Abstract
Background:
Hair follicles are among a handful of organs that exhibit immune privilege. Dysfunction of the hair follicle immune system underlies the development of inflammatory diseases, such as alopecia areata.
Methods:
Quantitative reverse transcription PCR and immunostaining was used to confirm the expression of major histocompatibility complex class I in human dermal papilla cells. Through transcriptomic analyses of human keratinocyte stem cells, major histocompatibility complex class I was identified as differentially expressed genes. Organ culture and patch assay were performed to assess the ability of WNT3a conditioned media to rescue immune privilege. Lastly, CD8+ T cells were detected near the hair bulb in alopecia areata patients through immunohistochemistry.
Results:
Inflammatory factors such as tumor necrosis factor alpha and interferon gamma were verified to induce the expression of major histocompatibility complex class I proteins in dermal papilla cells. Additionally, loss of immune privilege of hair follicles was rescued following treatment with conditioned media from outer root sheath cells. Transcriptomic analyses found 58 up-regulated genes and 183 down-regulated genes related in MHC class I+ cells. Using newborn hair patch assay, we demonstrated that WNT3a conditioned media with epidermal growth factor can restore hair growth. In alopecia areata patients, CD8+ T cells were increased during the transition from mid-anagen to late catagen.
Conclusion:
Identification of mechanisms governing epithelial and mesenchymal interactions of the hair follicle facilitates an improved understanding of the regulation of hair follicle immune privilege.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13770-021-00392-7.
Keywords: Immune privilege, Hair follicle, MHC molecule, Epithelial-mesenchymal interaction
Introduction
The hair follicle (HF) is a highly organized and complex system consisting of dermal papilla (DP) and dermal sheath (DS) cells derived from the mesenchyme and of epithelial components, such as the matrix, bulge, and outer root sheath (ORS) cells [1, 2]. Interactions between these two components and coordination of key signaling pathways are critical for the development, morphogenesis, and growth of hair [3–5]. One known property of the HF is the ability to cycle between phases of active growth and quiescence [1, 2]. During anagen, HFs actively produce a new hair shaft through the coordination of multiple cellular processes and display a unique property called immune privilege [6]. During catagen, HFs exhibit extremely high inflammatory signals, leading to massive apoptosis. In telogen, the HFs stop producing a hair shaft and enter a quiescent state [1]. The intrinsic regenerative cycle of mammalian HFs orchestrates the inflammatory expression patterns of cellular components in the skin such as adipose tissues, vascular cells, glands, and inflammatory cells [1, 2, 6–9]
The HF shows highly specialized temporal dynamics of its immune privilege especially during anagen. In 1971, Billingham and Silvers found that epidermal melanocytes from transplanted guinea pig skin can survive and migrate into bulb of the anagen HFs in genetically incompatible hosts [10]. Since then, the concept of HF immune privilege has been commonly accepted in the field of hair biology [6]. While major histocompatibility complex (MHC) class I glycoproteins are known to be expressed in most skin cells, its expression is significantly reduced in isthmus keratinocytes and is virtually absent in the proximal HF epithelium. By conferring immediate HF protection from immune attack, HF immune privilege might even promote peripheral self-tolerance [6]. Characterized by MHC class I negativity and an immunosuppressive cytokine milieu, anagen hair bulbs are one of the few immunologically privileged tissues of the mammalian body [6].
The process of cyclic hair regeneration involves the epithelial and mesenchymal interactions. Among them, bone morphogenetic proteins (BMPs), Wingless-INT (WNT), and epidermal growth factor (EGF) signalings are considered as the key players [2]. The BMP pathways act as the hair cycle inhibitor, whereas activation of WNT signaling plays a significant role in stimulating hair growth and rescuing the hyper-refractory skin domain [2, 11]. EGF regulates potent cell growth and differentiation in skin [12]. Additionally, the successful growth and maintenance of human hair follicles in vitro depends on EGF [13], while EGF also stimulates ORS cells to proliferate and migrate [14].
A collapse of immune privilege of the anagen hair bulb plays a crucial part in the pathogenesis of alopecia areata (AA), a disease in which the immune system begins attacking HFs, leaving round patches of hairless skin [15]. Abnormalities or dysfunction of the HF immune system explains the rather high incidence of folliculitis in immunocompromised patients [6]. Among various inflammatory cells in the skin, CD8+ T cells are localized in the distal HF epithelium and the distal perifollicular dermis [16]. Loss of HF immune privilege leads to macrophage-driven attacks on epithelial HF stem cells located in the bulge region of the outer root sheath. This attack is not always dependent on pathological circumstances, occurring under normal physiological circumstances as well. However, alteration of the immune nature of HFs in this disease, how they relate to the normal hair cycle, and whether these alterations can be reversed, remains poorly understood. Therefore, systemic characterization of the HF immune system in both healthy and diseased status will have significant clinical implications [6].
In our preliminary study, we observed an increased expression of MHC class I glycoproteins in cultured DP cells when compared to that of in vivo DP cells, suggesting that there may be a loss of immune privilege during in vitro culture [17, 18]. To verify this, we compared the expression of MHC class I glycoproteins in primary explanted DP cells without passage (DP P0) and after three passages (DP P3) using quantitative reverse transcription PCR (RT-qPCR) analysis and immunostaining. Additionally, we studied the effects of various cytokines and conditioned media (CM) from outer root sheath (ORS) cells and WNT3a-expressing cells on MHC class I glycoprotein expression in vitro to elucidate the molecular link between epithelial components and dermal components in the HF immune privilege. Transcriptomic analysis revealed differentially expressed genes associated with MHC class I levels. Furthermore, organ culture and patch assays were performed to investigate the effect of WNT3a CM and EGF supplemented WNT3a CM to restore hair growth, after interferon gamma (IFN-γ) treatment. Finally, we observed the distribution of CD8+ T cells in different hair cycles using hairs obtained from AA patients. These results advance our understanding of the communication between various cytokine and HF immune privilege.
Materials and methods
Human hair organ and DP cell culture
Hair biopsy specimens were obtained from the non-balding occipital scalp region. The Medical Ethics Committee of the Kyungpook National University Hospital (Daegu, Korea) approved all the described studies. Human DPs were then isolated from the bulbs of dissected anagen hair follicles, and DP cells were expanded in culture as described previously [19, 20]. Briefly, isolated primary human hair organ and DP cells [18, 21] were cultured in William E and DMEM low-glucose medium (Clonetics, Inc., San Diego, CA, USA) individually. The cells were maintained in a humidified incubator at 37 °C under 5% CO2.
Preparation of conditioned media
ORS cells are cultured as described previously [22]. Briefly, CM from ORS cells were obtained after 1 × 106 cells were incubated for 5–8 days in 10 cm diameter culture dishes containing EpiLife medium supplements according to manufacturer instructions (Thermo Fisher Scientific Korea Ltd, Seoul, Korea). Harvested CM was centrifuged at 1000 g for 10 min and sterilized by filtration. WNT3a secreting mouse L cells were purchased from ATCC (ATCC® CRL-2648™, Manassas, VA, USA) and cultured in DMEM supplemented with 0.4 mg/ml G-418 and 10% FBS. Also, CM from non-transfected L cells were collected as described above for control group. For the cytokine assay, DP cells in their third passage were plated overnight and incubated with various cytokines for 48 h. The cytokines used in this study are as follows: transforming growth factor beta (TGF-β) (10 ng/ml), insulin-like growth factor 2 (IGF-2) (50 ng/ml), EGF (100 ng/ml), IFN-γ (100 ng/ml), and tumor necrosis factor alpha (TNF-α) (100 ng/ml) (Immune Square, Inc., Daegu, Korea).
Immunostaining
Immunostaining was performed on cultured cell and cryostat sections of biopsied samples with the appropriate antibody and then visualized by microscopy [23]. Immunofluorescence (IF) of MHC class I was performed as follows: dried frozen sections on glass slides were immersed in MeOH for 20 min and washed in phosphate buffered saline (PBS) for 5 min. Sections were then blocked with 5% bovine serum albumin (BSA) for 1 h at room temperature. Following blocking, sections were incubated with primary human anti-MHC class I (1:100) (BD Pharmingen Inc., Franklin Lakes, NJ, USA) for overnight in 4ºC. Nonspecifically bounded primary antibodies were removed by three sequential washes in PBS for 5 min. After incubation of secondary antibody (anti-mouse immunoglobin Alexa Fluor 488 (1:1000)) (Agilent Dako, Santa Clara, CA, USA) for 1 h, three sequential PBS washes for 5 min were done, and then counterstained with DAPI (Thermo Scientific Inc., Rockford, IL, USA) before mounting in medium (Vector, Burlingame, CA, USA).
Immunohistochemistry (IHC) of CD8 (BD Pharmingen Inc.) with biopsied HFs specimens from AA patients and immunocytochemistry (ICC) of human anti-MHC class I with human DP cells were performed as above. After removed nonspecific binding, from primary antibody incubation, anti-mouse-HRP (Horseradish peroxidase) as secondary antibody (Agilent Dako) was applied for 1 h at room temperature. Sections were then washed two times in PBS for 5 min, and aminoethyl carbazole (AEC)(Agilent Dako) was applied for chromogen development of bound peroxidase activity. Sections were lightly counterstained with hematoxylin before mounting in aqueous medium (Agilent Dako).
Immunocytochemistry (ICC) was performed on cultured cells in the chamber slide. Cells were seeded 10,000 cells per well then incubated overnight at 37ºC in humidified atmosphere of 5% CO2. Cells were washed with PBS and immersed in chill 95% ethanol for 10 min and subsequent process was performed in the same manner as in IHC.
Quantitative real-time PCR
Total RNA was extracted from cultured primary human DP cell using RNAiso Plus (Takara Bio Inc., Shiga, Japan). Three micrograms of total RNA were used to synthesize cDNA using a First Strand cDNA Synthesis Kit (MBI Fermentas, Vilnius, Lithuania). Gene-specific forward and reverse primers were used in quantitative real time-PCR (RT-qPCR) using StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with SYBR Green PCR Master Mix (Takara Bio Inc.) according to the manufacturer’s instruction. MHC class I primers were synthesized at Genotech (Geno Tech Co., Daejeon, Korea). Since MHC class I have more than 169 polymorphisms [24, 25], we designed the primers to cover most polymorphisms of the MHC class I gene. Primer sequences were 5′-TGGAGGAGGAAGAGCTCAGAT-3′ and 5′-CTCTCCCCACCTCCTCACATT-3′. The primer sequences used for β-actin were 5′-GGACTTCGAGCAAGAGATGG-3′ and 5′-AGCACTGTGTTGGCGTACAG-3′. The PCR conditions were 95 °C for 2 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The data were normalized using comparative Ct method (ΔΔCt method) and relative levels of MHC class I were calculated to β-actin levels.
Bioinformatical analysis of MHC class I expression in human keratinocyte stem cell
Gene expression omnibus (GEO) information was used to analyze the expression of MHC Class I in human keratinocyte stem cells. We used GSE11089 for MHC class I gene list [26]. Heatmap and volcano plot were created using the R3.4 program. Information of R script were generated from ("https://github.com/HyunsuLee/R_nipple") as we previous reported [2, 27]. Bubble chart used a biological process of gene ontology (GO) associated MHC Class I in human keratinocyte stem cells. Inflammation and WNT signaling were used to compare differentially expressed genes (DEGs) with gene set enrichment analysis (GSEA) information. We analyzed about 2294 gene lists of the GSEA data using our bioinformatics pipeline [9, 27, 28].
Newborn epithelial cells and newborn dermal fibroblasts (NENF) patch assay
The hair-inductive capacity was assessed by the established NENF patch assay in nude mouse as described previously [22]. Briefly, isolated epithelial cells and dermal cells from newborn mouse dorsal skin (C57bl/6, Orient Bio, Seongnam, Korea) were resuspended in 100 ml of phosphate-buffered saline and co-implanted subcutaneously into 7-week-old female BALB/C nude mice (Orient Bio, Seongnam, Korea) after treated with the cytokines and CMs indicating concentration. Mice were sacrificed 2 weeks after cell implantation. The sites were excised, and then gross pictures were taken. HFs were counted by microscopic photography.
Results
DP exhibits low MHC class I in vivo but loses this property in vitro
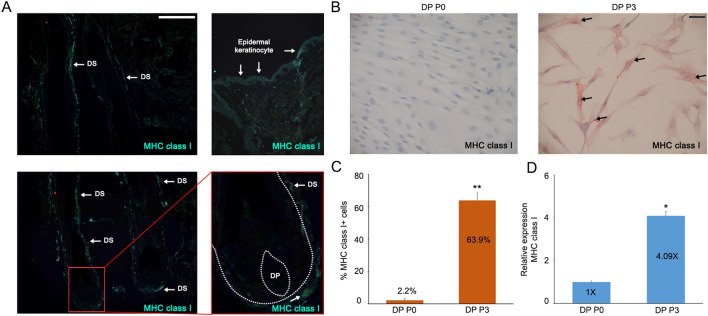
To assess the expression of MHC class I glycoproteins in vivo, cryo-sections of HFs were stained with human anti-MHC class I monoclonal antibodies. We detected the expression of MHC class I glycoproteins in dermal sheath and epidermal keratinocytes, whereas DP distinctly lacked MHC class I expression (Fig. 1A). Next, we examined the changes in expression of MHC class I glycoproteins when DP are cultured in vitro using RT-qPCR analysis and ICC (Fig. 1B–D). Our RT-qPCR results showed that the expression of MHC class I is significantly increased in DP P3 compared with DP P0 (Fig. 1D). Furthermore, DP P3 cells showed positive staining for MHC class I glycoproteins, whereas DP P0 cells did not (Fig. 1B). These results suggest that MHC class I expression in the DP may be modulated non-autonomously, such as by neighboring cells in vivo, since culturing DP alone resulted in increased MHC class I expression.
Fig. 1.
Loss of immune privilege in cultured DP. A Immunofluorescence (IF) was performed on cryosection biopsies of HFs using anti-MHC class I antibody. White arrows point in DS, and epidermal keratinocyte individually (scale bar = 100 µm, ×20). B ICC was performed on cultured DP cells for 0 passage (DP P0) or 3 passages (DP P3) with human anti-MHC class I antibody and quantified in C. Black arrows indicate stained cells with anti-MHC class I antibody. D Levels of MHC class I mRNA in cultured DP cells were measured by RT-qPCR analysis at each passage. Values were normalized to cultured DP at DP P0. Data are presented as the mean ± SD from three individuals. *p < 0.05 and **p < 0.001 compared with the control by Student’s t-test
Effects of cytokines on MHC class I expression in cultured DP cells
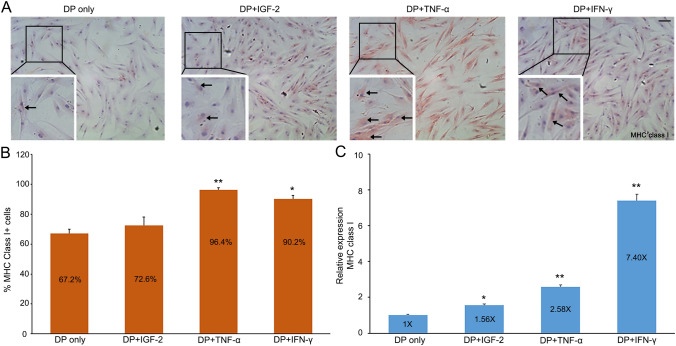
We investigated the influence of various cytokines on the expression of MHC class I glycoproteins in vitro. First, DP P3 cells were treated with IGF-2 and MHC class I inducers, TNF-α and IFN-γ (Fig. 2). Importantly, TNF-α is highly up-regulated in patients with alopecia areata, and its up-regulation is considered a potential indicator of this disease [29]. We performed immunocytochemistry and RT-qPCR to demonstrate their effects on the expression of MHC class I glycoproteins. In the case of IGF-2, a known catagen inducer, we found that its expression is up-regulated at the transcriptional level (1.56 times higher), but data from ICC was not statistically significant (p = 0.4137). After incubation with either TNF-α or IFN-γ for 48 h, DP P3 cells exhibited significantly up-regulated MHC class I gene expression (p = 0.0006 in TNF-α, 0.0035 in IFN-γ respectively), both transcriptionally and immunohistochemically, compared to the control group (Fig. 2B, C). When treated with TNF-α, almost every DP cell showed positive MHC class I stain (96.4%) (Fig. 2A, B). These results suggest that TNF-α and IFN-γ promote the expression of MHC class I proteins and may underlie a loss of HF immune privilege.
Fig. 2.
TNF-α and IFN-γ induce MHC class I expression of cultured DP cells. A Cultured DP (P3) cells were incubated with control media or media containing IGF-2, TNF-α, or IFN-γ for 48 h and then immunostained for MHC class I protein. Black arrows indicate stained cells with anti-MHC class I antibody (scale bar = 100 um, x20). B Percent of MHC class I+ cells in each media. C Their relative mRNA expression. Data are presented as the mean ± SD from three individuals after normalization to control. *p < 0.05 and **p < 0.001 compared with the control by Student’s t-test
Down-regulation of MHC class I expression in cultured DP cells by ORS CM and WNT3a conditioned media
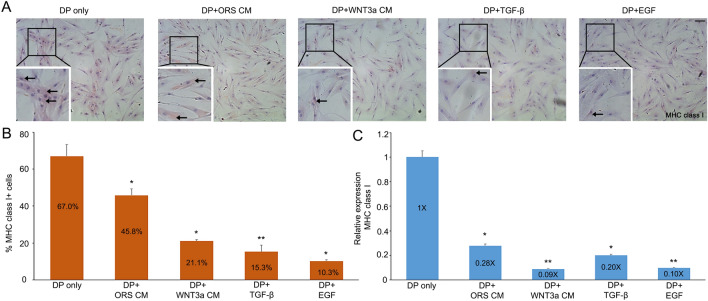
Since epithelial-mesenchymal interactions (EMIs) are critical for HF development and homeostasis, we examined the potential for MHC class I glycoprotein expression to be modulated by secreted factors from neighboring epithelial cells. ICC was performed on DP P3 cells following 24 h incubation with either ORS CM or WNT3a CM. DP P3 cells that were incubated in CMs showed significantly reduced (p = 0.0456 in ORS CM and 0.0019 in WNT3a CM) expression of MHC class I glycoproteins compared to the control (Fig. 3A, B). RT-qPCR analysis showed that when DP P3 cells are incubated with ORS CM or WNT3a CM, mRNA expression level of MHC class I is significantly reduced (p = 0.0153 in ORS CM and 0.0009 in WNT3a CM) compared to levels in control media (Fig. 3C). We also examined the effects of TGF-β or EGF on MHC class I glycoprotein and mRNA expression in the same way and then found that cultured DP cells treated with either TGF-β or EGF for 48 h showed significantly attenuated MHC class I expression (0.20 times and 0.10 times) (Fig. 3B, C). This demonstrates that TGF- β and EGF can regulate the expression of MHC class I protein in DP cells, thereby rescuing HF immune privilege.
Fig. 3.
Restoration of immune privilege. A DP P3 cells without treatment or incubated with either ORS CM, WNT3a CM, TGF-β, or EGF for 48 h were stained for MHC class I. Black arrows denote cells that stained for MHC class I expression (scale bar = 100 µm, ×20). B, C Percent of MHC class I+ cells in each media and their RT-qPCR analysis. Values were normalized to DP cultured without treatment. Data are presented as the mean ± SD from three individuals. *p < 0.05 and **p < 0.001 compared with the control by Student’s t-test
Restoration of hair growth in WNT3a CM using organ culture and NENF assay
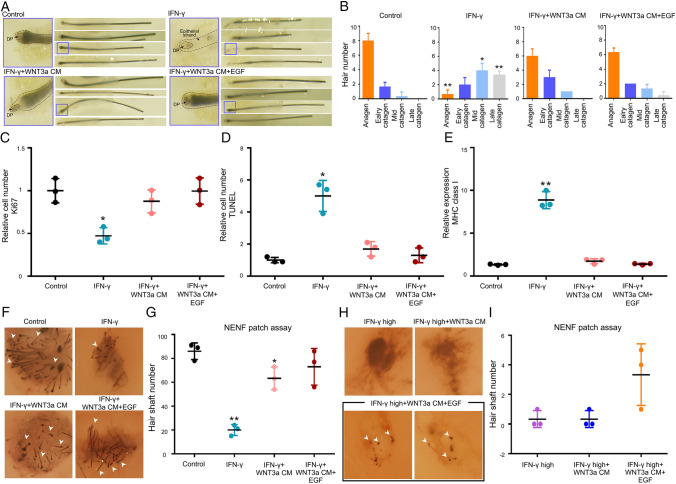
Based on Figs. 1 and 2, various cytokines influence the expression of MHC class I glycoproteins in vitro. Supplemented media containing PBS, IFN-γ, WNT3a CM and/or EGF CM by indicating concentration were treated in anagen hair follicle using organ culture individually (Fig. 4A). According to hair follicle morphology [1], the majority of the control group were still in anagen or early catagen (about 8–9 out of 10), whereas the IFN-γ treated group turned into mid/late catagen (6–8 out of 10). On the other hand, treatment with WNT3a and/or EGF-supplemented CM after IFN-γ treatment restored the proportion of hairs in anagen or early catagen comparable with the IFN-γ group (Fig. 4B).
Fig. 4.
The growing of hair follicle in organ culture and patch assay. A, B Biopsied HFs were cultured with PBS, IFN-γ, IFN-γ + WNT3a CM, and IFN-γ + WNT3a + EGF individually. The morphology and stage of HFs (anagen, early catagen, mid-catagen, or late catagen) were classified and counted from different media after 6 days. C The relative number of proliferating cells was measured using Ki67 staining. D Relative number of TUNEL positive cells were counted. E The relative mRNA expression of MHC class I was analyzed by RT-qPCR in same condition. F–I The neogenesis of HFs were tested by NENF patch assay with each condition. The gross picture of dorsal skins in nude mice were taken by microscopy in each condition and the number of hairs were measured. The white arrows indicate new hairs (×20). Data are presented as the mean ± SD from three individuals. *p < 0.05 and **p < 0.001 compared with the control by Student’s t-test
Moreover, Ki67 immunostaining and TUNEL assay were performed with hair follicles obtained from organ culture to test the effect these cytokines and CM have on the proliferation and apoptosis of HFs. The number of Ki67 positive cells decreased in IFN-γ treatment and recovered in WNT3a CM and EGF-supplemented Wnt3a CM groups (Fig. 4C). Conversely, the number of TUNEL positive cells exhibited the opposite pattern in the same samples (Fig. 4D). Importantly, increased MHC class I mRNA expression levels following IFN-γ treatment were reduced by WNT3a CM and EGF-supplemented Wnt3a CM (Fig. 4E).
To confirm the restoration of hair regeneration that was inhibited by IFN-γ in vivo, NENF patch assay was conducted in dorsal skin of nude mice using WNT3a CM and EGF-supplemented Wnt3a CM (Fig. 4F–I). Suppressed hair regeneration under 100 ng/ml of IFN-γ were overcome by WNT3a CM and EGF-supplemented Wnt3a CM (Fig. 4F, I). Additionally, in cases of high concentration of IFN-γ, both CM showed minimal rescue of hair regeneration (Fig. 4G, H). These results reinforce the effect that WNT3a CM and EGF-supplemented Wnt3a CM have on the restoration of hair growth.
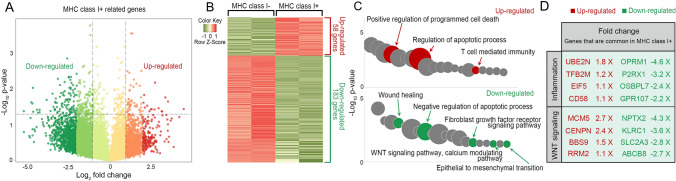
Analysis of signature genes of MHC class I expressed human keratinocyte stem cells
Our data showed that MHC class I expression can be regulated by secreted factors from neighboring epithelial cells. This led us to perform transcriptomic analysis of MHC class I+ and MHC class I- in human keratinocyte stem cell populations using bioinformatics. We used GSE11089 database to identify signature genes expressed and established DEGs firstly in the MHC class I+ cell populations (Table S1). All genes that are related MHC class I+ in epidermal stem cell (GSE11089) was visualized to identify populations (Fig. 5A). Among the DEGs in the MHC class I+ group, we found that 58 genes were up-regulated while 183 genes were down-regulated (Fig. 5B). Next, using GO analysis, the up-regulated genes in MHC class I+ are correlated with biological process such as positive regulation of programmed cell death, regulation of apoptosis, and T cell-mediated immunity (Fig. 5C). Additionally, more details about the other process were indicated in Table S2. To identify correlation between MHC class I+ and inflammation, genes related inflammation were compared with up-regulated genes and down-regulated genes, respectively. UBE2N, TFB2M, EIF5, and CD58 were selected by fold change in up-regulated genes of MHC class I+. In down-regulated genes of MHC class I+, OPRM1, P2RX1, OSBPL7, and GPR107 were selected. On the other hand, down-regulated genes were related to wound healing, negative regulation of apoptotic process, fibroblast growth factor receptor signaling pathway, WNT signaling pathway, calcium modulating pathway, and epithelial to mesenchymal transition. (Fig. 5C). In WNT signaling pathway, up and down-regulated genes of MHC class I+ were shown (Fig. 5D). Based on the GSEA data, we concluded that our finding factors were significantly meaningful in pathway signaling.
Fig. 5.
Bioinformatics analysis of MHC class I related genes from GEO. A Visualization of MHC class I+ related genes expression using microarray data from GSE 11089. Color was assigned to each range based on the log2 fold change value. Up-regulated genes belong in − log10 p-value > 1.3, log2 fold change > 1, while down-regulated genes belong in − log10 p-value > 1.3, log2 fold change > − 1. B From comparison MHC class I- versus MHC class I+ in keratinocyte stem cell, the up-regulated genes were shown in red color and down-regulated genes in green color. C Bubble chart displayed the GO biological process related to up-regulated and down-regulated genes of MHC class I+ group. Total 20 of biological process were listed based on − log10 p-value. Bubble size is the count of overlap genes from DEGs. D A total of 16 genes involved in inflammation and WNT signaling pathway were selected based on Gene-Set Enrichment Analysis (GSEA) information
The interaction of perifollicular CD8+ cells in Alopecia Areata
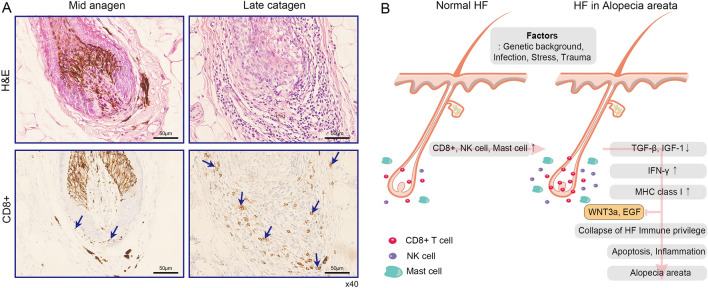
During the normal human hair cycle, immune cells can be detected around the bulb of anagen HFs [11]. To confirm the density of CD8+ T cells in transition from mid-anagen to late catagen in perifollicular hair, IHC was conducted with human anti-CD8 antibody in HFs from AA patients. Interestingly, increased CD8+ T cells were observed near to hair bulb and DP in both two different stages of HFs (Fig. 6A). Additionally, we observed CD8+ cells expression in nine patients (Supplementary figure S1). The prevalence of CD8+ T cells in HFs from AA patients suggest that the collapse of HF immune privilege due to altered immunoreactive cells may underlie hair loss observed in AA patients.
Fig. 6.
The interaction of perifollicular factors in AA. A H&E staining (top) and IHC (bottom) were performed with human anti-CD8 antibody in two different stages (left: mid anagen, right: late catagen) of biopsied HFs from AA patients. (Scale bar = 50 µm, ×40) A total nine individuals were tested (Fig. 6A and Fig. S1). Blue arrows indicate CD8+ T cells. B Schematic model for AA patient based on our results. Under normal conditions, immune cells such as CD8+ T cell, natural killer (NK) cells, and mast cells can be detected around HFs. Patients with a certain specific genetic background are predisposed to abnormalities in the micro-environment of the follicle. When various disruptions occur during anagen (e.g., infection, trauma, or stress), the clinical phenotype of alopecia areata results through above systemic mechanism (for more detailed explanation of this process, see the discussion section)
Discussion
MHC class I molecules play an essential role in the immune response by presenting cytosolic proteins to immune cells. When a cell is infected with a virus, viral proteins are broken down through the action of proteases and presented to immune cells that then recognize these “non-self” peptides and subsequently remove the virally transformed cells. MHC class I immunoreactivity is found in most skin cells; In hair, DP plays an integral role in the maintenance of a cycling HF; however, loss of immune privilege may leave the DP open to macrophage-driven attack, resulting in the loss of a critical component of the HF.
Meanwhile, the characteristics of cultured DP cells in 2D environment are expected to change when compared to in vivo condition. In effect, they lost their hair induction efficacy because some of cell surface signature proteins were significantly changed according to a hair regeneration study [30]. In our experiment for Figs. 1, 2 and 3, intact DPs are isolated from HF and they are mostly close to in vivo DP, and then intact DP were expanded to P0 cell. Therefore, P0 is not perfectly in vivo characteristic cells, rather, they are in vitro cells like in vivo. The cultured cells in P3 are changed by the alteration of the microenvironment in vitro condition.
Our data indicated that no immunoreactivity of MHC class I molecules was observed in DP cells in vivo (Fig. 1A). The anagen HF interacts with the immune system in a way that promotes immune privilege and prevents autoimmune attack of the shifting antigens into the growing HF. MHC class I expression on anagen keratinocytes is down-regulated [31] and a relative immunosuppression is observed in murine skin [32]. However, if HF immune privilege is compromised, the growing anagen HF is attacked as soon as it begins to produce the anagen-associated autoantigen, through disrupting anagen and driving the growing HF into premature catagen. For example, repeated truncation of the hair cycle by repeated attacks ultimately leads to nanogen, a state specific to AA characterized by an exhausted, dystrophic, miniaturized, and empty HF [31, 32].
Remarkably, we observed in our preliminary cDNA microarray analysis that when DP cells are cultured in vitro, they overexpress MHC class I glycoproteins compared to in vivo DP cells, suggesting that they might lose their immune privilege during in vitro cell culture. In this study, DP cells that were cultured in vitro and passaged three times (DP P3) showed a significantly increased expression of MHC class I glycoproteins compared to that of primary grown cells from explanted DP that were not passaged (DP P0). Additionally, DP P3 cells showed increased expression of MHC class I glycoproteins compared to DP P0 cells, reinforcing our results from RT-qPCR (Fig. 1). Because MHC class I glycoproteins play a critical role in the immune response as well as the HF immune system, our results support the idea that DP cells rapidly lose their immune privilege during in vitro culture.
To identify other factors that may influence immune privilege of the HF, we also investigated whether MHC class I expression is modulated by various cytokines. We treated cultured DP cells with either TGF-β or EGF because they are known to be essential for the maintenance of anagen [33], and we found that there was a down-regulation of MHC class I expression (Fig. 3), which suggests that the role of TGF- β and EGF in the anagen HF is to regulate the duration and to delay the catagen and telogen phases [34]. Moreover, treatment of cultured DP with either TNF-α or IFN-γ resulted in up-regulation of MHC class I expression (Fig. 2), consistent with the finding that pro-inflammatory cytokines, such as TNF-α and IFN-γ, promote the expression of MHC class I proteins [35]. We also investigated the influence of a known catagen inducer, IGF-2, on the expression of MHC class I in cultured DP cells and found that MHC class I expression is up-regulated following exposure to IGF-2 (Fig. 2). Our data strongly suggests that the property of immune privilege in DP depends on secreted cytokines during normal HF cycling. Furthermore, our data is in line with previous reports on the influence of cytokines on DP MHC class I expression [36, 37].
EMIs play a critical role in the morphogenesis of many organs and appendages [1, 2, 38, 39], and the HF is one notable example with EMIs occurring, not only in development but also as a part of normal hair follicle cycling. During HF development, extensive interactions occur between the HF’s epithelial and mesenchymal components, culminating in the formation of a hair shaft-producing mini-organ [1], which outcome that skin epithelial stem cell develop into a stratified epithelium in epidermis layer and entrenched into ORS and matrix cells while DP cells are also surrounded of them in HF successively. As the HF cycles through phases of active growth, involution, and rest, the epithelium and mesenchyme are regulated by a distinct set of molecular signals that are unique for each phase of the hair cycle [40]. For example, in telogen HFs, EMIs are characterized by a predominance of inhibitory signals that maintain the HF in a quiescent state. However, during anagen, a large variety of growth stimulatory pathways are activated in and coordinated between the epithelium and mesenchyme to produce a proper hair shaft. As the HF enters catagen, these activating signals exchanged between the epithelium and mesenchyme are terminated, leading to apoptosis in the HF epithelium, and other selected pathways are activated, promoting the transition of the DP into a quiescent state [41].
These close interactions between the DP and the HF epithelium led us to examine the possibility that the expression of MHC class I proteins in cultured DP cells may be modulated by ORS CM or WNT3a CM. We observed that MHC class I expression is significantly reduced when DP P3 cells were incubated with the CM (Figs. 2, 3), suggesting that MHC class I expression is modulated by secreted factors such as WNT3a. Furthermore, this result strongly suggests that the epithelium influences the immune privilege of in vivo hair through these secreted factors. Determining which secreted factors are involved in regulating the HF’s immune privilege can have significant impacts on our understanding of diseases such as alopecia areata, characterized by macrophage-driven attacks on the HF [39].
NENF patch assays have used to explore of the physiology of hair since it was established. It is composed of newborn epithelial keratinocyte and dermal fibroblast in heterogeneous implantation in mice, allowing for the immune privilege of mammalian hair and as a model for study morphogenic and molecular mechanism of EMIs. Therefore, it can be an useful tool to test trichogenicity in vivo. Furthermore, it has been adopted to generate new human hair follicle using 3D cultured human DP cells in nude mouse [42]. Here, hair inducing cellular component cells treated with supplemented media including IFN-γ, WNT3a CM and EGF-supplemented Wnt3a CM were implanted and then investigated their hair regeneration capacity. We assumed that increased MHC class I by IFN-γ in DP cells as potential suppressor for hair formation were restored by these conditioned media according to our investigation, (Fig. 4E, F), implying that WNT3a CM and EGF may play a significant role in rescuing the collapse of immune privilege by acting as an antagonist against external and internal factors (Fig. 4).
Using meta-analysis followed by gene transcriptional profiling, volcano plot, bubble chart and GSEA comparison, we organized the genes representing hair follicle stem cell in MHC class I+ and MHC class I- molecules. The expressional patterns of MHC class I related genes in hair follicle stem cell are very prominent in the global scale, and that allows us to investigate the signature genes specifically enhanced only in MHC class I+ compared with MHC class I-. Up-regulated genes of MHC class I+ were involved in the biological process of inflammation. Among them, UBE2N (Ubc13) is one of the up-regulated genes in MHC class I+ (Fig. 5D). It is a family of TNF receptor-associated factors (TRAFs) involved in signaling Toll-like receptors and TNF-family cytokine receptors, modulators of innate immunity and inflammation [43]. In hair biology, UBE2N gene is not yet well known. Meanwhile, down-regulated genes refered to proliferation and WNT signaling. In this study, we predicted the candidate genes involve during inflammation and proliferation in MHC class I+ cells. It will be helpful to find novel target for the treatment of AA.
In cooperation with the active disease, expression of MHC class I and II are increased along with the raised numbers of antigen presenting cells (APCs) in AA lesions [44]. CD8+ T cells are typically the first cells to penetrate intra‐follicular locations, closely followed by the dendritic cells/macrophages. High density of perifollicular macrophages and mast cells appear during catagen, while anagen is characterized by decreased MHC Class I and low numbers of natural killer (NK) cells. Meanwhile, the levels of CD8+ T cell staining measured in anagen stage in mammals support the hypothesis of HF immune privilege [6], but the difficulty of obtaining samples from AA patients precludes a more detailed study into the levels of CD8+ T cells in other stages of AA affected HFs. As shown in Fig. 6A, although immunoreactive cells did not meet the high standardization criteria (Fig. S1) in other stages and locations of AA affected HFs, CD8+ T cells in mid-anagen and late catagen were more abundant in AA patients than unaffected HFs [45].
Indeed, immune mediator cells such as macrophages, lymphocytes, T cells, B cells, NK cells, neutrophils, epithelial cells, fibroblast, and endothelial cells are related to WNT signal under inflammation response, but still controversial [44]. For example, TNF and WNT signaling can activate cell proliferation and differentiation. On the other hand, IL-6 signaling during inflammation are acting as negative factors in hair formation. In WNT signaling particularly, Wnt3a proteins were expressed during anagen to induce the target genes involved in the growth of the HF [46].
Human genetic and functional studies using model organisms have identified important pathways for AA development, suggesting a role for CD8+ T cells and IFN-γ in mediating HF damage [47]. In general, cyclic growth of hair is regulated by the diverse growth factors. Since EGF as a biological switch in hair growth cycle was suggested in 2003 [48], the hypothesis that continuous expression of EGF in HFs can arrest follicular development at the final stage of morphogenesis, adds credibility. On the other hand, Lasarte’s group reported that EGFR-engineered CD8+ lymphocytes are activated in response to EGF [49]. This research show that EGF may take a different role in normal and cancer cell which coincide with WNT signals. Based on our results in vitro condition, IGF-2, TNF-α, and IFN-γ may upregulate MHC class I, whereas ORS CM, Wnt3a CM, TGF-β and EGF can decrease it in HFs. Therefore, inhibiting MHC class I, CM may use as activator for hair regeneration (Fig. 4).
Taken together, our results suggest that DP cells gradually begin to lose their immune privilege due to negative microenvironmental factors. In an altered micro- and macro-environment, immune cells such as CD8+ T cells, NK cells, and mast cells are increased while anagen factors such as TGF-β and IGF-1 are decreased. The consequence of the micro- and macro-environmental change is the up-regulated IFN-γ and MHC class I proteins. Eventually, dysfunction of HFs immune privilege may occur, leading to the increased inflammation and apoptosis in HFs (Fig. 6B).
In conclusion, understanding the immune system of the HF and the ways to maintain its immune privilege is a key to find the therapeutic options for the immune mediated hair and skin disease. Thus, determining the effects of prolonged in vivo like culture conditions and the factors that impact DP immune privilege will help to improve our understanding of HF immune system.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1: Fig. S1. The expression pattern of CD8 + cells in nine AA patient. The comparison in different stage with H&E staining and IHC in AA patients.
Supplementary file 2: Table S1. The total gene list of the DEGs analyzed from MHC class I+ stem cell. The GEO data sets (GSE 11089) was used to select DEGs.
Supplementary file 3: Table S2. The terms of gene function used in the bubble chart. The bubble chart visualized biological functions of up-regulated genes and down-regulated genes.
Acknowledgement
This work was supported by Biomedical Research Institute Grant, Kyungpook National University Hospital (2016).
Author’s contributions
MK and JWO developed the concepts, designed the experimental methodology, analyzed the data, wrote, reviewed, and approved the final draft. JMP, MSJ, IS and JAK conducted the experiments, analyzed the data, wrote, and edited a draft under supervision of MK and JWO. NMM, TCH, AC, JCK, JYK and JK conducted the experiments and reviewed the draft. MK and JWO supervised the study.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The study protocol was approved by the institutional review board of Kyungpook National University, School of Medicine (IRB No. KNU 2013–01-037–004).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jung Min Park and Mee Sook Jun have contributed equally to this work.
Contributor Information
Moonkyu Kim, Email: moonkim@knu.ac.kr.
Ji Won Oh, Email: ohjiwon@knu.ac.kr.
References
- 1.Oh JW, Kloepper J, Langan EA, Kim Y, Yeo J, Kim MJ, et al. A guide to studying human hair follicle cycling in vivo. J Invest Dermatol. 2016;136:34–44. doi: 10.1038/JID.2015.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q, Oh JW, Lee HL, Dhar A, Peng T, Ramos R, et al. A multi-scale model for hair follicles reveals heterogeneous domains driving rapid spatiotemporal hair growth patterning. Elife. 2017;6:e22772. doi: 10.7554/eLife.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds AJ, Jahoda CA. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. 1992;115:587–593. doi: 10.1242/dev.115.2.587. [DOI] [PubMed] [Google Scholar]
- 4.Jahoda CA, Reynolds AJ, Oliver RF. Induction of hair growth in ear wounds by cultured dermal papilla cells. J Invest Dermatol. 1993;101:584–590. doi: 10.1111/1523-1747.ep12366039. [DOI] [PubMed] [Google Scholar]
- 5.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 6.Christoph T, Müller-Röver S, Audring H, Tobin DJ, Hermes B, Cotsarelis G, et al. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2000;142:862–873. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- 7.Oh JW, Lin SJ, Plikus MV. Regenerative metamorphosis in hairs and feathers: follicle as a programmable biological printer. Exp Dermatol. 2015;24:262–264. doi: 10.1111/exd.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, et al. Regeneration of fat cells from myofibroblasts during wound healing. Science. 2017;355:748–752. doi: 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu HJ, Oh JW, Spandau DF, Tholpady S, Diaz J, 3rd, Schroeder LJ, et al. Estrogen modulates mesenchyme-epidermis interactions in the adult nipple. Development. 2017;144:1498–1509. doi: 10.1242/dev.141630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billingham RE, Silvers WK. A biologist's reflections on dermatology. J Invest Dermatol. 1971;57:227–240. doi: 10.1111/1523-1747.ep12261543. [DOI] [PubMed] [Google Scholar]
- 11.Gentile P, Garcovich S. Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: wnt pathway, growth-factor, and mesenchymal stem cell signaling impact analysis on cell growth and hair follicle development. Cells. 2019;8:466. doi: 10.3390/cells8050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jost M, Kari C, Rodeck U. The EGF receptor - an essential regulator of multiple epidermal functions. Eur J Dermatol. 2000;10:505–510. [PubMed] [Google Scholar]
- 13.Philpott MP, Green MR, Kealey T. Human hair growth in vitro. J Cell Sci. 1990;97:463–471. doi: 10.1242/jcs.97.3.463. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Nan W, Wang S, Zhang T, Si H, Yang F, et al. Epidermal growth factor promotes proliferation and migration of follicular outer root sheath cells via Wnt/beta-catenin signaling. Cell Physiol Biochem. 2016;39:360–370. doi: 10.1159/000445630. [DOI] [PubMed] [Google Scholar]
- 15.Paus R, Bertolini M. The role of hair follicle immune privilege collapse in alopecia areata: status and perspectives. J Investig Dermatol Symp Proc. 2013;16:S25–S27. doi: 10.1038/jidsymp.2013.7. [DOI] [PubMed] [Google Scholar]
- 16.Paus R, Nickoloff BJ, Ito T. A 'hairy' privilege. Trends Immunol. 2005;26:32–40. doi: 10.1016/j.it.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Chen HF, Yu CY, Chen MJ, Chou SH, Chiang MS, Chou WH, et al. Characteristic expression of major histocompatibility complex and immune privilege genes in human pluripotent stem cells and their derivatives. Cell Transplant. 2015;24:845–864. doi: 10.3727/096368913X674639. [DOI] [PubMed] [Google Scholar]
- 18.Shin H, Kwack MH, Shin SH, Oh JW, Kang BM, Kim AA, et al. Identification of transcriptional targets of Wnt/beta-catenin signaling in dermal papilla cells of human scalp hair follicles: EP2 is a novel transcriptional target of Wnt3a. J Dermatol Sci. 2010;58:91–96. doi: 10.1016/j.jdermsci.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK, et al. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008;128:262–269. doi: 10.1038/sj.jid.5700999. [DOI] [PubMed] [Google Scholar]
- 20.Mali NM, Kim YH, Park JM, Kim D, Heo W, Le Dao B, et al. Characterization of human dermal papilla cells in alginate spheres. Appl Sci (Basel) 2018;8:1993. [Google Scholar]
- 21.Kang BM, Shin SH, Kwack MH, Shin H, Oh JW, Kim J, et al. Erythropoietin promotes hair shaft growth in cultured human hair follicles and modulates hair growth in mice. J Dermatol Sci. 2010;59:86–90. doi: 10.1016/j.jdermsci.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Bak SS, Park JM, Oh JW, Kim JC, Kim MK, Sung YK. Knockdown of FOXA2 impairs hair-inductive activity of cultured human follicular keratinocytes. Front Cell Dev Biol. 2020;8:575382. doi: 10.3389/fcell.2020.575382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwack MH, Ahn JS, Kim MK, Kim JC, Sung YK. Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J Invest Dermatol. 2012;132:43–49. doi: 10.1038/jid.2011.274. [DOI] [PubMed] [Google Scholar]
- 24.Bettinotti MP, Hadzikadic L, Ruppe E, Dhillon G, Stroncek DS, Marincola FM. New HLA-A, -B, and -C locus-specific primers for PCR amplification from cDNA: application in clinical immunology. J Immunol Methods. 2003;279:143–148. doi: 10.1016/s0022-1759(03)00233-3. [DOI] [PubMed] [Google Scholar]
- 25.Parham P. Function and polymorphism of human leukocyte antigen-A, B, C molecules. Am J Med. 1988;85:2–5. doi: 10.1016/0002-9343(88)90369-5. [DOI] [PubMed] [Google Scholar]
- 26.Kocer SS, Djurić PM, Bugallo MF, Simon SR, Matic M. Transcriptional profiling of putative human epithelial stem cells. BMC Genomics. 2008;9:359. doi: 10.1186/1471-2164-9-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JW, Kim YH, Oh JW. Comparative analyses of signature genes in acute rejection and operational tolerance. Immune Netw. 2017;17:237–249. doi: 10.4110/in.2017.17.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song SH, Jang HU, Oh JW, Kim JS. Gene expression analysis in nasal polyp using microarray. Korean J Otorhinolaryngol-Head Neck Surg. 2011;54:55. [Google Scholar]
- 29.Kasumagic-Halilovic E, Prohic A, Cavaljuga S. Tumor necrosis factor-alpha in patients with alopecia areata. Indian J Dermatol. 2011;56:494–496. doi: 10.4103/0019-5154.87124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- 31.Ruckert R, Hofmann U, van der Veen C, Bulfone-Paus S, Paus R. MHC class I expression in murine skin: developmentally controlled and strikingly restricted intraepithelial expression during hair follicle morphogenesis and cycling, and response to cytokine treatment in vivo. J Invest Dermatol. 1998;111:25–30. doi: 10.1046/j.1523-1747.1998.00228.x. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann U, Tokura Y, Rückert R, Paus R. The anagen hair cycle induces systemic immunosuppression of contact hypersensitivity in mice. Cell Immunol. 1998;184:65–73. doi: 10.1006/cimm.1998.1258. [DOI] [PubMed] [Google Scholar]
- 33.Mak AS, Westwood MJ, Ishiyama FI, Barker MC. Optimising conditions for learning sociocultural competencies for success. Int J Intercult Relat. 1999;23:77–90. [Google Scholar]
- 34.Rinaldi F, Sorbellini E, Castiglioni M, Bezzola P. The role of mimicking growth factors to control anagen phase: evaluation in vitro and in vivo. J Am Acad Dermatol. 2010;62:AB74. [Google Scholar]
- 35.Foitzik K, Paus R, Doetschman T, Dotto GP. The TGF-beta2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev Biol. 1999;212:278–289. doi: 10.1006/dbio.1999.9325. [DOI] [PubMed] [Google Scholar]
- 36.Blanar MA, Baldwin AS, Jr, Flavell RA, Sharp PA. A gamma-interferon-induced factor that binds the interferon response sequence of the MHC class I gene, H-2Kb. EMBO J. 1989;8:1139–1144. doi: 10.1002/j.1460-2075.1989.tb03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epperson DE, Arnold D, Spies T, Cresswell P, Pober JS, Johnson DR. Cytokines increase transporter in antigen processing-1 expression more rapidly than HLA class I expression in endothelial cells. J Immunol. 1992;149:3297–3301. [PubMed] [Google Scholar]
- 38.König A, Happle R, Hoffmann R. IFN-gamma-induced HLA-DR but not ICAM-1 expression on cultured dermal papilla cells is downregulated by TNF-alpha. Arch Dermatol Res. 1997;289:466–470. doi: 10.1007/s004030050222. [DOI] [PubMed] [Google Scholar]
- 39.Oh JW, Choi JY, Kim M, Abdi SIH, Lau HC, Kim M, et al. Fabrication and characterization of epithelial scaffolds for hair follicle regeneration. Tissue Eng Regen Med. 2012;9:147–156. [Google Scholar]
- 40.Hoffmann R, Eicheler W, Huth A, Wenzel E, Happle R. Cytokines and growth factors influence hair growth in vitro. Possible implications for the pathogenesis and treatment of alopecia areata. Arch Dermatol Res. 1996;288:153–6. doi: 10.1007/BF02505825. [DOI] [PubMed] [Google Scholar]
- 41.Bickenbach JR, Kulesz-Martin M. Introduction to JID symposium proceedings 52nd annual montagna symposium on the biology of skin “stem cells in skin” held at Snowmass, Colorado, USA, June 13–17, 2003. J Investig Dermatol Symp Proc. 2004;9:181–182. [Google Scholar]
- 42.Shin SH, Kim D, Hwang J, Kim MK, Kim JC, Sung YK. OVO homolog-like 1, a target gene of the Wnt/beta-catenin pathway, controls hair follicle neogenesis. J Invest Dermatol. 2014;134:838–840. doi: 10.1038/jid.2013.421. [DOI] [PubMed] [Google Scholar]
- 43.Fukushima T, Matsuzawa S, Kress CL, Bruey JM, Krajewska M, Lefebvre S, et al. Ubiquitin-conjugating enzyme Ubc13 is a critical component of TNF receptor-associated factor (TRAF)-mediated inflammatory responses. Proc Natl Acad Sci U S A. 2007;104:6371–6. doi: 10.1073/pnas.0700548104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo H, Cheng Y, Shapiro J, McElwee K. The role of lymphocytes in the development and treatment of alopecia areata. Expert Rev Clin Immunol. 2015;11:1335–1351. doi: 10.1586/1744666X.2015.1085306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito T, Ito N, Saatoff M, Hashizume H, Fukamizu H, Nickoloff BJ, et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128:1196–1206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y, Jin J, Feng M, Zhu D. Wnt signaling in inflammation in tissue repair and regeneration. Curr Protein Pept Sci. 2019;20:829–843. doi: 10.2174/1389203720666190507094441. [DOI] [PubMed] [Google Scholar]
- 47.Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mak KK, Chan SY. Epidermal growth factor as a biologic switch in hair growth cycle. J Biol Chem. 2003;278:26120–26126. doi: 10.1074/jbc.M212082200. [DOI] [PubMed] [Google Scholar]
- 49.Lozano T, Chocarro S, Martin C, Lasarte-Cia A, Del Valle C, Gorraiz M, et al. Genetic modification of CD8(+) T cells to express EGFR: potential application for adoptive T cell therapies. Front Immunol. 2019;10:2990. doi: 10.3389/fimmu.2019.02990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1: Fig. S1. The expression pattern of CD8 + cells in nine AA patient. The comparison in different stage with H&E staining and IHC in AA patients.
Supplementary file 2: Table S1. The total gene list of the DEGs analyzed from MHC class I+ stem cell. The GEO data sets (GSE 11089) was used to select DEGs.
Supplementary file 3: Table S2. The terms of gene function used in the bubble chart. The bubble chart visualized biological functions of up-regulated genes and down-regulated genes.