Aging is the primary risk factor for various chronic disorders, especially neurodegeneration diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD). It has been estimated that about one in ten individuals aged >65 are affected by AD, and this prevalence continues to increase with the aging of the population [1], causing a heavy burden on the family and society. As the aging population grows progressively around the globe, drug discoveries targeting the aging process have become a hot spot [2]. However, existing research is still in the exploratory stage [3], no effective intervention strategies are currently available in clinical practice. This is partially due to our limited understanding of aging. Recently, several single-cell and bulk RNA-seq studies of various organs at different ages across the mouse lifespan have revealed enrichment in the inflammatory and immune response processes in multiple organs [4, 5]. These findings emphasize the importance of alterations in immune function during organism aging. They also suggests that dysregulation of the immune system may play a key role in the aging process.
To test this hypothesis, researchers from the University of Minnesota investigated the contribution of the decline in immune function on systemic aging in vivo [6]. It has been reported that dysfunctional responses to DNA damage can result in the senescence of hematopoietic stem cells (HSCs) [7]. Thus, Yousefzadeh et al established an immunosenescence model by specifically removing the DNA-repairing enzyme ERCC1–XPF in mouse hematopoietic cells (Vav-iCre+/−;Ercc1−/fl mice), which would lead to the accumulation of endogenous DNA damage. They found that ERCC1–XPF knockout in hematopoietic cells did not affect mouse development as Ercc1 knock-out (KO) mice were healthy into adulthood. However, the mutant mice developed progressive peripheral leukopenia; the numbers of whole blood white cells gradually decreased by five months of age. Besides, immune cells in the bone marrow and spleen were also significantly reduced in this mouse model, and the cell number continued to decline as the mice aged. Moreover, the innate and adaptive immune functions were notably impaired in Ercc1 KO mice by five months of age. This evidence suggests that endogenous DNA damage accumulation in HSCs is sufficient to cause a decline in immune function.
To explore the mechanism of this functional decline, immune cells from spleen and bone marrow were isolated for further investigation. The results showed that the expression of senescence and senescence-associated secretory phenotype (SASP) markers were significantly increased in mutant mice B cells, T cells, NK cells, and macrophages compared to wild-type littermates. This indicates that increased endogenous DNA damage may accelerate immune cell aging.
Next, to determine whether immune senescence can affect non-lymphoid organs, tissues were collected from 8- to 11-month-old mice for verification. Widespread overexpression of p16 and p21 was detected in various tissues of Ercc1-KO mice. Reactive oxidative species levels were increased in the liver and kidney of mutant mice, along with an increased functional decline. Grip strength was diminished, indicating muscle degeneration. Levels of serum SASP factors such as β2-microglobulin and GDF-15 were also increased. Strikingly, the lifespan of Ercc1-KO mice was significantly reduced. These data demonstrated that an aged immune system is sufficient to drive the age-associated changes in multiple peripheral organs that might contribute to a reduced lifespan.
To further demonstrate that senescent immune cells are sufficient to drive secondary senescence and aging pathology, Yousefzadeh et al isolated splenocytes from 8- to 10-month-old Ercc1-KO mice and transplanted them into 3-month old p16Ink4a-luciferase reporter mice. They found that even one week after transplantation, recipient mice had significantly increased luciferase signal levels in multiple organs, along with the elevated levels of serum SASP factors. Besides that, transplantation of splenocytes from 19-month Ercc1-KO mice into Ercc1−/∆ mice significantly reduced the lifespan of the host mice. Overall, these data reinforce the conclusion that aged immune cells drive accelerated aging and death.
Since senescent immune cell transplantation leads to organism aging, can young immune cell transplantation alleviate the senescent phenotype? To test this idea, Yousefzadeh et al transplanted splenocytes from 2-month wild-type mice into 3-month Ercc1-KO mice, and found that this significantly promoted immune function and ameliorated kidney, spleen, and skin senescence one week after transplantation. Levels of circulating SASP factors and markers of tissue damage were also strikingly decreased. Numerous studies have revealed that inhibition of mTOR activity by rapamycin might have a positive impact on several model organisms and certain human age-related pathologies [2], thus the author presumed that rapamycin might also modify immunosenescence. To test this notion, 3-month Ercc1-KO mice were treated with 6 weeks of rapamycin, and enhanced immune function was verified. Taken together, their results indicate that targeting aging immune cells might be a novel anti-aging strategy.
During the past decades, investigations of the aging process and means to improve a healthy elderly life has become a global issue. It is widely accepted that inflammation plays an important role in aging since the aging process is always accompanied by immunosenescence. However, we still don’t know whether immune aging occurs before systemic aging. Is it the main cause of systemic aging? If so, what are the factors that induce immunosenescence? In this article, Yousefzadeh et al elaborate on these issues in detail. Their results show that the immune system is susceptible to endogenous DNA damage. If not repaired in time, the gradual accumulation of DNA damage accelerates the aging of the immune system and eventually causes widespread immune dysfunction. More importantly, aging of the immune system also leads to the premature aging of multiple organs (such as liver, kidney, and pancreas). This demonstrates that aging of the immune system is able to cause systemic aging, providing a potential target for the treatment of aging-related diseases.
In previous studies, scientists mainly focused on bone marrow when investigating the immune system. But in this article, the authors emphasize the function of the spleen, the largest secondary lymphoid tissue hosting a wide range of immunological functions. They demonstrated that transplantation of senescent splenocytes into young mice causes widespread functional impairments in tissue. More strikingly, they found that transplantation of young splenocytes into immunosenscent mice is enough to recover the overall functional decline. Thus, this study provides a potential alternative anti-aging intervention strategy by targeting senescent immune cells via splenocyte transplantation.
However, it is still unclear how the transplantation of young immune cells rejuvenates the whole body. The authors found that transplanted splenocytes appeared in several organs of the recipient mice, so it seems that transplanted splenocytes not only rebuild the immune system but also directly modulate other solid tissues. But this needs further investigation. Besides, the spleen is composed of various types of cells, including B cells, T cells, monocytes, and NK cells. Therefore, it is necessary to further explore which type of transplanted spleen cells play a major role, or whether it is the combined effects of various types of splenocytes.
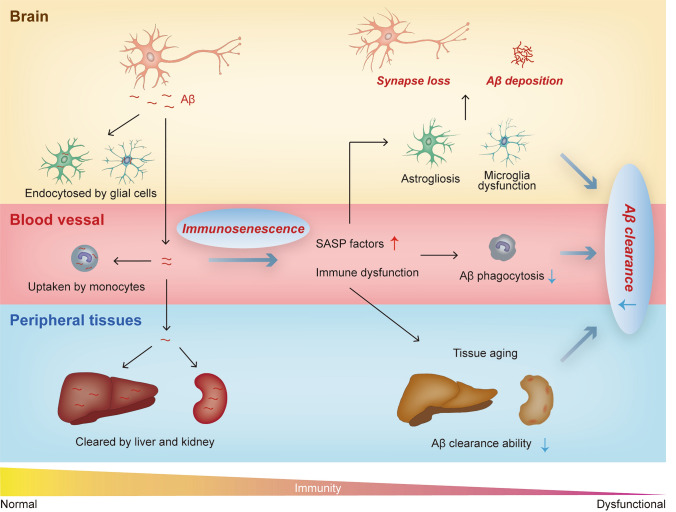
It is well recognized that aging is a key risk factor for AD [8]. Since immunosenscence can accelerate systemic aging, it may also play a critical role in the pathogenesis of AD (Fig. 1). Indeed, evidence has shown that an aging-induced increase of immune cell infiltration might lead to attenuate neurogenesis and cognition during normal brain aging [9]. Our results demonstrated that peripheral blood monocytes are capable of Aβ clearance and this clearance capacity diminishes with ageing [10]. These results suggest that rejuvenating the peripheral immune system may have potential in AD prevention and treatment. With the development and maturation of induced pluripotent stem cell technologies, the differentiation of young hematopoietic cells or splenocytes, and then transplantation into older people might be a promising direction. Besides that, modifying the cell senescence status with in vivo gene editing could also be an alternative strategy. Furthermore, as Yousefzadeh et al have shown, targeting the senescence-related pathway with a small molecular compound (rapamycin) could also be effective. In addition to AD, other neurodegenerative diseases (such as PD and amyotrophic lateral sclerosis) are also accompanied by immunosenescence. Therefore, investigating the therapeutic effect of peripheral immune system remodeling might be useful in the treatment of various neurodegenerative disorders.
Fig. 1.
Contribution of immunosenescence to the pathogenesis of AD. Under physiological conditions, around 40%–60% brain-born Aβ exits the brain and is cleared by peripheral tissues (i.e. kidney, liver and blood monocytes). In the circumstance of immunosenescence, immune system dysfunctions can lead to widespread premature aging of peripheral tissues. The Aβ clearance ability of aged tissues decreases, which might accelerate Aβ deposition in the brain. Besides, the secretion level of SASP factors of aged tissues is significantly increased, which might accelerate brain aging and neurodegeneration. SASP, senescence-associated secretory phenotype.
Acknowledgements
This highlight was supported by the National Natural Science Foundation of China (91749206 and 81930028).
Conflict of interest
The authors declare no conflict of interests.
References
- 1.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 2.Partridge L, Fuentealba M, Kennedy BK. The quest to slow ageing through drug discovery. Nat Rev Drug Discov. 2020;19:513–532. doi: 10.1038/s41573-020-0067-7. [DOI] [PubMed] [Google Scholar]
- 3.Ding XL, Lei P. Plasma replacement therapy for Alzheimer's disease. Neurosci Bull. 2020;36:89–90. doi: 10.1007/s12264-019-00394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaum N, Lehallier B, Hahn O, Pálovics R, Hosseinzadeh S, Lee SE, et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature. 2020;583:596–602. doi: 10.1038/s41586-020-2499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabula Muris Consortium A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature. 2020;583:590–595. doi: 10.1038/s41586-020-2496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594:100–105. doi: 10.1038/s41586-021-03547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akunuru S, Geiger H. Aging, clonality, and rejuvenation of hematopoietic stem cells. Trends Mol Med. 2016;22:701–712. doi: 10.1016/j.molmed.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan DY, Wang YJ, Wang YJ. Early intervention in Alzheimer's disease: How early is early enough? Neurosci Bull. 2020;36:195–197. doi: 10.1007/s12264-019-00429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuapio A, Ljunggren HG. Activated natural killer cells hit neurogenesis in the aging brain. Neurosci Bull. 2021;37:1072–1074. doi: 10.1007/s12264-021-00654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen SH, Tian DY, Shen YY, Cheng Y, Fan DY, Sun HL, et al. Amyloid-beta uptake by blood monocytes is reduced with ageing and Alzheimer's disease. Transl Psychiatry. 2020;10:423. doi: 10.1038/s41398-020-01113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]