Abstract
Given the growing number of cancer survivors, it is important to better understand socio-spatial mobility patterns of cancer patients after diagnosis that could have public health implications regarding post-diagnostic access to care for treatment and follow-up surveillance. In this exploratory study, residential histories from LexisNexis were linked to New Jersey colon cancer cases diagnosed from 2006 to 2011 to examine differences in socio-spatial mobility patterns after diagnosis by stage at cancer diagnosis, sex, and race/ethnicity. For the colon cancer cases, we summarized and compared the number of residences and changes in the residential census tract and neighborhood poverty after the diagnosis. We found only minor changes in neighborhood poverty among the cases during the follow-up period after diagnosis. During the follow-up period of up to 10 years after diagnosis, 67% of the patients did not move to a different residential census tract, and 10.8% moved from New Jersey to another state. Cases that moved to a different census tract changed after diagnosis were generally less wealthy than non-movers, but the destination of relocation varied by race/ethnicity and socioeconomic status. We also found a significant association between residential mobility and stage at diagnosis, whereby patients diagnosed with colon cancer at an early stage were more likely to be movers. This study contributes to understanding of the socio-spatial mobility patterns in colon cancer patients and may help to inform cancer research by summarizing the extent to which colon cancer patients move after diagnosis.
Keywords: Socio-spatial mobility, Social mobility, Geographic mobility, New Jersey, Colon cancer, Survival, Residential histories
Highlights
-
•
Post-diagnosis socio-spatial mobility is relatively low among colon cancer patients.
-
•
Post-diagnosis, ∼67% of all colon cancer patients in NJ did not change residence.
-
•
Movers spent more time living in high-poverty neighborhoods than non-movers.
-
•
Approximately 10% of all patients left New Jersey for other states.
-
•
Geographic destinations vary by race/ethnicity and socioeconomic status.
1. Introduction
There is growing evidence of associations between neighborhood social circumstances and health outcomes at the time a disease is diagnosed, including cancer (A. V. DiezRoux, 2001; Ana V. Diez Roux & Mair, 2010; Henry et al., 2014; Wiese et al., 2019; Wiese et al., 2020b). Studies of neighborhood socioeconomic conditions have shown that patients living in neighborhoods with high poverty or economic deprivation had a higher risk of death from cancer than patients living in wealthy neighborhoods (Aarts et al., 2010; Du et al., 2007; Gomez et al., 2007; Henry et al., 2014; Niu et al., 2010; Steinbrecher et al., 2012; Wiese et al., 2019, 2020b; Zhang et al., 2019), independent of known individual-level risk factors such as sex, race/ethnicity, and socioeconomic position.
Recently, more attention has been given to the potential impact of socio-spatial mobility on cancer outcomes through the integration of changes in residence and socioeconomic status (SES) when conducting neighborhood association studies (Namin et al., 2020; Wiese et al., 2020a, 2020b; Zhang et al., 2019). According to Gillespie (Gillespie, 2016), residential mobility affects individuals, families, and neighborhoods, resulting from changing sociodemographic structures of places over time. Residential mobility theories are also in line with biosocial frameworks and cancer theoretical frameworks positing that changes in exposures and behaviors based on where someone lives over the lifespan influence cancer risk and cancer health disparities (Lynch & Rebbeck, 2013; Warnecke et al., 2008; Wiese et al., 2018).
Results from several studies have concluded that residential history, including the number and distance of the moves from neighborhoods of high and/or low SES, play a role in cancer survival (Wiese et al., 2020a, 2020b; Zhang et al., 2019). These studies suggest that residential changes among individuals who live in high poverty areas (Wiese et al., 2020b) or experience high poverty for longer periods (Zhang et al., 2019) after diagnosis may be associated with increased cancer mortality. Additionally, a recent study by Liu et al. (2020) who analyzed a nationally representative sample of 185,637 non-institutionalized civilian adults from 2013 to 2018 using the National Health Interview Survey (which included 9% of cancer patients) concluded that younger age and being female or not having a family was associated with increased residential mobility among cancer survivors and patients. Lower SES, unemployment, and low perceived neighborhood social cohesion were significantly associated with changing residence and short residential tenure in this study, thus providing new insights into potential drivers for residential mobility among cancer patients (Liu et al., 2020).
In fact, relocation prior to cancer diagnosis or shortly afterward is an important psychosocial stressor that influences quality of life and cancer outcomes (Lix et al., 2006; McGrath & Rawson, 2013). Beyond negatively influencing mental and physical health conditions, residential mobility is often correlated with measurements of neighborhood satisfaction (Lin et al., 2012) and has been found to be associated with delayed cancer diagnosis and premature mortality (Muralidhar et al., 2016). Furthermore, residential relocation may cause a delay in access to treatment (Baugh & Verghese, 2013) or a loss of reduction in social networks that promote healthy behaviors (Cornwell & Waite, 2009).
Prior studies have also found that socio-spatial mobility can differ by race/ethnicity, often attributed to historical housing discrimination (Massey et al., 1994), originating from low social class (Crowder & South, 2005) and family background (e.g. parental education status) (Loury, 2005). And, although previous studies on colon cancer survival suggest that change of residency may play an important role in the racial/ethnic disparities in the risk of death (Wiese et al., 2020a, 2020b), to date, only a few studies have explored the impact of socio-spatial mobility within race/ethnic groups in cancer populations (Shvetsov et al., 2020).
This study examines racial/ethnic and sex differences in socio-spatial mobility patterns among New Jersey colon cancer patients diagnosed from 2006 to 2011 over a 10-year period after diagnosis, while also stratifying by stage of cancer at diagnosis. We describe the geographic relocation patterns of the New Jersey colon cancer patients by sex and race/ethnicity as well as census tract poverty (CT-poverty). We also summarize and compare the number of residences and CT-poverty changes during the follow-up period, and compare trajectories of CT-poverty of the cases that remain in the same residential census tract (non-movers) to those cases that change census tract by either moving within New Jersey or out-of-state (movers). The results from this exploratory study will help inform cancer research by summarizing the extent to which colon cancer patients move after diagnosis and by describing the socio-spatial mobility patterns that could have public health implications regarding post-diagnostic access to care for treatment and follow-up surveillance.
2. Study population
The study population included all colon cancer cases 18 years and older diagnosed between January 1, 2006 and December 31, 2011 in New Jersey and followed until December 31, 2016 (N = 10,133). The data were obtained from the New Jersey State Cancer Registry (NJSCR). The data included individual-level factors: sex (male, female), age and stage at the diagnosis (local, regional, distant), race/ethnicity [Non-Hispanic (NH) White, NH Black, NH Asian/Pacific Islander (API), NH Other, and Hispanic (any race)], as well as, the residency at the time of diagnosis geocoded to the 2010 Census Tract (CT), and vital status, including date of death and cause of death (if deceased) or date of last contact (if alive at the end of follow-up). The data for this study were initially collected and processed for a previously published case-control study on geographic clustering of cutaneous T-cell lymphoma in New Jersey (Henry et al., 2021). The study was reviewed and/or approved by Temple and Rutgers University Institutional Review Boards.
All cases were diagnosed with histologically confirmed, first primary colon cancer as defined according to the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3 C180–C189, C260; excluding histology codes 9050–9055, 9140, 9590–9992) (Percy et al., 1990). Cases were followed until their deaths or until December 31, 2016. Deaths attributed to colon cancer were coded based on ICD-10 code C18 (Percy et al., 1990). Early-stage cases were defined as those diagnosed with local or regional (direct extension only, lymph nodes only, direct extension and lymph nodes) SEER summary stages. Cases diagnosed at a distant stage cases were categorized as late-stage.
3. Residential histories
The residential histories of colon cancer cases were obtained through a data linkage with a commercial database, LexisNexis, Inc. (Miamisburg, Ohio, U.S.). LexisNexis residential histories for persons 18 years and older are based on numerous sources, including real estate/tax assessor records, deed transfers and mortgage records; motor vehicle, boat, and aircraft registrations; driver's license records; court filings; voter registrations; and Social Security Administration records (Hurley et al., 2017). LexisNexis returned a maximum of 20 residential addresses for each case.
All residential addresses were geocoded by the NJSCR using the NAACCR Aggie Geocoder (TexasA&M, 2016), and linked to 2010 CTs. To establish the residential history timeline, we followed the methods proposed by Stinchcomb and Roeser (Stinchcomb & Roeser, 2016) by selecting the CT of the most recent location in the timeline for each colon cancer case. This approach removes any overlap of newer and older addresses in the timeline.
The residential timeline of each patient included a sequence of time slices based on the start and end date of each residential CT throughout the follow-up period. The time at each residential CT for each patient was also summarized as a proportion of the total follow-up time of up to 10 years. Cases were categorized by mover status during the follow-up period: “non-movers” (remained in the same CT), and “movers” (changed residential CT). We also created a sub-category for movers, “out-of-state movers,” (moved to another state).
4. Neighborhood socioeconomic status data
Neighborhood socioeconomic status was defined using census tract poverty level (CT-poverty) (Boscoe, 2010; Henry et al., 2009), which has been linked to colon cancer survival and mortality in previous studies (Chien et al., 2013; Henry et al., 2009; Niu et al., 2010; Wiese et al., 2020a, 2020b).
CT-poverty was obtained from publicly available U.S. Census and American Community Survey (ACS) data. For addresses between 2006 and 2010, we used US Census 2010 and the ACS 5-year average data from 2006 to 2010, 2007 to 2011, 2008 to 2012, 2009 to 2013, and 2010 to 2014. ACS 2011–2015, 2012–2016, and 2013–2017 were used for addresses between 2011 and 2016. As suggested by other researchers (Krieger et al., 2002, 2003, 2005), we classified the CT-poverty into four categories: <5% (low poverty), 5%–<10%, 10%–<20%, and ≥20 (high poverty).
To account for residential mobility, we assigned corresponding CT-poverty values to every residential record. Additionally, we incorporated annual values to capture changes in CT-poverty for non-movers because of gentrification. We could not identify cases that moved within CTs because for this specific analysis, we did not have permission to access the residential addresses of the cases.
5. Statistical analysis
We examined the geographic relocation patterns among patients who left New Jersey after their diagnosis. We used the first residence outside New Jersey as the destination and their last known residence in New Jersey as the origin. The origin residential locations were grouped by counties for visualization, while the destinations were grouped by State or geographic region. The visualization of mobility patterns was accomplished using R package circlize (Gu et al., 2014), and mapped using QGIS version 3.10. We also compared the timing and type of residential relocation (move) after the diagnosis between early and late-stage patients by applying Chi-square statistical tests.
We calculated follow-up time as the difference in months between the date of diagnosis and the date of last contact or death. Cases missing follow-up time (i.e., only ascertained through death certificates or autopsy) were excluded from this analysis (n = 1,436). Cases were censored at the date of death if they died from causes other than colon cancer or the date the individual was lost to follow-up before the end of the follow-up period, whichever occurred first. Censoring was applied to generate a study population with similar attributes as it would have been used in cause-specific time-to-event survival analysis. We applied a pairwise Wilcoxon test to compare the average follow-up time and CT-poverty levels by mover status, cancer stage, sex, and race/ethnicity.
We applied logistic regression to examine associations between mover status and patient's individual and neighborhood CT characteristics. The multivariable generalized logistic regression model was used to assess the effect of individual-level variables and CT-poverty at the time of diagnosis on the odds of being mover versus non-mover during the follow-up period. The individual-level variables included in the model were age at diagnosis (continuous), race/ethnicity, sex, cancer stage at diagnosis, and CT-poverty at the time of diagnosis (continuous). Logistic regression was conducted using stats package in R software environment (version 4.1.0).
We also reorganized the follow-up time period into time intervals (months) based on the duration of residency at each CT. Each time interval was associated with a start and end date and the corresponding CT-poverty value and vital status (1-dead, 0-alive). We then calculated the proportion from the total follow-up time at every location and living in every CT-poverty category. The transformation of the dataset was implemented using R packages tidyvers and ggplot2 (Wickham et al., 2016; Wickham & Wickham, 2017).
CT-poverty sequence analysis was applied to estimate the average proportion of time cases were living in census tracts with different CT-poverty levels. A similar methodology was applied in a study by Vable et al. (2020) when analyzing educational trajectories (Vable et al., 2020). In our study, sequences were calculated using the amount of time at every CT and the corresponding CT-poverty category. For example, if an individual's total survival time was 120 months, 40 months of which was a resident in a CT with poverty level >20% (high), the percentage of time living in this CT-poverty category would be 30% (40 months/120 months). To achieve this, individual time intervals were summarized in non-chronological order by different CT-poverty categories (low to high). The results were then aggregated and plotted by mover status, cancer stage, sex, and race/ethnicity. We also estimated the number of individuals living in high CT-poverty areas during the entire follow-up period. All calculations and visualizations were completed using the R packages tidyvers and ggplot2 (Wickham et al., 2016; Wickham & Wickham, 2017). To compare the average time of residency in areas with highest CT-poverty between movers and non-movers, we used a pairwise t-test in R software environment (version 4.1.0).
Lastly, we developed spaghetti plots of CT-poverty trajectories with average values and confidence intervals based on a time-interval dataset of start and stop survival times and corresponding continuous CT-poverty values up to 120 months post-diagnosis. The spaghetti plots were generated by mover status, sex, cancer stage (early/late), and race/ethnicity. For spaghetti plots, the underlying model regressed CT-poverty on follow-up time. We used the pairwise Chow test (Chow, 1960) to estimate whether the regression coefficients for intercepts and slopes (trajectories) differed significantly between movers and non-movers within each race/ethnic group by cancer stage and sex.
All statistical tests were 2-sided with a significance level of p = .05, conducted using R software environment (version 4.1.0).
6. Results
6.1. Study population
The study population included 10,133 New Jersey residents diagnosed with colon cancer between 2006 and 2011: 48.6% male, 72.6% NHW, 12.9% NHB, 7.8% Hispanic, and 3.5% API. Although most cases were diagnosed at an early stage (37.5% local, 38.9% regional) with an average follow-up time of 60.6 months, about a third (32.3%) died from colon cancer by December 31, 2016. At the time of diagnosis, the average CT-poverty level was 8.17%, and 45.6% lived in the least impoverished neighborhoods (<5% CT-poverty level), and 8.6% lived in the most impoverished neighborhoods (>20% CT-poverty level). Over two-thirds (67.3%) remained in the same residential CT throughout the follow-up period (non-movers), while 21.9% and 10.8% changed residential CTs within New Jersey or out-of-state, respectively (Table 1).
Table 1.
Study population characteristics (N = 10,133).
| Overall n (%) | |
|---|---|
| Age (years) | |
| Mean (SD) | 65.7 (13.1) |
| Median [Min, Max] | 67.0 [20.0, 85.0] |
| Sex | |
| Male | 4,921 (48.6%) |
| Female | 5,213 (51.4%) |
| Race/Ethnicity | |
| NH-White | 7,357 (72.6%) |
| NH-Black | 1,308 (12.9%) |
| Hispanic | 790 (7.8%) |
| Asian/Pacific Islanders | 359 (3.5%) |
| Other | 320 (3.2%) |
| Stage at diagnosis | |
| Local | 3,801 (37.5%) |
| Regional, direct extension only | 1,339 (13.2%) |
| Regional, lymph nodes only | 1,269 (12.5%) |
| Regional, both | 1,342 (13.2%) |
| Distant | 2,383 (23.5%) |
| Vital status | |
| Censored | 6,857 (67.7%) |
| Colon cancer death | 3,277 (32.3%) |
| Follow-up time (months) | |
| Mean (SD) | 60.6 (39.6) |
| Median [Min, Max] | 65.0 [1.00, 140] |
| Mover status | |
| Non-Mover | 6,824 (67.3%) |
| In-State Mover | 2,218 (21.9%) |
| Out-State Mover | 1,092 (10.8%) |
| Census tractpoverty at diagnosis | |
| <5% (low) | 4,618 (45.6%) |
| 5% - <10% | 2,843 (28.1%) |
| 10% - <20% | 1,804 (17.8%) |
| ≥20% (high) | 869 (8.6%) |
| Census tractpoverty at diagnosis | |
| Mean % (SD) | 8.17 (8.20) |
| Median [Min, Max] | 5.53 [0, 70.4] |
6.2. Geographic mobility patterns
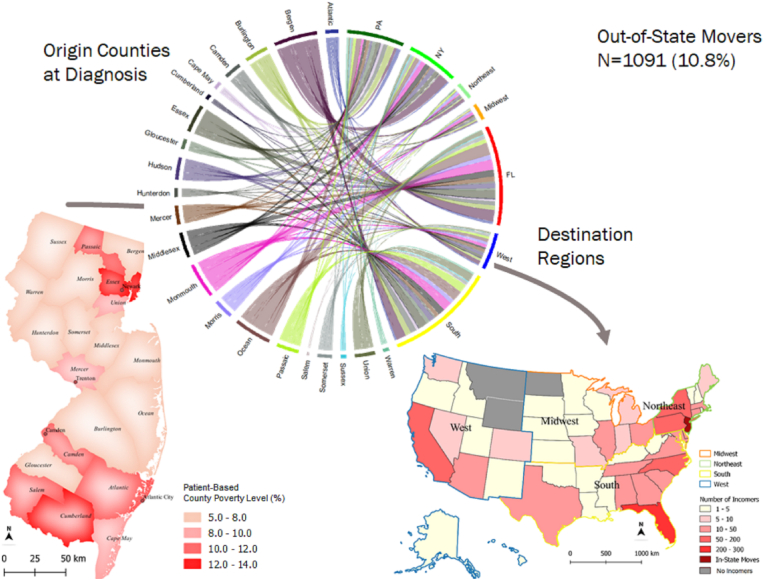
Among the movers, 2,218 (21.9%) changed residential CT within state boundaries, while 1,091 (10.8%) left the state sometime after diagnosis during the follow-up period. There were a total of 3,309 CT changes within the state, 279 moves to Florida (FL), 160 moves to Pennsylvania (PA), and 144 moves to New York (NY). There were also moves to the South and Northeast regions (Fig. 1).
Fig. 1.
Geographic mobility. Migration flows between the original counties (from the time of diagnosis) and destination States. New Jersey map shows origin counties by the average CT-poverty level at the time at diagnosis. U.S. map shows the migratory distribution by destination.
We detected a significant association (p<0.001) between staging and the timing of the move/relocation. There were significantly more late-stage colon cancer cases that were non-movers (79.7%) than cases diagnosed at an early stage (63.5%). Among late-stage cases who changed their residency, there were significantly fewer that moved after the first year (9.4%) in comparison to early-stage cases (24.6%) (Table 2).
Table 2.
Timing and type of residential relocation (move) after the diagnosis.
| Timing of residential relocationa | Early Stage |
Late Stage |
|---|---|---|
| n (%) | n (%) | |
| No moves | 4925 (63.54) | 1899 (79.72) |
| Moved within first year after diagnosis | 921 (11.88) | 259 (10.87) |
| Moved after 2–5 years post diagnosis | 1354 (17.47) | 200 (8.4) |
| Moved after year 5 after diagnosis | 551 (7.11) | 24 (1.01) |
| Total | 7751 | 2382 |
Chi-square p-value <0.001.
6.3. Socio-spatial mobility patterns
Results from the logistic regression analysis show that patients diagnosed at an older age (OR 0.41 95% CI 0.37–0.46) or with late-stage colon cancer (OR 0.98 95% CI 0.97–0.98) had a lower odds of changing census tracts (e.g., move to a different CT) during the follow-up period. Hispanic ethnicity compared to NH-Whites, and increasing CT-poverty were associated with higher odds of moving to a different CT (Hispanic OR 1.28 95% CI 1.09–1.5, CT-poverty OR 1.02 95% CI 1.01–1.02) (Table 3).
Table 3.
Logistic regression results. Odds of changing census tracts after diagnosis.
| Variable | Odds Ratio (95% Confidence Interval) | p-Value |
|---|---|---|
| Male | REF | |
| Female | 0.98 (0.9–1.07) | 0.64 |
| Non-Hispanic White | REF | |
| Non-Hispanic Black | 1.11 (0.96–1.27) | 0.15 |
| Hispanic | 1.28 (1.09–1.5) | 0.003 |
| Asian/Pacific Islander | 1.19 (0.95–1.49) | 0.13 |
| Others | 1.12 (0.88–1.43) | 0.34 |
| Early stage at diagnosis | REF | |
| Late stage at diagnosis | 0.41 (0.37–0.46) | <0.001 |
| Age at diagnosis* | 0.98 (0.97–0.98) | <0.001 |
| CT-poverty at diagnosis* | 1.02 (1.01–1.02) | <0.001 |
Note: *Odds Ratio estimate is per unit increase in age (for 1 year) and CT-poverty (for 1%).
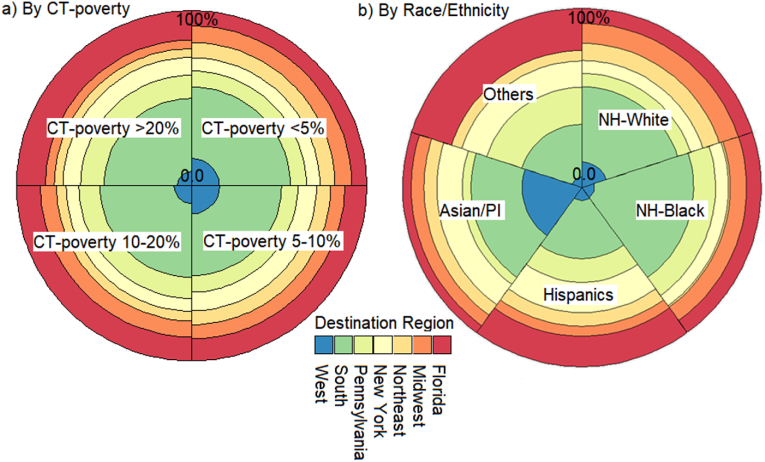
Fig. 2 shows the mobility of colon cancer cases by CT-poverty (a) and race/ethnicity (b). Patients from the wealthy areas moved more to the West, whereas those from the most deprived areas relocated to PA and NY (Fig. 2a). NH-Whites made up a majority (n = 747) of patients that moved out-of-state, followed by NH-Blacks (n = 156), Hispanics (n = 93), API (n = 51), and Others (n = 45). Florida was the top destination for NH-Whites and Hispanics. For NH-Blacks PA was the primary place for relocation, followed by several Southern states, including North Carolina, Georgia, and Florida. California was the top destination for API, followed by NY (Fig. 2b).
Fig. 2.
Relocation preference for cases who left New Jersey after the diagnosis based on the CT-Poverty at the diagnosis (a) and Race/Ethnicity (b).
6.4. Sequencing analysis
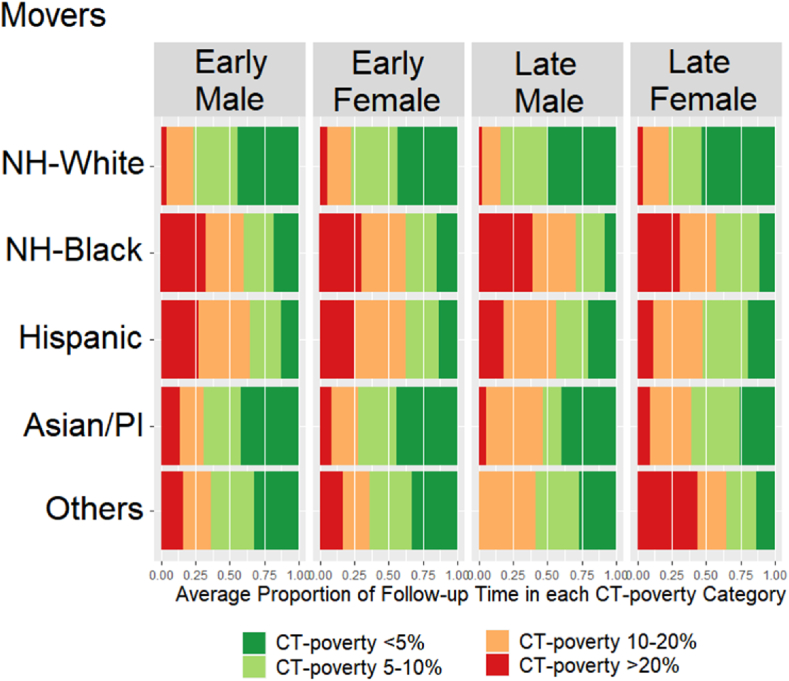
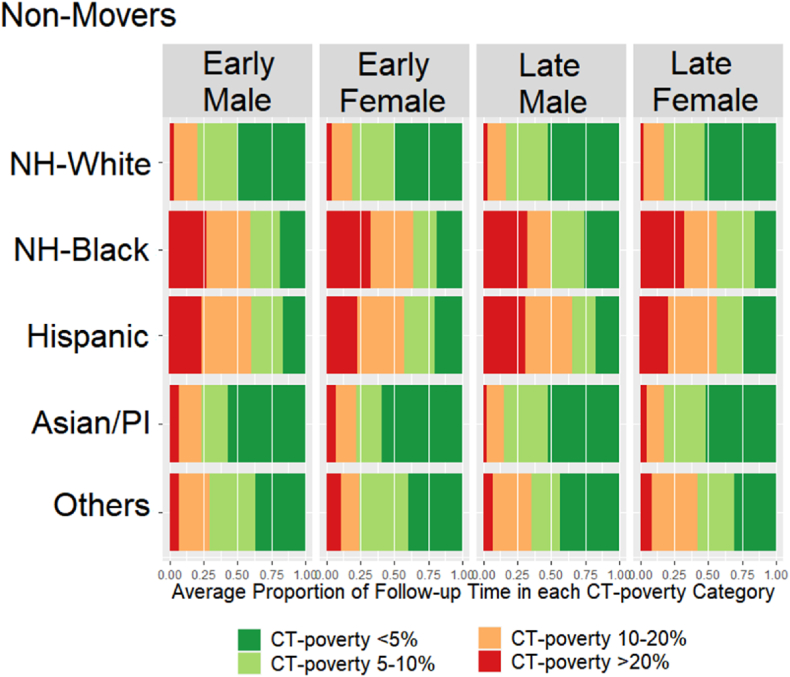
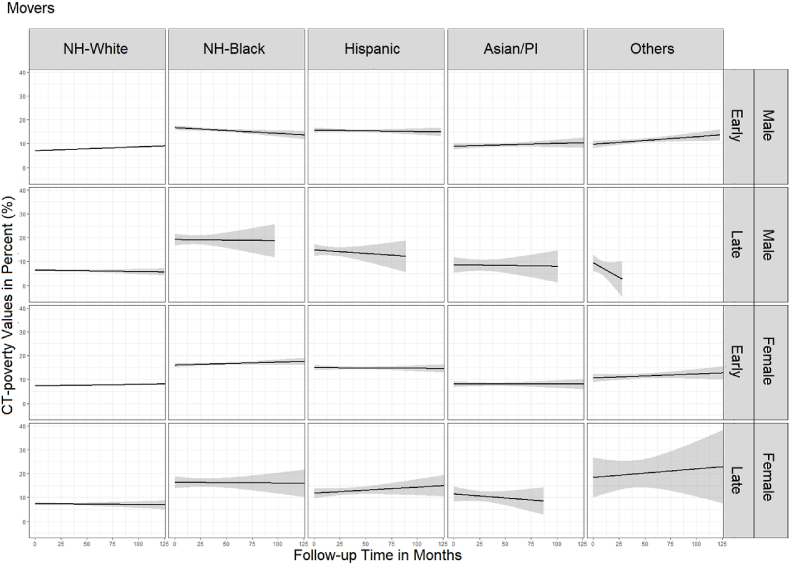
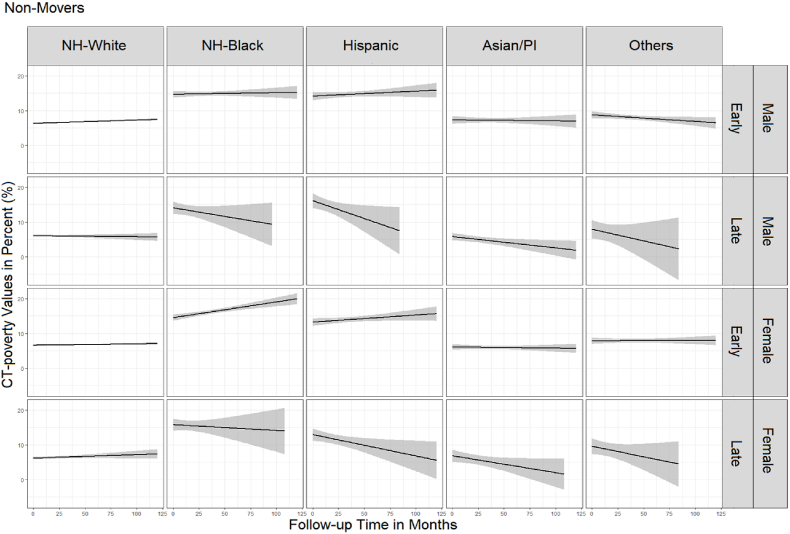
Fig. 3 (movers) and Fig. 4 (non-movers) show sequencing results. Movers spent on average slightly more time living in high poverty neighborhoods (11.3%) than non-movers (8.5%) (t-test p-value<0.01). NH-Whites lived the shortest proportion of time in high poverty neighborhoods regardless of stage, sex, and mover status. However, the average proportion of time living in high poverty neighborhoods among the NH-Whites was 1–2% higher for movers (3.5% for males, 5% for females) compared to non-movers (2% for males, 3% for females) (t-test p-value = 0.03). Female NH-Blacks lived on average the largest proportion of time in high poverty neighborhoods (30%), followed by NH-Black males (28%), regardless of the stage and mover status. Male and female Hispanics lived in high poverty neighborhoods on average approximately 23–25% of time (non-movers and movers). In contrast to male and female API non-movers who lived in poverty areas the least amount of time (5–6%), API male and female movers lived in high poverty neighborhoods on average 12% and 10% of the time, respectively. Among the Other races, the average proportion of time living in poverty varied by the stage at diagnosis, with patients diagnosed at late stage living substantially longer in high poverty compared to the early-stage patients. Additionally, there was a difference of almost 10 percentage points by mover status with non-movers averaging 6% for males and 9% for females and movers averaging 13.5% among males and 20% among females (t-test p-value = 0.03).
Fig. 3.
Average proportion of Time living in different CT-poverty categories during the follow-up period among Movers.
Fig. 4.
Average Proportion of Time living in different CT-poverty categories during the follow-up period among Non-Movers.
6.5. Trajectories analysis
Spaghetti plots in Fig. 5 (movers) and Fig. 6 (non-movers) show differences in the duration of follow-up time and CT-poverty trajectories by cancer stage, sex, and race/ethnicity. Generally, the average CT-poverty values for several groups (i.e., Other race, late-stage APIs) vary by ± 5 percentage points because of small sample sizes.
Fig. 5.
Average trajectory of census tract poverty during the follow-up period among movers.
Fig. 6.
Average trajectory and 95% confidence intervals of census tract poverty during the follow-up period among non-movers.
Unlike non-movers, movers generally experienced stagnation or a decrease in CT-poverty over the follow-up period (Fig. 5). However, movers had slightly higher average CT-poverty in comparison to non-movers with significant variation in early-stage NH-White males (6.7% vs 7.7, p<0.01), API males (7.2% vs 9.4%, p<0.01), NH-White females (6.8% vs 7.6%, p<0.01), API females (6.0% vs 8.0%, p+0.002), and females of Other races (7.9% vs 11.3%, p = 0.001) (Supplemental Table 1). We also found that, unlike their counterparts that did not move after their early-stage colon cancer diagnosis, CT-poverty decreased for NH-Black male movers (16.7%–13.1%) and increased for API male movers (8.9%–10.3%) (Fig. 5). NH-White colon cancer cases had stable, consistent CT-poverty trajectories ≤10% regardless of mover status, sex, and colon cancer stage at diagnosis. This was also true for API males and females diagnosed with colon cancer at an early stage (Fig. 5, Fig. 6).
Male and female NH-Black and Hispanic colon cancer patients diagnosed at early stage began and remained in neighborhoods above 10% CT-poverty throughout the follow-up period (Fig. 6). This was also true for NH-Black females diagnosed with late-stage colon cancer. Although there is large variability in CT-poverty toward the end of the follow-up period for racial/ethnic minorities in our study population. Trajectories suggest that NH-Black and Hispanic non-movers with early-stage colon cancer experience increasing CT-poverty while those diagnosed at late stage experienced decreasing CT-poverty. For example, for NH-Black male non-movers diagnosed at early stage, CT-poverty increased from 14.7% to 15.3% while for those diagnosed at late stage it decreased from 14.1% to 9.7%. For Hispanic females, those diagnosed at early stage, CT-poverty increased from 13.2% to 15.6%, while for those diagnosed at late stage, CT-poverty decreased from 12.9% to 5.6%. Trajectories were similar for male and female Hispanic non-movers diagnosed at late stage, starting at CT-poverty levels above 10% and declining to <10% within 5–10 years (Fig. 6).
Noteworthy is how NH-Black women, regardless of cancer stage and mover status, not only live in the highest CT-poverty areas at the time of their colon cancer diagnosis (14.5%–16.3% CT-average poverty) but their trajectories show increasing CT-poverty levels over time, some nearing 20% (e.g., NH-Black females diagnosed at early stages).
7. Discussion
The present study is among the first to incorporate residential histories with statewide cancer registry data to investigate the geographic and socio-spatial mobility among colon cancer cases after diagnosis. We found that as many as two-thirds of all colon cancer cases in New Jersey remained in the same CT, and out-of-state movers that make up approximately 11% of the patient pool appear to have a higher socioeconomic status then in-state/local movers. These results show active geographic residential mobility among colon cancer cases, which is also consistent with the U.S. population. A recent analysis of micro-census data on household mobility in the U.S. indicated that in comparison to other nations, U.S.-Americans are more flexible in changing place of residency and also over longer distances (Gillespie, 2016). Several factors may influence relocation: family, education, occupation, language, ancestry, ethnicity, and policy. Additionally, the selection of relocation destination and distance may be age-related as older populations are becoming more mobile with retirement (Litwak & Longino, 1987). Often, climate and neighborhood environment are influential factors. Our study shows that among the out-of-state movers, the primary destination were the Southern states, suggesting mild winter conditions may play a role in selection process. Additionally, Florida and other southern states are known for several retirement communities (Haas & Serow, 2002), which can offer a recreational environment (Glass & Skinner, 2013). However, we are not able to exclude that residential relocation to southern states is due to secondary (seasonal) residency. According to the National Association of Home Builders (NAHB), approximately 6% of the nationwide housing stock is secondary housing. Florida and some other coastal states are leading the list (http://eyeonhousing.org/2018/12/nations-stock-of-second-homes/). Thus, the residential relocation over longer distances (Gillespie, 2016) or the ownership of a seasonal property is associated with high SES status, and therefore, aligns with our findings that out-of-state movers are generally wealthier than non-movers or within-state movers.
Another important finding is the strong association between mover status and the stage of colon cancer at diagnosis. This suggests that patients diagnosed at an earlier stage (local/regional) would more likely become movers than patients diagnosed at a later stage (distant). This is likely due to greater opportunities to move due to longer survival times and successful completion of surgeries and other first course treatment after the first year of diagnosis. In contrast, there were substantially more non-movers among late-stage patients. This finding suggests that stage of disease is an important factor in understanding residential mobility after cancer diagnosis. More research is needed to better understand how stage of disease impacts geographic mobility among cancer patients.
7.1. Socio-spatial mobility
Evaluating socio-spatial residential mobility patterns often assumes that active changes in residential location (i.e., moves) result in concordant social or economic conditions such as CT-poverty. Consistent with the U.S. population (Thernstrom, 1968), we found that over 75% of New Jersey's colon cancer cases can be characterized as having low socio-spatial mobility (less than 10 percentage point changes in CT-poverty over time); but we also found strong evidence of changes in neighborhood CT-poverty among non-movers, suggesting ongoing deterioration or gentrification of the CTs during a relatively short time period (up to 10 years). How deterioration or gentrification of CTs impacts colon cancer survival remains to be investigated but is likely to significantly impact accessibility to cancer care and surveillance screening services for colon cancer patients.
Additionally, we found that on average non-movers lived a longer proportion of time in wealthier CTs than movers. This finding aligns with earlier research on household mobility in the U.S. using population-based survey data (Census and IPUMS), which concluded that wealthier populations appear to be less mobile than poor or less-affluent populations (Gillespie, 2016). This could be related to housing ownership and more stable employment.
Similar to earlier studies that examined social mobility in U.S. population, we found substantial differences in CT-poverty trajectories across race/ethnic groups. A larger proportion of NH-Blacks and Hispanics lived in high-poverty neighborhoods for longer periods than API and NH-Whites. Additionally, NH-Black women were the only group that experienced downward socio-spatial mobility regardless of whether they moved. These findings are consistent with prior literature (McLanahan & Kelly, 2006) and are mostly related to the limited socio-spatial mobility of Black populations given the growing economic inequality between Whites and Blacks in the U.S. and the socioeconomic segregation in neighborhoods, schools, workplaces, and social networks (Mijs & Roe, 2021). A study by Crowder and South (2005) also shows that Whites in comparison to Blacks are more likely to move into poor neighborhoods even when initially living in non-poor areas. Moreover, the proportion of single female-headed households is higher among Blacks than Whites, resulting in financial burden and fewer opportunities for upward social mobility (Crowder & South, 2005). Another study on residential socio-spatial mobility among race/ethnic groups shows that Latino population (especially Mexicans and Puerto Ricans) have the lowest odds for leaving high-poverty neighborhoods in comparison to Whites and Blacks (South et al., 2005), which could be related to education and employment or preference to live in areas with similar demographics of their own race/ethnicity (Farley et al., 1994).
8. Limitations
There are a few limitations in our study. The study population was limited to New Jersey residents and may not reflect the socio-spatial mobility patterns of colon cancer patients in other states. Additionally, we only investigated mobility and trajectories up to 10-years post-diagnosis. Results may differ if we followed cases longer or included data from pre-diagnosis residential histories. Future studies should evaluate pre-diagnosis socio-spatial mobility to assess the importance of residential location prior to colon cancer diagnosis. If incorporating pre-diagnosis time, however, there is also an increasing number of cases with a shorter residential history timeline as the years prior to the diagnosis increase. Since we took a cross-sectional approach to describing socio-spatial mobility patterns, our study was also limited by the unequal length of the follow-up time among the cases, which may have biased the estimates of the proportion of total follow-up time at each residential location. Post hoc analysis showed that unequal follow-up time was primarily a result of death among colon cancer patients with late-stage disease in the two years immediately following diagnosis. Stratifying by individual-level variables that are associated with the length of the follow-up including stage at diagnosis, mover status, sex, race/ethnicity, likely reduced some of this bias. Future studies should consider conducting multivariable analyses using longitudinal residential history data to fully account for the bias resulting from the unequal length of the follow-up time.
We were also unable to assess the race/ethnic mobility patterns in the general population and, therefore, unable to ascertain if the patterns found in our study population was a departure from what would be expected in a non-cancer population. Our comparisons to the social and geographic mobility in the general U.S. populations are solely discussed based on earlier findings using census data.
A major limitation of using commercially collected residential histories is related to secondary and vocational housing records. This is essential for estimating the number of out-of-state movers. In this study, we assumed that the relocation to another state took place only if the duration of residence in the non-New Jersey CT was longer than the duration at the New Jersey CT. When two in-State and out-of-state CTs were recorded simultaneously, we gave preference to the in-state CT. This could result in an underestimation of out-of-state movers. Another limitation was that we did not have access to self-reported information to validate the residential data. However, earlier studies found good concordance (82–92%) between LexisNexis addresses and addresses collected from study participants (Hurley et al., 2017; Jacquez et al., 2011). In our study, we could only validate the CTs at time of diagnosis reported by the NJSCR and LexisNexis. The data concordance was approximately 83% and increased to 93% when comparisons were limited to a 6-month window before and after the diagnosis date. We expect the unverified addresses to result in non-differential misclassification (equal misclassification of exposure among cases) in either direction but have a minimal impact on results.
The socio-spatial mobility was measured based on CT information. A change in geographic unit (census tract to census block group) may result in different conclusions (i.e., modifiable areal unit problem) (Buzzelli, 2020; Pawitan & Steel, 2009; Sahar et al., 2019). Future studies should consider repeating our analysis using the residential street addresses to measure within-CT moves. Additionally, inclusion of fine-grained mobile phone metadata (Bakker et al., 2019) may help to better understand the geographic and socio-economic mobility and integration based on daily mobility/movements. Results are based on neighborhood SES only. Examining socio-spatial mobility using individual-level SES could result in different patterns of associations.
Small sample sizes in race/ethnic minority groups resulted in a relatively large variation in the average CT-poverty values when estimating trajectories, which may vary by ± 5 percentage points. Small sample size also limited our ability to apply more stratifications by stage at diagnosis and vital status. A study with access to a larger sample size may result in different patterns in CT-poverty trajectories.
Finally, it is unknown whether other neighborhood socio-demographic (e.g., ethnic enclaves) or environmental (e.g., changes in the built environment, green spaces) factors would also vary between the time-varying and constant models and whether differences in race/ethnicity and sex would also affect associations in those models. Thus, future studies that continue to evaluate residential history and socio-spatial mobility, measured in terms of other socioeconomic indicators beyond poverty, are also warranted.
9. Conclusion
Our research contributes to understanding the socio-spatial mobility patterns among persons diagnosed with colon cancer and the differences by race/ethnicity and sex. Overall, the socio-spatial mobility patterns of New Jersey colon cancer cases are similar to those observed in the general U.S. population from earlier research when using census data. While the overall change in CT-poverty over a 5-10-year follow-up period was minor, differences in CT-poverty trajectories by cancer stage and move status were significant. Considering that residential mobility is arguably a stressful event regardless of the individual characteristics of the household members, and the reason for relocation (Gillespie, 2016), the incorporation of residential histories and adjustment for mover status is recommended for future research in cancer outcomes. Observing the difference in socio-spatial mobility by race/ethnicity suggest that analyzing the relationship between neighborhood effects at one time point and over time is complex and warrant more detailed investigations. Considering the growing number of cancer survivors, it is essential to understand the role of socio-spatial mobility in long-term follow-up studies.
Author statement
Daniel Wiese: Conceptualization, Methodology, Analysis, Writing- original draft preparation.
Shannon M. Lynch: Conceptualization, Methodology, Writing- Reviewing and Editing.
Antoinette M. Stroup: Supervision, Validation, Writing- Reviewing and Editing.
Aniruddha Maiti: Software, Data preparation, Writing- Reviewing and Editing.
Gerald Harris: Data curation, Writing- Reviewing.
Slobodan Vucetic: Supervision, Writing- Reviewing and Editing.
Kevin A. Henry: Conceptualization, Supervision, Validation, Writing- Reviewing and Editing.
Funding
This research was funded by the National Science Foundation (1560888). NJSCR data were collected using funding from NCI and the Surveillance, Epidemiology, and End Results (SEER) Program (HHSN261201300021I), and the CDC’s National Program of Cancer Registries (NPCR) (5U58DP003931-02/NU58DP006279-02-00), as well as the State of New Jersey and the Rutgers Cancer Institute of New Jersey. Publication of this article was funded in part by the Temple University Libraries Open Access Publishing Fund.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2022.101023.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aarts M.J., Lemmens V.E.P.P., Louwman M.W.J., Kunst A.E., Coebergh J.W.W. Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. European Journal of Cancer. 2010;46:2681–2695. doi: 10.1016/j.ejca.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Bakker M.A., Piracha D.A., Lu P.J., Bejgo K., Bahrami M., Leng Y., et al. Measuring fine-grained multidimensional integration using mobile phone metadata: The case of Syrian refugees in Turkey. Guide to Mobile Data Analytics in Refugee Scenarios. 2019:123–140. Springer. [Google Scholar]
- Baugh D.K., Verghese S. Vol. 3. Medicare Medicaid Res Rev; 2013. (Migration patterns for Medicaid enrollees 2005-2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscoe F.P. Towards the use of a census tract poverty indicator variable in cancer surveillance. Journal Registry Management. 2010;37:148–151. [PubMed] [Google Scholar]
- Buzzelli M. Modifiable areal unit problem. International Encyclopedia of Human Geography. 2020:169–173. [Google Scholar]
- Chien L.C., Schootman M., Pruitt S.L. The modifying effect of patient location on stage-specific survival following colorectal cancer using geosurvival models. Cancer Causes & Control. 2013;24:473–484. doi: 10.1007/s10552-012-0134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow G.C. Tests of equality between sets of coefficients in two linear regressions. Econometrica. 1960;28:591–605. [Google Scholar]
- Cornwell E.Y., Waite L.J. Social disconnectedness, perceived isolation, and health among older adults. Journal of Health and Social Behavior. 2009;50:31–48. doi: 10.1177/002214650905000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder K., South S.J. Race, class, and changing patterns of migration between poor and nonpoor neighborhoods. American Journal of Sociology. 2005;110:1715–1763. [Google Scholar]
- Diez Roux A.V. Investigating neighborhood and area effects on health. American Journal of Public Health. 2001;91:1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux A.V., Mair C. Neighborhoods and health. Annals of the New York Academy of Sciences. 2010;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- Du X.L., Fang S., Vernon S.W., El-Serag H., Shih Y.T., Davila J., et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- Farley R., Steeh C., Krysan M., Jackson T., Reeves K. Stereotypes and segregation: Neighborhoods in the detroit area. American Journal of Sociology. 1994;100:750–780. [Google Scholar]
- Gillespie B.J. Springer; 2016. Household mobility in America: Patterns, processes, and outcomes. [Google Scholar]
- Glass A.P., Skinner J.… Retirement communities: We know what they are or do we? Journal of Housing for the Elderly. 2013;27:61–88. [Google Scholar]
- Gomez S.L., O'Malley C.D., Stroup A., Shema S.J., Satariano W.A. Longitudinal, population-based study of racial/ethnic differences in colorectal cancer survival: Impact of neighborhood socioeconomic status, treatment and comorbidity. BMC Cancer. 2007;7:193. doi: 10.1186/1471-2407-7-193. 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Gu L., Eils R., Schlesner M., Brors B. Circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- Haas W.H., Serow W.J. The baby boom, amenity retirement migration, and retirement communities: Will the golden age of retirement continue? Research on Aging. 2002;24:150–164. [Google Scholar]
- Henry K.A., Niu X., Boscoe F.P. Geographic disparities in colorectal cancer survival. International Journal of Health Geographics. 2009;8:48. doi: 10.1186/1476-072X-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry K.A., Sherman R.L., McDonald K., Johnson C.J., Lin G., Stroup A.M., et al. Associations of census-tract poverty with subsite-specific colorectal cancer incidence rates and stage of disease at diagnosis in the United States. Journal Cancer Epidemiol. 2014;2014:823484. doi: 10.1155/2014/823484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry K.A., Wiese D., Maiti A., Harris G., Vucetic S., Stroup A.M. Geographic clustering of cutaneous T-cell lymphoma in New Jersey: An exploratory analysis using residential histories. Cancer Causes & Control. 2021;32:989–999. doi: 10.1007/s10552-021-01452-y. [DOI] [PubMed] [Google Scholar]
- Hurley S., Hertz A., Nelson D.O., Layefsky M., Von Behren J., Bernstein L., et al. Tracing a path to the past: Exploring the use of commercial credit reporting data to construct residential histories for epidemiologic studies of environmental exposures. American Journal of Epidemiology. 2017;185:238–246. doi: 10.1093/aje/kww108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquez G.M., Slotnick M.J., Meliker J.R., AvRuskin G., Copeland G., Nriagu J. Accuracy of commercially available residential histories for epidemiologic studies. American Journal of Epidemiology. 2011;173:236–243. doi: 10.1093/aje/kwq350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N., Chen J.T., Waterman P.D., Rehkopf D.H., Subramanian S.V. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: The public health disparities geocoding project. American Journal of Public Health. 2005;95:312–323. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N., Chen J.T., Waterman P.D., Soobader M.-J., Subramanian S.V., Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: Does the choice of area-based measure and geographic level matter?: The public health disparities geocoding project. American Journal of Epidemiology. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- Krieger N., Chen J.T., Waterman P.D., Soobader M.-J., Subramanian S., Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) Journal of Epidemiology & Community Health. 2003;57:186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K.C., Huang H.C., Bai Y.M., Kuo P.C. Lifetime residential mobility history and self-rated health at midlife. Journal of Epidemiology. 2012;22:113–122. doi: 10.2188/jea.JE20110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwak E., Longino C.F., Jr. Migration patterns among the elderly: A developmental perspective. The Gerontologist. 1987;27:266–272. doi: 10.1093/geront/27.3.266. [DOI] [PubMed] [Google Scholar]
- Liu B., Lee F.F., Boscoe F. Residential mobility among adult cancer survivors in the United States. BMC Public Health. 2020;20:1601. doi: 10.1186/s12889-020-09686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lix L.M., Hinds A., DeVerteuil G., Robinson J.R., Walker J., Roos L.L. Residential mobility and severe mental illness: A population-based analysis. Administration and Policy in Mental Health and Mental Health Services Research. 2006;33:160. doi: 10.1007/s10488-006-0035-5. [DOI] [PubMed] [Google Scholar]
- Loury G. In: Ethnicity, social mobility and public policy: Comparing the US and UK. Glenn T.M., Loury C., Teles S.M., editors. Cambridge University Press; Cambridge: 2005. Intergenerational mobility and racial inequality in education and earnings; pp. 160–177. [Google Scholar]
- Lynch S.M., Rebbeck T.R. Bridging the gap between biologic, individual, and macroenvironmental factors in cancer: A multilevel approach. Cancer Epidemiology Biomarkers & Prevention. 2013;22:485–495. doi: 10.1158/1055-9965.EPI-13-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey D.S., Gross A.B., Shibuya K. Migration, segregation, and the geographic concentration of poverty. American Sociological Review. 1994:425–445. [Google Scholar]
- McGrath P., Rawson N. The experience of relocation for specialist treatment for Indigenous women diagnosed with vulvar cancer in East Arnhem Land. Journal of Psychosocial Oncology. 2013;31:540–555. doi: 10.1080/07347332.2013.822051. [DOI] [PubMed] [Google Scholar]
- McLanahan S.S., Kelly E.L. The feminization of poverty. Handbook of the Sociology of Gender. 2006:127–145. Springer. [Google Scholar]
- Mijs J.J.B., Roe E.L. Is America coming apart? Socioeconomic segregation in neighborhoods, schools, workplaces, and social networks, 1970–2020. Sociology Compass. 2021;15 [Google Scholar]
- Muralidhar V., Nguyen P.L., Tucker-Seeley R.D. Recent relocation and decreased survival following a cancer diagnosis. Preventive Medicine. 2016;89:245–250. doi: 10.1016/j.ypmed.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namin S., Zhou Y., Neuner J., Beyer K. The role of residential history in cancer research: A scoping review. Social Science & Medicine. 2020:113657. doi: 10.1016/j.socscimed.2020.113657. [DOI] [PubMed] [Google Scholar]
- Niu X., Pawlish K.S., Roche L.M. Cancer survival disparities by race/ethnicity and socioeconomic status in New Jersey. Journal of Health Care for the Poor and Underserved. 2010;21:144–160. doi: 10.1353/hpu.0.0263. [DOI] [PubMed] [Google Scholar]
- Pawitan G., Steel D. 2009. Exploring the MAUP from a spatial perspective. [Google Scholar]
- Percy C., Holten V.v., Muir C.S., Organization W.H. 1990. International classification of diseases for oncology. Geneva. [Google Scholar]
- Sahar L., Foster S.L., Sherman R.L., Henry K.A., Goldberg D.W., Stinchcomb D.G., et al. GIScience and cancer: State of the art and trends for cancer surveillance and epidemiology. Cancer. 2019;125:2544–2560. doi: 10.1002/cncr.32052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvetsov Y.B., Shariff-Marco S., Yang J., Conroy S.M., Canchola A.J., Albright C.L., et al. Association of change in the neighborhood obesogenic environment with colorectal cancer risk: The Multiethnic Cohort Study. SSM - Population Health. 2020;10:100532. doi: 10.1016/j.ssmph.2019.100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South S.J., Crowder K., Chavez E. Exiting and entering high-poverty neighborhoods: Latinos, Blacks and Anglos compared. Social Forces. 2005;84:873–900. [Google Scholar]
- Steinbrecher A., Fish K., Clarke C.A., West D.W., Gomez S.L., Cheng I. Examining the association between socioeconomic status and invasive colorectal cancer incidence and mortality in California. Cancer Epidemiology and Prevention Biomarkers. 2012;21:1814–1822. doi: 10.1158/1055-9965.EPI-12-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb D., Roeser A. Westat, Inc; Rockville, MD: 2016. NCI/SEER residential history project technical report. [Google Scholar]
- Texas A&M N.a.N. NAACCR; 2016. NAACCR geocoder data dictionary. [Google Scholar]
- Thernstrom S. Notes on the historical study of social mobility. Comparative Studies in Society and History. 1968;10:162–172. [Google Scholar]
- Vable A.M., Duarte C.d., Cohen A.K., Glymour M.M., Ream R.K., Yen I.H. Does the type and timing of educational attainment influence physical health? A novel application of sequence analysis. American Journal of Epidemiology. 2020;189:1389–1401. doi: 10.1093/aje/kwaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke R.B., Oh A., Breen N., Gehlert S., Paskett E., Tucker K.L., et al. Approaching health disparities from a population perspective: The national institutes of health centers for population health and health disparities. American Journal of Public Health. 2008;98:1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H., Chang W., Wickham M.H. Vol. 2. Version; 2016. pp. 1–189. (Package ‘ggplot2’. Create elegant data visualisations Using the Grammar of graphics). [Google Scholar]
- Wickham H., Wickham M.H. 2017. Package tidyverse. Easily Install and Load the ‘tidyverse. [Google Scholar]
- Wiese D., Rodriguez Escobar J., Hsu Y., Kulathinal R.J., Hayes-Conroy A. The fluidity of biosocial identity and the effects of place, space, and time. Social Science & Medicine. 2018;198:46–52. doi: 10.1016/j.socscimed.2017.12.023. [DOI] [PubMed] [Google Scholar]
- Wiese D., Stroup A.M., Crosbie A., Lynch S.M., Henry K.A. Cancer Epidemiology Biomarkers & Prevention; 2019. The impact of neighborhood economic and racial inequalities on the spatial variation of breast cancer survival in New Jersey. [DOI] [PubMed] [Google Scholar]
- Wiese D., Stroup A.M., Maiti A., Harris G., Lynch S.M., Vucetic S., et al. Residential mobility and geospatial disparities in colon cancer survival. Cancer Epidemiology, Biomarkers & Prevention. 2020;29:2119–2125. doi: 10.1158/1055-9965.EPI-20-0772. [DOI] [PubMed] [Google Scholar]
- Wiese D., Stroup A.M., Maiti A., Harris G., Lynch S.M., Vucetic S., et al. Socioeconomic disparities in colon cancer survival: Revisiting neighborhood poverty using residential histories. Epidemiology. 2020;31:728–735. doi: 10.1097/EDE.0000000000001216. [DOI] [PubMed] [Google Scholar]
- Zhang D., Matthews C.E., Powell-Wiley T.M., Xiao Q. Ten-year change in neighborhood socioeconomic status and colorectal cancer. Cancer. 2019;125:610–617. doi: 10.1002/cncr.31832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.