Abstract
Background
To prevent unsolved problems of medical devices, we hypothesized that combinatorial effects of zwitterionic functional group and anti-bacterial metal ions can reduce effectively the thrombosis and bacterial infection of polymeric biomaterials. In this research, we designed a novel series of zwitterionic polyurethane (zPU) additives to impart anti-thrombotic properties to a polyvinyl chloride (PVC) matrix.
Methods
We have synthesized zPUs by combination of various components and zPUs complexed with metal ions. Zwitterion group was prepared by reaction with 1,3-propane sultone and Nmethyldiethanolamine and metal ions were incorporated into sulfobetaine chains via molecular complexation. These zPU additives were characterized using FT-IR, 1H-NMR, elemental analysis, and thermal analysis. The PVC film blended with zPU additives were prepared by utilizing a solvent casting and hot melting process.
Results
Water contact angle demonstrated that the introduction of zwitterion group has improved hydrophilicity of polyurethanes dramatically. Protein adsorption test resulted in improved anti-fouling effects dependent on additive concentration and decreases in their effects by metal complexation. Platelet adhesion test revealed anti-fouling effects by additive blending but not significant as compared to protein resistance results.
Conclusion
With further studies, the synthesized zPUs and zPUs complexed with metal ions are expected to be used as good biomaterials in biomedical fields. Based on our results, we can carefully estimate that the enhanced anti-fouling effect contributed to reduced platelet adhesion.
Graphic Abstract
Schematic explanation of the effect of zwitterionic polyurethane additives for blood-compatible and anti-bacterial bulk modification.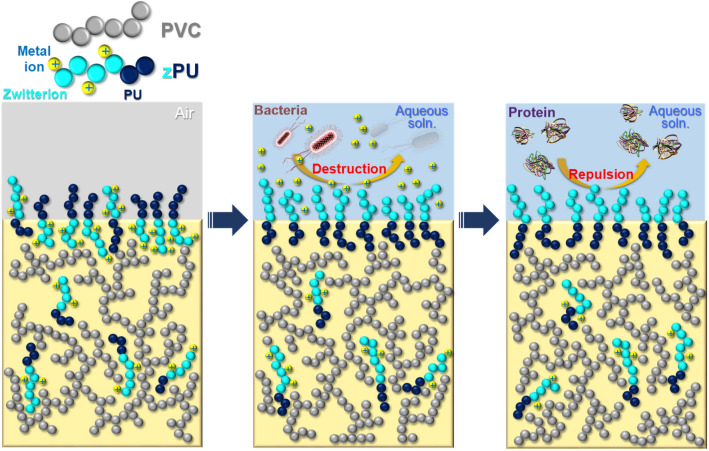
Keywords: Polymeric additive, Zwitterionic polymers, Anti-microbial metal ion, Anti-fouling, Anti-thrombosis
Introduction
Demands for blood-contacting medical devices, such as endovascular catheters and cardiovascular implants, is increasing with respect to the aging population [1, 2]. Although polymers have been widely applied to these devices due to merits of processability and controllable mechanical properties, better biocompatibility than its inherent one is required for a wider range of applications [3]. Despite considerable amounts of studies, in particular, the most vulnerable problems such as thrombosis and in-hospital infection are still not conclusive [4]. Thrombosis contains complex events related to protein adsorption, platelets adhesion and aggregation, immunogenic activation and coagulation cascade [5]. Considering these points, lots of studies have been conducted to improve anti-thrombotic property of polymer-based devices, including physical coating, chemical grafting and surface immobilization of biologically active agents [6–8]. Meanwhile, infection has been major themes to be solved for the purpose of blood contact as well as expanded implanting uses [9]. Causes of device-related infections are sequential processes of bacterial adhesion, spreading and biofilm formation [10].
In order to impart better blood compatibility of polymeric devices, physical networking or adsorption of a material is a relatively simple method [11]. However, it has been problematic because the adsorbed substances can be leached out by continuous hydraulic pressure and biological species in blood [12]. Chemical grafting is one of the most commonly used methods due to its stable surfaces. However, grafting process is complex or requires certain facilities such as UV curing machine. In addition, this technique can be difficult to apply to sophisticated surfaces [11, 13]. Using biological agents has shortcoming of physiological instability showing lowered activity and a short half-life in blood [7].
More effective method is to surface-modify a polymeric device by blending adequate amount of a certain oligomer with base polymer of a device [14]. In this technique, blended oligomers can migrate toward the surface from bulk at a glassy state in the process of thermal manufacturing such as extrusion and injection molding [15, 16]. Therefore, this method is quite efficient for practical application because it does not require post-manufacture unlike existing surface modifications.
A surface modification strategy for anti-fouling effect is effective way to achieve anti-thrombotic and/or -infectious properties. For the effect, zwitterionic oligomers have been investigated as a surface-modifying additive, which these with zwitterionic groups, such as phosphobetaine, sulfobetaine and carboxybetaine, can strongly attract water molecules via electrostatic interactions [17–21]. Sulfobetaine group has been of our interest as the best candidate to demonstrate resistance to fouling of blood plasma substances [22]. In practice, adsorption of plasma proteins on a device surface is major cause of platelet adhesion, coagulation cascade and bacterial adhesion, resulting in ultimately thrombosis and infection.
Several metal ions have been reported as representative anti-microbial agents, which exhibit high bactericidal effect with exceptionally small amount. Silver (Ag) ion is the most widely used metal ion for anti-bacterial purposes and, in particular, it is highly toxic to bacteria, exhibiting strong biocidal effects against various species because Ag ion itself damages bacterial wall and then penetrates into cytoplasmic region [23, 24]. Zinc (Zn) ion leads to interaction with bacteria by which destabilize the membrane and deactivate the respiratory system [25]. Copper (Cu) ion has strong biocidal property against a variety of bacteria and is cost-effective as compared to Ag ion [26]. Cerium (Ce) ion shows anti-bacterial property with very low minimum inhibitory concentration (MIC) and takes advantage of an antioxidant because of the mixed valence state [27]. All of mentioned metal ions can chelate with negatively charged groups so that these ions were employed in the complexation with zwitterionic sulfobetaine oligomers for anti-bacterial use in this study.
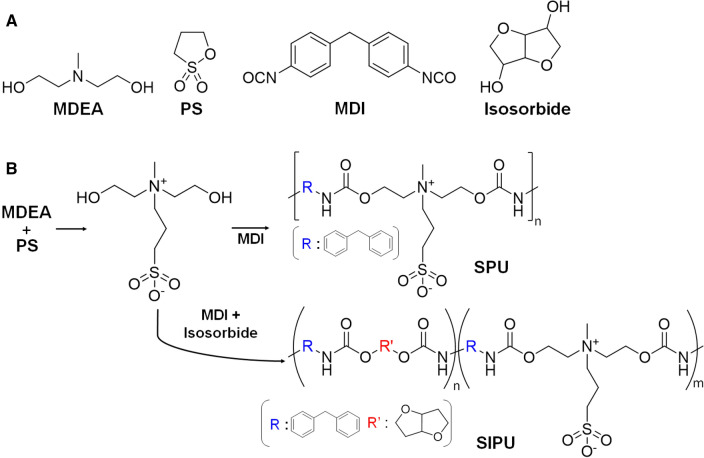
Herein, a series of oligomeric polyurethanes (PU) with a zwitterionic sulfobetaine group was synthesized and was complexed with anti-biotic metal ions (Fig. 1). a series of oligomers includes sulfobetaine-containing PU (SPU), sulfobetaine/isosorbide-containing PU (SIPU) and metal ions complexed SPU/SIPU. Isosorbide is an emerging eco-friendly material having cycloaliphatic ring, produced from dehydration of sorbitol, which used here to provide plasticity of PU. We hypothesized the first anti-biotic effect of chelated metal ions and the following second anti-fouling effect of SPU/SIPU without metal ions under surface-migration of polymeric additives after thermal processing. Investigation for proving our hypothesis has been conducted in aspects with wettability, protein adsorption, platelet adhesion, bacterial adhesion and microbial zone inhibition. Our results will suggest that metal ion chelating zwitterionic polymers and their use as surface-modifying additives can be a promising alternative way to existing coating and surface modification technologies for develop high performance medical devices, especially contacting blood.
Fig. 1.
A, B Synthesis of the polyurethanes with zwitterionic side chains
Materials and methods
Materials
N-methyldiethanolamine (MDEA), methylene diphenyl diisocyanate (MDI), 1,3-propanesultone (PS) and isosorbide were used for polyurethane synthesis and Tin (II) 2-ethylhexanoate was used for catalyst. Silver nitrate (AgNO3), zinc sulfate (ZnSO4), copper sulfate (CuSO4), and ammonium cerium nitrate (Ce(NH4)2(NO3)6) were selected as anti-microbial agents and used as reagents for chelating ions. Poly(vinyl chloride) (PVC) with weight average molecular weight of 42,000 was used as base polymer. All materials were purchased from Sigma-Aldrich (St. Louis, MO, USA). For protein adsorption test, fibrinogen from human plasma and bovine serum albumin (BSA) were purchased from Sigma-Aldrich.
Synthesis of sulfobetaine polyurethanes (SPUs)
Zwitterionic diol was prepared by reacting 0.2 M of MDEA and 0.2 M of PS in N,N-dimethylformamide (DMF) through vigorous stirring under N2 atmosphere at 50 °C for 5 h. After reaction completion, SPU was synthesized as the following: 0.27 M of MDI and 2.7 mM of Tin (II) 2-ethylhexanoate were added to 0.27 M of the synthesized diol in DMF and reacted at 80 °C for 20 min. For SIPU, 0.27 M of MDI, 0.09 M of isosorbide, and 2.7 mM of Tin (II) 2-ethylhexanoate were reacted with 0.27 M of the diol in DMF at the same condition as the above. The synthesized PU products were precipitated in anhydrous chloroform and washed 3 times with chloroform, and then dried under vacuum for 24 h.
Metal ions complexation with SPU/SIPU
Each compound (AgNO3, ZnSO4, Ce(NH4)2(NO3)6, CuSO4) in 0.005 M concentration was dissolved in distilled water and 0.01 M of the previously prepared SPU and SIPU was dissolved in anhydrous dimethyl sulfoxide (DMSO). Then, two solutions were poured into an Erlenmeyer flask and reacted at room temperature for 2 h with stirring. After the reaction, complexed product in the solution was precipitated in distilled water. After the process, precipitated product was rinsed three times with acetone and then dried under vacuum for 24 h.
Preparation of SPU and SIPU sec2blended PVC films
First, 1 g of PVC was dissolved in 3 ml of tetrahydrofuran (THF) using a homogenizer for 1 h. Then, SPU and SIPU (3 wt% and 10 wt%, respectively) and metal ions complexed SPU and SIPU (10 wt%) additives were dissolved in DMSO for 1 h under sonication. The PVC solution and the prepared additive solutions were mixed together and poured into a Teflon® mold and dried under vacuum at 80 °C for 24 h. Then, the product was processed with hot-press method. It was placed on between two stainless steel plates at 150 °C and pressurized at a pressure of 0.07 MPa for 2 min to obtain a homogeneous film of 0.2 mm thickness and then punched into a square (1 × 1 cm2).
Characterization of SPU/SIPU and their metal ions complexed ones
To evaluate the compositions and the structures of the zwitterionic additives, the attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) and proton nuclear magnetic resonance spectroscopy (1H NMR) were utilized. FT-IR spectra were measured on an FT/IR-4100 spectrophotometer (Jasco Analytical Instruments, Easton, MD, USA) and data were collected over 32 scans at 4 cm−1 resolution. The wavenumber range was 600–4000 cm−1. 1H NMR data was recorded with a Bruker AV400 NMR spectrometer (Bruker Instruments, Inc., Billerica, MA, USA) using DMSO as a solvent. Differential scanning calorimetry (DSC) was performed on a Q-2000 (TA Instruments, New Castle, DE, USA) differential scanning calorimeter under a nitrogen flow of 50 mL/min. Samples were rapidly heated to 210 °C and kept for 5 min to remove thermal history, then cooled to − 90 °C at a rate of 10 °C/min and finally reheated to 210 °C at the same rate. All DSC traces used data generated in the second heat to minimize the effect of thermal history. Static water contact angles of PVC film were measured on a goniometer (OCA40, Dataphysics, Filderstadt, Germany) at room temperature. A 3 µL drop of pure distilled water was placed on the film surface. Measurement was performed on five different points of each sample. In order to observe the change of contact angle after hydration, samples were placed in distilled water and dried after 24 h in vacuum for 6 h and then measured. The elemental compositions of PVC films blended with metal ions complexed additives were analyzed by an elemental analyzer (Flash 2000, Thermo Scientific, Waltham, MA, USA). The content of metal ions was calculated by subtracting organic elements from 100%.
Protein adsorption test
Protein adsorption test was performed using FITC-labeled bovine serum albumin (BSA) and fibrinogen. Films were placed in a 24-well plate and immersed in PBS at 37 °C for 2 h. Then, the films were treated with 2 mg/mL of FITC-BSA and 0.1 mg/mL of FITC-fibrinogen in PBS at 37 °C for 1 h, followed by five washing steps with PBS. The Micro BCA™ protein assay reagent kit (Pierce Biotechnology Inc., Waltham, MA, USA) was used to measure the amount of total proteins adsorbed on the surface of film. Absorbance at 562 nm was detected using UV–Vis spectrometer (Thermo Multiskan spectrum, Thermo Scientific).
Platelet adhesion
The prepared films were placed in a 24-well plate, immersed in PBS for 2 h, and 1 mL of human platelet-rich plasma was added to each well and cultured at 37 °C for 2 h. After washing with PBS, films were treated with 2% Triton-X solution (Sigma Aldrich) for 30 min. To quantify the activity of adhered platelets, Pierce™ LDH Cytotoxicity Assay kit (Thermo Scientific) was used as following the distributor’s instruction. The absorbance at 490 nm was measured with a multi-plate reader (Thermo Multiskan spectrum, Thermo Scientific).
Results and discussion
Characterizations of synthesized SPU and SIPU
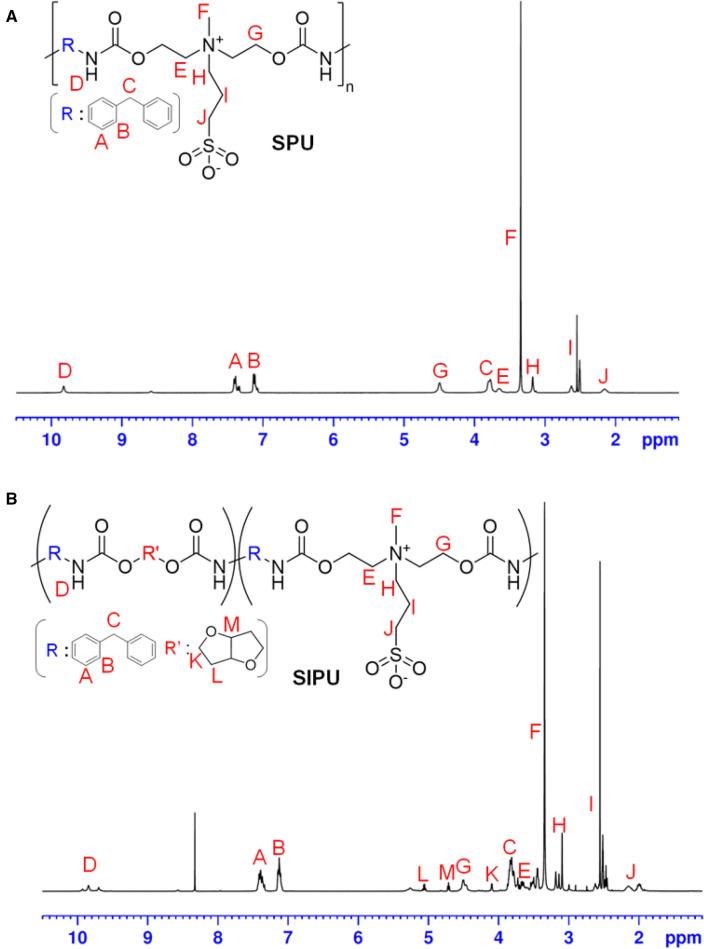
Two types of zwitterionic polyurethane (zPU) additives, sulfobetaine polyurethanes (SPU) and sulfobetaine isosorbide polyurethanes (SIPU), were synthesized via our optimized protocol. Furthermore, four different metal ions (Ag+, Zn2+, Cu2+, Ce4+) coordination was conducted to prepare the biocidal metal ions conjugated SPU and SIPU additives. The fabricated SPU, SIPU, and metal ions complexed SPU and SIPU were systematically investigated by FT-IR and 1H-NMR. FT-IR data shows the characteristic peak at 1730 cm−1 of C=O stretching vibration, suggesting the formation of urethane bond of SPU and SIPU. The characteristic peak appearing at 1038 cm−1 indicates the presence of SO3− groups and the quaternary ammonium adsorption band was detected at 967 cm−1, which demonstrates the successful incorporation of sulfobetaine groups to the polyurethane backbone [28, 29]. Figure 2 shows the 1H-NMR spectra of SPU and SIPU. The peaks appearing at 9.86 ppm is assigned as N–H bonding. The presence of sulfobetaine moiety was indicated by resonance signals at 3.35 ppm attributing to = N+–CH3 and 3.17 ppm, 2.50 ppm, and 2.12 ppm, corresponding to the proton signal from the –CH2 (Fig. 2A) [30]. Moreover, as shown in the Fig. 2B, the appearance of the new peak at 4.71 ppm, 5.13 ppm, and 4.09 ppm ascribing the proton signals from the –CH and − CH2, indicating the successful incorporation of isosorbide to the SIPU backbone [31]. After verifying the fabrication of SPU and SIPU, the amount of complexed metal ions was confirmed by the elemental analysis. Table 1 shows the elemental analysis results of C, H, N, O, S and metal ions in zPU complexation additives, in which C%, H%, N%, O% and S% exhibit the atomic percentage of carbon, hydrogen, nitrogen and sulfur atoms, respectively. The result related to S% shows that zwitterion was introduced into polyurethane and each metal% indicates that metal ions were successfully complexed with zPU additives. It was confirmed that zwitterion and metal ions were incorporated to the polyurethane backbones via the elemental composition of additives.
Fig. 2.
1H NMR spectra of A SPU and B SIPU
Table 1.
Elemental analysis of zPU additives complexed with metal ions
| Product | Elemental composition (%) | |||||
|---|---|---|---|---|---|---|
| C | H | N | O | S | Metal | |
| SPU | 61.57 | 6.68 | 9.97 | 14.81 | 6.96 | – |
| SPU_Ag | 59.15 | 5.76 | 9.75 | 17.42 | 3.23 | 4.69 |
| SPU_Zn | 57.89 | 5.78 | 9.06 | 19.71 | 4.94 | 2.62 |
| SPU_Cu | 43.80 | 5.42 | 6.83 | 29.25 | 6.91 | 7.79 |
| SPU_Ce | 58.02 | 5.77 | 9.71 | 19.76 | 3.18 | 3.56 |
| SIPU | 58.59 | 6.34 | 8.39 | 19.03 | 7.65 | – |
| SIPU_Ag | 55.41 | 5.68 | 8.43 | 22.07 | 3.08 | 5.34 |
| SIPU_Zn | 56.31 | 5.82 | 8.09 | 23.19 | 3.35 | 3.25 |
| SIPU_Cu | 39.39 | 5.11 | 5.90 | 31.37 | 5.87 | 12.36 |
| SIPU_Ce | 55.48 | 5.67 | 8.98 | 21.24 | 3.54 | 5.09 |
Thermal properties containing thermal phase transition and thermal stability were experimented with DSC. DSC thermograms of zPUs and zPUs complexed with metal ions were exhibited in Fig. 3. SPU and SIPU have the glass transition temperature (Tg) around − 50 °C and − 52 °C, indicating that these exhibit high elasticity at low temperature because the molecular interaction between amine group and urethane group can be weakened by sulfopropyl group. The Tg of SIPU containing isosorbide was little lower than SPU without isosorbide, meaning that isosorbide caused slight phase separation due to lowered linearity of chain [32]. After introducing metal ions into zPUs, The Tg lowered to − 32 ~ − 30 °C, showing higher Tg than corresponding that of zPUs. Complexed metal ions significantly increased the segmental mobility of zPUs chains, and the Tg differences between metal ions were nearly not observed.
Fig. 3.
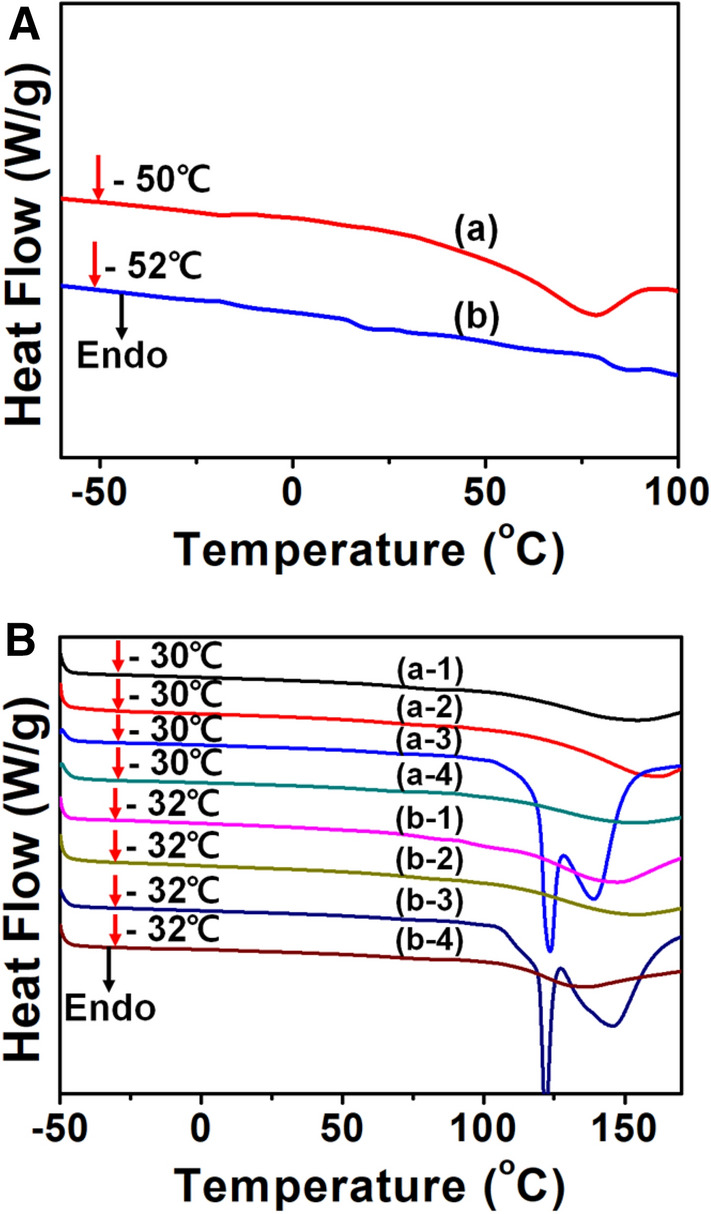
DSC thermograms of the A SPU, (a-1) SPU/Ag, (a-2) SPU/Zn, (a-3) SPU/Cu, (a-4) SPU/Ce, B SIPU, (b-1) SIPU/Ag, (b-2) SIPU/Zn, (b-3) SIPU/Cu, and (b-4) SIPU/Ce
Thermal property of zPUs-blended PVC films
Figure 4 shows DSC thermograms of additives-blended PVC and neat PVC films. Neat PVC film exhibited the glass transition temperature (Tg) at 80 °C. PVC/SPU3, PVC/SPU10, PVC/SIPU3, and PVC/SIPU10 showed Tg at around 78 °C, 77 °C, 78 °C, and 75 °C, respectively. It was demonstrated that Tg decreases, thereby the elasticity of the base polymer improves as the content of zPUs increases [33]. These results show that zPU additives affect intermolecular interactions of PVC and the reason of this phenomenon is the interaction between PVC backbones and zwitterionic segments which influences the mobility of polymer chains. Therefore, zPUs changed Tg from 80 to 77 °C, making it suitable for blending with base polymer in terms of material mechanics. In addition, the Tg of PVC/SIPU10 is lower than that in PVC/SPU10 probably because of the effect of isosorbide behaving as a plasticizer [34].
Fig. 4.
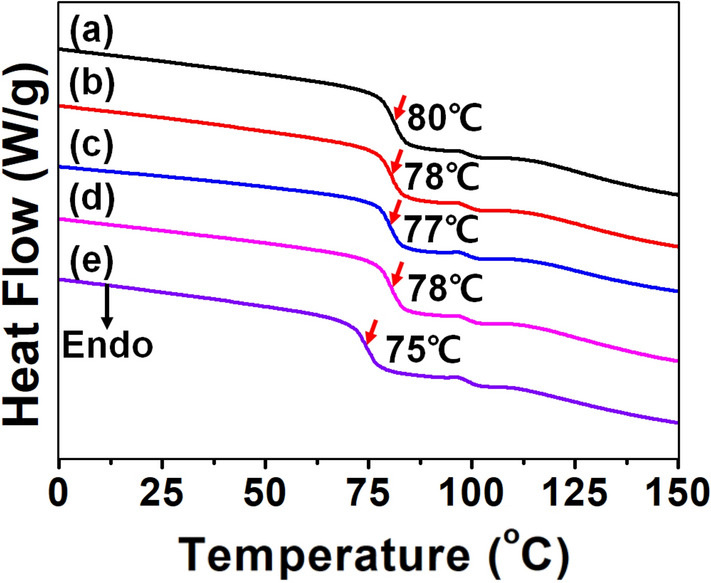
DSC thermograms of A neat PVC, B PVC/SPU3, C PVC/SPU10, D PVC/SIPU3, and E PVC/SIPU10
Surface wettability of zPUs-blended PVC films
The water contact angle of blended films was measured to characterize the surface wettability because it is an indicative parameter for anti-fouling effect [22]. Contact angles of bare PVC and blended PVC films containing zPUs or zPUs with metal ions are showed in Fig. 5. Contact angles of blended films gradually decrease as the amount of SPU and SIPU additives increases. This is because the presence of zPUs made the surface to be hydrophilic. For all films, hydrated state has lower contact angles than dried state. It means that blended PVC and even PVC only could be hydrated to contain water molecules and the water swollen PVC and blended PVCs were hydrophilic than dried those [35]. Furthermore, it can be confirmed that zwitterion functional groups of bulk modified film were oriented toward the surface to maximize the hydrophilicity under hydration conditions [36]. The films blending with zPU additives complexed with metal ions (SPU_metal and SIPU_metal samples) had a relatively lower hydrophilicity than films containing non-metal zPU additives because metal ions complexed with zwitterions influence balanced charge group, which could affect the hydrophilicity. Meanwhile, after releasing metal ions, it would be expected that the zPUs complexed with metal ions also exhibit high hydrophilicity and anti-fouling effect.
Fig. 5.
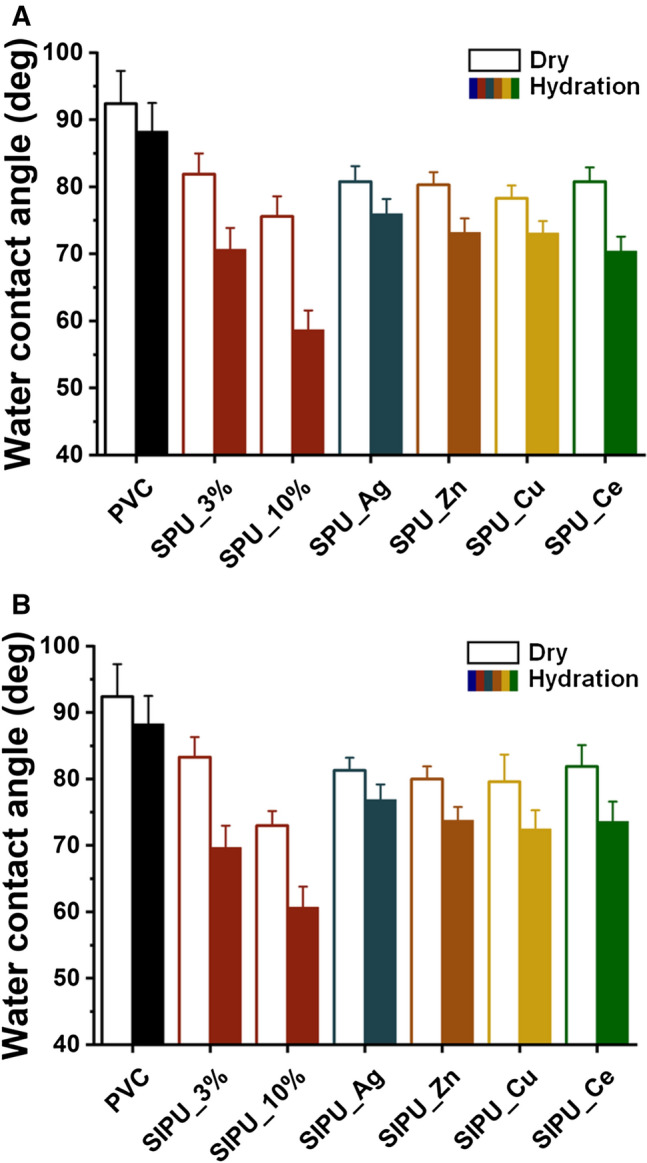
Water contact angle of films with additives contents
Anti-fouling effect of zPUs-blended PVC films
The protein resistance of the films has been examined by use of fibrinogen and bovine serum albumin (BSA), which play a vital role in evaluating the anti-fouling effect [37]. The adsorbed fibrinogen amount was determined by using the micro-BCA protein assay reagent kit (Fig. 6A, B) and FITC-labeled fibrinogen adsorption on films was attempted to confirm protein adsorption resistance (Fig. 6C). The fluorescein intensity of films decreased with increasing additives composite on the film, which is associated with the reduced deposition of the fibrinogen on the surface. Quantitative results showed that the amount of fibrinogen adsorbed compared to bare film substantially decreased by about 90% when zPU additives were added. These results indicate that films blended with additives have fibrinogen and better anti-fouling properties compared with the bare PVC film. Moreover, this tendency was consistent with the graph showing the hydrophilicity (Fig. 5) and the correlation of anti-fouling effects could be verified once again [38].
Fig. 6.
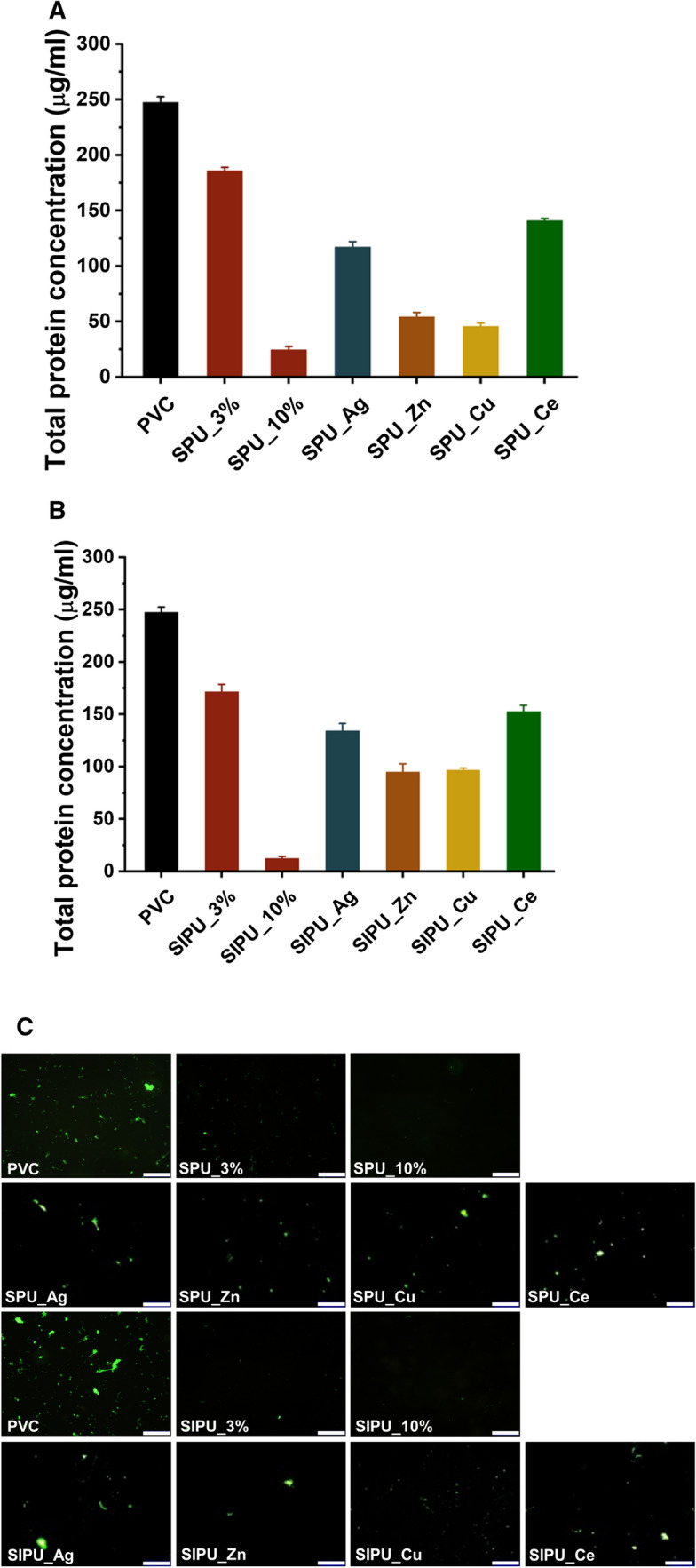
Fibrinogen adsorption. The quantified protein adsorption of fibrinogen on A SPU and B SIPU blended PVC films complexed with metal ions. C Fluorescence intensity and fluorescence microscopy images of fibrinogen adhesion on bare PVC and modified PVC films (bar = 200 μm)
FITC-labeled BSA adsorption was also experimented to evaluate the protein adsorption resistance. When adsorbed BSA was analyzed quantitatively, it was found that the adsorbed amount onto the treated film was gradually reduced by up to about 90% with increasing concentration of zPUs as compared with the bare film (Fig. 7A, B). Figure 7C shows the results that fluorescein intensity of films decreased with increasing additives composite on the film. Taking the results of protein adsorption into consideration, all the films containing additives have better anti-fouling effects than the bare PVC [16]. The amount of adsorbed fibrinogen and BSA substantially decreased with increasing content of the zPU additives in the films. However, the films processed with zPU additives complexed with metal ions showed different results, which was decreased anti-fouling effect due to metal ion complexed with zwitterion functional group. This is in the same context in which the metal ions combine with the zwitterion functional group, thus weakening the hydrophilicity of the material. However, after releasing metal ions, the zPUs combined with metal ion complex are expected to exhibit high protein adsorption resistance again as the water molecules would be attracted by a zwitterionic chains contained in polyurethane. For this reason, it is considered that the proteins are hardly approachable to the surface, and the improved antifouling property will be imparted to the bulk materials. Better hydrophilicity and inhibition of protein adsorption are expected to help inhibit thrombus formation.
Fig. 7.
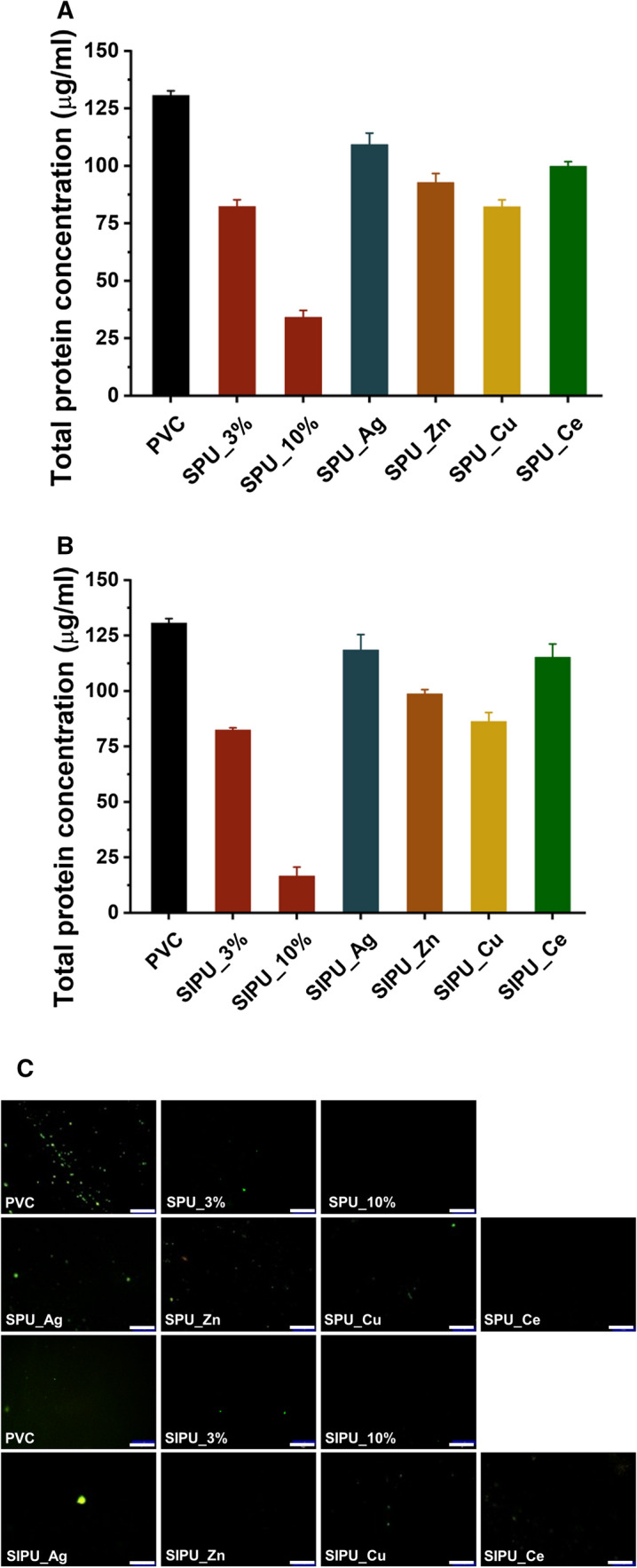
Fibrinogen adsorption. The quantified protein adsorption of bovine serum albumin (BSA) on A SPU and B SIPU blended PVC films complexed with metal ions. C Fluorescence intensity and fluorescence microscopy images of BSA adhesion on bare PVC and modified PVC films (bar = 200 μm)
Platelet adhesion on zPUs-blended PVC films
The lactate dehydrogenase (LDH) assay has been reported to provide a useful approach to research platelet activation adhesion on surface in vitro [39]. The LDH assay is one of the quantification methods that can detect adherent and activated platelets. Cellular integrity is indicated by routinely monitoring leakage of lactate dehydrogenase (LDH), a relatively stable cytoplasmic enzyme. LDH is normally stored within platelets and other blood cells. Therefore, the released LDH corresponds to the number of attached cells when the platelets were adhered to the polymer surface and the activity of LDH can be measured platelet activation and used to quantify platelet adhesion [40]. A graph comparing films using this LDH analysis method is shown in Fig. 8. It can be shown that zPU additives blended films exhibited lower platelet adhesion than non-treated PVC film. The platelet adhesion on surface tended to decrease with increasing SPU and SIPU concentration due to the anti-fouling effect of zwitterionic chains [16, 22, 41]. Furthermore, quantitative analysis of platelet adhesion showed that zPUs complexed with metal ions blended films also exhibits lower platelet adhesion than untreated PVC film. However, in the case of metal complexed films, the number of platelets adhered was equal to or slightly higher than the zPUs-blended films due to reduced hydrophilicity and anti-fouling effects, as noted in the preceding results. It could also be a reason that the surfaces of metal complexed films are more topographically complicated. However, after release of metal ions, zPUs are expected to perform low platelet adhesion. Eventually, it will be helpful to prevent protein adsorption on the film surface and to improve its anti-thrombus. The plasma protein adsorption is the first event in which a biomaterial contacts with blood in human body, accompanying platelet adhesion and activation. To our knowledge, the anti-fouling is the best strategy to reduce the adsorption of plasma proteins, especially fibrinogen closely related to the adhesion and activation of platelets. In this regard, the anti-fouling is different from anti-adhesion of cells such as platelets and bacteria.
Fig. 8.
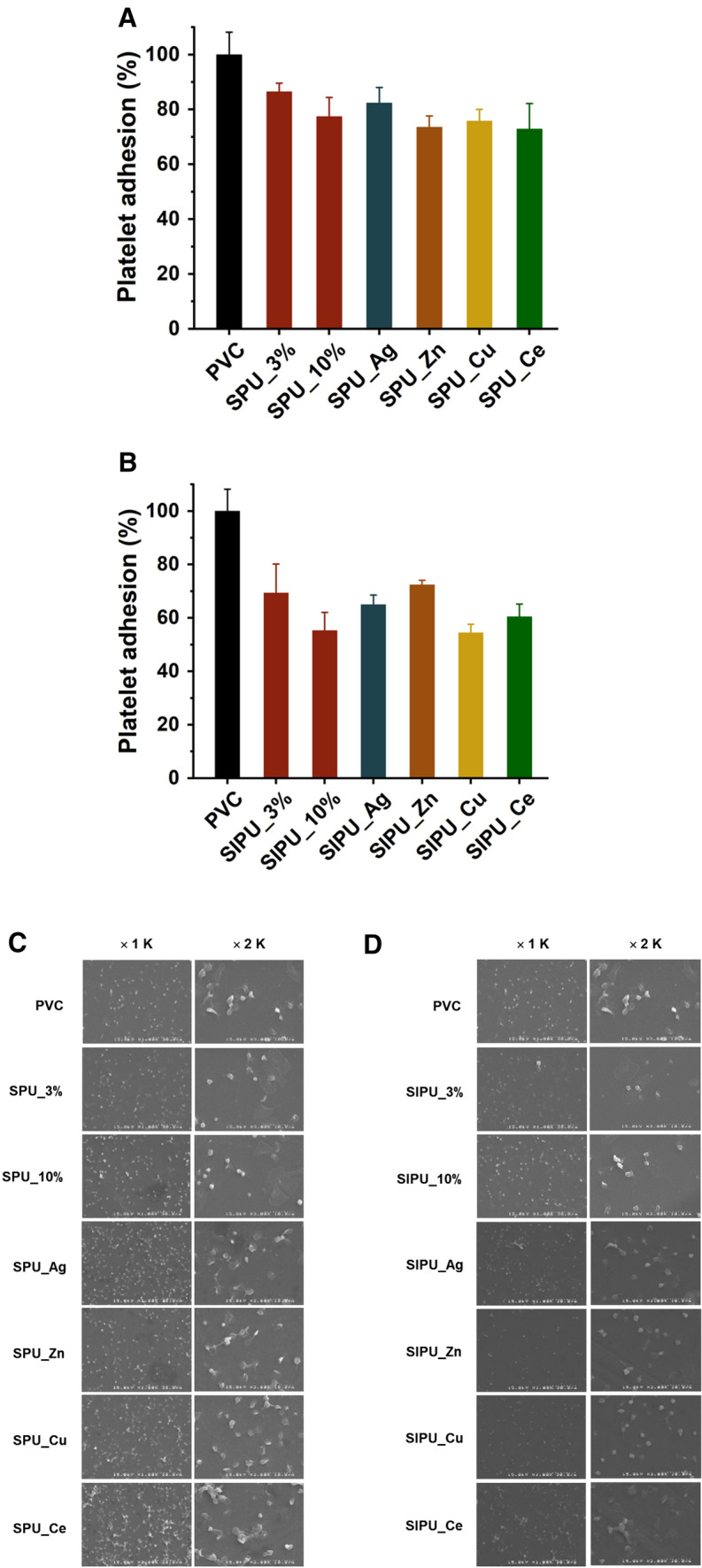
LDH quantitative results and adhesion morphology for the comparison of adhered platelets on PVC film surfaces blended with A, C SPU and B, D SIPU additives
Conclusion
We have synthesized zwitterionic polyurethanes (zPUs) by combination of various components and zPUs complexed with metal ions. Zwitterion group was prepared by reaction with 1,3-propane sultone and N-methyldiethanolamine (MDEA) and metal ions were incorporated into sulfobetaine chains via molecular complexation. The zPUs and zPUs complexed with metal ions were characterized by FT-IR, 1H NMR, and elemental analysis to confirm the successful synthesis. Water contact angle demonstrated that the introduction of zwitterion group has improved hydrophilicity of polyurethanes dramatically. Protein adsorption showed that zPUs and zPUs complexed with metal ions could prevent protein adsorption effectively on the surface. In addition, platelet adhesion analysis exhibited that zPUs and metal ion complexed zPUs could perform anti-thrombus effect on the surface of base polymer. As a result, the initial hydrophilicity and anti-fouling properties of metal ion complexed surface are slightly lowered, but after the metal ions are released showing antimicrobial effect, the hydrophilicity will be exhibited due to internal zwitterion chain, and it will show high resistance to protein and platelet. With further studies, the synthesized zPUs and zPUs complexed with metal ions are expected to be used as good biomaterials in biomedical fields. Based on our results, we can carefully estimate that the enhanced anti-fouling effect contributed to reduced platelet adhesion. However, this is not directly correlated with anti-bacterial effect due to reduced adhesion of bacteria.
Acknowledgements
This work was supported by the Next Generation Medical Device Platform Program (2015M3A9E2028580), the Basic Science Research Program (2020R1A2B5B0300234), and the Korea Medical Device Development Fund funded by the Korea Government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, and the Ministry of Food and Drug Safety, Republic of Korea) (202011B31 and 202011A05-05) through the National Research Foundation (NRF) of Korea funded by the Ministry of Science and ICT (MSIT), Republic of Korea.
Declaration
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dong-Heon Ga and Chung-Man Lim have contributed equally to this work.
Contributor Information
Dong Keun Han, Email: dkhan@cha.ac.kr.
Yoon Ki Joung, Email: ykjoung@kist.re.kr.
References
- 1.Frost MC, Reynolds MM, Meyerhoff ME. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials. 2005;26:1685–1693. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Hasebe T, Shimada A, Suzuki T, Matsuoka Y, Saito T, Yohena S, et al. Fluorinated diamond-like carbon as antithrombogenic coating for blood-contacting devices. J Biomed Mater Res A. 2006;76:86–94. doi: 10.1002/jbm.a.30512. [DOI] [PubMed] [Google Scholar]
- 3.Adipurnama I, Yang MC, Ciach T, Butruk-Raszeja B. Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: a review. Biomater Sci. 2017;5:22–37. doi: 10.1039/c6bm00618c. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, Park KD, Park HD, Lee WK, Han DK, Kim SH, et al. Platelet and bacterial repellence on sulfonated poly (ethylene glycol)-acrylate copolymer surfaces. Colloids Surf B Biointerfaces. 2000;18:355–370. doi: 10.1016/s0927-7765(99)00161-7. [DOI] [PubMed] [Google Scholar]
- 5.Courtney JM, Lamba NM, Sundaram S, Forbes CD. Biomaterials for blood-contacting applications. Biomaterials. 1994;15:737–744. doi: 10.1016/0142-9612(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 6.Desai NP, Hubbell JA. Surface physical interpenetrating networks of poly (ethylene terephthalate) and poly (ethylene oxide) with biomedical applications. Macromolecules. 1992;25:226–232. [Google Scholar]
- 7.Jo SB, Erdenebileg U, Dashnyam K, Jin GZ, Cha JR, El-Fiqi A, et al. Nano-graphene oxide/polyurethane nanofibers: mechanically flexible and myogenic stimulating matrix for skeletal tissue engineering. J Tissue Eng. 2020;11:2041731419900424. doi: 10.1177/2041731419900424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng G, Böker A, Zhang M, Krausch G, Müller AH. Amphiphilic cylindrical core− shell brushes via a “grafting from” process using ATRP. Macromolecules. 2001;34:6883–6888. [Google Scholar]
- 9.Flemming RG, Capelli CC, Cooper SL, Proctor RA. Bacterial colonization of functionalized polyurethanes. Biomaterials. 2000;21:273–281. doi: 10.1016/s0142-9612(99)00176-3. [DOI] [PubMed] [Google Scholar]
- 10.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 11.Tan J, Brash JL. Nonfouling biomaterials based on polyethylene oxide-containing amphiphilic triblock copolymers as surface modifying additives: Synthesis and characterization of copolymers and surface properties of copolymer–polyurethane blends. J Appl Polym Sci. 2008;108:1617–1628. [Google Scholar]
- 12.Li JT, Carlsson J, Lin JN, Caldwell KD. Chemical modification of surface active poly (ethylene oxide)−poly (propylene oxide) triblock copolymers. Bioconjug Chem. 1996;7:592–599. doi: 10.1021/bc960048v. [DOI] [PubMed] [Google Scholar]
- 13.Lim CM, Hur J, Jang H, Seo JH. Developing a thermal grafting process for zwitterionic polymers on cross-linked polyethylene with geometry-independent grafting thickness. Acta Biomater. 2019;85:180–191. doi: 10.1016/j.actbio.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Kober M, Wesslén B. Surface properties of a segmented polyurethane containing amphiphilic polymers as additives. J Appl Polym Sci. 1994;54:793–803. [Google Scholar]
- 15.Dmitrenko M, Penkova A, Kuzminova A, Missyul A, Ermakov S, Roizard D. Development and characterization of new pervaporation PVA membranes for the dehydration using bulk and surface modifications. Polymers (Basel) 2018;10:571. doi: 10.3390/polym10060571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaner P, Rubakh E, Kim DH, Asatekin A. Zwitterion-containing polymer additives for fouling resistant ultrafiltration membranes. J Memb Sci. 2017;533:141–159. [Google Scholar]
- 17.Sällström N, Capel A, Lewis MP, Engstrøm DS, Martin S. 3D-printable zwitterionic nano-composite hydrogel system for biomedical applications. J Tissue Eng. 2020;11:2041731420967294. doi: 10.1177/2041731420967294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moro T, Takatori Y, Ishihara K, Konno T, Takigawa Y, Matsushita T, et al. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat Mater. 2004;3:829–836. doi: 10.1038/nmat1233. [DOI] [PubMed] [Google Scholar]
- 19.Diez-Escudero A, Harlin H, Isaksson P, Persson C. Porous polylactic acid scaffolds for bone regeneration: a study of additively manufactured triply periodic minimal surfaces and their osteogenic potential. J Tissue Eng. 2020;11:2041731420956541. doi: 10.1177/2041731420956541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang S, Cao Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater. 2010;22:920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 21.Chen M, Briscoe WH, Armes SP, Klein J. Lubrication at physiological pressures by polyzwitterionic brushes. Science. 2009;323:1698–1701. doi: 10.1126/science.1169399. [DOI] [PubMed] [Google Scholar]
- 22.Lim CM, Seo J, Jang H, Seo JH. Optimizing grafting thickness of zwitterionic sulfobetaine polymer on cross-linked polyethylene surface to reduce friction coefficient. Appl Surf Sci. 2018;452:102–112. [Google Scholar]
- 23.Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl. 2013;52:1636–1653. doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Pasquet J, Chevalier Y, Pelletier J, Couval E, Bouvier D, Bolzinger MA. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloid Surf A Physicochem Eng Asp. 2014;457:263–274. [Google Scholar]
- 26.Paladini F, Pollini M, Sannino A, Ambrosio L. Metal-based antibacterial substrates for biomedical applications. Biomacromolecules. 2015;16:1873–1885. doi: 10.1021/acs.biomac.5b00773. [DOI] [PubMed] [Google Scholar]
- 27.Krishnamoorthy K, Veerapandian M, Zhang LH, Yun K, Kim SJ. Surface chemistry of cerium oxide nanocubes: toxicity against pathogenic bacteria and their mechanistic study. J Ind Eng Chem. 2014;20:3513–3517. [Google Scholar]
- 28.Weng X, Ji Y, Zhao F, An Q, Gao C. Tailoring the structure of polyamide thin film composite membrane with zwitterions to achieve high water permeability and antifouling property. RSC Adv. 2015;5:98730–98739. [Google Scholar]
- 29.Hu X, Lin X, Zhao H, Chen Z, Yang J, Li F, et al. Surface functionalization of polyethersulfone membrane with quaternary ammonium salts for contact-active antibacterial and anti-biofouling properties. Materials (Basel) 2016;9:376. doi: 10.3390/ma9050376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowe AB, Vamvakaki M, Wassall MA, Wong L, Billingham NC, Armes SP, et al. Well-defined sulfobetaine-based statistical copolymers as potential antibioadherent coatings. J Biomed Mater Res. 2000;52:88–94. doi: 10.1002/1097-4636(200010)52:1<88::aid-jbm11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Kasmi N, Roso M, Hammami N, Majdoub M, Boaretti C, Sgarbossa P, et al. Microwave-assisted synthesis of isosorbide-derived diols for the preparation of thermally stable thermoplastic polyurethane. Des Monomers Polym. 2017;20:547–563. doi: 10.1080/15685551.2017.1395502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Xiong Z, Zhang L, Tang Z, Zhang R, Zhu J. Isosorbide dioctoate as a “green” plasticizer for poly(lactic acid) Mater Des. 2016;91:262–268. [Google Scholar]
- 33.Kim BK, Lee SY, Xu M. Polyurethanes having shape memory effects. Polymer (Guildf) 1996;37:5781–5793. [Google Scholar]
- 34.Yin B, Hakkarainen M. Oligomeric isosorbide esters as alternative renewable resource plasticizers for PVC. J Appl Polym Sci. 2011;119:2400–2407. [Google Scholar]
- 35.Chang Y, Chang WJ, Shih YJ, Wei TC, Hsiue GH. Zwitterionic sulfobetaine-grafted poly(vinylidene fluoride) membrane with highly effective blood compatibility via atmospheric plasma-induced surface copolymerization. ACS Appl Mater Interfaces. 2011;3:1228–1237. doi: 10.1021/am200055k. [DOI] [PubMed] [Google Scholar]
- 36.Bengani-Lutz P, Converse E, Cebe P, Asatekin A. Self-Assembling zwitterionic copolymers as membrane selective layers with excellent fouling resistance: effect of zwitterion chemistry. ACS Appl Mater Interfaces. 2017;9:20859–20872. doi: 10.1021/acsami.7b04884. [DOI] [PubMed] [Google Scholar]
- 37.Zanini S, Müller M, Riccardi C, Orlandi M. Polyethylene glycol grafting on polypropylene membranes for anti-fouling properties. Plasma Chem Plasma Process. 2007;27:446–457. [Google Scholar]
- 38.Lee SY, Lee Y, Le Thi PL, Oh DH, Park KD. Sulfobetaine methacrylate hydrogel-coated anti-fouling surfaces for implantable biomedical devices. Biomater Res. 2018;22:3. doi: 10.1186/s40824-017-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamada Y, Kulik EA, Ikada Y. Simple method for platelet counting. Biomaterials. 1995;16:259–261. doi: 10.1016/0142-9612(95)92126-q. [DOI] [PubMed] [Google Scholar]
- 40.Sivaraman B, Latour RA. The relationship between platelet adhesion on surfaces and the structure versus the amount of adsorbed fibrinogen. Biomaterials. 2010;31:832–839. doi: 10.1016/j.biomaterials.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue WW, Li HJ, Xiang T, Qin H, Sun SD, Zhao CS. Grafting of zwitterion from polysulfone membrane via surface-initiated ATRP with enhanced antifouling property and biocompatibility. J Memb Sci. 2013;446:79–91. [Google Scholar]