Abstract
Ascorbate potentiates the response of nicotinic-acetylcholine-receptors containing α9 and α10 subunits found predominantly in the efferent systems of the inner ear, such as the efferent vestibular system (EVS). Prior mouse studies have shown that an attenuated EVS results in reduced vestibulo-ocular reflex (VOR) gain (=eye_velocity/head_velocity) plasticity in intact (VOR adaptation) and surgically-lesioned (VOR compensation) mice. We sought to determine whether ascorbate-treatment could improve VOR recovery after vestibular organ injury, possibly through potentiation of the EVS pathway. We tested 10 cba129 mice, 5 received ascorbate-treatment and 5 did not, but otherwise experienced the same conditions. Ascorbate-treatment comprised a once-daily intraperitoneal injection of L-form reduced ascorbate (4 g/kg) in 0.2 ml saline starting 1 week before, and ending 4 weeks after, unilateral labyrinthectomy surgery. These were deliberately high doses to determine the ascorbate effects on recovery. Baseline, acute, and chronic sinusoidal VOR gains (frequency and velocity ranges: 0.2–10 Hz, 20–100 deg/s) were measured 3–5 days before, 3–5 days after, and 28–31 days after labyrinthectomy. Mice treated with ascorbate had acute ipsilesional VOR gains 12 % higher compared to control mice (+45.2 ± 14.9 % from baseline versus +33.7 ± 15.4 %, P < 0.001). Similarly, chronic ipsilesional and contralesional VOR gains were respectively 16 % (+74.3 ± 16.3 % from baseline versus +58.1 ± 15.8 %, P < 0.001) and 13 % (+78.6 ± 16.0 % versus +65.6 ± 10.9 %, P < 0.001) higher compared to control mice. These data suggest ascorbate-treatment had a prophylactic effect reducing acute loss, and helped recovery during acute to chronic stages of compensation. One possible mechanism is that an ascorbate-enhanced EVS drives an increase in the number and sensitivity of irregular-discharging primary vestibular afferents, important for VOR plasticity.
Keywords: ascorbate, efferent vestibular system, vestibular compensation, vestibulo-ocular reflex, vestibular plasticity
INTRODUCTION
The vestibulo-ocular reflex (VOR) is the main vision-stabilising system during head movement. Ideally, the VOR gain (eye_velocity/head_velocity) equals 1.0, so that the eye movement perfectly compensates the head movement. When injury to a vestibular organ/nerve occurs, a natural process of recovery begins. After 2–3 days, the immediate symptoms of injury abate, but the VOR gain remains low, particularly during head rotations towards the lesioned side.
In primates, the efferent vestibular system (EVS) cell group comprises over 400 neurons located in the brainstem immediately lateral to the abducens nucleus (Goldberg and Fernandez 1980). These neurons project to the peripheral vestibular organs where they branch extensively to innervate the vestibular neuroepithelia (Lindeman 1969). The pattern of innervation in the periphery is diffuse with single efferent fibres terminating onto multiple hair cells and afferent nerve fibres of the vestibular sensory organs. Efferent terminals make direct contact with vestibular type II hair cells, which has an inhibitory effect in mammals (Poppi et al. 2018; Yu et al. 2020), but do not contact type I hair cells directly due to the intervening cup-like afferent terminal called the calyx. Rather, these efferent terminals contact the outside of the calyx and afferent fibres, which has a strong excitatory effect in mammals (Lindeman 1969; Goldberg and Fernandez 1980; Poppi et al. 2020; Ramkrishna et al. 2021).
Two recent behavioural studies suggest that the α9 subunit of the nicotinic acetylcholine receptor (nAChR) in the mammalian EVS plays an important role in VOR gain plasticity, specifically VOR adaptation in intact mice (Hübner et al. 2015) and VOR compensation in mice after unilateral labyrinthectomy (UL) (Hübner et al. 2017). These studies showed that α9-knockout mice had ~70 % reduced capacity for VOR adaptation and ~50 % reduced capacity for VOR compensation after UL compared to control (cba129) mice. Moreover, a study in aged mice (2-year-old mice equivalent to 70–80-year-old humans) showed similar characteristics to α9 knock-out mice where VOR plasticity was severely and selectively reduced. Indeed, reduced EVS activity due to ageing could explain why vestibular dysfunction is not well compensated later in life (Khan et al. 2017).
Ascorbic acid (ascorbate; vitamin C) has been shown to potentiate acetylcholine (ACh; the main neurotransmitter of the EVS) responses in Xenopus laevis oocytes expressing α9 and α10 (but not α4, β2, or α7) nAChRs in a concentration-dependent manner (Boffi et al. 2013). Since α9 and α10 nAChRs are largely confined to the auditory and vestibular efferent systems, they provide a promising therapeutic target (Elgoyhen et al. 1994; Hiel et al. 1996). Acute exposure of peripheral vestibular organs to ascorbic acid, at the cellular level, is not feasible because the α9 and α10 nAChRs cannot be specifically targeted without affecting other channels present, such as acid-sensing ion channels (ASICs). Attempts to block ASICs and other potentially affected channels would compromise results. The Xenopus oocyte study (employing an effective concentration range of 1–30 mM) found that ascorbate did not affect the receptor’s current-voltage profile or its apparent affinity for ACh, but it significantly enhanced the maximal evoked currents, increasing them by 140 %. This effect was specific to the L-form of reduced ascorbate. Ascorbate is a likely agonist of α9 and α10 nAChRs due to a positive allosteric mechanism of action rather than its better recognized antioxidant effect (Boffi et al. 2013) and is one of the few known agents that activates or potentiates α9 and α10 nAChRs and thus is likely to stimulate efferent pathways. The effect of an ascorbate-enhanced EVS on VOR compensation is unlikely to be immediate because compensation is a slow and long-term process. In addition, for VOR compensation to become evident, EVS enhancement must be accompanied by days of regular activity in a normal visual environment to provide the needed feedback to stimulate change.
The aim of this initial study was to determine whether high-dose ascorbate can enhance the EVS to significantly improve VOR recovery after vestibular organ injury. Specifically, we asked whether a once-daily high dose of intraperitoneal ascorbate provided for 1 week before and 4 weeks after unilateral labyrinthectomy (5 weeks in total) results in improved acute and chronic VOR compensation.
MATERIALS AND METHODS
Animal Groups and Surgical Preparation
All surgical and experimental procedures were approved by the Animal Care and Ethics Committee of the University of New South Wales. Data were obtained from 10 cba129 mice (both sexes, aged 11–14 weeks). The baseline, acute, and chronic responses for each mouse were respectively measured 3–5 days before, 3–5 days after, and 28–31 days after unilateral labyrinthectomy (UL). Each mouse was implanted with a head immobilisation device that consisted of a metal adapter plate permanently attached to the skull and a removable head pedestal attached via magnet and screw to the adapter plate before each experiment (for more details of the adapter plate surgery and setup see Hübner et al. 2014, 2017). UL was performed 4–7 days after adapter plate attachment (for more details on UL surgery, see Hübner et al. 2017). After UL, all animals were placed in a normal visual environment and closely monitored.
Ascorbate Treatment
Five mice were assigned to the intervention (ascorbate + surgery) group and five to the control (surgery only) group. The intervention group mice received ascorbate treatment for 1 week prior to UL and for 4 weeks after UL (5 weeks in total). Other than ascorbate treatment, living conditions were identical for both control and intervention mice. Ascorbate treatment consisted of a once daily (at 9 am each morning) intraperitoneal (IP) (0.2 ml, i.e., 1 % body weight) injection of L-form reduced ascorbate (4 g/kg, Sigma-Aldrich) in saline. The ascorbate concentration threshold required for significant potentiation of the EVS is unknown, so rather than multiple low dose injections of ascorbate each day, a single high-dose daily injection was used to obtain the highest peak plasma level. The trade-off with a single high dose was reduced duration of exposure, which is preferable to multiple low doses that may be below threshold, thus resulting in no effect. The dosage was based on two mouse studies that examined the effects of daily IP injections of ascorbate on tumour growth (4 g/kg once daily for 4 weeks: Pollard et al. 2010; 4 g/kg once daily for 5–7 weeks and 4 g/kg twice daily for 3–4 weeks: Yun et al. 2015). Neither study reported adverse events. Because ascorbate is water soluble, it is excreted rapidly from the body, so build-up of toxicity is generally not a problem (Elmore 2005). When blood ascorbate levels are high, additional treatment will only increase the level temporarily — it takes 24 h for blood serum levels to return to normal – which is why once-daily doses were administered.
Adaptation and Eye Movement Recording
During each of the three experimental sessions per mouse, the mouse was restrained in a cylindrical capsule attached to a platform driven by a high torque rotatory servomotor (GOLDLINE DDR D083, Danaher) (for more details on this high-speed mouse rotary system please see Hübner et al. 2013, 2014, 2015, 2017; Khan et al. 2017, 2019a, b). The VOR gain was measured for each eye in complete darkness using a three-dimensional video-oculography system that tracked a thin-filmed marker array placed on each eye. This technique allows accurate measurement of each component (horizontal, vertical, and torsional) of the VOR evoked eye movement (for more details on the high-speed video-oculography system, see Hübner et al. 2013, 2014; Migliaccio et al. 2005, 2010). The VOR was measured during whole-body horizontal sinusoidal oscillation at 0.2, 0.4, 0.5, 0.8, 1, 1.6, 2, 5, and 10 Hz with peak-velocities of 20, 50, and 100 °/s (i.e., 9 × 3 = 27 rotary stimuli).
Data Analysis
Three-dimensional eye movements measured in eye-coordinates were converted to rotation and velocity vectors in head-coordinates. The slow phase component of eye velocity was inverted so that the ideal VOR gain, calculated as eye velocity divided by head velocity, would yield unity (+1) (for more details on three-dimensional binocular eye movement recording and processing using video-oculography see Migliaccio et al. 2010; Hübner et al. 2013, 2014, 2015, 2017; Khan et al. 2017, 2019a, b). VOR gain responses were expected to vary between ipsilesional and contralesional rotations, so eye velocity traces were divided into positive and negative half-waves. The slow-phase VOR response to each individual half-wave of the head velocity stimulus and eye velocity response were least-square fit to pure sine waves. The amplitudes of the eye and head velocity fits were used to calculate the gain (for more detail on the half-wave analysis technique see Hübner et al. 2017; Khan et al. 2019b). Side asymmetry was calculated using a variation of the Jonkees formula so that positive (+) asymmetry indicated that the ipsilesional gain was smaller than the contralesional gain, side asymmetry = 0 denotes no asymmetry and 100 denotes complete asymmetry.
Statistical Analysis
Statistical analysis was performed using SPSS version 23 (IBM, Armonk, NY) and Excel 2013 (Microsoft) software. A linear mixed model (LMM) with repeated measures was used to analyse the data. The independent variables were mouse group (control, intervention), test time (baseline, acute, chronic), rotation side (ipsilesional, contralesional), rotation peak velocity (20, 50 or 100 °/s), and rotation frequency (0.2, 0.4, 0.5, 0.8, 1.0, 1.6, 2, 5, 10 Hz) with VOR gain as the dependent variable. A separate LMM, with the independent variable side removed, was performed on dependent variable side asymmetry. All variables were included in the model initially and those found insignificant were subsequently removed. Post hoc tests were performed using paired t-tests with multiple–comparison correction (least significant difference) in case of significant LMM results. Unless otherwise stated all results are reported as mean ± 1 SD.
RESULTS
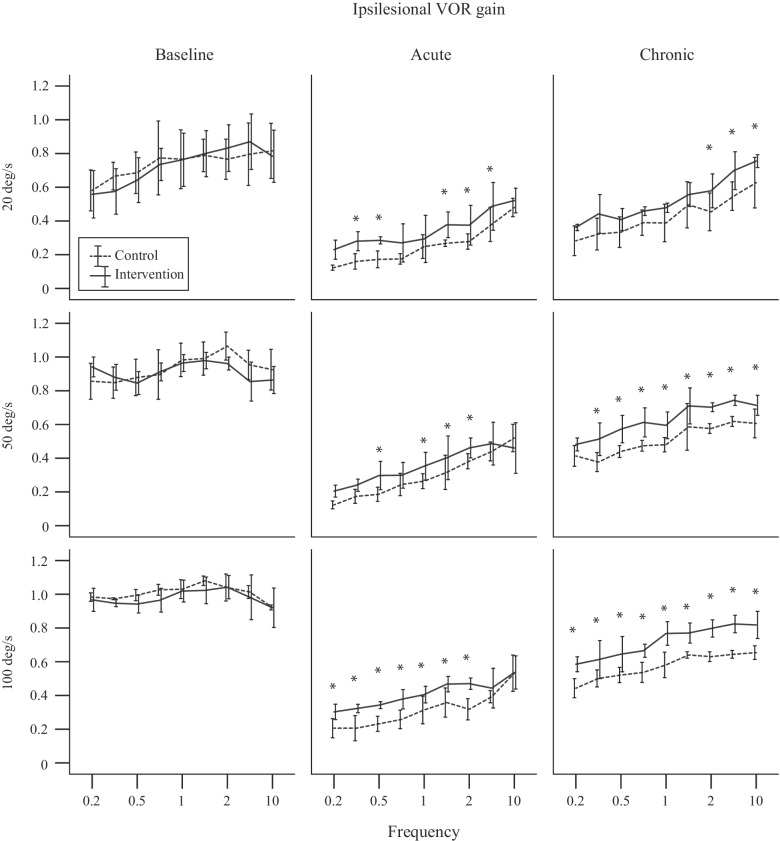
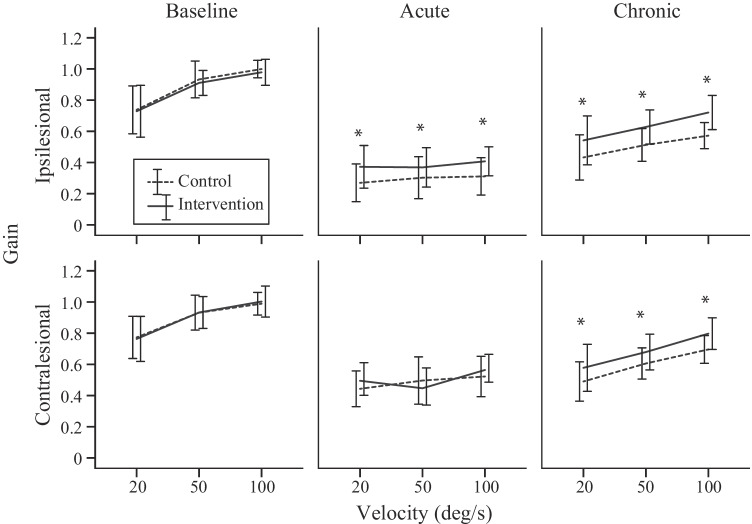
Linear mixed model (LMM) analysis revealed the factors that affected the VOR gain were mouse group (control, intervention) (LMM: F1, 462 = 86.02, P < 0.001), rotation side (ipsilesional, contralesional) (LMM: F1, 462 = 240.73, P < 0.001), rotation velocity (20–100 °/s) (LMM: F2, 462 = 341.94, P < 0.001), frequency (0.2–10 Hz) (LMM: F8, 459 = 90.57, P < 0.001), and time (baseline, acute, chronic) (LMM: F2, 668 = 3412.23, P < 0.001). There were also significant interactions between mouse group and time (LMM: F2, 668 = 54.53, P < 0.001), and between mouse group, side, and time (LMM: F2, 668 = 7.41, P = 0.001), suggesting that the VOR gain recovery time course was different between mouse groups, particularly on the ipsilesional side. Figure 1 shows the baseline (left column), acute (middle column), and chronic (right column) ipsilesional VOR gain across frequencies and peak velocities 20 (top row), 50 (middle row), and 100 °/s (bottom row) for each mouse group. Figure 2 shows the ipsilesional (top row) and contralesional (bottom row) baseline (left column), acute (middle column), and chronic (right column) VOR gains across peak velocities 20, 50, and 100 °/s pooled across frequencies for each mouse group. For both mouse groups, the VOR gain decreases acutely after unilateral labyrinthectomy surgery with evidence of recovery at the chronic stage.
Fig. 1.
Baseline (left column), acute (middle column), and chronic (right column) ipsilesional VOR gain across frequencies and peak velocities 20 (top row), 50 (middle row), and 100 °/s (bottom row) for each mouse group. The asterix (*) denotes a statistically significant difference of P < 0.05 between control and intervention mice
Fig. 2.
Ipsilesional (top row) and contralesional (bottom row) baseline (left column), acute (middle column), and chronic (right column) VOR gains across peak velocities 20, 50, and 100 °/s (pooled across frequencies) for each mouse group. The asterix (*) denotes a statistically significant difference of P < 0.05 between control and intervention mice
The baseline VOR gain was not significantly different between mouse group (LMM: F1, 405 = 1.11, P = 0.29) or between rotation side (LMM: F1, 405 = 2.91, P = 0.09) averaging 0.88 ± 0.15 (across mouse group, side, velocity, and frequency). As shown in prior mouse studies, VOR gain significantly increased with velocity (LMM: F2, 405 = 234.16, P < 0.001) and frequency (LMM: F8, 405 = 12.34, P < 0.001) (e.g., Migliaccio et al. 2010).
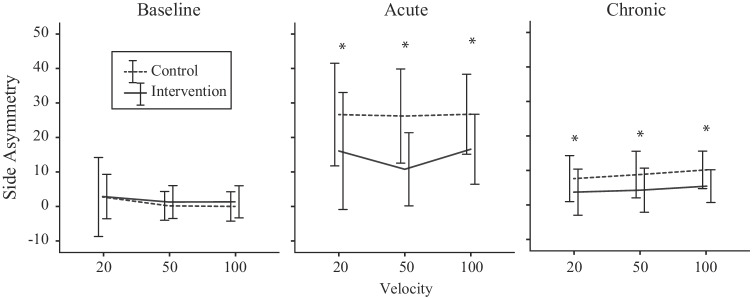
The VOR gain after acute injury was significantly different between mouse group (LMM: F1, 336 = 32.63, P < 0.001) and between rotation side (LMM: F1, 336 = 363.40, P < 0.001). For the control mouse, the respective ipsilesional and contralateral VOR gains (pooled across velocity and frequency) were 0.30 ± 0.13 (33.72 ± 15.36 % of baseline) and 0.49 ± 0.14 (54.09 ± 14.57 % of baseline), whereas for the intervention mouse, the gains were 0.38 ± 0.12 (45.21 ± 14.87 % of baseline) and 0.50 ± 0.12 (58.11 ± 15.47 % of baseline). There was a significant interaction between mouse group and side (LMM: F1, 336 = 18.94, P < 0.001), suggesting that the difference in gain between sides was greater in the control mouse. VOR gain side asymmetry index was significantly larger in the control mouse at 26.52 ± 13.28 compared to the intervention mouse at 14.44 ± 12.72 (LMM: F1, 168 = 55.52, P < 0.001). Figure 3 shows the baseline (left column), acute (middle column), and chronic (right column) side asymmetries across peak velocities 20, 50, and 100 °/s for each mouse group.
Fig. 3.
Baseline (left column), acute (middle column), and chronic (right column) side asymmetries across peak velocities 20, 50, and 100 °/s (pooled across frequencies) for each mouse group. The asterix (*) denotes a statistically significant difference of P < 0.05 between control and intervention mice
The VOR gain after chronic injury remained significantly different between mouse group (LMM: F1, 354 = 208.19, P < 0.001) and between rotation side (LMM: F1, 354 = 102.39, P < 0.001). For the control mouse, the respective ipsilesional and contralateral VOR gains (pooled across velocity and frequency for comparison purposes) were 0.51 ± 0.13 (58.06 ± 15.83 % of baseline) and 0.60 ± 0.14 (65.58 ± 10.89 % of baseline), whereas for the intervention mouse the gains were 0.63 ± 0.14 (74.28 ± 16.28 % of baseline) and 0.69 ± 0.15 (78.55 ± 16.00 % of baseline). The change in VOR gain from acute to chronic was significantly different between mouse group (LMM: F1, 432 = 1067.47, P < 0.001) and between rotation side (LMM: F1, 432 = 44.51, P < 0.001). For the control mouse, the respective ipsilesional and contralateral VOR gain changes from acute to chronic (pooled across velocity and frequency for comparison purposes) were 0.22 ± 0.18 (24.24 ± 13.66 %) and 0.12 ± 0.15 (11.16 ± 12.55 %), whereas for the intervention mouse, the gain changes were 0.27 ± 0.11 (30.54 ± 13.06 %) and 0.20 ± 0.12 (22.26 ± 12.48 %). The significant interaction between mouse group and side (LMM: F1, 354 = 6.19, P = 0.013) remained due to the difference between sides being greater in the control mouse. VOR gain asymmetry was significantly smaller in the intervention mouse at 4.29 ± 5.96 compared to the control mouse at 8.67 ± 6.37 (LMM: F1, 228 = 28.31, P < 0.001).
DISCUSSION
We sought to determine whether high dose ascorbate treatment could significantly improve VOR recovery after vestibular organ injury. Our findings suggest that mice treated with ascorbate for 1 week prior to unilateral labyrinthectomy (UL) had acute ipsilesional VOR gains that were 12 % higher compared to control mice, suggesting a prophylactic effect of ascorbate. In addition, chronic ipsilesional and contralesional VOR gains were respectively 16 % and 13 % higher compared to control mice, suggesting that ascorbate also helped recovery from the acute to chronic stage of recovery. Side asymmetry was smaller in the ascorbate treated mouse during both acute and chronic stages of compensation.
The present study is the first to examine the effects of a putatively enhanced EVS on VOR compensation. However, prior studies have examined the effect of an inhibited EVS on VOR adaptation and compensation using the α9 knock-out mouse model. Studies in α9 knock-out mice suggest that the EVS modifies the properties of the two types of primary vestibular afferent signals, referred to as the regular discharging and irregular discharging afferent pathways (Han et al. 2007; Hübner et al. 2015, 2017). Specifically, when the EVS action is attenuated by the loss of α9 receptors the regular pathway sensitivity is increased, while ‘irregular pathway’ sensitivity is decreased, and there is an apparent reduction in the proportion of irregular versus regular afferents (Han et al. 2007). Primate studies have shown that the VOR eye movement response has a component that matches the dynamic characteristics of the irregular discharging vestibular afferent pathway (Hullar and Minor 1999; Migliaccio et al. 2004, 2008). In addition, this ‘irregular’ component is highly adaptable, has a short latency, and appears to be responsible for most of the changes in VOR gain that occurs during adaptation training (Minor et al. 1999). Therefore, because ascorbate is a demonstrated agonist of α9 and α10 nAChRs that potentiates α9 and α10 nAChRs, it plausibly stimulates the EVS resulting in an increase in irregular afferent activity leading to improved compensation. This putative mechanism could explain why ascorbate worked both as a prophylactic and a treatment.
Pharmacological Treatment of Vestibular Compensation
The majority of pharmaceuticals used to treat vestibular conditions, control symptoms via vestibular suppressants (e.g., reduce intensity of vertigo and nystagmus evoked imbalance) or antiemetics (e.g., reduce vomiting and nausea) (e.g., Yacovino et al. 2016). There are also anti-inflammatory, anti-migraine, anti-convulsant, anti-Menieres’s, and antidepressant drugs (Yacovino et al. 2016; Strupp et al. 2011). However, most relevant to our study are pharmaceuticals that modify the main neurotransmitters/receptors in the vestibular system that affect compensation. Glutamate is an excitatory neurotransmitter for vestibular afferents, which interacts with several subreceptors including N-methyl-D-aspartic acid (NMDA) (Serafin et al. 1992; Soto et al. 2013). NMDA excitatory amino acids (EEA) receptors affect the basal discharge and tonic response of primary afferent input signals at vestibular nucleus (VN), whereas non-NMDA receptors affect responses to high-frequency head motion (Soto et al. 2013). EAA receptor-acting drugs, such as nermexane, block α9α10 nAChRs of rat inner ear cells and are thought to improve neuroprotection and compensation after lesions to the cochlea or balance organ (Plazas et al. 2007). Acetylcholine, centrally, mostly affects muscarninc receptors in VN, whereas peripherally, it mediates efferent synapses (Soto et al. 2013). Anticholinergics which affect muscarinic receptors, such as scopolamine, improve habituation to motion sickness in rats (Morita et al. 1990) and affect vestibular compensation, producing overcompensation if provided late during the natural compensation process (Zee 1988). Nitric oxide, affected by cholinergic neurons, also seems to play a role in vestibular compensation depending on the species (rat, frog, guinea pig: Smith et al. 2001). Antihistamines, such as betahistine, target histamine receptors in VN and are often used in conjuction with anticholinergics to prevent motion sickness and reduce symptom severity (Takeda et al, 1989; Serafin et al. 1992). Finally, activation of glucocorticoid receptors via neuroactive steroids, such as dexamethasone, have been shown to improve vestibular plasticity in lesioned rats (Cameron and Dutia 1999).
Experiment Limitations
Since this initial study design was meant to determine if there were effects of high dose ascorbate on VOR recovery, the individual effects of prophylacsis and treatment were not separated. To determine the effects of each, two further groups would need to be tested: one group that received ascorbate treatment only before UL, and another group that only received it after UL. Using this approach, one could determine the extent prophylactic treatment affected the compensation process, i.e., the period between acute and chronic stages of compensation. Another set of experiments would be to study compensation beyond one month after UL. Although unlikely, it is possible that the effects of ascorbate treatment plateau causing a halt in improvement, whereas non-treated mice continue to improve so that eventually recovery is the same in both mouse groups. However, based on our experience and data from other mouse studies, recovery seems to mostly occur during the first 20 days after labyrinthectomy (see figure 6 in Beraneck et al. 2008). We did not measure the effect of ascorbate on an unoperated animal; however, based on our studies in α9 knock-out mice, the EVS seems to mostly affect VOR plasticity rather than the baseline VOR (Hübner et al. 2015). In addition, we did not provide control mice with daily IP injections of 0.2 ml saline to control for the effect of saline alone. In both cases, we conjectured there would be no observable effects; however, those results will still need to be confirmed. Finally, to determine an effective therepautic dose, rather than using the maximum safe dose as determined by other non-related mouse studies (Pollard et al. 2010; Yun et al. 2015), in future experiments, measure of effective ascorbate blood serum levels will be needed.
Clinical Implications
Although the high doses of ascorbic acid used in this mouse study cannot be directly applied to humans, i.e., 4 g/kg/day translates to 320 g/day of ascorbate for an 80-kg human, it provides the first evidence that putatively stimulating the EVS results in significant improvement in VOR recovery. Our findings suggest the development of derivative drugs that stimulate the human EVS could result in improved VOR compensation if delivered prophylactically, e.g., patients about to undergo surgical (vestibular nerve section, canal plugging: Carey et al. 2007; Migliaccio et al. 2008) or chemical (gentamicin: e.g., Migliaccio et al. 2004) treatment that affect the vestibular organ, or as a treatment for patients where the vestibular loss was unforeseen. The results from this mouse study encourage the investigation of the efficacy of lower doses of ascorbate in humans; e.g., 5 g/day is considered completely safe in humans, which could be explored in a cohort of patients about to undergo a vestibular lesion.
CONCLUSION
This initial study using high-dose intraperitoneal ascorbate results in improved VOR gain recovery both during the acute and chronic stages of compensation. The likely mechanism of improvement is that ascorbate potentiates α9 and α10 subunits of the nicotinic acetylcholine receptors in the efferent vestibular system. This potentiation leads to efferent vestibular system stimulation that in turn drives an increase in the number and sensitivity of irregular primary vestibular afferents, which likely mediate most of the plasticity changes that occur during VOR compensation.
Funding
A. A. Migliaccio and this work were supported by a National Health and Medical Research Council of Australia (NHMRC) Biomedical Career Development Award CDA-568736 and NHMRC Project Grant APP1010896.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Beraneck M, McKee JL, Aleisa M, Cullen KE. Asymmetric recovery in cerebellar-deficient mice following unilateral labyrinthectomy. J Neurophysiol. 2008;100:945–958. doi: 10.1152/jn.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffi JC, Wedemeyer C, Lipovsek M, Katz E, Calvo DJ, Elgoyhen AB. Positive modulation of the α9α10 nicotinic cholinergic receptor by ascorbic acid. Br J Pharmacol. 2013;168:954–965. doi: 10.1111/j.1476-5381.2012.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron SA, Dutia MB. Lesion-induced plasticity in rat vestibular nucleus neurones dependent on glucocorticoid receptor activation. J Physiol. 1999;518:151–158. doi: 10.1111/j.1469-7793.1999.0151r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JP, Migliaccio AA, Minor LB. Semicircular canal function before and after surgery for superior canal dehiscence. Otol Neurotol. 2007;28:356–364. doi: 10.1097/01.mao.0000253284.40995.d8. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-X. [DOI] [PubMed] [Google Scholar]
- Elmore AR. Final report of the safety assessment of L-ascorbic acid, calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate as used in cosmetics. Int J Toxicol. 2005;24:51–111. doi: 10.1080/10915810590953851. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernanedez C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol. 1980;43:986–1025. doi: 10.1152/jn.1980.43.4.986. [DOI] [PubMed] [Google Scholar]
- Han GC, Lasker DM, Vetter DE, Minor LB (2007) Extracellular recordings from semicircular canal afferents in mice that lack the alpha 9 nicotinic acetylcholine receptor subunit. Assor Res Otolaryngol, Midwinter Meeting Abstracts. Denver 1–2
- Hiel H, Elgoyhen AB, Drescher DG, Morley BJ. Expression of nicotinic acetylcholine receptor mRNA in the adult rat peripheral vestibular system. Brain Res. 1996;738:347–352. doi: 10.1016/S0006-8993(96)01046-3. [DOI] [PubMed] [Google Scholar]
- Hübner PP, Khan SI, Migliaccio AA. Velocity-selective adaptation of the horizontal and cross-axis vestibulo-ocular reflex in the mouse. Exp Brain Res. 2014;232:3035–3046. doi: 10.1007/s00221-014-3988-8. [DOI] [PubMed] [Google Scholar]
- Hübner PP, Lim R, Brichta AM, Migliaccio AA. Glycine receptor deficiency and its effect on the horizontal vestibulo-ocular reflex: a study on the SPD1J mouse. J Assoc Res Otolaryngol. 2013;14:249–259. doi: 10.1007/s10162-012-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner PP, Khan SI, Migliaccio AA. The mammalian efferent vestibular system plays a crucial role in the high-frequency response and short-term adaptation of the vestibulo-ocular reflex. J Neurophysiol. 2015;114:3154–3165. doi: 10.1152/jn.00307.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner PP, Khan SI, Migliaccio AA. The mammalian efferent vestibular system plays a crucial role in vestibulo-ocular reflex compensation after unilateral labyrinthectomy. J Neurophysiol. 2017;117:1553–1568. doi: 10.1152/jn.01049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar TE, Minor LB. High-frequency dynamics of regularly discharging canal afferents provide a linear signal for angular vestibuloocular reflexes. J Neurophysiol. 1999;82:2000–2005. doi: 10.1152/jn.1999.82.4.2000. [DOI] [PubMed] [Google Scholar]
- Khan SI, Hübner PP, Brichta AM, Smith DW, Migliaccio AA. Ageing reduces the high-frequency and short-term adaptation of the vestibulo-ocular reflex in mice. Neurobiol Aging. 2017;51:122–131. doi: 10.1016/j.neurobiolaging.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Khan SI, Della Santina CC, Migliaccio AA (2019a) Angular vestibulo-ocular reflex responses in Otop1 mice. I. Otolith sensor input is essential for gravity context-specific adaptation. J Neurophysiol 121:2291–2299 [DOI] [PubMed]
- Khan SI, Della Santina CC, Migliaccio AA (2019b) Angular vestibulo-ocular reflex responses in Otop1 mice. II. Otolith sensor input improves compensation after unilateral labyrinthectomy. J Neurophysiol 121:2300–2307 [DOI] [PubMed]
- Lindeman HH. Regional differences in sensitivity of the vestibular sensory epithelia to ototoxic antibiotics. Acta Otolaryngol. 1969;67:177–189. doi: 10.3109/00016486909125441. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, MacDougall HG, Minor LB, Della Santina CC. Inexpensive system for real-time 3-dimensional video-oculography using a fluorescent marker array. J Neuroscie Methods. 2005;143:141–150. doi: 10.1016/j.jneumeth.2004.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AA, Meierhofer R, Della Santina CC. Characterization of the 3D angular vestibulo-ocular reflex in C57BL6 mice. Exp Brain Res. 2010;210:489–501. doi: 10.1007/s00221-010-2521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AA, Minor LB, Carey JP. Vergence-mediated modulation of the human horizontal vestibulo-ocular reflex is eliminated by a partial peripheral gentamicin lesion. Exp Brain Res. 2004;159:92–98. doi: 10.1007/s00221-004-1974-2. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Minor LB, Carey JP. Vergence-mediated modulation of the human angular vestibulo-ocular reflex is unaffected by canal plugging. Exp Brain Res. 2008;186:581–587. doi: 10.1007/s00221-007-1262-z. [DOI] [PubMed] [Google Scholar]
- Minor LB, Lasker DM, Backous DD, Hullar TE. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. I. Normal responses. J Neurophysiol. 1999;82:1254–1270. doi: 10.1152/jn.1999.82.3.1254. [DOI] [PubMed] [Google Scholar]
- Morita M, Takeda N, Hasegawa S, Yamatodani A, Wada H, Sakai S, Kubo T, Matsunaga T. Effects of anti-cholinergic and cholinergic drugs on habituation to motion in rats. Acta Otolaryngol. 1990;110:196–202. doi: 10.3109/00016489009122537. [DOI] [PubMed] [Google Scholar]
- Plazas PV, Savino J Kracun S, Gómez-Casati ME, Katz E, Parsons CG, Millar NS, Elgoyhen AB (2007) Inhibition of the alpha9-alpha10 nicotinic cholinergic receptor by neramexane, an open channel blocker of N-methyl-D-aspartate receptors. Eur J Pharmacol 566:11–19 [DOI] [PubMed]
- Pollard HB, Levine MA, Eidelman O, Pollard M. Pharmacological ascorbic acid suppresses syngeneic tumor growth and metastases in hormone-refractory prostate cancer. In Vivo. 2010;24:249–255. [PMC free article] [PubMed] [Google Scholar]
- Poppi LA, Tabatabaee H, Drury HR, Jobling P, Callister RJ, Migliaccio AA, Jordan PM, Holt JC, Rabbitt RD, Lim R, Brichta AM. ACh-induced hyperpolarization and decreased resistance in mammalian type II vestibular hair cells. J Neurophysiol. 2018;119:312–325. doi: 10.1152/jn.00030.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppi LA, Holt JC, Lim R, Brichta AM. A review of efferent cholinergic synaptic transmission in the vestibular periphery and its functional implications. J Neurophysiol. 2020;123:608–629. doi: 10.1152/jn.00053.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna Y, Manca M, Glowatzki E, Sadeghi SG. Cholinergic modulation of membrane properties of calyx terminals in the vestibular periphery. Neuroscience. 2021;452:98–110. doi: 10.1016/j.neuroscience.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafin M, Khateb A, de Waele C, Vidal PP, Mühlethaler M. Medial vestibular nucleus in the guinea-pig: NMDA-induced oscillations. Exp Brain Res. 1992;88:187–192. doi: 10.1007/BF02259140. [DOI] [PubMed] [Google Scholar]
- Smith PF, Zheng Y, Paterson S, Darlington CL (2001) The contribution of nitric oxide to vestibular compensation: are there species differences?. Acta Otolaryngol Suppl 545:57–60 [DOI] [PubMed]
- Soto E, Vega R, Seseña E. Neuropharmacological basis of vestibular system disorder treatment. J Vestib Res. 2013;23:119–137. doi: 10.3233/VES-130494. [DOI] [PubMed] [Google Scholar]
- Strupp M, Thurtell MJ, Shaikh AG, Brandt T, Zee DS, Leigh RJ. Pharmacotherapy of vestibular and ocular motor disorders, including nystagmus. J Neurol. 2011;258:1207–1222. doi: 10.1007/s00415-011-5999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Mashahiro M, Hasegawa S, Kubo T, Matsunaga T. Neurochemical mechanisms of motion sickness. Am J Otolaryngol. 1989;10:351–359. doi: 10.1016/0196-0709(89)90112-9. [DOI] [PubMed] [Google Scholar]
- Yacovino DA, Carrea R, Luis L (2016) Pharmacological treatment of vestibular disorders. Vestibular Disorders Association Article 050
- Yu Z, McIntosh JM, Sadeghi SG, Glowatzki E. Efferent synaptic transmission at the vestibular type II hair cell synapse. J Neurophysiol. 2020;124(2):360–374. doi: 10.1152/jn.00143.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, Muley A, Asara JM, Paik 9, Elemento O, Chen Z, Pappin DJ, Dow LE, Papadopoulos N, Gross SS, Cantley LC (2015) Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350:1391–1396 [DOI] [PMC free article] [PubMed]
- Zee DS. The management of patients with vestibular disorders. In: Barber HO, Sharpe JA, editors. Vestibular disorders. Chicago: Yearbook Med. Pub; 1988. pp. 254–274. [Google Scholar]