Abstract
We report a case of fatal pulmonary infection caused by Mycobacterium abscessus in a young patient with cystic fibrosis, who underwent bipulmonary transplantation after a 1-year history of severe lung disease. Fifteen days after surgery he developed septic fever with progressive deterioration in lung function. M. abscessus, initially isolated from a pleural fluid specimen, was then recovered from repeated blood samples, suggesting a disseminated nature of the mycobacterial disease. Drug susceptibility testing assay, performed on two sequential isolates of the microorganism, showed a pattern of multidrug resistance. Despite aggressive therapy with several antimycobacterial drugs, including clarithromycin, the infection persisted, and the patient died.
CASE REPORT
A 20-year-old man with cystic fibrosis (CF) underwent bilateral pulmonary transplantation in July of 1999. For approximately 1 year prior to admission he had been suffering from severe pulmonary disease with intermittent episodes of septic fever, and during this period he had been hospitalized several times. Sputum cultures had yielded Pseudomonas aeruginosa and Aspergillus fumigatus, whereas blood cultures (collected either via catheter and by venipuncture) were positive for Saccharomyces cerevisiae. On the basis of these microbiological findings, he had received several courses of antibiotic and antifungal therapy consisting of quinolones, third-generation cephalosporins, and amphotericin B, which had, however, produced only temporary remission of the symptoms. All cultures for mycobacteria (blood, sputum, urine, etc.) were repeatedly negative; sputum culture was contaminated by P. aeruginosa.
Bilateral lung transplantation was performed on 20 July 1999. There were no intraoperative complications, and the patient was started on immunosuppressive therapy.
On 5 August 1999, the patient developed a septic syndrome, and computed tomography (CT) performed on 7 August 1999 revealed a hyperdense lesion in the right paracardiac region with features of parenchymal involvement and a reactive pleural effusion. In light of the previous fungal recovery from clinical specimens, he was promptly started on intravenous amphotericin B therapy. On 6 August 1999 the pleural fluid was cultured for mycobacteria and was inoculated into Middlebrook 7H12 medium (BACTEC 12B; Becton Dickinson Diagnostic Instruments, Sparks, Md.) and Löwenstein-Jensen slant (BioMerieux, Marcy l'Etoile, France). On 8 August 1999, sputum and blood samples were also submitted for mycobacterial studies. A smear of respiratory samples showed many acid-fast bacilli.
All three specimens yielded a rapidly growing mycobacterium identified as Mycobacterium chelonae and subsequently as M. abscessus, and on 20 August 1999 the patient was started on intravenous ciprofloxacin, amikacin, and clarithromycin. Sensitivity studies soon revealed, however, that the isolates were clearly resistant to all three drugs, as well as to all of the others tested (Table 1). In fact, after 1 month of antibiotic therapy the patient was still febrile, and his pulmonary disease had worsened. Computed tomography performed at this point revealed diffuse nodular lesions throughout the entire pulmonary parenchyma. Because of the critical nature of the patient's clinical status, the treatment was empirically modified. Ciprofloxacin was replaced with levofloxacin and meropenem, which were subsequently changed to intravenous ethambutol and rifabutin, and later to streptomycin and ethambutol. The blood and pleural fluid specimens remained positive for acid-fast bacilli, and the patient died on 31 October 1999.
TABLE 1.
Drug susceptibility testing results of M. abscessus
| Drug | MIC (μg/ml) |
|---|---|
| Streptomycin | ≥256 |
| Isoniazid | ≥256 |
| Ceftazidime | ≥256 |
| Azithromycin | ≥256 |
| Clarithromycin | ≥256 |
| Levofloxacin | 32 |
| Vancomycin | ≥256 |
| Imipenem | 32 |
| Amoxicillin | ≥256 |
| Ethionamide | ≥256 |
| Trimethoprim-sulfamethoxazole | 32 |
| Ofloxacin | 32 |
| Amoxicillin-clavulanic acid | ≥256 |
| Rifampin | 2 |
Microbiology.
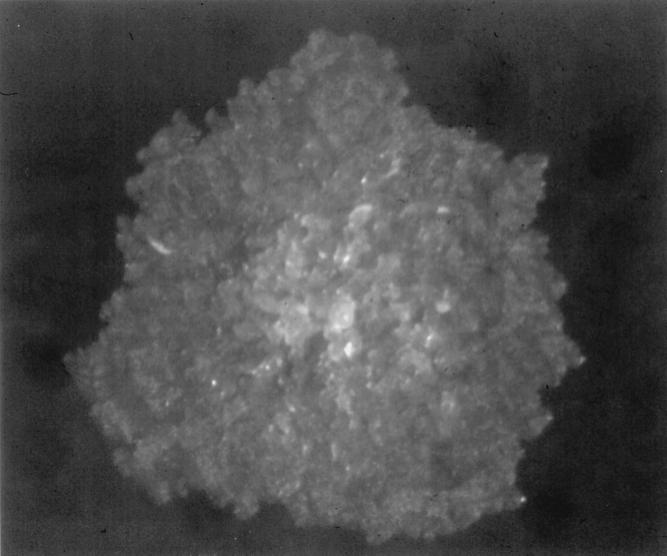
Prior to cultivation, sputum specimens were decontaminated by the NALC-NaOH method as previously described (15), whereas pleural fluid and blood specimens were directly cultured for mycobacteria. All of the specimens yielded a rapidly growing mycobacterium that was detected by the BACTEC 460 radiometric system (Becton Dickinson) and by visual observation of the Löwenstein-Jensen slants. Acid-fast staining was used to confirm that the isolates were mycobacteria; no contamination with other bacteria was observed. The isolates, subcultured on Middlebrook 7H11 agar (BBL Microbiology Systems, Cockeysville, Md.), grew within 3 days of incubation and appeared as a nonpigmented, large, flat, rough, and wrinkled colony, with a well-known morphotype (12) (Fig. 1). They were identified as M. chelonae on the basis of biochemical tests, including catalase, arylsulfatase 3-day, and nitrate reduction analyses and growth on MacConkey agar (12), and were sent to the Microbiology Laboratory of the Catholic University of the Sacred Heart for confirmation.
FIG. 1.
M. abscessus isolate grown on Middlebrook 7H11 agar. The colony appears large, flat, rough, and wrinkled, findings consistent with one of the described M. abscessus morphotypes.
The isolates were first subjected to PCR-reverse cross blot hybridization of the amplified 16S ribosomal DNA (15), which confirmed that they belonged to the M. chelonae group. The microorganisms were then analyzed by the hsp65 PCR-restriction method (18), which yielded a profile that matched that of M. abscessus obtained from Prasite (www.hospvd.ch:8005) (G. Prod'hom, M. M'Pandi, C. Taillard, M. Moser, A. Telenti, D. Blanc, V. Vincent, A. Strässle, G. Pfyffer, and J. Bille, Final Prog. Abstr. 20th Annu. Cong. Eur. Soc. Mycobacteriol., abstr. P59, p. 74, 1999). Alternatively, other biochemical tests, such as citrate utilization (16), could be used to differentiate the two organisms.
The MICs of 14 antimicrobial agents, including the first-line antimycobacterial drugs, were determined with the Etest diffusion agar method (PMD Epsilometer; AB Biodisk, Solna, Sweden). Briefly, a suspension obtained by emulsifying the microorganism's colonies in Mueller-Hinton broth to achieve a density equal to 1.0 McFarland turbidity standard, was used to inoculate the entire surface of three Mueller-Hinton agar plates supplemented with 5% sheep blood (BBL Microbiology Systems). Etest strips were applied on the plates according to the manufacturer's instructions. Plates were incubated at 30°C in ambient air, and the results were read after 72 h of incubation, as previously described (10). All of the M. abscessus isolates showed the same susceptibility pattern, with extremely high MIC values for all of the drugs tested (Table 1). Surprisingly, the isolates were resistant to clarithromycin, a drug commonly used for therapy of infections caused by rapidly growing mycobacteria.
Discussion.
Historically considered quite rare, the recovery of nontuberculous mycobacteria (NTM) from the pulmonary tracts of patients suffering from CF appears to be increasing (1, 9). Among NTM, M. abscessus, M. chelonae, and M. fortuitum are the species more frequently isolated from respiratory specimens in these patients; in particular, from a total of cases of cultures positive for mycobacteria, M. abscessus was found to be the second most common isolate after M. avium complex (5, 13). Although the significance of NTM in CF has not yet been firmly established (7), there are well-documented cases of clinically important pulmonary infections due to M. abscessus that clearly evidence the pathogenetic role of this organism (5). The case we presented addresses the severe nature of M. abscessus pulmonary infection complicating CF and the difficulty in eradicating the organism, particularly in patients with underlying lung disease.
M. abscessus belongs to the group of NTM, a heterogeneous group of microorganisms that can occasionally cause lung disease but more commonly affect patients with underlying chronic lung disease, such as bronchiectasis, pneumoconiosis, emphysema, or CF. As recently reported (6), patients with CF are at high risk for NTM disease, but in these individuals the clinical signs and symptoms of NTM infections are often undistinguishable from those of the chronic bacterial pulmonary infections that occur during the advanced stages of CF (5, 13). The situation is further complicated by the fact that other microorganisms, P. aeruginosa in particular, colonize the respiratory tract of CF patients, and their overgrowth often hampers the recovery of mycobacteria.
Misdiagnosis of mycobacterial infections can be a life-threatening error. In the CF patient described here, lung transplantation was followed by a severe pulmonary disease caused by a multidrug-resistant strain of M. abscessus. From the onset, the disease followed a fulminant course that led to irreversible and ultimately fatal lung damage. The dramatic outcome of the case highlights important issues.
Although M. chelonae, M. abscessus, and M. fortuitum are indeed the NTM species most frequently found in the respiratory tract of CF patients (7), the chronic nature of the pulmonary disease in CF makes it difficult to distinguish between NTM colonization and disease. In fact, with the emergence of high-resolution CT scanning, several cases of apparent “colonization” have had to be reclassified (5, 17). In our case, the CT documentation of a progressive decline in pulmonary function, together with the persistent positivity for mycobacteria in pleural fluid and blood cultures, were indicative of active NTM disease.
Bacterial overgrowth in clinical specimens, such as sputum, is a problem, and it leads to an underestimation of the prevalence of mycobacterial lung disease in CF patients. This phenomenon may explain the low rate (6 to 22%) of mycobacteria recovery observed, particularly in young patients (6, 8, 20). Improved methods are needed to detect mycobacteria in respiratory samples contaminated with other overgrowing microorganisms, such as P. aeruginosa, Staphylococcus aureus, Candida albicans, and Aspergillus fumigatus (2, 21). Molecular methods, able to detect and identify mycobacteria directly from clinical specimens, could improve the diagnosis of mycobacterial pulmonary infections. In particular, PCR-reverse cross blot hybridization, used in our case to identify pure culture isolates, has successfully been adopted for the direct analysis of clinical specimens from patients suspected of having mycobacterial disease (11, 14). Similarly, we have used this method on BACTEC broth cultures to quickly detect and identify several clinical important mycobacterial species (4, 15).
Lung transplantation is considered a therapeutic option in the CF patient population, but the posttransplantation immunosuppressive therapy increases the risk for opportunistic infections, including those caused by mycobacteria. Moreover, CF candidates for transplantation with true NTM colonization must be reliably identified because lung transplantation in a patient with acid-fast-bacillus-positive smears and cultures carries enormous risks. In fact, cervical adenitis and skin abscesses caused by M. chelonae have even been observed in a CF lung transplantation patient after a prolonged period of negative cultures (6, 19).
In our case, the question of whether or not the patient was colonized by the mycobacterium prior to transplantation remains unanswered. It is conceivable that the presence of P. aeruginosa could have prevented mycobacterial growth in the preoperative cultures, which were repeatedly negative, and that eradication of the P. aeruginosa infection with preoperative antibiotics allowed the NTM to emerge in postoperative cultures. On the other hand, the prolonged course of intravenous antibiotics that was administered for the bacterial infections may itself have predisposed the patient to nontuberculous colonization, as observed by Torrens et al. (20).
Although delayed diagnosis may have contributed to the fatal outcome of this case, the latter appears to be primarily attributable to the multidrug resistance of the M. abscessus isolate. To our knowledge, this is the first evidence of true resistance to clarithromycin, which is considered the most active drug against M. chelonae. In a study published in 1992, 100% of the isolates tested were susceptible to this drug at an MIC of ≤1 μg/ml (3). However, a more recent report has described a recurrent catheter-related infection due to a multidrug-resistant strain of M. chelonae, in which even clarithromycin displayed a significantly higher MIC (1.5 to 2 μg/ml) (8). The molecular genetic basis of the drug resistance of our isolate needs to be elucidated.
In conclusion, we illustrate here that M. abscessues infections may present with a severe and fatal course as a consequence of either late diagnosis and/or broad-spectrum drug resistance of the microorganism.
Acknowledgments
This work was supported in part by the National Tuberculosis Project (Istituto Superiore di Sanità-Ministero della Sanità), contract no. 96/D/T10.
We thank Marian Kent and Brunella Posteraro for their critical reading of the manuscript.
REFERENCES
- 1.Aitken M L, Burke W, McDonald G, Wallis C, Ramsey B, Nolan C. Nontuberculous mycobacterial disease in adult cystic fibrosis patients. Chest. 1993;103:1096–1099. doi: 10.1378/chest.103.4.1096. [DOI] [PubMed] [Google Scholar]
- 2.Bange F C, Kirschner P, Böttger E C. Recovery of mycobacteria from patients with cystic fibrosis. J Clin Microbiol. 1999;37:3761–3763. doi: 10.1128/jcm.37.11.3761-3763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown B A, Wallace R J, Jr, Onyi G, DeRosas V, Wallace R J., III Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and Mycobacterium chelonae-like organisms. Antimicrob Agents Chemother. 1992;36:180–184. doi: 10.1128/aac.36.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cingolani A, Sanguinetti M, Antinori A, Larocca L M, Ardito F, Posteraro B, Federico G, Fadda G, Ortona L. Brief report: disseminated mycobacteriosis caused by drug-resistant mycobacterium triplex in a human immunodeficiency virus-infected patient during highly active antiretroviral therapy. Clin Infect Dis. 2000;31:177–180. doi: 10.1086/313903. [DOI] [PubMed] [Google Scholar]
- 5.Cullen R A, Cannon C L, Mark E J, Colin A A. Mycobacterium abscessus infection in cystic fibrosis. Colonization or infection? Am J Respir Crit Care Med. 2000;161:641–645. doi: 10.1164/ajrccm.161.2.9903062. [DOI] [PubMed] [Google Scholar]
- 6.Fauroux B, Delaisi B, Clement A, Saizou C, Moissenet D, Truffot-Pernot C, Tournier G, Thien H V. Mycobacterial lung disease in cystic fibrosis: a prospective study. Pediatr Infect Dis J. 1997;16:354–358. doi: 10.1097/00006454-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Hjelt K, Højlyng N, Howitz P, Illum N, Munk E, Valerius N H, Fursted K, Hansen K N, Heltberg I, Koch C. The role of mycobacteria other than tuberculosis (MOTT) in patients with cystic fibrosis. Scand J Infect Dis. 1994;26:569–576. doi: 10.3109/00365549409011815. [DOI] [PubMed] [Google Scholar]
- 8.Hsueh P R, Teng L J, Yang P C, Chen Y C, Ho S W, Luh K T. Recurrent catheter-related infection caused by a single clone of Mycobacterium chelonae with two colonial morphotypes. J Clin Microbiol. 1998;36:1422–1424. doi: 10.1128/jcm.36.5.1422-1424.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilby J M, Gilligan P H, Yankaskas J R, Highsmith W E, Edwards L J, Knowles M R. Nontuberculous mycobacteria in adult patients with cystic fibrosis. Chest. 1992;102:70–75. doi: 10.1378/chest.102.1.70. [DOI] [PubMed] [Google Scholar]
- 10.Koontz F P, Erwin M E, Barrett M S, Jones R N. E-test for routine clinical antimicrobial susceptibility testing of rapid-growing mycobacteria isolates. Diagn Microbiol Infect Dis. 1994;19:183–186. doi: 10.1016/0732-8893(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 11.Kox L F, Jansen H M, Kuijper S, Kolk A H. Multiplex PCR assay for immediate identification of the infecting species in patients with mycobacterial disease. J Clin Microbiol. 1997;35:1492–1498. doi: 10.1128/jcm.35.6.1492-1498.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metchock B G, Nolte F S, Wallace R J., Jr . Mycobacterium. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. pp. 399–437. [Google Scholar]
- 13.Olivier K N, Yankaskas J R, Knowles M R. Non-tuberculous mycobacterial pulmonary disease in cystic fibrosis. Semin Respir Infect. 1996;11:272–284. [PubMed] [Google Scholar]
- 14.Posteraro B, Sanguinetti M, Garcovich A, Ardito F, Zampetti A, Masucci L, Sbordoni G, Cerimele D, Fadda G. PCR-reverse cross blot hybridization assay as an useful diagnostic tool in a case of Mycobacterium marinum sporotrichoid infection. Br J Dermatol. 1998;139:872–876. doi: 10.1046/j.1365-2133.1998.02516.x. [DOI] [PubMed] [Google Scholar]
- 15.Sanguinetti M, Posteraro B, Ardito F, Zanetti S, Cingolani A, Sechi LA, De Luca A, Ortona L, Fadda G. Routine use of PCR-reverse cross blot hybridization assay for rapid identification of Mycobacterium species growing in liquid media. J Clin Microbiol. 1998;36:1530–1533. doi: 10.1128/jcm.36.6.1530-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silcox V A, Good R C, Floyd M M. Identification of clinically significant Mycobacterium fortuitum complex isolates. J Clin Microbiol. 1981;14:686–691. doi: 10.1128/jcm.14.6.686-691.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka E, Amitani R, Niimi A, Suzuki K, Murayama T, Kuze F. Yield of computed tomography and bronchoscopy for the diagnosis of Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med. 1997;155:2041–2046. doi: 10.1164/ajrccm.155.6.9196113. [DOI] [PubMed] [Google Scholar]
- 18.Telenti A, Marchesi F, Balz M, Bally F, Böttger E C, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thiede S G, Olivier K N, Novotny D B, Molina P L. Mycobacterial bronchocentric granulomatosis in cystic fibrosis: CT-pathological correlation. Am J Respir Crit Care Med. 1996;153:A705. [Google Scholar]
- 20.Torrens J K, Dawkins P, Conway S P, Mora E. Non-tuberculous mycobacteria in cystic fibrosis. Thorax. 1998;53:182–185. doi: 10.1136/thx.53.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witthier S, Olivier K, Gilligan P, Knowles M, Della-Latta P The Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. Proficiency testing of clinical microbiology laboratories using modified decontamination procedures for detection of nontuberculous mycobacteria in sputum samples from cystic fibrosis patients. J Clin Microbiol. 1997;35:2706–2708. doi: 10.1128/jcm.35.10.2706-2708.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]