organic matter characteristics: A spectroscopic approach. Eur. J. Soil Sci. 2006;57:485. [Google Scholar]
- 38.Dick DP, Knicker H, Ávila LG, Inda AV, Jr, Giasson E, Bissani CA. Organic matter in constructed soils from a coal mining area in southern Brazil. Org. Geochem. 2006;37:1537. [Google Scholar]
- 39.Radev L, Hristov V, Michailova I, Helena M, Fernandes V, Miranda I. In vitro bioactivity of biphasic calcium phosphate silicate glass-ceramic in CaO-SiO2-P2O5 system. Process Appl. Ceram. 2010;4:15. [Google Scholar]
- 40.Pitman RM. Wood ash use in forestry—A review of the environmental impacts. Int. J. For. Res. 2006;79:563. [Google Scholar]
- 41.Shokouhimehr M, Kim JH, Lee YS. Heterogeneous Heck reaction catalyzed by recyclable polymer-supported N-heterocyclic carbene-palladium complex. Synlett. 2006;04:0618. doi: 10.1021/jo050721m. [DOI] [PubMed] [Google Scholar]
- 42.Anastas P, Eghbali N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010;39:301. doi: 10.1039/b918763b. [DOI] [PubMed] [Google Scholar]
- 43.Shokouhimehr M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts. 2015;5:534. [Google Scholar]
- 44.Nayebi B, Rabiee N, Nayebi B, Shahedi Asl M, Ramakrishna S, Jang HW, Varma RS, Shokouhimehr M. Boron nitride-palladium nanostructured catalyst: Efficient reduction of nitrobenzene derivatives in water. Nano Express. 2020;1:030012. [Google Scholar]
- 45.Zhang K, Suh JM, Lee TH, Cha JH, Choi JW, Jang HW, Varma RS, Shokouhimehr M. Copper oxide–graphene oxide nanocomposite: Efficient catalyst for hydrogenation of nitroaromatics in water. Nano Convergence. 2019;6:1. doi: 10.1186/s40580-019-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.